WO2020179919A1 - 薬剤分包装置 - Google Patents
薬剤分包装置 Download PDFInfo
- Publication number
- WO2020179919A1 WO2020179919A1 PCT/JP2020/009777 JP2020009777W WO2020179919A1 WO 2020179919 A1 WO2020179919 A1 WO 2020179919A1 JP 2020009777 W JP2020009777 W JP 2020009777W WO 2020179919 A1 WO2020179919 A1 WO 2020179919A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- drug
- tray
- cassette
- unit
- prescription
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61J—CONTAINERS SPECIALLY ADAPTED FOR MEDICAL OR PHARMACEUTICAL PURPOSES; DEVICES OR METHODS SPECIALLY ADAPTED FOR BRINGING PHARMACEUTICAL PRODUCTS INTO PARTICULAR PHYSICAL OR ADMINISTERING FORMS; DEVICES FOR ADMINISTERING FOOD OR MEDICINES ORALLY; BABY COMFORTERS; DEVICES FOR RECEIVING SPITTLE
- A61J3/00—Devices or methods specially adapted for bringing pharmaceutical products into particular physical or administering forms
Definitions
- the present invention relates to a drug packaging device, in which a predetermined drug is dispensed from a drug cassette containing a drug according to a prescription, and the prescribed drug for each dose is, for example, a blister pack type tray (hereinafter referred to as a tray). It is simply packaged in a "tray.”
- a predetermined drug is dispensed from a drug cassette containing a drug according to a prescription
- the prescribed drug for each dose is, for example, a blister pack type tray (hereinafter referred to as a tray). It is simply packaged in a "tray.”
- a tablet packaging device called a weekly calendar pack having storage recesses divided into 7 rows and 4 columns for automatically distributing and packaging tablets as a prescribed drug in individual storage recesses of a tray has been conventionally known.
- Patent Document 1 A tablet packaging device called a weekly calendar pack having storage recesses divided into 7 rows and 4 columns for automatically distributing and packaging tablets as a prescribed drug in individual storage recesses of a tray has been conventionally known.
- Patent Document 1 In the tray, tablets packaged according to the number of doses taken four times a day are stored in each storage recess for one row, and a total of seven rows can store one week's worth.
- the tablet packaging device of Patent Document 1 receives all the tablets for one tray by one carrier, moves the carrier onto the tray waiting at the discharge position, and presses the shutter forming the bottom wall of the carrier. When opened, it is a tablet packaging device that discharges tablets all at once into the storage recesses of the tray. According to this device, there are advantages that the packaging efficiency is high, there are few packaging errors, and the structure can be simplified.
- the drug packaging device If the drug cassette containing the prescription drug is not set in the drug packaging device, take out the corresponding drug cassette from the drug cassettes stored on the shelf and replace it with an unused drug cassette in the prescription. Then, the drug packaging device starts the packaging operation.
- Patent Document 2 When a drug tablet cassette corresponding to the drug to be prescribed is not set in the tablet packaging device, a drug packaging device that displays the mounting position of the tablet packaging device suitable for prescription by lighting a lamp has been conventionally known.
- an object of the present invention is to provide a drug packaging device capable of reducing the replacement work of the drug cassette, shortening the machine stop time as much as possible, and shortening the packaging work time as a whole.
- the drug packaging device has a drug supply unit capable of storing a plurality of types of drugs and delivering a drug based on prescription data, and the drug supply unit.
- a drug packaging device having a packaging unit that discharges the drug discharged from the unit from a discharge port and distributes and stores the drug in a tray having a plurality of storage recesses, and is provided with a plurality of the drug supply units.
- Drug data indicating a drug to be prescribed based on the drug storage unit, the prescription data storage unit that stores prescription data of a plurality of patients, and the prescription data stored in the prescription data storage unit was extracted and extracted.
- a control unit that controls the plurality of drug supply units based on drug data is provided, and each of the plurality of drug supply units detachably supports the drug cassette that houses the drug and the drug cassette that can be supplied.
- the drug feeder has an information recording unit that records identification information about the drug cassette, and the drug feeder has a driving unit that discharges the drug contained in the drug cassette, and the drug feeder. It has a reading unit that reads information from the information recording unit and an indicator that indicates the usage status of the drug cassette, and the control unit includes all drug data prescribed based on prescription data of a plurality of patients and all.
- the identification information from the reading unit that reads the identification information of the drug cassette mounted on the drug feeder is received, the indicator of the drug feeder is controlled, and the drug cassette mounted on the drug feeder The indicator is operated so that the medicine cassette that is not used for packaging can be discriminated from the inside.
- the drug packaging device extracts usable drug cassettes based on a plurality of input prescription data, and whether or not the set drug cassette is used in the plurality of prescription data. If there is a drug cassette that is not used in any of the prescription data, the indicator is controlled so that the drug cassette can be identified. This makes it possible to select a drug cassette that can be replaced based on multiple prescription data. By replacing the drug cassette with the required drug cassette, the replacement work of the drug cassette is reduced as much as possible, and the replacement work of the drug cassette is performed. It is possible to reduce the time when the machine is stopped and improve the efficiency of the packaging process.
- the drug packaging device preferably includes the following configuration.
- the indicator has an LED
- the control unit is configured to control the LED of the indicator of the drug feeder that supports the drug cassette used for packaging to light.
- the drug packaging device preferably includes the following configuration.
- the control unit extracts prescription data of a plurality of patients from the prescription data storage unit, and the drug attached to the drug feeder from the extracted prescription data of the plurality of patients.
- the drug cassettes used in the cassettes are sequentially selected, the number of selected drug cassettes is calculated, and the calculated number of drug cassettes mounted on the drug feeder is mounted on all the drug feeders. It is configured to select the drug cassette to be used based on the prescription data until the number of drug cassettes is exceeded.
- the packaging process can be performed efficiently.
- the drug packaging device preferably includes the following configuration.
- the control unit corresponds to the prescription data corresponding to the excess prescription data. Can be configured to release.
- packaging can be performed using the set drug cassette.
- the drug packaging device preferably includes the following configuration.
- the packaging unit is arranged adjacent to the tray loading unit on which the tray can be mounted, the tray discharging unit on which the tray containing the tablets can be mounted, and the tray can be detached.
- the tray drive unit which is supported by the tray, moves the supported tray at a position between the tray loading unit and the tray discharging unit, and moves the storage recess of the desired tray to the discharge port of the drug.
- the drug supply device includes a drug supply device that discharges the drug received from the drug supply unit from the discharge port, and the drug supply device has a drug transfer device that pushes the drug received from the drug supply unit to the discharge port.
- the drug transporting device has an extrusion case having a storage hole for storing the drug, and an extrusion spacer arranged below the extrusion case and having a storage hole for storing the drug.
- the extrusion spacer is configured to be replaceable according to the tray to be used.
- the extrusion case and the extrusion spacer can be replaced according to the tray to be used, so that the packaging process can be performed corresponding to various trays.
- the drug packaging device preferably includes the following configuration.
- the drug transport device is built in an extrusion path forming portion that guides the drug to the discharge port, and the extrusion case and the extrusion spacer are detachably attached to the extrusion path forming portion.
- packaging processing for various trays can be performed easily. Further, since the extruded spacer can be easily taken out, it is possible to easily clean the place where the drug is discharged.
- the drug packaging device preferably includes the following configuration.
- the adapter further includes an adapter for accommodating the tray, and the adapter includes a frame body formed in a rectangular frame shape and a bottom plate forming the bottom surface of the frame body, and the frame body corresponds to the storage recess of the tray.
- a pack adapter set plate having an opening is detachably attached.
- the drug packaging device preferably includes the following configuration.
- a pack spacer having an opening corresponding to the tray is arranged between the outlet of the drug supply device and the tray.
- packaging processing for various trays can be performed easily. Further, since the extruded spacer can be easily removed, it is possible to easily clean the place where the drug is discharged.
- the drug packaging device preferably includes the following configuration.
- the monitor further includes a monitor for displaying information on the drug packaging device, and the control unit causes the monitor to display the type of the tray according to the prescription and the type of the tray housed in the adapter.
- the drug packaging device preferably includes the following configuration.
- the extrusion case, the extrusion spacer, the pack adapter set plate, and the pack spacer are each provided with identification information for distinguishing the tray to be used, and the control unit displays the read identification information on the monitor.
- the extrusion case corresponding to the tray to be used, the extrusion spacer, the pack adapter set plate, and the pack spacer are configured to be distinguishable from each other.
- the pack adapter set plate corresponding to the tray, the pack spacer, the extrusion case and the extrusion spacer can be surely attached to the drug packaging device.
- the drug packaging device preferably includes the following configuration.
- the extrusion case, the extrusion spacer, the pack adapter set plate, and the back spacer are each provided with identification information for distinguishing the tray to be used, and the control unit displays the read identification information on the monitor. If the extrusion case, the extrusion spacer, the pack adapter set plate, and the back spacer corresponding to the tray to be used are identifiable, and the read identification information is different from the requested one. , Display an error on the monitor.
- the user can definitely replace the part corresponding to the tray 24 for prescription.
- the drug packaging device preferably includes the following configuration.
- the control unit determines whether or not each part is a desired part based on the identification information provided for each of the pack spacer, the extrusion case, the extrusion spacer, and the pack adapter set plate, and reads the identification. If the information is different from the desired part, the execution of the packaging process is prohibited. Further, the monitor may be configured to display an error and indicate that the execution of the packaging process is prohibited.
- the drug packaging device can select a drug cassette that can be replaced based on a plurality of prescription data, and by replacing the drug cassette with a necessary drug cassette, the replacement work of the drug cassette is reduced as much as possible. However, the time required for the machine to stop due to the replacement work of the drug cassette can be reduced, and the efficiency of the packaging process can be improved.
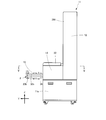
- FIG. 1 is a front view of the drug packaging device according to the embodiment of the present invention.
- FIG. 2 is a side view of the medicine packaging device of FIG.
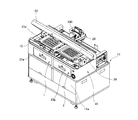
- FIG. 3 is a perspective view showing a state in which the drawer of the medicine packaging device of FIG. 1 is opened.
- FIG. 4 is a perspective view showing the arrangement configuration of the packaging unit of the drug packaging device of FIG. 1 with the lid of the packaging unit opened and the drug storage unit omitted.
- FIG. 5 is a cross-sectional view taken along the line VV of FIG. 2 with the lid removed.
- FIG. 6 is a perspective view showing the configuration of the drug supply device included in the drug storage unit of FIG.
