WO2021106952A1 - 移植片対宿主病に対する予防又は治療用組成物 - Google Patents
移植片対宿主病に対する予防又は治療用組成物 Download PDFInfo
- Publication number
- WO2021106952A1 WO2021106952A1 PCT/JP2020/043883 JP2020043883W WO2021106952A1 WO 2021106952 A1 WO2021106952 A1 WO 2021106952A1 JP 2020043883 W JP2020043883 W JP 2020043883W WO 2021106952 A1 WO2021106952 A1 WO 2021106952A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- genus
- bacteroides
- bacterium
- unclassified
- bacteria
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/66—Microorganisms or materials therefrom
- A61K35/74—Bacteria
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/66—Microorganisms or materials therefrom
- A61K35/74—Bacteria
- A61K35/741—Probiotics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/66—Microorganisms or materials therefrom
- A61K35/74—Bacteria
- A61K35/741—Probiotics
- A61K35/742—Spore-forming bacteria, e.g. Bacillus coagulans, Bacillus subtilis, clostridium or Lactobacillus sporogenes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/66—Microorganisms or materials therefrom
- A61K35/74—Bacteria
- A61K35/741—Probiotics
- A61K35/744—Lactic acid bacteria, e.g. enterococci, pediococci, lactococci, streptococci or leuconostocs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/66—Microorganisms or materials therefrom
- A61K35/74—Bacteria
- A61K35/741—Probiotics
- A61K35/744—Lactic acid bacteria, e.g. enterococci, pediococci, lactococci, streptococci or leuconostocs
- A61K35/745—Bifidobacteria
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/66—Microorganisms or materials therefrom
- A61K35/74—Bacteria
- A61K35/741—Probiotics
- A61K35/744—Lactic acid bacteria, e.g. enterococci, pediococci, lactococci, streptococci or leuconostocs
- A61K35/747—Lactobacilli, e.g. L. acidophilus or L. brevis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N1/00—Microorganisms, e.g. protozoa; Compositions thereof; Processes of propagating, maintaining or preserving microorganisms or compositions thereof; Processes of preparing or isolating a composition containing a microorganism; Culture media therefor
- C12N1/20—Bacteria; Culture media therefor
Definitions
- the present invention relates to a composition comprising a bacterial flora, in particular a prophylactic or therapeutic composition for graft-versus-host disease (GVHD).
- GVHD graft-versus-host disease
- Allogeneic hematopoietic stem cell transplantation is widely practiced as a curative treatment for various blood diseases, but acute GVHD associated with allo-HSCT is one of the important complications along with recurrence and infection.
- the drug used as the initial treatment (first-line treatment) for this GVHD is corticosteroid hormone (steroid), but about half of the drugs show the effect (Blood. 2007; 109 (10): 4119-4126. (Blood. 2007; 109 (10): 4119-4126.
- Non-Patent Document 1 there is no established second-line treatment.
- Non-Patent Document 2 fecal microbiota transplantation
- the present invention is particularly intended for a prophylactic or therapeutic composition for intestinal acute GVHD.
- the present inventor succeeded in preventing or treating GVHD by transplanting a composition containing a fecal-derived bacterial flora, and the fecal-derived bacterial flora.
- the present invention has been completed by identifying a bacterium effective for the prevention or treatment of GVHD contained in the above.
- the present invention is as follows. (1) Genus Brautia, Clostridium, unclassified Clostridias, Actinomyces, The genus Parabacteroides, The genus Lachnoclostridium, Bacteroides, The genus Faecalibacterium, unclassified Lachnospiraceae, The genus Rosebulia, The genus Ruminococcus, unclassified Firmicutes, The genus Dorea, The genus Phascolarctobacterium, Genus Sutterella, Genus Megamonas, The genus Collinsella, Eubacterium genus, Genus Coprococcus, The genus Shaaria, Genus Alitipes, Bifidobacterium genus, Lactobacillus, Veillonella genus, The genus Anaerostices, The genus Bilofila, The genus Butyricoccus, The genus Barnesiella, Genus Fusicatenibacter, Flavonifr, The
- composition according to (1) which comprises a bacterium belonging to any genus selected from the group consisting of the genus Lactococcus and the genus Lactococcus, or a combination thereof.
- composition according to (1) which comprises a bacterium belonging to any genus selected from the group consisting of the genus Barnesiera and the genus Fushicatenibacta, or a combination thereof.
- composition according to (3) which comprises a bacterium belonging to any genus selected from the group consisting of the genus Rak ⁇ Clostridium and the genus Bacteroides, or a combination thereof.
- Fecaribacterium genus unclassified Clostridias, unclassified Lachnospiraceae, The genus Rosebria, Ruminococcus, unclassified Firmicutes, Drea, Fascolarct Bacterium, Satela, Bacteroides, Genus Brautia, Parabacteroides, Genus Megamonas, Corinthella, The genus Eubacterium, Lactococcus, Anaerostipes, Vilovilla, Flavonifractor genus, unclassified Ruminococcaceae, unclassified Clostridiaceae, The genus Butyricoccus, Fecalicatena, Prevotella, Clostridium, Rak ⁇ Clostridium, Aristipes, Megasfaera, Robin Soniera, Genus Fushicatenibacta, The genus Barnesiera, Fecaritarea, The composition according to (1), which comprises a bacterium belonging to any genus selected from the group
- composition according to (5) which comprises a bacterium belonging to any genus selected from the group consisting of the genus Lactococcus and the genus Lactococcus, or a combination thereof.
