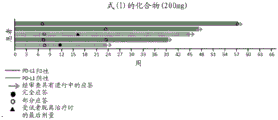
CN111386117A - 治疗癌症的组合物和方法 - Google Patents
治疗癌症的组合物和方法 Download PDFInfo
- Publication number
- CN111386117A CN111386117A CN201880077408.5A CN201880077408A CN111386117A CN 111386117 A CN111386117 A CN 111386117A CN 201880077408 A CN201880077408 A CN 201880077408A CN 111386117 A CN111386117 A CN 111386117A
- Authority
- CN
- China
- Prior art keywords
- cancer
- nivolumab
- formula
- administered
- compound
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 206010028980 Neoplasm Diseases 0.000 title claims abstract description 120
- 238000000034 method Methods 0.000 title claims abstract description 77
- 201000011510 cancer Diseases 0.000 title claims abstract description 76
- 239000000203 mixture Substances 0.000 title description 21
- 150000003839 salts Chemical class 0.000 claims abstract description 67
- 150000001875 compounds Chemical class 0.000 claims description 207
- 229960003301 nivolumab Drugs 0.000 claims description 163
- 238000011282 treatment Methods 0.000 claims description 92
- 230000004044 response Effects 0.000 claims description 73
- 229960005386 ipilimumab Drugs 0.000 claims description 62
- 206010005003 Bladder cancer Diseases 0.000 claims description 49
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 claims description 46
- 201000005112 urinary bladder cancer Diseases 0.000 claims description 45
- 238000002560 therapeutic procedure Methods 0.000 claims description 39
- 238000001990 intravenous administration Methods 0.000 claims description 38
- 238000001802 infusion Methods 0.000 claims description 34
- 208000002154 non-small cell lung carcinoma Diseases 0.000 claims description 31
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 claims description 31
- 229940045513 CTLA4 antagonist Drugs 0.000 claims description 26
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 claims description 22
- 230000000259 anti-tumor effect Effects 0.000 claims description 19
- 230000004083 survival effect Effects 0.000 claims description 19
- 206010061289 metastatic neoplasm Diseases 0.000 claims description 17
- 238000002512 chemotherapy Methods 0.000 claims description 16
- 201000001441 melanoma Diseases 0.000 claims description 16
- 208000000102 Squamous Cell Carcinoma of Head and Neck Diseases 0.000 claims description 15
- 201000000459 head and neck squamous cell carcinoma Diseases 0.000 claims description 15
- 230000001394 metastastic effect Effects 0.000 claims description 15
- 230000000306 recurrent effect Effects 0.000 claims description 13
- 206010008342 Cervix carcinoma Diseases 0.000 claims description 12
- 208000006265 Renal cell carcinoma Diseases 0.000 claims description 12
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 claims description 12
- 201000010881 cervical cancer Diseases 0.000 claims description 12
- 229910052697 platinum Inorganic materials 0.000 claims description 11
- 239000002246 antineoplastic agent Substances 0.000 claims description 10
- 229940127089 cytotoxic agent Drugs 0.000 claims description 9
- 208000003174 Brain Neoplasms Diseases 0.000 claims description 8
- 230000009977 dual effect Effects 0.000 claims description 8
- 208000031671 Large B-Cell Diffuse Lymphoma Diseases 0.000 claims description 7
- 206010059282 Metastases to central nervous system Diseases 0.000 claims description 7
- 206010012818 diffuse large B-cell lymphoma Diseases 0.000 claims description 7
- 206010061902 Pancreatic neoplasm Diseases 0.000 claims description 6
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 claims description 6
- 201000002528 pancreatic cancer Diseases 0.000 claims description 6
- 208000008443 pancreatic carcinoma Diseases 0.000 claims description 6
- 206010025323 Lymphomas Diseases 0.000 claims description 5
- 239000012458 free base Substances 0.000 claims description 5
- 230000001976 improved effect Effects 0.000 claims description 4
- 239000007788 liquid Substances 0.000 claims description 3
- 208000024334 diffuse gastric cancer Diseases 0.000 claims description 2
- 206010067482 No adverse event Diseases 0.000 claims 1
- 125000003854 p-chlorophenyl group Chemical group [H]C1=C([H])C(*)=C([H])C([H])=C1Cl 0.000 abstract 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 46
- 230000036470 plasma concentration Effects 0.000 description 45
- 201000010099 disease Diseases 0.000 description 40
- 230000002411 adverse Effects 0.000 description 37
- 239000000427 antigen Substances 0.000 description 28
- 108091007433 antigens Proteins 0.000 description 28
- 102000036639 antigens Human genes 0.000 description 28
- 239000003814 drug Substances 0.000 description 26
- -1 etc. Chemical class 0.000 description 22
- 102000008096 B7-H1 Antigen Human genes 0.000 description 21
- 108010074708 B7-H1 Antigen Proteins 0.000 description 21
- 102000008203 CTLA-4 Antigen Human genes 0.000 description 19
- 108010021064 CTLA-4 Antigen Proteins 0.000 description 18
- 210000003169 central nervous system Anatomy 0.000 description 18
- 206010027476 Metastases Diseases 0.000 description 16
- 238000002591 computed tomography Methods 0.000 description 16
- 230000034994 death Effects 0.000 description 16
- 231100000517 death Toxicity 0.000 description 16
- 201000004933 in situ carcinoma Diseases 0.000 description 16
- YGPSJZOEDVAXAB-UHFFFAOYSA-N kynurenine Chemical compound OC(=O)C(N)CC(=O)C1=CC=CC=C1N YGPSJZOEDVAXAB-UHFFFAOYSA-N 0.000 description 16
- 208000009458 Carcinoma in Situ Diseases 0.000 description 15
- 102100040678 Programmed cell death protein 1 Human genes 0.000 description 14
- 101710089372 Programmed cell death protein 1 Proteins 0.000 description 14
- 230000036210 malignancy Effects 0.000 description 14
- 229940124597 therapeutic agent Drugs 0.000 description 14
- 238000011156 evaluation Methods 0.000 description 13
- 101000889276 Homo sapiens Cytotoxic T-lymphocyte protein 4 Proteins 0.000 description 11
- 101001137987 Homo sapiens Lymphocyte activation gene 3 protein Proteins 0.000 description 11
- 239000003795 chemical substances by application Substances 0.000 description 11
- 230000007717 exclusion Effects 0.000 description 11
- 230000003902 lesion Effects 0.000 description 11
- 238000012552 review Methods 0.000 description 11
- 229950002916 avelumab Drugs 0.000 description 10
- 238000002648 combination therapy Methods 0.000 description 10
- 102000043321 human CTLA4 Human genes 0.000 description 10
- 210000001519 tissue Anatomy 0.000 description 10
- 210000002700 urine Anatomy 0.000 description 10
- 101000611936 Homo sapiens Programmed cell death protein 1 Proteins 0.000 description 9
- 238000004458 analytical method Methods 0.000 description 9
- 229960003852 atezolizumab Drugs 0.000 description 9
- 238000002619 cancer immunotherapy Methods 0.000 description 9
- 229940079593 drug Drugs 0.000 description 9
- 230000000694 effects Effects 0.000 description 9
- 102000048362 human PDCD1 Human genes 0.000 description 9
- 239000003112 inhibitor Substances 0.000 description 9
- 230000000670 limiting effect Effects 0.000 description 9
- 230000036961 partial effect Effects 0.000 description 9
- 101001117317 Homo sapiens Programmed cell death 1 ligand 1 Proteins 0.000 description 8
- 210000001744 T-lymphocyte Anatomy 0.000 description 8
- 210000004027 cell Anatomy 0.000 description 8
- 239000012634 fragment Substances 0.000 description 8
- 239000002207 metabolite Substances 0.000 description 8
- 229950007217 tremelimumab Drugs 0.000 description 8
- 208000023275 Autoimmune disease Diseases 0.000 description 7
- 101001037256 Homo sapiens Indoleamine 2,3-dioxygenase 1 Proteins 0.000 description 7
- 229940076838 Immune checkpoint inhibitor Drugs 0.000 description 7
- 102100040061 Indoleamine 2,3-dioxygenase 1 Human genes 0.000 description 7
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 7
- 208000005718 Stomach Neoplasms Diseases 0.000 description 7
- 230000002159 abnormal effect Effects 0.000 description 7
- 206010017758 gastric cancer Diseases 0.000 description 7
- 102000048776 human CD274 Human genes 0.000 description 7
- 238000003384 imaging method Methods 0.000 description 7
- 239000012274 immune-checkpoint protein inhibitor Substances 0.000 description 7
- 238000002595 magnetic resonance imaging Methods 0.000 description 7
- 229960002621 pembrolizumab Drugs 0.000 description 7
- 201000011549 stomach cancer Diseases 0.000 description 7
- 101000840545 Bacillus thuringiensis L-isoleucine-4-hydroxylase Proteins 0.000 description 6
- 101001037255 Saccharomyces cerevisiae (strain ATCC 204508 / S288c) Indoleamine 2,3-dioxygenase Proteins 0.000 description 6
- 238000001574 biopsy Methods 0.000 description 6
- 208000035475 disorder Diseases 0.000 description 6
- 230000006870 function Effects 0.000 description 6
- 230000003993 interaction Effects 0.000 description 6
- 238000009533 lab test Methods 0.000 description 6
- 238000007799 mixed lymphocyte reaction assay Methods 0.000 description 6
- 230000007170 pathology Effects 0.000 description 6
- 239000000546 pharmaceutical excipient Substances 0.000 description 6
- 238000001959 radiotherapy Methods 0.000 description 6
- 102000005962 receptors Human genes 0.000 description 6
- 108020003175 receptors Proteins 0.000 description 6
- 238000011160 research Methods 0.000 description 6
- 230000019491 signal transduction Effects 0.000 description 6
- 238000001356 surgical procedure Methods 0.000 description 6
- 230000009885 systemic effect Effects 0.000 description 6
- 102000004127 Cytokines Human genes 0.000 description 5
- 108090000695 Cytokines Proteins 0.000 description 5
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 5
- 241000725303 Human immunodeficiency virus Species 0.000 description 5
- 102000017578 LAG3 Human genes 0.000 description 5
- 101150030213 Lag3 gene Proteins 0.000 description 5
- 239000000090 biomarker Substances 0.000 description 5
- 210000004556 brain Anatomy 0.000 description 5
- 238000009826 distribution Methods 0.000 description 5
- 231100000371 dose-limiting toxicity Toxicity 0.000 description 5
- 229950009791 durvalumab Drugs 0.000 description 5
- 238000000684 flow cytometry Methods 0.000 description 5
- 229960002949 fluorouracil Drugs 0.000 description 5
- 238000009472 formulation Methods 0.000 description 5
- 208000006454 hepatitis Diseases 0.000 description 5
- 230000006698 induction Effects 0.000 description 5
- 238000007917 intracranial administration Methods 0.000 description 5
- 239000003446 ligand Substances 0.000 description 5
- KRTIYQIPSAGSBP-KLAILNCOSA-N linrodostat Chemical compound C1(CCC(CC1)C1=C2C=C(F)C=CC2=NC=C1)[C@@H](C)C(=O)NC1=CC=C(Cl)C=C1 KRTIYQIPSAGSBP-KLAILNCOSA-N 0.000 description 5
- 238000004519 manufacturing process Methods 0.000 description 5
- 238000011084 recovery Methods 0.000 description 5
- 208000024891 symptom Diseases 0.000 description 5
- 230000003442 weekly effect Effects 0.000 description 5
- 208000030507 AIDS Diseases 0.000 description 4
- 102100033793 ALK tyrosine kinase receptor Human genes 0.000 description 4
- 101710168331 ALK tyrosine kinase receptor Proteins 0.000 description 4
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 4
- 206010008909 Chronic Hepatitis Diseases 0.000 description 4
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 4
- 238000012286 ELISA Assay Methods 0.000 description 4
