EP2741761B1 - Modified osteopontin peptides having an inactivated rgd domain - Google Patents
Modified osteopontin peptides having an inactivated rgd domain Download PDFInfo
- Publication number
- EP2741761B1 EP2741761B1 EP12768879.4A EP12768879A EP2741761B1 EP 2741761 B1 EP2741761 B1 EP 2741761B1 EP 12768879 A EP12768879 A EP 12768879A EP 2741761 B1 EP2741761 B1 EP 2741761B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- hair
- polypeptide
- area
- osteopontin
- hair growth
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/1703—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- A61K38/1709—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/19—Cytokines; Lymphokines; Interferons
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/64—Proteins; Peptides; Derivatives or degradation products thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0014—Skin, i.e. galenical aspects of topical compositions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/14—Macromolecular materials
- A61L27/22—Polypeptides or derivatives thereof, e.g. degradation products
- A61L27/227—Other specific proteins or polypeptides not covered by A61L27/222, A61L27/225 or A61L27/24
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L27/54—Biologically active materials, e.g. therapeutic substances
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L27/58—Materials at least partially resorbable by the body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L27/60—Materials for use in artificial skin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/14—Drugs for dermatological disorders for baldness or alopecia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/24—Drugs for disorders of the endocrine system of the sex hormones
- A61P5/26—Androgens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q7/00—Preparations for affecting hair growth
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/74—Biological properties of particular ingredients
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/80—Process related aspects concerning the preparation of the cosmetic composition or the storage or application thereof
- A61K2800/91—Injection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/412—Tissue-regenerating or healing or proliferative agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2430/00—Materials or treatment for tissue regeneration
- A61L2430/18—Materials or treatment for tissue regeneration for hair reconstruction
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2430/00—Materials or treatment for tissue regeneration
- A61L2430/34—Materials or treatment for tissue regeneration for soft tissue reconstruction
Definitions
- the present invention relates to agents for stimulating hair growth in mammals.
- compositions comprising a modified osteopontin polypeptide for stimulating hair growth in mammals (including humans).
- Hair growth is cyclical, occurring in three stages: anagen, the active growth phase; catagen, the degenerative phase; and telogen, the resting phase. After telogen, the old hair fibre is shed and a new hair is generated as part of the repeating cycle.
- Alopecia areata Various types of hair loss are known, including alopecia areata, androgenetic alopecia, anagen effluvium, self-induced hair loss, telogen effluvium, and scarring alopecia.
- Alopecia areata thought to be an auto-immune disorder, begins with hair loss in a rounded patch on the scalp.
- Alopecia areata includes mild patchy hair loss on the scalp, as well the loss of all scalp hair, known as alopecia totalis, and the loss of all scalp and body hair, known as alopecia universalis.
- Androgenetic alopecia including male and female pattern baldness, is thought to be caused by a combination of genetic predisposition, aging, and androgen hormone levels.
- Androgenetic alopecia is associated with increased androgen stimulation, which adversely affects the hair follicles.
- Increased androgen stimulation can be produced by, among other mechanisms, elevated levels of 5-alpha-reductase, an enzyme that converts testosterone to dihydrotestosterone.
- Anagen effluvium is hair loss due to chemicals or radiation, such as chemotherapy or radiation treatment for cancer.
- Self-induced hair loss includes hair loss caused by conscious or unconscious self-inflicted damage to the hair. Two common types of self-induced hair loss are trichotillomania, or hair loss that results when someone continually pulls or plucks out his own hair, and traction alopecia, which is caused by hairstyles such as ponytails or braids that continually pull at the hair.
- compositions have been tested for their ability to stimulate hair growth, for example, by promoting or prolonging anagen.
- potassium channel openers such as minoxidil (Regaine RTM., Pharmacia Corp.) and diazoxide
- 5-alpha-reductase inhibitors such as finasteride (Propecia RTM., Merck & Co.)
- the immunosuppressant cyclosporin A include potassium channel openers, such as minoxidil (Regaine RTM., Pharmacia Corp.) and diazoxide; 5-alpha-reductase inhibitors, such as finasteride (Propecia RTM., Merck & Co.); and the immunosuppressant cyclosporin A.
- the first aspect of the invention provides a composition for stimulating hair growth in a mammal comprising:
- the active component of the compositions of the invention is derived from a naturally-occurring osteopontin protein (i . e .
- the polypeptide comprises an amino acid sequence corresponding to that of a modified, for example mutated, version of a naturally-occurring osteopontin protein).
- Osteopontin also known as bone sialoprotein I (BSP-1 or BNSP), early T-lymphocyte activation (ETA-1), secreted phosphoprotein 1 (SPP1), 2ar and Rickettsia resistance (Ric), is a gene product which is conserved in several mammalian species.
- the gene has seven exons, spans 5 kilobases in length and in humans it is located on the long arm of chromosome 4 region 13 (4q13).
- the protein is composed of ⁇ 300 amino acids residues and is rich in acidic residues: 30-36% are either aspartic or glutamic acid.
- Osteopontin has ⁇ 30 attached carbohydrate residues, including 10 sialic acid residues, which are attached to the protein during post-translational modification in the Golgi apparatus.
