WO2020085023A1 - 間葉系細胞の培養方法、活性化間葉系細胞の製造方法、毛包原基の製造方法、間葉系細胞の活性化方法、及び上皮系細胞の活性化方法 - Google Patents
間葉系細胞の培養方法、活性化間葉系細胞の製造方法、毛包原基の製造方法、間葉系細胞の活性化方法、及び上皮系細胞の活性化方法 Download PDFInfo
- Publication number
- WO2020085023A1 WO2020085023A1 PCT/JP2019/038934 JP2019038934W WO2020085023A1 WO 2020085023 A1 WO2020085023 A1 WO 2020085023A1 JP 2019038934 W JP2019038934 W JP 2019038934W WO 2020085023 A1 WO2020085023 A1 WO 2020085023A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- mesenchymal cells
- cells
- current
- hair follicle
- electrode
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0652—Cells of skeletal and connective tissues; Mesenchyme
- C12N5/0662—Stem cells
- C12N5/0666—Mesenchymal stem cells from hair follicles
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0625—Epidermal cells, skin cells; Cells of the oral mucosa
- C12N5/0627—Hair cells
- C12N5/0628—Hair stem cells; Hair progenitors
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2529/00—Culture process characterised by the use of electromagnetic stimulation
Definitions
- the present invention relates to a method for culturing mesenchymal cells, a method for producing activated mesenchymal cells, a method for producing hair follicle primordium, a method for activating mesenchymal cells, and a method for activating epithelial cells.
- Patent Document 1 a step of seeding mesenchymal cells and epithelial cells in a micro-engraved plate composed of regularly arranged micro-recesses and performing mixed culture while supplying oxygen to form a hair follicle primordium And a method for producing an aggregate of regenerated hair follicle primordia.
- Patent Document 2 discloses a method for culturing dermal papilla cells, which comprises culturing dermal papilla cells in the presence of basic fibroblast growth factor (bFGF), and subculturing the dermal papilla cells into spheres. Have been described.
- bFGF basic fibroblast growth factor
- Patent Document 3 discloses culturing dermal papilla cells maintaining hair-inducing ability, which comprises culturing dermal papilla cells in the presence of FGF-based material, BMP-2 / BMP-4-based material and WNT-based material. The method is described.
- the present invention has been made in view of the above problems, and a method for culturing mesenchymal cells that improves hair growth-related properties, a method for producing activated mesenchymal cells, a method for producing hair follicle primordia, and It is one of the objects to provide a method for activating leaflet cells and a method for activating epithelial cells.
- a method for culturing mesenchymal cells according to an embodiment of the present invention for solving the above-mentioned problem is to cultivate the mesenchymal cells adhered to the surface of an electrode while applying a current through the electrode. Including.
- the present invention provides a method for culturing mesenchymal cells, which improves hair growth-related properties of mesenchymal cells.
- the current may be a pulse current.
- the current may be applied via the electrode as the first electrode and the second electrode arranged at a position facing the first electrode.
- a method for producing activated mesenchymal cells according to an embodiment of the present invention for solving the above-mentioned problem is to culture mesenchymal cells adhered to the surface of an electrode while applying a current through the electrode. Thereby obtaining activated mesenchymal cells as compared to mesenchymal cells cultured by the same method except that no current is applied.
- the present invention provides a method for producing activated mesenchymal cells having improved hair growth-related properties.
- the current may be a pulse current.
- the current may be applied via the electrode as the first electrode and the second electrode arranged at a position facing the first electrode.
- the activated mesenchymal cells have an expression level of at least one hair growth-related gene increased while that of mesenchymal cells cultured by the same method except that no current is applied. It may be leafy cells.
- a method for producing a hair follicle primordium according to an embodiment of the present invention for solving the above-mentioned problem is that hair is obtained by co-culturing mesenchymal cells produced by any of the above methods and epithelial cells. Including forming a primordium. According to the present invention, there is provided a method for producing a hair follicle primordia with effectively improved hair growth-related properties.
- the hair follicle primordium may be formed by co-culturing the mesenchymal cells and the epithelial cells on a cell non-adhesive surface.
- the hair follicle primordia formed by the co-culture are the same method except that mesenchymal cells produced by the same method as the mesenchymal cells are used except that no current is applied.
- the activated epithelial cells may be included as compared with the epithelial cells contained in the hair follicle primordia formed in (4).
- the activated epithelial cells are mesenchymal cells produced by the same method as the mesenchymal cells except that the expression level of at least one hair growth-related gene is not applied with current.
- the number of epithelial cells contained in the hair follicle primordia formed by the same method may be higher than that of epithelial cells.
- the hair follicle primordia formed by the co-culture are the same method except that mesenchymal cells produced by the same method as the mesenchymal cells are used except that no current is applied.
- the activated mesenchymal cells may be included as compared with the mesenchymal cells contained in the hair follicle primordia formed in (1).
- the activated mesenchymal cells were formed by the same method except that mesenchymal cells produced by the same method as the mesenchymal cells were used except that no current was applied.
- the mesenchymal cells may have an increased expression level of at least one hair growth-related gene as compared with the mesenchymal cells contained in the hair follicle primordia.
- a method for activating mesenchymal cells according to an embodiment of the present invention for solving the above-mentioned problem is to culture the mesenchymal cells adhered to the surface of an electrode while applying a current through the electrode. Activates the mesenchymal cells.
- a method for activating mesenchymal cells which effectively improves hair growth-related properties of mesenchymal cells.
- the method for activating epithelial cells according to an embodiment of the present invention for solving the above-mentioned problems is a hair follicle primordium formed by co-culture of mesenchymal cells and epithelial cells, wherein the mesenchymal cells are By using the mesenchymal cells produced by any one of the above methods as the cells, the epithelial cells contained in the hair follicle primordia are activated.
- a method for activating epithelial cells which effectively improves hair growth-related properties of epithelial cells.
- a method for culturing mesenchymal cells that improves hair growth-related properties a method for producing activated mesenchymal cells, a method for producing hair follicle primordia, a method for activating mesenchymal cells, and epithelium
- a method for activating lineage cells is provided.
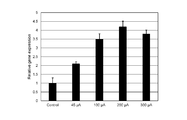
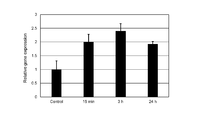
- Example 2 which concerns on this embodiment, it is explanatory drawing which shows the result of having evaluated the gene expression level of the hair papilla cell when changing the timing which starts applying an electric current.
- Example 2 which concerns on this embodiment, it is explanatory drawing which shows the result of having evaluated the gene expression level of the hair papilla cell when changing the applied electric current value.