- 206010014733 Endometrial cancer Diseases 0.000 description 4
- 206010014759 Endometrial neoplasm Diseases 0.000 description 4
- 208000005176 Hepatitis C Diseases 0.000 description 4
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 4
- 238000003556 assay Methods 0.000 description 4
- 230000008901 benefit Effects 0.000 description 4
- 239000002775 capsule Substances 0.000 description 4
- 230000008859 change Effects 0.000 description 4
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 4
- 229960004316 cisplatin Drugs 0.000 description 4
- 210000004443 dendritic cell Anatomy 0.000 description 4
- 230000002401 inhibitory effect Effects 0.000 description 4
- 229940043355 kinase inhibitor Drugs 0.000 description 4
- 229960000485 methotrexate Drugs 0.000 description 4
- 210000003205 muscle Anatomy 0.000 description 4
- 231100000252 nontoxic Toxicity 0.000 description 4
- 230000003000 nontoxic effect Effects 0.000 description 4
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 4
- 239000003757 phosphotransferase inhibitor Substances 0.000 description 4
- 238000000159 protein binding assay Methods 0.000 description 4
- 238000009801 radical cystectomy Methods 0.000 description 4
- 238000011269 treatment regimen Methods 0.000 description 4
- 102100031126 6-phosphogluconolactonase Human genes 0.000 description 3
- 108010029731 6-phosphogluconolactonase Proteins 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- 102100036475 Alanine aminotransferase 1 Human genes 0.000 description 3
- 108010082126 Alanine transaminase Proteins 0.000 description 3
- 108010003415 Aspartate Aminotransferases Proteins 0.000 description 3
- 102000004625 Aspartate Aminotransferases Human genes 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- 241000219198 Brassica Species 0.000 description 3
- 235000003351 Brassica cretica Nutrition 0.000 description 3
- 235000003343 Brassica rupestris Nutrition 0.000 description 3
- 206010006187 Breast cancer Diseases 0.000 description 3
- 208000026310 Breast neoplasm Diseases 0.000 description 3
- 210000001266 CD8-positive T-lymphocyte Anatomy 0.000 description 3
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 3
- 206010061818 Disease progression Diseases 0.000 description 3
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 3
- 108010018962 Glucosephosphate Dehydrogenase Proteins 0.000 description 3
- 101000686031 Homo sapiens Proto-oncogene tyrosine-protein kinase ROS Proteins 0.000 description 3
- 101000914484 Homo sapiens T-lymphocyte activation antigen CD80 Proteins 0.000 description 3
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 3
- 229940043367 IDO1 inhibitor Drugs 0.000 description 3
- 102000037984 Inhibitory immune checkpoint proteins Human genes 0.000 description 3
- 108091008026 Inhibitory immune checkpoint proteins Proteins 0.000 description 3
- 102000000588 Interleukin-2 Human genes 0.000 description 3
- 108010002350 Interleukin-2 Proteins 0.000 description 3
- 208000029523 Interstitial Lung disease Diseases 0.000 description 3
- OFOBLEOULBTSOW-UHFFFAOYSA-N Malonic acid Chemical compound OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 3
- 101100407308 Mus musculus Pdcd1lg2 gene Proteins 0.000 description 3
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 3
- 108700030875 Programmed Cell Death 1 Ligand 2 Proteins 0.000 description 3
- 102100024213 Programmed cell death 1 ligand 2 Human genes 0.000 description 3
- 102100023347 Proto-oncogene tyrosine-protein kinase ROS Human genes 0.000 description 3
- 206010039491 Sarcoma Diseases 0.000 description 3
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 3
- 208000000453 Skin Neoplasms Diseases 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 150000001413 amino acids Chemical group 0.000 description 3
- 208000007502 anemia Diseases 0.000 description 3
- 230000005875 antibody response Effects 0.000 description 3
- VSRXQHXAPYXROS-UHFFFAOYSA-N azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OC(=O)C1(C(O)=O)CCC1 VSRXQHXAPYXROS-UHFFFAOYSA-N 0.000 description 3
- QKSKPIVNLNLAAV-UHFFFAOYSA-N bis(2-chloroethyl) sulfide Chemical compound ClCCSCCCl QKSKPIVNLNLAAV-UHFFFAOYSA-N 0.000 description 3
- 229960004562 carboplatin Drugs 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 238000003745 diagnosis Methods 0.000 description 3
- 239000003085 diluting agent Substances 0.000 description 3
- 102000052116 epidermal growth factor receptor activity proteins Human genes 0.000 description 3
- 108700015053 epidermal growth factor receptor activity proteins Proteins 0.000 description 3
- 208000014829 head and neck neoplasm Diseases 0.000 description 3
- 229940088597 hormone Drugs 0.000 description 3
- 239000005556 hormone Substances 0.000 description 3
- 230000002519 immonomodulatory effect Effects 0.000 description 3
- 210000002865 immune cell Anatomy 0.000 description 3
- 210000000987 immune system Anatomy 0.000 description 3
- 238000003018 immunoassay Methods 0.000 description 3
- 230000001506 immunosuppresive effect Effects 0.000 description 3
- 238000009169 immunotherapy Methods 0.000 description 3
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 3
- 208000037819 metastatic cancer Diseases 0.000 description 3
- 208000011575 metastatic malignant neoplasm Diseases 0.000 description 3
- 150000007522 mineralic acids Chemical class 0.000 description 3
- 235000010460 mustard Nutrition 0.000 description 3
- 230000035772 mutation Effects 0.000 description 3
- YOHYSYJDKVYCJI-UHFFFAOYSA-N n-[3-[[6-[3-(trifluoromethyl)anilino]pyrimidin-4-yl]amino]phenyl]cyclopropanecarboxamide Chemical compound FC(F)(F)C1=CC=CC(NC=2N=CN=C(NC=3C=C(NC(=O)C4CC4)C=CC=3)C=2)=C1 YOHYSYJDKVYCJI-UHFFFAOYSA-N 0.000 description 3
- 238000007481 next generation sequencing Methods 0.000 description 3
- 150000007524 organic acids Chemical class 0.000 description 3
- 238000007911 parenteral administration Methods 0.000 description 3
- 238000002203 pretreatment Methods 0.000 description 3
- 208000037821 progressive disease Diseases 0.000 description 3
- 238000002271 resection Methods 0.000 description 3
- 238000012216 screening Methods 0.000 description 3
- 201000000849 skin cancer Diseases 0.000 description 3
- 239000003826 tablet Substances 0.000 description 3
- 230000008685 targeting Effects 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 3
- 230000009278 visceral effect Effects 0.000 description 3
- LMGGOGHEVZMZCU-FGJMKEJPSA-N (2s,4s)-4-[(2r,4s,5s,6s)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-2,5,7,12-tetrahydroxy-6,11-dioxo-3,4-dihydro-1h-tetracene-2-carboxylic acid Chemical compound C1[C@H](N)[C@H](O)[C@H](C)O[C@H]1O[C@@H]1C2=C(O)C(C(=O)C3=C(O)C=CC=C3C3=O)=C3C(O)=C2C[C@@](O)(C(O)=O)C1 LMGGOGHEVZMZCU-FGJMKEJPSA-N 0.000 description 2
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 2
- XMIIGOLPHOKFCH-UHFFFAOYSA-N 3-phenylpropionic acid Chemical compound OC(=O)CCC1=CC=CC=C1 XMIIGOLPHOKFCH-UHFFFAOYSA-N 0.000 description 2
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 206010003827 Autoimmune hepatitis Diseases 0.000 description 2
- 229940125565 BMS-986016 Drugs 0.000 description 2
- 102100036842 C-C motif chemokine 19 Human genes 0.000 description 2
- 102100036846 C-C motif chemokine 21 Human genes 0.000 description 2
- GAGWJHPBXLXJQN-UORFTKCHSA-N Capecitabine Chemical compound C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1[C@H]1[C@H](O)[C@H](O)[C@@H](C)O1 GAGWJHPBXLXJQN-UORFTKCHSA-N 0.000 description 2
- 201000009030 Carcinoma Diseases 0.000 description 2
- 208000024172 Cardiovascular disease Diseases 0.000 description 2
- 206010010356 Congenital anomaly Diseases 0.000 description 2
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 2
- RGHNJXZEOKUKBD-SQOUGZDYSA-N D-gluconic acid Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O RGHNJXZEOKUKBD-SQOUGZDYSA-N 0.000 description 2
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 2
- 108010010803 Gelatin Proteins 0.000 description 2
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 2
- 241000700721 Hepatitis B virus Species 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 101000713085 Homo sapiens C-C motif chemokine 21 Proteins 0.000 description 2
- 208000029663 Hypophosphatemia Diseases 0.000 description 2
- 108060003951 Immunoglobulin Proteins 0.000 description 2
- 102000008070 Interferon-gamma Human genes 0.000 description 2
- 108010074328 Interferon-gamma Proteins 0.000 description 2
- 102000013462 Interleukin-12 Human genes 0.000 description 2
- 108010065805 Interleukin-12 Proteins 0.000 description 2
- 102000003810 Interleukin-18 Human genes 0.000 description 2
- 108090000171 Interleukin-18 Proteins 0.000 description 2
- 108090001060 Lipase Proteins 0.000 description 2
- 102000004882 Lipase Human genes 0.000 description 2
- 239000004367 Lipase Substances 0.000 description 2
- 206010027457 Metastases to liver Diseases 0.000 description 2
- 206010027480 Metastatic malignant melanoma Diseases 0.000 description 2
- 108010061951 Methemoglobin Proteins 0.000 description 2
- GQPLMRYTRLFLPF-UHFFFAOYSA-N Nitrous Oxide Chemical compound [O-][N+]#N GQPLMRYTRLFLPF-UHFFFAOYSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 2
- LCTONWCANYUPML-UHFFFAOYSA-N Pyruvic acid Chemical compound CC(=O)C(O)=O LCTONWCANYUPML-UHFFFAOYSA-N 0.000 description 2
- 102100030086 Receptor tyrosine-protein kinase erbB-2 Human genes 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- 229920002472 Starch Polymers 0.000 description 2
- 235000021355 Stearic acid Nutrition 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 230000006044 T cell activation Effects 0.000 description 2
- 230000020385 T cell costimulation Effects 0.000 description 2
- 230000006052 T cell proliferation Effects 0.000 description 2
- NKANXQFJJICGDU-QPLCGJKRSA-N Tamoxifen Chemical compound C=1C=CC=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1 NKANXQFJJICGDU-QPLCGJKRSA-N 0.000 description 2
- MUMGGOZAMZWBJJ-DYKIIFRCSA-N Testostosterone Chemical compound O=C1CC[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 MUMGGOZAMZWBJJ-DYKIIFRCSA-N 0.000 description 2
- FOCVUCIESVLUNU-UHFFFAOYSA-N Thiotepa Chemical compound C1CN1P(N1CC1)(=S)N1CC1 FOCVUCIESVLUNU-UHFFFAOYSA-N 0.000 description 2
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 2
- 208000003721 Triple Negative Breast Neoplasms Diseases 0.000 description 2
- 210000001015 abdomen Anatomy 0.000 description 2
- 230000005856 abnormality Effects 0.000 description 2
- 238000009825 accumulation Methods 0.000 description 2
- RJURFGZVJUQBHK-UHFFFAOYSA-N actinomycin D Natural products CC1OC(=O)C(C(C)C)N(C)C(=O)CN(C)C(=O)C2CCCN2C(=O)C(C(C)C)NC(=O)C1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)NC4C(=O)NC(C(N5CCCC5C(=O)N(C)CC(=O)N(C)C(C(C)C)C(=O)OC4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-UHFFFAOYSA-N 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- 238000011374 additional therapy Methods 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- 208000037844 advanced solid tumor Diseases 0.000 description 2
- 238000011319 anticancer therapy Methods 0.000 description 2
- 230000001363 autoimmune Effects 0.000 description 2
- 239000002585 base Substances 0.000 description 2
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 2
