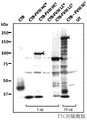
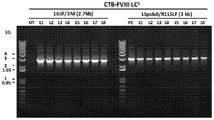
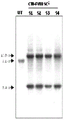
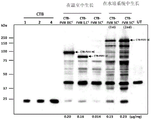
CN110603046B - 用于提高高等植物叶绿体中转基因表达的密码子优化和核糖体图谱分析 - Google Patents
用于提高高等植物叶绿体中转基因表达的密码子优化和核糖体图谱分析 Download PDFInfo
- Publication number
- CN110603046B CN110603046B CN201780031137.5A CN201780031137A CN110603046B CN 110603046 B CN110603046 B CN 110603046B CN 201780031137 A CN201780031137 A CN 201780031137A CN 110603046 B CN110603046 B CN 110603046B
- Authority
- CN
- China
- Prior art keywords
- codons
- codon
- protein
- replaced
- ctb
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/82—Vectors or expression systems specially adapted for eukaryotic hosts for plant cells, e.g. plant artificial chromosomes (PACs)
- C12N15/8241—Phenotypically and genetically modified plants via recombinant DNA technology
- C12N15/8242—Phenotypically and genetically modified plants via recombinant DNA technology with non-agronomic quality (output) traits, e.g. for industrial processing; Value added, non-agronomic traits
- C12N15/8257—Phenotypically and genetically modified plants via recombinant DNA technology with non-agronomic quality (output) traits, e.g. for industrial processing; Value added, non-agronomic traits for the production of primary gene products, e.g. pharmaceutical products, interferon
- C12N15/8258—Phenotypically and genetically modified plants via recombinant DNA technology with non-agronomic quality (output) traits, e.g. for industrial processing; Value added, non-agronomic traits for the production of primary gene products, e.g. pharmaceutical products, interferon for the production of oral vaccines (antigens) or immunoglobulins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0053—Mouth and digestive tract, i.e. intraoral and peroral administration
- A61K9/0056—Mouth soluble or dispersible forms; Suckable, eatable, chewable coherent forms; Forms rapidly disintegrating in the mouth; Lozenges; Lollipops; Bite capsules; Baked products; Baits or other oral forms for animals
- A61K9/0058—Chewing gums
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/02—Stomatological preparations, e.g. drugs for caries, aphtae, periodontitis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- C07K14/47—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
- C07K14/4701—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals not used
- C07K14/4723—Cationic antimicrobial peptides, e.g. defensins
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/575—Hormones
- C07K14/65—Insulin-like growth factors, i.e. somatomedins, e.g. IGF-1, IGF-2
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/745—Blood coagulation or fibrinolysis factors
- C07K14/755—Factors VIII, e.g. factor VIII C (AHF), factor VIII Ag (VWF)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N1/00—Microorganisms, e.g. protozoa; Compositions thereof; Processes of propagating, maintaining or preserving microorganisms or compositions thereof; Processes of preparing or isolating a composition containing a microorganism; Culture media therefor
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/82—Vectors or expression systems specially adapted for eukaryotic hosts for plant cells, e.g. plant artificial chromosomes (PACs)
- C12N15/8201—Methods for introducing genetic material into plant cells, e.g. DNA, RNA, stable or transient incorporation, tissue culture methods adapted for transformation
- C12N15/8214—Plastid transformation
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/82—Vectors or expression systems specially adapted for eukaryotic hosts for plant cells, e.g. plant artificial chromosomes (PACs)
- C12N15/8216—Methods for controlling, regulating or enhancing expression of transgenes in plant cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/82—Vectors or expression systems specially adapted for eukaryotic hosts for plant cells, e.g. plant artificial chromosomes (PACs)
- C12N15/8241—Phenotypically and genetically modified plants via recombinant DNA technology
