EP0903425B1 - Process for the electrolysis of brine - Google Patents
Process for the electrolysis of brine Download PDFInfo
- Publication number
- EP0903425B1 EP0903425B1 EP98402268A EP98402268A EP0903425B1 EP 0903425 B1 EP0903425 B1 EP 0903425B1 EP 98402268 A EP98402268 A EP 98402268A EP 98402268 A EP98402268 A EP 98402268A EP 0903425 B1 EP0903425 B1 EP 0903425B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- cathode
- membrane
- oxygen
- compartment
- temperature
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 238000000034 method Methods 0.000 title claims abstract description 17
- 230000008569 process Effects 0.000 title claims abstract description 13
- 238000005868 electrolysis reaction Methods 0.000 title claims description 22
- 239000012267 brine Substances 0.000 title description 7
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 title description 7
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 claims abstract description 66
- 239000012528 membrane Substances 0.000 claims abstract description 51
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 claims abstract description 50
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims abstract description 41
- 239000001301 oxygen Substances 0.000 claims abstract description 40
- 229910052760 oxygen Inorganic materials 0.000 claims abstract description 40
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims abstract description 38
- 239000007789 gas Substances 0.000 claims abstract description 35
- 239000011780 sodium chloride Substances 0.000 claims abstract description 24
- 238000005341 cation exchange Methods 0.000 claims abstract description 12
- 230000009467 reduction Effects 0.000 claims abstract description 6
- 239000000243 solution Substances 0.000 abstract description 9
- 239000007864 aqueous solution Substances 0.000 abstract description 7
- 210000004027 cell Anatomy 0.000 description 34
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 22
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 6
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 5
- 230000005587 bubbling Effects 0.000 description 5
- 239000001257 hydrogen Substances 0.000 description 5
- 229910052739 hydrogen Inorganic materials 0.000 description 5
- 239000003014 ion exchange membrane Substances 0.000 description 5
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 5
- 239000004810 polytetrafluoroethylene Substances 0.000 description 5
- WOCIAKWEIIZHES-UHFFFAOYSA-N ruthenium(iv) oxide Chemical compound O=[Ru]=O WOCIAKWEIIZHES-UHFFFAOYSA-N 0.000 description 5
- 239000000758 substrate Substances 0.000 description 5
- 239000010936 titanium Substances 0.000 description 5
- 229910052719 titanium Inorganic materials 0.000 description 5
- 229920000557 Nafion® Polymers 0.000 description 4
- 230000002209 hydrophobic effect Effects 0.000 description 4
- -1 polytetrafluoroethylene Polymers 0.000 description 4
- 239000011734 sodium Substances 0.000 description 4
- 208000031968 Cadaver Diseases 0.000 description 3
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 3
- DSVGQVZAZSZEEX-UHFFFAOYSA-N [C].[Pt] Chemical compound [C].[Pt] DSVGQVZAZSZEEX-UHFFFAOYSA-N 0.000 description 3
- 239000000460 chlorine Substances 0.000 description 3
- 229910052801 chlorine Inorganic materials 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- MOFOBJHOKRNACT-UHFFFAOYSA-N nickel silver Chemical compound [Ni].[Ag] MOFOBJHOKRNACT-UHFFFAOYSA-N 0.000 description 3
- 229910052708 sodium Inorganic materials 0.000 description 3
- 229910000679 solder Inorganic materials 0.000 description 3
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- 238000007792 addition Methods 0.000 description 2
- 239000003054 catalyst Substances 0.000 description 2
- 238000010349 cathodic reaction Methods 0.000 description 2
- 150000001768 cations Chemical class 0.000 description 2
- 210000005056 cell body Anatomy 0.000 description 2
- 238000003487 electrochemical reaction Methods 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 239000010956 nickel silver Substances 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 239000011347 resin Substances 0.000 description 2
- 229920005989 resin Polymers 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- MYMOFIZGZYHOMD-UHFFFAOYSA-N Dioxygen Chemical compound O=O MYMOFIZGZYHOMD-UHFFFAOYSA-N 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 239000003575 carbonaceous material Substances 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000006735 deficit Effects 0.000 description 1
- 238000005370 electroosmosis Methods 0.000 description 1
- 238000005265 energy consumption Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000005923 long-lasting effect Effects 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 238000000465 moulding Methods 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 229910001925 ruthenium oxide Inorganic materials 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B1/00—Electrolytic production of inorganic compounds or non-metals
- C25B1/01—Products
- C25B1/34—Simultaneous production of alkali metal hydroxides and chlorine, oxyacids or salts of chlorine, e.g. by chlor-alkali electrolysis
- C25B1/46—Simultaneous production of alkali metal hydroxides and chlorine, oxyacids or salts of chlorine, e.g. by chlor-alkali electrolysis in diaphragm cells
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B13/00—Diaphragms; Spacing elements
- C25B13/04—Diaphragms; Spacing elements characterised by the material
- C25B13/08—Diaphragms; Spacing elements characterised by the material based on organic materials
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B15/00—Operating or servicing cells
- C25B15/02—Process control or regulation
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B15/00—Operating or servicing cells
- C25B15/08—Supplying or removing reactants or electrolytes; Regeneration of electrolytes
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B9/00—Cells or assemblies of cells; Constructional parts of cells; Assemblies of constructional parts, e.g. electrode-diaphragm assemblies; Process-related cell features
- C25B9/17—Cells comprising dimensionally-stable non-movable electrodes; Assemblies of constructional parts thereof
- C25B9/19—Cells comprising dimensionally-stable non-movable electrodes; Assemblies of constructional parts thereof with diaphragms
Definitions
- the present invention relates to a method for electrolysis of a brine, and more specifically to an aqueous solution of sodium chloride by means of a membrane electrolysis cell and a gas electrode, said electrode being placed directly against the membrane and in a cathode compartment powered solely by gas.
- the present invention relates to a process for producing an aqueous solution of sodium hydroxide by electrolysis of an aqueous solution of sodium chloride using an "oxygen reduction cathode" having a yield of sodium hydroxide (current efficiency) and improved membrane life.
