WO2020004014A1 - エルゴチオネイン、アスコルビン酸2-グルコシド、アスコルビン酸、およびそれらの組み合わせの筋分化促進作用 - Google Patents
エルゴチオネイン、アスコルビン酸2-グルコシド、アスコルビン酸、およびそれらの組み合わせの筋分化促進作用 Download PDFInfo
- Publication number
- WO2020004014A1 WO2020004014A1 PCT/JP2019/023114 JP2019023114W WO2020004014A1 WO 2020004014 A1 WO2020004014 A1 WO 2020004014A1 JP 2019023114 W JP2019023114 W JP 2019023114W WO 2020004014 A1 WO2020004014 A1 WO 2020004014A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- ascorbic acid
- muscle
- medium
- differentiation
- egt
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0652—Cells of skeletal and connective tissues; Mesenchyme
- C12N5/0658—Skeletal muscle cells, e.g. myocytes, myotubes, myoblasts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L2/00—Non-alcoholic beverages; Dry compositions or concentrates therefor; Preparation or treatment thereof
- A23L2/52—Adding ingredients
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/15—Vitamins
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/40—Complete food formulations for specific consumer groups or specific purposes, e.g. infant formula
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/365—Lactones
- A61K31/375—Ascorbic acid, i.e. vitamin C; Salts thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/4164—1,3-Diazoles
- A61K31/4172—Imidazole-alkanecarboxylic acids, e.g. histidine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7048—Compounds having saccharide radicals and heterocyclic rings having oxygen as a ring hetero atom, e.g. leucoglucosan, hesperidin, erythromycin, nystatin, digitoxin or digoxin
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/17—Amino acids, peptides or proteins
- A23L33/175—Amino acids
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2002/00—Food compositions, function of food ingredients or processes for food or foodstuffs
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2500/00—Specific components of cell culture medium
- C12N2500/30—Organic components
- C12N2500/32—Amino acids
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2500/00—Specific components of cell culture medium
- C12N2500/30—Organic components
- C12N2500/38—Vitamins
Definitions
- the present invention relates to promotion of cell differentiation. Specifically, the present invention relates to a medium for promoting muscle differentiation and a medium additive containing ergothioneine, a pharmaceutical composition for promoting muscle differentiation, food and drink, and the like.
- the present inventors have conducted intensive studies to solve the above-mentioned problems, and have found that ergothionein promotes muscle differentiation, and that ergothioneine is used in combination with ascorbic acid 2-glucoside and / or ascorbic acid to greatly promote muscle differentiation. It has been found for the first time that ascorbic acid 2-glucoside alone promotes muscle differentiation, and that ascorbic acid alone promotes muscle differentiation, thereby completing the present invention.
- the present invention provides the following.
- a medium containing ergothioneine for promoting muscle differentiation (2) The medium according to (1), further comprising ascorbic acid 2-glucoside and / or ascorbic acid.
- a culture medium additive for promoting muscle differentiation comprising ergothioneine.
- the medium additive according to (3) further comprising ascorbic acid 2-glucoside and / or ascorbic acid.
- a kit for promoting muscle differentiation comprising ergothioneine.
- the kit according to (5) further comprising ascorbic acid 2-glucoside and / or ascorbic acid.
- a method for promoting muscle differentiation of cells comprising culturing the cells in a medium containing ergothioneine.
- a pharmaceutical composition for promoting muscle differentiation comprising ergothioneine.
- a medium for promoting muscle differentiation and a medium additive containing ergothioneine, a pharmaceutical composition for promoting muscle differentiation, food and drink, and the like are provided.
- the culture medium, culture medium additive, pharmaceutical composition, and food and drink of the present invention have excellent muscle differentiation promoting ability.
- the pharmaceutical composition and the food and drink of the present invention are particularly useful for elderly people, injured persons, sick / post-sick persons, and the like.
- Ergothioneine is a substance found in living organisms
- ascorbic acid 2-glucoside is a substance approved as a food additive and quasi-drug
- ascorbic acid is a substance widely found in nature such as fruits and vegetables. Therefore, the culture medium, culture medium additive, pharmaceutical composition, and food and drink of the present invention have high safety.
- FIG. 1 is a micrograph showing the effect of ergothioneine on muscle differentiation of C2C12 cells.
- the left photograph shows C2C12 cells before differentiation
- the middle photograph shows C2C12 cells differentiated in a differentiation medium without EGT (target)
- the right photograph shows C2C12 cells differentiated in a differentiation medium supplemented with 0.5 mM ⁇ EGT.
- FIG. 2 is a graph showing the effect of ergothioneine on MyoD mRNA expression levels in C2C12 cells.