- a composition for preventing or treating GVHD which comprises a bacterium containing DNA consisting of a base sequence having 94% or more homology with any of the base sequences shown in SEQ ID NOs: 1-120, or a combination thereof.
- the composition according to (7) which comprises a bacterium containing DNA consisting of a base sequence having 94% or more homology with any of the base sequences shown in SEQ ID NOs: 121 to 152, or a combination thereof.
- composition according to (9) which comprises a bacterium containing DNA consisting of a base sequence having 94% or more homology with any of the base sequences shown in SEQ ID NOs: 199 to 201, or a combination thereof.
- composition according to (7) which comprises a bacterium containing DNA consisting of a base sequence having 94% or more homology with any of the base sequences shown in SEQ ID NOs: 202 to 312, or a combination thereof.
- composition according to (11) which comprises a bacterium containing DNA consisting of a base sequence having 94% or more homology with any of the base sequences shown in SEQ ID NOs: 313 to 344, or a combination thereof.
- K-1 Bacteroides ovatus, Faecalibacterium prasnitzii, Megamonas funiformis, Bifidobacterium adolescentis, [Ruminococcus] torques, Collinsella aerofaciens, Parabacteroides merdae, butyrate-producing bacteria M104 / 1, Bifidobacterium phase, Clostridium sp. 826, Bacteroides coprocola, Roseburia faecis, Lachnospiraceae bacteria DJF_VP18k1, Clostridium sp. AT4, Lactobacillus rogosae, butyrate-producing bacteria A2-207, Roseburia sp.
- Flavoni preparationor plauti Eubacterium ventriosum, Clostridias bacteria 80/3, Butyricicoccus faecihominis, Bacteroides cellulosylitis, Coproccus catus, Parabacteroides jhonsiii, Lachnospiraceae bacteria DJF_RP14, [Ruminococcus] gnavus, Bifidobacterium longum, Bacteroides xylanisolvens, Prevotella stercore, Bacteroides prebeius, Firmicutes bacteria DJF_VR50, Suterella wadsworthensis, Lachnospiraceae bacteria 1_1_57FAA, Blautia obium, Barnesiella intestinihominis, [Eubacterium] eligens, Coproccus comes, Ruminococcus sp.
- DJF_VR70k1 butyrate-producing bacteria A2-175, Faecalitalea cylindriides, Blautia sp. YHC-4, [Clostridium] glycryrrhizinicium, Ruminococcus sp. 653, Ruminococcus lactaris, butyrate-producing bacteria SM6 / 1, butyrate-producing bacteria SS3 / 4, Clostridiaceae bacteria DJF_LS40, Bacteroides sp.
- composition according to (1) which is at least one selected from the group consisting of. (14) Bacteria Bacteroides vulgatus, Bacteroides stercoris, Bacteroides uniformis, Blautia wexlerae, Bacteroides sp.
- AR29 Dorea longicatena, Phascolarctobacterium faecium, Blautia massiliensis, Ruminococcus sp. K-1, Bacteroides ovatus, Faecalibacterium prasnitzii, Megamonas funiformis, Bifidobacterium adolescentis, [Ruminococcus] torques, Collinsella aerofaciens, Parabacteroides merdae, butyrate-producing bacteria M104 / 1, Bifidobacterium phase, Clostridium sp.
- a capsule preparation for the prevention or treatment of GVHD which comprises the composition according to any one of (1) to (17).
- a method for treating GVHD which comprises administering the composition according to any one of (1) to (17) or the capsule preparation according to (18) to a GVHD patient.
- a GVHD prevention method characterized by.
- (21) The presence of the bacterium described in any one of (1) to (15) is confirmed from the stool collected from humans, and the confirmed stool is suspended in an aqueous medium.
- a method for producing a suspension for transplanting a fecal bacterial flora is produced from the stool collected from humans, and the confirmed stool is suspended in an aqueous medium.
- a method for producing a bacterial mixture for transplantation of a fecal flora which comprises (23) The presence of the bacterium described in any one of (1) to (15) is confirmed from the stool collected from humans, the bacterium is isolated from the confirmed stool, and the isolated bacterium is prepared.
- a method for producing a fecal bacterial flora transplantation preparation which is characterized in that it is transformed into a fecal bacterial flora.
- the method according to any one of (21) to (24), wherein the presence of bacteria is confirmed by 16S rRNA gene analysis.
- the present inventor considers that intervention in the intestinal flora will lead to new preventive and therapeutic methods for GVHD after hematopoietic stem cell transplantation, and fecal microbiota transplantation (FMT) for GVHD patients.
- FMT fecal microbiota transplantation
- the FMT method is a treatment method in which a large amount of normal bacterial flora is administered by administering a fecal suspension of a healthy person into the digestive tract, and imbalance of the intestinal bacterial flora (dysbiosis) occurs. It is an attempted treatment for diseases that are thought to be involved.