- a conventional membrane electrolysis cell comprises a placed gas electrode in the cathode compartment of the electrolysis cell to divide this compartment into a solution compartment on the ion exchange membrane side and a gas compartment on the opposite side.
- the gas electrode is usually obtained by molding a mixture of a hydrophobic substance such as a polytetrafluoroethylene resin (hereinafter referred to as PTFE) and a supported catalyst or catalyst so that it presents hydrophobic properties preventing the passage of liquids.
- PTFE polytetrafluoroethylene resin
- a supported catalyst or catalyst so that it presents hydrophobic properties preventing the passage of liquids.
- PTFE polytetrafluoroethylene resin
- a supported catalyst or catalyst so that it presents hydrophobic properties preventing the passage of liquids.
- such a gas electrode progressively loses its hydrophobic properties when it is exposed to a high temperature of the order of 90 ° C and to an aqueous solution of sodium hydroxide of high concentration of about 32% or more.
- the gas electrode consists of a mixture comprising mainly a carbonaceous material and a resin, it has a mechanical fragility and tends to crack.
- the soda produced must have a titer of between 30 and 35%, otherwise the current efficiency will be reduced by increasing the back migration. hydroxyl ions in the membrane, and physically degrade the membrane. These specifications are given by chlor-alkali membrane manufacturers, and are valid for all types of membranes. This involves adding water to dilute the soda produced, 4.5 moles of water per mole of soda (to have a 33% soda).
- the electroosmotic flow through the membrane brings into the cathode compartment 3.5 moles of water per mole of Na + , when the concentration of NaCl in the anode compartment is 220 g / l.
- the amount of water available in contact with the membrane will be at most 3.5 moles of water per mole of sodium hydroxide, assuming that the water required for the electrochemical reaction is provided by the gas.
- a method of electrolysis of an aqueous solution of sodium chloride has now been found by means of an oxygen reduction membrane and cathode electrolysis cell comprising a cation exchange membrane which divides the cell into a compartment. anode and a cathode compartment in which said cathode is placed directly against the cation exchange membrane, said cathode compartment being fed with a humidified gas containing oxygen, characterized in that, to obtain a weight concentration of sodium hydroxide between the exchange membrane cation and the cathode less than 38.8%, an aqueous chloride solution is used.
- sodium (anolyte) having a sodium chloride concentration of less than 200 g / l and preferably of between 160 g / l and 190 g / l and that the water moistening the oxygen-containing gas is under form of water vapor.
- the temperature of the cathode compartment may be greater than the temperature of the anode compartment.
- the temperature of the cathode compartment may be greater than 5 ° C. to 20 ° C. at the temperature of the anode compartment and, preferably, greater than 10 ° C. to 15 ° C.
- the cathode compartment is fed with an oxygen-containing gas, previously wetted by bubbling in water heated to a temperature ranging from 50 ° C. to 100 ° C. and preferably at a temperature of between 80 ° C. and 100 ° C.
- the humidified oxygen will be introduced into the cathode compartment so that the water humidifying the oxygen is in the form of water vapor. This can be achieved by keeping the temperature of the bubbler lower than or equal to that of the cathode compartment.
- the volume content of water vapor of the humidified gas containing oxygen is between 10% and 80% and preferably between 20% and 60%.
- the oxygen-containing gas may be air, oxygen-enriched air or even oxygen. Preferably, oxygen will be used.
- the volume concentration of oxygen in the gas is less than 20%, and preferably at least 50%.
- the gases enriched with oxygen are preferably decarbonated beforehand.
- the weight concentration of sodium hydroxide between the cation exchange membrane and the cathode is less than 38.8%, preferably less than 37%.
- the method of the invention has the advantage of leading to a high yield of sodium hydroxide (current yield), to improve the lifetime of the cation exchange membranes and not to significantly disturb the voltage of the cell.
- the sodium hydroxide obtained by the process according to the present invention has a purity equivalent to the sodium hydroxide obtained by conventional methods of hydrogen evolution cathodes.
- the invention may be implemented with a device as described below.
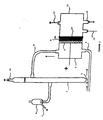
- a capillary placed between the cathodic seal and the membrane (not shown in FIG. 1) makes it possible to take sodium hydroxide between the membrane and the cathode in order to measure its concentration. The chlorine comes out at (15).
- An aqueous solution of NaCl is introduced into the anode compartment (1) by (3) at a weight concentration of NaCl as defined above and humidified gas containing oxygen in the cathode compartment (11) by (12); the water moistening the gas containing oxygen being in the form of water vapor.
- the temperature of the electrolysis is set to 80-90 ° C
- the temperature of the cathode compartment may be greater than the temperature of the anode compartment.
- the present invention it is advantageous to operate with a flow rate of oxygen which is greater than the consumption of the cathode.
- the temperature of the water in which the oxygen-containing gas is bubbling can be increased or decreased, as can the flow rate of humidified gas containing oxygen to adjust the titer of the soda at the outlet (14) of the cell. .
- the electrolytic cell of aqueous sodium chloride solution as shown in FIG. 1 is used.
- the electrolysis is carried out with a source of energy which is connected to the anode (+) and to the cathode (-) of the cell so as to apply to the cell a current density i of 3 to 4 k A / m 2 .
- the anode (8) consists of a titanium substrate coated with ruthenium oxide RuO 2 .
- the cathode (10) consists of platinum carbon shaped with PTFE on a nickel silver grid. (10% platinum on carbon, 0.56 mg Pt per cm 2 ).
- This cathode is marketed by E-TEK, Inc.
- the cation exchange membrane (9) is a Nafion N966 membrane, produced by the departments du Pont de Nemours.
- the gas used is pure oxygen.
- the operating conditions are identical to those of Example 3, except that the weight concentration of NaCl in the anolyte is 170 g / l.