- Osteopontin was first discovered as a novel sialoprotein in bone anchoring osteoclasts onto the mineralized bone matrix ( Franzen & Heinegard, 1985, Biochem. J. 232(3)715-24 ).
- the name osteopontin comes from the presence of the protein in bone ( osteo -) and its ability to form a bridge (- pons ) between bone cells and the mineral phase.
- Sequence analysis and subsequent structural studies showed osteopontin to be a 32 kDa glycoprotein composed of a highly acidic region of some ten aspartic acid residues thought to mediate the mineral binding properties of osteopontin.
- Osteopontin is constitutively expressed in osteoblasts and in several epithelial cell types, resulting in osteopontin being secreted into many body fluids. Bone is the only tissue type where osteopontin is deposited and from where it can be recovered in large amounts. The expression of osteopontin can also be induced In vascular smooth muscle cells, in different cancer cell types and among inflammatory cells (specifically macrophages and T lymphocytes). Several important cellular functions have been attributed to osteopontin such as adhesion, proliferation, migration, anti-apoptosis and chemo attraction.
- RGD cell-adhesion domain which interacts with different integrins, mainly with ⁇ v ⁇ 3 but also ⁇ v ⁇ 1, and ⁇ v ⁇ 5 (for review see Scatena et al., 2007, Arterio. Thromb. Vasc. Biol. 27:2302-2309 ).
- osteopontin has emerged as a potent cytokine capable of modulating several cell types involved in inflammation and immune responses.
- the broad range of functions being attributed to Osteopontin has been puzzling and cannot all be explained by the single cell-binding RGD sequence.
- the explanation came when an eleven amino acid peptide in osteopontin R 145 -G-D-S-L-A-Y-G-L-R-S 155 (SEQ ID NO:122) (corresponding to amino acids 144 to 154 of UniProt code P10923) was identified and later functionally mapped.
- a characterising feature of the osteopontin-derived polypeptide in the compositions of the invention is that the RGD domain naturally present in osteopontin is inactivated such that it is non-functional (at least in part).
- inactivation of the RGD domain may prevent the osteopontin-derived polypeptide from binding to one or more of the integrins which bind the naturally occurring osteopontin protein.
- modified osteopontin polypeptide we include polypeptides corresponding to a modified form of a naturally-occurring osteopontin protein in which the RGD domain is non-functional (at least in part), as well as fragments and variants thereof which retain a hair-stimulatory property of the 'full length' modified osteopontin.
- the non-functional RGD domain of the modified osteopontin polypeptide may be unable to bind integrin ⁇ v ⁇ 3 (see Scatena et al., supra ) .
- the naturally-occurring osteopontin protein is mammalian, e.g. human.
- the modified osteopontin polypeptides present in the compositions of the invention are capable of stimulating hair growth in mammals.
- the polypeptide is capable of stimulating the growth of human hair.
- the polypeptide is capable of stimulating the growth of hair in vivo.
- the polypeptide is capable of stimulating the growth of hair in vivo with greater efficacy than wildtype human osteopontin.
- greater efficacy in this context, we include a quicker onset of action and/or efficacy at a lower dose and/or greater maximum effect ( e . g . greater number of new follicles or density of hair).
- the polypeptide is capable of stimulating the growth of hair in vivo with a quicker onset of action and a greater maximum hair growth effect than wildtype human osteopontin at the same dose ( e.g . see Example 8).
- the stimulation of hair growth may be mediated by an effect of existing hair follicles and/or by inducing the formation of new hair follicles.
- the modified osteopontin polypeptide is capable of stimulating existing hair follicles (for example, by prolonging the anagen phase and/or by shortening the telogen phase such that the resting follicles become active).
- the polypeptide is capable of inducing the formation of new hair follicles, or stem cells for producing the same.
- modified osteopontin polypeptides lack the active tri-peptide sequence "arginine-glycine-aspartic acid" normally found in naturally-occurring osteopontin proteins. It will be appreciated that this RGD domain may be inactivated by a number of different strategies.
- Modified osteopontin polypeptides suitable for use in the compositions of the invention may be made by in vitro cell-based expression methods well known to persons skilled in the art (for example, see Sambrook & Russell, 2000, Molecular Cloning, A Laboratory Manual, Third Edition, Cold Spring Harbor, New York , which is incorporated herein by reference).
- the choice of expression vector and host cell to be used may depend on a number of factors. For example, if the modified osteopontin polypeptide is to be glycosylated, a mammalian expression system will be required.
- Suitable expression vectors and host cells are commercially available from many sources.
- the present invention also includes pharmaceutically and/or cosmetically acceptable acid or base addition salts of the above described modified osteopontin polypeptide.
- the acids which are used to prepare the pharmaceutically and/or cosmetically acceptable acid addition salts of the aforementioned base compounds useful in this invention are those which form non-toxic acid addition salts, i.e .
- Pharmaceutically and/or cosmetically acceptable base addition salts may also be used to produce pharmaceutically and/or cosmetically acceptable salt forms of the modified osteopontin polypeptides.