- FIG. 7 is a cross-sectional view showing the configuration of the drug extrusion portion of the drug supply device.
- FIG. 1 is a front view of the drug packaging device according to the embodiment of the present invention.
- FIG. 2 is a side view of the medicine packaging device of FIG.
- FIG. 3 is a perspective view showing a state
- FIG. 8 is a perspective view showing the configuration of a drug extrusion section for multidose.
- FIG. 9A is a perspective view showing a state in which the extrusion case of the drug extrusion section for multidose is removed.
- FIG. 9B is a perspective view showing a state in which the extrusion spacer of the drug extrusion portion for multidose is removed.
- FIG. 10A is a perspective view showing a state in which the extrusion case of the drug extrusion section for single dose is removed.
- FIG. 10B is a perspective view showing a state in which the extrusion spacer of the drug extrusion portion for single dose is removed.
- FIG. 11 is an enlarged front view showing a drug storage portion of the drug packaging device according to the embodiment of the present invention.
- FIG. 12 is a block diagram showing a control configuration of the medicine packaging device according to the embodiment of the present invention.
- FIG. 13 is a schematic diagram showing the structure of the drug supply unit of the drug packaging device according to the embodiment of the present invention.
- FIG. 14 is a flow chart showing the control operation of the medicine packing apparatus according to the embodiment of the present invention.
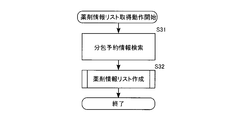
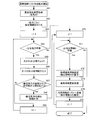
- FIG. 15 is a flow chart showing the control operation of the medicine packing apparatus according to the embodiment of the present invention, and shows the preparation operation of all medicine information lists.
- FIG. 16 is a flow chart showing a control operation of the drug packaging device according to the embodiment of the present invention, and shows a drug information list acquisition operation.
- FIG. 17 is a flow chart showing the control operation of the medicine packing apparatus according to the embodiment of the present invention, and shows the preparation operation of the medicine information list.
- FIG. 18 is an explanatory diagram showing the progress of the medicine packaging device displayed on the monitor.
- FIG. 19 is an explanatory diagram showing a drug information list screen displayed on the monitor.
- FIG. 20 is a front view of the medicine packaging device according to the second embodiment of the present invention.
- FIG. 21 is an enlarged front view showing a drug storage portion of the drug packaging device according to the second embodiment of the present invention.
- FIG. 22 is a flow chart showing the control operation of the medicine packing apparatus according to the second embodiment of the present invention, and shows the preparation operation of the medicine information list.
- FIG. 23 is an explanatory view showing a display example of the monitor of the drug packaging device according to the third embodiment of the present invention, and shows the operation of confirming the tray (pack) to be used.
- FIG. 24 is an explanatory view showing a display example of a monitor of the drug packaging device according to the third embodiment of the present invention, and shows information on replacement parts corresponding to trays (packs).
- FIG. 25 is an explanatory view showing a display example of a monitor of the drug packaging device according to the third embodiment of the present invention, and shows instruction information of a replacement operation of a replacement part corresponding to a tray (pack).
- FIG. 1 to 3 show the external configuration of the medicine packaging device 11 according to this embodiment.
- a drug storage unit 13 is provided in the upper half of the frame 12, and a packaging unit 14 (see FIG. 2) protruding forward from the upper half in the lower half.
- a storage portion 11a such as an empty tray is provided on the lower side thereof.
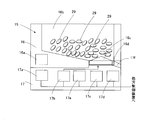
- a large number of drug supply units 15 are provided in the drug storage unit 13.
- the direction of the drug packaging device 11 according to this embodiment is defined as shown in FIGS. 1 and 2. That is, the width direction of the device is the X-axis, the front-back direction of the device is the Y-axis, and the height direction of the device is the Z-axis.
- the drug supply unit 15 is provided in multiple stages in the drug storage unit 13.
- the medicine supply unit 15 is composed of a medicine cassette 16 for accommodating medicines and a medicine feeder 17, and the medicine cassette 16 is set in the medicine feeder 17.
- the medicine cassette 16 is attachable to and detachable from the medicine feeder 17.
- the drug feeder 17 is set with a drug cassette 16 containing a drug required for packaging. Then, the drug cassette 16 unnecessary for the packaging can be removed from the drug feeder 17 and replaced with the drug cassette 16 containing the drug required for the packaging.
- the input device 100 is connected to the medicine packaging device 11.
- the input device 100 includes a code reader (not shown) that reads a barcode or QR code (registered trademark) written on a prescription.
- the input device 100 gives prescription data based on the read data to the medicine packaging device 11 by a code reader.
- the input device 100 includes an operation input device (not shown) such as a keyboard in addition to the code reader, and can give information such as prescription data to the drug packaging device 11 by using the operation input device.
- the drug packaging device 11 creates a prescription ID corresponding to the received prescription data.
- the prescription ID and prescription data are associated.
- the prescription data includes information necessary for packaging such as the type of drug, the number of drugs, the timing of administration, and patient information.
- the prescription ID is used when reading the associated prescription data.
- the input device 100 is composed of, for example, a personal computer.
- the personal computer includes a CPU, a ROM, a RAM, and the like.
- the CPU performs arithmetic processing according to various programs stored in the ROM.
- the input device 100 composed of a personal computer sends the prescription data to the medicine packaging device 11. Further, based on the state information during processing given by the drug packaging device 11, the monitor 101 displays the progress information of the drug packaging device 11, the procedure of the hand-spraying process, and the like.
- prescription data can be obtained from the system such as a pharmacy or hospital via the communication network. it can. Then, the input device 100 gives the prescription data obtained from a system such as a pharmacy or a hospital to the drug packaging device 11.
- the drug storage unit 13 includes a large number of drug supply units 15.
- the medicine supply unit 15 has a medicine cassette 16 and a medicine feeder 17 to which the medicine cassette 16 is attached.
- the multiple drug cassettes 16 store a plurality of types of drugs as drugs. Then, according to the prescription data corresponding to the prescription ID, the medicine is dispensed from the medicine cassette 16 by the medicine feeder 17, and the medicine falls onto the medicine supply device 19. The drug is counted as it passes through the passage of the drug feeder 17, and a predetermined number of drugs fall into the drug supply device 19.
- a drug supply device 19 is provided below the drug feeder 17.
- the medicine supply device 19 receives the medicine dropped from the medicine feeder 17.
- the medicines to be received are the number and kinds of medicines from the medicines in the medicine cassette 16 according to the prescription data.
- the drug supply device 19 collects the received drugs at the center and sends them to the drug extrusion section 20 extending in the front-rear direction. After the medicine is sent to the front side in the medicine extruding section 20, it is extruded by the extruding case 490 as described later, and the downward dispensing port 21 (FIG. 6, shown in FIG. (See Fig. 7).
- the drug supply device 19 dispenses the prescribed drug from the payout port 21 for each dose.
- the payout for example, four times a day for one week, three times a day for five days, three times a day for one month, etc. are intermittently paid out.
- the drug supply device 19 may be a so-called multi-dose case in which a plurality of drugs are dispensed for each dose according to a prescription from a discharge port 21, or a so-called single dose in which one drug is dispensed for each dose according to a prescription. ..
- the drug packaging device 11 stores a tray (pack sheet) 24 configured to store a plurality of drugs in one storage recess 24, and stores one drug in one storage recess.
- the tray 24 used according to the prescription such as the tray 24 configured as described above can be changed to a member used for the drug supply device 19 or the like. After storing the drug in the storage recess of the tray 24, a lid sheet is attached to form a blister pack according to the prescription.
- the drug cassette 16 and the drug feeder 17 of the drug supply unit 15 are housed in a drug cassette shelf 350 as shown in FIGS. 1 and 3.
- the medicine cassette shelf 350 is opened and closed in the vertical direction by the shutter 18 of the shutter.
- the drug in the drug cassette 16 housed in the drug cassette shelf 350 is dispensed by the drug feeder 17.
- the drug discharged from the drug cassette 16 falls downward and is introduced into the drug supply device 19 located on the lower side of the drug feeder 17. Then, after being carried to the drug extrusion section 20 inside the drug supply device 19, it is extruded from the drug extrusion section 20 to the discharge port 21 (see FIG. 6), and is packaged in the tray 24 by the packaging section 14.
- the configuration of the drug supply device 19 will be described later.
- the packaging portion 14 is usually covered with a cover lid 22 so that the inside can be observed from the peephole 22a.
- the tray loading unit 1 housed inside can be observed from the peephole 22a.
- a hand-spreading container is provided inside the packaging portion 14.
- the cover lid 22 of the packaging portion 14 is provided with a peep window 22a and a hand-pour lid 22b aligned in the X direction.
- the hand-spreading container is exposed to the outside, and the hand-spreading tablet can be put into the hand-spreading container.
- the tray 24 is placed on the cover lid 22 without using a hand-made container and the hand-made medicine is put therein. ..
- the packaging unit 14 is provided with a tray loading unit 1 and a tray discharging unit 2 as units that can be visually recognized from the outside of the device.
- the packaging unit 14 On the front surface of the packaging unit 14, as constituent members of the tray loading unit 1 and the tray discharging unit 2, the packaging unit 14 can be freely pulled out to the outside and pushed into the inside of the packaging unit 14, respectively.
- the input drawer 23a and the discharge drawer 23b are provided.
- the hand-spreading container when hand-spreading, the hand-spreading container is exposed by pulling up the hand-spreading lid 22b.
- the hand-spraying container is set to be located above the standby position of the tray discharge unit 2.
- the tray 24 is arranged on the cover lid 22 and the hand-spreading agent is charged.
- the tray loading unit 1 is a unit for loading the tray 24 before prescription into the apparatus.
- the tray loading unit 1 has a loading drawer 23a as described above, and the tray 24 is placed inside the loading drawer 23a.
- the packaging unit 14 includes a tray drive unit 4, a tray mounting unit 5, and a tray removing unit 6 inside the device.
- the tray loading unit 1 is a unit for loading the pre-prescription tray 24 into the apparatus.
- the tray loading unit 1 includes a loading drawer 23a as described above, and a first mounting table 30a on which the tray 24 is mounted inside the loading drawer 23a.
- the pack adapter set plate 10c is attached to the adapter 10, and the adapter 10 is mounted on the first mounting table 30a with the tray 24 set on the adapter set plate 10c.
- the loading drawer 23a of the tray loading unit 1 has the first loading table 30a in the tray handling position outside the packaging unit 14 when the loading drawer 23a is pulled out, and when the loading drawer 23a is pushed in, the inside of the packaging unit 14 is opened. It is set to be in the standby position.