Abstract
Description
La présente invention concerne un procédé d'électrolyse d'une saumure, et, plus précisément d'une solution aqueuse de chlorure de sodium au moyen d'une cellule d'électrolyse à membrane et d'une électrode à gaz, ladite électrode étant placée directement contre la membrane et dans un compartiment cathodique alimenté uniquement par du gaz.The present invention relates to a method for electrolysis of a brine, and more specifically to an aqueous solution of sodium chloride by means of a membrane electrolysis cell and a gas electrode, said electrode being placed directly against the membrane and in a cathode compartment powered solely by gas.
Plus particulièrement, la présente invention concerne un procédé de production d'une solution aqueuse d'hydroxyde de sodium par électrolyse d'une solution aqueuse de chlorure de sodium au moyen d'une "cathode à réduction d'oxygène" ayant un rendement en soude (rendement de courant) et une durée de vie de la membrane améliorés.More particularly, the present invention relates to a process for producing an aqueous solution of sodium hydroxide by electrolysis of an aqueous solution of sodium chloride using an "oxygen reduction cathode" having a yield of sodium hydroxide (current efficiency) and improved membrane life.
Des améliorations remarquables ont été obtenues récemment en ce qui concerne les membranes échangeuses d'ions fluorées et ont permis le développement de procédés d'électrolyse de solutions de chlorure de sodium au moyen de membranes échangeuses d'ions. Cette technique permet de produire de l'hydrogène et de l'hydroxyde de sodium dans le compartiment cathodique et du chlore dans le compartiment anodique d'une cellule d'électrolyse de saumure.Remarkable improvements have recently been achieved with respect to fluorinated ion exchange membranes and have enabled the development of processes for the electrolysis of sodium chloride solutions by means of ion exchange membranes. This technique makes it possible to produce hydrogen and sodium hydroxide in the cathode compartment and chlorine in the anode compartment of a brine electrolysis cell.
Pour réduire la consommation d'énergie, il a été proposé dans la demande de brevet
Théoriquement, il est possible de réduire la tension d'électrolyse de 1,23 V en utilisant la réaction cathodique avec apport d'oxygène représentée par (1) à la place de la réaction cathodique sans apport d'oxygène représentée par (2) :
2H2O + O2 + 4e- → 4OH- (1)
E = + 0,40V (par rapport à une électrode normale à hydrogène).
4H2O + 4e- → 2H2 + 4OH- (2)
E = -0,83 V (par rapport à une électrode normale à hydrogène).Theoretically, it is possible to reduce the electrolysis voltage by 1.23 V by using the cathodic reaction with oxygen supply represented by (1) in place of the cathodic reaction without oxygen supply represented by (2):
2H 2 O + O 2 + 4e - → 4OH - (1)
E = + 0.40V (relative to a normal hydrogen electrode).
4H 2 O + 4e - → 2H 2 + 4OH - (2)
E = -0.83 V (relative to a normal hydrogen electrode).
En général, une cellule conventionnelle d'électrolyse à membrane selon la technologie électrode à gaz, comprend une électrode à gaz placée dans le compartiment cathodique de la cellule d'électrolyse pour diviser ce compartiment en un compartiment à solution du côté de la membrane échangeuse d'ions et en un compartiment à gaz du côté opposé. L'électrode à gaz est habituellement obtenue en moulant un mélange d'une substance hydrophobe telle qu'une résine de polytétrafluoroéthylène (appelée dans la suite PTFE) et d'un catalyseur ou d'un catalyseur sur support, de manière qu'elle présente des propriétés hydrophobes empêchant le passage des liquides. Cependant, une telle électrode à gaz perd progressivement ses propriétés hydrophobes lorsqu'elle est exposée à une température élevée de l'ordre de 90°C et à une solution aqueuse d'hydroxyde de sodium de concentration élevée d'environ 32 % voir plus en masse au cours d'une électrolyse de longue durée. De ce fait, le liquide présent dans le compartiment à solution a tendance à pénétrer dans le compartiment à gaz. De plus, du fait que l'électrode à gaz est constituée par un mélange comprenant principalement une matière carbonée et une résine, elle présente une fragilité mécanique et a tendance à se fissurer. Ces inconvénients ont interdit l'utilisation pratique d'une telle électrode à gaz pour l'électrolyse d'une saumure.In general, a conventional membrane electrolysis cell according to the gas electrode technology comprises a placed gas electrode in the cathode compartment of the electrolysis cell to divide this compartment into a solution compartment on the ion exchange membrane side and a gas compartment on the opposite side. The gas electrode is usually obtained by molding a mixture of a hydrophobic substance such as a polytetrafluoroethylene resin (hereinafter referred to as PTFE) and a supported catalyst or catalyst so that it presents hydrophobic properties preventing the passage of liquids. However, such a gas electrode progressively loses its hydrophobic properties when it is exposed to a high temperature of the order of 90 ° C and to an aqueous solution of sodium hydroxide of high concentration of about 32% or more. mass during a long-lasting electrolysis. As a result, the liquid in the solution compartment tends to enter the gas compartment. In addition, because the gas electrode consists of a mixture comprising mainly a carbonaceous material and a resin, it has a mechanical fragility and tends to crack. These disadvantages have prohibited the practical use of such a gas electrode for the electrolysis of a brine.
Une telle configuration de cellule d'électrolyse est décrite dans la demande de brevet
Afin de résoudre les inconvénients mentionnés plus haut, il a été proposé dans le brevet
Si les problèmes de fragilité mécanique ont été ainsi résolus, il n'en reste pas moins que ce type de configuration de cellule présente des inconvénients tels que notamment le changement de la membrane et de la cathode.If the problems of mechanical fragility have been thus resolved, the fact remains that this type of cell configuration has disadvantages such as in particular the change of the membrane and the cathode.
Si on effectue le bilan eau dans une cellule d'électrolyse à membrane comprenant une cathode constituée de carbone platiné mis en forme avec du PTFE sur une grille de nickel argenté, on constate que la réaction électrochimique mise en jeu à la cathode-réaction (1)- consomme 2 moles d'eau pour 4 moles de soude produites, soit 0,5 mole d'eau pour une mole de soude.If the water balance is carried out in a membrane electrolysis cell comprising a cathode made of platinum carbon shaped with PTFE on a silver nickel grid, it is found that the electrochemical reaction involved at the cathode-reaction (1 ) - consumes 2 moles of water per 4 moles of sodium hydroxide produced, ie 0.5 moles of water per mole of sodium hydroxide.
