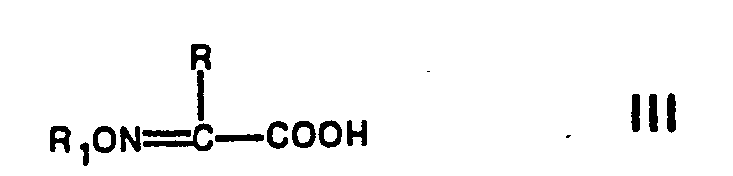EP0000005B1 - Nouveaux dérivés de céphalosporines, leurs préparations et leurs compositions pharmaceutiques - Google Patents
Nouveaux dérivés de céphalosporines, leurs préparations et leurs compositions pharmaceutiques Download PDFInfo
- Publication number
- EP0000005B1 EP0000005B1 EP78100078A EP78100078A EP0000005B1 EP 0000005 B1 EP0000005 B1 EP 0000005B1 EP 78100078 A EP78100078 A EP 78100078A EP 78100078 A EP78100078 A EP 78100078A EP 0000005 B1 EP0000005 B1 EP 0000005B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- salts
- formula
- compounds
- methyl
- hydrates
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
- 0 **(*[C@@]([C@@](C1*2)SCC(*(S*)=C)=C2C(O)=O)C1=S=C)=[N+][O-] Chemical compound **(*[C@@]([C@@](C1*2)SCC(*(S*)=C)=C2C(O)=O)C1=S=C)=[N+][O-] 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D501/00—Heterocyclic compounds containing 5-thia-1-azabicyclo [4.2.0] octane ring systems, i.e. compounds containing a ring system of the formula:, e.g. cephalosporins; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring
- C07D501/14—Compounds having a nitrogen atom directly attached in position 7
- C07D501/16—Compounds having a nitrogen atom directly attached in position 7 with a double bond between positions 2 and 3
- C07D501/20—7-Acylaminocephalosporanic or substituted 7-acylaminocephalosporanic acids in which the acyl radicals are derived from carboxylic acids
- C07D501/24—7-Acylaminocephalosporanic or substituted 7-acylaminocephalosporanic acids in which the acyl radicals are derived from carboxylic acids with hydrocarbon radicals, substituted by hetero atoms or hetero rings, attached in position 3
- C07D501/36—Methylene radicals, substituted by sulfur atoms
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
Definitions
- the present invention relates to new acyl derivatives of the general formula in which R is furyl, thienyl or phenyl which is optionally substituted by halogen, hydroxy, lower alkoxy or lower alkyl, R 1 is lower alkyl or aminocarbonylmethyl and X is a group of the formulas in which one of the two radicals R 2 and R 3 or R 4 and R 5 is hydrogen and the other is lower alkyl, carboxymethyl or sulfomethyl, and salts of these compounds and hydrates of these salts.
- salts of the compounds of formula 1 are alkali metal salts such as the sodium and potassium salt; the ammonium salt; Alkaline earth metal salts such as the calcium salt; Salts with organic bases, such as salts with amines, e.g. Salts with N-ethyl-piperidine, procaine, dibenzylamine, N, N'-dibenzyl-ethyl-ethylenediamine, alkylamines or dialkylamines, and salts with amino acids, such as e.g. Salts with arginine or lysine.
- the salts can be mono-salts or also disalts.
- the second salt formation takes place on the tautomeric enol form of the triazine residue b, which has an acid character.
- the compounds of formula I also form addition salts with organic or inorganic acids.
- such salts are hydrohalides, for example hydrochlorides, hydrobromides; Hydroiodides, as well as other minor acid salts, such as sulfates, nitrates, phosphates and the like, alkyl and mono-aryl sulfonates, such as ethanesulfonates, toluenesulfonates, benzenesulfonates and the like, and also other organic acid salts, such as acetates, tartrates, maleates, citrates, benzoates, salic , Abscorbate and the like
- the salts of the compounds of formula I can be hydrated.
- the hydration can take place in the course of the production process or can occur gradually as a result of hygroscopic properties of an initially anhydrous salt of a compound of the formula I.
- lower alkyl groups are either straight chain or branched and can contain up to 7 carbon atoms, e.g. Methyl, ethyl, n-propyl, isopropyl, n-pentyl, n-heptyl, lower alkoxy groups have an analogous meaning.