- the first mounting table 30a is provided on two first support members 36 arranged parallel to the X direction in which the tray 24 to be loaded into the apparatus is arranged.
- the first mounting table 30a supports both ends of the tray 24 in the X direction.
- the tray 24 is mounted on the first mounting table 30a of the first support member 36 while being accommodated in the adapter 10.
- the adapter 10 is configured in a frame shape in which the upper part is open and the tray 24 can be stored from above.
- the adapter 10 one that corresponds to the type of tray 24 to be set is used. That is, in the drug packaging device 11 of this embodiment, the tray 24 used can be changed according to the prescription. Then, parts for the adapter 10 corresponding to the tray 24 and parts used for the drug supply device 19 are prepared in advance. It can be replaced with a part for the adapter 10 used for the tray 24 corresponding to the prescription and used for the packaging process.
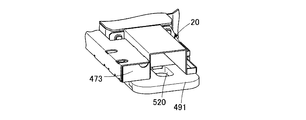
- the extrusion spacer 491 and the extrusion case 490 of the medicine supply device 19 are also configured to be replaceable according to the tray 24 to be used.
- the gap between the discharge port 21 of the drug supply device 19 and the tray 24 is maintained at a predetermined interval by the pack spacer 10d. Therefore, the pack spacer 10d corresponding to the tray 24 to be used is also prepared in advance.
- the pack spacer 10d is provided with an opening corresponding to the storage recess of the tray 24. The medicine is dispensed from the opening of the pack spacer 10d into the storage recess of the tray 24.
- the adapter 10 includes a frame body 10a formed in a rectangular frame shape and a bottom plate 10b forming the bottom surface of the frame body.
- a pack adapter set plate 10c provided with an opening corresponding to a storage recess of the tray 24 is attached to the frame body 10a.
- the tray 24 is housed in the adapter 10 by placing the tray 24 with the storage recess of the tray 24 and the opening of the pack adapter set plate 10c aligned from above the pack adapter set plate 10c mounted on the frame body 10a.
- the pack adapter set plate 10c is detachably attached to the adapter 10.
- the size and number of openings of the pack adapter set plate 10c differ depending on the tray 24 used. Therefore, a pack adapter set plate 10c is prepared according to the type of the tray 24.
- the pack adapter set plate 10c is determined according to the tray 24 selected according to the prescription.
- the pack adapter set plate 10c corresponding to the tray 24 is attached to the adapter 10. In other words, the pack adapter set plate 10c corresponding to the tray 24 is replaced and attached to the adapter 10.
- the pack spacer 10d corresponding to the tray 24 to be used is placed on the adapter 10.
- the pack spacer 10d set on the adapter 10 is sent to the tray drive unit 4 and mounted on the tray mounting unit 5.
- the gap between the discharge port 21 of the drug supply device 19 and the tray 24 is maintained at a predetermined interval by the pack spacer 10d.
- the pack spacer 10d is maintained in a state of being mounted on the tray mounting unit 5.
- the pack spacer 10d is configured to be moved onto the adapter 10.
- the adapter 10 itself has a plurality of recesses corresponding to the plurality of storage recesses of the tray 24 without using the pack adapter set plate 10c, the adapter 10 is replaced according to the type of the tray 24. do it.
- the tray discharge unit 2 is a unit on which the tray 24 in which the drug 29 is packaged is mounted and is taken out from the drug packaging device 11.
- the tray discharge unit 2 is provided in the packaging unit 14 so as to be aligned with the tray loading unit 1 in the X direction.
- the tray discharge unit 2 includes a discharge drawer 23b as described above, and a second mounting table 30b on which the tray 24 is placed inside the discharge drawer 23b.
- the discharge drawer 23b of the tray discharge unit 2 is arranged such that when the discharge drawer 23b is pulled out, the second mounting table 30b is in the tray handling position outside the packaging section 14 and is pushed inside the packaging section 14 when pushed. It is set to be in the standby position.
- the second mounting table 30b is provided on two second support members 37 arranged parallel to the X direction in which the tray 24 in which the drug is packaged is arranged.
- the second mounting table 30b supports both ends in the X direction of the adapter 10 in which the tray 24 is accommodated.
- the tray drive unit 4 includes a transport stage 48 configured to be horizontally movable in the X and Y directions.
- the transport stage 48 supports the tray 24 loaded into the tray loading unit 1 together with the adapter 10, and drives the tray so that the discharge port 21 for discharging the drug 29 corresponds to each storage recess 26 of the tray 24 to package the tray 24. After that, it is conveyed to the upper part of the tray discharge unit 2.
- the tray mounting unit 5 is a unit for mounting the adapter 10 accommodating the tray 24 mounted on the tray loading unit 1 to the tray drive unit 4.
- the tray removal unit 6 is a unit for removing the adapter 10 containing the tray 24 supported by the tray drive unit 4 from the tray drive unit 4 and mounting it on the tray discharge unit 2.
- the tray 24 loaded into the tray loading unit 1 is moved by the tray drive unit 4 so that the dispensing port 21 for discharging the drug corresponds to each storage recess of the tray 24.
- the tray 24 is conveyed to the upper part of the tray discharge unit 2 after being packaged.
- a plurality of drugs are housed in each of the plurality of drug supply units 15. That is, for example, when the drug is a tablet, a plurality of drugs 29 are contained in one drug cassette 16 (see FIG. 13).
- different drug supply units 15 When paying attention to a plurality of drug supply units 15, in principle, different drug supply units 15 contain different types of drugs. However, for frequently used drugs, the same type of drug may be accommodated across a plurality of drug supply units 15.
- Each of the plurality of drug supply units 15 can discharge the drug 29 stored in each drug cassette 16.
- the drug cassette 16 may be provided with a handle for the operator to hold when attaching / detaching.
- 60 drug supply units 15 are arranged in multiple stages above and below the drug storage unit 13, but this is just an example.
- the drug storage unit 13 is provided and the number and arrangement of the drug supply units 15 are provided. The method of is not limited to the one shown here.
- the drug cassette 16 and the drug feeder 17 of the drug supply unit 15 are housed in a drug cassette shelf 350 as shown in FIGS. 1 to 3.
- the medicine cassette shelf 350 is opened and closed in the vertical direction by the shutter 18 of the shutter.
- a part of the drug supply device 19 is communicated with a drug extrusion section 20 formed so as to extend to the packaging section 14. Further, the front end side of the drug extrusion section 20 of the drug supply device 19 is connected to the discharge port 21 to form a series of flow paths.
- the drug in the drug cassette 16 housed in the drug cassette shelf 350 is dispensed to the drug feeder 17.
- the drug discharged from the drug feeder 17 falls downward and is introduced into the drug supply device 19 located on the lower side of the drug feeder 17. Then, after being carried to the drug extrusion section 20 inside the drug supply device 19, it is pushed out from the drug extrusion section 20 to the discharge port 21, and is packaged in the tray 24 by the packaging section 14.
- the drug supply device 19 is roughly divided into a drug receiving part 365 (365a, 365b, 365c, 365d) (introducing part) located on the upper side, and two left and right lateral parts located on the lower side.
- a transport unit 366 (primary transport unit) and a drop path forming unit 367 (path forming unit) are provided.
- a drug extrusion unit 368 (secondary transport unit) is formed on the lower side of the drop path forming unit 367. Therefore, the drug supply device 19 includes a drug receiving unit 365 (introduction unit) having a horizontally extended shape, a lateral transport unit 366 (primary transport unit) having a shape extending horizontally, and a drug having a vertically extending shape. It has a three-stage stacking structure in which extrusion units 368 (secondary transport units) are vertically stacked.
- the uppermost drug receiving portion 365 (365a, 365b, 365c, 365d, 365e) is a portion for receiving the drug that has dropped from above, and functions as a drug introducing portion in the entire drug supply device 19. ..
- the medicine receiving portion 365 is adjacent to the end side medicine receiving portions 365a and 365d located on both end sides in the X direction (width direction of the medicine cassette shelf 350) and the end side medicine receiving portions 365a and 365d,
- the structure is provided with intermediate drug receiving portions 365b and 365c located on the intermediate side in the X direction and a central drug receiving portion 365e provided between the intermediate drug receiving portions 365b and 365c.
- the end-side drug receiving portions 365a and 365d, the interstitial drug receiving portions 365b and 365c, and the central drug receiving portion 365e are arranged so as to be connected in the X direction.
- Each of the drug introduction spaces 370 of the drug receiving portion 365 (365a, 365b, 365c, 365d, 365e) is a space that can communicate with the outside in the vertical direction, and has an opening surface serving as a drug introduction port on the upper side. ing.
- the end-side drug receiving portions 365a and 365d stand by in the drug receiving posture and receive the drug falling from the drug cassette 16 via the drug feeder 17. Then, the drug is supplied further downward by shifting to the drug discharge posture in a state where the drug is arranged inside the drug introduction space 370. In other words, the drug is temporarily stored inside the drug introduction space 370 and then supplied downward.
- the drug introduced into the drug introducing space 370 of the end side drug receiving parts 365a, 365d and the inter-part drug receiving parts 365b, 365c is one side of the lateral conveying part 366 in the intermediate stage. It is supplied to the drug extrusion unit 368 (secondary transfer unit) at the lowermost stage by being pushed and moved in the lateral direction and supplied to the drop path forming unit 367.
- the medicine introduced into the medicine introducing space 370 of the central medicine receiving portion 365e directly falls into the fall path forming portion 367 without passing through the intermediate lateral conveying portion 366, and the medicine pushing unit 368 (two Introduced to the next transport section).
- the horizontal transfer unit 366 at the intermediate stage will be described.
- two lateral transport units 366 symmetrical in the X direction and having the same structure are arranged side by side in the X direction.
- the drug discharge ports (not shown) of the two lateral transport units 366 are arranged adjacent to each other.
- the two drug discharge ports are arranged so as to sandwich the drop path forming portion 367.
- the lateral transport section 366 pushes and moves the drug laterally to supply the drug to the drop path forming section 367, and introduces the drug into the drug extrusion unit 368 (secondary transport section) at the lowermost stage.
- the shooter 480 connected to the drop path forming unit 367 is attached to the upper side of the extrusion path forming unit 473 of the drug extruding unit 20 and penetrates the inside in the vertical direction to supply the first medicine. 470 is formed.
- the opening portion located above the first drug supply hole 470 serves as an introduction port (drug introduction port 480a) when the drug is supplied to the drug extrusion unit 368.