La soude produite doit avoir un titre compris entre 30 et 35 % sous peine de diminuer le rendement en courant en augmentant la rétro-migration des ions hydroxyle dans la membrane, et de dégrader physiquement la membrane. Ces spécifications sont données par les fabricants de membrane chlore-soude, et sont valables pour tous les types de membranes. Cela implique un apport d'eau pour diluer la soude produite, de 4,5 moles d'eau par mole de soude (pour avoir une soude à 33 %).The soda produced must have a titer of between 30 and 35%, otherwise the current efficiency will be reduced by increasing the back migration. hydroxyl ions in the membrane, and physically degrade the membrane. These specifications are given by chlor-alkali membrane manufacturers, and are valid for all types of membranes. This involves adding water to dilute the soda produced, 4.5 moles of water per mole of soda (to have a 33% soda).
Le flux électro-osmotique à travers la membrane apporte dans le compartiment cathodique 3,5 moles d'eau par mole de Na+, lorsque la concentration en NaCl dans le compartiment anodique est de 220 g/l.The electroosmotic flow through the membrane brings into the cathode compartment 3.5 moles of water per mole of Na + , when the concentration of NaCl in the anode compartment is 220 g / l.
On consomme donc 0,5 + 4,5 = 5 moles d'eau pour une mole de soude. On apporte donc 3,5 moles d'eau par mole de soude, soit un déficit de 1,5 moles d'eau par mole de soude dans les conditions classiques de fonctionnement.0.5 + 4.5 = 5 moles of water are therefore consumed for one mole of soda. 3.5 moles of water are thus added per mole of sodium hydroxide, ie a deficit of 1.5 moles of water per mole of sodium hydroxide under the conventional conditions of operation.
Il a été proposé dans la demande de brevet
On a maintenant trouvé un procédé d'électrolyse d'une solution aqueuse de chlorure de sodium au moyen d'une cellule d'électrolyse à membrane et à cathode à réduction d'oxygène comprenant une membrane échangeuse de cations qui divise la cellule en un compartiment anodique et un compartiment cathodique dans lequel ladite cathode est placée directement contre la membrane échangeuse de cations, ledit compartiment cathodique étant alimenté par un gaz humidifié contenant de l'oxygène, caractérisé en ce que, pour obtenir une concentration pondérale en soude entre la membrane échangeuse de cation et la cathode inférieure à 38,8 %, on utilise une solution aqueuse de chlorure de sodium (anolyte) ayant une concentration en chlorure de sodium inférieure à 200 g/l et, de préférence comprise entre 160 g/l et 190 g/l et en ce que l'eau humidifiant le gaz contenant de l'oxygène est sous forme de vapeur d'eau.A method of electrolysis of an aqueous solution of sodium chloride has now been found by means of an oxygen reduction membrane and cathode electrolysis cell comprising a cation exchange membrane which divides the cell into a compartment. anode and a cathode compartment in which said cathode is placed directly against the cation exchange membrane, said cathode compartment being fed with a humidified gas containing oxygen, characterized in that, to obtain a weight concentration of sodium hydroxide between the exchange membrane cation and the cathode less than 38.8%, an aqueous chloride solution is used. of sodium (anolyte) having a sodium chloride concentration of less than 200 g / l and preferably of between 160 g / l and 190 g / l and that the water moistening the oxygen-containing gas is under form of water vapor.
En outre, selon la présente invention, la température du compartiment cathodique peut être supérieure à la température du compartiment anodique.In addition, according to the present invention, the temperature of the cathode compartment may be greater than the temperature of the anode compartment.
Selon la présente invention, la température du compartiment cathodique peut être supérieure de 5°C à 20°C à la température du compartiment anodique et, de préférence supérieure de 10°C à 15°C.According to the present invention, the temperature of the cathode compartment may be greater than 5 ° C. to 20 ° C. at the temperature of the anode compartment and, preferably, greater than 10 ° C. to 15 ° C.
Le compartiment cathodique est alimenté par un gaz contenant de l'oxygène, préalablement humidifié par barbotage dans de l'eau chauffée à une température allant de 50°C à 100°C et, de préférence, à une température comprise entre 80°C et 100°C.The cathode compartment is fed with an oxygen-containing gas, previously wetted by bubbling in water heated to a temperature ranging from 50 ° C. to 100 ° C. and preferably at a temperature of between 80 ° C. and 100 ° C.
Selon la présente invention, l'oxygène humidifié sera introduit dans le compartiment cathodique de façon à ce que l'eau humidifiant l'oxygène se trouve sous forme de vapeur d'eau. Ceci peut être obtenu en maintenant la température du barboteur inférieure ou égale à celle du compartiment cathodique.According to the present invention, the humidified oxygen will be introduced into the cathode compartment so that the water humidifying the oxygen is in the form of water vapor. This can be achieved by keeping the temperature of the bubbler lower than or equal to that of the cathode compartment.
La teneur volumique en vapeur d'eau du gaz humidifié contenant de l'oxygène est comprise entre 10 % et 80 % et, de préférence comprise entre 20 % et 60 %.The volume content of water vapor of the humidified gas containing oxygen is between 10% and 80% and preferably between 20% and 60%.
Le gaz contenant de l'oxygène peut être de l'air, de l'air enrichi en oxygène ou bien encore de l'oxygène. De préférence, on utilisera de l'oxygène. La concentration volumique en oxygène dans le gaz est moins égale à 20 %, et, de préférence, au moins égale à 50 %.The oxygen-containing gas may be air, oxygen-enriched air or even oxygen. Preferably, oxygen will be used. The volume concentration of oxygen in the gas is less than 20%, and preferably at least 50%.
Les gaz enrichis en oxygène, sont, de préférence, préalablement décarbonatés.The gases enriched with oxygen are preferably decarbonated beforehand.