- C12N15/8242—Phenotypically and genetically modified plants via recombinant DNA technology with non-agronomic quality (output) traits, e.g. for industrial processing; Value added, non-agronomic traits
- C12N15/8257—Phenotypically and genetically modified plants via recombinant DNA technology with non-agronomic quality (output) traits, e.g. for industrial processing; Value added, non-agronomic traits for the production of primary gene products, e.g. pharmaceutical products, interferon
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N7/00—Viruses; Bacteriophages; Compositions thereof; Preparation or purification thereof
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/24—Hydrolases (3) acting on glycosyl compounds (3.2)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/24—Hydrolases (3) acting on glycosyl compounds (3.2)
- C12N9/2402—Hydrolases (3) acting on glycosyl compounds (3.2) hydrolysing O- and S- glycosyl compounds (3.2.1)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/24—Hydrolases (3) acting on glycosyl compounds (3.2)
- C12N9/2402—Hydrolases (3) acting on glycosyl compounds (3.2) hydrolysing O- and S- glycosyl compounds (3.2.1)
- C12N9/2405—Glucanases
- C12N9/2408—Glucanases acting on alpha -1,4-glucosidic bonds
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/24—Hydrolases (3) acting on glycosyl compounds (3.2)
- C12N9/2402—Hydrolases (3) acting on glycosyl compounds (3.2) hydrolysing O- and S- glycosyl compounds (3.2.1)
- C12N9/2405—Glucanases
- C12N9/2451—Glucanases acting on alpha-1,6-glucosidic bonds
- C12N9/2454—Dextranase (3.2.1.11)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y302/00—Hydrolases acting on glycosyl compounds, i.e. glycosylases (3.2)
- C12Y302/01—Glycosidases, i.e. enzymes hydrolysing O- and S-glycosyl compounds (3.2.1)
- C12Y302/01084—Glucan 1,3-alpha-glucosidase (3.2.1.84), i.e. mutanase
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/54—Medicinal preparations containing antigens or antibodies characterised by the route of administration
- A61K2039/541—Mucosal route
- A61K2039/542—Mucosal route oral/gastrointestinal
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/545—Medicinal preparations containing antigens or antibodies characterised by the dose, timing or administration schedule
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/55566—Emulsions, e.g. Freund's adjuvant, MF59
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/55577—Saponins; Quil A; QS21; ISCOMS
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/57—Medicinal preparations containing antigens or antibodies characterised by the type of response, e.g. Th1, Th2
- A61K2039/575—Medicinal preparations containing antigens or antibodies characterised by the type of response, e.g. Th1, Th2 humoral response
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/60—Medicinal preparations containing antigens or antibodies characteristics by the carrier linked to the antigen
- A61K2039/6031—Proteins
- A61K2039/6037—Bacterial toxins, e.g. diphteria toxoid [DT], tetanus toxoid [TT]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2770/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses positive-sense
- C12N2770/00011—Details
- C12N2770/32011—Picornaviridae
- C12N2770/32611—Poliovirus
- C12N2770/32634—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Genetics & Genomics (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Zoology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Wood Science & Technology (AREA)
- General Health & Medical Sciences (AREA)
- Molecular Biology (AREA)
- Biotechnology (AREA)
- Medicinal Chemistry (AREA)
- Biomedical Technology (AREA)
- General Engineering & Computer Science (AREA)
- Biochemistry (AREA)
- Microbiology (AREA)
- Biophysics (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Virology (AREA)
- Physics & Mathematics (AREA)
- Cell Biology (AREA)
- Plant Pathology (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Gastroenterology & Hepatology (AREA)
- Toxicology (AREA)
- Immunology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Epidemiology (AREA)
- Communicable Diseases (AREA)
- Hematology (AREA)
- Oncology (AREA)
- Mycology (AREA)
- Diabetes (AREA)
