EP2734334B1 - Outil de meulage pour usiner des matières fragiles et procédé de fabrication d'un outil de meulage - Google Patents
Outil de meulage pour usiner des matières fragiles et procédé de fabrication d'un outil de meulage Download PDFInfo
- Publication number
- EP2734334B1 EP2734334B1 EP12817725.0A EP12817725A EP2734334B1 EP 2734334 B1 EP2734334 B1 EP 2734334B1 EP 12817725 A EP12817725 A EP 12817725A EP 2734334 B1 EP2734334 B1 EP 2734334B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- bonding agent
- grinding tool
- matrix
- metallic
- abrasive particles
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000000227 grinding Methods 0.000 title claims description 124
- 239000000463 material Substances 0.000 title claims description 36
- 238000003754 machining Methods 0.000 title claims description 9
- 238000004519 manufacturing process Methods 0.000 title claims description 8
- 239000002245 particle Substances 0.000 claims description 122
- 239000007767 bonding agent Substances 0.000 claims description 106
- 239000011159 matrix material Substances 0.000 claims description 66
- 229910052581 Si3N4 Inorganic materials 0.000 claims description 55
- HQVNEWCFYHHQES-UHFFFAOYSA-N silicon nitride Chemical compound N12[Si]34N5[Si]62N3[Si]51N64 HQVNEWCFYHHQES-UHFFFAOYSA-N 0.000 claims description 55
- 239000000843 powder Substances 0.000 claims description 30
- 239000010949 copper Substances 0.000 claims description 28
- 239000000203 mixture Substances 0.000 claims description 25
- 239000011135 tin Substances 0.000 claims description 23
- 229910000906 Bronze Inorganic materials 0.000 claims description 20
- 238000005245 sintering Methods 0.000 claims description 20
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 claims description 18
- 229910052802 copper Inorganic materials 0.000 claims description 18
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 claims description 15
- 229910052709 silver Inorganic materials 0.000 claims description 15
- 239000004332 silver Substances 0.000 claims description 15
- 238000000034 method Methods 0.000 claims description 14
- 239000010432 diamond Substances 0.000 claims description 13
- 239000000945 filler Substances 0.000 claims description 13
- 229910052718 tin Inorganic materials 0.000 claims description 13
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 claims description 12
- 239000004642 Polyimide Substances 0.000 claims description 11
- 229920001721 polyimide Polymers 0.000 claims description 11
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 claims description 10
- 229910052582 BN Inorganic materials 0.000 claims description 9
- PZNSFCLAULLKQX-UHFFFAOYSA-N Boron nitride Chemical compound N#B PZNSFCLAULLKQX-UHFFFAOYSA-N 0.000 claims description 9
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 8
- 229910002804 graphite Inorganic materials 0.000 claims description 8
- 239000010439 graphite Substances 0.000 claims description 8
- 229910003460 diamond Inorganic materials 0.000 claims description 6
- 239000011248 coating agent Substances 0.000 claims description 5
- 238000000576 coating method Methods 0.000 claims description 5
- 229910052759 nickel Inorganic materials 0.000 claims description 5
- 229920000642 polymer Polymers 0.000 claims description 3
- 238000002156 mixing Methods 0.000 claims 1
- 229910052751 metal Inorganic materials 0.000 description 33
- 239000002184 metal Substances 0.000 description 33
- 239000000919 ceramic Substances 0.000 description 11
- KUNSUQLRTQLHQQ-UHFFFAOYSA-N copper tin Chemical compound [Cu].[Sn] KUNSUQLRTQLHQQ-UHFFFAOYSA-N 0.000 description 9
- 239000010974 bronze Substances 0.000 description 8
- 238000012360 testing method Methods 0.000 description 7
- 230000006872 improvement Effects 0.000 description 6
- 238000007792 addition Methods 0.000 description 5
- UONOETXJSWQNOL-UHFFFAOYSA-N tungsten carbide Chemical compound [W+]#[C-] UONOETXJSWQNOL-UHFFFAOYSA-N 0.000 description 5
- TWNQGVIAIRXVLR-UHFFFAOYSA-N oxo(oxoalumanyloxy)alumane Chemical compound O=[Al]O[Al]=O TWNQGVIAIRXVLR-UHFFFAOYSA-N 0.000 description 4
- 229910000831 Steel Inorganic materials 0.000 description 3
- 238000010586 diagram Methods 0.000 description 3
- 230000001050 lubricating effect Effects 0.000 description 3
- 239000010959 steel Substances 0.000 description 3
- 239000010936 titanium Substances 0.000 description 3
- 239000004962 Polyamide-imide Substances 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- PQIJHIWFHSVPMH-UHFFFAOYSA-N [Cu].[Ag].[Sn] Chemical compound [Cu].[Ag].[Sn] PQIJHIWFHSVPMH-UHFFFAOYSA-N 0.000 description 2
- 239000003082 abrasive agent Substances 0.000 description 2
- 229910045601 alloy Inorganic materials 0.000 description 2
- 239000000956 alloy Substances 0.000 description 2
- 229910052782 aluminium Inorganic materials 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- 238000005520 cutting process Methods 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 238000005265 energy consumption Methods 0.000 description 2
- 239000012634 fragment Substances 0.000 description 2
- 239000007769 metal material Substances 0.000 description 2
- 238000003801 milling Methods 0.000 description 2
- 229920002312 polyamide-imide Polymers 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 230000003014 reinforcing effect Effects 0.000 description 2
- 229910001316 Ag alloy Inorganic materials 0.000 description 1
- 229920001342 Bakelite® Polymers 0.000 description 1
- 229910017938 Cu—Sn—Ti Inorganic materials 0.000 description 1
- 229910001128 Sn alloy Inorganic materials 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- ZJHIEQGQFJJLBM-UHFFFAOYSA-N [Co].[Sn].[Cu] Chemical compound [Co].[Sn].[Cu] ZJHIEQGQFJJLBM-UHFFFAOYSA-N 0.000 description 1
- VRUVRQYVUDCDMT-UHFFFAOYSA-N [Sn].[Ni].[Cu] Chemical compound [Sn].[Ni].[Cu] VRUVRQYVUDCDMT-UHFFFAOYSA-N 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 239000004637 bakelite Substances 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- 229910017052 cobalt Inorganic materials 0.000 description 1
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 1
- 229910052681 coesite Inorganic materials 0.000 description 1
- 229910052906 cristobalite Inorganic materials 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- -1 for example Substances 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- 238000007493 shaping process Methods 0.000 description 1
- HBMJWWWQQXIZIP-UHFFFAOYSA-N silicon carbide Chemical compound [Si+]#[C-] HBMJWWWQQXIZIP-UHFFFAOYSA-N 0.000 description 1
- 229910010271 silicon carbide Inorganic materials 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 238000002490 spark plasma sintering Methods 0.000 description 1
- 239000012798 spherical particle Substances 0.000 description 1
- 229910052682 stishovite Inorganic materials 0.000 description 1
- 210000001550 testis Anatomy 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 229910052905 tridymite Inorganic materials 0.000 description 1
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B24—GRINDING; POLISHING
- B24D—TOOLS FOR GRINDING, BUFFING OR SHARPENING
- B24D3/00—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents
- B24D3/02—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents the constituent being used as bonding agent
- B24D3/04—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents the constituent being used as bonding agent and being essentially inorganic
- B24D3/06—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents the constituent being used as bonding agent and being essentially inorganic metallic or mixture of metals with ceramic materials, e.g. hard metals, "cermets", cements
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B24—GRINDING; POLISHING
- B24D—TOOLS FOR GRINDING, BUFFING OR SHARPENING
- B24D3/00—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents
- B24D3/02—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents the constituent being used as bonding agent
- B24D3/04—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents the constituent being used as bonding agent and being essentially inorganic
- B24D3/06—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents the constituent being used as bonding agent and being essentially inorganic metallic or mixture of metals with ceramic materials, e.g. hard metals, "cermets", cements
- B24D3/08—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents the constituent being used as bonding agent and being essentially inorganic metallic or mixture of metals with ceramic materials, e.g. hard metals, "cermets", cements for close-grained structure, e.g. using metal with low melting point
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F1/00—Metallic powder; Treatment of metallic powder, e.g. to facilitate working or to improve properties
- B22F1/18—Non-metallic particles coated with metal
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B24—GRINDING; POLISHING
- B24D—TOOLS FOR GRINDING, BUFFING OR SHARPENING
- B24D18/00—Manufacture of grinding tools or other grinding devices, e.g. wheels, not otherwise provided for
- B24D18/0009—Manufacture of grinding tools or other grinding devices, e.g. wheels, not otherwise provided for using moulds or presses
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B24—GRINDING; POLISHING
- B24D—TOOLS FOR GRINDING, BUFFING OR SHARPENING
- B24D3/00—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents
- B24D3/02—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents the constituent being used as bonding agent
- B24D3/20—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents the constituent being used as bonding agent and being essentially organic
- B24D3/22—Rubbers synthetic or natural
- B24D3/24—Rubbers synthetic or natural for close-grained structure
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B24—GRINDING; POLISHING
- B24D—TOOLS FOR GRINDING, BUFFING OR SHARPENING
- B24D3/00—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents
- B24D3/02—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents the constituent being used as bonding agent
- B24D3/20—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents the constituent being used as bonding agent and being essentially organic
- B24D3/28—Resins or natural or synthetic macromolecular compounds
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B24—GRINDING; POLISHING
- B24D—TOOLS FOR GRINDING, BUFFING OR SHARPENING
- B24D3/00—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents
- B24D3/34—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents characterised by additives enhancing special physical properties, e.g. wear resistance, electric conductivity, self-cleaning properties
- B24D3/342—Physical features of abrasive bodies, or sheets, e.g. abrasive surfaces of special nature; Abrasive bodies or sheets characterised by their constituents characterised by additives enhancing special physical properties, e.g. wear resistance, electric conductivity, self-cleaning properties incorporated in the bonding agent
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B24—GRINDING; POLISHING
- B24D—TOOLS FOR GRINDING, BUFFING OR SHARPENING
- B24D5/00—Bonded abrasive wheels, or wheels with inserted abrasive blocks, designed for acting only by their periphery; Bushings or mountings therefor
- B24D5/02—Wheels in one piece
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B24—GRINDING; POLISHING
- B24D—TOOLS FOR GRINDING, BUFFING OR SHARPENING
- B24D5/00—Bonded abrasive wheels, or wheels with inserted abrasive blocks, designed for acting only by their periphery; Bushings or mountings therefor
- B24D5/06—Bonded abrasive wheels, or wheels with inserted abrasive blocks, designed for acting only by their periphery; Bushings or mountings therefor with inserted abrasive blocks, e.g. segmental
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B24—GRINDING; POLISHING
- B24D—TOOLS FOR GRINDING, BUFFING OR SHARPENING
- B24D7/00—Bonded abrasive wheels, or wheels with inserted abrasive blocks, designed for acting otherwise than only by their periphery, e.g. by the front face; Bushings or mountings therefor
- B24D7/06—Bonded abrasive wheels, or wheels with inserted abrasive blocks, designed for acting otherwise than only by their periphery, e.g. by the front face; Bushings or mountings therefor with inserted abrasive blocks, e.g. segmental
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B24—GRINDING; POLISHING
- B24D—TOOLS FOR GRINDING, BUFFING OR SHARPENING
- B24D99/00—Subject matter not provided for in other groups of this subclass
- B24D99/005—Segments of abrasive wheels
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C26/00—Alloys containing diamond or cubic or wurtzitic boron nitride, fullerenes or carbon nanotubes
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C32/00—Non-ferrous alloys containing at least 5% by weight but less than 50% by weight of oxides, carbides, borides, nitrides, silicides or other metal compounds, e.g. oxynitrides, sulfides, whether added as such or formed in situ
- C22C32/0047—Non-ferrous alloys containing at least 5% by weight but less than 50% by weight of oxides, carbides, borides, nitrides, silicides or other metal compounds, e.g. oxynitrides, sulfides, whether added as such or formed in situ with carbides, nitrides, borides or silicides as the main non-metallic constituents
- C22C32/0068—Non-ferrous alloys containing at least 5% by weight but less than 50% by weight of oxides, carbides, borides, nitrides, silicides or other metal compounds, e.g. oxynitrides, sulfides, whether added as such or formed in situ with carbides, nitrides, borides or silicides as the main non-metallic constituents only nitrides
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2998/00—Supplementary information concerning processes or compositions relating to powder metallurgy
- B22F2998/10—Processes characterised by the sequence of their steps
Definitions
- the invention relates to a grinding tool, in particular a grinding tool for grinding hard and/or brittle materials such as tungsten carbide.
- the grinding tool may in particular be a grinding wheel.
- the invention also relates to a method of making such a grinding tool.
- Grinding tools such as grinding wheels are used for machining of brittle materials.
- One area where such grinding tools are used is machining of tools that are made of hard metal (tungsten carbide).
- grinding tools may be used for machining operations in which drills or milling tools are shaped by grinding.
- the work piece which is to be shaped is made of a hard material such as tungsten carbide
- the abrasive tool must have abrasive particles of a very hard material. In practice, this normally means that the abrasive particles are diamond particles or grains of cubic boron nitride. Diamonds or cubic boron nitride grains for this purpose are commercially available and can be considered as standard components.
- Diamonds for this purpose may typically have an average or mean particle size of 50 ⁇ m (the size of the particles is of course varying) and have a plurality of sharp edges that can cut hard materials such as tungsten carbide.
- EP 1 144 160 A1 discloses an abrasive wheel for abrading very hard materials.
- a known type of grinding tool for this purpose is a grinding wheel with a core which may be made of, for example, a metallic material such as steel or aluminum.
- the core may also be made of a non-metallic material such as a polymeric material.
- the core can be shaped as a disc which can be mounted on a tool spindle for rotation about the axis of the disc-shaped metal core.
- An abrasive rim surrounds the core and is joined to the core.