Selon la présente invention, la concentration pondérale en soude entre la membrane échangeuse de cations et la cathode est inférieure à 38,8 %, de préférence inférieure à 37 %. Le procédé de l'invention présente l'avantage de conduire à un rendement en soude élevé (rendement du courant), d'améliorer la durée de vie des membranes échangeuses de cations et de ne pas perturber de façon significative la tension de la cellule.According to the present invention, the weight concentration of sodium hydroxide between the cation exchange membrane and the cathode is less than 38.8%, preferably less than 37%. The method of the invention has the advantage of leading to a high yield of sodium hydroxide (current yield), to improve the lifetime of the cation exchange membranes and not to significantly disturb the voltage of the cell.
En outre, la soude obtenue par le procédé selon la présente invention possède une pureté équivalente à la soude obtenue selon les procédés classiques des cathodes à dégagement d'hydrogène.In addition, the sodium hydroxide obtained by the process according to the present invention has a purity equivalent to the sodium hydroxide obtained by conventional methods of hydrogen evolution cathodes.
L'invention peut être mise en oeuvre avec un dispositif tel que décrit ci-après.The invention may be implemented with a device as described below.
La figure 1 schématise une cellule qui est constituée de :
- un compartiment anodique constitué d'un corps de cellule (1), d'un dégazeur (2). La solution de chlorure de sodium (saumure) est introduite par (3) et circule par gaz lift entre le corps de la cellule et le dégazeur (conduites (4) et (5)). Un trop plein (6) permet d'éliminer par (7) une partie de la saumure appauvrie. Des ajouts de saumure concentrée permettent de maintenir la concentration en NaCl dans l'anolyte à la valeur choisie ;
- une anode (8) qui peut être constituée par substrat en titane revêtu de RuO2,
- une membrane échangeuse de cations (9),
- une cathode (10) placée directement contre la membrane (9), qui peut être constituée d'une grille de nickel argenté recouverte de carbone platiné,
- un compartiment cathodique (11) constitué d'un corps de cellule. Le gaz humidifié contenant de l'oxygène est alimenté par le bas de la cellule (12) et ressort en partie haute (13) dans une colonne d'eau non représentée sur la figure 1 qui fixe la pression de travail. La soude est soutirée en (14) directement au titre voulue dans le bas de la cellule.
- an anode compartment consisting of a cell body (1), a degasser (2). The solution of sodium chloride (brine) is introduced by (3) and circulates by lift gas between the body of the cell and the degasser (lines (4) and (5)). An overflow (6) allows to eliminate (7) part of the depleted brine. Additions of concentrated brine make it possible to maintain the concentration of NaCl in the anolyte at the chosen value;
- an anode (8) which can be constituted by titanium substrate coated with RuO 2 ,
- a cation exchange membrane (9),
- a cathode (10) placed directly against the membrane (9), which may consist of a nickel-silver grid coated with platinum carbon,
- a cathode compartment (11) consisting of a cell body. The humidified gas containing oxygen is fed from the bottom of the cell (12) and exits at the top (13) in a water column not shown in Figure 1 which sets the working pressure. The soda is drawn at (14) directly to the desired title in the bottom of the cell.
Un capillaire placé entre le joint cathodique et la membrane (non représenté sur la figure 1 permet de prélever de la soude entre la membrane et la cathode afin de mesurer sa concentration. Le chlore sort en (15).A capillary placed between the cathodic seal and the membrane (not shown in FIG. 1) makes it possible to take sodium hydroxide between the membrane and the cathode in order to measure its concentration.The chlorine comes out at (15).
On introduit une solution aqueuse de NaCl dans le compartiment anodique (1) par (3) à une concentration pondérale en NaCl telle que définie précédemment et du gaz humidifié contenant de l'oxygène dans le compartiment cathodique (11) par (12) ; l'eau humidifiant le gaz contenant de l'oxygène étant sous forme de vapeur d'eau.An aqueous solution of NaCl is introduced into the anode compartment (1) by (3) at a weight concentration of NaCl as defined above and humidified gas containing oxygen in the cathode compartment (11) by (12); the water moistening the gas containing oxygen being in the form of water vapor.
Il n'y a ni ajout d'eau liquide, ni circulation de soude, dans le dispositif décrit ci-dessus.There is no addition of liquid water or circulation of soda in the device described above.
Selon la présente invention, la température de l'électrolyse est réglée vers 80-90°C, la température du compartiment cathodique pouvant être supérieure à la température du compartiment anodique.According to the present invention, the temperature of the electrolysis is set to 80-90 ° C, the temperature of the cathode compartment may be greater than the temperature of the anode compartment.
Lorsque l'on applique une densité de courant aux électrodes, du chlore provenant de l'électrolyse de la solution aqueuse de NaCl se dégage dans le compartiment anodique et est évacué via (4) et (15), les ions hydroxyles, formés par réduction de l'oxygène forment avec les cations alcalins circulant à travers la membrane, de la soude.When a current density is applied to the electrodes, chlorine resulting from the electrolysis of the aqueous NaCl solution is evolved in the anode compartment and is discharged via (4) and (15). Hydroxyl ions, formed by reduction of oxygen form with sodium cations circulating through the membrane, sodium hydroxide.
Selon la présente invention, on opère avantageusement avec un débit d'oxygène qui est supérieur à la consommation de la cathode. La température de l'eau dans laquelle barbote le gaz contenant de l'oxygène peut être augmentée ou diminuée, de même que le débit de gaz humidifié contenant de l'oxygène pour ajuster le titre de la soude en sortie (14) de la cellule.According to the present invention, it is advantageous to operate with a flow rate of oxygen which is greater than the consumption of the cathode. The temperature of the water in which the oxygen-containing gas is bubbling can be increased or decreased, as can the flow rate of humidified gas containing oxygen to adjust the titer of the soda at the outlet (14) of the cell. .
Les exemples qui suivent illustrent l'invention.The following examples illustrate the invention.
On utilise la cellule d'électrolyse de solution aqueuse de chlorure de sodium tel que représentée sur la figure 1.The electrolytic cell of aqueous sodium chloride solution as shown in FIG. 1 is used.
