WO2020004649A1 - 被膜形成組成物 - Google Patents
被膜形成組成物 Download PDFInfo
- Publication number
- WO2020004649A1 WO2020004649A1 PCT/JP2019/025927 JP2019025927W WO2020004649A1 WO 2020004649 A1 WO2020004649 A1 WO 2020004649A1 JP 2019025927 W JP2019025927 W JP 2019025927W WO 2020004649 A1 WO2020004649 A1 WO 2020004649A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- acid
- extract
- group
- hair
- comparative example
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 0 C*C(C(*)=O)NC(C(*)*C(*)=O)=C Chemical compound C*C(C(*)=O)NC(C(*)*C(*)=O)=C 0.000 description 2
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/33—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing oxygen
- A61K8/36—Carboxylic acids; Salts or anhydrides thereof
- A61K8/362—Polycarboxylic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/64—Proteins; Peptides; Derivatives or degradation products thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/02—Cosmetics or similar toiletry preparations characterised by special physical form
- A61K8/04—Dispersions; Emulsions
- A61K8/042—Gels
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/33—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing oxygen
- A61K8/36—Carboxylic acids; Salts or anhydrides thereof
- A61K8/361—Carboxylic acids having more than seven carbon atoms in an unbroken chain; Salts or anhydrides thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/33—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing oxygen
- A61K8/36—Carboxylic acids; Salts or anhydrides thereof
- A61K8/365—Hydroxycarboxylic acids; Ketocarboxylic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q17/00—Barrier preparations; Preparations brought into direct contact with the skin for affording protection against external influences, e.g. sunlight, X-rays or other harmful rays, corrosive materials, bacteria or insect stings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q5/00—Preparations for care of the hair
- A61Q5/02—Preparations for cleaning the hair
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q5/00—Preparations for care of the hair
- A61Q5/12—Preparations containing hair conditioners
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K5/00—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof
- C07K5/04—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing only normal peptide links
- C07K5/06—Dipeptides
- C07K5/06008—Dipeptides with the first amino acid being neutral
- C07K5/06017—Dipeptides with the first amino acid being neutral and aliphatic
- C07K5/06026—Dipeptides with the first amino acid being neutral and aliphatic the side chain containing 0 or 1 carbon atom, i.e. Gly or Ala
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D177/00—Coating compositions based on polyamides obtained by reactions forming a carboxylic amide link in the main chain; Coating compositions based on derivatives of such polymers
- C09D177/04—Polyamides derived from alpha-amino carboxylic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q1/00—Make-up preparations; Body powders; Preparations for removing make-up
- A61Q1/02—Preparations containing skin colorants, e.g. pigments
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
- A61Q19/10—Washing or bathing preparations
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q5/00—Preparations for care of the hair
- A61Q5/06—Preparations for styling the hair, e.g. by temporary shaping or colouring
Definitions
- the present invention relates to a film-forming composition, and more particularly, to a film-forming composition containing at least one lipid peptide type compound.
- a film formed on the skin or on the surface of the hair provides an effective barrier on the skin or on the surface of the hair, and thus suppresses evaporation of moisture in the skin or the hair, It plays various important roles in personal care products, such as enhancing the retention of active ingredients.
- ⁇ ⁇ ⁇ Recent health awareness, especially dry skin awareness, has led to a demand for a cosmetic composition having a high moisturizing effect.
- skin care preparations that exhibit a high moisturizing effect include coating cosmetics having a self-organized structure, such as those using a layered ⁇ -gel (Patent Document 1).
- the interlamellar lipids in the stratum corneum which is the outermost layer of the skin, also have a self-assembled lamellar structure, which suppresses invasion of substances from the outside and evaporation of moisture from the inside into the skin. It has the function of keeping moisturizing properties and skin flexibility.
- Patent Documents 2 and 3 hair preparations using polypeptides have been proposed.
- JP 2016-6030 A JP-A-10-77210 JP-A-2002-308756
- the layered ⁇ -gel In order to form the layered ⁇ -gel, it is necessary to mix a plurality of components at a specific ratio. Therefore, when the layered ⁇ -gel is used in combination with another component, the plurality of components and the other component are mixed with each other. Adjustment of the mixing ratio may be complicated. In addition, when a polymer such as a polypeptide is used as a film-forming agent, there is a sense of sharpness peculiar to the polymer.
- An object of the present invention is to provide a composition that can more easily form a film having excellent moisture retention. Further, an object of the present invention is to provide a composition capable of forming a coating film which has almost no feeling of crispness and has improved usability.
- the present inventors have found that a film is formed on the skin or the hair surface only by using at least one kind of lipid peptide type compound. 1. Excellent moisture retention. 2. suppression of moisture adsorption; 3. Promote penetration into the hair; 4. Adsorption of negatively charged components; The inventors have found that coalescence of oil components is suppressed, and have completed the present invention. In addition, the present inventors have found that the above-mentioned coating film has almost no crispness and the like, and has a good feeling of use, and thus completed the present invention.
- the present invention as a first aspect, relates to a film-forming composition
- a film-forming composition comprising at least one lipid peptide-type compound comprising a low-molecular lipid peptide or a pharmaceutically usable salt thereof.
- the lipid peptide-type compound is a compound in which a peptide portion having a repeating amino acid bonding structure is bonded to a lipid portion comprising an aliphatic group having 9 to 23 carbon atoms.
- the lipid peptide-type compound comprises at least one of the compounds represented by the following formulas (1) to (3) or a pharmaceutically usable salt thereof, A film-forming composition according to the second aspect.
- R 1 represents an aliphatic group having 9 to 23 carbon atoms
- R 2 represents a hydrogen atom or an alkyl group having 1 to 4 carbon atoms which may have a branched chain having 1 or 2 carbon atoms
- R 3 represents a — (CH 2 ) n —X group, n represents a number of 1 to 4, and X represents 1 to 3 amino groups, guanidino groups, —CONH 2 groups, or 1 to 3 nitrogen atoms.
- R 4 represents an aliphatic group having 9 to 23 carbon atoms
- R 5 to R 7 each independently represent a hydrogen atom or a carbon atom which may have a branched chain having 1 or 2 carbon atoms.
- R 8 represents an aliphatic group having 9 to 23 carbon atoms
- R 9 to R 12 each independently represent a hydrogen atom or a carbon atom which may have a branched chain having 1 or 2 carbon atoms.
- the present invention relates to a method for producing a film, comprising using the film-forming composition according to any one of the first to third aspects.
- the present invention relates to a film formed using the film-forming composition according to any one of the first to third aspects.
- a film excellent in moisture retention can be formed by containing at least one lipid peptide type compound. That is, since it is not necessary to adjust the ratio of each component in the composition excessively, it is possible to easily form a film having excellent moisture retention. Further, according to the present invention, when blended in cosmetics or the like, excellent moisture retention can be imparted to cosmetics. Furthermore, according to the present invention, it is possible to form a coating film having a good feeling of use with almost no feeling of snapping.
- the effect of suppressing the reflection of light, the effect of having water repellency, the effect of having fingerability and combability, the effect of preventing makeup twist, and the luster are imparted.
- the film having at least one or more of the following effects can be formed.
- the composition of the present invention has high biosafety and is particularly useful for pharmaceuticals and cosmetics. Useful.
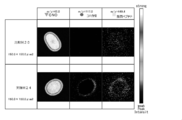
- FIG. 1 is a scanning electron microscope (SEM) image in Example 1. [(a) SEM image immediately after setting a sample on a scanning electron microscope (numbers in the figure are representative mineral oil droplets) Position)). (B) SEM image of the dried sample (numbers in the figure represent the positions of the mineral oil droplets shown in (a)). ]
- FIG. 2 is a photograph of the appearance of Example 3 and Comparative Example 3 [(a) is a photograph of the appearance of Example 3. (B) is an appearance photograph of Comparative Example 3. ].
- FIG. 3 is a scanning electron microscope (SEM) image in Example 3 and Comparative Example 3. [(a) SEM image of Example 3. (B) SEM image of Comparative Example 3. ].
- FIG. 1 is a scanning electron microscope (SEM) image in Example 1. [(a) SEM image immediately after setting a sample on a scanning electron microscope (numbers in the figure are representative mineral oil droplets) Position)). (B) SEM image of the dried sample (numbers in the figure
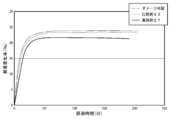
- FIG. 4 is a diagram showing the results of shampoo moisture retention tests (hair moisture retention) in Examples 4 to 8 and Comparative Example 4.
- FIG. 5 is a diagram showing the results of the contact angle measurement in Example 9 and Comparative Example 5 [(a) is a diagram showing the results of the contact angle measurement in Example 9; (B) It is a figure which shows the result of the contact angle measurement of the comparative example 5. ].
- FIG. 6 is a diagram showing the results (moisture retention of hair) of the conditioner moisture retention test in Example 10 and Comparative Example 6.
- FIG. 7 is a scanning electron microscope (SEM) image of the hair after the conditioner moisture retention test in Example 10 and Comparative Example 6. [(a) SEM image of Example 10. (B) SEM image of Comparative Example 6. ] FIG.
- SEM scanning electron microscope
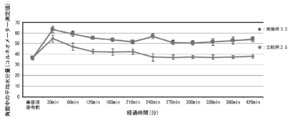
- FIG. 8 is a diagram showing measurement results of frictional resistance in Example 11 and Comparative Example 7.
- FIG. 9 is a diagram showing the results of the reflected light intensity measurement in Example 13 and Comparative Example 9.
- FIG. 10 is a photograph of the appearance of Example 14 and Comparative Example 10. [(a) A photograph of the appearance of Comparative Example 10. (B) A photograph of the appearance of Example 14. ].
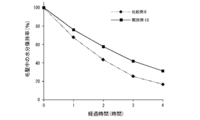
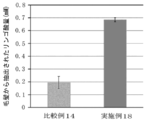
- FIG. 11 is a diagram showing the results of quantification of malic acid in Example 18 and Comparative Example 14.
- FIG. 12 is a scanning electron microscope (SEM) image of Example 19, Comparative Example 15, and the hair after the conditioner treatment on the damaged hair [(a) SEM image of damaged hair. (B) SEM image of Example 19. (C) An SEM image of Comparative Example 15. ]
- SEM scanning electron microscope
- FIG. 13 is a diagram showing the results of the dynamic friction coefficient measurement in Example 21 and Comparative Example 17.
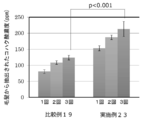
- FIG. 14 is a diagram showing the results of quantifying the amount of succinic acid in the conditioner-treated hair in Example 23 and Comparative Example 19.
- FIG. 15 is a graph showing the results of evaluation of the amounts of succinic acid and lipid peptides in human hair in Example 24 and Comparative Example 20.
- FIG. 16 is a diagram showing the evaluation results of the hardness of the conditioner-treated hair surface of Example 26, Comparative Example 22, and black and damaged hair.
- FIG. 17 is a diagram showing the results of evaluating water absorption and desorption after conditioner treatment on Example 27, Comparative Example 23, and damaged hair.
- FIG. 18 is a diagram showing the results of observing the time-dependent change of shine due to sebum floating in Example 28 and Comparative Example 24.
- FIG. 19 is a diagram illustrating the moisturizing effect of the serum in Example 30 and Comparative Example 26.
- FIG. 20 is a diagram showing the results of quantifying the amount of keratin in the hair treated with the conditioner in Example 33, Example 34, and Comparative Example 28.
