EP0890882B1 - Toner für Elektrophotographie - Google Patents
Toner für Elektrophotographie Download PDFInfo
- Publication number
- EP0890882B1 EP0890882B1 EP19980112261 EP98112261A EP0890882B1 EP 0890882 B1 EP0890882 B1 EP 0890882B1 EP 19980112261 EP19980112261 EP 19980112261 EP 98112261 A EP98112261 A EP 98112261A EP 0890882 B1 EP0890882 B1 EP 0890882B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- group
- zirconium dichloride
- methyl
- dimethylsilylbis
- ethylenebis
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 239000001993 wax Substances 0.000 claims description 29
- 229910052739 hydrogen Inorganic materials 0.000 claims description 11
- 239000001257 hydrogen Substances 0.000 claims description 11
- VPGLGRNSAYHXPY-UHFFFAOYSA-L zirconium(2+);dichloride Chemical compound Cl[Zr]Cl VPGLGRNSAYHXPY-UHFFFAOYSA-L 0.000 claims description 11
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 10
- -1 dimethylsilylbis-1-tetrahydroindenylzirconium dichloride diphenylsilylbis-1-indenylzirconium dichloride Chemical compound 0.000 claims description 10
- 150000001875 compounds Chemical class 0.000 claims description 9
- 125000005843 halogen group Chemical group 0.000 claims description 7
- 239000000155 melt Substances 0.000 claims description 7
- 238000006116 polymerization reaction Methods 0.000 claims description 7
- 229910052801 chlorine Inorganic materials 0.000 claims description 5
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 5
- 238000004519 manufacturing process Methods 0.000 claims description 5
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 5
- 229910052710 silicon Inorganic materials 0.000 claims description 5
- 125000006273 (C1-C3) alkyl group Chemical group 0.000 claims description 4
- 125000006656 (C2-C4) alkenyl group Chemical group 0.000 claims description 4
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 claims description 4
- QCWXUUIWCKQGHC-UHFFFAOYSA-N Zirconium Chemical compound [Zr] QCWXUUIWCKQGHC-UHFFFAOYSA-N 0.000 claims description 4
- 125000003118 aryl group Chemical group 0.000 claims description 4
- 125000004432 carbon atom Chemical group C* 0.000 claims description 4
- 229910052732 germanium Inorganic materials 0.000 claims description 4
- GNPVGFCGXDBREM-UHFFFAOYSA-N germanium atom Chemical compound [Ge] GNPVGFCGXDBREM-UHFFFAOYSA-N 0.000 claims description 4
- 229910052735 hafnium Inorganic materials 0.000 claims description 4
- VBJZVLUMGGDVMO-UHFFFAOYSA-N hafnium atom Chemical compound [Hf] VBJZVLUMGGDVMO-UHFFFAOYSA-N 0.000 claims description 4
- 239000010703 silicon Substances 0.000 claims description 4
- 239000010936 titanium Substances 0.000 claims description 4
- 229910052719 titanium Inorganic materials 0.000 claims description 4
- 229910052726 zirconium Inorganic materials 0.000 claims description 4
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 claims description 3
- 125000000217 alkyl group Chemical group 0.000 claims description 3
- 239000000460 chlorine Substances 0.000 claims description 3
- 229910052731 fluorine Inorganic materials 0.000 claims description 3
- 239000011737 fluorine Substances 0.000 claims description 3
- 125000000538 pentafluorophenyl group Chemical group FC1=C(F)C(F)=C(*)C(F)=C1F 0.000 claims description 3
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical group [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 claims description 2
- KZBUYRJDOAKODT-UHFFFAOYSA-N Chlorine Chemical compound ClCl KZBUYRJDOAKODT-UHFFFAOYSA-N 0.000 claims description 2
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 claims description 2
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 claims description 2
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 claims description 2
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 claims description 2
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 claims description 2
- FJMJPZLXUXRLLD-UHFFFAOYSA-L [Cl-].[Cl-].C1=CC2=CC=CC=C2C1[Zr+2]([SiH](C)C)C1C2=CC=CC=C2C=C1 Chemical compound [Cl-].[Cl-].C1=CC2=CC=CC=C2C1[Zr+2]([SiH](C)C)C1C2=CC=CC=C2C=C1 FJMJPZLXUXRLLD-UHFFFAOYSA-L 0.000 claims description 2
- 125000004429 atom Chemical group 0.000 claims description 2
- 125000001309 chloro group Chemical group Cl* 0.000 claims description 2
- 229910052804 chromium Inorganic materials 0.000 claims description 2
- 239000011651 chromium Substances 0.000 claims description 2
- 229910052751 metal Inorganic materials 0.000 claims description 2
- 239000002184 metal Substances 0.000 claims description 2
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 claims description 2
- 229910052750 molybdenum Inorganic materials 0.000 claims description 2
- 239000011733 molybdenum Substances 0.000 claims description 2
- 229910052758 niobium Inorganic materials 0.000 claims description 2
- 239000010955 niobium Substances 0.000 claims description 2