- the abrasive rim may comprise abrasive particles embedded in a matrix with one or more bonding agents.
- the material used in the abrasive rim is normally more expensive than the material of the core. For this reason, the abrasive rim has a smaller extension in the radial direction than the core (i.e. the abrasive rim is normally a smaller part of the grinding wheel since it is more expensive).
- the abrasive rim is gradually worn down until it is consumed and the grinding wheel can no longer be used.
- Known bonding agents for abrasive rims of grinding wheels include polymeric bonding agents such as, for example, Bakelite. Alternatively, the bonding agent may be a ceramic bonding agent. It is also known to use metallic bonding agents, in particular bonding agents of bronze that have been made by sintering. In such sintering operations, metal powder containing copper and tin is sintered together with abrasive particles such as diamond particles or grains of cubic boron nitride. Sometimes, silver can be added such that the bronze contains copper (Cu), tin (Sn) and silver (Ag). In the past, practical experience has showed that Cu/Sn/Ag alloys function well as bonding agents for abrasives and that such bonding agents function well during grinding.
- bronze alloys comprising silver function well as bonding agents for abrasives.
- silver since silver is expensive, other bronze alloys may be used in order to reduce the cost and the present invention is applicable also to bronze alloys without silver.
- bronze compositions for this purpose include copper/tin/cobalt (Cu/Sn/Co) and copper/tin/nickel (Cu/Sn/Ni). It has also been suggested that bronze compositions for this purpose may include copper/tin/titanium (Cu/Sn/Ti).
- Yet another known system includes hybrids of polymeric and metallic bonding agents in which metallic powder is sintered together with polymeric material such that a matrix is formed in which the polymeric bonding agent and the metallic bonding agent (typically a bronze alloy as described above) are closely intertwined with each other on a microscopic level.
- the metal bonding agent and the polymeric bonding agent each forms a network and the respective networks of the bonding agents penetrate each other.
- Such a hybrid matrix that comprises both a metal bonding agent and a polymeric bonding agent is disclosed in for example US patent No. 6063148 .
- such hybrids normally include one or several fillers.
- One such filler may be graphite which is used for its lubricating properties.
- the abrasive particles used may have different properties.
- the brittleness of diamonds may vary depending on the purpose for which the grinding tool is to be used.
- the properties of different diamonds may be matched to meet the properties of different bonding agents (or hybrids of bonding agents).
- the abrasive particles should be bonded in its matrix in such a way that the grinding tool functions as desired. It is desirable that the grinding tool have a good resistance to wear such that it can be used over an extended period. However, good wear resistance is not the only desired property and the grinding tool with the highest resistance to wear is not necessarily the best choice. Other desirable properties include low energy consumption (i.e. that the power required to drive the grinding tool is not excessively high) and constant or at least predictable performance properties. If the grinding effect of the abrasive rim varies too much over time, this causes problems. This is especially the case if the performance of the grinding tool varies in a way that is unpredictable.
- the extent of wear of the grinding tool under given circumstances depends to a very high degree on the properties of the matrix in which the abrasive particles are embedded. Therefore, the composition of the matrix is important.
- abrasive particles may be torn away from the matrix before they have been worn down.
- the work piece will come into direct contact with the relatively brittle matrix and wear down the matrix prematurely.
- power consumption drops momentarily until so much of the matrix has been worn down that fresh abrasive particles come to the surface.
- the abrasive rim of the grinding tool is worn out faster than it would otherwise have been. If the operation of the grinding tool has been programmed in advance, the consequence thereof may be that the grinding operation does not function properly since the grinding tool is set to operate based on an assumption of tool diameter that is now incorrect. This problem becomes more serious if the abrasive rim is worn out in a way that is difficult to predict, for example if wear occurs in sudden steps that come in irregular intervals.
- the required power for the grinding operation can be kept low such that the energy consumption during grinding can be minimized.
- the G-ratio expresses the ratio between the volume of the material removed by the grinding tool from a work piece and the volume lost by the grinding tool (the wear on the tool).
- a good grinding tool has a high G-ratio.
- the invention relates to a grinding tool as defined in claim 1.
- Preferred embodiments are defined in the dependent claims.
- the matrix may further optionally comprise a polymeric bonding agent that has been sintered together with the metallic bonding agent such that the polymeric bonding agent and the metallic bonding agent form a connected network.
- the silicon nitride constitutes 0.3 % - 5.0 % by volume of the metallic bonding agent.
- it may constitute 0.5 % - 5.0 % by volume of the metallic bonding agent, 1.0 % - 5.0 % by volume of the metallic bonding agent, or 0.5 % - 3.0 % by volume or 0.5 % - 2.0 % by volume.
- the silicon nitride may be present in the shape of grains having an average grain size which is preferably less than 10 ⁇ m but also preferably above 0.1 ⁇ m. Such particles may be 1250 Tyler mesh particles. The particles may thus include particles up to 10 ⁇ m even though average grain size is smaller.
- the polymeric bonding agent may comprise polyimide or be made entirely or almost entirely of polyimide.
- the matrix may optionally additionally comprise filler materials such as graphite.
- Graphite has lubricating properties which may be desirable during grinding.
- the metallic bonding agent is preferably a bronze alloy that comprises copper, tin and silver.
- the abrasive particles may be, for example, diamond particles or cubic boron nitride particles.
- the abrasive particles may have a mean particle size in the range of 4 ⁇ m - 181 ⁇ m. In many realistic embodiments, the abrasive particles may have a size in the range of 46 ⁇ m - 91 ⁇ m. In embodiments of the invention, the abrasive particles may have a coating of copper or nickel.
- the invention also relates to a method of making the inventive grinding tool, as defined in claim 11.
- the metallic powder may additionally comprise silver.
- the relative proportion of silicon nitride in the metal bonding agent refers to the volume proportion of the powder used in the manufacturing process.
- the method of manufacturing is such that, in the powder added before sintering, silicon nitride will constitute 0.02 % - 2.0% by volume of the metallic bonding agent (the silicon nitride being counted as part of the metal bonding agent). It is assumed that the silicon carbide particles will retain the same relative proportion of total volume also after sintering.
- a polymer is added to the metallic powder before sintering, preferably in the form of polyimide powder, such that also a polymeric bonding agent is formed which is a part of the matrix.
- the method may be carried out in such a way that the powder material for the bonding agents of the matrix is mixed with the abrasive particles to form a mixture.
- the mixture is then compacted in a cold press.
- the compacted mixture is then cured in a kiln at a temperature in the range of 380°C - 520°C, preferably 400°C - 500°C, for a period of 120 - 150 minutes. Thereafter, the compacted and cured mixture is placed in a press and subjected to a pressure of 1500 - 2000 kg/cm 2 . The pressure is then held until the mixture has reached a temperature below 300°C.
- filler material is added to the mixture of metallic powder and abrasive particles before the sintering operation.
- the filler material may optionally comprise graphite.
- the matrix of the inventive grinding tool may advantageously be a matrix which is a hybrid, i.e. a matrix having both a metal bonding agent and a polymeric bonding agent.