- 229910000019 calcium carbonate Inorganic materials 0.000 description 2
- 239000001506 calcium phosphate Substances 0.000 description 2
- 229910000389 calcium phosphate Inorganic materials 0.000 description 2
- 235000011010 calcium phosphates Nutrition 0.000 description 2
- 229940022399 cancer vaccine Drugs 0.000 description 2
- 229960005395 cetuximab Drugs 0.000 description 2
- 210000000038 chest Anatomy 0.000 description 2
- 229960004630 chlorambucil Drugs 0.000 description 2
- JCKYGMPEJWAADB-UHFFFAOYSA-N chlorambucil Chemical compound OC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1 JCKYGMPEJWAADB-UHFFFAOYSA-N 0.000 description 2
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 2
- 230000001684 chronic effect Effects 0.000 description 2
- 238000012790 confirmation Methods 0.000 description 2
- 229960004397 cyclophosphamide Drugs 0.000 description 2
- 238000009799 cystectomy Methods 0.000 description 2
- 238000002574 cystoscopy Methods 0.000 description 2
- 229960000684 cytarabine Drugs 0.000 description 2
- 230000002380 cytological effect Effects 0.000 description 2
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 2
- 230000007812 deficiency Effects 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 2
- 230000005750 disease progression Effects 0.000 description 2
- VLCYCQAOQCDTCN-UHFFFAOYSA-N eflornithine Chemical compound NCCCC(N)(C(F)F)C(O)=O VLCYCQAOQCDTCN-UHFFFAOYSA-N 0.000 description 2
- 239000002158 endotoxin Substances 0.000 description 2
- OVBPIULPVIDEAO-LBPRGKRZSA-N folic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-LBPRGKRZSA-N 0.000 description 2
- CHPZKNULDCNCBW-UHFFFAOYSA-N gallium nitrate Chemical compound [Ga+3].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O CHPZKNULDCNCBW-UHFFFAOYSA-N 0.000 description 2
- 229940044627 gamma-interferon Drugs 0.000 description 2
- 229920000159 gelatin Polymers 0.000 description 2
- 239000008273 gelatin Substances 0.000 description 2
- 235000019322 gelatine Nutrition 0.000 description 2
- 235000011852 gelatine desserts Nutrition 0.000 description 2
- 230000002949 hemolytic effect Effects 0.000 description 2
- 208000002672 hepatitis B Diseases 0.000 description 2
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 2
- 231100000844 hepatocellular carcinoma Toxicity 0.000 description 2
- FUZZWVXGSFPDMH-UHFFFAOYSA-N hexanoic acid Chemical compound CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 2
- 229960001101 ifosfamide Drugs 0.000 description 2
- HOMGKSMUEGBAAB-UHFFFAOYSA-N ifosfamide Chemical compound ClCCNP1(=O)OCCCN1CCCl HOMGKSMUEGBAAB-UHFFFAOYSA-N 0.000 description 2
- 102000018358 immunoglobulin Human genes 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- 239000004615 ingredient Substances 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- 238000007918 intramuscular administration Methods 0.000 description 2
- 230000002601 intratumoral effect Effects 0.000 description 2
- SUMDYPCJJOFFON-UHFFFAOYSA-N isethionic acid Chemical compound OCCS(O)(=O)=O SUMDYPCJJOFFON-UHFFFAOYSA-N 0.000 description 2
- 210000003734 kidney Anatomy 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 235000019421 lipase Nutrition 0.000 description 2
- 229920006008 lipopolysaccharide Polymers 0.000 description 2
- 210000004185 liver Anatomy 0.000 description 2
- 210000004072 lung Anatomy 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 238000012423 maintenance Methods 0.000 description 2
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- GLVAUDGFNGKCSF-UHFFFAOYSA-N mercaptopurine Chemical class S=C1NC=NC2=C1NC=N2 GLVAUDGFNGKCSF-UHFFFAOYSA-N 0.000 description 2
- 208000021039 metastatic melanoma Diseases 0.000 description 2
- 229940098779 methanesulfonic acid Drugs 0.000 description 2
- 229960004857 mitomycin Drugs 0.000 description 2
- TXXHDPDFNKHHGW-UHFFFAOYSA-N muconic acid Chemical compound OC(=O)C=CC=CC(O)=O TXXHDPDFNKHHGW-UHFFFAOYSA-N 0.000 description 2
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 2
- 238000002640 oxygen therapy Methods 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 210000004197 pelvis Anatomy 0.000 description 2
- 239000008194 pharmaceutical composition Substances 0.000 description 2
- 230000003285 pharmacodynamic effect Effects 0.000 description 2
- 230000000144 pharmacologic effect Effects 0.000 description 2
- 239000006187 pill Substances 0.000 description 2
- 230000035935 pregnancy Effects 0.000 description 2
- 230000000750 progressive effect Effects 0.000 description 2
- 235000018102 proteins Nutrition 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 108090000623 proteins and genes Proteins 0.000 description 2
- RXWNCPJZOCPEPQ-NVWDDTSBSA-N puromycin Chemical compound C1=CC(OC)=CC=C1C[C@H](N)C(=O)N[C@H]1[C@@H](O)[C@H](N2C3=NC=NC(=C3N=C2)N(C)C)O[C@@H]1CO RXWNCPJZOCPEPQ-NVWDDTSBSA-N 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 210000003289 regulatory T cell Anatomy 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 2
- 230000028327 secretion Effects 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
- 238000009097 single-agent therapy Methods 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 206010041823 squamous cell carcinoma Diseases 0.000 description 2
- 230000006641 stabilisation Effects 0.000 description 2
- 238000011105 stabilization Methods 0.000 description 2
- 239000008107 starch Substances 0.000 description 2
- 235000019698 starch Nutrition 0.000 description 2
- 239000008117 stearic acid Substances 0.000 description 2
- 230000004936 stimulating effect Effects 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 239000013589 supplement Substances 0.000 description 2
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 2
- 239000000454 talc Substances 0.000 description 2
- 235000012222 talc Nutrition 0.000 description 2
- 229910052623 talc Inorganic materials 0.000 description 2
- 229940066453 tecentriq Drugs 0.000 description 2
- 229960001196 thiotepa Drugs 0.000 description 2
- WYWHKKSPHMUBEB-UHFFFAOYSA-N tioguanine Chemical compound N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 2
- 230000000699 topical effect Effects 0.000 description 2
- 231100000419 toxicity Toxicity 0.000 description 2
- 230000001988 toxicity Effects 0.000 description 2
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 2
- 208000022679 triple-negative breast carcinoma Diseases 0.000 description 2
- 229940121358 tyrosine kinase inhibitor Drugs 0.000 description 2
- 239000005483 tyrosine kinase inhibitor Substances 0.000 description 2
- 150000004917 tyrosine kinase inhibitor derivatives Chemical class 0.000 description 2
- 210000003708 urethra Anatomy 0.000 description 2
- 229940055760 yervoy Drugs 0.000 description 2
- QBYIENPQHBMVBV-HFEGYEGKSA-N (2R)-2-hydroxy-2-phenylacetic acid Chemical compound O[C@@H](C(O)=O)c1ccccc1.O[C@@H](C(O)=O)c1ccccc1 QBYIENPQHBMVBV-HFEGYEGKSA-N 0.000 description 1
- ONKCBKDTKZIWHZ-MRWFHJSOSA-N (4r)-4-[[(2r)-6-amino-2-[[(2r)-2-[[4-(aminocarbamothioylamino)benzoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]hexanoyl]amino]-5-[[(2r)-1-amino-6-[bis[2-[[4-[2-(1h-imidazol-5-yl)ethylamino]-4-oxobutanoyl]amino]acetyl]amino]-1-oxohexan-2-yl]amino]-5-oxope Chemical compound C([C@H](C(=O)N[C@H](CCCCN)C(=O)N[C@H](CCC(O)=O)C(=O)N[C@H](CCCCN(C(=O)CNC(=O)CCC(=O)NCCC=1NC=NC=1)C(=O)CNC(=O)CCC(=O)NCCC=1NC=NC=1)C(N)=O)NC(=O)C=1C=CC(NC(=S)NN)=CC=1)C1=CC=C(O)C=C1 ONKCBKDTKZIWHZ-MRWFHJSOSA-N 0.000 description 1
- XRBSKUSTLXISAB-XVVDYKMHSA-N (5r,6r,7r,8r)-8-hydroxy-7-(hydroxymethyl)-5-(3,4,5-trimethoxyphenyl)-5,6,7,8-tetrahydrobenzo[f][1,3]benzodioxole-6-carboxylic acid Chemical compound COC1=C(OC)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@H](O)[C@@H](CO)[C@@H]2C(O)=O)=C1 XRBSKUSTLXISAB-XVVDYKMHSA-N 0.000 description 1
- XRBSKUSTLXISAB-UHFFFAOYSA-N (7R,7'R,8R,8'R)-form-Podophyllic acid Natural products COC1=C(OC)C(OC)=CC(C2C3=CC=4OCOC=4C=C3C(O)C(CO)C2C(O)=O)=C1 XRBSKUSTLXISAB-UHFFFAOYSA-N 0.000 description 1
- FPVKHBSQESCIEP-UHFFFAOYSA-N (8S)-3-(2-deoxy-beta-D-erythro-pentofuranosyl)-3,6,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol Natural products C1C(O)C(CO)OC1N1C(NC=NCC2O)=C2N=C1 FPVKHBSQESCIEP-UHFFFAOYSA-N 0.000 description 1
- FDKXTQMXEQVLRF-ZHACJKMWSA-N (E)-dacarbazine Chemical compound CN(C)\N=N\c1[nH]cnc1C(N)=O FDKXTQMXEQVLRF-ZHACJKMWSA-N 0.000 description 1
- LKJPYSCBVHEWIU-KRWDZBQOSA-N (R)-bicalutamide Chemical compound C([C@@](O)(C)C(=O)NC=1C=C(C(C#N)=CC=1)C(F)(F)F)S(=O)(=O)C1=CC=C(F)C=C1 LKJPYSCBVHEWIU-KRWDZBQOSA-N 0.000 description 1
- MIOPJNTWMNEORI-GMSGAONNSA-N (S)-camphorsulfonic acid Chemical compound C1C[C@@]2(CS(O)(=O)=O)C(=O)C[C@@H]1C2(C)C MIOPJNTWMNEORI-GMSGAONNSA-N 0.000 description 1
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 description 1
- AGNGYMCLFWQVGX-AGFFZDDWSA-N (e)-1-[(2s)-2-amino-2-carboxyethoxy]-2-diazonioethenolate Chemical compound OC(=O)[C@@H](N)CO\C([O-])=C\[N+]#N AGNGYMCLFWQVGX-AGFFZDDWSA-N 0.000 description 1
- WBYWAXJHAXSJNI-VOTSOKGWSA-M .beta-Phenylacrylic acid Natural products [O-]C(=O)\C=C\C1=CC=CC=C1 WBYWAXJHAXSJNI-VOTSOKGWSA-M 0.000 description 1
- AMMPLVWPWSYRDR-UHFFFAOYSA-N 1-methylbicyclo[2.2.2]oct-2-ene-4-carboxylic acid Chemical compound C1CC2(C(O)=O)CCC1(C)C=C2 AMMPLVWPWSYRDR-UHFFFAOYSA-N 0.000 description 1
- BTOTXLJHDSNXMW-POYBYMJQSA-N 2,3-dideoxyuridine Chemical compound O1[C@H](CO)CC[C@@H]1N1C(=O)NC(=O)C=C1 BTOTXLJHDSNXMW-POYBYMJQSA-N 0.000 description 1
- QCXJFISCRQIYID-IAEPZHFASA-N 2-amino-1-n-[(3s,6s,7r,10s,16s)-3-[(2s)-butan-2-yl]-7,11,14-trimethyl-2,5,9,12,15-pentaoxo-10-propan-2-yl-8-oxa-1,4,11,14-tetrazabicyclo[14.3.0]nonadecan-6-yl]-4,6-dimethyl-3-oxo-9-n-[(3s,6s,7r,10s,16s)-7,11,14-trimethyl-2,5,9,12,15-pentaoxo-3,10-di(propa Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N=C2C(C(=O)N[C@@H]3C(=O)N[C@H](C(N4CCC[C@H]4C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]3C)=O)[C@@H](C)CC)=C(N)C(=O)C(C)=C2O2)C2=C(C)C=C1 QCXJFISCRQIYID-IAEPZHFASA-N 0.000 description 1
- UXUZARPLRQRNNX-DXTOWSMRSA-N 2-amino-9-[(2r,3r,4r,5r)-3-fluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-3h-purin-6-one Chemical compound C1=NC=2C(=O)NC(N)=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1F UXUZARPLRQRNNX-DXTOWSMRSA-N 0.000 description 1
- YFBQXUGQIFAFMM-UHFFFAOYSA-N 2-chloro-n-methylethanamine Chemical compound CNCCCl YFBQXUGQIFAFMM-UHFFFAOYSA-N 0.000 description 1
- UPHOPMSGKZNELG-UHFFFAOYSA-N 2-hydroxynaphthalene-1-carboxylic acid Chemical compound C1=CC=C2C(C(=O)O)=C(O)C=CC2=C1 UPHOPMSGKZNELG-UHFFFAOYSA-N 0.000 description 1
- LGEXGKUJMFHVSY-UHFFFAOYSA-N 2-n,4-n,6-n-trimethyl-1,3,5-triazine-2,4,6-triamine Chemical compound CNC1=NC(NC)=NC(NC)=N1 LGEXGKUJMFHVSY-UHFFFAOYSA-N 0.000 description 1
- CTRPRMNBTVRDFH-UHFFFAOYSA-N 2-n-methyl-1,3,5-triazine-2,4,6-triamine Chemical compound CNC1=NC(N)=NC(N)=N1 CTRPRMNBTVRDFH-UHFFFAOYSA-N 0.000 description 1
- MLMQPDHYNJCQAO-UHFFFAOYSA-N 3,3-dimethylbutyric acid Chemical compound CC(C)(C)CC(O)=O MLMQPDHYNJCQAO-UHFFFAOYSA-N 0.000 description 1
- NDMPLJNOPCLANR-UHFFFAOYSA-N 3,4-dihydroxy-15-(4-hydroxy-18-methoxycarbonyl-5,18-seco-ibogamin-18-yl)-16-methoxy-1-methyl-6,7-didehydro-aspidospermidine-3-carboxylic acid methyl ester Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 NDMPLJNOPCLANR-UHFFFAOYSA-N 0.000 description 1
