EP0353540B2 - Fireproof doors insert with silica sol - Google Patents
Fireproof doors insert with silica sol Download PDFInfo
- Publication number
- EP0353540B2 EP0353540B2 EP89113178A EP89113178A EP0353540B2 EP 0353540 B2 EP0353540 B2 EP 0353540B2 EP 89113178 A EP89113178 A EP 89113178A EP 89113178 A EP89113178 A EP 89113178A EP 0353540 B2 EP0353540 B2 EP 0353540B2
- Authority
- EP
- European Patent Office
- Prior art keywords
- layer
- wall element
- hydroxide
- element according
- fire
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- RMAQACBXLXPBSY-UHFFFAOYSA-N silicic acid Chemical compound O[Si](O)(O)O RMAQACBXLXPBSY-UHFFFAOYSA-N 0.000 title claims abstract description 27
- XLYOFNOQVPJJNP-UHFFFAOYSA-M hydroxide Chemical compound [OH-] XLYOFNOQVPJJNP-UHFFFAOYSA-M 0.000 claims abstract description 29
- 239000011230 binding agent Substances 0.000 claims abstract description 24
- 239000000203 mixture Substances 0.000 claims abstract description 21
- 239000011490 mineral wool Substances 0.000 claims abstract description 15
- NTHWMYGWWRZVTN-UHFFFAOYSA-N sodium silicate Chemical compound [Na+].[Na+].[O-][Si]([O-])=O NTHWMYGWWRZVTN-UHFFFAOYSA-N 0.000 claims abstract description 14
- 235000019353 potassium silicate Nutrition 0.000 claims abstract description 10
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 32
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical group [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 claims description 23
- 238000000034 method Methods 0.000 claims description 13
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 claims description 12
- 239000000463 material Substances 0.000 claims description 11
- 229920001282 polysaccharide Polymers 0.000 claims description 11
- 239000005017 polysaccharide Substances 0.000 claims description 11
- 230000008569 process Effects 0.000 claims description 10
- 150000004676 glycans Chemical class 0.000 claims description 9
- 238000012856 packing Methods 0.000 claims description 8
- 239000000126 substance Substances 0.000 claims description 8
- 238000001035 drying Methods 0.000 claims description 7
- 239000002245 particle Substances 0.000 claims description 7
- 238000004519 manufacturing process Methods 0.000 claims description 6
- 239000000377 silicon dioxide Substances 0.000 claims description 6
- 230000009970 fire resistant effect Effects 0.000 claims description 5
- 239000007787 solid Substances 0.000 claims description 5
- 239000003365 glass fiber Substances 0.000 claims description 4
- 229910010272 inorganic material Inorganic materials 0.000 claims description 4
- 239000011147 inorganic material Substances 0.000 claims description 4
- 239000004033 plastic Substances 0.000 claims description 4
- 239000005995 Aluminium silicate Substances 0.000 claims description 3
- 235000012211 aluminium silicate Nutrition 0.000 claims description 3
- 229920003086 cellulose ether Polymers 0.000 claims description 3
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 claims description 3
- 239000004927 clay Substances 0.000 claims description 2
- 239000002734 clay mineral Substances 0.000 claims description 2
- 239000000470 constituent Substances 0.000 claims description 2
- 229910001679 gibbsite Inorganic materials 0.000 claims description 2
- 229920003002 synthetic resin Polymers 0.000 claims description 2
- 239000000057 synthetic resin Substances 0.000 claims description 2
- -1 polysaccharide ethers Chemical class 0.000 claims 2
- 229910021502 aluminium hydroxide Inorganic materials 0.000 claims 1
- 229910052681 coesite Inorganic materials 0.000 claims 1
- 238000010924 continuous production Methods 0.000 claims 1
- 229910052906 cristobalite Inorganic materials 0.000 claims 1
- 229910052500 inorganic mineral Inorganic materials 0.000 claims 1
- 238000003780 insertion Methods 0.000 claims 1
- 230000037431 insertion Effects 0.000 claims 1
- 239000011707 mineral Substances 0.000 claims 1
- 229920005989 resin Polymers 0.000 claims 1
- 239000011347 resin Substances 0.000 claims 1
- 230000000979 retarding effect Effects 0.000 claims 1
- 229910052682 stishovite Inorganic materials 0.000 claims 1
- 229910052905 tridymite Inorganic materials 0.000 claims 1
- 230000007704 transition Effects 0.000 abstract description 3
- 238000009413 insulation Methods 0.000 description 31
- 230000003578 releasing effect Effects 0.000 description 15
- 238000006243 chemical reaction Methods 0.000 description 6
- 230000000694 effects Effects 0.000 description 6
- 150000004679 hydroxides Chemical class 0.000 description 6
- 230000008901 benefit Effects 0.000 description 5
- YLGXILFCIXHCMC-JHGZEJCSSA-N methyl cellulose Chemical compound COC1C(OC)C(OC)C(COC)O[C@H]1O[C@H]1C(OC)C(OC)C(OC)OC1COC YLGXILFCIXHCMC-JHGZEJCSSA-N 0.000 description 5
- CPLXHLVBOLITMK-UHFFFAOYSA-N Magnesium oxide Chemical compound [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 4
- 239000010440 gypsum Substances 0.000 description 4
- 229910052602 gypsum Inorganic materials 0.000 description 4
- XEEYBQQBJWHFJM-UHFFFAOYSA-N iron Substances [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 4
- 239000000155 melt Substances 0.000 description 4
- 239000004115 Sodium Silicate Substances 0.000 description 3
- 229910000831 Steel Inorganic materials 0.000 description 3
- 239000003513 alkali Substances 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 239000011575 calcium Substances 0.000 description 3
- 239000008187 granular material Substances 0.000 description 3
- 239000011777 magnesium Substances 0.000 description 3
- 235000019795 sodium metasilicate Nutrition 0.000 description 3
- 229910052911 sodium silicate Inorganic materials 0.000 description 3
- 239000010959 steel Substances 0.000 description 3
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- 229910004298 SiO 2 Inorganic materials 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 239000000853 adhesive Substances 0.000 description 2
- 230000001070 adhesive effect Effects 0.000 description 2
- 125000000129 anionic group Chemical group 0.000 description 2
- 238000005336 cracking Methods 0.000 description 2
- 239000013078 crystal Substances 0.000 description 2
- 230000008030 elimination Effects 0.000 description 2
- 238000003379 elimination reaction Methods 0.000 description 2
- 239000003063 flame retardant Substances 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 239000012774 insulation material Substances 0.000 description 2
- 239000000395 magnesium oxide Substances 0.000 description 2
- 239000006259 organic additive Substances 0.000 description 2
- 235000012239 silicon dioxide Nutrition 0.000 description 2
- 239000002002 slurry Substances 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 229910018072 Al 2 O 3 Inorganic materials 0.000 description 1
- 239000010754 BS 2869 Class F Substances 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000032683 aging Effects 0.000 description 1
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 1
- 229910021486 amorphous silicon dioxide Inorganic materials 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 239000010425 asbestos Substances 0.000 description 1
- 229910001593 boehmite Inorganic materials 0.000 description 1
- 239000012267 brine Substances 0.000 description 1
- AXCZMVOFGPJBDE-UHFFFAOYSA-L calcium dihydroxide Chemical compound [OH-].[OH-].[Ca+2] AXCZMVOFGPJBDE-UHFFFAOYSA-L 0.000 description 1
- 239000000920 calcium hydroxide Substances 0.000 description 1
- 229910001861 calcium hydroxide Inorganic materials 0.000 description 1
- ODINCKMPIJJUCX-UHFFFAOYSA-N calcium oxide Inorganic materials [Ca]=O ODINCKMPIJJUCX-UHFFFAOYSA-N 0.000 description 1
- 239000000292 calcium oxide Substances 0.000 description 1
- 125000002091 cationic group Chemical group 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000001934 delay Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000011982 device technology Methods 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 239000008240 homogeneous mixture Substances 0.000 description 1
- FAHBNUUHRFUEAI-UHFFFAOYSA-M hydroxidooxidoaluminium Chemical compound O[Al]=O FAHBNUUHRFUEAI-UHFFFAOYSA-M 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 235000014413 iron hydroxide Nutrition 0.000 description 1
- NCNCGGDMXMBVIA-UHFFFAOYSA-L iron(ii) hydroxide Chemical compound [OH-].[OH-].[Fe+2] NCNCGGDMXMBVIA-UHFFFAOYSA-L 0.000 description 1
- 238000003475 lamination Methods 0.000 description 1
- VTHJTEIRLNZDEV-UHFFFAOYSA-L magnesium dihydroxide Chemical compound [OH-].[OH-].[Mg+2] VTHJTEIRLNZDEV-UHFFFAOYSA-L 0.000 description 1
- 239000000347 magnesium hydroxide Substances 0.000 description 1
- 229910001862 magnesium hydroxide Inorganic materials 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 239000002557 mineral fiber Substances 0.000 description 1
- TWNQGVIAIRXVLR-UHFFFAOYSA-N oxo(oxoalumanyloxy)alumane Chemical compound O=[Al]O[Al]=O TWNQGVIAIRXVLR-UHFFFAOYSA-N 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 230000002787 reinforcement Effects 0.000 description 1
- 229910052895 riebeckite Inorganic materials 0.000 description 1
- 238000005096 rolling process Methods 0.000 description 1
- 238000005245 sintering Methods 0.000 description 1
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 239000002341 toxic gas Substances 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 238000000844 transformation Methods 0.000 description 1
- 229920006186 water-soluble synthetic resin Polymers 0.000 description 1
Images
Classifications
-
- E—FIXED CONSTRUCTIONS
- E06—DOORS, WINDOWS, SHUTTERS, OR ROLLER BLINDS IN GENERAL; LADDERS
- E06B—FIXED OR MOVABLE CLOSURES FOR OPENINGS IN BUILDINGS, VEHICLES, FENCES OR LIKE ENCLOSURES IN GENERAL, e.g. DOORS, WINDOWS, BLINDS, GATES
- E06B5/00—Doors, windows, or like closures for special purposes; Border constructions therefor
- E06B5/10—Doors, windows, or like closures for special purposes; Border constructions therefor for protection against air-raid or other war-like action; for other protective purposes
- E06B5/16—Fireproof doors or similar closures; Adaptations of fixed constructions therefor
-
- E—FIXED CONSTRUCTIONS
- E04—BUILDING
- E04B—GENERAL BUILDING CONSTRUCTIONS; WALLS, e.g. PARTITIONS; ROOFS; FLOORS; CEILINGS; INSULATION OR OTHER PROTECTION OF BUILDINGS
- E04B1/00—Constructions in general; Structures which are not restricted either to walls, e.g. partitions, or floors or ceilings or roofs
- E04B1/62—Insulation or other protection; Elements or use of specified material therefor
- E04B1/74—Heat, sound or noise insulation, absorption, or reflection; Other building methods affording favourable thermal or acoustical conditions, e.g. accumulating of heat within walls
- E04B1/76—Heat, sound or noise insulation, absorption, or reflection; Other building methods affording favourable thermal or acoustical conditions, e.g. accumulating of heat within walls specifically with respect to heat only
- E04B1/78—Heat insulating elements
- E04B1/80—Heat insulating elements slab-shaped
-
- E—FIXED CONSTRUCTIONS
- E06—DOORS, WINDOWS, SHUTTERS, OR ROLLER BLINDS IN GENERAL; LADDERS
- E06B—FIXED OR MOVABLE CLOSURES FOR OPENINGS IN BUILDINGS, VEHICLES, FENCES OR LIKE ENCLOSURES IN GENERAL, e.g. DOORS, WINDOWS, BLINDS, GATES
- E06B3/00—Window sashes, door leaves, or like elements for closing wall or like openings; Layout of fixed or moving closures, e.g. windows in wall or like openings; Features of rigidly-mounted outer frames relating to the mounting of wing frames
- E06B3/70—Door leaves
- E06B3/7015—Door leaves characterised by the filling between two external panels
- E06B2003/7042—Door leaves characterised by the filling between two external panels with a fire retardant layer
Definitions
- the invention relates to a fire-resistant, heat transfer reducing wall element, in particular as an insert for a fire-retardant door, according to the preamble of claim 1 and a method for its production.