- Halogen represents all four halogens, i.e. Fluorine, chlorine, bromine and iodine; chlorine and bromine are preferred.
- Preferred R groups are furyl, thienyl and phenyl, especially furyl. Methyl is preferred as R 1 .
- Preferred X groups are group (c) and groups (a) and (b), in which one of the two radicals R 2 and R 3 or R 4 and R 5 is hydrogen and the other is methyl. Particularly preferred X groups are the 1,2,5,6-tetrahydro-2-methyl-5,6-dioxo-as-triazin-3-yl and the 1,4,5,6-tetrahydro-4-methyl - 5,6-dioxo-as-triazin-3-yl group.
- Preferred compounds are (7R) - 7 - [2 - (2 - furyl) - 2 - (methoxyimino) acetamido] - 3 - / [(1,2,5,6 - tetrahydro - 2 - methyl - 5,6 - dioxo - as - triazin - 3 - yl) thio] methyl / - 3 - cephem - 4 - carboxylic acid and its salts and the hydrates of these salts.
- the compounds of the formula I and their salts or hydrates of the salts can be in the synisomeric form or in the anti-isomeric form or as mixtures of these two forms.
- the syn-isomeric form or mixtures in which the syn-isomeric form predominates is preferred.
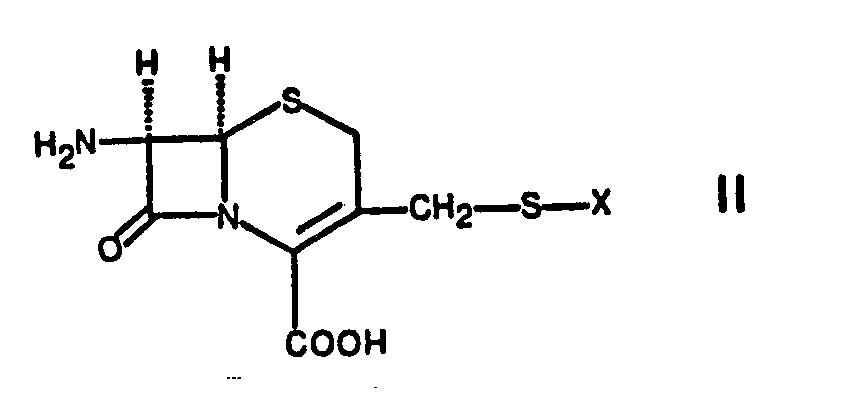
- the carboxy groups present in the starting compounds of formulas II and IV can optionally be protected, e.g. by esterification to an easily cleavable ester such as a silyl ester, e.g. the trimethylsilyl ester.
- the carboxy group can also be protected by salt formation with an inorganic or tertiary organic base such as triethylamine.
- Suitable reactive functional derivatives of acids of the formula III are e.g. Halides, i.e. Chlorides, bromides and fluorides; Azides; Anhydrides, especially mixed anhydrides with stronger acids; reactive esters, e.g. N-hydroxy succinimide esters, and amides, e.g. Imidazolides.
- Halogens e.g. Chlorine, bromine or iodine
- acyloxy residues e.g. lower alkanoyl radicals, such as acetoxy, lower alkyl or arylsulfonyloxy radicals, such as mesyloxy or tosyloxy, or the azidorest in question.
- a free acid of formula III can be condensed with one of the esters corresponding to formula II by means of a carbodiimide, such as dicyclohexylcarbodiimide, in an inert solvent, such as ethyl acetate, acetonitrile, dioxane, chloroform, methylene chloride, benzene or dimethylformamide, and then the ester group is split off .
- carbodiimides can be as Condensing agents also use oxazolium salts, for example N-ethyl-5-phenyl-isoxazolium-3'-sulfonate.
- an acid halide preferably the chloride of an acid of the formula III
- the reaction is preferably carried out in the presence of an acid binding agent, e.g. in the presence of aqueous alkali, preferably sodium hydroxide solution, or also in the presence of an alkali metal carbonate, such as potassium carbonate, or in the presence of a lower alkylated amine, such as triethylamine.