- GUCVJGMIXFAOAE-UHFFFAOYSA-N niobium atom Chemical compound [Nb] GUCVJGMIXFAOAE-UHFFFAOYSA-N 0.000 claims description 2
- 230000000737 periodic effect Effects 0.000 claims description 2
- 229920005606 polypropylene copolymer Polymers 0.000 claims description 2
- 229920005629 polypropylene homopolymer Polymers 0.000 claims description 2
- 229910052715 tantalum Inorganic materials 0.000 claims description 2
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 claims description 2
- 229910052718 tin Inorganic materials 0.000 claims description 2
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 claims description 2
- 229910052721 tungsten Inorganic materials 0.000 claims description 2
- 239000010937 tungsten Substances 0.000 claims description 2
- 229910052720 vanadium Inorganic materials 0.000 claims description 2
- GPPXJZIENCGNKB-UHFFFAOYSA-N vanadium Chemical compound [V]#[V] GPPXJZIENCGNKB-UHFFFAOYSA-N 0.000 claims description 2
- 125000006274 (C1-C3)alkoxy group Chemical group 0.000 claims 2
- 125000000229 (C1-C4)alkoxy group Chemical group 0.000 claims 1
- 239000000126 substance Substances 0.000 claims 1
- 238000005227 gel permeation chromatography Methods 0.000 description 11
- QQONPFPTGQHPMA-UHFFFAOYSA-N Propene Chemical compound CC=C QQONPFPTGQHPMA-UHFFFAOYSA-N 0.000 description 9
- 239000004743 Polypropylene Substances 0.000 description 8
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 6
- 239000003054 catalyst Substances 0.000 description 5
- 238000005259 measurement Methods 0.000 description 5
- 229920000642 polymer Polymers 0.000 description 5
- 229920001155 polypropylene Polymers 0.000 description 5
- VXNZUUAINFGPBY-UHFFFAOYSA-N 1-Butene Chemical compound CCC=C VXNZUUAINFGPBY-UHFFFAOYSA-N 0.000 description 4
- 238000002845 discoloration Methods 0.000 description 4
- 150000003254 radicals Chemical class 0.000 description 4
- 230000000052 comparative effect Effects 0.000 description 3
- 229920001577 copolymer Polymers 0.000 description 3
- 238000000034 method Methods 0.000 description 3
- 125000004805 propylene group Chemical group [H]C([H])([H])C([H])([*:1])C([H])([H])[*:2] 0.000 description 3
- 229920006395 saturated elastomer Polymers 0.000 description 3
- LIKMAJRDDDTEIG-UHFFFAOYSA-N 1-hexene Chemical compound CCCCC=C LIKMAJRDDDTEIG-UHFFFAOYSA-N 0.000 description 2
- KWKAKUADMBZCLK-UHFFFAOYSA-N 1-octene Chemical compound CCCCCCC=C KWKAKUADMBZCLK-UHFFFAOYSA-N 0.000 description 2
- WSSSPWUEQFSQQG-UHFFFAOYSA-N 4-methyl-1-pentene Chemical compound CC(C)CC=C WSSSPWUEQFSQQG-UHFFFAOYSA-N 0.000 description 2
- VQTUBCCKSQIDNK-UHFFFAOYSA-N Isobutene Chemical compound CC(C)=C VQTUBCCKSQIDNK-UHFFFAOYSA-N 0.000 description 2
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 2
- 125000003710 aryl alkyl group Chemical group 0.000 description 2
- 238000001816 cooling Methods 0.000 description 2
- 238000000113 differential scanning calorimetry Methods 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 239000012968 metallocene catalyst Substances 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- YWAKXRMUMFPDSH-UHFFFAOYSA-N pentene Chemical compound CCCC=C YWAKXRMUMFPDSH-UHFFFAOYSA-N 0.000 description 2
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 2
- 229920000098 polyolefin Polymers 0.000 description 2
- 125000000008 (C1-C10) alkyl group Chemical group 0.000 description 1
- 238000001644 13C nuclear magnetic resonance spectroscopy Methods 0.000 description 1
- YBYIRNPNPLQARY-UHFFFAOYSA-N 1H-indene Natural products C1=CC=C2CC=CC2=C1 YBYIRNPNPLQARY-UHFFFAOYSA-N 0.000 description 1
- YHQXBTXEYZIYOV-UHFFFAOYSA-N 3-methylbut-1-ene Chemical compound CC(C)C=C YHQXBTXEYZIYOV-UHFFFAOYSA-N 0.000 description 1
- LDTAOIUHUHHCMU-UHFFFAOYSA-N 3-methylpent-1-ene Chemical compound CCC(C)C=C LDTAOIUHUHHCMU-UHFFFAOYSA-N 0.000 description 1
- UQRONKZLYKUEMO-UHFFFAOYSA-N 4-methyl-1-(2,4,6-trimethylphenyl)pent-4-en-2-one Chemical group CC(=C)CC(=O)Cc1c(C)cc(C)cc1C UQRONKZLYKUEMO-UHFFFAOYSA-N 0.000 description 1
- SUWJESCICIOQHO-UHFFFAOYSA-N 4-methylhex-1-ene Chemical compound CCC(C)CC=C SUWJESCICIOQHO-UHFFFAOYSA-N 0.000 description 1
- 0 CC(C(*)C(*)C(*)C1*)C1*(C)(*)C(C(*)C(C)C(*)C(*)C1C)C1(C)*(C)(C)C Chemical compound CC(C(*)C(*)C(*)C1*)C1*(C)(*)C(C(*)C(C)C(*)C(*)C1C)C1(C)*(C)(C)C 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 1
- 239000005977 Ethylene Substances 0.000 description 1
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical group [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 1
- 238000004566 IR spectroscopy Methods 0.000 description 1
- 101001082628 Mus musculus H-2 class II histocompatibility antigen gamma chain Proteins 0.000 description 1
- 150000001336 alkenes Chemical class 0.000 description 1
- 125000002877 alkyl aryl group Chemical group 0.000 description 1
- 229940100198 alkylating agent Drugs 0.000 description 1
