EP0023184A1 - Procédé et installation de décapage thermochimique de pièces en acier inoxydable - Google Patents
Procédé et installation de décapage thermochimique de pièces en acier inoxydable Download PDFInfo
- Publication number
- EP0023184A1 EP0023184A1 EP80401096A EP80401096A EP0023184A1 EP 0023184 A1 EP0023184 A1 EP 0023184A1 EP 80401096 A EP80401096 A EP 80401096A EP 80401096 A EP80401096 A EP 80401096A EP 0023184 A1 EP0023184 A1 EP 0023184A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- bath
- parts
- sulfuric
- pickling
- rinsing
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 238000000034 method Methods 0.000 title claims abstract description 9
- 238000005554 pickling Methods 0.000 title claims description 19
- 238000009434 installation Methods 0.000 title claims description 11
- 229910001220 stainless steel Inorganic materials 0.000 title claims description 9
- 239000010935 stainless steel Substances 0.000 title claims description 8
- 230000008569 process Effects 0.000 title description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 claims abstract description 19
- 238000011282 treatment Methods 0.000 claims abstract description 7
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims abstract description 6
- 239000002253 acid Substances 0.000 claims description 11
- 238000007654 immersion Methods 0.000 claims description 6
- 230000009466 transformation Effects 0.000 claims description 4
- 229910000831 Steel Inorganic materials 0.000 claims description 2
- 239000010959 steel Substances 0.000 claims description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 claims 2
- 238000010438 heat treatment Methods 0.000 claims 1
- JOPOVCBBYLSVDA-UHFFFAOYSA-N chromium(6+) Chemical compound [Cr+6] JOPOVCBBYLSVDA-UHFFFAOYSA-N 0.000 abstract description 5
- 230000002378 acidificating effect Effects 0.000 abstract 1
- VWDWKYIASSYTQR-UHFFFAOYSA-N sodium nitrate Chemical compound [Na+].[O-][N+]([O-])=O VWDWKYIASSYTQR-UHFFFAOYSA-N 0.000 description 8
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 7
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 6
- 239000002351 wastewater Substances 0.000 description 6
- 229910052804 chromium Inorganic materials 0.000 description 5
- 239000011651 chromium Substances 0.000 description 5
- 229910052751 metal Inorganic materials 0.000 description 3
- 239000002184 metal Substances 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 235000010344 sodium nitrate Nutrition 0.000 description 3
- 239000004317 sodium nitrate Substances 0.000 description 3
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical class O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 2
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 2
- 238000010306 acid treatment Methods 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 1
- 101100165177 Caenorhabditis elegans bath-15 gene Proteins 0.000 description 1
- 229910000975 Carbon steel Inorganic materials 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 239000005569 Iron sulphate Substances 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 229940105847 calamine Drugs 0.000 description 1
- 239000010962 carbon steel Substances 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 238000010908 decantation Methods 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 229910052864 hemimorphite Inorganic materials 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 235000014413 iron hydroxide Nutrition 0.000 description 1
- 159000000014 iron salts Chemical class 0.000 description 1
- BAUYGSIQEAFULO-UHFFFAOYSA-L iron(2+) sulfate (anhydrous) Chemical compound [Fe+2].[O-]S([O-])(=O)=O BAUYGSIQEAFULO-UHFFFAOYSA-L 0.000 description 1
- NCNCGGDMXMBVIA-UHFFFAOYSA-L iron(ii) hydroxide Chemical compound [OH-].[OH-].[Fe+2] NCNCGGDMXMBVIA-UHFFFAOYSA-L 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 239000007800 oxidant agent Substances 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 238000005096 rolling process Methods 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052938 sodium sulfate Inorganic materials 0.000 description 1
- 235000011152 sodium sulphate Nutrition 0.000 description 1
- 238000005063 solubilization Methods 0.000 description 1
- 230000007928 solubilization Effects 0.000 description 1
- 238000004381 surface treatment Methods 0.000 description 1
- 238000010301 surface-oxidation reaction Methods 0.000 description 1
- 239000003643 water by type Substances 0.000 description 1
- 239000011787 zinc oxide Substances 0.000 description 1
- 235000014692 zinc oxide Nutrition 0.000 description 1
- CPYIZQLXMGRKSW-UHFFFAOYSA-N zinc;iron(3+);oxygen(2-) Chemical compound [O-2].[O-2].[O-2].[O-2].[Fe+3].[Fe+3].[Zn+2] CPYIZQLXMGRKSW-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23G—CLEANING OR DE-GREASING OF METALLIC MATERIAL BY CHEMICAL METHODS OTHER THAN ELECTROLYSIS
- C23G3/00—Apparatus for cleaning or pickling metallic material
- C23G3/02—Apparatus for cleaning or pickling metallic material for cleaning wires, strips, filaments continuously
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23G—CLEANING OR DE-GREASING OF METALLIC MATERIAL BY CHEMICAL METHODS OTHER THAN ELECTROLYSIS
- C23G1/00—Cleaning or pickling metallic material with solutions or molten salts
- C23G1/28—Cleaning or pickling metallic material with solutions or molten salts with molten salts
- C23G1/32—Heavy metals
Definitions
- the present invention relates to the pickling of raw foundry parts by thermochemical means.
