WO2019244934A1 - 新規抗pad2抗体 - Google Patents
新規抗pad2抗体 Download PDFInfo
- Publication number
- WO2019244934A1 WO2019244934A1 PCT/JP2019/024310 JP2019024310W WO2019244934A1 WO 2019244934 A1 WO2019244934 A1 WO 2019244934A1 JP 2019024310 W JP2019024310 W JP 2019024310W WO 2019244934 A1 WO2019244934 A1 WO 2019244934A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- pad2
- antibody
- present
- amino acid
- positions
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/40—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against enzymes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/78—Hydrolases (3) acting on carbon to nitrogen bonds other than peptide bonds (3.5)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K7/00—Peptides having 5 to 20 amino acids in a fully defined sequence; Derivatives thereof
- C07K7/04—Linear peptides containing only normal peptide links
- C07K7/08—Linear peptides containing only normal peptide links having 12 to 20 amino acids
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y305/00—Hydrolases acting on carbon-nitrogen bonds, other than peptide bonds (3.5)
- C12Y305/03—Hydrolases acting on carbon-nitrogen bonds, other than peptide bonds (3.5) in linear amidines (3.5.3)
- C12Y305/03006—Arginine deiminase (3.5.3.6)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/23—Immunoglobulins specific features characterized by taxonomic origin from birds
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/34—Identification of a linear epitope shorter than 20 amino acid residues or of a conformational epitope defined by amino acid residues
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/62—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising only variable region components
- C07K2317/622—Single chain antibody (scFv)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y305/00—Hydrolases acting on carbon-nitrogen bonds, other than peptide bonds (3.5)
- C12Y305/03—Hydrolases acting on carbon-nitrogen bonds, other than peptide bonds (3.5) in linear amidines (3.5.3)
- C12Y305/03015—Protein-arginine deiminase (3.5.3.15)
Definitions
- the present invention relates to a novel anti-PAD2 antibody.
- PAD2 Peptidylarginine deiminase 2
- PAD2 is known as an enzyme involved in citrullination of arginine in proteins. This citrullination is important for the structure and reaction of the protein, because arginine, which is the most basic of the amino acids constituting the protein, is converted to neutral citrulline.
- Non-Patent Document 1 describes that PAD2 is involved in TNF ⁇ -induced citrullination and arthritis (Abstract).
- Non-Patent Document 2 describes that citrullinated myelin basic protein represents a biochemical pathway for the development of multiple sclerosis (Abstract).
- Non-Patent Document 3 describes the relationship between citrullinated histones and the onset of arthritis (Abstract).
- Patent Document 1 discloses that a rabbit PAD2 was immunized to a mouse to prepare an anti-PAD2 antibody (Example 1), that the antibody bound to positions 1 to 165 of human PAD2 (Example 3), and that the antibody inhibited PAD2. It describes that the activity was examined (Example IV).
- the anti-PAD2 antibody of Patent Document 1 does not have a sufficiently strong PAD2 inhibitory activity and has room for improvement. In addition, no antibody having excellent PAD2 inhibitory activity has been reported.
- the present invention has been made in view of the above circumstances, and has as its object to provide an anti-PAD2 antibody having excellent PAD2 inhibitory activity.
- the inventors of the present invention prepared an anti-PAD2 antibody using a peptide having the amino acid sequence represented by SEQ ID NO: 1 (positions 341 to 357 of PAD2) as an antigen, as described in Examples below.
- SEQ ID NO: 1 positions 341 to 357 of PAD2
- the antibody had an excellent inhibitory activity on PAD2.
- this antibody unexpectedly exhibited significantly superior PAD2 inhibitory activity as compared to the anti-PAD2 antibodies (# 2 and # 34) prepared based on Patent Document 1.
- an anti-PAD2 antibody which specifically binds to positions 341 to 357 of PAD2 (Peptidylarginine deiminase 2). Using this antibody, PAD2 can be detected. The use of this antibody can inhibit the function of PAD2.
- an anti-PAD2 antibody that specifically binds to a peptide having the amino acid sequence represented by SEQ ID NO: 1. Using this antibody, PAD2 can be detected. The use of this antibody can inhibit the function of PAD2.
- an anti-PAD2 antibody which specifically binds to positions 344 to 357 of PAD2.
- PAD2 can be detected.
- the use of this antibody can inhibit the function of PAD2.
- a polynucleotide or a vector encoding the above-mentioned anti-PAD2 antibody there is provided a composition comprising the above anti-PAD2 antibody.
- a PAD2 citrullination activity inhibitor comprising the above-mentioned anti-PAD2 antibody.
- a method for producing an anti-PAD2 antibody comprising a step of growing a cell containing the polynucleotide or the vector.
- the KD (M) for PAD2 may be 9.0 ⁇ 10 ⁇ 8 or less, and the binding site may be a binding site identified by an alanine scan with three amino acid substitutions.
- the anti-PAD2 antibody may be a monoclonal antibody, and the anti-PAD2 antibody may be an antigen-binding fragment.
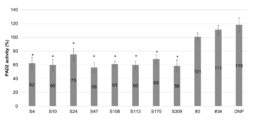
- FIG. 1 is a diagram showing the results of measuring the affinity of the anti-PAD2 antibody used in the examples.
- FIG. 2 is a diagram showing the results of measuring the citrullination activity of human PAD2 when the anti-PAD2 antibody used in the examples was added. * (p ⁇ 0.01) is shown in the results of the antibodies that showed a significant difference from the anti-DNP antibody (negative control) by t-test (two-sided).
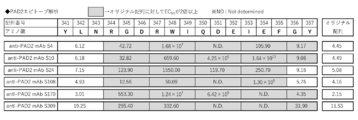
- FIG. 3 is a diagram showing the results of evaluating the epitope of the anti-PAD2 antibody used in the examples.
- One embodiment of the present invention is a novel anti-PAD2 antibody.
- This antibody is, for example, an anti-PAD2 antibody that specifically binds to positions 341 to 357 of PAD2.
- it may be an anti-PAD2 antibody that specifically binds to a peptide consisting of the amino acid sequence represented by SEQ ID NO: 1.
- it may be an anti-PAD2 antibody that specifically binds to positions 344 to 357 of PAD2. If this antibody is used, for example, the function of PAD2 can be inhibited.
- PAD2 is generally known as an enzyme involved in citrullination of arginine in proteins. Details of the amino acid sequence of PAD2 and the like can be found on a website such as NCBI (National Center for Biotechnology Information) or HGNC (HUGO Gene Nomenclature Corporation). The accession number of PAD2 described in NCBI is, for example, NP_031391.2. The amino acid sequence of PAD2 is, for example, SEQ ID NO: 2. PAD2 is not limited in its biological origin as long as it has PAD2 activity. PAD2 includes, for example, PAD2 from humans, monkeys, mice, rats, dogs, or cats. Positions 341 to 357 of PAD2 are typically Y, L, N, R, G, D, R, W, I, Q, D, E, I, E, F, G, Y (single amino acid) Notation) is located.
- anti-PAD2 antibody includes an antibody having a binding property to PAD2.
- the method for producing the anti-PAD2 antibody is not particularly limited.