- 239000002168 alkylating agent Substances 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 239000013522 chelant Substances 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 238000004140 cleaning Methods 0.000 description 1
- 238000007334 copolymerization reaction Methods 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 125000000058 cyclopentadienyl group Chemical group C1(=CC=CC1)* 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 238000001212 derivatisation Methods 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- ZSWFCLXCOIISFI-UHFFFAOYSA-N endo-cyclopentadiene Natural products C1C=CC=C1 ZSWFCLXCOIISFI-UHFFFAOYSA-N 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 125000003983 fluorenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3CC12)* 0.000 description 1
- 125000003709 fluoroalkyl group Chemical group 0.000 description 1
- 125000004407 fluoroaryl group Chemical group 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 238000004817 gas chromatography Methods 0.000 description 1
- 125000001188 haloalkyl group Chemical group 0.000 description 1
- 229910052736 halogen Inorganic materials 0.000 description 1
- 150000002367 halogens Chemical class 0.000 description 1
- GJTGYNPBJNRYKI-UHFFFAOYSA-N hex-1-ene;prop-1-ene Chemical compound CC=C.CCCCC=C GJTGYNPBJNRYKI-UHFFFAOYSA-N 0.000 description 1
- 150000002431 hydrogen Chemical class 0.000 description 1
- 230000004941 influx Effects 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 125000000555 isopropenyl group Chemical group [H]\C([H])=C(\*)C([H])([H])[H] 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- CPOFMOWDMVWCLF-UHFFFAOYSA-N methyl(oxo)alumane Chemical compound C[Al]=O CPOFMOWDMVWCLF-UHFFFAOYSA-N 0.000 description 1
- DVSDBMFJEQPWNO-UHFFFAOYSA-N methyllithium Chemical compound C[Li] DVSDBMFJEQPWNO-UHFFFAOYSA-N 0.000 description 1
- TVMXDCGIABBOFY-UHFFFAOYSA-N n-Octanol Natural products CCCCCCCC TVMXDCGIABBOFY-UHFFFAOYSA-N 0.000 description 1
- 125000002524 organometallic group Chemical group 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 239000002574 poison Substances 0.000 description 1
- 231100000614 poison Toxicity 0.000 description 1
- 230000037048 polymerization activity Effects 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 229920001897 terpolymer Polymers 0.000 description 1
- 125000003944 tolyl group Chemical group 0.000 description 1
- SQBBHCOIQXKPHL-UHFFFAOYSA-N tributylalumane Chemical compound CCCC[Al](CCCC)CCCC SQBBHCOIQXKPHL-UHFFFAOYSA-N 0.000 description 1
- VOITXYVAKOUIBA-UHFFFAOYSA-N triethylaluminium Chemical compound CC[Al](CC)CC VOITXYVAKOUIBA-UHFFFAOYSA-N 0.000 description 1
- MCULRUJILOGHCJ-UHFFFAOYSA-N triisobutylaluminium Chemical compound CC(C)C[Al](CC(C)C)CC(C)C MCULRUJILOGHCJ-UHFFFAOYSA-N 0.000 description 1
- JLTRXTDYQLMHGR-UHFFFAOYSA-N trimethylaluminium Chemical compound C[Al](C)C JLTRXTDYQLMHGR-UHFFFAOYSA-N 0.000 description 1
- 125000005023 xylyl group Chemical group 0.000 description 1
Classifications
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03G—ELECTROGRAPHY; ELECTROPHOTOGRAPHY; MAGNETOGRAPHY
- G03G9/00—Developers
- G03G9/08—Developers with toner particles
- G03G9/087—Binders for toner particles
- G03G9/08775—Natural macromolecular compounds or derivatives thereof
- G03G9/08782—Waxes
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03G—ELECTROGRAPHY; ELECTROPHOTOGRAPHY; MAGNETOGRAPHY
- G03G9/00—Developers
- G03G9/08—Developers with toner particles
- G03G9/087—Binders for toner particles
- G03G9/08702—Binders for toner particles comprising macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
- G03G9/08704—Polyalkenes
Definitions
- Waxes from thermal degradation of polypropylene plastic are hard waxes with a narrow molecular weight distribution.
- the type of production results partially colored products which have unsaturated end groups.
- Another option is the extreme cleaning of the waxes to remove all from the polymerization process derived residues. Often this is done Purification via a solution and filtration process or via a Fine distillation.
- the task was therefore to easily accessible temperature-stable waxes which are in an improved way for use as a component in toners can be used.
- waxes from the polymerization of propene together with Comonomers using metallocene catalysts in additional Use of hydrogen significantly improved temperature stability which are suitable for the use of these polypropylenes for the production of Toner is beneficial.
- the invention thus relates to the use of polypropylene homopolymer and copolymer waxes according to claim 1.
- Such PP waxes are therefore particularly suitable for use as a component in black and color toners in photocopiers and laser printers.
- Another advantage of using such polypropylene waxes is the Prevention of gel-like crosslinks, which in the further application to Inhomogeneities in the toner lead.