- Example 2 which concerns on this embodiment, it is explanatory drawing which shows the result of having evaluated the gene expression level of the hair papilla cell when changing the time which applies an electric current.
- Example 3 it is an explanatory view showing a photograph of hair growth from a hair follicle primordium formed using hair papilla cells cultured by applying an electric current and transplanted into a mouse. is there.
- Example 3 according to the present embodiment an explanatory view showing a photograph of hair growth from a hair follicle primordium formed using hair papilla cells cultured without applying an electric current and transplanted into a mouse. Is.
- Example 3 which concerns on this embodiment, it is explanatory drawing which shows the result of having evaluated the number of hair growth from the hair follicle primordium transplanted to the mouse.
- this method A method according to one embodiment of the present invention (hereinafter referred to as “this method”) will be described below.
- the present invention is not limited to this embodiment.
- This method includes a method for culturing mesenchymal cells, which comprises culturing mesenchymal cells adhered to the surface of an electrode while applying a current through the electrode. That is, the inventors of the present invention have conducted extensive studies on technical means for improving hair growth-related properties of mesenchymal cells, and as a result, by culturing the mesenchymal cells while applying electrical stimulation, The inventors have uniquely found that leaf cells are activated and their hair growth-related properties are effectively improved, and completed the present invention.
- the present method activates the mesenchymal cells by culturing the mesenchymal cells adhered to the surface of the electrode while applying a current through the electrode.
- the method of conversion is included.
- this method is a method in which mesenchymal cells adhered to the surface of an electrode are cultured while applying a current through the electrode, and mesenchymal cells cultured by the same method except that no current is applied.
- the method for producing activated mesenchymal cells which comprises obtaining the mesenchymal cells activated as compared with the method of 1.
- the present method includes a method for producing a hair follicle primordium, which comprises forming a hair follicle primordia by co-culturing the mesenchymal cells produced by the above-mentioned method and an epithelial cell. .
- the inventors of the present invention surprisingly include the activated mesenchymal cells and epithelial cells by co-culturing the cells to form hair follicle primordia, which is unexpectedly included in the hair follicle primordia. It was independently found that activated epithelial cells are activated.
- this method uses the mesenchymal cells produced by the above-mentioned method as the mesenchymal cells in the hair follicle primordia formed by co-culture of mesenchymal cells and epithelial cells.
- the method for activating the epithelial cells which activates the epithelial cells contained in the hair follicle primordia, is included.
- the mesenchymal cells used in the present method are those having hair growth-related properties (for example, expression of hair growth-related genes and / or formation of hair follicle primordia by co-culture with epithelial cells). It is not particularly limited.
- the mesenchymal cells may be cells derived from adult hair follicle tissue (for example, dermal papilla and / or hair root sheath), and skin tissue (whether fetus, juvenile, or adult skin tissue) Cells derived from stem cells (eg, induced pluripotent (iPS) stem cells, embryonic stem (ES) cells, or embryonic germ (EG) cells) in vitro. Cells may be used.
- stem cells eg, induced pluripotent (iPS) stem cells, embryonic stem (ES) cells, or embryonic germ (EG) cells
- the mesenchymal cells may be primary cells collected from a living body, or may be pre-cultured cells (eg, subcultured cells and / or established cells).
- Mesenchymal cells are identified as cells expressing, for example, Versican and ALP (alkaline phosphatase).
- Versican and ALP alkaline phosphatase
- hair papilla cells and / or hair bulb sheath cells are preferably used as the mesenchymal cells. Hair papilla cells and hair bulb sheath cells express Versican and ALP.
- the mesenchymal cells are preferably derived from humans, but may be derived from non-human animals (animals other than humans).
- the non-human animal is not particularly limited, but it is preferably a non-human vertebrate (vertebrate other than human).
- the non-human vertebrate is not particularly limited, but it is preferably a non-human mammal.
- Non-human mammals include, but are not limited to, primates (eg, monkeys), rodents (eg, mice, rats, hamsters, guinea pigs, rabbits), meats (eg, dogs, cats), or ungulates. (For example, pig, cow, horse, goat, sheep).
- the culture medium used for culturing the mesenchymal cells is not particularly limited as long as it has a composition suitable for survival of the mesenchymal cells and maintenance of hair growth-related properties.
- a culture medium for cell culture is preferably used.
- culture surface The surface to which the mesenchymal cells adhere (hereinafter referred to as “culture surface”) used in this method (hereinafter referred to as “culture electrode”) can be cultured by adhering the mesenchymal cells. Moreover, it is not particularly limited as long as it is a conductive surface to which an electric current can be applied.
- the culture electrode preferably contains a metal film.
- the metal constituting the metal film is not particularly limited as long as it imparts conductivity to the culture electrode, and for example, selected from the group consisting of gold, platinum, silver, copper, titanium, chromium and nickel. One or more of the above or alloys thereof are preferably used.
- the culture electrode may include a conductive polymer membrane. That is, for example, the culture electrode may include a metal film and a conductive polymer film formed on the metal film.
- the conductive polymer forming the conductive polymer film is not particularly limited, but, for example, polypyrrole-based polymer, polythiophene-based polymer, polyaniline-based polymer, polyacetylene-based polymer, and polyparaphenylene-based polymer One or more selected from the group consisting of is preferably used.
- the culture electrode may include a cell adhesive substance film. That is, for example, the culture electrode may include a metal film and a cell adhesive substance film formed on the metal film. Further, for example, the culture electrode may include a metal film, a conductive polymer film formed on the metal film, and a cell adhesive substance film formed on the conductive polymer film.
- the cell-adhesive substance is not particularly limited as long as it is a substance that promotes adhesion of mesenchymal cells to the electrode surface, and examples thereof include extracellular matrix components such as collagen, fibronectin, and laminin, and specific cells exhibiting cell adhesiveness. Amino acid sequences (eg, arginine, glycine, aspartic acid (so-called RGD) sequences, etc.) and specific sugar chain sequences exhibiting cell adhesiveness are preferably used.
- the culture electrode does not include a cell adhesive substance film, for example, by adsorbing the cell adhesive substance contained in the culture solution in contact with the culture surface of the culture electrode on the culture surface, mesenchyme
- the lineage cell can adhere to the culture surface. That is, the culture surface is not particularly limited as long as it is a conductive surface that exhibits cell adhesiveness in contact with the culture solution.
- Adhesion of mesenchymal cells to the culture surface is performed by seeding the mesenchymal cells on the culture surface and retaining the mesenchymal cells on the culture surface. Specifically, a culture solution containing mesenchymal cells is dropped onto the culture surface, and the mesenchymal cells precipitated on the culture surface in the culture solution are treated under conditions suitable for culturing the mesenchymal cells. (For example, the mesenchymal cells are adhered to the culture surface by holding at a temperature suitable for culturing the mesenchymal cells, a gas phase composition in contact with the culture solution, a composition of the culture solution) for a predetermined time. Although the mesenchymal cells before adhering to the culture surface are spherical, the mesenchymal cells adhering to the culture surface have a flatter shape than before adhesion.
- the culture of mesenchymal cells in this method is performed while applying an electric current through the culture electrode to which the mesenchymal cells adhere.
- the conditions for applying the current are not particularly limited as long as the effects of the present invention can be obtained, but, for example, the expression level of at least one hair growth-related gene in the mesenchymal cells after culturing is determined by applying the current.
- conditions that increase more than that of mesenchymal cells cultured by the same method are preferably used.
- the timing of applying the current is not particularly limited as long as the effect of the present invention can be obtained, but for example, it may be 6 hours or more after the mesenchymal cells are seeded on the electrode surface. It is preferable that the time is 10 hours or more, more preferably 15 hours or more, and even more preferably 20 hours or more.
- the timing of starting the application of the electric current is preferably, for example, 170 hours before the mesenchymal cells are seeded on the electrode surface, more preferably 120 hours before, and 80 hours before. Is more preferable, and it is particularly preferable that it is before 50 hours have elapsed.
- the timing to start applying the current may be specified by arbitrarily combining any one of the lower limit values and any one of the upper limit values.
- the total time of applying the electric current (when the electric current is applied in a plurality of times during the culture period, the total of the plural times of applying the electric current) is not particularly limited as long as the effect of the present invention can be obtained. Is, for example, preferably 5 minutes or longer, more preferably 10 minutes or longer, even more preferably 15 minutes or longer, and particularly preferably 20 minutes or longer.
- the total time for applying the current is, for example, preferably 120 hours or less, more preferably 80 hours or less, further preferably 50 hours or less, and particularly preferably 20 hours or less. preferable.
- the total time for applying the current may be specified by arbitrarily combining any of the above lower limit values and any of the above upper limit values.
- the time for applying the current in one electrical stimulation (when applying the current in multiple times during the culture period, the time for continuously applying the current in one of the multiple current applications) is It is not particularly limited as long as the effect of the present invention can be obtained, but is preferably 5 minutes or more, more preferably 10 minutes or more, still more preferably 15 minutes or more, 20 Especially preferably, it is not less than minutes.
- the time for applying the current in one electrical stimulation is, for example, preferably 120 hours or less, more preferably 80 hours or less, further preferably 50 hours or less, and 20 hours or less. Is particularly preferable.
- the time for which the current is applied in one electrical stimulation may be specified by arbitrarily combining any of the above lower limit values and any of the above upper limit values.
- the current value applied per unit area (cm 2 ) of the culture surface (electrode surface) is not particularly limited as long as the effect of the present invention can be obtained, but is preferably 10 ⁇ A / cm 2 or more, for example. , more preferably 20 .mu.A / cm 2 or more, even more preferably at 30 .mu.A / cm 2 or more, and particularly preferably 40 .mu.A / cm 2 or more.
- the current value to be applied is preferably 1000 ⁇ A / cm 2 or less, more preferably 700 ⁇ A / cm 2 or less, even more preferably at 400 .mu.A / cm 2 or less, 200 .mu.A / cm 2
- the applied current value may be specified by arbitrarily combining any of the above lower limit values and any of the above upper limit values.
- the current to be applied is preferably a pulse current.
- the pulse current is not particularly limited as long as it is a pulsed current.
- one pulse is composed of the following (1), (2) and (3), and the following (1) and (2) ) Or may consist of the following (1) and (3): (1) first applying a positive or negative current of a first value for a first time; (2) then A second value of negative or positive current is applied for a second time ;, (3) no current is applied for a subsequent third time.
- the description of "plus current or minus current" in (1) and "minus current or plus current” in (2) means that when plus current is applied in (1), minus current is applied in (2).
- the negative current is applied in (1), it means that the positive current is applied in (2).
- the first value and the second value of the pulse current described above may be different, but are preferably the same.
- the first time and the second time may be different, but are preferably the same.
- the third time may be different or the same as the first time and / or the second time.
- the frequency of the pulse current is not particularly limited as long as the effects of the present invention are obtained, but is preferably 2 Hz or higher, more preferably 3 Hz or higher, and further preferably 4 Hz or higher. It is preferably 5 Hz or more, and particularly preferably. Note that 1 Hz means that the pulse current is applied once per second.
- the frequency of the pulse current is, for example, preferably 100 Hz or less, more preferably 50 Hz or less, even more preferably 25 Hz or less, and particularly preferably 10 Hz or less.
- the frequency of the pulse current may be specified by arbitrarily combining any of the above lower limit values and any of the above upper limit values.
- Applying an electric current through the culture electrode is performed using, for example, the culture electrode as the first electrode and the second electrode.
- the first electrode (culture electrode) is used as the working electrode and the second electrode is used as the counter electrode.
- a reference electrode may be used.
- the positional relationship between the first electrode and the second electrode is not particularly limited as long as the first electrode and the second electrode are in contact with the culture medium containing mesenchymal cells.
- a current may be applied via a culture electrode as the first electrode and a second electrode arranged at a position facing the first electrode. That is, in this case, between the first electrode and the second electrode, the mesenchymal cells adhered to the culture surface of the first electrode, and contact the first electrode and the second electrode A culture medium containing mesenchymal cells is arranged.
- FIG. 1 schematically shows an example of a culture device for culturing mesenchymal cells while applying an electric current.
- the culture device 1 is used as a counter electrode which is arranged at a position facing a first electrode 10 which is a culture electrode used as a working electrode and a culture surface 11 of the first electrode 10.
- a second electrode 20 which is provided.
- the first electrode 10 is composed of a metal film such as a gold thin film formed on a substrate 12 composed of a glass plate or the like.
- the second electrode 20 is made of a metal such as platinum.
- the culture device 1 includes a plurality (four) of culture wells 30.
- Each culture well 30 has a partition wall 31 for holding the culture solution M on the first electrode 10.
- the mesenchymal cells C adhere to the culture surface 11 and are cultured in the culture medium M.
- the first electrode 10 and the second electrode 20 are each connected to a power supply 40. That is, the first electrode 10 and the second electrode 20 are connected to the power source 40 by the first wiring 41 and the second wiring 42, respectively.
- the second electrode 20 of each of the four culture wells 30 is connected to the power source 40 by the wiring 42, it is possible to apply a current to the four culture wells 30 under different conditions. .