- the fire resistance is determined by the duration at which a certain temperature rise on one side of the wall element, the other side of the wall element below a certain limit temperature, for. B. 180 ° C remains.
- the service life of the wall element until this limit temperature is reached on the cold side in minutes gives the fire resistance class, whereby according to DIN 4102, Part 5 z. B. the classification in fire resistance class F 30 means a 30-minute service life, F 90 a 90-minute service life etc.
- thermal insulation measures alone can only achieve a limited delay in the rise in temperature on the cold side, and this requires relatively large wall thicknesses.
- thermal insulation materials with a correspondingly high thermal resistance on the one hand and sufficient temperature resistance on the other hand are not available, with the exception of asbestos, which should not be used due to its health-threatening effects.
- Mineral wool such as rock wool, sinters together from the hot side under the high fire temperatures and thus loses its effectiveness as a thermal insulation material relatively quickly on the hot side, so that relatively large wall thicknesses are required;
- mineral wool has a relatively low heat capacity and can therefore only delay the temperature rise on the cold side by its own heat absorption with small wall thicknesses.
- Gypsum in particular is in practical use as a material for a layer that can delay the rise in temperature on the cold side due to the storage of latent heat as a result of phase change.
- the relatively high enthalpy is used when the crystal water is split off, which occurs from approx. 50 ° C.
- gypsum can only be handled as a layer with the dimensions required in practice in the form of so-called gypsum plasterboard, with a layer of gypsum being laminated on both sides with cardboard.
- the cardboard lining naturally increases the fire load and promotes the formation of flammable smoldering and decomposition gases.
- DE-OS 30 23 632 sodium metasilicate has become known, a substance that melts at around 48 ° C. while absorbing heat in its own crystal water and is stable in the molten state.
- the melt with a relatively low viscosity is formed even at the lowest temperatures and, of course, tends to accumulate in the lower region of the available space.
- the hot upper area is completely exposed from the material of the layered fire protection zone, so that the temperature on the cold side rises practically abruptly there.
- the sodium metasilicate is to be embedded in an open-pore support structure made of a wettable material, for example granulated mineral wool, to avoid draining away too quickly.
- a generic fire resistant, heat transfer reducing wall element according to the preamble of claim 1 is known.
- the two insulation layers made of bound mineral wool are combined with at least one fire protection zone made of a granulate made of alkali or alkaline earth carbonate, the granulate being coated with an inorganic binder, e.g. B. magnesia binder.
- This known wall element offers sufficient resistance to a fire; however, since the material of the fire protection zone consists of alkali or alkaline earth carbonate in the form of granules, the fire protection zone is relatively wide, which leads to a relatively large structural thickness of the wall element. In addition, the known wall element is very heavy and prone to breakage.
- a method for producing the wall element is also known from this document.
- Another fire resistant wall element having the features of the preamble of claim 1 is known from GB-A-1 558 073.
- a layer of kaolin is provided as the inorganic material.
- the invention has for its object to provide a wall element of the type specified in the preamble of claim 1 and a method for its production, which in combination with mineral fiber insulation layers a high fire resistance class such as F 90 according to DIN 4102, part 5 in comparison with the stand the technology of reduced thickness and weight can easily achieve.
- Substances are used as hydroxides or binders to ensure that no flammable and / or toxic gases are generated in the event of a fire.
- As water-releasing hydroxides aluminum hydroxide Al (OH) 3 or z.
- Magnesium hydroxide Mg (OH) 2 , calcium hydroxide Ca (OH) 2 , iron hydroxide Fe (OH) 3 or FeO (OH) can be used, which with elimination of water in the corresponding oxides Al 2 O 3 , MgO, CaO, Fe 2 O 3 etc. are transferred.
- the conversion of the hydroxides into the corresponding oxides is an endothermic reaction, whereby heat is absorbed. Furthermore, water is released in a heat-consuming endothermic reaction, which escapes in the form of water vapor.
- the binder has the function of binding the water-releasing hydroxide on at least one insulation layer in the form of a layer.
- Aluminum hydroxide Al (OH) 3 (hydrargillite) changes to AIO (OH) (boehmite) when heated to approx. 150 ° C, which changes to gamma-aluminum oxide at approx. 400 ° C with elimination of water: 2 Al (OH) 3rd ---- ⁇ 2 AlO (OH) ---- ⁇ Al 2nd O 3rd
- aluminum hydroxide is available in a variety of different preparations, e.g. B. available as a martinal (registered trademark of the Martinswerk, Bergheim) on the market.
- the measure of using the water-releasing hydroxide in different fractions with at least two particle size ranges leads to a higher packing density of the hydroxide.
- increasing the packing density has the advantage that more mass with endothermic and water-releasing properties can be introduced into the layer and, on the other hand, that the proportion of the more expensive binder can be reduced if necessary.
- the increase in the packing density leads to a lower shrinkage of the layer during drying, as a result of which cracking can be largely suppressed.
- the average grain size of the finest fraction is less than 2 microns ensures that this fraction fits well into the packing gaps formed by the spherical packing of the coarsest fraction with an average grain size of less than 100 microns and in particular lower 30 microns and thereby a tightly packed Compound of the dehydrating hydroxide, e.g. B. the aluminum hydroxide is formed.
- Silica sol is a colloidal solution of amorphous silicon dioxide in water, to which small quantities of alkali are added to stabilize it in technical qualities.
- Commercial silica sols which occur as anionic or as cationic silica sols contain, for example, 15 to 45% by weight of solids, calculated as SiO 2 .
- An anionic silica sol with a solids content of 30 to 45% by weight, in particular 40% by weight, is preferably added to the layer between the insulating layers of bound mineral wool of the wall element according to the invention as silica sol.
- silica sol serves as a binder and also splits off water upon transition into the crosslinked gel state and upon aging and during heating, with the formation of silicon dioxide according to the following equation: Si (OH) 4th ---- ⁇ SiO (OH) 2nd ---- ⁇ SiO 2nd .
- Aluminum hydroxide in the form of the commercial article Martinal is inert in the pH range between 3.5 and 10.5 and is insoluble in acids and alkalis. Therefore the mixture of Al (OH) 3 (pH about 9.1) and silica sol (pH about 9 to 10) is stable.
- a water glass for example potassium or sodium water glass in aqueous solution
- a water glass for example potassium or sodium water glass in aqueous solution
- Such solutions are strongly basic so that they slowly attack aluminum hydroxide. If this reaction with water glass has a disruptive effect, Mg (OH) 2 , Ca (OH) 2 , Fe (OH) 3 or FeO (OH) or another hydroxide instead of aluminum hydroxide or a mixture of such hydroxides together with water glass can be used as the water-releasing hydroxide will.
- any water-releasing hydroxide for example Mg (OH) 2 , Ca (OH) 2 , Fe (OH) 3 , FeO (OH) or a mixture of such hydroxides can be used together with silica sol.
- a mass is made from a suitable water-releasing agent Hydroxide, e.g. B. aluminum hydroxide, and a silica sol processed into a plastic mass which is applied to one side of an insulation layer in a uniform layer.
- Hydroxide e.g. B. aluminum hydroxide
- silica sol processed into a plastic mass which is applied to one side of an insulation layer in a uniform layer.
- the silica sol solidifies in the layer over a period of 2 to 8 hours. It can e.g. B. can be significantly shortened by heating or microwave drying.
- the water from the damp mixture of hydroxide and a water glass or silica sol is distributed to a small extent in the underlying insulation layer and can evaporate over time.
- the strength of the layer is not dependent on complete removal of the water, since it is primarily due to the transition of the water glass or silica sol to the crosslinked gel state.
- a second layer of insulation can also be placed on top of the still wet layer and evenly bonded to the layer of plastic mass by applying slight pressure.