- the chemical bases that may be used as reagents to prepare pharmaceutical and/or cosmetically acceptable base salts of the present compounds that are acidic in nature are those that form non-toxic base salts with such compounds.
- Such non-toxic base salts include, but are not limited to those derived from such pharmaceutical and/or cosmetically acceptable cations such as alkali metal cations (e.g. potassium and sodium) and alkaline earth metal cations (e.g. calcium and magnesium), ammonium or water-soluble amine addition salts such as N-methylglucamine-(meglumine), and the lower alkanolammonium and other base salts of pharmaceutically acceptable organic amines, among others.
- modified osteopontin polypeptide of the invention may be lyophilised for storage and reconstituted in a suitable carrier prior to use. Any suitable lyophilisation method (e.g . spray drying, cake drying) and/or reconstitution techniques can be employed. It will be appreciated by those skilled in the art that lyophilisation and reconstitution can lead to varying degrees of activity loss and that use levels may have to be adjusted upward to compensate.
- lyophilisation and reconstitution can lead to varying degrees of activity loss and that use levels may have to be adjusted upward to compensate.
- the lyophilised (freeze dried) polypeptide loses no more than about 20%, or no more than about 25%, or no more than about 30%, or no more than about 35%, or no more than about 40%, or no more than about 45%, or no more than about 50% of its activity (prior to lyophilisation) when rehydrated.
- the modified osteopontin polypeptide is provided in the form of a composition comprising the polypeptide and a pharmaceutically acceptable and/or cosmetically acceptable excipient, carrier or diluent, selected with regard to the intended route of administration and standard pharmaceutical or cosmetic practice (for example, see Remington: The Science and Practice of Pharmacy, 19th edition, 1995, Ed. Alfonso Gennaro, Mack Publishing Company, Pennsylvania, USA , which is incorporated herein by reference).
- pharmaceutically acceptable is included that the formulation is sterile and pyrogen free.
- Suitable pharmaceutical carriers are well known in the art of pharmacy.
- the carrier(s) must be “acceptable” in the sense of being compatible with the compound of the invention and not deleterious to the recipients thereof.
- the carriers will be water or saline which will be sterile and pyrogen free; however, other acceptable carriers may be used.
- pharmaceutically acceptable carrier and pharmaceutically acceptable excipient includes any compound(s) used in forming a part of the formulation that is intended to act merely as a carrier, i.e., not intended to have biological activity itself.
- the pharmaceutically acceptable carrier or excipient is generally safe, non-toxic, and neither biologically nor otherwise undesirable.
- a pharmaceutically acceptable carrier or excipient as used herein includes both one and more than one such carrier or excipient.
- cosmetically acceptable is used to denote formulations suitable for use as cosmetic products.
- Suitable cosmetic carriers are well known in the art, such as those commonly used in shampoos, lotions, creams and other such products.
- the excipient may be one or more of carbohydrates, polymers, lipids and minerals.
- carbohydrates include lactose, sucrose, mannitol, and cyclodextrines, which are added to the composition, e.g.,, for facilitating lyophilisation.
- lipids are fatty acids, phospholipids, mono-, di-, and triglycerides, ceramides, sphingolipids and glycolipids, all of different acyl chain lenght and saturation, egg lecithin, soy lecithin, hydrogenated egg and soy lecithin, which are added to the composition for reasons similar to those for polymers.
- minerals are talc, magnesium oxide, zinc oxide and titanium oxide, which are added to the composition to obtain benefits such as reduction of liquid accumulation or advantageous pigment properties.
- the composition may comprise an adjuvant.
- adjuvant is intended to mean any compound added to the formulation to increase the biological effect of the peptide.
- the adjuvant may be one or more of zinc, copper or silver salts with different anions, for example, but not limited to fluoride, chloride, bromide, iodide, tiocyanate, sulfde, hydroxide, phosphate, carbonate, lactate, glycolate, citrate, borate, tartrate, and acetates of different acyl composition.
- compositions of the invention may also be in the form of biodegradable microspheres.
- Aliphatic polyesters such as poly(lactic acid) (PLA), poly(glycolic acid) (PGA), copolymers of PLA and PGA (PLGA) or poly(carprolactone) (PCL), and polyanhydrides have been widely used as biodegradable polymers in the production of microspheres. Preparations of such microspheres can be found in US 5,851,451 and in EP0213303 .
- compositions of the invention may also be in the form of polymer gels, where polymers such as starch, cellulose ethers, cellulose carboxymethylcellulose, hydroxypropylmethyl cellulose, hydroxyethyl cellulose, ethylhydroxyethyl cellulose, alginates, carageenans, hyaluronic acid and derivatives thereof, polyacrylic acid, polysulphonate,
- polymers such as starch, cellulose ethers, cellulose carboxymethylcellulose, hydroxypropylmethyl cellulose, hydroxyethyl cellulose, ethylhydroxyethyl cellulose, alginates, carageenans, hyaluronic acid and derivatives thereof, polyacrylic acid, polysulphonate,
- compositions of the invention may be administered by a variety of routes, for example topical, transdermal, parenteral or oral administration.
- compositions of the invention are suitable for topical administration or intracutaneous administration.