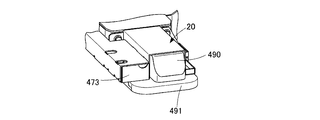
- the drug delivery device 474 is built in the extrusion path forming unit 473. As shown in FIG. 7, the drug transfer device 474 has a structure including an extrusion case 490 and an extrusion spacer 491, and is linearly moved in the drug extrusion section 20 by a drive device (not shown).
- the extrusion case 490 is provided with a storage hole 490a into which a plurality of chemicals can be inserted.
- the storage hole 490a is formed corresponding to the size of the storage recess of the tray 24.
- the extruded spacer 491 is partially formed with a storage hole 520 penetrating from the top surface to the bottom surface to convey the drug.
- the storage hole 520 is formed corresponding to the size of the storage recess of the tray 24.
- the extrusion case 490 and the extrusion spacer 491 are formed corresponding to the tray 24 to be used.
- the extrusion case 490 and the extrusion spacer 491 are interchangeably provided with respect to the drug transfer device 474 and are attached corresponding to the tray 24 used.
- Extrusion case 490 and extrusion spacer 491 will be described with reference to FIGS. 7 to 10B.
- the extrusion case 490 and the extrusion spacer 491 are formed corresponding to the tray 24 to be used, as described above.
- the tray 24 has a plurality of storage recesses, and there are a so-called single-dose type tray in which only one tablet is stored in each storage recess, and a so-called multi-dose type tray in which a plurality of tablets are stored in the storage recess. Further, there are various trays of multi-dose type in which the size and shape of the storage recess are different. As described above, the tray 24 has different types such as the size and shape of the storage recesses and the number of storage recesses.
- the adapter 10, the extrusion case 490, and the extrusion spacer 491 are configured in a size and shape corresponding to the tray 24 to be used. Therefore, the pack adapter set plate 10c, the pack spacer 10d, the extrusion case 490, and the extrusion spacer 491 are replaced according to the tray 24 to be used.
- FIGS. 9A and 9B show an extrusion case 490 and an extrusion spacer 491 when a multi-dose type tray (multi-dose pack) is used. That is, the extrusion case 490 and the extrusion spacer 491 used when inserting a plurality of chemicals into one storage recess of the tray are shown.
- the extruding case 490 and the extruding spacer 491 are formed in a size capable of extruding a plurality of medicines.
- the extrusion case 490 and the extrusion spacer 491 are attached to the extrusion path forming portion 473. Even when a plurality of drugs are stored in one storage recess, the shape of the storage recess differs depending on the tray 24.
- the storage holes 490a and 520 are formed to correspond to the size of the storage recess of the tray 24.
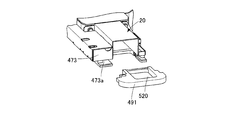
- the extrusion case 490 and the extrusion spacer 491 are detachably provided as shown in FIG. 9B.
- the push-out path forming portion 473 is provided with an engaging portion 473a with which the push-out spacer 491 is engaged.
- the pushing spacer 491 is attached to the pushing path forming portion 473 by engaging the pushing spacer 491 with the engaging portion 473a.
- 10A and 10B show an extrusion case 490 and an extrusion spacer 491 when a single dose type tray (single dose pack) is used. That is, the extrusion case 490 and the extrusion spacer 491 used when inserting one drug into one storage recess of the tray are shown. Since the single-dose type stores one drug in one storage recess, the extrusion case 490 and the extrusion spacer 491 have a shape suitable for extruding one drug, and the extrusion case 490 for multi-dose and the extrusion case 490. It is formed smaller than the extruded spacer 491 to reduce the chemical path.
- the pack adapter set plate 10c for the adapter 10, the pack spacer 10d, the extrusion case 490, and the extrusion spacer 491 are attached with a burgund or the like as identification information corresponding to the prescription.
- the barcode or the like is read and the adapter 10 corresponding to the tray 24 is used.
- the pack adapter set plate 10c, the pack spacer 10d, the extrusion case 490, and the extrusion spacer 491 are configured so that they can be attached to the device without fail.
- RFID and the like are attached to the pack adapter set plate 10c for the adapter 10, the pack spacer 10d, the extrusion case 490, and the extrusion spacer 491, and the pack adapter set plate 10c for the adapter 10 and the pack spacer 10d are wirelessly attached. And, it can be configured to confirm whether the extrusion case 490 and the extrusion spacer 491 are parts corresponding to the prescription.
- the extrusion spacer 491 since the extrusion spacer 491 is detachably configured, it can be easily replaced corresponding to the tray 24 using the extrusion spacer 492. As a result, packaging for various trays can be easily performed. Further, since the extruded spacer 491 can be easily removed, it is possible to easily clean the place where the medicine is discharged.
- FIG. 11 a part of the medicine container 13 is shown in an enlarged manner.
- a plurality of drug supply units 15 are arranged in a multi-layer structure in the drug storage unit 13.
- the medicine cassette 16 is set in the medicine feeder 17.
- the medicine feeder 17 has an indicator 17e.
- the indicator 17e displays whether or not the drug cassette 16 is in use, for example, the drug cassette 16 used for packaging.
- the indicator 17e is composed of, for example, an LED (Light Emitting Diode), and the prescription control device 200 described later controls lighting, non-lighting, blinking, and the like of the LED.
- the operator can grasp the state of the medicine cassette 16 and the like by checking the indicator (LED) 17e.
- the indicator 17e By configuring the indicator 17e with a plurality of LEDs such as red, green, and blue, the color of the light emitting LED may be changed so that the operator can grasp the state of the drug cassette 16.
- FIG. 13 is a schematic view showing the structure of the drug supply unit 15.
- the drug supply unit 15 includes a drug cassette 16 for accommodating the drug and a drug feeder 17.
- the drug cassette 16 includes an information recording unit 16a.
- the drug feeder 17 includes a reading unit 17a.
- the information recording unit 16a records identification information regarding the drug cassette 16.
- the information recording unit 16a uses, for example, an RF (Radio Frequency) ID.
- the information recording unit 16a may be any one that records identification information regarding the drug cassette 16, and may be a bar code or a QR code (registered trademark), not limited to RFID.
- the reading unit 17a provided on the drug feeder 17 reads the information of the information recording unit 16a.
- the reading unit 17a since the information recording unit 16a uses RFID, the reading unit 17a uses an RF reading device that reads RFID.
- the read information of the medicine cassette 16 is sent to the prescription control device 200 described later.
- the drug cassette 16 has a storage space 16b inside. A plurality of drugs (tablets) 29 are stored in the storage space 16b.
- the medicine cassette 16 includes a rotor 16c for feeding the medicine (tablet) 29 from the accommodation space 16b by a predetermined amount.
- the drug cassette 16 has a discharge port 16d for discharging the drug (tablet) 29.
- the drug feeder 17 includes a drive unit 17b.
- the drive unit 17b drives the rotor 16c under the control of the prescription control device 200 described later, and dispenses the drug (tablet) 29 in a desired amount.
- the drive unit 17b rotates the rotor 16c, holds the contained drug 29 in a pocket (not shown) provided in the rotor 16c while stirring, and sequentially discharges the held drug 29 from the discharge port 16d.
- the discharged drug 29 falls from the passage 17f into the drug supply device 19.
- the amount of the drug 29 discharged is counted by a sensor provided in the passage 17f, and when the predetermined quantity is reached, the drive unit 17b is stopped and the rotation of the rotor 16c is stopped.
- the drug feeder 17 includes a cassette mounting detection unit 17c, a cassette detachment detection unit 17d, and an indicator 17e composed of an LED.
- the cassette loading detection unit 17c and the cassette detachment detection unit 17d are shown only schematically.
- the cassette mounting detection unit 17c and the cassette detachment detection unit 17d may be combined by the same sensor.
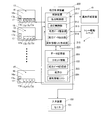
- the drug packaging device 11 includes a prescription control device 200.
- the input device 100 is connected to the prescription control device 200 of the medicine packaging device 11.
- the prescription data read by the code reader of the input device 100 is given to the prescription control device 200.
- the prescription data is given to the prescription control device 200 via the communication network.
- the prescription control device 200 performs payout control of the drug supply unit 15, drug supply control of the drug supply device 19, drive control of the tray drive unit 4, and the like.
- the prescription control device 200 includes a control device 210 and a data storage unit 220.
- the control device 210 includes a CPU, a ROM, a RAM, and the like.
- the CPU executes various arithmetic processes according to various programs stored in a recording medium such as a hard disk or a semiconductor memory to perform various control operations.
- FIG. 13 various control operations performed by the CPU executing a program suitable for each operation are represented as functional blocks.
- Each functional block represents a control operation performed by arithmetic processing of the CPU as a function in order to facilitate understanding, and is realized by the CPU and various programs.
- the control device 210 includes a payout control unit 211, a lighting control unit 212, a prescription data matching unit 213, a prescription data extraction unit 214, and a drug information list creation unit 215.
- the control device 210 obtains the RFID of the drug cassette 16 read by the reading unit 17a of the drug feeder 17, and gives the information of the obtained drug cassette 16 to the data storage unit 220. Then, the information on the drug cassette 16 given to the data storage unit 220 is stored in the cassette information storage unit 221.
- the control device 210 reads the cassette information from the cassette information storage unit 221 to grasp the type of the drug in the drug cassette 16 set in the drug feeder 17.
- the control device 210 gives prescription data of a plurality of patients input via the input device 100 or a communication line to the data storage unit 220, and the prescription data of the plurality of patients is stored in the prescription data storage unit 222 of the data storage unit 220. It will be remembered.
- control device 210 creates a prescription ID corresponding to the received prescription data, and the created prescription ID is given to the data storage unit 220.
- the given prescription ID is given to the prescription ID storage unit 223 in association with the prescription data.
- the prescription data includes information such as the type of drug, the number of drugs, the timing of taking the drug, and the patient information.
- the prescription ID is used when reading the associated prescription data.
- the payout control unit 211 controls the drive unit 17b of the drug feeders 17 of the plurality of drug supply units 15 to discharge the drug from the drug cassette 16 and store the drug in the corresponding storage recess of the tray 24 according to the prescription data.
- the lighting control unit 212 controls the lighting of the indicator 17e, which is an LED provided on the medicine feeder 17.
- the indicator 17e of the drug feeder 17 in which the drug cassette 16 used by the prescription data based on a plurality of prescriptions is set is controlled to be turned on.
- the operator can determine which drug cassette 16 may be removed by selecting the drug feeder 17 whose indicator 17e is not lit.