- the anti-PAD2 antibody may be produced by immunizing mammals or birds with PAD2.
- An anti-PAD2 antibody that specifically binds to positions 341 to 357 of PAD2 may be produced, for example, by immunizing a mammal or bird with a peptide consisting of the amino acid sequence shown in SEQ ID NO: 1.
- An anti-PAD2 antibody that specifically binds to positions 344 to 357 or 344 to 355 of PAD2 is, for example, a peptide consisting of the amino acid sequence represented by SEQ ID NO: 1, or a position 4 to 17 or 4 to 15 of SEQ ID NO: 1.
- the peptide may be produced by immunizing a mammal or bird with a peptide consisting of the amino acid sequence of position 1.
- An anti-PAD2 antibody that specifically binds to positions 344 to 357 or 344 to 355 of PAD2 shows, for example, binding to wild-type PAD2 and to Ala mutant PAD2 at positions 344 to 357 or 344 to 355. It may be obtained through a step of selecting an anti-PAD2 antibody that does not show binding property.
- the anti-PAD2 antibody according to one embodiment of the present invention may be an antibody that inhibits the function of PAD2. Function inhibition includes, for example, inhibition of citrullination activity.
- the anti-PAD2 antibody according to one embodiment of the present invention may be an antibody that binds to calcium-bound PAD2 or an antibody that inhibits the function of calcium-bound PAD2.
- the anti-PAD2 antibody according to one embodiment of the present invention may have a function of inhibiting PAD2 activity.
- the anti-PAD2 antibody according to one embodiment of the present invention may have an antibody that binds to calcium-bound PAD2 or a function of inhibiting calcium-bound PAD2 activity.
- the anti-PAD2 antibody according to one embodiment of the present invention may be a monoclonal antibody. If it is a monoclonal antibody, it can act on PAD2 more efficiently than a polyclonal antibody. From the viewpoint of efficiently producing an anti-PAD2 monoclonal antibody having a desired effect, it is preferable to immunize chickens with PAD2.
- PAD2 used as an antigen includes PAD2 full length or PAD2 peptide fragment unless otherwise specified.
- the antibody class of the anti-PAD2 antibody according to one embodiment of the present invention is not particularly limited, and may be, for example, IgM, IgD, IgG, IgA, IgE, or IgY.
- the antibody subclass is not particularly limited, but may be, for example, IgG1, IgG2, IgG3, IgG4, IgA1, or IgA2.
- the anti-PAD2 antibody according to one embodiment of the present invention may be an antibody fragment having PAD2 binding activity (hereinafter, may be referred to as “antigen-binding fragment”).
- antigen-binding fragment an antibody fragment having PAD2 binding activity
- there are effects such as an increase in stability or an antibody production efficiency.
- the anti-PAD2 antibody according to one embodiment of the present invention may be a fusion protein.
- This fusion protein may be one in which a polypeptide or oligopeptide is bound to the N or C terminus of PAD2.
- the oligopeptide may be a His tag.
- the fusion protein may be a fusion of a mouse, human, or chicken antibody partial sequence. Such fusion proteins are also included in one form of the anti-PAD2 antibody according to the present embodiment.
- the anti-PAD2 antibody according to one embodiment of the present invention may be an antibody obtained through a step of immunizing chickens with PAD2.
- the anti-PAD2 antibody has a KD (M) of, for example, 9.0 ⁇ 10 ⁇ 8 , 7.0 ⁇ 10 ⁇ 8 , 5.0 ⁇ 10 ⁇ 8 , 3.0 ⁇ 10 ⁇ 8 , 1.0 ⁇ 10 ⁇ 8. , 9.0 ⁇ 10 ⁇ 9 , 7.0 ⁇ 10 ⁇ 9 , 5.0 ⁇ 10 ⁇ 9 , 3.0 ⁇ 10 ⁇ 9 or less, and may be in the range of any two of them.
- the anti-PAD2 antibody according to one embodiment of the present invention may be an antibody that binds to PAD2 wild type or mutant type. Mutants include those resulting from DNA sequence differences between individuals, such as SNPs.
- the amino acid sequence of the wild-type or mutant PAD2 is preferably at least 80%, more preferably at least 90%, more preferably at least 95%, particularly preferably at least 98%, the amino acid sequence shown in SEQ ID NO: 2. Has the property.
- the “anti-PAD2 antibody that specifically binds to positions 341 to 357 of PAD2” includes an antibody that binds within the amino acid region of positions 341 to 357 of PAD2. As long as this antibody has, for example, a binding property to the peptide having the amino acid sequence represented by SEQ ID NO: 1, the site recognized by the antibody within positions 341 to 357 of PAD2 is not limited.
- This antibody includes, for example, an antibody that specifically binds to an epitope containing one or more amino acids at positions 341 to 357 of PAD2.
- This antibody includes, for example, an antibody that specifically binds to positions 344 to 357 of PAD2.
- anti-PAD2 antibody that specifically binds to positions 344 to 357 of PAD2 includes an antibody that binds within the amino acid region of positions 344 to 357 of PAD2.
- This antibody includes, for example, an antibody that specifically binds to an epitope containing one or more amino acids at positions 344 to 357 of PAD2.
- the antibodies include, for example, antibodies that specifically bind to PAD2 at positions 344-346, 347-349, 350-352, 353-355, or 356-357.
- the antibodies also include, for example, antibodies that specifically bind to positions 344 to 355 of PAD2.
- anti-PAD2 antibody that specifically binds to positions 344 to 355 of PAD2 includes an antibody that binds within the amino acid region of positions 344 to 355 of PAD2.
- This antibody includes, for example, an antibody that specifically binds to an epitope containing one or more amino acids at positions 344 to 355 of PAD2.
- Such antibodies include, for example, antibodies that specifically bind to positions 344-346, 347-349, 350-352, or 353-355 of PAD2.
- the antibodies also include, for example, antibodies that specifically bind to positions 347 to 355 of PAD2.
- anti-PAD2 antibody that specifically binds to positions 347 to 355 of PAD2 includes an antibody that binds within the amino acid region of positions 347 to 355 of PAD2.
- This antibody includes, for example, an antibody that specifically binds to an epitope containing one or more amino acids at positions 347 to 355 of PAD2.
- This antibody includes, for example, an antibody that specifically binds to positions 347 to 349, 350 to 352, or 353 to 355 of PAD2.
- the anti-PAD2 antibody according to one embodiment of the present invention may be an antibody that has binding to wild-type PAD2 and does not have binding to Ala mutant PAD2 at positions 344 to 357.
- “Ala mutant PAD2 at positions 344 to 357” is a PAD2 at positions 344 to 346, 347 to 349, 350 to 352, 353 to 355, 356 to 357, or 344 to Includes Ala variant antibody at position 355.
- the Ala mutant PAD2 at positions 344 to 357 may not be substituted at positions other than positions 344 to 346, 347 to 349, 350 to 352, 353 to 355, or 356 to 357.
- "having no binding” means that it does not substantially have binding. Or, it includes the case where it has no significant binding property. Or, in comparison with the EC 50 for wild-type, EC 50 for evaluation may be evaluated as having no binding to the case of more than two times. At this time, if the EC 50 is Not determined, it may be evaluated that it has no binding property. Alternatively, when the binding to the evaluation target is 50% or less as compared with the binding to the wild type, the binding may be evaluated as having no binding.
