EP2475387B1 - Vap-1 inhibitors for use in treating fibrotic conditions - Google Patents
Vap-1 inhibitors for use in treating fibrotic conditions Download PDFInfo
- Publication number
- EP2475387B1 EP2475387B1 EP10815041.8A EP10815041A EP2475387B1 EP 2475387 B1 EP2475387 B1 EP 2475387B1 EP 10815041 A EP10815041 A EP 10815041A EP 2475387 B1 EP2475387 B1 EP 2475387B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- fibrosis
- vap
- seq
- nos
- fibrotic
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000003112 inhibitor Substances 0.000 title claims description 62
- 230000003176 fibrotic effect Effects 0.000 title claims description 51
- 101000774560 Crotalus atrox Zinc metalloproteinase-disintegrin-like VAP1 Proteins 0.000 title 1
- 101710132836 Membrane primary amine oxidase Proteins 0.000 claims description 89
- 241000282414 Homo sapiens Species 0.000 claims description 63
- 206010016654 Fibrosis Diseases 0.000 claims description 59
- 230000004761 fibrosis Effects 0.000 claims description 53
- 208000019425 cirrhosis of liver Diseases 0.000 claims description 28
- 102100024092 Aldo-keto reductase family 1 member C4 Human genes 0.000 claims description 24
- 238000011282 treatment Methods 0.000 claims description 21
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 20
- 210000001519 tissue Anatomy 0.000 claims description 19
- 208000006454 hepatitis Diseases 0.000 claims description 17
- 201000010099 disease Diseases 0.000 claims description 16
- 229920001184 polypeptide Polymers 0.000 claims description 16
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 16
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 16
- 102000008186 Collagen Human genes 0.000 claims description 15
- 108010035532 Collagen Proteins 0.000 claims description 15
- 208000007342 Diabetic Nephropathies Diseases 0.000 claims description 15
- 229920001436 collagen Polymers 0.000 claims description 15
- 208000033679 diabetic kidney disease Diseases 0.000 claims description 15
- 201000002793 renal fibrosis Diseases 0.000 claims description 12
- 238000009825 accumulation Methods 0.000 claims description 11
- 208000006545 Chronic Obstructive Pulmonary Disease Diseases 0.000 claims description 10
- 206010067125 Liver injury Diseases 0.000 claims description 10
- 231100000753 hepatic injury Toxicity 0.000 claims description 10
- 210000000056 organ Anatomy 0.000 claims description 10
- 208000005069 pulmonary fibrosis Diseases 0.000 claims description 10
- 102000056133 human AOC3 Human genes 0.000 claims description 9
- 210000000651 myofibroblast Anatomy 0.000 claims description 9
- 230000037390 scarring Effects 0.000 claims description 9
- 206010008909 Chronic Hepatitis Diseases 0.000 claims description 7
- 206010057469 Vascular stenosis Diseases 0.000 claims description 7
- 230000001154 acute effect Effects 0.000 claims description 7
- 208000018631 connective tissue disease Diseases 0.000 claims description 7
- 208000037803 restenosis Diseases 0.000 claims description 7
- 206010054793 Arterial fibrosis Diseases 0.000 claims description 6
- 206010058029 Arthrofibrosis Diseases 0.000 claims description 6
- 206010005042 Bladder fibrosis Diseases 0.000 claims description 6
- 206010006253 Breast fibrosis Diseases 0.000 claims description 6
- 108091035707 Consensus sequence Proteins 0.000 claims description 6
- 208000014919 IgG4-related retroperitoneal fibrosis Diseases 0.000 claims description 6
- 206010025180 Lymph node fibrosis Diseases 0.000 claims description 6
- 206010048654 Muscle fibrosis Diseases 0.000 claims description 6
- 206010035600 Pleural fibrosis Diseases 0.000 claims description 6
- 206010038979 Retroperitoneal fibrosis Diseases 0.000 claims description 6
- 206010039710 Scleroderma Diseases 0.000 claims description 6
- 206010050207 Skin fibrosis Diseases 0.000 claims description 6
- 206010068107 Thyroid fibrosis Diseases 0.000 claims description 6
- 230000009787 cardiac fibrosis Effects 0.000 claims description 6
- 230000004968 inflammatory condition Effects 0.000 claims description 6
- 206010028537 myelofibrosis Diseases 0.000 claims description 6
- 231100000331 toxic Toxicity 0.000 claims description 6
- 230000002588 toxic effect Effects 0.000 claims description 6
- 238000004519 manufacturing process Methods 0.000 claims description 4
- 239000003814 drug Substances 0.000 claims description 3
- 125000003275 alpha amino acid group Chemical group 0.000 claims 2
- 102100027159 Membrane primary amine oxidase Human genes 0.000 description 144
- 108010028700 Amine Oxidase (Copper-Containing) Proteins 0.000 description 61
- 241000699670 Mus sp. Species 0.000 description 50
- 230000000694 effects Effects 0.000 description 44
- 210000004185 liver Anatomy 0.000 description 29
- 239000003981 vehicle Substances 0.000 description 22
- 206010012601 diabetes mellitus Diseases 0.000 description 21
- 239000011159 matrix material Substances 0.000 description 21
- 241001465754 Metazoa Species 0.000 description 18
- 210000002966 serum Anatomy 0.000 description 18
- 238000000034 method Methods 0.000 description 15
- 210000004024 hepatic stellate cell Anatomy 0.000 description 14
- 230000009467 reduction Effects 0.000 description 13
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 12
- 150000001413 amino acids Chemical group 0.000 description 12
- 230000005764 inhibitory process Effects 0.000 description 12
- 238000010172 mouse model Methods 0.000 description 12
- 241000699666 Mus <mouse, genus> Species 0.000 description 11
- 238000004458 analytical method Methods 0.000 description 11
- 238000012360 testing method Methods 0.000 description 11
- 102000016942 Elastin Human genes 0.000 description 10
- 108010014258 Elastin Proteins 0.000 description 10
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Chemical compound OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 10
- 235000001014 amino acid Nutrition 0.000 description 10
- 229940024606 amino acid Drugs 0.000 description 10
- 238000003556 assay Methods 0.000 description 10
- 229920002549 elastin Polymers 0.000 description 10
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 9
- 230000001434 glomerular Effects 0.000 description 9
- 210000003734 kidney Anatomy 0.000 description 9
- 229910052717 sulfur Inorganic materials 0.000 description 9
- 229910052727 yttrium Inorganic materials 0.000 description 9
- 108010088751 Albumins Proteins 0.000 description 8
- 102000009027 Albumins Human genes 0.000 description 8
- BAVYZALUXZFZLV-UHFFFAOYSA-N Methylamine Chemical compound NC BAVYZALUXZFZLV-UHFFFAOYSA-N 0.000 description 8
- 210000004027 cell Anatomy 0.000 description 8
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 8
- 150000001875 compounds Chemical class 0.000 description 8
- DDRJAANPRJIHGJ-UHFFFAOYSA-N creatinine Chemical compound CN1CC(=O)NC1=N DDRJAANPRJIHGJ-UHFFFAOYSA-N 0.000 description 8
- 239000000243 solution Substances 0.000 description 8
- 238000010186 staining Methods 0.000 description 8
- 238000002560 therapeutic procedure Methods 0.000 description 8
- 210000001124 body fluid Anatomy 0.000 description 7
- 239000003795 chemical substances by application Substances 0.000 description 7
- 230000006378 damage Effects 0.000 description 7
- 239000000203 mixture Substances 0.000 description 7
- 239000000758 substrate Substances 0.000 description 7
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 6
- 208000034827 Neointima Diseases 0.000 description 6
- 230000003510 anti-fibrotic effect Effects 0.000 description 6
- WGQKYBSKWIADBV-UHFFFAOYSA-N benzylamine Chemical compound NCC1=CC=CC=C1 WGQKYBSKWIADBV-UHFFFAOYSA-N 0.000 description 6
- 210000004369 blood Anatomy 0.000 description 6
- 239000008280 blood Substances 0.000 description 6
- 230000007882 cirrhosis Effects 0.000 description 6
- 229960003957 dexamethasone Drugs 0.000 description 6
- 229910052731 fluorine Inorganic materials 0.000 description 6
- 239000008103 glucose Substances 0.000 description 6
- 238000002347 injection Methods 0.000 description 6
- 239000007924 injection Substances 0.000 description 6
- 208000014674 injury Diseases 0.000 description 6
- 206010053219 non-alcoholic steatohepatitis Diseases 0.000 description 6
- 230000002829 reductive effect Effects 0.000 description 6
- ZSJLQEPLLKMAKR-GKHCUFPYSA-N streptozocin Chemical compound O=NN(C)C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O ZSJLQEPLLKMAKR-GKHCUFPYSA-N 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- VZGDMQKNWNREIO-UHFFFAOYSA-N tetrachloromethane Chemical compound ClC(Cl)(Cl)Cl VZGDMQKNWNREIO-UHFFFAOYSA-N 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- 238000011740 C57BL/6 mouse Methods 0.000 description 5
- 101100222276 Drosophila melanogaster cuff gene Proteins 0.000 description 5
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 5
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 5
- 102000010909 Monoamine Oxidase Human genes 0.000 description 5
- 108010062431 Monoamine oxidase Proteins 0.000 description 5
- 241000208125 Nicotiana Species 0.000 description 5
- 235000002637 Nicotiana tabacum Nutrition 0.000 description 5
- ZSJLQEPLLKMAKR-UHFFFAOYSA-N Streptozotocin Natural products O=NN(C)C(=O)NC1C(O)OC(CO)C(O)C1O ZSJLQEPLLKMAKR-UHFFFAOYSA-N 0.000 description 5
- 208000004608 Ureteral Obstruction Diseases 0.000 description 5
- 208000027418 Wounds and injury Diseases 0.000 description 5
- 238000000540 analysis of variance Methods 0.000 description 5
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 5
- 238000011813 knockout mouse model Methods 0.000 description 5
- 230000003902 lesion Effects 0.000 description 5
- 210000004072 lung Anatomy 0.000 description 5
- 230000008692 neointimal formation Effects 0.000 description 5
- 238000001543 one-way ANOVA Methods 0.000 description 5
- 239000008194 pharmaceutical composition Substances 0.000 description 5
- 108090000623 proteins and genes Proteins 0.000 description 5
- 239000000523 sample Substances 0.000 description 5
- 239000000779 smoke Substances 0.000 description 5
- 239000011780 sodium chloride Substances 0.000 description 5
- 229960001052 streptozocin Drugs 0.000 description 5
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 4