- FIG. 21 is a scanning electron microscope (SEM) image of the hair of Example 36, Comparative Example 30, and damaged hair after the conditioner treatment [(a) SEM image of Example 36. (B) SEM image of Comparative Example 30. (C) SEM image of damaged hair. ]
- the present invention relates to a film-forming composition
- a film-forming composition comprising at least one lipid peptide-type compound comprising a low-molecular lipid peptide or a pharmaceutically usable salt thereof.
- the film-forming composition according to the present invention means all those which bring industrial utility value by utilizing film-forming properties, and in particular, for forming a film on the surface of skin or hair which is a film-forming object. It means a composition, but does not limit the application to paints, coating materials, sealers, primers, correction fluids and the like.
- the above-mentioned lipid peptide-type compound is preferably a compound in which a peptide portion having a repeating bonding structure of amino acids is bonded to a lipid portion composed of an aliphatic group having 9 to 23 carbon atoms.
- lipid peptide-type compound examples include compounds (lipid peptides) represented by the following formulas (1) to (3) or a pharmaceutically usable salt thereof (a lipid portion which is a hydrophobic portion and a hydrophilic portion). Or a low molecular weight compound having a peptide moiety as a site).
- R 1 represents an aliphatic group having 9 to 23 carbon atoms
- R 1 is a linear aliphatic group having 11 to 23 carbon atoms which can have 0 to 2 unsaturated bonds.
- Specific examples of the lipid moiety (acyl group) composed of R 1 and an adjacent carbonyl group include lauroyl, dodecylcarbonyl, myristoyl, tetradecylcarbonyl, palmitoyl, margaroyl, oleoyl, and elideyl.
- R 2 contained in the peptide moiety represents a hydrogen atom or an alkyl group having 1 to 4 carbon atoms and having a branched chain having 1 or 2 carbon atoms.
- the alkyl group having 1 to 4 carbon atoms which may have a branched chain having 1 or 2 carbon atoms means that the main chain has 1 to 4 carbon atoms and the branched chain having 1 or 2 carbon atoms.
- alkyl group which may have, and specific examples thereof include a methyl group, an ethyl group, an n-propyl group, an i-propyl group, an n-butyl group, an i-butyl group, a sec-butyl group and a tert-butyl group. And the like.
- R 2 is preferably a hydrogen atom or an alkyl group having 1 to 3 carbon atoms which may have a branched chain having 1 carbon atom, and more preferably a hydrogen atom.
- An alkyl group having 1 to 3 carbon atoms which may have a branched chain having 1 carbon atom means an alkyl group having 1 to 3 carbon atoms in the main chain and having a branched chain having 1 carbon atom.
- methyl group an ethyl group, an n-propyl group, an i-propyl group, an i-butyl group and a sec-butyl group, and preferably a methyl group, an i-propyl group, It is an i-butyl group or a sec-butyl group.
- R 3 represents a — (CH 2 ) nX group.
- n represents a number of 1 to 4
- X represents an amino group, a guanidino group, a —CONH 2 group, or a 5-membered ring having 1 to 3 nitrogen atoms or Represents a 6-membered ring or a fused heterocyclic ring composed of a 5-membered ring and a 6-membered ring.
- X is preferably an amino group, a guanidino group, a carbamoyl group (—CONH 2 group), a pyrrole group, an imidazole group, a pyrazole group, or an indole group. Preferably it is an imidazole group.
- n is preferably 1 or 2, and more preferably 1.
- the — (CH 2 ) n- group is preferably an aminomethyl group, a 2-aminoethyl group, a 3-aminopropyl group, a 4-aminobutyl group, a carbamoylmethyl group, a 2-carbamoylethyl group, or a 3-carbamoyl group.
- a lipid peptide particularly suitable as a lipid peptide type compound is a compound formed from the following lipid part and peptide part (amino acid assembly part).
- abbreviations of amino acids include alanine (Ala), asparagine (Asn), glutamine (Gln), glycine (Gly), histidine (His), isorosine (Ile), leucine (Leu), and lysine (Lysine). Lys), tryptophan (Trp), and valine (Val).
- Lauroyl-Gly-His Lauroyl-Gly-Gln, Lauroyl-Gly-Asn, Lauroyl-Gly-Trp, Lauroyl-Gly-Lys, Lauroyl-Ala-His, Lauroyl-Ala-Gln, Lauroyl-Ala-Asn, Lauroyl -Ala-Trp, Lauroyl-Ala-Lys; Myristoyl-Gly-His, Myristoyl-Gly-Gln, Myristoyl-Gly-Asn, Myristoyl-Gly-Trp, Myristoyl-Gly-Lys, Myristoyl-Ala-His, Myristoyl-Ala -Gln, myristoyl-Ala-Asn, myristoyl-Ala-Trp, myristoyl-Ala-Lys; palmitoyl-Gly-His, palmitoyl-Gly-Gln, palmi
- lauroyl-Gly-His lauroyl-Ala-His, myristoyl-Gly-His, myristoyl-Ala-His; palmitoyl-Gly-His, palmitoyl-Ala-His; stearoyl-Gly-His, stearoyl-Ala. -His.
- R 4 represents an aliphatic group having 9 to 23 carbon atoms, and specific preferred examples thereof include the same groups as those defined for R 1 above.
- R 5 to R 7 are each independently a hydrogen atom, an alkyl group having 1 to 4 carbon atoms, which may have a branched chain having 1 or 2 carbon atoms, or-( CH 2 ) nX, and at least one of R 5 to R 7 represents a — (CH 2 ) nX group.
- n a number from 1 to 4
- X represents an amino group, a guanidino group, a —CONH 2 group, or a 5- or 6-membered ring having 1 to 3 nitrogen atoms, or a 5- and 6-membered ring Represents a fused heterocyclic ring composed of
- preferred specific examples of R 5 to R 7 include the same groups as defined for R 2 and R 3 above.
- a preferred lipid peptide is a compound formed from the following lipid part and peptide part (amino acid assembly part). Lauroyl-Gly-Gly-His, Myristoyl-Gly-Gly-His, Myristoyl-Gly-Gly-Gln, Myristoyl-Gly-Gly-Asn, Myristoyl-Gly-Gly-Trp, Myristoyl-Gly-Gly-Lys, Myristoyl Gly-Ala-His, Myristoyl-Gly-Ala-Gln, Myristoyl-Gly-Ala-Asn, Myristoyl-Gly-Ala-Trp, Myristoyl-Gly-Ala-Lys, Myristoyl-Aly-Gly-His, Myristoyl-Ala- Gly-Gln, myristoyl-Ala-Gly-Asn, myristoyl-Ala-Asn, myristoyl-A
- lauroyl-Gly-Gly-His myristoyl-Gly-Gly-His, palmitoyl-Gly-Gly-His, palmitoyl-Gly-His-Gly, palmitoyl-His-Gly-Gly, stearoyl. -Gly-Gly-His.
- R 8 represents an aliphatic group having 9 to 23 carbon atoms, and preferred specific examples include the same groups as defined for R 1 above.
- R 9 to R 12 each independently represent a hydrogen atom, an alkyl group having 1 to 4 carbon atoms, which may have a branched chain having 1 or 2 carbon atoms, or-( CH 2 ) nX, and at least one of R 9 to R 12 represents a — (CH 2 ) nX group.
- n a number from 1 to 4
- X represents an amino group, a guanidino group, a —CONH 2 group, or a 5- or 6-membered ring having 1 to 3 nitrogen atoms, or a 5- and 6-membered ring Represents a fused heterocyclic ring composed of
- preferred specific examples of R 9 to R 12 include the same groups as defined for R 2 and R 3 above.
- preferred lipid peptide-type compounds include lauroyl-Gly-Gly-Gly-His, myristoyl-Gly-Gly-Gly-His, and palmitoyl-Gly-Gly-Gly-Gly-His. , Palmitoyl-Gly-Gly-His-Gly, palmitoyl-Gly-His-Gly-Gly, palmitoyl-His-Gly-Gly-Gly, stearoyl-Gly-Gly-Gly-Gly-Gly-His and the like.
- the content of the lipid peptide type compound is, for example, 0.01 to 30% by mass, preferably 0.02 to 10% by mass, more preferably 0.03% by mass, based on the total mass of the film-forming composition. To 5% by mass.
- the lipid peptide type compound at least one of the compounds (lipid peptides) represented by the above formulas (1) to (3) or pharmaceutically usable salts thereof may be used. More preferably, these compounds can be used alone or in combination of two or more.
- composition of the present invention may contain water, alcohol, polyhydric alcohol, or a mixed solution thereof in addition to at least one kind of the above-mentioned lipid peptide type compound.
- Examples of the water include purified water, purified water, hard water, soft water, natural water, deep sea water, electrolytic alkaline ionized water, electrolytic acidic ionized water, ionized water, and cluster water.
- the alcohol is a monohydric alcohol, for example, an alcohol having 1 to 6 carbon atoms which is dissolved in water at an arbitrary ratio, specifically, methanol, ethanol, 2-propanol, i-butanol and the like, and higher alcohols. Alcohol, specifically, oleyl alcohol, phenoxy alcohol and the like can be mentioned.
- the polyhydric alcohol is a dihydric or higher alcohol, such as propylene glycol, 1,3-butanediol, 2-ethyl-1,3-hexanediol, glycerin, isopentyldiol, ethylhexanediol, and erythrulose.
- a polyhydric alcohol when a polyhydric alcohol is contained, its content can be, for example, 1% by mass to 60% by mass, and preferably 1% by mass to 30% by mass, based on the total mass of the composition. In the present invention, when a polyhydric alcohol is contained, the polyhydric alcohol can be used alone or in combination of two or more.
- composition of the present invention can contain, if necessary, a cosmetic additive, a quasi-drug additive, an additive usable as a pharmaceutical additive, and the like.
- additional components such as physiologically active substances and functional substances to be added to skin external preparations such as cosmetics, quasi-drugs or pharmaceuticals include, for example, pigments, oily bases, humectants, feel improvers, surfactants other than the above.
- Agents polymers, thickening / gelling agents, solvents, antioxidants, reducing agents, oxidizing agents, preservatives, antibacterial agents, bactericides, chelating agents, pH adjusters, acids, alkalis, powders, inorganic salts, UV absorbers, whitening agents, vitamins and their derivatives, hair growth agents, blood circulation promoters, stimulants, hormones, anti-wrinkles, anti-aging agents, tightening agents, cooling agents, warming agents, promoting wound healing Agent, stimulant, analgesic, cell activator, plant / animal / microbial extract, antipruritic, exfoliating / dissolving agent, antiperspirant, freshener, astringent, enzyme, nucleic acid, fragrance, pigment, coloring agent, Dye, anti-inflammatory, anti-inflammatory, anti-asthma, anti-chronic obstructive pulmonary disease, anti-allergy, immune Modifiers, anti-infective agents and antifungal agents.
- the pigment examples include inorganic white pigments such as titanium dioxide and zinc oxide; inorganic red pigments such as red iron oxide (iron oxide) and iron titanate; inorganic brown pigments such as ⁇ -iron oxide; Inorganic yellow pigments; inorganic black pigments such as black iron oxide and low titanium oxide; inorganic purple pigments such as mango violet and cobalt violet; inorganic green pigments such as chromium oxide, chromium hydroxide, and cobalt titanate; Pearl pigments such as titanium oxide coated mica, titanium oxide coated bismuth oxychloride, titanium oxide coated talc, colored titanium oxide coated mica, bismuth oxychloride, fish scale foil; talc, sericite, mica, Extender pigments such as kaolin, calcium carbonate, magnesium carbonate, silicic anhydride, barium sulfate, aluminum hydroxide; Aluminum powder, copper powder, metal powder pigments such as gold; surface treated inorganic and metallic powder pigments; zirconium, organic pigments such as barium
- oil base examples include higher (polyhydric) alcohols such as oleyl alcohol, jojoba alcohol, chimyl alcohol, seraky alcohol, butyl alcohol, hexyldecanol, isostearyl alcohol, 2-octyldodecanol, and dimer diol; benzyl alcohol and the like.