- Anti PAD2 antibody according to an embodiment of the present invention the EC 50 for 344 to 357 positions of Ala mutant PAD2, compared to EC 50 for wild-type PAD2, may be an antibody is more than twice.
- ⁇ double or more '' may be, for example, 2, 3, 4, 5 , 10, 100, 10 5 , 10 10 , or 10 15 , and any two of those values may be used. It may be within the range.
- anti-PAD2 polyclonal antibody includes, for example, antiserum. Binding or reactivity includes affinity.
- the anti-PAD2 antibody according to one embodiment of the present invention may be an antibody having binding to Ala mutant PAD2 at positions 344 to 357 of 50% or less as compared to binding to wild-type PAD2.
- the anti-PAD2 antibody according to one embodiment of the present invention is an antibody whose binding to Ala mutant PAD2 at positions 344 to 357 is 50% or less as compared with the binding using an anti-PAD2 polyclonal antibody. You may.
- “50% or less” may be, for example, 50, 45, 40, 35, 30, 25, 20, 15, 10, 5, 1, or 0%, and any of them. It may be between two values.
- the anti-PAD2 antibody comprises a step of selecting an antibody showing significant binding to wild-type PAD2, or an antibody showing no binding to Ala mutant PAD2 at positions 344 to 357. And a step of selecting.
- the anti-PAD2 antibody according to one embodiment of the present invention may have binding to other amino acid residues within the epitope as long as it has specific binding to positions 341 to 357 of PAD2.
- An antibody that specifically binds to a specific site may be an antibody that recognizes a specific site.
- the anti-PAD2 antibody according to one embodiment of the present invention may be an antibody that specifically binds to an epitope within positions 341 to 357 of PAD2.
- the anti-PAD2 antibody according to one embodiment of the present invention may be an antibody that specifically binds to an epitope including positions 341 to 357 of PAD2.
- the anti-PAD2 antibody according to one embodiment of the present invention may have binding to amino acid residues other than positions 341 to 357 of PAD2 in the epitope.
- the anti-PAD2 antibody according to one embodiment of the present invention has a binding property to the peptide having the amino acid sequence represented by SEQ ID NO: 1, and has at least one kind of the peptide having the amino acid sequence represented by SEQ ID NO: 3 to 8. An antibody having no binding property may be used.
- the anti-PAD2 antibody according to one embodiment of the present invention may be an antibody having no binding to the deleted PAD2 that does not include positions 341 to 357 of PAD2.
- Anti PAD2 antibody according to an embodiment of the present invention the EC 50 s against one or more peptides of the amino acid sequence shown in SEQ ID NO: 3-8, as compared to the EC 50 for wild-type PAD2, is more than twice antibody It may be.
- Anti PAD2 antibody according to an embodiment of the present invention SEQ ID NO: 3 ⁇ EC 50 against one or more peptides of the amino acid sequence represented by 8, as compared to the EC 50 for the case of using the anti PAD2 polyclonal antibodies, 2 The antibody may be more than twice as many.
- the anti-PAD2 antibody according to one embodiment of the present invention is an antibody whose binding to one or more of the peptides having the amino acid sequences represented by SEQ ID NOs: 3 to 8 is 50% or less as compared to the binding to wild-type PAD2. It may be.
- the anti-PAD2 antibody according to one embodiment of the present invention has a binding property to one or more of the peptides having the amino acid sequences represented by SEQ ID NOS: 3 to 8 compared to the binding property using the anti-PAD2 polyclonal antibody. % Or less.
- the anti-PAD2 antibody may be an antibody that specifically binds to positions 4 to 17 or 4 to 15 of the peptide having the amino acid sequence represented by SEQ ID NO: 1.
- This antibody has binding to other amino acid residues within the epitope as long as it has binding at positions 4 to 17 or 4 to 15 of the peptide having the amino acid sequence represented by SEQ ID NO: 1. Is also good.
- an antibody that specifically binds to a peptide includes an antibody having specific binding property to a peptide.
- An antibody that specifically binds to a peptide is one in which one of the binding properties of the antibody is specified.
- the antibody may have binding property to a peptide and may have binding property to another compound.
- An antibody that specifically binds to the peptide having the amino acid sequence represented by SEQ ID NO: 1 includes an antibody having specific binding to the peptide and binding to full-length PAD2. The same applies to an antibody that specifically binds to positions 4 to 17 or 4 to 15 of the peptide having the amino acid sequence represented by SEQ ID NO: 1.
- the site to which the anti-PAD2 antibody according to one embodiment of the present invention binds may be evaluated by, for example, alanine scanning.
- the “alanine scan” is a technique of, for example, substituting a certain amino acid in a protein with alanine and examining the properties of an antibody binding to the protein.
- Evaluation by alanine scan for example, (i) a step of preparing an Ala mutant by replacing 3 amino acid residues of the antigen with Ala, (ii) a step of measuring the affinity of the Ala mutant and the test antibody, Alternatively, a binding site that can be evaluated through (iii) a step of evaluating an amino acid residue before Ala substitution as a binding site in an Ala mutant in which the test antibody did not show significant reactivity.
- the above step (i) may include a step of substituting 3 amino acid residues of a plurality of antigens with Ala to produce a plurality of Ala mutants.
- This evaluation method may include the step of (iv) measuring the affinity of the test antibody to wild-type PAD2, or measuring the affinity between the Ala mutant and the anti-PAD2 polyclonal antibody.
- This evaluation method is based on (v) the EC 50 value of the test antibody when measuring the affinity between the test antibody and the Ala mutant, the test value when measuring the affinity between the test antibody and the wild type.
- the method may include a step of evaluating that the compound did not show any reactivity.
- Alanine scanning may be performed by three amino acid substitutions. At this time, if the number of amino acids contained in the antigen is not divisible by 3, one end of the antigen may be substituted with 2 amino acids.
- the antigen used for the alanine scan may be PAD2 or a peptide fragment thereof. Affinity may be assessed, for example, by ELISA or surface plasmon resonance analysis.
- the anti-PAD2 antibody according to one embodiment of the present invention may be an antibody that specifically binds to an epitope including positions 347 to 354 of PAD2.
- the anti-PAD2 antibody according to one embodiment of the present invention may be an antibody that specifically binds to an epitope including positions 351, 352, 353, or 354 of PAD2. This antibody may have binding to other amino acid residues within the epitope as long as it has binding at positions 351, 352, 353 or 354 of PAD2.
- the anti-PAD2 antibody according to one embodiment of the present invention may be an antibody that specifically binds to positions 8, 9, 10, or 11 of the peptide having the amino acid sequence shown in SEQ ID NO: 1.
- the anti-PAD2 antibody may be an antibody that does not have specific binding to a mutant PAD2 having an Ala mutation at positions 351, 352, 353, or 354 of PAD2. . From the viewpoint of the ability to inhibit citrullination, these antibodies are particularly preferably antibodies that recognize positions 351, 352, 353 or 354 of PAD2. At this time, the binding may be evaluated by an alanine scan in which one amino acid residue is substituted with Ala.