- XLZYKTYMLBOINK-UHFFFAOYSA-N 3-(4-hydroxybenzoyl)benzoic acid Chemical compound OC(=O)C1=CC=CC(C(=O)C=2C=CC(O)=CC=2)=C1 XLZYKTYMLBOINK-UHFFFAOYSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- ZRPLANDPDWYOMZ-UHFFFAOYSA-N 3-cyclopentylpropionic acid Chemical compound OC(=O)CCC1CCCC1 ZRPLANDPDWYOMZ-UHFFFAOYSA-N 0.000 description 1
- AOJJSUZBOXZQNB-VTZDEGQISA-N 4'-epidoxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-VTZDEGQISA-N 0.000 description 1
- DODQJNMQWMSYGS-QPLCGJKRSA-N 4-[(z)-1-[4-[2-(dimethylamino)ethoxy]phenyl]-1-phenylbut-1-en-2-yl]phenol Chemical compound C=1C=C(O)C=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1 DODQJNMQWMSYGS-QPLCGJKRSA-N 0.000 description 1
- QCQCHGYLTSGIGX-GHXANHINSA-N 4-[[(3ar,5ar,5br,7ar,9s,11ar,11br,13as)-5a,5b,8,8,11a-pentamethyl-3a-[(5-methylpyridine-3-carbonyl)amino]-2-oxo-1-propan-2-yl-4,5,6,7,7a,9,10,11,11b,12,13,13a-dodecahydro-3h-cyclopenta[a]chrysen-9-yl]oxy]-2,2-dimethyl-4-oxobutanoic acid Chemical compound N([C@@]12CC[C@@]3(C)[C@]4(C)CC[C@H]5C(C)(C)[C@@H](OC(=O)CC(C)(C)C(O)=O)CC[C@]5(C)[C@H]4CC[C@@H]3C1=C(C(C2)=O)C(C)C)C(=O)C1=CN=CC(C)=C1 QCQCHGYLTSGIGX-GHXANHINSA-N 0.000 description 1
- RJWBTWIBUIGANW-UHFFFAOYSA-N 4-chlorobenzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=C(Cl)C=C1 RJWBTWIBUIGANW-UHFFFAOYSA-N 0.000 description 1
- IDPUKCWIGUEADI-UHFFFAOYSA-N 5-[bis(2-chloroethyl)amino]uracil Chemical compound ClCCN(CCCl)C1=CNC(=O)NC1=O IDPUKCWIGUEADI-UHFFFAOYSA-N 0.000 description 1
- NMUSYJAQQFHJEW-KVTDHHQDSA-N 5-azacytidine Chemical compound O=C1N=C(N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 NMUSYJAQQFHJEW-KVTDHHQDSA-N 0.000 description 1
- WYXSYVWAUAUWLD-SHUUEZRQSA-N 6-azauridine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C=N1 WYXSYVWAUAUWLD-SHUUEZRQSA-N 0.000 description 1
- 229960005538 6-diazo-5-oxo-L-norleucine Drugs 0.000 description 1
- YCWQAMGASJSUIP-YFKPBYRVSA-N 6-diazo-5-oxo-L-norleucine Chemical compound OC(=O)[C@@H](N)CCC(=O)C=[N+]=[N-] YCWQAMGASJSUIP-YFKPBYRVSA-N 0.000 description 1
- VVIAGPKUTFNRDU-UHFFFAOYSA-N 6S-folinic acid Natural products C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)NC(CCC(O)=O)C(O)=O)C=C1 VVIAGPKUTFNRDU-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 201000004384 Alopecia Diseases 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 241000223600 Alternaria Species 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 102100029361 Aromatase Human genes 0.000 description 1
- 108010078554 Aromatase Proteins 0.000 description 1
- NOWKCMXCCJGMRR-UHFFFAOYSA-N Aziridine Chemical compound C1CN1 NOWKCMXCCJGMRR-UHFFFAOYSA-N 0.000 description 1
- 208000004736 B-Cell Leukemia Diseases 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- VGGGPCQERPFHOB-MCIONIFRSA-N Bestatin Chemical compound CC(C)C[C@H](C(O)=O)NC(=O)[C@@H](O)[C@H](N)CC1=CC=CC=C1 VGGGPCQERPFHOB-MCIONIFRSA-N 0.000 description 1
- 108010006654 Bleomycin Proteins 0.000 description 1
- 208000019838 Blood disease Diseases 0.000 description 1
- 206010005949 Bone cancer Diseases 0.000 description 1
- 208000018084 Bone neoplasm Diseases 0.000 description 1
- COVZYZSDYWQREU-UHFFFAOYSA-N Busulfan Chemical compound CS(=O)(=O)OCCCCOS(C)(=O)=O COVZYZSDYWQREU-UHFFFAOYSA-N 0.000 description 1
- 101710112622 C-C motif chemokine 19 Proteins 0.000 description 1
- 108010029697 CD40 Ligand Proteins 0.000 description 1
- 102100032937 CD40 ligand Human genes 0.000 description 1
- 101100504320 Caenorhabditis elegans mcp-1 gene Proteins 0.000 description 1
- 101100314454 Caenorhabditis elegans tra-1 gene Proteins 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- GAGWJHPBXLXJQN-UHFFFAOYSA-N Capecitabine Natural products C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1C1C(O)C(O)C(C)O1 GAGWJHPBXLXJQN-UHFFFAOYSA-N 0.000 description 1
- AOCCBINRVIKJHY-UHFFFAOYSA-N Carmofur Chemical compound CCCCCCNC(=O)N1C=C(F)C(=O)NC1=O AOCCBINRVIKJHY-UHFFFAOYSA-N 0.000 description 1
- 108010012236 Chemokines Proteins 0.000 description 1
- 102000019034 Chemokines Human genes 0.000 description 1
- JWBOIMRXGHLCPP-UHFFFAOYSA-N Chloditan Chemical compound C=1C=CC=C(Cl)C=1C(C(Cl)Cl)C1=CC=C(Cl)C=C1 JWBOIMRXGHLCPP-UHFFFAOYSA-N 0.000 description 1
- 208000006332 Choriocarcinoma Diseases 0.000 description 1
- WBYWAXJHAXSJNI-SREVYHEPSA-N Cinnamic acid Chemical compound OC(=O)\C=C/C1=CC=CC=C1 WBYWAXJHAXSJNI-SREVYHEPSA-N 0.000 description 1
- 206010009944 Colon cancer Diseases 0.000 description 1
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 1
- 229920002261 Corn starch Polymers 0.000 description 1
- 108010007218 Cytochromes b6 Proteins 0.000 description 1
- RGHNJXZEOKUKBD-UHFFFAOYSA-N D-gluconic acid Natural products OCC(O)C(O)C(O)C(O)C(O)=O RGHNJXZEOKUKBD-UHFFFAOYSA-N 0.000 description 1
- 108010092160 Dactinomycin Proteins 0.000 description 1
- WEAHRLBPCANXCN-UHFFFAOYSA-N Daunomycin Natural products CCC1(O)CC(OC2CC(N)C(O)C(C)O2)c3cc4C(=O)c5c(OC)cccc5C(=O)c4c(O)c3C1 WEAHRLBPCANXCN-UHFFFAOYSA-N 0.000 description 1
- 206010061819 Disease recurrence Diseases 0.000 description 1
- 101710121366 Disintegrin and metalloproteinase domain-containing protein 11 Proteins 0.000 description 1
- 208000030453 Drug-Related Side Effects and Adverse reaction Diseases 0.000 description 1
- HTIJFSOGRVMCQR-UHFFFAOYSA-N Epirubicin Natural products COc1cccc2C(=O)c3c(O)c4CC(O)(CC(OC5CC(N)C(=O)C(C)O5)c4c(O)c3C(=O)c12)C(=O)CO HTIJFSOGRVMCQR-UHFFFAOYSA-N 0.000 description 1
- JOYRKODLDBILNP-UHFFFAOYSA-N Ethyl urethane Chemical compound CCOC(N)=O JOYRKODLDBILNP-UHFFFAOYSA-N 0.000 description 1
- 208000010201 Exanthema Diseases 0.000 description 1
- MPJKWIXIYCLVCU-UHFFFAOYSA-N Folinic acid Natural products NC1=NC2=C(N(C=O)C(CNc3ccc(cc3)C(=O)NC(CCC(=O)O)CC(=O)O)CN2)C(=O)N1 MPJKWIXIYCLVCU-UHFFFAOYSA-N 0.000 description 1
- 241000735332 Gerbera Species 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- BLCLNMBMMGCOAS-URPVMXJPSA-N Goserelin Chemical compound C([C@@H](C(=O)N[C@H](COC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1[C@@H](CCC1)C(=O)NNC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H]1NC(=O)CC1)C1=CC=C(O)C=C1 BLCLNMBMMGCOAS-URPVMXJPSA-N 0.000 description 1
- 108010069236 Goserelin Proteins 0.000 description 1
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 1
- 102100039620 Granulocyte-macrophage colony-stimulating factor Human genes 0.000 description 1
- 102000009465 Growth Factor Receptors Human genes 0.000 description 1
- 108010009202 Growth Factor Receptors Proteins 0.000 description 1
- 206010019233 Headaches Diseases 0.000 description 1
- 206010019851 Hepatotoxicity Diseases 0.000 description 1
- 101000713106 Homo sapiens C-C motif chemokine 19 Proteins 0.000 description 1
- 101100166600 Homo sapiens CD28 gene Proteins 0.000 description 1
- 101001019455 Homo sapiens ICOS ligand Proteins 0.000 description 1
- 101001042104 Homo sapiens Inducible T-cell costimulator Proteins 0.000 description 1
- 101001046686 Homo sapiens Integrin alpha-M Proteins 0.000 description 1
- 101001002634 Homo sapiens Interleukin-1 alpha Proteins 0.000 description 1
- 101001012157 Homo sapiens Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 description 1
- 101000738771 Homo sapiens Receptor-type tyrosine-protein phosphatase C Proteins 0.000 description 1
- 101000611183 Homo sapiens Tumor necrosis factor Proteins 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical class Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 1
- VSNHCAURESNICA-UHFFFAOYSA-N Hydroxyurea Chemical compound NC(=O)NO VSNHCAURESNICA-UHFFFAOYSA-N 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- 206010020772 Hypertension Diseases 0.000 description 1
- 102100034980 ICOS ligand Human genes 0.000 description 1
- MPBVHIBUJCELCL-UHFFFAOYSA-N Ibandronate Chemical compound CCCCCN(C)CCC(O)(P(O)(O)=O)P(O)(O)=O MPBVHIBUJCELCL-UHFFFAOYSA-N 0.000 description 1
- XDXDZDZNSLXDNA-TZNDIEGXSA-N Idarubicin Chemical compound C1[C@H](N)[C@H](O)[C@H](C)O[C@H]1O[C@@H]1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2C[C@@](O)(C(C)=O)C1 XDXDZDZNSLXDNA-TZNDIEGXSA-N 0.000 description 1
- XDXDZDZNSLXDNA-UHFFFAOYSA-N Idarubicin Natural products C1C(N)C(O)C(C)OC1OC1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2CC(O)(C(C)=O)C1 XDXDZDZNSLXDNA-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 1
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 1
- 206010062016 Immunosuppression Diseases 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 102100022338 Integrin alpha-M Human genes 0.000 description 1
- 102000014150 Interferons Human genes 0.000 description 1
- 108010050904 Interferons Proteins 0.000 description 1
- 102100020881 Interleukin-1 alpha Human genes 0.000 description 1
- 102000003816 Interleukin-13 Human genes 0.000 description 1
- 108090000176 Interleukin-13 Proteins 0.000 description 1
- 108090000172 Interleukin-15 Proteins 0.000 description 1
- 108010002386 Interleukin-3 Proteins 0.000 description 1
- 102000000646 Interleukin-3 Human genes 0.000 description 1
- 108090000978 Interleukin-4 Proteins 0.000 description 1
- 208000008839 Kidney Neoplasms Diseases 0.000 description 1
- WTDRDQBEARUVNC-LURJTMIESA-N L-DOPA Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C(O)=C1 WTDRDQBEARUVNC-LURJTMIESA-N 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 229920001491 Lentinan Polymers 0.000 description 1
- 240000007472 Leucaena leucocephala Species 0.000 description 1
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 1
- 108010000817 Leuprolide Proteins 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 229940125568 MGD013 Drugs 0.000 description 1
- 241000282567 Macaca fascicularis Species 0.000 description 1
- 108010046938 Macrophage Colony-Stimulating Factor Proteins 0.000 description 1
- 102000007651 Macrophage Colony-Stimulating Factor Human genes 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 201000009906 Meningitis Diseases 0.000 description 1
- 206010027406 Mesothelioma Diseases 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 108700037088 Methemoglobin Reductase Deficiency Proteins 0.000 description 1
- 229930192392 Mitomycin Natural products 0.000 description 1
- TXXHDPDFNKHHGW-CCAGOZQPSA-N Muconic acid Natural products OC(=O)\C=C/C=C\C(O)=O TXXHDPDFNKHHGW-CCAGOZQPSA-N 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- 101100519207 Mus musculus Pdcd1 gene Proteins 0.000 description 1
- 208000009525 Myocarditis Diseases 0.000 description 1
- OVBPIULPVIDEAO-UHFFFAOYSA-N N-Pteroyl-L-glutaminsaeure Natural products C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)NC(CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-UHFFFAOYSA-N 0.000 description 1
- ZDZOTLJHXYCWBA-VCVYQWHSSA-N N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol Chemical compound O([C@H]1[C@H]2[C@@](C([C@H](O)C3=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=4C=CC=CC=4)C[C@]1(O)C3(C)C)=O)(C)[C@@H](O)C[C@H]1OC[C@]12OC(=O)C)C(=O)C1=CC=CC=C1 ZDZOTLJHXYCWBA-VCVYQWHSSA-N 0.000 description 1
- MBBZMMPHUWSWHV-BDVNFPICSA-N N-methylglucamine Chemical compound CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO MBBZMMPHUWSWHV-BDVNFPICSA-N 0.000 description 1
- UIBHKZCMOPHOOT-UHFFFAOYSA-N NP(N)(=O)NCl Chemical compound NP(N)(=O)NCl UIBHKZCMOPHOOT-UHFFFAOYSA-N 0.000 description 1
- 208000002454 Nasopharyngeal Carcinoma Diseases 0.000 description 1
- 206010061306 Nasopharyngeal cancer Diseases 0.000 description 1
