EP1720504B1 - Compression apparatus - Google Patents
Compression apparatus Download PDFInfo
- Publication number
- EP1720504B1 EP1720504B1 EP05713935A EP05713935A EP1720504B1 EP 1720504 B1 EP1720504 B1 EP 1720504B1 EP 05713935 A EP05713935 A EP 05713935A EP 05713935 A EP05713935 A EP 05713935A EP 1720504 B1 EP1720504 B1 EP 1720504B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- sleeve
- expandable chamber
- compression apparatus
- recited
- chambers
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 230000006835 compression Effects 0.000 title claims abstract description 84
- 238000007906 compression Methods 0.000 title claims abstract description 84
- 239000012530 fluid Substances 0.000 claims abstract description 61
- 238000004891 communication Methods 0.000 claims abstract description 11
- 210000000689 upper leg Anatomy 0.000 claims description 36
- 210000003127 knee Anatomy 0.000 claims description 30
- 210000003414 extremity Anatomy 0.000 claims description 26
- 210000002414 leg Anatomy 0.000 claims description 21
- 244000309466 calf Species 0.000 claims description 18
- 230000037361 pathway Effects 0.000 claims description 14
- 210000003423 ankle Anatomy 0.000 claims description 12
- 238000009423 ventilation Methods 0.000 claims description 6
- 238000011282 treatment Methods 0.000 abstract description 2
- 238000002560 therapeutic procedure Methods 0.000 description 9
- 230000002792 vascular Effects 0.000 description 9
- 238000011321 prophylaxis Methods 0.000 description 8
- 238000000034 method Methods 0.000 description 7
- 206010051055 Deep vein thrombosis Diseases 0.000 description 4
- 206010047249 Venous thrombosis Diseases 0.000 description 4
- 238000001356 surgical procedure Methods 0.000 description 4
- 239000000853 adhesive Substances 0.000 description 3
- 230000001070 adhesive effect Effects 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 230000017531 blood circulation Effects 0.000 description 3
- 238000001816 cooling Methods 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 210000003462 vein Anatomy 0.000 description 3
- 206010002091 Anaesthesia Diseases 0.000 description 2
- 206010030124 Oedema peripheral Diseases 0.000 description 2
- 239000004698 Polyethylene Substances 0.000 description 2
- 230000037005 anaesthesia Effects 0.000 description 2
- 230000006837 decompression Effects 0.000 description 2
- 239000004744 fabric Substances 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 229920000915 polyvinyl chloride Polymers 0.000 description 2
- 239000004800 polyvinyl chloride Substances 0.000 description 2
- 238000011084 recovery Methods 0.000 description 2
- 208000005189 Embolism Diseases 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 208000008589 Obesity Diseases 0.000 description 1
- 208000003251 Pruritus Diseases 0.000 description 1
- 229920002334 Spandex Polymers 0.000 description 1
- 208000001435 Thromboembolism Diseases 0.000 description 1
- 208000007536 Thrombosis Diseases 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 230000023555 blood coagulation Effects 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 230000004087 circulation Effects 0.000 description 1
- 230000035602 clotting Effects 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 230000002526 effect on cardiovascular system Effects 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 230000007803 itching Effects 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 210000003141 lower extremity Anatomy 0.000 description 1
- 230000036210 malignancy Effects 0.000 description 1
- 235000020824 obesity Nutrition 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 210000004197 pelvis Anatomy 0.000 description 1
- -1 polyethylene Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 210000001147 pulmonary artery Anatomy 0.000 description 1
- 230000002685 pulmonary effect Effects 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 239000004759 spandex Substances 0.000 description 1
- 230000003068 static effect Effects 0.000 description 1
- 150000003673 urethanes Chemical class 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61H—PHYSICAL THERAPY APPARATUS, e.g. DEVICES FOR LOCATING OR STIMULATING REFLEX POINTS IN THE BODY; ARTIFICIAL RESPIRATION; MASSAGE; BATHING DEVICES FOR SPECIAL THERAPEUTIC OR HYGIENIC PURPOSES OR SPECIFIC PARTS OF THE BODY
- A61H23/00—Percussion or vibration massage, e.g. using supersonic vibration; Suction-vibration massage; Massage with moving diaphragms
- A61H23/04—Percussion or vibration massage, e.g. using supersonic vibration; Suction-vibration massage; Massage with moving diaphragms with hydraulic or pneumatic drive
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61H—PHYSICAL THERAPY APPARATUS, e.g. DEVICES FOR LOCATING OR STIMULATING REFLEX POINTS IN THE BODY; ARTIFICIAL RESPIRATION; MASSAGE; BATHING DEVICES FOR SPECIAL THERAPEUTIC OR HYGIENIC PURPOSES OR SPECIFIC PARTS OF THE BODY
- A61H9/00—Pneumatic or hydraulic massage
- A61H9/005—Pneumatic massage
- A61H9/0078—Pneumatic massage with intermittent or alternately inflated bladders or cuffs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61H—PHYSICAL THERAPY APPARATUS, e.g. DEVICES FOR LOCATING OR STIMULATING REFLEX POINTS IN THE BODY; ARTIFICIAL RESPIRATION; MASSAGE; BATHING DEVICES FOR SPECIAL THERAPEUTIC OR HYGIENIC PURPOSES OR SPECIFIC PARTS OF THE BODY
- A61H2201/00—Characteristics of apparatus not provided for in the preceding codes
- A61H2201/16—Physical interface with patient
- A61H2201/1602—Physical interface with patient kind of interface, e.g. head rest, knee support or lumbar support
- A61H2201/1645—Physical interface with patient kind of interface, e.g. head rest, knee support or lumbar support contoured to fit the user
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61H—PHYSICAL THERAPY APPARATUS, e.g. DEVICES FOR LOCATING OR STIMULATING REFLEX POINTS IN THE BODY; ARTIFICIAL RESPIRATION; MASSAGE; BATHING DEVICES FOR SPECIAL THERAPEUTIC OR HYGIENIC PURPOSES OR SPECIFIC PARTS OF THE BODY
- A61H2201/00—Characteristics of apparatus not provided for in the preceding codes
- A61H2201/16—Physical interface with patient
- A61H2201/1683—Surface of interface
- A61H2201/169—Physical characteristics of the surface, e.g. material, relief, texture or indicia
- A61H2201/1697—Breathability of the material
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61H—PHYSICAL THERAPY APPARATUS, e.g. DEVICES FOR LOCATING OR STIMULATING REFLEX POINTS IN THE BODY; ARTIFICIAL RESPIRATION; MASSAGE; BATHING DEVICES FOR SPECIAL THERAPEUTIC OR HYGIENIC PURPOSES OR SPECIFIC PARTS OF THE BODY
- A61H2201/00—Characteristics of apparatus not provided for in the preceding codes
- A61H2201/50—Control means thereof
- A61H2201/5002—Means for controlling a set of similar massage devices acting in sequence at different locations on a patient
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61H—PHYSICAL THERAPY APPARATUS, e.g. DEVICES FOR LOCATING OR STIMULATING REFLEX POINTS IN THE BODY; ARTIFICIAL RESPIRATION; MASSAGE; BATHING DEVICES FOR SPECIAL THERAPEUTIC OR HYGIENIC PURPOSES OR SPECIFIC PARTS OF THE BODY
- A61H2201/00—Characteristics of apparatus not provided for in the preceding codes
- A61H2201/50—Control means thereof
- A61H2201/5007—Control means thereof computer controlled
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61H—PHYSICAL THERAPY APPARATUS, e.g. DEVICES FOR LOCATING OR STIMULATING REFLEX POINTS IN THE BODY; ARTIFICIAL RESPIRATION; MASSAGE; BATHING DEVICES FOR SPECIAL THERAPEUTIC OR HYGIENIC PURPOSES OR SPECIFIC PARTS OF THE BODY
- A61H2201/00—Characteristics of apparatus not provided for in the preceding codes
- A61H2201/50—Control means thereof
- A61H2201/5058—Sensors or detectors
- A61H2201/5071—Pressure sensors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61H—PHYSICAL THERAPY APPARATUS, e.g. DEVICES FOR LOCATING OR STIMULATING REFLEX POINTS IN THE BODY; ARTIFICIAL RESPIRATION; MASSAGE; BATHING DEVICES FOR SPECIAL THERAPEUTIC OR HYGIENIC PURPOSES OR SPECIFIC PARTS OF THE BODY
- A61H2205/00—Devices for specific parts of the body
- A61H2205/06—Arms
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61H—PHYSICAL THERAPY APPARATUS, e.g. DEVICES FOR LOCATING OR STIMULATING REFLEX POINTS IN THE BODY; ARTIFICIAL RESPIRATION; MASSAGE; BATHING DEVICES FOR SPECIAL THERAPEUTIC OR HYGIENIC PURPOSES OR SPECIFIC PARTS OF THE BODY
- A61H2205/00—Devices for specific parts of the body
- A61H2205/10—Leg
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61H—PHYSICAL THERAPY APPARATUS, e.g. DEVICES FOR LOCATING OR STIMULATING REFLEX POINTS IN THE BODY; ARTIFICIAL RESPIRATION; MASSAGE; BATHING DEVICES FOR SPECIAL THERAPEUTIC OR HYGIENIC PURPOSES OR SPECIFIC PARTS OF THE BODY
- A61H2205/00—Devices for specific parts of the body
- A61H2205/12—Feet
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61H—PHYSICAL THERAPY APPARATUS, e.g. DEVICES FOR LOCATING OR STIMULATING REFLEX POINTS IN THE BODY; ARTIFICIAL RESPIRATION; MASSAGE; BATHING DEVICES FOR SPECIAL THERAPEUTIC OR HYGIENIC PURPOSES OR SPECIFIC PARTS OF THE BODY
- A61H2209/00—Devices for avoiding blood stagnation, e.g. Deep Vein Thrombosis [DVT] devices
Definitions
- the present disclosure generally relates to the field of vascular therapy for application to a limb of a body, and more particularly, to a compression apparatus having removable portions.
- a major concern for immobile patients and persons alike are medical conditions that form clots in the blood, such as, deep vein thrombosis (DVT) and peripheral edema.
- DVT deep vein thrombosis
- Such patients and persons include those undergoing surgery, anesthesia, extended periods of bed rest, etc.
- These blood clotting conditions generally occur in the deep veins of the lower extremities and/or pelvis.
- These veins such as the iliac, femoral, popiteal and tibial return deoxygenated to the heart.
- a static pool of blood is ideal for clot formations.
- a major risk associated with this condition is interference with cardiovascular circulation. Most seriously, a fragment of the blood clot can break loose and migrate.
- a pulmonary emboli can form blocking a main pulmonary artery, which may be life threatening.
- the conditions and resulting risks associated with patient immobility may be controlled or alleviated by applying intermittent pressure to a patient's limb, such as, for example, a leg, to assist in blood circulation.
- a patient's limb such as, for example, a leg
- Known devices have been employed to assist in blood circulation, such as, one piece pads and compression boots. See, for example, U.S. Patent Nos. 6,290,662 and 6,494,852 .
- sequential compression devices consist of an air pump connected to a disposable wraparound pad by a series of air tubes.
- the wraparound pad is placed around the patient's leg. Air is then forced into different parts of the wraparound pad in sequence, creating pressure around the calves and improving venous return.
- a compression apparatus according to the preamble of claim 1 is known from US-A-4 624 248 .
- prophylaxis sequential compression apparatus that reduces bulk and is not cumbersome during use to improve comfort and compliance to a patient. It would be desirable if the prophylaxis sequential compression apparatus includes a removable portion to achieve the advantages of the present disclosure. It would be highly desirable if the prophylaxis sequential compression apparatus has a valve connector that facilitates quick disconnect from a pressurized fluid source. It is contemplated that the prophylaxis sequential compression apparatus is easily and efficiently manufactured.
- the invention provides a compression apparatus according to claim 1.
- a compression apparatus that reduces bulk and is not cumbersome during use to improve comfort and compliance to a patient for overcoming the disadvantages and drawbacks of the prior art.
- the compression apparatus includes a removable portion to achieve the advantages of the present disclosure.
- the compression apparatus has a valve connector that facilitates quick disconnect from a pressurized fluid source. The compression apparatus is easily and efficiently fabricated.
- the compression apparatus in accordance with the principles of the present disclosure, includes a thigh length compression sleeve that converts to a knee length sleeve via tearing away and removing the thigh bladder and disconnecting the thigh bladder air supply line.
- the thigh bladder air supply line will remove easily along with the thigh bladder, attaching at or near the point where the thigh bladder is removed from the sleeve. This would allow for a single motion to accomplish both removing of the thigh bladder and the thigh bladder supply line.
- the convertible sleeve allows the patient to use a more comfortable sleeve (knee vs. thigh) as risk for DVT decreases after surgery. This provides practitioners with various options while using a single apparatus.
- the compression apparatus is perforated for improved compliance and comfort with the patient during the overall length of time for wearing the apparatus. It is contemplated that the apparatus can be used with both nomadic and/or stationary compression systems.
- a pressurized fluid source continues to deliver pressurized fluid after removal of the valve.
- the pressurized fluid source can signal a high alarm if there are kinks in the tubing and a low alarm if there are leaks in the tubing.
- the compression apparatus is sequentially activated by increasing pressure through the tubes to correspond with the three portions of the sleeve.
- the distal end is the ankle bladder (high pressure)
- the proximal end is the thigh bladder (low pressure).
- the pressurized fluid source pumps air to the sleeve in a 60 second cycle with 11 seconds being compression and the rest being decompression.
- the compression apparatus includes a sleeve configured for disposal about a limb.
- the sleeve includes a first portion defining a first expandable chamber and a second portion defining a second expandable chamber and a third expandable chamber.
- the second portion includes a connector in fluid communication with a pressurized fluid source and the first expandable chamber, the second expandable chamber and the third expandable chamber thereby facilitating fluid communication between the pressurized fluid source and the chambers.
- the first portion is removable from the second portion.
- the first portion is connected to the second portion via a perforated attachment.
- the first portion may be configured for disposal about a first part of the limb and the second portion is configured for disposal about a second part of the limb.
- the second expandable chamber may be disposed with the second portion for disposal about a second part of the limb and the third expandable chamber is disposed with the second portion for disposal about a third part of the limb.
- the compression apparatus can include a variety of welds and bladders forming a quilting effect.
- the first, second and third expandable chambers can each define at least one sub-chamber.