- compositions of the invention may be administered topically to the skin (e.g. scalp).
- the composition may be provided in the form of an ointment containing the active polypeptide suspended or dissolved in, for example, a mixture with one or more of the following: mineral oil, liquid petrolatum, white petrolatum, propylene glycol, polyoxyethylene polyoxypropylene compound, emulsifying wax and water.
- the polypeptide can be formulated as a suitable lotion or cream, suspended or dissolved in, for example, a mixture of one or more of the following: mineral oil, sorbitan monostearate, a polyethylene glycol, liquid paraffin, polysorbate 60, cetyl esters wax, cetearyl alcohol, 2-octyldodecanol, benzyl alcohol and water.
- compositions of the invention may be administered parenterally, for example intracutaneously.
- Such compositions are best used in the form of a sterile aqueous solution which may contain other substances, for example, enough salts or glucose to make the solution isotonic with blood.
- the aqueous solutions should be suitably buffered (preferably to a pH of from 3 to 9), if necessary.
- suitable parenteral formulations under sterile conditions is readily accomplished by standard pharmaceutical techniques well known to those skilled in the art.
- sustained-release system such as microsphere formulations, for delivering the modified osteopontin polypeptides.
- compositions can be administered by a surgically implanted device that releases the active polypeptide directly to the required site (i.e . the epidermis).
- compositions of the invention may also be delivered by transdermal methodologies.
- EPT electroporation therapy
- iontophoresis systems can be employed for the administration of proteins and polypeptides.
- a device is used to deliver a pulsed electric field to cells, resulting in the increased permeability of the cell membranes to the drug and significant enhancement of intracellular drug delivery.
- An alternative transdermal method utilises small particles of up to 30 microns in diameter on the surface of the skin experience electrical pulses identical or similar to those used in electroporation.
- the particles are driven through the stratum corneum and into deeper layers of the skin.
- the particles can be loaded or coated with drugs or genes or can simply act as "bullets" that generate pores in the skin through which the drugs can enter.
- Suitable methods for administration of the polypeptides and compositions of the invention are well known in the art, for example, see Therapeutic Protein and Peptide Formulation and Delivery, Zahra Shahrokh et a/. (Eds), 1997, American Chemical Society, ISBN13: 9780841235281 .
- a second aspect of the invention provides a polypeptide consisting of the amino acid sequence of SEQ ID NO: 63 63.
- the polypeptide is isolated ( e.g. outside the mammalian body).
- the polypeptide of the invention may be for medical use in the treatment or prevention of a disease or condition associated with hair loss (as described in detail below).
- a polypeptide of the invention for stimulating hair growth in a mammal, wherein the use is cosmetic or commercial (as described in detail below).
- compositions according to the first aspect of the invention for use in stimulating hair growth in a mammal.
- composition may be for use in the treatment or prevention of a disease or condition associated with hair loss, such as alopecia.
- compositions of the invention may also be used for treatment of conditions associated with the loss of telogen hairs.
- the alopecia is selected from the group consisting of:
- the alopecia may be androgenic alopecia.
- the alopecia may be anagen effluvium. This condition, resulting from the early entry of hairs into the telogen phase, may be due to a variety of causes, including eating disorders, fever, childbirth, chronic illness, major surgery, anemia, severe emotional disorders, crash diets, hypothyroidism, and drugs.
- the hair loss may be induced by radiotherapy and/or chemotherapy agents.
- hair loss is a common and distressing side effect of treatment with chemotherapeutic drugs such as cisplatin, etoposide and paclitaxel.
- the mammal is a human.
- a modified osteopontin polypeptide as defined above in relation to the first aspect of the invention in the preparation of a medicament for stimulating hair growth in a mammal.
- the medicament may be for stimulating existing hair follicles and/or inducing the growth of new hair follicles (or stem cells for producing the same).
- the method may be for stimulating existing hair follicles and/or inducing the growth of new hair follicles (or stem cells for producing the same).
- the polypeptide composition of the invention can be administered to the patient in an effective amount.
- a 'therapeutically effective amount', or 'effective amount', or 'therapeutically effective', as used herein, refers to that amount which provides a stimulatory effect on hair growth. This is a predetermined quantity of active material calculated to produce the desired therapeutic effect. As is appreciated by those skilled in the art, the amount of a compound may vary depending on its specific activity. Suitable dosage amounts may contain a predetermined quantity of active composition calculated to produce the desired therapeutic effect in association with the required diluent. In the methods and use for manufacture of compositions of the invention, a therapeutically effective amount of the active component is provided.
- a therapeutically effective amount can be determined by the ordinary skilled medical or veterinary worker based on patient characteristics, such as age, weight, sex, condition, complications, other diseases, etc ., as is well known in the art.
- composition of the first aspect of the invention are not limited to medical uses but may also be used as cosmetic agents (in the sense that they do not provide any physical health improvement, as such, but merely provide an aesthetic benefit to the mammal).
- composition according to the first aspect of the invention for stimulating hair growth in a mammal, wherein the use is cosmetic.
- the cosmetic composition may be for stimulating existing hair follicles and/or inducing the growth of new hair follicles (or stem cells for producing the same).
