WO2024166757A1 - 低次酸化チタン粉末 - Google Patents
低次酸化チタン粉末 Download PDFInfo
- Publication number
- WO2024166757A1 WO2024166757A1 PCT/JP2024/002994 JP2024002994W WO2024166757A1 WO 2024166757 A1 WO2024166757 A1 WO 2024166757A1 JP 2024002994 W JP2024002994 W JP 2024002994W WO 2024166757 A1 WO2024166757 A1 WO 2024166757A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- low
- titanium oxide
- oxide powder
- order titanium
- less
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01G—COMPOUNDS CONTAINING METALS NOT COVERED BY SUBCLASSES C01D OR C01F
- C01G23/00—Compounds of titanium
- C01G23/04—Oxides; Hydroxides
- C01G23/043—Titanium sub-oxides
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01G—COMPOUNDS CONTAINING METALS NOT COVERED BY SUBCLASSES C01D OR C01F
- C01G23/00—Compounds of titanium
- C01G23/04—Oxides; Hydroxides
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2002/00—Crystal-structural characteristics
- C01P2002/70—Crystal-structural characteristics defined by measured X-ray, neutron or electron diffraction data
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/51—Particles with a specific particle size distribution
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2006/00—Physical properties of inorganic compounds
- C01P2006/12—Surface area
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2006/00—Physical properties of inorganic compounds
- C01P2006/60—Optical properties, e.g. expressed in CIELAB-values
- C01P2006/62—L* (lightness axis)
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2006/00—Physical properties of inorganic compounds
- C01P2006/60—Optical properties, e.g. expressed in CIELAB-values
- C01P2006/63—Optical properties, e.g. expressed in CIELAB-values a* (red-green axis)
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2006/00—Physical properties of inorganic compounds
- C01P2006/60—Optical properties, e.g. expressed in CIELAB-values
- C01P2006/64—Optical properties, e.g. expressed in CIELAB-values b* (yellow-blue axis)
Definitions
- the present invention relates to low-order titanium oxide powder.
- Patent Document 1 describes a method in which titanium dioxide (white titanium oxide) is heated and reduced to 600° C. or higher in a reducing atmosphere of hydrogen gas, ammonia gas, or the like to form black low-order titanium oxide, and this sintered body is mechanically pulverized to obtain black titanium oxide powder (paragraph 0002 of Patent Document 1, etc.).
- paragraph 0057 of Patent Document 2 describes that titanium oxides with a higher degree of reduction (such as Ti 3 O 5 and Ti 4 O 7 ) generally have a black color.
- Ti 3 O 5 which is one of the low-order titanium oxides, undergoes five phase transitions, namely, ⁇ phase, ⁇ phase, ⁇ phase, ⁇ phase, and ⁇ phase.
- TiO2 is fired with a reducing agent at a high temperature of about 1000°C or higher, high-temperature stable phases called ⁇ -phase and ⁇ -phase are formed, and ⁇ - Ti3O5 and ⁇ - Ti3O5 are obtained.
- the present inventors have found that when TiO2 is fired with a reducing agent, the grain growth of particles containing Ti3O5 is suppressed, thereby stabilizing the metastable ⁇ -phase ( ⁇ - Ti3O5 ), and therefore the L * value of the resulting low-order titanium oxide can be made lower.
- the inventors discovered that by adding fine particles composed of a composition that is not easily substituted for Ti in Ti3O5 during the reduction and firing of TiO2 , the grain growth of particles containing Ti3O5 can be suppressed, thereby obtaining a low-order titanium oxide powder containing ⁇ -Ti3O5 having a sufficiently low L * value and excellent blackness, and that at this time, the fine particles remain attached to the surface of the base particles containing ⁇ - Ti3O5 , which led to the completion of the present invention.
- the following low-order titanium oxide powder is provided.
- the low-order titanium oxide powder according to 1. The low-order titanium oxide powder, wherein the fine particles contain one or more elements selected from the group consisting of SiO 2 , Si 3 N 4 , B 4 C, and MgO. 3.