- an "antibody” comprises a molecule or a population thereof capable of specifically binding to a particular epitope on an antigen.
- the antibody may be a polyclonal antibody or a monoclonal antibody.
- the antibody can exist in various forms, for example, a full-length antibody (an antibody having a Fab region and an Fc region), an Fv antibody, a Fab antibody, an F (ab ') 2 antibody, a Fab' antibody, a diabody, and a single antibody.
- Antibodies include modified or unmodified antibodies.
- the modified antibody may be a combination of an antibody and various molecules such as polyethylene glycol.
- the modified antibody can be obtained by chemically modifying the antibody using a known technique.
- the amino acid sequence, class, or subclass of the antibody may be derived, for example, from a human, a non-human mammal (eg, rat, mouse, rabbit, cow, monkey, etc.), or a bird (eg, chicken).
- Antibodies also include, for example, isolated, purified, or recombinant antibodies. Antibodies can be used, for example, in vitro or in vivo.
- a “polyclonal antibody” is used to administer an immunogen containing an antigen of interest to mammals (eg, rats, mice, rabbits, cows, monkeys, etc.) or birds (eg, chickens). It can be generated by Administration of the immunogen may be infused with one or more immunizing agents or adjuvants. Adjuvants are sometimes used to increase the immune response and may include Freund's adjuvant (complete or incomplete), mineral gels (such as aluminum hydroxide), or surfactants (such as lysolecithin) and the like. . Immunization protocols are known in the art and may be performed by any method that elicits an immune response, depending on the host organism selected (Protein Experiment Handbook, @Yodosha (2003): 86-91). .).
- “monoclonal antibody” includes an antibody in which individual antibodies constituting a population react to substantially the same epitope. Alternatively, the antibodies may be those in which the individual antibodies constituting the population are substantially the same (naturally occurring mutations are allowed). Monoclonal antibodies are highly specific, differing from ordinary polyclonal antibodies, which typically include different antibodies corresponding to different epitopes.
- the method for producing the monoclonal antibody is not particularly limited. For example, a method similar to the hybridoma method described in “Kohler G, Milstein C., Nature. 1975 Aug 7; 256 (5517): 495-497.” May be produced.
- monoclonal antibodies may be made by methods similar to recombinant methods as described in US Pat. No. 4,816,567.
- the monoclonal antibody is "Clackson et al., Nature. 1991 Aug 15; 352 (6336): 624-628.”
- it may be prepared by the method described in "Protein Experiment Handbook, @Yodosha (2003): 92-96.”
- the “Fv antibody” is an antibody including an antigen recognition site.
- This region contains a non-covalently linked dimer of one heavy and one light chain variable domain.
- the three CDRs of each variable domain can interact to form an antigen binding site on the surface of the VH-VL dimer.
- the ⁇ Fab antibody '' is, for example, a fragment obtained by treating an antibody containing a Fab region and an Fc region with the protease papain, and the N-terminal half of the H chain and the entire L chain. Is an antibody bound via some disulfide bonds.
- Fab can be obtained, for example, by treating the above-described anti-PAD2 antibody containing an Fab region and an Fc region according to the embodiment of the present invention with the protease papain.
- the ⁇ F (ab ') 2 antibody'' for example, of the fragment obtained by treating the antibody containing the Fab region and the Fc region with the protease pepsin, the site corresponding to the Fab 2 Antibodies.
- F (ab ′) 2 can be obtained, for example, by treating the above-mentioned anti-PAD2 antibody containing an Fab region and an Fc region according to the embodiment of the present invention with the protease pepsin. Further, for example, it can be produced by making the following Fab ′ a thioether bond or a disulfide bond.
- the “Fab ′ antibody” is, for example, an antibody obtained by cleaving a disulfide bond in the hinge region of F (ab ′) 2 .
- it can be obtained by treating F (ab ') 2 with a reducing agent dithiothreitol.
- the “scFv antibody” is an antibody in which VH and VL are linked via an appropriate peptide linker.
- the scFv antibody for example, to obtain a cDNA encoding the VH and VL of the anti-PAD2 antibody according to the embodiment of the present invention, construct a polynucleotide encoding a VH-peptide linker-VL, and vector the polynucleotide And can be produced using cells for expression.
- diabody is an antibody having a bivalent antigen-binding activity.
- the divalent antigen-binding activities can be the same, or one can be a different antigen-binding activity.
- a diabody is constructed, for example, by constructing a polynucleotide encoding scFv such that the amino acid sequence of the peptide linker has a length of 8 residues or less, incorporating the resulting polynucleotide into a vector, and using cells for expression. it can.
- dsFv is an antibody in which a polypeptide having a cysteine residue introduced into VH and VL is bound via a disulfide bond between the cysteine residues.
- the position to be introduced into the cysteine residue should be selected based on the three-dimensional structure prediction of the antibody according to the method shown by Reiter et al. (Reiter et al., Protein Eng. 1994 May; 7 (5): 697-704.) Can be.
- the "peptide or polypeptide having antigen-binding property" is an antibody comprising VH or VL of an antibody, or CDR1, 2, or 3 thereof. Peptides containing multiple CDRs can be linked directly or via a suitable peptide linker.
- the above Fv antibody, Fab antibody, F (ab ') 2 antibody, Fab' antibody, scFv antibody, diabody, dsFv antibody, peptide or polypeptide having antigen-binding property (hereinafter, also referred to as "Fv antibody etc.”
- the production method is not particularly limited. For example, it can be produced using expression cells by incorporating a DNA encoding a region such as an Fv antibody in the anti-PAD2 antibody according to the embodiment of the present invention into an expression vector. Alternatively, it may be produced by a chemical synthesis method such as the Fmoc method (fluorenylmethyloxycarbonyl method) and the tBOC method (t-butyloxycarbonyl method).
- the antigen-binding fragment according to the embodiment of the present invention may be one or more of the Fv antibodies and the like.
- a “chimeric antibody” is one in which the variable region of an antibody between heterologous organisms and the constant region of the antibody are linked, and can be constructed by genetic recombination technology.
- a mouse-human chimeric antibody, a chicken-human chimeric antibody, a chicken-mouse chimeric antibody and the like can be mentioned.
- a mouse-human chimeric antibody can be prepared, for example, by the method described in "Roguska et al., Proc Natl Acad Sci U U S A.
- Basic methods for producing mouse-human chimeric antibodies include, for example, encoding mouse leader and variable region sequences present in cloned cDNAs, encoding human antibody constant regions already present in mammalian cell expression vectors. Ligated to the sequence. Alternatively, a mouse leader sequence and a variable region sequence present in the cloned cDNA may be ligated to a sequence encoding a human antibody constant region, and then ligated to a mammalian cell expression vector.
- Fragments of the human antibody constant region can be those of the H chain constant region of any human antibody and the L chain constant region of the human antibody, such as C ⁇ 1, C ⁇ 2, C ⁇ 3 or C ⁇ 4 for those of the human H chain, For the L chain, C ⁇ or C ⁇ can be mentioned, respectively.
