EP0903425B1 - Vefahren zur Elektrolyse einer Salzlösung - Google Patents
Vefahren zur Elektrolyse einer Salzlösung Download PDFInfo
- Publication number
- EP0903425B1 EP0903425B1 EP98402268A EP98402268A EP0903425B1 EP 0903425 B1 EP0903425 B1 EP 0903425B1 EP 98402268 A EP98402268 A EP 98402268A EP 98402268 A EP98402268 A EP 98402268A EP 0903425 B1 EP0903425 B1 EP 0903425B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- cathode
- membrane
- oxygen
- compartment
- temperature
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 238000000034 method Methods 0.000 title claims abstract description 17
- 230000008569 process Effects 0.000 title claims abstract description 13
- 238000005868 electrolysis reaction Methods 0.000 title claims description 22
- 239000012267 brine Substances 0.000 title description 7
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 title description 7
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 claims abstract description 66
- 239000012528 membrane Substances 0.000 claims abstract description 51
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 claims abstract description 50
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims abstract description 41
- 239000001301 oxygen Substances 0.000 claims abstract description 40
- 229910052760 oxygen Inorganic materials 0.000 claims abstract description 40
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims abstract description 38
- 239000007789 gas Substances 0.000 claims abstract description 35
- 239000011780 sodium chloride Substances 0.000 claims abstract description 24
- 238000005341 cation exchange Methods 0.000 claims abstract description 12
- 230000009467 reduction Effects 0.000 claims abstract description 6
- 239000000243 solution Substances 0.000 abstract description 9
- 239000007864 aqueous solution Substances 0.000 abstract description 7
- 210000004027 cell Anatomy 0.000 description 34
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 22
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 6
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 5
- 230000005587 bubbling Effects 0.000 description 5
- 239000001257 hydrogen Substances 0.000 description 5
- 229910052739 hydrogen Inorganic materials 0.000 description 5
- 239000003014 ion exchange membrane Substances 0.000 description 5
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 5
- 239000004810 polytetrafluoroethylene Substances 0.000 description 5
- WOCIAKWEIIZHES-UHFFFAOYSA-N ruthenium(iv) oxide Chemical compound O=[Ru]=O WOCIAKWEIIZHES-UHFFFAOYSA-N 0.000 description 5
- 239000000758 substrate Substances 0.000 description 5
- 239000010936 titanium Substances 0.000 description 5
- 229910052719 titanium Inorganic materials 0.000 description 5
- 229920000557 Nafion® Polymers 0.000 description 4
- 230000002209 hydrophobic effect Effects 0.000 description 4
- -1 polytetrafluoroethylene Polymers 0.000 description 4
- 239000011734 sodium Substances 0.000 description 4
- 208000031968 Cadaver Diseases 0.000 description 3
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 3
- DSVGQVZAZSZEEX-UHFFFAOYSA-N [C].[Pt] Chemical compound [C].[Pt] DSVGQVZAZSZEEX-UHFFFAOYSA-N 0.000 description 3
- 239000000460 chlorine Substances 0.000 description 3
- 229910052801 chlorine Inorganic materials 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- MOFOBJHOKRNACT-UHFFFAOYSA-N nickel silver Chemical compound [Ni].[Ag] MOFOBJHOKRNACT-UHFFFAOYSA-N 0.000 description 3
- 229910052708 sodium Inorganic materials 0.000 description 3
- 229910000679 solder Inorganic materials 0.000 description 3
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- 238000007792 addition Methods 0.000 description 2
- 239000003054 catalyst Substances 0.000 description 2
- 238000010349 cathodic reaction Methods 0.000 description 2
- 150000001768 cations Chemical class 0.000 description 2
- 210000005056 cell body Anatomy 0.000 description 2
- 238000003487 electrochemical reaction Methods 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 239000010956 nickel silver Substances 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 239000011347 resin Substances 0.000 description 2
- 229920005989 resin Polymers 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- MYMOFIZGZYHOMD-UHFFFAOYSA-N Dioxygen Chemical compound O=O MYMOFIZGZYHOMD-UHFFFAOYSA-N 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 239000003575 carbonaceous material Substances 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000006735 deficit Effects 0.000 description 1
