WO2022019319A1 - Agent antimicrobien et son procédé de fabrication - Google Patents
Agent antimicrobien et son procédé de fabrication Download PDFInfo
- Publication number
- WO2022019319A1 WO2022019319A1 PCT/JP2021/027238 JP2021027238W WO2022019319A1 WO 2022019319 A1 WO2022019319 A1 WO 2022019319A1 JP 2021027238 W JP2021027238 W JP 2021027238W WO 2022019319 A1 WO2022019319 A1 WO 2022019319A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- antibacterial
- antibacterial agent
- plant
- derived
- hydrolase
- Prior art date
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N25/00—Biocides, pest repellants or attractants, or plant growth regulators, characterised by their forms, or by their non-active ingredients or by their methods of application, e.g. seed treatment or sequential application; Substances for reducing the noxious effect of the active ingredients to organisms other than pests
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N25/00—Biocides, pest repellants or attractants, or plant growth regulators, characterised by their forms, or by their non-active ingredients or by their methods of application, e.g. seed treatment or sequential application; Substances for reducing the noxious effect of the active ingredients to organisms other than pests
- A01N25/02—Biocides, pest repellants or attractants, or plant growth regulators, characterised by their forms, or by their non-active ingredients or by their methods of application, e.g. seed treatment or sequential application; Substances for reducing the noxious effect of the active ingredients to organisms other than pests containing liquids as carriers, diluents or solvents
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N65/00—Biocides, pest repellants or attractants, or plant growth regulators containing material from algae, lichens, bryophyta, multi-cellular fungi or plants, or extracts thereof
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N65/00—Biocides, pest repellants or attractants, or plant growth regulators containing material from algae, lichens, bryophyta, multi-cellular fungi or plants, or extracts thereof
- A01N65/40—Liliopsida [monocotyledons]
- A01N65/44—Poaceae or Gramineae [Grass family], e.g. bamboo, lemon grass or citronella grass
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01P—BIOCIDAL, PEST REPELLANT, PEST ATTRACTANT OR PLANT GROWTH REGULATORY ACTIVITY OF CHEMICAL COMPOUNDS OR PREPARATIONS
- A01P3/00—Fungicides
Definitions
- the present invention relates to an antibacterial agent and a method for producing the same.
- Human hands, furniture, daily necessities, etc. are sprayed with ethanol solution or hypochlorite water, or wiped with a non-woven cloth or cloth soaked with ethanol solution or hypochlorite water.
- Ethanol and hypochlorite water have a sufficient antibacterial effect, but the search for an antibacterial agent with an excellent antibacterial effect has not stopped, and research and development are being promoted every day.
- the ethanol solution depends on the concentration of ethanol in the solution, it is highly volatile, so that it volatilizes immediately even if it is applied to furniture or the like, for example. Therefore, in a short time until the ethanol solution is applied and volatilized, the desired excellent antibacterial effect may not always be obtained. Therefore, although an antibacterial agent having a certain degree of sustainability is desired, it has been difficult to achieve both excellent antibacterial effect and sustainability with a conventional ethanol solution as an antibacterial agent.
- the present invention advantageously solves the above problems, and an object of the present invention is to provide an antibacterial agent that achieves both excellent antibacterial effect and sustainability at a high level.
- the present inventors can achieve both excellent antibacterial effect and sustainability at a high level in an aqueous solution containing a plant-derived antibacterial component and a hydrolase, and can solve the above-mentioned problems.
- the present invention is as follows [1] to [10].
- any antibacterial agent according to any one of [1] to [4] which contains an essential oil.
- a method for producing an antibacterial agent comprising a step of adding a plant-derived antibacterial component and a hydrolase to water or an aqueous solution having a pH of 6.5 to 10.
- an antibacterial agent that achieves both excellent antibacterial effect and sustainability at a high level.
- the antibacterial agent of the present invention contains a plant-derived antibacterial component and a hydrolase.