- 101000694615 Homo sapiens Membrane primary amine oxidase Proteins 0.000 description 4
- -1 aSMA Proteins 0.000 description 4
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 4
- 230000027455 binding Effects 0.000 description 4
- 239000000090 biomarker Substances 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 230000036772 blood pressure Effects 0.000 description 4
- 230000037396 body weight Effects 0.000 description 4
- 235000012000 cholesterol Nutrition 0.000 description 4
- 229940109239 creatinine Drugs 0.000 description 4
- 238000011161 development Methods 0.000 description 4
- 238000003745 diagnosis Methods 0.000 description 4
- 208000035475 disorder Diseases 0.000 description 4
- 230000029142 excretion Effects 0.000 description 4
- 231100000283 hepatitis Toxicity 0.000 description 4
- OAKJQQAXSVQMHS-UHFFFAOYSA-N hydrazine Substances NN OAKJQQAXSVQMHS-UHFFFAOYSA-N 0.000 description 4
- 230000004054 inflammatory process Effects 0.000 description 4
- 230000003907 kidney function Effects 0.000 description 4
- 210000004698 lymphocyte Anatomy 0.000 description 4
- 210000002540 macrophage Anatomy 0.000 description 4
- 108020004999 messenger RNA Proteins 0.000 description 4
- 239000002480 mineral oil Substances 0.000 description 4
- 235000010446 mineral oil Nutrition 0.000 description 4
- 208000008338 non-alcoholic fatty liver disease Diseases 0.000 description 4
- KHIWWQKSHDUIBK-UHFFFAOYSA-N periodic acid Chemical compound OI(=O)(=O)=O KHIWWQKSHDUIBK-UHFFFAOYSA-N 0.000 description 4
- 235000018102 proteins Nutrition 0.000 description 4
- 102000004169 proteins and genes Human genes 0.000 description 4
- 230000004044 response Effects 0.000 description 4
- MNDBXUUTURYVHR-UHFFFAOYSA-N roflumilast Chemical compound FC(F)OC1=CC=C(C(=O)NC=2C(=CN=CC=2Cl)Cl)C=C1OCC1CC1 MNDBXUUTURYVHR-UHFFFAOYSA-N 0.000 description 4
- 229960002586 roflumilast Drugs 0.000 description 4
- 230000008719 thickening Effects 0.000 description 4
- 230000002792 vascular Effects 0.000 description 4
- VXLBSYHAEKDUSU-JXMROGBWSA-N (2e)-2-(fluoromethylidene)-4-(4-fluorophenyl)butan-1-amine Chemical compound NC\C(=C\F)CCC1=CC=C(F)C=C1 VXLBSYHAEKDUSU-JXMROGBWSA-N 0.000 description 3
- 102100032752 C-reactive protein Human genes 0.000 description 3
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- 239000004471 Glycine Substances 0.000 description 3
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 3
- 206010020772 Hypertension Diseases 0.000 description 3
- 206010061218 Inflammation Diseases 0.000 description 3
- PIWKPBJCKXDKJR-UHFFFAOYSA-N Isoflurane Chemical compound FC(F)OC(Cl)C(F)(F)F PIWKPBJCKXDKJR-UHFFFAOYSA-N 0.000 description 3
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 3
- 208000031481 Pathologic Constriction Diseases 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 238000011529 RT qPCR Methods 0.000 description 3
- 206010061481 Renal injury Diseases 0.000 description 3
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 3
- 229930006000 Sucrose Natural products 0.000 description 3
- 229940127282 angiotensin receptor antagonist Drugs 0.000 description 3
- 239000000872 buffer Substances 0.000 description 3
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 3
- 239000003153 chemical reaction reagent Substances 0.000 description 3
- 230000001684 chronic effect Effects 0.000 description 3
- 230000007423 decrease Effects 0.000 description 3
- 239000008121 dextrose Substances 0.000 description 3
- UQLDLKMNUJERMK-UHFFFAOYSA-L di(octadecanoyloxy)lead Chemical compound [Pb+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O UQLDLKMNUJERMK-UHFFFAOYSA-L 0.000 description 3
- 238000013118 diabetic mouse model Methods 0.000 description 3
- 230000008030 elimination Effects 0.000 description 3
- 238000003379 elimination reaction Methods 0.000 description 3
- 210000003979 eosinophil Anatomy 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 210000002744 extracellular matrix Anatomy 0.000 description 3
- 210000002950 fibroblast Anatomy 0.000 description 3
- 239000012530 fluid Substances 0.000 description 3
- 239000012634 fragment Substances 0.000 description 3
- 238000010353 genetic engineering Methods 0.000 description 3
- 238000007490 hematoxylin and eosin (H&E) staining Methods 0.000 description 3
- 210000003494 hepatocyte Anatomy 0.000 description 3
- 150000002429 hydrazines Chemical class 0.000 description 3
- 238000003384 imaging method Methods 0.000 description 3
- 230000005847 immunogenicity Effects 0.000 description 3
- 238000007912 intraperitoneal administration Methods 0.000 description 3
- 239000007928 intraperitoneal injection Substances 0.000 description 3
- 208000017169 kidney disease Diseases 0.000 description 3
- 239000008101 lactose Substances 0.000 description 3
- 239000003550 marker Substances 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 230000001338 necrotic effect Effects 0.000 description 3
- 210000000440 neutrophil Anatomy 0.000 description 3
- 229910052757 nitrogen Inorganic materials 0.000 description 3
- 239000012188 paraffin wax Substances 0.000 description 3
- 239000000546 pharmaceutical excipient Substances 0.000 description 3
- 229920001223 polyethylene glycol Polymers 0.000 description 3
- 238000010149 post-hoc-test Methods 0.000 description 3
- 230000001084 renoprotective effect Effects 0.000 description 3
- 238000013424 sirius red staining Methods 0.000 description 3
- 230000007863 steatosis Effects 0.000 description 3
- 231100000240 steatosis hepatitis Toxicity 0.000 description 3
- 230000036262 stenosis Effects 0.000 description 3
- 208000037804 stenosis Diseases 0.000 description 3
- 239000005720 sucrose Substances 0.000 description 3
- 229910052721 tungsten Inorganic materials 0.000 description 3
- 238000007473 univariate analysis Methods 0.000 description 3
- 210000002700 urine Anatomy 0.000 description 3
- 229910052720 vanadium Inorganic materials 0.000 description 3
- 230000003442 weekly effect Effects 0.000 description 3
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 2
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 2
- PKYCWFICOKSIHZ-UHFFFAOYSA-N 1-(3,7-dihydroxyphenoxazin-10-yl)ethanone Chemical compound OC1=CC=C2N(C(=O)C)C3=CC=C(O)C=C3OC2=C1 PKYCWFICOKSIHZ-UHFFFAOYSA-N 0.000 description 2
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 2
- 101150014240 AOC3 gene Proteins 0.000 description 2
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 2
- 102000008128 Apolipoprotein E3 Human genes 0.000 description 2
- 108010060215 Apolipoprotein E3 Proteins 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- BPYKTIZUTYGOLE-IFADSCNNSA-N Bilirubin Chemical compound N1C(=O)C(C)=C(C=C)\C1=C\C1=C(C)C(CCC(O)=O)=C(CC2=C(C(C)=C(\C=C/3C(=C(C=C)C(=O)N\3)C)N2)CCC(O)=O)N1 BPYKTIZUTYGOLE-IFADSCNNSA-N 0.000 description 2
- 108010006654 Bleomycin Proteins 0.000 description 2
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 2
- WZUVPPKBWHMQCE-UHFFFAOYSA-N Haematoxylin Chemical compound C12=CC(O)=C(O)C=C2CC2(O)C1C1=CC=C(O)C(O)=C1OC2 WZUVPPKBWHMQCE-UHFFFAOYSA-N 0.000 description 2
- 206010019668 Hepatic fibrosis Diseases 0.000 description 2
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 2
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 2
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 2
- 101150046735 LEPR gene Proteins 0.000 description 2
- 101150063827 LEPROT gene Proteins 0.000 description 2
- 241001529936 Murinae Species 0.000 description 2
- 229930040373 Paraformaldehyde Natural products 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- 238000008050 Total Bilirubin Reagent Methods 0.000 description 2
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- 150000001412 amines Chemical class 0.000 description 2
- 229940044094 angiotensin-converting-enzyme inhibitor Drugs 0.000 description 2
- 230000003367 anti-collagen effect Effects 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 239000003125 aqueous solvent Substances 0.000 description 2
- 230000003143 atherosclerotic effect Effects 0.000 description 2
- 230000033228 biological regulation Effects 0.000 description 2
- 229960001561 bleomycin Drugs 0.000 description 2
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 description 2
- 230000000903 blocking effect Effects 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 238000004624 confocal microscopy Methods 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- 210000002919 epithelial cell Anatomy 0.000 description 2
- 210000001105 femoral artery Anatomy 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- 238000003209 gene knockout Methods 0.000 description 2
- 230000024924 glomerular filtration Effects 0.000 description 2
- 231100000853 glomerular lesion Toxicity 0.000 description 2
- 235000013922 glutamic acid Nutrition 0.000 description 2
- 239000004220 glutamic acid Substances 0.000 description 2
- 230000009931 harmful effect Effects 0.000 description 2
- 230000002440 hepatic effect Effects 0.000 description 2
- 238000007489 histopathology method Methods 0.000 description 2
- 229910052739 hydrogen Inorganic materials 0.000 description 2
- 230000001771 impaired effect Effects 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 238000010253 intravenous injection Methods 0.000 description 2
- 229960002725 isoflurane Drugs 0.000 description 2
- 229950010854 mofegiline Drugs 0.000 description 2
- 238000000491 multivariate analysis Methods 0.000 description 2
- 230000007935 neutral effect Effects 0.000 description 2
- 150000002923 oximes Chemical class 0.000 description 2
- 229920002866 paraformaldehyde Polymers 0.000 description 2
- 230000008506 pathogenesis Effects 0.000 description 2
- 230000007170 pathology Effects 0.000 description 2
- 239000002953 phosphate buffered saline Substances 0.000 description 2
- DHRLEVQXOMLTIM-UHFFFAOYSA-N phosphoric acid;trioxomolybdenum Chemical compound O=[Mo](=O)=O.O=[Mo](=O)=O.O=[Mo](=O)=O.O=[Mo](=O)=O.O=[Mo](=O)=O.O=[Mo](=O)=O.O=[Mo](=O)=O.O=[Mo](=O)=O.O=[Mo](=O)=O.O=[Mo](=O)=O.O=[Mo](=O)=O.O=[Mo](=O)=O.OP(O)(O)=O DHRLEVQXOMLTIM-UHFFFAOYSA-N 0.000 description 2
- 239000000902 placebo Substances 0.000 description 2
- 229940068196 placebo Drugs 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 2
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 2
- LWIHDJKSTIGBAC-UHFFFAOYSA-K potassium phosphate Substances [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 230000000750 progressive effect Effects 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 238000010561 standard procedure Methods 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 208000037816 tissue injury Diseases 0.000 description 2
- 150000003626 triacylglycerols Chemical class 0.000 description 2
- 208000001072 type 2 diabetes mellitus Diseases 0.000 description 2
- 230000002485 urinary effect Effects 0.000 description 2
- UZSBWSNIXUXPDF-LSDHHAIUSA-N (1r,2s)-2-[amino(methyl)amino]-1,2-diphenylethanol Chemical compound C1([C@@H](O)[C@@H](N(N)C)C=2C=CC=CC=2)=CC=CC=C1 UZSBWSNIXUXPDF-LSDHHAIUSA-N 0.000 description 1
- UDOPVWKLUOHIID-WPRPVWTQSA-N (1r,2s)-2-[amino(methyl)amino]-1-phenylpropan-1-ol Chemical compound CN(N)[C@@H](C)[C@H](O)C1=CC=CC=C1 UDOPVWKLUOHIID-WPRPVWTQSA-N 0.000 description 1
- UZSBWSNIXUXPDF-CABCVRRESA-N (1s,2r)-2-[amino(methyl)amino]-1,2-diphenylethanol Chemical compound C1([C@H](O)[C@H](N(N)C)C=2C=CC=CC=2)=CC=CC=C1 UZSBWSNIXUXPDF-CABCVRRESA-N 0.000 description 1
- DYPFWPZTPLZNEM-UWVGGRQHSA-N (1s,2s)-2-[amino(methyl)amino]-2,3-dihydro-1h-inden-1-ol Chemical compound C1=CC=C2[C@H](O)[C@@H](N(N)C)CC2=C1 DYPFWPZTPLZNEM-UWVGGRQHSA-N 0.000 description 1
- PLTXFYKTRPWVHP-LBPRGKRZSA-N (2s)-n,3-dihydroxy-2-[[4-(2-methylbutan-2-yl)phenyl]sulfonylamino]propanamide Chemical compound CCC(C)(C)C1=CC=C(S(=O)(=O)N[C@@H](CO)C(=O)NO)C=C1 PLTXFYKTRPWVHP-LBPRGKRZSA-N 0.000 description 1
- LVDIGZBRJXVHMH-RMKNXTFCSA-N (e)-3-fluoro-4-(4-methylsulfonylphenoxy)but-2-en-1-amine Chemical compound CS(=O)(=O)C1=CC=C(OC\C(F)=C/CN)C=C1 LVDIGZBRJXVHMH-RMKNXTFCSA-N 0.000 description 1
- KRMXWOUKQUUWEU-RUDMXATFSA-N (e)-3-fluoro-4-[(2-methyl-1,3-benzothiazol-5-yl)oxy]but-2-en-1-amine Chemical compound NC\C=C(F)/COC1=CC=C2SC(C)=NC2=C1 KRMXWOUKQUUWEU-RUDMXATFSA-N 0.000 description 1
- JXGONOWMPBTGBN-UHFFFAOYSA-N 1-[amino(methyl)amino]-3-(3-methoxyphenoxy)propan-2-ol Chemical compound COC1=CC=CC(OCC(O)CN(C)N)=C1 JXGONOWMPBTGBN-UHFFFAOYSA-N 0.000 description 1
- QUBIUECSHYHMOK-UHFFFAOYSA-N 2,3-dihydro-1h-indene;hydrazine Chemical class NN.C1=CC=C2CCCC2=C1 QUBIUECSHYHMOK-UHFFFAOYSA-N 0.000 description 1
- IZXIZTKNFFYFOF-UHFFFAOYSA-N 2-Oxazolidone Chemical class O=C1NCCO1 IZXIZTKNFFYFOF-UHFFFAOYSA-N 0.000 description 1
- CEMZPAYDPVUXTQ-UHFFFAOYSA-N 2-[4-[2-(2-acetamido-1,3-thiazol-4-yl)ethyl]phenyl]-n-(diaminomethylidene)acetamide Chemical compound S1C(NC(=O)C)=NC(CCC=2C=CC(CC(=O)NC(N)=N)=CC=2)=C1 CEMZPAYDPVUXTQ-UHFFFAOYSA-N 0.000 description 1
- MZMLXSFLDADQLO-UHFFFAOYSA-N 2-[4-[2-[5-(4-acetylpiperazin-1-yl)pyridin-2-yl]ethyl]phenyl]acetohydrazide Chemical compound C1CN(C(=O)C)CCN1C(C=N1)=CC=C1CCC1=CC=C(CC(=O)NN)C=C1 MZMLXSFLDADQLO-UHFFFAOYSA-N 0.000 description 1
- MZTVYRBWPUOPQJ-UHFFFAOYSA-N 2-aminooxy-1-(3,4-dimethoxyphenyl)ethanol Chemical compound COC1=CC=C(C(O)CON)C=C1OC MZTVYRBWPUOPQJ-UHFFFAOYSA-N 0.000 description 1
- ZLBWPYIKHLGYQC-UHFFFAOYSA-N 2-aminooxy-1-phenylethanol Chemical compound NOCC(O)C1=CC=CC=C1 ZLBWPYIKHLGYQC-UHFFFAOYSA-N 0.000 description 1
- URMVFILWXLQJIP-UHFFFAOYSA-N 4,5,6,7-tetrahydro-3h-imidazo[4,5-c]pyridine Chemical class C1NCCC2=C1NC=N2 URMVFILWXLQJIP-UHFFFAOYSA-N 0.000 description 1
- VVEBXHIYHLNGCX-UHFFFAOYSA-N 4-(4-methoxyphenyl)but-3-yn-1-amine Chemical compound COC1=CC=C(C#CCCN)C=C1 VVEBXHIYHLNGCX-UHFFFAOYSA-N 0.000 description 1
- ZVFVOMFIUGHFMF-LFIBNONCSA-N 4-[(e)-4-amino-2-fluorobut-2-enoxy]-n-(1-phenylethyl)benzenesulfonamide Chemical compound C=1C=CC=CC=1C(C)NS(=O)(=O)C1=CC=C(OC\C(F)=C/CN)C=C1 ZVFVOMFIUGHFMF-LFIBNONCSA-N 0.000 description 1
- VICFISWUVIINPR-UHFFFAOYSA-N 4-phenylbut-3-yn-1-amine Chemical compound NCCC#CC1=CC=CC=C1 VICFISWUVIINPR-UHFFFAOYSA-N 0.000 description 1
- GPXRFEBTDGYHMW-UHFFFAOYSA-N 5-amino-2-hydroxy-n-[(2-hydroxyphenyl)methyl]benzamide Chemical compound NC1=CC=C(O)C(C(=O)NCC=2C(=CC=CC=2)O)=C1 GPXRFEBTDGYHMW-UHFFFAOYSA-N 0.000 description 1
- WPZOWTYSBMBSMN-UHFFFAOYSA-N 5-phenoxypenta-2,3-dien-1-amine Chemical compound NCC=C=CCOC1=CC=CC=C1 WPZOWTYSBMBSMN-UHFFFAOYSA-N 0.000 description 1
- UKJCHPVMXDSGAD-UHFFFAOYSA-N 6-ethoxy-5-(hydroxyiminomethyl)-1,3-dimethylpyrimidine-2,4-dione Chemical compound CCOC1=C(C=NO)C(=O)N(C)C(=O)N1C UKJCHPVMXDSGAD-UHFFFAOYSA-N 0.000 description 1
- 239000005541 ACE inhibitor Substances 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 102000007469 Actins Human genes 0.000 description 1
- 108010085238 Actins Proteins 0.000 description 1
- 102100036475 Alanine aminotransferase 1 Human genes 0.000 description 1
- 108010082126 Alanine transaminase Proteins 0.000 description 1
- 206010001580 Albuminuria Diseases 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 102000013918 Apolipoproteins E Human genes 0.000 description 1
- 108010025628 Apolipoproteins E Proteins 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- 108010003415 Aspartate Aminotransferases Proteins 0.000 description 1
- 102000004625 Aspartate Aminotransferases Human genes 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 108010074051 C-Reactive Protein Proteins 0.000 description 1
- 238000011746 C57BL/6J (JAX™ mouse strain) Methods 0.000 description 1
- UWGDXLSOVZQIRL-UHFFFAOYSA-N CSC1=C(C=NO)C(=O)N(C)C(=O)N1C Chemical compound CSC1=C(C=NO)C(=O)N(C)C(=O)N1C UWGDXLSOVZQIRL-UHFFFAOYSA-N 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 102000008954 Copper amine oxidases Human genes 0.000 description 1
- 241000699802 Cricetulus griseus Species 0.000 description 1
- 235000015655 Crocus sativus Nutrition 0.000 description 1
- 244000124209 Crocus sativus Species 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- 238000001061 Dunnett's test Methods 0.000 description 1
- 208000032928 Dyslipidaemia Diseases 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical class OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 229910052693 Europium Inorganic materials 0.000 description 1
- 102000008857 Ferritin Human genes 0.000 description 1
- 108050000784 Ferritin Proteins 0.000 description 1
- 238000008416 Ferritin Methods 0.000 description 1
- 108020004206 Gamma-glutamyltransferase Proteins 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 101000669513 Homo sapiens Metalloproteinase inhibitor 1 Proteins 0.000 description 1
- AVXURJPOCDRRFD-UHFFFAOYSA-N Hydroxylamine Chemical compound ON AVXURJPOCDRRFD-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 206010022489 Insulin Resistance Diseases 0.000 description 1
- 206010023421 Kidney fibrosis Diseases 0.000 description 1
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- 208000017170 Lipid metabolism disease Diseases 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 102100039364 Metalloproteinase inhibitor 1 Human genes 0.000 description 1
- 238000011887 Necropsy Methods 0.000 description 1
- 208000008589 Obesity Diseases 0.000 description 1
- 206010033307 Overweight Diseases 0.000 description 1
- 102000004316 Oxidoreductases Human genes 0.000 description 1
- 108090000854 Oxidoreductases Proteins 0.000 description 1
- 206010033372 Pain and discomfort Diseases 0.000 description 1
- DPWPWRLQFGFJFI-UHFFFAOYSA-N Pargyline Chemical compound C#CCN(C)CC1=CC=CC=C1 DPWPWRLQFGFJFI-UHFFFAOYSA-N 0.000 description 1
- 206010035664 Pneumonia Diseases 0.000 description 1
- RVGRUAULSDPKGF-UHFFFAOYSA-N Poloxamer Chemical compound C1CO1.CC1CO1 RVGRUAULSDPKGF-UHFFFAOYSA-N 0.000 description 1
- 229920001213 Polysorbate 20 Polymers 0.000 description 1
- 208000004880 Polyuria Diseases 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 102000004669 Protein-Lysine 6-Oxidase Human genes 0.000 description 1
- 108010003894 Protein-Lysine 6-Oxidase Proteins 0.000 description 1
- 102000016611 Proteoglycans Human genes 0.000 description 1
- 108010067787 Proteoglycans Proteins 0.000 description 1
- 206010061924 Pulmonary toxicity Diseases 0.000 description 1
- 238000010240 RT-PCR analysis Methods 0.000 description 1
- 206010062104 Renal mass Diseases 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 208000034189 Sclerosis Diseases 0.000 description 1
- 108010090804 Streptavidin Proteins 0.000 description 1
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical compound C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 description 1
- 239000013504 Triton X-100 Substances 0.000 description 1
- 229920004890 Triton X-100 Polymers 0.000 description 1
- 229930003451 Vitamin B1 Natural products 0.000 description 1
- 235000021068 Western diet Nutrition 0.000 description 1
- CJGJSZAVWIBYJO-TWGQIWQCSA-N [(e)-3-fluoro-2-phenylprop-2-enyl]hydrazine Chemical compound NNC\C(=C\F)C1=CC=CC=C1 CJGJSZAVWIBYJO-TWGQIWQCSA-N 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- OFLXLNCGODUUOT-UHFFFAOYSA-N acetohydrazide Chemical class C\C(O)=N\N OFLXLNCGODUUOT-UHFFFAOYSA-N 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 230000002730 additional effect Effects 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 230000001668 ameliorated effect Effects 0.000 description 1
- 125000002344 aminooxy group Chemical group [H]N([H])O[*] 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 238000001949 anaesthesia Methods 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 230000003042 antagnostic effect Effects 0.000 description 1
- 230000003276 anti-hypertensive effect Effects 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- 239000008365 aqueous carrier Substances 0.000 description 1
- 239000007900 aqueous suspension Substances 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 150000003975 aryl alkyl amines Chemical class 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 239000012131 assay buffer Substances 0.000 description 1
- 238000011888 autopsy Methods 0.000 description 1
- 210000002469 basement membrane Anatomy 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- BTNNPSLJPBRMLZ-LGMDPLHJSA-N benfotiamine Chemical compound C=1C=CC=CC=1C(=O)SC(/CCOP(O)(O)=O)=C(/C)N(C=O)CC1=CN=C(C)N=C1N BTNNPSLJPBRMLZ-LGMDPLHJSA-N 0.000 description 1
- 229960002873 benfotiamine Drugs 0.000 description 1
- 229960000686 benzalkonium chloride Drugs 0.000 description 1