- the silica sol By transitioning the silica sol into the cross-linked gel state, the two insulation layers are irreversibly connected or glued to one another in this procedure.
- the binder is an organic substance, such as a polysaccharide, a polysaccharide derivative, in particular a polysaccharide ether, preferably a cellulose ether, such as. B. Tylose (registered trademark) or adding a synthetic resin has the advantage that drastic savings in binder can be achieved by this measure if necessary.
- the organic additive for example tylose, even if it is used in fire-irrelevant concentrations of far below 1% by weight, based on the mixture of hydroxide and binder, still has such a bondability or adhesive strength of the hydroxide / binder Mixture has the effect that the usual binder concentration can be reduced by a factor of approx. 2 to 5. This results in an economical saving of binders, especially the relatively expensive silica brine.
- the wall element produced in this way has sufficient fire resistance for normal applications. If there is an increased requirement for fire resistance, one or more insulation layers can be stacked in the same way with the wall element produced in this way.
- the thickest possible insulation layers made from bound mineral wool can be used.
- the thickness of the insulation layers is limited by the prescribed maximum thickness of fire protection doors and by the high weight of fire protection doors with very thick insulation layers.
- panels with a thickness of 20 to 40 mm, in particular 30 mm and a bulk density of 140 to 280 kg / m 2 , in particular 200 kg / m 2 have proven to be good insulation layers.
- the layer of inorganic material is applied in a thickness of 2 to 5 mm, in particular 3 mm, so that the layer 3 has a basis weight of 5 to 10 kg / m 2 , in particular 7 to 8 kg / m 2 .
- the outer insulation layer made of mineral wool facing the respective fire initially insulates the layer of inorganic material, which also serves as a fire protection zone, and protects it from direct flame access, so that the latter is only later exposed to the full flame effect .
- the second insulation layer made of mineral wool remains, which is arranged on the cold side in order to effect the required insulation on the cold side. This insulation layer is protected by the layer acting as a fire protection zone and can therefore fully develop its insulation effect over a long period of time.
- the layer has a nonwoven, in particular a nonwoven made of glass fibers, the cracking in the layer of water-releasing hydroxide and binder during the drying process and in particular during rapid drying, for. B. reduced or prevented under 4 min at 600 ° C.
- the fleece is integrated into the solidifying mass so that a heterogeneous layer is formed which contains water-releasing hydroxide, e.g. B. aluminum hydroxide, inorganic binder, e.g. B. contains silica sol and the fleece.
- Another advantage of this measure is that the layer has a reinforcement due to the fleece lying on it, which improves handling during production.
- rock wool as mineral wool has the advantage that it is very temperature-resistant on the one hand and, on the other hand, inexpensive in the required packaging, e.g. B. is to be produced as a plate.
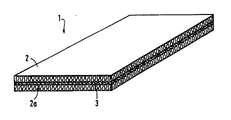
- the figure shows a view of the wall element according to the invention.
- the wall element 1 consists of two insulation layers 2, 2a made of mineral wool, between which a layer 3, made of a mixture of a water-releasing hydroxide and a silica sol, is arranged.
- Layer 3 serves as a fire protection layer since, in the event of a fire, it converts to aluminum oxide or silicon dioxide when heated and not only releases water vapor but also a large one during this endothermic conversion
- the layer 3 forms a tight protective shield for the insulation layer 2 or 2a, which faces away from the fire. While the insulation layer facing the fire sinters together relatively quickly under the action of heat, layer 3 delays the sintering together of the insulation layer 2a or 2 facing away from the fire, as a result of which a rapid rise in temperature on the cold side can be avoided.
- the wall element according to the invention serves in particular as an insert for a fire-retardant door.
- the wall element 1 can be prefabricated on the one hand in the form of a double-layer plate sandwich and inserted between the sheet steel shells of a fire protection door.
- aluminum hydroxide is used as the water-releasing hydroxide in two fractions with different particle size ranges and silica sol as the inorganic binder.
- silica sol for example, dissolved water glass can also be used as the inorganic binder.
- the ingredients are mixed e.g. B. in a screw conveyor and brings the homogeneous mixture obtained onto one of the two insulation layers in a uniform layer. Then layer 3 is dried or, if necessary, the second insulation layer is applied to the still moist layer, pressed on and dried.
- tylose is used as an organic additive
- a further advantageous embodiment uses a glass fiber fleece to reinforce layer 3, which for example has a weight of 50 g / m 2 .
- the fleece is on the layer 3 of aluminum hydroxide and binder z. B. applied by rolling, the fleece is connected to the layer 3 after drying and curing.
- the use of a fleece applied to the hydroxide mixture reduces the formation of cracks, especially when the layer 3 is subjected to rapid drying, e.g. B. 3 minutes at 600 ° C.
- the embodiment of the process according to the invention is particularly economical since the process can be carried out continuously or semi-continuously and the mixture can be produced in accordance with its consumption for the production of the layer.
Abstract
Description
Die Erfindung betrifft ein feuerwiderstandsfähiges, den Wärmedurchgang reduzierendes Wandelement, insbesondere als Einlage für eine feuerhemmende Tür, nach dem Oberbegriff des Anspruchs 1 sowie ein Verfahren zu seiner Herstellung.The invention relates to a fire-resistant, heat transfer reducing wall element, in particular as an insert for a fire-retardant door, according to the preamble of claim 1 and a method for its production.
Zur Erzielung einer hohen Feuerwiderstandsklasse bei wandartigen Bauelementen ist man bestrebt, Wärmedämmlagen mit Feuerschutzzonen zu kombinieren, deren Wärmeaufnahmevermögen dadurch wesentlich erhöht ist, daß beim Temperaturanstieg im Brandfalle endotherme chemische Reaktionen oder Phasenumwandlungen ablaufen. Bekanntlich bestimmt sich die Feuerwiderstandsfähigkeit nach der Dauer, bei der bei einem bestimmten Temperaturanstieg auf der einen Seite des Wandelements die andere Seite des Wandelements unter einer bestimmten Grenztemperatur, z. B. 180°C, bleibt. Die Standzeit des Wandelementes bis zum Erreichen dieser Grenztemperatur auf der kalten Seite in Minuten ergibt die Feuerwiderstandsklasse, wobei nach DIN 4102, Teil 5 z. B. die Einstufung in die Feuerwiderstandsklasse F 30 eine 30-minütige Standzeit bedeutet, F 90 eine 90-minütige Standzeit usw. Es liegt auf der Hand, daß durch Wärmedämmaßnahmen allein nur eine begrenzte Verzögerung des Temperaturanstieges auch auf der kalten Seite erzielt werden kann, und hierzu relativ große Wanddicken erforderlich sind. Darüberhinaus stehen Wärmedämmstoffe mit entsprechend hohem Wärmedurchlaßwiderstand einerseits und unter den Wandtemperaturen ausreichender Temperaturbeständigkeit andererseits nicht zur Verfügung, mit Ausnahme von Asbest, das infolge seiner gesundheitsgefährdenden Auswirkungen nicht verwendet werden soll. Mineralwolle, wie Steinwolle sintert unter den hohen Brandtemperaturen ausgehend von der heißen Seite zusammen und büßt so bei geringer Wanddicke auf der heißen Seite relativ schnell ihre Wirksamkeit als Wärmedämmaterial ein, so daß relativ große Wanddicken erforderlich sind; weiterhin weist Mineralwolle eine relativ geringe Wärmekapazität auf und kann daher den Temperaturanstieg auf der kalten Seite durch eigene Wärmeaufnahme bei geringen Wanddicken nur unwesentlich verzögern.In order to achieve a high fire resistance class for wall-like components, efforts are being made to combine thermal insulation layers with fire protection zones, the heat absorption capacity of which is significantly increased by the fact that endothermic chemical reactions or phase transformations take place when the temperature rises in the event of a fire. As is known, the fire resistance is determined by the duration at which a certain temperature rise on one side of the wall element, the other side of the wall element below a certain limit temperature, for. B. 180 ° C remains. The service life of the wall element until this limit temperature is reached on the cold side in minutes gives the fire resistance class, whereby according to DIN 4102, Part 5 z. B. the classification in fire resistance class F 30 means a 30-minute service life, F 90 a 90-minute service life etc. It is obvious that thermal insulation measures alone can only achieve a limited delay in the rise in temperature on the cold side, and this requires relatively large wall thicknesses. In addition, thermal insulation materials with a correspondingly high thermal resistance on the one hand and sufficient temperature resistance on the other hand are not available, with the exception of asbestos, which should not be used due to its health-threatening effects. Mineral wool, such as rock wool, sinters together from the hot side under the high fire temperatures and thus loses its effectiveness as a thermal insulation material relatively quickly on the hot side, so that relatively large wall thicknesses are required; Furthermore, mineral wool has a relatively low heat capacity and can therefore only delay the temperature rise on the cold side by its own heat absorption with small wall thicknesses.
Als Material für eine Schicht, die durch Speicherung latenter Wärme infolge Phasenumwandlung den Temperaturanstieg an der kalten Seite verzögern kann, ist insbesondere Gips im praktischen Einsatz. Dabei wird die relativ hohe Enthalpie bei Abspaltung des Kristallwassers genutzt, was ab ca. 50°C erfolgt. Jedoch kann Gips als eine Lage mit den in der Praxis benötigten Abmessungen nur in Form sogenannter Gipskartonbauplatten gehandhabt werden, wobei eine Gipsschicht beidseitig mit Karton kaschiert ist. Die Kartonkaschierung erhöht natürlich die Brandlast und fördert die Entstehung brennbarer Schwel- und Zersetzungsgase.Gypsum in particular is in practical use as a material for a layer that can delay the rise in temperature on the cold side due to the storage of latent heat as a result of phase change. The relatively high enthalpy is used when the crystal water is split off, which occurs from approx. 50 ° C. However, gypsum can only be handled as a layer with the dimensions required in practice in the form of so-called gypsum plasterboard, with a layer of gypsum being laminated on both sides with cardboard. The cardboard lining naturally increases the fire load and promotes the formation of flammable smoldering and decomposition gases.