- 206010028813 Nausea Diseases 0.000 description 1
- 206010061309 Neoplasm progression Diseases 0.000 description 1
- 208000012902 Nervous system disease Diseases 0.000 description 1
- 208000025966 Neurological disease Diseases 0.000 description 1
- 206010029350 Neurotoxicity Diseases 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- 108091034117 Oligonucleotide Proteins 0.000 description 1
- 229930187135 Olivomycin Natural products 0.000 description 1
- 108700020796 Oncogene Proteins 0.000 description 1
- 206010033128 Ovarian cancer Diseases 0.000 description 1
- 206010061535 Ovarian neoplasm Diseases 0.000 description 1
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 1
- 102000004316 Oxidoreductases Human genes 0.000 description 1
- 108090000854 Oxidoreductases Proteins 0.000 description 1
- 229930012538 Paclitaxel Natural products 0.000 description 1
- 206010033645 Pancreatitis Diseases 0.000 description 1
- 208000037581 Persistent Infection Diseases 0.000 description 1
- 102000017343 Phosphatidylinositol kinases Human genes 0.000 description 1
- 108050005377 Phosphatidylinositol kinases Proteins 0.000 description 1
- 108091000080 Phosphotransferase Proteins 0.000 description 1
- KMSKQZKKOZQFFG-HSUXVGOQSA-N Pirarubicin Chemical compound O([C@H]1[C@@H](N)C[C@@H](O[C@H]1C)O[C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1CCCCO1 KMSKQZKKOZQFFG-HSUXVGOQSA-N 0.000 description 1
- 206010035664 Pneumonia Diseases 0.000 description 1
- 206010035742 Pneumonitis Diseases 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 102100024216 Programmed cell death 1 ligand 1 Human genes 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- 201000004681 Psoriasis Diseases 0.000 description 1
- 206010061924 Pulmonary toxicity Diseases 0.000 description 1
- 206010037660 Pyrexia Diseases 0.000 description 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-N R-2-phenyl-2-hydroxyacetic acid Natural products OC(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-N 0.000 description 1
- 206010037868 Rash maculo-papular Diseases 0.000 description 1
- 101710100968 Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 description 1
- 102100037422 Receptor-type tyrosine-protein phosphatase C Human genes 0.000 description 1
- 206010038389 Renal cancer Diseases 0.000 description 1
- 206010040047 Sepsis Diseases 0.000 description 1
- 206010042033 Stevens-Johnson syndrome Diseases 0.000 description 1
- 231100000168 Stevens-Johnson syndrome Toxicity 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- UCKMPCXJQFINFW-UHFFFAOYSA-N Sulphide Chemical compound [S-2] UCKMPCXJQFINFW-UHFFFAOYSA-N 0.000 description 1
- 108091008874 T cell receptors Proteins 0.000 description 1
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 229940123237 Taxane Drugs 0.000 description 1
- 208000024313 Testicular Neoplasms Diseases 0.000 description 1
- 208000033133 Testicular seminomatous germ cell tumor Diseases 0.000 description 1
- 206010057644 Testis cancer Diseases 0.000 description 1
- 208000024770 Thyroid neoplasm Diseases 0.000 description 1
- 206010044221 Toxic encephalopathy Diseases 0.000 description 1
- 206010070863 Toxicity to various agents Diseases 0.000 description 1
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 1
- FYAMXEPQQLNQDM-UHFFFAOYSA-N Tris(1-aziridinyl)phosphine oxide Chemical compound C1CN1P(N1CC1)(=O)N1CC1 FYAMXEPQQLNQDM-UHFFFAOYSA-N 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- 206010064390 Tumour invasion Diseases 0.000 description 1
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 1
- 206010046851 Uveitis Diseases 0.000 description 1
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 1
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 206010047642 Vitiligo Diseases 0.000 description 1
- 206010047700 Vomiting Diseases 0.000 description 1
- XZSRRNFBEIOBDA-CFNBKWCHSA-N [2-[(2s,4s)-4-[(2r,4s,5s,6s)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-3,4-dihydro-1h-tetracen-2-yl]-2-oxoethyl] 2,2-diethoxyacetate Chemical compound O([C@H]1C[C@](CC2=C(O)C=3C(=O)C4=CC=CC(OC)=C4C(=O)C=3C(O)=C21)(O)C(=O)COC(=O)C(OCC)OCC)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 XZSRRNFBEIOBDA-CFNBKWCHSA-N 0.000 description 1
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 229960005339 acitretin Drugs 0.000 description 1
- RJURFGZVJUQBHK-IIXSONLDSA-N actinomycin D Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)N[C@@H]4C(=O)N[C@@H](C(N5CCC[C@H]5C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-IIXSONLDSA-N 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 230000003044 adaptive effect Effects 0.000 description 1
- 230000001919 adrenal effect Effects 0.000 description 1
- 201000005188 adrenal gland cancer Diseases 0.000 description 1
- 208000024447 adrenal gland neoplasm Diseases 0.000 description 1
- 229940009456 adriamycin Drugs 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 229910001413 alkali metal ion Inorganic materials 0.000 description 1
- 229910001420 alkaline earth metal ion Inorganic materials 0.000 description 1
- 229940045714 alkyl sulfonate alkylating agent Drugs 0.000 description 1
- 150000008052 alkyl sulfonates Chemical class 0.000 description 1
- 229940100198 alkylating agent Drugs 0.000 description 1
- 239000002168 alkylating agent Substances 0.000 description 1
- IHUNBGSDBOWDMA-AQFIFDHZSA-N all-trans-acitretin Chemical compound COC1=CC(C)=C(\C=C\C(\C)=C\C=C\C(\C)=C\C(O)=O)C(C)=C1C IHUNBGSDBOWDMA-AQFIFDHZSA-N 0.000 description 1
- SHGAZHPCJJPHSC-YCNIQYBTSA-N all-trans-retinoic acid Chemical compound OC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-YCNIQYBTSA-N 0.000 description 1
- 208000026935 allergic disease Diseases 0.000 description 1
- 231100000360 alopecia Toxicity 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Natural products OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 229960000473 altretamine Drugs 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 229950010817 alvocidib Drugs 0.000 description 1
- BIIVYFLTOXDAOV-YVEFUNNKSA-N alvocidib Chemical compound O[C@@H]1CN(C)CC[C@@H]1C1=C(O)C=C(O)C2=C1OC(C=1C(=CC=CC=1)Cl)=CC2=O BIIVYFLTOXDAOV-YVEFUNNKSA-N 0.000 description 1
- 235000001014 amino acid Nutrition 0.000 description 1
- 229940024606 amino acid Drugs 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 238000012863 analytical testing Methods 0.000 description 1
- 239000003098 androgen Substances 0.000 description 1
- 229940030486 androgens Drugs 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- PYKYMHQGRFAEBM-UHFFFAOYSA-N anthraquinone Natural products CCC(=O)c1c(O)c2C(=O)C3C(C=CC=C3O)C(=O)c2cc1CC(=O)OC PYKYMHQGRFAEBM-UHFFFAOYSA-N 0.000 description 1
- 150000004056 anthraquinones Chemical class 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000002280 anti-androgenic effect Effects 0.000 description 1
- 230000005911 anti-cytotoxic effect Effects 0.000 description 1
- 229940046836 anti-estrogen Drugs 0.000 description 1
- 230000001833 anti-estrogenic effect Effects 0.000 description 1
- 230000000340 anti-metabolite Effects 0.000 description 1
- 239000000051 antiandrogen Substances 0.000 description 1
- 229940030495 antiandrogen sex hormone and modulator of the genital system Drugs 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 230000009464 antigen specific memory response Effects 0.000 description 1
- 210000000612 antigen-presenting cell Anatomy 0.000 description 1
- 239000013059 antihormonal agent Substances 0.000 description 1
- 229940100197 antimetabolite Drugs 0.000 description 1
- 239000002256 antimetabolite Substances 0.000 description 1
- 229940045687 antimetabolites folic acid analogs Drugs 0.000 description 1
- 230000004596 appetite loss Effects 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 229960002756 azacitidine Drugs 0.000 description 1
- 229950011321 azaserine Drugs 0.000 description 1
- 150000001541 aziridines Chemical class 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 238000013476 bayesian approach Methods 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-N benzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-N 0.000 description 1
- 229940092714 benzenesulfonic acid Drugs 0.000 description 1
- HFACYLZERDEVSX-UHFFFAOYSA-N benzidine Chemical compound C1=CC(N)=CC=C1C1=CC=C(N)C=C1 HFACYLZERDEVSX-UHFFFAOYSA-N 0.000 description 1
- 125000005605 benzo group Chemical group 0.000 description 1
- 229940049706 benzodiazepine Drugs 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 229960000997 bicalutamide Drugs 0.000 description 1
- 238000009809 bilateral salpingo-oophorectomy Methods 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 229960000074 biopharmaceutical Drugs 0.000 description 1
- 229960001561 bleomycin Drugs 0.000 description 1
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 230000017531 blood circulation Effects 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 238000010322 bone marrow transplantation Methods 0.000 description 1
- 210000000481 breast Anatomy 0.000 description 1
- 201000005389 breast carcinoma in situ Diseases 0.000 description 1
- SXDBWCPKPHAZSM-UHFFFAOYSA-N bromic acid Chemical compound OBr(=O)=O SXDBWCPKPHAZSM-UHFFFAOYSA-N 0.000 description 1
- 229960002092 busulfan Drugs 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 210000004899 c-terminal region Anatomy 0.000 description 1
- 108700002839 cactinomycin Proteins 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229930195731 calicheamicin Natural products 0.000 description 1
- HXCHCVDVKSCDHU-LULTVBGHSA-N calicheamicin Chemical compound C1[C@H](OC)[C@@H](NCC)CO[C@H]1O[C@H]1[C@H](O[C@@H]2C\3=C(NC(=O)OC)C(=O)C[C@](C/3=C/CSSSC)(O)C#C\C=C/C#C2)O[C@H](C)[C@@H](NO[C@@H]2O[C@H](C)[C@@H](SC(=O)C=3C(=C(OC)C(O[C@H]4[C@@H]([C@H](OC)[C@@H](O)[C@H](C)O4)O)=C(I)C=3C)OC)[C@@H](O)C2)[C@@H]1O HXCHCVDVKSCDHU-LULTVBGHSA-N 0.000 description 1
- 230000009400 cancer invasion Effects 0.000 description 1
- 238000009566 cancer vaccine Methods 0.000 description 1
- 229960004117 capecitabine Drugs 0.000 description 1
- 229960003261 carmofur Drugs 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 230000022131 cell cycle Effects 0.000 description 1
- 230000003915 cell function Effects 0.000 description 1
- 238000002659 cell therapy Methods 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 210000001175 cerebrospinal fluid Anatomy 0.000 description 1
- 210000003679 cervix uteri Anatomy 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 229940044683 chemotherapy drug Drugs 0.000 description 1
- 238000002192 cholecystectomy Methods 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- ZYVSOIYQKUDENJ-WKSBCEQHSA-N chromomycin A3 Chemical compound O([C@@H]1C[C@@H](O[C@H](C)[C@@H]1OC(C)=O)OC=1C=C2C=C3C[C@H]([C@@H](C(=O)C3=C(O)C2=C(O)C=1C)O[C@@H]1O[C@H](C)[C@@H](O)[C@H](O[C@@H]2O[C@H](C)[C@@H](O)[C@H](O[C@@H]3O[C@@H](C)[C@H](OC(C)=O)[C@@](C)(O)C3)C2)C1)[C@H](OC)C(=O)[C@@H](O)[C@@H](C)O)[C@@H]1C[C@@H](O)[C@@H](OC)[C@@H](C)O1 ZYVSOIYQKUDENJ-WKSBCEQHSA-N 0.000 description 1
- 235000013985 cinnamic acid Nutrition 0.000 description 1
- 229930016911 cinnamic acid Natural products 0.000 description 1
- 235000015165 citric acid Nutrition 0.000 description 1
- 210000001072 colon Anatomy 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 238000013329 compounding Methods 0.000 description 1