- the content of ⁇ -Ti 3 O 5 contained in the low-order titanium oxide powder is 10 mass% or more based on a total of 100 mass% of Ti 2 O 3 , ⁇ -Ti 3 O 5 , ⁇ -Ti 3 O 5 , ⁇ -Ti 3 O 5 , ⁇ -Ti 3 O 5 and Ti 4 O 7 .
- the low-order titanium oxide powder according to any one of 1. to 3. A low-order titanium oxide powder having an L * value of 15.0 or less, an a * value of 5.0 or less, and ab * value of 1.0 or less in an L * a * b * color space. 5.
- a low-order titanium oxide powder in which, when the particle diameter at which the cumulative value is 50% in the volume frequency particle size distribution of the low-order titanium oxide powder measured by a laser diffraction scattering method is defined as d50, the particle diameter is 0.001 ⁇ m or more and 3.0 ⁇ m or less.
- the present invention provides a low-order titanium oxide powder with excellent blackness.
- FIG. 1 is an SEM image of the low-order titanium oxide powder of Example 1.
- 1 is an SEM image of the low-order titanium oxide powder of Comparative Example 2.
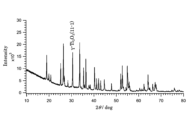
- FIG. 2 is a diagram showing the X-ray diffraction pattern of the low-order titanium oxide powder of Example 1.
- FIG. 2 is a diagram showing the X-ray diffraction pattern of the low-order titanium oxide powder of Comparative Example 2.
- the low-order titanium oxide powder of this embodiment is a powder (particle group) containing TiO x (where X is in the range of 1.50 ⁇ X ⁇ 1.75), and is configured to include base particles containing ⁇ -Ti 3 O 5 and fine particles having a particle size smaller than the particle size of the base particles, with multiple fine particles attached to the surface of one base particle.
- ⁇ -Ti 3 O 5 has a lower L * value than any of ⁇ , ⁇ , or ⁇ -Ti 3 O 5. Therefore, it has been found that a low-order titanium oxide powder containing ⁇ -Ti 3 O 5 with excellent blackness can be obtained.
- the low-order titanium oxide powder of this embodiment can be used for various purposes, but can also be used as a black pigment (black filler) to be added to a dispersion medium such as a resin.
- the low-order titanium oxide powder is less likely to scatter into space than carbon black, a common black pigment, and therefore has excellent low dust properties.
- the low-order titanium oxide powder has a composition represented by TiO2X .
- X in TiO x is a value in the range of 1.50 ⁇ X ⁇ 1.75.
- X in TiO x is expressed as a weighted average, for example, with the mass ratio of the crystal composition contained in the low-order titanium oxide powder being used as the weight.
- TiO x means their average composition.
- the mass ratios of the crystal compositions contained in the low-order titanium oxide powder can be calculated by Rietveld analysis of the X-ray diffraction pattern of the low-order titanium oxide powder.
- Rietveld method software for example, Rigaku Corporation's integrated powder X-ray analysis software PDXL2
- the crystal structures are obtained from the crystal structure database (Pearson's Crystal Data) as follows: Ti 2 O 3 is 1243140 (Journal of Applied Physics 119, 014905(2016)), ⁇ -Ti 3 O 5 is 1127327 (Chemistry An Asian Journal 6, 1886(2011), ⁇ -Ti 3 O 5 is 1944823 (Journal of Solid State Chemistry 192, 356(2012)), ⁇ -Ti 3 O 5 is 1900755 (Journal of Solid State Chemistry 20, 29(1977)), ⁇ -Ti 3 O 5 is 1900755 (Journal of Solid State Chemistry 20, 29(1977)), and ⁇ -Ti 3 O 5 is 1900755 (Journal of Solid State Chemistry 20, 29(1977)).