- the cosmetic composition may be used for the treatment or prevention of baldness, which may be associated with a receding hairline and/or thinning hair.
- compositions are not limited to use on the scalp, but may also be applied elsewhere on the body (including to the face to encourage the growth of a beard, eyelashes, eyebrows, etc. )
- the mammal is a human.
- compositions of the invention may be used in vivo, ex vivo or in vitro.
- compositions may be used to stimulate hair growth ex vivo, for example in a skin explant prior to grafting of the skin on to the mammal.
- compositions may be used to grow hair follicles in vitro, e.g. in cell culture, which may then be transplanted to a patient.
- polypeptide according to the first aspect of the invention for stimulating hair growth in vitro or ex vivo.
- Also described herein is a method for making a composition according to the first aspect of the invention, the method comprising admixing a modified osteopontin polypeptide in which an RGD domain is inactivated with a pharmaceutically acceptable and/or cosmetically acceptable excipient, carrier or diluent.
- Polypeptides produced by pCEP4 plasmid contain an inserted His-tag, which is used to facilitate the isolation with Ni-sepharose gel chromatography (Invitrogen). Collected medium was diluted with binding buffer (20mM sodium phosphate, 500mM sodium chloride, pH 7.8), Ni-sepharose suspension was added before incubation on shaker overnight at 4°C. The Ni-sepharose gel was pelleted by centrifugation at 1000g for seven minutes and poured into a BioRad disposable mini column. Unbound proteins were removed with binding buffer followed by washing buffer (20mM sodium phosphate, 500mM sodium chloride, pH 6.0). Polypeptides were eluated from the column with 500mM imidazole.
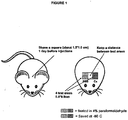
- the study consisted of four experimental groups each containing five male wtC57BL/6N mice.
- each animal received four intracutaneous injections of 25 ⁇ l each at separate locations (each approx. 0.5 x 0.5 cm) within the shaved rectangle (see Figure 1 ).
- Test polypeptide + PBS 25 ⁇ l Necropsy after 24h (day 1) 5 2
- Test polypeptide + PBS 25 ⁇ l Necropsy after 48h (day 2) 5 3
- Test polypeptide + PBS 25 ⁇ l Necropsy after 96h (day 4) 5 4
- the marked skin areas in the neck were removed and processed as follows: One of the polypeptide-treated skin samples and one of the PBS-treated control skin samples were fixed in 4% paraformaldehyde and subsequently embedded in paraffin. The two other skin samples (polypeptide-treated and PBS-treated) were snap-frozen in liquid nitrogen and stored appropriately at -80°C.
- the data show that treatment with the exemplary modified osteopontin polypeptide of SEQ ID NO: 1 increases the number of hair follicles/mm epidermis by about 60%.
- mice (C57BL/6) were used at an age of 6 to 8 weeks.
- Exemplary polypeptides FOL-004 (SEQ ID NO: 5) and FOL-005 (SEQ ID NO: 63) both induced rapid hair growth, which was evident from day 5 and maintained until the end of the assessment period (day 16)..
- a 'full length' polypeptide of the invention, (“Cx”) also induced pronounced hair growth, albeit with a slower onset than the FOL-004 (SEQ ID NO: 5) or FOL-005 (SEQ ID NO: 63).
- This sequence is a fragment of wildtype mouse osteopontin, and corresponds to the region of the protein from which "FOL-004" is derived (i.e. "FOL-004" is a mutated version of wildtype fragment "FOL-001").
- Peptide FOL-001 (SEQ ID NO: 121) produced a delayed but detectable hair growth effect in mice, which became evident at about day 9 and reached a plateau at a score of about 1.3.
- Example B Following completion of the term of hair growth assessment for Example B, the animals were euthanized and dorsal skin peeled and removed for observation of the internal surface.
- Table 8 Observation of melanogenesis Group Skin colour (externa) % anagen induction* Black colour in peeled skin (day 16) % anagen induction** No.of animals showing hair growth Cx(60nM Black (5/5 animals) 100 3/5 60 5/5 FOL-004 (60nM) Black (5/5 animals) 100 1/5 20 5/5 FOL-005 (60nM) Black (5/5 animals) 100 3/5 60 5/5 Vehicle Pink (4/5 animals) 20 1/5 20 1/5
- the hair growth cycle consists of three phases: a resting telogen phase (C57BL/6 skin is a pale pink colour), an active hair growth anagen phase (where the skin becomes dark gray or black), and finally a catagen phase (where hair growth stops, and the skin transitions back to the telogen phase, returning to a pale pink colour).
- a resting telogen phase C57BL/6 skin is a pale pink colour
- an active hair growth anagen phase where the skin becomes dark gray or black
- a catagen phase where hair growth stops, and the skin transitions back to the telogen phase, returning to a pale pink colour.
- telogen phase the test polypeptide was administered by subcutaneous/topical route.
- a good hair growth promoter triggers the transition from the resting telogen phase to the active anagen phase, and thus a transition from light skin to dark skin. This dark pigmentation may result from the collection of melanin in the hair follicles, in preparation for new hair growth during the anagen phase. Melanin synthesis of follicular melanocytes is strictly coupled to the anagen phase, ceases during catagen and is absent in telogen phase. Hence a good hair growth promoter causes blackening of dorsal skin.