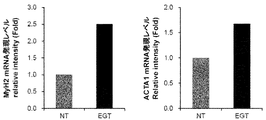
- FIG. 3 is a graph showing the effect of ergothioneine on the expression levels of MyH2 mRNA and ACTA1 mRNA in C2C12 cells.
- FIG. 1 is a micrograph showing the effect of ergothioneine on muscle differentiation of C2C12 cells.
- the left photograph shows C2C12 cells before differentiation
- the middle photograph shows C2C12 cells differentiated in a differentiation medium without EGT (target)
- the right photograph shows C2C12
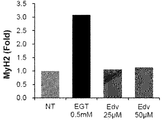
- FIG. 4 is a graph showing that the antioxidant edaravone does not affect MyH2M mRNA expression levels in C2C12 cells.
- FIG. 5 shows the effect of the combined use of ergothioneine and ascorbic acid 2-glucoside and the use of ascorbic acid 2-glucoside alone on the expression levels of MyH2 mRNA (upper panel) and ACTA1 mRNA (lower panel) in C2C12 cells. It is a graph shown.
- EGT indicates the use of a medium supplemented with ergothioneine
- AG indicates the use of a medium supplemented with ascorbic acid 2-glucoside
- E + AG indicates the use of a medium supplemented with ergothioneine and ascorbic acid 2-glucoside.
- NT indicates that a medium without the addition of ergothioneine and ascorbic acid 2-glucoside was used.
- FIG. 6 is a graph showing the effects of the combination use of ergothioneine and ascorbic acid and the use of ascorbic acid alone on the expression levels of MyH2 mRNA (upper panel) and ACTA1 mRNA (lower panel) in C2C12 cells.
- EGT indicates the use of ergothioneine-added medium
- VC indicates the use of ascorbic acid-added medium
- E + VC indicates the use of ergothionein and ascorbine-added medium.
- NT indicates that a medium without the addition of ergothioneine and ascorbic acid was used.
- the present inventors have found for the first time that ergothioneine promotes muscle differentiation.
- EGT Ergothioneine
- EGT Ergothioneine
- its antioxidant capacity is higher than that of vitamin C, vitamin E, cysteine, and glutathione.
- EGT has an ultraviolet absorbing effect, a melanin production inhibitory effect, an ability to eliminate reactive oxygen species, an elastase activity inhibitory effect to suppress the formation of wrinkles and sagging, and a tyrosinase activity inhibitory effect to suppress the formation of spots.
- EGT is one of the compounds that have attracted particular attention in the beauty and food industries. However, it was first found by the present inventors that EGT promotes muscle differentiation.
- muscle differentiation refers to the differentiation of stem cells or muscle progenitor cells having the ability to differentiate into skeletal muscle into myotube cells.
- the medium of the present invention is used to promote the differentiation of these cells.
- Stem cells are mesodermal stem cells.
- the mesodermal stem cells may be present in a living body, or may be derived from iPS cells or ES cells.
- Muscle progenitor cells of skeletal muscle are known, and include satellite cells and myoblasts.
- myogenic differentiation refers to the differentiation of satellite cells or myoblasts into myotubes. Satellite cells have somatic stem cell-like properties and are precursor cells of muscle cells. Satellite cells are activated and differentiate into myoblasts.
- EG EGT used in the present invention may be in a free form or a salt form.
- a salt of EGT may be formed between the negatively charged oxygen of the carboxyl group of the EGT and, for example, a hydrogen ion or an alkali metal ion, and may be formed between the positively charged nitrogen of the trimethylamino group and, for example, a halide ion. May be formed.
- the EGT used in the present invention may be a hydrate.
- the differentiation into muscle can be confirmed by the expression of myosin, a muscle-specific protein.
- promotion of muscle differentiation refers to an increase in the level of myosin gene expression in stem cells or muscle progenitor cells. In a particular embodiment, it refers to the transformation of satellite cells or myoblasts into cells that overexpress myosin mRNA.
- promotion of muscle differentiation means that a satellite cell or a myoblast-derived muscle cell increases the gene expression level of myosin mRNA as a muscle marker by 1.5 times or more, preferably 2 times or more, and more preferably 3 times or more. It may be defined as increasing.
- promotion of muscle differentiation refers to increasing the expression of MyoD mRNA in satellite cells or myoblasts by 1.5 times or more, preferably by 2 times or more, in the absence of EGT.
- the present invention provides a medium additive for promoting muscle differentiation, comprising EGT.
- the additive of the present invention is added at the time of preparing the medium or to the prepared medium.
- the additive of the present invention may be added to a medium during culturing.
- the form of the additive of the present invention may be any form, and may be a solid such as a powder, a granule or a tablet, a semi-solid such as a paste, or a liquid such as a concentrated liquid.
- the present invention provides, in the fourth aspect, a method for promoting muscle differentiation of cells, comprising culturing the cells in a medium containing EGT.
- a method for promoting muscle differentiation of cells comprising culturing the cells in a medium containing EGT.
- satellite cells or myoblasts are cultured.
- Culture conditions such as medium composition, culture temperature, and culture time are known to those skilled in the art.
- the present invention provides a pharmaceutical composition for promoting muscle differentiation, comprising EGT.
- the subject of administration of the pharmaceutical composition of the present invention is not particularly limited as long as it is an animal that can benefit from muscle regeneration.
- the animal to which the pharmaceutical composition of the present invention is administered is preferably a human, but may be a non-human animal such as a dog, cat, horse, or cow.
- Examples of methods for measuring muscle mass, particularly skeletal muscle mass include, but are not limited to, BIA, CT and MRI, DXA, ultrasonic echo, and visual observation.
- Methods for measuring muscular strength, particularly skeletal muscle strength include, but are not limited to, methods for measuring grip strength, back muscle strength, leg extension strength, walking speed, body-raising, standing long jump, ball throwing, and the like.
- the pharmaceutical composition of the present invention can be produced using known means and methods such as mixing, kneading, stirring, drying, pulverizing, tableting, and solubilizing.
- the pharmaceutical composition of the present invention usually contains a carrier or excipient. Carriers or excipients are known to those skilled in the art and can be selected according to the administration site, administration route, amount of EGT contained, and the like.
- the dosage form of the pharmaceutical composition of the present invention may be any dosage form and is not particularly limited.
- the dosage form may be, for example, a liquid such as an injection, an infusion, an oral solution, a lotion, or a semi-solid such as a cream, a pasta, an ointment, a tablet, a powder, a granule, a troche, a suppository, and the like. Or a freeze-dried powder that can be prepared at the time of use.
- the administration route of the pharmaceutical composition of the present invention may be any route, for example, intramuscular injection, subcutaneous injection, intradermal injection, intravenous injection, infusion, transdermal administration, administration from cheek mucosa, transanal administration, oral Although administration is mentioned, it is not particularly limited.
- administration for skeletal muscle regeneration, preferably the pharmaceutical composition of the present invention is injected intramuscularly.
- the dose of EGT by the pharmaceutical composition of the present invention is an amount capable of promoting muscle differentiation in vivo or an amount capable of regenerating muscle.
- the dose of EGT with the pharmaceutical composition of the present invention varies depending on the desired degree of muscle regeneration, the site of administration, the route of administration, and the like. For example, when administered orally to an adult, it is usually 1 mg to 5000 mg, preferably 10 mg to 5000 mg per day. It may be 1000 mg, but is not limited to these amounts.
- the dose of EGT can be appropriately determined by a doctor while checking the size and amount of muscle, for example.
- the pharmaceutical composition of the present invention may be administered once to several times a day.
- the pharmaceutical composition of the present invention may be administered daily or every one to several days.
- the administration of the pharmaceutical composition of the present invention may be continued until the desired muscle regeneration effect appears.
- the present invention provides a food or drink for promoting muscle differentiation.
- the food and drink of the present invention is provided to animals that benefit from muscle regeneration.
- the animal species is not particularly limited, but is preferably human.
- the animal species may be non-human animals such as dogs, cats and horses.
- the food and drink of the present invention include not only general foods but also health foods, specified health foods, nutritionally functional foods, functionally labeled foods and the like.
- the food and drink of the present invention may be provided as a supplement.
- the form of the supplement may be any, and is not particularly limited.
- the form of the food and drink of the present invention may be any form, and is not particularly limited.
- the food and drink of the present invention may be one obtained by adding EGT to, for example, existing food and drink.
- it may be in the form of tablets, powders, granules, troches, candies, juices, nectars, drinks, seasonings and the like.
- the supplement can be produced by a method according to a known method for producing a pharmaceutical composition or a method according to a known method for producing a food or drink.
- the food and drink of the present invention can be used to promote muscle regeneration, especially skeletal muscle regeneration. You may. Specific uses of the food and drink of the present invention include suppression of sarcopenia and promotion of muscle regeneration at the time of injury, but are not limited to these uses.
- the muscle to be regenerated may be at any site.
- the amount of EGT ingested by the food or drink of the present invention is an amount capable of promoting muscle differentiation or an amount capable of regenerating muscle in vivo.
- the amount of EGT ingested by the food or drink of the present invention varies depending on the desired degree of muscle regeneration, the site of muscle regeneration, and the like. For example, when administered orally to an adult, it is usually 1 mg to 5000 mg, preferably 10 mg to 1000 mg per day. However, it is not limited to these amounts.
- the food or drink of the present invention may be taken once to several times a day.
- the food or drink of the present invention may be taken daily or every one to several days.
- the administration of the food or drink of the present invention may be continued until the desired muscle regeneration effect appears.