- Endocrinology (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662310788P | 2016-03-20 | 2016-03-20 | |
| US62/310,788 | 2016-03-20 | ||
| PCT/US2017/023263 WO2017165320A1 (en) | 2016-03-20 | 2017-03-20 | Codon optimization and ribosome profiling for increasing transgene expression in chloroplasts of higher plants |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN110603046A CN110603046A (zh) | 2019-12-20 |
| CN110603046B true CN110603046B (zh) | 2022-10-18 |
Family
ID=59899714
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201780031137.5A Active CN110603046B (zh) | 2016-03-20 | 2017-03-20 | 用于提高高等植物叶绿体中转基因表达的密码子优化和核糖体图谱分析 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US11332754B2 (enExample) |
| EP (1) | EP3432902B8 (enExample) |
| JP (1) | JP7145083B2 (enExample) |
| CN (1) | CN110603046B (enExample) |
| CA (1) | CA3055900A1 (enExample) |
| WO (1) | WO2017165320A1 (enExample) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN117224709A (zh) * | 2016-05-12 | 2023-12-15 | 宾夕法尼亚州立大学托管会 | 用于抑制生物膜沉积和产生的组合物和方法 |
| WO2020041599A1 (en) * | 2018-08-22 | 2020-02-27 | The Trustees Of The University Of Pennsylvania | Compositions and methods for production of antibiotic free biopharmaceuticals in lettuce chloroplasts |
| CN112195226B (zh) * | 2019-07-08 | 2022-05-24 | 清华大学 | 极小量细胞核糖体保护的rna片段测序的方法 |
| CN111635935A (zh) * | 2020-06-10 | 2020-09-08 | 华中农业大学 | 一种低成本、高通量间接量化蛋白表达水平的方法及其应用 |
| CN120266212A (zh) * | 2022-11-24 | 2025-07-04 | 南京金斯瑞生物科技有限公司 | 密码子优化 |
| CN117511978B (zh) * | 2024-01-08 | 2024-03-22 | 深圳粒影生物科技有限公司 | 一种提高外源蛋白表达量的方法 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1886512A (zh) * | 2002-04-23 | 2006-12-27 | 斯克里普斯研究所 | 多肽在叶绿体中的表达以及用于表达多肽的组合物和方法 |
| WO2011063284A1 (en) * | 2009-11-19 | 2011-05-26 | Sapphire Energy, Inc. | Production of therapeutic proteins in photosynthetic organisms |
| CN102146344A (zh) * | 2010-11-02 | 2011-08-10 | 上海师范大学 | 优化密码子提高外源基因表达量和莱茵衣藻制氢量的方法 |
Family Cites Families (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4946676A (en) * | 1986-08-27 | 1990-08-07 | De Staat Der Nederlanden | Vaccine comprising an immunogenic protein and, as an adjuvant, a substantially non-immunogenic, sequentially homologous peptide |
| JPH10201483A (ja) * | 1996-11-25 | 1998-08-04 | Lion Corp | ムタナーゼ遺伝子 |
| US20110179530A1 (en) | 2001-01-23 | 2011-07-21 | University Of Central Florida Research Foundation, Inc. | Pharmaceutical Proteins, Human Therapeutics, Human Serum Albumin Insulin, Native Cholera Toxin B Subunit on Transgenic Plastids |
| US7741536B2 (en) | 1997-08-07 | 2010-06-22 | University Of Central Florida Research Foundation, Inc. | Expression of human serum albumin in plastids |
| NL1009834C2 (nl) * | 1998-08-10 | 2000-02-11 | Stanislaus Laurens Johan Woute | Antistoffen voor toepassing in de gerichte en tijdelijke behandeling van mens en dier. |
| BR9915919A (pt) | 1998-10-07 | 2001-08-21 | Syngenta Participations Ag | Proteìnas terapeuticamente ativas em plantas |
| JP2005185182A (ja) * | 2003-12-25 | 2005-07-14 | Lion Corp | α−1,3−グルコオリゴ糖の製造方法、該製造方法により得られるα−1,3−グルコオリゴ糖およびこれを含有する製剤組成物 |
| US8043833B2 (en) * | 2005-10-31 | 2011-10-25 | Novo Nordisk A/S | Expression of soluble therapeutic proteins |
| WO2007133558A2 (en) * | 2006-05-09 | 2007-11-22 | The Scripps Research Institute | Robust expression of a bioactive mammalian protein in chlamydomonas chloroplast |
| DK2134853T3 (en) * | 2007-04-03 | 2018-10-08 | Oxyrane Uk Ltd | Glycosylation of molecules |
| GB0911870D0 (en) * | 2009-07-08 | 2009-08-19 | Ucl Business Plc | Optimised coding sequence and promoter |
| US8326547B2 (en) * | 2009-10-07 | 2012-12-04 | Nanjingjinsirui Science & Technology Biology Corp. | Method of sequence optimization for improved recombinant protein expression using a particle swarm optimization algorithm |
| WO2012006623A1 (en) | 2010-07-09 | 2012-01-12 | Biogen Idec Hemophilia Inc. | Systems for factor viii processing and methods thereof |
| WO2013063049A1 (en) * | 2011-10-24 | 2013-05-02 | University Of Central Florida Research Foundation, Inc. | Orally-administered plastid expressed cholera toxin b subunit-exendin 4 as treatment for type 2 diabetes |
| WO2013170031A1 (en) * | 2012-05-09 | 2013-11-14 | The Regents Of The University Of California | Method for in silico modeling of gene product expression and metabolism |
| FI3889173T3 (fi) | 2013-02-15 | 2023-10-02 | Bioverativ Therapeutics Inc | Optimoitu tekijä viii:n geeni |
| WO2015073988A1 (en) * | 2013-11-15 | 2015-05-21 | Trustees Of The University Of Pennsylvania | Compositions and methods for suppression of inhibitor formation against factor viii in hemophilia a patients |
-
2017
- 2017-03-20 CN CN201780031137.5A patent/CN110603046B/zh active Active
- 2017-03-20 JP JP2018568189A patent/JP7145083B2/ja active Active
- 2017-03-20 EP EP17770930.0A patent/EP3432902B8/en active Active
- 2017-03-20 WO PCT/US2017/023263 patent/WO2017165320A1/en not_active Ceased
- 2017-03-20 US US16/086,416 patent/US11332754B2/en active Active
- 2017-03-20 CA CA3055900A patent/CA3055900A1/en active Pending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1886512A (zh) * | 2002-04-23 | 2006-12-27 | 斯克里普斯研究所 | 多肽在叶绿体中的表达以及用于表达多肽的组合物和方法 |
| WO2011063284A1 (en) * | 2009-11-19 | 2011-05-26 | Sapphire Energy, Inc. | Production of therapeutic proteins in photosynthetic organisms |
| CN102146344A (zh) * | 2010-11-02 | 2011-08-10 | 上海师范大学 | 优化密码子提高外源基因表达量和莱茵衣藻制氢量的方法 |
Non-Patent Citations (2)
| Title |
|---|
| Codon usage influences the local rate of translation elongation to regulate co-translational protein folding;Chien-Hung Yu等;《Mol Cell》;20150903;第59卷(第5期);全文 * |
| Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling;Nicholas T. Ingolia等;《Science》;20090410;第324卷;摘要 * |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3432902A4 (en) | 2019-08-28 |
| EP3432902A1 (en) | 2019-01-30 |
| EP3432902B1 (en) | 2025-11-05 |
| US20190194679A1 (en) | 2019-06-27 |
| CA3055900A1 (en) | 2017-09-28 |
| CN110603046A (zh) | 2019-12-20 |
| JP7145083B2 (ja) | 2022-09-30 |
| JP2019509064A (ja) | 2019-04-04 |
| EP3432902B8 (en) | 2025-12-17 |
| WO2017165320A1 (en) | 2017-09-28 |
| US11332754B2 (en) | 2022-05-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN110603046B (zh) | 用于提高高等植物叶绿体中转基因表达的密码子优化和核糖体图谱分析 | |
| US11085049B2 (en) | Influenza virus-like particle production in plants | |
| DK2445928T3 (en) | CHEMICAL INFLUENZA VIRUS-LIKE PARTICLES INCLUDING HEMAGGLUTIN | |
| Daniell et al. | Cold chain and virus‐free oral polio booster vaccine made in lettuce chloroplasts confers protection against all three poliovirus serotypes | |
| Kwon et al. | Codon optimization to enhance expression yields insights into chloroplast translation | |
| CN101820903A (zh) | 预防性和治疗性流感疫苗、抗原、组合物和方法 | |
| IL263643B (en) | Air intake system for engines | |
| CA2658217A1 (en) | Expression system | |
| US20260014243A1 (en) | Crimean-congo hemorrhagic fever virus replicon particles and use thereof | |
| EP2169054A1 (en) | Vaccine for swine edema disease | |
| KR20080091759A (ko) | 신규 식물 바이러스 입자 및 그의 불활성화 방법 | |
| JP2023539356A (ja) | 改変コロナウイルス構造タンパク質 | |
| CN111565747B (zh) | 与猪fc片段融合的抗原,以及包含该抗原的疫苗组合物 | |
| KR101680903B1 (ko) | 돼지 유행성 설사병 바이러스의 에피토프와 로타바이러스 유전자를 발현하는 형질전환체 및 이를 포함하는 백신 조성물 | |
| Barzigar et al. | Transient recombinant expression of highly immunogenic CagA, VacA and NapA in Nicotiana benthamiana | |
| HK40017237B (en) | Codon optimization and ribosome profiling for increasing transgene expression in chloroplasts of higher plants | |
| HK40017237A (en) | Codon optimization and ribosome profiling for increasing transgene expression in chloroplasts of higher plants | |
| KR101671528B1 (ko) | 돼지 유행성 설사병 바이러스의 에피토프와 점막면역보조제를 발현하는 형질전환체 및 이를 포함하는 백신 조성물 | |
| US12467058B2 (en) | Influenza virus-like particle production in plants | |
| US20250277007A1 (en) | Cwp2 protein as an effective vaccine against clostridioides difficile infection | |
| CA2907591C (en) | Influenza virus-like particle production in plants | |
| CN119947996A (zh) | 修饰的乙型流感病毒血球凝集素 | |
| HK1220729B (en) | Influenza virus-like particle production in plants |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: DE Ref document number: 40017237 Country of ref document: HK |
|
| GR01 | Patent grant | ||
| GR01 | Patent grant |