- an acid binding agent e.g. in the presence of aqueous alkali, preferably sodium hydroxide solution, or also in the presence of an alkali metal carbonate, such as potassium carbonate, or in the presence of a lower alkylated amine, such as triethylamine.
- Water is preferably used as the solvent; but it can also be in an aprotic organic solvent such as e.g. Dimethylformamide, dimethyl sulfoxide, or hexamethylphosphoric triamide can be worked.
- reaction of a compound of formula II with a compound of formula III or a reactive functional derivative thereof can be advantageously carried out at temperatures between about -40 ° C and room temperature, for example at about 0-10 o C, take place.
- reaction of a compound of formula IV with a thiol of formula V can be carried out in a manner known per se, e.g. at a temperature between about 40 and 80 ° C, suitably at about 60 ° C, in a polar solvent, for example in an alcohol, e.g. in a lower alkanol such as ethanol, propanol and the like, in dimethylformamide or dimethyl sulfoxide, preferably in water or in a buffer solution with a pH of about 6 to 7, preferably 6.5.
- a polar solvent for example in an alcohol, e.g. in a lower alkanol such as ethanol, propanol and the like, in dimethylformamide or dimethyl sulfoxide, preferably in water or in a buffer solution with a pH of about 6 to 7, preferably 6.5.
- the protective group is a silyl group (silyl ester), this group can be split off particularly easily by treating the reaction product with water. If the carboxyl group of an acid of formula IV is protected by salt formation (e.g. with triethylamine), this salt-forming protective group can be split off by treatment with acid.
- the acid can be e.g. Hydrochloric acid, sulfuric acid, phosphoric acid or citric acid can be used.
- the 7-amino compounds of the formula 11 used as starting products can be started from a compound of the formula in which Y represents a leaving group and the carboxy group can be in protected form, are prepared with a thiol of the formula V.
- the reaction can be carried out under the same conditions as those of the starting compounds IV and V.
- syn / anti mixture of a compound of the formula I obtained can be separated into the corresponding syn and anti forms in the customary manner, for example by recrystallization or by chromatographic methods using a suitable solvent or solvent mixture.
- 2,275,215 and 2,280,381 cephalosporin derivatives which can carry a substituted thiomethyl radical of the present type ⁇ CH 2 ⁇ S ⁇ X in the 3-position. It has now been found that the new cephalosporin derivatives of the formula I and their salts and the hydrates of these salts, in which the substituents mentioned in positions 7 and 3 are present simultaneously for the first time, have valuable antibiotic, in particular bactericidal activity.
- ß-lactamase-forming staphylococci and various ß-lactamase-forming gram-negative bacteria, such as, for example, Haemophilus influenzae, Escherichia coli, Proteus and Klebsiella species.
- the compounds of formula I can be used for the treatment and prophylaxis of infectious diseases.
- a daily dose of approximately 1 g to approximately 4 g is suitable for the adult.
- the parenteral administration of the compounds according to the invention is particularly preferred.
- mice are infected intraperitoneally with an aqueous suspension of Proteus mirabilis.
- the test substance is applied subcutaneously one hour after the infection.
- the number of surviving animals is determined on the 4th day.
- Different doses are applied and the dose at which 50% of the test animals survived (CD sa , mg / kg) is determined by interpolation.
- compositions can contain the compounds of formula I, their salts or hydrated forms of these salts, possibly in a mixture with another therapeutically valuable substance. They are expediently mixed with a pharmaceutical, inorganic or organic inert carrier material which is particularly suitable for parenteral administration, such as e.g. with water, gum arabic.