- the present inventors have found that by using EGT in combination with ascorbic acid 2-glucoside (hereinafter, referred to as AG) and / or ascorbic acid (hereinafter, referred to as VC), muscle differentiation can be achieved. For the first time, they find it to be greatly facilitated. Therefore, the medium, medium additive, kit, pharmaceutical composition, and food and drink of the present invention described above may contain AG and / or VC in addition to EGT. In the method for promoting muscle differentiation of cells of the present invention described above, EGT may be used in combination with AG and / or VC. The combined use of EGT and AG and / or VC can further promote muscle differentiation and further promote muscle regeneration.
- AG is a vitamin C derivative, and is a substance in which glucose is bonded to the 2-position hydroxyl group of ascorbic acid by ⁇ -coordination. AG is a substance having high stability and high safety for living organisms. AG is recognized as an active ingredient in food additives and quasi-drugs, and is used, for example, as a main ingredient in whitening cosmetics or as a nutritional enhancer in foods.
- VC also known as vitamin C, is a substance having an antioxidant action widely found in nature. VC is used in foods, pharmaceuticals, quasi-drugs, cosmetics, and the like as antioxidants and nutritional enhancers.
- the medium of the present invention containing AG and / or VC, a culture medium additive, a kit, a pharmaceutical composition and a food and drink, and the method of promoting muscle differentiation of cells of the present invention using AG and / or VC can be used for cells and living organisms.
- the VC used in the present invention may be in a free form or a salt form.
- VC salts include, but are not limited to, the sodium, potassium and calcium salts.
- the present inventors have found that AG alone promotes muscle differentiation, that VC alone promotes muscle differentiation, and that both AG and VC promote muscle differentiation. It is heading for the first time. Accordingly, the present invention provides the following.
- A A medium for promoting muscle differentiation, comprising AG and / or VC.
- B A medium additive for promoting muscle differentiation, comprising AG and / or VC.
- C A kit for promoting muscle differentiation, comprising AG and / or VC.
- D A method for promoting muscle differentiation of cells, comprising culturing the cells in a medium containing AG and / or VC.
- E A pharmaceutical composition for promoting muscle differentiation, comprising AG and / or VC.
- F The pharmaceutical composition according to (e), for promoting muscle regeneration.
- G A food or drink for promoting muscle differentiation, comprising AG and / or VC.
- H The food or drink according to (g), for promoting muscle regeneration.
- the amount of AG to be added to the medium of the present invention depends on the types of stem cells and muscle progenitor cells (for example, satellite cells or myoblasts) to be cultured, culture conditions such as culture temperature and culture time, and the composition of the medium. Can be appropriately determined. For example, about 0.05 mM to about 5 mM, preferably about 0.2 mM to about 2 mM, of AG may be added to a known low serum medium for muscle differentiation.
- the amount of VC added to the medium of the present invention is the same as the amount of AG added.
- the dose of AG in the pharmaceutical composition of the present invention varies depending on the desired degree of muscle regeneration, administration site and administration route. For example, when administered orally to an adult, it is usually about 10 mg to about 10,000 mg, preferably about 10 mg per day. It may be from 100 mg to about 1000 mg.
- the dose of AG can be appropriately determined by a doctor while checking the size and amount of muscle, for example.
- the dose of VC according to the pharmaceutical composition of the present invention is the same as the dose of AG.
- the amount of AG taken by the food and drink of the present invention varies depending on the desired degree of muscle regeneration, the site of muscle regeneration, and the like. For example, when taken by an adult, usually about 1 mg to about 10000 mg, preferably about 10 mg to about 10,000 mg per day. It may be 1000 mg.
- the intake of VC by the food and drink of the present invention is the same as the intake of AG.
- the present invention provides the following.
- (1) A method for promoting muscle differentiation of a cell comprising culturing the cell in a medium containing EGT.
- (M) A method for promoting muscle differentiation in a subject requiring administration of a pharmaceutical composition containing EGT to the subject in need of promoting muscle differentiation.
- EGT and AG and / or VC may be used in combination.
- the present invention provides the following.
- Q Use of EGT for production of a medium for promoting muscle differentiation.
- R Use of EGT for the production of a media additive to promote muscle differentiation.
- S Use of EGT for the manufacture of a kit for promoting muscle differentiation.
- T Use of EGT to promote muscle differentiation of cells in medium.
- U Use of EGT for the manufacture of a medicament for promoting muscle differentiation in a subject.
- V the use, wherein the medicament is for regenerating muscle in the subject;
- W Use of ergoonein for the manufacture of a food or drink to promote muscle differentiation in a subject.
- X The use according to (w), wherein the food or drink is for regenerating muscle in the subject.
- Z Use of EGT to promote muscle differentiation in a subject.
- EGT may be used in combination with AG and / or VC.