- the above mass ratio (%) is calculated by using the mass ratio (%) of 1127327 (Chemistry An Asian Journal 6, 1886 (2011)
- the crystal composition of the low-order titanium oxide powder needs to contain at least ⁇ -Ti 3 O 5 , and may contain two or more selected from the group consisting of Ti 2 O 3 , ⁇ -Ti 3 O 5 , ⁇ -Ti 3 O 5 , ⁇ -Ti 3 O 5 , ⁇ -Ti 3 O 5 , and Ti 4 O 7 .
- the lower limit of the content of ⁇ -Ti 3 O 5 contained in the low-order titanium oxide powder is, for example, 10 mass % or more, preferably 20 mass % or more, and more preferably 30 mass % or more , based on a total of 100 mass % of Ti 2 O 3 , ⁇ -Ti 3 O 5 , ⁇ -Ti 3 O 5 , ⁇ -Ti 3 O 5, ⁇ -Ti 3 O 5, and Ti 4 O 7. This makes it possible to further reduce the L * value of the low-order titanium oxide powder.
- the upper limit of the content of the above-mentioned ⁇ -Ti 3 O 5 is not particularly limited, but may be, for example, 90 mass % or less, 85 mass % or less, or 80 mass % or less, relative to 100 mass % in total of Ti 2 O 3 , ⁇ -Ti 3 O 5 , ⁇ - Ti 3 O 5 , ⁇ -Ti 3 O 5 , ⁇ -Ti 3 O 5 , and Ti 4 O 7. This makes it possible to adjust the saturation, and to adjust the powder to have the desired blackness and saturation.
- the low-order titanium oxide powder may contain other low-order titanium oxides besides the above Ti2O3, Ti3O5, and Ti4O7 , for example , one or more of other low-order titanium oxides such as Ti2.5O4 , TiO, and Ti3O , as long as the effects of the present invention are not impaired.
- the total content of Na, K and P contained in the low-order titanium oxide powder is, for example, 2000 mass ppm or less, preferably 1000 mass ppm or less, more preferably 500 mass ppm or less, and further preferably 100 mass ppm or less. This improves reactivity and makes it easier to obtain a desired crystal phase.
- the total content of Pb, Cd and Cr contained in the low-order titanium oxide powder may be, for example, 200 mass ppm or less, preferably 100 mass ppm or less, more preferably 50 mass ppm or less, and further preferably 30 mass ppm or less. This improves reactivity and makes it easier to obtain a desired crystal phase.
- the content (mass conversion) of elements contained in the low-order titanium oxide powder can be calculated from the analysis results of the elemental composition obtained by ICP optical emission spectrometry using, for example, Agilent 5110 ICP-OES (manufactured by Agilent Technologies, Inc.).
- the low-order titanium oxide powder contains base particles containing ⁇ -Ti 3 O 5 as particles, and fine particles having a composition different from that of the base particles.
- the base particle may be configured to include primary particles and/or secondary particles.
- the shape of the primary particles contained in the base particle may be any of spherical, plate-like, needle-like, polygonal and irregular shapes, and one or more of these may be contained.
- the secondary particles may include aggregates formed by aggregating a plurality of primary particles, or links formed by linking a plurality of primary particles, etc.
- the fine particles in the low order titanium oxide powder may include particles that are attached to a portion of the surface of the base particle and/or particles that are not attached to the base particle.
- one or more of the microparticles attached to a portion of the surface of the base particle are present discontinuously on that portion of the surface of the base particle.
- the microparticles are scattered on the surface of the base particle.
- the microparticles do not cover the entire surface of the base particle continuously.
- the fine particles are considered to be attached to the base particles if they are simply in physical contact with each other.
- the base particles and the fine particles may or may not be chemically bonded to each other.
- the fine particles may be any particles that do not contain any metal easily substituted for Ti, and examples thereof include SiO 2 , Si 3 N 4 , B 4 C, and MgO. These may be used alone or in combination of two or more kinds. Although the detailed mechanism is unclear, it is presumed that since Si, B, or Mg are metal elements that are difficult to replace with Ti in Ti3O5 , oxides or nitrides of Si, B, or Mg adhere to the particle surfaces containing Ti3O5 during reduction and firing of TiO2 , thereby suppressing the grain growth of these particles.