- the present inventors have an antibacterial agent containing both a plant-derived antibacterial component and a hydrolyzing enzyme, which has an excellent antibacterial effect and excellent durability as compared with an antibacterial agent containing a plant-derived antibacterial component alone. It was found that the present invention was made. Hereinafter, each component will be described.
- Plants having an antibacterial effect can be exemplified by bear zasa, wasabi, ginger, shiso, dokudami, grapefruit, pomelo, cherry, mango, kiwi, etc., and for grapefruit, pomelo, cherry, mango, kiwi, peel, fruit or seed. Can also be selected and used, and one kind or a combination of two or more kinds of extracts extracted from these plants (hereinafter, simply referred to as "extracted extracts”) can be used as a plant-derived antibacterial ingredient. Plants rich in flavonoids such as Kumazasa (collectively referred to as "flavonoids”) are preferable plants.
- an antibacterial agent having a composition containing only a plant-derived antibacterial component as an antibacterial component can be an antibacterial agent having high safety to the human body.
- an extract can be obtained by a known production method using the above-mentioned plants as raw materials.
- a method for producing such a known extract for example, a method of immersing a plant or crushed material in water, boiling water or alcohol to extract the extract, or a method of applying pressure to the plant or crushed material to extract the extract. Examples thereof include a method of crushing a plant and dissolving the crushed product in water, boiling water, or alcohol.
- the extract can be used as a vegetable antibacterial component. Further, a commercially available antibacterial agent containing a plant extract as a main component can also be used.
- Kumazasa extract is considered to have polysaccharides as the main component of its antibacterial effect. Allyl isothiocyanate is considered to be the main component of the antibacterial effect of wasabi extract. Gingerol or trans-6-shogaol is considered to be the main component of the antibacterial effect of the ginger extract. Perillaldehyde, which is a kind of polyphenol, is considered to be the main component of the antibacterial effect of the shiso extract. Decanoyl acetaldehyde is considered to be the main component of the antibacterial effect of Houttuynia cordata extract. Flavonoids are considered to be the main component of the antibacterial effect of flavonoid extracts. These extracts are also used as food products, and are common in that they have an excellent antibacterial effect while having a small toxicity to the human body and a small environmental load.
- Kumazasa extract, Wasabi extract and Ginger extract are particularly excellent in antibacterial effect, so at least one of these Kumazasa extract, Wasabi extract and Ginger extract. It is preferable to include.
- the plant-derived antibacterial component is preferably contained in an antibacterial agent in the form of an aqueous solution in an amount of about 0.01 to 0.1% by mass. If it is less than 0.01% by mass, it is difficult to obtain the desired antibacterial effect, and even if it is contained in excess of 0.1% by mass, the antibacterial effect is saturated. More preferably, 0.05 to 0.1% by mass.
- hydrolase is an enzyme that catalyzes a hydrolysis reaction.
- a typical reaction catalyzed by a hydrolase is a reaction that digests and decomposes proteins, lipids, and polysaccharides ⁇ carbohydrates> into amino acids, fatty acids, glucose, etc., and the addition of a hydrolase enhances the antibacterial effect and enhances the sustainability. be able to.
- hydrolyzing enzyme examples include proteolytic enzymes, lipid-degrading enzymes, saccharide-degrading enzymes and the like, and proteolytic enzymes and lipid-degrading enzymes are preferable. The reason is considered to be the effect of proteolytic enzymes and lipolytic enzymes acting on bacteria and viruses to decompose them.
- hydrolase one type may be used, or two or more types of enzymes may be used in combination.
- proteolytic enzymes include alkaline proteases, neutral proteases, acidic proteases and peptidases.
- lipolytic enzymes include lipase, phospholipase, esterase and phosphatase.
- hydrolase a commercially available enzyme can be used.
- proteases HBI's Orientase AY, Tetrase S (A feed), Orientase 10NL, Orientase 90N, Orientase OP, Nucresin, Orientase 22BF, Sumiteam AP, Sumiteam manufactured by Shin Nihon Kagaku Kogyo Co., Ltd.
- peptidase examples include Novozymes Japan Flavorzyme 1000 L, Flavorzyme 500 MG, Amano Enzyme Protease, Peptidase R, DSM Maxipro FPC, Maxipro CPP, and Toyobo PSP-101. ..
- Lipase Entyron LP manufactured by Rakuto Kasei Kogyo Co., Ltd., Sumiteam NLS manufactured by Shin Nihon Kagaku Kogyo Co., Ltd., Neurose F3G manufactured by Amano Enzyme Co., Ltd., Lipase R “Amano”, Lipase A “Amano” 6, Lipase AY “Amano” 30SD, Lipase MER “Amano”, Lipase G “Amano” 50, Lipase DF “Amano” 15, DSM Panamore Golden, Panamore Spring, Picantase R800, Picantase A, Bakezyme L80.000B, Cakezyme , Maxapearl A2, Paratase 20000L manufactured by Novozymes Japan, Lipase 50BG, Lipase SL manufactured by Meishu Sangyo Co., Ltd., Lipase MY, Lipase OF, Lipase QLM,
- Esterases include Sumiteam PHY manufactured by Shin Nihon Kagaku Kogyo Co., Ltd., Phytase manufactured by DSM Co., Ltd., Sumiteam PM manufactured by Shin Nihon Kagaku Kogyo Co., Ltd., nuclease "Amano” G manufactured by Amano Enzyme, and Danisco Japan.
- Examples include OPTIMASE AE manufactured by Toyobo, LPP-209, COE-301, COE-302, COE-311, and COE-313 manufactured by Toyobo.
- tecanose manufactured by Tres Bio Research Institute can be mentioned as a substance containing both peptidase and lipase.
- the hydrolase can include a hydrolase other than lipase and protease.
- it can contain glucoamylase, which decomposes starch.
- the hydrolase is preferably contained in an antibacterial agent in the form of an aqueous solution in an amount of about 0.1 to 1.5% by mass. If it is less than 0.1% by mass, it is difficult to obtain the desired antibacterial effect, and even if it is contained in excess of 1.5% by mass, the antibacterial effect is saturated. It is preferably 0.5 to 1.0% by mass.
- An antibacterial agent containing a plant-derived antibacterial component and a hydrolase is preferably used in the form of an aqueous solution.
- the pH of the aqueous solution is 6.5 to 10, more preferably 7 to 9, and even more preferably about 7 to 8. Since the aqueous solution is weakly alkaline, a good antibacterial effect can be obtained.
- the pH adjuster for example, sodium hydrogen carbonate, so-called baking soda, can be exemplified.
- Sodium hydrogen carbonate is a highly safe component for the human body, and is preferable because the pH of the aqueous solution can be easily adjusted to about 7 to 8.
- the pH adjuster is not limited to sodium hydrogen carbonate, and a material capable of making the pH of the aqueous solution weakly alkaline can be appropriately selected.
- a material capable of making the pH of the aqueous solution weakly alkaline can be appropriately selected.
- an amount capable of adjusting the pH of the aqueous solution within the above-mentioned suitable range is added depending on the raw material of the pH adjuster.
- the antibacterial agent can further contain essential oils in addition to the extracts extracted from the plants described above.
- the essential oil refers to a volatile oil derived from a plant, and among the essential oils, the essential oil having antibacterial and antiviral effects can be used as an antibacterial agent.
- the essential oil having antibacterial and antiviral effects include hinoki essential oil, hiba essential oil, mint essential oil (hakka oil), eucalyptus essential oil, rabensala essential oil, lavender essential oil, lemon essential oil, rosemary essential oil and the like.
- Essential oils can be produced by extracting from these raw materials, or commercially available essential oils can be obtained and used as antibacterial agents.
- the blending ratio of the essential oil in the antibacterial agent in the form of an aqueous solution is not particularly limited, but preferably contains about 0.1 to 1.0% by mass.