- Hybrid bonding solutions can combine the best properties of metal bonding agents with the best properties of polymeric bonding agents. If re-sharpening by a sharpening tool is needed, a grinding tool with a hybrid matrix can be re-sharpened easier than a pure metal matrix. At the same time, a grinding tool with a hybrid matrix has better resistance to wear than a matrix using only a polymeric bonding agent.
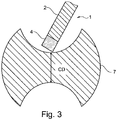
- the grinding tool may in particular be a grinding wheel which is intended for machining hard and/or brittle materials such as tungsten carbide. Such materials may be present in work pieces for tools such as for example drills or milling tools and the grinding tool 1 of the present invention may be a grinding wheel that is used for shaping such tools.
- the grinding tool 1 comprises a core 2 and an abrasive rim 4.
- the core 2 may be made of a less expensive material such as steel or some other metal.
- the core could be made of, for example, a polymeric material.
- the core could also comprise more than one material. For example, it could be made partially by metal such as steel or aluminum and partially be a polymeric material.
- the core 2 may be provided with a through-hole or cavity 3 such that the grinding tool 1 may be mounted on a spindle (not shown) for rotary movement.
- the abrasive rim 4 comprises abrasive particles 5 embedded in a matrix 6.
- the matrix 6 in turn comprises a metallic bonding agent which is a sintered bronze alloy.
- the metallic bonding agent constitutes 50 % - 100 % by volume of the matrix 6 and embodiments are thus conceivable in which the entire matrix 6 is made up of the metallic bonding agent.
- the matrix 6 normally comprises at least some other component. For example, it may comprise filler such as graphite that has lubricating properties. In most embodiments, the matrix 6 would also comprise a polymeric bonding agent that may be formed by polyimide.
- the abrasive particles 5 will emit small fragments and be worn down gradually.
- the wear on the abrasive rim 4 will be relatively slow such that the diameter of the grinding tool 1 can be kept substantially constant during a longer period.
- wear on the abrasive rim 4 will be kept at an even pace and the power during operation will not vary so much.
- the matrix 6 is instead incapable of holding the abrasive particles 5 firmly, it may happen that abrasive particles come loose well before they have been fragmented. As a consequence, they will be lost before their entire abrasive potential has been used.
- the abrasive tool 1 will be worn out faster and the diameter of the grinding tool (such as a grinding wheel) will decrease faster. A smaller diameter of the grinding tool 1 may result in a less accurate machining of the work pieces.
- a grinding tool 1 acts on a work piece 7.
- the work piece 7 may be, for example, a work piece that shall be shaped to a drill.
- the grinding tool 1 is rotated by means of a power source acting through for example a spindle (not shown).
- the abrasive rim 4 of the grinding tool acts on the work piece 7 to cut a groove in the work piece.
- the work piece has a core diameter CD which is determined by the action of the grinding tool 1. If the grinding tool 1 is worn down such that its diameter decreases, the core diameter CD will grow unless the wear is compensated (for example by repositioning of the grinding tool 1 in relation to the work piece 7). It is therefore very desirable that the wear can be kept low and that the wear that does take place does not come in sudden unpredictable leaps.
- the inventors of the present invention have considered what steps can be taken to improve the ability of the matrix to hold the abrasive particles. Without wishing to be bound by theory, the inventors believe that one reason that metal bonding agents release the abrasive particles embedded therein may be that dislocations inside the metal bonding agent weaken the metallic bonding agent. Assuming this theory to be correct, the inventors first speculated that it should be possible to improve the matrix by reinforcing it with particles blocking dislocations in the metal bonding agent. Consequently, the inventors tried different additions to the metal powder that was used for sintering the metal bonding agent. One additive that was tried was aluminum oxide which was added to an extent corresponding to 1.0 % by volume of the metal bonding agent. This resulted in a certain improvement but the improvement was not as good as the inventors had hoped. The inventors also tried addition of 0.01 % by volume of silicon nitride. The improvement of that addition was even less than the improvement achieved by the aluminum oxide.
- the inventors investigated whether increased amounts of silicon nitride would produce better results. This was confirmed in testes carried out by the inventors. When silicon nitride was added in quantities significantly larger than 0.01 % by volume of the metal bonding agent, it was discovered that a very substantial improvement was obtained.
- a grinding tool with this composition was then compared to a standard grinding tool using a hybrid matrix and which did not contain silicon nitride (Si 3 N 4 ).
- the grinding tools were both grinding wheels in which the abrasive rim 4 was shaped as a ring surrounding the core 2.
- the diameter of the standard tool was worn down by 136 ⁇ m while the diameter of the grinding tool with the experimental composition was worn down by only 58 ⁇ m.
- the G-ratio for the tool with 1.0 % silicon nitride was 2335.
- a tool using 0.01 % by volume of silicon nitride was worn down 94 ⁇ m while a tool using 1.0 % by volume aluminum oxide was worn down 84 ⁇ m.
- the resistance to wear was still good but not quite as good as for the grinding tool with 1.0% by volume silicon nitride.
- the tool with 5.0 % silicon nitride had higher power consumption.
- the G-ratio was good but not quite as good as for the tools with 1.0% and 0.1 % by volume.
- the inventors have also tested a grinding wheel which had a shape and a composition similar to the other tools tested but in which the silicon nitride constituted 0.1 % by volume of the metal bonding agent. It was found that, under the same test conditions as the other tools that were tested, the wear of the tool with 0.1 % by volume silicon nitride was 62 ⁇ m and the G-ration was 2084. While this was inferior to the results obtained at 1 % by volume, it was still a very substantial improvement compared to the standard grinding tool.
- the inventors have also tested a grinding wheel which had a silicon nitride content of 0.02 % by volume of the metal bonding agent but which was otherwise similar to the other grinding wheels tested. Under similar test conditions, the grinding wheel with 0.02 % by volume of silicon nitride has a wear (diameter reduction) of 58 ⁇ m and a G-ratio of 2283. The results were thus slightly better than the results obtained at a ratio of 0.1 % by volume.
- Tests of resistance to wear and test of G-ratio have been carried out at 0 % by volume, 0.01 % by volume, 0.02 % by volume, 1.0 % by volume and 5.0 % by volume silicon nitride.
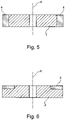
- the tools that were tested were grinding wheels of substantially the kind shown in Figure 5 , i.e. grinding tools with an abrasive rim 4 that surrounds a core 2 and where the grinding tool 1 rotates about the axis A during operation.
- Resistance to wear as a function of silicon nitride content can be seen in Figure 7 .
- the resistance to wear is expressed in Figure 7 as diameter reduction.
- resistance to wear increased significantly when the content of silicon nitride was increased from 0.01 % to 0.02 %.
- the wear resistance continued to be high up to a silicon nitride content of 5.0 % by volume of the metallic bonding agent.
- the G-ratio as a function of silicon nitride content can be seen in Figure 8 . As can be seen in the figure, the best values are obtained at a silicon nitride content in the range of 0.02 % - 5.0%. From Figure 8 , it can also be derived that the G-ratio is sinking towards the right in the figure even though the G-ratio at 5.0% by volume is still good.