- higher (polyhydric) alcohols such as oleyl alcohol, jojoba alcohol, chimyl alcohol, seraky alcohol, butyl alcohol, hexyldecanol, isostearyl alcohol, 2-octyldodecanol, and dimer diol
- benzyl alcohol and the like.
- Aralkyl alcohols and derivatives thereof isostearic acid, behenic acid, undecylenic acid, 12-hydroxystearic acid, palmitooleic acid, oleic acid, linoleic acid, linoleic acid, erucic acid, docosahexaenoic acid, eicosapentaenoic acid, isohexadecanoic acid, Anteisohenicosanoic acid, long-chain branched fatty acid, dimer acid, hydrogenated dimer acid, etc .; liquid paraffin (mineral oil), heavy liquid isoparaffin, light liquid isoparaffin, ⁇ -olefin Rigomer, polyisobutene, hydrogenated polyisobutene, polybutene, squalane, olive-derived squalane, squalene, petrolatum, hydrocarbons such as solid paraffin; candelilla wax, carnauba wax, rice wax, wood wax, beeswax,
- humectant / feel improver examples include polyols such as glycerin, trimethylolpropane, pentaerythritol, hexylene glycol, diglycerin, polyglycerin, diethylene glycol, dipropylene glycol, polypropylene glycol, and ethylene glycol / propylene glycol copolymer.
- polyols such as glycerin, trimethylolpropane, pentaerythritol, hexylene glycol, diglycerin, polyglycerin, diethylene glycol, dipropylene glycol, polypropylene glycol, and ethylene glycol / propylene glycol copolymer.
- glycol alkyl ethers such as diethylene glycol monoethyl ether (ethoxydiglycol), ethylene glycol monoethyl ether, ethylene glycol monobutyl ether, and diethylene glycol dibutyl ether; (eicosane diacid / tetradecane diacid) polyglyceryl-10, tetradecane di -Soluble esters such as polyglyceryl-10 acid; sorbitol, xylitol, erythritol, man Sugar alcohols such as toll and maltitol; glucose, fructose, galactose, mannose, threose, xylose, arabinose, fucose, ribose, deoxyribose, maltose, trehalose, lactose, raffinose, gluconic acid, glucuronic acid, cyclodextrins ( ⁇ -, ⁇
- Preferred examples of the surfactant include an anionic surfactant, a nonionic surfactant, a cationic surfactant, an amphoteric surfactant, and a polymer surfactant.
- Preferred examples of the surfactant include, for example, fatty acid salts such as potassium laurate and potassium myristate; alkyl sulfate salts such as sodium lauryl sulfate, triethanolamine lauryl sulfate and ammonium lauryl sulfate; Polyoxyethylene alkyl sulfates such as sodium laureth sulfate and triethanolamine laureth sulfate; cocoyl methyl taurine sodium, cocoyl methyl taurine potassium, lauroyl methyl taurine sodium, myristoyl methyl taurine sodium, lauroyl methyl alanine sodium, lauroyl sarcosine sodium, lauroyl sarcosine tri Acyl N-methyl amino acid salts such as ethanolamine
- Polyoxyethylene alkyl ethers having various polyoxyethylene addition numbers such as poly (ethylene behenyl ether) s, isosteares (polyoxyethylene isostearyl ether), octyldodeces (polyoxyethylene octyldodecyl ether); polyoxyethylene alkylphenyl Ether: polyoxyethylene hydrogenated castor oil, polyoxyethylene castor oil, polyoxyethylene hydrogenated castor oil monoisostearate, polyoxyethylene hydrogenated castor oil triisostearate, polyoxyethylene hydrogenated castor oil monopyroglutamic acid monoisostearate diester Oil and hydrogenated castor oil derivatives, such as polyoxyethylene hydrogenated castor oil and maleic acid; polyoxyethylene phytosterol; polyoxyethylene cholesterol Polyoxyethylene cholestanol; polyoxyethylene lanolin; polyoxyethylene reduced lanolin; polyoxyethylene / polyoxypropylene cetyl ether, polyoxyethylene / polyoxypropylene 2-decyltetradecy
- Alkyl glyceryl ethers polyhydric alcohol alkyl ethers; polyoxyethylene alkylamines; condensates of tetrapolyoxyethylene / tetrapolyoxypropylene-ethylenediamine; natural surfactants such as saponins and sophorolipids; polyoxyethylene fatty acid amides;
- coconut oil fatty acid monoethanolamide cocamide MEA
- coconut oil fatty acid diethanolamide cocamide DEA
- lauric acid monoethanolamide Lauramide MEA
- lauric acid diethanolamide lauric acid diethanolamide
- lauramide MIPA lauric acid monoisopropanolamide
- palmitic acid monoethanolamide partamide MEA
- palmitic acid diethanolamide partamide DEA
- coconut oil fatty acid methylethanolamide Fatty acid alkanolamides such as cocamidomethyl MEA
- alkyl dimethylamine oxides such as lauramine oxide, cocamine oxide, stearamine oxide, and behen
- Silicone-based nonionic surfactants include alkyltrimethylammonium chlorides such as behentrimonium chloride, stearyltrimonium chloride, cetrimonium chloride, lauryltrimonium chloride; stearyltrimonium bromide Alkyltrimethylammonium bromide; dialkyldimethylammonium chlorides such as distearyldimonium chloride and dicocodimonium chloride; fatty acid amidoamines such as stearamidopropyldimethylamine and stearamidoethyldiethylamine and salts thereof; alkyl ethers such as stearoxypropyldimethylamine Amines and salts or quaternary salts thereof; ethyl sulfate long-chain branched fatty acid (12-31) aminopropylethyldimethylammonium, ethyl Fatty acid amide type quaternary ammonium salts such as lanolin
- -Dimethylamino acid betaine imidazoline betaines such as sodium cocoamphoacetate and sodium lauroamphoacetate; alkylsulfobetaines such as alkyldimethyltaurine; sulfuric acid betaines such as alkyldimethylaminoethanol sulfate; phosphoric acids such as alkyldimethylaminoethanol phosphate; Betaine; sphingolipids such as phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, sphingomyelin, lysolecithin, hydrogenated soybean phospholipids, Phospholipids such as hydrogenated soybean phospholipids, hydrogenated egg yolk phospholipids, partially hydrogenated egg yolk phospholipids, hydroxylated lecithin; silicone amphoteric surfactants; polymer surfactants such as polyvinyl alcohol and sodium alginate , Starch derivatives, tragacanth gum, acrylic acid / alky
- the solvent examples include lower alcohols such as ethanol, 2-propanol (isopropyl alcohol), butanol and isobutyl alcohol; glycols such as propylene glycol, diethylene glycol, dipropylene glycol and isopentyl diol; diethylene glycol monoethyl ether (ethoxydiglycol).
- lower alcohols such as ethanol, 2-propanol (isopropyl alcohol), butanol and isobutyl alcohol
- glycols such as propylene glycol, diethylene glycol, dipropylene glycol and isopentyl diol
- diethylene glycol monoethyl ether ethoxydiglycol
- Glycol ethers such as ethylene glycol monoethyl ether, ethylene glycol monobutyl ether, triethylene glycol monoethyl ether, diethylene glycol diethyl ether, diethylene glycol dibutyl ether, propylene glycol monoethyl ether and dipropylene glycol monoethyl ether; ethylene glycol monoethyl ether Acetate, diethylene glycol Glycol ether esters such as ethyl ether acetate and propylene glycol monoethyl ether acetate; glycol esters such as diethoxyethyl succinate and ethylene glycol disuccinate; benzyl alcohol, benzyloxyethanol, propylene carbonate, dialkyl carbonate, acetone, and acetic acid Preferred examples include ethyl, N-methylpyrrolidone and toluene.
- tocopherol derivatives such as tocopherol (vitamin E) and tocopherol acetate; BHT, BHA; gallic acid derivatives such as propyl gallate; vitamin C (ascorbic acid) and / or derivatives thereof; erythorbic acid and derivatives thereof; Sulfites such as sodium sulfite; bisulfites such as sodium bisulfite; thiosulfates such as sodium thiosulfate; metabisulfite; thiotaurine, hypotaurine; thioglycerol, thiourea, thioglycolic acid, and cysteine hydrochloride are preferred.
- thioglycolic acid As the reducing agent, thioglycolic acid, cysteine, cysteamine and the like are preferable.
- oxidizing agent preferred are aqueous hydrogen peroxide, ammonium persulfate, sodium bromate, percarbonate and the like.
- Preservatives, antibacterial agents, and bactericides include hydroxybenzoic acid such as methylparaben, ethylparaben, propylparaben, and butylparaben and salts or esters thereof; salicylic acid; sodium benzoate; phenoxyethanol; methylchloroisothiazolinone; Isothiazolinone derivatives such as linone; imidazolinium urea; dehydroacetic acid and salts thereof; phenols; halogenated bisphenols such as triclosan, acid amides, quaternary ammonium salts; trichlorocarbanide, zinc pyrithione, benzalkonium chloride, chloride Benzethonium, sorbic acid, chlorhexidine, chlorhexidine gluconate, halocarban, hexachlorophen, hinokitiol; phenol, isopropylphenol, cresol, Mall, para chlorophenol, pheny
- the chelating agent examples include edetates (ethylenediaminetetraacetate) such as EDTA, EDTA2Na, EDTA3Na, and EDTA4Na; hydroxyethylethylenediaminetriacetate such as HEDTA3Na; pentetate (diethylenetriaminepentaacetate); phytic acid; etidronic acid and the like Phosphonic acid and salts thereof such as sodium salt thereof; polyaspartic acid, polyamino acids such as polyglutamic acid; sodium polyphosphate, sodium metaphosphate, phosphoric acid; sodium citrate, citric acid, alanine, dihydroxyethylglycine, gluconic acid, Ascorbic acid, succinic acid and tartaric acid are preferred.
- edetates ethylenediaminetetraacetate
- HEDTA3Na EDTA3Na
- EDTA4Na hydroxyethylethylenediaminetriacetate
- pH adjusters, acids and alkalis include ascorbic acid, citric acid, sodium citrate, lactic acid, sodium lactate, potassium lactate, glycolic acid, succinic acid, acetic acid, sodium acetate, malic acid, tartaric acid, fumaric acid, phosphoric acid, Hydrochloric acid, sulfuric acid, monoethanolamine, diethanolamine, triethanolamine, isopropanolamine, triisopropanolamine, 2-amino-2-methyl-1,3-propanediol, 2-amino-2-hydroxymethyl-1,3-propane Preferred examples include diol, arginine, sodium hydroxide, potassium hydroxide, aqueous ammonia, guanidine carbonate, and ammonium carbonate.
- Powders include mica, talc, kaolin, sericite, montmorillonite, kaolinite, mica, muscovite, phlogopite, synthetic mica, biotite, biotite, permiculite, magnesium carbonate, calcium carbonate, aluminum silicate, silicate Barium, calcium silicate, magnesium silicate, strontium silicate, metal tungstate, magnesium, zeolite, barium sulfate, calcined calcium sulfate, calcium phosphate such as tricalcium phosphate, fluorine apatite, hydroxyapatite, ceramic powder, bentonite, smectite , Clay, mud, metal soap (eg, zinc myristate, calcium palmitate, aluminum stearate), calcium carbonate, red iron oxide, yellow iron oxide, black iron oxide, ultramarine, navy blue, carbon black, acid Titanium, fine and ultrafine titanium oxide, zinc oxide, fine and ultrafine zinc oxide, alumina, silica, fumed
- Copolymer resin powder polyester powder, benzoguanamine resin powder
- Organic powders and surface-treated powders of various sizes and shapes such as polyethylene terephthalate / polymethyl methacrylate laminate powder, polyethylene terephthalate / aluminum / epoxy laminate powder, urethane powder, silicone powder, Teflon (registered trademark) powder, etc.
- Organic-inorganic composite powders are preferred.
- sodium chloride-containing salts such as common salt, normal salt, rock salt, sea salt, natural salt
- sodium phosphates such as 1Na / 2Na / 3Na phosphate, phosphoric acid Potassiums, calcium phosphates, and magnesium phosphates are preferred.
- the ultraviolet absorber examples include para-aminobenzoic acid, para-aminobenzoic acid monoglycerin ester, N, N-dipropoxypara-aminobenzoic acid ethyl ester, N, N-diethoxypara-aminobenzoic acid ethyl ester, N, N-dimethylpara-aminobenzoic acid ethyl ester Benzoic acid-based ultraviolet absorbers such as esters, N, N-dimethylparaaminobenzoic acid butyl ester, N, N-dimethylparaaminobenzoic acid methyl ester; anthranilic acid-based ultraviolet absorbers such as homomenthyl-N-acetylanthranilate; salicylic acid And salicylic acids such as sodium salts thereof, amyl salicylate, menthyl salicylate, homomenthyl salicylate, octyl salicylate, phenyl salicylate, benzyl sal
- whitening agents include hydroquinone glycosides such as arbutin and ⁇ -arbutin and esters thereof; ascorbic acid phosphates such as ascorbic acid, sodium ascorbic acid phosphate and magnesium ascorbic acid phosphate; ascorbic acid Fatty acid esters of ascorbic acid such as tetraisopalmitic acid ester, alkyl ethers of ascorbic acid such as ethyl ether of ascorbic acid, glucosides of ascorbic acid such as ascorbic acid-2-glucoside and fatty acid esters thereof, ascorbic acid sulfate ester, tocopheryl ascorbyl phosphate And other ascorbic acid derivatives; kojic acid, ellagic acid, tranexamic acid and its derivatives, ferulic acid and its derivatives, placenta extract, glutathione, oryzanol, butylreso Shinoru, oil-soluble Kamomiraekisu, oil-soluble phosphat
- vitamin A such as retinol, retinol acetate, retinol palmitate; thiamine hydrochloride, thiamine sulfate, riboflavin, riboflavin acetate, pyridoxine hydrochloride, pyridoxine dioctanoate, pyridoxine dipalmitate, Flavin adenine dinucleotide, cyanocobalamin, folic acids, nicotinic acids such as nicotinamide / benzyl nicotinate, vitamin Bs such as choline; vitamin Cs such as ascorbic acid and salts thereof such as sodium; vitamin D; vitamins E such as ⁇ , ⁇ , ⁇ -tocopherol; other vitamins such as pantothenic acid and biotin; phosphate salts of ascorbic acid such as sodium salt of ascorbic acid phosphate and magnesium salt of ascorbic acid phosphate; Fatty acid esters of ascorbic
- hair-growth agents examples include plant extracts and tinctures such as assembly extract, pepper tincture, ginger tincture, ginger extract, and cantharis tincture; capsaicin, nonylate valenylamide, zingerone, ictamol, tannic acid , Borneol, cyclanderate, cinnarizine, tolazoline, acetylcholine, verapamil, cepharanthin, ⁇ -oryzanol, vitamin E and derivatives such as tocopherol nicotinate / tocopherol acetate, ⁇ -oryzanol, nicotinic acid and nicotinamide / nicotinic acid benzyl ester Derivatives such as inositol hexanicotinate, nicotine alcohol, etc., allantoin, photosensitizer 301, photosensitizer 401, capronium chloride, pentadecan
- estradiol estrone
- ethinyl estradiol cortisone
- hydrocortisone hydrocortisone
- prednisone prednisone and the like are preferred.
- Other therapeutic agents such as anti-wrinkle agents, anti-aging agents, tightening agents, cooling agents, warming agents, wound healing promoters, stimulants, analgesics, cell activators, etc. include retinols, retinoic acids, retinoin Acid tocopheryl; lactic acid, glycolic acid, gluconic acid, fruit acid, salicylic acid and derivatives such as glycosides / esterified products thereof, hydroxycapric acid, long-chain ⁇ -hydroxy fatty acid, ⁇ - such as long-chain ⁇ -hydroxy fatty acid cholesteryl and the like.
- ⁇ -hydroxy acids and derivatives thereof ⁇ -aminobutyric acid, ⁇ -amino- ⁇ -hydroxybutyric acid; carnitine; carnosine; creatine; ceramides, sphingosines; caffeine, xanthine, and derivatives thereof; coenzyme Q10, carotene, lycopene , Astaxanthin, lutein, ⁇ -lipoic acid, nanocolloidal platinum, fullerenes, etc.
- Catechins flavones such as quercetin; isoflavones; gallic acid and ester sugar derivatives; tannins, sesamin, protoanthocyanidins, chlorogenic acid, polyphenols such as apple polyphenols; rutin and glycosides Derivatives such as hesperidin and glycosides; lignan glycosides; licorice extract-related substances such as glabridine, glabrene, liquiritin, isoliquiritin; lactoferrin; shogaol, gingerol; fragrance substances such as menthol, cedrol and derivatives thereof; Preferred are capsaicin, vanillin and the like and derivatives; insect repellents such as diethyltoluamide; complexes of physiologically active substances with cyclodextrins.
- Plant / animal / microbial extracts include iris extract, ashitaba extract, asnaro extract, asparagus extract, avocado extract, amateur extract, almond extract,retea extract, arnica extract, aloe extract, apricot extract, apricot nucleus extract, ginkgo extract , Inchiko extract, Fennel extract, Turmeric extract, Oolong tea extract, Owaurushi extract, Eijitsu extract, Echinashi leaf extract, Emisoso extract, Japanese gourd extract, Oubaku extract, Ouren extract, Barley extract, Panax ginseng extract, Hypericum perforatum extract, Odorikosou extract, Ononisu extract , Dutch mustard extract, orange extract, seawater dried product, seaweed extract, oyster leaf extract, oyster extract, hydrolyzed elastin, hydrolyzed wheat powder, water content Silk, cuckoo extract, chamomile extract, oil-soluble chamomile extract, carrot extract, sagebrush extract, oats wheat extract, calcade extract,
- antipruritic agents examples include diphenhydramine hydrochloride, chlorpheniramine maleate, camphor, substance-P inhibitor and the like.
- exfoliating / dissolving agent examples include salicylic acid, sulfur, resorcin, selenium sulfide, and pyridoxine.
- antiperspirants examples include chlorohydroxyaluminum, aluminum chloride, zinc oxide, zinc paraphenolsulfonate, and the like.
- cooling agent examples include menthol, methyl salicylate, and the like.
- Astringents include citric acid, tartaric acid, lactic acid, aluminum / potassium sulfate, tannic acid and the like.
- Enzymes include superoxide dismutase, catalase, lysozyme chloride, lipase, papain, pancreatin, protease and the like.
- nucleic acids As the nucleic acids, ribonucleic acids and salts thereof, deoxyribonucleic acids and salts thereof, and disodium adenosine triphosphate are preferred.
- acetyl cedrene, amyl cinnamaldehyde, allyl amyl glycolate, ⁇ -ionone, iso-e super isobutyl quinoline, iris oil, iron, indole, ylang-ylang oil, undecanaal, undecenal, ⁇ -undecalactone, Estragole, Eugenol, Oak Moss, Opoponax Resinoid, Orange Oil, Eugenol, Orlanthol, Garrack Solid, Carvacrol, L-Carbon, Camphor, Cannon, Carrot Seed Oil, Clove Oil, Methyl Cinnarate, Geraniol, Geranilnitrile , Isobornyl acetate, geranyl acetate, dimethylbenzylcarbinyl acetate, styryl acetate, ceryl acetate, terpinel acetate, pt-butylcyclohexyl acetate, vetivery
- Pigments, colorants and dyes include Brown No. 201, Black No. 401, Purple No. 201, Purple No. 401, Blue No. 1, Blue No. 2, Blue No. 201, Blue No. 202, Blue No. 203, Blue No. 204, Blue No.
- Yellow No. 202-2 Yellow No. 203, Yellow No. 204, Yellow No. 205, Yellow No. 4, Yellow No. 401, Yellow No. 402, Yellow No. 403-1, Yellow No. 404, Yellow No. 405, Yellow No. 406, Yellow No. 407, yellow No.
- anti-inflammatory and anti-inflammatory agents include glycyrrhizic acid and derivatives thereof, glycyrrhetinic acid derivatives, salicylic acid derivatives, hinokitiol, guaiazulene, allantoin, indomethacin, ketoprofen, ibuprofen, diclofenac, loxoprofen, celecoxib, infliximab, etanercorcept, zinc oxide, hydrochloride, and hydrochloride.
- Prednisone, diphedramine hydrochloride, chlorpheniramine maleate; and plant extracts such as a peach leaf extract and a pine leaf extract are preferred.
- Anti-asthma, anti-chronic obstructive pulmonary disease, anti-allergy, immunomodulators include aminophylline, theophylline, steroids (fluticasone, beclomethasone, etc.), leukotriene antagonists, thromboxane inhibitors, intal, ⁇ 2 agonist (Formoterol, salmeterol, albuterol, tulobuterol, clenbuterol, epinephrine, etc.), tiotropium, ipratropium, dextromethorphan, dimethorphan, bromhexine, tranilast, ketotifen, azelastine, cetirizine, chlorpheniramine, mexetalimscrotaline, mextrezine , Cytokine regulators, interferon, omalizumab, and protein / antibody preparations are preferred.
- Oseltamivir, zanamivir and itraconazole are preferred as anti-infectives and antifungals.
- cosmetic raw material standards cosmetic varieties compounding component standards, Japanese Cosmetic Industry Federation component label name list, INCI dictionary (The International Cosmetic Ingredient Dictionary and Handbook), quasi-drug raw material standards, Japanese Pharmacopoeia, pharmaceutical additive standards Ingredients listed in the Official Compendium of Food Additives, etc., and listed in Japanese and foreign patent publications and patent publications (including published publications and republication) whose international patent classification IPC belongs to the classification of A61K7 and A61K8 It is possible to incorporate known cosmetic components, pharmaceutical components, food components, and the like, such as those described above, in a known combination and in a blending ratio and blending amount.
- the film-forming composition of the present invention can be produced, for example, by mixing and stirring at least one kind of lipid peptide type compound, water and, if desired, other components while heating, and then allowing the mixture to stand and cool.
- the present invention also relates to a method for producing a film, characterized by using the above-mentioned film-forming composition.
- the method for producing the film is not particularly limited as long as the film can be produced, and examples include a method in which the above-mentioned film-forming composition is applied to an object to be applied and then dried by natural drying or heat drying.