- thermochemical pickling of carbon steel parts first involves immersing these parts in a molten soda bath, maintained at around 450 ° C and generally containing a lower proportion of sodium nitrate as an oxidizing agent, to dissolve a certain number of oxides of elements present in stainless steels.
- Certain other oxides which are not soluble in soda must be eliminated in a subsequent acid treatment bath, which generally includes a sulfuric bath to neutralize the last traces of base and reduce part of the iron salts, then a fluonitric bath to complete the solubilization of the last metal salts covering the surface of the parts to be stripped.
- the wastewater from this rinsing operation poses a serious pollution problem because it contains soluble chromium in the hexavalent state considered to be particularly harmful and which cannot be discharged into the sewer.
- the object of the present invention is to provide an improvement to this technique for pickling raw stainless steel parts and it consists in passing these parts directly from the molten soda bath to the subsequent acid treatment bath, by eliminating the intermediate step of rinse with water.
- thermochemical pickling of stainless steel parts in which there is direct immersion of the parts coming from the molten soda bath in the acid bath.
- the Applicant has verified by chemical analysis that there occurs, within the acid bath, a self-reduction of the hexavalent chromium formed in the sodium hydroxide bath.
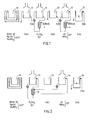
- FIG. 1 shows a conventional installation for thermochemical pickling
- FIG. 2 an installation according to the invention.
- This installation differs essentially from that shown in Figure 1 on the one hand by the elimination of the rinsing bath 11 and on the other hand by the addition of a heat exchanger 16; this exchanger 16 takes heat from the circulation circuit of the sulfuric pickling bath 12 to transmit it to the circulation circuit of the fluonitric pickling bath 14.
- the method according to the invention therefore makes it possible to save energy while considerably simplifying the treatment of waste water from a thermochemical pickling installation.
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Mechanical Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Cleaning And De-Greasing Of Metallic Materials By Chemical Methods (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR7919021 | 1979-07-24 | ||
| FR7919021A FR2461765A1 (fr) | 1979-07-24 | 1979-07-24 | Procede et installation de decapage thermochimique de pieces en acier inoxydable |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| EP0023184A1 true EP0023184A1 (fr) | 1981-01-28 |
Family
ID=9228211
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP80401096A Withdrawn EP0023184A1 (fr) | 1979-07-24 | 1980-07-23 | Procédé et installation de décapage thermochimique de pièces en acier inoxydable |
Country Status (4)
| Country | Link |
|---|---|
| EP (1) | EP0023184A1 (enExample) |
| JP (1) | JPS5620171A (enExample) |
| ES (2) | ES493624A0 (enExample) |
| FR (1) | FR2461765A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0507006A1 (en) * | 1991-04-02 | 1992-10-07 | Unitika Ltd. | Method of treating salt bath liquid |
| CN105483739A (zh) * | 2015-12-08 | 2016-04-13 | 无锡华工薄板有限公司 | 一种带钢酸洗槽用浓度控制系统 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1035596A (en) * | 1965-06-28 | 1966-07-13 | Allegheny Ludlum Steel | Improvements in or relating to a process of descaling stainless steel |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5157641A (en) * | 1974-11-18 | 1976-05-20 | Nippon Steel Corp | Sutenresukono datsusukeeruhoho |
-
1979
- 1979-07-24 FR FR7919021A patent/FR2461765A1/fr active Granted
-
1980
- 1980-07-23 JP JP10103780A patent/JPS5620171A/ja active Pending
- 1980-07-23 ES ES493624A patent/ES493624A0/es active Granted
- 1980-07-23 EP EP80401096A patent/EP0023184A1/fr not_active Withdrawn
- 1980-09-15 ES ES495055A patent/ES8107332A1/es not_active Expired
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1035596A (en) * | 1965-06-28 | 1966-07-13 | Allegheny Ludlum Steel | Improvements in or relating to a process of descaling stainless steel |
Non-Patent Citations (2)
| Title |
|---|
| THE MAKING, SHAPING AND TREATING OF STEEL Ed. H.E. McGANNON, 9eme edition, 1971,pages 1172-1173 Herbick & Held Pittsburgh US * |
| WERKSTOFFE UND KORROSION, Vol. 25, No 9, 1974, page 695, nr. 74-1643. * |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0507006A1 (en) * | 1991-04-02 | 1992-10-07 | Unitika Ltd. | Method of treating salt bath liquid |
| US5496449A (en) * | 1991-04-02 | 1996-03-05 | Unitika, Ltd. | Method of treating salt bath liquid |
| CN105483739A (zh) * | 2015-12-08 | 2016-04-13 | 无锡华工薄板有限公司 | 一种带钢酸洗槽用浓度控制系统 |
Also Published As
| Publication number | Publication date |
|---|---|
| ES8104434A1 (es) | 1981-04-16 |
| ES493624A0 (es) | 1981-04-16 |
| FR2461765B1 (enExample) | 1981-12-04 |
| ES495055A0 (es) | 1981-08-16 |
| JPS5620171A (en) | 1981-02-25 |
| ES8107332A1 (es) | 1981-08-16 |
| FR2461765A1 (fr) | 1981-02-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| FI101981B (fi) | Menetelmä ruostumattoman teräksen peittaukseen ja passivointiin ilman typpihappoa | |
| US5490908A (en) | Annealing and descaling method for stainless steel | |
| KR890001379B1 (ko) | 산화물 스케일형성조절 및 스케일제거를 포함하는 금속 제품의 제조공정 | |
| US3951681A (en) | Method for descaling ferrous metals | |
| EP0023184A1 (fr) | Procédé et installation de décapage thermochimique de pièces en acier inoxydable | |
| RU2085616C1 (ru) | Способ травления высококачественной стали | |
| JPH0696800B2 (ja) | 冷間圧延ステンレス鋼シートおよびストリップの処理法 | |
| Hudson | Pickling and descaling | |
| KR100437641B1 (ko) | 페라이트계 스테인레스강의 산세방법 | |
| US3778309A (en) | Descaling process for alloys containing chromium | |
| JPH11279781A (ja) | 表面に模様のないオーステナイト系ステンレス鋼板の製造方法 | |
| JPH01162786A (ja) | 高強度オーステナイト系ステンレス鋼の酸洗方法 | |
| JPS61167000A (ja) | 連続焼鈍鋼帯の酸化膜除去方法 | |
| JP2517353B2 (ja) | ステンレス鋼帯の脱スケ―ル方法 | |
| JP3299389B2 (ja) | Ni系ステンレス鋼板の酸洗方法 | |
| JPH09324286A (ja) | ステンレス鋼帯,耐熱鋼帯の脱スケール方法 | |
| JP3169872B2 (ja) | 冷間圧延ステンレス鋼帯の連続焼鈍酸洗方法及び装置 | |
| JPH0390600A (ja) | 耐食性と外観性の優れたCr含有冷延鋼板の製造法 | |
| KR100514781B1 (ko) | 환원방식의 전처리에 의한 마르텐사이트계 스테인레스강선재의 산세 방법 | |
| JP2003119551A (ja) | 極めて良好な表面光沢度を有するステンレス鋼冷間圧延鋼帯 | |
| JPS5920752B2 (ja) | オ−ステナイト系ステンレス鋼板の酸洗方法 | |
| KR950004239B1 (ko) | 오스테나이트계 스텐레스 냉연소둔강판의 전해산세방법 | |
| SU872579A1 (ru) | Способ изготовлени труб из нержавеющих сталей и сплавов | |
| JPS5953685A (ja) | ステンレス鋼帯の酸洗方法 | |
| JPH05331699A (ja) | フェライト系ステンレス焼鈍鋼帯のデスケーリング方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR GB IT LU NL SE |
|
| 17P | Request for examination filed |
Effective date: 19801211 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION IS DEEMED TO BE WITHDRAWN |
|
| 18D | Application deemed to be withdrawn |
Effective date: 19841107 |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: ANTOINE, LOUIS Inventor name: DUBOURG, JEAN-CLAUDE |