- 210000002808 connective tissue Anatomy 0.000 description 1
- 239000008120 corn starch Substances 0.000 description 1
- DMSZORWOGDLWGN-UHFFFAOYSA-N ctk1a3526 Chemical compound NP(N)(N)=O DMSZORWOGDLWGN-UHFFFAOYSA-N 0.000 description 1
- 238000009109 curative therapy Methods 0.000 description 1
- 229960003901 dacarbazine Drugs 0.000 description 1
- 229960000640 dactinomycin Drugs 0.000 description 1
- 229960000975 daunorubicin Drugs 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 229950003913 detorubicin Drugs 0.000 description 1
- 239000000032 diagnostic agent Substances 0.000 description 1
- 229940039227 diagnostic agent Drugs 0.000 description 1
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 1
- 239000003534 dna topoisomerase inhibitor Substances 0.000 description 1
- 229960003668 docetaxel Drugs 0.000 description 1
- MOTZDAYCYVMXPC-UHFFFAOYSA-N dodecyl hydrogen sulfate Chemical compound CCCCCCCCCCCCOS(O)(=O)=O MOTZDAYCYVMXPC-UHFFFAOYSA-N 0.000 description 1
- 230000003828 downregulation Effects 0.000 description 1
- 229960004679 doxorubicin Drugs 0.000 description 1
- 230000037437 driver mutation Effects 0.000 description 1
- NOTIQUSPUUHHEH-UXOVVSIBSA-N dromostanolone propionate Chemical compound C([C@@H]1CC2)C(=O)[C@H](C)C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H](OC(=O)CC)[C@@]2(C)CC1 NOTIQUSPUUHHEH-UXOVVSIBSA-N 0.000 description 1
- 229950004683 drostanolone propionate Drugs 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 239000012636 effector Substances 0.000 description 1
- 229960002759 eflornithine Drugs 0.000 description 1
- 229940056913 eftilagimod alfa Drugs 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 230000002357 endometrial effect Effects 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 229940116977 epidermal growth factor Drugs 0.000 description 1
- 206010015037 epilepsy Diseases 0.000 description 1
- 229960001904 epirubicin Drugs 0.000 description 1
- 229930013356 epothilone Natural products 0.000 description 1
- 150000003883 epothilone derivatives Chemical class 0.000 description 1
- 210000003238 esophagus Anatomy 0.000 description 1
- ITSGNOIFAJAQHJ-BMFNZSJVSA-N esorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)C[C@H](C)O1 ITSGNOIFAJAQHJ-BMFNZSJVSA-N 0.000 description 1
- 229950002017 esorubicin Drugs 0.000 description 1
- 229960001842 estramustine Drugs 0.000 description 1
- FRPJXPJMRWBBIH-RBRWEJTLSA-N estramustine Chemical compound ClCCN(CCCl)C(=O)OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 FRPJXPJMRWBBIH-RBRWEJTLSA-N 0.000 description 1
- 239000000328 estrogen antagonist Substances 0.000 description 1
- AFAXGSQYZLGZPG-UHFFFAOYSA-N ethanedisulfonic acid Chemical compound OS(=O)(=O)CCS(O)(=O)=O AFAXGSQYZLGZPG-UHFFFAOYSA-N 0.000 description 1
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 1
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 1
- 201000005884 exanthem Diseases 0.000 description 1
- 206010016256 fatigue Diseases 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 238000009093 first-line therapy Methods 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- ODKNJVUHOIMIIZ-RRKCRQDMSA-N floxuridine Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(F)=C1 ODKNJVUHOIMIIZ-RRKCRQDMSA-N 0.000 description 1
- 229960000961 floxuridine Drugs 0.000 description 1
- 229960000390 fludarabine Drugs 0.000 description 1
- GIUYCYHIANZCFB-FJFJXFQQSA-N fludarabine phosphate Chemical compound C1=NC=2C(N)=NC(F)=NC=2N1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@@H]1O GIUYCYHIANZCFB-FJFJXFQQSA-N 0.000 description 1
- MKXKFYHWDHIYRV-UHFFFAOYSA-N flutamide Chemical compound CC(C)C(=O)NC1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1 MKXKFYHWDHIYRV-UHFFFAOYSA-N 0.000 description 1
- 229960002074 flutamide Drugs 0.000 description 1
- 235000019152 folic acid Nutrition 0.000 description 1
- 239000011724 folic acid Substances 0.000 description 1
- 229960000304 folic acid Drugs 0.000 description 1
- 150000002224 folic acids Chemical class 0.000 description 1
- 235000008191 folinic acid Nutrition 0.000 description 1
- 239000011672 folinic acid Substances 0.000 description 1
- VVIAGPKUTFNRDU-ABLWVSNPSA-N folinic acid Chemical compound C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 VVIAGPKUTFNRDU-ABLWVSNPSA-N 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 235000011087 fumaric acid Nutrition 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 210000000232 gallbladder Anatomy 0.000 description 1
- 229940044658 gallium nitrate Drugs 0.000 description 1
- 238000013110 gastrectomy Methods 0.000 description 1
- 229960005277 gemcitabine Drugs 0.000 description 1
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 1
- 230000037442 genomic alteration Effects 0.000 description 1
- 208000005017 glioblastoma Diseases 0.000 description 1
- 239000000174 gluconic acid Substances 0.000 description 1
- 235000012208 gluconic acid Nutrition 0.000 description 1
- 229940097042 glucuronate Drugs 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 229930182470 glycoside Natural products 0.000 description 1
- 230000013595 glycosylation Effects 0.000 description 1
- 238000006206 glycosylation reaction Methods 0.000 description 1
- 229960002913 goserelin Drugs 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 239000007902 hard capsule Substances 0.000 description 1
- 201000010536 head and neck cancer Diseases 0.000 description 1
- 231100000869 headache Toxicity 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 208000014951 hematologic disease Diseases 0.000 description 1
- 208000018706 hematopoietic system disease Diseases 0.000 description 1
- 230000003258 hemophagocytic effect Effects 0.000 description 1
- 230000002440 hepatic effect Effects 0.000 description 1
- 231100000283 hepatitis Toxicity 0.000 description 1
- 231100000304 hepatotoxicity Toxicity 0.000 description 1
- 230000007686 hepatotoxicity Effects 0.000 description 1
- UUVWYPNAQBNQJQ-UHFFFAOYSA-N hexamethylmelamine Chemical compound CN(C)C1=NC(N(C)C)=NC(N(C)C)=N1 UUVWYPNAQBNQJQ-UHFFFAOYSA-N 0.000 description 1
- 201000008298 histiocytosis Diseases 0.000 description 1
- 229940125697 hormonal agent Drugs 0.000 description 1
- 238000001794 hormone therapy Methods 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 229960001330 hydroxycarbamide Drugs 0.000 description 1
- 230000009610 hypersensitivity Effects 0.000 description 1
- 210000003026 hypopharynx Anatomy 0.000 description 1
- 208000003532 hypothyroidism Diseases 0.000 description 1
- 230000002989 hypothyroidism Effects 0.000 description 1
- 238000009802 hysterectomy Methods 0.000 description 1
- 229940015872 ibandronate Drugs 0.000 description 1
- 229960000908 idarubicin Drugs 0.000 description 1
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Substances C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 230000005847 immunogenicity Effects 0.000 description 1
- 229940072221 immunoglobulins Drugs 0.000 description 1
- 230000003308 immunostimulating effect Effects 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 102000006639 indoleamine 2,3-dioxygenase Human genes 0.000 description 1
- 108020004201 indoleamine 2,3-dioxygenase Proteins 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 238000011221 initial treatment Methods 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 229940079322 interferon Drugs 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 230000009545 invasion Effects 0.000 description 1
- 230000002262 irrigation Effects 0.000 description 1
- 238000003973 irrigation Methods 0.000 description 1
- 150000004715 keto acids Chemical class 0.000 description 1
- 201000010982 kidney cancer Diseases 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 210000000867 larynx Anatomy 0.000 description 1
- 229940115286 lentinan Drugs 0.000 description 1
- 229960001691 leucovorin Drugs 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- GFIJNRVAKGFPGQ-LIJARHBVSA-N leuprolide Chemical compound CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=CC=C(O)C=C1 GFIJNRVAKGFPGQ-LIJARHBVSA-N 0.000 description 1
- 229960004338 leuprorelin Drugs 0.000 description 1
- 201000007270 liver cancer Diseases 0.000 description 1
- 208000014018 liver neoplasm Diseases 0.000 description 1
- 229960003538 lonidamine Drugs 0.000 description 1
- WDRYRZXSPDWGEB-UHFFFAOYSA-N lonidamine Chemical compound C12=CC=CC=C2C(C(=O)O)=NN1CC1=CC=C(Cl)C=C1Cl WDRYRZXSPDWGEB-UHFFFAOYSA-N 0.000 description 1
- 208000019017 loss of appetite Diseases 0.000 description 1
- 235000021266 loss of appetite Nutrition 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- 208000012965 maculopapular rash Diseases 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 239000001630 malic acid Substances 0.000 description 1
- 235000011090 malic acid Nutrition 0.000 description 1
- 208000026037 malignant tumor of neck Diseases 0.000 description 1
- 229960002510 mandelic acid Drugs 0.000 description 1
- MQXVYODZCMMZEM-ZYUZMQFOSA-N mannomustine Chemical compound ClCCNC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CNCCCl MQXVYODZCMMZEM-ZYUZMQFOSA-N 0.000 description 1
- 229950008612 mannomustine Drugs 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 231100000682 maximum tolerated dose Toxicity 0.000 description 1
- HAWPXGHAZFHHAD-UHFFFAOYSA-N mechlorethamine Chemical class ClCCN(C)CCCl HAWPXGHAZFHHAD-UHFFFAOYSA-N 0.000 description 1
- 229960004961 mechlorethamine Drugs 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 229960001924 melphalan Drugs 0.000 description 1
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 1
- 229960001428 mercaptopurine Drugs 0.000 description 1
- 108020004999 messenger RNA Proteins 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- 230000009401 metastasis Effects 0.000 description 1
- 208000010208 methemoglobin reductase deficiency Diseases 0.000 description 1
- 229930182817 methionine Natural products 0.000 description 1
- WBYWAXJHAXSJNI-UHFFFAOYSA-N methyl p-hydroxycinnamate Natural products OC(=O)C=CC1=CC=CC=C1 WBYWAXJHAXSJNI-UHFFFAOYSA-N 0.000 description 1
- CXKWCBBOMKCUKX-UHFFFAOYSA-M methylene blue Chemical compound [Cl-].C1=CC(N(C)C)=CC2=[S+]C3=CC(N(C)C)=CC=C3N=C21 CXKWCBBOMKCUKX-UHFFFAOYSA-M 0.000 description 1
- 229960000907 methylthioninium chloride Drugs 0.000 description 1
- HPNSFSBZBAHARI-UHFFFAOYSA-N micophenolic acid Natural products OC1=C(CC=C(C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-UHFFFAOYSA-N 0.000 description 1
- 239000011325 microbead Substances 0.000 description 1
- 229960000350 mitotane Drugs 0.000 description 1
- 229960001156 mitoxantrone Drugs 0.000 description 1
- KKZJGLLVHKMTCM-UHFFFAOYSA-N mitoxantrone Chemical compound O=C1C2=C(O)C=CC(O)=C2C(=O)C2=C1C(NCCNCCO)=CC=C2NCCNCCO KKZJGLLVHKMTCM-UHFFFAOYSA-N 0.000 description 1
- 201000010225 mixed cell type cancer Diseases 0.000 description 1
- 208000029638 mixed neoplasm Diseases 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 210000000214 mouth Anatomy 0.000 description 1
- 210000004877 mucosa Anatomy 0.000 description 1
- 201000003731 mucosal melanoma Diseases 0.000 description 1
- 201000006417 multiple sclerosis Diseases 0.000 description 1
- 229960000951 mycophenolic acid Drugs 0.000 description 1