- the fine particles do not contain easily-substitutable metals for Ti means that the main raw material constituting the fine particles is not easily-substitutable metals for Ti, but the fine particles may contain easily-substitutable metals for Ti that are unavoidably mixed in the raw materials and during the production process, and specifically, the total content of easily-substitutable metals for Ti in terms of oxide in the entire low-order titanium oxide powder may be defined as, for example, 3 mass% or less.
- the lower limit of the content of the Si element contained in the low-order titanium oxide powder is, for example, 0.04 mass% or more, preferably 0.5 mass% or more, and more preferably 0.7 mass% or more. This allows the L * value of the low-order titanium oxide powder to be further reduced.
- the upper limit of the content of the Si element is, for example, 20 mass % or less, preferably 15 mass % or less, and more preferably 8 mass % or less. This allows the particle size to be made small.
- the content of the B element contained in the low-order titanium oxide powder may be, for example, 0.02% by mass or more and 20% by mass or less.
- the content of the Mg element contained in the low-order titanium oxide powder may be, for example, 0.01% by mass or more and 20% by mass or less.
- the content of the element Si, B, or Mg contained in the low-order titanium oxide powder can be measured by the above-mentioned ICP emission spectroscopic analysis.
- the lower limit of the specific surface area of the low-order titanium oxide powder as measured by the BET method is, for example, 1.0 m 2 /g or more, preferably 3.0 m 2 /g or more, and more preferably 5.0 m 2 /g or more. This allows the L * value of the low-order titanium oxide powder to be further reduced.
- the upper limit of the specific surface area of the low-order titanium oxide powder as measured by the BET method is, for example, 30 m 2 /g or less, preferably 20 m 2 /g or less, and more preferably 10 m 2 /g or less. This can improve the handleability of the powder.
- a specific surface area measuring device e.g., Macsorb HM model-1201, manufactured by Mountech
- the particle size at which the cumulative value is 50% is defined as d50.
- the upper limit of d50 of the low-order titanium oxide powder is, for example, 3.0 ⁇ m or less, preferably 1.5 ⁇ m or less, and more preferably 1.0 ⁇ m or less. This allows the L * value of the low-order titanium oxide powder to be further reduced.
- the lower limit of d50 of the low-order titanium oxide powder is, for example, 0.001 ⁇ m or more, preferably 0.01 ⁇ m or more, and more preferably 0.23 ⁇ m or more. This can improve the coloring power when mixed with a medium.
- the particle size distribution of the low-order titanium oxide powder is measured by the following procedure. First, 100 mg of low-order titanium oxide powder and 50 mL of ion-exchanged water are placed in a polystyrene sample bottle (volume: 100 mL (e.g., AS ONE, PS-100)), and an ultrasonic homogenizer (e.g., Branson Ultrasonics Corporation, model: DIGITALSONIFIER450) is used to perform ultrasonic dispersion treatment for 60 seconds at an output of 10% amplitude.
- an ultrasonic homogenizer e.g., Branson Ultrasonics Corporation, model: DIGITALSONIFIER450
- a particle size distribution measuring device using a laser diffraction scattering method (e.g., Beckman Coulter, model LS 13 320) is used to measure the volume-based particle size distribution of the dispersed low-order titanium oxide powder under the following measurement conditions.
- the low-order titanium oxide powder may be configured so that the L * value in the L * a * b * color space is 15.0 or less, the a * value is 5.0 or less, and the b * value is 1.0 or less. This results in a low-order titanium oxide powder with excellent blackness.
- the upper limit of the L * value may be, for example, 15.0 or less, 14.0 or less, or 13.0 or less, preferably 12.0 or less, more preferably 11.6 or less, and even more preferably 11.2.
- the lower limit of the L * value may be, for example, 8.0 or more, 8.5 or more, or 9.0 or more.
- the upper limit of the a * value is, for example, 5.0 or less, preferably 2.0 or less, and more preferably 1.5 or less, while the lower limit of the a * value is, for example, -2.0 or more, preferably -1.5 or more, and more preferably -1.0 or more.
- the upper limit of the b * value is, for example, 1.0 or less, preferably -0.1 or less, more preferably -1.0 or less, and even more preferably -2.0 or less, while the lower limit of the b * value is, for example, -6.0 or more, preferably -5.0 or more, and more preferably -4.0 or more.
- a colorimeter e.g., ZE-2000 (manufactured by Nippon Denshoku Industries Co., Ltd.)
- the low-order titanium oxide powder of this embodiment is suitable for use as a pigment (colored filler) such as a black pigment, but the use is not limited to this.
- the pigment (colored filler) is used, for example, in cosmetics, electronic components such as semiconductors, coating materials such as paints and inks, etc.
- the low-order titanium oxide powder may be used, for example, dispersed in a dispersion medium. That is, the dispersion of this embodiment contains the low-order titanium oxide powder and a dispersion medium described above. This makes it possible to sufficiently improve the blackness of the dispersion even with the addition of a small amount.
- the dispersion medium is appropriately selected depending on the application of the dispersion, and may be, for example, water, alcohol, ketone, ester, resin, etc.
- resins include epoxy resin, silicone resin, phenol resin, melamine resin, urea resin, unsaturated polyester, fluororesin, polyimide, polyamideimide, polyetherimide, polybutylene terephthalate, polyethylene terephthalate, polyphenylene sulfide, wholly aromatic polyester, polysulfone, liquid crystal polymer, polyethersulfone, polycarbonate, maleimide-modified resin, ABS (acrylonitrile butadiene styrene) resin, AAS (acrylonitrile acrylic rubber styrene) resin, AES (acrylonitrile ethylene propylene diene rubber styrene) resin, etc.
- An example of a method for producing a low-order titanium oxide powder includes a firing step in which a mixture containing, as raw materials, TiO2 powder and fine powder not containing easily replaceable metals such as SiO2 , and TiH2 powder as a reducing agent is heated under an inert gas atmosphere.
- the firing process reduces TiO2 to produce low-order titanium oxide.
- the fine particles that do not contain metals that are easily substituted for Ti, such as SiO2 can suppress the grain growth of Ti3O5 particles contained in the low-order titanium oxide powder.
- the powder is classified into large fine powders having a particle size of more than 5 ⁇ m and not more than 100 ⁇ m, medium fine powders having a particle size of more than 0.1 ⁇ m and not more than 5 ⁇ m, and small fine powders having a particle size of 0.1 ⁇ m or less.
- the particle size is the median diameter (the particle size at which the cumulative value is 50% in the volume frequency particle size distribution measured by the laser diffraction scattering method is d50).
- the particle size and specific surface area of the raw material, reducing agent, etc. can be selected according to the particle size of the desired low-order titanium oxide powder.
- the raw material and fine powder not containing a metal easily replaceable with Ti may be selected so that the particle size of the TiO2 powder is greater than the particle size of the SiO2 powder, or the specific surface area of the TiO2 powder is less than the specific surface area of the SiO2 powder.
- the fine particles do not contain the above-mentioned Ti-substitutable metal, they can be used without being limited to SiO 2 powder.
- powder containing one or more selected from the group consisting of SiO 2 , Si 3 N 4 , B 4 C, and MgO can be used.
- the molar ratio of TiO2 to TiH2 contained in the mixture is, for example, 3.2 or more and 6.0 or less, preferably 3.5 or more and 5.5 or less, and more preferably 3.8 or more and 5.2 or less.
- the heating temperature in the firing step is, for example, 800°C or higher and 1200°C or lower, preferably 900°C or higher and 1150°C or lower, and more preferably 950°C or higher and 1100°C or lower.
- the mixture is placed in a known firing furnace such as an electric furnace, and the firing step is carried out.
- the inert gas atmosphere may contain, for example, Ar gas or He gas, and preferably contains Ar gas. Note that instead of the inert gas atmosphere, a vacuum atmosphere may be used. If necessary, a gaseous reducing agent may be introduced.
- the heating time in the calcination step may be, for example, 1 hour or more, 2 hours or more, or 4 hours or more in order to allow the reduction reaction to proceed sufficiently, and may be, for example, 24 hours or less, 18 hours or less, or 12 hours or less in order to appropriately suppress the growth of the low-order titanium oxide powder and make it easy to collect it in a powder state.
- the method for producing a low-order titanium oxide powder of the present embodiment may further include a washing step of washing the low-order titanium oxide powder obtained in the firing step.
- the washing step can remove impurities in the low-order titanium oxide powder.
- the washing is carried out with at least one selected from the group consisting of hot water, alcohol, and organic acid.
- the alcohol may be, for example, methanol, ethanol, or a mixture thereof.
- the organic acid may be, for example, acetic acid. From the viewpoint of suppressing the incorporation of ionic impurities such as halide ions into the powder of low-order titanium oxide, washing with an organic acid is preferred.
- the method for producing a low-order titanium oxide powder of this embodiment may further include a particle size adjustment step of pulverizing and classifying the low-order titanium oxide powder after the firing step, if necessary.
- the pulverization method may be a method using various pulverizers such as a mortar, a ball mill, a jet mill, or a fine mill.
- the pulverization step may be performed once or may be performed two or more times. When the pulverization step is performed two or more times, the pulverization method used in each pulverization step may be different from each other. By performing the pulverization step, the chromaticity and specific surface area of the low-order titanium oxide powder can be adjusted.
- this manufacturing method may include a firing step, a washing step, and a pulverization step in this order, or may include a firing step, a pulverization step, and a washing step in this order.
- a step of drying the low-order titanium oxide powder may be further carried out between the washing step and the pulverization step.
- the drying temperature in the drying step may be, for example, 100°C or higher and 200°C or lower.
- the drying time may be, for example, 10 hours or higher and 20 hours or lower.
- Eirich mixer Nippon Eirich Co., Ltd.
- the obtained mixture was transferred to an alumina crucible and heated in an electric furnace (Fuji Denpa Kogyo Co., Ltd., Hi-Multi 10000) under an Ar atmosphere at a temperature increased from room temperature at a rate of 10°C/min to 1100°C (calcination temperature) for 12 hours (calcination time). After heating, the obtained powder was pulverized in a mortar for 5 minutes to obtain a low-order titanium oxide powder.
- an electric furnace Fluji Denpa Kogyo Co., Ltd., Hi-Multi 10000
- Example 2 to 16 A low-order titanium oxide powder was obtained in the same manner as in Example 1, except that the molar ratio of TiO2 : TiH2 , the proportion (wt%) of fine powder added to the mixture of TiO2 and TiH2 , the firing temperature, and the firing time were changed to the values shown in Table 1.
- Himulti 5000 was used in the electric furnace instead of Himulti 10000.
- the obtained low-order titanium oxide powder was subjected to powder X-ray diffraction measurement. Specifically, the X-ray diffraction pattern was measured under the following measurement conditions using a horizontal sample type multipurpose X-ray diffractometer (Rigaku Corporation, RINT-Ultima IV). The obtained X-ray diffraction patterns confirmed that each low-order titanium oxide powder had the crystal composition shown in Table 1.
- the results of the X-ray diffraction patterns showed that the low-order titanium oxide powders of Examples 1 to 16 contained a peak corresponding to ⁇ -Ti 3 O 5 (a peak with a diffraction angle 2 ⁇ in the range of 30.0° to 31.0°), but the low-order titanium oxide powders of Comparative Examples 1 to 7 did not contain a peak corresponding to ⁇ -Ti 3 O 5 .
- the X-ray diffraction patterns of Example 1 and Comparative Example 2 are shown in FIG. 3 and FIG. 4, respectively.