- the prescription data matching unit 213 matches the drug type of the prescription data to be prescribed with the drug of the drug cassette 16 set in the drug supply unit 15, and selects the drug cassette 16 corresponding to the drug type of the prescription data.
- the lighting control unit 212 of the control device 210 lights the LED of the indicator 17e of the drug feeder 17 corresponding to the drug cassette 16 selected by the prescription data matching unit 213.
- the prescription data extraction unit 214 extracts prescription data to be packaged / paid out from a plurality of prescription data stored in the data storage unit 220.
- the drug information list creation unit 215 creates a drug information list representing the drugs used for performing the packaging process based on the extracted prescription data.
- the control device 210 gives the created drug information list to the data storage unit 220.
- the drug information list given to the data storage unit 220 is stored in the drug information list storage unit 224.
- the packaging process of the drug packaging device 11 will be described.
- the control device 210 of the prescription control device 200 associates the prescription data stored in the prescription data storage unit 222 with the cassette information stored in the cassette information storage unit 221. Then, the prescription control device 200 performs payout control for the plurality of drug feeders 17. Since each drug feeder 17 is provided with a drive unit 17b, the payout control unit 211 of the control device 210 controls the plurality of drive units 17b.
- the medicine dispensed from the medicine cassette 16 by the medicine feeder 17 drops into the medicine supply device 19.
- the prescription control device 200 controls the drug supply device 19, and the drug is charged from the drug supply device 19 into the storage recess of the tray 24.
- the control device 210 of the prescription control device 200 controls the operation of the tray drive unit 4 and moves the tray 24 so that the corresponding storage recess of the tray 24 is located at the position of the discharge port 21 of the drug supply device 19. Then, the prescription control device 200 also controls the control device 210 so that the drug is charged from the drug supply device 19 into the storage recess. When the charging is completed, the control device 210 controls the operation of the tray drive unit 4 so that the next storage recess moves to the position of the discharge port of the drug supply device 19.
- the control device 210 of the prescription control device 200 repeats the above operation until the medicine is loaded into all the storage recesses of the tray 24. After that, the control device 210 of the prescription control device 200 controls the tray drive unit 4 to move the tray 24 to the tray discharge unit 2.
- the operator confirms that the discharge drawer 23b of the tray discharge unit 2 can be pulled out, and pulls out the discharge drawer 23b from the tray discharge unit 2. In this way, the drug packaging operation is performed.
- the prescription data stored in the prescription data storage unit 222 is read out, and the drug feeder 17 and the drive unit 4 are controlled based on the prescription data to perform the packaging processing. Will be.
- the control device 210 of the prescription control device 200 operates the payout control unit 211, and executes a supply operation of supplying the drug from the drug cassette 16 based on the prescription data.
- the drive unit 17b of the corresponding drug feeder 17 is operated so that the drug of the type and quantity corresponding to the prescription data is supplied based on the prescription data and the cassette information. If the prescription data is exchanged, the target of the drive unit 17b of the corresponding drug feeder 17 also changes.
- the prescription control device 200 displays a drug information list for each prescription on the monitor 101 of the input device 100. By displaying a list of drugs used for prescription on the monitor 101, the operator can grasp the type, number, and the like of the drugs corresponding to the prescription. Further, the prescription control device 200 displays various information regarding the prescription, such as the progress status of each prescription and the hand-spraying work format used in the hand-spraying work.
- the number of drug feeders 17 is the upper limit for the drug cassettes 16 that can be set in the drug packing device 11. That is, the drug packaging device 11 including the 60 drug feeders 17 can set the 60 drug cassettes 16. For example, when there are 120 drug cassettes, 60 drug cassettes 16 can be set in the drug packaging device 11. However, the remaining 60 drug cassettes 16 will be stored on a shelf or the like separate from the drug packaging device 11.
- the drug cassette 16 corresponding to the drug to be prescribed is not set in the drug packaging device 11, it is necessary to take out the corresponding drug cassette 16 stored on the shelf and replace it with the unused drug cassette 16 in the prescription. is there. If the prescription control device 200 is configured to notify the cassette information of the medicine or the like stored in the medicine cassette 16 stored on the shelf by using a communication means or the like, it corresponds to the time when the medicine cassette 16 is replaced. The drug cassette 16 can be easily found.
- a plurality of prescriptions are given to the drug packaging device 11 of this embodiment, a plurality of prescription data are extracted by the prescription data extraction unit 214, and packaging processing is performed based on the prescription data of each prescription sequentially extracted. ..
- the drug cassette is used when the drug used for the packaging process is not set in the drug packaging device each time. Will be replaced and the packaging process will be performed. For this reason, the time during which the machine is stopped during replacement of the drug cassette becomes long.
- the present invention extracts usable drug cassettes 16 based on a plurality of input prescription data, determines whether or not the set drug cassette 16 is used in the plurality of prescription data, and determines which one is used. If there is a drug cassette 16 that is not used even in the prescription data, the indicator 17e is controlled so that the drug cassette 16 can be identified. Then, a drug cassette 16 that can be replaced is selected based on a plurality of prescription data. By replacing the drug cassette 16 with a necessary drug cassette 16, the replacement work of the drug cassette 16 is reduced as much as possible, the time when the machine is stopped due to the replacement work of the drug cassette 16 is reduced, and the efficiency of the packaging process is improved. ..
- the prescription control device 200 of this embodiment controls so that the progress status of the drug packaging device 11 and the like are displayed on the monitor 101 of the input device 100.
- FIG. 18 is an explanatory diagram showing the progress of the medicine packaging device displayed on the monitor.
- the packing progress display area 1100 displays the progress and status of packing.
- the package list display area 1110 is an area for displaying a list of prescription data.
- the packaging device operation area 1140 is an area for operating the packaging device.
- the sprinkling position / sprinkling amount guidance display area 1150 of the hand-spraying drug used in the hand-spraying prescription is an area for displaying the sprinkling position of the hand-spreading drug and the amount of the drug.
- the function key display area 1130 is an area for performing various processes.
- the processing operations displayed in each area are performed by clicking the corresponding areas with a mouse or the like.
- the packaging progress display area 1100 includes an L-Drawer area 1101 indicating the status of the input / drawer 23a or work instruction information to the operator, and an R-Drawer area 1102 indicating the status of the discharge drawer 23b or the work instruction information to the operator.
- the Dispensing area 1103 that displays the payout state is displayed.
- control device 200 analyzes the operation of the packaging and updates the progress status when there is a change in the items related to the progress status of the packaging.
- the L-Drawer area 1101 includes a prescription ID display area 1101a for displaying the prescription ID packaged in the tray set in the input drawer 23a, and a notification area 1101b for notifying the operator of the state of the input drawer 23a.
- the operator can confirm the progress status of the drawer 23a by confirming the display in the L-Drawer area 1101.
- the tray 24 since the tray 24 is set in the loading drawer 23a, nothing is displayed in the notification area 1011b, but when the tray 24 is not set in the loading drawer 23a, the tray is set. A notification is displayed asking you to do so.
- the prescription ID display area 1101a of the L-Drawer area 1101 displays the prescription ID number when there is a prescription to be packaged next. Next, if there is no prescription to be packaged, the display is cleared.
- the notification area 1101b of the L-Drawer area 1101 displays the operation instruction content corresponding to the following display conditions when there is a prescription to be packaged next. If the tray 24 is not set, the message "Please set the tray.” is displayed. If the loading drawer 23a is not closed, the message "Please close the drawer.” Is displayed.
- the R-Drawer area 1102 includes a prescription ID display area 1102a for displaying the prescription data packaged in the tray 24 set in the discharge drawer 23b, and a notification area 1102b for notifying the operator of the state of the discharge drawer 23b.
- the operator can confirm the progress of the discharge drawer 23b by confirming the display of the R-Drawer area 1102.
- the packaging of the drug is completed in the tray 24 set in the discharge drawer 23b, and the notification area 1102b displays a notification that the tray should be taken out.
- the R-Drawer area 1102 notification area 1102b displays the operation instruction content corresponding to the following display conditions. If a plurality of display conditions are satisfied, the latest operation instruction content is displayed.
- the message "Please remove the tray.” is displayed. If the discharge drawer 23b is not closed, the message "Please close the drawer.” Is displayed.
- the Dispensing area 1103 includes an area 1103c indicating that the prescription is being paid out, a prescription ID display area 1103a for displaying the prescription data of the prescription currently being paid out, and a payout notification area 1103b for displaying the processing requiring handing.
- the area 1103c indicating that the product is being paid out displays the operation instruction content corresponding to the following display conditions when there is a prescription to be packaged. If there is a hand medicine in the prescription and the hand medicine completion registration is not registered, a message “Please complete all hand medicine” is displayed.
- the package list display area 1110 displays the prescription ID and the prescription data input to the drug packaging device 11 in the order in which they are extracted by the prescription data extraction unit 214. Then, it may be configured to be displayed differently from other displays so that the prescription ID currently prescribed can be known.
- a tab 1160 with a cassette and a tab 1161 without a cassette indicating a packaged prescription and a hand-sprayed prescription using a drug cassette 16 containing a drug to be packaged from the prescription data based on the prescription ID are displayed. Will be done.
- the operator's work can be performed easily and safely. That is, the worker can sprinkle the correct number of drugs in the correct place without making a mistake in the target drug (drug), and the safety is improved. For example, if the work is to be taken three times a day in the morning, noon, and evening, the drug (drug) to be taken in the morning, then the drug (drug) to be taken in the afternoon, and then in order from the upper right of the matrix. In addition, it is sprinkled with a drug to be taken in the evening.
- a display may be displayed on the monitor 101 so that it can be discriminated whether the tab currently selected is “with cassette” or “without cassette”.
- the start button of the packaging device In the packaging device operation area 1140, the start button of the packaging device, the stop button of the packaging device, the pause button of the packaging device, the unlock button of the discharge drawer 23b, etc. are displayed, and when each button is clicked, the corresponding button is displayed. The operation is performed.
- the drug list display area 1120 includes a selection line label for displaying the selected line of the package list, a patient name label for displaying the patient name of the selected line of the package list, a prescription drug list for displaying a list of prescription drugs, and a prescription. It has a drug number label that displays the drugs displayed in the internal drug list.
- the drug list display area 1120 displays all drug data corresponding to the given prescription data in the package reservation operation described later.
- the display mode of the above-mentioned package list display area 1110 changes according to the command input from the input device 100 in the package reservation operation described later. For example, when the target prescription data is selected from a plurality of prescription data displayed as a list, an asterisk or the like is added to the target prescription data so that the selected prescription data is displayed in a different manner.