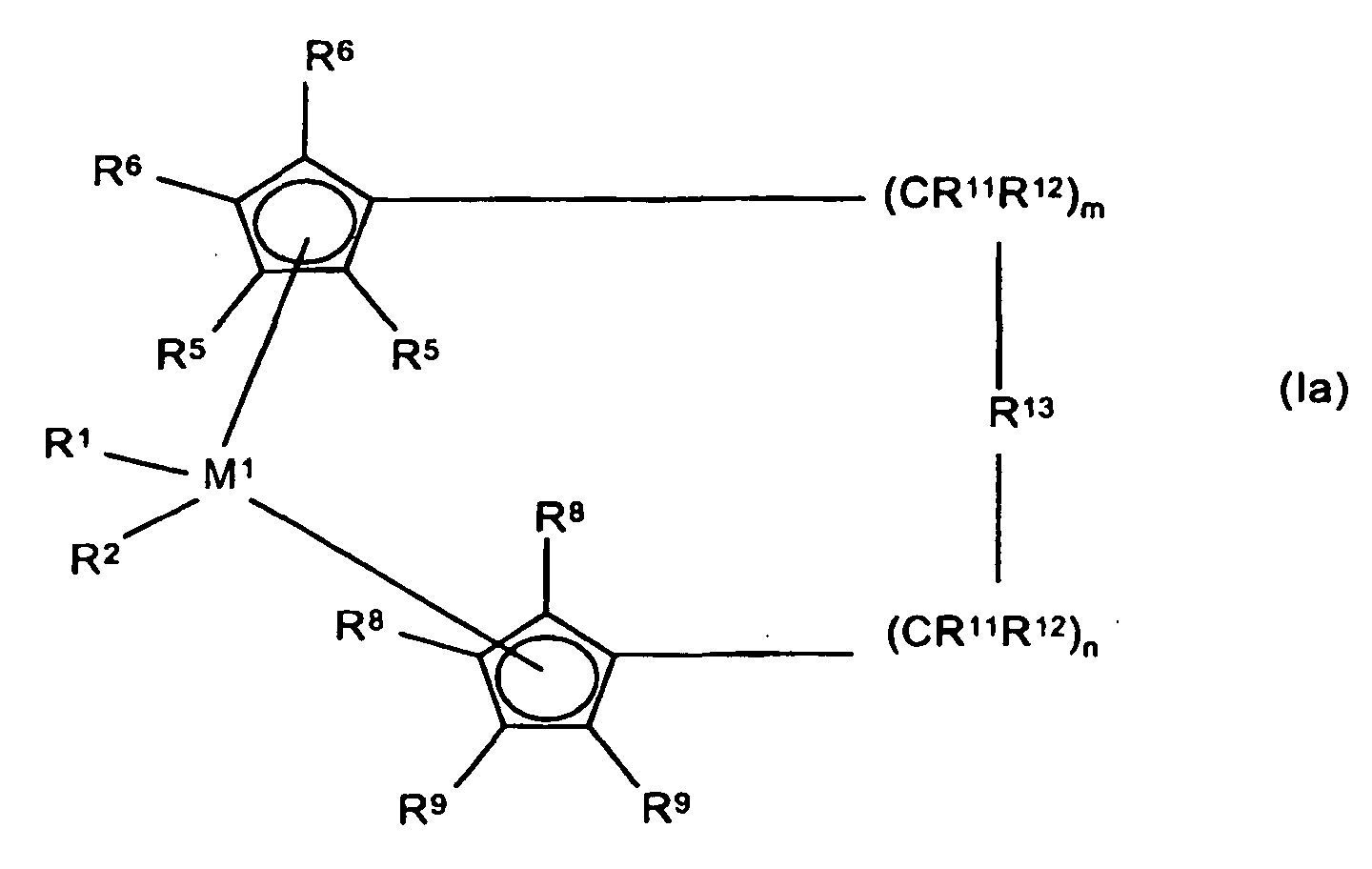
- the polyolefin waxes are formed by the use of sandwich chelate compounds, the metallocene compounds of formula Ia.
- M 1 is a metal of group IVb, Vb or VIb of the Periodic Table, for example titanium, zirconium, hafnium, vanadium, niobium, tantalum, chromium, molybdenum, tungsten, preferably titanium, zirconium and hafnium.
- R 1 and R 2 are identical or different and denote a hydrogen atom, a C 1 -C 10 -, preferably C 1 -C 3 -alkyl group, in particular methyl, a C 1 -C 10 -, preferably C 1 -C 3 -alkoxy group , a C 6 -C 10 -, preferably C 6 -C 8 -aryl group, a C 6 -C 10 -, preferably C 6 -C 8 -aryloxy group, a C 2 -C 10 -, preferably C 2 -C 4 - Alkenyl group, a C 7 -C 40 , preferably C 7 -C 10 arylalkyl group, a C 7 -C 40 , preferably C 7 -C 12 alkylaryl group, a C 8 -C 40 -, preferably C 8 -C 12 -Arylalkenyl distr or a halogen atom, preferably chlorine.
- R 5 , R 6 , R 8 and R 9 are identical or different and denote a hydrogen atom, a halogen atom, preferably a fluorine, chlorine or bromine atom, a C 1 -C 10 -, preferably C 1 -C 4 -alkyl group, a C 6 -C 10 , preferably C 6 -C 8, aryl group, a C 1 -C 10 , preferably C 1 -C 3 alkoxy group, an -NR 16 2 -, -SR 16 -, -OSiR 16 3 , -SiR 16 3 - or -PR 16 2 radical, in which R 16 is a C 1 -C 10 -, preferably C 1 -C 3 -alkyl group or a C 6 -C 10 -, preferably C 6 -C 8 - Aryl group, or in the case of Si or P-containing radicals is also a halogen atom, preferably chlorine atom, or two adjacent radicals R 5
- M 2 is silicon, germanium or tin, preferably silicon and germanium.
- R 11 and R 12 are the same or different and have the meaning given for R 17 .
- m and n are the same or different and are zero, 1 or 2, preferably zero or 1, where m plus n is zero, 1 or 2, preferably zero or 1.
- R 15 has the meaning of R 17 and R 18 .
- Suitable cocatalysts for metallocenes of the formula I are organoaluminum compounds, in particular alumoxanes or else aluminum-free systems such as R 22 ⁇ NH 4 -x BR 23 4 , R 22 ⁇ PH 4-x BR 23 4 , R 22 3 CBR 23 4 or BR 23 3 ,
- x is a number from 1 to 4
- the radicals R 22 are identical or different, preferably identical, and are C 1 -C 10 -alkyl or C 6 -C 18 -aryl or two radicals R 22 form together with the they join a ring
- R 23 are the same or different, preferably the same, and are C 6 -C 18 -aryl which may be substituted by alkyl, haloalkyl or fluorine.