- a ⁇ humanized antibody '' has, for example, one or more CDRs from a non-human species, and a framework region from a human immunoglobulin, and a constant region from a human immunoglobulin. Is an antibody that binds to the antigen.
- the "human antibody” is, for example, a region comprising the variable region and the constant region of the heavy chain, the variable region and the constant region of the light chain constituting the antibody is derived from a gene encoding human immunoglobulin.
- Antibody Various techniques known in the art may be used for producing human antibodies.
- the anti-PAD2 antibody according to one embodiment of the present invention may be in the form of scFv, and in that case, may have a linker between the heavy chain and the light chain.
- the linker include, but are not limited to, a sequence of 0 to 5 amino acids typically consisting of G and P. A linker is not required and may not be present.
- amino acid is a generic term for organic compounds having an amino group and a carboxyl group.
- any of the amino acids in the amino acid sequence may be chemically modified. Further, any of the amino acids in the amino acid sequence may form a salt or a solvate. Further, any of the amino acids in the amino acid sequence may be L-type or D-type. Even in such cases, it can be said that the antibody according to the embodiment of the present invention includes the above “specific amino acid sequence”.
- Examples of the chemical modification of amino acids contained in proteins in vivo include N-terminal modification (eg, acetylation, myristoylation, etc.), C-terminal modification (eg, amidation, glycosylphosphatidylinositol addition, etc.), or side chain Modifications (eg, phosphorylation, sugar chain addition, etc.) are known.
- N-terminal modification eg, acetylation, myristoylation, etc.
- C-terminal modification eg, amidation, glycosylphosphatidylinositol addition, etc.
- side chain Modifications eg, phosphorylation, sugar chain addition, etc.
- One embodiment of the present invention is a polynucleotide or a vector encoding the anti-PAD2 antibody according to the above embodiment of the present invention.
- a transformant can be prepared by introducing this polynucleotide or vector into a cell.
- the transformant may be a cell of a human or a non-human mammal (eg, rat, mouse, guinea pig, rabbit, cow, monkey, etc.).
- Examples of the mammalian cells include Chinese hamster ovary cells (CHO cells), monkey cells COS-7, human embryo-derived kidney cells (eg, HEK293 cells), and the like.
- the transformant may be a genus Escherichia, yeast, or the like.
- the polynucleotide or the vector may be constructed so that an anti-PAD2 antibody can be expressed.
- the polynucleotide or vector may include components necessary for protein expression, such as, for example, a promoter, an enhancer, an origin of replication, or an antibiotic resistance gene.
- the polynucleotide or vector may have a heterologous base sequence.
- the heterologous nucleotide sequence is, for example, two or more organisms selected from the group consisting of humans and non-human organisms (eg, bacteria, archaea, yeast, insects, birds, viruses, mammals other than humans, and the like) It may contain a base sequence derived from it.
- Examples of the above vectors include Escherichia coli-derived plasmids (eg, pET-Blue), Bacillus subtilis-derived plasmids (eg, pUB110), yeast-derived plasmids (eg, pSH19), and animal cell expression plasmids (eg, pA1-11, pcDNA3.1- V5 / His-TOPO), bacteriophage such as ⁇ phage, virus-derived vectors, and the like.
- the vector may be an expression vector or may be circular.
- a calcium phosphate method, a lipofection method, an electroporation method, a method using an adenovirus, a method using a retrovirus, or microinjection can be used (revised 4th edition) Niigata Genetic Engineering Handbook, Yodosha (2003): 152-179.).
- a method for producing an antibody using cells for example, the method described in "Protein Experiment Handbook, Yodosha (2003): 128-142.” Can be used.
- One embodiment of the present invention is a method for producing an anti-PAD2 antibody, comprising a step of growing cells containing the polynucleotide or the vector according to the above-described embodiment of the present invention.
- the step of growing includes a step of culturing.
- the production method may include a step of recovering the anti-PAD2 antibody.
- the production method may include a step of preparing a cell culture solution.
- the production method may include a step of purifying the anti-PAD2 antibody.
- the purification of the antibody comprises, for example, ammonium sulfate, ethanol precipitation, protein A, protein G, gel filtration chromatography, anion, cation exchange chromatography, phosphocellulose chromatography, hydrophobic interaction chromatography. Chromatography, affinity chromatography, hydroxylapatite chromatography, or lectin chromatography can be used (Protein Experiment Handbook, @Yodosha (2003): 27-52.).
- One embodiment of the present invention is a composition comprising the anti-PAD2 antibody according to the above embodiment of the present invention.
- PAD2 can be detected efficiently.
- it can efficiently inhibit citrullination of PAD2.
- the components contained in the composition are not particularly limited, and may include, for example, a buffer.
- one or more of the various embodiments of the inhibitor and pharmaceutical composition described below may be applied.
- One embodiment of the present invention is a PAD2 citrullination activity inhibitor comprising the anti-PAD2 antibody according to the above embodiment of the present invention.
- Use of this inhibitor can efficiently inhibit citrullination by PAD2.
- the rate of decrease in citrullination activity by the inhibitor may be 20, 30, 40, 60, 80% or more, or may be in the range of any two of them. This reduction rate may be represented by, for example, a relative rate when the reduction rate when using PBS is 0%.
- “agent” includes a composition used for research or treatment, for example.
- the inhibitors include, for example, therapeutic agents.
- the inhibitors can be used, for example, in vitro or inovo.
- the inhibitor may include the composition according to the embodiment of the present invention described above.
- One embodiment of the present invention is a method for inhibiting PAD2 citrullination activity, which comprises a step of contacting the anti-PAD2 antibody according to the above embodiment of the present invention with PAD2.
- One embodiment of the present invention is a method of inhibiting PAD2 citrullination activity, comprising the step of administering the above-mentioned anti-PAD2 antibody according to the embodiment of the present invention to a patient.
- the above-mentioned inhibition method includes an inhibition method performed for research or treatment.
- One embodiment of the present invention is the use of an anti-PAD2 antibody according to the above embodiment of the present invention for producing an inhibitor of citrullination activity of PAD2.
- the protein to be citrullinated is not particularly limited, and includes, for example, a protein having arginine. This protein includes, for example, histone and myelin basic proteins.
- One embodiment of the present invention is a pharmaceutical composition comprising the anti-PAD2 antibody according to the above embodiment of the present invention.
- the pharmaceutical composition may include one or more pharmacologically acceptable carriers.
- Pharmaceutical compositions include, for example, pharmaceutical compositions for the treatment of arthritis, rheumatoid arthritis, or multiple sclerosis.
- the pharmaceutical composition may include the composition according to the embodiment of the present invention described above.
- One embodiment of the present invention is a method for treating a disease, comprising a step of administering the above-mentioned anti-PAD2 antibody (or a pharmaceutical composition containing the anti-PAD2 antibody) according to the above-mentioned embodiment of the present invention to a patient.
- Treatment includes, for example, treatment of arthritis, rheumatoid arthritis, multiple sclerosis, or Sjogren's syndrome.
- Anti-PAD2 antibodies such as S4 described in Examples below are particularly effective in treating arthritis or rheumatoid arthritis because they strongly inhibit histone citrullination.
- anti-PAD2 antibodies such as S4 strongly inhibit citrullination of myelin basic protein, and are therefore particularly effective for treating multiple sclerosis.
- One embodiment of the present invention is the use of an anti-PAD2 antibody according to the above embodiments of the present invention for producing a pharmaceutical composition.
- One embodiment of the present invention is a PAD2 detection reagent containing the anti-PAD2 antibody according to the above embodiment of the present invention. If this reagent is used, PAD can be detected efficiently.
- One embodiment of the present invention is a method for detecting PAD2, comprising a step of bringing a test sample into contact with the anti-PAD2 antibody according to the above embodiment of the present invention.
- One embodiment of the present invention is a kit including the anti-PAD2 antibody according to the above embodiment of the present invention. Using this kit, for example, treatment, diagnosis, or detection of PAD2 can be performed.
- This kit may include, for example, the composition according to the above-described embodiment of the present invention, an inhibitor, a pharmaceutical composition, a diagnostic agent, or a detection reagent, and may include an instruction manual, a buffer solution, a container, or a package. May be included.
- “treatment” includes the ability to exert a symptom-ameliorating effect, a suppressing effect, or a prophylactic effect of a patient's disease or one or more symptoms associated with the disease.
- the “therapeutic agent” may be a pharmaceutical composition comprising the active ingredient and one or more pharmacologically acceptable carriers.
- the “pharmaceutical composition” may be produced, for example, by mixing an active ingredient and the above-mentioned carrier and by any method known in the technical field of pharmaceuticals. The form of use of the pharmaceutical composition is not limited as long as it is used for treatment, and the active ingredient may be a single active ingredient or a mixture of the active ingredient and an optional ingredient.
- the shape of the carrier is not particularly limited, and may be, for example, a solid or a liquid (for example, a buffer).
- the content of the carrier may be, for example, a pharmaceutically effective amount.
- An effective amount can be, for example, an amount sufficient for pharmaceutically stability or delivery of the active ingredient.
- buffers are effective in stabilizing the active ingredients in vials.
- the dose, administration interval, and administration method are not particularly limited, and may be appropriately selected depending on the patient's age, body weight, symptoms, target organ, and the like.
- the pharmaceutical composition preferably contains a therapeutically effective amount, or an effective amount of an active ingredient that exhibits a desired effect.
- One embodiment of the present invention provides a method for promoting the ability of a composition to inhibit citrullination activity, comprising increasing the proportion of an anti-PAD2 antibody that specifically binds to positions 341 to 357 of PAD2 in the composition. is there.
- One embodiment of the present invention is a composition comprising an anti-PAD2 antibody, wherein 90% or more of the anti-PAD2 antibody molecules in the composition are anti-PAD2 antibodies that specifically bind to positions 341-357 of PAD2.
- One embodiment of the invention is an antibody population comprising an anti-PAD2 antibody, wherein 90% or more of the anti-PAD2 antibody molecules in the antibody population are anti-PAD2 antibodies that specifically bind to positions 341-357 of PAD2.
- There is an antibody population The above 90% or more may be, for example, 90%, 95, 96, 97, 98, 99% or more, or 100%, or may be in the range of any two values.
- One embodiment of the present invention is a peptide consisting of the amino acid sequence represented by SEQ ID NO: 1.
- an antibody that binds to PAD2 can be produced. Further, an antibody that binds to PAD2 can be detected.
- the peptide consisting of the amino acid sequence represented by SEQ ID NO: 1 may have undergone chemical modification (for example, KLH modification), and such a chemically modified peptide is also a form of the peptide consisting of the amino acid sequence represented by SEQ ID NO: 1. included.
- the peptide having the amino acid sequence represented by SEQ ID NO: 1 may be an isolated, purified, or concentrated peptide.
- One embodiment of the present invention is an antigen composition comprising a peptide having the amino acid sequence represented by SEQ ID NO: 1.
- the antigen composition may include, for example, a buffer or an adjuvant.
- One embodiment of the present invention is a method for producing an anti-PAD2 antibody, comprising a step of immunizing a mammal or bird with a peptide consisting of the amino acid sequence represented by SEQ ID NO: 1.
- One embodiment of the present invention is a method for producing an anti-PAD2 antibody, comprising a step of bringing an antibody or an antibody library into contact with a peptide consisting of the amino acid sequence represented by SEQ ID NO: 1.
- One embodiment of the present invention is a method for detecting an antibody that binds to PAD2, comprising a step of contacting a peptide consisting of the amino acid sequence represented by SEQ ID NO: 1 with a test sample containing an anti-PAD2 antibody.
- One embodiment of the present invention is a composition for detecting an antibody that binds to active PAD2, comprising a peptide having the amino acid sequence represented by SEQ ID NO: 1.
- bond may be a covalent bond or a non-covalent bond, and may be, for example, an ionic bond, a hydrogen bond, a hydrophobic interaction, or a hydrophilic interaction.
- "significantly” means that, for example, a statistically significant difference is evaluated using a Student's t-test (one-sided or two-sided), and p ⁇ 0.05 or p ⁇ 0.01 is satisfied. Is also good. Alternatively, a state in which a difference is substantially generated may be employed.
- Example 1 Preparation of Anti-PAD2 Antibody 333 ⁇ g of a KLH-modified peptide antigen (SEQ ID NO: 1, positions 341 to 357 of PAD2) was immunized intraperitoneally to three 3-month-old Boris Brown birds. Antigen was immunized with complete Freund's adjuvant (014-09541, Wako) for primary immunization and incomplete Freund's adjuvant (011-09551, Wako) for secondary and tertiary immunization. For the fourth immunization, an antigen diluted in PBS (phosphate buffered saline) was injected intravenously. Blood was collected from the subwing vein every other week, and the antibody titer was confirmed by ELISA.
- PBS phosphate buffered saline
- the fourth immunization was defined as the final immunization.
- chicken spleens were collected, lymphocytes were isolated by density gradient centrifugation using Ficoll paque PLUS (17-1440-03, GE Healthcare), and RNA was isolated using TRIzole Reagent (15596026, Life Technologies). Was extracted.
- CDNA was synthesized from the extracted RNA by RT-PCR using PrimeScript II 1st Strand cDNA Synthesis Kit (6210A, TAKARA) to prepare a scFv phage library.
- An expression vector of a type in which a chicken ⁇ chain was inserted instead of the mouse ⁇ chain of pPDS was used.
- the scFv phage library was prepared according to the method described in the reference: "Nakamura et al., J Vet Med Sci. 2004 Ju; 66 (7): 807-814".
- scFv phage antibody library panning was performed on a plate on which full-length PAD2 was immobilized. Panning was performed according to the method described in the reference: "Nakamura et al., J Vet Med Sci. 2004 Ju; 66 (7): 807-814". After performing panning five times, the reactivity of the library was confirmed by ELISA using a plate on which the synthetic peptide was immobilized, and phage was screened from the library where the reactivity began to increase.
- phage was infected with Escherichia coli, plated on 2 ⁇ YT Agar plate containing ampicillin (50 ⁇ g / ml, Nacalai), and the obtained colonies were cultured in 2 ⁇ YT liquid medium containing ampicillin. After infection with helper phage, phage induction was performed in 2X YT liquid medium containing ampicillin (50 ⁇ g / ml), kanamycin (25 ⁇ g / ml, Meiji Seika Co., Ltd.) and IPTG (100 ⁇ g / ml, nacalai). went.
- the reactivity of the scFv phage antibody in the obtained culture supernatant was confirmed by ELISA using an antigen-immobilized plate.
- the obtained positive clones were sequenced using a DNA sequencer (ABI PRISM 3100-Genetic Analyzer, Applied Biosystems) to determine the sequence.
- Clones having different sequences were subjected to amplification of the chicken antibody gene H chain variable region and L chain variable region by PCR using the DNA chain encoding the scFv antibody as a template, and then treating the PCR product with SacII and NheI restriction enzymes ( R0157S, R0131S, BioLabs), and a mouse / chicken chimeric antibody (IgG1) expression vector (H chain expression vector: pcDNA4 / myc-His), which was similarly treated with restriction enzymes for the H chain variable region and the L chain variable region.
- ELISA ELISA was performed under the following conditions to evaluate the reactivity of S4 and the like to human PAD2.
- Material / antibody anti-PAD2 antibody (S4, S10, S24, S47, S108, S113, S170, S309), WO / 2014/086365 anti-PAD2 antibody (# 2, # 34), anti-PAD2 antibody Dinitrophenyl (DNP) antibody (negative control antibody).
- # 2 and # 34 those prepared based on the amino acid sequence of the variable region described in the examples of WO / 2014/086365 were used (the same applies to the following examples).
- -Antigen full-length human PAD2 antigen
- Example 3 Evaluation of ability of anti-PAD2 antibody to inhibit citrullination activity Under the following conditions, the ability of anti-PAD2 antibody to inhibit citrullination activity of PAD2 was measured.
- Table 4 shows the antibody concentration (EC 50 ) that inhibits human PAD2 by 50%. When the antibody concentration that gives 50% activity inhibition at 600 nM or less could not be calculated, it was expressed as ND (Not determined). It was shown that antibodies such as S4 have higher inhibitory activity than # 34.
- Example 4 Evaluation of ability of anti-PAD2 antibody to inhibit citrullination activity Under the following conditions, the ability of anti-PAD2 antibody to inhibit the citrullination activity of calcium conjugate PAD2 was measured.
- Table 5 shows antibody concentrations (EC 25 ) that give 25% inhibition of human PAD2 activity.
- concentration of the antibody that gives 25% activity inhibition at 600 nM or less could not be calculated, it was expressed as ND (Not determined).
- citrullination activity of the calcium conjugate PAD2 it was shown that antibodies such as S4 had a higher inhibitory activity than # 2.
- Example 5 Evaluation of ability of anti-PAD2 antibody to inhibit citrullination activity Under the following conditions, the ability of anti-PAD2 antibody to inhibit citrullination activity of PAD2 was measured. At this time, calf thymus-derived histone was used as a substrate.
- the total volume of the solution was 50 ⁇ L, final concentration 20 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1 mM DTT, 1.8 mg / mL histone, 1 mM CaCl 2 , 5 nM human PAD2, and 0.1-600 nM Each antibody.
- the solution was allowed to stand at 37 ° C. for 4 hours, and 12.5 ⁇ L of 5M perchloric acid was added to stop the reaction. Histones derived from calf thymus which were contained in this solution and were citrullinated were calorimetrically quantified.
- Table 6 shows antibody concentrations (EC 50 ) that give 50% inhibition of human PAD2 activity. When the antibody concentration that gives 50% activity inhibition at 600 nM or less could not be calculated, it was expressed as ND (Not determined). It was shown that antibodies such as S4 have higher inhibitory activity than # 2 and # 34.
- Example 6 Evaluation of ability of anti-PAD2 antibody to inhibit citrullination activity Under the following conditions, the ability of anti-PAD2 antibody to inhibit citrullination activity of PAD2 was measured. At this time, bovine myelin basic protein was used as a substrate.
- the total volume of the solution was 50 ⁇ L, final concentration 20 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1 mM DTT, 1.8 mg / mL bovine myelin basic protein, 1 mM CaCl 2 , 5 nM human PAD2, And 600 nM of each antibody.
- the solution was allowed to stand at 37 ° C. for 4 hours, and 12.5 ⁇ L of 5M perchloric acid was added to stop the reaction.
- the citrullinated bovine myelin basic protein contained in this solution was colorimetrically determined.
- FIG. 2 shows the results of measuring the citrullination activity of human PAD2 upon addition of each antibody.
- the values in FIG. 2 are the average value and the standard deviation when the TBS value is 100%.
- the antibodies # 2 and # 34 did not show any inhibitory activity.
- antibodies such as S4 showed high inhibitory activity.
- * (P ⁇ 0.01) is shown for the antibody that showed a significant difference from the anti-DNP antibody (negative control).
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Genetics & Genomics (AREA)
- General Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Medicinal Chemistry (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Molecular Biology (AREA)
- General Engineering & Computer Science (AREA)
- Immunology (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biotechnology (AREA)
- Microbiology (AREA)
- Biomedical Technology (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Physical Education & Sports Medicine (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Rheumatology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Peptides Or Proteins (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Enzymes And Modification Thereof (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2020525771A JP7084057B2 (ja) | 2018-06-20 | 2019-06-19 | 新規抗pad2抗体 |
| CA3102360A CA3102360A1 (en) | 2018-06-20 | 2019-06-19 | Novel anti-pad2 antibody |
| EP19823371.0A EP3816289A4 (en) | 2018-06-20 | 2019-06-19 | NEW ANTI-PAD2 ANTIBODIES |
| US16/972,832 US20210246225A1 (en) | 2018-06-20 | 2019-06-19 | Novel anti-pad2 antibody |
| CN201980038636.6A CN112262213A (zh) | 2018-06-20 | 2019-06-19 | 新型抗pad2抗体 |
| JP2022085941A JP7411264B2 (ja) | 2018-06-20 | 2022-05-26 | 新規抗pad2抗体 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2018-117142 | 2018-06-20 | ||
| JP2018117142 | 2018-06-20 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2019244934A1 true WO2019244934A1 (ja) | 2019-12-26 |
Family
ID=68984058
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2019/024310 Ceased WO2019244934A1 (ja) | 2018-06-20 | 2019-06-19 | 新規抗pad2抗体 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20210246225A1 (enExample) |
| EP (1) | EP3816289A4 (enExample) |
| JP (2) | JP7084057B2 (enExample) |
| CN (1) | CN112262213A (enExample) |
| CA (1) | CA3102360A1 (enExample) |
| WO (1) | WO2019244934A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023191035A1 (ja) | 2022-03-31 | 2023-10-05 | 株式会社ファーマフーズ | 線維化の抑制のための組成物又は方法 |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN118001407B (zh) * | 2024-04-02 | 2024-06-21 | 北京大学人民医院 | Pad2在制备治疗缺血缺氧性恶性心律失常药物中的应用 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| WO2014086365A1 (en) | 2012-12-03 | 2014-06-12 | Rigshospitalet | Anti-pad2 antibodies and treatment of autoimmune diseases |
| WO2016155745A1 (en) * | 2015-03-27 | 2016-10-06 | Rigshospitalet | Cross-reactive anti-pad antibodies |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040110938A1 (en) * | 2000-02-24 | 2004-06-10 | Parekh Rajesh Bhikhu | Proteins, genes and their use for diagnosis and treatment of schizophrenia |
| CA2561742A1 (en) * | 2004-04-01 | 2005-10-27 | Sequenom, Inc. | Methods for identifying risk of osteoarthritis and treatments thereof |
| WO2007095250A2 (en) * | 2006-02-13 | 2007-08-23 | The Cleveland Clinic Foundation | Compositions and methods for inhibiting optic nerve damage |
| WO2009033743A1 (en) * | 2007-09-13 | 2009-03-19 | University Of Zurich Prorektorat Forschung | Monoclonal amyloid beta (abeta)-specific antibody and uses thereof |
| ES2652127T3 (es) * | 2008-06-04 | 2018-01-31 | Modiquest B.V. | Agentes anti-inflamatorios |
| US8975033B2 (en) * | 2012-11-05 | 2015-03-10 | The Johns Hopkins University | Human autoantibodies specific for PAD3 which are cross-reactive with PAD4 and their use in the diagnosis and treatment of rheumatoid arthritis and related diseases |
| CN104360070B (zh) * | 2014-11-28 | 2017-02-22 | 山东新创生物科技有限公司 | 肽基精氨酸脱亚胺酶2在制备肿瘤临床血液诊断试剂中的应用 |
-
2019
- 2019-06-19 US US16/972,832 patent/US20210246225A1/en active Pending
- 2019-06-19 WO PCT/JP2019/024310 patent/WO2019244934A1/ja not_active Ceased
- 2019-06-19 EP EP19823371.0A patent/EP3816289A4/en active Pending
- 2019-06-19 CN CN201980038636.6A patent/CN112262213A/zh active Pending
- 2019-06-19 JP JP2020525771A patent/JP7084057B2/ja active Active
- 2019-06-19 CA CA3102360A patent/CA3102360A1/en active Pending
-
2022
- 2022-05-26 JP JP2022085941A patent/JP7411264B2/ja active Active
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| WO2014086365A1 (en) | 2012-12-03 | 2014-06-12 | Rigshospitalet | Anti-pad2 antibodies and treatment of autoimmune diseases |
| WO2016155745A1 (en) * | 2015-03-27 | 2016-10-06 | Rigshospitalet | Cross-reactive anti-pad antibodies |
Non-Patent Citations (13)
| Title |
|---|
| "NCBI", Database accession no. NP_031391.2 |
| "New Gene Engineering Handbook", 2003, YODOSHA CO .,LTD., pages: 128 - 142 |
| BAWADEKAR ET AL.: "Peptidylarginine deiminase 2 is required for tumor necrosis factor alpha-induced citrullination and arthritis, but not neutrophil extracellular trap formation", J AUTOIMMUN, vol. 80, June 2017 (2017-06-01), pages 39 - 47, XP029997133, DOI: 10.1016/j.jaut.2017.01.006 |
| CLACKSON ET AL., NATURE, vol. 352, no. 6336, 15 August 1991 (1991-08-15), pages 624 - 628 |
| KOHLER GMILSTEIN C., NATURE, vol. 256, no. 5517, 7 August 1975 (1975-08-07), pages 495 - 497 |
| MARKS ET AL., J MOL BIOL., vol. 222, no. 3, 5 December 1991 (1991-12-05), pages 581 - 597 |
| MOSCARELLO ET AL.: "The role of citrullinated proteins suggests a novel mechanism in the pathogenesis of multiple sclerosis", NEUROCHEM RES, vol. 32, no. 2, 22 September 2006 (2006-09-22), pages 251 - 6, XP019483205 |
| NAKAMURA ET AL., J VET MED SCI, vol. 66, no. 7, June 2004 (2004-06-01), pages 807 - 814 |
| REITER ET AL., PROTEIN ENG., vol. 7, no. 5, May 1994 (1994-05-01), pages 697 - 704 |
| ROGUSKA ET AL., PROC NATL ACAD SCI USA., vol. 91, no. 3, 1 February 1994 (1994-02-01), pages 969 - 973 |
| See also references of EP3816289A4 |
| SOHN ET AL.: "Local Joint inflammation and histone citrullination in a murine model of the transition from preclinical autoimmunity to inflammatory arthritis", ARTHRITIS RHEUMATOL, vol. 67, no. 11, November 2015 (2015-11-01), pages 2877 - 87 |
| TATEISHI ET AL., J VET MED SCI, vol. 70, no. 4, April 2008 (2008-04-01), pages 397 - 400 |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023191035A1 (ja) | 2022-03-31 | 2023-10-05 | 株式会社ファーマフーズ | 線維化の抑制のための組成物又は方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP7411264B2 (ja) | 2024-01-11 |
| CA3102360A1 (en) | 2019-12-26 |
| JP7084057B2 (ja) | 2022-06-14 |
| EP3816289A1 (en) | 2021-05-05 |
| EP3816289A4 (en) | 2022-03-02 |
| US20210246225A1 (en) | 2021-08-12 |
| JPWO2019244934A1 (ja) | 2021-06-24 |
| JP2022119899A (ja) | 2022-08-17 |
| CN112262213A (zh) | 2021-01-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US12448464B2 (en) | Anti-PAD4 antibody | |
| CN110769851B (zh) | 兽用抗il31抗体 | |
| JP2021526022A (ja) | 抗インターロイキン17a抗体、医薬組成物、およびその使用 | |
| TWI635097B (zh) | 新穎抗人類tslp受體抗體 | |
| JP7624185B2 (ja) | 新規抗pad4抗体 | |
| JP7179743B2 (ja) | 抗SIRPg抗体の新規の使用 | |
| WO2014194274A2 (en) | Oncostatin m receptor antigen binding proteins | |
| CN107522783B (zh) | 一种抗白介素17a的抗体、其制备方法和应用 | |
| US20220185879A1 (en) | IL17A Antibodies and Antagonists for Veterinary Use | |
| JP2022512646A (ja) | スタフィロコッカス・アウレウス(Staphylococcus aureus)ロイコトキシンに対して向けられた抗体 | |
| CN114555121A (zh) | 兽用抗il31抗体 | |
| TW202035459A (zh) | 抗人類Fn14抗體 | |
| JP7411264B2 (ja) | 新規抗pad2抗体 | |
| CN105518024A (zh) | 人抗il-32抗体 | |
| WO2013001819A1 (ja) | 可溶性インテグリンα4変異体 | |
| WO2023191035A1 (ja) | 線維化の抑制のための組成物又は方法 | |
| TW201139459A (en) | Osteopontin-specific monoclonal antibodies | |
| HK1243095A (en) | An anti-il-17a antibody, the preparation method and application thereof | |
| HK1243095A1 (en) | An anti-il-17a antibody, the preparation method and application thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 19823371 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2020525771 Country of ref document: JP |
|
| ENP | Entry into the national phase |
Ref document number: 3102360 Country of ref document: CA |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2019823371 Country of ref document: EP Effective date: 20210120 |