- the present invention provides the following.
- Aa A method for producing a culture medium for promoting muscle differentiation, comprising adding AG and / or VC.
- Bb A method for producing a medium additive for promoting muscle differentiation, comprising adding AG and / or VC.
- Cc A method for producing a kit for promoting muscle differentiation, comprising adding AG and / or VC.
- Dd A method for promoting muscle differentiation of a cell, comprising culturing the cell in a medium containing AG and / or VC.
- Ee A method for promoting muscle differentiation in a subject requiring promotion of muscle differentiation, comprising administering a pharmaceutical composition comprising AG and / or VC to the subject.
- the present invention provides the following.
- (Mm) Use of AG and / or VC for the manufacture of a medicament for promoting muscle differentiation in a subject.
- C2C12 cells were suspended in Dulbecco's modified Eagle's medium containing 10% fetal calf serum (hereinafter referred to as growth medium) and adjusted to a cell density of 65 ⁇ 10 4 cells / ml. 1.5 ml of the cell suspension was added to a 6-well plate, and cultured in a 5% CO 2 incubator for 24 hours. After removing the growth medium, 2 ml of Dulbecco's modified Eagle medium (hereinafter referred to as differentiation medium) containing 0.5 mM EGT and containing 2% horse serum was added. The differentiation medium was replaced with a new one every other day, and the culture was continued. The control group performed exactly the same operation except that it did not contain EGT.
- Dulbecco's modified Eagle's medium containing 10% fetal calf serum
- C2C12 cells were suspended in growth medium and adjusted to a cell density of 65 ⁇ 10 4 cells / ml. 1.5 ml of the cell suspension was added to a 6-well plate, and cultured in a 5% CO 2 incubator for 24 hours. After removing the growth medium, 2 ml of a differentiation medium containing 0.1 mM or 0.5 mM of EGT was added. The control group (hereinafter abbreviated as NT) performed exactly the same operation except that it did not contain EGT. Two days later, the medium was removed, and TRIzol (Invitrogen) was added in 1 ml portions to lyse the cells. The cell lysate was collected in an Eppendorf tube, and RNA was collected according to the TRIzol protocol.
- NT a differentiation medium containing 0.1 mM or 0.5 mM of EGT
- RNA was used to obtain a complementary strand DNA of mRNA using a reverse transcription kit (Takara Bio).
- a reverse transcription kit (Takara Bio)
- MyoD is known as a master regulator that induces muscle differentiation.
- PPIA is known as a housekeeping gene, and the expression level of the PPIA gene was used as an internal standard.
- the expression level of MyoD mRNA was corrected by dividing the expression level of MyoD mRNA by the expression level of PPIA mRNA, and the MyoD mRNA expression level was calculated.
- FIG. 2 shows the gene expression level (relative value) when EGT was added, with the MyoD mRNA expression level of NT being 1. MyoD mRNA expression levels increased depending on the concentration of EGT.
- C2C12 cells were suspended in growth medium and adjusted to a cell density of 65 ⁇ 10 4 cells / ml. 1.5 ml of the cell suspension was added to a 6-well plate, and cultured in a 5% CO 2 incubator for 24 hours. After removing the growth medium, 2 ml of a differentiation medium containing 0.5 mM EGT was added. The control group (NT) performed exactly the same operation except that EGT was not included. Two days later, the medium was removed, 1 ml of TRIzol (Invitrogen) was added, and RNA was recovered according to the TRIzol protocol. The recovered RNA was converted to a complementary strand DNA using a reverse transcription kit (Takara Bio).
- TRIzol Invitrogen
- FIG. 3 shows the gene expression level (relative value) when EGT was added, assuming that the mRNA expression level of NT was 1. The addition of EGT increased the expression levels of MyH2 mRNA and ACTA1 mRNA.
- C2C12 cells were suspended in growth medium and adjusted to a cell density of 65 ⁇ 10 4 cells / ml. 1.5 ml of the cell suspension was added to a 6-well plate, and cultured in a 5% CO 2 incubator for 24 hours. After removing the growth medium, 2 ml of a differentiation medium containing 0.5 mM EGT or 25 ⁇ M edaravone (hereinafter abbreviated as Edv) and 50 ⁇ M Edv was added. Edv is a powerful synthetic antioxidant. The medium was replaced with a new one every other day and the culture was continued. The control group (NT) performed exactly the same operation except that EGT was not included.
- a reverse transcription kit (Takara Bio)
- the following primer DNA (MyH2 [NM — 001039545]: forward 5′GAGCAAGATGCAGGGAAAG3 ′ (SEQ ID NO: 5), reverse to the obtained complementary strand DNA according to the protocol, according to the protocol.