- the mass fraction (mass%) of each crystal composition in the obtained low-order titanium oxide powder was calculated using Rietveld method software (Rigaku Corporation, integrated powder X-ray analysis software PDXL2).
- the crystal structure is given in the crystal structure database (Pearson's Crystal Data) as 1243140 for Ti 2 O 3 (Journal of Applied Physics 119, 014905(2016)), 1127327 for ⁇ -Ti 3 O 5 (Chemistry An Asian Journal 6, 1886(2011)), 1944823 for ⁇ -Ti 3 O 5 (Journal of Solid State Chemistry 192, 356(2012)), 1900755 for ⁇ -Ti 3 O 5 (Journal of Solid State Chemistry 20, 29(1977)), and 1127327 for ⁇ -Ti 3 O 5 (Chemistry An Asian Journal 6, 1886 (2011) was used.
- Table 1 ⁇ -Ti 3 O 5 was not confirmed in each of the examples and comparative examples.
- the particle size distribution of the obtained low-order titanium oxide powder was measured by the following procedure.
- the measurement results of the particle diameter (d50) at which the cumulative value becomes 50% are shown in Table 1.
- 100 mg of low-order titanium oxide powder and 50 mL of ion-exchanged water are placed in a polystyrene sample bottle (volume: 100 mL (e.g., AS ONE, PS-100)), and an ultrasonic homogenizer (e.g., Branson Ultrasonics Corporation, model: DIGITALSONIFIER450) is used to perform ultrasonic dispersion treatment for 60 seconds at an output of 10% amplitude.
- an ultrasonic homogenizer e.g., Branson Ultrasonics Corporation, model: DIGITALSONIFIER450
- a particle size distribution measuring device using a laser diffraction scattering method (e.g., Beckman Coulter, model LS 13 320) was used to measure the volumetric particle size distribution of the dispersed low-order titanium oxide powder under the following measurement conditions.
- the obtained low-order titanium oxide powder was subjected to elemental analysis using an Agilent 5110 ICP-OES (manufactured by Agilent Technologies, Inc.). Specifically, 0.1 g of the powder was weighed into a platinum crucible, 1 ml each of HF and HCl was added, and pressure acid decomposition was carried out at 150°C for 4 hours. Thereafter, the volume was adjusted to 6 ml, and after confirming that there was no unnecessary residue, ICP emission spectrometry was carried out. The results are shown in Table 1.
- the low-order titanium oxide powders in each of Examples 1 to 16 had a lower L * value in the L * a * b * color space than Comparative Examples 1 to 7, and therefore showed excellent blackness.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Environmental & Geological Engineering (AREA)
- General Life Sciences & Earth Sciences (AREA)
- Geology (AREA)
- Inorganic Chemistry (AREA)
- Inorganic Compounds Of Heavy Metals (AREA)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020257027474A KR20250135864A (ko) | 2023-02-10 | 2024-01-31 | 저차 산화 타이타늄 분말 |
| CN202480011559.6A CN120677127A (zh) | 2023-02-10 | 2024-01-31 | 低价氧化钛粉末 |
| JP2024576266A JPWO2024166757A1 (enExample) | 2023-02-10 | 2024-01-31 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2023-019372 | 2023-02-10 | ||
| JP2023019372 | 2023-02-10 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2024166757A1 true WO2024166757A1 (ja) | 2024-08-15 |
Family
ID=92262473
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2024/002994 Ceased WO2024166757A1 (ja) | 2023-02-10 | 2024-01-31 | 低次酸化チタン粉末 |
Country Status (5)
| Country | Link |
|---|---|
| JP (1) | JPWO2024166757A1 (enExample) |
| KR (1) | KR20250135864A (enExample) |
| CN (1) | CN120677127A (enExample) |
| TW (1) | TW202440467A (enExample) |
| WO (1) | WO2024166757A1 (enExample) |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2022039111A1 (ja) * | 2020-08-21 | 2022-02-24 | デンカ株式会社 | 特定の低次酸化チタンの結晶組成を有する粒子、並びにその製造方法 |
| WO2022158390A1 (ja) * | 2021-01-25 | 2022-07-28 | デンカ株式会社 | 特定の低次酸化チタンの結晶組成を有する粒子及びその製造方法、並びに分散体 |
| WO2023276761A1 (ja) * | 2021-07-01 | 2023-01-05 | デンカ株式会社 | 粉体及び分散体 |
-
2024
- 2024-01-31 JP JP2024576266A patent/JPWO2024166757A1/ja active Pending
- 2024-01-31 KR KR1020257027474A patent/KR20250135864A/ko active Pending
- 2024-01-31 WO PCT/JP2024/002994 patent/WO2024166757A1/ja not_active Ceased
- 2024-01-31 CN CN202480011559.6A patent/CN120677127A/zh active Pending
- 2024-02-07 TW TW113104844A patent/TW202440467A/zh unknown
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2022039111A1 (ja) * | 2020-08-21 | 2022-02-24 | デンカ株式会社 | 特定の低次酸化チタンの結晶組成を有する粒子、並びにその製造方法 |
| WO2022158390A1 (ja) * | 2021-01-25 | 2022-07-28 | デンカ株式会社 | 特定の低次酸化チタンの結晶組成を有する粒子及びその製造方法、並びに分散体 |
| WO2023276761A1 (ja) * | 2021-07-01 | 2023-01-05 | デンカ株式会社 | 粉体及び分散体 |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20250135864A (ko) | 2025-09-15 |
| CN120677127A (zh) | 2025-09-19 |
| JPWO2024166757A1 (enExample) | 2024-08-15 |
| TW202440467A (zh) | 2024-10-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7634015B2 (ja) | 特定の低次酸化チタンの結晶組成を有する粒子、並びにその製造方法 | |
| EP3636591B1 (en) | Zirconium nitride powder and production method therefor | |
| JP6591948B2 (ja) | 窒化ジルコニウム粉末及びその製造方法 | |
| EP4282825A1 (en) | Particles having specific low-order titanium oxide crystal composition, method for producing same, and dispersion | |
| JP2007522062A (ja) | 火炎加水分解により製造され、広い表面積を有する酸化アルミニウム粉末 | |
| EP3546426A1 (en) | Black-film-forming mixed powder and production method therefor | |
| JP7682271B2 (ja) | 粉体及び分散体 | |
| WO2023032986A1 (ja) | 電子材料用シリカ及びその製造方法 | |
| JP2019104651A (ja) | 窒化ジルコニウム系黒色フィラー及びその製造方法、そのフィラーを含有する塗料組成物及びその塗膜 | |
| JP2003192452A (ja) | ジルコニア粉末およびその焼結体 | |
| KR100818469B1 (ko) | 복합 흑색 산화물 입자, 그 제조방법, 흑색 도료 및 블랙매트릭스 | |
| WO2024166757A1 (ja) | 低次酸化チタン粉末 | |
| WO2024166758A1 (ja) | 低次酸化チタン粉末 | |
| WO2024166756A1 (ja) | 低次酸化チタン粉末 | |
| EP1683763A1 (en) | Titanium dioxide powder and method for production thereof | |
| JP5829386B2 (ja) | 結晶性の高い微細なito粉末とその用途および製造方法等 | |
| KR20240012486A (ko) | 무기 산화물 분말 및 그 제조 방법, 그리고 수지 조성물 | |
| JP6952051B2 (ja) | 赤外線遮蔽材、及び酸化スズ粒子の製造方法 | |
| CN1863735A (zh) | 复合黑色氧化物粒子、其制造方法、黑色涂料及黑色矩阵 | |
| WO2025182878A1 (ja) | 球状シリカ粒子粉体及び球状シリカ粒子粉体の製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 24753201 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2024576266 Country of ref document: JP Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2024576266 Country of ref document: JP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 202480011559.6 Country of ref document: CN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1020257027474 Country of ref document: KR |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWP | Wipo information: published in national office |
Ref document number: 202480011559.6 Country of ref document: CN |