- R 22 is ethyl, propyl, butyl or phenyl and R 23 is phenyl, pentafluorophenyl, 3,5-bistrifluoromethylphenyl, mesityl, xylyl or tolyl.
- cocatalysts are particularly suitable in combination with metallocenes of the formula I when R 1 and R 2 are a C 1 -C 10 -alkyl group or an aryl or benzyl group, preferably methyl group.
- Derivatization to the metallocenes of the formula I can be carried out by literature methods, for example by reaction with alkylating agents such as methyllithium (see Organometallics 9 (1990) 1539, J. Am. Chem. Soc., 95 (1973) 6263).
- organoaluminum Compounds such as e.g. Triethylaluminum, tributylaluminum and others as well Mixtures thereof suitable.
- supported single-center catalysts may also be used Use come. Preference is given to catalyst systems in which by a high polymerization activity, the residual contents of support material and cocatalyst do not exceed a concentration of 100 ppm in the product.
- Propene is prepared in the presence of Hydrogen and optionally further olefins having 2 to 18 carbon atoms as comonomers polymerized.
- useful comonomers are ethylene, 1-butene, 1-pentene, 1-hexene, 1-octene, 2-methyl-1-propene, 3-methyl-1-butene, 3-methyl-1-pentene, 4-methyl-1-pentene, 4-methyl-1-hexene, styrene.
- copolymer waxes are Propylene / ethylene, propylene / 1-butene and propylene / 1-hexene waxes.
- Copolymer waxes contain 0 to 20 wt .-% of the comonomer based on the Total polymer.
- Terpolymer waxes contain at least 80% by weight of the Main polymer, wherein the two comonomers each up to 19 wt .-%, in Sum of both comonomers, however, a maximum of 20 wt .-% of the total polymer are included.
- the waxes of the invention contain less than 10% of unsaturated End groups, preferably less than 5% unsaturated end groups.
- the waxes of the invention may be in any suitable type of reactor be prepared, such as a loop reactor, autoclave or gas phase.
- the regulation the molecular weight is preferably not on the variation of the Polymerization temperature, but at a constant temperature by changing the hydrogen pressure.
- the polypropylene homo- and copolymer waxes have a viscosity of 10 to 100,000 mPas at 170 ° C., the molecular weight distribution is narrow and is M w / M n ⁇ 5, preferably less than 3.
- Example 1 was repeated, but no hydrogen metered.
- the properties are summarized in Table 1.
- the GPC measurement gives an M w of 12640 and M w / M n equal to 2.5.
- Example 1 was carried out with 26 mg of dimethylsilylbisindenylzirconium dichloride using 230 mbar of hydrogen. This results in 9.9 kg of PP wax.
- the GPC measurement gives an M w of 6920 and M w / M n equal to 2.9.
- the properties are summarized in Table 1.
- Example 1 was repeated, but metered instead of hydrogen 200 mmol of trimethylaluminum as a solution in toluene.
- the properties are summarized in Table 1.
- the GPC measurement gives an M w of 24300 and M w / M n equal to 2.8.
Landscapes
- Physics & Mathematics (AREA)
- Spectroscopy & Molecular Physics (AREA)
- General Physics & Mathematics (AREA)
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
- Transition And Organic Metals Composition Catalysts For Addition Polymerization (AREA)
- Developing Agents For Electrophotography (AREA)
Description
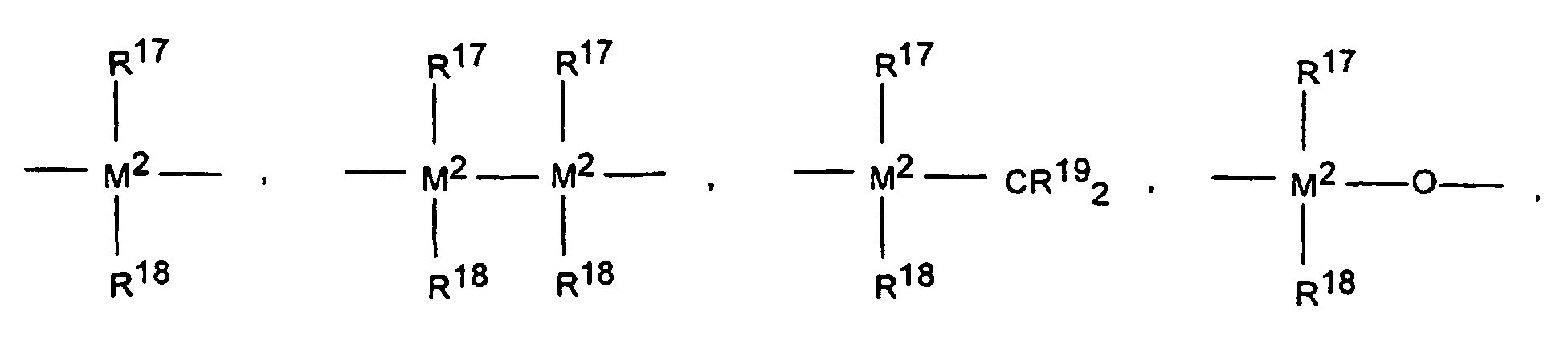
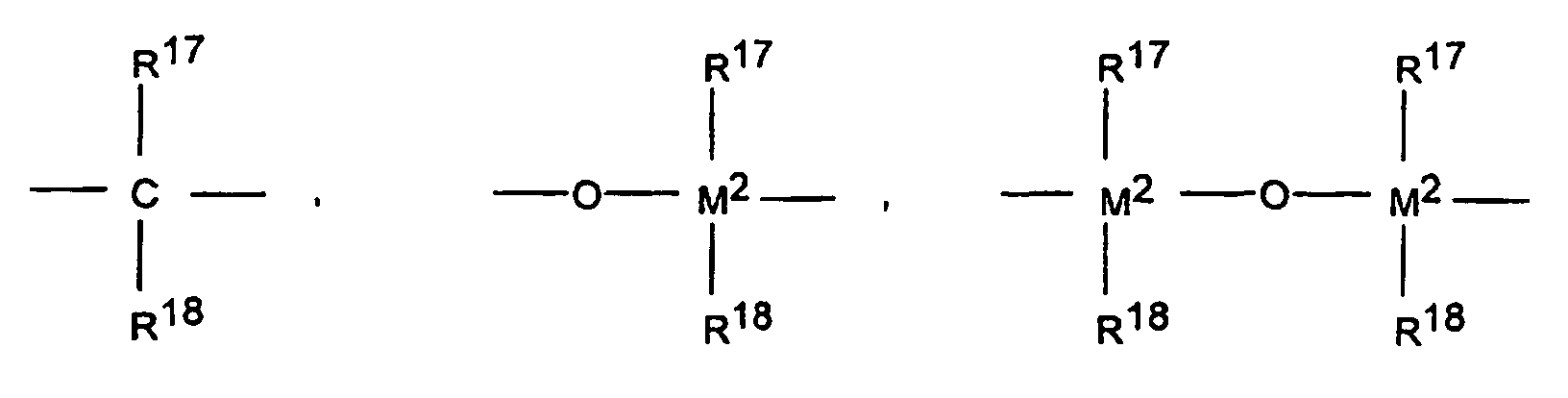
R13 ist vorzugsweise =CR17R18, =SiR17R18, =GeR17R18, -O-, -S-, =SO, =PR17 oder =P(O)R17.