- the expression levels of MyH2 and ACTA1 mRNA in C2C12 cells in the case of using a differentiation medium containing only EGT, the case of using a differentiation medium containing EGT and AG, and the case of using a differentiation medium containing only AG were determined.
- Examined. AA2G (registered trademark) made by Hayashibara was used as AG.
- the concentration of EGT in the differentiation medium was 0.5 mM, and the concentration of AG was 1.0 mM.
- the experimental procedure was the same as in Example 3.
- the control group (NT) performed exactly the same operation except that EGT and AG were not included.
- FIG. 5 shows the results. It was confirmed that the addition of EGT and AG to the medium significantly increased the expression of MyH2 mRNA and ACTA1 mRNA.
- the expression levels of MyH2 and ACTA1 mRNA in C2C12 cells in the case of using a differentiation medium containing only EGT, in the case of using a differentiation medium containing EGT and VC, and in the case of using a differentiation medium containing only VC were determined. Examined. The concentration of EGT in the differentiation medium was 0.5 mM, and the concentration of VC was 0.5 mM. The experimental procedure was the same as in Example 3. The control group (NT) performed exactly the same operation except that it did not contain EGT and VC. FIG. 6 shows the results. It was confirmed that the addition of EGT and VC to the medium significantly increased the expression of MyH2 mRNA and ACTA1 mRNA.
- the present invention can be used in the fields of pharmaceuticals, foods, etc. and research reagents.
- SEQ ID NO: 1 shows the nucleotide sequence of a forward primer for amplifying MyoD gene.
- SEQ ID NO: 2 shows a base sequence of a reverse primer for amplifying MyoD gene.
- SEQ ID NO: 3 shows a base sequence of a forward primer for amplifying PPIA gene.
- SEQ ID NO: 4 shows a base sequence of a reverse primer for amplifying PPIA gene.
- SEQ ID NO: 5 shows a base sequence of a forward primer for amplifying MyH2 gene.
- SEQ ID NO: 6 shows a base sequence of a reverse primer for amplifying MyH2 gene.
- SEQ ID NO: 7 shows a base sequence of a forward primer for amplifying ACTA1 gene.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Epidemiology (AREA)
- Nutrition Science (AREA)
- Polymers & Plastics (AREA)
- Food Science & Technology (AREA)
- Biomedical Technology (AREA)
- Mycology (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Zoology (AREA)
- Rheumatology (AREA)
- Genetics & Genomics (AREA)
- Biotechnology (AREA)
- Wood Science & Technology (AREA)
- Molecular Biology (AREA)
- Pediatric Medicine (AREA)
- Microbiology (AREA)
- Cell Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biochemistry (AREA)
- General Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Physical Education & Sports Medicine (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Neurology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Coloring Foods And Improving Nutritive Qualities (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US17/255,779 US12454678B2 (en) | 2018-06-29 | 2019-06-11 | Muscle differentiation-promoting actions of ergothioneine, ascorbic acid 2-glucoside, ascorbic acid, and combination thereof |
| EP19826692.6A EP3816278A4 (en) | 2018-06-29 | 2019-06-11 | MYOGENIC DIFFERENTIATION PROMOTING ACTIVITIES OF ERGOTHIONEINE, ASCORBIC ACID-2-GLUCOSIDE, ASCORBIC ACID AND THEIR COMBINATION |
| JP2020527369A JP7759724B2 (ja) | 2018-06-29 | 2019-06-11 | エルゴチオネイン、アスコルビン酸2-グルコシド、アスコルビン酸、およびそれらの組み合わせの筋分化促進作用 |
| CN201980043514.6A CN112352046A (zh) | 2018-06-29 | 2019-06-11 | 麦角硫因、抗坏血酸2-葡糖苷、抗坏血酸和它们的组合的成肌分化促进作用 |
| JP2023203550A JP2024026241A (ja) | 2018-06-29 | 2023-12-01 | エルゴチオネイン、アスコルビン酸2-グルコシド、アスコルビン酸、およびそれらの組み合わせの筋分化促進作用 |
| JP2025114256A JP2025148417A (ja) | 2018-06-29 | 2025-07-07 | エルゴチオネイン、アスコルビン酸2-グルコシド、アスコルビン酸、およびそれらの組み合わせの筋分化促進作用 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2018-124613 | 2018-06-29 | ||
| JP2018124613 | 2018-06-29 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2020004014A1 true WO2020004014A1 (ja) | 2020-01-02 |
Family
ID=68986429
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2019/023114 Ceased WO2020004014A1 (ja) | 2018-06-29 | 2019-06-11 | エルゴチオネイン、アスコルビン酸2-グルコシド、アスコルビン酸、およびそれらの組み合わせの筋分化促進作用 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US12454678B2 (enExample) |
| EP (1) | EP3816278A4 (enExample) |
| JP (3) | JP7759724B2 (enExample) |
| CN (1) | CN112352046A (enExample) |
| TW (1) | TWI815907B (enExample) |
| WO (1) | WO2020004014A1 (enExample) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2022230487A1 (ja) * | 2021-04-26 | 2022-11-03 | サントリーホールディングス株式会社 | 赤血球及び/又はヘモグロビン増加用組成物 |
| JP2023132710A (ja) * | 2022-03-11 | 2023-09-22 | キッコーマン株式会社 | エルゴチオネインを光に対し安定化させた液体組成物 |
| WO2024172003A1 (ja) * | 2023-02-16 | 2024-08-22 | サントリーホールディングス株式会社 | エルゴチオネインを含有する筋萎縮抑制用組成物及びSrcチロシンキナーゼ阻害用組成物 |
| DE102023131771B3 (de) | 2023-09-15 | 2025-02-06 | Esspen Gmbh | Verfahren und Vorrichtung zur Verarbeitung medizintechnischer Bilddaten im Zusammenhang mit einer Trepanation |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2022242711A1 (en) * | 2021-05-20 | 2022-11-24 | Nanjing Nutrabuilding Bio-Tech Co., Ltd. | Methods for ameliorating and preventing age‐related muscle degeneration |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009167169A (ja) * | 2007-11-30 | 2009-07-30 | Lvmh Recherche | アスコルビン酸−2−グルコシド及びエルゴチオネインを含んでなる化粧組成物 |
| US20150157648A1 (en) * | 2012-06-26 | 2015-06-11 | Entia Biosciences, Inc. | Nutritional approach to improving athletic performance and reducing injury with l-ergothioneine and/or vitamin d2 |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU2003243266A1 (en) * | 2002-05-20 | 2003-12-12 | Oxis International, Inc. | Method of reducing incidence diabetic embryopathy with l-ergothioneine |
| CA2683858A1 (en) * | 2007-04-12 | 2008-10-23 | Oxis International, Inc. | Ergothioneine and/or its derivatives as a cell preservative |
| US20110262965A1 (en) * | 2010-04-23 | 2011-10-27 | Life Technologies Corporation | Cell culture medium comprising small peptides |
| US20120141611A1 (en) | 2010-12-05 | 2012-06-07 | Oxis International Inc. | Methods and compositions using ergothioneine to treat a variety of health related factors |
| TWI549696B (zh) * | 2014-09-05 | 2016-09-21 | 中國醫藥大學 | 麥角硫因應用於活化細胞之Nrf2訊息途徑之用途 |
-
2019
- 2019-06-11 US US17/255,779 patent/US12454678B2/en active Active
- 2019-06-11 EP EP19826692.6A patent/EP3816278A4/en active Pending
- 2019-06-11 CN CN201980043514.6A patent/CN112352046A/zh active Pending
- 2019-06-11 JP JP2020527369A patent/JP7759724B2/ja active Active
- 2019-06-11 WO PCT/JP2019/023114 patent/WO2020004014A1/ja not_active Ceased
- 2019-06-13 TW TW108120402A patent/TWI815907B/zh active
-
2023
- 2023-12-01 JP JP2023203550A patent/JP2024026241A/ja active Pending
-
2025
- 2025-07-07 JP JP2025114256A patent/JP2025148417A/ja active Pending
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009167169A (ja) * | 2007-11-30 | 2009-07-30 | Lvmh Recherche | アスコルビン酸−2−グルコシド及びエルゴチオネインを含んでなる化粧組成物 |
| US20150157648A1 (en) * | 2012-06-26 | 2015-06-11 | Entia Biosciences, Inc. | Nutritional approach to improving athletic performance and reducing injury with l-ergothioneine and/or vitamin d2 |
Non-Patent Citations (4)
| Title |
|---|
| MITSUMOTO Y. ET AL.: "A LONG-LASTING VITAMIN C DERIVATIVE, ASCRBIC ACID 2-PHOSPHATE, INCREASES MYOGENIN GENE EXPRESSION AND PROMOTES DIFFERENTIATION IN L6 MUSCLE CELLS", BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS, vol. 199, no. 1, 28 February 1994 (1994-02-28), pages 394 - 402, XP024764982, ISSN: 0006-291X, DOI: 10.1006/bbrc.1994.1242 * |