- the dorsal skin of the animals was then excised out by peeling and the peeled skin reversed and observed for the induction of melanogenesis indicated by visual blackening. Histological analysis reveals number of follicles and skin thickness. A good hair growth promoter increases no. of follicles in sub cutis and skin thickness.
- the exemplary mutated osteopontin polypeptides of the invention showed pronounced induction of anagen, as evidenced by an increase in the number of hair follicles in the subcutis and/or enhanced skin thickness.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Medicinal Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Dermatology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Gastroenterology & Hepatology (AREA)
- Zoology (AREA)
- Immunology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Birds (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Biomedical Technology (AREA)
- Molecular Biology (AREA)
- Diabetes (AREA)
- Endocrinology (AREA)
- Marine Sciences & Fisheries (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
- Cosmetics (AREA)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PL16169698T PL3087999T3 (pl) | 2011-08-10 | 2012-08-10 | Zmodyfikowane peptydy osteopontyny ze zdezaktywowaną domeną rgd i ich zastosowanie |
| DK16169698.4T DK3087999T3 (da) | 2011-08-10 | 2012-08-10 | Modificerede osteopontinpeptider med et inaktiveret RGD-domæne og anvendelser deraf |
| EP16169698.4A EP3087999B1 (en) | 2011-08-10 | 2012-08-10 | Modified osteopontin peptides having an inactivated rgd domain and uses thereof |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB1113770.0A GB2493540A (en) | 2011-08-10 | 2011-08-10 | Agents for stimulating hair growth in mammals |
| PCT/GB2012/051955 WO2013021212A2 (en) | 2011-08-10 | 2012-08-10 | Novel compositions and uses thereof |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP16169698.4A Division EP3087999B1 (en) | 2011-08-10 | 2012-08-10 | Modified osteopontin peptides having an inactivated rgd domain and uses thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP2741761A2 EP2741761A2 (en) | 2014-06-18 |
| EP2741761B1 true EP2741761B1 (en) | 2016-05-18 |
Family
ID=44735704
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP16169698.4A Active EP3087999B1 (en) | 2011-08-10 | 2012-08-10 | Modified osteopontin peptides having an inactivated rgd domain and uses thereof |
| EP12768879.4A Active EP2741761B1 (en) | 2011-08-10 | 2012-08-10 | Modified osteopontin peptides having an inactivated rgd domain |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP16169698.4A Active EP3087999B1 (en) | 2011-08-10 | 2012-08-10 | Modified osteopontin peptides having an inactivated rgd domain and uses thereof |
Country Status (15)
| Country | Link |
|---|---|
| US (3) | US9381149B2 (enExample) |
| EP (2) | EP3087999B1 (enExample) |
| JP (1) | JP6049718B2 (enExample) |
| KR (1) | KR101992282B1 (enExample) |
| CN (1) | CN103930125B (enExample) |
| AU (1) | AU2012293448B2 (enExample) |
| BR (1) | BR112014003051B1 (enExample) |
| CA (1) | CA2844749C (enExample) |
| DK (2) | DK3087999T3 (enExample) |
| ES (2) | ES2740826T3 (enExample) |
| GB (1) | GB2493540A (enExample) |
| PL (2) | PL2741761T3 (enExample) |
| RU (1) | RU2604797C2 (enExample) |
| TR (1) | TR201910685T4 (enExample) |
| WO (1) | WO2013021212A2 (enExample) |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2493540A (en) | 2011-08-10 | 2013-02-13 | Follicum Ab | Agents for stimulating hair growth in mammals |
| GB201406989D0 (en) * | 2014-04-17 | 2014-06-04 | Follicum Ab | Novel treatments |
| JP6548126B2 (ja) * | 2014-11-27 | 2019-07-24 | 国立大学法人大阪大学 | Iii型コラーゲン産生促進剤 |
| KR101906557B1 (ko) | 2015-08-06 | 2018-10-11 | 서울대학교산학협력단 | 합성 펩타이드를 이용한 유도만능줄기세포의 제조방법 |
| WO2018175630A1 (en) * | 2017-03-23 | 2018-09-27 | The Regents Of The University Of California | Stimulation of hair growth by senescent cells and senescence associated secretory phenotype |
| BR112019021886A2 (pt) * | 2017-05-04 | 2020-06-02 | Follicum Ab | Peptídeos para o tratamento de diabetes |
| KR102091567B1 (ko) * | 2018-06-15 | 2020-03-20 | 인하대학교 산학협력단 | 오스테오폰틴 단백질 단편을 유효성분으로 함유하는 뇌손상 예방 또는 치료용 약학 조성물 |
| AU2019374981A1 (en) * | 2018-11-07 | 2021-05-27 | Follicum Ab | Peptide fragments for treatment of diabetes |
| ES2953088T3 (es) * | 2019-04-24 | 2023-11-08 | Follicum Ab | Formulación tópica |
| CN115397458B (zh) * | 2020-02-25 | 2024-09-13 | 安普力菲卡控股集团公司 | 用于刺激毛发生长的组合物和方法 |
| JP2024532616A (ja) * | 2021-08-25 | 2024-09-05 | アンプリフィカ・ホールディングス・グループ・インコーポレーテッド | 毛髪の成長を刺激するための組成物および方法 |
| WO2023218095A1 (en) * | 2022-05-13 | 2023-11-16 | Coegin Pharma Ab | Agents for stimulating tissue regeneration |
| WO2023218094A1 (en) * | 2022-05-13 | 2023-11-16 | Coegin Pharma Ab | Agents inducing vascularisation |
| WO2024263662A2 (en) * | 2023-06-20 | 2024-12-26 | Amplifica Holdings Group, Inc. | Compositions and methods for stimulating hair growth |
Family Cites Families (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| SE459005B (sv) | 1985-07-12 | 1989-05-29 | Aake Rikard Lindahl | Saett att framstaella sfaeriska polymerpartiklar |
| US5643872A (en) | 1989-10-23 | 1997-07-01 | Smithkline Beecham Corporation | Cyclic anti-aggregatory peptides |
| US5756094A (en) | 1991-03-27 | 1998-05-26 | Trustees Of The University Of Pennsylvania | Methods for stimulating follicular growth |