- HPNSFSBZBAHARI-RUDMXATFSA-N mycophenolic acid Chemical compound OC1=C(C\C=C(/C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-RUDMXATFSA-N 0.000 description 1
- KVBGVZZKJNLNJU-UHFFFAOYSA-N naphthalene-2-sulfonic acid Chemical compound C1=CC=CC2=CC(S(=O)(=O)O)=CC=C21 KVBGVZZKJNLNJU-UHFFFAOYSA-N 0.000 description 1
- 201000011216 nasopharynx carcinoma Diseases 0.000 description 1
- 230000008693 nausea Effects 0.000 description 1
- 230000017074 necrotic cell death Effects 0.000 description 1
- 238000010984 neurological examination Methods 0.000 description 1
- 231100000228 neurotoxicity Toxicity 0.000 description 1
- 230000007135 neurotoxicity Effects 0.000 description 1
- XWXYUMMDTVBTOU-UHFFFAOYSA-N nilutamide Chemical compound O=C1C(C)(C)NC(=O)N1C1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1 XWXYUMMDTVBTOU-UHFFFAOYSA-N 0.000 description 1
- 229960002653 nilutamide Drugs 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 239000001272 nitrous oxide Substances 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- CZDBNBLGZNWKMC-MWQNXGTOSA-N olivomycin Chemical compound O([C@@H]1C[C@@H](O[C@H](C)[C@@H]1O)OC=1C=C2C=C3C[C@H]([C@@H](C(=O)C3=C(O)C2=C(O)C=1)O[C@H]1O[C@@H](C)[C@H](O)[C@@H](OC2O[C@@H](C)[C@H](O)[C@@H](O)C2)C1)[C@H](OC)C(=O)[C@@H](O)[C@@H](C)O)[C@H]1C[C@H](O)[C@H](OC)[C@H](C)O1 CZDBNBLGZNWKMC-MWQNXGTOSA-N 0.000 description 1
- 229950005848 olivomycin Drugs 0.000 description 1
- 210000002747 omentum Anatomy 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 150000007530 organic bases Chemical class 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 210000003300 oropharynx Anatomy 0.000 description 1
- 210000001672 ovary Anatomy 0.000 description 1
- 210000003101 oviduct Anatomy 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 229960001592 paclitaxel Drugs 0.000 description 1
- 210000000496 pancreas Anatomy 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 210000003695 paranasal sinus Anatomy 0.000 description 1
- 238000012753 partial hepatectomy Methods 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 229960002340 pentostatin Drugs 0.000 description 1
- FPVKHBSQESCIEP-JQCXWYLXSA-N pentostatin Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(N=CNC[C@H]2O)=C2N=C1 FPVKHBSQESCIEP-JQCXWYLXSA-N 0.000 description 1
- 210000005259 peripheral blood Anatomy 0.000 description 1
- 239000011886 peripheral blood Substances 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- QPFYXYFORQJZEC-UHFFFAOYSA-N phenazopyridine Chemical compound NC1=NC(N)=CC=C1N=NC1=CC=CC=C1 QPFYXYFORQJZEC-UHFFFAOYSA-N 0.000 description 1
- 102000020233 phosphotransferase Human genes 0.000 description 1
- 229960001221 pirarubicin Drugs 0.000 description 1
- IUGYQRQAERSCNH-UHFFFAOYSA-N pivalic acid Chemical compound CC(C)(C)C(O)=O IUGYQRQAERSCNH-UHFFFAOYSA-N 0.000 description 1
- 238000011518 platinum-based chemotherapy Methods 0.000 description 1
- 231100000374 pneumotoxicity Toxicity 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 235000019422 polyvinyl alcohol Nutrition 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- WSHYKIAQCMIPTB-UHFFFAOYSA-M potassium;2-oxo-3-(3-oxo-1-phenylbutyl)chromen-4-olate Chemical compound [K+].[O-]C=1C2=CC=CC=C2OC(=O)C=1C(CC(=O)C)C1=CC=CC=C1 WSHYKIAQCMIPTB-UHFFFAOYSA-M 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- OIGNJSKKLXVSLS-VWUMJDOOSA-N prednisolone Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 OIGNJSKKLXVSLS-VWUMJDOOSA-N 0.000 description 1
- 229960005205 prednisolone Drugs 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- CPTBDICYNRMXFX-UHFFFAOYSA-N procarbazine Chemical compound CNNCC1=CC=C(C(=O)NC(C)C)C=C1 CPTBDICYNRMXFX-UHFFFAOYSA-N 0.000 description 1
- 229960000624 procarbazine Drugs 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 239000000092 prognostic biomarker Substances 0.000 description 1
- 230000035755 proliferation Effects 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- 210000002307 prostate Anatomy 0.000 description 1
- 230000007047 pulmonary toxicity Effects 0.000 description 1
- 150000003212 purines Chemical class 0.000 description 1
- 229950010131 puromycin Drugs 0.000 description 1
- 150000003230 pyrimidines Chemical class 0.000 description 1
- 229940107700 pyruvic acid Drugs 0.000 description 1
- IUVKMZGDUIUOCP-BTNSXGMBSA-N quinbolone Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1 IUVKMZGDUIUOCP-BTNSXGMBSA-N 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 238000011127 radiochemotherapy Methods 0.000 description 1
- 229960004622 raloxifene Drugs 0.000 description 1
- GZUITABIAKMVPG-UHFFFAOYSA-N raloxifene Chemical compound C1=CC(O)=CC=C1C1=C(C(=O)C=2C=CC(OCCN3CCCCC3)=CC=2)C2=CC=C(O)C=C2S1 GZUITABIAKMVPG-UHFFFAOYSA-N 0.000 description 1
- ZAHRKKWIAAJSAO-UHFFFAOYSA-N rapamycin Natural products COCC(O)C(=C/C(C)C(=O)CC(OC(=O)C1CCCCN1C(=O)C(=O)C2(O)OC(CC(OC)C(=CC=CC=CC(C)CC(C)C(=O)C)C)CCC2C)C(C)CC3CCC(O)C(C3)OC)C ZAHRKKWIAAJSAO-UHFFFAOYSA-N 0.000 description 1
- 206010037844 rash Diseases 0.000 description 1
- 230000008707 rearrangement Effects 0.000 description 1
- 108091006082 receptor inhibitors Proteins 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 229940121484 relatlimab Drugs 0.000 description 1
- 229930002330 retinoic acid Natural products 0.000 description 1
- 206010039073 rheumatoid arthritis Diseases 0.000 description 1
- 231100000279 safety data Toxicity 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- 210000003079 salivary gland Anatomy 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 239000004576 sand Substances 0.000 description 1
- 230000000276 sedentary effect Effects 0.000 description 1
- 230000001235 sensitizing effect Effects 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- 229960002930 sirolimus Drugs 0.000 description 1
- QFJCIRLUMZQUOT-HPLJOQBZSA-N sirolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 QFJCIRLUMZQUOT-HPLJOQBZSA-N 0.000 description 1
- 210000003491 skin Anatomy 0.000 description 1
- 208000020352 skin basal cell carcinoma Diseases 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910001467 sodium calcium phosphate Inorganic materials 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 239000001488 sodium phosphate Substances 0.000 description 1
- 239000007901 soft capsule Substances 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 230000000392 somatic effect Effects 0.000 description 1
- 229950007213 spartalizumab Drugs 0.000 description 1
- 210000000952 spleen Anatomy 0.000 description 1
- 238000010911 splenectomy Methods 0.000 description 1
- 238000011272 standard treatment Methods 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 238000011476 stem cell transplantation Methods 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 229960001052 streptozocin Drugs 0.000 description 1
- ZSJLQEPLLKMAKR-GKHCUFPYSA-N streptozocin Chemical compound O=NN(C)C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O ZSJLQEPLLKMAKR-GKHCUFPYSA-N 0.000 description 1
- 150000003890 succinate salts Chemical class 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 208000014794 superficial urinary bladder carcinoma Diseases 0.000 description 1
- 230000000153 supplemental effect Effects 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 230000001360 synchronised effect Effects 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 238000011521 systemic chemotherapy Methods 0.000 description 1
- 238000009121 systemic therapy Methods 0.000 description 1
- 229960001603 tamoxifen Drugs 0.000 description 1
- 238000002626 targeted therapy Methods 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 1
- NRUKOCRGYNPUPR-QBPJDGROSA-N teniposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@@H](OC[C@H]4O3)C=3SC=CC=3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 NRUKOCRGYNPUPR-QBPJDGROSA-N 0.000 description 1
- 229960001278 teniposide Drugs 0.000 description 1
- 201000003120 testicular cancer Diseases 0.000 description 1
- 208000024662 testicular seminoma Diseases 0.000 description 1
- 229960003604 testosterone Drugs 0.000 description 1
- 125000005207 tetraalkylammonium group Chemical group 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 201000002510 thyroid cancer Diseases 0.000 description 1
- 210000001685 thyroid gland Anatomy 0.000 description 1
- 229960003087 tioguanine Drugs 0.000 description 1
- 239000004408 titanium dioxide Substances 0.000 description 1
- 235000010215 titanium dioxide Nutrition 0.000 description 1
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 1
- 229940044693 topoisomerase inhibitor Drugs 0.000 description 1
- 229960005026 toremifene Drugs 0.000 description 1
- XFCLJVABOIYOMF-QPLCGJKRSA-N toremifene Chemical compound C1=CC(OCCN(C)C)=CC=C1C(\C=1C=CC=CC=1)=C(\CCCl)C1=CC=CC=C1 XFCLJVABOIYOMF-QPLCGJKRSA-N 0.000 description 1
- 239000003053 toxin Substances 0.000 description 1
- 231100000765 toxin Toxicity 0.000 description 1
- 108700012359 toxins Proteins 0.000 description 1
- 206010044412 transitional cell carcinoma Diseases 0.000 description 1
- 229960000575 trastuzumab Drugs 0.000 description 1
- 229950001353 tretamine Drugs 0.000 description 1
- IUCJMVBFZDHPDX-UHFFFAOYSA-N tretamine Chemical compound C1CN1C1=NC(N2CC2)=NC(N2CC2)=N1 IUCJMVBFZDHPDX-UHFFFAOYSA-N 0.000 description 1
- 229960001727 tretinoin Drugs 0.000 description 1
- NOYPYLRCIDNJJB-UHFFFAOYSA-N trimetrexate Chemical compound COC1=C(OC)C(OC)=CC(NCC=2C(=C3C(N)=NC(N)=NC3=CC=2)C)=C1 NOYPYLRCIDNJJB-UHFFFAOYSA-N 0.000 description 1
- 229960001099 trimetrexate Drugs 0.000 description 1
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 1
- 229960001005 tuberculin Drugs 0.000 description 1
- 230000004565 tumor cell growth Effects 0.000 description 1
- 230000005751 tumor progression Effects 0.000 description 1
- 229950009811 ubenimex Drugs 0.000 description 1
- 231100000402 unacceptable toxicity Toxicity 0.000 description 1
- 229960001055 uracil mustard Drugs 0.000 description 1
- 210000000626 ureter Anatomy 0.000 description 1
- 210000001635 urinary tract Anatomy 0.000 description 1
- VBEQCZHXXJYVRD-GACYYNSASA-N uroanthelone Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(C)C)[C@@H](C)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)CNC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CS)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O)C(C)C)[C@@H](C)CC)C1=CC=C(O)C=C1 VBEQCZHXXJYVRD-GACYYNSASA-N 0.000 description 1
- 208000023747 urothelial carcinoma Diseases 0.000 description 1
- 210000004291 uterus Anatomy 0.000 description 1
- 229960005486 vaccine Drugs 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 239000003981 vehicle Substances 0.000 description 1
- 229960003048 vinblastine Drugs 0.000 description 1
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 1
- 229960004528 vincristine Drugs 0.000 description 1
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 1
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 1
- UGGWPQSBPIFKDZ-KOTLKJBCSA-N vindesine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(N)=O)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1N=C1[C]2C=CC=C1 UGGWPQSBPIFKDZ-KOTLKJBCSA-N 0.000 description 1
- 229960004355 vindesine Drugs 0.000 description 1
- GBABOYUKABKIAF-GHYRFKGUSA-N vinorelbine Chemical compound C1N(CC=2C3=CC=CC=C3NC=22)CC(CC)=C[C@H]1C[C@]2(C(=O)OC)C1=CC([C@]23[C@H]([C@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC GBABOYUKABKIAF-GHYRFKGUSA-N 0.000 description 1
- 229960002066 vinorelbine Drugs 0.000 description 1
- 238000011179 visual inspection Methods 0.000 description 1
- 230000008673 vomiting Effects 0.000 description 1
- 229940053867 xeloda Drugs 0.000 description 1
- 229960000641 zorubicin Drugs 0.000 description 1
- FBTUMDXHSRTGRV-ALTNURHMSA-N zorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(\C)=N\NC(=O)C=1C=CC=CC=1)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 FBTUMDXHSRTGRV-ALTNURHMSA-N 0.000 description 1
- PAPBSGBWRJIAAV-UHFFFAOYSA-N ε-Caprolactone Chemical compound O=C1CCCCCO1 PAPBSGBWRJIAAV-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/39541—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against normal tissues, cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/04—Antineoplastic agents specific for metastasis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2818—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against CD28 or CD152
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2300/00—Mixtures or combinations of active ingredients, wherein at least one active ingredient is fully defined in groups A61K31/00 - A61K41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Immunology (AREA)
- Organic Chemistry (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Molecular Biology (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Urology & Nephrology (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Biomedical Technology (AREA)
- Dermatology (AREA)
- Oncology (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Applications Claiming Priority (13)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201762565819P | 2017-09-29 | 2017-09-29 | |
| US62/565819 | 2017-09-29 | ||
| US201762569766P | 2017-10-09 | 2017-10-09 | |
| US62/569766 | 2017-10-09 | ||
| US201762580219P | 2017-11-01 | 2017-11-01 | |
| US62/580219 | 2017-11-01 | ||
| US201762609210P | 2017-12-21 | 2017-12-21 | |
| US62/609210 | 2017-12-21 | ||
| US201862642711P | 2018-03-14 | 2018-03-14 | |
| US62/642711 | 2018-03-14 | ||
| US201862678490P | 2018-05-31 | 2018-05-31 | |
| US62/678490 | 2018-05-31 | ||
| PCT/US2018/053443 WO2019067913A1 (en) | 2017-09-29 | 2018-09-28 | COMPOSITIONS AND METHODS OF TREATING CANCER |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN111386117A true CN111386117A (zh) | 2020-07-07 |
Family
ID=65902748
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201880077408.5A Pending CN111386117A (zh) | 2017-09-29 | 2018-09-28 | 治疗癌症的组合物和方法 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US11351163B2 (enExample) |
| EP (1) | EP3691643A4 (enExample) |
| JP (1) | JP7341130B2 (enExample) |
| KR (1) | KR102760684B1 (enExample) |
| CN (1) | CN111386117A (enExample) |
| WO (1) | WO2019067913A1 (enExample) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013173223A1 (en) * | 2012-05-15 | 2013-11-21 | Bristol-Myers Squibb Company | Cancer immunotherapy by disrupting pd-1/pd-l1 signaling |
| EP3288980B1 (en) * | 2015-04-28 | 2021-03-10 | Bristol-Myers Squibb Company | Treatment of pd-l1-positive melanoma using an anti-pd-1 antibody |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20160137652A1 (en) * | 2014-11-05 | 2016-05-19 | Flexus Biosciences, Inc. | Immunoregulatory agents |
| CN105960415A (zh) * | 2014-02-04 | 2016-09-21 | 辉瑞大药厂 | 用于治疗癌症的pd-1拮抗剂和vegfr抑制剂的组合 |
| WO2017079669A1 (en) * | 2015-11-04 | 2017-05-11 | Incyte Corporation | Pharmaceutical compositions and methods for indoleamine 2,3-dioxygenase inhibition and indications therefor |
| CN106714839A (zh) * | 2014-05-15 | 2017-05-24 | 百时美施贵宝公司 | 使用抗pd‑1抗体和另一种抗癌剂的组合治疗肺癌 |
Family Cites Families (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5851795A (en) | 1991-06-27 | 1998-12-22 | Bristol-Myers Squibb Company | Soluble CTLA4 molecules and uses thereof |
| US6051227A (en) | 1995-07-25 | 2000-04-18 | The Regents Of The University Of California, Office Of Technology Transfer | Blockade of T lymphocyte down-regulation associated with CTLA-4 signaling |
| SG143018A1 (en) | 1998-12-23 | 2008-06-27 | Pfizer | Human monoclonal antibodies to ctla-4 |
| EE05627B1 (et) | 1998-12-23 | 2013-02-15 | Pfizer Inc. | CTLA-4 vastased inimese monoklonaalsed antikehad |
| WO2001014557A1 (en) | 1999-08-23 | 2001-03-01 | Dana-Farber Cancer Institute, Inc. | Pd-1, a receptor for b7-4, and uses therefor |
| US7605238B2 (en) | 1999-08-24 | 2009-10-20 | Medarex, Inc. | Human CTLA-4 antibodies and their uses |
| EP1212422B1 (en) | 1999-08-24 | 2007-02-21 | Medarex, Inc. | Human ctla-4 antibodies and their uses |
| US7034121B2 (en) | 2000-01-27 | 2006-04-25 | Genetics Institue, Llc | Antibodies against CTLA4 |
| BR0316880A (pt) | 2002-12-23 | 2005-10-25 | Wyeth Corp | Anticorpos contra pd-1 e usos dos mesmos |
| EP2439273B1 (en) | 2005-05-09 | 2019-02-27 | Ono Pharmaceutical Co., Ltd. | Human monoclonal antibodies to programmed death 1(PD-1) and methods for treating cancer using anti-PD-1 antibodies alone or in combination with other immunotherapeutics |
| EP2007423A2 (en) | 2006-04-05 | 2008-12-31 | Pfizer Products Incorporated | Ctla4 antibody combination therapy |
| NZ600758A (en) | 2007-06-18 | 2013-09-27 | Merck Sharp & Dohme | Antibodies to human programmed death receptor pd-1 |
| US8168757B2 (en) | 2008-03-12 | 2012-05-01 | Merck Sharp & Dohme Corp. | PD-1 binding proteins |
| WO2012122444A1 (en) | 2011-03-10 | 2012-09-13 | Provectus Pharmaceuticals, Inc. | Combination of local and systemic immunomodulative therapies for enhanced treatment of cancer |
| ES2669310T3 (es) | 2011-04-20 | 2018-05-24 | Medimmune, Llc | Anticuerpos y otras moléculas que se unen con B7-H1 y PD-1 |
| SG11201508528TA (en) | 2013-05-02 | 2015-11-27 | Anaptysbio Inc | Antibodies directed against programmed death-1 (pd-1) |
| CN111423511B (zh) | 2013-05-31 | 2024-02-23 | 索伦托药业有限公司 | 与pd-1结合的抗原结合蛋白 |
| CN104250302B (zh) | 2013-06-26 | 2017-11-14 | 上海君实生物医药科技股份有限公司 | 抗pd‑1抗体及其应用 |
| PT3702373T (pt) | 2013-09-13 | 2022-09-27 | Beigene Switzerland Gmbh | Anticorpos anti-pd1 e a sua utilização como agentes terapêuticos e de diagnóstico |
| CR20160319A (es) | 2013-12-12 | 2016-11-08 | Jiangsu Hengrui Medicine Co | Anticuerpo pd-1, fragmento de union al antigeno de este y uso médico de este |
| TWI681969B (zh) | 2014-01-23 | 2020-01-11 | 美商再生元醫藥公司 | 針對pd-1的人類抗體 |
| JOP20200094A1 (ar) | 2014-01-24 | 2017-06-16 | Dana Farber Cancer Inst Inc | جزيئات جسم مضاد لـ pd-1 واستخداماتها |
| AR102537A1 (es) | 2014-11-05 | 2017-03-08 | Flexus Biosciences Inc | Agentes inmunomoduladores |
| DK3237446T3 (en) | 2014-12-22 | 2021-07-26 | Pd 1 Acquisition Group Llc | Anti-PD-1-antistoffer |
| US10144779B2 (en) | 2015-05-29 | 2018-12-04 | Agenus Inc. | Anti-CTLA-4 antibodies and methods of use thereof |
| EP3307777A4 (en) | 2015-06-11 | 2019-02-13 | Wuxi Biologics (Shanghai) Co. Ltd. | NOVEL ANTI-PD-L1 ANTIBODIES |
| WO2017020291A1 (en) | 2015-08-06 | 2017-02-09 | Wuxi Biologics (Shanghai) Co. Ltd. | Novel anti-pd-l1 antibodies |
| WO2017024465A1 (en) | 2015-08-10 | 2017-02-16 | Innovent Biologics (Suzhou) Co., Ltd. | Pd-1 antibodies |
| HRP20241381T1 (hr) | 2015-08-11 | 2024-12-20 | WuXi Biologics Ireland Limited | Nova anti-pd-1 protutijela |
| WO2017024515A1 (en) | 2015-08-11 | 2017-02-16 | Wuxi Biologics (Cayman) Inc. | Novel anti-pd-1 antibodies |
| KR20220131277A (ko) | 2015-09-01 | 2022-09-27 | 아게누스 인코포레이티드 | 항-pd-1 항체 및 이를 이용하는 방법 |
| AU2016370376B2 (en) | 2015-12-14 | 2023-12-14 | Macrogenics, Inc. | Bispecific molecules having immunoreactivity with PD-1 and CTLA-4, and methods of use thereof |
| WO2017123557A1 (en) | 2016-01-11 | 2017-07-20 | Armo Biosciences, Inc. | Interleukin-10 in production of antigen-specific cd8+ t cells and methods of use of same |
| WO2017132827A1 (en) | 2016-02-02 | 2017-08-10 | Innovent Biologics (Suzhou) Co., Ltd. | Pd-1 antibodies |
| US10731128B2 (en) * | 2016-11-22 | 2020-08-04 | Alloplex Biotherapeutics, Inc. | Compositions and methods for in vitro activation and expansion of serial killer T cell populations and passive immunization of a cancer patient with tumor cell killing cells |
-
2018
- 2018-09-28 CN CN201880077408.5A patent/CN111386117A/zh active Pending
- 2018-09-28 WO PCT/US2018/053443 patent/WO2019067913A1/en not_active Ceased
- 2018-09-28 US US16/649,277 patent/US11351163B2/en active Active
- 2018-09-28 JP JP2020517983A patent/JP7341130B2/ja active Active
- 2018-09-28 KR KR1020207011965A patent/KR102760684B1/ko active Active
- 2018-09-28 EP EP18861598.3A patent/EP3691643A4/en active Pending
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN105960415A (zh) * | 2014-02-04 | 2016-09-21 | 辉瑞大药厂 | 用于治疗癌症的pd-1拮抗剂和vegfr抑制剂的组合 |
| CN106714839A (zh) * | 2014-05-15 | 2017-05-24 | 百时美施贵宝公司 | 使用抗pd‑1抗体和另一种抗癌剂的组合治疗肺癌 |
| US20160137652A1 (en) * | 2014-11-05 | 2016-05-19 | Flexus Biosciences, Inc. | Immunoregulatory agents |
| WO2017079669A1 (en) * | 2015-11-04 | 2017-05-11 | Incyte Corporation | Pharmaceutical compositions and methods for indoleamine 2,3-dioxygenase inhibition and indications therefor |
Non-Patent Citations (1)
| Title |
|---|
| LILLIAN L.SIU等: "Abstract CT116:BMS-986205,an optimized indoleamine 2,3-dioxygenase 1(IDO1) inhibitor,is well tolerated with potent pharmacodynamic(PD) activity,alone and in combination with nivolumab(nivo) in advanced cancers in a phase 1/2a trial", 《CANCER RESEARCH》 * |
Also Published As
| Publication number | Publication date |
|---|---|
| KR102760684B1 (ko) | 2025-01-24 |
| JP7341130B2 (ja) | 2023-09-08 |
| US20210030739A1 (en) | 2021-02-04 |
| JP2020536068A (ja) | 2020-12-10 |
| WO2019067913A1 (en) | 2019-04-04 |
| US11351163B2 (en) | 2022-06-07 |
| EP3691643A1 (en) | 2020-08-12 |
| EP3691643A4 (en) | 2021-06-16 |
| KR20200061382A (ko) | 2020-06-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN110536703B (zh) | 使用抗axl抗体-药物缀合物的组合疗法 | |
| CN113271945A (zh) | 包含3-(1-氧代异吲哚啉-2-基)哌啶-2,6-二酮衍生物的给药方案和药物组合 | |
| CN106456753A (zh) | 用于治疗癌症的pd‑1拮抗剂和ido1抑制剂的组合 | |
| CN113507963A (zh) | 用于晚期实体瘤癌症的治疗性rna及抗pd1抗体 | |
| JP2020517652A5 (enExample) | ||
| CN115916199A (zh) | 包含3-(1-氧代异吲哚啉-2-基)哌啶-2,6-二酮衍生物的给药方案 | |
| JP2024028805A (ja) | がん処置のための坑il-8抗体及び坑pd-1抗体を用いる組合せ治療 | |
| US20220160718A1 (en) | Compositions and methods of treating cancer | |
| US20230131598A1 (en) | Combination treatment for cancer | |
| CN115698075A (zh) | 涉及抗icos和抗pd1抗体,任选地进一步涉及抗tim3抗体的癌症的组合治疗 | |
| CN111386117A (zh) | 治疗癌症的组合物和方法 | |
| JP2021533090A (ja) | 併用療法 | |
| CN112839644A (zh) | 用pd-1轴结合拮抗剂、抗代谢物和铂剂治疗肺癌的方法 | |
| CN115461362A (zh) | 基于ICOS抗体和PD-L1抗体TGF-β受体融合蛋白的癌症组合疗法 | |
| JP2022535034A (ja) | 抗原結合タンパク質 | |
| CN113226369A (zh) | 给药 | |
| JP2025505642A (ja) | Egfr/met二重特異性抗体で処置された患者における注入関連反応を低減するための方法 | |
| TW202305009A (zh) | 以皮下投予抗pd1抗體治療癌症之方法 | |
| US20230340136A1 (en) | Treatment of cll | |
| US20230365691A1 (en) | Use of anti-pd-1 antibody in treatment of nasopharyngeal carcinoma | |
| WO2025245489A1 (en) | Treatment of tumors in subjects having fgl-1 positive samples | |
| HK40053012A (en) | Methods of treating lung cancer with a pd-1 axis binding antagonist, an antimetabolite, and a platinum agent |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination |