EP3411121B1 - Glucose conjugates of triptolide, analogs and uses thereof - Google Patents
Glucose conjugates of triptolide, analogs and uses thereof Download PDFInfo
- Publication number
- EP3411121B1 EP3411121B1 EP17748283.3A EP17748283A EP3411121B1 EP 3411121 B1 EP3411121 B1 EP 3411121B1 EP 17748283 A EP17748283 A EP 17748283A EP 3411121 B1 EP3411121 B1 EP 3411121B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- cancer
- substituted
- unsubstituted
- syndrome
- disease
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/365—Lactones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/54—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic compound
- A61K47/549—Sugars, nucleosides, nucleotides or nucleic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D493/00—Heterocyclic compounds containing oxygen atoms as the only ring hetero atoms in the condensed system
- C07D493/22—Heterocyclic compounds containing oxygen atoms as the only ring hetero atoms in the condensed system in which the condensed system contains four or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07H—SUGARS; DERIVATIVES THEREOF; NUCLEOSIDES; NUCLEOTIDES; NUCLEIC ACIDS
- C07H15/00—Compounds containing hydrocarbon or substituted hydrocarbon radicals directly attached to hetero atoms of saccharide radicals
- C07H15/26—Acyclic or carbocyclic radicals, substituted by hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07J—STEROIDS
- C07J73/00—Steroids in which the cyclopenta[a]hydrophenanthrene skeleton has been modified by substitution of one or two carbon atoms by hetero atoms
- C07J73/001—Steroids in which the cyclopenta[a]hydrophenanthrene skeleton has been modified by substitution of one or two carbon atoms by hetero atoms by one hetero atom
- C07J73/003—Steroids in which the cyclopenta[a]hydrophenanthrene skeleton has been modified by substitution of one or two carbon atoms by hetero atoms by one hetero atom by oxygen as hetero atom
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Definitions
- the invention relates generally to anti-cancer compounds and more specifically to glucose conjugates of triptolide and analogs thereof, and methods of treating cancer using such compounds.
- Triptolide is an active component from the traditional Chinese medicinal plant Thunder God Vine. It has been shown to possess anti-inflammatory, immunosuppressive and anticancer activities, among others. Its molecular target has been identified as the XPB (ERCC3) subunit of the general transcription factor TFIIH. It works by blocking RNAPII transcription initiation and nucleoside excision repair. Triptolide and analogs have been developed as new anticancer and immunosuppressive drugs. Unfortunately, dose-limiting toxicity and insolubility have been major hurdles for its development as a new drug.
- the present invention is based on the seminal discovery that conjugation of triptolide with glucose to form glucose-triptolide conjugates provides compounds with effective anti-proliferative activity and improved tolerability as compared to naturally occurring triptolide compounds.
- the invention provides an anti-proliferative effective amount of a glucose-triptolide conjugate compound for use in the treatment of cancer.
- glucose-triptolide conjugate compounds have the structure of Formula I: T&A-L 1 -Sugar (I)
- the cancer may be one of the following, but not limited to bladder cancer, breast cancer, ovarian cancer, pancreatic cancer, and gastric cancer, cervical cancer, colon cancer, endometrial cancer, head and neck cancer, lung cancer, melanoma, multiple myeloma, leukemia, non-hodgkin's lymphoma, prostate cancer, rectal cancer, malignant melanomas, alimentary/gastrointestinal tract cancer, liver cancer, skin cancer, lymphoma, kidney cancer, muscle cancer, bone cancer, brain cancer, eye or ocular cancer, rectal cancer, colon cancer, cervical cancer, bladder cancer, oral cancer, benign and malignant tumors, stomach cancer, corpus uteri, testicular cancer, renal cancer, throat cancer, acute lymphocytic leukemia, acute myelogenous leukemia, Ewing's Sarcoma, Kaposi's Sarcoma, basal cell carcinoma and squamous cell carcinoma, small cell lung cancer, choriocarcinoma, rhabdomyosarcoma,
- the cancer is prostate cancer.
- the cancer is metastatic cancer.
- T&A moiety is compound 1 and the sugar is compound 19 , 20 , 37 or 38 .
- L 1 is a direct bond, -COCH 2 CH 2 CO-, or -CH 2 -.
- T&A moiety is compound 1 and the sugar is compound 19 .
- glucose triptolide conjugate compound has a Formula G1-9.
- the compound is Formula G4.
- Formula G1-9 are illustrated in the structures provided herein.
- the invention also provides a pharmaceutical composition comprising the compounds listed above.
- the method of treatment includes administration intravenously, such as at a dosage of about 0.1 mg/kg to 2 mg/kg per dosage.
- the compound is administered once daily for up to about 4 weeks.
- the method may further include administering a chemotherapeutic compound, for example, prior to, simultaneously with, or following administration of a compound of the invention.
- the method of synthesizing a glucose-triptolide conjugate compound includes synthetic scheme II or III. These schemes are provided herein.
- an anti-proliferative effective amount of a glucose-triptolide conjugate compound is administered to a subject in a method to treat possible organ rejection in subjects that have undergone an organ transplant.
- an anti-proliferative effective amount of a glucose-triptolide conjugate compound is administered to a subject in a method to treat autoimmune diseases.
- immune related diseases include but are not limited to: Acute disseminated encephalomyelitis (ADEM), Addison's disease, Ankylosing spondylitis, Antiphospholipid antibody syndrome, Autoimmune hemolytic anemia, Autoimmune hepatitis, Autoimmune inner ear disease, Autoimmune Lymphoproliferative Syndrome (ALPS), Autoimmune polyendocrine/polyglandular syndrome, Autoimmune thrombocytoipenia purpura, Balo disease, Behcet disease, Bullous pemphigoid, Cardiomyopathy, Celiac sprue-dermatitis herpetiformis, Chronic fatigue immune dysfunction syndrome (CFIDS), Chronic inflammatory demyelinating neuropathy, Cicatrical pemphigoid, Coeliac disease, Cold agglutinin disease, CREST syndrome, Crohn's disease, Cystic fibrosis, Degos disease, Dermatomyositis, Diabetes (Type I or Ju
- a library of glucose conjugates of triptolide and analogs thereof is used to screen for compounds for treating cancer.
- a library of glucose conjugates of triptolide and analogs thereof is used to screen for compounds for treating possible organ rejection.
- a library of glucose conjugates of triptolide and analogs thereof is used to screen for compounds for treating autoimmune disease.
- the present invention is based on trying to solve the issues of solubility and toxicity associated with triptolide. It was hypothesized that if triptolide could be conjugated to glucose, the two aforementioned problems associated with triptolide could be addressed.
- the glucose-triptolide conjugates would be preferentially taken up by cancer cells and they should also exhibit much higher water solubility due to the water solubility of glucose moiety.
- cancer or "cancerous growth” means the uncontrolled, abnormal growth of cells and includes within its scope all the well-known diseases that are caused by the uncontrolled and abnormal growth of cells.
- Non-limiting examples of common cancers include bladder cancer, breast cancer, ovarian cancer, pancreatic cancer, and gastric cancer, cervical cancer, colon cancer, endometrial cancer, head and neck cancer, lung cancer, melanoma, multiple myeloma, leukemia (e.g. myeloid, lymphocytic, myelocytic and lymphoblastic leukemias), non-hodgkin's lymphoma, prostate cancer, rectal cancer, and malignant melanomas.
- leukemia e.g. myeloid, lymphocytic, myelocytic and lymphoblastic leukemias
- non-hodgkin's lymphoma prostate cancer, rectal cancer, and malignant melanomas.
- chemotherapeutic agents can be used prior to, simultaneously with or following treatment with invention compounds.
- Illustrative agents include but are not limited to, taxol, cytochalasin B, gramicidin D, ethidium bromide, emetine, mitomycin, etoposide, tenoposide, vincristine, vinblastine, colchicin, doxorubicin, daunorubicin, dihydroxy anthracin dione, mitoxantrone, mithramycin, actinomycin D, 1-dehydrotestosterone, glucocorticoids, procaine, tetracaine, lidocaine, propranolol, and puromycin and analogs or homologs thereof.
- Therapeutic antibodies or other proteins are also envisioned in combination therapies of the invention.
- This example illustrates the synthesis of glucose-conjugated triptolide compounds.
- Five glucose conjugates with different types of linkers connecting glucose to triptolide were synthesized, designated G1--G5 (schemes I-III).
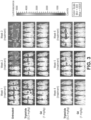
- the Octanol-water partition coefficient, LogP were significantly improved over triptolide itself ( Fig. 1 ).
- the activity of the different glucose-triptolide conjugates was assessed in vitro ( Fig. 2 ). It was found that they differ in their anti-proliferative activity as measured by tritiated thymidine incorporation with G4 and G3 showing moderate activity, while G1, G2 and G5 exhibiting significantly reduced activity. The ability of the glucose conjugates to inhibit the ATPase activity of XPB in the TFIIH complex was also addressed. Interestingly, none of the five glucose conjugates showed appreciable inhibitory activity, suggesting that the cellular activity was a result of breakdown of the conjugates either in cell culture or upon entry into cells.
- the in vivo antitumor activity of the glucose-triptolide conjugate G4 was determined using an established metastatic prostate cancer model (Reference 1).
- the luciferase---expressing prostate cancer line PC3/ML was injected into NOD/SCID/IL2r ⁇ null (NSG) mice through the tail vein.
- NSG NOD/SCID/IL2r ⁇ null mice
- the metastasis of the injected prostate cancer cells into liver, kidney, lung and bone can be monitored in live animals by bioluminescent imaging (BLI). It was shown that this is a reliable model with reproducible liver metastasis and all animals succumb by Week 7 after injection of cells (or Week 4 after initiation of treatments).
- mice treated with 11 had lower tumor burden during Weeks 1 and 2 compared with those treated with triptolide.
- both treatment groups showed undetectable tumor cells while all animals in the untreated groups died.
- tumors immediately returned in animals treated with triptolide. But no tumor cells were detectable in those treated with G4 till the end of the experiment, revealing sustained anticancer activity of G4 in vivo.
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Biotechnology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Genetics & Genomics (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Saccharide Compounds (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662291416P | 2016-02-04 | 2016-02-04 | |
| PCT/US2017/016527 WO2017136739A1 (en) | 2016-02-04 | 2017-02-03 | Glucose conjugates of triptolide, analogs and uses thereof |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP3411121A1 EP3411121A1 (en) | 2018-12-12 |
| EP3411121A4 EP3411121A4 (en) | 2019-12-18 |
| EP3411121B1 true EP3411121B1 (en) | 2024-09-11 |
Family
ID=59500284
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP17748283.3A Active EP3411121B1 (en) | 2016-02-04 | 2017-02-03 | Glucose conjugates of triptolide, analogs and uses thereof |
Country Status (8)
| Country | Link |
|---|---|
| US (2) | US10695319B2 (enExample) |
| EP (1) | EP3411121B1 (enExample) |
| JP (1) | JP7042744B2 (enExample) |
| CN (1) | CN108601952B (enExample) |
| AU (1) | AU2017214572A1 (enExample) |
| CA (1) | CA3013619A1 (enExample) |
| MX (1) | MX2018009406A (enExample) |
| WO (1) | WO2017136739A1 (enExample) |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN109942665B (zh) * | 2018-04-02 | 2021-04-06 | 欣凯医药化工中间体(上海)有限公司 | 雷公藤内酯醇衍生物及其制备方法和应用 |
| CN109897053B (zh) * | 2019-03-28 | 2020-05-29 | 中国医学科学院医药生物技术研究所 | 雷斯吲哚甲及其制备方法和抗丙型肝炎病毒用途 |
| CN111040018A (zh) * | 2019-12-30 | 2020-04-21 | 广东省中医院(广州中医药大学第二附属医院、广州中医药大学第二临床医学院、广东省中医药科学院) | 丙烯酸雷公藤甲素酯、其制备方法及其应用 |
| EP4086271B1 (en) | 2019-12-30 | 2023-12-13 | Guangdong Provincial Hospital of TCM | Triptolide acrylate, preparation method therefor and use thereof |
| EP4114468A4 (en) * | 2020-03-02 | 2025-04-09 | The Johns Hopkins University | GLUCOSETRIPTOLIDE CONJUGATES AND USES THEREOF |
| JP2023538424A (ja) * | 2020-08-21 | 2023-09-07 | ルヤン コーポレイション | トリプトリドコンジュゲートおよびその使用 |
| CN115286684A (zh) * | 2022-05-20 | 2022-11-04 | 福建医科大学 | 一种糖与雷公藤内酯醇或其衍生物的缀合物及其制备方法与应用 |
| ES3035530T3 (en) | 2023-03-09 | 2025-09-04 | Minneamrita Therapeutics Llc | Drug combination for the treatment of stomach cancer |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6218367B1 (en) * | 1998-09-15 | 2001-04-17 | Organomed Corporation | Paclitaxel-carbohydrate conjugates: design, synthesis and biological evaluations |
| US6620843B2 (en) | 2001-01-19 | 2003-09-16 | Pharmagenesis | Anticancer treatment using triptolide prodrugs |
| CA2448795A1 (en) | 2001-03-15 | 2002-09-26 | Pharmagenesis, Inc. | Amino acid derivatives of triptolide compounds as immune modulators and anticancer agent |
| US9150600B2 (en) * | 2009-05-07 | 2015-10-06 | Regents Of The University Of Minnesota | Triptolide prodrugs |
-
2017
- 2017-02-03 CN CN201780010159.3A patent/CN108601952B/zh active Active
- 2017-02-03 EP EP17748283.3A patent/EP3411121B1/en active Active
- 2017-02-03 JP JP2018540128A patent/JP7042744B2/ja active Active
- 2017-02-03 AU AU2017214572A patent/AU2017214572A1/en not_active Abandoned
- 2017-02-03 MX MX2018009406A patent/MX2018009406A/es unknown
- 2017-02-03 US US16/074,750 patent/US10695319B2/en active Active
- 2017-02-03 WO PCT/US2017/016527 patent/WO2017136739A1/en not_active Ceased
- 2017-02-03 CA CA3013619A patent/CA3013619A1/en not_active Abandoned
-
2020
- 2020-06-11 US US16/899,226 patent/US20200297693A1/en not_active Abandoned
Non-Patent Citations (4)
| Title |
|---|
| DATABASE MEDLINE [online] US NATIONAL LIBRARY OF MEDICINE (NLM), BETHESDA, MD, US; 2013, HAMADA HIROKI ET AL: "Chemo-enzymatic Synthesis of Propionyl-ester-linked Taxol-monosaccharide Conjugate and its Drug Delivery System Using Hybrid-Bio-nanocapsules Targeting Brain Glioma Cells.", Database accession no. NLM24665217 * |
| DATABASE MEDLINE [online] US NATIONAL LIBRARY OF MEDICINE (NLM), BETHESDA, MD, US; 25 March 2013 (2013-03-25), LIU PENGXING ET AL: "Highly water-soluble platinum(II) complexes as GLUT substrates for targeted therapy: improved anticancer efficacy and transporter-mediated cytotoxic properties.", Database accession no. NLM23420130 * |
| HAMADA HIROKI ET AL: "Chemo-enzymatic Synthesis of Propionyl-ester-linked Taxol-monosaccharide Conjugate and its Drug Delivery System Using Hybrid-Bio-nanocapsules Targeting Brain Glioma Cells.", CLINICAL MEDICINE INSIGHTS. WOMEN'S HEALTH 2013, vol. 6, 2013, pages 71 - 75, XP002793229, ISSN: 1179-562X, DOI: 10.4137/CMWH.S8213 * |
| LIU PENGXING ET AL: "Highly water-soluble platinum(II) complexes as GLUT substrates for targeted therapy: improved anticancer efficacy and transporter-mediated cytotoxic properties.", CHEMICAL COMMUNICATIONS (CAMBRIDGE, ENGLAND) 25 MAR 2013, vol. 49, no. 24, 25 March 2013 (2013-03-25), pages 2421 - 2423, XP002793228, ISSN: 1364-548X, DOI: 10.1039/c3cc38589b * |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2019509986A (ja) | 2019-04-11 |
| US20200297693A1 (en) | 2020-09-24 |
| EP3411121A1 (en) | 2018-12-12 |
| CA3013619A1 (en) | 2017-08-10 |
| MX2018009406A (es) | 2018-11-09 |
| CN108601952A (zh) | 2018-09-28 |
| CN108601952B (zh) | 2022-03-04 |
| EP3411121A4 (en) | 2019-12-18 |
| US10695319B2 (en) | 2020-06-30 |
| AU2017214572A1 (en) | 2018-08-16 |
| JP7042744B2 (ja) | 2022-03-28 |
| WO2017136739A1 (en) | 2017-08-10 |
| US20190038596A1 (en) | 2019-02-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3411121B1 (en) | Glucose conjugates of triptolide, analogs and uses thereof | |
| Nakada et al. | Novel antibody drug conjugates containing exatecan derivative-based cytotoxic payloads | |
| JP2019504100A5 (enExample) | ||
| ES2717898T3 (es) | Efecto potenciador para agentes antitumorales | |
| JP2019529444A5 (enExample) | ||
| CN111836814A (zh) | 稠环化合物、其制备方法及用途 | |
| KR101993963B1 (ko) | 항 종양제 및 항 종양 효과 증강제 | |
| KR101848131B1 (ko) | 저용량 이리노테칸염산염 수화물을 함유하는 항종양제 | |
| JP2019509986A5 (enExample) | ||
| JP2019524713A5 (enExample) | ||
| WO2016024595A1 (ja) | エピルビシン複合化ブロック共重合体と、抗癌剤とを含むミセル、及び当該ミセルを含む癌又は耐性癌、転移癌の治療に適用可能な医薬組成物 | |
| TWI615145B (zh) | 含伊立替康鹽酸鹽水合物之抗腫瘤劑 | |
| WO2007023778A1 (ja) | 新規抗癌併用薬 | |
| CN108498518B (zh) | 七元环小檗碱类似物及其药物组合物在制备治疗多发性骨髓瘤的药物中的应用 | |
| ES2910659T3 (es) | Nuevo derivado de PEG | |
| JP6488280B2 (ja) | タキサン系化合物を含有する抗腫瘍剤及び抗腫瘍効果増強剤 | |
| CN111773388A (zh) | A-失碳-5α雄甾烷化合物类药物与抗癌药物的联合应用 | |
| CN117138054A (zh) | 雷公藤甲素的多肽缀合物及其用途 | |
| JPWO2020011724A5 (enExample) | ||
| BR112023022531B1 (pt) | CONJUGADO DE FÁRMACO E ANTICORPO ANTI-c-Met, COMPOSIÇÃO FARMACÊUTICA COMPREENDENDO OS MESMOS E SEU USO NO TRATAMENTO DE CÂNCER PULMONAR DE CÉLULAS NÃO PEQUENAS | |
| JP2015199678A (ja) | ラパチニブトシル酸塩水和物を含有する抗腫瘍剤及び抗腫瘍効果増強剤 | |
| HK1240958A1 (en) | Peg derivative | |
| CN102370986A (zh) | 含羟甲基酞胺哌啶酮衍生物的药物组合物及其制备和应用 | |
| MY144013A (en) | N-phenyl-2-pyrimidine-amine derivatives and process for the preparation thereof | |
| TW201336493A (zh) | 卡巴利他索(cabazitaxel)之新穎小兒藥用途 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE INTERNATIONAL PUBLICATION HAS BEEN MADE |
|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20180815 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: POMPER, MARTIN, G. Inventor name: HE, QINGLI Inventor name: LIU, JUN Inventor name: YU, BIAO Inventor name: MINN, II Inventor name: WANG, QIAOLING |
|
| DAV | Request for validation of the european patent (deleted) | ||
| DAX | Request for extension of the european patent (deleted) | ||
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: A61P 35/00 20060101AFI20190808BHEP Ipc: A61K 31/365 20060101ALI20190808BHEP Ipc: A61P 37/06 20060101ALI20190808BHEP Ipc: C07D 493/22 20060101ALI20190808BHEP |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: DE Ref document number: 1259645 Country of ref document: HK |
|
| A4 | Supplementary search report drawn up and despatched |
Effective date: 20191120 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: A61P 35/00 20060101AFI20191114BHEP Ipc: A61P 37/06 20060101ALI20191114BHEP Ipc: A61K 31/365 20060101ALI20191114BHEP Ipc: C07D 493/22 20060101ALI20191114BHEP |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: HE, QINGLI Inventor name: LIU, JUN Inventor name: YU, BIAO Inventor name: MINN, II Inventor name: POMPER, MARTIN, G. Inventor name: WANG, QIAOLING |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| 17Q | First examination report despatched |
Effective date: 20201112 |
|
| P01 | Opt-out of the competence of the unified patent court (upc) registered |
Effective date: 20230515 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R079 Ipc: A61K0031365000 Ref document number: 602017084794 Country of ref document: DE Free format text: PREVIOUS MAIN CLASS: A61P0035000000 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: C07D 493/22 20060101ALI20231109BHEP Ipc: A61P 37/06 20060101ALI20231109BHEP Ipc: A61P 35/00 20060101ALI20231109BHEP Ipc: A61K 31/365 20060101AFI20231109BHEP |
|
| INTG | Intention to grant announced |
Effective date: 20231121 |
|
| GRAJ | Information related to disapproval of communication of intention to grant by the applicant or resumption of examination proceedings by the epo deleted |
Free format text: ORIGINAL CODE: EPIDOSDIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| INTC | Intention to grant announced (deleted) | ||
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20240429 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602017084794 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG9D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241211 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MP Effective date: 20240911 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241212 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240911 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240911 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240911 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240911 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240911 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241211 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241211 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241211 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240911 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240911 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241212 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240911 Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240911 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240911 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 1722086 Country of ref document: AT Kind code of ref document: T Effective date: 20240911 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240911 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20250111 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20250113 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240911 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240911 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240911 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240911 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240911 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240911 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240911 Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240911 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20250227 Year of fee payment: 9 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602017084794 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240911 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20250612 |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: WD Ref document number: 1259645 Country of ref document: HK |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 602017084794 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240911 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240911 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20250203 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20250228 |