- the antibacterial agent can further contain an antibacterial component other than the plant-derived antibacterial component.
- an antibacterial component other than the plant-derived antibacterial component By containing an antibacterial component other than the plant-derived antibacterial component, the antibacterial effect can be further enhanced.
- antibacterial components other than plant-derived antibacterial components include copper, silver, cetylpyridinium chloride (CPC), isopropylmethylphenol (IPMP), lauroylsarcosine sodium (LSS), chlorhexidine hydrochloride (CHX), triclosan (TC), etc. It can be exemplified.
- the blending ratio of the antibacterial component other than the plant-derived antibacterial component in the antibacterial agent in the form of an aqueous solution is not particularly limited, but preferably contains about 0.01 to 0.2% by mass.
- the antibacterial agent can further include a cellulose derivative.
- the cellulose derivative is cellulose or a compound obtained by substituting or chemically modifying cellulose or a salt thereof.
- the degree of substitution of cellulose is not particularly limited. Examples of the cellulose derivative include cellulose, methyl cellulose, carboxymethyl cellulose and sodium carboxymethyl cellulose, and carboxymethyl cellulose or a salt thereof (for example, sodium carboxymethyl cellulose) is preferable.
- Commercially available sodium carboxymethyl cellulose can be used as a thickener or the like. There are food additive grade and industrial grade, but any of them can be used depending on the use of the dispersion.
- the particle size of the powder is not particularly limited, and a commercially available cellulose derivative can be used as it is.
- the content of sodium carboxymethyl cellulose is 2% by mass or less, preferably 0.1 to 2% by mass, in the antibacterial agent in the form of an aqueous solution.
- the antibacterial agent can further include hydroxyapatite.
- Hydroxyapatite is porous and can enhance the antibacterial effect by adsorbing pathogens or viruses.
- hydroxyapatite commercially available hydroxyapatite can be used.
- Commercially available hydroxyapatite includes those derived from minerals and those derived from living organisms, and any hydroxyapatite derived from minerals or living organisms can be used, but hydroxyapatite derived from living organisms is preferable.
- Organism-derived hydroxyapatite is hydroxyapatite obtained by using biological components (bone, coral, shell, eggshell, etc.) as a calcium source for obtaining hydroxyapatite.
- Hydroxyapatite derived from eggshell has the trade name Bioapatite TM of Bioapatite Co., Ltd.
- the amount of hydroxyapatite is preferably 1% by mass or more, more preferably 5% by mass or more, still more preferably 10% by mass or more, and 30% by mass or less in the antibacterial agent in the form of an aqueous solution.
- the antibacterial agent may contain other components as needed.
- antibacterial agents having a suitable composition examples include an antibacterial agent containing Kumazasa extract as a plant-derived antibacterial component and a protease as a hydrolase, and a hydrolase containing peppermint oil and xylitol as plant-derived antibacterial components.
- antibacterial agents containing proteases There are antibacterial agents containing proteases.
- a suitable blending ratio of mentha oil and xylitol in an antibacterial agent containing mentha oil and xylitol as the above plant-derived antibacterial components and protease as a hydrolase is 0.3 to 0.5% by mass of mentha oil and xylitol. It is 0.1 to 5.0% by mass.
- An antibacterial agent is produced by adding a plant-derived antibacterial component and a hydrolase to water.
- the pH of water is preferably pH 6.5 to 10, more preferably 7 to 9, and even more preferably 7 to 8.
- a pH regulator such as sodium bicarbonate
- the order of addition to water is preferably sodium hydrogen carbonate first, followed by the plant-derived antibacterial component and the hydrolyzing enzyme, but sodium hydrogencarbonate, the plant-derived antibacterial component and the hydrolyzing enzyme may be added at the same time.
- the plant-derived antibacterial component and the hydrolyzing enzyme may be added first, and then sodium hydrogen carbonate may be added promptly.