- the sleeve may define at least one ventilation opening.
- the at least one opening can include openings formed in a surface of the expandable chambers.
- the at least one opening may include a slit disposed between the second expandable chamber and the third expandable chamber.
- the connector can communicate with the chambers via a tubular pathway.
- the tubular pathway of the first expandable chamber may be removable from the connector.
- a pressurized fluid may be delivered to the chambers for expansion thereof in a sequential time interval such that, for example, the first expandable chamber is expanded, followed by (2.5 seconds later) the second expandable chamber, followed by (3 seconds later) the third expandable chamber to a total of 11 seconds from the start of the first expandable chamber.
- the chambers are then all simultaneously vented to the atmosphere.
- the compression apparatus includes a sleeve configured to wrap about a leg and defining a plurality of ventilation openings.
- the sleeve includes a thigh portion defining a first inflatable chamber having sub-chambers.
- the sleeve further includes a calf portion defining a second inflatable chamber having sub-chambers and an ankle portion defining a third inflatable chamber having sub-chambers.
- the ankle portion includes a valve connector that fluidly communicates both a pressurized fluid source and the chambers via a tubular pathway to facilitate inflation of the chambers.
- the thigh portion is removably connected to the calf portion via a perforated attachment and the tubular pathway of the first inflatable chamber is removable from the valve connector.
- the compression apparatus includes an expandable sleeve that is configured for disposal about a leg.
- the sleeve extends a length from below a knee of the leg to above the knee.
- the sleeve is convertible from the length extending from below the knee to above the knee, to a length extending solely below the knee.
- the length of the sleeve extending from below the knee to above the knee may include a first portion disposed about a thigh of the leg, the first portion being removable from the sleeve. The first portion is connected to the sleeve via perforations.
- the ankle bladder is compressed for 21 ⁇ 2 seconds, the mid-section bladder is compressed for 21 ⁇ 2 seconds and the proximal section bladder is also compressed for 2 1 ⁇ 2 seconds. After the 11 th second elapses, all bladders are vented simultaneously.
- the thigh portion may be tom away, thereby converting from a full leg to a knee length.
- a ventilation slit is disposed on the back of the calf portion. This dissipates heat, relieves itching and accommodates movement.
- a knit or hosiery under the compressive sleeve may be used.
- a disclosed method of performing compression on a limb of a body includes the steps of providing a sleeve configured for disposal about the limb, the sleeve includes a first portion defining a first inflatable chamber and a second portion defining a second inflatable chamber and a third inflatable chamber, the second portion includes a connector in fluid communication with a pressurized fluid source and the chambers thereby facilitating fluid communication between the pressurized fluid source and the chambers, the first portion is removable from the second portion; disposing the sleeve about the limb; delivering pressurized fluid to the first inflatable chamber; delivering pressurized fluid to the second inflatable chamber; delivering pressurized fluid to the third inflatable chamber; deflating the chambers; and removing the first portion from the second portion.
- the steps of delivering may each be performed for a duration of 2.5 seconds.
- the step of removing may include disconnecting the first inflatable chamber from the connector.
- the step of removing includes tearing the first portion from the second portion via a perforated attachment.
- a disclosed method of performing compression on a limb of a body includes the steps of providing an expandable sleeve configured for disposal about a leg; disposing the sleeve about the limb such that the sleeve extends a length from below a knee of the leg to above the knee; delivering pressurized fluid to the sleeve; deflating the sleeve; and converting the sleeve from the length extending from below the knee to above the knee, to a length extending solely below the knee.
- the step of disposing the sleeve about the limb such that the sleeve extends a length from below a knee of the leg to above the knee can include a first portion of the sleeve being disposed about a thigh of the leg.
- the step of converting includes tearing the first portion from the sleeve.
- the exemplary embodiments of the compression apparatus and methods of operation disclosed are discussed in terms of vascular therapy including a prophylaxis compression apparatus for application to a limb of a body and more particularly in terms of a compression apparatus having removable portions. It is contemplated that the compression apparatus may be employed for preventing and overcoming the risks associated with patient immobility. It is further contemplated that the compression apparatus alleviates the conditions arising from patient immobility to prevent for example, DVT, peripheral edema, etc. It is contemplated that the compression apparatus according to the present disclosure may be attributable to all types of venous compression systems, including, but not limited to a prophylaxis sequential compression apparatus.
- prophylaxis sequential shall not be construed as limiting the general venous compression apparatus described herein. It is envisioned that the present disclosure, however, finds application with a wide variety of immobile conditions of persons and patients alike, such as, for example, those undergoing surgery, anesthesia, extended periods of bed rest, obesity, advanced age, malignancy, prior thromboembolism, etc.
- proximal refers to a portion of a structure that is closer to a torso of a subject and the term “distal” refers to a portion that is further from the torso.
- distal refers to a portion that is further from the torso.
- subject refers to a patient undergoing vascular therapy using the compression apparatus.
- the term “practitioner” refers to an individual administering the compression apparatus and may include support personnel.
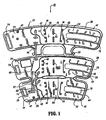
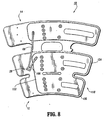
- Compression apparatus 10 includes a sleeve 12 configured for disposal about a limb, such as, for example, a leg L ( FIGS. 4-6 ) of a subject's body. It is contemplated that sleeve 12 and other parts of compression apparatus 10 may be disposed, wrapped, mounted, etc., with various limbs, extremities, etc. of as subject's body, such as, for example, legs, arms, etc.
- Sleeve 12 includes a first portion, such as, for example, thigh portion 14 that defines a first expandable chamber, such as, for example, first inflatable chamber 16.
- a second portion 18 of sleeve 12 defines a second expandable chamber, such as, for example, second inflatable chamber 20 and a third expandable chamber, such as, for example, third inflatable chamber 22.
- the first portion 14 and the second portion 18 may include one or a plurality of expandable chambers.
- sleeve 12 or portions thereof may be disposable.
- Second portion 18 has a calf portion 24 that includes second inflatable chamber 20 and an ankle portion 26 that includes third inflatable chamber 22. It is contemplated that the first portion 14 and second portion 18 may be disposed about various portions of a subject's limb, according to the requirements of a particular vascular therapy application.
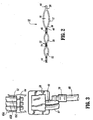
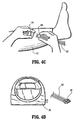
- Ankle portion 26 includes a valve connector 64 in fluid communication with a pressurized fluid source 30 via valve connector 28 and tubing 62 ( FIGS. 4C and 4D ) and chambers 16, 20 and 22 via a fluid pathway including tubing, as will be discussed below (see, for example, the valve connector described in U.S. Patent Application Serial No. 10/784,639, filed on February 23, 2004 and entitled Fluid Conduit Connector Apparatus).
- Tubing 62 is made up of three separate tubes or lumens 65A, 65B and 65C. This configuration facilitates fluid communication between pressurized fluid 30 and chambers 16, 20 and 22.
- Thigh portion 14 is removable from second portion 18.
- calf portion 24 is removably connected to thigh portion 14 via a perforated attachment 32, as will be discussed.
- This removable configuration advantageously reduces the bulk of compression apparatus 10 via facile manipulation to increase comfort and compliance to a subject.
- Compression apparatus 10 also provides a subject with increased mobility.
- sleeve 12 may include flexible sections, such as, elastic or spandex materials, disposed between the portions to facilitate mobility of a limb during use.