- CADWTSSKOVRVJC-UHFFFAOYSA-N benzyl(dimethyl)azanium;chloride Chemical compound [Cl-].C[NH+](C)CC1=CC=CC=C1 CADWTSSKOVRVJC-UHFFFAOYSA-N 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- 235000020958 biotin Nutrition 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- 239000010839 body fluid Substances 0.000 description 1
- 229940098773 bovine serum albumin Drugs 0.000 description 1
- RMRJXGBAOAMLHD-IHFGGWKQSA-N buprenorphine Chemical compound C([C@]12[C@H]3OC=4C(O)=CC=C(C2=4)C[C@@H]2[C@]11CC[C@]3([C@H](C1)[C@](C)(O)C(C)(C)C)OC)CN2CC1CC1 RMRJXGBAOAMLHD-IHFGGWKQSA-N 0.000 description 1
- 229960001736 buprenorphine Drugs 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 150000003857 carboxamides Chemical class 0.000 description 1
- 238000012754 cardiac puncture Methods 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 230000020411 cell activation Effects 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 1
- 229960004926 chlorobutanol Drugs 0.000 description 1
- 208000037976 chronic inflammation Diseases 0.000 description 1
- 230000006020 chronic inflammation Effects 0.000 description 1
- 208000020832 chronic kidney disease Diseases 0.000 description 1
- 230000007012 clinical effect Effects 0.000 description 1
- BTFHLQRNAMSNLC-UHFFFAOYSA-N clorgyline Chemical compound C#CCN(C)CCCOC1=CC=C(Cl)C=C1Cl BTFHLQRNAMSNLC-UHFFFAOYSA-N 0.000 description 1
- 238000004737 colorimetric analysis Methods 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 230000001010 compromised effect Effects 0.000 description 1
- 210000002808 connective tissue Anatomy 0.000 description 1
- 238000012937 correction Methods 0.000 description 1
- 238000010219 correlation analysis Methods 0.000 description 1
- 239000003246 corticosteroid Substances 0.000 description 1
- 229960001334 corticosteroids Drugs 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 239000002577 cryoprotective agent Substances 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 238000004925 denaturation Methods 0.000 description 1
- 230000036425 denaturation Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 150000004985 diamines Chemical class 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 239000003651 drinking water Substances 0.000 description 1
- 235000020188 drinking water Nutrition 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 208000028208 end stage renal disease Diseases 0.000 description 1
- 201000000523 end stage renal failure Diseases 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 238000006911 enzymatic reaction Methods 0.000 description 1
- 238000001952 enzyme assay Methods 0.000 description 1
- OGPBJKLSAFTDLK-UHFFFAOYSA-N europium atom Chemical compound [Eu] OGPBJKLSAFTDLK-UHFFFAOYSA-N 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 238000013401 experimental design Methods 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 235000021323 fish oil Nutrition 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 102000006640 gamma-Glutamyltransferase Human genes 0.000 description 1
- 230000002641 glycemic effect Effects 0.000 description 1
- 108020004445 glyceraldehyde-3-phosphate dehydrogenase Proteins 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 150000002357 guanidines Chemical class 0.000 description 1
- 229940083094 guanine derivative acting on arteriolar smooth muscle Drugs 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 230000013632 homeostatic process Effects 0.000 description 1
- 150000002443 hydroxylamines Chemical class 0.000 description 1
- 230000000260 hypercholesteremic effect Effects 0.000 description 1
- 201000001421 hyperglycemia Diseases 0.000 description 1
- 238000010191 image analysis Methods 0.000 description 1
- 125000004857 imidazopyridinyl group Chemical class N1C(=NC2=C1C=CC=N2)* 0.000 description 1
- 238000007654 immersion Methods 0.000 description 1
- 238000003125 immunofluorescent labeling Methods 0.000 description 1
- 238000003364 immunohistochemistry Methods 0.000 description 1
- 239000003018 immunosuppressive agent Substances 0.000 description 1
- 229940124589 immunosuppressive drug Drugs 0.000 description 1
- 238000002513 implantation Methods 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 208000027866 inflammatory disease Diseases 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 238000012317 liver biopsy Methods 0.000 description 1
- 201000007270 liver cancer Diseases 0.000 description 1
- 208000019423 liver disease Diseases 0.000 description 1
- 208000014018 liver neoplasm Diseases 0.000 description 1
- 210000005228 liver tissue Anatomy 0.000 description 1
- 238000007477 logistic regression Methods 0.000 description 1
- 231100000516 lung damage Toxicity 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 238000013227 male C57BL/6J mice Methods 0.000 description 1
- 229910052748 manganese Inorganic materials 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- 238000000386 microscopy Methods 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 210000003205 muscle Anatomy 0.000 description 1
- WHHVXDOFHSRUTI-UHFFFAOYSA-N n-[(5-bromo-1,3-benzodioxol-4-yl)methylidene]hydroxylamine Chemical compound C1=C(Br)C(C=NO)=C2OCOC2=C1 WHHVXDOFHSRUTI-UHFFFAOYSA-N 0.000 description 1
- MXNVSFOCVLXIFP-UHFFFAOYSA-N n-[2-(hydroxyamino)-2-oxoethyl]-2-(2-methyl-1h-indol-3-yl)acetamide Chemical compound C1=CC=C2C(CC(=O)NCC(=O)NO)=C(C)NC2=C1 MXNVSFOCVLXIFP-UHFFFAOYSA-N 0.000 description 1
- LMDXCMJWFMSUAI-UHFFFAOYSA-N n-[4-[2-[4-(diaminomethylideneamino)phenyl]ethyl]-1,3-thiazol-2-yl]acetamide Chemical compound S1C(NC(=O)C)=NC(CCC=2C=CC(NC(N)=N)=CC=2)=C1 LMDXCMJWFMSUAI-UHFFFAOYSA-N 0.000 description 1
- KGHOAUPVYIZJSU-UHFFFAOYSA-N n-[4-[2-[4-(diaminomethylideneamino)phenyl]ethyl]-5-[(4-methylsulfonylphenyl)methyl]-1,3-thiazol-2-yl]acetamide Chemical compound C=1C=C(S(C)(=O)=O)C=CC=1CC=1SC(NC(=O)C)=NC=1CCC1=CC=C(NC(N)=N)C=C1 KGHOAUPVYIZJSU-UHFFFAOYSA-N 0.000 description 1
- VXAPNCLMTSOGLM-UHFFFAOYSA-N n-[4-[2-[4-[(2-amino-1h-imidazol-5-yl)methyl]phenyl]ethyl]-1,3-thiazol-2-yl]acetamide Chemical compound S1C(NC(=O)C)=NC(CCC=2C=CC(CC=3N=C(N)NC=3)=CC=2)=C1 VXAPNCLMTSOGLM-UHFFFAOYSA-N 0.000 description 1
- 230000017074 necrotic cell death Effects 0.000 description 1
- 230000001537 neural effect Effects 0.000 description 1
- 239000002547 new drug Substances 0.000 description 1
- 235000020925 non fasting Nutrition 0.000 description 1
- 230000009871 nonspecific binding Effects 0.000 description 1
- 239000000346 nonvolatile oil Substances 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 235000020824 obesity Nutrition 0.000 description 1
- 150000002895 organic esters Chemical class 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 210000001672 ovary Anatomy 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 229960001779 pargyline Drugs 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- WVDDGKGOMKODPV-ZQBYOMGUSA-N phenyl(114C)methanol Chemical compound O[14CH2]C1=CC=CC=C1 WVDDGKGOMKODPV-ZQBYOMGUSA-N 0.000 description 1
- GVKCHTBDSMQENH-UHFFFAOYSA-L phloxine B Chemical compound [Na+].[Na+].[O-]C(=O)C1=C(Cl)C(Cl)=C(Cl)C(Cl)=C1C1=C2C=C(Br)C(=O)C(Br)=C2OC2=C(Br)C([O-])=C(Br)C=C21 GVKCHTBDSMQENH-UHFFFAOYSA-L 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 229920001992 poloxamer 407 Polymers 0.000 description 1
- 229940044476 poloxamer 407 Drugs 0.000 description 1
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 1
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 1
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 1
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 1
- 229940068977 polysorbate 20 Drugs 0.000 description 1
- 229920000053 polysorbate 80 Polymers 0.000 description 1
- 229940068968 polysorbate 80 Drugs 0.000 description 1
- 238000010837 poor prognosis Methods 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 235000011009 potassium phosphates Nutrition 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 230000003449 preventive effect Effects 0.000 description 1
- 239000000651 prodrug Substances 0.000 description 1
- 229940002612 prodrug Drugs 0.000 description 1
- 239000000092 prognostic biomarker Substances 0.000 description 1
- JKANAVGODYYCQF-UHFFFAOYSA-N prop-2-yn-1-amine Chemical class NCC#C JKANAVGODYYCQF-UHFFFAOYSA-N 0.000 description 1
- MILWSGRFEGYSGM-UHFFFAOYSA-N propane-1,2-diol;propane-1,2,3-triol Chemical compound CC(O)CO.OCC(O)CO MILWSGRFEGYSGM-UHFFFAOYSA-N 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 201000001474 proteinuria Diseases 0.000 description 1
- 230000008817 pulmonary damage Effects 0.000 description 1
- 150000003236 pyrrolines Chemical class 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 239000000700 radioactive tracer Substances 0.000 description 1
- VMXUWOKSQNHOCA-UKTHLTGXSA-N ranitidine Chemical compound [O-][N+](=O)\C=C(/NC)NCCSCC1=CC=C(CN(C)C)O1 VMXUWOKSQNHOCA-UKTHLTGXSA-N 0.000 description 1
- 230000009103 reabsorption Effects 0.000 description 1
- 230000008439 repair process Effects 0.000 description 1
- 230000029058 respiratory gaseous exchange Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 206010039073 rheumatoid arthritis Diseases 0.000 description 1
- 235000013974 saffron Nutrition 0.000 description 1
- 239000004248 saffron Substances 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 231100000241 scar Toxicity 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- DUIOPKIIICUYRZ-UHFFFAOYSA-N semicarbazide Chemical compound NNC(N)=O DUIOPKIIICUYRZ-UHFFFAOYSA-N 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 230000000405 serological effect Effects 0.000 description 1
- 208000037974 severe injury Diseases 0.000 description 1
- 230000009528 severe injury Effects 0.000 description 1
- 210000003491 skin Anatomy 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 239000001488 sodium phosphate Substances 0.000 description 1
- 235000011008 sodium phosphates Nutrition 0.000 description 1
- 239000012453 solvate Substances 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 239000008223 sterile water Substances 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 229940124530 sulfonamide Drugs 0.000 description 1
- 150000003456 sulfonamides Chemical class 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 238000012385 systemic delivery Methods 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 229960003495 thiamine Drugs 0.000 description 1
- DPJRMOMPQZCRJU-UHFFFAOYSA-M thiamine hydrochloride Chemical compound Cl.[Cl-].CC1=C(CCO)SC=[N+]1CC1=CN=C(C)N=C1N DPJRMOMPQZCRJU-UHFFFAOYSA-M 0.000 description 1
- 230000000451 tissue damage Effects 0.000 description 1
- 231100000827 tissue damage Toxicity 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- 210000000626 ureter Anatomy 0.000 description 1
- 208000019206 urinary tract infection Diseases 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 238000012795 verification Methods 0.000 description 1
- 235000010374 vitamin B1 Nutrition 0.000 description 1
- 239000011691 vitamin B1 Substances 0.000 description 1
- 239000008215 water for injection Substances 0.000 description 1
- 230000029663 wound healing Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/13—Amines
- A61K31/135—Amines having aromatic rings, e.g. ketamine, nortriptyline
- A61K31/137—Arylalkylamines, e.g. amphetamine, epinephrine, salbutamol, ephedrine or methadone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/3955—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against proteinaceous materials, e.g. enzymes, hormones, lymphokines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/18—Drugs for disorders of the alimentary tract or the digestive system for pancreatic disorders, e.g. pancreatic enzymes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/10—Drugs for disorders of the urinary system of the bladder
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
- A61P15/14—Drugs for genital or sexual disorders; Contraceptives for lactation disorders, e.g. galactorrhoea
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/02—Drugs for dermatological disorders for treating wounds, ulcers, burns, scars, keloids, or the like
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/14—Drugs for disorders of the endocrine system of the thyroid hormones, e.g. T3, T4
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/08—Vasodilators for multiple indications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/40—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against enzymes
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/531—Production of immunochemical test materials
- G01N33/532—Production of labelled immunochemicals
- G01N33/533—Production of labelled immunochemicals with fluorescent label
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/573—Immunoassay; Biospecific binding assay; Materials therefor for enzymes or isoenzymes
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/577—Immunoassay; Biospecific binding assay; Materials therefor involving monoclonal antibodies binding reaction mechanisms characterised by the use of monoclonal antibodies; monoclonal antibodies per se are classified with their corresponding antigens
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6893—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids related to diseases not provided for elsewhere
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/90—Enzymes; Proenzymes
- G01N2333/902—Oxidoreductases (1.)
- G01N2333/906—Oxidoreductases (1.) acting on nitrogen containing compounds as donors (1.4, 1.5, 1.7)
- G01N2333/90605—Oxidoreductases (1.) acting on nitrogen containing compounds as donors (1.4, 1.5, 1.7) acting on the CH-NH2 group of donors (1.4)
- G01N2333/90633—Oxidoreductases (1.) acting on nitrogen containing compounds as donors (1.4, 1.5, 1.7) acting on the CH-NH2 group of donors (1.4) with oxygen as acceptor (1.4.3) in general
- G01N2333/90638—Oxidoreductases (1.) acting on nitrogen containing compounds as donors (1.4, 1.5, 1.7) acting on the CH-NH2 group of donors (1.4) with oxygen as acceptor (1.4.3) in general with a definite EC number (1.4.3.-)
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/08—Hepato-biliairy disorders other than hepatitis
- G01N2800/085—Liver diseases, e.g. portal hypertension, fibrosis, cirrhosis, bilirubin
Definitions
- Fibrotic conditions usually occur as a result of a disturbed woundhealing process after trauma or chronic inflammation.
- the fibrotic pathology is especially prevalent in organs that are on a regular basis exposed to chemical and biological insults, e.g. liver, lung, skin and kidney. Regardless if the disorder is acute or chronic they share a common characteristic of abnormal fibroblast activation and accumulation of extracellular matrix (ECM), leading to a loss of organ function as the normal tissue is replaced by scar tissue.
- ECM extracellular matrix
- said anti-VAP-1 antibody has a heavy chain polypeptide comprising a first CDR sequence selected from SEQ ID NOs 4 to 8, a second CDR sequence selected from SEQ ID NOs 9 to 13, and a third CDR sequence selected from SEQ ID NOs 14 to 18, and/or a light chain polypeptide comprising a first CDR sequence selected from SEQ ID NOs 27 to 31, a second CDR sequence selected from SEQ ID NOs 32 to 36 and a third CDR sequence selected from SEQ ID NOs 37 to 41.
- hepatitis acute and chronic hepatitis, biliary disease and toxic liver injury, pulmonary fibrosis, renal fibrosis, including that resulting from diabetic nephropathy, myelofibrosis, pancreatic fibrosis, scleroderma, connective tissue diseases, scarring, skin fibrosis, cardiac fibrosis, organ transplant, vascular stenosis, restenosis, arterial fibrosis, arthrofibrosis, breast fibrosis, muscle fibrosis, retroperitoneal fibrosis, thyroid fibrosis, lymph node fibrosis, bladder fibrosis, pleural fibrosis and COPD, a disease in which airway walls are fibrotic with the accumulation of myofibroblasts and collagen, and like all fibrotic tissues, are contracted.
- anti-VAP-1 antibody or “monoclonal anti-VAP-1 antibody” (MAb) is meant to include intact antibodies as well as antibody fragments, such as Fab and F(ab')2 fragments, which are capable of specifically binding to VAP-1 protein.
- Other preferred anti-VAP-1 antibodies include those having a heavy chain polypeptide comprising a first CDR sequence selected from SEQ ID NOs 4 to 8, a second CDR sequence selected from SEQ ID NOs 9 to 13, and a third CDR sequence selected from SEQ ID NOs 14 to 18 and/or a light chain polypeptide comprising a first CDR sequence selected from SEQ ID NOs 27 to 31, a second CDR sequence selected from SEQ ID NOs 32 to 36 and a third CDR sequence selected from SEQ ID NOs 37 to 41.
- SSAO inhibitors disclosed herein include, but are not limited to hydrazine derivatives such as allylhydrazines, especially phenylallylhydrazines; and hydroxylamine (i.e. aminoxy) derivatives. More specific examples of phenylallylhydrazines include but are not limited to 2-(phenyl-allyl)-hydrazine, N-[2-(4'-fluorophenyl)-allyl]-hydrazine and (E)-1-fluoro-2-phenyl-3-hydrazinopropene, whereas more specific examples of hydroxylamine derivatives include but are not limited to 2-aminooxyl-1-phenyl-ethanol, and 2-aminooxyl-1-(3',4'-dimethoxy-phenyl)-ethanol.
- SSAO inhibitors include any stereoisomer, mixture of stereoisomers, E or Z forms, mixture of E and Z forms, prodrug, metabolite, crystalline form, non-crystalline form, hydrate, solvate or salt thereof having an ability to inhibit or block the SSAO activity of VAP-1.
- anti-VAP-1 antibodies may be provided as a pharmaceutical composition comprising a pharmaceutically acceptable carrier or diluent and, as active ingredient, an anti-VAP-1 antibody.
- the composition contains the anti-VAP-1 antibody in an amount sufficient to antagonize (fully or partially) the patient's SSAO activity or native VAP-1 binding to the biological ligands of VAP-1 in patients in need of such antagonizing.
- Amounts and regimens for the administration of anti-VAP-1 antibodies may be determined readily by those with ordinary skill in the clinical art of treating fibrosis-related disorders.
- the dosage of the anti-VAP-1 antibody treatment will vary depending on considerations such as: age, gender and general health of the patient to be treated; kind of concurrent treatment, if any; frequency of treatment and nature of the effect desired; extent of tissue damage; duration of the symptoms; and other variables to be adjusted by the individual physician.
- a desired dose can be administered in one or more applications to obtain the desired results.
- Pharmaceutical compositions according to the present embodiments may be provided in unit dosage forms.
- Aqueous compositions according to the embodiments may comprise suitable buffer agents, such as sodium and potassium phosphates, citrate, acetate, carbonate or glycine buffers depending on the targeted pH-range.
- suitable buffer agents such as sodium and potassium phosphates, citrate, acetate, carbonate or glycine buffers depending on the targeted pH-range.
- sodium chloride as a tonicity adjuster is also useful.
- Compositions may include other excipients, such as stabilizing agents or preservatives.
- the therapeutically useful anti-VAP-1 antibodies may be conjugated, either chemically or by genetic engineering, to other agents, which provide targeting of the antibodies to a desired site of action.
- other compounds may be conjugated, either chemically or by genetic engineering, to the antibodies, so as to enhance or provide additional properties to the antibodies, especially properties, which enhance the antibodies' ability to promote alleviation of harmful effects mediated by VAP-1 binding.
- Level of sVAP-1 in a body fluid sample such as serum may be determined by the following method:
- TR-IFMA time-resolved one-step immunofluorometric assay
- DELFIA time-resolved one-step immunofluorometric assay
- TK8-14 Biotie Therapies Corp.
- Detection of bound soluble VAP-1 is done using europium-conjugated mouse anti-human VAP-1 antibody TK8-18 (Biotie Therapies Corp.) as a tracer.
- the label is detected by measuring the time-resolved fluorescence (Victor3 multilabel counter) at 615 nm.
- the fluorescence counts directly correlate with how much soluble VAP-1 is present in the sample.
- the sample data are then analyzed in comparison to the standard curve of a reference.
- the present disclosure provides a kit for use in diagnosis of fibrosis.
- the kit comprises one or more reagents for assessing the amount sVAP-1, such as a specific anti-VAP-1 antibody, e.g. one of the anti-VAP-1 antibodies mentioned above.
- the kit comprises one or more reagents for assessing the SSAO activity in a bodily fluid such as serum or plasma.
- the kit may comprise a substrate for VAP-1 SSAO, such as benzylamine, methylamine, aminocetone or other aliphatic or aromatic monoamines, together with a suitable SSAO enzyme activity assay buffer, and a set of reagents and method for detecting SSAO activity.
- SSAO activity may be detected using a coupled assay in which the generation of hydrogen peroxide from the action of SSAO activity on monoamine substrates is measured, or it may be measured directly by the monitoring the conversion of a water soluble amine to an organic solvent soluble aldehyde using a 14 C labelled amine substrate, such as benzylamine.
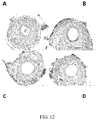
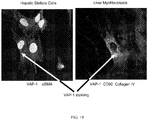
- the aim of the study was to assess the effect of VAP-1 inhibitors on fibrotic liver injury in mouse.
- mice All mice were maintained and housed under conventional conditions at the Biomedical services unit at the University of Birmingham, according to Home Office regulations. Four mice were housed per cage and acclimated to the housing situation for one week before the experiments.
- Female C57BL/6 and VAP-1-/- mice (AOC3 gene knockout mice lacking VAP-1) of the age of 8-10 weeks were used in the study.
- C57BL/6 mice were obtained from a stock colony from the Biomedical services unit at the University of Birmingham, whereas VAP-1-/- (AOC3 gene knockout) mice were obtained from the contract breeder Taconic, Denmark.
- a mouse model of chronic hepatic fibrosis was established by i.p. administration of carbon tetrachloride (CCI4; Aldrich Chemical) at a dose of 1 ml/kg dissolved in mineral oil bi-weekly for 8 weeks, whereas the control group only received mineral oil.
- Mice treated with a mouse anti-mouse VAP-1 antibody BTT-1029 received weekly i.v. injections two weeks prior to and during the CCI4 administration. Animals were terminated 96 h after the ultimate dose of CCI4. Blood samples were withdrawn by cardiac puncture during isoflurane anaesthesia, after which mice were culled by cervical dislocation. Livers were dissected and cut into 4 pieces for different processing.
- HSC hepatic stellate cells
- Activated HSC are regarded as the principal source for synthesising ECM components in fibrotic liver, including elastin.
- CCI4 administered wild type livers showed a profound increase in ⁇ SMA and elastin mRNA levels, indicating an accumulation of ⁇ SMA expressing HSCs and deposition of elastin ( Figure 4 ).
- the mRNA levels of both ⁇ SMA and elastin in BTT-1029 treated wild type and VAP-1-/- livers were significantly lower compared to the wild type liver.
- the differences in elastin and collagen IV expression were also confirmed by confocal microscopy, whereas laminin levels remained unchanged ( Figure 3 ).
- SSAO activities of the serum and liver tissue samples were assayed radiochemically using [7- 14 C]-benzylamine hydrochloride (spec. act. 57 mCi/mmol) as a substrate ( Figure 5 ).
- Serum (40 mg/ml protein) or tissue preparations (2 mg/ml protein) were preincubated with 5 ⁇ M clorgyline and pargyline, and with non-specific binding tubes also with 1 mM semicarbazide at 37°C for 30 min. The assay was performed at 37°C for one hour in the final volume of 200 ⁇ l of 0.2 mM Na-phosphate buffer (pH 7.4) containing [7- 14 C]-benzylamine as a substrate.
- Kidneys were fixed in 4 % paraformaldehyde, embedded in paraffin and cut into 4 ⁇ m sections. Histopathological analysis was done on Sirius red and H&E stained sections. Staining was performed according to standard procedures. The amount of Sirius red fibrils were quantified by threshold analysis using Image J software.
- the tobacco smoke induced mouse model of COPD was employed to assess the effect of VAP-1 inhibitors on the treatment of COPD.
- C57BL/6J mice were exposed once daily to tobacco smoke (TS) for 11 consecutive days resulting in pulmonary inflammation 24 hours following the final TS exposure. After 11 days the response comprised of significant increases in macrophages, epithelial cells, eosinophils, neutrophils and lymphocytes.
- TS tobacco smoke
- Neointimal and medial thickening is an early and essential stage in the development of atherosclerotic lesions and an essential component of restenosis. It is accompanied by fibrotic changes in the neointima and media of the vascular wall.
- This study evaluated the role of blocking SSAO in fibrotic disease by evaluating the effect of systemic delivery (by daily ip injection) of a small molecule SSAO inhibitor (mofegiline, BTT-2089) on cuff-induced neointimal thickening (cuff-induced stenosis) in the femoral artery of ApoE3 Leiden mice that received a moderate western type diet.
- the group treated with the SSAO inhibitor BTT-2079 10mg/kg i.p. daily also resulted in a significant reduction in neointima formation. No significant changes between all groups were seen in vessel wall diameter, media and lumen area.
- the intima media ratios of the BTT-2079 10 mg/kg and BTT-2089 30 mg/kg were significantly less compared to the control group, but percentage lumen stenosis was only significantly less in the BTT-2089 30 mg/kg group compared to the control group. Vascular integrity was not affected.
- mice at the age of 8 weeks are treated systemically with bleomycin (100 mg/kg) for 7 days by Alzet osmotic minipumps to elicit pulmonary damage.
- Non-pulmonary toxicity is observed for days 7-21 after pump implantation.
- 21 days there is 12-15% fibrosis in the lungs as evaluated histopathologically. This is followed by clinical lung damage which may be observed by increased breathing rate and dramatic loss in body weight and eventually lead death within 42 days (if not terminated before).
- the mesangial matrix includes the basement membrane and associated polyanionic proteoglycans and other molecules which are stained red to purple by the periodic acid Schiff (PAS) method.
- PAS periodic acid Schiff
- mice Male mice aged 6-7 weeks (20-25g body weight) are anesthetised with isoflurane (2-chloro-2-(difluoromethoxy)-1, 1,1-trifluoro-ethane) inhalation and injected subcutaneously with 0,05-0,1 mg/kg buprenorphine preoperatively.
- the mice are subjected to unilateral ureteral obstruction (UUO) or a sham operation.
- UUO operated mice the left ureter is ligated with a 4-0 silk suture at two points and cut between the ligatures in order to prevent retrograde urinary tract infection.
- the mice are sacrificed 7 days post-operatively.
- GFR glomerular filtration rate
- ACE angiotensin converting enzyme
- ARA angiotensin receptor antagonist
- diabetic nephropathy which can include treatment with an ACE inhibitor and/or ARA, antihypertensive therapy with a blood pressure target of less than 130/80, and tight glycemic control with appropriately set targets for HbA1C.
- sVAP-1 level was used as a lone biomarker to predict the presence of significant liver fibrosis (Stages F2-4), a level of ⁇ 1000ng/ml had a positive predictive value of 88.9%.
- the area under the receiver operating characteristic curve (AUROC) for predicting significant fibrosis (F2-4), advanced fibrosis (F3-4) and cirrhosis (F4) was 0.71 (95% Cl 0.62-0.80), 0.68 (95% Cl 0.58-0.78) and 0.75 (95% Cl 0.58-0.92) respectively ( Figure 18 ).
- a fibrosis score (calculated from the regression equation) of factors independently associated with liver fibrosis on multivariate analysis (sVAP-1, Diabetic status and AST/ALT ratio), had an AUROC for predicting significant fibrosis (F2-4), advanced fibrosis (F3-4) and cirrhosis (F4) of 0.79 (95% Cl 0.71-0.87), 0.80 (95% Cl 0.71-0.88) and 0.89 (95% Cl 0.74-1.02) ( Figure 19 ).
- the VAP-1 protein has a monoamine oxidase enzyme activity called SSAO (semicarbazide-sensitive amine oxidase).
- SSAO monoamine oxidase enzyme activity
- SSAO enzyme activity is an integral part of the VAP-1 protein it follows that levels of sVAP-1 in bodily fluids can also be determined by measuring the amount of SSAO activity in a bodily fluid (such as serum or plasma).
- SSAO is the principal monoamine oxidase activity in human serum and plasma acting on SSAO substrates such as benzylamine or methylamine.