- water high-purity water, for example, reverse osmosis membrane water can be used, but tap water can also be used depending on the application.
- a plant-derived antibacterial component such as Kumazasa extract, ginger extract, and wasabi extract
- the liquid may be emulsified and the liquid may become cloudy.
- cloudiness and emulsification can be suppressed, and a transparent antibacterial agent can be obtained.
- the antibacterial agent can be contained in a container and applied to an object to be disinfected by spraying, or can be wiped with a non-woven fabric or cloth soaked with the antibacterial agent.
- a sample of an example of an antibacterial agent, a sample of sterile physiological saline as Comparative Example 1, and a sample containing a plant-derived antibacterial component and not containing a hydrolase were prepared as Comparative Example 2.
- the sample of the example contains 0.1% by mass of Kumazasa extract as a plant-derived antibacterial component and 0.1% by mass of a protease as a hydrolase. Sodium hydrogen carbonate was added to pure water to adjust the pH to 7.8, and then the above-mentioned Kumazasa extract and protease were added to prepare the pH.
- the sample of Comparative Example 2 contains 0.1% by mass of Kumazasa extract as a plant-derived antibacterial component. It was prepared by adding the above-mentioned Kumazasa extract to pure water.
- Test bacteria A total of 5 types of test bacteria were prepared. Escherichia coli NBRC3972 (E. coli) Staphylococcus aureus NBRC13276 (Staphylococcus aureus) Streptococcus mutans NBRC13955 (Caries fungus) Porphyromonas gingivalis JCM12257 (Periodontal disease bacteria) Candida albicans NBRC1594
- E. coli Escherichia coli
- S. aureus Staphylococcus aureus
- E. coli Escherichia coli
- S. aureus Staphylococcus aureus
- S. mutans Streptococcus mutans
- the test bacteria were precultured (anaerobic culture) at 35 ° C. for 24 hours on GAM agar medium.
- Suspension before culture in sterile saline which was adjusted to about 10 8 cells / mL was tested bacterial solution.
- Porphyromonas gingivalis is a test bacterium supplemented with hemin and menadione (hereinafter referred to as "H / M +”) at 35 ° C. for 48 hours in GAM agar medium (anaerobic culture). Did. The pre-pre-culture solution was inoculated into the H / M + GAM bouillon and pre-cultured (anaerobic culture) at 35 ° C. for 48 hours. The preculture was diluted with H / M + GAM broth, was tested bacterial solution which was adjusted to about 10 8 / mL.
- hemin and menadione were made by adding 1 mL each of a hemin solution and a menadione solution to 100 mL of the medium.
- hemin solution 50 mg of hemin was dissolved in 1 mL of 1NNaOH, and purified water was added to make 100 mL.
- the menadione solution is prepared by dissolving 5 mg of menadione in 1 mL of ethanol and adding purified water to make 100 mL.
- Candida albicans (hereinafter, "C. Albicans" hereinafter.) Is, 25 ° C.
- the test bacteria in potato dextrose agar medium were cultured 48 hours before, were suspended before culture in sterile saline, about 10 8 cells /
- the solution prepared in mL was used as the test bacterial solution.
- Test operation 19.8 g of the sample of the example was taken in a sterile vial and inoculated with a 1% amount (0.2 mL) of the test bacterial solution. This was stored under the operating conditions described below, and after a specified time, 1 g thereof was collected and diluted with 9 mL of the diluted solution. This diluted solution was further diluted in stages, and the viable cell count was measured by the agar plate pour method.
- the diluent was LP diluent for E. co1i, S. aureus, S. mutans, and C. albicans, and H / M + GAM bouillon for P. gingivalis.
- the LP diluted solution is 1 g of polypeptone, 7 g of eggrecithin, 20 g of polysorbate, and 980 mL of purified water.
- the samples of Comparative Example 1 and Comparative Example 2 were operated in the same manner, and the viable cell count was measured immediately after inoculation and after the final action time.
- E. coli and S. aureus were prepared on SCDLP agar medium at 30 ° C. for 3 days.
- S. mutans was prepared on GAM agar medium at 35 ° C. for 3 days (anaerobic culture).
- P. gingivalis was set on H / M + GAM agar medium at 35 ° C. for 3 to 5 days (anaerobic culture).
- C. albicans was GPLP agar medium at 25 ° C. for 3 days.
- Test results The test results are shown in Table 1.
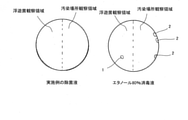
- the left side of the screen of each petri dish is the observation area for airborne bacteria
- the right side of the screen is the observation area for various germs in the contaminated area.
- the agar medium at the lower left of the screen is a sample coated with the disinfectant solution of the example
- the agar medium at the lower right of the screen is a sample coated with an 80% ethanol disinfectant solution.
- FIG. 2 shows a sketch of the breeding state of the fungus on each agar medium. From FIGS. 1 and 2, the growth of floating bacteria 1 and 2 was observed in the sample coated with the 80% ethanol disinfectant solution, whereas the growth of the floating bacteria was observed in the sample coated with the disinfectant solution of the example. As a result, it was found that the disinfectant solution of the example was superior in sustainability to the 80% ethanol disinfectant solution.
- test pieces made of photographic paper that had been exposed to light were prepared. Of each test piece, one test piece is dropped with the disinfectant solution of the example to the tip portion, another test piece is dropped with 80% ethanol disinfectant solution to the tip portion, and the other test piece is further. A household sodium hypochlorite bleaching agent (30 mL of bleaching agent diluted in 5 L of water) was added dropwise to the tip portion. The surface texture of the test piece before dropping is shown in FIG. 3, and the surface texture of the test piece after soaking for 30 minutes is shown in FIG. In FIGS. 3 and 4, the test piece No. 1 was dropped with the disinfectant solution of the example, the test piece No. 2 was dropped with 80% ethanol disinfectant, and the test piece No.
- Example 2 As an antibacterial agent, the bactericidal effect was investigated for another example (Example 2) having a component composition different from that of the above-mentioned example.
- the sample of the example contains 0.3% by mass of menthol-based peppermint oil, 0.3% by mass of xylitol, and 0.1% by mass of protease as a hydrolase as plant-derived antibacterial components. .. After adding sodium hydrogen carbonate to pure water to adjust the pH to 7.8, the above-mentioned peppermint oil, xylitol and protease were added to prepare the pH.
- Test bacteria A total of 5 types of test bacteria were prepared. Escherichia coli NBRC3972 (E. coli) Pseudomonas aeruginosa BRC13275 (Pseudomonas aeruginosa) Staphylococcus aureus NBRC13276 (Staphylococcus aureus) Candida albicans NBRC1594 Trichophyton mentagrophytes NBRC6124 (ringworm)
- E. coli Escherichia coli
- S. aureus Staphylococcus aureus
- C. albicans Candida albicans
- P. aeruginosa Pseudomonas aeruginosa
- Trichophyton mentagrophytes (hereinafter referred to as "T. mentagrophytes”) is a polysorbet in which the test bacteria are pre-cultured in Sabouraud-dextrose agar medium at 25 ° C for 10 to 14 days, and then the spores and hyphae are scraped off with a platinum loop. Suspend in 0.05% sterile saline of ⁇ 80 and grind with a homogenizer. After the solution was filtered through sterile gauze to fourfold, those prepared approximately 10 7 cells / mL was tested bacterial solution.
- Test operation 19.8 g of the sample of Example 2 was placed in a sterile vial and inoculated with a 1% amount (0.2 mL) of the test bacterial solution. This was stored under the same operating conditions as in the example described above, the same amount was collected after the same specified time, and diluted with the same amount of the same diluted solution. This diluted solution was further diluted in stages, and the viable cell count was measured by the agar plate pour method.
- the medium for measuring the viable cell count and the culture conditions were as follows. E. coli, P. aeruginosa, and S. aureus were prepared on SCDLP agar medium at 30 ° C. for 3 days. C. albicans was GPLP agar medium at 25 ° C. for 3 days. T. mentagrophytes was Sabouraud-glucose LP agar medium at 25 ° C. for 10-14 days.
- E. coli Esscherichia coli
- P. aeruginosa Pseudomonas aeruginosa
- S. aureus S. aureus
- C. albicans Ringworm
- T. mentagrophytes T. mentagrophytes
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Environmental Sciences (AREA)
- Plant Pathology (AREA)
- Dentistry (AREA)
- Agronomy & Crop Science (AREA)
- Mycology (AREA)
- Microbiology (AREA)
- Pest Control & Pesticides (AREA)
- Biotechnology (AREA)
- Natural Medicines & Medicinal Plants (AREA)
- Toxicology (AREA)
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
Abstract
La présente invention concerne un agent antimicrobien qui permet d'obtenir à la fois un excellent effet antimicrobien et une persistance à un niveau élevé et comprend un composant antimicrobien dérivé de plante et une hydrolase.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2022538033A JPWO2022019319A1 (fr) | 2020-07-21 | 2021-07-20 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2020-124687 | 2020-07-21 | ||
| JP2020124687 | 2020-07-21 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2022019319A1 true WO2022019319A1 (fr) | 2022-01-27 |
Family
ID=79729194
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2021/027238 WO2022019319A1 (fr) | 2020-07-21 | 2021-07-20 | Agent antimicrobien et son procédé de fabrication |
Country Status (3)
| Country | Link |
|---|---|
| JP (1) | JPWO2022019319A1 (fr) |
| TW (1) | TW202222160A (fr) |
| WO (1) | WO2022019319A1 (fr) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN117814267A (zh) * | 2024-02-29 | 2024-04-05 | 山东夙沙高科生物有限公司 | 一种植物源杀菌剂、制备方法及应用 |
| CN117814267B (zh) * | 2024-02-29 | 2024-06-07 | 山东夙沙高科生物有限公司 | 一种植物源杀菌剂、制备方法及应用 |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2013180956A (ja) * | 2012-02-29 | 2013-09-12 | Sunstar Engineering Inc | 殺菌剤組成物 |
| JP2015501330A (ja) * | 2011-11-03 | 2015-01-15 | ザ トラスティーズ オブ コロンビア ユニバーシティ イン ザ シティー オブ ニューヨーク | 抗菌性組成物、治癒組成物、洗浄組成物、抗カビ性局所用クリーム、食用消毒クレンザー、表面消毒剤、殺虫剤 |
| JP2017141164A (ja) * | 2016-02-08 | 2017-08-17 | イビデン株式会社 | 抗微生物剤 |
| CN107548928A (zh) * | 2017-09-22 | 2018-01-09 | 界首市靳寨乡红星家庭农场 | 一种温室有机菠菜的种植方法 |
| JP2018504434A (ja) * | 2015-02-06 | 2018-02-15 | オースフェリクス・リミテッドAuspherix Limited | オーラノフィンを使用してバイオフィルムを抑制及び分散する方法 |
| JP2020048499A (ja) * | 2018-09-27 | 2020-04-02 | 多木化学株式会社 | 液状微生物資材 |
-
2021
- 2021-07-20 JP JP2022538033A patent/JPWO2022019319A1/ja active Pending
- 2021-07-20 WO PCT/JP2021/027238 patent/WO2022019319A1/fr active Application Filing