- the drug The list displayed in the list display area 1120 is displayed so that the drug cassette 16 corresponding to the selected prescription data and the drug cassette 16 housed in another shelf can be seen.
- the drug list display area 1120 displays the status of the displayed drug. That is, it is displayed so that it can be identified whether the drug is contained in the set drug cassette 16, the drug stored in the stored drug cassette 16, or the drug to be hand-sprayed.
- FIG. 19 is an explanatory diagram showing an example of displaying a drug information list in the drug list display area 1120.
- drug information based on the prescription data is displayed in the drug information list 1120a.
- the drug information list 1120a displays a list of drugs to be paid out based on the selected prescription data.
- the Close button 1120b is used to close the drug information list 1120a.
- the Refill button 1120c is used when displaying detailed information regarding the filling of the medicine.
- the hand-spraying extraction button 1120d is used when displaying the hand-spraying drug on the drug information list 1120a.
- the hand-spraying extraction button 1120d By clicking the hand-spraying extraction button 1120d, the hand-spreading drug is displayed in the drug information list 1120a. Thereby, the operator can know that the medicine to be sprinkled in the sprinkling container is present in the target prescription. Then, the operator can confirm the necessary medicines when preparing the medicines by hand or by pre-dispersing the medicines on a tray (not shown) for preparation.
- the cassette extraction button 1120e is used when displaying the medicine dispensed using the medicine cassette 16 in the medicine information list 1120a.
- the all drug extraction button 1120f is used when displaying all the drugs extracted in the drug information list 1120a.
- the non-mounted button 1120 g is used when displaying the drug information of the non-mounted drug cassette 16.
- the drug information list 1120a shows the drug information contained in all the drug cassettes 16 that are not mounted in the drug packaging device 11 and are stored in another shelf or the like. Is displayed. That is, the drug information list 1120a displays drug information of only all drug cassettes 16 stored in another shelf or the like.
- the operator can confirm only the drug cassette 16 that is dispensed from the drug cassette 16 with the target prescription and is not attached to the drug packaging device 11. Then, the worker can collect the target drug cassette 16 from a place such as a shelf that is separately stored. The operator may replace the collected drug cassette 16 with the drug cassette 16 mounted on the drug packaging device 11. At this time, if there is a drug cassette 16 that is not used in any of the prescription data, the LED of the indicator 17e is controlled to be in a non-lighting state so that the drug cassette 16 can be identified. Therefore, the worker may remove the medicine cassette 16 of the medicine feeder 17 that is not lit and replace it with the collected medicine cassette 16.
- the drug inventory shortage button 1120h is used when displaying the drug information in which the drug cassette 16 mounted has a drug inventory shortage in the drug information list 1120a.
- the LED of the indicator 17e of the medicine feeder 17 is controlled to blink. The operator can recognize that the drug cassette 16 in which the LED of the indicator 17e is blinking is out of stock. Further, the LED of the indicator 17e of the drug feeder 17 is kept lit because the drug cassette 16 must not be removed when the operator does not click the drug inventory shortage button 1120h.
- the lighting state of the LED of the indicator 17e is controlled so that the drug cassette 16 can be identified.
- the LED of the indicator 17e of the drug feeder 17 in which the drug cassette 16 used in this packaging reservation is set is turned on.
- the LED of the indicator 17e of the medicine feeder 17 is turned off.
- a replaceable drug cassette 16 can be selected, and the drug cassette 16 can be replaced with a necessary drug cassette 16.
- the replacement work of the medicine cassette 16 is reduced as much as possible, and the packaging process can be performed.
- the medicine list display area 1120 also displays information related to manual work.
- the total number of the medicines dispensed from the medicine cassette 16 and the hand-sprayed medicines is displayed in each square of the hand-spraying position / amount guidance display area 1150 related to the hand-spraying work.
- this all-drug display button is clicked, both the prescription drug corresponding to the hand-sprayed drug and the drug dispensed from the drug cassette 16 in the prescription are displayed in the sprinkling position / sprinkling amount guidance display area 1150 of the hand-spreading drug. Is displayed. Information about the total quantity of all the drugs contained is then displayed in each cell. As a result, when all the medicines have been dispensed and hand-sprayed and the discharge drawer 23b is pulled out, the displayed quantity and the actual quantity of each square in the tray 24 can be visually inspected.
- the total number of each cell of the hand-sprayed drug is displayed in each cell in the spraying position / spray amount guidance display area 1150 of the hand-spraying drug related to the hand-spraying work.
- a display button for only the hand-delivered medicine for displaying the information contained in the hand-delivered medicine cell is displayed. Clicking the Show Only Hand-Sprayed Drugs button causes each cell to display information on the total number of prescription drugs sprinkled as hand-sprinkled.
- the hand-spraying skip button is displayed, and when this hand-spreading skip button is clicked, the confirmation of the completion of the hand-spraying drug is skipped.
- the hand-spraying drug sprinkling position / sprinkling amount guidance display area 1150 displays the display contents of the hand-spreading drug sprinkling position and the sprinkling amount guidance.
- the monitor 101 includes a lot number text box for inputting the lot number of the drug and an expiration date text box 1151 for inputting the expiration date of the drug.
- the prescription control device 200 controls so that the sprinkling completion button is not activated. Therefore, the lot number cannot be input.
- the list of prescriptions is displayed in the package list display area 1110, but the items that are being packaged or are in turn and those that are waiting in line are displayed.
- the completion command is sent to the prescription control device 200.
- the hand-spraying set button is pressed and the lid is closed, the drug drops onto the tray 24.
- the tray 24 reaches the discharge drawer 23b, the collation dialog of the sheet to be attached to the tray 24 is displayed, and the barcode of the sheet is read.
- the collation is completed correctly, the lock of the right discharge drawer 23b is released, and the packaging operation is completed.
- the prescription control device 200 of this embodiment can be controlled to display the progress status of the medicine packaging device 11 on the monitor 101 of the input device 100, and the operator can receive the packaging progress information. It can be notified sequentially. Based on this display, the operator can cancel the extracted prescription while confirming the display on the monitor 101 when creating a drug list for collectively using the input plurality of prescription data.
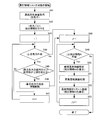
- FIG. 14 is a flow chart for explaining the operation of the present invention.
- processing based on a plurality of prescriptions is performed. Therefore, the control device 210 of the prescription control device 200 reserves and processes a plurality of packaging processes. That is, in the control device 210, the prescription data extraction unit 214 extracts a plurality of prescription data from the prescription data storage unit 222 in response to the reservation of the plurality of packaging processes.
- the control device 210 displays the package list display area 1110 of the monitor 101 in the order in which the prescription data stored in the data storage unit 220 is extracted (see FIG. 18). A list of prescription data is displayed in the package list display area 1110.
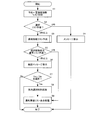
- the control device 210 creates a reservation list information list from the selected prescription data (step S1). .. Then, the selected prescription data is displayed on the monitor 101. For example, the display mode of the number (No.) display of the prescription data selected from the list of prescription data displayed in the package list display area 1110 is changed, and the selected prescription data is displayed so as to be understood.
- control device 210 determines the number of prescription data in the selected reservation list information list (step S2), and if the selected prescription data is 0, displays that there is no prescription data (step S2). Step S4), the operation is terminated. If the number of selected prescription data is one or more, the process proceeds to step S3.
- step S3 the operation of creating a list of all drug information corresponding to the selected prescription data is started. That is, all the medicines used in a plurality of prescription data are extracted, the prescription data matching unit 213 matches the medicines stored in the medicine cassette 16, and the medicine cassette 16 is set to accommodate the medicines. A list of the medicines stored in the medicine cassettes 16 that are not set and the medicines to be sprinkled is created. The operation of creating all the drug information lists will be described later according to FIG.
- the worker may select prescription data that delays the packaging process based on the created drug information list, depending on the efficiency of the packaging process. If there is prescription data that delays the packaging process during the drug information list creation operation, the worker sets to cancel the corresponding prescription data.
- control device 210 determines from the reservation list information whether or not there is prescription data to be released from this work (step S5).
- step S5 if the list to be canceled is 0, proceed to step S9. Then, in step S9, the drug information list based on the selected prescription data is displayed on the monitor 101, and the operation is terminated.
- step S5 if there is more than one list to be released, the process proceeds to step S6, a message confirming the release of the selected prescription list is displayed on the monitor 101, and the process proceeds to step S7.
- step S7 if the release is performed, that is, if the release is OK (S7: Yes), the process proceeds to step S8. If it is not canceled (S7: No), the process proceeds to step S9. Then, in step S9, the drug information list based on the selected prescription data is displayed on the monitor 101, and the operation is terminated.
- step 8 the selected prescription data is released, and the process proceeds to step S9.
- step S9 a drug information list based on the selected prescription data is created. That is, all the medicines used in the selected plurality of prescription data are extracted, and the medicine cassette 16 in which the medicines are stored and the medicines stored in the medicine cassette 16 not set are hand-sprayed. Make a list of drugs to do. Then, the medicine cassette 16 to be used ends the operation by turning on the LED of the indicator 17e.
- the unused drug cassette 16 can be determined by the LED of the indicator 17e of the drug feeder 17 not lit. Then, the medicine cassette 16 is removed from the medicine feeder 17 in which the LED of the indicator 17e is not lit, and the necessary medicine cassette 16 is set. The LED of the indicator 17e of the medicine feeder 17 in which the medicine cassette 16 for replacement is set is turned on according to the information of the medicine cassette 16.
- the operator can confirm that the LED of the indicator 17e of the drug feeder 17 is lit or not lit, so that the drug cassette 16 can be replaced without making a mistake.
- the packaging process operation is started, and packaging is performed sequentially according to the prescription data. Then, when hand-spraying is required, the hand-spreading work is performed, and when all the work is completed, the packaging processing operation is completed.
- the drug information list creation operation will be described with reference to the flowchart of FIG.
- the drug information list creating operation first, in order to turn on the LED of the indicator 17e of the drug feeder 17 in which the drug cassette 16 to be used is set, the LED flag is turned on in step S11. Then, a load screen indicating that drug information is being loaded is displayed on the monitor 101 (step S12).
- the control device 210 determines whether or not packaging is in progress (step S13). If it is determined that packaging is in progress (S13: Yes), the process proceeds to step S14, and the LED flag is turned off. That is, during packaging, the LED of the indicator 17e of the medicine feeder 17 may be used for an error display or the like, so the LED lighting control for discriminating the replaceable medicine cassette 16 is not performed. When the packaging operation is completed, the LED lighting control of the indicator 17e for discriminating the replaceable drug cassette 16 is performed.