Es hat daher auch nicht an Versuchen gefehlt, derartige latente wärmeaufnehmende Feuerschutzzonen aus anderen Stoffen herzustellen, die keine Kaschierungen aus organischen Stoffen oder dergleichen benötigen.There has therefore been no lack of attempts to produce such latent heat-absorbing fire protection zones from other substances which do not require laminations from organic substances or the like.
Hierzu ist aus der DE-OS 30 23 632 Natrium-Metasilicat bekannt geworden, ein Stoff, der bei ca. 48°C unter Wärmeaufnahme im eigenen Kristallwasser aufschmilzt und im schmelzflüssigen Zustand beständig ist. Hier besteht jedoch das Problem, daß die Schmelze mit relativ niedriger Viskosität bereits bei niedrigsten Temperaturen gebildet wird und natürlich die Tendenz hat, sich im unteren Bereich des zur Verfügung stehenden Raumes anzusammeln. Hierdurch wird gerade der heiße obere Bereich vollständig von dem Material der schichtförmigen Feuerschutzzone entblößt, so daß dort die Temperatur an der kalten Seite praktisch sprunghaft ansteigt. Nach der Lehre der DE-OS 30 23 632 soll das Natrium-Metasilicat zur Vermeidung eines allzu schnellen Abfließens in ein offenporiges Stützgerüst aus einem benetzbaren Material, beispielsweise granulierte Mineralwolle, eingebettet werden. Nach einer ähnlichen Lehre der DE-OS 30 22 945 soll zusätzlich ein Stoff mit eingebracht werden, der die Schmelze bindet und mit dieser eine teigige oder feste Masse bildet. Es hat sich gezeigt, daß mit derartigen Maßnahmen das Abfließen der Schmelze verzögert werden kann. Eine andere Frage ist der niedrige Schmelzpunkt von Natrium-Metasilicat von ca. 50°C, der auch ohne Brand unter ungünstigen Einflüssen erzielt werden kann, so daß sich in einem ungünstigen Fall auch ohne Brandausbruch Schmelze bilden und im Bodenbereich ansammeln kann, was aber im Brandfall die Feuerwiderstandsfähigkeit des Wandelementes in der Regel nicht stark verringert.For this purpose, DE-OS 30 23 632 sodium metasilicate has become known, a substance that melts at around 48 ° C. while absorbing heat in its own crystal water and is stable in the molten state. Here, however, there is the problem that the melt with a relatively low viscosity is formed even at the lowest temperatures and, of course, tends to accumulate in the lower region of the available space. As a result, the hot upper area is completely exposed from the material of the layered fire protection zone, so that the temperature on the cold side rises practically abruptly there. According to the teaching of DE-OS 30 23 632, the sodium metasilicate is to be embedded in an open-pore support structure made of a wettable material, for example granulated mineral wool, to avoid draining away too quickly. According to a similar teaching of DE-OS 30 22 945, a substance is also to be introduced which binds the melt and forms a doughy or solid mass with it. It has been shown that such measures can delay the outflow of the melt. Another question is the low melting point of sodium metasilicate of approx. 50 ° C, which can also be achieved without fire under unfavorable influences, so that in an unfavorable case, melt can form and accumulate in the floor area without fire, which is, however, in the In the event of fire, the fire resistance of the wall element is generally not greatly reduced.
Aus der DE-OS 35 10 935 ist ein gattungsgemäßes feuerwiderstandsfähiges, den Wärmedurchgang reduzierendes Wandelement gemäß dem Oberbegriff des Anspruchs 1 bekannt. Beim bekannten Wandelement sind die beiden Dämmlagen aus gebundener Mineralwolle mit mindestens einer Feuerschutzzone aus einem Granulat aus Alkali- oder Erdalkalikarbonat kombiniert, wobei das Granulat durch ein anorganisches Bindemittel, z. B. Magnesiabinder, gebunden ist. Dieses bekannte Wandelement setzt zwar einem Brand einen ausreichenden Widerstand entgegen; da das Material der Feuerschutzzone jedoch aus Alkali- oder Erdalkalikarbonat in Form von Granulat besteht, ist die Feuerschutzzone relativ breit, was zu einer relativ großen Baudicke des Wandelements führt. Außerdem ist das bekannte Wandelement sehr schwer und bruchanfällig.From DE-OS 35 10 935 a generic fire resistant, heat transfer reducing wall element according to the preamble of claim 1 is known. In the known wall element, the two insulation layers made of bound mineral wool are combined with at least one fire protection zone made of a granulate made of alkali or alkaline earth carbonate, the granulate being coated with an inorganic binder, e.g. B. magnesia binder. This known wall element offers sufficient resistance to a fire; however, since the material of the fire protection zone consists of alkali or alkaline earth carbonate in the form of granules, the fire protection zone is relatively wide, which leads to a relatively large structural thickness of the wall element. In addition, the known wall element is very heavy and prone to breakage.
Aus diesem Dokument ist auch ein Verfahren zur Herstellung des Wandelements bekannt.A method for producing the wall element is also known from this document.
Ein weiteres feuerwiderstandsfähiges Wandelement mit den Merkmalen des Oberbegriffs des Anspruchs 1 ist durch GB-A-1 558 073 bekannt. Dabei ist als anorganisches Material eine Schicht aus Kaolin vorgesehen.Another fire resistant wall element having the features of the preamble of claim 1 is known from GB-A-1 558 073. A layer of kaolin is provided as the inorganic material.
Demgegenüber liegt der Erfindung die Aufgabe zugrunde, ein Wandelement der im Oberbegriff des Anspruches 1 angegebenen Gattung und ein Verfahren zu seiner Herstellung zu schaffen, welches in Kombination mit Mineralfaser-Dämmlagen eine hohe Feuerwiderstandsklasse wie F 90 gemäß DIN 4102, Teil 5 bei gegenüber dem Stand der Technik verminderter Baudicke und vermindertem Gewicht problemlos erreichen kann.In contrast, the invention has for its object to provide a wall element of the type specified in the preamble of claim 1 and a method for its production, which in combination with mineral fiber insulation layers a high fire resistance class such as F 90 according to DIN 4102, part 5 in comparison with the stand the technology of reduced thickness and weight can easily achieve.
Die Lösung dieser Aufgabe erfolgt vorrichtungstechnisch durch die kennzeichnenden Merkmale des Anspruchs 1 und verfahrenstechnisch durch die Merkmale des Anspruchs 14.This object is achieved in terms of device technology by the characterizing features of claim 1 and in terms of process technology by the features of claim 14.
Als Hydroxide bzw. Bindemittel werden Stoffe verwendet, die sicherstellen, daß im Brandfalle keine brennbaren und/oder giftigen Gase erzeugt werden. Als wasserabspaltende Hydroxide können Aluminiumhydroxid Al(OH)3 oder z. B. Magnesiumhydroxid Mg(OH)2, Kalziumhydroxid Ca(OH)2,-Eisenhydroxid Fe(OH)3 bzw. FeO(OH) eingesetzt werden, die unter Wasserabspaltung in die entsprechenden Oxide Al2O3, MgO, CaO, Fe2O3 usw. überführt werden. Die Umwandlung der Hydroxide in die entsprechenden Oxide ist eine endotherme Reaktion, wodurch Wärme aufgenommen wird. Weiterhin wird in einer Wärme verbrauchenden endothermen Reaktion Wasser freigesetzt, das in Form von Wasserdampf entweicht.Substances are used as hydroxides or binders to ensure that no flammable and / or toxic gases are generated in the event of a fire. As water-releasing hydroxides, aluminum hydroxide Al (OH) 3 or z. B. Magnesium hydroxide Mg (OH) 2 , calcium hydroxide Ca (OH) 2 , iron hydroxide Fe (OH) 3 or FeO (OH) can be used, which with elimination of water in the corresponding oxides Al 2 O 3 , MgO, CaO, Fe 2 O 3 etc. are transferred. The conversion of the hydroxides into the corresponding oxides is an endothermic reaction, whereby heat is absorbed. Furthermore, water is released in a heat-consuming endothermic reaction, which escapes in the form of water vapor.
Das Bindemittel hat hierbei die Funktion das wasserabspaltende Hydroxid auf wenigstens einer Dämmlage in Form einer Schicht zu binden.The binder has the function of binding the water-releasing hydroxide on at least one insulation layer in the form of a layer.
Aluminiumhydroxid Al(OH)3 (Hydrargillit) geht bei Erwärmung auf ca. 150°C in AIO(OH) (Böhmit) über, der bei ca. 400°C unter Wasserabspaltung in Gamma-Aluminiumoxid übergeht:
Die Maßnahme, das wasserabspaltende Hydroxid in unterschiedlichen Fraktionen mit mindestens zwei Korngrößenbereichen einzusetzen, führt zu einer größeren Packungsdichte des Hydroxids. Die Erhöhung der Packungsdichte hat einerseits den Vorteil, daß mehr Masse mit endothermen und wasserabspaltenden Eigenschaften in die Schicht eingetragen werden kann und andererseits, daß der Anteil des teureren Bindemittels bei Bedarf gesenkt werden kann.The measure of using the water-releasing hydroxide in different fractions with at least two particle size ranges leads to a higher packing density of the hydroxide. On the one hand, increasing the packing density has the advantage that more mass with endothermic and water-releasing properties can be introduced into the layer and, on the other hand, that the proportion of the more expensive binder can be reduced if necessary.
Selbstverständlich können - insbesondere zur weiteren Einsparung von Bindemittel - mehrere hinsichtlich ihrer Korngröße aufeinander abgestimmte Fraktionen unter Umständen auch unterschiedlicher wasserabspaltender Hydroxide verwendet werden.Of course, several fractions which are coordinated with respect to their grain size can also be used under certain circumstances, in particular to further save on binders, and under certain circumstances also different water-releasing hydroxides.