- the metallic bonding agent may contain silicon nitride in an amount that constitutes 0.02 % - 5.0 % by volume of the metallic bonding agent. Since power consumption was higher at 5.0 % by volume, the inventors have concluded that values lower than 5.0% will have good resistance to wear but lower power consumption compared to tools with a silicon nitride content of 5% by volume. Therefore, a preferred range may be 0.02 % by volume to 3.0% by volume, 0.5 % - 3.0 % by volume, 0.5 % - 2.0 % by volume or 1.0 % - 2.0 % by volume of the metallic bonding agent.
- power consumption was generally lower than at 0.02 % by volume.
- power consumption was higher than at a content of 0.02 % but the power consumption at 5.0 % by volume was more even, the power consumption was more predictable than at 0.02 % by volume.
- the silicon nitride particles should preferably have a size up to 10 ⁇ m (1250 Tyler mesh). For sieved particles, this will normally mean that average grain size is less than 10 ⁇ m.
- the average particle size (D50) of the silicon nitride particles may then be about 2 ⁇ m - 3 ⁇ m (depending on how average particle size is measured).
- the specific surface area of the silicon nitride particles may advantageously be in the range of 5 m 2 /g - 6 m 2 /g. If the particles used are too small, this may result in clogging and difficulties during manufacturing. Moreover, for giving optimized strength to the metal bonding agent, it is believed by the inventors that particles up to 10 ⁇ m should preferably be included.
- the matrix 6 should further comprise a polymeric bonding agent that has been sintered together with the metallic bonding agent such that the polymeric bonding agent and the metallic bonding agent form a connected network (even though such a polymeric bonding agent is optional).
- the use of a polymeric agent makes it possible to fine tune the properties of the matrix and adapt it to different kinds of abrasive particles.
- the polymeric bonding agent may suitably be polyimide or comprise polyimide. The reason for this is that polyimide is heat resistant and can withstand the high temperatures during sintering. If a polymeric bonding agent is used, the polymeric bonding agent may be present in an amount of up to 50% by volume of the matrix (i.e. the amount of polymeric bonding agent is in the range of 0% - 50% by volume of the matrix). For example, the polymeric bonding agent may represent 10 % - 40 % or 10% - 30% by volume of the matrix.
- the polymeric bonding agent could be formed by some other polymeric material.
- it could be formed by polyamide-imide which is also capable of withstanding high temperatures.
- polyimide is preferred since it has better grinding properties than polyamide-imide.
- the metallic bonding agent is a preferably a bronze alloy that comprises copper, tin and silver. Silver improves the desirable properties of the metal bonding agent.
- the abrasive particles 5 may be either diamond particles or cubic boron nitride particles. Diamonds are harder and have better abrasive properties but cubic boron nitride is more temperature resistant. Moreover, diamonds may react chemically with certain materials.
- the abrasive particles 5 may be are diamond particles or particles of cubic boron nitride.
- the particles may be in the range of 4 ⁇ m - 181 ⁇ m even though particles outside this range may be considered depending on the requirements in each specific case.
- the abrasive particles 5 may have a mean particle size in the range of 46 ⁇ m - 91 ⁇ m which is a range that is suitable for many grinding operations.
- the abrasive particles 5 may optionally have a coating of copper or nickel.
- a coating of copper or nickel can improve the bond between the abrasive particles 5 and the matrix 6. However, the abrasive properties of the particles 5 will be somewhat reduced if the particles have such a coating.
- the relative proportion of abrasive particles 5 in relation to the bonding agents and fillers in the matrix 6 may vary depending on the requirements in each case.
- the amount of abrasive particles may represent a 10 % - 50 % of the total volume of the abrasive rim (i.e. the total volume of the abrasive particles and the matrix). If the relative proportion of abrasive particles is higher than 50 %, there is a substantial danger that the matrix will no longer be able to hold the abrasive particles. If the relative proportion of abrasive particles is less than 10 %, the grinding effect may become too small.
- the relative proportion of abrasive particles may preferably be in the range of 15 % - 30 % and a suitable value may be 25 %.
- the silicon nitride is present in the shape of grains having an average grain size which is equal to or less than 10 ⁇ m but above 0.1 ⁇ m.
- they may have a mean size in the range of 1 ⁇ m - 10 ⁇ m or 2 ⁇ m - 9 ⁇ m . It is believed by the inventors that silicon nitride particles smaller than 0.1 ⁇ m may result in clogging of the silicon nitride particles which reduces their reinforcing effect.
- the silicon nitride particles may have three different crystallographic structures designated as ⁇ , ⁇ and ⁇ phases (also known as trigonal phase, hexagonal phase and cubic phase).
- the ⁇ and ⁇ phases are the most common.
- the ⁇ phase can only be synthesized under high pressure and high temperature. Any of these phases can be used.
- the phase used is the ⁇ phase.
- the silicon nitride particles added may also be a mixture of particles of different phases.
- a grinding tool according to the invention is compared with a standard grinding tool.
- the vertical axis represents power consumption while the horizontal axis represents number of work pieces upon which the respective grinding tool has acted.
- B5 represents a grinding tool according to the invention while EZ represents a standard grinding tool.
- the tool represented as B5 has a power consumption that first rises steeply and thereafter remains substantially constant.
- the conventional tool as represented by EZ has a power consumption that rises steeply and then suddenly drops before it rises again. This indicates that the abrasive particles of the B5 tool are slowly fragmented while EZ represents a grinding tool where the abrasive particles are suddenly torn away. The wear on the tool will therefore be faster.
- B5 represents a tool with both a metal bonding agent and a polymeric bonding agent.
- the metal bonding agent is a bronze that has copper, tin and silver. It has been sintered using a metal powder that contains 45 % by volume copper, 45 % by volume tin and 10 % by volume silver.
- the polymeric bonding agent constitutes 1.0 % by volume of the total amount of bonding agent.
- the grinding tool of Figure 1 may have a cross section as shown in Figure 5 .
- the abrasive rim 4 is placed radially outside the core 2 such that the rim 4 completely surrounds the core 2.
- the tests explained with reference to Figure 4 , Figure 7 and Figure 8 have been carried out on such a grinding tool.
- the invention is not limited to such an embodiment.
- the core 2 may extend at least as much in the radial direction as the abrasive rim 4.
- the grinding tool has an abrasive rim 4 that does not extend beyond the core 2 in the radial direction.
- the abrasive rim 4 has an extension in the axial direction that is different from that of the core 2 (the axial direction being the axis of rotation A of the grinding tool 1 when it is driven by a spindle, see Figure 5 and Figure 6 ).
- the grinding tool 1 is not necessarily designed for rotation. Instead, it could act on work pieces in a reciprocating movement.
- the term "core” should thus be understood broadly as any kind of carrier body for the abrasive rim.
- the term “rim” should also be understood broadly as any kind of layer secured to the core 2 such that abrasive particles can act on a work piece.
- the invention further comprises a method of making the inventive grinding tool.
- the method comprises sintering abrasive particles together with metallic powder that comprises copper and tin such that the sintering results in a matrix in which the abrasive particles 5 are embedded.