- SSAO activity may be used as an equivalent marker of liver fibrosis.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Immunology (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Molecular Biology (AREA)
- Urology & Nephrology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Hematology (AREA)
- Biomedical Technology (AREA)
- Epidemiology (AREA)
- Microbiology (AREA)
- Biochemistry (AREA)
- Pathology (AREA)
- Biotechnology (AREA)
- General Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- Physics & Mathematics (AREA)
- Food Science & Technology (AREA)
- Cell Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Endocrinology (AREA)
- Mycology (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Emergency Medicine (AREA)
- Heart & Thoracic Surgery (AREA)
- Rheumatology (AREA)
- Physical Education & Sports Medicine (AREA)
- Cardiology (AREA)
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PL10815041T PL2475387T3 (pl) | 2009-09-08 | 2010-09-07 | Inhibitory vap-1 do zastosowania w leczeniu stanów zwłóknieniowych |
| SI201031624T SI2475387T1 (en) | 2009-09-08 | 2010-09-07 | VAP-1 INHIBITORS FOR USE IN PHIBROTIC FLUOR TREATMENT |
| RS20180101A RS56809B1 (sr) | 2009-09-08 | 2010-09-07 | Vap-1 inhibitori za upotrebu u lečenju fibroznih stanja |
| HRP20180131TT HRP20180131T1 (hr) | 2009-09-08 | 2018-01-23 | Inhibitori vap-1, namijenjeni upotrebi u liječenju fibrotičnih stanja |
| MEP-2018-21A ME02999B (me) | 2009-09-08 | 2018-02-28 | Vap-1inhibitori za upotrebu u lečenju fibroznih stanja |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US24040209P | 2009-09-08 | 2009-09-08 | |
| US32303210P | 2010-04-12 | 2010-04-12 | |
| PCT/FI2010/050689 WO2011029996A1 (en) | 2009-09-08 | 2010-09-07 | Use of vap-1 inhibitors for treating fibrotic conditions |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP2475387A1 EP2475387A1 (en) | 2012-07-18 |
| EP2475387A4 EP2475387A4 (en) | 2015-05-13 |
| EP2475387B1 true EP2475387B1 (en) | 2017-11-01 |
Family
ID=43732037
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP10815041.8A Active EP2475387B1 (en) | 2009-09-08 | 2010-09-07 | Vap-1 inhibitors for use in treating fibrotic conditions |
Country Status (26)
| Country | Link |
|---|---|
| US (2) | US9795671B2 (ru) |
| EP (1) | EP2475387B1 (ru) |
| JP (3) | JP6009355B2 (ru) |
| KR (2) | KR101837117B1 (ru) |
| CN (2) | CN107661500A (ru) |
| AU (1) | AU2010294123B2 (ru) |
| CA (1) | CA2773218C (ru) |
| CY (1) | CY1119867T1 (ru) |
| DK (1) | DK2475387T3 (ru) |
| ES (1) | ES2657943T3 (ru) |
| HR (1) | HRP20180131T1 (ru) |
| HU (1) | HUE035890T2 (ru) |
| IL (1) | IL218535A (ru) |
| LT (1) | LT2475387T (ru) |
| ME (1) | ME02999B (ru) |
| NO (1) | NO2475387T3 (ru) |
| NZ (3) | NZ706740A (ru) |
| PL (1) | PL2475387T3 (ru) |
| PT (1) | PT2475387T (ru) |
| RS (1) | RS56809B1 (ru) |
| RU (2) | RU2667963C1 (ru) |
| SG (1) | SG179012A1 (ru) |
| SI (1) | SI2475387T1 (ru) |
| UA (1) | UA112154C2 (ru) |
| WO (1) | WO2011029996A1 (ru) |
| ZA (1) | ZA201202515B (ru) |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| UA112154C2 (uk) * | 2009-09-08 | 2016-08-10 | Біоті Терапіс Корп. | Застосування повністю людського анти-vap-1-антитіла для лікування фіброзних станів |
| JP5953035B2 (ja) * | 2011-11-28 | 2016-07-13 | 学校法人慶應義塾 | 病理診断支援装置、病理診断支援方法、及び病理診断支援プログラム |
| SG11201406948PA (en) | 2012-05-02 | 2014-11-27 | Boehringer Ingelheim Int | Substituted 3-haloallylamine inhibitors of ssao and uses thereof |
| WO2014024820A1 (ja) * | 2012-08-06 | 2014-02-13 | 塩野義製薬株式会社 | 新規慢性腎臓病治療用医薬組成物及び新規慢性腎臓病治療薬のスクリーニング方法 |
| KR102205845B1 (ko) | 2013-10-28 | 2021-01-22 | 삼성전자주식회사 | 입자에 기반한 모델링 방법 및 장치 |
| WO2015189534A1 (en) * | 2014-06-12 | 2015-12-17 | Proximagen Limited | Vap-1 inhibitors for treating muscular dystrophy |
| JP2018080114A (ja) * | 2015-05-29 | 2018-05-24 | 株式会社アールテック・ウエノ | 抗ヒトvap−1モノクローナル抗体 |
| KR102340352B1 (ko) * | 2017-06-27 | 2021-12-21 | 주식회사 뉴라클사이언스 | 섬유증의 치료를 위한 항-fam19a5 항체의 용도 |
| WO2023164548A1 (en) * | 2022-02-23 | 2023-08-31 | Yale University | Il-6 inhibitor as treatment for nephropathy |
Family Cites Families (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4997854A (en) * | 1989-08-25 | 1991-03-05 | Trustees Of Boston University | Anti-fibrotic agents and methods for inhibiting the activity of lysyl oxidase in-situ using adjacently positioned diamine analogue substrates |
| US5580780A (en) | 1992-06-09 | 1996-12-03 | Jalkanen; Sirpa | Vascular adhesion protein-(VAP-1) and VAP-1-specific antibodies |
| EP0979271A1 (en) * | 1997-05-23 | 2000-02-16 | Biotie Therapies Ltd. | Vascular adhesion protein-1 having amine oxidase activity |
| WO2002002541A2 (en) | 2000-07-05 | 2002-01-10 | Biotie Therapies Corp. | Inhibitors of copper-containing amine oxidases |
| WO2002038153A1 (en) | 2000-11-09 | 2002-05-16 | Biovitrum Ab | New use of 4, 5, 6, 7-tetrahydroimidazo-[4,5-c]pyridine derivatives |
| WO2002053161A1 (en) * | 2000-12-29 | 2002-07-11 | Alteon, Inc. | Method for treating fibrotic diseases or other indications |
| US6982286B2 (en) | 2001-07-12 | 2006-01-03 | Biotie Therapies Corp. | Carbocyclic hydrazino inhibitors of copper-containing amine oxidases |
| FI20020807A0 (fi) | 2002-04-29 | 2002-04-29 | Biotie Therapies Oyj | Uusia humanisoituja anti-VAP-1 monoklonaalisia vasta-aineita |
| EP1587800A1 (en) | 2003-01-27 | 2005-10-26 | Astellas Pharma Inc. | Thiazole derivatives and their use as vap-1 inhibitors |
| JP4758337B2 (ja) | 2003-03-31 | 2011-08-24 | 株式会社アールテック・ウエノ | 血管透過性亢進疾患を治療する方法 |
| JP2007501802A (ja) | 2003-08-08 | 2007-02-01 | ラ ホヤ ファーマシューティカル カンパニー | 疾患の処置に有用な、セミカルバジド感受性アミンオキシダーゼ(ssao)およびvap−1媒介性接着のインヒビター |
| WO2005072738A1 (en) | 2004-01-30 | 2005-08-11 | Faron Pharmaceuticals Oy | Compositions useful especially for treatment or prevention of metabolic syndrome |
| FI20040270A0 (fi) | 2004-02-20 | 2004-02-20 | Biotie Therapies Oyj | Kuparia sisältävien amiinioksidaasien imhibiittoreina käyttökelpoiset hydratsinoalkoholijohdannaiset |
| AU2005216955A1 (en) | 2004-02-25 | 2005-09-09 | La Jolla Pharmaceutical Company | Amines and amides for the treatment of diseases |
| KR101206319B1 (ko) | 2004-03-18 | 2012-11-29 | 가부시키가이샤 아루떼꾸 우에노 | 티아졸 유도체를 포함하는 수성 조성물 |
| CA2575411A1 (en) | 2004-07-27 | 2006-02-02 | Astellas Pharma Inc. | Thiazole derivatives having vap-1 inhibitory activity |
| US20080269282A1 (en) | 2004-08-02 | 2008-10-30 | Genmedica Therapeutics Sl | Compounds for Inhibiting Copper-Containing Amine Oxidases and Uses Thereof |
| EP1791835A2 (en) | 2004-09-09 | 2007-06-06 | Astellas Pharma Inc. | Thiazole derivatives having vap-1 inhibitory activity |
| WO2006094201A2 (en) | 2005-03-02 | 2006-09-08 | La Jolla Pharmaceutical Company | Semicarbazide-sensitive amide oxidase inhibitors |
| WO2007005737A2 (en) | 2005-07-01 | 2007-01-11 | Case Western Reserve University | Amine oxidase inhibitors |
| US20070066646A1 (en) | 2005-08-02 | 2007-03-22 | Genmedica Therapeutics Sl | Compounds for Inhibiting Copper-Containing Amine Oxidases and Uses Thereof |
| US20080058922A1 (en) * | 2006-08-31 | 2008-03-06 | Cardiac Pacemakers, Inc. | Methods and devices employing vap-1 inhibitors |
| FI20075278A0 (fi) * | 2007-04-20 | 2007-04-20 | Biotie Therapies Corp | Uudet täysin ihmisperäiset anti-VAP-1 monoklonaaliset vasta-aineet |
| WO2009055002A1 (en) | 2007-10-24 | 2009-04-30 | La Jolla Pharmaceutical Company | Combined inhibitors of cyclooxygenase and semicarbazide-sensitive amine oxidase (ssao) (vascular adhesion protein, vap-1) |
| EP2222160B1 (en) | 2007-11-21 | 2015-03-18 | Pharmaxis Ltd. | Haloallylamine inhibitors of ssao/vap-1 and uses therefor |
| TWI437986B (zh) * | 2008-01-31 | 2014-05-21 | R Tech Ueno Ltd | 噻唑衍生物及使用該衍生物作為vap-1抑制劑之用途 |
| TWI490214B (zh) | 2008-05-30 | 2015-07-01 | 艾德克 上野股份有限公司 | 苯或噻吩衍生物及該等作為vap-1抑制劑之用途 |
| HUP0800498A2 (en) | 2008-08-06 | 2010-03-29 | Semmelweis Egyetem | Use of dihydralazine for the preparation of medicaments for treatment of diseases related to ssao level |
| HUE025381T2 (en) | 2008-09-11 | 2016-02-29 | Pecsi Tudomanyegyetem | Compounds for the inhibition of SSAO / VAP-1 and their use for the treatment and prevention of diseases |
| CA2737527C (en) | 2008-09-16 | 2015-12-29 | Proximagen Limited | 4,5,6,7-tetrahydroimidazo[4,5-c]pyridine compounds useful as inhibitors of ssao activity |
| ES2403633T3 (es) | 2008-12-04 | 2013-05-20 | Proximagen Limited | Compuestos de imidazopiridina |
| UA112154C2 (uk) * | 2009-09-08 | 2016-08-10 | Біоті Терапіс Корп. | Застосування повністю людського анти-vap-1-антитіла для лікування фіброзних станів |
-
2010
- 2010-07-09 UA UAA201204308A patent/UA112154C2/uk unknown
- 2010-09-07 SG SG2012015772A patent/SG179012A1/en unknown
- 2010-09-07 NZ NZ706740A patent/NZ706740A/en not_active IP Right Cessation
- 2010-09-07 CN CN201710879422.4A patent/CN107661500A/zh active Pending
- 2010-09-07 KR KR1020127009075A patent/KR101837117B1/ko active IP Right Grant
- 2010-09-07 AU AU2010294123A patent/AU2010294123B2/en not_active Ceased
- 2010-09-07 RU RU2016107043A patent/RU2667963C1/ru active
- 2010-09-07 SI SI201031624T patent/SI2475387T1/en unknown
- 2010-09-07 PL PL10815041T patent/PL2475387T3/pl unknown
- 2010-09-07 DK DK10815041.8T patent/DK2475387T3/en active
- 2010-09-07 JP JP2012528399A patent/JP6009355B2/ja not_active Expired - Fee Related
- 2010-09-07 KR KR1020187006357A patent/KR20180026808A/ko not_active IP Right Cessation
- 2010-09-07 NO NO10815041A patent/NO2475387T3/no unknown
- 2010-09-07 RU RU2012113561/15A patent/RU2580626C2/ru active
- 2010-09-07 ES ES10815041.8T patent/ES2657943T3/es active Active
- 2010-09-07 PT PT108150418T patent/PT2475387T/pt unknown
- 2010-09-07 NZ NZ598920A patent/NZ598920A/en not_active IP Right Cessation
- 2010-09-07 EP EP10815041.8A patent/EP2475387B1/en active Active
- 2010-09-07 NZ NZ61643210A patent/NZ616432A/en not_active IP Right Cessation
- 2010-09-07 CN CN2010800503481A patent/CN102740886A/zh active Pending
- 2010-09-07 WO PCT/FI2010/050689 patent/WO2011029996A1/en active Application Filing
- 2010-09-07 RS RS20180101A patent/RS56809B1/sr unknown
- 2010-09-07 LT LTEP10815041.8T patent/LT2475387T/lt unknown
- 2010-09-07 CA CA2773218A patent/CA2773218C/en not_active Expired - Fee Related
- 2010-09-07 HU HUE10815041A patent/HUE035890T2/en unknown
- 2010-09-07 US US13/395,029 patent/US9795671B2/en active Active
-
2012
- 2012-03-07 IL IL218535A patent/IL218535A/en active IP Right Grant
- 2012-04-05 ZA ZA2012/02515A patent/ZA201202515B/en unknown
-
2016
- 2016-01-08 JP JP2016003089A patent/JP6290940B2/ja not_active Expired - Fee Related
-
2017
- 2017-09-21 US US15/711,828 patent/US10576148B2/en not_active Expired - Fee Related
-
2018
- 2018-01-23 HR HRP20180131TT patent/HRP20180131T1/hr unknown
- 2018-01-31 CY CY20181100116T patent/CY1119867T1/el unknown
- 2018-02-08 JP JP2018020789A patent/JP2018104445A/ja active Pending
- 2018-02-28 ME MEP-2018-21A patent/ME02999B/me unknown
Non-Patent Citations (1)
| Title |
|---|
| None * |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10576148B2 (en) | Use of VAP-1 inhibitors for treating fibrotic conditions | |
| Kashiwagi et al. | Locally activated renin-angiotensin system associated with TGF-β1 as a major factor for renal injury induced by chronic inhibition of nitric oxide synthase in rats | |
| Zimmermann et al. | Endothelin and subarachnoid hemorrhage: an overview | |
| Boffa et al. | Regression of renal vascular and glomerular fibrosis: role of angiotensin II receptor antagonism and matrix metalloproteinases | |
| CN102183656B (zh) | 用于检测肾小管细胞损伤的早发的方法和试剂盒 | |
| Granchi et al. | Expression and regulation of endothelin-1 and its receptors in human penile smooth muscle cells | |
| Mizuiri et al. | Urinary angiotensin‐converting enzyme 2 in patients with CKD | |
| DK2717906T3 (en) | APPLICATION OF ALKALISK PHOSPHATASE FOR THE CONSERVATION OF Kidney Function | |