- the present inventor performed patient intestinal flora analysis before and after FMT in patients who developed GVHD after hematopoietic stem cell transplantation, and identified bacteria useful for prevention and / or treatment of GVHD. As a result of the analysis, it was found that there is a clear difference in the colonized flora distribution between the patients whose GVHD has improved and the other patients.
- the stool processed product can be used as the composition of the present invention.
- the processed stool includes a suspension in which the collected stool is suspended in an appropriate solvent (for example, physiological saline or buffer), or a suitable sieve, gauze, filter or the like (for example, pore size). It includes those filtered through 0.1 mm to 0.5 mm), or precipitates after centrifugation. Further, these compositions may be frozen in a freezer or liquid nitrogen, or freeze-dried or spray-dried. When a suspension is made using the above solvent, it can be suspended in 1 to 20 ml of liquid per 1 g of stool. When a suspension is prepared and then centrifuged to isolate and use the bacteria, the suspension can be resuspended in a liquid volume of 0.2-1 mL with respect to 1 g of the bacterial volume.
- lyophilization agents such as various sugars (sucrose, fructose, lactose, mannitol, etc.), glycerol, polyethylene glycol (PEG), trehalose, glycine, glucose, dextran, erythritol, etc. and / or lyophilization protection Agents can also be added.
- the collected stool or a processed product thereof can be stored for 6 to 10 hours after collecting the stool or after processing.
- the storage temperature is not particularly limited, but it is preferably stored in a refrigerator (for example, 4 ° C.).
- the composition thus prepared is used as an FMT material.
- the prepared FMT material is preferably stored under anaerobic conditions (eg, anaerobic unit, anaerobic bag, etc.) until use.
- the storage is preferably refrigerated storage (for example, 4 ° C.).
- a composition containing a fecal flora (untreated or treated fecal material) is transplanted between different individuals, such as between humans or between animals.
- the composition used for FMT can be returned to the same individual as the individual from which it was collected and transplanted, or the fecal bacterial flora collected from one individual can be transplanted to another individual.
- the target disease is GVHD, and examples thereof include GVHD by hematopoietic stem cell transplantation.
- GVHD includes, but is not limited to, intestinal acute GVHD.
- the transplantation method may be oral administration or parenteral administration, and is not particularly limited.
- transplantation with a gastroduodenal tube, oral administration filled in a capsule or the like, administration of a suppository, transplantation into the large intestine using a large intestine fiber or a high-pressure enema, and the like can be mentioned.
- the transplant amount of one transplant is 150 ml to 300 ml in the case of liquid, and is performed once a day. If it is repeated depending on the condition of the recipient, it should be repeated 2 to 4 times every 4 days to 2 weeks.
- GVHD can be treated by transplanting (administering) the composition of the present invention to a GVHD patient.
- the response rate (CR + PR) which is the sum of the complete response (CR) and the partial response (PR) is evaluated using the result of the maximum effect 4 weeks after the final administration and within the observation period.
- the bacterial flora in the patients (group) judged to be CR or PR and the bacterial flora in the patients (group) judged to be other groups are compared and analyzed. Analysis of the gut microbiota at each time point was performed using the donor's stool preparation solution and a part of the patient's stool (for example, before FMT, 1 to 3 days after the last FMT, 1, 2, 4 weeks). To do.
- the method of narrowing down using the heterogeneity of bacterial abundance between CR or PR group and Others group as an index is as follows. It's a street.
- Method 1 Narrowing down by median (1) At each time point of stool collection, CR or PR group (referred to as “CR or PR group”), and other groups (referred to as “Average group”) of each genus or OTU (operational taxonomic unit). The average value of the samples in the reactive abundance group is calculated, and the genus or OTU having a larger average value of 0.1% or more of the "CR or PR group” or the “Others group” is used for the analysis. (2) At each time point, the median of the sample in the genus or OTU reactive abundance group is calculated in the CR or PR group and the Others group, respectively.
- the Log 2 found change here refers to the ratio obtained by dividing the median of the CR or PR group by the median of the Others group, and the logarithmic value (base 2) of the ratio. Since the median may include 0, the Log2 found change is calculated after adding 0.0001 to the median.
- a genus or OTU having a Log2 fold change of 0.5 or more is considered to be abundant in the CR or PR group, and a genus of -0.5 or less is considered to be abundant in the Others group.
- step 5 Count the number of times that the Log2 fold change was 0.5 or more or -0.5 or less at the time point when the stool was collected after the stool transplantation. (6) In step 5, it is preferable that the number of times of 0.5 or more exceeds 1, and it is determined that the higher the number of times, the more the bacteria contribute to the effect of FMT. If the number of counts exceeds the majority of the number of fecal collections after transplantation, it is considered to contribute more to the effect of FMT. However, if the number of times of -0.5 or less exceeds the number of times of 0.5 or more, it is not judged that there are many times in the CR or PR group and it is excluded.