- DSC =
- Differential Scanning Calorimetry
- GPC =
- Gel-Permeations-Chromatographie
- GC =
- Gaschromatographie
- Mw =
- Molmassengewichtsmittel in g/mol nach GPC
- Mn =
- Molmassenzahlenmittel in g/mol nach GPC
- Mw/Mn =
- Polydispersität
Die Schmelzviskositäten (SV) sind bei 170°C mit einem Rotationsviskosimeter bestimmt worden.
Die Bestimmung des Isotaktischen Index (I.I.) erfolgt durch IR-Spektroskopie nach J.P. Luongo, J. Appl. Polym. Chem., 3, 302 (1960).
Die Untersuchung der Kettenenden der Polymere erfolgt über 13C-NMR wie in Polymer, 1989, Vol. 30, S. 428 beschrieben. Sofern weniger als 10 % aller Endgruppen als Isopropenyl-Endgruppen vorliegen, findet sich in Tabelle 1 die Angabe "gesättigt".
Parallel hierzu werden 31 mg Ethylen-bis-1,1'-(tetrahydroindenyl)-zirkondichlorid in 15 ml 10 Gew.-% toluolischer Lösung von Methylaluminoxan gelöst und gerührt. Die Polymerisation wird durch portionsweise Zugabe der Katalysator-Lösung gestartet und die Innentemperatur des Reaktors auf 70°C geregelt. Wasserstoff wurde gemäß GC-Kontrolle nachdosiert und auf den Anfangswert konstant gehalten. Nach 1 Stunde wurde die Polymerisation durch Zugabe von CO2 gestoppt, der Reaktor entspannt und das Produkt als Schmelze abgelassen. Es resultieren 12,1 kg PP-Wachs. Die GPC-Messung ergibt ein Mw von 3528 und Mw/Mn gleich 2,1. Die Eigenschaften sind in Tabelle 1 zusammengefaßt.
| Bsp. | SV (170°C) | Smp [°C] | ΔH [J/g] | I.I. | Kettenende | Farbnote |
| 1 | 30 | 122 | 91 | 86 | gesättigt | 1-2 |
| V1 | 443 | 121 | 94 | 83,7 | ungesättigt | 5-6 |
| 2 | 760 | 134 | 91 | 84 | gesättigt | 1 |
| V2 | 3540 | 133 | 90 | 79 | ungesättigt | 5-6 |
Claims (2)
- Verwendung von Polypropylen-Homopolymer und -Copolymer Wachsen, welche weniger als 10 % ungesättigte Kettenenden und eine Schmelzviskosität von 10 bis 100 000 mPa·s gemessen bei 170°C besitzen, hergestellt durch Polymerisation unter Verwendung von Metallocen-Verbindungen in Gegenwart von Wasserstoff, als Komponente für die Herstellung von Tonern, dadurch gekennzeichnet, dass als Metallocen-Verbindungen rac-Isomere mit der allgemeinen chemischen Formel I a eingesetzt werden, in der M1 ein Metall der Gruppe IVb, Vb oder VIb des Periodensystems bedeutet, insbesondere Titan, Zirkon, Hafnium, Vanadium, Niob, Tantal, Chrom, Molybdän, Wolfram, vorzugsweise Titan, Zirkon und Hafnium;
in der R1 und R2 gleiche oder verschiedene Bedeutung haben können und ein Wasserstoffatom, eine C1-C10-, vorzugsweise C1-C3-Alkylgruppe, insbesondere Methyl, eine C1- C10-, vorzugsweise C1-C3-Alkoxygruppe, eine C6-C10-, vorzugsweise C6-C8-Arylgruppe, eine C6-C10-, vorzugsweise C6-C8-Aryloxygruppe, eine C2-C10-, vorzugsweise C2-C4-Alkenylgruppe, eine C7-C40-, vorzugsweise C7-C10-Arylalkylgruppe, eine C7-C40-, vorzugsweise C7-C12-Alkylarylgruppe, eine C8-C40-, vorzugsweise C8-C12-Arylalkenylgruppe oder ein Halogenatom sein können;
in der R5, R6, R8, R9 gleiche oder verschiedene Bedeutung haben können und ein Wasserstoffatom, ein Halogenatom, vorzugsweise ein Fluor-, Chlor- oder Bromatom, eine C1-C10-, vorzugsweise C1-C4-Alkylgruppe, eine C6-C10-, vorzugsweise C6-C8-Arylgruppe, eine C1-C10-, vorzugsweise C1-C3-Alkoxygruppe, einen -NR16 2-, -SR16-, -OSiR16 3-, -SiR16 3- oder -PR16 2-Rest, worin R16 eine C1-C10-, vorzugsweise C1-C3-Alkylgruppe oder eine C6-C10-, vorzugsweise C6-C8-Arylgruppe, oder im Falle Si oder P enthaltender Reste auch ein Halogenatom, vorzugsweise Chloratom, sein können, oder je zwei benachbarte Reste R5, R6, R8, R9 mit den sie verbindenden C-Atomen einen Ring bilden können;
in der R13 ein Rest mit nachfolgender Bedeutung ist: =BR17, =AlR17, -Ge-, -Sn-, -O-, -S-, =SO, =SO2, =NR15, =CO, =PR15 oder =P(O)R15, wobei R17, R18 und R19 gleich oder verschieden sein können und ein Wasserstoffatom, ein Halogenatom, eine C1-C30-, vorzugsweise C1-C4-Alkylgruppe, insbesondere eine Methylgruppe, eine C1-C10-Fluoralkylgruppe, vorzugsweise CF3-Gruppe, eine C6-C10-Fluorarylgruppe, vorzugsweise eine Pentafluorphenylgruppe, eine C6-C10-, vorzugsweise C6-C8-Arylgruppe, eine C1-C10, vorzugsweise C1-C4-Alkoxygruppe, insbesondere eine Methoxygruppe, eine C2-C10-, vorzugsweise C2-C4-Alkenylgruppe, eine C7-C40-, vorzugsweise C7-C10-Arylalkylgruppe, eine C8-C40-, vorzugsweise C8-C12-Arylalkenylgruppe oder eine C7-C40-, vorzugsweise C7-C12-Alkylarylgruppe bedeuten, oder in der R17 und R18 oder R17 und R19 jeweils zusammen mit den sie verbindenden Atomen einen Ring bilden;
in der M2 Silizium, Germanium oder Zinn, bevorzugt Silizium und Germanium ist;
in der R11 und R12 gleiche oder verschiedene Bedeutung haben können und die für R17 genannte Bedeutung besitzen, m und n gleich oder verschieden sein können und null, 1 oder 2 sind;
und in der R15 die Bedeutung von R17 und R18 hat. - Verwendung gemäß Anspruch 1, dadurch gekennzeichnet, dass es sich bei den Metallocen-Verbindungen um rac-Isomere folgender Verbindungen handelt:Dimethylsilyl-bis-1-(2,3,5-trimethylcyclopentadienyl)zirkoniumdichlorid,Dimethylsilyl-bis-1-(2,4-dimethyl-cyclopentadienyl)zirkoniumdichlorid,Dimethylsilyl-bis-1-(2-methyl-4,5-benzoindenyl)-zirkoniumdichlorid,Dimethylsilyl-bis-1-(2-methyl-4-ethylindenyl)-zirkoniumdichlorid,Dimethylsilyl-bis-1-(2-methyl-4-i-propylindenyl)-zirkoniumdichlorid,Dimethylsilyl-bis-1-(2-methyl-4-phenylindenyl)-zirkoniumdichlorid,Dimethylsilyl-bis-1-(2-methyl-indenyl)-zirkoniumdichlorid,Dimethylsilyl-bis-1-(2-methyltetrahydroindenyl)-zirkoniumdichlorid,Dimethylsilyl-bis-1-indenylzirkoniumdichlorid,Dimethylsilyl-bis-1-indenylzirkoniumdimethyl,Dimethylsilyl-bis-1-tetrahydroindenylzirkoniumdichlorid,Diphenylsilyl-bis-1-indenylzirkoniumdichlorid,Ethylen-bis-1-(2-methyl-4,5-benzoindenyl)-zirkoniumdichlorid,Ethylen-bis-1-(2-methyl-4-phenylindenyl)-zirkoniumdichlorid,Ethylen-bis-1-(4,7-dimethyl-indenyl)-zirkoniumdichlorid,Ethylen-bis-1-indenylzirkoniumdichlorid,Ethylen-bis-1-tetrahydroindenylzirkoniumdichlorid,Ethylen-bis-1-(2-methyl-tetrahydroindenyl)-zirkoniumdichlorid,Ethylen-bis-1-(2-methyl-4,5-benzo-6,7-dihydroindenyl)-zirkoniumdichlorid,Ethylen-bis-1-(2-methyl-indenyl)-zirkoniumdichlorid,sowie jeweils die Alkyl- oder Aryl-Derivate dieser Metallocendichloride.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE1997129834 DE19729834A1 (de) | 1997-07-11 | 1997-07-11 | Toner für Elektrophotographie |
| DE19729834 | 1997-07-11 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0890882A1 EP0890882A1 (de) | 1999-01-13 |
| EP0890882B1 true EP0890882B1 (de) | 2005-11-23 |
Family
ID=7835450
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP19980112261 Expired - Lifetime EP0890882B1 (de) | 1997-07-11 | 1998-07-02 | Toner für Elektrophotographie |
Country Status (4)
| Country | Link |
|---|---|
| EP (1) | EP0890882B1 (de) |
| JP (1) | JPH1184718A (de) |
| DE (2) | DE19729834A1 (de) |
| ES (1) | ES2252806T3 (de) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE10159955A1 (de) | 2001-12-06 | 2003-06-26 | Clariant Gmbh | Verwendung von polar modifizierten Polyolefinwachsen in Fototonern |
| CN104204002B (zh) | 2012-03-28 | 2016-08-17 | 三井化学株式会社 | 丙烯·α-烯烃共聚物及其用途 |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0563834B1 (de) * | 1992-04-01 | 1995-10-11 | MITSUI TOATSU CHEMICALS, Inc. | Syndiotaktischen Polypropylen, Wachs, Verfahren zur Herstellung derselbe und ihre Anwendung in eine Wärmefixiertonerzusammensetzung für geheizte Walze |
| JP3467659B2 (ja) * | 1995-03-07 | 2003-11-17 | コニカミノルタホールディングス株式会社 | トナー及びこのトナーの定着方法 |
| JP2806368B2 (ja) * | 1995-06-26 | 1998-09-30 | 富士ゼロックス株式会社 | 画像形成方法 |
-
1997
- 1997-07-11 DE DE1997129834 patent/DE19729834A1/de not_active Withdrawn
-
1998
- 1998-07-02 ES ES98112261T patent/ES2252806T3/es not_active Expired - Lifetime
- 1998-07-02 EP EP19980112261 patent/EP0890882B1/de not_active Expired - Lifetime
- 1998-07-02 DE DE59813209T patent/DE59813209D1/de not_active Expired - Fee Related
- 1998-07-10 JP JP19627198A patent/JPH1184718A/ja not_active Ceased
Also Published As
| Publication number | Publication date |
|---|---|
| DE19729834A1 (de) | 1999-01-14 |
| EP0890882A1 (de) | 1999-01-13 |
| ES2252806T3 (es) | 2006-05-16 |
| JPH1184718A (ja) | 1999-03-30 |
| DE59813209D1 (de) | 2005-12-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0890584B1 (de) | Polypropylenwachs | |
| AU700256B2 (en) | Metallocene compounds, process for their prepration, and their use in catalysts for the polymerization of olefins | |