L'électrolyse est réalisée avec une source d'énergie qui est reliée à l'anode (+) et à la cathode (-) de la cellule de façon à appliquer à la cellule une densité de courant i de 3 à 4 k A/m2.The electrolysis is carried out with a source of energy which is connected to the anode (+) and to the cathode (-) of the cell so as to apply to the cell a current density i of 3 to 4 k A / m 2 .
L'anode (8) est constituée par un substrat en titane revêtu d'oxyde de ruthénium RuO2.The anode (8) consists of a titanium substrate coated with ruthenium oxide RuO 2 .
La cathode (10) est constituée par du carbone platiné mis en forme avec du PTFE sur une grille de nickel argenté. (10 % de platine sur le carbone ; 0,56 mg de Pt par cm2).The cathode (10) consists of platinum carbon shaped with PTFE on a nickel silver grid. (10% platinum on carbon, 0.56 mg Pt per cm 2 ).
Cette cathode est commercialisée par la Société E-TEK, Inc.This cathode is marketed by E-TEK, Inc.
La membrane changeuse de cations (9) est une membrane Nafion N966, produite par la Société du Pont de Nemours.The cation exchange membrane (9) is a Nafion N966 membrane, produced by the Société du Pont de Nemours.
Le gaz utilisé est de l'oxygène pur.The gas used is pure oxygen.
- Membrane Nafion® N966 ; anode substrat de titane recouvert de Ru02.Nafion ® N966 membrane; anode titanium substrate covered with Ru02.
- Température anodique = Température cathodique = 80°C.Anode temperature = Cathode temperature = 80 ° C.
- Densité de courant i = 3 kA/m2.Current density i = 3 kA / m 2 .
- L'oxygène est humidifié par barbotage dans de l'eau à 80°C avant son entrée dans la cellule. Son débit est de 5 l/h.The oxygen is wetted by bubbling in water at 80 ° C before entering the cell. Its flow rate is 5 l / h.
- La teneur volumique en vapeur d'eau de l'oxygène humidifié est d'environ 55 %.The volume content of water vapor of humidified oxygen is about 55%.
- Concentration pondérale en NaCl dans l'anolyte = 220 g/l.NaCl concentration in the anolyte = 220 g / l.
- Concentration pondérale de la soude en sortie de cellule = 30 %.Weight concentration of sodium hydroxide at the outlet of the cell = 30%.
- Concentration pondérale de la soude entre la membrane et la cathode = 40 %.Weight concentration of the soda between the membrane and the cathode = 40%.
- Tension de cellule = 2,2 V.Cell voltage = 2.2 V.
- Rendement en soude = 93 % (bilan réalisé sur 24 heures de fonctionnement continu).Soda yield = 93% (balance achieved over 24 hours of continuous operation).
On constate que le titre soude en sortie de cellule est correct, mais le rendement est largement inférieur aux valeurs attendues avec ce type de membrane.It is found that the solder titre at the cell outlet is correct, but the yield is much lower than the expected values with this type of membrane.
- Membrane Nafion® N966 ; anode substrat de titane recouvert de Ru02.Nafion ® N966 membrane; anode titanium substrate covered with Ru02.
- Température anodique = Température cathodique = 80°C.Anode temperature = Cathode temperature = 80 ° C.
- Densité de courant i = 3 kA/m2.Current density i = 3 kA / m 2 .
- L'oxygène est humidifié par barbotage dans de l'eau à 80°C avant son entrée dans la cellule, son débit est doublé par rapport à l'exemple 1.The oxygen is humidified by bubbling in water at 80 ° C before entering the cell, its flow rate is doubled compared to Example 1.
- Concentration pondérale en NaCl dans l'anolyte = 220 g/l.NaCl concentration in the anolyte = 220 g / l.
- Concentration pondérale de la soude en sortie de cellule 28,5 %.Weight concentration of sodium hydroxide at the outlet of the cell 28.5%.
- Concentration pondérale de la soude entre la membrane et la cathode = 39 %.Weight concentration of the soda between the membrane and the cathode = 39%.
- Tension de cellule Ecell = 2,2 V.Ecell cell voltage = 2.2 V.
- Rendement en soude = 93,4 % (bilan réalisé sur 24 heures de fonctionnement continu).Soda yield = 93.4% (balance achieved over 24 hours of continuous operation).
On constate que le titre soude en sortie de cellule est trop faible, la concentration en soude à l'interface membrane/cathode est inchangée et est élevée, le rendement quasi-identique : l'eau apportée par l'oxygène ne traverse pas la cathode pour diluer la soude à l'interface membrane/cathode, elle ne sert donc qu'à diluer la soude à l'arrière de la cathode.It is found that the solder titer at the cell outlet is too low, the sodium concentration at the membrane / cathode interface is unchanged and is high, the almost identical yield: the water supplied by the oxygen does not pass through the cathode to dilute the soda at the membrane / cathode interface, it is only used to dilute the soda at the rear of the cathode.
- Membrane Nafion® N966 ; anode substrat de titane recouvert de RuO2.Nafion ® N966 membrane; anode titanium substrate covered with RuO 2 .
- Température anodique = Température cathodique = 80°C.Anode temperature = Cathode temperature = 80 ° C.
- Densité de courant i = 3 kA/m2.Current density i = 3 kA / m 2 .
- L'oxygène est humidifié par barbotage dans de l'eau à 80°C avant son entrée dans la cellule, le débit d'oxygène est identique à celui de l'exemple 1.The oxygen is wetted by bubbling in water at 80 ° C before entering the cell, the flow rate of oxygen is identical to that of Example 1.
- Concentration pondérale en NaCl dans l'anolyte = 190 g/l.Weight concentration of NaCl in the anolyte = 190 g / l.
- Concentration pondérale de la soude en sortie de cellule = 30 %.Weight concentration of sodium hydroxide at the outlet of the cell = 30%.
- Concentration pondérale de la soude entre la membrane et la cathode = 37,5 %.Weight concentration of the soda between the membrane and the cathode = 37.5%.