- Method 2 Narrowing down bacteria by mean and prevalence
- CR or PR group (referred to as “CR or PR group") at each time point of fecal collection, and other groups (referred to as “Others group”) of each genus or OTU
- the average value of the samples in the reactive abundance group is calculated, and the genus or OTU having a larger average value of 0.1% or more of the "CR or PR group” or the “Others group” is used for the analysis.
- the mean of the sample in the genus or OTU reactive abundance group is calculated in the CR or PR group and the Others group, respectively.
- the prevalence of the genus or OTU of each group (the proportion of donors or recipients whose reactive awareness is greater than zero) is calculated.
- Log 2 found change refers to the logarithmic value (base 2) of the ratio obtained by dividing the mean of the CR or PR group by the mean of the Others group to obtain the ratio. Since the mean may contain 0, 0.0001 is added to the mean, and then the Log2 found change is calculated.
- the Log2 Folder change of the ratio of the recipient's mean before FMT is a negative value, or the prevalence of bacteria belonging to the corresponding genus or OTU in the donor is 0. Those that become are excluded because it cannot be determined that they were transplanted from a donor by FMT.
- the sequence of the v1-v2 region contains DNA having 94% or more homology with any of the nucleotide sequences shown in SEQ ID NOs: 1 to 120. It is a fungus that has.
- "94% or more homology” means, for example, 94% or more, 95% or more, 96% or more, 97% or more, 98% or more, 99% or more, 99.5% or more, 99.8% or more. , 99.9% or more, or 100% homology (the same applies hereinafter). More preferably, it is a bacterium having a DNA having 94% or more homology with any of the nucleotide sequences shown in SEQ ID NOs: 121 to 152.
- the 16S rRNA gene of the bacterium extracted by the narrowing down of methods 1 and 2 for example, the sequence of the v1-v2 region is 97% or more of any of the nucleotide sequences shown in SEQ ID NOs: 1-120. It is a bacterium having DNA having homology. More preferably, it is a bacterium having a DNA having 97% or more homology with any of the nucleotide sequences shown in SEQ ID NOs: 121 to 152.
- the bacteria determined to be preferable by method 1 have a sequence of the v1-v2 region of the 16S rRNA gene of 94% or more of any of the nucleotide sequences shown in SEQ ID NOs: 153 to 198. It is a bacterium having DNA having homology. More preferably, it is a bacterium having a DNA having 94% or more homology with any of the nucleotide sequences shown in SEQ ID NOs: 199 to 201.
- the bacteria determined to be preferable by method 1 have a sequence of the v1-v2 region of the 16S rRNA gene with any of the nucleotide sequences shown in SEQ ID NOs: 153 to 198. It is a bacterium having DNA having 97% or more homology. More preferably, it is a bacterium having a DNA having 97% or more homology with any of the nucleotide sequences shown in SEQ ID NOs: 199 to 201.
- the bacteria determined to be preferable by method 2 have the sequence of the v1-v2 region of the 16S rRNA gene with any of the nucleotide sequences shown in SEQ ID NOs: 202 to 312 and 94. It is a bacterium having DNA having a homology of% or more. More preferably, it is a bacterium having a DNA having 94% or more homology with any of the nucleotide sequences shown in SEQ ID NOs: 313 to 344.
- the bacteria determined to be preferable by method 2 have any of the sequences of the v1-v2 region of the 16S rRNA gene shown in SEQ ID NOs: 202 to 312. It is a bacterium having DNA having 97% or more homology with the base sequence. More preferably, it is a bacterium having a DNA having 97% or more homology with any of the nucleotide sequences shown in SEQ ID NOs: 313 to 344.
- Bacteria belonging to the "unclassified taxonomy name" are bacteria having a DNA having a DNA homology of 94% or more with the base sequence of the 16S rRNA gene of the bacteria listed in the Species name shown in Table 1A below.
- the bacteria judged to be more preferable from the length of the colonization period in the recipient belong to the following genera.
- Clostridium genus Clostridium unclassified Clostridiales Actinomyces genus Parabacteroides genus Lachnoclostridium genus Bacteroides genus
- the bacteria determined to be preferable by the method 2 belong to the following genera.
- the bacterium belonging to the "Unclassified taxonomy name" is a bacterium having a DNA having a DNA homology of 94% or more with the base sequence of the 16S rRNA gene of the bacterium listed in the Species name in Table 1A.
- the bacteria judged to be more preferable from the length of the colonization period in the recipient belong to the following genera. Faecalibacterium genus unclassified Clostridiales unclassified Lachnospiraceae Rosebria genus Ruminococcus genus unclassified Firmicutes
- the genus of bacteria extracted by the above two narrowing down is as follows.
- the bacteria belonging to the "Unclassified taxonomy name" are the nucleotide sequences of the 16S rRNA genes of the bacteria listed in the Species name in Table 1A and 94% or more. It is a bacterium having DNA having homology.
- compositions of the present invention include Brautia, Clostridium, unclasified Clostridias, Actinomyces, Parabacteroides, Lacnoclostridium, Bacteroides, Fecaribacterium, unclasfied Lachnospiraceae, Rosebriae.