| ONITSUKA Y: "Investigation of an efficient culture condition for the differentiation of murine skeletal myoblasts", JPN. J. VET. RES., vol. 61, no. 1, 2, 2013, pages 39 |
| ÖZTÜRKLER Y. ET AL.: "The Effects of L-Ergothioneine and L-Ascorbic Acid on the In Vitro Maturation (IVM) and Embryonic Development (IVC) of Sheep Oocytes", KAFKAS UNIV VET FAK DERG, vol. 16, no. 5, 1 January 2010 (2010-01-01), pages 757 - 763, XP055666935, ISSN: 1300-6045, DOI: 10.9775/kvfd.2009.1646 * |
| See also references of EP3816278A4 |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2022230487A1 (ja) * | 2021-04-26 | 2022-11-03 | サントリーホールディングス株式会社 | 赤血球及び/又はヘモグロビン増加用組成物 |
| JPWO2022230487A1 (enExample) * | 2021-04-26 | 2022-11-03 | ||
| JP2023132710A (ja) * | 2022-03-11 | 2023-09-22 | キッコーマン株式会社 | エルゴチオネインを光に対し安定化させた液体組成物 |
| JP7569818B2 (ja) | 2022-03-11 | 2024-10-18 | キッコーマン株式会社 | エルゴチオネインを光に対し安定化させた液体組成物 |
| WO2024172003A1 (ja) * | 2023-02-16 | 2024-08-22 | サントリーホールディングス株式会社 | エルゴチオネインを含有する筋萎縮抑制用組成物及びSrcチロシンキナーゼ阻害用組成物 |
| DE102023131771B3 (de) | 2023-09-15 | 2025-02-06 | Esspen Gmbh | Verfahren und Vorrichtung zur Verarbeitung medizintechnischer Bilddaten im Zusammenhang mit einer Trepanation |
Also Published As
| Publication number | Publication date |
|---|---|
| CN112352046A (zh) | 2021-02-09 |
| US20210130787A1 (en) | 2021-05-06 |
| EP3816278A4 (en) | 2022-05-04 |
| JP2024026241A (ja) | 2024-02-28 |
| TW202015549A (zh) | 2020-05-01 |
| TWI815907B (zh) | 2023-09-21 |
| JP7759724B2 (ja) | 2025-10-24 |
| JPWO2020004014A1 (ja) | 2021-07-15 |
| EP3816278A1 (en) | 2021-05-05 |
| US12454678B2 (en) | 2025-10-28 |
| JP2025148417A (ja) | 2025-10-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7759724B2 (ja) | エルゴチオネイン、アスコルビン酸2-グルコシド、アスコルビン酸、およびそれらの組み合わせの筋分化促進作用 | |
| JP2020528085A (ja) | 幹細胞から抽出されたエキソソームを有効成分として含む骨粗鬆症の予防または治療用の組成物 | |
| JP7379152B2 (ja) | 筋線維化抑制用組成物 | |
| EP2913059A1 (en) | Novel method for treating spinal cord injury using hmgb1 fragment | |
| US20180104269A1 (en) | Composition for suppressing muscular fatty change | |
| CN104271735B (zh) | 含有海藻糖的用于预防肺栓塞形成的哺乳动物细胞悬浮液 | |
| CN109963574B (zh) | 用于预防或治疗肝纤维化的包含脂肪干细胞来源外排体作为活性成分的组合物 | |
| US20190038769A1 (en) | Micro-rna profiling, compositions, and methods of treating diseases | |
| US20180071248A1 (en) | Composition for improving circadian rhythm | |
| KR102187681B1 (ko) | 히알루론산 나노입자를 함유하는 섬유증의 예방 또는 치료용 약학 조성물 | |
| US11414663B2 (en) | Micro-RNA profiling, compositions, and methods of treating diseases | |
| EP3216858B1 (en) | Agent for maintaining stem cells in undifferentiated state and agent for promoting growth thereof | |
| KR20210132050A (ko) | I형 콜라겐 또는 엘라스틴의 산생 촉진제 | |
| JP6468895B2 (ja) | 幹細胞の未分化状態維持剤及び増殖促進剤 | |
| US10744151B1 (en) | Micro-RNA profiling, compositions, and methods of treating diseases | |
| KR20230026096A (ko) | 줄기세포 유래 세포외소포체를 유효성분으로 포함하는 조성물 | |
| JP7083141B2 (ja) | 筋肉量減少又は筋力低下を改善するための組成物及びその利用 | |
| JP6840376B2 (ja) | 幹細胞の未分化状態維持剤及び増殖促進剤 | |
| US20230040823A1 (en) | Micro-rna profiling, compositions, and methods of treating diseases | |
| JP6587899B2 (ja) | 幹細胞の未分化状態維持剤及び増殖促進剤 | |
| CN114642662B (zh) | Mk-5046的药物用途 | |
| JP2014015428A (ja) | サテライト細胞分化促進剤 | |
| JP2020143008A (ja) | 新規褐色脂肪細胞分化誘導剤 | |
| US20190040466A1 (en) | Micro-rna profiling, compositions, and methods of treating diseases | |
| JP5923404B2 (ja) | Trpv4活性抑制剤 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 19826692 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2020527369 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWG | Wipo information: grant in national office |
Ref document number: 17255779 Country of ref document: US |