- step S15 the drug information list is acquired.
- the operation of acquiring the drug information list to be acquired will be described later with reference to FIG.
- step S16 the control device 210 determines the number of acquired drug information lists. If the drug information list is 0, the process proceeds to step S17. Then, in step S17, the fact that there is no drug information list is displayed on the monitor 101, and the operation ends.
- step S16 If there is one or more drug information lists in step S16, the process proceeds to step S18. Then, in step S18, the medicine information list is set to be displayed on the monitor 101, and the process proceeds to step S19.
- step S19 the control device 210 calculates the number of drug cassettes 16 to be used in the acquired total drug list so that the number of drug cassettes 16 based on the drug information list does not exceed the number of drug feeders 17. Then, when the calculated number of drug cassettes 16 exceeds the number of drug feeders 17, the number of drug cassettes 16 used is counted in the order of input of prescription data, and the prescription data in which the drug cassette 16 exceeds the number of drug feeders 17 and thereafter. A selection release number list for releasing the prescription data following is created and displayed on the monitor 101. Then, the load screen of the monitor 101 is hidden (step S20), the operation ends, and the process returns to step S5 of FIG.
- step S31 all prescription data reserved for packaging is searched
- step S32 a routine for creating a drug information list based on the packaging reservation based on all the searched prescription data.
- step S32 the drug information list acquisition operation is completed, and the process returns to step S16 in FIG.
- the information of the drug cassette 16 for each drug of all the prescription data reserved for the package, the information of the drugs not contained in the drug cassette 16, and the drug feeder 17 Acquires the type of the drug cassette 16 set in (step S41).
- step S42 for each prescription, information on the drug cassette 16 for each drug in the prescription data, information on the drug not contained in the drug cassette 16, and the type of the drug cassette 16 set in the drug feeder 17 are extracted. Then, the prescription data is classified in the order of the packaging process (step S42), and the process proceeds to step S43.
- the order (i) of prescription data to be packaged is initialized to 1 (step S43), and the process proceeds to step S44.
- step S44 it is determined whether or not the order (i) of prescription data currently processed is less than or equal to the number of all prescription data. If the order of prescription data (i) is less than or equal to the total number of prescription data, the process proceeds to step S45 (S44: Yes), and if the order of prescription (i) exceeds the total number of prescriptions (S44: No). ), Proceed to step S48.
- step S45 it is determined whether or not the i-th prescription data exceeds the upper limit of the number of drug cassettes 16 that can be set in the drug feeder 17 (step S45). If it exceeds (S45: YES), the process proceeds to step S46, the extraction information of the i-th prescription data currently being processed is deleted, and the process proceeds to step S48.
- step S46 the control device 210 stores information on the prescription data such as the order of prescriptions from which the prescription extraction information has been deleted, acquires cassette information for displaying the replaceable drug cassette 16, and steps. Proceed to S48.
- the process when the number of drug cassettes 16 that can be set in the drug feeder 17 exceeds the upper limit is performed as follows, for example. All of the drug cassettes 16 set at the time of prescription selection are required for prescription data, and when there is no drug cassette 16 that can be replaced, each prescription is confirmed, and in this embodiment, the maximum of 60 types of drug cassettes 16 is exceeded. Check if it is.
- a plurality of prescription data are sequentially processed, and when the prescription data exceeds the upper limit of the number of drug cassettes 16, the prescription data is excluded from the packaging target and based on the plurality of prescription data not exceeding the upper limit.
- the drug cassette 16 may be replaced or the like.
- eight prescription data are for eight different drugs. It is assumed that the drug is contained in the drug cassette 16.
- the number of drug cassettes 16 that can be set is 60.
- the 8th prescription lacks the types of drug cassettes 16. Therefore, at the 8th prescription, the message "Is it OK to select the following prescriptions so that the number of drug feeders is less than or equal to the number of drug feeders after that?" Then, the prescription data of the eighth prescription is canceled, and if there is the same medicine as the medicine cassette 16 already selected in the next prescription, the upper limit of the number of medicine cassettes 16 may not be exceeded. You can continue processing the data. The released prescription data is sorted and displayed later in the package list display area 1110.
- step S45 If the upper limit of the number of drug cassettes 16 that can be set is not exceeded in step S45 (S45: No), the process proceeds to step S47, the order (i) is incremented by one in step S47, and the process returns to step S44. The above operation is repeated.
- step S48 in order to proceed to the processing of the prescription data extracted and not deleted, the count number (j) of the extracted prescription data is initialized to 1, and the process proceeds to step S50.
- step S49 Whether or not the current prescription data count number (j) exceeds the total number of prescription data extracted and not deleted in step S49, that is, the total number of prescription data subject to the drug information list (A). to decide. If the current prescription data count number (j) does not exceed the total number of prescription data (A) (S49: Yes), the process proceeds to step S50.
- step S50 information on the new drug is set based on the prescription data of the extracted count number (j). Then, the process proceeds to step S51.
- step S51 the drug information is updated. Then, it progresses to step S52. Then, in step S52, the drug information of the extracted count number (j) prescription data is registered in the list, and the process proceeds to step S53.
- step S53 the count number (j) is incremented by one, the process returns to step S49, and the current prescription data count number (j) is calculated from the total number of prescriptions (A) extracted and not deleted in step S49. The above operation is repeated until is exceeded.
- the current prescription data count number (j) exceeds the total number of prescriptions extracted and not deleted (A)
- the drug information list creation operation ends. Then, the routine of step S32 shown in FIG. 9 ends, and the process returns to step S16 of FIG.
- the control device 210 displays a list of drug lists in the drug list display area 1120 of the monitor 101. Then, among the drugs selected from the drug information list, those contained in the drug cassette 16 set in the drug feeder 17 are extracted. The LED of the indicator 17e of the extracted drug feeder 17 is turned on to make the operator recognize the drug cassette 16 used for the extracted prescription data.
- the drug cassette 16 in which the LED of the indicator 17e of the drug feeder 17 is not lit is taken out and the drug cassette 16 is replaced.
- the drug cassette 16 can be replaced by the number of times the LED of the indicator 17e of the drug feeder 17 is not lit.
- the packaging process is started.
- a plurality of prescription data can be obtained without replacing the drug cassette 16, so that the time for stopping the device is reduced and the efficiency of the packaging process is improved.
- the monitor 101 displays the position of the storage recess (mass) of the tray 24 to be hand-spread. In this way, by hand-spreading without passing through the drug packaging device 11, the hand-spraying can be performed in parallel while the prescription of only the drug cassette 16 is being dispensed, and the time for the machine to stop can be reduced.
- the hand-spraying instruction is displayed on the monitor 101, the hand-spraying place can be grasped.
- the hand-spraying is performed in the storage recess of the tray 24 without using the drug packaging device 11, so that the monitor 101 is instructed to hand-spread.
- the hand-spreading process can be easily performed.
- the drug cassettes 16 have the same size.
- the drug packaging device 11 there is a device configured such that the size of the drug cassette 16 is different and the corresponding drug feeder 17 is also different.
- 20 and 21 show a drug packaging device 11 using two types of drug cassettes, large and small.
- a drug supply unit 15 including a large drug cassette 16 and a drug feeder 17 is arranged at the bottom.
- a drug supply unit 15 including a small drug cassette 16 and a drug feeder 17 is arranged.
- the number of the drug supply unit 15 including the large drug cassette 16 and the drug feeder 17 is smaller than the number of the drug supply unit 15 including the small drug cassette 16 and the drug feeder 17.
- the routine of the drug information list creating operation is different. Since the other control flows are the same as those of the drug packaging device 11 using a single size, the routine of the drug information list creation operation will be described with reference to FIG.
- the drug packaging device 11 starts the operation of creating the drug information list based on the package reservation, the information and the drug of the drug cassette 16 for each drug of all the prescription data reserved for packaging are started. Information on the medicine not contained in the cassette 16 and the type of the medicine cassette 16 set in the medicine feeder 17 are acquired (step S61).
- step S62 for each prescription, information on the drug cassette 16 for each drug in the prescription data, information on the drug not contained in the drug cassette 16, and the type of the drug cassette 16 set in the drug feeder 17 are extracted. Then, the prescription data is classified in the order of the packaging process (step S62), and the process proceeds to step S63.
- step S64 it is determined whether or not the order (i) of the prescription data currently being processed is equal to or less than the total number of prescription data. If the order of prescription data (i) is less than or equal to the total number of prescription data, the process proceeds to step S65 (S64: Yes), and if the order of prescription (i) exceeds the total number of prescriptions (S64: No). ), and proceeds to step S70.
- step S65 it is determined whether the number of large drug cassettes 16 used has reached the upper limit, and if the number reaches the upper limit, the drugs using the large drug cassette 16 are distributed to the hand-spreading operation. Then, the process proceeds to step S66.
- step S66 the number of large drug cassettes 16 and small drug cassettes 16 used is counted, and the process proceeds to step S67.
- step S66 the total number of drug cassettes 16 used in the extracted prescription data is counted.
- step S67 it is determined whether or not the i-th prescription data exceeds the upper limit of the number of drug cassettes 16 that can be set in the drug feeder 17 (step S67). If it exceeds (S67: YES), the process proceeds to step S68, the extraction information of the i-th prescription data currently being processed is deleted, and the process proceeds to step S70.
- step S68 the control device 210 stores information on the prescription data such as the order of prescriptions from which the prescription extraction information has been deleted, acquires cassette information for displaying the replaceable drug cassette 16, and proceeds to step S70. move on.
- step S67 If the upper limit of the number of drug cassettes 16 that can be set is not exceeded in step S67 (S67: No), the process proceeds to step S69, the order (i) is incremented by one in step S69, and the process returns to step S64. The above operation is repeated.
- step S70 in order to proceed to the processing of the prescription data extracted and not deleted, the count number (j) of the extracted prescription data is initialized to 1, and the process proceeds to step S71.
- step S71 Whether or not the current prescription data count number (j) exceeds the total number of prescription data extracted and not deleted in step S71, that is, the total number of prescription data subject to the drug information list (A). to decide. If the current prescription data count number (j) does not exceed the total number of prescription data (A) (S71: Yes), the process proceeds to step S72.
- step S72 information on a new drug is set based on the extracted count number (j) prescription data. Then, the process proceeds to step S73.
- step S73 the drug information is updated. Then, it progresses to step S74. Then, in step S74, the drug information of the extracted count number (j) prescription data is registered in the list, and the process proceeds to step S75.
- step S75 the count number (j) is incremented by one, the process returns to step S71, and the current prescription data count number (j) is calculated from the total number of prescriptions (A) extracted and not deleted in step S49. The above operation is repeated until is exceeded.
- the current prescription data count number (j) exceeds the total number of prescriptions extracted and not deleted (A)
- the drug information list creation operation ends. Then, the routine of step S32 shown in FIG. 9 described above is completed, and the process returns to step S16 of FIG. 8 described above.
- the control device 210 displays a list of drug lists in the drug list display area 1120 of the monitor 101. Then, among the drugs selected from the drug information list, those contained in the drug cassette 16 set in the drug feeder 17 are extracted. The LED of the indicator 17e of the extracted drug feeder 17 is turned on to make the operator recognize the drug cassette 16 used for the extracted prescription data.
- the type of the drug cassette 16 is not limited to two, and the types such as large, medium, and small sizes are appropriately selected according to the application.
- the prescription control device 200 controls the processing and the like corresponding to the type of tray of the drug packaging device 11 to be displayed on the monitor 101 of the input device 100.
- FIG. 23 is an explanatory diagram showing the progress of the medicine packaging device displayed on the monitor.
- the monitor 101 displays a processing status display area 1200, a pack setting area 1210, a package list display area 1250, a drug list display area 1260, a function key display area 1130, a selected prescription number message box 1240, and the like.
- the processing status display area 1200 displays a pack list to indicate the processable status.
- the pack setting area 1210 includes an area 1211 for displaying the pack (tray) used in the selected prescription, and an area 1212 for displaying the pack (tray) corresponding to the parts currently mounted on the drug packaging device 11.
- a selection icon (selectable process that can be paid out) 1213 for selecting a prescription that can be paid out is displayed.
- the packing list display area 1250 is an area for displaying a list of prescription data.
- the packaging device operation area 1140 is an area for operating the packaging device.
- the function key display area 1130 is an area for performing various processes.
- the prescription control device 200 changes the display of the selected prescription column to shading or the like. This allows the user to confirm the selected prescription.
- the prescription control device 200 displays the number of selected prescriptions in the message box 1240. Then, the prescription control device 200 determines the tray 24 to be used for the packaging process based on the prescription data. The prescription control device 200 confirms the set parts on the adapter 10 or the like for the tray (pack) set in the drug packaging device 11.
- the set component is a tray (pack) for single dose. Area 1212 is displayed as a single dose pack.
- the prescription control device 200 displays in the area 1211 that the tray 24 used for the selected prescription in the pack setting area 1210 is a tray for multi-dose (multi-dose pack).
- the tray 24 used for packaging and the tray parts set in the drug packaging device 11 are different. Therefore, it is necessary to replace the tray parts set in the medicine packaging device 11.
- the prescription control device 200 displays on the monitor 101 prompting the user to replace the tray parts, as shown in FIG. 24.
- the prescription control device 200 displays on the monitor for changing the pack spacer 10d, the extrusion case 490, the extrusion spacer 491, and the pack adapter set plate 10c to a multi-dose pack (tray).
- the user prepares a pack spacer 10d for a multi-dose pack, an extrusion case 490, an extrusion spacer 491, and a pack adapter set plate 10c. Bar codes for identifying the corresponding trays 24 are provided on the pack spacer 10d, the extrusion case 490, the extrusion spacer 491, and the pack adapter set plate 10c, respectively.
- the prescription control device 200 reads the barcodes of the pack spacer 10d, the extrusion case 490, the extrusion spacer 491, and the pack adapter set plate 10c, respectively, and displays a display prompting the monitor 101 to replace them.
- the user reads the barcodes of the pack spacer 10d, the extrusion case 490, the extrusion spacer 491, and the pack adapter set plate 10c.
- the packed spacer 10d, the extrusion case 490, the extrusion spacer 491, and the pack adapter set plate 10c are selected according to the barcodes of the read pack spacer 10d, extrusion case 490, extrusion spacer 491, and pack adapter set plate 10c. It is determined whether the component is the desired component. When the information read by the barcode is different from the desired component, an error display is displayed.
- the prescription control device 200 determines that the pack spacer 10d, the extrusion case 490, the extrusion spacer 491, and the pack adapter set plate 10c are desired parts
- the pack spacer 10d, the extrusion case 490, and the extrusion spacer 491 the pack adapter set plate 10c is displayed on the monitor 101 so as to be replaced.
- the monitor 101 displays the relevant portion in a way that is easy for the user to see, such as by shading the relevant portion.
- the prescription control device 200 executes the packaging process.
- the prescription control device 200 uses the barcodes of the read pack spacer 10d, extrusion case 490, extrusion spacer 491, and pack adapter set plate 10c to select the pack spacer 10d, extrusion case 490, extrusion spacer 491, and pack adapter set plate. It is determined whether 10c is the desired component. If the information read by the barcode is different from the desired component, the execution of the packaging process is prohibited.
- the pack spacer 10d, the extrusion case 490, the extrusion spacer 491, and the pack adapter set plate 10c are each provided with wireless identification information such as RFID, and it is authenticated when the main body is attached that each is a correct part. , Confirmation may be performed, and similarly, if it is determined that the component is not the target component, an error display may be displayed on the monitor.
- the type of drug is not limited as long as it is a drug that can be packaged such as a capsule.
- the packaging container is not limited to this, and can also be used as a drug packaging device for packaging in a packaging paper or a vial bottle. This invention can be applied.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Basic Packing Technique (AREA)
- Medical Preparation Storing Or Oral Administration Devices (AREA)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2021503672A JPWO2020179919A1 (enExample) | 2019-03-06 | 2020-03-06 | |
| AU2020231749A AU2020231749B2 (en) | 2019-03-06 | 2020-03-06 | Drug packaging device |
| JP2024080980A JP2024109719A (ja) | 2019-03-06 | 2024-05-17 | 薬剤分包装置 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2019-040633 | 2019-03-06 | ||
| JP2019040633 | 2019-03-06 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2020179919A1 true WO2020179919A1 (ja) | 2020-09-10 |
Family
ID=72338702
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2020/009777 Ceased WO2020179919A1 (ja) | 2019-03-06 | 2020-03-06 | 薬剤分包装置 |
Country Status (3)
| Country | Link |
|---|---|
| JP (2) | JPWO2020179919A1 (enExample) |
| AU (1) | AU2020231749B2 (enExample) |
| WO (1) | WO2020179919A1 (enExample) |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6082130A (ja) * | 1983-10-08 | 1985-05-10 | Tokyo Shokai:Kk | 錠剤供給装置 |
| JPH04339701A (ja) * | 1991-05-16 | 1992-11-26 | Sanyo Electric Co Ltd | 薬剤包装機 |
| JP2003237710A (ja) * | 2002-02-20 | 2003-08-27 | Sanyo Electric Co Ltd | 薬剤供給装置 |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102583628B1 (ko) * | 2013-01-18 | 2023-09-27 | 가부시키가이샤 유야마 세이사쿠쇼 | 약품 불출 장치, 약품 불출 방법, 약품 불출 프로그램, 기록 매체 |
| US9977871B2 (en) * | 2014-01-14 | 2018-05-22 | Capsa Solutions Llc | Cassette control including presence sensing and verification |
| KR20170068285A (ko) * | 2015-12-09 | 2017-06-19 | 에이치엠에이치 주식회사 | 약제의 블리스터 포장 장치 |
| JP7358686B2 (ja) * | 2017-05-12 | 2023-10-11 | 株式会社湯山製作所 | 薬剤払出装置、薬剤払出装置の制御プログラム、および制御プログラムを記録した記録媒体 |
| EP3741687B1 (en) * | 2018-03-09 | 2022-05-04 | Yuyama Mfg. Co., Ltd. | Individual packaging device for tablets |
| KR20180043215A (ko) * | 2018-03-30 | 2018-04-27 | 에이치엠에이치 주식회사 | 약제의 블리스터 포장 장치 |
-
2020
- 2020-03-06 JP JP2021503672A patent/JPWO2020179919A1/ja active Pending
- 2020-03-06 AU AU2020231749A patent/AU2020231749B2/en active Active
- 2020-03-06 WO PCT/JP2020/009777 patent/WO2020179919A1/ja not_active Ceased
-
2024
- 2024-05-17 JP JP2024080980A patent/JP2024109719A/ja active Pending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6082130A (ja) * | 1983-10-08 | 1985-05-10 | Tokyo Shokai:Kk | 錠剤供給装置 |
| JPH04339701A (ja) * | 1991-05-16 | 1992-11-26 | Sanyo Electric Co Ltd | 薬剤包装機 |
| JP2003237710A (ja) * | 2002-02-20 | 2003-08-27 | Sanyo Electric Co Ltd | 薬剤供給装置 |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2020231749B2 (en) | 2025-04-24 |
| AU2020231749A1 (en) | 2021-10-07 |
| JP2024109719A (ja) | 2024-08-14 |
| JPWO2020179919A1 (enExample) | 2020-09-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6798639B2 (ja) | 錠剤分包装置 | |
| JP7518432B2 (ja) | 薬品払出装置、薬品払出プログラム | |
| KR100852493B1 (ko) | 약제 공급 장치 | |
| WO2011102491A1 (ja) | 薬剤払出装置及び装着カセット管理方法 | |
| JP2022000165A (ja) | 秤量装置 | |
| JP6044270B2 (ja) | 薬剤包装システム、情報処理装置、及びその制御方法とプログラム。 | |
| JP4484097B2 (ja) | 調剤システム | |
| WO2020179919A1 (ja) | 薬剤分包装置 | |
| JP2019051100A (ja) | 秤量装置 | |
| JP2019118657A (ja) | 薬剤分包機 | |
| JP7593695B2 (ja) | 薬剤分包機 | |
| JP7460084B2 (ja) | 薬剤分包機 | |
| JP2019051102A (ja) | 秤量装置 | |
| JP2019051103A (ja) | 秤量装置 | |
| JP2019051106A (ja) | 秤量装置 | |
| JP2023143826A (ja) | 薬剤払出し装置 | |
| JP2022182942A (ja) | 薬剤供給装置、薬剤供給装置の制御プログラム、およびこの制御プログラムを記録した記録媒体 | |
| JP2020037015A (ja) | 秤量装置 | |
| JP2016198581A (ja) | 調剤システム |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 20767255 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2021503672 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2020231749 Country of ref document: AU Date of ref document: 20200306 Kind code of ref document: A |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 20767255 Country of ref document: EP Kind code of ref document: A1 |