- compositions of the present invention include Brautia, Clostridium, unclasified Clostridiales, Actinomyces, Parabacteroides, Lacnoclostridium, Bacteroides, Fecaribacterium, Unclasfied Lachnospiraceae, Roceliae.
- Bacteria belonging to any genus selected from the group consisting of the genus Clostridium, unclasfied Firmicutes, Drea, Fascolarctobacterium, Satella, Megamonas, Corinthella, Eubacterium, and Coprococcus are included.
- the bacteria determined to be preferable by Method 2 are the following species.
- Group B Bacteroides vulgatus Bacteroides stercoris Bacteroides uniformis Blautia wexlerae Bacteroides sp. AR29 Dorea longicatena Phascolarctobacterium faecium Blautia massiliensis Ruminococcus sp. K-1 Bacteroides ovatus Faecalibacterium prasnitzii Megamonas funiformis Bifidobacterium adolescentis [Ruminococcus] torques Collinsella aerofaciens Parabacteroides merdae butyrate-producing bacteria M104 / 1 Bifidobacterium faecale Clostridium sp.
- Group C Bacteroides vulgatus Bacteroides stercoris Bacteroides uniformis Blautia wexlerae Bacteroides sp. AR29 Dorea longicatena Phascolarctobacterium faecium Blautia massiliensis Ruminococcus sp. K-1 Bacteroides ovatus
- composition of the present invention may contain bacteria belonging to the above-listed genera alone or in combination of a plurality of bacteria.
- a combination of a plurality of bacteria 40 or less types of bacteria are preferable, 30 or less types of bacteria are more preferable, and 20 or less types of bacteria are most preferable. If there are 20 or less types, for example, 20, 19, 18, 17, 16, 15, 14, 13, 12, 11, 10, 9, 8, 7, 6, 5, 4, 3, 2 types are arbitrary. You can choose a combination of bacteria.
- composition of the present invention may be in any powder, solid or liquid form for appropriate use in allo-HSCT or for long-term use, and these powder, solid or liquid forms. Can also be used as a capsule preparation. By using a capsule preparation, it is possible to avoid complications such as tube insertion and bleeding due to colon fibers, and there is an advantage that the burden on the patient is reduced in implementation.
- the composition of the present invention may be a live bacterium or a dead bacterium, or may be a mixture of a live bacterium and a dead bacterium.
- compositions of the present invention may include at least one selected from pH stabilizers, acidifiers, preservatives, vitamins, minerals, nutritional supplements, prebiotics and probiotics.
- GVHD can be prevented or treated by administering the composition of the present invention to the transplant target patient before, after, or both times of hematopoietic stem cell transplantation. Examples of GVHD include steroid-resistant or steroid-dependent GVHD, and intestinal acute GVHD.
- treatment is not limited to the degree of suppression as long as the onset of GVHD can be suppressed. Therefore, “treatment” includes both complete response (CR) and partial response (PR).
- Complete response (CR) means the disappearance of all gastrointestinal GVHD-related symptoms
- partial response (PR) means down staging of one or more gastrointestinal GVHD. In the present invention, it was considered effective (treated) when PR or CR was reached in steroid-resistant cases, and when the steroid dose was successfully reduced by 40% or more in steroid-dependent cases as compared with before treatment.
- prevention means to suppress the onset of GVHD in advance, prevent the condition of GVHD that has already occurred from becoming worse than that, and prevent the recurrence of subsided GVHD.
- the present invention provides a method for treating GVHD, which comprises administering the composition or capsule preparation to a GVHD patient.
- the present invention provides a method for preventing GVHD, which comprises administering the composition or capsule preparation to a patient subject to hematopoietic stem cell transplantation before, after, or both of the hematopoietic stem cell transplantation.
- Bacterial mixture, preparation The present invention is characterized in that the presence of the bacterium is confirmed from stool collected from humans, and the confirmed stool is suspended in a solvent (for example, physiological saline, buffer solution). , Provided is a method for producing a suspension for transplanting a fecal bacterial flora.
- the present invention is also characterized in that the presence of the bacterium is confirmed from the stool collected from a human, the bacterial flora is obtained from the confirmed stool, and the bacterial flora is suspended in a solvent.
- a method for producing a suspension for transplanting a fecal bacterial flora is provided.
- the present invention is characterized in that the presence of the bacterium is confirmed from the stool collected from a human, the bacterium is separated from the stool whose presence is confirmed, and the separated bacterium is mixed.
- a method for producing a bacterial mixture for flora transplantation is provided.
- the type of bacteria contained in the sample may be one type or two or more types.
- the presence of the bacterium is confirmed from the stool collected from a human, and the bacterial flora of the stool whose presence is confirmed or the bacterium isolated from the stool whose presence is confirmed is formulated.
- a method for producing a preparation for transplanting a fecal bacterial flora which is characterized by the above.
- a formulation a method of encapsulation in a capsule is exemplified.
- the stool can be collected according to the method described in the above section "1. FMT".
- the method for confirming the presence of bacteria is not particularly limited, and examples thereof include 16S rRNA gene analysis method, qPCR and Microarray using a DNA sequence of a region specific to the target bacterium, and MALDI-TOF MS method. ..