- a predetermined potential is applied between the first electrode 10 and the second electrode 20 in each culture well 30 by the power supply 40, so that the first electrode 10 and the second electrode 20 are interposed.
- An electric current can be applied.
- the mesenchymal cells adhered to the culture surface of the culture electrode may be cultured while applying an electric current using such a culture device.
- the time for culturing the mesenchymal cells on the culture surface is not particularly limited as long as the effects of the present invention can be obtained, but is preferably 5 hours or more, and more preferably 20 hours or more. It is more preferably 50 hours or more, still more preferably 70 hours or more.
- the time for culturing the mesenchymal cells on the culture surface is, for example, preferably 400 hours or less, more preferably 300 hours or less, further preferably 200 hours or less, and 100 hours. The following is particularly preferable.
- the time for culturing the mesenchymal cells on the culture surface may be specified by arbitrarily combining any one of the above lower limits and any one of the above upper limits.
- the mesenchymal cells are activated by culturing the mesenchymal cells attached to the culture surface of the culture electrode while applying a current through the culture electrode. That is, activated mesenchymal cells can be obtained by culturing while applying an electric current.
- activation of mesenchymal cells means improvement of hair growth-related properties of the mesenchymal cells.
- the improvement of hair growth-related properties of mesenchymal cells was, for example, increased by increasing the expression level of hair growth-related genes of the mesenchymal cells, and / or was formed using the mesenchymal cells. It includes improving the hair-growth properties of the hair follicle primordia (for example, increasing the number and / or the length of hairs growing from the hair follicle primordia implanted in the living body).
- activated mesenchymal cells obtained by this method for example, the expression level of at least one hair growth-related gene, that of mesenchymal cells cultured by the same method except that no current is applied. There are more mesenchymal cells.
- the hair growth-related gene expressed in mesenchymal cells is not particularly limited as long as it is a gene related to the contribution of the mesenchymal cells to hair growth, and includes, for example, Versican, ALP, BMP4, Nexin and Notch1. It may be one or more selected from the group.
- the activated mesenchymal cells obtained by the present method are, for example, mesenchymal cells cultured by the same method except that the expression amount of the ALP gene measured by RT-PCR is not applied with current. Is 1.5 times or more, or 2.0 times or more.
- the activated mesenchymal cells produced by this method can be used for various purposes. That is, the activated mesenchymal cells are provided, for example, in a state of being directly adhered to the culture surface of the culture electrode or recovered from the culture surface and used for research purposes or medical uses such as transplantation.
- activated mesenchymal cells are preferably used for forming hair follicle primordia in vitro. That is, by co-culturing the activated mesenchymal cells produced by the present method and the epithelial cells, it is possible to form a hair follicle primordium having excellent hair growth-related properties.
- the epithelial cells used in the formation of the hair follicle primordia in the present method have hair growth-related properties (eg, expression of hair growth-related genes and / or formation of hair follicle primordia by co-culture with mesenchymal cells).
- the epithelial cell may be a cell derived from hair follicle tissue (for example, the outermost layer of the outer root sheath of the bulge region of the hair follicle tissue, and / or the hair matrix), and is a cell derived from skin tissue.
- it may be a cell derived from a stem cell (eg, iPS stem cell, ES cell, or EG cell) in vitro.
- the epithelial cells may be primary cells collected from a living body or may be pre-cultured cells (eg, subcultured cells and / or established cells). Epithelial cells are identified, for example, as cells that express cytokeratin. The epithelial cells are preferably epithelial stem cells. Epithelial stem cells are identified as cells that express, for example, cytokeratin 15 and / or CD34.
- the epithelial cells are preferably derived from humans, but may be derived from non-human animals (animals other than humans).
- the non-human animal is not particularly limited, but it is preferably a non-human vertebrate (vertebrate other than human).
- the non-human vertebrate is not particularly limited, but it is preferably a non-human mammal.
- Non-human mammals include, but are not limited to, primates (eg, monkeys), rodents (eg, mice, rats, hamsters, guinea pigs, rabbits), meats (eg, dogs, cats), or ungulates. (For example, pig, cow, horse, goat, sheep).
- the co-culture of mesenchymal cells and epithelial cells is performed by mixing mesenchymal cells recovered after being cultured while applying an electric current and epithelial cells prepared separately from the mesenchymal cells. Culture.
- the method of co-culture is not particularly limited as long as it is a method in which mesenchymal cells and epithelial cells aggregate to form hair follicle primordia, but, for example, the mesenchymal cells and the epithelial cells are It is preferred to form hair follicle primordia by co-culturing on a non-adhesive surface.
- the cell non-adhesive surface is not particularly limited as long as it is a surface to which mesenchymal cells and epithelial cells do not substantially adhere. That is, the cell non-adhesive surface is, for example, a surface in which mesenchymal cells and epithelial cells are not adhered and is maintained in a floating state, or the mesenchymal cells and epithelial cells are loosely adhered, but treated with trypsin. It is a surface on which the mesenchymal cells and epithelial cells are easily detached by an operation of flowing a culture solution such as pipetting without performing enzyme treatment such as.
- the culture container having a cell-non-adhesive surface for example, a commercially available multi-well plate having a cell-non-adhesive coating on the bottom surface of each well can be used.
- the micro intaglio described in International Publication No. 2017/073625 can also be preferably used.
- the mesenchymal cells and the epithelial cells are co-culturing the mesenchymal cells and the epithelial cells (for example, the mesenchymal cells and the epithelial cells are dispersed and mixed in a culture solution and cultured), the mesenchymal cells and the epithelial cells are
- the hair follicle primordia are formed by spontaneous aggregation.
- the hair follicle primordium formed is, for example, a mesenchymal cell aggregation portion formed by spontaneously aggregating mesenchymal cells with each other, and is formed by spontaneously aggregating epithelial cells with each other, and A part of the mesenchymal cell agglutination site is combined with the epithelial cell agglutination site.
- the mesenchymal cell agglutinating part constitutes the inner core part
- the epithelial cell agglutinating part may constitute the outer layer covering the outer periphery of the mesenchymal cell agglomerate ( So-called core-shell hair follicle primordia).
- mesenchymal cells and epithelial cells may be dispersed and arranged (so-called random type hair follicle primordia).
- non-adherent hair follicle primordia are produced on the non-cell-adhesive surface.
- the hair follicle primordia in the non-adhesive state is a hair follicle primordium in a floating state that does not adhere to the cell non-adhesive surface, or loosely adheres to the cell non-adhesive surface, but is not treated with trypsin or the like. It is a hair follicle primordium that is easily detached from the cell non-adhesive surface by an operation of flowing a culture solution such as pipetting without performing enzyme treatment.