- 2021-07-21 TW TW110126769A patent/TW202222160A/zh unknown
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2015501330A (ja) * | 2011-11-03 | 2015-01-15 | ザ トラスティーズ オブ コロンビア ユニバーシティ イン ザ シティー オブ ニューヨーク | 抗菌性組成物、治癒組成物、洗浄組成物、抗カビ性局所用クリーム、食用消毒クレンザー、表面消毒剤、殺虫剤 |
| JP2013180956A (ja) * | 2012-02-29 | 2013-09-12 | Sunstar Engineering Inc | 殺菌剤組成物 |
| JP2018504434A (ja) * | 2015-02-06 | 2018-02-15 | オースフェリクス・リミテッドAuspherix Limited | オーラノフィンを使用してバイオフィルムを抑制及び分散する方法 |
| JP2017141164A (ja) * | 2016-02-08 | 2017-08-17 | イビデン株式会社 | 抗微生物剤 |
| CN107548928A (zh) * | 2017-09-22 | 2018-01-09 | 界首市靳寨乡红星家庭农场 | 一种温室有机菠菜的种植方法 |
| JP2020048499A (ja) * | 2018-09-27 | 2020-04-02 | 多木化学株式会社 | 液状微生物資材 |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN117814267A (zh) * | 2024-02-29 | 2024-04-05 | 山东夙沙高科生物有限公司 | 一种植物源杀菌剂、制备方法及应用 |
| CN117814267B (zh) * | 2024-02-29 | 2024-06-07 | 山东夙沙高科生物有限公司 | 一种植物源杀菌剂、制备方法及应用 |
Also Published As
| Publication number | Publication date |
|---|---|
| TW202222160A (zh) | 2022-06-16 |
| JPWO2022019319A1 (fr) | 2022-01-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR102476983B1 (ko) | 감광 분산액 및 이의 용도 | |
| JP6803896B2 (ja) | 次亜塩素酸を含む抗微生物剤 | |
| JP2012072134A (ja) | 抗菌用銀イオン生成液、その液から生成された銀イオン抗菌液及びその抗菌液を生成する生成方法 | |
| US20060075922A1 (en) | Controlled-acidity composition | |
| CN108391673A (zh) | 一种纳米银/季铵盐复合消毒剂的制备方法 | |
| WO1997027879A1 (fr) | Solution permettant de preserver et de steriliser des lentilles de contact | |
| CN108157358A (zh) | 一种消毒液及其制备方法 | |
| JP2010531320A (ja) | 治療薬または消毒薬としての相乗的組成物の使用 | |
| KR20060133122A (ko) | 고추냉이 추출물이 봉입된 알긴산염 마이크로캡슐 및 그제조방법 | |
| JP7058490B2 (ja) | ウイルス、細菌および真菌を抑える抗菌組成物 | |
| TWI277393B (en) | Acidic solution of sparingly-soluble group IIA complexes | |
| WO2022019319A1 (fr) | Agent antimicrobien et son procédé de fabrication | |
| KR20080081633A (ko) | 항균비누 조성물 | |
| KR102201176B1 (ko) | 천연 과일 추출물 및 유기산이 함유된 살균소독제의 제조방법 | |
| KR102127417B1 (ko) | 과산화수소를 이용한 살균소독제 조성물 | |
| JP5314555B2 (ja) | 腐植抽出液の製造方法 | |
| CN1647643A (zh) | 一种消毒剂 | |
| JP2010126710A (ja) | バイオフィルム除去剤組成物 | |
| Lisa et al. | The synergistic antioxidant effect and antimicrobial efficacity of propolis, myrrh and chlorhexidine as beneficial toothpaste components | |
| JPS5940122B2 (ja) | オキシジアセトアルデヒドソセイブツ | |
| KR20200084035A (ko) | 생물막의 제거 방법 | |
| KR101390347B1 (ko) | 가시박 추출물을 유효성분으로 함유하는 조성물을 이용한 손 세정제 제조방법 | |
| CN101548683B (zh) | 一种液体消毒剂及其制备方法 | |
| KR102199015B1 (ko) | 탈취성 및 살균성이 우수한 소독제 조성물 및 그 제조방법 | |
| JP2014024780A (ja) | 殺菌剤 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 21845206 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2022538033 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 21845206 Country of ref document: EP Kind code of ref document: A1 |