- Tension de cellule = 2,2 V.Cell voltage = 2.2 V.
- Rendement en soude = 95,9 % (bilan réalisé sur 24 heures de fonctionnement continu).Soda yield = 95.9% (balance achieved over 24 hours of continuous operation).
On constate que le titre soude en sortie de cellule est inchangé, le rendement est nettement supérieur à celui obtenu dans l'exemple 1, la tension de cellule n'est pas perturbée.It is found that the solder titer at the cell outlet is unchanged, the yield is significantly higher than that obtained in Example 1, the cell voltage is not disturbed.
Les conditions opératoires sont identiques à celles de l'exemple 3, excepté que la concentration pondérale en NaCl dans l'anolyte est de 170 g/l.The operating conditions are identical to those of Example 3, except that the weight concentration of NaCl in the anolyte is 170 g / l.
Les résultats sont les suivants :
- concentration pondérale de la soude sortie cellule : 32 %,
- concentration pondérale de la soude entre la membrane et la cathode : 35 %,
- rendement en soude : 96 %.
- weight concentration of the soda output cell: 32%,
- weight concentration of the soda between the membrane and the cathode: 35%,
- soda yield: 96%.
Claims (8)
- Process for the electrolysis of an aqueous sodium chloride solution by means of a membrane electrolysis cell having an oxygen reduction cathode, comprising a cation exchange membrane that divides the cell into an anode compartment and a cathode compartment, in which said cathode is placed directly against the cation exchange membrane, said cathode compartment being supplied with an oxygen-containing humidified gas, characterized in that, to obtain a sodium hydroxide weight concentration between the cation exchange membrane and the cathode of less than 38.8%, an aqueous sodium chloride solution (anolyte) having a sodium chloride weight concentration of less than 200 g/l is used and in that the water humidifying the oxygen-containing gas is in the form of water vapour.
- Process according to Claim 1, characterized in that the sodium chloride weight concentration of the aqueous sodium chloride solution is between 160 g/l and 190 g/l.
- Process according to either of Claims 1 and 2, characterized in that the gas is oxygen.
- Process according to one of Claims 1 to 3, characterized in that the volume content of water vapour of the oxygen-containing humidified gas is between 10% and 80%.
- Process according to Claim 4, characterized in that the volume content of water vapour of the oxygen-containing humidified gas is between 20% and 60%.
- Process according to one of Claims 1 to 5, characterized in that, in addition, the temperature of the cathode compartment is above the temperature of the anode compartment.
- Process according to Claim 6, characterized in that the temperature of the cathode compartment is 5°C to 20°C above the temperature of the anode compartment.
- Process according to Claim 7, characterized in that the temperature of the cathode compartment is 10°C to 15°C above the temperature of the anode compartment.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR9711795 | 1997-09-23 | ||
| FR9711795A FR2768751B1 (en) | 1997-09-23 | 1997-09-23 | ELECTROLYSIS OF A BRINE |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0903425A1 EP0903425A1 (en) | 1999-03-24 |
| EP0903425B1 true EP0903425B1 (en) | 2007-10-31 |
Family
ID=9511354
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP98402268A Expired - Lifetime EP0903425B1 (en) | 1997-09-23 | 1998-09-15 | Process for the electrolysis of brine |
Country Status (12)
| Country | Link |
|---|---|
| US (1) | US6080298A (en) |
| EP (1) | EP0903425B1 (en) |
| JP (1) | JP3073968B2 (en) |
| KR (1) | KR100313259B1 (en) |
| CN (1) | CN1107744C (en) |
| AT (1) | ATE377100T1 (en) |
| BR (1) | BR9803590A (en) |
| CA (1) | CA2245144C (en) |
| DE (1) | DE69838632T2 (en) |
| ES (1) | ES2296325T3 (en) |
| FR (1) | FR2768751B1 (en) |
| NO (1) | NO322395B1 (en) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050011753A1 (en) * | 2003-06-23 | 2005-01-20 | Jackson John R. | Low energy chlorate electrolytic cell and process |
| FR2930541B1 (en) | 2008-04-29 | 2010-05-21 | Solvay | PROCESS FOR PURIFYING AQUEOUS SOLUTIONS |
| KR101239145B1 (en) | 2009-03-17 | 2013-03-06 | 김영준 | Device to electrolysis of aquous solution of sodium chloride contained in food waste |
| CN102134724B (en) * | 2010-12-31 | 2012-06-20 | 北京化工大学 | Method for desalting waste liquor in sodium carbonate production by using anion-exchange membrane electrolyzer |
| CN106148992A (en) * | 2015-04-20 | 2016-11-23 | 李坚 | Ionic membrane catalysis method or electrodialysis catalysis method water hydrogen manufacturing and application thereof |
| WO2018180726A1 (en) | 2017-03-30 | 2018-10-04 | 株式会社カネカ | Method for manufacturing sodium hydroxide and/or chlorine and 2 chamber type saltwater electrolytic cell |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4221644A (en) * | 1979-08-14 | 1980-09-09 | Diamond Shamrock Corporation | Air-depolarized chlor-alkali cell operation methods |
| JPS5641392A (en) * | 1979-09-11 | 1981-04-18 | Toyo Soda Mfg Co Ltd | Electrolytic method of alkali chloride aqueous solution |
| JP3400508B2 (en) * | 1993-10-27 | 2003-04-28 | ペルメレック電極株式会社 | Brine electrolysis method and electrolyzer |
| JP3344828B2 (en) * | 1994-06-06 | 2002-11-18 | ペルメレック電極株式会社 | Saltwater electrolysis method |
| JPH08333693A (en) * | 1995-06-05 | 1996-12-17 | Permelec Electrode Ltd | Electrolytic cell |
-
1997
- 1997-09-23 FR FR9711795A patent/FR2768751B1/en not_active Expired - Fee Related
-
1998
- 1998-09-15 EP EP98402268A patent/EP0903425B1/en not_active Expired - Lifetime
- 1998-09-15 ES ES98402268T patent/ES2296325T3/en not_active Expired - Lifetime
- 1998-09-15 AT AT98402268T patent/ATE377100T1/en not_active IP Right Cessation
- 1998-09-15 DE DE69838632T patent/DE69838632T2/en not_active Expired - Lifetime
- 1998-09-17 NO NO19984306A patent/NO322395B1/en not_active IP Right Cessation
- 1998-09-21 KR KR1019980038977A patent/KR100313259B1/en not_active IP Right Cessation
- 1998-09-21 JP JP10266805A patent/JP3073968B2/en not_active Expired - Fee Related
- 1998-09-22 CA CA002245144A patent/CA2245144C/en not_active Expired - Fee Related
- 1998-09-22 BR BR9803590-8A patent/BR9803590A/en not_active IP Right Cessation
- 1998-09-23 CN CN98120557A patent/CN1107744C/en not_active Expired - Fee Related