- the hair follicle primordia formed in this method may be, for example, hair follicle primordia spheroids.
- Hair follicle primordia spheroids are roughly spherical cell aggregates. Hair follicle primordia spheroids are formed in a non-adherent state and are easily recovered. Therefore, the hair follicle primordia spheroid is preferably used for transplantation into a living body.
- the hair follicle primordia formed by the co-culture in this method are formed by the same method except that the mesenchymal cells produced by the same method as the activated mesenchymal cells are used except that no current is applied.
- the activated hair follicle primordia contains activated epithelial cells as compared to the epithelial cells.
- mesenchymal cells cultured while applying a current by this method are called activated mesenchymal cells, and were cultured in the same manner as the activated mesenchymal cells except that no current was applied.
- the mesenchymal cells are called control mesenchymal cells
- the hair follicle primordium formed by co-culture of the control mesenchymal cells and epithelial cells is called control hair follicle primordia
- the activated mesenchyme The hair follicle primordia formed by the co-culture of lineage cells and epithelial cells are control hairs formed by the same method except that the control mesenchymal cells are used in place of the activated mesenchymal cells. It contains activated epithelial cells as compared to the epithelial cells contained in the envelope.
- epithelial cells co-cultured with mesenchymal cells cultured while applying an electric current by this method were co-cultured with mesenchymal cells cultured under the same conditions except that the current was not applied. It is activated compared to epithelial cells.
- activated mesenchymal cells it is possible to produce hair follicle primordia containing activated epithelial cells as compared with the case of using control mesenchymal cells.
- activation of epithelial cells means improvement of hair growth-related properties of the epithelial cells.
- the improvement of hair growth-related properties of epithelial cells is, for example, an increase in the expression level of hair growth-related genes of the epithelial cells, and / or a hair follicle progenitor formed using the epithelial cells.
- Including improved hair growth properties of the group eg, increased number and / or length of hair that grows from the hair follicle primordium implanted in the body).
- the activated epithelial cells contained in the hair follicle primordium formed by the present method are, for example, activated mesenchymal cells except that the expression level of at least one hair growth-related gene is not applied with current. Except that mesenchymal cells produced by the same method as described above were used, the number of epithelial cells included in the hair follicle primordium formed by the same method was higher than that of the epithelial cells.
- the hair growth-related gene expressed in epithelial cells is not particularly limited as long as it is a gene related to the contribution of the epithelial cells to hair growth.
- Wnt10b, LEF1 (Lymphoid Enhancer Factor-1), Shh ( Sonic hedgehog) and ⁇ -catenin may be one or more selected from the group consisting of.
- the activated epithelial cells contained in the hair follicle primordia produced by the present method are the same method except that the expression level of the Wnt10b gene measured by RT-PCR is the same except that no current is applied. It may be 1.5 times or more, 2.0 times or more, and 2.5 times or more that of the epithelial cells co-cultured with the cultured mesenchymal cells. Or may be 3.0 times or more.
- the hair follicle primordia formed by the co-culture in the present method are the same method except that the mesenchymal cells produced by the same method as the activated mesenchymal cells are used except that the current is not applied.
- the activated mesenchymal cells are included as compared with the mesenchymal cells contained in the hair follicle primordia formed in (1). That is, the activated mesenchymal cells produced by this method maintain the activated state even after forming hair follicle primordia by co-culture with epithelial cells.
- activated mesenchymal cells contained in the hair follicle primordia formed by this method are mesenchymal cells produced by the same method as activated mesenchymal cells except that no current is applied.
- mesenchymal cells in which the expression level of at least one hair growth-related gene is increased compared to the mesenchymal cells contained in the hair follicle primordia formed by the same method except that cells were used. is there.
- the activated mesenchymal cells contained in the hair follicle primordia produced by the present method are the same method except that the expression level of the Versican gene measured by RT-PCR is the same as that when no current is applied. It may be 1.5 times or more, and may be 2.0 times or more, that of the mesenchymal cells that have been cultivated in (1) and have formed the hair follicle primordia.
- activated mesenchymal cells contained in the hair follicle primordia produced by this method are cultured by the same method except that the ALP gene expression level measured by RT-PCR is not applied with current. It may be 1.5 times or more, 2.0 times or more, or 2.5 times or more that of the mesenchymal cells forming the hair follicle primordia. , 3.0 times or more.
- the production of the hair follicle primordia by the present method is the production of the hair follicle primordia containing activated epithelial cells and / or activated mesenchymal cells, that is, activated. It can be said that it is the production of hair follicle primordia.
- the hair follicle primordia produced by this method can be directly used for research purposes, but are also preferably used for transplantation into a living body. That is, according to this method, a hair follicle primordia that produces hair by being transplanted into a living body is produced.
- the living body is not particularly limited and may be a human or a non-human animal.
- the non-human animal is not particularly limited, but it is preferably a non-human mammal.
- Non-human mammals include, but are not limited to, for example, primates (eg, monkeys), rodents (eg, mice, rats, hamsters, guinea pigs, rabbits), meats (eg, dogs, cats), or It may be a hoof (eg, pig, cow, horse, goat, sheep).
- the transplantation of the hair follicle primordia into the living body is preferably transplantation into the skin of the living body.
- the transplant to the skin may be, for example, subcutaneous transplant or intradermal transplant.
- hair follicle primordia and their transplantation into animals may be for medical or research purposes. That is, for example, for the treatment or prevention of a disease associated with hair loss, a hair follicle progenitor is produced for the purpose of transplanting into a human patient suffering from or possibly suffering from the disease, or the hair follicle progenitor is It may be transplanted to the human patient.
- the diseases associated with hair loss are not particularly limited, and examples thereof include androgenetic alopecia (AGA), female androgenetic alopecia (Female Androgenetic Alopecia: FAGA), postpartum alopecia, diffuse alopecia, and seborrhea.
- Alopecia degeneration, alopecia pityroides, tractive alopecia, dysmetabolic alopecia, compressive alopecia, alopecia areata, neuronal alopecia, alopecia, systemic alopecia, and symptomatic alopecia It may be one or more selected from the group.
- a hair follicle progenitor is produced, or the hair follicle precursor is produced.
- the group may be transplanted to a non-human animal.
- a culture device having the same structure as the culture device 1 shown in FIG. 1 was manufactured as follows. First, the substrate was washed. That is, a cleaning solution was prepared by mixing an ammonia solution (25%) and a hydrogen peroxide solution (30%) at a volume ratio of 3: 1. Then, the slide glass used as the substrate was immersed in a cleaning liquid boiled at 300 ° C. for 5 minutes to remove impurities attached to the surface of the slide glass. Then, the operation of immersing the slide glass in ultrapure water boiled at 300 ° C. for 5 minutes was repeated twice to wash away the cleaning liquid.
- an electrode for culturing mesenchymal cells on the surface was prepared. That is, a chromium thin film was formed on the surface of the slide glass washed as described above by using a sputtering device, and further a gold thin film was formed on the chromium thin film.
- the slide glass on which the gold thin film is formed is immersed in a mixed solution of a 0.1 M polypyrrole solution and a 0.1 M p-toluenesulfonic acid solution, and a current of 0.25 mA / cm 2 is applied to the mixed solution for 4 minutes. Then, a polypyrrole film (conductive polymer film) was formed on the gold thin film. Then, the slide glass on which the polypyrrole film was formed was washed with ultrapure water and air-dried.
- a culture well was prepared. That is, by adhering a 4-well chamber (Lab Tek II Chamber Slide) to a part of the surface of the slide glass on which the polypyrrole film has been formed, the frame (partition) of the 4-well chamber is partitioned and the polypyrrole film is separated.
- Four culture wells were formed with a bottom consisting of the formed culture surface.
- each culture well was washed with ethanol for sterilization. Then, a 0.03% collagen type I solution was added to each culture well and air-dried to form a collagen thin film on the polypyrrole film on the bottom surface of the culture well. In this way four culture surfaces were obtained, each area being about 2 cm 2 .
- a resin base material of a size that closes the upper opening of each culture well was prepared, and one surface of the resin base material was covered with a platinum mesh.
- this resin substrate was used as a lid for the culture wells, and the platinum mesh surface was fitted into the upper opening of each culture well so that the platinum mesh surface faces the bottom surface of each culture well. .
- a galvanostat device was used as the power source. That is, on the surface of the slide glass on which the polypyrrole film is formed, the portion where the culture well is not formed (the portion where the frame body of the 4-well chamber is not arranged) is electrically connected to the power source through the metal wiring. The platinum mesh of each of the four resin substrates used as lids for the four culture wells was electrically connected to the power supply via metal wiring. Thus, a culture device used for culturing mesenchymal cells was manufactured.
- Hair papilla cells were used as the mesenchymal cells. That is, commercially available human dermal papilla cells (PromoCell) were subcultured, and the human dermal papilla cells of the 4th to 6th passages (P4 to P6) were used for the experiment. As the culture medium, a commercially available culture medium for dermal papilla cells (PromoCell) was used.
- Example 1-1 dermal papilla cells were seeded in each culture well, and the dermal papilla cells were cultured on the bottom surface (culture surface of the culture electrode) of each culture well for 3 days. During this culture period, a pulse current was applied to each culture well for 16 minutes from the time point 24 hours after seeding the hair papilla cells.
- pulse current stimulation (1) first, a current of “+200 ⁇ A (current value per unit area of culture surface: about +100 ⁇ A / cm 2 )” was applied to each culture chamber for 0.05 seconds. Then, (2) then a current of “ ⁇ 200 ⁇ A (current value per unit area of the culture surface: about ⁇ 100 ⁇ A / cm 2 )” was applied for 0.05 seconds, and (3) thereafter, the current was not applied ( A current of 0 ⁇ A) for 0.1 second was applied as a pulse of the (1), (2) and (3).
- Comparative Example 1-1 dermal papilla cells were cultured for 3 days by the same method except that no current was applied. Further, as Comparative Example 1-2, dermal papilla cells were cultured for 3 days by the same method except that no current was applied, using the culture apparatus manufactured by the same method except that the polypyrrole film was not formed.
- each culture well was washed with a phosphate buffer (PBS). Then, the stained dermal papilla cells were observed with a phase contrast microscope and a fluorescence microscope.
- PBS phosphate buffer
- Example 1-1 As a result of observing the stained hair papilla cells with a phase-contrast microscope and a fluorescence microscope, it was found that almost all hairs in Example 1-1, Comparative Example 1-1 and Comparative Example 1-2 were irrespective of the presence or absence of electrical stimulation.
- the papillary cells were stained with FDA, which confirmed that they were alive. That is, it was confirmed that the pulsed current stimulation used in this experiment did not affect the survival of dermal papilla cells.
- Hair papilla cells were used as the mesenchymal cells. That is, commercially available human dermal papilla cells (PromoCell) were subcultured, and the human dermal papilla cells of P4 to P6 were used for the experiment. A culture medium for dermal papilla cells (PromoCell) was used as the culture medium.
- Example 2-1 1.2 ⁇ 10 4 hair papilla cells were seeded in each culture well, and the hair papilla cells were cultured on the bottom surface (culture surface of the culture electrode) of each culture well for 3 days. did.
- Example 2-1 the timing of starting to apply the pulse current (5 hours, 29 hours, 53 hours after seeding the hair papilla cells), the magnitude of the pulse current to be applied (0 ⁇ A) (0 ⁇ A / cm 2 -culture surface), 45 ⁇ A (22.5 ⁇ A / cm 2 ), 100 ⁇ A (50 ⁇ A / cm 2 ), 200 ⁇ A (100 ⁇ A / cm 2 ), and 300 ⁇ A (150 ⁇ A / cm 2 )), continue pulse current
- the hair papilla cells were cultured while applying a pulsed current under a plurality of conditions in which the application time (16 minutes, 3 hours, 24 hours) was different.
- Comparative Example 2-1 dermal papilla cells were cultured for 3 days by the same method except that no current was applied.
- PCR analysis of hair growth-related genes The gene expression level of dermal papilla cells cultured for 3 days was analyzed by RT-PCR. Regarding the nucleotide sequences of the primers used in RT-PCR, for ALP (alkaline phosphatase), Forward Primer was “5′-ATTGACCACGGGCACCAT-3 ′” and Reverse Primer was “5′-CTCCACCGCCTCATGCA-3 ′”, which was used as a control. Regarding GAPDH, the Forward Primer was “5′-GCACCGTCAAGGCTgAGAAC-3 ′”, and the Reverse Primer was “5′-TGGTGAAGACGCCACGTGGA-3 ′”.
- ALP alkaline phosphatase
- FIG. 2A shows the results of analysis of the expression level of ALP gene in dermal papilla cells cultured while changing the timing at which the pulse current is applied. Specifically, FIG. 2A shows the timing when the pulse current was started to be applied to the papilla cells in Comparative Example 2-1 (“Control” in the figure) in which no current was applied and Example 2-1. , An example that was 5 hours after the hair papilla cells were seeded (“Day 0” in the figure), an example that was 29 hours (“Day 1” in the figure), and 53 For each of the examples (“Day 2” in the figure) at which time has elapsed, the relative gene expression level (vertical in the figure) when the gene expression level in Comparative Example 2-1 is set to “1”. Axis "Relative gene expression").