- the matrix comprises a metallic bonding agent which is a sintered bronze alloy.
- silicon nitride in the form of a powder is added to the metallic powder before sintering to such an extent that the silicon nitride will constitute 0.1% - 5.0% by volume of the metallic bonding agent.
- the metal powder used is preferably metal powder with particles that are smaller than 44 ⁇ m but they should preferably be larger than the silicon nitride particles. Preferably they should be at least twice as large. An average size in the range of 15 ⁇ m - 44 ⁇ m may be suitable.
- the metallic powder may optionally also comprise silver.
- the metal powder may come in the shape of pre-alloyed particles or as particles of pure copper, pure tin, pure silver etc.
- a polymer may be added to the metallic powder before sintering, preferably in the form of polyimide powder, such that also a polymeric bonding agent is formed which is a part of the matrix 6.
- the sintering method may be carried out such that the powder material for the bonding agents of the matrix 6 is mixed with the abrasive particles 5.
- the mixture is compacted in a cold press. Thereafter the compacted is mixture is cured in a kiln at a temperature in the range of 380°C - 520°C, preferably 400°C - 500°C or 440°C - 460°C, for a period of 120 - 150 minutes.
- the time required depends on size. In a larger press form, more time is required.
- the compacted and cured mixture is placed in a press and subjected to a pressure of 1500 - 2000 kg/cm 2. The pressure is then maintained until the mixture has reached a temperature below 300°C.
- the inventors have made grinding tools according to this method in a process where the temperature in the kiln was 450°C.
- the abrasive rim 4 may also be manufactured by means of spark plasma sintering (SPS). By this technique, the abrasive rim 4 may be manufactured very fast.
- SPS spark plasma sintering
- the rim with the matrix containing abrasive particles may be sintered separately and subsequently fastened (e.g. glued) onto the core 2.
- the abrasive rim 5 may be sintered directly onto the core 2 such that it is bonded to the core as it is formed.
- the core 2 may be electrolytically plated with copper on at least one surface of the core which will meet the abrasive rim 4.
- the abrasive rim 4 can then be sintered onto the copper-plated surface such that a seam is formed.
- Filler material may optionally be added to the mixture of metallic powder and abrasive particles 5 before the sintering operation.
- the filler material may comprise graphite.
- Other possible filler materials may include, for example, spheres of aluminum oxide.
- the bronze used in the metal bonding agent is selected from the group including copper - tin (Cu/Sn), copper - tin - cobalt (Cu/Sn/Co), copper - tin - nickel (Cu/Sn/Ni) or copper - tin - silver (Cu/Sn/Ag). Even more preferred, the bronze is a copper - tin - silver bronze. Other bronze alloys can also be considered.
- the inventive grinding tool can be used for machining hard and/or brittle materials. This does not exclude the possibility that the grinding tool can be used also for other materials.
- the matrix 6 may optionally also comprise at least one ceramic component in the shape of ceramic particles.
- the ceramic component may be, for example, frit and contain SiO 2 .
- Ceramic particles for the matrix may be frit in the shape of spherical particles having a particle size of 50 ⁇ m - 500 ⁇ m depending on the size of the abrasive particles. For larger abrasive particles, larger ceramic particles will be used.
- the abrasive particles may be embedded in the ceramic particles while the ceramic particles are embedded in a hybrid matrix with a metallic bonding agent and a polymeric bonding agent.
- the ceramic particles may be held stronger by the matrix than the abrasive particles would be held. The free-cutting properties of the abrasive rim are thus improved.
- the ceramic component does not have such a good resistance to wear as the metallic bonding agent. By combining ceramics, metal and polymeric bonding agents, it is possible to combine the best properties of these bonding agents.
Landscapes
- Engineering & Computer Science (AREA)
- Mechanical Engineering (AREA)
- Chemical & Material Sciences (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Ceramic Engineering (AREA)
- Inorganic Chemistry (AREA)
- Manufacturing & Machinery (AREA)
- Polishing Bodies And Polishing Tools (AREA)
Claims (15)
- Outil de meulage (1) pour usiner des matériaux durs et/ou cassants, lequel outil de meulage (1) comprend un cœur (2) et un rebord abrasif (4), le rebord abrasif (4) comprenant des particules abrasives (5) intégrées dans une matrice (6), la matrice (6) comprenant un agent de liaison métallique qui est un alliage de bronze fritté, l'agent de liaison métallique constituant 50 % à 100 % en volume de la matrice, l'agent de liaison métallique contenant du nitrure de silicium en une quantité qui constitue 0,02 % à 2,0 % en volume de l'agent de liaison métallique dans lequel le nitrure de silicium est présent sous la forme de grains ayant une taille moyenne de grain qui est inférieure à 10 µm et supérieure à 0,1 µm.
- Outil de meulage (1) selon la revendication 1, dans lequel la matrice (6) comprend en outre un agent de liaison polymère qui a été fritté conjointement avec l'agent de liaison métallique de telle sorte que l'agent de liaison polymère et l'agent de liaison métallique forment un réseau connecté.
- Outil de meulage (1) selon la revendication 1 ou la revendication 2, dans lequel le nitrure de silicium constitue 0,3 % à 2,0 % par volume de l'agent de liaison métallique.
- Outil de meulage (1) selon la revendication 1 ou la revendication 2, dans lequel le nitrure de silicium constitue 0,5 % à 2 % par volume de l'agent de liaison métallique.
- Outil de meulage selon la revendication 2, dans lequel l'agent de liaison polymère comprend du polyimide.
- Outil de meulage selon la revendication 1 ou la revendication 2, dans lequel la matrice comprend en outre des matériaux de remplissage tels que du graphite.
- Outil de meulage selon l'une quelconque des revendications 1 à 6, dans lequel l'agent de liaison métallique est un alliage de bronze qui comprend du cuivre, de l'étain et de l'argent.
- Outil de meulage selon l'une quelconque des revendications 1 à 7, dans lequel les particules abrasives (5) sont des particules de diamant ou des particules de nitrure de bore cubiques.
- Outil de meulage selon la revendication 8, dans lequel les particules abrasives (5) ont une taille moyenne de particule dans la plage de 4 µm à 181 µm et de préférence dans la plage de 46 µm à 91 µm.
- Outil de meulage selon la revendication 9, dans lequel les particules abrasives (5) ont un revêtement de cuivre ou de nickel.
- Procédé de fabrication d'un outil de meulage (1) lequel procédé comprend le frittage de particules abrasives conjointement avec de la poudre métallique qui comprend du cuivre et de l'étain de telle sorte que le frittage donne une matrice (6) dans laquelle les particules abrasives (6) sont intégrées, la matrice comprenant un agent de liaison métallique qui est un alliage de bronze fritté, dans lequel du nitrure de silicium sous la forme d'une poudre est ajouté à la poudre métallique avant le frittage et dans une telle mesure que le nitrure de silicium constitue 0,02 % à 2,0 % par volume de l'agent de liaison métallique et dans lequel le nitrure de silicium qui est ajouté est sous la forme de grains ayant une taille moyenne de grain qui est inférieure à 10 µm et supérieure à 0,1 µm.