| US5753612A (en) | 1992-10-27 | 1998-05-19 | Yissum Research Development Co. Of The Hebrew University Of Jerusalem | Pharmaceutical composition and method for inhibiting hair growth by administration of activin or activin agonists |
| US6008058A (en) | 1993-06-18 | 1999-12-28 | University Of Louisville | Cyclic peptide mixtures via side chain or backbone attachment and solid phase synthesis |
| JPH11504016A (ja) * | 1995-04-20 | 1999-04-06 | ザ・トラスティーズ・オブ・ザ・ユニバーシティ・オブ・ペンシルバニア | 毛髪の成長を調節する組成物及び方法 |
| CA2192782C (en) | 1995-12-15 | 2008-10-14 | Nobuyuki Takechi | Production of microspheres |
| EP1222913B1 (en) | 1999-10-21 | 2005-09-28 | Kao Corporation | Depilating method |
| AU4256901A (en) | 2000-03-23 | 2001-10-03 | Glaxo Group Limited | Method of screening for inhibitors of osteopontin |
| US6428967B1 (en) * | 2000-05-01 | 2002-08-06 | Board Of Regents, The University Of Texas System | LDL receptor signaling pathways |
| WO2002046465A2 (en) * | 2000-12-08 | 2002-06-13 | Oxford Biomedica (Uk) Limited | Method for identification of genes involved in specific diseases |
| DE60229509D1 (de) * | 2001-04-05 | 2008-12-04 | Astellas Pharma Inc | Anti-osteopontin-antikörper und dessen verwendung |
| PL211763B1 (pl) * | 2001-05-17 | 2012-06-29 | Serono Lab | Zastosowanie osteopontyny, cząsteczki kwasu nukleinowego, komórki zmodyfikowanej genetycznie oraz kompozycja farmaceutyczna |
| MXPA06006746A (es) | 2003-12-18 | 2006-08-18 | Novo Nordisk As | Analogos de glp-1 novedosos ligados a agentes similares a albumina. |
| EP1709165B1 (en) | 2004-01-06 | 2014-04-23 | Novozymes A/S | Polypeptides of alicyclobacillus |
| AR048024A1 (es) * | 2004-03-05 | 2006-03-22 | Bayer Cropscience Gmbh | Plantas con actividad aumentada de distintas enzimas fosforilantes del almidon |
| RU2309730C2 (ru) * | 2006-04-18 | 2007-11-10 | Леонид Юрьевич Брежнев | Средство для стимуляции роста волос, его варианты и способ его получения |
| WO2008086449A2 (en) * | 2007-01-09 | 2008-07-17 | Oregon Health & Science University | Synthetic osteopontin peptides and methods of use |
| GB2493540A (en) | 2011-08-10 | 2013-02-13 | Follicum Ab | Agents for stimulating hair growth in mammals |
-
2011
- 2011-08-10 GB GB1113770.0A patent/GB2493540A/en not_active Withdrawn
-
2012
- 2012-08-10 ES ES16169698T patent/ES2740826T3/es active Active
- 2012-08-10 DK DK16169698.4T patent/DK3087999T3/da active
- 2012-08-10 PL PL12768879T patent/PL2741761T3/pl unknown
- 2012-08-10 WO PCT/GB2012/051955 patent/WO2013021212A2/en not_active Ceased
- 2012-08-10 BR BR112014003051-0A patent/BR112014003051B1/pt active IP Right Grant
- 2012-08-10 AU AU2012293448A patent/AU2012293448B2/en active Active
- 2012-08-10 CA CA2844749A patent/CA2844749C/en active Active
- 2012-08-10 US US14/237,108 patent/US9381149B2/en active Active
- 2012-08-10 PL PL16169698T patent/PL3087999T3/pl unknown
- 2012-08-10 TR TR2019/10685T patent/TR201910685T4/tr unknown
- 2012-08-10 JP JP2014524449A patent/JP6049718B2/ja active Active
- 2012-08-10 DK DK12768879.4T patent/DK2741761T3/en active
- 2012-08-10 EP EP16169698.4A patent/EP3087999B1/en active Active
- 2012-08-10 KR KR1020147006108A patent/KR101992282B1/ko active Active
- 2012-08-10 CN CN201280049850.XA patent/CN103930125B/zh active Active
- 2012-08-10 EP EP12768879.4A patent/EP2741761B1/en active Active
- 2012-08-10 RU RU2014108819/10A patent/RU2604797C2/ru active
- 2012-08-10 ES ES12768879.4T patent/ES2576299T3/es active Active
-
2016
- 2016-06-16 US US15/184,290 patent/US10137169B2/en active Active
-
2018
- 2018-10-31 US US16/176,201 patent/US10517932B2/en active Active
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2741761B1 (en) | Modified osteopontin peptides having an inactivated rgd domain | |
| US20170143605A1 (en) | Compositions Comprising Osteopontin Derivatives for the Inhibition of Hair Growth | |
| JP2025011085A (ja) | プラスミノーゲンアクチベーターインヒビター1(plasminogen activator inhibitor 1)(PAI-1)阻害剤(inhibitor)およびその使用 | |
| AU2019339488B2 (en) | Uses of plasminogen activator inhibitor 1 (PAI-1) inhibitors | |
| KR102890438B1 (ko) | 탈모 증상 완화능을 갖는 두피 유래 펩타이드 | |
| HK1197540B (en) | Modified osteopontin peptides having an inactivated rgd domain | |
| KR101113806B1 (ko) | 트레오네이트를 유효성분으로 하는 탈모 예방 또는 치료용 조성물 | |
| HK1230068B (en) | Modified osteopontin peptides having an inactivated rgd domain and uses thereof | |
| HK1230068A1 (en) | Modified osteopontin peptides having an inactivated rgd domain and uses thereof | |
| JP5051471B2 (ja) | 休止期脱毛症治療剤 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20140130 |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: DE Ref document number: 1197540 Country of ref document: HK |
|
| 17Q | First examination report despatched |
Effective date: 20150211 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| INTG | Intention to grant announced |
Effective date: 20150930 |
|
| INTG | Intention to grant announced |
Effective date: 20151202 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Ref country code: AT Ref legal event code: REF Ref document number: 799823 Country of ref document: AT Kind code of ref document: T Effective date: 20160615 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2576299 Country of ref document: ES Kind code of ref document: T3 Effective date: 20160706 Ref country code: NL Ref legal event code: FP |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602012018674 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: NV Representative=s name: STOLMAR AND PARTNER INTELLECTUAL PROPERTY S.A., CH Ref country code: FR Ref legal event code: PLFP Year of fee payment: 5 Ref country code: DK Ref legal event code: T3 Effective date: 20160808 |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: TRGR |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| REG | Reference to a national code |