- FIG. 2B shows the results of analysis of ALP gene expression in dermal papilla cells cultured by changing the magnitude of applied pulse current.
- the magnitude of the pulse current applied to the dermal papilla cells in Comparative Example 2-1 (“Control” in the figure) in which no current was applied and Example 2-1 were different.
- the pulse current was applied for 16 minutes from the time point when 29 hours had elapsed after seeding the hair papilla cells.
- a pulse current (1) first, a positive current having a predetermined value (for example, a current of “+45 ⁇ A (about +22.5 ⁇ A / cm 2 )”) is applied for 0.05 seconds, and (2) then a predetermined current is applied.
- a negative current of a value (for example, a current of “ ⁇ 45 ⁇ A (about ⁇ 22.5 ⁇ A / cm 2 )”) is applied for 0.05 seconds, and (3) after that, no current is applied (0 ⁇ A) for 0.1 seconds.
- An electric current was applied to hold (1), (2), and (3) as one pulse.
- the expression level of ALP gene in dermal papilla cells was increased as compared with the case where no current was applied.
- the expression level of ALP gene in dermal papilla cells was significantly increased as compared with the case where no current was applied.
- FIG. 2C shows the results of analysis of the ALP gene expression level of dermal papilla cells cultured by changing the duration of continuous pulse current application (which is also the total duration of pulse current application during the culture period).
- the duration of continuous pulse current application which is also the total duration of pulse current application during the culture period.
- the time when the pulse current was applied to the hair papilla cells was 16 minutes.
- the comparative example for each of the examples (“16 min” in the figure), 3 hours (“3h” in the figure), and 24 hours (“24h” in the figure)
- the relative gene expression level (the vertical axis "Relative gene expression” in the figure) is shown when the gene expression level in 2-1 is "1".
- the expression level of ALP gene in the hair papilla cells was significantly increased as compared with the case where the current was not applied.
- Hair papilla cells were used as the mesenchymal cells. That is, commercially available human dermal papilla cells (PromoCell) were subcultured, and the human dermal papilla cells of P4 to P6 were used for the experiment. A culture medium for dermal papilla cells (PromoCell) was used as the culture medium.
- Example 3-1 1.2 ⁇ 10 4 hair papilla cells were seeded in each culture well, and the hair papilla cells were cultured on the bottom surface (culture surface of the culture electrode) of each culture well for 3 days. did. During this culture period, a pulse current of 200 ⁇ A (100 ⁇ A / cm 2 ) was applied to each culture well for 16 minutes from the point 29 hours after seeding the hair papilla cells.
- the hair papilla cells and the epithelial cells are mixed so that the cell density of the hair papilla cells becomes 5 ⁇ 10 3 cells / well and the cell density of the epithelial cells becomes 1 ⁇ 10 4 cells / well. And seeded into each well of a 96-multiwell plate.
- a mixed medium prepared by mixing mesenchymal cell culture medium (DMEM + 10% FBS + 1% penicillin / streptomycin) with HuMedia-KG2 at a volume ratio of 1: 1 was used.
- this hair follicle primordium was formed by culturing hair papilla cells and epithelial cells in a mixed state for 3 days. That is, as the culture time passed, the cells spontaneously aggregated in each well to form spherical and floating hair follicle primordia.
- this hair follicle primordia includes a hair papilla cell aggregation portion formed mainly by aggregation of hair papilla cells, and an epithelial cell aggregation portion formed mainly by aggregation of epithelial cells. A part of the hair papilla cell aggregation part and a part of the epithelial cell aggregation part were bonded.
- Comparative Example 3-1 the same method was used except that hair papilla cells cultured by the same method were used in place of applying no current instead of the hair papilla cells cultured while applying current.
- the hair follicle primordium was formed by co-culturing in.
- PCR analysis of hair growth-related genes The gene expression level of the hair follicle primordium formed by the culture for 3 days was analyzed by RT-PCR.
- the nucleotide sequences of the primers used in the RT-PCR were “5′-CCAGCAAGCACAAAATTTTCA-3 ′” for Reverse Primer, “5′-TGCACTGGATCTGTTTCTTCA-3 ′” for Reverse Primer, and LEF1 (Lymphr enhance Factor-1 ),
- Forward Primer is “5′-CCCGATGACGGAAAGCAT-3 ′”
- Reverse Primer is “5′-TCGAGTAGGAGGGTCCCTTGT-3 ′”
- ALP Forward Primer is “5′-CATravermCGergic”.
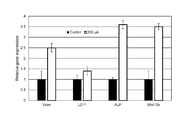
- FIG. 3 shows the results of analysis of gene expression levels in the hair follicle primordia.
- each of the genes (Versican, LEF1, ALP, and Wnt10b) shown on the horizontal axis was formed using dermal papilla cells cultured in Comparative Example 3-1 without applying an electric current.
- the expression level (black bar graph) of the hair follicle primordium (“Control” in the figure) thus treated was set to “1”
- hair papilla cells cultured by applying an electric current in Example 3-1 were used.
- the expression level of the hair follicle primordium (“200 ⁇ A” in the figure) formed using the above method (vertical axis “Relative gene expression” in the figure) (open bar graph) is shown.
- both Versican and ALP which are characteristic of dermal papilla cells, showed that the gene expression level of the hair follicle primordia containing dermal papilla cells cultured by applying an electric current was It was significantly larger than that of cultured hair follicle primordia containing hair papilla cells.
- the hair papilla cells cultured by applying an electric current are not only not only formed before the formation of hair follicle primordia after the culture, but also after formation of the hair follicle primordia by co-culture with epithelial cells. It was confirmed that the expression level of hair-growth-related genes was increased in the hair follicle primordia.
- LEF1 and Wnt10b which are characteristic of epithelial cells, were also cultured without application of current, as the gene expression level of epithelial cells that formed hair follicle primordia along with hair papilla cells cultured with current application. It was larger than that of epithelial cells that formed hair follicle primordia with hair papilla cells, and in particular, the gene expression level of Wnt10b was remarkably large.
- the epithelial cells that formed hair follicle primordia by co-culture with hair papilla cells cultured by applying an electric current showed a marked increase in the expression level of the hair growth-related gene in the hair follicle primordia. That is, an unexpected remarkable effect was confirmed.
- FIGS. 4A and 4B respectively show hair follicle primordia formed using hair papilla cells cultured by applying an electric current and hair papilla cells cultured without applying an electric current.
