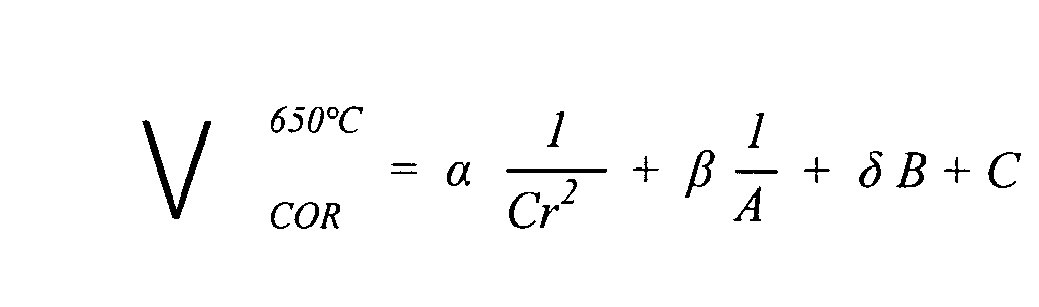EP2027300B1 - Steel compositions for special uses - Google Patents
Steel compositions for special uses Download PDFInfo
- Publication number
- EP2027300B1 EP2027300B1 EP20070788846 EP07788846A EP2027300B1 EP 2027300 B1 EP2027300 B1 EP 2027300B1 EP 20070788846 EP20070788846 EP 20070788846 EP 07788846 A EP07788846 A EP 07788846A EP 2027300 B1 EP2027300 B1 EP 2027300B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- steel composition
- contents
- weight
- composition according
- corrosion
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Revoked
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/02—Ferrous alloys, e.g. steel alloys containing silicon
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/04—Ferrous alloys, e.g. steel alloys containing manganese
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/22—Ferrous alloys, e.g. steel alloys containing chromium with molybdenum or tungsten
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/40—Ferrous alloys, e.g. steel alloys containing chromium with nickel
- C22C38/44—Ferrous alloys, e.g. steel alloys containing chromium with nickel with molybdenum or tungsten
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S148/00—Metal treatment
- Y10S148/902—Metal treatment having portions of differing metallurgical properties or characteristics
- Y10S148/909—Tube
Definitions
- the invention relates to a new steel composition for special purposes, in particular high performance in the presence of corrosion by oxidizing media such as, for example, fumes or water vapor, under high pressure and / or temperature.
- oxidizing media such as, for example, fumes or water vapor
- the present invention improves the situation.
- the invention generally provides a steel composition for special applications, which is in the zone comprising, in terms of weight content, about 1.8 to 11% of chromium (and preferably between about 2.3 and 10% of chromium ), less than 1% of silicon, and between 0.20 and 0.45% of manganese. It has been found possible to adjust the contents of the composition according to a predetermined pattern chosen to obtain substantially optimal corrosion characteristics under given high performance conditions. temperature. This model can involve as addition or as residual at least one element selected from molybdenum, tungsten, cobalt, and nickel.
- the composition has a silicon content by weight of between about 0.20 and 0.50%, preferably between about 0.30 and 0.50%. It may also comprise a manganese content by weight of between about 0.25 and 0.45%, and more preferably between about 0.25 and 0.40%.
- said model comprises at least one contribution term of chromium, and a contribution term of manganese alone.
- the manganese contribution term alone may include a second degree polynomial function of the manganese content.
- the term chromium contribution may include a quadratic term in inverse of the chromium content, and a term in inverse of a quantity containing the chromium content.
- the invention also covers a seamless tube or its accessory, consisting essentially of a proposed steel composition, the application of the steel composition to seamless tubes and accessories, intended to generate, convey or condition water vapor under high pressure and temperature, as well as the technique described for optimizing the properties of special steel compositions, in particular for their application to seamless tubes and accessories, intended to generate, convey or condition water vapor under high pressure and temperature.
- the steel X 20 Cr Mo V 12 - 1 to 12% Cr according to the German standard DIN 17.175 is not very fashionable because its implementation is very delicate and its creep characteristics are exceeded.
- the oxidation phenomena by the oxidizing fumes occur outside the tubes and more particularly outside the superheater tubes taking into account the flow of fumes that see these tubes.
- a metal having slow oxidation kinetics and capable of forming fine and adherent calamines is therefore highly desirable.
- a characteristic of identical creep resistance, a steel tube resistant to oxidation by steam can thus superheat steam at a higher temperature than a steel tube less resistant to oxidation by steam.
- the boiler calculation codes do not take into account the characteristics of resistance to hot oxidation (empirical rules are used which define too pessimistically an extra thickness for hot oxidation by both smoked only by water vapor).
- This composition is commercially designated VM12. It has surprised the inventors with regard to the resistance to hot oxidation by steam at 600 ° C. and 650 ° C., which is much greater than that of the 9% Cr steels, equal to or even greater than that of the X 20 Cr Mo V12-1 steel also containing 12% Cr and almost as good as that of TP 347 FG austenitic grade containing 18% Cr.
- the figure 1 illustrates the mechanism conventionally governing the hot oxidation of 9-12% Cr steels. As can be seen, the oxide germs homogeneously over the entire surface.
- the mechanism of figure 2 relates to the VM 12 grade, to certain X20 Cr Mo V 12-1 steel compositions and to TP 347 FG fine-grained austenitic grade: here, the oxide is produced in the form of isolated seeds which must develop on the surface before forming a layer and developing in depth. This mechanism leads to slow oxidation kinetics and adherent calamines.
- the Applicant has sought to do better, and in particular to obtain quantitative elements to improve existing steels, including those with 9% Cr whose resistance to oxidation is considered until now insufficient and those to 2.25 % Cr.
- the autoimmune des Mines de Douai first developed, on the occasion of a study contract with the Applicant, a formula for predicting the loss of metal thickness (determined after etching of the oxide formed without metal attack) over one year from a modeling of the influence of all elements of the chemical composition.
- LPL Lower Protective Layer of Scale
- the Applicant has therefore resumed measurements of the hot oxidation kinetics by water vapor at 650 ° C presented at the conference Les Embiez 2004 (see above) on 16 samples of steels with a ferritic structure (ferrite + perlite, bainite revenue, martensite revenue) whose Cr content ranges from 2.25% (T22 - T23) to 13%.
- the figure 4 is a table of composition of the steels tested with, in last column, the values of the corrosion measurements corresponding to the loss of thickness of metal over a year (corrosion rate Vcor) for these steels.
- ND in the table of the figure 4 means "not available”.
- the Applicant has performed on these experimental results a multidimensional statistical analysis. It is based on a plurality of terms reflecting a reasoned empirical approach of certain mechanisms or influences, which determine the Vcor corrosion rate.
- the formula [21] gives the average loss of metal thickness (in mm) over one year of exposure to water vapor at 650 ° C. This average loss of thickness is it even deduced from a weight loss of the metal after selective etching of the oxide, under standard conditions.
- Formula [21] has various terms as follows:
- Term Influence represented 1 / Cr 2 mainly represents the influence of the chromium content, here a inverse dependence on the square of the chromium content 1 / A mainly represents the influence of molybdenum, tungsten, nickel and cobalt contents, given an interaction with the chromium content B mainly represents the influence of the silicon content, also in view of an interaction with the chromium content VS mainly represents the influence of the manganese content, taking into account interactions with the tungsten and nickel contents
- the invention is not limited to the expression of the formula [21], which is known to write equivalents of different appearance.
- the formula [21] was set at 650 ° C, it is naturally valid for other temperatures, lower or higher. For example, a steel grade having a rather high corrosion rate at 650 ° C may be acceptable at lower temperatures, if it has interesting properties from any point of view, including a lower manufacturing cost.
- the Applicant has found a strong detrimental influence of the Mn content above about 0.25%, according to the indications of the formula [21] (grade range studied: 0.2 - 0.53% ). It also found that the Si content plays little when Si is greater than or equal to 0.20% (grade range studied: 0.09-0.47%). It also noted the absence of significant influence of the carbon content within the limits studied (0.1-0.2%).
- the Applicant was then interested in searching among the ferritic performance grades of the specifications ASTM, A213 and A335 for use in boilers (T91, P91, T92, P92, T23, P23, T24, P24) particular areas of chemical composition that lead with thin and very adherent calamines to make the tubes work better at steam temperatures of the order of 600 ° or 650 ° C and vapor pressures of the order of 300 bar.
- the steel grades proposed here for seamless tubes for conveying water vapor under high pressure and temperature include (by weight) 1.8 to 13% chromium (Cr), less than 1% silicon (Si) and between 0.10 and 0.45% manganese (Mn).
- the steel comprises an addition of at least 1 element chosen from molybdenum (Mo), tungsten (W), cobalt (Co), vanadium (V), niobium (Nb), titanium ( Ti), boron (B) and nitrogen (N).
- grades E10 allows a gain of between 18% (for E10-ax) and 42% (for E10-min), relative to the corrosion rate of the composition "reference" R10.
- the steel has between 2.3 and 2.6% Cr.
- the steel of mode E10 comprises an Si content of between 0.20 and 0.50% and very preferably between 0.30 and 0.50%.
- the steel comprises an Mn content of between 0.30 and 0.45%.
- the steel according to this mode E10 preferably comprises between 0.87 and 1% Mo. It does not include a voluntary addition of W, tungsten being a residual steel and its content of the order of 0.01% .
- the steel according to the mode E10 has contents of Cr, Mn, Si, Mo, W, Ni, Co whose Vcor value calculated according to the equation [21] is at most equal to about 0.9 mm / year, preferably 0.85 mm / year. Better results are obtained for Vcor at most equal to about 0.7 mm / year.
- grades E11 allows a gain of between 12% (for E11-max) and 51% (for E11-min), compared to the corrosion rate of the composition "reference".
- the steel comprises between 2.3 and 2.6% Cr.
- the mode E11 steel has an Si content between 0.20 and 0.50% and very preferably between 0.30 and 0.50%.
- the steel comprises an Mn content of between 0.25 and 0.45%.
- the steel according to this mode E11 preferably comprises between 1.45% and 1.60% W and between 0.05 and 0.20% Mo.
- the steel according to the mode E11 has contents of Cr, Mn, Si, Mo, W, Ni, Co whose Vcor value calculated according to the equation [21] is less than about 1.4 mm / year, preferably at most equal to about 1.25 mm / year. Better results are obtained for Vcor at most equal to about 0.9 mm / year.
- Table T12 below is constructed similarly to Tables T10 and T11. ⁇ b> Table T12 ⁇ / b> mn Yes Cr MB W Or Co Vcor measured Calculated Vcor Reference (R12) 0.50 0.25 2.30 0.85 - 0.05 - ND 0.83 E12 - max 0.45 0.25 2.40 0.90 - 0.10 - ND 0.76 E12 - min 0.30 0.45 2.60 0.70 - 0.02 - ND 0.58 E12 - med 0.40 0.30 2.50 0.80 - 0.05 - ND 0.67
- the gain is more limited on the selection according to the invention: from 9% (E12-max) to 30% (E12-min). It is believed that this is mainly because the margin on the Cr content is lower than for the embodiments E10 or E11.
- the steel comprises between 2.4 and 2.6% Cr.
- the steel has an Si content of between 0.20 and 0.45% and very preferably between 0.30 and 0.45%.
- the steel comprises an Mn content of between 0.30 and 0.45%.
- the steel according to this mode E12 does not include any addition of W (residual tungsten content of the order of 0.01%); its Mo content is preferably between 0.70 and 0.9%.
- the steel according to this mode E12 has contents of Cr, Mn, Si, Mo, W, Ni, Co whose Vcor value calculated according to the equation [21] is at most equal to about 0.8 mm / and preferably at most equal to about 0.75 mm / year. Better results are obtained for Vcor at most equal to about 0.7 mm / year.
- modes E10, E11 and E12 are quite similar in terms of chromium, manganese and silicon content.
- other contents Cr, Mn and / or Si of one of these modes E1 can be applied at least partially to another mode E1.
- the steels according to embodiment E20 do not contain microadditions of V, Nb, N or B.
- Formula [21] has been derived from the indications for different grades of steel of this embodiment E20. These grades are represented by three examples, denoted E20-max, E20-med, and E20-min, according to the corrosion rate obtained.
- grades E20 allows a gain of between 16% (for E20-max) and 89% (for E20-min), compared to the corrosion rate of the "reference" composition R20.
- the steel has between 9.2 and 10.00% Cr.
- the steel of the mode E20 has an Si content of between 0.25 and 0.50% and very preferably between 0.30 and 0.40%.
- the steel comprises an Mn content of between 0.30 and 0.45%.
- the steel according to this mode E20 preferably comprises between 0.90 and 1.00% Mo. It does not include a voluntary addition of W, the tungsten being a residual of the steel and its content of the order of 0, 01%.
- the steel according to the mode E20 has contents of Cr, Mn, Si, Mo, W, Ni, Co whose Vcor value calculated according to the equation [21] is at most equal to about 0.09 mm / year, preferably 0.06 mm / year. Better results are obtained for Vcor at most equal to about 0.04 mm / year.
- Table T21 below is constructed similarly to Table T10. ⁇ b> Table T21 ⁇ / b> mn Yes Cr MB W Or Co Vcor measured Vcor calculated Reference (R21) 0.46 0.31 8.73 0.99 0.01 0.26 - 0.094 0.106 E21-max 0.45 0.3 8.90 0.95 - 0.20 - ND 0,095 E21- min 0.30 0.50 9.50 0.85 - 0.02 - ND 0,021 E21 - med 0.40 0.35 9.00 0.90 - 0.05 - ND 0.066
- E21 ranges from 10% (E21-max) to 80% (E21-min). It is remarkable that for E21-min, the value obtained is five times lower than the reference value.
- the steel comprises between 8.9 and 9.5% Cr.
- the steel comprises an Si content of between 0.20 and 0.50% and very preferably between 0.30 and 0.50%.
- the steel comprises an Mn content of between 0.30 and 0.45%. It preferably comprises between 0.85% and 0.95% Mo.
- the steel according to embodiment E21 comprises at most 0.2% Ni (and very preferably at most 0.1%), and practically no tungsten (residual of the order of 0.01%).
- the steel according to the mode E21 has contents of Cr, Mn, Si, Mo, W, Ni, Co whose Vcor value calculated according to the equation [21] is less than about 0.1 mm / year. Better results are obtained for Vcor at most equal to about 0.07 mm / year.
- Table T22 below is constructed similarly to Table T10. ⁇ b> Table T22 ⁇ / b> mn Yes Cr MB W Or Co Vcor measured Vcor calculated Reference (R21) 0.41 0.22 8.51 0.44 1.69 0.13 - 0.113 0.113 E22 - max 0.40 0.25 8.90 0.45 1.70 0.20 - ND 0.11 E22 - min 0.30 0.50 9.50 0.30 1.50 0.02 - ND 0,055 E22 - med 0.35 0.30 9.20 0.40 1.70 0.1 - ND 0.082
- the gain on the selection of these embodiments E22 ranges from 2% (E22-max) to 52% (E22-min).
- the steel comprises between 8.9 and 9.5% Cr.
- the steel of mode E22 has an Si content of between 0.20 and 0.50% and very preferably between 0.30 and 0.50%.
- the steel of mode E22 comprises an Mn content of between 0.30 and 0.45% and more preferably between 0.30 and 0.40%.
- the steel according to the mode E22 preferably comprises between 0.30% and 0.45% Mo. It comprises between 1.50 and 1.75% W.
- the steel according to the mode E22 comprises at most 0.2% Ni and very preferably at most 0.1%.
- the steel according to the mode E22 has contents of Cr, Mn, Si, Mo, W, Ni, Co which, according to the equation [21], give a value Vcor at most equal to about 0.11 mm / year. Better results are obtained for Vcor at most equal to about 0.08 mm / year.
- the modes E21 and E22 are quite similar in terms of chromium, manganese and silicon content.
- the other contents of Cr, Mn and / or Si of one of these modes E2 can be applied at least partially to the other.
- the model used leads to increasing the content of certain alphagenic elements such as Cr, Si and to reducing the content of certain gamma-containing elements such as Mn and Ni, which may favor the appearance of delta ferrite.
- the proposed technique for optimizing special steels includes the following elements. It starts from a known steel grade or grade with known properties other than hot corrosion, which we want to optimize from the point of view of hot corrosion. A long-term corrosion property is calculated according to a model such as that of formula [21] on a reference composition. In the vicinity of the known steel, a particular range of composition of the steel grade is sought, leading to a better value of the corrosion property according to the same model.
- the steel according to the invention can also be used without the list being exhaustive as sheet to manufacture welded tubes, fittings, reactors, boiler parts, as molded part for manufacturing turbine bodies or valve bodies as forging for making turbine shafts and rotors, fittings, as metal powder for making various components in powder metallurgy, as solder metal and other similar applications.
- V HORN 650 ⁇ ° C ⁇ ⁇ 1 VS ⁇ r 2 + ⁇ ⁇ 1 AT + ⁇ B + VS
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Mechanical Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Heat Treatment Of Steel (AREA)
- Treatment Of Steel In Its Molten State (AREA)
- Heat Treatment Of Articles (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Catalysts (AREA)
- Carbon Steel Or Casting Steel Manufacturing (AREA)
- Soft Magnetic Materials (AREA)
Abstract
Description
L'invention concerne une nouvelle composition d'acier pour usages spéciaux, en particulier à hautes performances en présence de corrosion par des milieux oxydants tels que, par exemple, des fumées ou la vapeur d'eau, sous pression et/ou température élevées.The invention relates to a new steel composition for special purposes, in particular high performance in the presence of corrosion by oxidizing media such as, for example, fumes or water vapor, under high pressure and / or temperature.
Des ambiances de pression et température élevées en présence de vapeur d'eau existent notamment en production industrielle de l'électricité. La génération, le conditionnement (notamment la surchauffe et la resurchauffe) et le transport de la vapeur d'eau se font à l'aide d'éléments en acier, en particulier des tubes sans soudure. Malgré une longue histoire de solutions envisagées ou mises en oeuvre, sur laquelle on reviendra, de sérieux problèmes demeurent en termes de tenue dans l'ambiance concernée, de même que dans le temps.High atmospheres of pressure and temperature in the presence of water vapor exist in particular in the industrial production of electricity. The generation, conditioning (including overheating and reheating) and transport of water vapor are done using steel elements, especially seamless tubes. Despite a long history of solutions envisaged or implemented, on which we will return, serious problems remain in terms of holding in the environment concerned, as well as in time.
Ces problèmes sont particulièrement difficiles à résoudre, du fait notamment de la variabilité significative des propriétés des aciers en fonction de leurs constituants, et de la lourdeur des essais de corrosion à chaud sur une longue période.These problems are particularly difficult to solve, in particular because of the significant variability of the properties of the steels according to their constituents, and the heaviness of the hot corrosion tests over a long period.
Dans la suite du présent document on emploiera le terme « corrosion » ou « corrosion à chaud » pour désigner les phénomènes de perte de métal par oxydation à chaud.In the rest of this document, the term "corrosion" or "hot corrosion" will be used to denote the phenomena of loss of metal by hot oxidation.
La présente invention vient améliorer la situation.The present invention improves the situation.
L'invention propose en général une composition d'acier pour applications spéciales, qui se situe dans la zone comprenant, en teneur en poids, environ 1,8 à 11 % de Chrome (et préférentiellement entre environ 2,3 et 10 % de Chrome), moins de 1 % de Silicium, et entre 0,20 et 0,45 % de Manganèse. Il s'est avéré possible d'ajuster les teneurs de la composition selon un modèle prédéterminé, choisi pour obtenir des caractéristiques de corrosion sensiblement optimales dans des conditions données de performances à haute température. Ce modèle peut faire intervenir comme addition ou comme résiduel au moins un élément choisi parmi le molybdène, le tungstène, le cobalt, et le nickel.The invention generally provides a steel composition for special applications, which is in the zone comprising, in terms of weight content, about 1.8 to 11% of chromium (and preferably between about 2.3 and 10% of chromium ), less than 1% of silicon, and between 0.20 and 0.45% of manganese. It has been found possible to adjust the contents of the composition according to a predetermined pattern chosen to obtain substantially optimal corrosion characteristics under given high performance conditions. temperature. This model can involve as addition or as residual at least one element selected from molybdenum, tungsten, cobalt, and nickel.
Plus particulièrement, la composition comporte une teneur de silicium en poids comprise entre environ 0,20 et 0,50 %, de préférence entre environ 0,30 et 0,50%. Elle peut comporter aussi une teneur de manganèse en poids comprise entre 0,25 et 0,45 % environ, et plus préférentiellement entre 0,25 et 0,40% environ.More particularly, the composition has a silicon content by weight of between about 0.20 and 0.50%, preferably between about 0.30 and 0.50%. It may also comprise a manganese content by weight of between about 0.25 and 0.45%, and more preferably between about 0.25 and 0.40%.
Selon l'invention, ledit modèle comporte au moins un terme de contribution du chrome, et un terme de contribution du manganèse seul. Le terme de contribution du manganèse seul peut comprendre une fonction polynomiale du second degré de la teneur en manganèse. Le terme de contribution du chrome peut comprendre un terme quadratique en inverse de la teneur en chrome, et un terme en inverse d'une quantité contenant la teneur en chrome.According to the invention, said model comprises at least one contribution term of chromium, and a contribution term of manganese alone. The manganese contribution term alone may include a second degree polynomial function of the manganese content. The term chromium contribution may include a quadratic term in inverse of the chromium content, and a term in inverse of a quantity containing the chromium content.
Selon des modes de réalisation, que l'on décrira plus en détail :
- la composition d'acier comporte entre 2,3 et 2,6 % en poids de Chrome, environ.
- la composition d'acier comporte entre 8,9 et 9,5 % à 10 %, en poids de Chrome, environ.
- the steel composition comprises between 2.3 and 2.6% by weight of chromium, approximately.
- the steel composition comprises from about 8.9 to about 9.5% to about 10% by weight of chromium.
L'invention couvre également un tube sans soudure ou son accessoire, essentiellement constitué d'une composition d'acier proposée, l'application de la composition d'acier à des tubes sans soudure et accessoires, destinés à générer, à véhiculer ou à conditionner de la vapeur d'eau sous pression et température élevées, ainsi que la technique décrite pour optimiser les propriétés des compositions d'aciers spéciaux, en particulier pour leur application à des tubes sans soudure et accessoires, destinés à générer, à véhiculer ou à conditionner de la vapeur d'eau sous pression et température élevées.The invention also covers a seamless tube or its accessory, consisting essentially of a proposed steel composition, the application of the steel composition to seamless tubes and accessories, intended to generate, convey or condition water vapor under high pressure and temperature, as well as the technique described for optimizing the properties of special steel compositions, in particular for their application to seamless tubes and accessories, intended to generate, convey or condition water vapor under high pressure and temperature.
D'autres caractéristiques et avantages de l'invention apparaîtront mieux à la lecture de la description détaillée ci-après, faite en référence aux dessins annexés, sur lesquels :
- La
figure 1 illustre schématiquement le déroulement dans le temps d'un premier mécanisme d'oxydation, dit ici de <type 1> ; - La
figure 2 illustre schématiquement le déroulement dans le temps d'un second mécanisme d'oxydation, dit ici de <type 2> ; - La
figure 3 est un graphe illustratif de propriétés de compositions d'acier ; - La
figure 4 est un tableau de compositions d'acier, ayant fait l'objet de mesures de corrosion à long terme à 650°C, lesquelles figurent dans la dernière colonne du tableau ; - La
figure 5 est un graphe représentant une correspondance entre des données mesurées et des données calculées ; et - La
figure 6 est un graphe formant détail partiel de lafigure 5 .
- The
figure 1 schematically illustrates the course in time of a first oxidation mechanism, here called <type 1>; - The
figure 2 schematically illustrates the course in time of a second oxidation mechanism, here called <type 2>; - The
figure 3 is an illustrative graph of properties of steel compositions; - The
figure 4 is a table of steel compositions that have been subjected to long-term corrosion measurements at 650 ° C, which are shown in the last column of the table; - The
figure 5 is a graph representing a correspondence between measured data and calculated data; and - The
figure 6 is a graph forming partial detail of thefigure 5 .
Les dessins, la description ci-après et ses annexes contiennent, pour l'essentiel, des éléments de caractère certain. Ils pourront donc non seulement servir à mieux faire comprendre la présente invention, mais aussi contribuer à sa définition, le cas échéant.The drawings, the description below and its annexes contain, for the most part, elements of a certain character. They can therefore not only serve to better understand the present invention, but also contribute to its definition, if any.
On examine maintenant les conditions dans lesquelles l'invention peut s'appliquer.The conditions under which the invention can be applied are now examined.
On considère par exemple le cas d'une centrale thermique à combustible fossile, qui comprend une chaudière de puissance délivrant de la vapeur d'eau surchauffée à une turbine à vapeur accouplée à un alternateur. On connaît le bon rendement thermique de ce genre de centrales thermiques, que l'on cherche par ailleurs à rendre de moins en moins polluantes, en limitant les rejets tant de fumées que de gaz nocifs tels que le SO2, les NOx et le CO2, ce dernier étant plus particulièrement responsable de l'effet de serre. Or la réduction de la quantité relative de CO2 produite lors de la combustion passe par l'augmentation du rendement de la chaudière, laquelle est liée à la température et à la pression de la vapeur délivrée à la turbine.Consider for example the case of a fossil fuel power plant, which includes a power boiler delivering superheated steam to a steam turbine coupled to an alternator. We know the good thermal efficiency of this kind of thermal power plants, which we also try to make less and less polluting, by limiting the emissions of both fumes and harmful gases such as SO 2 , NO x and CO 2 , the latter being more particularly responsible for the greenhouse effect. But the reduction in the relative amount of CO 2 produced during the combustion passes through the increase of the efficiency of the boiler, which is related to the temperature and the pressure of the steam delivered to the turbine.
La vapeur d'eau étant essentiellement confinée dans des tubes sans soudure en acier, on a cherché depuis de nombreuses années à améliorer les caractéristiques de résistance à long terme des tubes à la pression intérieure de fluide à haute température en améliorant leur résistance au fluage et notamment leur résistance à la rupture par fluage en 100 000 heures.Since water vapor is essentially confined in seamless steel tubes, attempts have been made for many years to improve the long-term strength characteristics of the tubes at the high temperature internal pressure of the fluid. by improving their resistance to creep and in particular their resistance to creep rupture in 100,000 hours.
Le groupe dit American Society for Testing and Materials ("ASTM") a établi des normes ou spécifications dans lesquelles puisent les hommes du métier pour le choix de leurs aciers. S'agissant d'aciers spéciaux pour usage à haute température, ce sont :
- la spécification A213, intitulée "Standard Specification for Seamless Ferritic and Austenitic Alloy-Steel Boiler, Superheater and Heat-Exchanger Tubes", et
- la spécification A335 : "Standard Specification for Seamless Ferritic Alloy-Steel Pipe for High-Temperature Service".
- Specification A213, entitled "Standard Specification for Seamless Ferritic and Austenitic Alloy-Steel Boiler, Superheater and Heat-Exchanger Tubes", and
- Specification A335: "Standard Specification for Seamless Ferritic Alloy-Steel Pipe for High-Temperature Service".
Les chaudières des années 1960 mettaient en oeuvre des aciers non alliés pour les panneaux d'écran de la chaudière et des nuances à 2,25 % Cr et 1 % Mo (nuances T22 de l'ASTM A213 et P22 de l'ASTM A335) pour les parties chaudes des tubes de surchauffeurs et les conduites de vapeur surchauffée (160 bars - 560° C).Boilers from the 1960s used unalloyed steels for boiler screen panels and grades of 2.25% Cr and 1% Mo (ASTM A313 ASTM A213 and P22 grades T22) for the hot parts of the superheater tubes and the superheated steam lines (160 bar - 560 ° C).
Les aciers inoxydables austénitiques à 18 % Cr et 10 % Ni possèdent intrinsèquement de meilleures caractéristiques de résistance au fluage que les nuances plus faiblement alliées à structure ferritique mais possèdent de graves inconvénients du fait qu'une même chaudière doit alors comprendre des parties en acier à structure austénitique et d'autres à structure ferritique : il en découle d'une part des différences de coefficients de dilatation thermique, et d'autre part la nécessité de réaliser des jonctions soudées entre tubes de structure métallurgique différente.Austenitic stainless steels at 18% Cr and 10% Ni intrinsically have better creep characteristics than the lower alloyed grades with a ferritic structure, but have serious drawbacks because the same boiler must then comprise parts made of carbon steel. austenitic structure and others ferritic structure: it follows on the one hand differences in coefficients of thermal expansion, and on the other hand the need to make welded joints between tubes of different metallurgical structure.
La tendance a donc été à l'amélioration des matériaux à structure ferritique.The trend has therefore been to improve materials with a ferritic structure.
L'acier X 20 Cr Mo V 12 - 1 à 12 % Cr selon la nonne allemande DIN 17.175 n'est plus très en vogue car sa mise en oeuvre est très délicate et ses caractéristiques de fluage sont dépassées.The steel X 20 Cr Mo V 12 - 1 to 12% Cr according to the German standard DIN 17.175 is not very fashionable because its implementation is very delicate and its creep characteristics are exceeded.
Les années 1980 ont vu l'apparition dans les normes de nuances à 9 % Cr microalliées (T91 et P91, T92 et P92 selon ASTM A213 et A335) possédant à la fois une bonne résistance au fluage et d'excellentes propriétés de mise en oeuvre.The 1980s saw the emergence in the standards of microalloyed 9% Cr grades (T91 and P91, T92 and P92 according to ASTM A213 and A335) with both good creep resistance and excellent processing properties. .
En parallèle, sont apparues dans les années 1990, des nuances à 2,25 % Cr microalliées (T23, P23, T24, P24) pour améliorer les performances des panneaux d'écrans et/ou de certaines parties des surchauffeurs.In parallel, appeared in the 1990s, grades to 2.25% Cr microallied (T23, P23, T24, P24) to improve the performance of the display panels and / or parts of the superheaters.
Se sont alors posés des problèmes de tenue à l'oxydation à chaud, notamment dans le cas des aciers à 9 % Cr en comparaison à l'acier X 20 Cr Mo V 12 - 1 contenant 12 % Cr. On sait en effet que le Cr et également Si et Al sont des éléments qui réduisent l'oxydation à chaud.Then there were problems of resistance to hot oxidation, especially in the case of 9% Cr steels in comparison with X 20 Cr Mo V 12 - 1 steel containing 12% Cr. It is known that Cr and also Si and Al are elements that reduce hot oxidation.
Le terme <oxydation à chaud> regroupe 2 types de phénomènes :
- l'oxydation par les fumées oxydantes, et
- l'oxydation par la vapeur d'eau.
- oxidation by oxidizing fumes, and
- oxidation by water vapor.
Les phénomènes d'oxydation par les fumées oxydantes se produisent à l'extérieur des tubes et plus particulièrement à l'extérieur des tubes de surchauffeurs compte-tenu des flux de fumées que voient passer ces tubes.The oxidation phenomena by the oxidizing fumes occur outside the tubes and more particularly outside the superheater tubes taking into account the flow of fumes that see these tubes.
Ils se traduisent par une perte d'épaisseur de métal et de ce fait par une augmentation de la contrainte tangentielle σ dans le tube que l'on peut écrire par la relation [11] annexée, où D est le diamètre extérieur, e est l'épaisseur et P la pression intérieure de vapeur à l'intérieur des tubes.They result in a loss of thickness of metal and thus by an increase of the tangential stress σ in the tube that can be written by the attached relation [11], where D is the outside diameter, e is the thickness and P internal vapor pressure inside the tubes.
La cinétique d'oxydation est d'autant plus rapide que la couche d'oxyde (ou calamine) est mince. On pourrait donc croire qu'elle s'autolimite avec la croissance de la couche de calamine. Malheureusement, lorsque la couche de calamine est épaisse, elle perd de l'adhérence et se détache en feuilles (exfoliation). Il en découle que l'oxydation reprend à grande vitesse là où le métal est à nu.The kinetics of oxidation is even faster than the oxide layer (or calamine) is thin. One might think that it is self-limiting with the growth of the calamine layer. Unfortunately, when the calamine layer is thick, it loses adhesion and breaks off into leaves (exfoliation). It follows that the oxidation resumes at high speed where the metal is exposed.
Un métal ayant une cinétique d'oxydation lente et apte à former des calamines fines et adhérentes est donc hautement souhaitable.A metal having slow oxidation kinetics and capable of forming fine and adherent calamines is therefore highly desirable.
Il en est de même pour d'autres raisons pour les phénomènes d'oxydation par la vapeur d'eau qui se manifestent à l'intérieur des tubes et qui ont été plus récemment étudiés. En effet, la calamine formée à l'intérieur des tubes de surchauffeurs constitue un isolant thermique entre les fumées (source de chaleur) et la vapeur d'eau à surchauffer. Et une calamine épaisse côté vapeur (intérieur du tube) se traduit par une température plus élevée du métal que lorsque la calamine est mince. Or l'influence négative de la température sur la résistance au fluage est exponentielle.The same is true for other reasons for the phenomena of oxidation by water vapor which are manifested inside the tubes and which have been more recently studied. Indeed, the scale formed inside the superheater tubes is a thermal insulator between the fumes (heat source) and the water vapor to overheat. And a thick calamine on the steam side (inside the tube) results in a higher temperature of the metal than when the calamine is thin. However, the negative influence of temperature on creep resistance is exponential.
A caractéristique de résistance au fluage identique, un tube en acier résistant à l'oxydation par la vapeur pourra donc surchauffer la vapeur à une plus haute température qu'un tube en acier moins résistant à l'oxydation par la vapeur.A characteristic of identical creep resistance, a steel tube resistant to oxidation by steam can thus superheat steam at a higher temperature than a steel tube less resistant to oxidation by steam.
En outre, en cas de calamine épaisse et/ou peu adhérente, une exfoliation de celle-ci peut avoir pour conséquence :
- dans le cas des tubes dé surchauffeurs une accumulation de la calamine exfoliée dans les épingles des serpentins de surchauffeurs, entravant la circulation de vapeur et pouvant causer des éclatements de tubes surchauffeurs par surchauffe catastrophique,
- un entraînement de la calamine exfoliée, issue tant des tubes surchauffeurs que des collecteurs de vapeur ou des conduites de vapeur, dans les aubes de la turbine avec un risque d'érosion et/ou abrasion et de destruction de celles-ci.
- in the case of superheater tubes, an accumulation of exfoliated calamine in the pins of the superheater coils, hindering the flow of steam and causing superheater tubes to burst due to catastrophic overheating,
- entrainment of the exfoliated calamine, resulting from both superheater tubes and steam collectors or steam lines, in the blades of the turbine with a risk of erosion and / or abrasion and destruction thereof.
Pour le moment, les codes de calcul de chaudière ne prennent pas en compte de manière fine les caractéristiques de résistance à l'oxydation à chaud (on utilise des règles empiriques définissant de manière trop pessimiste une surépaisseur pour l'oxydation à chaud tant par les fumées que par la vapeur d'eau).For the time being, the boiler calculation codes do not take into account the characteristics of resistance to hot oxidation (empirical rules are used which define too pessimistically an extra thickness for hot oxidation by both smoked only by water vapor).
Dans
Cette composition est désignée commercialement VM12. Elle a surpris les inventeurs en ce qui concerne la résistance à l'oxydation à chaud par la vapeur à 600°C et 650°C, qui est très supérieure à celle des aciers à 9 % Cr, égale voire supérieure à celle de l'acier X 20 Cr Mo V12-1 contenant également 12 % Cr et presque aussi bonne que celle de la nuance austénitique TP 347 FG contenant 18 % Cr.This composition is commercially designated VM12. It has surprised the inventors with regard to the resistance to hot oxidation by steam at 600 ° C. and 650 ° C., which is much greater than that of the 9% Cr steels, equal to or even greater than that of the X 20 Cr Mo V12-1 steel also containing 12% Cr and almost as good as that of TP 347 FG austenitic grade containing 18% Cr.
Des résultats expérimentaux obtenus à l'Ecole des Mines de Douai ont été présentés à la conférence "High Temperature Corrosion and Protection of Materiaux 6, les Embiez 2004, et ont été publiés dans
Les auteurs (V. Lepingle et al.) ont observé qu'il est difficile de prévoir de manière quantitative la cinétique d'oxydation à chaud, les éléments de la composition chimique de l'acier pouvant avoir une influence non linéaire, voire fonctionner en synergie.The authors (V. Lepingle et al.) Have observed that it is difficult to predict in a quantitative way the kinetics of hot oxidation, the elements of the chemical composition of steel being able to have a non-linear influence, or even to work with synergy.
Ils ont notamment fait apparaître l'existence de deux types distincts de mécanismes de croissance intervenant dans l'oxydation à chaud, illustrés sur les
La
Le mécanisme de la
D'autres travaux se sont également intéressés à prédire la cinétique d'oxydation à chaud par la vapeur d'eau.Other studies have also focused on predicting the kinetics of hot oxidation by water vapor.
Une communication de Zurek et al. a été également présentée à la conférence Les Embiez et publiée dans "
dans laquelle Δm est l'accroissement de masse par oxydation et t le temps, tandis que z est généralement pris égal à 1/2. La constante Kp présentant une décroissance brutale au-delà d'une certaine teneur en chrome.A paper by Zurek et al. was also presented at the conference Embiez and published in "
where Δm is the oxidation mass increase and t is the time, while z is usually 1/2. The constant Kp exhibiting a sudden decrease beyond a certain chromium content.
Les principales conclusions que l'on peut tirer de Zurek et al. sont les suivantes (voir
- L'ajout de manganèse déplace vers la droite la zone de forte décroissance de Kp en fonction de la teneur en chrome ; Selon ce travail, addition de Mn tend à contrarier l'effet bénéfique du Cr ;
- L'ajout de silicium ou de cobalt déplace au contraire vers la gauche la zone de forte décroissance de Kp en fonction de la teneur en chrome. Selon ce travail, Si et Co ont une influence bénéfique qui étend le domaine d'action du Cr.
- The addition of manganese shifts to the right the zone of strong decay of Kp as a function of the chromium content; According to this work, addition of Mn tends to thwart the beneficial effect of Cr;
- The addition of silicon or cobalt displaces on the contrary to the left the zone of strong decay of Kp as a function of the chromium content. According to this work, Si and Co have a beneficial influence that extends the field of action of Cr.
On comprend qu'il est difficile d'en tirer des indications précises sur les propriétés de tel ou tel alliage.It is understood that it is difficult to draw precise indications on the properties of any particular alloy.
Osgerby et al. (
Globalement, les conclusions de ces travaux sont diverses, et même opposées en ce qui concerne le cas du manganèse dans les aciers ferritiques.Overall, the conclusions of this work are diverse, and even opposite as regards the case of manganese in ferritic steels.
La Demanderesse a cherché à mieux faire, et en particulier à obtenir des éléments quantitatifs permettant d'améliorer les aciers existants, notamment ceux à 9 % Cr dont la résistance à l'oxydation est considérée jusqu'à présent insuffisante et ceux à 2,25 % Cr.The Applicant has sought to do better, and in particular to obtain quantitative elements to improve existing steels, including those with 9% Cr whose resistance to oxidation is considered until now insufficient and those to 2.25 % Cr.
L'Ecole des Mines de Douai a tout d'abord mis au point à l'occasion d'un contrat d'étude avec la Demanderesse une formule de prévision de la perte d'épaisseur de métal (déterminée après décapage de l'oxyde formé sans attaque du métal) sur un an à partir d'une modélisation de l'influence de l'ensemble des éléments de la composition chimique.The Ecole des Mines de Douai first developed, on the occasion of a study contract with the Applicant, a formula for predicting the loss of metal thickness (determined after etching of the oxide formed without metal attack) over one year from a modeling of the influence of all elements of the chemical composition.
Cette formule dite LPL (Lowerest Protective Layer of Scale) n'est pas publique et les termes n'en sont pas connus de la Demanderesse.This so-called LPL (Lower Protective Layer of Scale) is not public and the terms are unknown to the Applicant.
La Demanderesse a simplement pu constater des écarts notables entre les résultats expérimentaux et les résultats obtenus par application de la formule LPL, qui lui ont été communiqués.The Applicant has simply been able to note significant differences between the experimental results and the results obtained by application of the LPL formula, which were communicated to him.
La Demanderesse a donc repris les mesures de la cinétique d'oxydation à chaud par la vapeur d'eau à 650°C présentées à la conférence Les Embiez 2004 (voir ci-dessus) sur 16 échantillons d'aciers à structure ferritique (ferrite + perlite, bainite revenue, martensite revenue) dont la teneur en Cr va de 2,25 % (T22 - T23) à 13 %. La
Le terme « ND » dans le tableau de la
La Demanderesse a réalisé sur ces résultats expérimentaux une analyse statistique multidimensionnelle. Elle s'est fondée sur une pluralité de termes traduisant une approche empirique raisonnée de certains mécanismes ou influences, qui déterminent la vitesse de corrosion Vcor.The Applicant has performed on these experimental results a multidimensional statistical analysis. It is based on a plurality of terms reflecting a reasoned empirical approach of certain mechanisms or influences, which determine the Vcor corrosion rate.
Après plusieurs essais, la Demanderesse a obtenu la formule [21] annexée, qui exprime la vitesse de corrosion Vcor à 650°C, sur le long terme, c'est-à-dire sur une période de l'ordre d'une année.After several tests, the Applicant has obtained the formula [21] attached, which expresses the corrosion rate Vcor at 650 ° C, in the long term, that is to say over a period of about a year .
La formule [21] donne la perte d'épaisseur moyenne de métal (en mm) sur un an d'exposition à la vapeur d'eau à 650°C. Cette perte d'épaisseur moyenne est elle même déduite d'une perte de poids du métal après décapage sélectif de l'oxyde, en conditions standard. La formule [21] comporte différents termes précisés comme suit :
Les teneurs de la formule [21] sont exprimées en % en poids (ou en masse).The contents of formula [21] are expressed as% by weight (or by weight).
Les coefficients α (alpha), β (béta) et δ (delta) et ceux qui interviennent dans les expressions B et C ont sensiblement les valeurs indiquées en annexe 1, Section 3, expressions [31] à [36].The coefficients α (alpha), β (beta) and δ (delta) and those involved in the expressions B and C have substantially the values indicated in
A côté de cela, si l'on examine la formule [21] globalement, il apparaît qu'elle comporte notamment :
- une fonction de la teneur en chrome qui comprend un terme en 1/Cr2 avec un terme d'allure en 1/Cr (
terme 1/A), et un terme correctif en Cr (terme B), - une fonction polynomiale (ici du second degré) de la teneur en Manganèse (terme C),
- une contribution conjointe (notée q) de W + Ni (tungstène + nickel) qui est d'une part en 1/-q dans le terme A, et d'autre part en q dans le terme C.
- les autres teneurs n'interviennent qu'une seule fois, d'une manière qui se lit directement sur la formule.
- a function of the chromium content which comprises a term in 1 / Cr 2 with a term of appearance in 1 / Cr (
term 1 / A), and a corrective term in Cr (term B), - a polynomial function (here of the second degree) of the Manganese content (term C),
- a joint contribution (denoted q) of W + Ni (tungsten + nickel) which is on the one hand in 1 / -q in the term A, and on the other hand in q in the term C.
- the other grades only occur once, in a way that reads directly on the formula.
Les
- en
figure 5 (partie de droite), que la correspondance est excellente pour des teneurs en Chrome voisines de 2,25 %, - en
figure 5 (partie de gauche), ainsi qu'enfigure 6 , qui est un détail de la partie de gauche de lafigure 5 , que la correspondance est également excellente pour des teneurs en Chrome voisines de 9 % et 12 %.
- in
figure 5 (right part), that the correspondence is excellent for chromium contents close to 2.25%, - in
figure 5 (left part), as well asfigure 6 , which is a detail of the left part of thefigure 5 , that the correspondence is also excellent for chromium contents close to 9% and 12%.
En bref, la modélisation et l'expérience donnent des résultats remarquablement concordants. Bien évidemment, l'invention n'est pas limitée à l'expression de la formule [21], dont on sait écrire des équivalents d'allure différente. On peut également en écrire des équivalents simplifiés, d'usage plus local (en termes de fourchettes de teneurs), compte-tenu des propriétés de variation de chacun des termes, ou de leurs éléments. Enfin, si la formule [21] a été établie à 650°C, elle est naturellement valable pour d'autres températures, inférieures ou supérieures. Par exemple, une nuance d'acier ayant une vitesse de corrosion plutôt élevée à 650 °C pourra être acceptable à des températures inférieures, si elle a des propriétés intéressantes d'un point de vue quelconque, y compris un coût de fabrication moindre.In short, modeling and experience yield remarkably consistent results. Obviously, the invention is not limited to the expression of the formula [21], which is known to write equivalents of different appearance. One can also write simplified equivalents, of more local use (in terms of ranges of contents), taking into account the properties of variation of each of the terms, or their elements. Finally, if the formula [21] was set at 650 ° C, it is naturally valid for other temperatures, lower or higher. For example, a steel grade having a rather high corrosion rate at 650 ° C may be acceptable at lower temperatures, if it has interesting properties from any point of view, including a lower manufacturing cost.
Plus finement, la Demanderesse a constaté une forte influence néfaste de la teneur en Mn au-dessus d'environ 0,25 %, conformément aux indications de la formule [21] (fourchette de teneurs étudiée : 0,2 - 0,53%). Elle a également constaté que la teneur en Si joue peu lorsque Si est supérieur ou égal à 0,20 % (fourchette de teneurs étudiée : 0,09 - 0,47%). Elle a également noté l'absence d'influence significative de la teneur en carbone dans les limites étudiées (0,1-0,2%).More finely, the Applicant has found a strong detrimental influence of the Mn content above about 0.25%, according to the indications of the formula [21] (grade range studied: 0.2 - 0.53% ). It also found that the Si content plays little when Si is greater than or equal to 0.20% (grade range studied: 0.09-0.47%). It also noted the absence of significant influence of the carbon content within the limits studied (0.1-0.2%).
La Demanderesse s'est alors intéressée à rechercher parmi les nuances performantes ferritiques des spécifications ASTM, A213 et A335 pour usage en chaudières (T91, P91, T92, P92, T23, P23, T24, P24) des domaines particuliers de composition chimique qui conduisent à des calamines minces et très adhérentes permettant de faire mieux travailler les tubes à des températures de vapeur de l'ordre de 600°, voire 650° C et des pressions de vapeur de l'ordre de 300 bars.The Applicant was then interested in searching among the ferritic performance grades of the specifications ASTM, A213 and A335 for use in boilers (T91, P91, T92, P92, T23, P23, T24, P24) particular areas of chemical composition that lead with thin and very adherent calamines to make the tubes work better at steam temperatures of the order of 600 ° or 650 ° C and vapor pressures of the order of 300 bar.
En général, les fabricants de tubes commandent jusqu'à présent leur acier dans le bas des fourchettes de teneur en chrome, compte tenu du coût de cet élément et de son caractère alphagène de cet élément. Par exemple, pour une fourchette théorique de 8,00 à 9,50 % pour le grade T91 de l'ASTM A213, les fabricants de tubes commandent un acier contenant autour de 8,5% Cr, ce qui minimise le risque de présence de ferrite delta sur produit.In general, pipe manufacturers have so far ordered their steel at the lower chromium range, given the cost of this element and its alphagenic nature of this element. For example, for a theoretical range of 8.00 to 9.50% for ASTM A213 grade T91, tube manufacturers control a steel containing around 8.5% Cr, which minimizes the risk of ferrite delta on product.
Quant au manganèse, on sait qu'il permet de fixer le soufre de l'acier, et que cette fixation évite des problèmes de forgeabilité (brûlure de l'acier). Ainsi, alors que la fourchette de l'ASTM A213 est de 0,30 - 0, 60% pour le grade T91, il est habituel d'élaborer les aciers pour usage à haute température avec des teneurs en manganèse voisines de 0,50 %, donc dans le haut de cette fourchette.As for the manganese, it is known that it makes it possible to fix the sulfur of the steel, and that this fixation avoids problems of forgeability (burning of the steel). Thus, while the range of ASTM A213 is 0.30-0.60% for T91 grade, it is usual to develop steels for high temperature use with manganese contents close to 0.50%. , so at the top of this range.
En général, les nuances d'acier proposées ici pour des tubes sans soudure destinés à véhiculer de la vapeur d'eau sous pression et température élevées comprennent (en poids) 1,8 à 13 % de chrome (Cr), moins de 1 % de silicium (Si) et entre 0,10 et 0,45 % de manganèse (Mn). En option, l'acier comprend une addition d'au moins 1 élément choisi parmi le molybdène (Mo), le tungstène (W), le cobalt (Co), le vanadium (V), le niobium (Nb), le titane (Ti), le bore (B) et l'azote (N).In general, the steel grades proposed here for seamless tubes for conveying water vapor under high pressure and temperature include (by weight) 1.8 to 13% chromium (Cr), less than 1% silicon (Si) and between 0.10 and 0.45% manganese (Mn). As an option, the steel comprises an addition of at least 1 element chosen from molybdenum (Mo), tungsten (W), cobalt (Co), vanadium (V), niobium (Nb), titanium ( Ti), boron (B) and nitrogen (N).
Au vu de l'expérience acquise, la Demanderesse s'est focalisée sur deux groupes de nuances performantes en fluage car alliées au Mo ou au W et microalliées (Nb, V, N et éventuellement B et Ti) et améliorables du point de vue oxydation à chaud. Ce sont :
- premier groupe les aciers à 2,25 % Cr : grades T/P22, T/P23, T/P24
- second groupe les aciers à 9 %. Cr : grades T/P91, T/P92
- first group steels at 2.25% Cr: grades T / P22, T / P23, T / P24
- second group the 9% steels. Cr: T / P91, T / P92 grades
Il en a découlé l'identification de nuances d'aciers spéciaux particulièrement avantageuses en termes de vitesse de corrosion, comme on va le voir maintenant.This resulted in the identification of special grades of steels particularly advantageous in terms of corrosion rate, as will be seen now.
Les normes ASTM A213 et A335 définissent respectivement les grades T22 et P22 comme contenant :
- 0,30 à 0,60 % Mn
- au plus 0,50 % Si
- 1,90 à 2,60 % Cr
- 0,87 à 1,13 % Mo
- 0,05 à 0,15 % C
- au plus 0,025 % S
- au plus 0,025 % P
- 0.30 to 0.60% Mn
- at most 0.50% Si
- 1.90 to 2.60% Cr
- 0.87 to 1.13% Mo
- 0.05 to 0.15% C
- not more than 0.025% S
- not more than 0.025% P
S'agissant de grades anciens, ils ne contiennent pas de microadditions de Ti, Nb, V et B.Since they are old grades, they do not contain microadditions of Ti, Nb, V and B.
Dans le tableau T10 ci-après, les colonnes 2 à 7 précisent les compositions pour un acier de référence du domaine, et pour trois autres aciers proposés (désignés en colonne 1). Dans la colonne Vcor mesurée, « ND » signifie non disponible. On comprendra que les essais requis pour déterminer une vitesse de corrosion fiable et précise à haute température sur un an sont particulièrement longs, délicats et dispendieux.In Table T10 below,
Pour l'acier de référence (R10), on voit que la valeur mesurée et la valeur prédite par la formule [21] se correspondent presque exactement. La formule [21] étant ainsi vérifiée, on en tire des indications sur d'autres nuances d'acier de ce mode de réalisation E10. Ces autres nuances sont représentées par trois exemples, notés E10-max, E10-med, et E10-min, d'après la vitesse de corrosion obtenue.
La sélection des nuances E10 permet un gain compris entre 18 % (pour E10-ax) et 42 % (pour E10-min), par rapport à la vitesse de corrosion de la composition « référence » R10.The selection of grades E10 allows a gain of between 18% (for E10-ax) and 42% (for E10-min), relative to the corrosion rate of the composition "reference" R10.
Dans ce mode E10, l'acier comporte entre 2,3 et 2,6 % Cr.In this mode E10, the steel has between 2.3 and 2.6% Cr.
Préférentiellement, l'acier du mode E10 comporte une teneur en Si comprise entre 0,20 et 0,50 % et très préférentiellement entre 0,30 et 0,50 %. Préférentiellement, l'acier comprend une teneur en Mn comprise entre 0,30 et 0,45 %.Preferably, the steel of mode E10 comprises an Si content of between 0.20 and 0.50% and very preferably between 0.30 and 0.50%. Preferably, the steel comprises an Mn content of between 0.30 and 0.45%.
L'acier selon ce mode E10 comporte préférentiellement entre 0,87 et 1% Mo. Il ne comporte pas d'addition volontaire de W, le tungstène étant un résiduel de l'acier et sa teneur de l'ordre de 0,01%.The steel according to this mode E10 preferably comprises between 0.87 and 1% Mo. It does not include a voluntary addition of W, tungsten being a residual steel and its content of the order of 0.01% .
Très préférentiellement, l'acier selon le mode E10 possède des teneurs en Cr, Mn, Si, Mo, W, Ni, Co dont la valeur Vcor calculée selon l'équation [21] est au plus égale à environ 0,9 mm/an, de préférence 0,85 mm/an. De meilleurs résultats sont obtenus pour Vcor au plus égale à environ 0,7 mm/an.Very preferably, the steel according to the mode E10 has contents of Cr, Mn, Si, Mo, W, Ni, Co whose Vcor value calculated according to the equation [21] is at most equal to about 0.9 mm / year, preferably 0.85 mm / year. Better results are obtained for Vcor at most equal to about 0.7 mm / year.
Les normes ASTM A213 et A335 définissent respectivement les grades T23 et P23 comme contenant :
- 0,10 à 0,60 % Mn
- au plus 0,50 % Si
- 1,90 à 2,60 % Cr
- 0,05 à 0,30 % Mo
- 1,45 à 1,75 % W
- 0,04 à 0,10 % C
- au plus 0,030 % P
- au plus 0,010 % S
- 0,20 à 0,30 % V
- 0,02 à 0,08 % Nb
- 0,0005 à 0,006 % B
- au plus 0,030 % de N
- au plus 0,030 % d'Al
- 0.10 to 0.60% Mn
- at most 0.50% Si
- 1.90 to 2.60% Cr
- 0.05 to 0.30% Mo
- 1.45 to 1.75% W
- 0.04 to 0.10% C
- not more than 0.030% P
- not more than 0.010% S
- 0.20 to 0.30% V
- 0.02 to 0.08% Nb
- 0.0005 to 0.006% B
- not more than 0.030% of N
- not more than 0.030% of Al
Le remplacement d'une grosse partie du molybdène par le tungstène et les microadditions donnent à ces grades des caractéristiques de résistance au fluage très améliorées par rapport aux grades T/P22. Une telle amélioration ne permet par contre pas d'augmenter la limite supérieure de tenue en température vis à vis de l'oxydation à chaud.The replacement of a large proportion of molybdenum by tungsten and microadditions gives these grades very improved creep characteristics compared to T / P22 grades. Such an improvement does not, on the other hand, make it possible to increase the upper temperature resistance limit with respect to hot oxidation.
Dans le tableau T11 ci-après, les colonnes 2 à 7 précisent les compositions pour un acier de référence du domaine, et pour trois autres aciers proposés (désignés en colonne 1). Pour l'acier de référence, on voit que la valeur mesurée et la valeur prédite par la formule [21] se correspondent exactement. La formule [21] étant ainsi vérifiée, on en tire des indications sur les trois autres nuances d'acier de ce mode de réalisation E11, notées E11-max, E11-med, et E11-min, d'après la vitesse de corrosion obtenue.
La sélection des nuances E11 permet un gain compris entre 12 % (pour E11-max) et 51 % (pour E11-min), par rapport à la vitesse de corrosion de la composition « référence ».The selection of grades E11 allows a gain of between 12% (for E11-max) and 51% (for E11-min), compared to the corrosion rate of the composition "reference".
Dans ce mode E11, l'acier comporte entre 2,3 et 2,6 % Cr.In this mode E11, the steel comprises between 2.3 and 2.6% Cr.
Préférentiellement, l'acier du mode E11 comporte une teneur en Si comprise entre 0,20 et 0,50 % et très préférentiellement entre 0,30 et 0,50 %. Préférentiellement, l'acier comprend une teneur en Mn comprise entre 0,25 et 0,45 %.Preferably, the mode E11 steel has an Si content between 0.20 and 0.50% and very preferably between 0.30 and 0.50%. Preferably, the steel comprises an Mn content of between 0.25 and 0.45%.
L'acier selon ce mode E11 comporte préférentiellement entre 1,45 % et 1,60 % W et entre 0,05 et 0,20 % Mo.The steel according to this mode E11 preferably comprises between 1.45% and 1.60% W and between 0.05 and 0.20% Mo.
Très préférentiellement, l'acier selon le mode E11 possède des teneurs en Cr, Mn, Si, Mo, W, Ni, Co dont la valeur Vcor calculée selon l'équation [21] est inférieure à environ 1,4 mm/an, de préférence au plus égale à environ 1,25 mm/an. De meilleurs résultats sont obtenus pour Vcor au plus égale à environ 0,9 mm/an.Very preferably, the steel according to the mode E11 has contents of Cr, Mn, Si, Mo, W, Ni, Co whose Vcor value calculated according to the equation [21] is less than about 1.4 mm / year, preferably at most equal to about 1.25 mm / year. Better results are obtained for Vcor at most equal to about 0.9 mm / year.
Ces aciers contiennent selon la norme ASTM A213
- 0,30 à 0,70 % Mn
- 0,15 à 0,45 % Si
- 2,20 à 2,60 % Cr
- 0,70 à 1,10 % Mo
- 0,04à0,10%C
- au plus 0,020 % P
- au plus 0,010 % S
- 0,20 à 0,30 % V
- 0,06 à 0,10 % Ti
- 0,0015 à 0,0020 % B
- au plus 0,012 % N
- au plus 0,020 % Al
- 0.30 to 0.70% Mn
- 0.15 to 0.45% Si
- 2.20 to 2.60% Cr
- 0.70 to 1.10% MB
- 0,04à0,10% C
- not more than 0.020% P
- not more than 0.010% S
- 0.20 to 0.30% V
- 0.06 to 0.10% Ti
- 0.0015 to 0.0020% B
- not more than 0.012% N
- not more than 0.020% Al
Le tableau T12 ci-après est construit de manière semblables aux tableaux T10 et T11.
Le gain est plus limité sur la sélection selon l'invention : de 9 % (E12-max) à 30 % (E12-min). Il est estimé que cela tient essentiellement au fait que la marge sur la teneur en Cr est plus faible que pour les modes de réalisation E10 ou E11.The gain is more limited on the selection according to the invention: from 9% (E12-max) to 30% (E12-min). It is believed that this is mainly because the margin on the Cr content is lower than for the embodiments E10 or E11.
Selon ce mode E12, l'acier comporte entre 2,4 et 2,6 % Cr. Préférentiellement, l'acier comporte une teneur en Si comprise entre 0,20 et 0,45 % et très préférentiellement entre 0,30 et 0,45 %. Préférentiellement, l'acier comprend une teneur en Mn comprise entre 0,30 et 0,45 %.According to this mode E12, the steel comprises between 2.4 and 2.6% Cr. Preferably, the steel has an Si content of between 0.20 and 0.45% and very preferably between 0.30 and 0.45%. Preferably, the steel comprises an Mn content of between 0.30 and 0.45%.
L'acier selon ce mode E12 ne comporte pas d'addition de W (teneur en tungstène résiduelle de l'ordre de 0,01%) ; sa teneur en Mo est de préférence comprise entre 0,70 et 0,9 %.The steel according to this mode E12 does not include any addition of W (residual tungsten content of the order of 0.01%); its Mo content is preferably between 0.70 and 0.9%.
Très préférentiellement, l'acier selon ce mode E12 possède des teneurs en Cr, Mn, Si, Mo, W, Ni, Co dont la valeur Vcor calculée selon l'équation [21] est au plus égale à environ 0,8 mm/an et de préférence au plus égale à environ 0,75 mm/an. De meilleurs résultats sont obtenus pour Vcor au plus égale à environ 0,7 mm/an.Very preferably, the steel according to this mode E12 has contents of Cr, Mn, Si, Mo, W, Ni, Co whose Vcor value calculated according to the equation [21] is at most equal to about 0.8 mm / and preferably at most equal to about 0.75 mm / year. Better results are obtained for Vcor at most equal to about 0.7 mm / year.
On observera que les modes E10, E11 et E12 (globalement notés E1) sont assez proches, en termes de teneur en Chrome, Manganèse et Silicium. Ainsi, d'autres teneurs en Cr, Mn et/ou Si de l'un de ces modes E1 peuvent être appliquées au moins partiellement à un autre mode E1.It will be observed that the modes E10, E11 and E12 (generally noted E1) are quite similar in terms of chromium, manganese and silicon content. Thus, other contents Cr, Mn and / or Si of one of these modes E1 can be applied at least partially to another mode E1.
Les normes ASTM A213 et A335 définissent respectivement les grades T9 et P9 comme contenant :
- 0,30 à 0,60 % Mn
- 0,25 à 1,00 % Si
- 8,00 à 10,00 % Cr
- 0,90 à 1,10% Mo
- au plus 0,15 % C
- au plus 0,025 % P
- au plus 0,025 % S
- 0.30 to 0.60% Mn
- 0.25 to 1.00% Si
- 8.00 to 10.00% Cr
- 0.90 to 1.10% MB
- not more than 0.15% C
- not more than 0.025% P
- not more than 0.025% S
Par rapport aux modes de réalisation E21 et E22 exposés plus loin dans le texte, les aciers selon le mode de réalisation E20 ne contiennent pas de microadditions de V, Nb, N ou B.With respect to the embodiments E21 and E22 discussed later in the text, the steels according to embodiment E20 do not contain microadditions of V, Nb, N or B.
Dans le tableau T20 ci-après, les colonnes 2 à 7 précisent les compositions pour un acier de référence du domaine, et pour trois autres aciers proposés (désignés en colonne 1). Dans la colonne Vcor mesurée, « ND » signifie non disponible. On comprendra que les essais requis pour déterminer une vitesse de corrosion fiable et précise à haute température sur un an sont particulièrement longs, délicats et dispendieux.In Table T20 below,
On a tiré de la formule [21] des indications sur différentes nuances d'acier de ce mode de réalisation E20. Ces nuances sont représentées par trois exemples, notés E20-max, E20-med, et E20-min, d'après la vitesse de corrosion obtenue.
La sélection des nuances E20 permet un gain compris entre 16 % (pour E20-max) et 89 % (pour E20-min), par rapport à la vitesse de corrosion de la composition « référence » R20.The selection of grades E20 allows a gain of between 16% (for E20-max) and 89% (for E20-min), compared to the corrosion rate of the "reference" composition R20.
Dans ce mode E20, l'acier comporte entre 9.2 et 10.00 % Cr.In this mode E20, the steel has between 9.2 and 10.00% Cr.
Préférentiellement, l'acier du mode E20 comporte une teneur en Si comprise entre 0,25 et 0,50 % et très préférentiellement entre 0,30 et 0,40 %. Préférentiellement, l'acier comprend une teneur en Mn comprise entre 0,30 et 0,45 %.Preferably, the steel of the mode E20 has an Si content of between 0.25 and 0.50% and very preferably between 0.30 and 0.40%. Preferably, the steel comprises an Mn content of between 0.30 and 0.45%.
L'acier selon ce mode E20 comporte préférentiellement entre 0,90 et 1,00% Mo. Il ne comporte pas d'addition volontaire de W, le tungstène étant un résiduel de l'acier et sa teneur de l'ordre de 0,01%.The steel according to this mode E20 preferably comprises between 0.90 and 1.00% Mo. It does not include a voluntary addition of W, the tungsten being a residual of the steel and its content of the order of 0, 01%.
Très préférentiellement, l'acier selon le mode E20 possède des teneurs en Cr, Mn, Si, Mo, W, Ni, Co dont la valeur Vcor calculée selon l'équation [21] est au plus égale à environ 0,09 mm/an, de préférence 0,06 mm/an. De meilleurs résultats sont obtenus pour Vcor au plus égale à environ 0,04 mm/an.Very preferably, the steel according to the mode E20 has contents of Cr, Mn, Si, Mo, W, Ni, Co whose Vcor value calculated according to the equation [21] is at most equal to about 0.09 mm / year, preferably 0.06 mm / year. Better results are obtained for Vcor at most equal to about 0.04 mm / year.
Ces aciers contiennent selon les normes ASTM A213 et A335 :
- 0,30 à 0,60 % Mn
- 0,20 à 0,50 % Si
- 8,00 à 9,50 % Cr
- 0,85 à 1,05 % Mo
- au plus 0,40 % Ni
- 0,08 à 0,12 % C
- au plus 0,020 % P
- au plus 0,010 % S
- 0,18 à 0,25 % V
- 0,06 à 0,1 % Nb
- 0,030 à 0,070 % N
- au plus 0,040 % Al
- 0.30 to 0.60% Mn
- 0.20 to 0.50% Si
- 8.00 to 9.50% Cr
- 0.85 to 1.05% MB
- not more than 0.40% Ni
- 0.08 to 0.12% C
- not more than 0.020% P
- not more than 0.010% S
- 0.18 to 0.25% V
- 0.06 to 0.1% Nb
- 0.030 to 0.070% N
- not more than 0.040% Al
Le tableau T21 ci-après est construit de manière semblable au tableau T10.
Le gain sur la sélection de ces modes de réalisations E21 va de 10 % (E21-max) à 80 % (E21-min). Il est remarquable que, pour E21-min, la valeur obtenue est cinq fois plus faible que la valeur de référence.The gain on the selection of these embodiments E21 ranges from 10% (E21-max) to 80% (E21-min). It is remarkable that for E21-min, the value obtained is five times lower than the reference value.
Selon ce mode E21, l'acier comporte entre 8,9 et 9,5 % Cr.According to this mode E21, the steel comprises between 8.9 and 9.5% Cr.
Préférentiellement, l'acier comporte une teneur en Si comprise entre 0,20 et 0,50 % et très préférentiellement entre 0,30 et 0,50 %.Preferably, the steel comprises an Si content of between 0.20 and 0.50% and very preferably between 0.30 and 0.50%.
Préférentiellement, l'acier comprend une teneur en Mn comprise entre 0,30 et 0,45 %. Il comporte préférentiellement entre 0,85 % et 0,95 % Mo.Preferably, the steel comprises an Mn content of between 0.30 and 0.45%. It preferably comprises between 0.85% and 0.95% Mo.
Préférentiellement, l'acier selon le mode de réalisation E21 comprend au plus 0,2 % Ni (et très préférentiellement au plus 0,1%), et pratiquement pas de tungstène (résiduel de l'ordre de 0,01%).Preferably, the steel according to embodiment E21 comprises at most 0.2% Ni (and very preferably at most 0.1%), and practically no tungsten (residual of the order of 0.01%).
Très préférentiellement l'acier selon le mode E21 possède des teneurs en Cr, Mn, Si, Mo, W, Ni, Co dont la valeur Vcor calculée selon l'équation [21] est inférieure à environ 0,1 mm/an. De meilleurs résultats sont obtenus pour Vcor au plus égale à environ 0,07 mm/an.Very preferably, the steel according to the mode E21 has contents of Cr, Mn, Si, Mo, W, Ni, Co whose Vcor value calculated according to the equation [21] is less than about 0.1 mm / year. Better results are obtained for Vcor at most equal to about 0.07 mm / year.
Ces aciers contiennent selon les normes ASTM A213 et A335 :
- au plus 0,30 à 0,60 % Mn
- au plus 0,50 % Si
- 8,50 à 9,50 % Cr
- 0,30 à 0,60 % Mo
- 1,50 à 2,00 % W
- au plus 0,40 % Ni
- 0,07 à 0,13 % C
- au plus 0,020 % P
- au plus 0,010 % S
- 0,15 à 0,25 % V
- 0,04 à 0,09 % Nb
- 0,001 à 0,006 % B
- 0,030 à 0,070 % N
- au plus 0,040 % Al
- not more than 0.30 to 0.60% Mn
- at most 0.50% Si
- 8.50 to 9.50% Cr
- 0.30 to 0.60% Mo
- 1.50 to 2.00% W
- not more than 0.40% Ni
- 0.07 to 0.13% C
- not more than 0.020% P
- not more than 0.010% S
- 0.15 to 0.25% V
- 0.04 to 0.09% Nb
- 0.001 to 0.006% B
- 0.030 to 0.070% N
- not more than 0.040% Al
Le tableau T22 ci-après est construit de manière semblable au tableau T10.
Ici, le gain sur la sélection de ces modes de réalisations E22 va de 2 % (E22-max) à 52 % (E22-min).Here, the gain on the selection of these embodiments E22 ranges from 2% (E22-max) to 52% (E22-min).
Selon ce mode de réalisation E22, l'acier comporte entre 8,9 et 9,5 % Cr.According to this embodiment E22, the steel comprises between 8.9 and 9.5% Cr.
Préférentiellement, l'acier du mode E22 comporte une teneur en Si comprise entre 0,20 et 0,50 % et très préférentiellement entre 0,30 et 0,50 %.Preferably, the steel of mode E22 has an Si content of between 0.20 and 0.50% and very preferably between 0.30 and 0.50%.
Préférentiellement, l'acier du mode E22 comprend une teneur en Mn comprise entre 0,30 et 0,45 % et plus préférentiellement entre 0,30 et 0,40%.Preferably, the steel of mode E22 comprises an Mn content of between 0.30 and 0.45% and more preferably between 0.30 and 0.40%.
L'acier selon le mode E22 comporte préférentiellement entre 0,30 % et 0,45 % Mo. Il comporte entre 1,50 et 1,75 % W.The steel according to the mode E22 preferably comprises between 0.30% and 0.45% Mo. It comprises between 1.50 and 1.75% W.
Préférentiellement, l'acier selon le mode E22 comprend au plus 0,2 % Ni et très préférentiellement au plus 0, 1 %.Preferably, the steel according to the mode E22 comprises at most 0.2% Ni and very preferably at most 0.1%.
Très préférentiellement l'acier selon le mode E22 possède des teneurs en Cr, Mn, Si, Mo, W, Ni, Co qui, selon l'équation [21], donnent une valeur Vcor au plus égale à environ 0,11 mm/an. De meilleurs résultats sont obtenus pour Vcor au plus égale à environ 0,08 mm/an.Very preferably, the steel according to the mode E22 has contents of Cr, Mn, Si, Mo, W, Ni, Co which, according to the equation [21], give a value Vcor at most equal to about 0.11 mm / year. Better results are obtained for Vcor at most equal to about 0.08 mm / year.
On observera que les modes E21 et E22 (globalement notés E2) sont assez proches, en termes de teneur en Chrome, Manganèse et Silicium. Ainsi, les autres teneurs en Cr, Mn et/ou Si de l'un de ces modes E2 peuvent être appliquées au moins partiellement à l'autre.It will be observed that the modes E21 and E22 (generally noted E2) are quite similar in terms of chromium, manganese and silicon content. Thus, the other contents of Cr, Mn and / or Si of one of these modes E2 can be applied at least partially to the other.
On considèrera maintenant une situation intermédiaire.We will now consider an intermediate situation.
Le modèle utilisé conduit à augmenter la teneur en certains éléments alphagènes tels que Cr, Si et à réduire la teneur en certains éléments gammagènes tels que Mn et Ni, ce qui peut favoriser l'apparition de ferrite delta.The model used leads to increasing the content of certain alphagenic elements such as Cr, Si and to reducing the content of certain gamma-containing elements such as Mn and Ni, which may favor the appearance of delta ferrite.
Si la réduction de teneur en Mo et/ou W (éléments alphagènes) est insuffisante pour compenser l'augmentation de teneur en Cr, Si et la réduction de celle en Mn et Ni du point de vue de l'apparition de ferrite delta, il y aura lieu d'ajuster la teneur en éléments gammagènes comme N et C qui n'interviennent pas dans le présent modèle. On utilisera à cet égard des formules connues de prévision de ferrite delta en fonction des teneurs en chrome équivalent et nickel équivalent.If the reduction in Mo and / or W content (alphagenic elements) is insufficient to compensate for the increase in Cr, Si content and the reduction of that in Mn and Ni from the point of view of the appearance of delta ferrite, it It will be necessary to adjust the content of gamma-like elements such as N and C that are not involved in the present model. In this respect, it will be possible to use known formulas for prediction of delta ferrite as a function of the contents of equivalent chromium and equivalent nickel.
La technique proposée pour optimiser des aciers spéciaux comprend les éléments suivants. On part d'une nuance ou grade d'acier connu ayant des propriétés connues autres que la corrosion à chaud, que l'on cherche à optimiser du point de vue corrosion à chaud. On calcule une propriété de corrosion à long terme selon un modèle tel que celui de la formule [21] sur une composition de référence. On cherche au voisinage de l'acier connu une fourchette particulière de composition de la nuance d'acier conduisant à une meilleure valeur de la propriété de corrosion selon le même modèle.The proposed technique for optimizing special steels includes the following elements. It starts from a known steel grade or grade with known properties other than hot corrosion, which we want to optimize from the point of view of hot corrosion. A long-term corrosion property is calculated according to a model such as that of formula [21] on a reference composition. In the vicinity of the known steel, a particular range of composition of the steel grade is sought, leading to a better value of the corrosion property according to the same model.
Dès lors que le modèle est de grande fiabilité, cette technique a de nombreux avantages, dont :
- éviter de fabriquer des aciers inhabituels seulement pour des tests de corrosion,
- éviter les délicats et coûteux tests de corrosion à long terme et haute température.
- avoid making unusual steels only for corrosion tests,
- avoid the delicate and costly tests of long-term and high temperature corrosion.
Cette technique permet surtout d'utiliser des données ciblées et non outrageusement pessimistes pour la conception de chaudières ou de tuyauteries de vapeur et par là de minimiser la surépaisseur de corrosion prise en compte dans les calculs de conception.This technique makes it possible especially to use targeted and not outrageously pessimistic data for the design of boilers or piping systems. steam and thereby minimize the extra thickness of corrosion taken into account in the design calculations.
Elle permet en outre d'augmenter la température de vapeur à température de métal donné et d'éviter des exfoliations de calamine en favorisant la germination hétérogène et discontinue de l'oxyde en surface de l'acier du côté vapeur.It also makes it possible to increase the vapor temperature at a given metal temperature and to avoid calamine exfoliations by promoting the heterogeneous and discontinuous germination of the oxide on the surface of the steel on the vapor side.
L'acier selon l'invention peut également être utilisé sans que la liste soit exhaustive comme tôle pour fabriquer des tubes soudés, des raccords, des réacteurs, des pièces de chaudronnerie, comme pièce moulée pour fabriquer des corps de turbine ou des corps de vannes de sécurité, comme pièce forgée pour fabriquer des arbres et des rotors de turbine, des raccords, comme poudre métalliques pour réaliser des composants divers en métallurgie des poudres, comme métal d'apport de soudure et d'autres applications similaires.The steel according to the invention can also be used without the list being exhaustive as sheet to manufacture welded tubes, fittings, reactors, boiler parts, as molded part for manufacturing turbine bodies or valve bodies as forging for making turbine shafts and rotors, fittings, as metal powder for making various components in powder metallurgy, as solder metal and other similar applications.
Claims (16)
- Steel composition according to specifications ASTM A213 and A335 defining respectively grades T23 and P23 displaying high hot corrosion performance due to oxidising environments such as fumes or water vapour, characterised in that it comprises, by weight, about 2.3 to 2.6 % of chromium, at most 0.5 % of silicon, between 0.20 and 0.45 % of manganese, between 1.45 and 1.6 % of tungsten, and between 0.05 and 0.2 % of molybdenum, the contents of the steel composition being adjusted based on a predetermined model, selected to obtain substantially optimum properties for resistance to hot oxidation under given conditions for high-temperature performance over a long period, the model being:
contents by weight of Cr, Mn, Si, Mo, W, Ni, Co being such that the corrosion value Vcor is less than 1.4. - Steel composition according to claim 1, characterised by contents by weight of Cr, Mn, Si, Mo, W, Ni, Co being such that the corrosion value Vcor is at most equal to 1.25.
- Steel composition according to specifications ASTM A213 and A335 defining respectively grades T22 and P22 displaying high hot corrosion performance due to oxidising environments such as fumes or water vapour, characterised in that it comprises, by weight, about 2.3 to 2.6 % of chromium, at most 0.5 % of silicon, between 0.20 and 0.45 % of manganese, between 0.87 and 1 % of molybdenum and very little tungsten, the contents of the steel composition being adjusted based on a predetermined model, selected to obtain substantially optimum properties for resistance to hot oxidation under given conditions for high-temperature performance over a long period, the model being:
contents by weight of Cr, Mn, Si, Mo, W, Ni, Co being such that the corrosion value Vcor is at most equal to 0.9. - Steel composition according to claim 3, characterised by contents by weight of Cr, Mn, Si, Mo, W, Ni, Co being such that the corrosion value Vcor is at most equal to 0.85.
- Steel composition according to specifications ASTM A213 and A335 defining respectively grades T24 and P24 displaying high hot corrosion performance due to oxidising environments such as fumes or water vapour, characterised in that it comprises, by weight, about 2.4 to 2.6 % of chromium, between 0.15 to 0.45 % of silicon, between 0.30 and 0.45 % of manganese, between 0.7 and 0.9 % of molybdenum and almost no tungsten, the contents of the steel composition being adjusted based on a predetermined model, selected to obtain substantially optimum properties for resistance to hot oxidation under given conditions for high-temperature performance over a long period, the model being:
contents by weight of Cr, Mn, Si, Mo, W, Ni, Co being such that the corrosion value Vcor is at most equal to 0.8. - Steel composition according to claim 5, characterised by contents by weight of Cr, Mn, Si, Mo, W, Ni, Co being such that the corrosion value Vcor is at most equal to 0.75.
- Steel composition according to specifications ASTM A213 and A335 defining respectively grades T91 and P91 displaying high hot corrosion performance due to oxidising environments such as fumes or water vapour, characterised in that it comprises, by weight, about 8.9 to 9.5 % of chromium, between 0.2 to 0.5 % of silicon, between 0.85 and 0.95 % of molybdenum, between 0.3 and 0.45 % of manganese and substantially an absence of W, the contents of the steel composition being adjusted based on a predetermined model, selected to obtain substantially optimum properties for resistance to hot oxidation under given conditions for high-temperature performance over a long period, the model being:
contents by weight of Cr, Mn, Si, Mo, W, Ni, Co being such that the corrosion value Vcor is less than 0.1. - Steel composition according to claim 7, characterised by contents by weight of Cr, Mn, Si, Mo, W, Ni, Co being such that the corrosion value Vcor is at most equal to 0.07.
- Steel composition according to specifications ASTM A213 and A335 defining respectively grades T91 and P91 displaying high hot corrosion performance due to oxidising environments such as fumes or water vapour, characterised in that it comprises, by weight, about 8.9 to 9.5 % of chromium, at most 0.5 % of silicon, between 0.3 and 0.45 % of manganese, between 1.5 and 1.75 % of tungsten, and between 0.3 and 0.45 % of molybdenum, the contents of the steel composition being adjusted based on a predetermined model, selected to obtain substantially optimum properties for resistance to hot oxidation under given conditions for high-temperature performance over a long period, the model being:
contents by weight of Cr, Mn, Si, Mo, W, Ni, Co being such that the corrosion value Vcor is at most equal to 0.11. - Steel composition according to claim 9, characterised by contents by weight of Cr, Mn, Si, Mo, W, Ni, Co being such that the corrosion value Vcor is at most equal to 0.08.
- Steel composition according to one of claims 7 to 10, characterised in that it comprises less than 0.2 % of nickel.
- Steel composition according to specifications ASTM A213 and A335 defining respectively grades T9 and P9 displaying high hot corrosion performance due to oxidising environments such as fumes or water vapour, characterised in that it comprises, by weight, about 9.2 to 10 % of chromium, between 0.25 and 1 % of silicon, between 0.9 and 1 % of molybdenum, between 0.3 and 0.45 % of manganese, and substantially an absence of W, the contents of the steel composition being adjusted based on a predetermined model, selected to obtain substantially optimum properties for resistance to hot oxidation under given conditions for high-temperature performance over a long period, the model being:
contents by weight of Cr, Mn, Si, Mo, W, Ni, Co being such that the corrosion value Vcor is at most equal to 0.09. - Steel composition according to one of claims 1 to 4 and 7 to 11, characterised in that it comprises a content of silicon between 0.20 and 0.50 %, preferably between 0.30 and 0.50 %.
- Steel composition according to one of claims 1 to 4, characterised in that it comprises a content of manganese between 0.25 and 0.45 %.
- Seamless or accessory tube, basically consisting of a steel composition according to one of the preceding claims.
- Application of the steel composition to seamless and accessory tubes, intended to generate, to convey or to condition water vapour under elevated pressure and temperature.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PL07788846T PL2027300T3 (en) | 2006-06-09 | 2007-06-07 | Steel compositions for special uses |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR0605133A FR2902111B1 (en) | 2006-06-09 | 2006-06-09 | STEEL COMPOSITIONS FOR SPECIAL PURPOSES |
| PCT/FR2007/000941 WO2007141427A2 (en) | 2006-06-09 | 2007-06-07 | Steel compositions for special uses |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP2027300A2 EP2027300A2 (en) | 2009-02-25 |
| EP2027300B1 true EP2027300B1 (en) | 2011-08-17 |
| EP2027300B8 EP2027300B8 (en) | 2012-11-14 |
Family
ID=37635762
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP07788846A Revoked EP2027300B8 (en) | 2006-06-09 | 2007-06-07 | Steel compositions for special uses |
Country Status (17)
| Country | Link |
|---|---|
| US (1) | US9005520B2 (en) |
| EP (1) | EP2027300B8 (en) |
| JP (1) | JP2009540118A (en) |
| KR (1) | KR20090023475A (en) |
| CN (1) | CN101466859B (en) |
| AT (1) | ATE520796T1 (en) |
| AU (1) | AU2007255279B2 (en) |
| BR (1) | BRPI0712148B1 (en) |
| CA (1) | CA2654521C (en) |
| EA (1) | EA015633B1 (en) |
| ES (1) | ES2371534T3 (en) |
| FR (1) | FR2902111B1 (en) |
| HR (1) | HRP20110850T1 (en) |
| MX (1) | MX2008015740A (en) |
| PL (1) | PL2027300T3 (en) |
| UA (1) | UA97368C2 (en) |
| WO (1) | WO2007141427A2 (en) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| IT1403688B1 (en) * | 2011-02-07 | 2013-10-31 | Dalmine Spa | STEEL TUBES WITH THICK WALLS WITH EXCELLENT LOW TEMPERATURE HARDNESS AND RESISTANCE TO CORROSION UNDER TENSIONING FROM SULFUR. |
| DE102011054718B4 (en) * | 2011-10-21 | 2014-02-13 | Hitachi Power Europe Gmbh | Method for generating a voltage reduction in erected tube walls of a steam generator |
| US20130202908A1 (en) * | 2012-02-08 | 2013-08-08 | Grzegorz Jan Kusinski | Equipment for use in corrosive environments and methods for forming thereof |
| CN102747287A (en) * | 2012-07-31 | 2012-10-24 | 宝山钢铁股份有限公司 | High-temperature resistant pipe suitable for delayed coking process and producing method of high-temperature resistant pipe |
| CN102994888A (en) * | 2012-11-27 | 2013-03-27 | 天津大学 | Novel high-chromium ferritic heat resistant steel and thermo-mechanical treatment process |
| US11162457B2 (en) | 2017-08-11 | 2021-11-02 | General Electric Company | Turbine fan system and method |
Family Cites Families (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| SU773130A1 (en) * | 1978-09-11 | 1980-10-23 | Центральный Ордена Трудового Красного Знамени Научно-Исследовательский Институт Черной Металлургии Им. И.П. Бардина | Martensite-ferrite steel |
| SU857293A1 (en) * | 1978-12-25 | 1981-08-23 | Челябинский политехнический институт им. Ленинского комсомола | Steel |
| JPS5672156A (en) * | 1979-11-15 | 1981-06-16 | Japan Steel Works Ltd:The | Low-alloy heat-resistant steel for high temperature use |
| JPS5915977B2 (en) * | 1980-06-16 | 1984-04-12 | 住友金属工業株式会社 | Seamless steel for pipes with excellent corrosion resistance |
| JPS6167757A (en) * | 1984-09-08 | 1986-04-07 | Nisshin Steel Co Ltd | Chromium steel having superior oxidation resistance |
| JPS6376854A (en) * | 1986-09-18 | 1988-04-07 | Kawasaki Steel Corp | Heat resistant ferritic steel having superior strength at high temperature |
| JPH062926B2 (en) | 1989-02-20 | 1994-01-12 | 住友金属工業株式会社 | Heat resistant steel with high temperature creep strength |
| JPH02217483A (en) * | 1989-02-20 | 1990-08-30 | Furukawa Electric Co Ltd:The | Copper sheet for ornament |
| JPH0353045A (en) * | 1989-07-19 | 1991-03-07 | Kawasaki Steel Corp | Low-alloy heat-resisting steel excellent in toughness at low temperature and strength at high temperature |
| JP3237137B2 (en) | 1991-08-12 | 2001-12-10 | 住友金属工業株式会社 | High chromium ferritic heat-resistant steel with small decrease in strength of weld heat affected zone |
| JP2687067B2 (en) * | 1992-06-17 | 1997-12-08 | 新日本製鐵株式会社 | Method for producing high Cr ferritic steel sheet having excellent creep strength and good workability |
| NO303695B1 (en) * | 1994-03-09 | 1998-08-17 | Mannesmann Ag | Steel with high heat resistance for boiler construction |
| JP3096959B2 (en) | 1996-02-10 | 2000-10-10 | 住友金属工業株式会社 | Low Mn and low Cr ferrite heat resistant steel with excellent high temperature strength |
| JP3214350B2 (en) * | 1996-04-09 | 2001-10-02 | 住友金属工業株式会社 | Method for producing Cr-Mo based seamless steel pipe excellent in high temperature strength |
| DE69705167T2 (en) * | 1996-06-24 | 2001-11-15 | Mitsubishi Jukogyo K.K., Tokio/Tokyo | Ferritic steels with a low Cr content and ferritic cast steels with a low Cr content, which have excellent high-temperature strength and weldability |
| JP3454027B2 (en) | 1996-07-29 | 2003-10-06 | Jfeスチール株式会社 | Boiler steel and seamless steel pipe for boilers with excellent hot workability and creep resistance |
| JP3687249B2 (en) * | 1997-01-29 | 2005-08-24 | Jfeスチール株式会社 | 2.25Cr steel softening heat treatment method |
| JP3457834B2 (en) * | 1997-04-09 | 2003-10-20 | 三菱重工業株式会社 | Weld metal for low Cr ferritic heat resistant steel with excellent toughness |
| JPH1161342A (en) | 1997-08-08 | 1999-03-05 | Mitsubishi Heavy Ind Ltd | High chromium ferritic steel |
| SE516137C2 (en) * | 1999-02-16 | 2001-11-19 | Sandvik Ab | Heat-resistant austenitic steel |
| JP3565331B2 (en) | 1999-08-18 | 2004-09-15 | 三菱重工業株式会社 | High strength low alloy heat resistant steel |
| JP3514182B2 (en) * | 1999-08-31 | 2004-03-31 | 住友金属工業株式会社 | Low Cr ferritic heat resistant steel excellent in high temperature strength and toughness and method for producing the same |
| JP2001271141A (en) * | 2000-03-24 | 2001-10-02 | Kawasaki Steel Corp | HIGH Cr STEEL FOR SEAMLESS PIPE |
| JP3518515B2 (en) | 2000-03-30 | 2004-04-12 | 住友金属工業株式会社 | Low / medium Cr heat resistant steel |
| JP2002069588A (en) * | 2000-08-29 | 2002-03-08 | Sumitomo Metal Ind Ltd | Ferritic heat-resistant steel |
| JP3570379B2 (en) * | 2000-12-28 | 2004-09-29 | 住友金属工業株式会社 | Low alloy heat resistant steel |
| FR2823226B1 (en) * | 2001-04-04 | 2004-02-20 | V & M France | STEEL AND STEEL TUBE FOR HIGH TEMPERATURE USE |
| JP3711959B2 (en) | 2001-06-15 | 2005-11-02 | 住友金属工業株式会社 | Heat resistant low alloy steel pipe and method for producing the same |
| JP3690325B2 (en) | 2001-07-26 | 2005-08-31 | Jfeスチール株式会社 | Fe-Cr-Al alloy foil excellent in oxidation resistance and high temperature deformation resistance and method for producing the same |
| US6890393B2 (en) * | 2003-02-07 | 2005-05-10 | Advanced Steel Technology, Llc | Fine-grained martensitic stainless steel and method thereof |
-
2006
- 2006-06-09 FR FR0605133A patent/FR2902111B1/en not_active Expired - Fee Related
-
2007
- 2007-06-07 US US12/303,764 patent/US9005520B2/en active Active
- 2007-06-07 EA EA200870608A patent/EA015633B1/en not_active IP Right Cessation
- 2007-06-07 JP JP2009513729A patent/JP2009540118A/en active Pending
- 2007-06-07 CA CA 2654521 patent/CA2654521C/en active Active
- 2007-06-07 ES ES07788846T patent/ES2371534T3/en active Active
- 2007-06-07 PL PL07788846T patent/PL2027300T3/en unknown
- 2007-06-07 KR KR1020097000521A patent/KR20090023475A/en not_active Application Discontinuation
- 2007-06-07 CN CN2007800213275A patent/CN101466859B/en not_active Expired - Fee Related
- 2007-06-07 AU AU2007255279A patent/AU2007255279B2/en not_active Ceased
- 2007-06-07 AT AT07788846T patent/ATE520796T1/en active
- 2007-06-07 BR BRPI0712148A patent/BRPI0712148B1/en not_active IP Right Cessation
- 2007-06-07 UA UAA200900138A patent/UA97368C2/en unknown
- 2007-06-07 WO PCT/FR2007/000941 patent/WO2007141427A2/en active Application Filing
- 2007-06-07 EP EP07788846A patent/EP2027300B8/en not_active Revoked
- 2007-06-07 MX MX2008015740A patent/MX2008015740A/en active IP Right Grant
-
2011
- 2011-11-15 HR HR20110850T patent/HRP20110850T1/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| HRP20110850T1 (en) | 2011-12-31 |
| MX2008015740A (en) | 2009-03-02 |
| ES2371534T3 (en) | 2012-01-04 |
| WO2007141427A2 (en) | 2007-12-13 |
| BRPI0712148A2 (en) | 2012-02-22 |
| CN101466859B (en) | 2012-08-22 |
| EP2027300B8 (en) | 2012-11-14 |
| EA015633B1 (en) | 2011-10-31 |
| US20100307430A1 (en) | 2010-12-09 |
| US9005520B2 (en) | 2015-04-14 |
| FR2902111A1 (en) | 2007-12-14 |
| CA2654521C (en) | 2014-10-14 |
| EP2027300A2 (en) | 2009-02-25 |
| CN101466859A (en) | 2009-06-24 |
| FR2902111B1 (en) | 2009-03-06 |
| KR20090023475A (en) | 2009-03-04 |
| AU2007255279B2 (en) | 2011-10-13 |
| UA97368C2 (en) | 2012-02-10 |
| BRPI0712148B1 (en) | 2018-09-11 |
| CA2654521A1 (en) | 2007-12-13 |
| PL2027300T3 (en) | 2012-01-31 |
| JP2009540118A (en) | 2009-11-19 |
| WO2007141427A3 (en) | 2008-07-31 |
| EA200870608A1 (en) | 2009-04-28 |
| ATE520796T1 (en) | 2011-09-15 |
| AU2007255279A1 (en) | 2007-12-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2027300B1 (en) | Steel compositions for special uses | |
| CA2442299C (en) | Steel and steel tube for high-temperature use | |
| EP2418295B1 (en) | Low thermal expansion Ni-base superalloy | |
| EP1818422B2 (en) | Ferritic stainless steel with 19% of chromium stabilised with niobium | |
| EP1717330B1 (en) | Metal tube for use in carburizing gas atmosphere | |
| KR101256268B1 (en) | Austenitic stainless steel | |
| AU2003200351B2 (en) | Duplex stainless steel for urea manufacturing plants | |
| WO2009150989A1 (en) | Process for producing high-alloy seamless pipe | |
| CN101171351A (en) | Stainless steel pipe for oil well excellent in enlarging characteristics | |
| WO2007091535A1 (en) | Ferritic heat-resistant steel | |
| JP3650951B2 (en) | Seamless steel pipe for oil wells with excellent stress corrosion cracking resistance | |
| FR2605908A1 (en) | ROLLER ROLLER WITH NO PILGRIM FOR TUBE MANUFACTURE | |
| EP2449143B1 (en) | Cryogenic treatment of martensitic steel with mixed hardening | |
| EP0430754B1 (en) | Stainless shape memory alloy and process for producing the same | |
| EP2128278B1 (en) | Process for producing bend pipe for line pipe and bend pipe for line pipe | |
| EP3578676B1 (en) | Austenitic alloy with high aluminum content and associated design process | |
| EP2257652B1 (en) | Method of manufacturing sheets of austenitic stainless steel with high mechanical properties | |
| EP1885900A1 (en) | Steel for submarine hulls with improved weldability | |
| EP0845544A1 (en) | Steel product made from bainitic steel and process for making the steel product | |
| EP2742165B1 (en) | Steel for manufacturing carburized steel parts, carburized steel parts produced with said steel, and method for manufacturing same | |
| CA3162447A1 (en) | Solid metallic component and method for producing same | |
| EP0892076A1 (en) | Nickel based alloy and welding electrode made from a nickel based alloy | |
| EP3411509B1 (en) | Elemental composition for steel with improved anti-coking properties | |
| WO2000023632A1 (en) | Case hardening structural steel, method for obtaining same and parts formed with same | |
| FR2741360A1 (en) | TWO-PHASE SUPERPLASTIC STAINLESS STEEL HAVING LOW DEFORMATION RESISTANCE AND EXCELLENT LENGTH PROPERTIES |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20081118 |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LI LT LU LV MC MT NL PL PT RO SE SI SK TR |
|
| AX | Request for extension of the european patent |
Extension state: AL BA HR MK RS |
|
| 17Q | First examination report despatched |
Effective date: 20090812 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| RAX | Requested extension states of the european patent have changed |
Extension state: HR Payment date: 20081118 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: PETELOT, EMILIE Inventor name: FOUQUET, ANNIE Inventor name: PETELOT, CATHELINE Inventor name: LEPINGLE, VIVIANE Inventor name: PETELOT, ADELINE Inventor name: LOUIS, GHISLAIN |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LI LT LU LV MC MT NL PL PT RO SE SI SK TR |
|
| AX | Request for extension of the european patent |
Extension state: HR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: LANGUAGE OF EP DOCUMENT: FRENCH |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602007016606 Country of ref document: DE Effective date: 20111027 |
|
| REG | Reference to a national code |
Ref country code: RO Ref legal event code: EPE |
|
| REG | Reference to a national code |
Ref country code: HR Ref legal event code: TUEP Ref document number: P20110850 Country of ref document: HR |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: TRGR |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: VDEP Effective date: 20110817 |
|
| REG | Reference to a national code |
Ref country code: HR Ref legal event code: T1PR Ref document number: P20110850 Country of ref document: HR |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2371534 Country of ref document: ES Kind code of ref document: T3 Effective date: 20120104 |
|
| LTIE | Lt: invalidation of european patent or patent extension |
Effective date: 20110817 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20111219 Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110817 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110817 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20111217 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110817 |
|
| REG | Reference to a national code |
Ref country code: PL Ref legal event code: T3 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110817 Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110817 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110817 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20111118 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FD4D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110817 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110817 |
|
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110817 |
|
| 26 | Opposition filed |
Opponent name: SUMITOMO METAL INDUSTRIES, LTD. Effective date: 20120516 |
|
| PLAX | Notice of opposition and request to file observation + time limit sent |
Free format text: ORIGINAL CODE: EPIDOSNOBS2 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110817 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R026 Ref document number: 602007016606 Country of ref document: DE Effective date: 20120516 |
|
| PLAF | Information modified related to communication of a notice of opposition and request to file observations + time limit |
Free format text: ORIGINAL CODE: EPIDOSCOBS2 |
|
| RIN2 | Information on inventor provided after grant (corrected) |
Inventor name: VANDENBERGHE BRUNO Inventor name: LOUIS, GHISLAIN Inventor name: LEPINGLE, VIVIANE Inventor name: FOUQUET, ANNIE Inventor name: PETELOT, ADELINE Inventor name: LEYER JEAN Inventor name: PETELOT DANIEL (DECEDE) Inventor name: PETELOT, CATHELINE Inventor name: PETELOT, EMILIE |
|
| BERE | Be: lapsed |
Owner name: V & M FRANCE Effective date: 20120630 |
|
| PLAB | Opposition data, opponent's data or that of the opponent's representative modified |
Free format text: ORIGINAL CODE: 0009299OPPO |
|
| PLBB | Reply of patent proprietor to notice(s) of opposition received |
Free format text: ORIGINAL CODE: EPIDOSNOBS3 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120630 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| R26 | Opposition filed (corrected) |
Opponent name: NIPPON STEEL & SUMITOMO METAL CORPORATION Effective date: 20120516 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120630 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120630 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120630 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20111117 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110817 |
|
| PLAB | Opposition data, opponent's data or that of the opponent's representative modified |
Free format text: ORIGINAL CODE: 0009299OPPO |
|
| R26 | Opposition filed (corrected) |
Opponent name: SUMITOMO METAL INDUSTRIES, LTD. Effective date: 20120516 |
|
| PLAB | Opposition data, opponent's data or that of the opponent's representative modified |
Free format text: ORIGINAL CODE: 0009299OPPO |
|
| R26 | Opposition filed (corrected) |
Opponent name: NIPPON STEEL & SUMITOMO METAL CORPORATION Effective date: 20120516 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110817 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120607 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20070607 |
|
| REG | Reference to a national code |
Ref country code: HR Ref legal event code: ODRP Ref document number: P20110850 Country of ref document: HR Payment date: 20160524 Year of fee payment: 10 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20160614 Year of fee payment: 10 Ref country code: DE Payment date: 20160621 Year of fee payment: 10 Ref country code: GB Payment date: 20160621 Year of fee payment: 10 Ref country code: CZ Payment date: 20160606 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20160627 Year of fee payment: 10 Ref country code: RO Payment date: 20160601 Year of fee payment: 10 Ref country code: AT Payment date: 20160621 Year of fee payment: 10 Ref country code: SE Payment date: 20160620 Year of fee payment: 10 Ref country code: PL Payment date: 20160603 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20160628 Year of fee payment: 10 |
|
| RDAF | Communication despatched that patent is revoked |
Free format text: ORIGINAL CODE: EPIDOSNREV1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R064 Ref document number: 602007016606 Country of ref document: DE Ref country code: DE Ref legal event code: R103 Ref document number: 602007016606 Country of ref document: DE |
|
| RDAG | Patent revoked |
Free format text: ORIGINAL CODE: 0009271 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: PATENT REVOKED |
|
| 27W | Patent revoked |
Effective date: 20161231 |
|
| GBPR | Gb: patent revoked under art. 102 of the ep convention designating the uk as contracting state |
Effective date: 20161231 |
|
| REG | Reference to a national code |
Ref country code: HR Ref legal event code: PNEV Ref document number: P20110850 Country of ref document: HR |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MA03 Ref document number: 520796 Country of ref document: AT Kind code of ref document: T Effective date: 20161231 |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: ECNC |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: PATENT REVOKED |