- sleeve 12 includes a top sheet 34 and a bottom sheet 36 that are overlaid to form the sleeve.
- Top sheet 34 and bottom sheet 36 are fixedly joined at seams that define inflatable chambers 16, 20 and 22.
- a seam 38 defines chamber 16
- a seam 40 defines chamber 20
- a seam 42 defines chamber 22.
- An edge 44 extends beyond seams 38, 40 and 42 about sleeve 12.
- sleeve 12 includes a plurality of seams, disposed variously thereabout, that join top sheet 34 and bottom sheet 36. It is further contemplated that the seams may be welded, sewn, formed by adhesive, heat sealed, etc.
- Top sheet 34 and bottom sheet 36 are fabricated from materials suitable for inflation of chambers 16, 20 and 22, such as, for example, films and fabrics, such as PVC (polyvinyl chloride) and PE (polyethylene), depending on the particular vascular therapy application and/or preference. Semi-flexible and flexible fabrics, such as urethanes and silicones may also be used.
- Sleeve 12 may include separate structure that include chambers 16, 20 and 22 and are disposed with or mounted to sheets 34, 36.
- Sleeve 12 defines vent openings, such as, for example sleeve apertures 46 that provide cooling to an adjacent portion of the limb of the subject.
- Sleeve apertures 46 pass completely through top sheet 34 and bottom sheet 36. This advantageously improves comfort to the subject during use.
- Sleeve 12 includes a weld portion 48 that surrounds sleeve aperture 46 to seal off the respective chamber from the aperture and prevent fluid communication therebetween.
- Sleeve 12 also includes vent holes 47 to provide cooling. It is envisioned that sleeve 12 may include a plurality of vent openings disposed variously thereabout.
- vent slit 50 is disposed between inflatable chamber 20 and inflatable chamber 22. Vent slit 50 passes completely through top sheet 34 and bottom sheet 36.
- the vent slit advantageously provides cooling to the subject and increases mobility of the calf and ankle during use. It is contemplated that vent slit 50 may extend various length.
- Thigh portion 14 includes an axial line of spot welds 52 that define sub-chambers 54 of inflatable chamber 16.
- Calf portion 24 similarly includes an axial line of spot welds 55 that define sub-chambers 56 of inflatable chamber 20 and ankle portion 26 includes spot welds 58 that define sub-chambers 60 of inflatable chamber 22.
- the sub-chambers may be alternatively formed via a continuous weld, adhesive, hot seal, etc.
- welds 58 may be disposed in various orientations to create alternative configurations for the sub-chambers.
- Valve connector 28 communicates with chambers 16, 20 and 22 via a fluid pathway.
- the fluid pathway includes tubing 62 that connects valve connector 28 to pressurized fluid source 30, which may include a pump (see, for example, the controller pump described in U.S. Patent Application Serial No. 10/784,323, filed on February 23, 2004 and entitled Compression Treatment System).
- Pressurized fluid source 30 may be stationary or portable. It is contemplated that pressurized fluid source 30 may include the necessary electronics, computer software, etc. to carry out vascular therapy, in accordance with the principles of the present disclosure.
- Tubing 62 attaches to valve connector 28 via a coupler 64, as shown in FIG. 3 .
- Tubing 66 extends from valve connector 28 and fluidly connects to inflatable chamber 20.
- Tubing 67 extends from valve connector 28 and fluidly connects to inflatable chamber 22.
- Tubing 68 extends from valve connector 28 and fluidly connects to inflatable chamber 16.
- Tubing 68 includes a quick disconnect port 70.
- Port 70 attaches with valve connector 28 and is easily removable to facilitate removal of thigh portion 14 from calf portion 24.
- Tubing 62 and lumens 65A, 65B and 65C correspond with tubes 67, 66 and 68, respectively.
- valve connector 28 may be fixed with sleeve 12, removable, tethered, etc.
- port 70 may be fixed with valve connector 28 and tubing 68 is removable from thigh portion 14.
- Sleeve 12 includes securing parts, such as, for example, hook and loop pads 72 mounted in an orientation for engagement with corresponding hook and loop pads 74. Hook and loop pads 72, 74 enable secure mounting of sleeve 12 with leg L of a subject. It is contemplated that one or a plurality of securing parts may be variously disposed about sleeve 12. It is further contemplated that the securing parts may include for example, clips, adhesive, pins, etc.
- compression apparatus 10 similar to that described above, is assembled, sterilized and packaged for use.
- compression apparatus 10 is provided and manipulated for disposal about leg L of the subject.
- Tubing 66 is connected with calf portion 24 and tubing 67 is connected with ankle portion 26.
- Tubing 68 is connected to thigh portion 14.
- Tubing 66, 67 and 68 is connected to valve connector 28, which is connected with tubing 62 and pressurized fluid source 30 ( FIGS. 4C and 4D ). Therefore, the fluid pathway of compression apparatus 10 establishes fluid communication between pressurized fluid source 30 and chambers 16, 20 and 22.
- Sleeve 12 is wrapped about leg L and secured thereto via hook and loop pads 72, 74, discussed above, as shown in FIGS. 4A and 4B .
- Sleeve 12 extends a length from below a knee of leg L, via second portion 18, to above the knee, via thigh portion 14.
- Compression apparatus 10 is sequentially activated by delivering pressurized fluid to chambers 16, 20 and 22 via the fluid pathway.
- pressurized fluid source 30 delivers air to sleeve 12 in a 60 second cycle including 11 seconds in compression and 49 seconds in decompression. Compressed air is delivered to inflatable chamber 22 for 2.5 seconds. Then, compressed air is delivered to inflatable chamber 20 for 2.5 seconds. Compressed air is then delivered to inflatable chamber 16 for 2.5 seconds.
- Compression apparatus 10 maintains inflation for several seconds until the 11 th second and then chambers 16, 20 and 22 are deflated simultaneously. It is contemplated that this sequential compression may continue for a plurality of cycles, according to the requirements of a particular vascular therapy application. Other sequential compression cycles are also contemplated. It is envisioned that various forms of fluid may be delivered to sleeve 12, such as, for example, liquid, gases, etc.
- thigh portion 14 may be removed from second portion 18.
- sleeve 12 is convertible from the length extending from below the knee to above the knee, to a length extending solely below the knee.
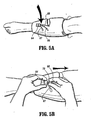
- Sleeve 12 is manipulated such that thigh portion 14 is removed and tom from calf portion 24 via perforations 32, as shown in FIGS. 6A and 6B .
- Port 70 connected to tubing 68, is easily manipulated to quick disconnect from valve connector 28, as shown in FIG. 5B .
- sleeve 12 The remaining portion of sleeve 12, second portion 18 including calf portion 24 and ankle portion 26, is stand alone and continues to operate as described above. This converts sleeve 12 from a full leg length apparatus to a knee length apparatus. Compression apparatus 10 may be employed to completion of a desired vascular therapy application. Other methods of use are also contemplated, for example, the thigh portion 14 may not be removed and remain with the sleeve 12.
- Connector 28 (and optionally disconnect port 70) is configured such that upon separation of tube 68 from connector 28, a desired amount of fluid flow from fluid source 30 is continuously achieved through the connector 28. Such continued fluid flow is desirable to maintain continuity with the pressurized fluid source 30. That is, fluid flow adjustments to the fluid source 30 need not be made if a user or practitioner decides to remove thigh portion 14 from the compression apparatus 10. Even after removal of thigh portion 14, the pressurized fluid source 30 will continue to deliver the same amount of fluid and pressure through tubing 65C into connector 28 and out into the atmosphere.