- the stool of the collection source contains the bacterium. Diluted suspension of feces is subjected to various culture conditions, and a single colony is collected to isolate the fungus.
- the dosage form of the preparation of the present invention is not particularly limited as long as it contains the bacterium of the present invention, but it is preferably an orally administered preparation.
- an excipient, a disintegrant, a lubricant, a colorant, a flavoring and odorant, etc. are added to the main drug as necessary, and then tablets and coated tablets are prepared by a conventional method. , Granules, fine granules, powders, capsules and the like.
- an acid resistant capsule is used as the capsule. Examples of acid-resistant capsules include DR Caps (registered trademark) (Capsugel).
- Excipients include, for example, lactose, corn starch, sucrose, glucose, sorbit, crystalline cellulose, silicon dioxide and the like
- binders include, for example, polyvinyl alcohol, ethyl cellulose, methyl cellulose, gum arabic, hydroxypropyl cellulose, hydroxypropyl methyl cellulose and the like.
- magnesium stearate, talc, silica, etc. are permitted to be added to pharmaceuticals as a coloring agent, but as a flavoring and flavoring agent, cocoa powder, peppermint brain, aromatic acid, etc. , Hakka oil, dragon brain, cellulose powder, etc. are used.
- the above-mentioned preparation is preferably a capsule preparation.
- the bacteria contained in the preparation are equivalent to dried cells, for example, 1 to 2000 mg, preferably 10 to 500 mg, more preferably 100 to 250 mg, and per day depending on the weight, symptoms, etc. of the patient. 150-200 mg, preferably 175 mg can be administered.
- the bacterium according to the present invention can be used both as a drug and as a quasi-drug.
- FMT for screening of preferred bacterial flora Subjects of FMT FMT was performed on 15 cases of steroid-resistant or addictive intestinal acute GVHD.
- the skin and liver are the target organs for acute GVHD, but since the intestinal acute GVHD is more severe than other organs, the intestinal tract was targeted this time.
- Steroid resistance is a case in which the condition remains unchanged after 5 days after the start of treatment despite a sufficient amount of steroid treatment (prednisolone 1 mg / kg or more), and a steroid-dependent case responds to steroid treatment once.
- the exclusion criteria are as follows. 1) GVHD responding to steroid treatment 2) GVHD that worsens after first-line treatment with steroids (in this case, regardless of the affected organ) 3) Cases with uncontrollable infections 4) When diarrhea is thought to be due to a cause other than GVHD
- the administration rate was set so as not to exceed 30 seconds per 50 mL. After all the suspensions had been added, the inside of the tube was washed with 50 mL of physiological saline, the tube was removed, and the administration was completed. When no side effect of grade 3 (defined by Non-Patent Document 3) or more related to FMT was observed, one additional administration was performed between the first FMT and 4 to 14 days while observing the effect. In this case, it was decided to collect feces from the same donor as the first time. Fecal transplantation was to be performed within 12 hours, and as much as possible, within 8 hours of the donor's stool collection.
- the therapeutic effect was evaluated 4 weeks after the final FMT.
- the criteria for determining the therapeutic effect are as follows.
- the intestinal bacterial flora was analyzed using the prepared fecal preparation solution and a part of the patient's feces (before FMT, 1 to 3 days after the final FMT, 1, 2 and 4 weeks). Bacterial DNA was extracted from each sample according to a conventional method. Primers designed to cover the variable region of the 16S rRNA gene, v1-v2 (27Fmod: 5'-agrgttgtgattiggttag-3'(SEQ ID NO: 345)), 338R: 5'-tgcttgtccccgtggtag-3'(SEQ ID NO: 346).
- bacterial species were identified by homology search using glsarch against the 16S rRNA gene database (RefSeq, RDP, GRD, CORE).
- the threshold of homology in genus identification was 94%.
- the threshold of homology in the identification of Species was 97%.
- FIG. 2 shows a graph showing the composition ratio of the fecal bacterial flora at the genus level before and after FMT.
- the diversity of the flora was reduced (disbiosis), and Staphylococcus and Enterococcus were predominant.
- the diversity of the flora was restored, and the flora was changed to a flora containing various bacteria such as Bacteroides and Bifidobacterium.
- the Others group cases in which Escherichia became dominant after 4 weeks were also observed.
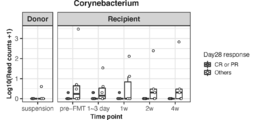
- the changes over time for each genus are shown in FIGS. 3 to 9. It was confirmed that all the genera shown in FIGS. 3 to 9 were abundant in the CR or PR group as compared with the Others group. In addition, it was confirmed that Corynebacterium shown in FIG. 10 was abundantly present in the Others group at all time points.