- 238000005370 electroosmosis Methods 0.000 description 1
- 238000005265 energy consumption Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000005923 long-lasting effect Effects 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 238000000465 moulding Methods 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 229910001925 ruthenium oxide Inorganic materials 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B1/00—Electrolytic production of inorganic compounds or non-metals
- C25B1/01—Products
- C25B1/34—Simultaneous production of alkali metal hydroxides and chlorine, oxyacids or salts of chlorine, e.g. by chlor-alkali electrolysis
- C25B1/46—Simultaneous production of alkali metal hydroxides and chlorine, oxyacids or salts of chlorine, e.g. by chlor-alkali electrolysis in diaphragm cells
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B13/00—Diaphragms; Spacing elements
- C25B13/04—Diaphragms; Spacing elements characterised by the material
- C25B13/08—Diaphragms; Spacing elements characterised by the material based on organic materials
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B15/00—Operating or servicing cells
- C25B15/02—Process control or regulation
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B15/00—Operating or servicing cells
- C25B15/08—Supplying or removing reactants or electrolytes; Regeneration of electrolytes
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B9/00—Cells or assemblies of cells; Constructional parts of cells; Assemblies of constructional parts, e.g. electrode-diaphragm assemblies; Process-related cell features
- C25B9/17—Cells comprising dimensionally-stable non-movable electrodes; Assemblies of constructional parts thereof
- C25B9/19—Cells comprising dimensionally-stable non-movable electrodes; Assemblies of constructional parts thereof with diaphragms
Definitions
- the present invention relates to a method for electrolysis of a brine, and more specifically to an aqueous solution of sodium chloride by means of a membrane electrolysis cell and a gas electrode, said electrode being placed directly against the membrane and in a cathode compartment powered solely by gas.
- the present invention relates to a process for producing an aqueous solution of sodium hydroxide by electrolysis of an aqueous solution of sodium chloride using an "oxygen reduction cathode" having a yield of sodium hydroxide (current efficiency) and improved membrane life.
- a conventional membrane electrolysis cell comprises a placed gas electrode in the cathode compartment of the electrolysis cell to divide this compartment into a solution compartment on the ion exchange membrane side and a gas compartment on the opposite side.
- the gas electrode is usually obtained by molding a mixture of a hydrophobic substance such as a polytetrafluoroethylene resin (hereinafter referred to as PTFE) and a supported catalyst or catalyst so that it presents hydrophobic properties preventing the passage of liquids.
- PTFE polytetrafluoroethylene resin
- a supported catalyst or catalyst so that it presents hydrophobic properties preventing the passage of liquids.
- PTFE polytetrafluoroethylene resin
- a supported catalyst or catalyst so that it presents hydrophobic properties preventing the passage of liquids.
- such a gas electrode progressively loses its hydrophobic properties when it is exposed to a high temperature of the order of 90 ° C and to an aqueous solution of sodium hydroxide of high concentration of about 32% or more.
- the gas electrode consists of a mixture comprising mainly a carbonaceous material and a resin, it has a mechanical fragility and tends to crack.
- the soda produced must have a titer of between 30 and 35%, otherwise the current efficiency will be reduced by increasing the back migration. hydroxyl ions in the membrane, and physically degrade the membrane. These specifications are given by chlor-alkali membrane manufacturers, and are valid for all types of membranes. This involves adding water to dilute the soda produced, 4.5 moles of water per mole of soda (to have a 33% soda).
- the electroosmotic flow through the membrane brings into the cathode compartment 3.5 moles of water per mole of Na + , when the concentration of NaCl in the anode compartment is 220 g / l.
- the amount of water available in contact with the membrane will be at most 3.5 moles of water per mole of sodium hydroxide, assuming that the water required for the electrochemical reaction is provided by the gas.
- a method of electrolysis of an aqueous solution of sodium chloride has now been found by means of an oxygen reduction membrane and cathode electrolysis cell comprising a cation exchange membrane which divides the cell into a compartment. anode and a cathode compartment in which said cathode is placed directly against the cation exchange membrane, said cathode compartment being fed with a humidified gas containing oxygen, characterized in that, to obtain a weight concentration of sodium hydroxide between the exchange membrane cation and the cathode less than 38.8%, an aqueous chloride solution is used.
- sodium (anolyte) having a sodium chloride concentration of less than 200 g / l and preferably of between 160 g / l and 190 g / l and that the water moistening the oxygen-containing gas is under form of water vapor.
- the temperature of the cathode compartment may be greater than the temperature of the anode compartment.
- the temperature of the cathode compartment may be greater than 5 ° C. to 20 ° C. at the temperature of the anode compartment and, preferably, greater than 10 ° C. to 15 ° C.
- the cathode compartment is fed with an oxygen-containing gas, previously wetted by bubbling in water heated to a temperature ranging from 50 ° C. to 100 ° C. and preferably at a temperature of between 80 ° C. and 100 ° C.
- the humidified oxygen will be introduced into the cathode compartment so that the water humidifying the oxygen is in the form of water vapor. This can be achieved by keeping the temperature of the bubbler lower than or equal to that of the cathode compartment.
- the volume content of water vapor of the humidified gas containing oxygen is between 10% and 80% and preferably between 20% and 60%.
- the oxygen-containing gas may be air, oxygen-enriched air or even oxygen. Preferably, oxygen will be used.
- the volume concentration of oxygen in the gas is less than 20%, and preferably at least 50%.
- the gases enriched with oxygen are preferably decarbonated beforehand.
- the weight concentration of sodium hydroxide between the cation exchange membrane and the cathode is less than 38.8%, preferably less than 37%.
- the method of the invention has the advantage of leading to a high yield of sodium hydroxide (current yield), to improve the lifetime of the cation exchange membranes and not to significantly disturb the voltage of the cell.
- the sodium hydroxide obtained by the process according to the present invention has a purity equivalent to the sodium hydroxide obtained by conventional methods of hydrogen evolution cathodes.
- the invention may be implemented with a device as described below.
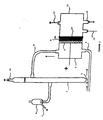
- a capillary placed between the cathodic seal and the membrane (not shown in FIG. 1) makes it possible to take sodium hydroxide between the membrane and the cathode in order to measure its concentration. The chlorine comes out at (15).
- An aqueous solution of NaCl is introduced into the anode compartment (1) by (3) at a weight concentration of NaCl as defined above and humidified gas containing oxygen in the cathode compartment (11) by (12); the water moistening the gas containing oxygen being in the form of water vapor.
- the temperature of the electrolysis is set to 80-90 ° C
- the temperature of the cathode compartment may be greater than the temperature of the anode compartment.
- the present invention it is advantageous to operate with a flow rate of oxygen which is greater than the consumption of the cathode.
- the temperature of the water in which the oxygen-containing gas is bubbling can be increased or decreased, as can the flow rate of humidified gas containing oxygen to adjust the titer of the soda at the outlet (14) of the cell. .
- the electrolytic cell of aqueous sodium chloride solution as shown in FIG. 1 is used.
- the electrolysis is carried out with a source of energy which is connected to the anode (+) and to the cathode (-) of the cell so as to apply to the cell a current density i of 3 to 4 k A / m 2 .
- the anode (8) consists of a titanium substrate coated with ruthenium oxide RuO 2 .
- the cathode (10) consists of platinum carbon shaped with PTFE on a nickel silver grid. (10% platinum on carbon, 0.56 mg Pt per cm 2 ).
- This cathode is marketed by E-TEK, Inc.
- the cation exchange membrane (9) is a Nafion N966 membrane, produced by the departments du Pont de Nemours.
- the gas used is pure oxygen.
- the operating conditions are identical to those of Example 3, except that the weight concentration of NaCl in the anolyte is 170 g / l.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Automation & Control Theory (AREA)
- Electrolytic Production Of Non-Metals, Compounds, Apparatuses Therefor (AREA)
- Water Treatment By Electricity Or Magnetism (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
Claims (8)
- Verfahren zur Elektrolyse einer wässrigen Natriumchloridlösung mittels einer Elektrolysezelle mit Membran und mit einer Sauerstoffreduktionskathode, die eine Kationenaustauschmembran umfasst, die die Zelle in ein Anodenkompartiment und ein Kathodenkompartiment, in dem die Kathode direkt gegen die Kationenaustauschmembran angeordnet ist, teilt, wobei das Kathodenkompartiment mit einem befeuchteten, Sauerstoff enthaltenden Gas versorgt wird, dadurch gekennzeichnet, dass, um eine Gewichtskonzentration der Sole zwischen der Kationenaustauschmembran und der Kathode unterhalb von 38,8 % zu erhalten, eine wässrige Natriumchloridlösung (Anolyt) verwendet wird, die eine Gewichtskonzentration an Natriumchlorid von weniger als 200 g/l aufweist und dadurch, dass das Wasser, das das Sauerstoff enthaltende Gas befeuchtet, in Form von Wasserdampf vorliegt.
- Verfahren nach Anspruch 1, dadurch gekennzeichnet, dass die Gewichtskonzentration an Natriumchlorid der wässrigen Natriumchloridlösung zwischen 160 g/l und 190 g/l liegt.
- Verfahren nach einem der Ansprüche 1 oder 2, dadurch gekennzeichnet, dass das Gas Sauerstoff ist.
- Verfahren nach einem der Ansprüche 1 bis 3, dadurch gekennzeichnet, dass der Volumengehalt an Wasserdampf des befeuchteten, Sauerstoff enthaltenden Gases zwischen 10 % und 80 % liegt.
- Verfahren nach Anspruch 4, dadurch gekennzeichnet, dass der Volumengehalt an Wasserdampf des befeuchteten, Sauerstoff enthaltenden Gases zwischen 20 % und 60 % liegt.
- Verfahren nach einem der Ansprüche 1 bis 5, ferner dadurch gekennzeichnet, dass die Temperatur des Kathodenkompartiments über der Temperatur des Anodenkompartiments liegt.
- Verfahren nach Anspruch 6, dadurch gekennzeichnet, dass die Temperatur des Kathodenkompartiments um 5 °C bis 20 °C über der Temperatur des Anodenkompartiments liegt.
- Verfahren nach Anspruch 7, dadurch gekennzeichnet, dass die Temperatur des Kathodenkompartiments um 10 °C bis 15 °C über der Temperatur des Anodenkompartiments liegt.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR9711795 | 1997-09-23 | ||
| FR9711795A FR2768751B1 (fr) | 1997-09-23 | 1997-09-23 | Procede d'electrolyse d'une saumure |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0903425A1 EP0903425A1 (de) | 1999-03-24 |
| EP0903425B1 true EP0903425B1 (de) | 2007-10-31 |
Family
ID=9511354
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP98402268A Expired - Lifetime EP0903425B1 (de) | 1997-09-23 | 1998-09-15 | Vefahren zur Elektrolyse einer Salzlösung |
Country Status (12)
| Country | Link |
|---|---|
| US (1) | US6080298A (de) |
| EP (1) | EP0903425B1 (de) |
| JP (1) | JP3073968B2 (de) |
| KR (1) | KR100313259B1 (de) |
| CN (1) | CN1107744C (de) |
| AT (1) | ATE377100T1 (de) |
| BR (1) | BR9803590A (de) |
| CA (1) | CA2245144C (de) |
| DE (1) | DE69838632T2 (de) |
| ES (1) | ES2296325T3 (de) |
| FR (1) | FR2768751B1 (de) |
| NO (1) | NO322395B1 (de) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050011753A1 (en) * | 2003-06-23 | 2005-01-20 | Jackson John R. | Low energy chlorate electrolytic cell and process |
| FR2930541B1 (fr) * | 2008-04-29 | 2010-05-21 | Solvay | Procede d'epuration de solutions aqueuses |
| KR101239145B1 (ko) | 2009-03-17 | 2013-03-06 | 김영준 | 음식물 쓰레기에 포함된 염화나트륨 수용액을 전기 분해하는 장치 |
| CN102134724B (zh) * | 2010-12-31 | 2012-06-20 | 北京化工大学 | 阴离子膜电解槽装置用于纯碱生产中废液脱盐的方法 |
| CN106148992A (zh) * | 2015-04-20 | 2016-11-23 | 李坚 | 离子膜催化法或电渗析催化法水制氢及其应用 |
| WO2018180726A1 (ja) | 2017-03-30 | 2018-10-04 | 株式会社カネカ | 水酸化ナトリウム及び/又は塩素の製造方法、並びに2室法型食塩水電解槽 |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4221644A (en) * | 1979-08-14 | 1980-09-09 | Diamond Shamrock Corporation | Air-depolarized chlor-alkali cell operation methods |
| JPS5641392A (en) * | 1979-09-11 | 1981-04-18 | Toyo Soda Mfg Co Ltd | Electrolytic method of alkali chloride aqueous solution |
| JP3400508B2 (ja) * | 1993-10-27 | 2003-04-28 | ペルメレック電極株式会社 | 塩水電解方法及び電解槽 |
| JP3344828B2 (ja) * | 1994-06-06 | 2002-11-18 | ペルメレック電極株式会社 | 塩水の電解方法 |
| JPH08333693A (ja) * | 1995-06-05 | 1996-12-17 | Permelec Electrode Ltd | 電解槽 |
-
1997
- 1997-09-23 FR FR9711795A patent/FR2768751B1/fr not_active Expired - Fee Related
-
1998
- 1998-09-15 ES ES98402268T patent/ES2296325T3/es not_active Expired - Lifetime
- 1998-09-15 EP EP98402268A patent/EP0903425B1/de not_active Expired - Lifetime
- 1998-09-15 AT AT98402268T patent/ATE377100T1/de not_active IP Right Cessation
- 1998-09-15 DE DE69838632T patent/DE69838632T2/de not_active Expired - Lifetime
- 1998-09-17 NO NO19984306A patent/NO322395B1/no not_active IP Right Cessation
- 1998-09-21 JP JP10266805A patent/JP3073968B2/ja not_active Expired - Fee Related
- 1998-09-21 KR KR1019980038977A patent/KR100313259B1/ko not_active Expired - Fee Related
- 1998-09-22 CA CA002245144A patent/CA2245144C/fr not_active Expired - Fee Related
- 1998-09-22 BR BR9803590-8A patent/BR9803590A/pt not_active IP Right Cessation
- 1998-09-23 US US09/158,889 patent/US6080298A/en not_active Expired - Lifetime
- 1998-09-23 CN CN98120557A patent/CN1107744C/zh not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| FR2768751B1 (fr) | 1999-10-29 |
| CN1107744C (zh) | 2003-05-07 |
| CA2245144C (fr) | 2002-08-13 |
| JP3073968B2 (ja) | 2000-08-07 |
| ES2296325T3 (es) | 2008-04-16 |
| FR2768751A1 (fr) | 1999-03-26 |
| DE69838632T2 (de) | 2008-08-28 |
| CN1219610A (zh) | 1999-06-16 |
| NO984306D0 (no) | 1998-09-17 |
| US6080298A (en) | 2000-06-27 |
| JPH11152591A (ja) | 1999-06-08 |
| KR100313259B1 (ko) | 2002-02-19 |
| NO984306L (no) | 1999-03-24 |
| NO322395B1 (no) | 2006-10-02 |
| KR19990029993A (ko) | 1999-04-26 |
| DE69838632D1 (de) | 2007-12-13 |
| ATE377100T1 (de) | 2007-11-15 |
| CA2245144A1 (fr) | 1999-03-23 |
| BR9803590A (pt) | 1999-12-14 |
| EP0903425A1 (de) | 1999-03-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP3182216B2 (ja) | ガス減極電極構造体並びにそれを用いて電気化学的反応を行うための方法及び装置 | |
| US4221644A (en) | Air-depolarized chlor-alkali cell operation methods | |
| US9273404B2 (en) | Process for electrolysis of alkali metal chlorides with oxygen-consuming electrodes | |
| EP0113931B1 (de) | Kathode zur elektrolytischen Herstellung von Wasserstoff und ihre Anwendung | |
| FR2711675A1 (fr) | Procédé et cellule d'électrolyse de saumure. | |
| JP3344828B2 (ja) | 塩水の電解方法 | |
| EP0903425B1 (de) | Vefahren zur Elektrolyse einer Salzlösung | |
| US9150970B2 (en) | Process for electrolysis of alkali metal chlorides with oxygen-consuming electrodes in micro-gap arrangement | |
| JP2002275670A (ja) | イオン交換膜電解槽および電解方法 | |
| CA1155792A (en) | Air-depolarized chlor-alkali cell operation methods | |
| JP7602548B2 (ja) | エチレンオキサイドの製造方法 | |
| JP2699793B2 (ja) | 過酸化水素の製造方法 | |
| JP3538271B2 (ja) | 塩酸電解装置 | |
| EP0221790A1 (de) | Verfahren zur Herstellung von Glyoxylsäure durch elektrochemische Reduktion von Oxalsäure | |
| RU2785846C1 (ru) | Электролиз воды с перекрёстным потоком | |
| JP3395416B2 (ja) | 過酸化水素の製造方法 | |
| JP4251432B2 (ja) | 塩化水素水溶液から塩素を電気化学的に製造する方法 | |
| JP4251432B6 (ja) | 塩化水素水溶液から塩素を電気化学的に製造する方法 | |
| JPH10110283A (ja) | 食塩電解方法 | |
| JP3420790B2 (ja) | 塩化アルカリ電解用電解槽及び電解方法 | |
| JP3706716B2 (ja) | 電解方法 | |
| RU2086706C1 (ru) | Способ получения хлорной кислоты | |
| BE872632A (fr) | Electrocatalyseur et electrode a base d'oxydes de metaux du groupe du platine, reduits et thermiquement stabilises. | |
| JPS6112033B2 (de) | ||
| JPH05339771A (ja) | 食塩電解用隔膜、並びに、食塩電解方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 19981005 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE |
|
| AX | Request for extension of the european patent |
Free format text: AL;LT;LV;MK;RO;SI |
|
| AKX | Designation fees paid |
Free format text: AT BE CH DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: ATOFINA |
|
| 17Q | First examination report despatched |
Effective date: 20010523 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: ARKEMA |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: ARKEMA FRANCE |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| RBV | Designated contracting states (corrected) |
Designated state(s): AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: ARKEMA FRANCE |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: LANGUAGE OF EP DOCUMENT: FRENCH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REF | Corresponds to: |
Ref document number: 69838632 Country of ref document: DE Date of ref document: 20071213 Kind code of ref document: P |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 20080206 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2296325 Country of ref document: ES Kind code of ref document: T3 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20080131 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20080331 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FD4D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20071031 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20071031 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20080801 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20071031 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20080201 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20071031 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20080930 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20071031 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20080930 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20080930 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20080915 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20110907 Year of fee payment: 14 Ref country code: FR Payment date: 20110922 Year of fee payment: 14 Ref country code: GB Payment date: 20110914 Year of fee payment: 14 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20110916 Year of fee payment: 14 Ref country code: NL Payment date: 20110922 Year of fee payment: 14 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20111017 Year of fee payment: 14 Ref country code: BE Payment date: 20110913 Year of fee payment: 14 |
|
| BERE | Be: lapsed |
Owner name: ARKEMA FRANCE Effective date: 20120930 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: V1 Effective date: 20130401 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20120915 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20130531 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130403 Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120915 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120930 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20121001 Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120915 Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130401 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 69838632 Country of ref document: DE Effective date: 20130403 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20131018 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120916 |