| US5296434A (en) | Soluble catalyst systems for the preparation of polyalk-1-enes having high molecular weights | |
| DE69315187T2 (de) | Ataktisches Polypropylen | |
| US5331054A (en) | Propylene copolymer composition | |
| CA1264399A (en) | Process for the preparation of polyolefins | |
| EP0943631B1 (de) | Polymere des Propens | |
| DE69823031T3 (de) | Olefin polymerisationsverfahren | |
| DE3879806T2 (de) | Polymerisierung von Äthylen. | |
| DE68915616T2 (de) | Verfahren zur Herstellung von Ethylen-alpha-Olefin-Elastomeren mit hohem Molekulargewicht unter Anwendung eines Metallocen-Alumoxan-Katalysators. | |
| US6177377B1 (en) | Polymer blends and process for preparation | |
| DE69629227T2 (de) | Verfahren zur Herstellung ataktischer Copolymeren aus Propylene mit Ethylen | |
| DE69021991T2 (de) | Verfahren zur Herstellung von Polyolefinpulver mit kontrollierter Morphologie unter Anwendung eines metallocenhaltigen Katalytorsystems. | |
| US20050203255A1 (en) | Low molecular weight polypropylene derivatives | |
| DE3788652T2 (de) | Hochdruck- und hochtemperatur-olefinpolymerisationsverfahren. | |
| EP0625995B1 (de) | Katalysatorsysteme zur polymerisation von c 2- bis c 10-alkenen | |
| AU774353B2 (en) | High-molecular polypropylene with a broad distribution of the molecular weight and a short isotactic sequence length | |
| EP0890882B1 (de) | Toner für Elektrophotographie | |
| JP3210039B2 (ja) | プロピレン共重合体組成物 | |
| JP2005206625A (ja) | 安定化されたポリマーの製造方法 | |
| Minami et al. | Development of low isotactic polyolefin | |
| Hungenberg et al. | α‐olefin oligomers and polymers with metallocene catalysts | |
| DE68915456T2 (de) | Katalysator und verfahren zur polymerisierung von olefinen. | |
| KR100242209B1 (ko) | 올레핀 중합용 지글러-나타와 메탈로센의 공담지촉매 조성물을 이용한 폴리프로필렌의 제조방법 | |
| KR19990039714A (ko) | 폴리프로필렌의 제조방법 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): DE ES FR GB IT NL |
|
| AX | Request for extension of the european patent |
Free format text: AL;LT;LV;MK;RO;SI |
|
| 17P | Request for examination filed |
Effective date: 19990713 |
|
| AKX | Designation fees paid |
Free format text: DE ES FR GB IT NL |
|
| 17Q | First examination report despatched |
Effective date: 20030424 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): DE ES FR GB IT NL |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REF | Corresponds to: |
Ref document number: 59813209 Country of ref document: DE Date of ref document: 20051229 Kind code of ref document: P |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 20060222 |
|
| RAP2 | Party data changed (patent owner data changed or rights of a patent transferred) |
Owner name: CLARIANT PRODUKTE (DEUTSCHLAND) GMBH |
|
| NLT2 | Nl: modifications (of names), taken from the european patent patent bulletin |
Owner name: CLARIANT PRODUKTE (DEUTSCHLAND) GMBH Effective date: 20060315 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2252806 Country of ref document: ES Kind code of ref document: T3 |
|
| ET | Fr: translation filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20060824 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: CD |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20080625 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20080709 Year of fee payment: 11 Ref country code: DE Payment date: 20080620 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20080714 Year of fee payment: 11 Ref country code: FR Payment date: 20080612 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20080630 Year of fee payment: 11 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20090702 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee |
Effective date: 20100201 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20100331 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090731 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090702 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20100202 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20090703 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090703 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090702 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20100201 |