- 1998-09-23 US US09/158,889 patent/US6080298A/en not_active Expired - Lifetime
Also Published As
| Publication number | Publication date |
|---|---|
| KR19990029993A (en) | 1999-04-26 |
| NO322395B1 (en) | 2006-10-02 |
| NO984306D0 (en) | 1998-09-17 |
| JPH11152591A (en) | 1999-06-08 |
| DE69838632D1 (en) | 2007-12-13 |
| FR2768751A1 (en) | 1999-03-26 |
| FR2768751B1 (en) | 1999-10-29 |
| CA2245144A1 (en) | 1999-03-23 |
| CA2245144C (en) | 2002-08-13 |
| KR100313259B1 (en) | 2002-02-19 |
| NO984306L (en) | 1999-03-24 |
| BR9803590A (en) | 1999-12-14 |
| CN1107744C (en) | 2003-05-07 |
| ATE377100T1 (en) | 2007-11-15 |
| ES2296325T3 (en) | 2008-04-16 |
| US6080298A (en) | 2000-06-27 |
| DE69838632T2 (en) | 2008-08-28 |
| CN1219610A (en) | 1999-06-16 |
| JP3073968B2 (en) | 2000-08-07 |
| EP0903425A1 (en) | 1999-03-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP3182216B2 (en) | Gas depolarized electrode structure and method and apparatus for performing an electrochemical reaction using the same | |
| US4221644A (en) | Air-depolarized chlor-alkali cell operation methods | |
| US9273404B2 (en) | Process for electrolysis of alkali metal chlorides with oxygen-consuming electrodes | |
| EP0113931B1 (en) | Cathode for the electrolytic production of hydrogen, and its use | |
| JP3344828B2 (en) | Saltwater electrolysis method | |
| FR2711675A1 (en) | Brine electrolysis process and cell. | |
| EP0903425B1 (en) | Process for the electrolysis of brine | |
| FR2803856A1 (en) | SYNTHESIS OF TETRAMETHYLAMMONIUM HYDROXIDE | |
| US9150970B2 (en) | Process for electrolysis of alkali metal chlorides with oxygen-consuming electrodes in micro-gap arrangement | |
| JP2002275670A (en) | Ion exchange membrane electrolytic cell and electrolysis method | |
| CA1155792A (en) | Air-depolarized chlor-alkali cell operation methods | |
| JP2699793B2 (en) | Method for producing hydrogen peroxide | |
| JP3538271B2 (en) | Hydrochloric acid electrolyzer | |
| RU2785846C1 (en) | Water electrolysis with cross flow | |
| EP0221790A1 (en) | Process for the production of glyoxylic acid by the electrochemical reduction of oxalic acid | |
| JP3395416B2 (en) | Method for producing hydrogen peroxide | |
| JP4251432B2 (en) | Method for electrochemical production of chlorine from aqueous hydrogen chloride solution | |
| JP4251432B6 (en) | Method for electrochemical production of chlorine from aqueous hydrogen chloride solution | |
| JP3420790B2 (en) | Electrolyzer and electrolysis method for alkali chloride electrolysis | |
| JPH10110283A (en) | Electrolytic soda process | |
| JP3706716B2 (en) | Electrolysis method | |
| RU2086706C1 (en) | Method of producing perchloric acid | |
| FI65281B (en) | FOERFARANDE FOER DRIFT AV EN KLOR-ALKALIELEKTROLYSCELL | |
| JPS6112033B2 (en) | ||
| JPH05339771A (en) | Diaphragm for sodium chloride electrolysis and method for electrolyzing sodium chloride |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 19981005 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE |
|
| AX | Request for extension of the european patent |
Free format text: AL;LT;LV;MK;RO;SI |
|
| AKX | Designation fees paid |
Free format text: AT BE CH DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: ATOFINA |
|
| 17Q | First examination report despatched |
Effective date: 20010523 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: ARKEMA |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: ARKEMA FRANCE |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| RBV | Designated contracting states (corrected) |
Designated state(s): AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: ARKEMA FRANCE |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: LANGUAGE OF EP DOCUMENT: FRENCH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REF | Corresponds to: |
Ref document number: 69838632 Country of ref document: DE Date of ref document: 20071213 Kind code of ref document: P |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 20080206 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2296325 Country of ref document: ES Kind code of ref document: T3 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20080131 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20080331 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FD4D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20071031 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20071031 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20080801 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20071031 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20080201 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20071031 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20080930 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20071031 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20080930 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20080930 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20080915 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20110907 Year of fee payment: 14 Ref country code: FR Payment date: 20110922 Year of fee payment: 14 Ref country code: GB Payment date: 20110914 Year of fee payment: 14 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20110916 Year of fee payment: 14 Ref country code: NL Payment date: 20110922 Year of fee payment: 14 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20111017 Year of fee payment: 14 Ref country code: BE Payment date: 20110913 Year of fee payment: 14 |
|
| BERE | Be: lapsed |
Owner name: ARKEMA FRANCE Effective date: 20120930 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: V1 Effective date: 20130401 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20120915 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20130531 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130403 Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120915 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120930 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20121001 Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120915 Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130401 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 69838632 Country of ref document: DE Effective date: 20130403 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20131018 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120916 |