- Procédé selon la revendication 11 dans lequel la poudre métallique comprend en outre de l'argent.
- Procédé selon la revendication 11 ou la revendication 12, dans lequel un polymère est ajouté à la poudre métallique avant le frittage, de préférence sous la forme de poudre de polyimide, de telle sorte que également un agent de liaison polymère est formé qui est une partie de la matrice (6).
- Procédé selon l'une quelconque des revendications 11 à 13, dans lequel le procédé comprend ; mélanger le matériau en poudre pour les agents de liaison de la matrice (6) avec les particules abrasives (5) ; compacter le mélange dans une presse à froid ; durcir le mélange compacté dans un four à une température dans la plage de 380 °C à 520 °C, de préférence de 400 °C à 500 °C, pendant une période de 120 à 150 minutes ; ensuite placer le mélange compacté et durci dans une presse et le soumettre à une pression de 1500 à 2000 kg/cm2 ; et maintenir la pression jusqu'à ce que le mélange ait atteint une température inférieure à 300 °C.
- Procédé selon l'une quelconque des revendications 11 à 14, dans lequel un matériau de remplissage est ajouté au mélange de poudre métallique et de particules abrasives (5) avant l'opération de frittage et dans lequel le matériau de remplissage comprend du graphite.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| SE1150720A SE537723C2 (sv) | 2011-07-22 | 2011-07-22 | Ett slipverktyg för bearbetning av spröda material samt ettförfarande för framställning av ett slipverktyg |
| PCT/SE2012/050842 WO2013015737A1 (fr) | 2011-07-22 | 2012-07-18 | Outil de meulage pour usiner des matières fragiles et procédé de fabrication d'un outil de meulage |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP2734334A1 EP2734334A1 (fr) | 2014-05-28 |
| EP2734334A4 EP2734334A4 (fr) | 2015-11-11 |
| EP2734334B1 true EP2734334B1 (fr) | 2022-11-02 |
Family
ID=47601365
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP12817725.0A Active EP2734334B1 (fr) | 2011-07-22 | 2012-07-18 | Outil de meulage pour usiner des matières fragiles et procédé de fabrication d'un outil de meulage |
Country Status (15)
| Country | Link |
|---|---|
| US (1) | US20140227952A1 (fr) |
| EP (1) | EP2734334B1 (fr) |
| JP (1) | JP5982725B2 (fr) |
| KR (1) | KR101861890B1 (fr) |
| CN (1) | CN103781596B (fr) |
| AU (1) | AU2012287547B2 (fr) |
| BR (1) | BR112014001447A2 (fr) |
| CA (1) | CA2842534A1 (fr) |
| IL (1) | IL230524A (fr) |
| MX (1) | MX358578B (fr) |
| MY (1) | MY169695A (fr) |
| RU (1) | RU2594923C2 (fr) |
| SE (1) | SE537723C2 (fr) |
| WO (1) | WO2013015737A1 (fr) |
| ZA (1) | ZA201400915B (fr) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN107428014B (zh) * | 2015-06-22 | 2019-06-04 | 京瓷株式会社 | 刀具 |
| JP6687231B2 (ja) * | 2015-07-15 | 2020-04-22 | 三井研削砥石株式会社 | 研磨工具及びその製造方法並びに研磨物の製造方法 |
| CN109571291A (zh) * | 2018-12-20 | 2019-04-05 | 江苏友美工具有限公司 | 多功能磨切金刚石磨轮及其制备工艺 |
| WO2021161332A1 (fr) * | 2020-02-11 | 2021-08-19 | INDIAN INSTITUTE OF TECHNOLOGY MADRAS (IIT Madras) | Système et procédé de développement de meules brasées à couche unique par placement de grains dans un réseau prédéfini |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2003094341A (ja) * | 2001-09-27 | 2003-04-03 | Allied Material Corp | メタルボンド超砥粒砥石 |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| SU1021586A1 (ru) * | 1982-01-27 | 1983-06-07 | Всесоюзный научно-исследовательский конструкторско-технологический институт природных алмазов и инструмента | Металлическа св зка дл изготовлени алмазного инструмента |
| JPS61100374A (ja) * | 1984-10-23 | 1986-05-19 | Toyota Banmotsupusu Kk | 研削工具 |
| JPS61111885A (ja) * | 1984-11-06 | 1986-05-29 | Showa Denko Kk | 研削用成形体 |
| RU2101164C1 (ru) * | 1995-11-22 | 1998-01-10 | Институт сверхтвердых материалов им.В.Н.Бакуля НАН Украины | Связка абразивного инструмента |
| AT403671B (de) * | 1996-02-14 | 1998-04-27 | Swarovski Tyrolit Schleif | Schleifwerkzeug mit einem metall-kunstharzbindemittel und verfahren zu seiner herstellung |
| JP3052896B2 (ja) * | 1997-06-13 | 2000-06-19 | 日本電気株式会社 | 研磨布表面のドレス治具及びその製造方法 |
| US6066189A (en) * | 1998-12-17 | 2000-05-23 | Norton Company | Abrasive article bonded using a hybrid bond |
| US6200208B1 (en) * | 1999-01-07 | 2001-03-13 | Norton Company | Superabrasive wheel with active bond |
| JP2002001668A (ja) * | 2000-06-19 | 2002-01-08 | Mitsubishi Materials Corp | メタルボンド砥石 |
| AU2003230237A1 (en) * | 2002-04-11 | 2003-10-20 | Showa Denko, K. K. | Metal-coated abrasives, grinding wheel using metal-coated abrasives and method of producing metal-coated abrasives |
| KR20070008717A (ko) * | 2004-05-03 | 2007-01-17 | 쓰리엠 이노베이티브 프로퍼티즈 컴파니 | 미세마무리를 위한 지지 슈 및 방법 |
| CN1788931A (zh) * | 2005-12-23 | 2006-06-21 | 湖南大学 | 工程陶瓷材料高效深磨磨削工艺 |
| JP5338107B2 (ja) * | 2008-03-28 | 2013-11-13 | 東レ株式会社 | 砥石およびその製造方法 |
-
2011
- 2011-07-22 SE SE1150720A patent/SE537723C2/sv not_active IP Right Cessation
-
2012
- 2012-07-18 CN CN201280036226.6A patent/CN103781596B/zh active Active
- 2012-07-18 MY MYPI2014000176A patent/MY169695A/en unknown
- 2012-07-18 MX MX2014000837A patent/MX358578B/es active IP Right Grant
- 2012-07-18 US US14/233,932 patent/US20140227952A1/en not_active Abandoned
- 2012-07-18 JP JP2014521596A patent/JP5982725B2/ja not_active Expired - Fee Related
- 2012-07-18 RU RU2014106604/02A patent/RU2594923C2/ru not_active IP Right Cessation
- 2012-07-18 KR KR1020147004205A patent/KR101861890B1/ko active IP Right Grant
- 2012-07-18 EP EP12817725.0A patent/EP2734334B1/fr active Active
- 2012-07-18 WO PCT/SE2012/050842 patent/WO2013015737A1/fr active Application Filing
- 2012-07-18 CA CA2842534A patent/CA2842534A1/fr not_active Abandoned
- 2012-07-18 BR BR112014001447A patent/BR112014001447A2/pt not_active IP Right Cessation
- 2012-07-18 AU AU2012287547A patent/AU2012287547B2/en not_active Ceased
-
2014
- 2014-01-19 IL IL230524A patent/IL230524A/en active IP Right Grant
- 2014-02-06 ZA ZA2014/00915A patent/ZA201400915B/en unknown
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2003094341A (ja) * | 2001-09-27 | 2003-04-03 | Allied Material Corp | メタルボンド超砥粒砥石 |
Also Published As
| Publication number | Publication date |
|---|---|
| SE1150720A1 (sv) | 2013-01-23 |
| SE537723C2 (sv) | 2015-10-06 |
| CN103781596A (zh) | 2014-05-07 |
| MX2014000837A (es) | 2014-07-09 |
| MX358578B (es) | 2018-08-27 |
| US20140227952A1 (en) | 2014-08-14 |
| EP2734334A4 (fr) | 2015-11-11 |
| CN103781596B (zh) | 2016-10-19 |
| ZA201400915B (en) | 2014-11-26 |
| AU2012287547B2 (en) | 2017-02-02 |
| WO2013015737A1 (fr) | 2013-01-31 |
| WO2013015737A9 (fr) | 2013-04-04 |
| KR101861890B1 (ko) | 2018-05-28 |
| MY169695A (en) | 2019-05-13 |
| KR20140061415A (ko) | 2014-05-21 |
| IL230524A0 (en) | 2014-03-31 |
| BR112014001447A2 (pt) | 2017-02-21 |
| IL230524A (en) | 2017-10-31 |
| CA2842534A1 (fr) | 2013-01-31 |
| RU2594923C2 (ru) | 2016-08-20 |
| NZ620302A (en) | 2015-12-24 |
| AU2012287547A1 (en) | 2014-03-06 |
| RU2014106604A (ru) | 2015-09-10 |
| JP2014522740A (ja) | 2014-09-08 |
| JP5982725B2 (ja) | 2016-08-31 |
| EP2734334A1 (fr) | 2014-05-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8882868B2 (en) | Abrasive slicing tool for electronics industry | |
| TWI449601B (zh) | 硬性及/或脆性材料之研磨處理 | |
| EP2699387B1 (fr) | Roue de meulage liée à la résine | |
| JP2006346857A (ja) | 研磨工具 | |
| EP2734334B1 (fr) | Outil de meulage pour usiner des matières fragiles et procédé de fabrication d'un outil de meulage | |
| JP2014237892A (ja) | 立方晶窒化ホウ素成形体 | |
| CN109093122B (zh) | 一种切削型金刚石刀具及其制备方法 | |
| CN108119065B (zh) | 孕镶金刚石钻头切削齿及其制造方法 | |
| CN108818329B (zh) | 一种金刚石砂轮及其制备方法 | |
| KR100407227B1 (ko) | 복합본드숫돌 및 수지결합상을 보유하는 숫돌 | |
| JP5033814B2 (ja) | 切削工具用切削チップ及び切削チップの製造方法並びに切削工具 | |
| CN104175237A (zh) | 稀土改性钨基结合剂金刚石磨轮及其制造方法 | |
| CN108818331B (zh) | 一种青铜基cbn砂轮及其制备方法 | |
| CN104128605A (zh) | 稀土改性钨基结合剂金刚石圆锯片及其制造方法 | |
| NZ620302B2 (en) | A grinding tool for machining brittle materials and a method of making a grinding tool | |
| JPH10296636A (ja) | メタルボンド砥石 | |
| JP2020504684A (ja) | 研磨物品を形成するプロセス | |
| JPH10202533A (ja) | ダイヤモンド切断砥石 | |
| CN104148639A (zh) | 稀土改性钨基结合剂金刚石砂轮及其制造方法 | |
| CN104148641A (zh) | 稀土改性钨基结合剂金刚石角磨片及其制造方法 | |
| MXPA00009489A (en) | Abrasive tools | |
| JP2011088263A (ja) | メタルボンドホイールおよび工具の製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20140120 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: THOLIN, MICHAEL Inventor name: JOHANSSON, IDA Inventor name: WESTBERG, FREDRIK Inventor name: BERGH, STEFAN |
|
| DAX | Request for extension of the european patent (deleted) | ||
| RA4 | Supplementary search report drawn up and despatched (corrected) |
Effective date: 20151014 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: B22F 3/16 20060101ALI20151008BHEP Ipc: B24D 3/06 20060101ALI20151008BHEP Ipc: B24D 3/34 20060101AFI20151008BHEP Ipc: B24D 3/28 20060101ALI20151008BHEP Ipc: B22F 7/06 20060101ALI20151008BHEP Ipc: B24D 99/00 20100101ALI20151008BHEP |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: 3M INNOVATIVE PROPERTIES COMPANY |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| 17Q | First examination report despatched |
Effective date: 20181128 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: B22F 1/17 20220101ALN20220429BHEP Ipc: C22C 32/00 20060101ALI20220429BHEP Ipc: C22C 26/00 20060101ALI20220429BHEP Ipc: B24D 18/00 20060101ALI20220429BHEP Ipc: B24D 7/06 20060101ALI20220429BHEP Ipc: B24D 5/06 20060101ALI20220429BHEP Ipc: B24D 5/02 20060101ALI20220429BHEP Ipc: B24D 3/08 20060101ALI20220429BHEP Ipc: B22F 3/16 20060101ALI20220429BHEP Ipc: B22F 7/06 20060101ALI20220429BHEP Ipc: B24D 3/06 20060101ALI20220429BHEP Ipc: B24D 3/28 20060101ALI20220429BHEP Ipc: B24D 99/00 20100101ALI20220429BHEP Ipc: B24D 3/34 20060101AFI20220429BHEP |
|
| INTG | Intention to grant announced |
Effective date: 20220530 |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: JOHANSSON, IDA Inventor name: BERGH, STEFAN Inventor name: WESTBERG, FREDRIK Inventor name: THOLIN, MICHAEL |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP Ref country code: AT Ref legal event code: REF Ref document number: 1528420 Country of ref document: AT Kind code of ref document: T Effective date: 20221115 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602012078955 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG9D |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MP Effective date: 20221102 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 1528420 Country of ref document: AT Kind code of ref document: T Effective date: 20221102 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20221102 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230302 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230202 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20221102 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20221102 Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20221102 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20221102 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20221102 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20221102 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20221102 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230302 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20221102 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230203 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20221102 |
|
| P01 | Opt-out of the competence of the unified patent court (upc) registered |
Effective date: 20230530 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20221102 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20221102 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20221102 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20221102 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20221102 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20230621 Year of fee payment: 12 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602012078955 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20221102 Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20221102 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20230803 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20221102 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20230620 Year of fee payment: 12 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20221102 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20221102 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20230731 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230718 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20230718 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230718 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MM4A |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230718 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230731 |