Desweiteren führt die Erhöhung der Packungsdichte zu einer geringeren Schwindung der Schicht während des Trockens, wodurch eine Rißbildung weitgehend unterdrückt werden kann.Furthermore, the increase in the packing density leads to a lower shrinkage of the layer during drying, as a result of which cracking can be largely suppressed.
Dadurch, daß die mittlere Korngröße der feinsten Fraktion unter 2 µm liegt, ist gewährleistet, daß diese Fraktion sich gut in die durch die Kugelpackung der gröbsten Fraktion mit einer mittleren Korngröße unter 100 µm und insbesondere untere 30 µm ausgebildeten Packungslücken einfügt und hierdurch ein dicht gepackter Verbund des wasserabspaltenden Hydroxides, z. B. des Aluminiumhydroxids gebildet wird.The fact that the average grain size of the finest fraction is less than 2 microns ensures that this fraction fits well into the packing gaps formed by the spherical packing of the coarsest fraction with an average grain size of less than 100 microns and in particular lower 30 microns and thereby a tightly packed Compound of the dehydrating hydroxide, e.g. B. the aluminum hydroxide is formed.
Kieselsol ist eine kolloide Lösung von amorphem Siliziumdioxid in Wasser, der bei technischen Qualitäten geringfügige Mengen Alkali zur Stabilisierung zugesetzt sind. Handelsübliche Kieselsole, die als anionische oder als kationische Kieselsole vorkommen, enthalten z.B. 15 bis 45 Gew.-% Feststoff, berechnet als SiO2. Der Schicht zwischen den Dämmlagen aus gebundener Mineralwolle des erfindungsgemäßen Wandelementes wird als Kieselsol vorzugsweise ein anionisches Kieselsol mit einem Feststoffgehalt von 30 bis 45 Gew.-%, insbesondere 40 Gew.-% zugesetzt. Kieselsol dient in dem erfindungsgemäß eingesetzten Gemisch als Bindemittel und spaltet beim übergang in den vernetzten Gelzustand und beim Altern sowie beim Erwärmen ebenfalls Wasser ab unter Bildung von Siliziumdioxid gemäß der folgenden Gleichung:
Aluminiumhydroxid in Form des Handelsartikels Martinal ist im pH-Bereich zwischen 3,5 und 10,5 inert und unlöslich in Säuren und Laugen. Deshalb ist die Mischung von Al(OH)3 (pH etwa 9,1) und Kieselsol (pH etwa 9 bis 10) beständig.Aluminum hydroxide in the form of the commercial article Martinal is inert in the pH range between 3.5 and 10.5 and is insoluble in acids and alkalis. Therefore the mixture of Al (OH) 3 (pH about 9.1) and silica sol (pH about 9 to 10) is stable.
Statt Kieselsol kann jedoch als Mischungsbestandteil ein Wasserglas, z.B. Kalium- oder Natriumwasserglas in wäßriger Lösung eingesetzt werden. Solche Lösungen sind stark basisch, so daß sie Aluminiumhydroxid langsam angreifen. Wenn diese Reaktion mit Wasserglas störend wirkt, kann als wasserabspaltendes Hydroxid Mg(OH)2, Ca(OH)2, Fe(OH)3 bzw. FeO(OH) oder ein anderes Hydroxid anstatt Aluminiumhydroxid oder eine Mischung solcher Hydroxide zusammen mit Wasserglas eingesetzt werden.Instead of silica sol, however, a water glass, for example potassium or sodium water glass in aqueous solution, can be used as the constituent of the mixture. Such solutions are strongly basic so that they slowly attack aluminum hydroxide. If this reaction with water glass has a disruptive effect, Mg (OH) 2 , Ca (OH) 2 , Fe (OH) 3 or FeO (OH) or another hydroxide instead of aluminum hydroxide or a mixture of such hydroxides together with water glass can be used as the water-releasing hydroxide will.
Es kann aber auch ein beliebiges wasserabspaltendes Hydroxid, z.B. Mg(OH)2, Ca(OH)2, Fe(OH)3, FeO(OH) oder eine Mischung solcher Hydroxide zusammen mit Kieselsol verwendet werden.However, any water-releasing hydroxide, for example Mg (OH) 2 , Ca (OH) 2 , Fe (OH) 3 , FeO (OH) or a mixture of such hydroxides can be used together with silica sol.
Zur Herstellung des Wandelements wird eine Masse aus einem geeigneten wasserabspaltenden Hydroxid, z. B. Aluminiumhydroxid, und einem Kieselsol zu einer plastischen Masse verarbeitet, die auf eine Seite einer Dämmlage in gleichmäßiger Schicht aufgetragen wird.To produce the wall element, a mass is made from a suitable water-releasing agent Hydroxide, e.g. B. aluminum hydroxide, and a silica sol processed into a plastic mass which is applied to one side of an insulation layer in a uniform layer.
Das Verfestigen des Kieselsols in der Schicht erfolgt in einem Zeitraum von 2 bis 8 Stunden. Es kann z. B. durch Heizen oder Mikrowellentrocknung erheblich abgekürzt werden. Das Wasser aus dem feuchten Gemisch aus Hydroxid und einem Wasserglas oder Kieselsol verteilt sich zu einem geringen Teil in der unterliegenden Dämmlage und kann im Verlauf der Zeit verdunsten. Die Festigkeit der Schicht ist jedoch nicht von einer vollständigen Enffernung des Wassers abhängig, da sie vor allem auf dem übergang des Wasserglases bzw. Kieselsols in den vernetzten Gelzustand beruht.The silica sol solidifies in the layer over a period of 2 to 8 hours. It can e.g. B. can be significantly shortened by heating or microwave drying. The water from the damp mixture of hydroxide and a water glass or silica sol is distributed to a small extent in the underlying insulation layer and can evaporate over time. However, the strength of the layer is not dependent on complete removal of the water, since it is primarily due to the transition of the water glass or silica sol to the crosslinked gel state.
Es kann jedoch auch auf die noch feuchte Schicht eine zweite Dämmlage aufgelegt und durch leichten Druck mit der Schicht aus der plastischen Masse gleichmäßig verbunden werden. Durch übergang des Kieselsols in den vernetzten Gelzustand werden bei dieser Vorgehensweise die beiden Dämmlagen miteinander irreversibel verbunden bzw. verklebt.However, a second layer of insulation can also be placed on top of the still wet layer and evenly bonded to the layer of plastic mass by applying slight pressure. By transitioning the silica sol into the cross-linked gel state, the two insulation layers are irreversibly connected or glued to one another in this procedure.
Dem Bindemittel einen organischen Stoff, wie ein Polysaccharid, ein Polysaccharidderivat, insbesondere ein Polysaccharidether, vorzugsweise ein Celluloseether, wie z. B. Tylose (eingetragenes Warenzeichen) oder ein Kunstharz zuzusetzen, hat den Vorteil, daß durch diese Maßnahme bei Bedarf drastische Einsparungen an Bindemittel erzielt werden können. Dies liegt darin begründet, daß der organische Zusatz, beispielsweise Tylose, auch wenn er in brandtechnisch irrelevanten Konzentrationen von weit unter 1 Gew.-% bezogen auf die Mischung aus Hydroxid und Bindemittel eingesetzt wird, noch eine solche Bindefähigkeit bzw. Klebekraft des Hydroxid/Bindemittel-Gemisches bewirkt, daß die sonst übliche Bindemittelkonzentration um einen Faktor von ca. 2 bis 5 vermindert werden kann. Hierdurch ergibt sich eine kostengünstige Einsparung von Bindemittel, insbesondere des relativ teuren Kieselsoles.The binder is an organic substance, such as a polysaccharide, a polysaccharide derivative, in particular a polysaccharide ether, preferably a cellulose ether, such as. B. Tylose (registered trademark) or adding a synthetic resin has the advantage that drastic savings in binder can be achieved by this measure if necessary. This is due to the fact that the organic additive, for example tylose, even if it is used in fire-irrelevant concentrations of far below 1% by weight, based on the mixture of hydroxide and binder, still has such a bondability or adhesive strength of the hydroxide / binder Mixture has the effect that the usual binder concentration can be reduced by a factor of approx. 2 to 5. This results in an economical saving of binders, especially the relatively expensive silica brine.
Vorzugsweise werden 70 bis 95 Gew.-% Aluminiumhydroxid und 30 bis 5 Gew.-% Kieselsol, berechnet als SiO2, insbesondere 80 bis 90 Gew.-% Aluminiumhydroxid und 20 bis 10 Gew.-% Kieselsol, z.B. 85 Gew.-% Aluminiumhydroxid und 15 Gew.-% Kieselsol, eingesetzt. Es ist auch möglich, anstelle eines Teils des Aluminiumhydroxids oder zusätzlich zu Aluminiumhydroxid Ton oder Tonerde oder ein Tonmineral in Form eines Pulvers oder einer wäßrigen Aufschlämmung zu verwenden.70 to 95% by weight of aluminum hydroxide and 30 to 5% by weight of silica sol, calculated as SiO 2 , in particular 80 to 90% by weight of aluminum hydroxide and 20 to 10% by weight of silica sol, for example 85% by weight, are preferably used. Aluminum hydroxide and 15 wt .-% silica sol. It is also possible to use clay or alumina or a clay mineral in the form of a powder or an aqueous slurry instead of a part of the aluminum hydroxide or in addition to aluminum hydroxide.
Für gewöhnliche Anwendungen besitzt das so hergestellte Wandelement ausreichende Feuerwiderstandsfähigkeit. Bei einer erhöhten Anforderung an die Feuerwiderstandsfähigkeit können noch eine oder mehrere Dämmlagen in gleicher Weise mit dem so hergestellten Wandelement sandwichartig gestapelt werden.The wall element produced in this way has sufficient fire resistance for normal applications. If there is an increased requirement for fire resistance, one or more insulation layers can be stacked in the same way with the wall element produced in this way.
Zur Erhöhung der Feuerwiderstandswerte können möglichst dicke Dämmlagen aus gebundener Mineralwolle eingesetzt werden. Die Dicke der Dämmlagen ist jedoch durch die vorgeschriebene Höchstdicke von Feuerschutztüren sowie durch das hohe Gewicht von Feuerschutztüren mit sehr dicken Dämmlagen begrenzt. In der Praxis haben sich als Dämmlagen Platten mit einer Dicke von 20 bis 40 mm, insbesondere 30 mm und einer Rohdichte von 140 bis 280 kg/m2, insbesondere 200 kg/m2 gut bewährt. Die Schicht aus anorganischem Material wird in einer Dicke von 2 bis 5 mm, insbesondere 3 mm aufgetragen, so daß die Schicht 3 ein Flächengewicht von 5 bis 10 kg/m2, insbesondere 7 bis 8 kg/m2 besitzt. Mit einem solchen Wandelement 1, welches bei Bedarf symmetrisch aufgebaut sein kann, lassen sich hervorragende Feuerwiderstandswerte erzielen. Der Grund hierfür liegt vermutlich darin, daß die in diesem Falle dem jeweiligen Brand zugekehrte äußere Dämmlage aus Mineralwolle zunächst die Schicht aus anorganischem Material, die gleichzeitig als Feuerschutzzone dient, dämmt und vor unmittelbarem Flammenzutritt schützt, so daß letztere erst später der vollen Flammenwirkung ausgesetzt ist. In diesem Fall verbleibt die zweite Dämmlage aus Mineralwolle, die auf der kalten Seite angeordnet ist, um die erforderliche Dämmung der kalten Seite zu bewirken. Diese Dämmlage ist durch die als Feuerschutzzone wirkende Schicht geschützt und kann somit ihre Dämmwirkung über lange Zeit hinweg voll entfalten.To increase the fire resistance values, the thickest possible insulation layers made from bound mineral wool can be used. However, the thickness of the insulation layers is limited by the prescribed maximum thickness of fire protection doors and by the high weight of fire protection doors with very thick insulation layers. In practice, panels with a thickness of 20 to 40 mm, in particular 30 mm and a bulk density of 140 to 280 kg / m 2 , in particular 200 kg / m 2, have proven to be good insulation layers. The layer of inorganic material is applied in a thickness of 2 to 5 mm, in particular 3 mm, so that the layer 3 has a basis weight of 5 to 10 kg / m 2 , in particular 7 to 8 kg / m 2 . With such a wall element 1, which can be constructed symmetrically if required, excellent fire resistance values can be achieved. The reason for this is probably that in this case the outer insulation layer made of mineral wool facing the respective fire initially insulates the layer of inorganic material, which also serves as a fire protection zone, and protects it from direct flame access, so that the latter is only later exposed to the full flame effect . In this case, the second insulation layer made of mineral wool remains, which is arranged on the cold side in order to effect the required insulation on the cold side. This insulation layer is protected by the layer acting as a fire protection zone and can therefore fully develop its insulation effect over a long period of time.
Dadurch, daß die Schicht ein Vlies, insbesondere ein Vlies aus Glasfasern aufweist, wird die Rißbildung in der Schicht aus wasserabspaltendem Hydroxid und Bindemittel während des Trocknungsvorganges und insbesondere während einer Schnelltrocknung, z. B. unter 4 min bei 600°C vermindert bzw. verhindert. Das Vlies wird hiebei in die erstarrende Masse integriert, so daß eine heterogene Schicht gebildet wird, die wasserabspaltendes Hydroxid, z. B. Aluminiumhydroxid, anorganisches Bindemittel, z. B. Kieselsol und das Vlies enthält.The fact that the layer has a nonwoven, in particular a nonwoven made of glass fibers, the cracking in the layer of water-releasing hydroxide and binder during the drying process and in particular during rapid drying, for. B. reduced or prevented under 4 min at 600 ° C. The fleece is integrated into the solidifying mass so that a heterogeneous layer is formed which contains water-releasing hydroxide, e.g. B. aluminum hydroxide, inorganic binder, e.g. B. contains silica sol and the fleece.
Ein weiterer Vorteil dieser Maßnahme ist es, daß die Schicht aufgrund des ihr aufliegenden Vlieses eine Armierung aufweist, die die Handhabbarkeit während der Produktion verbessert.Another advantage of this measure is that the layer has a reinforcement due to the fleece lying on it, which improves handling during production.
Steinwolle als Mineralwolle einzusetzen, hat den Vorteil, daß diese zum einen sehr temperaturbeständig ist und zum anderen kostengünstig in der erforderlichen Konfektionierung, z. B. als Platte herzustellen ist.Using rock wool as mineral wool has the advantage that it is very temperature-resistant on the one hand and, on the other hand, inexpensive in the required packaging, e.g. B. is to be produced as a plate.
Weitere Einzelheiten, Merkmale und Vorteile der vorliegenden Erfindung ergeben sich aus der nachfolgenden Beschreibung einer Ausführungsform anhand der Zeichnung.Further details, features and advantages of the present invention result from the following description of an embodiment based on the drawing.
Es zeigt die Figur eine Ansicht des erfindungsgemäßen Wandelements.The figure shows a view of the wall element according to the invention.
Das Wandelement 1 besteht aus zwei Dämmlagen 2, 2a aus Mineralwolle, zwischen denen eine Schicht 3, hergestellt aus einem Gemisch aus einem wasserabspaltendem Hydroxid und einem Kieselsol, angeordnet ist. Die Schicht 3 dient dabei als Feuerschutzschicht, da sie im Brandfalle bei ihrer Erwärmung sich zu Aluminiumoxid bzw. Siliziumdioxid umwandelt und bei dieser endothermen Umwandlung nicht nur Wasserdampf freisetzt, sondern auch eine großeThe wall element 1 consists of two
Menge an Energie verbraucht. Ferner bildet die Schicht 3 nach ihrer Erhitzung einen dichten Schutzschild für die Dämmlage 2 oder 2a, die dem Feuer abgewandt ist. Während die dem Feuer zugewandte Dämmlage unter der Hitzeeinwirkung relativ rasch zusammensintert, verzögert die Schicht 3 das Zusammensintern der dem Feuer abgewandten Dämmlage 2a bzw. 2, wodurch ein schneller Temperaturanstieg an der kalten Seite vermieden werden kann.Amount of energy consumed. Furthermore, the layer 3 forms a tight protective shield for the
Das erfindungsgemäße Wandelement dient insbesondere als Einlage für eine feuerhemmende Tür. Zur Herstellung einer solchen Tür kann das Wandelement 1 einerseits in Form eines doppellagigen Plattensandwiches vorgefertigt und zwischen den Stahlblechschalen einer Feuerschutztür eingefügt werden. Andererseits ist es möglich, eine Dämmlage in eine Stahlblechschale der Feuerschutztür einzulegen, die Schicht aus dem Gemisch aus einem wasserabspaltenden Hydroxid und einem Kieselsol auf die Dämmlage aufzubringen und dann die zweite Dämmlage auf die Schicht in die Stahlblechschale für die Feuerschutztür einzupassen, wonach die beiden Dämmlagen getrocknet und ausgehärtet werden.The wall element according to the invention serves in particular as an insert for a fire-retardant door. To produce such a door, the wall element 1 can be prefabricated on the one hand in the form of a double-layer plate sandwich and inserted between the sheet steel shells of a fire protection door. On the other hand, it is possible to insert an insulation layer in a sheet steel shell of the fire protection door, to apply the layer of the mixture of a water-releasing hydroxide and a silica sol to the insulation layer and then to fit the second insulation layer onto the layer in the steel sheet shell for the fire protection door, after which the two insulation layers be dried and cured.
Weiterhin ist auch die Vorfertigung einer über die Schicht 3 verbundenen Verbundplatte möglich, die dann nach Aushärtung der Schicht 3 als einstückiges Wandelement 1 dient.Furthermore, it is also possible to prefabricate a composite panel connected via the layer 3, which then serves as an integral wall element 1 after the layer 3 has hardened.
Nach einem besonders bevorzugten Verfahren setzt man Aluminiumhydroxid als wasserabspaltendes Hydroxid in zwei Fraktionen mit unterschiedlichen Korngrößenbereichen sowie Kieselsol als anorganisches Bindemittel einBevorzugt ist hierbei eine Mischung von 15,3 Gew.-% Aluminiumhydroxid mit einem Korngrößenbereich von 0,9 bis 1,3 µm, 61,2 Gew.-% Aluminiumhydroxid mit einem Korngrößenbereich von 15 bis 25 µm in fester Form oder in Form einer plastischen wäßrigen Aufschlämmung sowie 23,5 Gew.-% Kieselsol. Anstelle des Kieselsols kann auch beispielsweise gelöstes Wasserglas als anorganisches Bindemittel verwendet werden.According to a particularly preferred process, aluminum hydroxide is used as the water-releasing hydroxide in two fractions with different particle size ranges and silica sol as the inorganic binder. A mixture of 15.3% by weight aluminum hydroxide with a particle size range of 0.9 to 1.3 µm, 61 is preferred , 2 wt .-% aluminum hydroxide with a grain size range of 15 to 25 microns in solid form or in the form of a plastic aqueous slurry and 23.5 wt .-% silica sol. Instead of the silica sol, for example, dissolved water glass can also be used as the inorganic binder.
Die Verwendung eines wasserabspaltenden Hydroxids mit zwei unterschiedlichen Korngrößenfraktionen bewirkt durch das Ausfüllen der Pakkungslücken der gröberen Fraktion durch die Partikel der kleineren Fraktion eine dichtere Packung des sich endotherm umwandelnden Hydroxid-Materials. Ferner kann hierdurch relativ teures Bindemittel eingespart werden.The use of a water-releasing hydroxide with two different particle size fractions results in the packing of the coarser fraction being filled by the particles of the smaller fraction in a denser packing of the endothermic converting hydroxide material. It also saves relatively expensive binders.
Aufgrund des angegebenen Konzentrationsverhältnisses zwischen Hydroxid und Bindemittel wird eine Durchfeuchtung der unterliegenden bzw. der ggf. aufgelegten Dämmlage weitgehend vermieden, da das wasserabspaltende Hydroxid nicht mehr absedimentiert und damit nicht in die Fasern der Dämmlage eingespült wird.Due to the specified concentration ratio between hydroxide and binding agent, moisture penetration of the underlying or possibly applied insulation layer is largely avoided, since the water-releasing hydroxide is no longer sedimented and is therefore not washed into the fibers of the insulation layer.
Die Bestandteile vermischt man z. B. in einer Förderschnecke und bringt das erhaltene homogene Gemisch auf eine der beiden Dämmlagen in einer gleichmäßigen Schicht auf. Anschließend wird die Schicht 3 getrocknet oder ggf. die zweite Dämmlage auf die noch feuchte Schicht aufgebracht, angedrückt und getrocknet.The ingredients are mixed e.g. B. in a screw conveyor and brings the homogeneous mixture obtained onto one of the two insulation layers in a uniform layer. Then layer 3 is dried or, if necessary, the second insulation layer is applied to the still moist layer, pressed on and dried.
Es ist jedoch auch vorteilhaft möglich, durch einen geringen, brandtechnisch hinsichtlich seiner Menge irrelevanten Zusatz eines organischen Stoffes, wie z B. Tylose oder Stärke, Kieselsol oder Wasserglas aus Kostengründen zu ersetzen, da solche Polysaccharide bzw. Polysaccharidderivate schon in geringen Mengen eine relativ hohe Klebekraft haben und somit das anorganische Bindemittel zum Teil ersetzen können. Dies gilt ebenfalls für eine Vielzahl von wasserlöslichen Kunstharzen.However, it is also advantageously possible to replace it with a small addition of an organic substance, such as tylose or starch, silica sol or water glass, which is irrelevant in terms of fire, for cost reasons, since such polysaccharides or polysaccharide derivatives are relatively high even in small amounts Have adhesive power and can thus partially replace the inorganic binder. This also applies to a large number of water-soluble synthetic resins.
Wenn Tylose als organischer Zusatz Verwendung findet, wird bevorzugt eine Mischung aus 96,5 Gew.-% Aluminiumhydroxid, 3,45 Gew.-% Kieselsol und 0,05 Gew.-% Tylose, bezogen auf die trockene Mischung, zur Herstellung der Schicht 3 verwendet. Dies entspricht einer Einsparung von weit mehr als der Hälfte der üblichen Kieselsolmenge.If tylose is used as an organic additive, a mixture of 96.5% by weight aluminum hydroxide, 3.45% by weight silica sol and 0.05% by weight tylose, based on the dry mixture, is preferably used to produce the layer 3 used. This corresponds to a saving of far more than half of the usual amount of silica sol.
Eine weitere vorteilhafte Ausführungsform verwendet zur Armierung der Schicht 3 ein Glasfaservlies, welches beispielsweise ein Gewicht von 50 g/m2 aufweist. Das Vlies wird auf die Schicht 3 aus Aluminiumhydroxid und Bindemittel z. B. durch Aufwalzen aufgebracht, wobei das Vlies nach Trocknung und Aushärtung mit der Schicht 3 verbunden ist.A further advantageous embodiment uses a glass fiber fleece to reinforce layer 3, which for example has a weight of 50 g / m 2 . The fleece is on the layer 3 of aluminum hydroxide and binder z. B. applied by rolling, the fleece is connected to the layer 3 after drying and curing.
Weiterhin bewirkt die Verwendung eines auf die Hydroxidmischung aufgebrachten Vlieses eine Verminderung der Rißbildung, insbesondere wenn die Schicht 3 einer Schnelltrocknung z. B. 3 min bei 600°C unterzogen wird.Furthermore, the use of a fleece applied to the hydroxide mixture reduces the formation of cracks, especially when the layer 3 is subjected to rapid drying, e.g. B. 3 minutes at 600 ° C.
Die Ausführungsform des erfindungsgemäßen Verfahrens ist besonders wirtschaftlich, da das Verfahren kontinuierlich oder halbkontinuierlich durchgeführt werden kann und die Mischung nach Maßgabe ihres Verbrauches zur Herstellung der Schicht hergestellt werden kann.The embodiment of the process according to the invention is particularly economical since the process can be carried out continuously or semi-continuously and the mixture can be produced in accordance with its consumption for the production of the layer.
Claims (19)
- Fire resistant wall element reducing the passing through of heat in particular as insertion for a fire retarding door with at least two insulating layers (2, 2a) made of bound mineral wool between which a layer (3) made of a mixture of at least mainly inorganic material is disposed, characterised in that the layer (3) is made of a water separating hydroxide other than kaolin and a binding material, and
whereby the water separating hydroxide is inserted in different fractions with at least two grain size fractions. - Wall element according to claim 1, characterised in that the water separating hydroxide is aluminum hydroxide.
- Wall element according to claim 1 or 2, characterised in that the average grain size of the finest fraction is under 2µm.
- Wall element according to claim 1 or 2, characterised in that the average grain size of the coarsest fraction is under 100 µm and preferably under 30µm.
- Wall element according to one of the claims 1 to 4, characterised in that the binding material is silicic sol and/or water glass.
- Wall element according to one of the claims 1 to 5, characterised in that the binding material contains an organic addition selected from the group consisting of: polysaccharides, polysaccharide derivatives in particular polysaccharide ethers preferably cellulose ethers and synthetic resins in fine technically irrelevant concentration.
- Wall element according to one of the claims 1 to 6, characterised in that the layer is made of a mixture of 70 to 95 weight % aluminum hydroxide calculated at Al(OH)3 and 30 to 5 weight % silicic sol calculated as SiO2.
- Wall element according to claim 7, characterised in that the mixture consists of about 85 parts by weight of aluminum hydroxide and about 15 parts by weight of silicic sol.
- Wall element according to one of the claims 1 to 8, characterised in that the mixture contains, instead of a part of the water separating hydroxide or additionally to the water separating hydroxide, a clay or a clay mineral.
- Wall element according to one of the claims 1 to 9, characterised in that the layer has a smaller thickness than each insulating layer.
- Wall element according to claim 10, characterised in that the layer is 1 to 5mm in particular 3mm thick.
- Wall element according to claim 10 or 11, characterised in that the layer has a fleece in particular a fleece of glass fibres.
- Wall element according to claims 1 to 12, characterised in that the mineral wall is rock wool.
- Process for the manufacture of a fire resistant wall element reducing the passing through of heat, according to claim 1, characterised in that in a continuous process a binding material is added to a water separating hydroxide other than kaolin in solid form or in aqueous slimewhereby the hydroxide is inserted in different fractions with at least two grain size fractions for achieving a larger packing density;both constituents are mixed;the mixture obtained brought to one of the two insulating layers (2, 2a) in uniform layer (3) and the binding material can be consolidated by drying and the second insulating layer (2a) brought onto the consolidated layer (3) or the second insulating layer (2a) is brought on to the still moist layer (3) and the binding material can then be consolidated.
- A process according to Claim 14, characterised in that the binder used is silica sol or dissolved water glass.
- A process according to Claim 14 or 15, characterised in that an organic substance consisting of: polysaccharides, polysaccharide derivatives, especially polysaccharide ethers, preferably cellulose ethers and plastic resins, is added to the binder in a concentration which is irrelevant with regard to burning properties.
- A process according to Claim 14, characterised in that a finest fraction with an average particle size of less than 2 µm is used.
- A process according to Claim 14, characterised in that a coarsest fraction with an average particle size of less than 100 µm and preferably less than 30 µm is used.
- A process according to Claims 14 to 18, characterised in that the layer is reinforced with a matting, in particular a matting of glass fibres.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE3824598 | 1988-07-19 | ||
| DE3824598A DE3824598A1 (en) | 1988-07-19 | 1988-07-19 | INSERT FOR FIRE PROTECTION DOORS WITH PEBBLE SOL |
Publications (4)
| Publication Number | Publication Date |
|---|---|
| EP0353540A2 EP0353540A2 (en) | 1990-02-07 |
| EP0353540A3 EP0353540A3 (en) | 1991-12-11 |
| EP0353540B1 EP0353540B1 (en) | 1994-10-05 |
| EP0353540B2 true EP0353540B2 (en) | 1997-12-29 |
Family
ID=6359119
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP89113178A Expired - Lifetime EP0353540B2 (en) | 1988-07-19 | 1989-07-18 | Fireproof doors insert with silica sol |
Country Status (4)
| Country | Link |
|---|---|
| EP (1) | EP0353540B2 (en) |
| AT (1) | ATE112601T1 (en) |
| DE (2) | DE3824598A1 (en) |
| DK (1) | DK354289A (en) |
Families Citing this family (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE4036088A1 (en) * | 1990-11-13 | 1992-05-14 | Gruenzweig & Hartmann | METAL HYDROXIDE AND MAGNESIABINDER FIRE PROTECTION AGENTS AND THEIR USE |
| DE4313820C2 (en) * | 1993-04-27 | 2003-04-10 | Woellner Silikat Gmbh | Coating compound and its use |
| DE4338619C5 (en) * | 1993-11-11 | 2007-12-27 | Saint-Gobain Isover G+H Ag | Coated mineral wool product and process for its production |
| NL9400839A (en) * | 1994-05-24 | 1996-01-02 | Isobouw Systems Bv | Panel. |
| DE29507498U1 (en) * | 1995-05-05 | 1995-07-13 | Gruenzweig & Hartmann | Fire protection element with a layered structure, in particular as an insert for fire protection doors, and semi-finished products therefor |
| ES2102969B1 (en) * | 1995-06-27 | 1998-04-01 | Andreu Barbera Jose Vicente | PERFECTED INSULATION FOR FIREWALL DOORS |
| DE19525961A1 (en) * | 1995-07-17 | 1997-01-23 | Webu Spezialtueren Gmbh | Fire door with improved fire resistance |
| DE19546980C2 (en) * | 1995-12-15 | 1999-08-19 | Gruenzweig & Hartmann | Fire protection element |
| DE29915463U1 (en) * | 1999-09-03 | 2001-01-18 | Rockwool Mineralwolle | Insulating element for thermal and / or acoustic insulation of building walls |
| DE19952931A1 (en) * | 1999-11-03 | 2001-05-10 | Saint Gobain Isover G & H Ag | Bound mineral wool product with fire protection function and fire protection element with the bound mineral wool product |
| EP1239093A3 (en) * | 2001-02-21 | 2003-08-06 | Deutsche Rockwool Mineralwoll GmbH & Co. OHG | Fire protection element, particularly for fireproof doors |
| DE10212331A1 (en) * | 2002-03-20 | 2003-10-16 | Rockwool Mineralwolle | Fire protection element, especially for fire protection doors |
| DE10212332B4 (en) | 2002-03-20 | 2004-02-12 | Deutsche Rockwool Mineralwoll Gmbh & Co. Ohg | Fire protection element, especially for fire protection doors |
| BE1014759A6 (en) * | 2002-04-15 | 2004-03-02 | Wienerberger Bricks N V | Process for the prefabrication of a wall element. |
| ITVI20060160A1 (en) * | 2006-05-25 | 2007-11-26 | Giampaolo Nelzi | FIRE DOOR WITH VERTICAL AND / OR HORIZONTAL OPENING |
| EP1900884B1 (en) * | 2006-07-19 | 2008-08-13 | Pavatex SA | Soft woodfibre panel for internal insulation |
| CH706060B1 (en) * | 2012-01-16 | 2015-09-15 | Rwd Schlatter Ag | Door-layer board, door, use the door and kit. |
| ITMO20120297A1 (en) * | 2012-11-28 | 2014-05-29 | Basic & Co Srl | FIREWAY DOOR |
| NL2016698B1 (en) * | 2016-04-29 | 2017-11-20 | M J Vroegop Holding B V | Fire resistant panel. |
| US20230282924A1 (en) * | 2022-03-01 | 2023-09-07 | GM Global Technology Operations LLC | Intumescent inorganic composites for mitigating a thermal runaway event in a battery |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE7321823U (en) * | 1973-09-27 | Bolliger W | Fire protection component | |
| DK364375A (en) * | 1975-08-12 | 1977-02-13 | Rockwool As | BRANDDROJ PLADE |
| DE3220821C2 (en) * | 1982-06-03 | 1985-02-07 | Karl 6902 Sandhausen Serwane | Fire-proof waterproofing membrane |
| DE3510935A1 (en) * | 1985-03-26 | 1986-10-09 | Grünzweig + Hartmann und Glasfaser AG, 6700 Ludwigshafen | Fire-resistant wall element which reduces the passage of heat and is intended in particular as an insert for a fire-retardant door, process for the production thereof and fire-retardant door equipped therewith |
| DE3540524A1 (en) * | 1985-11-15 | 1987-05-27 | Bayer Ag | FUEL-CONTAINING INTUMESCENT MATERIALS BASED ON EPOXY RESIN |
| DE3607839A1 (en) * | 1986-03-10 | 1987-09-17 | Paul Couwenbergs | Production and process of a double-sided flame-retardant, insulating, sound-absorbing compartment panel for walls and ceilings and for cable partitioning - with the use of hot-dip galvanised general purpose mounts |
-
1988
- 1988-07-19 DE DE3824598A patent/DE3824598A1/en not_active Withdrawn
-
1989
- 1989-07-18 AT AT89113178T patent/ATE112601T1/en not_active IP Right Cessation
- 1989-07-18 EP EP89113178A patent/EP0353540B2/en not_active Expired - Lifetime
- 1989-07-18 DK DK354289A patent/DK354289A/en not_active Application Discontinuation
- 1989-07-18 DE DE58908472T patent/DE58908472D1/en not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| EP0353540A3 (en) | 1991-12-11 |
| EP0353540A2 (en) | 1990-02-07 |
| DK354289A (en) | 1990-01-20 |
| EP0353540B1 (en) | 1994-10-05 |
| ATE112601T1 (en) | 1994-10-15 |
| DK354289D0 (en) | 1989-07-18 |
| DE58908472D1 (en) | 1994-11-10 |
| DE3824598A1 (en) | 1990-01-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0353540B2 (en) | Fireproof doors insert with silica sol | |
| EP0114965B1 (en) | Coated insulating slabs for façades and roofings based on mineral fibres, and method for their production | |
| EP0485867B1 (en) | Fire-proofing composition comprising metallic hydroxyde and magnesia-binders and its use | |
| DE60009853T2 (en) | Binder compositions for binding particulate material | |
| DE2725779A1 (en) | SELF-HOLDING COMPOUND FOR A FIRE-RESISTANT LAYER | |
| DE2842858C3 (en) | Composite panel with two cover sheets and a core | |
| EP1097807B1 (en) | Fire protecting bound mineral wool product and fire protection element comprising said product | |
| DE3325467A1 (en) | CELLULAR GLASS COATED WITH A HEAT-INSULATING MATERIAL | |
| DE2043238A1 (en) | Process for the production of lightweight foamed moldings | |
| EP0741003B1 (en) | Fire protection element with layered structure, particularly as insert for fireproof doors and semi-product for use in the element | |
| AT391107B (en) | COMPOSITE COMPONENT, CONSISTING OF AT LEAST TWO PARTS OF DIFFERENT FIBER MATERIALS | |
| DE69627897T2 (en) | HEAT-INSULATING COMPONENTS | |
| DE4401983C2 (en) | Insulation material made of straw, its manufacture and use | |
| DE2831505C2 (en) | Refractory, exothermic, thermally insulating article, its use and method for its manufacture | |
| DE3105593C2 (en) | Process for the production of plastic masses for further processing into fire-resistant or refractory materials, masses produced by the process and their use | |
| DD202040A5 (en) | PROCESS FOR PRODUCING CERAMIC FIBERS CONTAINING, FIRE-RESISTANT OR FIRE-RESISTANT MASSES | |
| EP0310138A1 (en) | Building element and process for its production | |
| DE2217315B2 (en) | Injectable refractory masses and methods of making them | |
| EP0829459A1 (en) | Non-inflammable heat insulating panels based on expanded perlite granules | |
| DE3248664A1 (en) | Coated facade or roof insulating panel of mineral fibres and process for its production | |
| CH691541A5 (en) | Fire-resistant substance mixture. | |
| DE3413679A1 (en) | HEAT STORAGE BLOCKS AND THEIR PRODUCTION AND AN ELECTRIC HEAT STORAGE UNIT CONTAINING THESE BLOCKS | |
| DE4313820C2 (en) | Coating compound and its use | |
| CH618917A5 (en) | Mineral fibre board adhesion-bonded to a metal foil | |
| DE19546980C2 (en) | Fire protection element |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): AT BE CH DE ES FR GB IT LI NL SE |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| RHK1 | Main classification (correction) |
Ipc: E04B 1/94 |
|
| AK | Designated contracting states |
Kind code of ref document: A3 Designated state(s): AT BE CH DE ES FR GB IT LI NL SE |
|
| 17P | Request for examination filed |
Effective date: 19920214 |
|
| 17Q | First examination report despatched |
Effective date: 19930319 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE ES FR GB IT LI NL SE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRE;WARNING: LAPSES OF ITALIAN PATENTS WITH EFFECTIVE DATE BEFORE 2007 MAY HAVE OCCURRED AT ANY TIME BEFORE 2007. THE CORRECT EFFECTIVE DATE MAY BE DIFFERENT FROM THE ONE RECORDED.SCRIBED TIME-LIMIT Effective date: 19941005 Ref country code: ES Free format text: THE PATENT HAS BEEN ANNULLED BY A DECISION OF A NATIONAL AUTHORITY Effective date: 19941005 Ref country code: BE Effective date: 19941005 Ref country code: NL Effective date: 19941005 Ref country code: GB Effective date: 19941005 Ref country code: FR Effective date: 19941005 |
|
| REF | Corresponds to: |
Ref document number: 112601 Country of ref document: AT Date of ref document: 19941015 Kind code of ref document: T |
|
| REF | Corresponds to: |
Ref document number: 58908472 Country of ref document: DE Date of ref document: 19941110 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Effective date: 19950105 |
|
| EN | Fr: translation not filed | ||
| NLV1 | Nl: lapsed or annulled due to failure to fulfill the requirements of art. 29p and 29m of the patents act | ||
| GBV | Gb: ep patent (uk) treated as always having been void in accordance with gb section 77(7)/1977 [no translation filed] |
Effective date: 19941005 |
|
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Effective date: 19950718 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Effective date: 19950731 Ref country code: LI Effective date: 19950731 |
|
| 26 | Opposition filed |
Opponent name: ROCKWOOL INTERNATIONAL A/S Effective date: 19950630 |
|
| PLBF | Reply of patent proprietor to notice(s) of opposition |
Free format text: ORIGINAL CODE: EPIDOS OBSO |
|
| PLBF | Reply of patent proprietor to notice(s) of opposition |
Free format text: ORIGINAL CODE: EPIDOS OBSO |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PLAW | Interlocutory decision in opposition |
Free format text: ORIGINAL CODE: EPIDOS IDOP |
|
| PLAW | Interlocutory decision in opposition |
Free format text: ORIGINAL CODE: EPIDOS IDOP |
|
| PUAH | Patent maintained in amended form |
Free format text: ORIGINAL CODE: 0009272 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: PATENT MAINTAINED AS AMENDED |
|
| 27A | Patent maintained in amended form |
Effective date: 19971229 |
|
| AK | Designated contracting states |
Kind code of ref document: B2 Designated state(s): AT BE CH DE ES FR GB IT LI NL SE |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: AEN Free format text: AUFRECHTERHALTUNG DES PATENTES IN GEAENDERTER FORM |
|
| EN | Fr: translation not filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20020806 Year of fee payment: 14 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20040203 |