- Sleeve 12 similar to that described above, includes thigh portion 14 and a second portion 118.
- Second portion 118 has a calf portion 124 and an ankle portion 126 that include an inflatable chamber 122.
- Pressurized fluid source 30 ( FIG. 1 ) fluidly communicates with sleeve 12 via valve connector 28 and tubing 62 ( FIG. 1 ).
- Valve connector 28 fluidly communicates with chambers 16 and 122 via separate tubes 68 and 166, respectively, for employment similar to that described above, including the optional removal of thigh portion 14 via perforations 32.
Landscapes
- Health & Medical Sciences (AREA)
- Public Health (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Rehabilitation Therapy (AREA)
- Epidemiology (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Pain & Pain Management (AREA)
- Physical Education & Sports Medicine (AREA)
- Massaging Devices (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
- Surgical Instruments (AREA)
- Separation By Low-Temperature Treatments (AREA)
- Electron Tubes For Measurement (AREA)
- Nonmetallic Welding Materials (AREA)
- Orthopedics, Nursing, And Contraception (AREA)
- Finger-Pressure Massage (AREA)
- Loading And Unloading Of Fuel Tanks Or Ships (AREA)
- Superconductors And Manufacturing Methods Therefor (AREA)
- Prostheses (AREA)
- Fluid-Pressure Circuits (AREA)
- Polarising Elements (AREA)
- Accessory Devices And Overall Control Thereof (AREA)
- Quick-Acting Or Multi-Walled Pipe Joints (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Tea And Coffee (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Non-Positive Displacement Air Blowers (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PL05713935T PL1720504T3 (pl) | 2004-02-23 | 2005-02-23 | Aparat uciskowy |
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/784,607 US7871387B2 (en) | 2004-02-23 | 2004-02-23 | Compression sleeve convertible in length |
| US10/784,639 US7490620B2 (en) | 2004-02-23 | 2004-02-23 | Fluid conduit connector apparatus |
| US10/784,604 US7282038B2 (en) | 2004-02-23 | 2004-02-23 | Compression apparatus |
| US10/784,323 US7354410B2 (en) | 2004-02-23 | 2004-02-23 | Compression treatment system |
| PCT/US2005/005600 WO2005082315A1 (en) | 2004-02-23 | 2005-02-23 | Compression apparatus |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP1720504A1 EP1720504A1 (en) | 2006-11-15 |
| EP1720504B1 true EP1720504B1 (en) | 2010-05-26 |
Family
ID=34916527
Family Applications (6)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP05713935A Active EP1720504B1 (en) | 2004-02-23 | 2005-02-23 | Compression apparatus |
| EP05713933.9A Active EP1722738B1 (en) | 2004-02-23 | 2005-02-23 | Compression treatment system |
| EP10185262.2A Withdrawn EP2319476A3 (en) | 2004-02-23 | 2005-02-23 | Compression treatment system |
| EP10185260.6A Active EP2314268B1 (en) | 2004-02-23 | 2005-02-23 | Compression treatment system |
| EP05723526A Not-in-force EP1720505B1 (en) | 2004-02-23 | 2005-02-23 | Compression apparatus |
| EP05713934A Not-in-force EP1718894B1 (en) | 2004-02-23 | 2005-02-23 | Fluid conduit connector apparatus |
Family Applications After (5)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP05713933.9A Active EP1722738B1 (en) | 2004-02-23 | 2005-02-23 | Compression treatment system |
| EP10185262.2A Withdrawn EP2319476A3 (en) | 2004-02-23 | 2005-02-23 | Compression treatment system |
| EP10185260.6A Active EP2314268B1 (en) | 2004-02-23 | 2005-02-23 | Compression treatment system |
| EP05723526A Not-in-force EP1720505B1 (en) | 2004-02-23 | 2005-02-23 | Compression apparatus |
| EP05713934A Not-in-force EP1718894B1 (en) | 2004-02-23 | 2005-02-23 | Fluid conduit connector apparatus |
Country Status (15)
| Country | Link |
|---|---|
| EP (6) | EP1720504B1 (es) |
| JP (4) | JP4602996B2 (es) |
| KR (5) | KR100868148B1 (es) |
| CN (1) | CN102614074B (es) |
| AT (3) | ATE468834T1 (es) |
| AU (4) | AU2005216934B2 (es) |
| CA (4) | CA2552355C (es) |
| DE (2) | DE602005021460D1 (es) |
| DK (1) | DK1720504T3 (es) |
| ES (4) | ES2806930T3 (es) |
| HK (1) | HK1091390A1 (es) |
| IL (4) | IL176409A (es) |
| NO (4) | NO20064256L (es) |
| PL (2) | PL1720504T3 (es) |
| WO (4) | WO2005083313A1 (es) |
Families Citing this family (63)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB0515294D0 (en) | 2005-07-26 | 2005-08-31 | Novamedix Distrib Ltd | Limited durability closure means for an inflatable medical garment |
| GB0523249D0 (en) * | 2005-11-15 | 2005-12-21 | Huntleigh Technology Plc | Identification means |
| US8029451B2 (en) | 2005-12-12 | 2011-10-04 | Tyco Healthcare Group Lp | Compression sleeve having air conduits |
| GB0601453D0 (en) * | 2006-01-24 | 2006-03-08 | Bristol Myers Squibb Co | Pressurised medical device |
| JP4710758B2 (ja) * | 2006-08-28 | 2011-06-29 | パナソニック電工株式会社 | マッサージ機 |
| GB0622415D0 (en) | 2006-11-10 | 2006-12-20 | Huntleigh Technology Plc | Compression system |
| US8016779B2 (en) * | 2007-04-09 | 2011-09-13 | Tyco Healthcare Group Lp | Compression device having cooling capability |
| US8506508B2 (en) | 2007-04-09 | 2013-08-13 | Covidien Lp | Compression device having weld seam moisture transfer |
| US8016778B2 (en) | 2007-04-09 | 2011-09-13 | Tyco Healthcare Group Lp | Compression device with improved moisture evaporation |
| US8029450B2 (en) | 2007-04-09 | 2011-10-04 | Tyco Healthcare Group Lp | Breathable compression device |
| US8162861B2 (en) | 2007-04-09 | 2012-04-24 | Tyco Healthcare Group Lp | Compression device with strategic weld construction |
| US8128584B2 (en) | 2007-04-09 | 2012-03-06 | Tyco Healthcare Group Lp | Compression device with S-shaped bladder |
| US8070699B2 (en) | 2007-04-09 | 2011-12-06 | Tyco Healthcare Group Lp | Method of making compression sleeve with structural support features |
| US8034007B2 (en) | 2007-04-09 | 2011-10-11 | Tyco Healthcare Group Lp | Compression device with structural support features |
| US8109892B2 (en) | 2007-04-09 | 2012-02-07 | Tyco Healthcare Group Lp | Methods of making compression device with improved evaporation |
| US8021388B2 (en) | 2007-04-09 | 2011-09-20 | Tyco Healthcare Group Lp | Compression device with improved moisture evaporation |
| US8182437B2 (en) | 2007-05-08 | 2012-05-22 | Wright Therapy Products, Inc. | Pneumatic compression therapy system and methods of using same |
| US8388557B2 (en) | 2007-06-20 | 2013-03-05 | Remo Moomiaie-Qajar | Portable compression device |
| GB0712764D0 (en) | 2007-07-02 | 2007-08-08 | Smith & Nephew | Carrying Bag |
| US8246028B2 (en) | 2007-11-08 | 2012-08-21 | Tyco Healthcare Group Lp | Telescopingly adjustable clamp |