- the solid obtained here was dissolved in a mixed solution of 600 g of water and 750 g of methanol, and 30.5 mL (183.2 mmol) of 6N hydrochloric acid was added to neutralize the solid to precipitate a solid, which was filtered.
- the obtained solid was dissolved in a mixed solution of 120 g of tetrahydrofuran and 30 g of water at 60 ° C, 150 g of ethyl acetate was added, and the mixture was cooled from 60 ° C to 30 ° C. Thereafter, the precipitated solid was filtered.
- the obtained solid was dissolved in a solvent of 120 g of tetrahydrofuran and 60 g of acetonitrile, heated to 60 ° C., stirred for 1 hour, cooled, and filtered.
- the obtained solid was washed with 120 g of water, filtered, and dried under reduced pressure to obtain 26.9 g of white crystals of N-palmitoyl-Gly-His free body (hereinafter, also simply referred to as Pal-GH) (yield: 65). %).
- Preparation Example 1 Preparation of premix
- Pal-GH, stearic acid, 1,2-hexanediol, polyoxyethylene lauryl ether (manufactured by Nikko Chemicals) and water as additives were added to a 300 mL beaker at the ratio shown in Table 1 below, and the liquid temperature was 80 ° C.
- the mixture was heated and stirred at 200 rpm to obtain a uniform solution.
- the mixture was cooled with stirring, and when the liquid temperature reached 60 ° C., the mixture was allowed to stand and cooled to prepare an ES-01 premix.
- Example 1 Gelation test and observation of gel morphology According to Table 2, propylene glycol alginate, POE60-hardened castor oil, mineral oil and pure water were placed in a 300 mL tall beaker, and heated and stirred at 80 ° C. The stirring was performed at 200 rpm using a LABORATORY HIGH MIXER manufactured by AS ONE Corporation. Next, the ES-01 premix heated to 80 ° C. was added, and the mixture was further heated and stirred for 5 minutes. After the completion of the heating and stirring, the mixture was stirred and cooled until the liquid temperature reached about 50 ° C., and gel formation was confirmed. Gel formation was confirmed by the test tube inversion method, and the state where the fluidity of the dispersion liquid was lost and the liquid did not flow down even when the tall beaker was inverted was determined to be gelation.
- FIG. 1 shows observation images of a sample immediately after being placed in the scanning electron microscope and a sample that has been placed in the scanning electron microscope for a predetermined time and dried.
- Example 2 According to Table 3, propylene glycol alginate, POE60 hydrogenated castor oil, and pure water were placed in a 300 mL tall beaker, and heated and stirred at 80 ° C. The stirring was performed at 200 rpm using a LABORATORY HIGH MIXER manufactured by AS ONE Corporation. Next, the ES-01 premix heated to 80 ° C. was added, and the mixture was further heated and stirred for 5 minutes. After completion of the heating and stirring, the mixture was stirred and cooled until the liquid temperature reached about 50 ° C., and gel formation was confirmed. Gel formation was confirmed by the test tube inversion method, and the state where the fluidity of the dispersion liquid was lost and the liquid did not flow down even when the tall beaker was inverted was determined to be gelation.
- Example 3 and Comparative Example 3 Reflection light intensity measurement According to Table 4, the gel obtained in Example 2 or the liquid of Comparative Example 2 was weighed, and squalane was added thereto. After the addition, a uniform thickener or liquid was obtained using a spatula. Then, the thickened material of Example 3 or the liquid of Comparative Example 3 shown in Table 4 was applied to Bioskin (manufactured by Bureaux Co., Ltd.) at a concentration of 10 mg / cm 2, and was placed in a thermostat at 35 ° C. And dried.
- Bioskin manufactured by Bureaux Co., Ltd.
- the coating obtained by the composition of the present invention has about half the reflected light intensity as compared with the coating obtained by the composition not containing the lipid peptide type compound (Comparative Example 3). (Example 3).
- the film-forming composition of the present invention was able to form a film capable of suppressing light reflection.
- Example 4 and Comparative Example 4 Moisture retention test of shampoo According to Table 5, A phase was weighed in a 300 mL tall beaker. On the other hand, ES-01 premix similarly heated and dissolved in purified water heated to 70 ° C. was weighed to prepare phase B. Phase B was added to Phase A heated to 70 ° C. Thereafter, the mixture was stirred and cooled until the liquid temperature reached about 40 ° C.
- a human hair bundle (black hair, 1 g, 10 cm) (manufactured by Kadoya Co., Ltd.) was reciprocated 10 times with a cyclon sponge, and was similarly reciprocated 10 times with a non-cyclon sponge contact surface.
- 10 g of the composition of Example 4 or Comparative Example 4 was weighed into a 50-mL sample tube, and the hair bundle subjected to the above-described treatment was added thereto, followed by stirring with a mix rotor for 3 minutes. About 300 mL of pure water was put into a 300 mL tall beaker, and a hair bundle immersed in shampoo was put therein, and washed for 30 seconds.
- Examples 5 to 8 Moisture retention test of shampoo According to Table 6, each component was weighed in a 300 mL tall beaker. The mixture was heated to 70 ° C., and when a uniform solution was obtained, the mixture was stirred and cooled until the liquid temperature reached about 40 ° C. Then, the moisture retention was determined in the same procedure as in Example 4. The result is shown in FIG. Here, Pal-H represents palmitoyl histidine.
- the shampoo to which the lipid peptide type compound was added had an improved hair moisturizing rate as compared to the shampoo without the lipid peptide type compound (Comparative Example 4) (Examples 4 to 8). ).
- the moisturizing rate of hair was particularly improved and the moisturizing effect was excellent.
- Example 9 and Comparative Example 5 According to Table 7, the phases B and C were dissolved by heating. After heating the phase D and performing a homomixer (5000 rpm, 3 minutes) treatment, the above-mentioned heated phases B and C were added, and a homomixer (5000 rpm, 3 minutes) treatment was further performed. After the homomixer treatment, the phase A was slowly added, and the mixture was further treated with a homomixer (5000 rpm, 3 minutes). Thereafter, the mixture was stirred and cooled until the liquid temperature reached about 40 ° C.
- a homomixer 5000 rpm, 3 minutes
- Example 9 100 ⁇ L of the composition of Example 9 or Comparative Example 5 was uniformly applied to artificial leather cut to 2 cm ⁇ 2 cm, dried at room temperature for 30 minutes, and a contact angle meter Drop @ Master @ DMC-MCS (manufactured by Kyowa Kogyo Co., Ltd.) was used. To evaluate the contact angle. The result is shown in FIG.
- the contact angle of the coating obtained by the composition of the present invention was larger than that of the coating obtained by the composition not containing the lipid peptide type compound (Comparative Example 5).
- Example 9 The film forming composition of the present invention was able to form a film having good water repellency.
- Example 10 and Comparative Example 6 Moisture retention test of conditioner Phase A was weighed according to Table 8, and heated to 75 ° C to obtain a uniform solution. On the other hand, components other than the ES-01 premix of the phase B were heated to 75 ° C., and the ES-01 premix heated to 75 ° C. was added thereto. Next, the phase B was added to the phase A heated to 75 ° C., and the mixture was stirred and cooled to around room temperature.
- a human hair bundle (black hair, 1 g, 10 cm) (manufactured by Kadoya Co., Ltd.) was reciprocated 10 times with a cyclon sponge, and was similarly reciprocated 10 times with a non-cyclon sponge contact surface.
- 4.0 g of the composition (conditioner) of Example 10 or Comparative Example 6 was placed on the hair bundle that had been subjected to the above-mentioned treatment, and after being hand-adapted, about 300 mL of pure water was placed in a 300 mL tall beaker. Washing was performed for 30 seconds by immersion and shaking in the object. Further, rewashing was performed in a separately prepared 300 mL tall beaker containing about 300 mL of pure water.
- FIG. 6 shows the result.
- SEM scanning electron microscope
- the conditioner to which the lipid peptide-type compound was added was improved in the moisturizing rate of the hair and superior in the moisturizing effect as compared to the conditioner without the lipid peptide-type compound (Comparative Example 6) ( Example 10). Further, the SEM image shown in FIG. 7 confirmed that the conditioner to which the lipid peptide type compound was added formed a film on the hair. On the other hand, the conditioner to which no lipid peptide type compound was added did not form a film on the hair.
- Formulation Example 2 Liquid Crystal Cream According to the composition shown in Table 10, A, B, C, D and E were prepared and mixed to prepare a liquid crystal cream.
- UV milk (O / W emulsion) According to the composition shown in Table 11, A, B, C and D were prepared and mixed to prepare a UV milk (O / W emulsion).
- Formulation Example 6 Shampoo According to the composition shown in Table 14, A, B, C and D were prepared and mixed to prepare a shampoo.
- Example 11 and Comparative Example 7 Evaluation of Hair After Conditioner Treatment as Fingers As Example 11, a composition having the same composition as the composition of Example 10 was prepared according to Table 8 above. Further, as Comparative Example 7, a composition having the same composition as the composition of Comparative Example 6 was prepared. 10 g of the composition (conditioner) of Example 11 or Comparative Example 7 was uniformly applied to the entire human hair bundle (black hair, 1 g, 10 cm) (manufactured by Kadoya), and after 5 minutes, the applied conditioner was washed. . After standing at room temperature overnight, the frictional resistance was measured using a tactile TYPE33 (Shinto Kagaku Co., Ltd.) and used as an index for the finger. FIG. 8 shows the frictional resistance of Example 11 and Comparative Example 7. As Reference Example 1, the frictional resistance of the untreated human hair bundle was also measured under the same conditions.
- the hair treated with the conditioner had lower friction resistance than the hair not treated with the conditioner (Example 11 and Comparative Example 7).
- the hair treated with the conditioner to which the lipid peptide-type compound was added had remarkably small frictional resistance, and had good fingering properties to the hair (Example 11).
- the film-forming composition of the present invention was able to form a film having low frictional resistance, that is, a film having good finger piercing properties and comb piercing properties.
- Example 12 Preparation of O / W Liquid Foundation According to Table 15 below, an O / W liquid foundation in which 17% by mass of the pigment was uniformly dispersed was prepared. In addition, the amount of each component in Table 15 represents mass% (wt%) based on the total mass of the O / W liquid foundation.
- phase A In a 200 mL beaker (manufactured by HARIO Corporation), 4.0 g of NIKKOL Nikolurs 41, 14.0 g of NIKKOL NATURAL OILS, and 1.0 g of NIKKOL Nicogard 88 were added, and the mixture was heated and stirred at 75 ° C. for 10 minutes (phase A). The phase A is added to the phase B while stirring slowly, and the resulting mixture is emulsified at 5,000 rpm for 3 minutes at 75 ° C. using a homomixer (QUICK HOMO MIXER LR-1A manufactured by Mizuho Industry Co., Ltd.). Was. Further, 1.0 g (phase D) of ES-01 premix heated to 75 ° C.
- phase B 10 minutes
- a 200 mL beaker manufactured by HARIO Corporation
- 4.0 g of NIKKOL Nikolurs 41, 14.0 g of NIKKOL NATURAL OILS, and 1.0 g of NIKKOL Nicogard 88 were added, and the mixture was heated and stirred at 75 ° C. for 10 minutes (phase A).
- the phase A is added to the phase B while stirring slowly, and the resulting mixture is emulsified at 5,000 rpm for 3 minutes at 75 ° C. using a homomixer (QUICK HOMO MIXER LR-1A manufactured by Mizuho Industry Co., Ltd.).
- QUICK HOMO MIXER LR-1A manufactured by Mizuho Industry Co., Ltd.
- Example 13 and Comparative Example 9 Reflection Light Intensity Measurement Reflection was performed using the O / W liquid foundation of Example 12 as Example 13 and the O / W liquid foundation of Comparative Example 8 as Comparative Example 9. The light intensity was measured.
- the ⁇ / W liquid foundation of Example 13 or the ⁇ / W liquid foundation of Comparative Example 9 was applied to Bioskin (manufactured by Beulux Co., Ltd.) to a concentration of 10 mg / cm 2 and placed in a thermostat at 35 ° C. And dried.
- a variable-angle photometer GP-5 manufactured by Murakami Color Research Laboratory Co., Ltd.
- FIG. 9 shows the measurement results.
- Example 14 and Comparative Example 10 Evaluation of cosmetic twist
- the O / W liquid foundation of Example 12 was used as the composition of Example 14, and the O / W liquid foundation of Comparative Example 8 was used as the composition of Comparative Example 10.
- On human skin 0.5 g of the ⁇ / W liquid foundation of Example 14 or ⁇ / W liquid foundation of Comparative Example 10 was applied, and after evenly spreading, 0.5 g of artificial sebum was dropped and allowed to stand at room temperature for 30 minutes. Placed.
- FIG. 10 shows external appearance photographs of Example 14 and Comparative Example 10 after standing at room temperature for 30 minutes.
- Example 15 and Comparative Example 11 Preparation of Conditioner Containing Malic Acid According to Table 16 below, a foundation containing 1.5% by mass (wt%) of malic acid was prepared. Phase A was heated and stirred to 75 ° C. in a 200 mL beaker (manufactured by HARIO Corporation). Phase B and phase C were mixed in a 200 mL beaker, and heated and stirred to 75 ° C. The A phase was added to the mixed phase of the B phase and the C phase, and emulsification was performed at 75 rpm at 5,000 rpm for 5 minutes using a homomixer (QUICK HOMO MIXER LR-1A manufactured by Mizuho Industry Co., Ltd.).
- a homomixer QUICK HOMO MIXER LR-1A manufactured by Mizuho Industry Co., Ltd.
- phase D heated to 75 ° C. was added, and the mixture was heated and stirred at 75 ° C. for 5 minutes, and then stirred and cooled until the temperature reached 40 ° C. In the above steps, all stirring was performed at 200 rpm.
- Example 16 and Comparative Example 12 Moisturizing test of human hair (1) Preparation procedure of damaged hair sample (damaged hair) 50 bundles of human hair (BS-BA, manufactured by Belux Inc.) (per bundle) , About 10 cm, about 1 g), apply one set of Gatsby EX High Bleach (manufactured by Mandom Co., Ltd.) (mixture of 18 g of powder, 70 mL of water, 35 g of cream) and leave it at room temperature for 1 hour to decolorize the hair Processing was performed. Thereafter, the hair was washed with distilled water, and dried for 30 minutes using a constant temperature dryer (OF-300B, manufactured by AS ONE Corporation) set at 65 ° C. The above operation was repeated three times in total.
- BS-BA manufactured by Belux Inc.
- the hair was immersed in 1 L of an aqueous solution in which 1% by mass of sodium dodecyl sulfate (manufactured by Fujifilm Wako Pure Chemical Industries, Ltd.) was dissolved in distilled water, and washed with distilled water. Damaged hair samples (bleach hair) were prepared by removing excess water with K-dry (manufactured by Nippon Paper Crecia Co., Ltd.) and then drying overnight at room temperature.
- sodium dodecyl sulfate manufactured by Fujifilm Wako Pure Chemical Industries, Ltd.
- Damaged hair samples were prepared by removing excess water with K-dry (manufactured by Nippon Paper Crecia Co., Ltd.) and then drying overnight at room temperature.
- the black hair treated with the conditioner had a lower water loss rate than the black hair not treated with the conditioner (Reference Examples 2 and 3) (Example 16 and Comparative Example 12).
- the black hair treated with the conditioner to which the lipid peptide type compound was added had a lower water loss rate and improved the moisturizing effect of human hair.
- Nystack registered trademark
- NW-10 manufactured by Nichiban Co., Ltd.
- the black hair treated with the conditioner had higher reflected light intensity than the black hair not treated with the conditioner, and gloss was imparted to the hair (the gloss recovered) (Example 17 and Comparative Example) 13).
- the black hair treated with the conditioner to which the lipid peptide type compound was added had particularly high reflected light intensity, and the gloss of the hair was further improved (the gloss was recovered more).
- Example 18 and Comparative Example 14 Quantification of Malic Acid in Human Hair After Conditioner Treatment As Example 18, human hair treated in (2) of Example 16 was used, and as Comparative Example 14, Comparative Example 12 was used. Using the human hair treated in (2), the amount of malic acid in the human hair was evaluated. The quantification of malic acid was evaluated using the EnzyChrom TM Malate Assay Kit (EMAL-100) manufactured by BioAssay Systems. 200 milligrams of the human hair of Example 18 or Comparative Example 14 was weighed into a 50 mL sample tube, and 4 mL of purified water was added thereto, followed by ultrasonication for 60 minutes to extract malic acid.
- EEL-100 EnzyChrom TM Malate Assay Kit
- the film-forming composition of the present invention was able to form a film having a high retention of the active ingredient (a coating that could contain a large amount of the active ingredient).
- Example 19 Comparative Example 15; SEM measurement of conditioner-treated hair
- 1 g of the conditioner prepared in Example 10 and Comparative Example 6 was applied to a bundle of damaged hair (10 cm, 50 pieces) prepared in the same procedure as (1) of Example 16, It was left still for 5 minutes. Thereafter, the resultant was washed by shaking in a 300 mL tall beaker containing 300 mL of purified water, and excess purified water was removed with K-dry (manufactured by Nippon Paper Crecia Co., Ltd.), and dried overnight at room temperature.
- K-dry manufactured by Nippon Paper Crecia Co., Ltd.
- Example 20 Comparative Example 16; Evaluation of moisture absorption and desorption of hair treated with conditioner
- Example 20 a bundle of hair (approximately 10 cm, approximately 1 g) produced by the same procedure as in Example 16 (1) was subjected to the same treatment as in Example 16 (2). After shearing to 1 cm, 16 mg of hair was weighed, and the amount of moisture absorption / desorption was evaluated using a moisture absorption / desorption measuring device IGAsorp (manufactured by Heiden Isokeyama). After measuring the wet weight of each sample under the condition of a temperature of 25 ° C. and a humidity of 40%, the sample is dried at a temperature of 25 ° C.
- IGAsorp manufactured by Heiden Isokeyama
- the relative humidity was increased stepwise from 0% to 90% in increments of 10%, and the moisture content in the hair was calculated from the equilibrium weight of the sample at each relative humidity point. Further, even when the relative humidity was gradually decreased from 90% to 0% in steps of 10%, the moisture content in the hair was calculated from the equilibrium weight of the sample at each relative humidity point.
- the maximum equilibration time at each humidity was 360 minutes, the above procedure was performed by automatic measurement by a program, and the equilibrium weight of the sample was determined by the asymptote method based on the LDF (linear propulsion approximation) model.
- the equilibrium weight of the sample in the case where the LDF model was not applied was calculated by moving-averaging the measured value after 180 minutes of measurement.
- the results of the obtained moisture percentage are shown in Table 19 (when the humidity rises) and Table 20 (when the humidity falls).
- Comparative Example 16 the results of human hair treated in (2) of Comparative Example 12 and the results of damaged hair are also shown in Table 19. In comparison with the damaged hair not subjected to the conditioner treatment, Comparative Example 16 suppressed the adsorption of moisture, and Example 19 tended to further suppress the adsorption of moisture.
- Example 21, Comparative Example 17; Dynamic friction coefficient of hair treated with conditioner As Example 21, one bundle (about 10 cm, about 1 g) of damaged hair prepared by the same procedure as in Example 16 (1) was subjected to the same treatment as in Example 16 (2). Was measured and used as an index for the finger. The kinetic friction coefficient was measured using a KES-SE friction tester (manufactured by Kato Tech Co., Ltd.), and a 10 mm square silicon sensor was used as the sensor. The hair bundle treated in the same manner as (2) of Comparative Example 12 was also measured, and Comparative Example 17 was obtained. FIG. 13 shows the results of both. The hair provided in Example 21 tended to have a lower coefficient of kinetic friction on the surface of the hair as compared with Comparative Example 17.
- Example 22, Comparative Example 18; Preparation of Conditioner Containing Succinic Acid According to the following Table 21, a conditioner containing 1.5% by mass of succinic acid was prepared. Phase A was heated and stirred to 75 ° C. in a 200 mL beaker (manufactured by HARIO Corporation). The phase B and the phase C were mixed in a 200 mL beaker, and heated and stirred to 75 ° C. The A phase was added to the B + C phase, and emulsified at 5,000 rpm for 5 minutes at 75 ° C. using a homomixer (QUICK HOMO MIXER LR-1A manufactured by Mizuho Industry Co., Ltd.). Thereafter, phase D heated to 75 ° C. was added, and the mixture was heated and stirred at 75 ° C. for 5 minutes, and then stirred and cooled until the temperature reached 40 ° C. In the above steps, all stirring was performed at 200 rpm.
- Example 23 Comparative Example 19; Evaluation of succinic acid content of conditioner-treated hair
- Example 23 and Comparative Example 19 one gram of the conditioner prepared in Example 22 and Comparative Example 18 was taken by hand, and one bundle (about 10 cm) of damaged hair made in the same procedure as (1) of Example 16 was taken. , About 1 g) and allowed to stand for 5 minutes. Thereafter, the resultant was washed by rocking in a 300 mL tall beaker containing 300 mL of water, excess water was removed with K-dry (manufactured by Nippon Paper Crecia Co., Ltd.), and dried overnight at room temperature. The above process was performed once, twice, and three times to produce three types of samples.
- Each of the prepared damaged hair samples was cut into a length of 1 cm.
- the quantification of succinic acid was evaluated using a Succinate colorimetric Assay Kit (manufactured by Megazyme). 200 mg of each of the human hairs treated in Example 23 and Comparative Example 19 was weighed in a 50 mL sample tube, and 4 mL of purified water was added thereto, followed by sonication for 60 minutes to extract succinic acid.
- Example 24, Comparative Example 20; Evaluation of penetration amount of succinic acid and lipid peptide in human hair As Example 24 and Comparative Example 20, 1 g of the conditioner prepared in Example 22 and Comparative Example 18 was taken by hand, and a bundle of damaged hair (about 10 cm, About 1 g) and allowed to stand for 5 minutes. Thereafter, the resultant was washed by rocking in a 300 mL tall beaker containing 300 mL of water, excess water was removed with K-dry (manufactured by Nippon Paper Crecia Co., Ltd.), and dried overnight at room temperature. The above processing was repeated for three days, and the time-of-flight secondary ion mass spectrometer TOF.
- Example 25 Comparative Example 21; Measurement of zeta potential of hair surface treated with conditioner
- Example 25 and Comparative Example 21 one gram of the conditioner prepared in Example 22 and Comparative Example 18 was taken by hand, and a bundle of damaged hair (approximately 10 cm) prepared in the same procedure as (1) of Example 16 was taken. , About 1 g) and allowed to stand for 5 minutes. Thereafter, the resultant was washed by rocking in a 300 mL tall beaker containing 300 mL of water, excess water was removed with K-dry (manufactured by Nippon Paper Crecia Co., Ltd.), and dried overnight at room temperature.
- K-dry manufactured by Nippon Paper Crecia Co., Ltd.
- the surface zeta potential of the above hair sample was evaluated by a solid surface zeta potential device SurPASS (manufactured by Anton-Paar). 1 mM KCl was prepared for the electrolyte, and the measurement was performed at a temperature of 23 ° C. The results are shown in Table 22. In Comparative Example 21, it was confirmed that the surface potential was more cationic than that in the bleached hair subjected to the bleaching treatment, and in Example 25, the value became more cationic.
- Example 26 Comparative Example 22; Hardness evaluation of conditioner-treated hair surface
- Example 26 and Comparative Example 22 one gram of the conditioner prepared in Example 22 and Comparative Example 18 was taken by hand, and a bundle of damaged hair (approximately 10 cm) made in the same procedure as (1) of Example 16 was taken. , About 1 g) and allowed to stand for 5 minutes. Thereafter, the resultant was washed by rocking in a 300 mL tall beaker containing 300 mL of water, excess water was removed with K-dry (manufactured by Nippon Paper Crecia Co., Ltd.), and dried overnight at room temperature.
- K-dry manufactured by Nippon Paper Crecia Co., Ltd.
- the hardness of the surface of each of the hair samples was measured with a Hyditron TI980 tribo indenter (manufactured by Bruker).
- the indentation depth was set to 1 ⁇ m, the indenter was a Berkovich type, and the average value measured ten times was calculated.
- FIG. 16 shows the results. It was shown that the bleached damaged hair had a harder hair surface than untreated black hair. Comparative Example 22 showed that the hair surface became softer than that of the damaged hair, and Example 26 showed that the surface became softer.
- Example 27 Comparative Example 23; Evaluation of moisture absorption and desorption of hair treated with conditioner
- Example 27 and Comparative Example 23 one gram of the conditioner prepared in Example 22 and Comparative Example 18 was taken by hand, and a bundle (about 10 cm) of damaged hair made in the same procedure as (1) of Example 16 was taken. , About 1 g) and allowed to stand for 5 minutes. Thereafter, the resultant was washed by rocking in a 300 mL tall beaker containing 300 mL of water, excess water was removed with K-dry (manufactured by Nippon Paper Crecia Co., Ltd.), and dried overnight at room temperature.
- K-dry manufactured by Nippon Paper Crecia Co., Ltd.
- the prepared treated sample was sheared to 1 cm, and the amount of water adsorbed and desorbed on 3 mg of human hair was evaluated using a water adsorption and desorption measuring device IGAsorp (manufactured by Heiden Isokeyama).
- the hair was dried for 2 days in a relative humidity of 0%, and the amount of mass change when the humidity was increased to 90% relative humidity at a stretch was measured.
- the above procedure was performed by automatic measurement by a program. The results are shown in FIG. As compared with the damaged hair that was not subjected to the conditioner treatment, Comparative Example 23 suppressed the adsorption of moisture, and Example 27 tended to further suppress the adsorption.
- Example 28 Comparative Example 24; Temporal change of shine due to sebum floating
- O / W liquid foundation of Example 12 As Example 28 and the O / W liquid foundation of Comparative Example 8 as Comparative Example 24, the change of shine over time due to sebum floating was evaluated.
- the O / W liquid foundations of Example 12 and Comparative Example 8 were applied to the face of the subject, living as usual, and the surface of the face was applied from 1 hour after application of each foundation to 6 hours after every other hour.
- the gloss was measured using Glossymeter GL200 (manufactured by Courage + khazaka electronic).
- FIG. 18 shows the results. In Example 28, as compared with Comparative Example 24, even after 6 hours, shine caused by sebum floating over time was suppressed.
- Example 29, Comparative Example 25; Preparation of serum According to Table 23 below, a serum containing 0.005% of hyaluronic acid was prepared. Phase A was heated and stirred to 75 ° C. in a 200 mL beaker (manufactured by HARIO Corporation). The B, C, and D phases were mixed in a 200 mL beaker, and heated and stirred to 75 ° C. Phase A was added to phase B + C + D and stirred at 75 ° C. for 5 minutes. Thereafter, the mixture was stirred and cooled to 45 ° C., phase E was added, and the mixture was stirred and cooled to 35 ° C. In the above steps, all stirring was performed at 200 rpm.
- Example 30, Comparative Example 26; Moisturizing effect of serum The moisturizing effect was evaluated using the serum of Example 29 as Example 30 and the serum of Comparative Example 25 as Comparative Example 26.
- the serum of Example 29 and Comparative Example 25 was applied to the inside of the forearm of the subject, and in an environment of 50% humidity and a temperature of 24 ° C., the serum was applied from 1 hour to 7 hours every 1 hour.
- the horny water content of the forearm was measured using Corneometer CM825 (Courage + Khazaka electronic). Ten places were randomly extracted from the applied forearm and measured, and the average was taken as the measurement result. The result is shown in FIG. In Example 30, as compared with Comparative Example 26, a high water content was retained in the keratin even after 7 hours, and it was confirmed that there was a moisturizing effect.
- Example 31, Example 32, Comparative Example 27; Preparation of keratin-containing conditioner According to Table 24 below, a conditioner containing 1.5% by mass of keratin was prepared. Phase A was heated and stirred to 75 ° C. in a 200 mL beaker (manufactured by HARIO Corporation). The phase B and the phase C were mixed in a 200 mL beaker, and heated and stirred to 75 ° C. The A phase was added to the B + C phase, and the mixture was emulsified at 5,000 rpm for 5 minutes at 75 ° C. using a homomixer (QUICK HOMO MIXER LR-1A manufactured by Mizuho Industry Co., Ltd.).
- a homomixer QUICK HOMO MIXER LR-1A manufactured by Mizuho Industry Co., Ltd.
- phase D heated to 75 ° C. was added, and the mixture was heated and stirred at 75 ° C. for 5 minutes, and then stirred and cooled until the temperature reached 40 ° C. In the above steps, all stirring was performed at 200 rpm.
- Example 33, Example 34, Comparative Example 28; Evaluation of keratin content in hair treated with conditioner As Example 33, Example 34, and Comparative Example 28, the conditioners adjusted in Example 31, Example 32, and Comparative Example 27 were prepared by hand in the same procedure as (1) of Example 16 by taking each 1 g of the conditioner. It was applied to one bundle (about 10 cm, about 1 g) of damaged hair and allowed to stand for 5 minutes. After that, it is washed by shaking in a 300 mL tall beaker containing 300 mL of methanol (manufactured by Junsei Chemical Co., Ltd.), excess methanol is removed with K-dry (manufactured by Nippon Paper Crecia Co., Ltd.), and dried overnight at room temperature. Was.
- Each of the prepared damaged hair samples was cut into a length of 1 cm.
- the quantification of keratin was evaluated using Keratin Orange ⁇ -Keratin Assay Kit (manufactured by biocolor).
- 5 mg of each of the human hairs treated in Examples and Comparative Examples was weighed into 1.5 mL microtubes (manufactured by Eppendorf), 1 mL of Digestion reagent was added, and vortexed for 1 hour.
- Reference standard ⁇ -Keratin 5.0 mg / mL
- Digestion reagent was diluted with Digestion reagent to prepare a sample for a calibration curve (0, 1, 2, 3, 4, 5 mg / mL).
- Neutralizing solution (1 M HCl) was added to 200 ⁇ L of the hair sample and 200 ⁇ L of the calibration curve sample to neutralize the pH of each solution.
- 50 ⁇ L of Dye reagent was added to the sample, mixed by vortex, and allowed to stand for 30 minutes to react.
- 450 ⁇ L of Saturated solution ((NH 4 ) 2 SO 4 ) was added to the sample.
- Saturated solution ((NH 4 ) 2 SO 4 ) was added to the sample.
- the sample was centrifuged at 12,000 rpm for 10 minutes, the supernatant was discarded, and 50 ⁇ L of Digestion reagent was added, and the mixture was vortexed sufficiently to solubilize keratin.
- FIG. 20 shows the results of the test. Compared with Comparative Example 28, a higher amount of keratin was extracted from the hairs of Example 33 and Example 34.
- Example 35 Comparative Example 29; Preparation of 18-MEA-containing conditioner
- a conditioner containing 1.5% by mass of 18-methyleicoic acid (18-MEA) was prepared.
- Phase A was heated and stirred to 75 ° C. in a 200 mL beaker (manufactured by HARIO Corporation).
- the phase B and the phase C were mixed in a 200 mL beaker, and heated and stirred to 75 ° C.
- the A phase was added to the B + C phase, and emulsification was performed at 75 rpm at 5,000 rpm for 5 minutes using a homomixer (QUICK HOMO MIXER LR-1A manufactured by Mizuho Industry Co., Ltd.).
- phase D heated to 75 ° C. was added, and the mixture was heated and stirred at 75 ° C. for 5 minutes, and then stirred and cooled until the temperature reached 40 ° C. In the above steps, all stirring was performed at 200 rpm.
- Example 36 Comparative Example 30; SEM measurement of hair treated with conditioner
- Example 36 and Comparative Example 30 one gram of the conditioner prepared in Example 35 and Comparative Example 29 was taken by hand, and one bundle (about 10 cm) of damaged hair made in the same procedure as (1) of Example 16 was taken. , About 1 g) and allowed to stand for 5 minutes. Thereafter, the resultant was washed by shaking in a 300 mL tall beaker containing 300 mL of purified water, and excess purified water was removed with K-dry (manufactured by Nippon Paper Crecia Co., Ltd.), and dried overnight at room temperature.
- K-dry manufactured by Nippon Paper Crecia Co., Ltd.
- FIG. 21 shows the measurement results of the damaged hair.
- the structure of the cuticle surface can be observed, whereas in Example 36, the cuticle surface was covered with the coating.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical & Material Sciences (AREA)
- Epidemiology (AREA)
- Birds (AREA)
- Dermatology (AREA)
- Organic Chemistry (AREA)
- Emergency Medicine (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Wood Science & Technology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Dispersion Chemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Molecular Biology (AREA)
- Medicinal Chemistry (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Cosmetics (AREA)
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2020527698A JP7460962B2 (ja) | 2018-06-29 | 2019-06-28 | 被膜形成組成物 |
| KR1020217002238A KR20210025070A (ko) | 2018-06-29 | 2019-06-28 | 피막형성 조성물 |
| US17/256,729 US20210259941A1 (en) | 2018-06-29 | 2019-06-28 | Coating film-forming composition |
| EP19827474.8A EP3808329A4 (en) | 2018-06-29 | 2019-06-28 | COATING FILM FORMING COMPOSITION |
| EP22155607.9A EP4014958A1 (en) | 2018-06-29 | 2019-06-28 | Coating film-forming composition |
| CN201980056353.4A CN112638359A (zh) | 2018-06-29 | 2019-06-28 | 被膜形成用组合物 |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2018125136 | 2018-06-29 | ||
| JP2018-125136 | 2018-06-29 | ||
| JP2018233469 | 2018-12-13 | ||
| JP2018-233469 | 2018-12-13 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2020004649A1 true WO2020004649A1 (ja) | 2020-01-02 |
Family
ID=68984647
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2019/025927 Ceased WO2020004649A1 (ja) | 2018-06-29 | 2019-06-28 | 被膜形成組成物 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US20210259941A1 (enExample) |
| EP (2) | EP4014958A1 (enExample) |
| JP (1) | JP7460962B2 (enExample) |
| KR (1) | KR20210025070A (enExample) |
| CN (1) | CN112638359A (enExample) |
| TW (1) | TW202017558A (enExample) |
| WO (1) | WO2020004649A1 (enExample) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2022065433A1 (ja) * | 2020-09-28 | 2022-03-31 | 日産化学株式会社 | 毛髪用化粧料 |
| WO2022092284A1 (ja) * | 2020-10-30 | 2022-05-05 | 日産化学株式会社 | 脂質ペプチドとショ糖エステルを含む組成物 |
| KR20220106873A (ko) * | 2021-01-22 | 2022-08-01 | 주식회사 코스메카코리아 | 미세먼지 흡착방지용 화장료 조성물의 제조방법 및 그에 의해 제조된 미세먼지 흡착방지용 화장료 조성물 |
| CN116744892A (zh) * | 2020-10-30 | 2023-09-12 | 日产化学株式会社 | 包含脂质肽和蔗糖酯的组合物 |
| WO2025216117A1 (ja) * | 2024-04-08 | 2025-10-16 | 日産化学株式会社 | 脂質ペプチド及びショ糖エステルを含む組成物 |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN115612321B (zh) * | 2022-09-27 | 2024-01-19 | 常州君合科技股份有限公司 | 一种环保型水性锌铝防腐涂液及其制备方法 |
| CN115975483B (zh) * | 2022-12-19 | 2023-08-22 | 浦诺菲新材料有限公司 | 自修复涂层涂布液的制备方法,以及汽车漆面保护膜 |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH1077210A (ja) | 1996-09-06 | 1998-03-24 | Nakanihon Seni Kogyo Kyodo Kumiai | 化粧料用組成物及びその製造方法並びにこれを配合した化粧品 |
| JP2002308756A (ja) | 2001-03-05 | 2002-10-23 | L'oreal Sa | ポリアミノ酸の保湿剤としての用途とそれを含有する化粧品用又は製薬用組成物 |
| WO2010013555A1 (ja) * | 2008-08-01 | 2010-02-04 | 日産化学工業株式会社 | 新規脂質ジペプチド並びにゲル |
| JP2010047534A (ja) * | 2008-08-22 | 2010-03-04 | Kyushu Univ | 酵素応答性ゲル |
| WO2010106981A1 (ja) * | 2009-03-16 | 2010-09-23 | 日産化学工業株式会社 | 低分子ゲル化剤からなるスプレー用基材 |
| JP2016006030A (ja) | 2014-05-30 | 2016-01-14 | 花王株式会社 | 皮膚化粧料 |
Family Cites Families (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2760634B1 (fr) * | 1997-03-14 | 2002-10-11 | Oreal | Composition aqueuse topique solide ayant l'aspect d'un gel permettant la formation d'un film lors de son application |
| EP1816998B1 (en) * | 2004-11-22 | 2009-03-18 | Symrise GmbH & Co. KG | Formulations comprising ceramides and/or pseudoceramides and (alpha-)bisabolol for combating skin damage |
| FR2911779B1 (fr) * | 2007-01-30 | 2009-04-24 | Lvmh Rech | Composition contenant un extrait d'ambre |
| JP2009073764A (ja) * | 2007-09-20 | 2009-04-09 | Fujifilm Corp | 生体用粘着ゲルシートおよびそれを用いたシート状化粧料 |
| EP2494953B1 (en) * | 2009-10-26 | 2017-09-27 | Nissan Chemical Industries, Ltd. | Composition for use as cosmetic or external skin preparation |
| CN103635231A (zh) * | 2011-01-10 | 2014-03-12 | 道康宁法国公司 | 局部用组合物 |
| TW201907903A (zh) * | 2013-12-25 | 2019-03-01 | 日商日產化學工業股份有限公司 | 含有脂質肽型化合物之條狀基材 |
| JP2016027035A (ja) * | 2014-06-30 | 2016-02-18 | ロート製薬株式会社 | 皮膚外用組成物、化粧料及び皮膚外用組成物の変色抑制方法 |
| JP6975516B2 (ja) * | 2014-06-30 | 2021-12-01 | ロート製薬株式会社 | 外用組成物、化粧料、経皮吸収促進用組成物、外用組成物における有効成分の経皮吸収性を高める方法、経皮投与型医薬及び点眼用組成物 |
| EP3212688B1 (en) * | 2014-10-31 | 2022-04-13 | Lubrizol Advanced Materials, Inc. | Thermoplastic polyurethane film for delivery of active agents to skin surfaces |
| DE102014225582A1 (de) * | 2014-12-11 | 2015-10-01 | Henkel Ag & Co. Kgaa | Stylinggel mit Spinnen-Seidenprotein und Polyacrylsäure |
| EP3851093B1 (en) * | 2015-08-10 | 2024-05-22 | Mary Kay, Inc. | Topical compositions |
| CN106109358A (zh) * | 2016-08-25 | 2016-11-16 | 安迪美生物科技(北京)有限公司 | 一种多肽蚕丝补水修护面膜及其制备方法 |
| DE102017218599A1 (de) * | 2017-10-18 | 2019-04-18 | Henkel Ag & Co. Kgaa | "Wasserarme Pflege-Shampoos im Pouch" |
| CN108836901A (zh) * | 2018-09-26 | 2018-11-20 | 嫦娥创新(武汉)生物科技有限公司 | 一种含酵母菌发酵产物滤液的面膜精华液及其制备方法 |
-
2019
- 2019-06-28 EP EP22155607.9A patent/EP4014958A1/en not_active Withdrawn
- 2019-06-28 TW TW108122947A patent/TW202017558A/zh unknown
- 2019-06-28 WO PCT/JP2019/025927 patent/WO2020004649A1/ja not_active Ceased
- 2019-06-28 EP EP19827474.8A patent/EP3808329A4/en active Pending
- 2019-06-28 US US17/256,729 patent/US20210259941A1/en active Pending
- 2019-06-28 JP JP2020527698A patent/JP7460962B2/ja active Active
- 2019-06-28 KR KR1020217002238A patent/KR20210025070A/ko active Pending
- 2019-06-28 CN CN201980056353.4A patent/CN112638359A/zh active Pending
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH1077210A (ja) | 1996-09-06 | 1998-03-24 | Nakanihon Seni Kogyo Kyodo Kumiai | 化粧料用組成物及びその製造方法並びにこれを配合した化粧品 |
| JP2002308756A (ja) | 2001-03-05 | 2002-10-23 | L'oreal Sa | ポリアミノ酸の保湿剤としての用途とそれを含有する化粧品用又は製薬用組成物 |
| WO2010013555A1 (ja) * | 2008-08-01 | 2010-02-04 | 日産化学工業株式会社 | 新規脂質ジペプチド並びにゲル |
| JP2010047534A (ja) * | 2008-08-22 | 2010-03-04 | Kyushu Univ | 酵素応答性ゲル |
| WO2010106981A1 (ja) * | 2009-03-16 | 2010-09-23 | 日産化学工業株式会社 | 低分子ゲル化剤からなるスプレー用基材 |
| JP2016006030A (ja) | 2014-05-30 | 2016-01-14 | 花王株式会社 | 皮膚化粧料 |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2022065433A1 (ja) * | 2020-09-28 | 2022-03-31 | 日産化学株式会社 | 毛髪用化粧料 |
| WO2022092284A1 (ja) * | 2020-10-30 | 2022-05-05 | 日産化学株式会社 | 脂質ペプチドとショ糖エステルを含む組成物 |
| CN116744892A (zh) * | 2020-10-30 | 2023-09-12 | 日产化学株式会社 | 包含脂质肽和蔗糖酯的组合物 |
| EP4230265A4 (en) * | 2020-10-30 | 2024-05-08 | Nissan Chemical Corporation | Composition containing lipid peptide and sucrose ester |
| KR20220106873A (ko) * | 2021-01-22 | 2022-08-01 | 주식회사 코스메카코리아 | 미세먼지 흡착방지용 화장료 조성물의 제조방법 및 그에 의해 제조된 미세먼지 흡착방지용 화장료 조성물 |
| KR102552912B1 (ko) | 2021-01-22 | 2023-07-10 | 주식회사 코스메카코리아 | 미세먼지 흡착방지용 화장료 조성물의 제조방법 및 그에 의해 제조된 미세먼지 흡착방지용 화장료 조성물 |
| WO2025216117A1 (ja) * | 2024-04-08 | 2025-10-16 | 日産化学株式会社 | 脂質ペプチド及びショ糖エステルを含む組成物 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20210259941A1 (en) | 2021-08-26 |
| KR20210025070A (ko) | 2021-03-08 |
| EP4014958A1 (en) | 2022-06-22 |
| EP3808329A1 (en) | 2021-04-21 |
| JP7460962B2 (ja) | 2024-04-03 |
| EP3808329A4 (en) | 2021-08-18 |
| CN112638359A (zh) | 2021-04-09 |
| TW202017558A (zh) | 2020-05-16 |
| JPWO2020004649A1 (ja) | 2021-07-08 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5971487B2 (ja) | 化粧料の製造方法、化粧料用ゲルの調製方法及び化粧料原料に配合される高分子増粘剤の使用量を軽減する方法 | |
| JP7460962B2 (ja) | 被膜形成組成物 | |
| EP3747911A1 (en) | Functional polysaccharide particle | |
| JP6624380B2 (ja) | 脂質ペプチド型化合物を含有するスティック状基材 | |
| JP6734568B2 (ja) | 脂質ペプチド型化合物を含有するスティック状基材 | |
| US20210401734A1 (en) | Transdermally absorbable base material containing lipid peptide compound | |
| WO2011052613A1 (ja) | 化粧料及び皮膚外用剤、並びに医療用機器 | |
| JPWO2015005219A1 (ja) | 分散液及びヒドロゲル形成方法 | |
| JP6802711B2 (ja) | 脂質ペプチド型化合物を含有する保湿基材 | |
| JP2022189546A (ja) | 脂質の流動化を高める組成物 | |
| US20220251393A1 (en) | Pigments containing cellulose | |
| JP2013053171A (ja) | 浸透促進剤 | |
| JP4931568B2 (ja) | 表面処理粉体を含有する化粧料 | |
| JP6694166B2 (ja) | 脂質ペプチド型化合物を含有するスティック状基材の硬度調整方法 | |
| WO2021132653A1 (ja) | 汚染防止材料 | |
| KR20200108447A (ko) | 분체를 포함하는 조성물 | |
| WO2022092284A1 (ja) | 脂質ペプチドとショ糖エステルを含む組成物 | |
| JP2023091264A (ja) | 付着抑制方法 | |
| JPWO2018207947A1 (ja) | 多糖含有スティック状基材 | |
| JPWO2018190383A1 (ja) | 顔料又は粉体用分散剤を含有する分散液 | |
| JP2023048158A (ja) | 脂質ペプチドとノニオン性界面活性剤を含む機能性液状組成物 | |
| JP2024037077A (ja) | シクロペンタシロキサン代替組成物及びそれを含む化粧料 | |
| WO2021132668A1 (ja) | 浸透促進材料 | |
| JPWO2018207948A1 (ja) | 高含水スティック状基材 | |
| WO2015194054A1 (ja) | フェニルエチルアミン誘導体又はシネフリン含有デスモプラキン発現誘導剤又はデスモプラキン細胞膜局在誘導剤 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 19827474 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2020527698 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20217002238 Country of ref document: KR Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2019827474 Country of ref document: EP Effective date: 20210113 |