- SEQ ID NO: 345 Synthetic DNA
- SEQ ID NO: 346 Synthetic DNA
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- Immunology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Organic Chemistry (AREA)
- Zoology (AREA)
- Transplantation (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Genetics & Genomics (AREA)
- Biotechnology (AREA)
- Wood Science & Technology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Biomedical Technology (AREA)
- Virology (AREA)
- Biochemistry (AREA)
- General Engineering & Computer Science (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2021561461A JPWO2021106952A1 (enExample) | 2019-11-26 | 2020-11-25 | |
| US17/779,286 US20230024068A1 (en) | 2019-11-26 | 2020-11-25 | Prophylactic or therapeutic composition for graft-versus-host disease |
| EP20892673.3A EP4085916A4 (en) | 2019-11-26 | 2020-11-25 | PROPHYLACTIC OR THERAPEUTIC COMPOSITION FOR GRAFT VERSUS HOST DISEASE |
| CN202080082543.6A CN115461064A (zh) | 2019-11-26 | 2020-11-25 | 针对移植物抗宿主病的预防或治疗用组合物 |
| US19/080,046 US20250281549A1 (en) | 2019-11-26 | 2025-03-14 | Prophylactic or therapeutic composition for graft-versus-host disease |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2019-213436 | 2019-11-26 | ||
| JP2019213436 | 2019-11-26 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US17/779,286 A-371-Of-International US20230024068A1 (en) | 2019-11-26 | 2020-11-25 | Prophylactic or therapeutic composition for graft-versus-host disease |
| US19/080,046 Division US20250281549A1 (en) | 2019-11-26 | 2025-03-14 | Prophylactic or therapeutic composition for graft-versus-host disease |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2021106952A1 true WO2021106952A1 (ja) | 2021-06-03 |
Family
ID=76129434
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2020/043883 Ceased WO2021106952A1 (ja) | 2019-11-26 | 2020-11-25 | 移植片対宿主病に対する予防又は治療用組成物 |
Country Status (5)
| Country | Link |
|---|---|
| US (2) | US20230024068A1 (enExample) |
| EP (1) | EP4085916A4 (enExample) |
| JP (1) | JPWO2021106952A1 (enExample) |
| CN (1) | CN115461064A (enExample) |
| WO (1) | WO2021106952A1 (enExample) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2023016081A (ja) * | 2021-07-21 | 2023-02-02 | 国立大学法人東海国立大学機構 | 腸内細菌叢に占めるコリンセラ属の情報を提供する方法、当該情報を用いたcovid-19重症化予測方法およびサイトカインストーム重症化予測方法 |
| WO2023069655A1 (en) * | 2021-10-20 | 2023-04-27 | City Of Hope | Clostridium butyricum compositions and methods of using the same |
| WO2025239419A1 (ja) * | 2024-05-17 | 2025-11-20 | 株式会社バイオパレット | 人工腸内細菌叢の製造方法 |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2025104216A1 (en) * | 2023-11-15 | 2025-05-22 | Helmholtz Zentrum Muenchen - Deutsches Forschungszentrum Für Gesundheit Und Umwelt (Gmbh) | Bacteriophage compositions |
| CN118987051A (zh) * | 2024-09-13 | 2024-11-22 | 南方医科大学南方医院 | 粪副拟杆菌在制备治疗移植物抗宿主病药物中的应用 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS648092B2 (enExample) | 1985-06-07 | 1989-02-13 | Maruchiitetsukusu Purodakutsu Corp | |
| JP2013537531A (ja) | 2010-08-04 | 2013-10-03 | トーマス・ジュリアス・ボロディ | 糞便細菌叢移植のための組成物ならびにそれを作製および使用する方法ならびにそれを送達するためのデバイス |
| JP2016501852A (ja) | 2012-11-26 | 2016-01-21 | トーマス・ジュリアス・ボロディ | 糞便微生物叢の回復のための組成物、ならびにそれらを作製および使用する方法 |
| JP2017537979A (ja) * | 2014-11-25 | 2017-12-21 | メモリアル スローン−ケタリング キャンサー センター | 腸内微生物叢及びgvhd |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| MA41020A (fr) * | 2014-11-25 | 2017-10-03 | Evelo Biosciences Inc | Compositions probiotiques et prébiotiques, et leurs procédés d'utilisation pour la modulation du microbiome |
| US20180185421A1 (en) * | 2016-08-29 | 2018-07-05 | Tokyo Metropolitan Government | Composition comprising fecal microbiota |
| CN110191946A (zh) * | 2016-12-23 | 2019-08-30 | 学校法人庆应义塾 | 诱导cd8+t细胞的组合物及方法 |
-
2020
- 2020-11-25 JP JP2021561461A patent/JPWO2021106952A1/ja active Pending
- 2020-11-25 EP EP20892673.3A patent/EP4085916A4/en active Pending
- 2020-11-25 CN CN202080082543.6A patent/CN115461064A/zh active Pending
- 2020-11-25 WO PCT/JP2020/043883 patent/WO2021106952A1/ja not_active Ceased