| US8114117B2 (en) | 2008-09-30 | 2012-02-14 | Tyco Healthcare Group Lp | Compression device with wear area |
| US8235923B2 (en) | 2008-09-30 | 2012-08-07 | Tyco Healthcare Group Lp | Compression device with removable portion |
| US8133039B2 (en) | 2009-01-26 | 2012-03-13 | Tyco Healthcare Group Lp | Mount for a compression control unit |
| US8419666B2 (en) * | 2009-09-23 | 2013-04-16 | Caremed Supply, Inc. | Compression sleeve |
| US8506507B2 (en) * | 2010-03-09 | 2013-08-13 | Covidien Lp | Venous augmentation system |
| US8652079B2 (en) | 2010-04-02 | 2014-02-18 | Covidien Lp | Compression garment having an extension |
| USD675741S1 (en) | 2010-08-16 | 2013-02-05 | Covidien Lp | Pneumatic compression controller |
| USD659839S1 (en) | 2010-08-16 | 2012-05-15 | Tyco Healthcare Group Lp | Support for a pneumatic compression controller |
| US10751221B2 (en) | 2010-09-14 | 2020-08-25 | Kpr U.S., Llc | Compression sleeve with improved position retention |
| US8398572B2 (en) * | 2010-09-21 | 2013-03-19 | Covidien Lp | Bladder tube connection |
| US8758282B2 (en) | 2010-09-29 | 2014-06-24 | Covidien Lp | Compression garment apparatus having support bladder |
| US8753300B2 (en) * | 2010-09-29 | 2014-06-17 | Covidien Lp | Compression garment apparatus having baseline pressure |
| CA2778395A1 (en) * | 2011-06-10 | 2012-12-10 | Tyco Healthcare Group Lp | Compression device having a pause feature |
| US10828402B2 (en) * | 2011-10-14 | 2020-11-10 | Alcon Inc. | Collar connector |
| US10195102B2 (en) | 2012-03-12 | 2019-02-05 | Tactile Systems Technology, Inc. | Compression therapy device with multiple simultaneously active chambers |
| US9889063B2 (en) * | 2012-06-11 | 2018-02-13 | Wright Therapy Products, Inc. | Methods and systems for determining use compliance of a compression therapy device |
| US9205021B2 (en) | 2012-06-18 | 2015-12-08 | Covidien Lp | Compression system with vent cooling feature |
| WO2014021267A1 (ja) * | 2012-07-30 | 2014-02-06 | 国立大学法人高知大学 | 生体内アセチルコリン産生促進装置 |
| CA2882299C (en) | 2012-08-18 | 2023-09-19 | Wright Therapy Products, Inc. | Methods for determining the size of body parts as part of compression therapy procedures |
| US9872812B2 (en) * | 2012-09-28 | 2018-01-23 | Kpr U.S., Llc | Residual pressure control in a compression device |
| USD764654S1 (en) | 2014-03-13 | 2016-08-23 | Smith & Nephew, Inc. | Canister for collecting wound exudate |
| US9295605B2 (en) | 2013-12-02 | 2016-03-29 | Wright Therapy Products, Inc. | Methods and systems for auto-calibration of a pneumatic compression device |
| US10470967B2 (en) | 2014-01-20 | 2019-11-12 | Tactile Systems Technology, Inc. | Bespoke compression therapy device |
| US10292894B2 (en) | 2014-02-11 | 2019-05-21 | Tactile Systems Technology, Inc. | Compression therapy device and compression therapy protocols |
| USD764653S1 (en) | 2014-05-28 | 2016-08-23 | Smith & Nephew, Inc. | Canister for collecting wound exudate |
| USD764048S1 (en) | 2014-05-28 | 2016-08-16 | Smith & Nephew, Inc. | Device for applying negative pressure to a wound |
| USD764047S1 (en) | 2014-05-28 | 2016-08-16 | Smith & Nephew, Inc. | Therapy unit assembly |
| USD765830S1 (en) | 2014-06-02 | 2016-09-06 | Smith & Nephew, Inc. | Therapy unit assembly |
| USD770173S1 (en) | 2014-06-02 | 2016-11-01 | Smith & Nephew, Inc. | Bag |
| US10071011B2 (en) * | 2014-06-30 | 2018-09-11 | Kpr U.S., Llc | Compression garment inflation |
| CA2959031A1 (en) | 2014-08-27 | 2016-03-03 | Covidien Lp | Compression garment inflation |
| CA2973748C (en) | 2015-01-27 | 2020-04-14 | Medivance Incorporated | Improved medical pad and system for thermotherapy |
| DE102015105371A1 (de) * | 2015-04-09 | 2016-10-13 | Kongsberg Automotive Ab | Massagevorrichtung für einen Fahrzeugsitz |
| US10166164B2 (en) | 2016-04-27 | 2019-01-01 | Radial Medical, Inc. | Adaptive compression therapy systems and methods |
| EP4079278A1 (en) | 2016-05-26 | 2022-10-26 | Huntleigh Technology Limited | Compression therapy system and method |
| WO2018230930A1 (ko) * | 2017-06-16 | 2018-12-20 | 주식회사 뷰엘리스 | 공기압 마사지용 다리커프 |
| AU2019237526B2 (en) | 2018-03-23 | 2024-09-26 | TurnCare, Inc. | Inflatable perfusion enhancement apparatuses and associated devices, systems and methods |
| US11504927B2 (en) | 2018-03-23 | 2022-11-22 | TurnCare, Inc. | Systems and methods for controlling and monitoring inflatable perfusion enhancement apparatus for mitigating contact pressure |
| JP6813531B2 (ja) * | 2018-05-16 | 2021-01-13 | 日東工器株式会社 | 空気圧式のマッサージ装置及びその空気供給チューブ |
| US10893998B2 (en) | 2018-10-10 | 2021-01-19 | Inova Labs Inc. | Compression apparatus and systems for circulatory disorders |
| KR20220019182A (ko) | 2020-08-07 | 2022-02-16 | 유인종 | 하지 정맥류 치료장치용 압박 스타킹에 사용되는 공기압 조절 장치 및 이를 포함하는 하지 정맥류 치료장치 |
| KR20220019180A (ko) | 2020-08-07 | 2022-02-16 | 유인종 | 하지 정맥류 치료장치용 압박 스타킹 및 그 제조방법, 그리고 이를 포함하는 하지 정맥류 치료장치 |
| JP7283816B1 (ja) * | 2022-02-17 | 2023-05-30 | 株式会社テクノ高槻 | 給排気装置 |
Family Cites Families (47)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1883240A (en) * | 1925-11-27 | 1932-10-18 | Honeywell Regulator Co | Magnetically operated valve |
| US1608239A (en) * | 1925-12-09 | 1926-11-23 | Rosett Joshua | Therapeutic device |
| US2628850A (en) * | 1949-03-19 | 1953-02-17 | Donald V Summerville | Releasable conduit connection with automatic valving |
| US4013069A (en) * | 1975-10-28 | 1977-03-22 | The Kendall Company | Sequential intermittent compression device |
| US4091804A (en) * | 1976-12-10 | 1978-05-30 | The Kendall Company | Compression sleeve |
| US4355632A (en) * | 1980-08-06 | 1982-10-26 | Jobst Institute, Inc. | Anti-shock pressure garment |
| US4374518A (en) * | 1980-10-09 | 1983-02-22 | Raul Villanueva | Electronic device for pneumomassage to reduce lymphedema |
| JPS5942030Y2 (ja) * | 1980-12-23 | 1984-12-06 | 株式会社日器 | 空圧式マツサ−ジ器用圧迫袋 |
| IL63574A (en) * | 1981-08-14 | 1985-07-31 | Mego Afek | Massaging sleeve for body limbs |
| JPS5892926U (ja) * | 1981-12-17 | 1983-06-23 | 日東工器株式会社 | 空圧マツサ−ジ器の圧縮空気供給装置 |
| US4624248A (en) * | 1983-02-07 | 1986-11-25 | David Clark Company Incorporated | Transparent pressure garment |
| US4696289C1 (en) * | 1983-06-22 | 2002-09-03 | Novamedix Distrib Ltd | Method of stimulating the venous-pump mechanism of the foot and for enhancement of arterial flow to the foot |
| US5989204A (en) * | 1991-09-27 | 1999-11-23 | Kinetic Concepts, Inc. | Foot-mounted venous compression device |
| US5186163A (en) * | 1991-11-25 | 1993-02-16 | The Kendall Company | Compression device |
