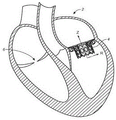
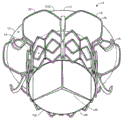
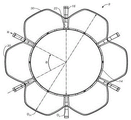
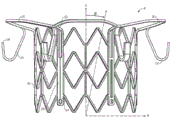
CN107233146B - 适用心脏二尖瓣和三尖瓣带瓣支架置换瓣膜 - Google Patents
适用心脏二尖瓣和三尖瓣带瓣支架置换瓣膜 Download PDFInfo
- Publication number
- CN107233146B CN107233146B CN201710675846.9A CN201710675846A CN107233146B CN 107233146 B CN107233146 B CN 107233146B CN 201710675846 A CN201710675846 A CN 201710675846A CN 107233146 B CN107233146 B CN 107233146B
- Authority
- CN
- China
- Prior art keywords
- valve
- stent
- heart
- metal
- tricuspid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 210000004115 mitral valve Anatomy 0.000 title claims abstract description 38
- 210000002216 heart Anatomy 0.000 title claims abstract description 33
- 210000000591 tricuspid valve Anatomy 0.000 title claims abstract description 18
- 238000000034 method Methods 0.000 claims abstract description 22
- 239000002184 metal Substances 0.000 claims description 69
- 229910052751 metal Inorganic materials 0.000 claims description 69
- 210000005003 heart tissue Anatomy 0.000 claims description 25
- 239000008280 blood Substances 0.000 claims description 23
- 210000004369 blood Anatomy 0.000 claims description 23
- 238000002513 implantation Methods 0.000 claims description 12
- 230000000747 cardiac effect Effects 0.000 claims description 9
- 230000008569 process Effects 0.000 claims description 5
- 230000001174 ascending effect Effects 0.000 claims description 4
- 230000008859 change Effects 0.000 claims description 3
- 210000003709 heart valve Anatomy 0.000 abstract description 22
- 238000013461 design Methods 0.000 description 15
- 230000006870 function Effects 0.000 description 13
- 230000017531 blood circulation Effects 0.000 description 11
- 210000001519 tissue Anatomy 0.000 description 11
- 206010027727 Mitral valve incompetence Diseases 0.000 description 10
- 238000001356 surgical procedure Methods 0.000 description 10
- 230000008439 repair process Effects 0.000 description 9
- 230000010339 dilation Effects 0.000 description 7
- 238000006073 displacement reaction Methods 0.000 description 6
- 210000002837 heart atrium Anatomy 0.000 description 6
- 210000005240 left ventricle Anatomy 0.000 description 6
- 210000003540 papillary muscle Anatomy 0.000 description 6
- 210000001765 aortic valve Anatomy 0.000 description 5
- 239000004744 fabric Substances 0.000 description 4
- 229910001092 metal group alloy Inorganic materials 0.000 description 4
- 150000002739 metals Chemical class 0.000 description 4
- 238000002560 therapeutic procedure Methods 0.000 description 4
- 210000000709 aorta Anatomy 0.000 description 3
- 210000003698 chordae tendineae Anatomy 0.000 description 3
- 230000006835 compression Effects 0.000 description 3
- 238000007906 compression Methods 0.000 description 3
- 230000006386 memory function Effects 0.000 description 3
- 238000010992 reflux Methods 0.000 description 3
- 230000002792 vascular Effects 0.000 description 3
- 206010056370 Congestive cardiomyopathy Diseases 0.000 description 2
- 201000010046 Dilated cardiomyopathy Diseases 0.000 description 2
- 229910000640 Fe alloy Inorganic materials 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- 206010067171 Regurgitation Diseases 0.000 description 2
- 230000002411 adverse Effects 0.000 description 2
- 230000036760 body temperature Effects 0.000 description 2
- 230000001627 detrimental effect Effects 0.000 description 2
- 230000004064 dysfunction Effects 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 230000003090 exacerbative effect Effects 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 239000007943 implant Substances 0.000 description 2
- 208000014674 injury Diseases 0.000 description 2
- 231100000518 lethal Toxicity 0.000 description 2
- 230000001665 lethal effect Effects 0.000 description 2
- 230000007257 malfunction Effects 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 210000004165 myocardium Anatomy 0.000 description 2
- 230000007170 pathology Effects 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 210000003102 pulmonary valve Anatomy 0.000 description 2
- 239000010935 stainless steel Substances 0.000 description 2
- 229910001220 stainless steel Inorganic materials 0.000 description 2
- 230000008733 trauma Effects 0.000 description 2
- 230000000472 traumatic effect Effects 0.000 description 2
- 238000011282 treatment Methods 0.000 description 2
- 208000031481 Pathologic Constriction Diseases 0.000 description 1
- 208000035965 Postoperative Complications Diseases 0.000 description 1
- 206010052428 Wound Diseases 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- 238000007792 addition Methods 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 230000001746 atrial effect Effects 0.000 description 1
- 238000010009 beating Methods 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 210000005242 cardiac chamber Anatomy 0.000 description 1
- 230000002612 cardiopulmonary effect Effects 0.000 description 1
- 210000000038 chest Anatomy 0.000 description 1
- 230000004087 circulation Effects 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 210000002808 connective tissue Anatomy 0.000 description 1
- 210000003748 coronary sinus Anatomy 0.000 description 1
- 238000002716 delivery method Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 229910003460 diamond Inorganic materials 0.000 description 1
- 239000010432 diamond Substances 0.000 description 1
- 230000010247 heart contraction Effects 0.000 description 1
- 208000019622 heart disease Diseases 0.000 description 1
- 230000007770 heart valve development Effects 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 230000005923 long-lasting effect Effects 0.000 description 1
- 238000002324 minimally invasive surgery Methods 0.000 description 1
- 208000005907 mitral valve insufficiency Diseases 0.000 description 1
- 230000002685 pulmonary effect Effects 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- 230000036262 stenosis Effects 0.000 description 1
- 208000037804 stenosis Diseases 0.000 description 1
- 238000011144 upstream manufacturing Methods 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- 230000002861 ventricular Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/24—Heart valves ; Vascular valves, e.g. venous valves; Heart implants, e.g. passive devices for improving the function of the native valve or the heart muscle; Transmyocardial revascularisation [TMR] devices; Valves implantable in the body
- A61F2/2412—Heart valves ; Vascular valves, e.g. venous valves; Heart implants, e.g. passive devices for improving the function of the native valve or the heart muscle; Transmyocardial revascularisation [TMR] devices; Valves implantable in the body with soft flexible valve members, e.g. tissue valves shaped like natural valves
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/24—Heart valves ; Vascular valves, e.g. venous valves; Heart implants, e.g. passive devices for improving the function of the native valve or the heart muscle; Transmyocardial revascularisation [TMR] devices; Valves implantable in the body
- A61F2/2412—Heart valves ; Vascular valves, e.g. venous valves; Heart implants, e.g. passive devices for improving the function of the native valve or the heart muscle; Transmyocardial revascularisation [TMR] devices; Valves implantable in the body with soft flexible valve members, e.g. tissue valves shaped like natural valves