- 2020-11-25 US US17/779,286 patent/US20230024068A1/en not_active Abandoned
-
2025
- 2025-03-14 US US19/080,046 patent/US20250281549A1/en active Pending
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS648092B2 (enExample) | 1985-06-07 | 1989-02-13 | Maruchiitetsukusu Purodakutsu Corp | |
| JP2013537531A (ja) | 2010-08-04 | 2013-10-03 | トーマス・ジュリアス・ボロディ | 糞便細菌叢移植のための組成物ならびにそれを作製および使用する方法ならびにそれを送達するためのデバイス |
| JP2016501852A (ja) | 2012-11-26 | 2016-01-21 | トーマス・ジュリアス・ボロディ | 糞便微生物叢の回復のための組成物、ならびにそれらを作製および使用する方法 |
| JP2017537979A (ja) * | 2014-11-25 | 2017-12-21 | メモリアル スローン−ケタリング キャンサー センター | 腸内微生物叢及びgvhd |
Non-Patent Citations (5)
| Title |
|---|
| BLOOD, vol. 109, no. 10, 2007, pages 4119 - 4126 |
| BLOOD, vol. 124, no. 7, 2014, pages 1174 - 1182 |
| BONE MARROW TRANSPLANTATION, vol. 15, 1995, pages 825 - 828 |
| KAITO S. ET AL.: "Fecal microbiota transplantation with frozen capsules for a patient with refractory acute gut graft-versus-host disease", BLOOD ADV., vol. 2, no. 22, 2018, pages 3097 - 3101, XP023541807 * |
| See also references of EP4085916A4 |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2023016081A (ja) * | 2021-07-21 | 2023-02-02 | 国立大学法人東海国立大学機構 | 腸内細菌叢に占めるコリンセラ属の情報を提供する方法、当該情報を用いたcovid-19重症化予測方法およびサイトカインストーム重症化予測方法 |
| JP7783588B2 (ja) | 2021-07-21 | 2025-12-10 | 国立大学法人東海国立大学機構 | 腸内細菌叢に占めるコリンセラ属の情報を提供する方法、当該情報を用いたcovid-19重症化予測方法およびサイトカインストーム重症化予測方法 |
| WO2023069655A1 (en) * | 2021-10-20 | 2023-04-27 | City Of Hope | Clostridium butyricum compositions and methods of using the same |
| WO2025239419A1 (ja) * | 2024-05-17 | 2025-11-20 | 株式会社バイオパレット | 人工腸内細菌叢の製造方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20250281549A1 (en) | 2025-09-11 |
| JPWO2021106952A1 (enExample) | 2021-06-03 |
| EP4085916A4 (en) | 2024-03-27 |
| US20230024068A1 (en) | 2023-01-26 |
| CN115461064A (zh) | 2022-12-09 |
| EP4085916A1 (en) | 2022-11-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2021106952A1 (ja) | 移植片対宿主病に対する予防又は治療用組成物 | |
| CN113073066B (zh) | 罗伊氏乳杆菌及其应用、组合物、药物和食品 | |
| CN111343972B (zh) | 包含细菌的口服药物制剂 | |
| EP3107553A1 (en) | Methods and compositions for intestinal microenvironment transfer | |
| WO2012142605A1 (en) | Rapid recolonization deployment agent | |
| EP3563837B9 (en) | Bacteriophage therapy | |
| US20200038459A1 (en) | Composition comprising fecal microbiota | |
| AU2018209227A1 (en) | Autologous fecal sample for use in the treatment of microbial dysbiosis | |
| WO2025036176A1 (zh) | 一株肠道菌及其在抗肿瘤免疫治疗中的应用 | |
| US20200281991A1 (en) | Methods and compositions for treating disorders related to a gut dysbiosis | |
| CN112336753A (zh) | 一种肠道菌群胶囊及其制备方法和应用 | |
| WO2023222098A1 (en) | A new streptomyces spororaveus strain and a new antibiotics against bacteria and fungi | |
| US20200197449A1 (en) | Compositions and Methods for Treating Alopecia and Related Disorders | |
| Amin et al. | Isolation and identification of Lactobacillus species from the vagina and their antimicrobial properties | |
| CN113164527A (zh) | 用于治疗癫痫和相关障碍的组合物和方法 | |
| CN117568180A (zh) | 一种载菌微藻、制备方法及其应用 | |
| Abd El-Mongy et al. | In vitro effect of chitosan lactobacillus acidophilus nanoparticles on vancomycin-resistant multidrug-resistant Enterococcus faecalis | |
| EP2887949B1 (en) | Therapeutic bacteriophages | |
| Ramona et al. | The POTENTIAL OF LACTIC ACID BACTERIA, ISOLATED FROM SEVERAL SOURCES, TO INHIBIT THE GROWTH OF Candida albicans ATCC10231 | |
| TWI625124B (zh) | 嗜熱鏈球菌tci633菌株及其代謝產物於預防及/或治療骨質疏鬆症之應用 | |
| EP4606380A1 (en) | Method for fecal microbiome purification and uses thereof | |
| JP2023165053A (ja) | 治療用組成物、治療方法、糞便の評価方法、診断方法、及び予後評価方法 | |
| WO2020214739A1 (en) | Methods and compositions for treating inflammatory diseases | |
| US11364269B2 (en) | Methods and compositions for reducing Listeria monocytogenes infection or colonization | |
| JP2025153503A (ja) | 糞便細菌叢を含む医薬組成物 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 20892673 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2021561461 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2020892673 Country of ref document: EP Effective date: 20220627 |