| AU696794B2 (en) * | 1991-12-17 | 1998-09-17 | Covidien Ag | Pneumatic compression device and methods for use in the medical field |
| GB2295458B (en) * | 1992-09-15 | 1996-08-14 | Huntleigh Technology Plc | DVT prevention apparatus and method |
| JPH06245949A (ja) * | 1993-02-22 | 1994-09-06 | Hokoku Kogyo Co Ltd | 温冷マッサージ治療装置 |
| US5354260A (en) * | 1993-05-13 | 1994-10-11 | Novamedix, Ltd. | Slipper with an inflatable foot pump |
| US5443440A (en) * | 1993-06-11 | 1995-08-22 | Ndm Acquisition Corp. | Medical pumping apparatus |
| US5478119A (en) * | 1993-09-16 | 1995-12-26 | The Kendall Company | Polarized manifold connection device |
| US5795312A (en) * | 1993-09-27 | 1998-08-18 | The Kendall Company | Compression sleeve |
| US5575762A (en) * | 1994-04-05 | 1996-11-19 | Beiersdorf-Jobst, Inc. | Gradient sequential compression system and method for reducing the occurrence of deep vein thrombosis |
| US5591200A (en) * | 1994-06-17 | 1997-01-07 | World, Inc. | Method and apparatus for applying pressure to a body limb for treating edema |
| CA2153375C (en) * | 1994-07-26 | 2000-09-12 | Arnold Tobler | Attachment of hook and loop fastener to a compression sleeve |
| US5876359A (en) | 1994-11-14 | 1999-03-02 | Bock; Malcolm G. | Sequential compression device controller |
| GB9507328D0 (en) * | 1995-04-08 | 1995-05-31 | Novamedix Ltd | A medical device |
| JPH09313557A (ja) * | 1996-05-31 | 1997-12-09 | Megoafeck Ind Measuring Instr | 空圧マッサージ装置 |
| IL120935A0 (en) * | 1996-06-07 | 1997-09-30 | Bibi Roni | Medical apparatus for facilitating blood circulation in the lower limbs |
| US5681339A (en) * | 1996-08-12 | 1997-10-28 | Mcewen; James A. | Apparatus and method for monitoring the patency of tubing in a pneumatic medical device |
| JP4124503B2 (ja) * | 1997-06-06 | 2008-07-23 | 株式会社アドバンス | 血行促進装置 |
| JP4059956B2 (ja) * | 1997-06-30 | 2008-03-12 | 日東工器株式会社 | 空圧式マッサージ器 |
| JP2001514047A (ja) * | 1997-08-31 | 2001-09-11 | メデイカル・コンプレツシヨン・システムズ(デイ・ビー・エヌ) | 四肢圧迫装置 |
| US6494852B1 (en) | 1998-03-11 | 2002-12-17 | Medical Compression Systems (Dbn) Ltd. | Portable ambulant pneumatic compression system |
| US6007559A (en) * | 1998-06-12 | 1999-12-28 | Aci Medical | Vascular assist methods and apparatus |
| JP2002521137A (ja) * | 1998-07-30 | 2002-07-16 | メディカル ダイナミックス ユーエスエイ, エルエルシー | 周期的な治療動作を人の足に適用するための医療用デバイス |
| US6062244A (en) * | 1998-08-13 | 2000-05-16 | Aci Medical | Fluidic connector |
| DE19846922C2 (de) * | 1998-10-12 | 2003-12-11 | Manuel Fernandez | Behandlungsvorrichtung |
| JP3909789B2 (ja) * | 1998-12-28 | 2007-04-25 | 日東工器株式会社 | エアマッサージ器 |
| KR100563403B1 (ko) * | 1999-01-11 | 2006-03-23 | 훼미리 가부시키가이샤 | 마사지기 |
| FR2789811B1 (fr) | 1999-02-11 | 2001-05-18 | Radiall Sa | Raccord coaxial pour relier deux cartes de circuit imprime |
| US6290662B1 (en) | 1999-05-28 | 2001-09-18 | John K. Morris | Portable, self-contained apparatus for deep vein thrombosis (DVT) prophylaxis |
| US6592534B1 (en) * | 1999-12-27 | 2003-07-15 | Aircast, Inc. | Inflatable medical appliance for prevention of DVT |
| JP2001286521A (ja) * | 2000-04-10 | 2001-10-16 | Nippon Colin Co Ltd | 静脈血栓塞栓症防止装置 |
| US6558338B1 (en) * | 2000-11-20 | 2003-05-06 | Mego Afek Industrial Measuring Instruments | System for and method of applying pressure to human body |
| JP3951637B2 (ja) * | 2001-06-14 | 2007-08-01 | 松下電工株式会社 | エアーマッサージ機 |
| JP2003319988A (ja) * | 2002-05-07 | 2003-11-11 | Marutaka Co Ltd | 腕用エアマッサージ機 |
| US20050242315A1 (en) | 2002-07-27 | 2005-11-03 | Lund Niels K | Rapid coupling device and method for assembling a coupling socket |
-
2005
- 2005-02-23 KR KR1020067016842A patent/KR100868148B1/ko active IP Right Grant
- 2005-02-23 EP EP05713935A patent/EP1720504B1/en active Active
- 2005-02-23 CA CA002552355A patent/CA2552355C/en not_active Expired - Fee Related
- 2005-02-23 JP JP2006554297A patent/JP4602996B2/ja not_active Expired - Fee Related
- 2005-02-23 DE DE602005021460T patent/DE602005021460D1/de active Active
- 2005-02-23 WO PCT/US2005/005599 patent/WO2005083313A1/en active Application Filing
- 2005-02-23 AT AT05713935T patent/ATE468834T1/de not_active IP Right Cessation
- 2005-02-23 WO PCT/US2005/005600 patent/WO2005082315A1/en active Application Filing
- 2005-02-23 CA CA002552331A patent/CA2552331C/en not_active Expired - Fee Related
- 2005-02-23 EP EP05713933.9A patent/EP1722738B1/en active Active
- 2005-02-23 AU AU2005216934A patent/AU2005216934B2/en not_active Ceased
- 2005-02-23 AU AU2005216924A patent/AU2005216924B2/en active Active
- 2005-02-23 PL PL05713935T patent/PL1720504T3/pl unknown
- 2005-02-23 ES ES10185260T patent/ES2806930T3/es active Active
- 2005-02-23 DE DE602005022165T patent/DE602005022165D1/de active Active
- 2005-02-23 EP EP10185262.2A patent/EP2319476A3/en not_active Withdrawn
- 2005-02-23 CA CA002552353A patent/CA2552353C/en not_active Expired - Fee Related
- 2005-02-23 EP EP10185260.6A patent/EP2314268B1/en active Active
- 2005-02-23 KR KR1020067016843A patent/KR20070001964A/ko active Search and Examination
- 2005-02-23 JP JP2006554298A patent/JP4686485B2/ja not_active Expired - Fee Related
- 2005-02-23 KR KR1020067016796A patent/KR100914569B1/ko active IP Right Grant
- 2005-02-23 AT AT05713934T patent/ATE473390T1/de active
- 2005-02-23 EP EP05723526A patent/EP1720505B1/en not_active Not-in-force
- 2005-02-23 AU AU2005216923A patent/AU2005216923B2/en active Active
- 2005-02-23 EP EP05713934A patent/EP1718894B1/en not_active Not-in-force
- 2005-02-23 WO PCT/US2005/005598 patent/WO2005082314A1/en active Application Filing
- 2005-02-23 PL PL05723526T patent/PL1720505T3/pl unknown
- 2005-02-23 JP JP2006554310A patent/JP2007522892A/ja active Pending
- 2005-02-23 ES ES05713933T patent/ES2414880T3/es active Active
- 2005-02-23 AU AU2005217424A patent/AU2005217424B2/en not_active Ceased
- 2005-02-23 DK DK05713935.4T patent/DK1720504T3/da active
- 2005-02-23 ES ES05713935T patent/ES2346546T3/es active Active
- 2005-02-23 KR KR1020067016795A patent/KR100873540B1/ko active IP Right Grant
- 2005-02-23 JP JP2006554296A patent/JP4571156B2/ja active Active
- 2005-02-23 ES ES05723526T patent/ES2378886T3/es active Active
- 2005-02-23 CN CN201210098027.XA patent/CN102614074B/zh active Active
- 2005-02-23 AT AT05723526T patent/ATE536851T1/de active
- 2005-02-23 KR KR1020087023566A patent/KR100918718B1/ko active IP Right Grant
- 2005-02-23 CA CA2552354A patent/CA2552354C/en not_active Expired - Fee Related
- 2005-02-23 WO PCT/US2005/005679 patent/WO2005082316A2/en active Application Filing
-
2006
- 2006-06-19 IL IL176409A patent/IL176409A/en active IP Right Grant
- 2006-06-19 IL IL176410A patent/IL176410A/en active IP Right Grant
- 2006-06-20 IL IL176432A patent/IL176432A0/en unknown
- 2006-06-20 IL IL176433A patent/IL176433A/en active IP Right Grant
- 2006-09-20 NO NO20064256A patent/NO20064256L/no not_active Application Discontinuation
- 2006-09-20 NO NO20064255A patent/NO20064255L/no not_active Application Discontinuation
- 2006-09-21 NO NO20064281A patent/NO20064281L/no not_active Application Discontinuation
- 2006-09-22 NO NO20064310A patent/NO20064310L/no not_active Application Discontinuation
- 2006-12-04 HK HK06113291.3A patent/HK1091390A1/xx not_active IP Right Cessation
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1720504B1 (en) | Compression apparatus | |
| US7871387B2 (en) | Compression sleeve convertible in length | |
| EP1795168B1 (en) | Compression apparatus | |
| US8216165B2 (en) | Compression garments with heel elevation | |
| US7967766B2 (en) | Compression garment with heel elevation | |
| US8142343B2 (en) | Suprapatellar external counterpulsation apparatus | |
| JP5197821B2 (ja) | 嚢管の接続 | |
| US10258536B2 (en) | External peripheral vascular occlusion for enhanced cardiopulmonary resuscitation | |
| WO2015196190A2 (en) | Intermittent and sequential compression device and method | |
| JP2012071130A (ja) | グリップを有する圧迫ガーメント | |
| US20220387248A1 (en) | External counterpulsation device | |
| NZ548557A (en) | Compression apparatus | |
| CN116636666A (zh) | 病号服 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20060623 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LI LT LU MC NL PL PT RO SE SI SK TR |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: BOCK, MALCOLM Inventor name: TESLUK, CHRISTOPHER Inventor name: TORDELLA, ELISE |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: DE Ref document number: 1091390 Country of ref document: HK |
|
| DAX | Request for extension of the european patent (deleted) | ||
| 17Q | First examination report despatched |
Effective date: 20080808 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LI LT LU MC NL PL PT RO SE SI SK TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REF | Corresponds to: |
Ref document number: 602005021460 Country of ref document: DE Date of ref document: 20100708 Kind code of ref document: P |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: T3 |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: T3 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2346546 Country of ref document: ES Kind code of ref document: T3 |
|
| LTIE | Lt: invalidation of european patent or patent extension |
Effective date: 20100526 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100526 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100526 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100526 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100926 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100526 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100526 |
|
| REG | Reference to a national code |
Ref country code: PL Ref legal event code: T3 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100526 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100827 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100927 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100526 |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: GR Ref document number: 1091390 Country of ref document: HK |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100526 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100526 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100526 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20110301 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602005021460 Country of ref document: DE Effective date: 20110228 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20110228 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20110228 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20110228 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: TR Payment date: 20120209 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DK Payment date: 20120224 Year of fee payment: 8 Ref country code: BE Payment date: 20120227 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20120228 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20130227 Year of fee payment: 9 Ref country code: IE Payment date: 20130226 Year of fee payment: 9 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20110223 |
|
| BERE | Be: lapsed |
Owner name: TYCO HEALTHCARE GROUP LP Effective date: 20130228 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: V1 Effective date: 20130901 |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: EBP |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100826 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130901 Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20100526 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130228 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130228 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: PL Payment date: 20140203 Year of fee payment: 10 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 602005021460 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 602005021460 Country of ref document: DE Effective date: 20140902 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MM4A |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20140223 Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20140902 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130223 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 12 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20150223 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 13 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: 732E Free format text: REGISTERED BETWEEN 20180118 AND 20180124 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 14 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: CD Owner name: COVIDIEN LP, US Effective date: 20181012 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: PC2A Owner name: COVIDIEN LP Effective date: 20190117 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: PC2A Owner name: KPR U.S., LLC Effective date: 20190118 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20240301 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20240227 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20240222 Year of fee payment: 20 Ref country code: FR Payment date: 20240226 Year of fee payment: 20 |