- Fig. 3 shows a photograph of hair regenerated from the hair follicle primordia taken 30 days after the transplantation of the hair follicle primordia into the mouse.
- a hair follicle primordia formed using hair papilla cells cultured by applying an electric current (“Example 3-1” in the figure), and cultured without applying an electric current.
- Hair follicle primordia formed by using dermal papilla cells (“Comparative Example 3-1” in the figure) 30 days after transplantation into mice, The results of counting the number of hairs (vertical axis “number of hair growth” in the figure) are shown.
- the number of hairs formed from the hair follicle primordia formed using hair papilla cells cultured by applying an electric current was about 40.
- the number of hairs formed from the hair follicle primordia formed by using hair papilla cells cultured without applying an electric current was about 18 It was a book.
- the hair follicle primordia produced using hair papilla cells cultured by applying an electric current compared with the hair follicle primordia formed using hair papilla cells cultured without applying an electric current. It was confirmed that the hair growth ability when transplanted into a living body was remarkably excellent.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Zoology (AREA)
- Organic Chemistry (AREA)
- Biotechnology (AREA)
- Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Genetics & Genomics (AREA)
- Wood Science & Technology (AREA)
- Developmental Biology & Embryology (AREA)
- Microbiology (AREA)
- Cell Biology (AREA)
- Biochemistry (AREA)
- General Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Rheumatology (AREA)
- Dermatology (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2018201545A JP7246629B2 (ja) | 2018-10-26 | 2018-10-26 | 間葉系細胞の培養方法、活性化間葉系細胞の製造方法、毛包原基の製造方法、間葉系細胞の活性化方法、及び上皮系細胞の活性化方法 |
| JP2018-201545 | 2018-10-26 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2020085023A1 true WO2020085023A1 (ja) | 2020-04-30 |
Family
ID=70331106
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2019/038934 Ceased WO2020085023A1 (ja) | 2018-10-26 | 2019-10-02 | 間葉系細胞の培養方法、活性化間葉系細胞の製造方法、毛包原基の製造方法、間葉系細胞の活性化方法、及び上皮系細胞の活性化方法 |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JP7246629B2 (enExample) |
| WO (1) | WO2020085023A1 (enExample) |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2020099793A (ja) * | 2020-04-01 | 2020-07-02 | 株式会社三洋物産 | 遊技機 |
| JP2020099794A (ja) * | 2020-04-01 | 2020-07-02 | 株式会社三洋物産 | 遊技機 |
| US20250163382A1 (en) * | 2022-03-01 | 2025-05-22 | National University Corporation Yokohama National University | Method for producing proliferated hair follicle mesenchymal cells, and use thereof |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017073625A1 (ja) * | 2015-10-30 | 2017-05-04 | 国立大学法人横浜国立大学 | 再生毛包原基の集合体の製造方法、毛包組織含有シート、及び毛包組織含有シートの製造方法 |
| JP2018082638A (ja) * | 2016-11-21 | 2018-05-31 | 国立大学法人横浜国立大学 | 毛細血管構造を有する再生毛包原基の集合体の製造方法、毛包組織含有シート、及び毛包組織含有シートの製造方法 |
-
2018
- 2018-10-26 JP JP2018201545A patent/JP7246629B2/ja active Active
-
2019
- 2019-10-02 WO PCT/JP2019/038934 patent/WO2020085023A1/ja not_active Ceased
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017073625A1 (ja) * | 2015-10-30 | 2017-05-04 | 国立大学法人横浜国立大学 | 再生毛包原基の集合体の製造方法、毛包組織含有シート、及び毛包組織含有シートの製造方法 |
| JP2018082638A (ja) * | 2016-11-21 | 2018-05-31 | 国立大学法人横浜国立大学 | 毛細血管構造を有する再生毛包原基の集合体の製造方法、毛包組織含有シート、及び毛包組織含有シートの製造方法 |
Non-Patent Citations (2)
| Title |
|---|
| AIZAWA, MASUO: "New-Production by Electrically Controlled Cell Culture", JOURNAL OF SYNTHETIC ORGANIC CHEMISTRY, vol. 48, no. 6, 1990, Japan, pages 571 - 576, XP055709114 * |
| BLOISE, N. ET AL.: "The effect of pulsed electromagnetic field exposure on osteoinduction of human mesenchymal stem cells cultured on nano- Ti02 surfaces", PLOS ONE, vol. 13, no. 6, 14 June 2018 (2018-06-14), pages 1/19 - 19/19, XP081620213 * |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2020065503A (ja) | 2020-04-30 |
| JP7246629B2 (ja) | 2023-03-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5932671B2 (ja) | ガイドを有する移植用再生器官原基の製造方法、当該方法によって製造される、ガイドを有する移植用再生器官原基を含む組成物、およびガイドを有する移植用再生器官原基の移植方法 | |
| JP5887333B2 (ja) | 毛色が制御された移植用再生毛包原基の製造方法、当該移植用再生毛包原基を含む組成物、およびその移植方法 | |
| Martins et al. | The current status of iPS cells in cardiac research and their potential for tissue engineering and regenerative medicine | |
| EP3124600A1 (en) | Method for fabricating cell aggregate for self-organization | |
| Rak-Raszewska et al. | Organ in vitro culture: what have we learned about early kidney development? | |
| WO2020085023A1 (ja) | 間葉系細胞の培養方法、活性化間葉系細胞の製造方法、毛包原基の製造方法、間葉系細胞の活性化方法、及び上皮系細胞の活性化方法 | |
| JP7427180B2 (ja) | 毛包原基及びその製造方法 | |
| WO2023058429A1 (ja) | 毛髪再生能を有する細胞凝集塊の製造方法及びこれに関連する方法 | |
| JP7444367B2 (ja) | 増幅毛包間葉系細胞の製造方法及びその使用 | |
| JP7246595B2 (ja) | 毛包原基、毛包原基の製造方法、及び毛包原基に含まれる細胞の活性化方法 | |
| JP2023063011A (ja) | 間葉系細胞及び/又は毛包原基の活性化に関する方法及びキット | |
| Bidez III et al. | Enhanced cardiac differentiation of mouse embryonic stem cells by electrical stimulation | |
| JP2023056592A (ja) | 移植用毛様組織及びこれに関連する方法 | |
| Foldes et al. | Differentiation and Use of Cardiomyocytes Derived from Human Pluripotent Stem Cells | |
| Luna | Biomimetic Components Aiming to Improve in vitro Cardiac Culture Systems as Building Blocks to Reshape Failing Hearts | |
| Cormack | Studies on the cardiomyocytic potential of dermal stem cells |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 19874810 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 19874810 Country of ref document: EP Kind code of ref document: A1 |