Ref country code: NO Ref legal event code: T2 Effective date: 20160518 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160518 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160819 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160518 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160518 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160518 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160919 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160518 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160518 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160518 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160518 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602012018674 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160518 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R082 Ref document number: 602012018674 Country of ref document: DE |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160518 |
|
| 26N | No opposition filed |
Effective date: 20170221 |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: GR Ref document number: 1197540 Country of ref document: HK |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 6 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160810 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PCAR Free format text: NEW ADDRESS: RUE DU CENDRIER 15 CP 1489, 1201 GENEVE (CH) |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20120810 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160518 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160518 Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160518 Ref country code: MT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160831 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160518 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 7 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160518 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: UEP Ref document number: 799823 Country of ref document: AT Kind code of ref document: T Effective date: 20160518 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160518 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20220721 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: TR Payment date: 20220725 Year of fee payment: 11 Ref country code: SE Payment date: 20220810 Year of fee payment: 11 Ref country code: NO Payment date: 20220811 Year of fee payment: 11 Ref country code: IT Payment date: 20220721 Year of fee payment: 11 Ref country code: IE Payment date: 20220810 Year of fee payment: 11 Ref country code: FI Payment date: 20220729 Year of fee payment: 11 Ref country code: ES Payment date: 20220901 Year of fee payment: 11 Ref country code: DK Payment date: 20220812 Year of fee payment: 11 Ref country code: AT Payment date: 20220729 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: PL Payment date: 20220722 Year of fee payment: 11 Ref country code: BE Payment date: 20220721 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 20220901 Year of fee payment: 11 |
|
| P01 | Opt-out of the competence of the unified patent court (upc) registered |
Effective date: 20230510 |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: EBP Effective date: 20230831 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MM Effective date: 20230901 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MM01 Ref document number: 799823 Country of ref document: AT Kind code of ref document: T Effective date: 20230810 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230810 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230810 Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230810 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230831 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20230831 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230901 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MM4A |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230811 Ref country code: NO Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230831 Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230901 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230810 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230831 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230810 Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230810 Ref country code: DK Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230831 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230831 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20240927 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230811 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230810 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230810 Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230811 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20250717 Year of fee payment: 14 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20250819 Year of fee payment: 14 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20250819 Year of fee payment: 14 |