| Lu et al. | Effects of atorvastatin on progression of diabetic nephropathy and local RAGE and soluble RAGE expressions in rats | |
| CN1791797A (zh) | 用于检测肾小管细胞损伤的早发的方法和试剂盒 | |
| Gross et al. | Renoprotective effect of a dopamine D3 receptor antagonist in experimental type II diabetes | |
| MX2013009647A (es) | Metodos para tratar y diagnosticar enfermedades. | |
| Marahrens et al. | Knockout of aminopeptidase A in mice causes functional alterations and morphological glomerular basement membrane changes in the kidneys | |
| JP2008512463A (ja) | マトリックスメタロプロテイナーゼ−12に関連する糸球体基底膜疾患の治療 | |
| TWI594764B (zh) | 治療用途 | |
| WO2022064069A1 (en) | Mmp-9 as a predictive marker of chronic allograft dysfunction | |
| 真壁志帆 | Urinary aquaporin 2 as a potential indicator predicting tolvaptan response in patients with ADPKD | |
| Huart et al. | GUT MICROBIOTA AND FAECAL LEVELS OF SHORT CHAIN FATTY ACIDS DIFFER UPON BLOOD PRESSURE LEVELS IN MAN | |
| Anguiano Gómez | Circulating ACE2 and kidney disease: modulation of expression and value as a biomarker of atheromatosis progression | |
| KR20200045910A (ko) | 섬유화 질환을 예방 또는 치료하는 조성물 | |
| CA2654020A1 (en) | Methods for inhibiting cardiac pai-1 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20120405 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME RS |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: DE Ref document number: 1169042 Country of ref document: HK |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: A61P 17/02 20060101ALI20141127BHEP Ipc: A61K 39/395 20060101AFI20141127BHEP Ipc: A61P 43/00 20060101ALI20141127BHEP |
|
| RA4 | Supplementary search report drawn up and despatched (corrected) |
Effective date: 20150415 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: A61K 39/395 20060101AFI20150409BHEP Ipc: A61P 17/02 20060101ALI20150409BHEP Ipc: A61P 43/00 20060101ALI20150409BHEP |
|
| 17Q | First examination report despatched |
Effective date: 20161202 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| INTG | Intention to grant announced |
Effective date: 20170606 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: BIOTIE THERAPIES CORP. Owner name: THE UNIVERSITY OF BIRMINGHAM |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME RS |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 941374 Country of ref document: AT Kind code of ref document: T Effective date: 20171115 Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602010046422 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: HR Ref legal event code: TUEP Ref document number: P20180131 Country of ref document: HR |
|
| REG | Reference to a national code |
Ref country code: RO Ref legal event code: EPE |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: T3 Effective date: 20180130 |
|
| REG | Reference to a national code |
Ref country code: PT Ref legal event code: SC4A Ref document number: 2475387 Country of ref document: PT Date of ref document: 20180206 Kind code of ref document: T Free format text: AVAILABILITY OF NATIONAL TRANSLATION Effective date: 20180129 |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: TRGR |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: FP |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2657943 Country of ref document: ES Kind code of ref document: T3 Effective date: 20180307 |
|
| REG | Reference to a national code |
Ref country code: EE Ref legal event code: FG4A Ref document number: E014862 Country of ref document: EE Effective date: 20180129 |
|
| REG | Reference to a national code |
Ref country code: NO Ref legal event code: T2 Effective date: 20171101 |
|
| REG | Reference to a national code |
Ref country code: HR Ref legal event code: T1PR Ref document number: P20180131 Country of ref document: HR |
|
| REG | Reference to a national code |
Ref country code: HU Ref legal event code: AG4A Ref document number: E035890 Country of ref document: HU |
|
| REG | Reference to a national code |
Ref country code: GR Ref legal event code: EP Ref document number: 20180400275 Country of ref document: GR Effective date: 20180518 |
|
| REG | Reference to a national code |
Ref country code: SK Ref legal event code: T3 Ref document number: E 26794 Country of ref document: SK |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: GR Ref document number: 1169042 Country of ref document: HK |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602010046422 Country of ref document: DE |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 9 |
|
| 26N | No opposition filed |
Effective date: 20180802 |
|
| REG | Reference to a national code |
Ref country code: HR Ref legal event code: ODRP Ref document number: P20180131 Country of ref document: HR Payment date: 20190806 Year of fee payment: 10 |
|
| REG | Reference to a national code |
Ref country code: HR Ref legal event code: ODRP Ref document number: P20180131 Country of ref document: HR Payment date: 20200807 Year of fee payment: 11 |
|
| REG | Reference to a national code |
Ref country code: HR Ref legal event code: ODRP Ref document number: P20180131 Country of ref document: HR Payment date: 20210811 Year of fee payment: 12 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: MC Payment date: 20210922 Year of fee payment: 12 Ref country code: NL Payment date: 20210920 Year of fee payment: 12 Ref country code: LU Payment date: 20210920 Year of fee payment: 12 Ref country code: SM Payment date: 20210910 Year of fee payment: 12 Ref country code: LT Payment date: 20210820 Year of fee payment: 12 Ref country code: IT Payment date: 20210924 Year of fee payment: 12 Ref country code: IE Payment date: 20210916 Year of fee payment: 12 Ref country code: HR Payment date: 20210811 Year of fee payment: 12 Ref country code: FR Payment date: 20210914 Year of fee payment: 12 Ref country code: CY Payment date: 20210809 Year of fee payment: 12 Ref country code: CH Payment date: 20210917 Year of fee payment: 12 Ref country code: BG Payment date: 20210928 Year of fee payment: 12 Ref country code: CZ Payment date: 20210810 Year of fee payment: 12 Ref country code: AT Payment date: 20210921 Year of fee payment: 12 Ref country code: EE Payment date: 20210927 Year of fee payment: 12 Ref country code: FI Payment date: 20210921 Year of fee payment: 12 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DK Payment date: 20210916 Year of fee payment: 12 Ref country code: GB Payment date: 20210917 Year of fee payment: 12 Ref country code: BE Payment date: 20210920 Year of fee payment: 12 Ref country code: DE Payment date: 20210921 Year of fee payment: 12 Ref country code: NO Payment date: 20210921 Year of fee payment: 12 Ref country code: RO Payment date: 20210811 Year of fee payment: 12 Ref country code: IS Payment date: 20210910 Year of fee payment: 12 Ref country code: GR Payment date: 20210927 Year of fee payment: 12 Ref country code: PL Payment date: 20210810 Year of fee payment: 12 Ref country code: HU Payment date: 20210829 Year of fee payment: 12 Ref country code: LV Payment date: 20210917 Year of fee payment: 12 Ref country code: SK Payment date: 20210809 Year of fee payment: 12 Ref country code: SE Payment date: 20210916 Year of fee payment: 12 Ref country code: SI Payment date: 20210810 Year of fee payment: 12 Ref country code: TR Payment date: 20210813 Year of fee payment: 12 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: UEP Ref document number: 941374 Country of ref document: AT Kind code of ref document: T Effective date: 20171101 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: PT Payment date: 20210809 Year of fee payment: 12 Ref country code: MK Payment date: 20210811 Year of fee payment: 12 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20211008 Year of fee payment: 12 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: MT Payment date: 20210902 Year of fee payment: 12 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: AL Payment date: 20210907 Year of fee payment: 12 |
|
| REG | Reference to a national code |
Ref country code: HR Ref legal event code: PBON Ref document number: P20180131 Country of ref document: HR Effective date: 20220907 |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MM4D Effective date: 20220907 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 602010046422 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: EE Ref legal event code: MM4A Ref document number: E014862 Country of ref document: EE Effective date: 20220930 |
|
| REG | Reference to a national code |
Ref country code: NO Ref legal event code: MMEP |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230320 Ref country code: RO Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220907 Ref country code: PT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230307 Ref country code: MC Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220930 Ref country code: LT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220907 Ref country code: FI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220907 Ref country code: CZ Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220907 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: EBP Effective date: 20220930 |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: EUG |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MM Effective date: 20221001 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MM01 Ref document number: 941374 Country of ref document: AT Kind code of ref document: T Effective date: 20220907 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20220907 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20220930 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LV Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220907 Ref country code: HR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220907 Ref country code: CY Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220907 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20221001 Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220907 |
|
| REG | Reference to a national code |
Ref country code: SI Ref legal event code: KO00 Effective date: 20230517 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NO Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220930 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220930 Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220907 Ref country code: HU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220908 Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220930 Ref country code: EE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220930 Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230401 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220930 Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220907 |
|
| REG | Reference to a national code |
Ref country code: SK Ref legal event code: MM4A Ref document number: E 26794 Country of ref document: SK Effective date: 20220907 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220908 Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220908 Ref country code: GR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230406 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220930 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20231027 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220907 Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220907 Ref country code: DK Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220930 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220907 Ref country code: PL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220907 Ref country code: AL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220907 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220908 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220908 |