- the pharmaceutical preparations are preferably in liquid form, e.g. as solutions, suspensions or emulsions. If necessary, they are sterilized and / or contain auxiliaries, such as preservatives, stabilizers, wetting agents or emulsifiers, salts for changing the osmotic pressure or buffers.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Communicable Diseases (AREA)
- Pharmacology & Pharmacy (AREA)
- Oncology (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Cephalosporin Compounds (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Saccharide Compounds (AREA)
Claims (17)
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| LU77485 | 1977-06-03 | ||
| LU77485 | 1977-06-03 | ||
| CH314278 | 1978-03-22 | ||
| CH3142/78 | 1978-03-22 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0000005A1 EP0000005A1 (fr) | 1978-12-20 |
| EP0000005B1 true EP0000005B1 (fr) | 1980-10-29 |
Family
ID=25692293
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP78100078A Expired EP0000005B1 (fr) | 1977-06-03 | 1978-06-01 | Nouveaux dérivés de céphalosporines, leurs préparations et leurs compositions pharmaceutiques |
Country Status (26)
| Country | Link |
|---|---|
| EP (1) | EP0000005B1 (fr) |
| JP (1) | JPS5412394A (fr) |
| AR (1) | AR225134A1 (fr) |
| AT (2) | AT362501B (fr) |
| AU (1) | AU518648B2 (fr) |
| BR (1) | BR7803565A (fr) |
| CA (1) | CA1114808A (fr) |
| CU (1) | CU21118A (fr) |
| DE (2) | DE2860248D1 (fr) |
| DK (1) | DK246878A (fr) |
| ES (2) | ES470442A1 (fr) |
| FI (1) | FI781754A7 (fr) |
| FR (1) | FR2393000A1 (fr) |
| GB (1) | GB1599232A (fr) |
| GR (1) | GR73554B (fr) |
| HU (1) | HU182498B (fr) |
| IE (1) | IE46903B1 (fr) |
| IL (1) | IL54803A (fr) |
| IT (1) | IT1098306B (fr) |
| MC (1) | MC1195A1 (fr) |
| NL (1) | NL7805715A (fr) |
| NO (1) | NO781934L (fr) |
| NZ (1) | NZ187392A (fr) |
| PH (1) | PH14653A (fr) |
| PT (1) | PT68134A (fr) |
| SE (1) | SE7806465L (fr) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4200745A (en) * | 1977-12-20 | 1980-04-29 | Eli Lilly And Company | 7[2-(2-Aminothiazol-4-yl)-2-alkoxyimino]acetamido 3[4-alkyl-5-oxo-6-hydroxy-3,4 dihydro 1,2,4-triazin 3-yl]thio methyl cephalosporins |
| FR2432521A1 (fr) * | 1978-03-31 | 1980-02-29 | Roussel Uclaf | Nouvelles oximes o-substituees derivees de l'acide 7-amino thiazolyl acetamido cephalosporanique, leur procede de preparation et leur application comme medicaments |
| MC1259A1 (fr) * | 1978-05-30 | 1980-01-14 | Hoffmann La Roche | Derives acyles |
| NO782998L (no) * | 1978-07-19 | 1980-01-22 | Hoffmann La Roche | Cephalosporinestere og -etere. |
| US4472574A (en) * | 1981-05-22 | 1984-09-18 | Hoffman-La Roche Inc. | Process for the manufacture of a cephem carboxylic acid derivative |
| US4698338A (en) * | 1986-02-19 | 1987-10-06 | Eli Lilly And Company | 7[2-(2-aminothiazol-4-yl)-2-benzyloximino]acetamido-3[4-alkyl-5-oxo-6-hydroxy-3,4-dihydro-1,2,4-triazin-3-yl]thiomethyl cephalosporins |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1399086A (en) * | 1971-05-14 | 1975-06-25 | Glaxo Lab Ltd | Cephalosporin compounds |
| CH609989A5 (en) * | 1974-06-21 | 1979-03-30 | Hoffmann La Roche | Process for the preparation of acyl derivatives |
| CA1100129A (fr) * | 1974-08-02 | 1981-04-28 | William H.W. Lunn | Traduction non-disponible |
| GB1555471A (en) * | 1975-06-19 | 1979-11-14 | Glaxo Lab Ltd | 7 carbamoylalkoxyimino acetamido 3 em 4 carboxylic acidsand derivatives thereof |
| GB1576625A (en) * | 1976-04-12 | 1980-10-08 | Fujisawa Pharmaceutical Co | Syn isomer 3,7 disubstituted 3 cephem 4 carboxylic acid compounds and processes for the preparation thereof |
| GB1596278A (en) * | 1976-11-30 | 1981-08-26 | Glaxo Operations Ltd | 7-(-oxyimino-acetamino)cephalosporin derivatives |
-
1978
- 1978-05-25 NL NL7805715A patent/NL7805715A/xx not_active Application Discontinuation
- 1978-05-25 GB GB22499/78A patent/GB1599232A/en not_active Expired
- 1978-05-26 MC MC781305A patent/MC1195A1/fr unknown
- 1978-05-26 NZ NZ187392A patent/NZ187392A/en unknown
- 1978-05-26 IE IE1056/78A patent/IE46903B1/en unknown
- 1978-05-29 AU AU36588/78A patent/AU518648B2/en not_active Expired
- 1978-05-29 IL IL54803A patent/IL54803A/xx unknown
- 1978-05-31 HU HU78HO2077A patent/HU182498B/hu unknown
- 1978-06-01 DE DE7878100078T patent/DE2860248D1/de not_active Expired
- 1978-06-01 EP EP78100078A patent/EP0000005B1/fr not_active Expired
- 1978-06-01 JP JP6501078A patent/JPS5412394A/ja active Pending
- 1978-06-01 FR FR7816468A patent/FR2393000A1/fr active Granted
- 1978-06-01 FI FI781754A patent/FI781754A7/fi not_active Application Discontinuation
- 1978-06-01 SE SE7806465A patent/SE7806465L/xx unknown
- 1978-06-01 DE DE19782824065 patent/DE2824065A1/de not_active Withdrawn
- 1978-06-01 GR GR56409A patent/GR73554B/el unknown
- 1978-06-02 ES ES470442A patent/ES470442A1/es not_active Expired
- 1978-06-02 DK DK246878A patent/DK246878A/da not_active Application Discontinuation
- 1978-06-02 NO NO781934A patent/NO781934L/no unknown
- 1978-06-02 PH PH21225A patent/PH14653A/en unknown
- 1978-06-02 PT PT68134A patent/PT68134A/pt unknown
- 1978-06-02 BR BR787803565A patent/BR7803565A/pt unknown
- 1978-06-02 AT AT403578A patent/AT362501B/de not_active IP Right Cessation
- 1978-06-02 AR AR272437A patent/AR225134A1/es active
- 1978-06-02 CA CA304,644A patent/CA1114808A/fr not_active Expired
- 1978-06-02 IT IT24165/78A patent/IT1098306B/it active
- 1978-07-03 CU CU7834929A patent/CU21118A/es unknown
-
1979
- 1979-02-27 ES ES478115A patent/ES478115A1/es not_active Expired
-
1980
- 1980-07-02 AT AT0345980A patent/AT365197B/de not_active IP Right Cessation
Also Published As
| Publication number | Publication date |
|---|---|
| IT7824165A0 (it) | 1978-06-02 |
| NL7805715A (nl) | 1978-12-05 |
| ES478115A1 (es) | 1979-06-01 |
| DE2824065A1 (de) | 1978-12-14 |
| GB1599232A (en) | 1981-09-30 |
| AT362501B (de) | 1981-05-25 |
| ATA403578A (de) | 1980-10-15 |
| FI781754A7 (fi) | 1978-12-04 |
| CA1114808A (fr) | 1981-12-22 |
| IE46903B1 (en) | 1983-11-02 |
| AU518648B2 (en) | 1981-10-15 |
| PT68134A (en) | 1978-07-01 |
| HU182498B (en) | 1984-01-30 |
| MC1195A1 (fr) | 1979-02-23 |
| GR73554B (fr) | 1984-03-14 |
| SE7806465L (sv) | 1978-12-04 |
| NZ187392A (en) | 1984-05-31 |
| AU3658878A (en) | 1979-12-06 |
| ES470442A1 (es) | 1979-10-01 |
| NO781934L (no) | 1978-12-05 |
| IE781056L (en) | 1978-12-03 |
| DK246878A (da) | 1978-12-04 |
| IL54803A0 (en) | 1978-07-31 |
| PH14653A (en) | 1981-10-14 |
| DE2860248D1 (en) | 1981-01-29 |
| BR7803565A (pt) | 1979-02-20 |
| IT1098306B (it) | 1985-09-07 |
| CU21118A (es) | 1983-04-06 |
| FR2393000B1 (fr) | 1982-10-29 |
| JPS5412394A (en) | 1979-01-30 |
| ATA345980A (de) | 1981-05-15 |
| AR225134A1 (es) | 1982-02-26 |
| AT365197B (de) | 1981-12-28 |
| EP0000005A1 (fr) | 1978-12-20 |
| FR2393000A1 (fr) | 1978-12-29 |
| IL54803A (en) | 1982-01-31 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| DE2462736C2 (de) | 7-Aminothiazolylacetamido-3-cephem-4-carbonsäuren und Verfahren zur Herstellung derselben | |
| DE2727753C2 (fr) | ||
| CH630632A5 (de) | Verfahren zur herstellung neuer cephalosporinderivate. | |
| EP0030294B1 (fr) | Procédé de préparation de dérivés de céphalosporine; intermédiaires et leur préparation | |
| DE2922036C2 (de) | 7-[2-(2-Amino-4-thiazolyl)-2-methoxyimino-acetamido]- cephalosporansäure-Derivate | |
| CH633558A5 (de) | Verfahren zur herstellung neuer cephalosporinderivate. | |
| DE2555858A1 (de) | 3-thiosubstituierte cephalosporinverbindungen und verfahren zu ihrer herstellung | |
| CH639666A5 (de) | Cephalosporansaeurederivate, diese verbindungen enthaltende arzneimittel und verfahren zu ihrer herstellung. | |
| CH655118A5 (de) | Cephalosporinverbindungen, verfahren zu ihrer herstellung und antibakterielle mittel, die diese verbindungen enthalten. | |
| CH641468A5 (de) | Cephemderivate. | |
| DE2736471C2 (fr) | ||
| DE3307550C2 (fr) | ||
| EP0000005B1 (fr) | Nouveaux dérivés de céphalosporines, leurs préparations et leurs compositions pharmaceutiques | |
| EP0049855A2 (fr) | Dérivés de céphalosporine, leur préparation, compositions pharmaceutiques les contenant et intermédiaires pour leur préparation | |
| DE69109913T2 (de) | Cephalosporinzwischenverbindungen, Verfahren zu ihrer Herstellung sowie zur Herstellung ihrer Endprodukte. | |
| DE2527291C2 (fr) | ||
| DE2521063A1 (de) | Phenylacetamidocephalosporin- derivate | |
| EP0029966A2 (fr) | Dérivés de céphalosporine, leurs préparations et compositions pharmaceutiques qui les contiennent | |
| EP0553792B1 (fr) | Procédé pour la préparation de l'hémiheptahydrate du sel disodé de ceftriaxone | |
| EP0036652A2 (fr) | Dérivés de céphalosporine, leur préparation, leurs compositions, leur utilisation dans le traitement contre des maladies et leurs intermédiaires | |
| CH666037A5 (de) | Verfahren zur herstellung von cephalosporin-syn-isomeren. | |
| EP0008372B1 (fr) | Esters et éthers de céphalosporines facilement hydrolysables, leur préparation et compositions les contenant | |
| CH634326A5 (de) | 2-hydroxyiminoacetamido-cephemcarbonsaeuren und ihre herstellung. | |
| EP0049499B1 (fr) | Dérivés de céphalosporine, leur préparation, compositions pharmaceutiques les contenant et intermédiaires pour leur préparation | |
| DD201142A5 (de) | Verfahren zur herstellung von bistetrazolyl-methylsubstituierten cephalosporiantibiotika |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): BE CH DE FR LU NL SE |
|
| 17P | Request for examination filed | ||
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): BE CH DE FR LU NL SE |
|
| REF | Corresponds to: |
Ref document number: 2860248 Country of ref document: DE Date of ref document: 19810129 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19810630 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 19821220 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19831212 Year of fee payment: 7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: LU Payment date: 19840104 Year of fee payment: 7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19840507 Year of fee payment: 7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 19840630 Year of fee payment: 7 Ref country code: NL Payment date: 19840630 Year of fee payment: 7 Ref country code: BE Payment date: 19840630 Year of fee payment: 7 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Effective date: 19850602 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Effective date: 19850630 Ref country code: BE Effective date: 19850630 |
|
| BERE | Be: lapsed |
Owner name: F. HOFFMANN-LA ROCHE & CO. A.G. Effective date: 19850601 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Effective date: 19860101 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19860228 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Effective date: 19860301 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee | ||
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GR Payment date: 19911230 Year of fee payment: 6 |
|
| EUG | Se: european patent has lapsed |
Ref document number: 78100078.1 Effective date: 19860728 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |