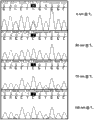
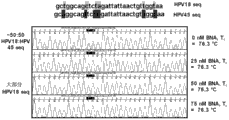
CN103517993A - 用于对混合群体中的靶dna进行测序的试剂盒和方法 - Google Patents
用于对混合群体中的靶dna进行测序的试剂盒和方法 Download PDFInfo
- Publication number
- CN103517993A CN103517993A CN201280020801.3A CN201280020801A CN103517993A CN 103517993 A CN103517993 A CN 103517993A CN 201280020801 A CN201280020801 A CN 201280020801A CN 103517993 A CN103517993 A CN 103517993A
- Authority
- CN
- China
- Prior art keywords
- nucleic acid
- chain
- sealing
- reference sequences
- sequence
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 238000012163 sequencing technique Methods 0.000 title claims abstract description 131
- 238000000034 method Methods 0.000 title claims abstract description 74
- 230000002438 mitochondrial effect Effects 0.000 claims abstract description 10
- 230000035772 mutation Effects 0.000 claims abstract description 10
- 108091028043 Nucleic acid sequence Proteins 0.000 claims abstract description 8
- 150000007523 nucleic acids Chemical class 0.000 claims description 182
- 102000039446 nucleic acids Human genes 0.000 claims description 178
- 108020004707 nucleic acids Proteins 0.000 claims description 178
- 238000007789 sealing Methods 0.000 claims description 126
- 230000008859 change Effects 0.000 claims description 60
- 238000006243 chemical reaction Methods 0.000 claims description 50
- 239000011541 reaction mixture Substances 0.000 claims description 49
- 125000003729 nucleotide group Chemical group 0.000 claims description 48
- 230000000295 complement effect Effects 0.000 claims description 46
- 108020004414 DNA Proteins 0.000 claims description 45
- 239000002773 nucleotide Substances 0.000 claims description 44
- 238000012360 testing method Methods 0.000 claims description 40
- 101100193693 Kirsten murine sarcoma virus K-RAS gene Proteins 0.000 claims description 32
- 238000001514 detection method Methods 0.000 claims description 25
- 230000036425 denaturation Effects 0.000 claims description 16
- 238000004925 denaturation Methods 0.000 claims description 16
- 238000002844 melting Methods 0.000 claims description 13
- 230000008018 melting Effects 0.000 claims description 13
- 108091027305 Heteroduplex Proteins 0.000 claims description 12
- 238000006366 phosphorylation reaction Methods 0.000 claims description 11
- 238000001712 DNA sequencing Methods 0.000 claims description 10
- 238000009396 hybridization Methods 0.000 claims description 10
- 108010014303 DNA-directed DNA polymerase Proteins 0.000 claims description 9
- 102000016928 DNA-directed DNA polymerase Human genes 0.000 claims description 9
- 108060002716 Exonuclease Proteins 0.000 claims description 9
- 101000984753 Homo sapiens Serine/threonine-protein kinase B-raf Proteins 0.000 claims description 9
- 102100027103 Serine/threonine-protein kinase B-raf Human genes 0.000 claims description 9
- 102000013165 exonuclease Human genes 0.000 claims description 9
- 230000004048 modification Effects 0.000 claims description 9
- 238000012986 modification Methods 0.000 claims description 9
- 108700024394 Exon Proteins 0.000 claims description 8
- 238000011534 incubation Methods 0.000 claims description 8
- 108020004705 Codon Proteins 0.000 claims description 7
- 108091093037 Peptide nucleic acid Proteins 0.000 claims description 7
- 108091032973 (ribonucleotides)n+m Proteins 0.000 claims description 6
- 102000004190 Enzymes Human genes 0.000 claims description 5
- 108090000790 Enzymes Proteins 0.000 claims description 5
- 230000004087 circulation Effects 0.000 claims description 4
- 238000004519 manufacturing process Methods 0.000 claims description 4
- HDVCHBLHEICPPP-UHFFFAOYSA-N O=P(=O)C1=CC=NC(P(=O)=O)=C1P(=O)=O Chemical class O=P(=O)C1=CC=NC(P(=O)=O)=C1P(=O)=O HDVCHBLHEICPPP-UHFFFAOYSA-N 0.000 claims description 3
- 230000000593 degrading effect Effects 0.000 claims description 3
- 239000000975 dye Substances 0.000 claims description 2
- 229910052708 sodium Inorganic materials 0.000 claims description 2
- 239000011734 sodium Substances 0.000 claims description 2
- 208000035177 MELAS Diseases 0.000 claims 1
- 230000015572 biosynthetic process Effects 0.000 claims 1
- 238000006722 reduction reaction Methods 0.000 claims 1
- 206010028980 Neoplasm Diseases 0.000 abstract description 18
- 201000011510 cancer Diseases 0.000 abstract description 13
- 238000011275 oncology therapy Methods 0.000 abstract description 2
- 230000003612 virological effect Effects 0.000 abstract 1
- 239000000523 sample Substances 0.000 description 40
- 239000000203 mixture Substances 0.000 description 22
- 210000004027 cell Anatomy 0.000 description 13
- 238000002474 experimental method Methods 0.000 description 12
- 239000000047 product Substances 0.000 description 11
- 241000701806 Human papillomavirus Species 0.000 description 8
- 108091034117 Oligonucleotide Proteins 0.000 description 8
- 102200055464 rs113488022 Human genes 0.000 description 8
- 102200006531 rs121913529 Human genes 0.000 description 8
- 206010069754 Acquired gene mutation Diseases 0.000 description 7
- 238000005259 measurement Methods 0.000 description 7
- 230000000869 mutational effect Effects 0.000 description 7
- 230000037439 somatic mutation Effects 0.000 description 7
- 102000053602 DNA Human genes 0.000 description 6
- 238000012408 PCR amplification Methods 0.000 description 6
- 239000002299 complementary DNA Substances 0.000 description 6
- 238000013461 design Methods 0.000 description 6
- 238000003199 nucleic acid amplification method Methods 0.000 description 6
- 102200055537 rs121913355 Human genes 0.000 description 6
- 102200006540 rs121913530 Human genes 0.000 description 6
- 230000003321 amplification Effects 0.000 description 5
- 238000000137 annealing Methods 0.000 description 5
- 108090000623 proteins and genes Proteins 0.000 description 5
- 239000007787 solid Substances 0.000 description 5
- 210000001519 tissue Anatomy 0.000 description 5
- 108020004682 Single-Stranded DNA Proteins 0.000 description 4
- 230000008901 benefit Effects 0.000 description 4
- 201000010099 disease Diseases 0.000 description 4
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- 238000002156 mixing Methods 0.000 description 4
- 238000004393 prognosis Methods 0.000 description 4
- 238000011282 treatment Methods 0.000 description 4
- 108700028369 Alleles Proteins 0.000 description 3
- 206010009944 Colon cancer Diseases 0.000 description 3
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 3
- 108020005196 Mitochondrial DNA Proteins 0.000 description 3
- 206010058799 Mitochondrial encephalomyopathy Diseases 0.000 description 3
- 238000003556 assay Methods 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 230000008034 disappearance Effects 0.000 description 3
- 238000006073 displacement reaction Methods 0.000 description 3
- 208000006443 lactic acidosis Diseases 0.000 description 3
- 239000013612 plasmid Substances 0.000 description 3
- 102000040430 polynucleotide Human genes 0.000 description 3
- 108091033319 polynucleotide Proteins 0.000 description 3
- 239000002157 polynucleotide Substances 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 230000002194 synthesizing effect Effects 0.000 description 3
- 230000007704 transition Effects 0.000 description 3
- 241000894006 Bacteria Species 0.000 description 2
- 108020004635 Complementary DNA Proteins 0.000 description 2
- 108010008286 DNA nucleotidylexotransferase Proteins 0.000 description 2
- 102100033215 DNA nucleotidylexotransferase Human genes 0.000 description 2
- 206010059866 Drug resistance Diseases 0.000 description 2
- 108010090804 Streptavidin Proteins 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 239000011324 bead Substances 0.000 description 2
- 238000010804 cDNA synthesis Methods 0.000 description 2
- 229960005395 cetuximab Drugs 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 239000005546 dideoxynucleotide Substances 0.000 description 2
- 239000003814 drug Substances 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 230000002969 morbid Effects 0.000 description 2
- 238000005457 optimization Methods 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 238000012882 sequential analysis Methods 0.000 description 2
- 210000004881 tumor cell Anatomy 0.000 description 2
- 108090001008 Avidin Proteins 0.000 description 1
- 201000009030 Carcinoma Diseases 0.000 description 1
- 108091026890 Coding region Proteins 0.000 description 1
- 239000003298 DNA probe Substances 0.000 description 1
- 229940122558 EGFR antagonist Drugs 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- 206010064571 Gene mutation Diseases 0.000 description 1
- 206010027476 Metastases Diseases 0.000 description 1
- 201000002169 Mitochondrial myopathy Diseases 0.000 description 1
- 206010068052 Mosaicism Diseases 0.000 description 1
- 108020005202 Viral DNA Proteins 0.000 description 1
- 108020000999 Viral RNA Proteins 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 238000003149 assay kit Methods 0.000 description 1
- 230000002902 bimodal effect Effects 0.000 description 1
- 239000012472 biological sample Substances 0.000 description 1
- 239000000090 biomarker Substances 0.000 description 1
- 229960000074 biopharmaceutical Drugs 0.000 description 1
- 238000001574 biopsy Methods 0.000 description 1
- 230000006287 biotinylation Effects 0.000 description 1
- 238000007413 biotinylation Methods 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 210000001175 cerebrospinal fluid Anatomy 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 230000035572 chemosensitivity Effects 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 230000001186 cumulative effect Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 102000052116 epidermal growth factor receptor activity proteins Human genes 0.000 description 1
- 108700015053 epidermal growth factor receptor activity proteins Proteins 0.000 description 1
- ZMMJGEGLRURXTF-UHFFFAOYSA-N ethidium bromide Chemical compound [Br-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CC)=C1C1=CC=CC=C1 ZMMJGEGLRURXTF-UHFFFAOYSA-N 0.000 description 1
- 229960005542 ethidium bromide Drugs 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 210000003677 hemocyte Anatomy 0.000 description 1
- 229940000351 hemocyte Drugs 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 208000023692 inborn mitochondrial myopathy Diseases 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 210000002751 lymph Anatomy 0.000 description 1
- 201000001441 melanoma Diseases 0.000 description 1
- 108020004999 messenger RNA Proteins 0.000 description 1
- 230000009401 metastasis Effects 0.000 description 1
- 230000002906 microbiologic effect Effects 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 239000003068 molecular probe Substances 0.000 description 1
- YOHYSYJDKVYCJI-UHFFFAOYSA-N n-[3-[[6-[3-(trifluoromethyl)anilino]pyrimidin-4-yl]amino]phenyl]cyclopropanecarboxamide Chemical compound FC(F)(F)C1=CC=CC(NC=2N=CN=C(NC=3C=C(NC(=O)C4CC4)C=CC=3)C=2)=C1 YOHYSYJDKVYCJI-UHFFFAOYSA-N 0.000 description 1
- 238000007899 nucleic acid hybridization Methods 0.000 description 1
- 238000002515 oligonucleotide synthesis Methods 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 229960001972 panitumumab Drugs 0.000 description 1
- 244000045947 parasite Species 0.000 description 1
- 239000002831 pharmacologic agent Substances 0.000 description 1
- 125000002467 phosphate group Chemical group [H]OP(=O)(O[H])O[*] 0.000 description 1
- 150000003014 phosphoric acid esters Chemical class 0.000 description 1
- 230000026731 phosphorylation Effects 0.000 description 1
- 210000002381 plasma Anatomy 0.000 description 1
- 238000003752 polymerase chain reaction Methods 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 210000002345 respiratory system Anatomy 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 210000003296 saliva Anatomy 0.000 description 1
- 239000013535 sea water Substances 0.000 description 1
- 238000011896 sensitive detection Methods 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 238000011451 sequencing strategy Methods 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- 239000002689 soil Substances 0.000 description 1
- 239000011343 solid material Substances 0.000 description 1
- 230000000392 somatic effect Effects 0.000 description 1
- 108010068698 spleen exonuclease Proteins 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 239000008399 tap water Substances 0.000 description 1
- 235000020679 tap water Nutrition 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 239000002023 wood Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6806—Preparing nucleic acids for analysis, e.g. for polymerase chain reaction [PCR] assay
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6869—Methods for sequencing
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/70—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving virus or bacteriophage
- C12Q1/701—Specific hybridization probes
- C12Q1/708—Specific hybridization probes for papilloma
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2563/00—Nucleic acid detection characterized by the use of physical, structural and functional properties
- C12Q2563/173—Nucleic acid detection characterized by the use of physical, structural and functional properties staining/intercalating agent, e.g. ethidium bromide
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Engineering & Computer Science (AREA)
- Analytical Chemistry (AREA)
- Immunology (AREA)
- Biotechnology (AREA)
- Microbiology (AREA)
- Molecular Biology (AREA)
- Physics & Mathematics (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Virology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161447490P | 2011-02-28 | 2011-02-28 | |
| US61/447,490 | 2011-02-28 | ||
| US201161532887P | 2011-09-09 | 2011-09-09 | |
| US61/532,887 | 2011-09-09 | ||
| PCT/US2012/026938 WO2012118802A1 (en) | 2011-02-28 | 2012-02-28 | Kit and method for sequencing a target dna in a mixed population |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN103517993A true CN103517993A (zh) | 2014-01-15 |
Family
ID=45815993
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201280020801.3A Pending CN103517993A (zh) | 2011-02-28 | 2012-02-28 | 用于对混合群体中的靶dna进行测序的试剂盒和方法 |
Country Status (8)
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN107709557A (zh) * | 2015-04-15 | 2018-02-16 | 通用医疗公司 | 基于lna的突变型富集下一代测序测定 |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102859003B (zh) | 2010-03-08 | 2014-07-30 | 达纳-法伯癌症研究院有限公司 | 使用参考封闭序列的全cold-pcr富集 |
| US11130992B2 (en) | 2011-03-31 | 2021-09-28 | Dana-Farber Cancer Institute, Inc. | Methods and compositions to enable multiplex COLD-PCR |
| WO2014089797A1 (zh) * | 2012-12-13 | 2014-06-19 | 深圳华大基因科技服务有限公司 | 用于高通量测序的锁核酸修饰的dna片段 |
| US10913977B2 (en) | 2013-07-24 | 2021-02-09 | Dana-Farber Cancer Institute, Inc. | Methods and compositions to enable enrichment of minor DNA alleles by limiting denaturation time in PCR or simply enable enrichment of minor DNA alleles by limiting the denaturation time in PCR |
| KR20160003444A (ko) * | 2014-07-01 | 2016-01-11 | 주식회사 유진셀 | 핵산말단분해효소를 이용한 서열-특이적 핵산 검출 방법 및 장치와 이에 사용되는 키트 |
| CA2978709C (en) * | 2015-03-06 | 2024-06-04 | Pillar Biosciences Inc. | Selective amplification of overlapping amplicons |
| CN107849606A (zh) * | 2015-04-20 | 2018-03-27 | 尼欧基因组学实验室股份有限公司 | 提高下一代测序灵敏度的方法 |
| WO2017070339A1 (en) * | 2015-10-20 | 2017-04-27 | Richardson Katherine | Microfluidic device for enrichment of nucleic acid sequence alterations |
| EP3551756A4 (en) | 2016-12-12 | 2020-07-15 | Dana Farber Cancer Institute, Inc. | COMPOSITIONS AND METHODS FOR MOLECULAR BARCODE CODING OF DNA MOLECULES BEFORE ENRICHMENT OF MUTATIONS AND / OR DETECTION OF MUTATIONS |
| US11174511B2 (en) | 2017-07-24 | 2021-11-16 | Dana-Farber Cancer Institute, Inc. | Methods and compositions for selecting and amplifying DNA targets in a single reaction mixture |
| CN115927566B (zh) * | 2017-09-20 | 2025-02-18 | 深圳华大智造科技股份有限公司 | 一种用于Small RNA的测序方法、测序试剂和应用 |
| ES2981912T3 (es) * | 2019-07-11 | 2024-10-11 | Univ Tokyo Science Found | Método para amplificar un ácido nucleico usando un portador en fase sólida |
| CN113567404A (zh) * | 2021-06-11 | 2021-10-29 | 上海交通大学 | 一种分析肿瘤细胞耐药性的方法和试剂盒 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2293238A (en) * | 1994-09-13 | 1996-03-20 | Inceltec Ltd | Primers for replication and/or amplification reactions |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5849497A (en) * | 1997-04-03 | 1998-12-15 | The Research Foundation Of State University Of New York | Specific inhibition of the polymerase chain reaction using a non-extendable oligonucleotide blocker |
| FR2779154B1 (fr) * | 1998-05-27 | 2002-07-12 | Bio Merieux | Procede d'amplification d'au moins une sequence nucleotidique particuliere et amorces de mise en oeuvre |
| US6045993A (en) * | 1998-05-30 | 2000-04-04 | Visible Genetics Inc. | Method, reagent and kit for genotyping of human papillomavirus |
| DE10012540B4 (de) * | 2000-03-15 | 2004-09-23 | Vermicon Ag | Oligonukleotide und Verfahren zum spezifischen Nachweis von Mikroorganismen durch Polymerase-Kettenreaktion |
| CA2421078A1 (en) * | 2000-08-30 | 2002-03-07 | Haplogen, Llc | Method for determining alleles |
| GB0406863D0 (en) * | 2004-03-26 | 2004-04-28 | Qiagen As | Nucleic acid sequencing |
| PT2183379E (pt) * | 2007-08-01 | 2015-09-25 | Dana Farber Cancer Inst Inc | Enriquecimento de uma sequência alvo |
| US8071338B2 (en) * | 2007-08-08 | 2011-12-06 | Roche Molecular Systems, Inc. | Suppression of amplification using an oligonucleotide and a polymerase significantly lacking 5′-3′ nuclease activity |
| ES2359058B1 (es) * | 2009-07-02 | 2012-03-27 | Consejo Superior De Investigaciones Cient�?Ficas (Csic) | Quimera de adn polimerasa del fago ph1 29. |
| CN103626777B (zh) | 2009-07-29 | 2015-10-21 | 内尔维阿诺医学科学有限公司 | Plk抑制剂的盐类 |
| CN102859003B (zh) | 2010-03-08 | 2014-07-30 | 达纳-法伯癌症研究院有限公司 | 使用参考封闭序列的全cold-pcr富集 |
-
2012
- 2012-02-28 KR KR1020137025264A patent/KR20140010093A/ko not_active Withdrawn
- 2012-02-28 CA CA2828535A patent/CA2828535A1/en not_active Abandoned
- 2012-02-28 EP EP12708466.3A patent/EP2681332A1/en not_active Withdrawn
- 2012-02-28 CN CN201280020801.3A patent/CN103517993A/zh active Pending
- 2012-02-28 US US13/407,274 patent/US20120225421A1/en not_active Abandoned
- 2012-02-28 JP JP2013556801A patent/JP2014507950A/ja active Pending
- 2012-02-28 AU AU2012223438A patent/AU2012223438A1/en not_active Abandoned
- 2012-02-28 WO PCT/US2012/026938 patent/WO2012118802A1/en active Application Filing
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2293238A (en) * | 1994-09-13 | 1996-03-20 | Inceltec Ltd | Primers for replication and/or amplification reactions |
Non-Patent Citations (1)
| Title |
|---|
| MILBURY COREN A ET AL: "Ice-COLD-PCR enables rapid amplification and robust enrichment for low-abundance unknown DNA mutations", 《NUCLEIC ACIDS RESEARCH》, 11 October 2010 (2010-10-11), pages 1 - 10 * |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN107709557A (zh) * | 2015-04-15 | 2018-02-16 | 通用医疗公司 | 基于lna的突变型富集下一代测序测定 |
| US10876151B2 (en) | 2015-04-15 | 2020-12-29 | The General Hospital Corporation | LNA-based mutant enrichment next-generation sequencing assays |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2681332A1 (en) | 2014-01-08 |
| KR20140010093A (ko) | 2014-01-23 |
| WO2012118802A1 (en) | 2012-09-07 |
| CA2828535A1 (en) | 2012-09-07 |
| AU2012223438A1 (en) | 2013-09-26 |
| JP2014507950A (ja) | 2014-04-03 |
| US20120225421A1 (en) | 2012-09-06 |
| WO2012118802A9 (en) | 2013-07-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN103517993A (zh) | 用于对混合群体中的靶dna进行测序的试剂盒和方法 | |
| JP7384459B2 (ja) | ヌクレアーゼ協同pcr原理に基づいて低存在比のdna突然変異を濃縮する検出技術システムおよび使用 | |
| US10968487B2 (en) | Oligonucleotides and methods for detecting KRAS and PIK3CA mutations | |
| JP5777340B2 (ja) | Igf2遺伝子の対立遺伝子特異的な発現を判定するための一塩基多型ならびに新規および公知の多型の組み合わせ | |
| CN103717750A (zh) | 少数核酸物质的定量 | |
| JP2018512878A (ja) | 次世代シークエンシングの感度を高めるための方法 | |
| KR20100063050A (ko) | 디지털 pcr에 의한 다양한 길이의 핵산의 분석 | |
| EP4081661B1 (en) | Multiplex drop-off digital polymerase chain reaction methods | |
| JP2014500028A (ja) | ヒト上皮成長因子受容体遺伝子内の突然変異を検出するための方法及び組成物 | |
| US20170009279A1 (en) | Detection of Neighboring Variants | |
| JP7541586B2 (ja) | 対立遺伝子の区分性を高めるpcr方法及びpcrキット | |
| Liu et al. | Development of a POCT detection platform based on a locked nucleic acid-enhanced ARMS-RPA-GoldMag lateral flow assay | |
| JP6153515B2 (ja) | Hla−a*24:02を検出する方法、及び検出キット | |
| CN110964833B (zh) | 一种一管检测血浆游离dna中kras和braf基因突变的试剂盒 | |
| Wong et al. | Real-time quantitative polymerase chain reaction analysis of mitochondrial DNA point mutation | |
| Chen et al. | Allelic discrimination of cis-trans relationships by digital polymerase chain reaction: GJB2 (p. V27I/p. E114G) and CFTR (p. R117H/5T) | |
| Liu et al. | Identification of DNA variants at ultra-low variant allele frequencies via Taq polymerase cleavage of wild-specific blockers | |
| Hamasaki et al. | A novel loop-mediated isothermal amplification-based genotyping method and its application for identifying proprotein convertase subtilisin/kexin type 9 variants in familial hypercholesterolemia | |
| Song et al. | Real-time bidirectional pyrophosphorolysis-activated polymerization for quantitative detection of somatic mutations | |
| EP3592860A1 (en) | Rhd gene allele associated with a weak d phenotype and its uses | |
| KR20250057696A (ko) | 헬리코박터 파일로리 제균 후의 환자에 있어서 위암의 발증 리스크를 평가하는 방법, 폴리뉴클레오티드 및 키트 | |
| Hobson-Peters et al. | A whole-blood homogeneous assay for the multiplex detection of the factor V G1691A and the prothrombin G20210A mutations | |
| JP6754202B2 (ja) | K−ras遺伝子増幅用フォワードプライマーセット、K−ras遺伝子増幅用キット、K−ras遺伝子増幅方法、多型解析方法、及び薬効判定方法 | |
| KR20220085748A (ko) | 유방암 유전자 돌연변이 초고감도 선택적 증폭 방법 및 이를 위한 조성물 | |
| CN114250277A (zh) | 一种新的检测和定量单核苷酸变异的方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| AD01 | Patent right deemed abandoned | ||
| AD01 | Patent right deemed abandoned |
Effective date of abandoning: 20171013 |