- A61F2/2418—Scaffolds therefor, e.g. support stents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2220/00—Fixations or connections for prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2220/0008—Fixation appliances for connecting prostheses to the body
- A61F2220/0016—Fixation appliances for connecting prostheses to the body with sharp anchoring protrusions, e.g. barbs, pins, spikes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2310/00—Prostheses classified in A61F2/28 or A61F2/30 - A61F2/44 being constructed from or coated with a particular material
- A61F2310/00005—The prosthesis being constructed from a particular material
- A61F2310/00011—Metals or alloys
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Cardiology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Prostheses (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US15/272,213 US10052201B2 (en) | 2016-09-21 | 2016-09-21 | Valved stent for mitral and tricuspid heart valve replacement |
| US15/272,213 | 2016-09-21 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN107233146A CN107233146A (zh) | 2017-10-10 |
| CN107233146B true CN107233146B (zh) | 2020-04-10 |
Family
ID=59858967
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201710675846.9A Active CN107233146B (zh) | 2016-09-21 | 2017-08-09 | 适用心脏二尖瓣和三尖瓣带瓣支架置换瓣膜 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US10052201B2 (enExample) |
| EP (1) | EP3298987B1 (enExample) |
| JP (1) | JP7036556B2 (enExample) |
| CN (1) | CN107233146B (enExample) |
| WO (1) | WO2018057257A1 (enExample) |
Families Citing this family (29)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8579964B2 (en) | 2010-05-05 | 2013-11-12 | Neovasc Inc. | Transcatheter mitral valve prosthesis |
| EP2688516B1 (en) * | 2011-03-21 | 2022-08-17 | Cephea Valve Technologies, Inc. | Disk-based valve apparatus |
| US9554897B2 (en) | 2011-04-28 | 2017-01-31 | Neovasc Tiara Inc. | Methods and apparatus for engaging a valve prosthesis with tissue |
| US9308087B2 (en) | 2011-04-28 | 2016-04-12 | Neovasc Tiara Inc. | Sequentially deployed transcatheter mitral valve prosthesis |
| US9345573B2 (en) | 2012-05-30 | 2016-05-24 | Neovasc Tiara Inc. | Methods and apparatus for loading a prosthesis onto a delivery system |
| US9572665B2 (en) | 2013-04-04 | 2017-02-21 | Neovasc Tiara Inc. | Methods and apparatus for delivering a prosthetic valve to a beating heart |
| EP4112006A1 (en) | 2015-12-15 | 2023-01-04 | Neovasc Tiara Inc. | Transseptal delivery system |
| DE202017007326U1 (de) | 2016-01-29 | 2020-10-20 | Neovasc Tiara Inc. | Klappenprothese zum Verhindern einer Abflussobstruktion |
| JP2019535415A (ja) | 2016-11-21 | 2019-12-12 | ニオバスク ティアラ インコーポレイテッド | 経カテーテル心臓弁送達システムの急速退却のための方法およびシステム |
| US10390953B2 (en) * | 2017-03-08 | 2019-08-27 | Cardiac Dimensions Pty. Ltd. | Methods and devices for reducing paravalvular leakage |
| US11666444B2 (en) | 2017-08-03 | 2023-06-06 | The Regents Of The University Of California | Atrial cage for placement, securing and anchoring of atrioventricular valves |
| CN111263622A (zh) | 2017-08-25 | 2020-06-09 | 内奥瓦斯克迪亚拉公司 | 顺序展开的经导管二尖瓣假体 |
| CN109806028B (zh) * | 2017-11-21 | 2021-02-26 | 先健科技(深圳)有限公司 | 心脏瓣膜 |
| CN109966023B (zh) * | 2017-12-28 | 2024-09-27 | 上海微创心通医疗科技有限公司 | 心脏瓣膜假体及其支架 |
| CN110101486B (zh) * | 2018-02-01 | 2024-02-27 | 上海微创心通医疗科技有限公司 | 心脏瓣膜假体及其输送器 |
| US12232955B2 (en) | 2018-10-06 | 2025-02-25 | The Foundry | Modular valve replacement systems and associated devices and methods of use |
| CA3118599C (en) | 2018-11-08 | 2025-04-22 | Neovasc Tiara Inc | VENTRICULAR DEPLOYMENT OF A TRANSCATHETER MITRAL VALVE PROSTHESIS |
| WO2020185597A1 (en) | 2019-03-08 | 2020-09-17 | Neovasc Tiara Inc. | Retrievable prosthesis delivery system |
| CN109938879A (zh) | 2019-03-19 | 2019-06-28 | 上海欣吉特生物科技有限公司 | 一种手术瓣膜及其植入装置 |
| JP7438236B2 (ja) | 2019-04-01 | 2024-02-26 | ニオバスク ティアラ インコーポレイテッド | 制御可能に展開可能な補綴弁 |
| AU2020271896B2 (en) | 2019-04-10 | 2022-10-13 | Neovasc Tiara Inc. | Prosthetic valve with natural blood flow |
| WO2020236931A1 (en) | 2019-05-20 | 2020-11-26 | Neovasc Tiara Inc. | Introducer with hemostasis mechanism |
| JP7520897B2 (ja) | 2019-06-20 | 2024-07-23 | ニオバスク ティアラ インコーポレイテッド | 薄型人工補綴僧帽弁 |
| CN111281604B (zh) * | 2020-01-22 | 2022-04-22 | 沛嘉医疗科技(苏州)有限公司 | 人工生物瓣膜及其处理方法 |
| CN111265329A (zh) * | 2020-04-17 | 2020-06-12 | 王宇森 | 一种个性化分支血管支架 |
| CA3201702A1 (en) | 2020-12-14 | 2022-06-23 | Casey Torrance | Modular pre-loaded medical implants and delivery systems |
| CN113940787A (zh) * | 2021-09-03 | 2022-01-18 | 中国医学科学院阜外医院 | 一种应用于主动脉的人工血管及其制备方法 |
| CN114569184A (zh) * | 2022-02-24 | 2022-06-03 | 广东海思卡尔医疗科技有限公司 | 一种用于人体腔道内的封堵器械 |
| AU2023265163A1 (en) | 2022-05-05 | 2024-11-21 | Magnolia Medical Corp. | Prosthetic heart valve, systems, and methods |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2777616A1 (en) * | 2013-03-14 | 2014-09-17 | CardiAQ Valve Technologies, Inc. | Prosthesis for atraumatically grasping intralumenal tissue |
| US9078751B2 (en) * | 2009-03-17 | 2015-07-14 | Mitrassist Medical Ltd. | Heart valve prosthesis with collapsible valve and method of delivery thereof |
| CN104771247A (zh) * | 2014-01-15 | 2015-07-15 | 赛诺医疗科学技术有限公司 | 一种治疗二尖瓣返流的装置及方法 |
| CN105188611A (zh) * | 2013-02-04 | 2015-12-23 | 爱德华兹生命科学公司 | 用于置换二尖瓣的假体瓣膜 |
Family Cites Families (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1893131A1 (en) | 2005-04-20 | 2008-03-05 | The Cleveland Clinic Foundation | Apparatus and method for replacing a cardiac valve |
| US20090276040A1 (en) | 2008-05-01 | 2009-11-05 | Edwards Lifesciences Corporation | Device and method for replacing mitral valve |
| US8323335B2 (en) | 2008-06-20 | 2012-12-04 | Edwards Lifesciences Corporation | Retaining mechanisms for prosthetic valves and methods for using |
| AU2009295960A1 (en) | 2008-09-29 | 2010-04-01 | Cardiaq Valve Technologies, Inc. | Heart valve |
| US8449599B2 (en) | 2009-12-04 | 2013-05-28 | Edwards Lifesciences Corporation | Prosthetic valve for replacing mitral valve |
| US20110319988A1 (en) | 2009-12-08 | 2011-12-29 | Avalon Medical, Ltd. | Device and System for Transcatheter Mitral Valve Replacement |
| US8579964B2 (en) | 2010-05-05 | 2013-11-12 | Neovasc Inc. | Transcatheter mitral valve prosthesis |
| EP3459500B1 (en) | 2010-09-23 | 2020-09-16 | Edwards Lifesciences CardiAQ LLC | Replacement heart valves and delivery devices |
| EP2688516B1 (en) | 2011-03-21 | 2022-08-17 | Cephea Valve Technologies, Inc. | Disk-based valve apparatus |
| CA2840084C (en) | 2011-06-21 | 2019-11-05 | Foundry Newco Xii, Inc. | Prosthetic heart valve devices and associated systems and methods |
| US9039757B2 (en) | 2011-10-19 | 2015-05-26 | Twelve, Inc. | Prosthetic heart valve devices, prosthetic mitral valves and associated systems and methods |
| FR2985659B1 (fr) | 2012-01-13 | 2015-03-06 | Assist Publ Hopitaux De Paris | Dispositif d'ancrage d'une valve cardiaque prothetique. |
| US20130184811A1 (en) * | 2012-01-13 | 2013-07-18 | Tendyne Holdings, Inc. | Device and Method for Replacing Mitral Valve |
| LT2852354T (lt) * | 2012-05-20 | 2020-09-25 | Tel Hashomer Medical Research Infrastructure And Services Ltd. | Mitralinio vožtuvo protezas |
| US9561103B2 (en) | 2013-07-17 | 2017-02-07 | Cephea Valve Technologies, Inc. | System and method for cardiac valve repair and replacement |
| US9820852B2 (en) | 2014-01-24 | 2017-11-21 | St. Jude Medical, Cardiology Division, Inc. | Stationary intra-annular halo designs for paravalvular leak (PVL) reduction—active channel filling cuff designs |
| US10064719B2 (en) | 2014-03-11 | 2018-09-04 | Highlife Sas | Transcatheter valve prosthesis |
| US9763779B2 (en) | 2014-03-11 | 2017-09-19 | Highlife Sas | Transcatheter valve prosthesis |
| CA2941398C (en) | 2014-03-26 | 2018-05-01 | St. Jude Medical, Cardiology Division, Inc. | Transcatheter mitral valve stent frames |
| CA3161000A1 (en) | 2014-05-19 | 2015-11-26 | Edwards Lifesciences Cardiaq Llc | Replacement mitral valve with annular flap |
| US9662203B2 (en) * | 2014-06-11 | 2017-05-30 | Medtronic Vascular, Inc. | Prosthetic valve with vortice-inducing baffle |
| CA2914094C (en) * | 2014-06-20 | 2021-01-05 | Edwards Lifesciences Corporation | Surgical heart valves identifiable post-implant |
| US10441416B2 (en) * | 2015-04-21 | 2019-10-15 | Edwards Lifesciences Corporation | Percutaneous mitral valve replacement device |
| WO2017041029A1 (en) * | 2015-09-02 | 2017-03-09 | Edwards Lifesciences Corporation | Spacer for securing a transcatheter valve to bioprosthetic cardiac structure |
| CN108992209B (zh) * | 2015-11-06 | 2022-03-04 | 麦克尔有限公司 | 二尖瓣假体 |
-
2016
- 2016-09-21 US US15/272,213 patent/US10052201B2/en active Active
-
2017
- 2017-08-09 CN CN201710675846.9A patent/CN107233146B/zh active Active
- 2017-08-30 WO PCT/US2017/049421 patent/WO2018057257A1/en not_active Ceased
- 2017-09-14 EP EP17191047.4A patent/EP3298987B1/en active Active
- 2017-09-20 JP JP2017180239A patent/JP7036556B2/ja active Active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9078751B2 (en) * | 2009-03-17 | 2015-07-14 | Mitrassist Medical Ltd. | Heart valve prosthesis with collapsible valve and method of delivery thereof |
| CN105188611A (zh) * | 2013-02-04 | 2015-12-23 | 爱德华兹生命科学公司 | 用于置换二尖瓣的假体瓣膜 |
| EP2777616A1 (en) * | 2013-03-14 | 2014-09-17 | CardiAQ Valve Technologies, Inc. | Prosthesis for atraumatically grasping intralumenal tissue |
| CN104771247A (zh) * | 2014-01-15 | 2015-07-15 | 赛诺医疗科学技术有限公司 | 一种治疗二尖瓣返流的装置及方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2018057257A1 (en) | 2018-03-29 |
| US10052201B2 (en) | 2018-08-21 |
| JP7036556B2 (ja) | 2022-03-15 |
| JP2018047242A (ja) | 2018-03-29 |
| CN107233146A (zh) | 2017-10-10 |
| EP3298987A1 (en) | 2018-03-28 |
| US20180078365A1 (en) | 2018-03-22 |
| EP3298987B1 (en) | 2020-11-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN107233146B (zh) | 适用心脏二尖瓣和三尖瓣带瓣支架置换瓣膜 | |
| CN109199640B (zh) | 一种人工瓣膜假体 | |
| CN108578016B (zh) | 一种经心尖植入式二尖瓣瓣膜装置 | |
| US11395734B2 (en) | Prosthetic valve and prosthetic valve implanting method | |
| CN105658179B (zh) | 用于二尖瓣反流方法的装置和方法 | |
| RU2727458C1 (ru) | Протез сердечного клапана, прикрепляемый к межжелудочковой перегородке, а также способ его доставки и отсоединения | |
| US8758430B2 (en) | Medical apparatus for the therapeutic treatment of an insufficient cardiac valve | |
| JP5356499B2 (ja) | 複オリフィス移植可能心臓弁及び移植方法 | |
| JP2018047242A5 (enExample) | ||
| WO2018184225A1 (zh) | 一种人工瓣膜 | |
| WO2019062366A1 (zh) | 心脏瓣膜假体 | |
| EP2063807A1 (en) | Prosthetic heart valves, support structures and systems and methods for implanting the same | |
| WO2022267851A1 (zh) | 一种用于阻止瓣膜返流的修复装置 | |
| US20250339298A1 (en) | Compliance-enhancing blood vessel grafting | |
| CN113712708B (zh) | 一种用于阻止瓣膜返流的修复器械 | |
| CN216221854U (zh) | 一种用于阻止瓣膜返流的修复装置 | |
| US20220304800A1 (en) | Valve Reshaping Device, System, and Related Methods | |
| CN216124622U (zh) | 一种用于阻止瓣膜返流的修复器械 | |
| JP2025525212A (ja) | 幾何学形態対応型心臓弁置換装置 | |
| US20240091003A1 (en) | Valve Reshaping Device, System, and Related Methods | |
| US20250143873A1 (en) | Self-expanding biological valve | |
| CN118512284A (zh) | 经股静脉瓣膜支架及瓣膜装置 | |
| RU122287U1 (ru) | Биологический протез аортального клапана | |
| HK1256493B (en) | A transapical replacement for mitral valve | |
| HK1256493A1 (zh) | 一种经心尖植入式二尖瓣瓣膜装置 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |