WO2019159887A1 - 垂直配向液晶硬化膜 - Google Patents
垂直配向液晶硬化膜 Download PDFInfo
- Publication number
- WO2019159887A1 WO2019159887A1 PCT/JP2019/004844 JP2019004844W WO2019159887A1 WO 2019159887 A1 WO2019159887 A1 WO 2019159887A1 JP 2019004844 W JP2019004844 W JP 2019004844W WO 2019159887 A1 WO2019159887 A1 WO 2019159887A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- liquid crystal
- film
- group
- cured film
- crystal cured
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- ORFAEUZTGBHBGO-XXUXADGJSA-N C=CC(OCCOC(CCC(OCCc(cc1)ccc1OC([C@H](CC1)CC[C@@H]1C(Oc(ccc(OC([C@H](CC1)CC[C@@H]1C(Oc1ccc(CCOC(CCC(OCCOC(C=C)=O)=O)=O)cc1)=O)=O)c1S2)c1SC2=C(C#N)C#N)=O)=O)=O)=O)=O Chemical compound C=CC(OCCOC(CCC(OCCc(cc1)ccc1OC([C@H](CC1)CC[C@@H]1C(Oc(ccc(OC([C@H](CC1)CC[C@@H]1C(Oc1ccc(CCOC(CCC(OCCOC(C=C)=O)=O)=O)cc1)=O)=O)c1S2)c1SC2=C(C#N)C#N)=O)=O)=O)=O)=O ORFAEUZTGBHBGO-XXUXADGJSA-N 0.000 description 1
Images
Classifications
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B5/00—Optical elements other than lenses
- G02B5/30—Polarising elements
- G02B5/3016—Polarising elements involving passive liquid crystal elements
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B7/00—Layered products characterised by the relation between layers; Layered products characterised by the relative orientation of features between layers, or by the relative values of a measurable parameter between layers, i.e. products comprising layers having different physical, chemical or physicochemical properties; Layered products characterised by the interconnection of layers
- B32B7/02—Physical, chemical or physicochemical properties
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B7/00—Layered products characterised by the relation between layers; Layered products characterised by the relative orientation of features between layers, or by the relative values of a measurable parameter between layers, i.e. products comprising layers having different physical, chemical or physicochemical properties; Layered products characterised by the interconnection of layers
- B32B7/02—Physical, chemical or physicochemical properties
- B32B7/023—Optical properties
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K19/00—Liquid crystal materials
- C09K19/04—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit
- C09K19/06—Non-steroidal liquid crystal compounds
- C09K19/34—Non-steroidal liquid crystal compounds containing at least one heterocyclic ring
- C09K19/3402—Non-steroidal liquid crystal compounds containing at least one heterocyclic ring having oxygen as hetero atom
- C09K19/3405—Non-steroidal liquid crystal compounds containing at least one heterocyclic ring having oxygen as hetero atom the heterocyclic ring being a five-membered ring
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K19/00—Liquid crystal materials
- C09K19/04—Liquid crystal materials characterised by the chemical structure of the liquid crystal components, e.g. by a specific unit
- C09K19/06—Non-steroidal liquid crystal compounds
- C09K19/34—Non-steroidal liquid crystal compounds containing at least one heterocyclic ring
- C09K19/3491—Non-steroidal liquid crystal compounds containing at least one heterocyclic ring having sulfur as hetero atom
- C09K19/3497—Non-steroidal liquid crystal compounds containing at least one heterocyclic ring having sulfur as hetero atom the heterocyclic ring containing sulfur and nitrogen atoms
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B1/00—Optical elements characterised by the material of which they are made; Optical coatings for optical elements
- G02B1/08—Optical elements characterised by the material of which they are made; Optical coatings for optical elements made of polarising materials
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B5/00—Optical elements other than lenses
- G02B5/30—Polarising elements
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B5/00—Optical elements other than lenses
- G02B5/30—Polarising elements
- G02B5/3025—Polarisers, i.e. arrangements capable of producing a definite output polarisation state from an unpolarised input state
- G02B5/3033—Polarisers, i.e. arrangements capable of producing a definite output polarisation state from an unpolarised input state in the form of a thin sheet or foil, e.g. Polaroid
-
- G—PHYSICS
- G09—EDUCATION; CRYPTOGRAPHY; DISPLAY; ADVERTISING; SEALS
- G09F—DISPLAYING; ADVERTISING; SIGNS; LABELS OR NAME-PLATES; SEALS
- G09F9/00—Indicating arrangements for variable information in which the information is built-up on a support by selection or combination of individual elements
- G09F9/30—Indicating arrangements for variable information in which the information is built-up on a support by selection or combination of individual elements in which the desired character or characters are formed by combining individual elements
-
- H—ELECTRICITY
- H05—ELECTRIC TECHNIQUES NOT OTHERWISE PROVIDED FOR
- H05B—ELECTRIC HEATING; ELECTRIC LIGHT SOURCES NOT OTHERWISE PROVIDED FOR; CIRCUIT ARRANGEMENTS FOR ELECTRIC LIGHT SOURCES, IN GENERAL
- H05B33/00—Electroluminescent light sources
- H05B33/02—Details
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K59/00—Integrated devices, or assemblies of multiple devices, comprising at least one organic light-emitting element covered by group H10K50/00
- H10K59/50—OLEDs integrated with light modulating elements, e.g. with electrochromic elements, photochromic elements or liquid crystal elements
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2323/00—Functional layers of liquid crystal optical display excluding electroactive liquid crystal layer characterised by chemical composition
- C09K2323/02—Alignment layer characterised by chemical composition
- C09K2323/023—Organic silicon compound, e.g. organosilicon
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K59/00—Integrated devices, or assemblies of multiple devices, comprising at least one organic light-emitting element covered by group H10K50/00
- H10K59/80—Constructional details
- H10K59/8791—Arrangements for improving contrast, e.g. preventing reflection of ambient light
Definitions
- the present invention relates to a vertically aligned liquid crystal cured film, a laminate, an elliptically polarizing plate, and an organic EL display device.
- An elliptically polarizing plate is an optical member in which a polarizing plate and a retardation plate are laminated.
- a polarizing plate and a retardation plate are laminated.
- a so-called ⁇ / 4 plate is used as the retardation plate.
- the phase difference plate used for such an elliptically polarizing plate one showing reverse wavelength dispersion is suitable in that it exhibits the same phase difference performance in a wide wavelength range of visible light.
- a retardation plate As a retardation plate exhibiting reverse wavelength dispersion, a retardation plate comprising a horizontally aligned liquid crystal cured film obtained by polymerizing and curing a polymerizable liquid crystal compound exhibiting reverse wavelength dispersion in a horizontal direction is known. . There is also a need for a polarizing plate with an optical compensation function having a function of compensating for the same optical performance as seen from the front direction when viewed from an oblique direction.
- a polarizing plate with an optical compensation function a polarizing plate further comprising a vertically aligned liquid crystal cured film obtained by polymerizing and curing a polymerizable liquid crystal compound in a vertically aligned state together with a horizontally aligned liquid crystal cured film having reverse wavelength dispersion is known. It has been. Further, among these vertically aligned liquid crystal cured films, Patent Document 1 proposes a vertically aligned liquid crystal cured film using a polymerizable liquid crystal compound exhibiting reverse wavelength dispersion.
- a liquid crystal compound exhibiting reverse wavelength dispersion has an unstable molecular centroid, so that a large number of alignment defects are generated with the liquid crystal compound alone, and vertical alignment is difficult. Therefore, an alignment film for vertical alignment is required for the production of a vertically aligned liquid crystal cured film. However, in that case, a process of forming an alignment film for vertical alignment is required, which causes a problem that productivity is lowered.
- the present invention has been made in view of the above problems, and an object thereof is to provide a vertically aligned liquid crystal cured film in which the occurrence of alignment defects is suppressed without an alignment film.
- the present invention includes the following aspects.
- a vertically aligned liquid crystal cured film that is aligned in a direction perpendicular to the in-plane direction and includes at least one selected from the group consisting of a nonionic silane compound and an ionic compound.
- the nonionic silane compound is a silane coupling agent having an alkoxysilyl group and a polar group.
- RthC (550) represents the retardation value in the thickness direction of the vertically aligned liquid crystal cured film at a wavelength of 550 nm
- the vertically aligned liquid crystal cured film according to any one of [1] to [5], wherein [7]
- RthC (450) represents the retardation value in the thickness direction at a wavelength of 450 nm of the vertically aligned liquid crystal cured film
- RthC (550) represents the retardation in the thickness direction of the vertically aligned liquid crystal cured film at a wavelength of 550 nm.
- the phase difference value at a wavelength of 450 nm when rotated by 40 ° is shown]
- R0 (450) represents the in-plane retardation value of the laminate at a wavelength of 450 nm
- R0 (550) represents the in-plane retardation value of the laminate at a wavelength of 550 nm
- R40 ( 450) shows the retardation value at a wavelength of 450 nm when rotated by 40 ° around the fast axis direction of the film oriented in the horizontal direction of the laminate, and R40 (550) is oriented in the horizontal direction of the laminate.
- the polarizing film includes a horizontally aligned liquid crystal cured film B oriented in a horizontal direction with respect to the film plane of the polarizing film, and the horizontally aligned liquid crystal cured film B includes a dichroic dye.
- the elliptically polarizing plate according to any one of [17]. [19] The elliptically polarizing plate according to [18], wherein the dichroic dye has an azo group. [20]
- the horizontal alignment liquid crystal cured film B is a cured film obtained by curing the liquid crystal compound in a smectic phase in which the liquid crystal compound is aligned in the horizontal direction with respect to the in-plane direction of the film. Elliptical polarizing plate.
- An organic EL display device comprising the elliptically polarizing plate according to any one of [15] to [20].
- acryl and methacryl may be referred to as “(meth) acryl”.
- “system” is added after the compound name, and the compound and its derivatives may be collectively referred to.
- the name of a polymer is expressed by adding “system” after the compound name, the repeating unit of the polymer is derived from the compound or a derivative thereof, or the chemical modification after the polymerization to the repeating unit derived from the compound or the derivative thereof, etc. It means that the polymer is subjected to.
- the vertically aligned liquid crystal cured film of the present invention is aligned in a direction perpendicular to the in-plane direction and includes at least one selected from the group consisting of a nonionic silane compound and an ionic compound.
- the vertically aligned liquid crystal cured film is aligned in a direction perpendicular to the in-plane direction. That is, it includes a liquid crystal compound and / or a polymer of the liquid crystal compound that is aligned in a direction perpendicular to the in-plane direction of the vertically aligned liquid crystal cured film.
- the three-dimensional refractive index ellipsoid formed by the vertically aligned liquid crystal cured film may have biaxiality, but preferably has uniaxiality.
- the vertical alignment liquid crystal cured film of the present invention suppresses the occurrence of alignment defects even without the alignment film.
- the reason is presumed as follows.
- the vertically aligned liquid crystal cured film of the present invention includes at least one selected from the group consisting of a nonionic silane compound and an ionic compound.
- a composition for forming a vertically aligned liquid crystal cured film is applied to a substrate to form a coating film, the coating film is heated and dried to form a dry film.
- the ionic compound Due to the affinity between the functional silane compound and the liquid crystal compound and / or the affinity between the ionic compound and the liquid crystal compound, the ionic compound is present on the substrate surface side, and / or the surface side of the dry film (from the substrate surface)
- the distribution is such that a nonionic silane compound is present on the far side. Since such distribution increases the vertical alignment regulating force, the liquid crystal compound tends to be aligned in the direction perpendicular to the substrate surface in the dry film. For this reason, a cured film can be formed while maintaining a state in which the liquid crystal compound is vertically aligned. Therefore, the vertical alignment liquid crystal cured film of the present invention is considered to suppress the occurrence of alignment defects even without the alignment film.
- nonionic silane compound and an ionic compound As at least one selected from the group consisting of a nonionic silane compound and an ionic compound, three embodiments of a nonionic silane compound, an ionic compound, and a nonionic silane compound and an ionic compound can be mentioned. Only one of the nonionic silane compound and the ionic compound has the effect of increasing the vertical alignment regulating force, but from the viewpoint of further increasing the vertical alignment regulating force, both the nonionic silane compound and the ionic compound are included. Preferably it is.
- the vertically aligned liquid crystal cured film deteriorates the oblique reflection hue of the display including the elliptically polarizing plate including the vertically aligned liquid crystal cured film (for example, the problem that coloring such as red and blue is observed in the oblique hue of the display) )
- RthC (550) represents the retardation value in the thickness direction of the vertically aligned liquid crystal cured film at a wavelength of 550 nm] It is preferable to satisfy.
- the retardation value RthC (550) in the thickness direction of the vertically aligned liquid crystal cured film is more preferably from ⁇ 100 nm to ⁇ 40 nm, and more preferably from ⁇ 80 nm to from the viewpoint of further suppressing the deterioration of the oblique reflection hue of the display. More preferably, it is ⁇ 40 nm or less.
- the retardation value RthC (550) in the thickness direction of the vertically aligned liquid crystal cured film can be adjusted by the thickness dC of the vertically aligned liquid crystal cured film.
- NzC (550) indicates the refractive index at a wavelength of 550 nm in the thickness direction of the vertically aligned liquid crystal cured film, and dC indicates the film thickness of the vertically aligned liquid crystal cured film.] Therefore, in order to obtain a desired retardation value RthC (550) in the thickness direction, the three-dimensional refractive index and the film thickness dC may be adjusted.
- the vertically aligned liquid crystal cured film is used from the viewpoint of suppressing a decrease in ellipticity when viewed from the oblique side on the short wavelength side in the elliptically polarizing plate including the vertically aligned liquid crystal cured film.
- RthC (450) represents the retardation value in the thickness direction at a wavelength of 450 nm of the vertically aligned liquid crystal cured film
- RthC (550) represents the retardation in the thickness direction of the vertically aligned liquid crystal cured film at a wavelength of 550 nm. Show value] It is preferable to satisfy.
- RthC (450) / RthC (550) of the vertically aligned liquid crystal cured film is more preferably 0.95 or less, and further preferably 0.90 or less.
- the retardation value RthC (450) in the thickness direction of the vertically aligned liquid crystal cured film can be adjusted by the thickness dC of the vertically aligned liquid crystal cured film, similarly to RthC (550).
- the upper limit of the thickness of the vertically aligned liquid crystal cured film is preferably 3 ⁇ m or less, more preferably 2.5 ⁇ m or less, further preferably 2.0 ⁇ m or less, and particularly preferably 1.5 ⁇ m or less from the viewpoint of thinning.
- the lower limit of the thickness of the vertically aligned liquid crystal cured film is preferably 0.1 ⁇ m or more, more preferably 0.3 ⁇ m or more, and further preferably 0.4 ⁇ m or more.
- the thickness of the vertically aligned liquid crystal cured film can be measured using an ellipsometer or a contact-type film thickness meter.
- the nonionic silane compound is a compound that is nonionic and contains Si element.
- the nonionic silane compound sufficiently improves the vertical alignment of the liquid crystal compound (I) -1 in the production of a vertically aligned liquid crystal cured film, and further improves the vertical alignment of the liquid crystal compound in combination with the ionic compound. be able to.
- the nonionic silane compound can easily reduce the surface tension of the composition for forming a vertically aligned liquid crystal cured film, and can improve the wettability of the composition to the substrate.
- Nonionic silane compounds include, for example, silicon polymers such as polysilanes, silicone resins such as silicone oils and silicone resins, and organic inorganic silane compounds such as silicone oligomers, silses siloxanes, and alkoxy silanes (more specifically, Include silane coupling agents and the like.
- the nonionic silane compound may be a silicone monomer type or a silicone oligomer (polymer) type.
- Silicone oligomers are shown in the form of (monomer)-(monomer) copolymer: 3-mercaptopropyltrimethoxysilane-tetramethoxysilane copolymer, 3-mercaptopropyltrimethoxysilane-tetraethoxysilane copolymer, 3-mercapto Mercaptopropyl group-containing copolymers such as propyltriethoxysilane-tetramethoxysilane copolymer and 3-mercaptopropyltriethoxysilane-tetraethoxysilane copolymer; mercaptomethyltrimethoxysilane-tetramethoxysilane copolymer, mercaptomethyltrimethoxysilane- Tetraethoxysilane copolymer, mercaptomethyltriethoxys
- nonionic silane compounds may be used individually by 1 type, or may be used in combination of 2 or more type. Moreover, the silane containing compound illustrated to the term of a leveling agent can also be used. Of these nonionic silane compounds, a silane coupling agent is preferable from the viewpoint of further improving the adhesion.
- the silane coupling agent is selected from the group consisting of a vinyl group, an epoxy group, a styryl group, a methacryl group, an acrylic group, an amino group, an isocyanurate group, a ureido group, a mercapto group, an isocyanate group, a carboxy group, and a hydroxy group at the terminal. And a compound containing Si element having at least one functional group and at least one alkoxysilyl group or silanol group.
- the silane coupling agent is preferably a silane coupling agent having an alkoxysilyl group and another different reactive group (for example, the above functional group) from the viewpoint of further improving adhesion. Furthermore, the silane coupling agent is preferably a silane coupling agent having an alkoxysilyl group and a polar group. When the silane coupling agent has at least one alkoxysilyl group and at least one polar group in the molecule, the vertical alignment property of the liquid crystal compound is further improved, and the vertical alignment promoting effect is remarkably obtained.
- Examples of the polar group include an epoxy group, an amino group, an isocyanurate group, a mercapto group, a carboxy group, and a hydroxy group.
- the polar group may have a substituent or a protective group as appropriate in order to control the reactivity of the silane coupling agent.
- silane coupling agent examples include vinyltrimethoxysilane, vinyltriethoxysilane, vinyltris (2-methoxyethoxy) silane, N- (2-aminoethyl) -3-aminopropylmethyldimethoxysilane, N- (2- Aminoethyl) -3-aminopropyltrimethoxysilane, 3-aminopropyltriethoxysilane, 3-triethoxysilyl-N- (1,3-dimethyl-butylidene) propylamine, 3-glycidoxypropyltrimethoxysilane, 3-glycidoxypropylmethyldimethoxysilane, 2- (3,4-epoxycyclohexyl) ethyltrimethoxysilane, 3-chloropropylmethyldimethoxysilane, 3-chloropropyltrimethoxysilane, 3-methacryloyloxypropyltrimethoxysilane
- silane coupling agents examples include KP321, KP323, KP324, KP326, KP340, KP341, X22-161A, KF6001, KBM-1003, KBE-1003, KBM-303, KBM-402, KBM-403. , KBE-402, KBE-403, KBM-1403, KBM-502, KBM-503, KBE-502, KBE-503, KBM-5103, KBM-602, KBM-603, KBM-903, KBE-903, KBE Silane coupling agents manufactured by Shin-Etsu Chemical Co., Ltd. such as -9103, KBM-573, KBM-575, KBM-9659, KBE-585, KBM-802, KBM-803, KBE-846, and KBE-9007 Is mentioned.
- the content of the nonionic silane compound is preferably 0.01% by mass to 5% by mass, and preferably 0.05% by mass to 4% by mass with respect to the solid content of the composition for forming a vertically aligned liquid crystal cured film. %, More preferably 0.1% by mass to 3% by mass.
- the content of the nonionic silane compound is 0.01% by mass or more based on the solid content of the composition, the vertical alignment of the liquid crystal compound is further improved, and the content of the nonionic silane compound is If it is contained in an amount of 5% by mass or less based on the solid content, the applicability of the composition is hardly lowered.
- the ionic compound sufficiently improves the vertical alignment of the liquid crystal compound (I) -1 in the production of a vertically aligned liquid crystal cured film, and the vertical alignment of the liquid crystal compound (I) -1 by combination with a nonionic silane compound. The property can be further improved.
- the ionic compound examples include an onium salt (more specifically, a quaternary ammonium salt, a tertiary sulfonium salt in which a nitrogen atom has a positive charge, and a quaternary phosphonium in which a phosphorus atom has a positive charge. Salt).
- an onium salt more specifically, a quaternary ammonium salt, a tertiary sulfonium salt in which a nitrogen atom has a positive charge, and a quaternary phosphonium in which a phosphorus atom has a positive charge. Salt.
- an onium salt more specifically, a quaternary ammonium salt, a tertiary sulfonium salt in which a nitrogen atom has a positive charge, and a quaternary phosphonium in which a phosphorus atom has a positive charge. Salt.
- a quaternary onium salt is preferable from the viewpoint of further improving the vertical alignment
- the molecular weight of the ionic compound is preferably 100 or more from the viewpoint of further improving the vertical alignment of the liquid crystal compound (I) -1.
- the molecular weight of the ionic compound is preferably 10,000 or less, more preferably 5000 or less, and more preferably 3000 or less from the viewpoint of further improving the coating properties of the vertically aligned liquid crystal cured film forming composition. Is more preferable.
- the molecular weight of the ionic compound is more preferably 100 or more and 10,000 or less from the viewpoint of further improving the vertical alignment property of the liquid crystal compound (I) -1 and further improving the coating property of the composition.
- Examples of the cation component of the ionic compound include inorganic cations and organic cations. Of the cationic components of these ionic compounds, organic cations are preferred from the viewpoint of suppressing the occurrence of alignment defects in the liquid crystal compound. Examples of the organic cation include an imidazolium cation, a pyridinium cation, an ammonium cation, a sulfonium cation, and a phosphonium cation.
- ionic compounds generally have a counter anion.
- an anion component used as the counter ion of the said cation component an inorganic anion or an organic anion is mentioned, for example.
- an organic anion is preferable from the viewpoint of suppressing the occurrence of alignment defects in the liquid crystal compound.
- the cation and the anion do not necessarily have a one-to-one correspondence. Examples of the anion component include the following.
- ionic compound can be appropriately selected from the combination of the cation component and the anion component.
- Specific examples of the compound that is a combination of a cation component and an anion component include the following.
- (Imidazolium salt) 1-ethyl-3-methylimidazolium hexafluorophosphate, 1-ethyl-3-methylimidazolium bis (fluorosulfonyl) imide, 1-ethyl-3-methylimidazolium bis (trifluoromethanesulfonyl) imide, 1-ethyl-3-methylimidazolium p-toluenesulfonate, 1-butyl-3-methylimidazolium methanesulfonate, etc.
- the ionic compound may be used alone or in combination of two or more.
- the ionic compound preferably has a Si element and / or an F element in the molecular structure of the cation moiety. This is because if the ionic compound has Si element and / or F element in the molecular structure of the cation site, the ionic compound can be segregated on the surface of the vertically aligned liquid crystal cured film.
- ionic compounds whose constituent elements are all nonmetallic elements are preferred.
- a method for improving the vertical alignment property of the liquid crystal compound for example, there is a method of treating the surface of the substrate using a surfactant having an alkyl group having a long chain length to some extent.
- This method is described, for example, in “Liquid Crystal Handbook”, Chapter 2, Liquid Crystal Orientation and Physical Properties (issued by Maruzen Co., Ltd.).
- the method of improving the vertical alignment property of a liquid crystal compound with a surfactant can be applied to an ionic compound. That is, as a method for improving the vertical alignment property of the liquid crystal compound, for example, a method of treating the substrate surface with an ionic compound having an alkyl group having a long chain length to some extent can be mentioned.
- the ionic compound preferably satisfies the following formula (10) from the viewpoint of improving the vertical alignment of the liquid crystal compound. 5 ⁇ M ⁇ 16 (10)
- M is represented by the following formula (11).
- M (the number of covalent bonds from the positively charged atom to the end of the molecular chain of the substituent having the largest number of covalent bonds from the end of the molecular chain to the end of the molecular chain among the substituents directly bonded on the positively charged atom ) ⁇ (number of positively charged atoms) (11)
- the substituent having two or more positively charged atoms is counted from the positively charged atoms considered as the base point.
- the number of covalent bonds to the closest other positively charged atom is defined as “the number of covalent bonds from the positively charged atom to the end of the molecular chain” described in the definition of M above.
- the ionic compound is an oligomer or polymer having two or more repeating units, the structural unit is considered as one molecule, and the above M is calculated.
- the number of covalent bonds leading to the atom having the same positive charge via the ring structure, or the end of a substituent bonded to the ring structure Of the number of covalent bonds, the larger number of covalent bonds is defined as “the number of covalent bonds from the positively charged atom to the end of the molecular chain” described in the definition of M above.
- the content of the ionic compound is usually preferably 0.01 to 5% by mass, more preferably 0.05 to 4% by mass, based on the solid content of the composition for forming a vertically aligned liquid crystal cured film.
- the content is preferably 0.1 to 3% by mass.
- liquid crystal compound examples include the following formula (I) -1:
- Ar represents a divalent group having two or more ring structures, and one of the two or more ring structures is a 6-membered ring
- G 1 and G 2 each independently represent a divalent aromatic group or a divalent alicyclic hydrocarbon group, and are included in the divalent aromatic group and the divalent alicyclic hydrocarbon group.
- the carbon atoms contained in the divalent aromatic group and the divalent alicyclic hydrocarbon group may each independently be substituted with an oxygen atom, a sulfur atom, or a nitrogen atom, * Represents a bond]
- the liquid crystal compound (I) -1 represents a divalent group in which Ar in the formula (I) -1 has two or more ring structures, and one of the two or more ring structures is a 6-membered ring. There is a tendency to adopt a T-shaped structure because L 1 and L 2 are bonded to positions 1 and 4 of the 6-membered ring. A compound having such a structure generally tends to exhibit reverse wavelength dispersion. Therefore, the liquid crystal compound (I) -1 exhibits reverse wavelength dispersion. On the other hand, since the liquid crystal compound (I) -1 has a T-shaped structure, it is usually difficult to perform vertical alignment alone.
- the vertical alignment property of the liquid crystal compound (I) -1 is sufficiently improved by including a nonionic silane compound or an ionic compound in the composition for forming a vertically aligned liquid crystal cured film.
- a nonionic silane compound or an ionic compound in the composition for forming a vertically aligned liquid crystal cured film.
- the composition for forming a vertically aligned liquid crystal cured film preferably contains both a nonionic silane compound and an ionic compound.
- Ar represents a divalent group having two or more ring structures.
- the unit of the ring structure that Ar has is a single ring.
- Ar has four, two, and three ring structures, respectively.
- two or more monocycles may be condensed and adjacent to each other, two or more monocycles may be bonded to each other via a chemical bond, They may be adjacent to each other without condensing or via chemical bonds.
- the first aspect may be referred to as a condensed type
- the second aspect may be referred to as a bonded type
- the third aspect may be referred to as a spiro ring type.
- a single ring and a condensed ring (polycycle) in which a single ring is condensed may be bonded to each other via a chemical bond, and the polycycle and the polycycle may be bonded to each other via a chemical bond.
- a bond condensation type Liquid crystal compounds A, (A) -2, and (A) -3, which will be described later, are a bond condensation type, a condensation type, and a bond type, respectively.
- the chemical bond that connects two or more rings in the bond type and the bond condensation type includes, for example, a conjugated double bond (more specifically, —C ⁇ C— and —C ⁇ N— etc.) and a carbonyl group. May include a bond or group that expands the spatial extent of the conjugated system.
- Examples of the monocycle include monocyclic hydrocarbon rings (more specifically, cycloalkane rings and benzene rings) and monocyclic heterocycles.
- Examples of monocyclic heterocycles include 5-membered heterocycles (more specifically, pyrrole ring, furan ring, thiophene ring, oxazole ring, imidazole ring, pyrazole ring, thiazole ring, triazole).
- the polycycle is a structure having two or more ring structures, and the ring structure may be an aromatic ring or a hydrocarbon ring. Examples of the polycycle include those containing a condensed ring and a monocyclic heterocycle.
- the condensed ring is, for example, a ring obtained by condensing two or more of the same kind of monocycles, and a ring obtained by condensing two or more different kinds of single rings.
- the condensed ring include polycyclic hydrocarbon rings (more specifically, naphthalene ring, anthracene ring, and phenanthrene ring), and polycyclic heterocyclic rings (more specifically, quinoline ring, Quinoxaline ring, benzofuran ring, benzothiophene ring, fluorene ring, indole ring, carbazole ring, benzimidazole ring, benzothiazole ring, thienothiazole ring, benzoxazole ring, 1,3-benzodithiol ring, phenanthroline ring, etc.) Is mentioned.
- a polycyclic structure is preferable from the viewpoint of developing reverse wavelength dispersion characteristics, and a polycyclic heterocyclic
- the monocycle and polycycle may have a substituent.
- substituents that the monocyclic ring and polycyclic ring may have include a hydrogen atom, an alkyl group having 1 to 20 carbon atoms, and an alkoxy group having 1 to 20 carbon atoms.
- Group, imino group, alkapolyenyl group, cyano group, or amino group, and a carbon atom in the substituent may be further substituted with an oxygen atom, a nitrogen atom, or a sulfur atom ( In this case, the hydrogen atom bonded to the carbon atom may be increased or decreased in accordance with the valence of the atom to be substituted).
- the spatial spread of the conjugated system may be expanded like an imino group, alkapolyenyl group, cyano group, hydroxy group, and amino group. These substituents may be further substituted.
- Examples of the 6-membered ring possessed by Ar include a benzene ring and a cyclohexane ring.
- the 6-membered ring may contain a hetero atom as a ring member atom.
- Examples of condensed rings including 6-membered rings include quinoline ring, quinoxaline ring, benzofuran ring, benzothiophene ring, fluorene ring, indole ring, carbazole ring, benzimidazole ring, benzothiazole ring, thienothiazole ring, benzoxazole ring, Examples thereof include a 1,3-benzodithiol ring and a phenanthroline ring.
- Ar is preferably a divalent group containing a ring structure having one or more sulfur atoms as ring member atoms from the viewpoint of further improving the reverse wavelength dispersion of the polarizing plate.
- Ar preferably represents a divalent group represented by the following formula from the viewpoint of further improving the reverse wavelength dispersion. * Indicates a bond.
- X 1 , X 2 , and X 3 are each independently selected from CR 1X, R 2X , NR 3X , a sulfur atom, and an oxygen atom.
- R 1X , R 2X , and R 3X each independently represent a hydrogen atom or an alkyl group having 1 to 4 carbon atoms.
- U includes at least one ring structure, and examples of the ring structure include a structure including a monocyclic ring and / or a polycyclic ring described in paragraphs 0041 to 0043 above.
- Y may be an arbitrary substituent, but preferably contains at least one ring structure from the viewpoint of improving reverse wavelength dispersion, and the ring structure is described in the above paragraphs 0041 to 0043.
- L 10 is a divalent linking group, which is a single bond, —O—CO—O—, —N ⁇ N—, —C ⁇ C—, —CR a ⁇ CR b —, —CH ⁇ NN ⁇ CH— or —CR c ⁇ N—.
- R c to R g each independently represents a hydrogen atom or an alkyl group having 1 to 10 carbon atoms, and the carbon atom in the alkyl group may be substituted with a nitrogen atom, an oxygen atom, or a sulfur atom.
- Good in this case, the number of hydrogen atoms is appropriately increased or decreased according to the valence number).
- Z represents a hydrogen atom or a nonmetallic atom of Groups 14 to 16 to which a substituent may be bonded.
- Z is a structure that expands the spatial spread of the conjugated system (more specifically, a double bond site, a triple bond site, and an aromatic ring that satisfies the Hückel rule from the viewpoint of improving reverse wavelength dispersion.
- a heterocyclic ring and at least one selected from the group consisting of atoms selected from nitrogen atoms and sulfur atoms.
- Examples of the divalent linking group represented by L 1 and L 2 include an alkylene group having 1 to 4 carbon atoms, —O—, —S—, —R a1 OR a2 —, —R a3 COOR a4 —, —R a5 OCOR a6 —, R a7 OC ⁇ OOR a8 —, —N ⁇ N—, —CR c ⁇ CR d —, and —C ⁇ C—.
- R a1 to R a8 each independently represents a single bond or an alkylene group having 1 to 4 carbon atoms (more specifically, a methylene group, an ethylene group, a propylene group, a butylene group, or the like), and R c and R d each independently represents an alkyl group having 1 to 4 carbon atoms (more specifically, a methyl group, an ethyl group, a propyl group, a butyl group, or the like) or a hydrogen atom.
- L 1 and L 2 are each independently preferably a single bond, an alkylene group having 1 to 4 carbon atoms, —O—, —S—, —R a1 OR a2 —, —R a3 COOR a4 —, —R a5 OCOR a6 —, R a7 OC ⁇ OOR a8 —, —N ⁇ N—, —CR c ⁇ CR d —, or —C ⁇ C—.
- L 1 and L 2 are each independently more preferably a single bond, —OR a2-1 —, —CH 2 —, —CH 2 CH 2 —, —COOR a4-1 —, or OCOR a6-1 —. Represents.
- R a2-1 , R a4-1 , and R a6-1 each independently represents a single bond, —CH 2 —, or —CH 2 CH 2 —.
- L 1 and L 2 each independently represents a single bond, —O—, —CH 2 CH 2 —, —COO—, —COOCH 2 CH 2 —, or OCO—.
- Examples of the divalent aromatic group represented by G 1 and G 2 include a phenylenediyl group and a naphthylenediyl group.
- the divalent aromatic group may be substituted with a substituent such as a halogen atom (more specifically, a fluorine atom, a chlorine atom, a bromine atom, etc.) or an alkyl group having 1 to 4 carbon atoms.
- the divalent aromatic group may have a hetero atom (more specifically, an oxygen atom, a sulfur atom, a nitrogen atom, etc.) as a ring member atom.
- Examples of the divalent alicyclic hydrocarbon group represented by G 1 and G 2 include a cyclopentanediyl group, a cyclohexanediyl group, and a cycloheptanediyl group.
- the divalent alicyclic hydrocarbon group may be substituted with a substituent such as a halogen atom and an alkyl group having 1 to 4 carbon atoms.
- the aromatic group is a group having a planar structure having a planarity
- the number of ⁇ electrons of the cyclic structure is [4n + 2] according to the Hückel rule (n is a positive number of 1 or more). Indicates an integer).
- G 1 and G 2 are each independently preferably a 1,4-phenylenediyl group optionally substituted with at least one substituent selected from the group consisting of a halogen atom and an alkyl group having 1 to 4 carbon atoms.
- 1,4-cyclohexanediyl group optionally substituted with at least one substituent selected from the group consisting of a halogen atom and an alkyl group having 1 to 4 carbon atoms, more preferably 1 substituted with a methyl group , 4-phenylenediyl group, unsubstituted 1,4-phenylenediyl group, or unsubstituted 1,4-trans-cyclohexanediyl group, particularly preferably unsubstituted 1,4-phenylenediyl group or Substituted 1,4-trans-cyclohexanediyl group.
- At least one of a plurality of G 1 and G 2 is preferably a divalent alicyclic hydrocarbon group, and at least one of G 1 and G 2 bonded to L 1 or L 2. More preferably, it is a divalent alicyclic hydrocarbon group.
- the liquid crystal compound (I) -1 preferably has a maximum absorption in a wavelength range of 260 to 400 nm.
- the liquid crystal compound (I) -1 has an aromatic group having a hetero atom or a structure that expands the conjugated system, the absorption in the near ultraviolet region is shifted to the longer wavelength side than the benzene ring, so that the wavelength is 260 nm or more. In this way, it is preferable to have maximum absorption in the wavelength region of 260 nm or more from the viewpoint of improving reverse wavelength dispersion.
- the liquid crystal compound (I) -1 preferably has a maximum absorption in a region of a wavelength of 400 nm or less because coloring may occur when the absorption has a maximum in a wavelength region longer than the wavelength of 400 nm. Furthermore, from the viewpoint of further improving the wavelength dispersibility, it is more preferable to have a maximum absorption in a region of a wavelength of 280 nm to 400 nm, and it is more preferable to have a maximum absorption in a region of a wavelength of 300 nm to 400 nm.
- Liquid crystal compound (I) -1 is represented by the following formula (I) -2:
- liquid crystal compound (I) -2) A liquid crystal compound having a structure represented by the formula (hereinafter sometimes referred to as liquid crystal compound (I) -2) is preferred.
- Ar represents a divalent group having two or more ring structures, and one of the two or more ring structures is a 6-membered ring
- G 1 , G 2 , and G 3 each independently represent a divalent aromatic group or a divalent alicyclic hydrocarbon group, and the divalent aromatic group and the divalent alicyclic carbon group
- the hydrogen atom contained in the hydrogen group is independently a halogen atom, an alkyl group having 1 to 4 carbon atoms, a fluoroalkyl group having 1 to 4 carbon atoms, an alkoxy group having 1 to 4 carbon atoms, a cyano group, or a nitro group.
- the carbon atoms contained in the divalent aromatic group and the divalent alicyclic hydrocarbon group are each independently substituted with an oxygen atom, a sulfur atom, or a
- Formula (I) in -2 Ar, L 1, L 2 , G 1, and G 2 has the formula (I) in -1 Ar, L 1, L 2 , G 1, and G 2 and respectively synonymous is there.
- B 1 is preferably a single bond, an alkylene group having 1 to 4 carbon atoms, —O—, —S—, —R a1 OR a2 —, —R a3 COOR a4 —, —R a5 OCOR a6 —, R a7.
- L 1 and L 2 are each independently more preferably a single bond, —OR a2-1 —, —CH 2 —, —CH 2 CH 2 —, —COOR a4-1 —, or OCOR a6-1 —. Represents.
- R a2-1 , R a4-1 , and R a6-1 each independently represent a single bond, —CH 2 —, or —CH 2 CH 2 —.
- L 1 and L 2 each independently represents a single bond, —O—, —CH 2 CH 2 —, —COO—, —COOCH 2 CH 2 —, or OCO—.
- Examples of the divalent aromatic group represented by G 3 include a phenylenediyl group and a naphthylylenediyl group.
- the divalent aromatic group may be substituted with a substituent such as a halogen atom (more specifically, a fluorine atom, a chlorine atom, a bromine atom, etc.) or an alkyl group having 1 to 4 carbon atoms.
- Examples of the divalent alicyclic hydrocarbon group represented by G 3 include a cyclopentanediyl group, a cyclohexanediyl group, and a cycloheptanediyl group.
- the divalent alicyclic hydrocarbon group may be substituted with a substituent such as a halogen atom or an alkyl group having 1 to 4 carbon atoms.
- G 3 each independently preferably represents a 1,4-phenylenediyl group which may be substituted with at least one substituent selected from the group consisting of a halogen atom and an alkyl group having 1 to 4 carbon atoms, a halogen atom And a 1,4-cyclohexanediyl group optionally substituted with at least one substituent selected from the group consisting of alkyl groups having 1 to 4 carbon atoms, more preferably 1,4-cyclohexanediyl group substituted with a methyl group A phenylenediyl group, an unsubstituted 1,4-phenylenediyl group, or an unsubstituted 1,4-trans-cyclohexanediyl group, and particularly preferably an unsubstituted 1,4-phenylenediyl group or an unsubstituted 1 , 4-trans-cyclohexanediyl group.
- Liquid crystal compound (I) -1 is represented by the following formula (I) -3:
- liquid crystal compound (I) -3 It is more preferable that the liquid crystal compound has a structure represented by the formula (hereinafter sometimes referred to as “liquid crystal compound (I) -3”).
- Ar represents a divalent group having two or more ring structures, and one of the two or more ring structures is a 6-membered ring
- Binds L 1 and L 2 at the 4th and L 1 , L 2 , B 1 and B 2 each independently represent a single bond or a divalent linking group
- G 1 , G 2 , G 3 , and G 4 each independently represent a divalent aromatic group or a divalent alicyclic hydrocarbon group, and the divalent aromatic group or the divalent fat
- the hydrogen atom contained in the cyclic hydrocarbon group is a halogen atom, an alkyl group having 1 to 4 carbon atoms, a fluoroalkyl group having 1 to 4 carbon atoms, an alkoxy group having 1 to 4 carbon atoms, a cyano group, or a nitro group.
- the carbon atom contained in the divalent aromatic group or the divalent alicyclic hydrocarbon group may be substituted with an oxygen atom, a sulfur atom, or a
- Formula (I) in -3 Ar L 1, L 2 , G 1, and G 2 has the formula (I) in -1 Ar, L 1, L 2 , G 1, and G 2 and respectively synonymous is there.
- B 1 and G 3 in formula (I) -3 has the same meaning as B 1 and G 3, respectively formula (I) in -2.
- B 2 is preferably a single bond, an alkylene group having 1 to 4 carbon atoms, —O—, —S—, —R a1 OR a2 —, —R a3 COOR a4 —, —R a5 OCOR a6 —, R a7.
- L 1 and L 2 are each independently more preferably a single bond, —OR a2-1 —, —CH 2 —, —CH 2 CH 2 —, —COOR a4-1 —, or OCOR a6-1 —. Represents.
- R a2-1 , R a4-1 , and R a6-1 each independently represent a single bond, —CH 2 —, or —CH 2 CH 2 —.
- L 1 and L 2 each independently represents a single bond, —O—, —CH 2 CH 2 —, —COO—, —COOCH 2 CH 2 —, or OCO—.
- Examples of the divalent aromatic group represented by G 4 include a phenylenediyl group or a naphthylylenediyl group.
- the divalent aromatic group may be substituted with a substituent such as a halogen atom (more specifically, a fluorine atom, a chlorine atom, a bromine atom, etc.) or an alkyl group having 1 to 4 carbon atoms.
- Examples of the divalent alicyclic hydrocarbon group represented by G 3 include a cyclopentanediyl group, a cyclohexanediyl group, and a cycloheptanediyl group.
- the divalent alicyclic hydrocarbon group may be substituted with a substituent such as a halogen atom or an alkyl group having 1 to 4 carbon atoms.
- G 4 is preferably a 1,4-phenylenediyl group which may be substituted with at least one substituent selected from the group consisting of a halogen atom and an alkyl group having 1 to 4 carbon atoms, a halogen atom and 1 carbon atom.
- 1,4-cyclohexanediyl group optionally substituted with at least one substituent selected from the group consisting of ⁇ 4 alkyl groups, more preferably a 1,4-phenylenediyl group substituted with a methyl group, An unsubstituted 1,4-phenylenediyl group or an unsubstituted 1,4-trans-cyclohexanediyl group, particularly preferably an unsubstituted 1,4-phenylenediyl group or an unsubstituted 1,4-trans -A cyclohexanediyl group.
- the liquid crystal compound (I) -1 may have one or more polymerizable groups.
- a polymerizable group means a group that can participate in a polymerization reaction by an active species such as an active radical or an acid generated from a photopolymerization initiator.
- the polymerizable group include an epoxy group, vinyl group, vinyloxy group, 1-chlorovinyl group, isopropenyl group, 4-vinylphenyl group, acryloyloxy group, methacryloyloxy group, oxiranyl group, and oxetanyl group. .
- an acryloyloxy group and a methacryloyloxy group are preferable.
- the liquid crystal compound (I) -1 having a polymerizable group can form a polymer by a polymerization reaction.
- liquid crystal compound (I) -1 examples include liquid crystal compounds having structures represented by the formulas (A) -1 to (A) -5.
- the content of the liquid crystal compound (I) -1 (the total of the contents when a plurality of liquid crystal compounds are included) is 50 to 99 with respect to 100 parts by mass of the solid content of the vertically aligned liquid crystal cured film forming composition. 0.5 part by mass is preferable, 60 to 99 parts by mass is more preferable, and 70 to 99 parts by mass is still more preferable.
- the mass of the solid content of the composition means the total mass of the components excluding the solvent from the composition.
- the vertically aligned liquid crystal cured film is a cured product of the composition for forming a vertically aligned liquid crystal cured film.
- the method for producing a vertically aligned liquid crystal cured film includes a coating film forming step in which a composition for forming a vertically aligned liquid crystal cured film is applied to a substrate, a coating film is formed on the substrate, the coating film is dried, and a dry film is formed.
- the laminate produced by this production method is composed of a substrate and a vertically aligned liquid crystal cured film.
- the case where the liquid crystal compound has one or more polymerizable groups and the composition further contains a photopolymerization initiator will be described as an example.
- the composition is applied to a base material using a printing apparatus to form a coating film on the base material.
- the application method include printing methods such as a gravure coating method, a die coating method, and a flexo method.
- the coating film is dried using a heating device to form a dry film. After the coating film is heated and the solvent in the coating film is removed, the liquid crystal compound is vertically aligned and converted into a dry film.
- the heating temperature is preferably equal to or higher than the phase transition temperature of the liquid crystal compound so that the solvent can be removed.
- the dry film is irradiated with active energy rays (more specifically, ultraviolet rays or the like) to form a vertically aligned liquid crystal cured film.
- active energy rays more specifically, ultraviolet rays or the like
- the liquid crystal compound maintains a liquid crystal state that is aligned perpendicular to the substrate plane in the dry film.
- the liquid crystal compound is photopolymerized while maintaining a vertically aligned liquid crystal state. Thereby, a vertically aligned liquid crystal cured film can be directly formed on the substrate.
- the vertically aligned liquid crystal cured film can be directly formed on the substrate without forming the alignment film.
- the method for producing a vertically aligned liquid crystal cured film may further include a vertical alignment film forming step of forming a vertical alignment film for the purpose of further improving the orientation of the vertically aligned liquid crystal cured film.
- the vertically aligned liquid crystal cured film is indirectly formed on the substrate via the vertically aligned film.
- the vertical alignment film forming process is a process executed before the coating film forming process, and forms a vertical alignment film.
- the alignment film forming process includes a second coating film forming process, a second dry coating forming process, and an alignment film forming process.
- a vertical alignment film forming composition is applied onto the substrate using a printing apparatus to form the second coating film.
- the composition for forming a vertical alignment film includes, for example, an alignment polymer described later and the solvent described above.
- the second dry film forming step for example, the second coating film is heated using a heating device to dry the second coating film, thereby forming the second dry film.
- a vertical alignment film is formed by irradiating and curing the second dry film by using a UV irradiation apparatus.
- the manufacturing method of a vertical alignment liquid crystal cured film includes a vertical alignment film formation process, a vertical alignment liquid crystal cured film is formed on a vertical alignment film.
- composition for forming a vertically aligned liquid crystal cured film includes, for example, either a nonionic silane compound or an ionic compound, or both, and an additive added as necessary.
- the additive include a liquid crystal compound, a photopolymerization initiator, a leveling agent, a solvent, a polymerization inhibitor, and an adhesion improver.
- the liquid crystal compound include liquid crystal compound (I) -1. These additives may be used individually by 1 type, or may be used in combination of 2 or more type.
- a nonionic silane compound and an ionic compound and, if necessary, other components are agitated at a predetermined temperature to disperse these components substantially uniformly. Alternatively, it can be obtained by dissolving.
- the composition for forming a vertically aligned liquid crystal cured film is usually applied to a substrate or the like in a state dissolved in a solvent, it preferably contains a solvent.
- the solvent include water; methanol, ethanol, ethylene glycol, isopropyl alcohol, propylene glycol, ethylene glycol methyl ether, ethylene glycol butyl ether, 1-methoxy-2-propanol, 2-butoxyethanol, and propylene glycol monomethyl ether.
- Alcohol solvents such as ethyl acetate, butyl acetate, ethylene glycol methyl ether acetate, ⁇ -butyrolactone, propylene glycol methyl ether acetate and ethyl lactate; acetone, methyl ethyl ketone, cyclopentanone, cyclohexanone, 2-heptanone, and methyl Ketone solvents such as isobutyl ketone; aliphatic hydrocarbon solvents such as pentane, hexane, and heptane; Cycloaliphatic hydrocarbon solvents such as cyclohexane; aromatic hydrocarbon solvents such as toluene and xylene; nitrile solvents such as acetonitrile; ether solvents such as tetrahydrofuran and dimethoxyethane; chlorine-containing solvents such as chloroform and chlorobenzene Amide solvents such as dimethylacetamide, dimethyl
- solvent may be used individually by 1 type, or may be used in combination of 2 or more type.
- solvents alcohol solvents, ester solvents, ketone solvents, chlorine-containing solvents, amide solvents, and aromatic hydrocarbon solvents are preferable.
- solvent may be used individually by 1 type, or may be used in combination of 2 or more type.
- the content of the solvent is preferably 50 to 98 parts by mass, more preferably 70 to 95 parts by mass with respect to 100 parts by mass of the composition for forming a vertically aligned liquid crystal cured film. Therefore, the solid content in 100 parts by mass of the composition is preferably 2 to 50 parts by mass.
- the solid content of the composition is 50 parts by mass or less, since the viscosity of the composition is low, the thickness of the vertically aligned liquid crystal cured film becomes substantially uniform, and unevenness is hardly generated in the vertically aligned liquid crystal cured film. Tend.
- the solid content can be appropriately determined in consideration of the thickness of the vertically aligned liquid crystal cured film to be produced.
- the composition for forming a vertically aligned liquid crystal cured film may contain a photopolymerization initiator for the purpose of advancing the polymerization reaction.
- a photoinitiator provides an active species that absorbs active energy rays and initiates a polymerization reaction.
- the photopolymerization initiator can be used as a curable composition that cures by radical polymerization, such as (meth) acrylate or urethane (meth) acrylate, as a curable material.
- a photocationic polymerization initiator can be used.
- Examples of the photopolymerization initiator include a photoradical polymerization initiator and a photocationic polymerization initiator.
- Examples of the photo radical polymerization initiator include benzoin compounds, benzophenone compounds, benzyl ketal compounds, ⁇ -hydroxy ketone compounds, ⁇ -amino ketone compounds, triazine compounds, and the like.
- Examples of the cationic photopolymerization initiator include aromatic diazonium salts, onium salts such as aromatic iodonium salts and aromatic sulfonium salts, and iron-arene complexes.
- photopolymerization initiator examples include Irgacure (registered trademark) 907, Irgacure 184, Irgacure 651, Irgacure 819, Irgacure 250, Irgacure 369, Irgacure 379, Irgacure 127, Irgacure 2959, Irgacure 754, and Irgacure 379E.
- Photopolymerization initiators manufactured by BASF Japan, Inc . photopolymerization initiators manufactured by Seiko Chemical Co., Ltd., such as Sequol BZ, Sequol Z, and Sequol BEE, kayacure BP100 (manufactured by Nippon Kayaku Co., Ltd.), And Adeka optomer SP-152, Adeka optomer SP-170, Adeka optomer N-1717, Ade Photopolymerization initiators made by ADEKA Corporation, such as Optomer N-1919, Adeka Arcles NCI-831, Adeka Arcles NCI-930; Photopolymerization made by Nippon Siebel Hegner, such as TAZ-A and TAZ-PP Initiator; Photopolymerization initiator manufactured by Sanwa Chemical Co., such as TAZ-104; Photopolymerization initiator manufactured by Nippon Kayaku Co., Ltd., such as Kayrad (registered trademark) series; Dow Chemical Co., Ltd.
- Photoinitiators such as Cyracure UVI series Photopolymerization initiators manufactured by San-Apro Co., Ltd. such as CPI series; Photopolymerization initiators manufactured by Midori Chemical Co., Ltd. such as TAZ, BBI, and DTS; such as RHODORSIL (registered trademark) Examples include photopolymerization initiators manufactured by Rhodia Co., Ltd. These photoinitiators may be used individually by 1 type, or may be used in combination of 2 or more type. A photoinitiator can be suitably selected and used according to the material to be used.
- the maximum absorption wavelength of the photopolymerization initiator is preferably 300 nm to 400 nm, more preferably 300 nm to 380 nm.
- these photopolymerization initiators ⁇ -acetophenone polymerization initiators and oxime photopolymerization initiators are preferred.
- Examples of the ⁇ -acetophenone polymerization initiator include 2-methyl-2-morpholino-1- (4-methylsulfanylphenyl) propan-1-one, 2-dimethylamino-1- (4-morpholinophenyl) -2 -Benzylbutan-1-one (2-dimethylamino-2-benzyl-1- (4-morpholinophenyl) butan-1-one) and 2-dimethylamino-1- (4-morpholinophenyl) -2- ( 4-methylphenylmethyl) butan-1-one.
- ⁇ -acetophenone polymerization initiators include 2-methyl-2-morpholino-1- (4-methylsulfanylphenyl) propan-1-one and 2-dimethylamino-1- (4-morpholinophenyl) -2-benzyl Butan-1-one is preferred.
- Commercially available products of ⁇ -acetophenone compounds include ⁇ -acetophenone polymerization initiators manufactured by BASF Japan Ltd. such as Irgacure 369, 379EG, and 907, and ⁇ -acetophenone systems manufactured by Seiko Chemical Co., Ltd. such as Seikol BEE. A polymerization initiator etc. are mentioned.
- An oxime photopolymerization initiator generates radicals when irradiated with light. Polymerization of the composition for forming a vertically aligned liquid crystal cured film in the deep part of the coating film suitably proceeds by this radical. Moreover, it is preferable to use the oxime type photoinitiator which can utilize an ultraviolet-ray with a wavelength of 350 nm or more efficiently from a viewpoint of making a polymerization reaction in the deep part of a coating film progress more efficiently. Examples of the oxime photopolymerization initiator that can efficiently use ultraviolet rays having a wavelength of 350 nm or more include triazine compounds and oxime ester type carbazole compounds.
- Type carbazole compounds examples include 1,2-octanedione, 1- [4- (phenylthio) -2- (O-benzoyloxime)], and ethanone, 1- [9-ethyl-6- (2 -Methylbenzoyl) -9H-carbazol-3-yl] -1- (O-acetyloxime).
- oxime ester type carbazole compounds examples include, for example, oxime ester type carbazole compounds manufactured by BASF Japan, such as Irgacure OXE-01, Irgacure OXE-02, and Irgacure OXE-03, and Adekaoptomer N-1919, An oxime ester type carbazole compound manufactured by ADEKA Corporation, such as Adeka Arcles NCI-831, can be mentioned.
- the content of the photopolymerization initiator is usually 0 when the solid content (excluding the content of the solvent from the composition) contained in the vertically aligned liquid crystal cured film forming composition is 100 parts by mass.
- the amount is preferably 1 to 20 parts by mass, more preferably 0.5 to 10 parts by mass, and still more preferably 1 to 7 parts by mass.
- the photopolymerization initiator is 0.1 to 20 parts by mass with respect to 100 parts by mass of the composition, the polymerization reaction is likely to proceed sufficiently.
- the leveling agent is used for the purpose of adjusting the coating property of the composition for forming a vertically aligned liquid crystal cured film, i.e., adjusting the fluidity of the composition for coating, and further improving the surface of the layer obtained by coating the composition. You may add to this composition in order to make it flat.
- the leveling agent include a silicone leveling agent such as a silane coupling agent, a polyacrylate leveling agent, and a fluoroalkyl leveling agent. Of these leveling agents, silicone-based leveling agents and fluoroalkyl-based leveling agents are preferred from the viewpoint of further improving the vertical alignment of the liquid crystal compound.
- leveling agents examples include DC3PA, SH7PA, DC11PA, SH28PA, SH29PA, SH30PA, ST80PA, ST86PA, SH8400, SH8700, and FZ2123; leveling agents manufactured by Toray Dow Corning Corp .; , KP326, KP340, KP341, X22-161A, KF6001, KBM-1003, KBE-1003, KBM-303, KBM-402, KBM-403, KBE-402, KBE-403, KBM-1403, KBM-502, KBM -503, KBE-502, KBE-503, KBM-5103, KBM-602, KBM-603, KBM-903, KBE-903, KBE-9103, KBM-573, KBM Leveling agents manufactured by Shin-Etsu Chemical Co., Ltd.
- TSF400 TSF401, TSF410, TSF4300, TSF4440, TSF4445 Momentive Performance Materials Japan GK leveling agent such as TSF-4446, TSF4452, and TSF4460; Fluorinert (registered trademark) FC-72, FC-40, FC-43, and FC-3283 Leveling agents manufactured by Sumitomo 3M Co., Ltd .; Megafac® R-08, R-30, R-90, F-410, F-411, F-443, F- 445, F-470, F-4 7.
- Leveling agents manufactured by DIC Corporation such as F-479, F-482, F-482, and F-556; Ftop (trade name) EF301, EF303, EF351, and EF352 Leveling agent manufactured by Mitsubishi Materials Electronic Chemical Co., Ltd .; Surflon (registered trademark) S-381, S-382, S-383, S-393, SC-101, SC-105, KH-40 Leveling agents manufactured by AGC Seimi Chemical Co., Ltd., such as SA-100; Leveling agents manufactured by Daikin Fine Chemical Laboratory, Inc., such as trade names E1830 and E5844; BM-1000, BM-1100, BYK-352, Chemie leveling agents such as BYK-353 and BYK-361N (both trade names: BM). These leveling agents may be used individually by 1 type, or may be used in combination of 2 or more type.
- the leveling agent preferably has a content of 0.001 to 3% by mass, more preferably 0.01 to 3% by mass in the solid content of the composition for forming a vertically aligned liquid crystal cured film. ⁇ 3% by weight is more preferred.
- the content of the leveling agent in the solid content of the composition is 0.001 to 3% by mass, the coating property of the composition is further improved.
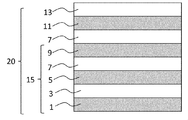
- the laminate includes the vertically aligned liquid crystal cured film.
- the laminate includes a base material, an alignment film for vertical alignment (hereinafter sometimes referred to as a vertical alignment film), an alignment film for horizontal alignment (hereinafter sometimes referred to as a horizontal alignment film), an adhesive layer, And / or a film oriented in the horizontal direction with respect to the in-plane direction of the vertical alignment liquid crystal cured film described later (hereinafter sometimes referred to as a horizontal alignment film).
- a laminate comprising the vertical alignment liquid crystal cured film, a horizontal alignment film and a horizontal alignment film, a laminate comprising the vertical alignment liquid crystal cured film and a substrate, and the vertical alignment liquid crystal curing.
- a laminate including a film, a horizontal alignment film, a horizontal alignment film, and a substrate can be mentioned.
- the laminate since the vertically aligned liquid crystal cured film can be formed without an alignment film for vertical alignment, the laminate may not include the vertical alignment film.
- the vertically aligned liquid crystal cured film can be adjacent to the substrate.
- the vertical alignment liquid crystal cured film adjacent to the base material produced by the above-mentioned method can transfer only the vertical alignment liquid crystal cured film through the adhesive layer, and remove the base material to produce a laminate.
- Base material As a base material, a glass base material and a film base material are mentioned, for example, A film base material is preferable from a workability viewpoint, and a long roll-shaped film is more preferable at the point which can be manufactured continuously.
- the resin constituting the film substrate examples include polyolefins such as polyethylene, polypropylene, and norbornene-based polymers; cyclic olefin-based resins; polyvinyl alcohol; polyethylene terephthalate; polymethacrylic acid esters; And cellulose esters such as diacetyl cellulose and cellulose acetate propionate; polyethylene naphthalate; polycarbonate; polysulfone; polyethersulfone; polyetherketone; and plastics such as polyphenylene sulfide and polyphenylene oxide.
- the bonding surface of the base material with the adhesive layer may be subjected to a release treatment such as a silicone treatment.
- a commercially available cellulose ester base material for example, a cellulose ester base material manufactured by Fuji Photo Film Co., Ltd. such as Fujitac Film; manufactured by Konica Minolta Opto Co., Ltd.
- the cellulose ester base material of this is mentioned.
- Such a resin can be formed into a substrate by known methods such as a solvent casting method and a melt extrusion method.
- cyclic olefin resins examples include cyclic olefin resins manufactured by Ticona (Germany) such as “Topas (registered trademark); cyclic olefins manufactured by JSR Corporation such as“ Arton (registered trademark) ”. Type olefin resin; “ZEONOR (registered trademark)” and “ZEONEX (registered trademark)” cyclic olefin resin manufactured by Nippon Zeon Co., Ltd .; “Apel” (registered trademark) Mitsui Examples include cyclic olefin-based resins manufactured by Chemical Co., Ltd. Commercially available cyclic olefin resin base materials can also be used.
- cyclic olefin resin substrates examples include cyclic olefin resin substrates manufactured by Sekisui Chemical Co., Ltd., such as “ESCINA (registered trademark)” and “SCA40 (registered trademark)”; “ZEONOR FILM (registered trademark)”. And the cyclic olefin resin substrate made by JSR Corporation such as “Arton Film (registered trademark)”.
- the substrate has a thickness that facilitates laminating each layer and is easy to peel off.
- the thickness of such a substrate is usually 5 to 300 ⁇ m, preferably 10 to 150 ⁇ m.
- the alignment film is a film having an alignment regulating force that aligns the liquid crystal compound of the liquid crystal cured film in a predetermined direction.
- various alignments such as vertical alignment, horizontal alignment, hybrid alignment, and tilt alignment can be controlled depending on the type of alignment film material, rubbing conditions, and light irradiation conditions.
- Such processing for expressing the orientation regulating force is called orientation processing.
- the vertical alignment film is an alignment film having an alignment regulating force for aligning the liquid crystal compound in the vertical direction. For this reason, a vertical alignment liquid crystal film can be formed by using a vertical alignment film.
- the vertical alignment film preferably has a solvent resistance that does not dissolve when the composition for forming a vertical alignment liquid crystal cured film is applied, and has heat resistance in heat treatment for removing the solvent and aligning the liquid crystal compound.
- the vertical alignment film is preferably made of a material that lowers the surface tension of the surface of the substrate or the like.
- orientation polymers such as polyimide, polyamide, polyamic acid that is a hydrolyzate thereof, and fluoropolymers of perfluoroalkyl, silane compounds, and polysiloxane compounds obtained by a condensation reaction thereof.
- a composition containing such a material and a solvent for example, the solvent exemplified in the section of the vertical alignment liquid crystal cured film (hereinafter, also referred to as a composition for forming a vertical alignment film) is formed on a substrate or the like. After coating and removing the solvent, the coating film can be obtained by heating or the like.
- the vertical alignment film is composed of Si element and C element as constituent elements from the viewpoint of easily reducing the surface tension and improving the adhesion with the layer adjacent to the vertical alignment film.
- membrane containing the compound containing is preferable, and a silane compound can be used conveniently.
- the silane compound the nonionic silane compound described above or a silane-containing ionic compound exemplified in the section of the ionic compound can be used.
- the vertical alignment is regulated. You can increase your power.
- These silane compounds may be used individually by 1 type, may be used in combination of 2 or more type, and may be used in mixture with other materials.
- silane compound is a nonionic silane compound
- a silane compound having an alkyl group at the molecular terminal is preferable from the viewpoint of easily increasing the vertical alignment regulating force, and a silane compound having an alkyl group having 3 to 30 carbon atoms is more preferable.
- the thickness of the vertical alignment film is preferably 5 ⁇ m or less, more preferably 3 ⁇ m or less, still more preferably 2 ⁇ m or less, preferably 1 nm or more, more preferably 5 nm, from the viewpoint of expressing the alignment regulating force. Or more, more preferably 10 nm or more, and particularly preferably 30 nm or more.
- the film thickness of the vertical alignment film can be measured using an ellipsometer or a contact-type film thickness meter.
- the horizontal alignment film has an alignment regulating force that aligns the liquid crystal compound in the horizontal direction.
- the horizontal alignment film can form a horizontal alignment state of the horizontal alignment liquid crystal cured film when the horizontal alignment liquid crystal cured film forming composition is formed on the horizontal alignment film.
- the alignment regulating force can be arbitrarily adjusted depending on, for example, the type of the alignment film, the surface state, and the rubbing conditions. It is possible. Such processing for expressing the orientation regulating force is called orientation processing.
- the horizontal alignment film preferably has a solvent resistance that does not dissolve when the liquid crystal composition is applied or the like, and has heat resistance in heat treatment for removing the solvent or aligning the liquid crystal compound.
- the horizontal alignment film examples include a rubbing alignment film, a photo alignment film, and a groove alignment film having a concavo-convex pattern and a plurality of grooves on the surface.
- a photo-alignment film is preferable in that the alignment direction can be easily controlled.
- a rubbing alignment film is usually formed by applying a composition containing an orientation polymer and a solvent (hereinafter sometimes referred to as a rubbing alignment film forming composition) to a substrate and removing the solvent to form a coating film. Then, the alignment regulating force can be imparted by rubbing the coating film.
- a composition containing an orientation polymer and a solvent hereinafter sometimes referred to as a rubbing alignment film forming composition
- orientation polymer examples include polyamides and gelatins having amide bonds, polyimides having imide bonds, and polyamic acids, polyvinyl alcohols, alkyl-modified polyvinyl alcohols, polyacrylamides, polyoxazoles, polyethyleneimines, polystyrenes having imide bonds. , Polyvinyl pyrrolidone, polyacrylic acid, and polyacrylic acid esters. These orientation polymers may be used alone or in combination of two or more.
- the concentration of the alignment polymer in the composition for forming a rubbing alignment film may be in a range where the alignment polymer is completely dissolved in the solvent.
- the content of the alignment polymer is preferably 0.1 to 20 parts by mass, and more preferably 0.1 to 10 parts by mass with respect to 100 parts by mass of the rubbing alignment film forming composition.
- compositions for forming a rubbing alignment film include, for example, a composition for forming a rubbing alignment film manufactured by Nissan Chemical Industries, Ltd. such as Sanever (registered trademark), and a JSR (such as Optomer (registered trademark)).
- the composition for forming a rubbing alignment film manufactured by Co., Ltd. may be mentioned.
- Examples of the rubbing treatment include a method in which a rubbing cloth is wound and the coating film is brought into contact with a rotating rubbing roll. If masking is performed when the rubbing treatment is performed, a plurality of regions (patterns) having different orientation directions can be formed in the alignment film.
- a composition containing a polymer or monomer having a photoreactive group and a solvent (hereinafter sometimes referred to as a photo-alignment film-forming composition) is applied to a substrate, and the solvent is removed. It is obtained by irradiation with polarized light (preferably polarized UV) later.
- polarized light preferably polarized UV
- the photo-alignment film can arbitrarily control the direction of the alignment regulating force by selecting the polarization direction of the polarized light to be irradiated.
- the photoreactive group refers to a group that generates alignment ability when irradiated with light. Specific examples include groups that are involved in photoreactions that are the origin of alignment ability, such as alignment-induced reactions, isomerization reactions, photodimerization reactions, photocrosslinking reactions, or photodecomposition reactions of molecules generated by light irradiation.
- an unsaturated bond particularly a group having a double bond is preferable, and a carbon-carbon double bond (C ⁇ C bond), a carbon-nitrogen double bond (C ⁇ N bond), and nitrogen-nitrogen.
- a group having at least one double bond selected from the group consisting of a double bond (N ⁇ N bond) and a carbon-oxygen double bond (C ⁇ O bond) is particularly preferred.
- Examples of the photoreactive group having a C ⁇ C bond include a vinyl group, a polyene group, a stilbene group, a stilbazole group, a stilbazolium group, a chalcone group, and a cinnamoyl group.
- Examples of the photoreactive group having a C ⁇ N bond include groups having a structure such as an aromatic Schiff base and an aromatic hydrazone.
- Examples of the photoreactive group having a C ⁇ O bond include a benzophenone group, a coumarin group, an anthraquinone group, and a maleimide group. These groups may have a substituent such as an alkyl group, an alkoxy group, an aryl group, an allyloxy group, a cyano group, an alkoxycarbonyl group, a hydroxyl group, a sulfonic acid group, and a halogenated alkyl group.
- a group involved in the photodimerization reaction or the photocrosslinking reaction is preferable from the viewpoint of excellent orientation.
- a photoreactive group involved in the photodimerization reaction is preferable, and a cinnamoyl group is preferable in that a photoalignment film having a relatively small amount of polarized light irradiation necessary for alignment and having excellent thermal stability and stability over time can be easily obtained.
- chalcone groups are preferred.
- the polymer having a photoreactive group a polymer having a cinnamoyl group in which the terminal portion of the polymer side chain has a cinnamic acid structure or a cinnamic acid ester structure is particularly preferable.
- Content of the polymer or monomer which has a photoreactive group can be adjusted with the kind of polymer or monomer, or the thickness of the target photo-alignment film, and it is 0.2 with respect to 100 mass parts of compositions for photo-alignment film formation.
- the amount is preferably at least part by mass, more preferably 0.3 to 10 parts by mass.
- the composition for forming a photo-alignment film applied on a substrate may be directly irradiated with polarized light after removing the solvent.
- the polarized light is preferably substantially parallel light.
- the wavelength of the polarized light to be irradiated should be in a wavelength range where the photoreactive group of the polymer or monomer having the photoreactive group can absorb light energy. Specifically, UV (ultraviolet light) having a wavelength of 250 to 400 nm is particularly preferable.
- Examples of the light source for irradiating the polarized light include a xenon lamp, a high-pressure mercury lamp, an ultrahigh-pressure mercury lamp, a metal halide lamp, and an ultraviolet laser such as KrF and ArF.
- a high-pressure mercury lamp, an ultrahigh-pressure mercury lamp, and a metal halide lamp are preferable because of high emission intensity of ultraviolet rays having a wavelength of 313 nm.
- the polarizing element include a polarizing prism such as a polarizing filter, Glan Thompson, and Grand Taylor, and a wire grid.
- a wire grid is preferable from the viewpoint of increasing the area and resistance to heat.
- the groove alignment film is a film having a concavo-convex pattern or a plurality of grooves (grooves) on the film surface.
- the composition is applied to a film having a plurality of linear grooves arranged at equal intervals, the liquid crystal compound is aligned in the direction along the groove.
- a method for obtaining a groove alignment film a method of forming a concavo-convex pattern by performing development and rinsing after exposure through an exposure mask having a pattern-shaped slit on the photosensitive polyimide film surface, a plate having grooves on the surface A layer of a pre-curing UV curable resin is formed on the substrate, and a method of curing the resin layer after transferring the formed resin layer to the base material, and a pre-curing UV curable resin film formed on the base material, Examples include a method in which a roll-shaped master having a plurality of grooves is pressed to form irregularities and then cured.
- the film thickness of the horizontal alignment film is preferably 1 ⁇ m or less, more preferably 0.5 ⁇ m or less, and even more preferably 0.3 ⁇ m or less, from the viewpoint of realizing a thin film and exhibiting alignment regulating power.
- the thickness of the horizontal alignment film is preferably 1 nm or more, more preferably 5 nm or more, still more preferably 10 nm or more, and particularly preferably 30 nm or more.
- the film thickness of the horizontal alignment film can be measured using an ellipsometer or a contact-type film thickness meter.
- a film oriented in the horizontal direction with respect to the in-plane direction of the vertically aligned liquid crystal cured film is a retardation film.
- the horizontal alignment film include a stretched film and a horizontal alignment liquid crystal cured film A.
- the optical properties of the horizontal alignment film can be adjusted by the alignment state of the polymerizable liquid crystal compound or the stretching method. From the viewpoint of thinning the horizontal alignment film, the horizontal alignment liquid crystal cured film A is preferable.
- the horizontal alignment liquid crystal cured film A is a liquid crystal cured film cured in a state where the polymerizable liquid crystal compound is aligned in the horizontal direction with respect to the in-plane direction of the vertical alignment liquid crystal cured film.
- the optical axis of the polymerizable liquid crystal compound is aligned in the horizontal direction with respect to the in-plane direction of the vertically aligned liquid crystal cured film.
- the polymerizable liquid crystal compound include liquid crystal compound (I) -1 having at least one polymerizable group.
- the horizontally aligned liquid crystal cured film included in the laminate of the present invention is referred to as a horizontally aligned liquid crystal cured film A
- the horizontally aligned liquid crystal cured film included in the polarizing film of the polarizing plate described later is horizontally aligned liquid crystal cured.
- membranes B are distinguished from each other.
- the horizontally aligned liquid crystal cured film A is a cured product of a composition (hereinafter sometimes referred to as a composition for forming a horizontally aligned liquid crystal cured film A).
- the manufacturing method of the horizontal alignment liquid crystal cured film A is different from the above-described manufacturing method of the vertical alignment liquid crystal cured film in that it is formed on the horizontal alignment film.
- the composition is applied onto a previously prepared horizontal alignment film to form a coating film, and the coating film is dried to form a dry film.
- stretched film examples of the stretched film include a stretched film made of a polycarbonate-based resin. Examples of commercially available stretched films include stretched films manufactured by Teijin Limited such as “Pure Ace (registered trademark) WR”.
- the stretched film is usually obtained by stretching a base film.
- a method of stretching the base film for example, a wound body in which the base film is wound on a roll is prepared, and the base film is continuously unwound from the wound body, and the unwound base
- the material film is conveyed to a heating furnace.
- the set temperature of the heating furnace is preferably in the range of near the glass transition temperature of the base film to the glass transition temperature + 50 ° C.
- the base film is stretched in the transport direction or in a direction orthogonal to the transport direction.
- stretching the conveyance direction and tension
- the delay axis direction of the stretched film varies depending on the stretching method, and the delay axis or the optical axis is determined according to the stretching method.
- the stretched film and the laminate of the present invention can be bonded via an adhesive layer.
- the laminated body of the present invention has the following relational expression (3) from the viewpoint of suppressing a decrease in ellipticity on the short wavelength side in the elliptically polarizing plate including the laminated body: ReA (450) / ReA (550) ⁇ 1 (3)
- ReA (450) represents the in-plane retardation value at a wavelength of 450 nm of the film oriented in the horizontal direction with respect to the in-plane direction of the vertically aligned liquid crystal cured film
- ReA (550) In-plane retardation value at a wavelength of 550 nm of a film oriented in the horizontal direction with respect to the in-plane direction of the vertically aligned liquid crystal cured film It is preferable to satisfy.
- ReA (450) / ReA (550) is more preferably 0.95 or less, and further preferably 0.90 or less.
- the following (3) -2 120 nm ⁇ ReA (550) ⁇ 170 nm (3) -2 It is preferable to satisfy From the viewpoint of improving the ellipticity of the elliptically polarizing plate provided with the laminate, preferably 130 nm ⁇ ReA (550) ⁇ 160 nm.
- the laminate including the vertically aligned liquid crystal cured film and the film oriented in the horizontal direction with respect to the in-plane direction of the vertically aligned liquid crystal cured film is obtained from the phase difference value and the oblique direction in the front direction.
- the thickness is more preferably 8 nm or less, and further preferably 4 nm or less.
- a laminate including a vertically aligned liquid crystal cured film and a film oriented in a horizontal direction with respect to the in-plane direction of the vertically aligned liquid crystal cured film includes an elliptically polarizing plate including the laminate.
- the following relational expression (5)
- R0 (450) represents the in-plane retardation value of the laminate at a wavelength of 450 nm
- R40 (450) is aligned in the horizontal direction with respect to the in-plane direction of the vertical alignment liquid crystal cured film. Shows the retardation value at a wavelength of 450 nm when the film is rotated 40 ° around the fast axis direction] It is preferable to satisfy.
- the thickness is more preferably 8 nm or less, and further preferably 4 nm or less.
- a laminate including a vertically aligned liquid crystal cured film and a film oriented in a horizontal direction with respect to the in-plane direction of the vertically aligned liquid crystal cured film includes an elliptically polarizing plate including the laminate.
- the manufacturing method of the laminated body of this invention includes a vertical alignment liquid crystal cured film formation process.
- the vertically aligned liquid crystal cured film forming step is the above-described method for producing a vertically aligned liquid crystal cured film.
- a laminate composed of a substrate and a vertically aligned liquid crystal cured film, and a laminate composed of the substrate, the alignment film, and the vertically aligned liquid crystal cured film can be produced. it can.
- the manufacturing method of a laminated body further includes a stretched film bonding process or a horizontal alignment liquid crystal cured film A formation process.
- the stretched film bonding step the stretched film is bonded to, for example, a vertically aligned liquid crystal cured film using an adhesive.
- the manufacturing method of a laminated body provided with the horizontal alignment liquid crystal cured film A may be manufactured, for example, by bonding a vertical alignment liquid crystal cured film and a horizontal alignment liquid crystal cured film via an adhesive layer, The horizontally aligned liquid crystal cured film A may be formed on the vertically aligned liquid crystal cured film. Further, a vertically aligned liquid crystal cured film may be formed on the stretched film or on the horizontally aligned liquid crystal cured film A.
- Adhesive examples include pressure-sensitive adhesives, dry-solidifying adhesives, and chemically reactive adhesives.
- Examples of the chemically reactive adhesive include an active energy ray curable adhesive.
- the pressure-sensitive adhesive usually contains a polymer and may contain a solvent.
- the polymer include acrylic polymers, silicone polymers, polyesters, polyurethanes, and polyethers.
- pressure-sensitive pressure-sensitive adhesives containing acrylic polymers have excellent optical transparency, moderate wettability and cohesive strength, excellent adhesion, and weather resistance and heat resistance. It is preferable because of its high properties and the like, and is unlikely to float or peel off under heating or humidification conditions.
- acrylic polymer examples include (meth) acrylates in which the alkyl group in the ester moiety is an alkyl group having 1 to 20 carbon atoms such as a methyl group, an ethyl group, or a butyl group, and (meth) acrylic acid or hydroxyethyl (A copolymer with a (meth) acrylic monomer having a functional group such as (meth) acrylate is preferred.
- the pressure-sensitive adhesive containing such a copolymer has excellent adhesiveness, and even when removed after being bonded to the transfer object, it is relatively easy to cause no adhesive residue on the transfer object. This is preferable because it can be removed.
- the glass transition temperature of the acrylic polymer is preferably 25 ° C. or less, and more preferably 0 ° C. or less.
- the mass average molecular weight of such an acrylic polymer is preferably 100,000 or more.
- the solvent examples include the solvents mentioned as the above solvent.
- the pressure-sensitive adhesive may contain a light diffusing agent.
- the light diffusing agent is an additive that imparts light diffusibility to the pressure-sensitive adhesive and may be fine particles having a refractive index different from that of the polymer contained in the pressure-sensitive adhesive.
- Examples of the light diffusing agent include fine particles made of an inorganic compound and fine particles made of an organic compound (polymer). Many of the polymers that pressure sensitive adhesives, including acrylic polymers, contain as an active ingredient have a refractive index of about 1.4 to 1.6, so that the light diffusion is 1.2 to 1.8. It is preferable to select appropriately from the agents.
- the refractive index difference between the polymer and the light diffusing agent contained in the pressure-sensitive adhesive as an active ingredient is usually 0.01 or more, and from the viewpoint of the brightness and display properties of the display device, 0.01 to 0.2. preferable.
- the fine particles used as the light diffusing agent are preferably spherical fine particles, or fine particles close to monodisperse, and more preferably fine particles having an average particle diameter of 2 to 6 ⁇ m.
- the refractive index is measured by a general minimum deviation method or Abbe refractometer.
- Examples of the fine particles made of an inorganic compound include aluminum oxide (refractive index 1.76) and silicon oxide (refractive index 1.45).
- Examples of fine particles made of an organic compound (polymer) include melamine beads (refractive index 1.57), polymethyl methacrylate beads (refractive index 1.49), methyl methacrylate / styrene copolymer resin beads (refractive index 1). .50 to 1.59), polycarbonate beads (refractive index 1.55), polyethylene beads (refractive index 1.53), polystyrene beads (refractive index 1.6), polyvinyl chloride beads (refractive index 1.46), And silicone resin beads (refractive index 1.46).
- the content of the light diffusing agent is usually 3 to 30 parts by mass with respect to 100 parts by mass of the polymer.
- the thickness of the pressure-sensitive pressure-sensitive adhesive is determined according to its adhesion and the like and is not particularly limited, but is usually 1 ⁇ m to 40 ⁇ m. From the viewpoint of processability and durability, the thickness is preferably 3 ⁇ m to 25 ⁇ m, and more preferably 5 ⁇ m to 20 ⁇ m. By adjusting the thickness of the pressure-sensitive adhesive layer to 5 ⁇ m to 20 ⁇ m, the brightness of the display device when viewed from the front or obliquely is maintained, and blurring or blurring of the display image occurs. Can be difficult.
- the dry-solidifying adhesive may contain a solvent.
- a solvent for example, a polymer of a monomer having a protonic functional group such as a hydroxyl group, a carboxyl group, or an amino group and an ethylenically unsaturated group, or a urethane polymer as a main component
- a crosslinking agent or a curable compound such as a polyvalent aldehyde, an epoxy compound, an epoxy resin, a melamine compound, a zirconia compound, and a zinc compound is included.
- Examples of the polymer of a monomer having a protonic functional group such as a hydroxyl group, a carboxyl group or an amino group and an ethylenically unsaturated group include, for example, an ethylene-maleic acid copolymer, an itaconic acid copolymer, and an acrylic acid copolymer. , Acrylamide copolymer, saponified polyvinyl acetate, and polyvinyl alcohol resin.
- polyvinyl alcohol resin examples include polyvinyl alcohol, partially saponified polyvinyl alcohol, fully saponified polyvinyl alcohol, carboxyl group-modified polyvinyl alcohol, acetoacetyl group-modified polyvinyl alcohol, methylol group-modified polyvinyl alcohol, and amino group-modified polyvinyl alcohol. Can be mentioned.
- the content of the polyvinyl alcohol-based resin in the water-based adhesive is usually 1 to 10 parts by weight, preferably 1 to 5 parts by weight with respect to 100 parts by weight of water.
- the urethane resin examples include a polyester ionomer type urethane resin.
- the polyester ionomer type urethane resin here is a urethane resin having a polyester skeleton, and a resin in which a small amount of an ionic component (hydrophilic component) is introduced. Since such an ionomer type urethane resin is emulsified in water without using an emulsifier and becomes an emulsion, it can be an aqueous adhesive. When a polyester ionomer type urethane resin is used, it is effective to blend a water-soluble epoxy compound as a crosslinking agent.
- the epoxy resin examples include a polyamide epoxy resin obtained by reacting a polyalkylene polyamine such as diethylenetriamine or triethylenetetramine with a dicarboxylic acid such as adipic acid and epichlorohydrin.
- examples of such commercially available polyamide epoxy resins include “Smiles Resin (registered trademark) 650” and “Smiles Resin 675” manufactured by Sumika Chemtex Co., Ltd., and “WS-525” manufactured by Japan PMC Co., Ltd. It is done.
- the content thereof is usually 1 to 100 parts by mass, preferably 1 to 50 parts by mass with respect to 100 parts by mass of the polyvinyl alcohol resin.
- the thickness of the adhesive layer formed from the dry-solidifying adhesive is usually 0.001 to 5 ⁇ m, preferably 0.01 to 2 ⁇ m, from the viewpoint of suppressing the appearance failure. Preferably, it is 0.01 to 0.5 ⁇ m.
- the active energy ray curable adhesive may contain a solvent.
- An active energy ray-curable adhesive is an adhesive that cures upon irradiation with an active energy ray.
- Examples of the active energy ray curable adhesive include a cationic polymerizable adhesive containing an epoxy compound and a cationic polymerization initiator, a radical polymerizable adhesive containing an acrylic curing component and a radical polymerization initiator, An adhesive containing both a cationic polymerizable curing component such as an epoxy compound and a radical polymerizable curing component such as an acrylic compound, and further containing a cationic polymerization initiator and a radical polymerization initiator, and these Examples thereof include an adhesive that is cured by irradiation with an electron beam without containing a polymerization initiator.
- active energy ray-curable adhesives contains radical polymerizable active energy ray-curable adhesives containing an acrylic curing component and a photoradical polymerization initiator, and epoxy compounds and photocationic polymerization initiators.
- Cationic polymerizable active energy ray curable adhesives are preferred.
- the acrylic curing component include (meth) acrylates such as methyl (meth) acrylate and hydroxyethyl (meth) acrylate, and (meth) acrylic acid.
- the active energy ray-curable adhesive containing an epoxy compound may further contain a compound other than the epoxy compound. Examples of compounds other than the epoxy compound include oxetane compounds and acrylic compounds.
- Examples of the photo radical polymerization initiator and the photo cation polymerization initiator include the above-mentioned photo radical polymerization initiator and photo cation polymerization initiator.
- the content of the radical polymerization initiator and the cationic polymerization initiator is usually 0.5 to 20 parts by mass, preferably 1 to 15 parts by mass with respect to 100 parts by mass of the active energy ray-curable adhesive.
- the active energy ray-curable adhesive further contains an ion trap agent, an antioxidant, a chain transfer agent, a tackifier, a thermoplastic resin, a filler, a flow regulator, a plasticizer, and an antifoaming agent. May be.
- the elliptically polarizing plate includes the laminate and a polarizing film.
- the elliptically polarizing plate may further include an arbitrary layer (more specifically, a protective layer, an adhesive, etc.) as necessary.
- the laminate and the polarizing film are bonded via, for example, an adhesive.
- FIG. 1 is a schematic cross-sectional view showing an example of the layer configuration of an elliptically polarizing plate.
- An elliptically polarizing plate 20 shown in FIG. 1 includes a laminate 15, an adhesive 7, a polarizing film 11, and a protective layer 13.
- the laminate 15 is composed of the base material 1, the horizontal alignment film 3, the horizontal alignment liquid crystal cured film A 5, the adhesive 7, and the vertical alignment liquid crystal cured film 9.
- the angle formed by the slow axis of the horizontally aligned liquid crystal cured film A 5 and the absorption axis of the polarizing film 11 is 45 ⁇ 5 °.
- the polarizing film is a film having a polarizing function.
- the polarizing film include a film containing a dichroic dye and oriented in the horizontal direction with respect to the film surface of the polarizing film (more specifically, a stretched film on which the dichroic dye is adsorbed (hereinafter referred to as polarizing film A).
- polarizing film A a stretched film on which the dichroic dye is adsorbed
- a horizontally aligned liquid crystal cured film B containing a dichroic dye hereinafter sometimes referred to as a polarizing film B)
- a horizontally aligned liquid crystal cured film B containing a dichroic dye is preferable.
- the dichroic dye means a dye which exhibits absorption anisotropy and has a property that the absorbance in the major axis direction of the molecule of the dichroic dye is different from the absorbance in the minor axis direction.
- the horizontally aligned liquid crystal cured film B is a cured product of a composition containing a dichroic dye and a polymerizable liquid crystal compound (B) (hereinafter sometimes referred to as a composition for forming a polarizing film B).
- the horizontal alignment liquid crystal cured film B is a liquid crystal cured film that contains a dichroic dye and is cured in a state where the polymerizable liquid crystal compound (B) is aligned in the horizontal direction with respect to the in-plane direction.
- the horizontally aligned liquid crystal cured film B is preferably a cured film in which the polymerizable liquid crystal compound (B) is cured in a smectic phase aligned in the horizontal direction with respect to the in-plane direction of the film. That is, when the polymerizable liquid crystal compound (B) is a thermotropic liquid crystal, it may be a thermotropic liquid crystal compound showing a nematic liquid crystal phase or a thermotropic liquid crystal compound showing a smectic liquid crystal phase. .
- the liquid crystal state exhibited by the polymerizable liquid crystal compound is preferably a smectic phase, and a higher order smectic phase is more preferable from the viewpoint of high performance.
- higher-order smectic liquid crystal compounds that form a smectic B phase, a smectic D phase, a smectic E phase, a smectic F phase, a smectic G phase, a smectic H phase, a smectic I phase, a smectic J phase, a smectic K phase, or a smectic L phase.
- higher-order smectic liquid crystal compounds that form a smectic B phase, a smectic F phase, or a smectic I phase.
- the liquid crystal phase formed by the polymerizable liquid crystal compound (B) is a high-order smectic phase
- a polarizing film having higher polarization performance can be produced.
- such a polarizing film having high polarization performance can obtain a Bragg peak derived from a higher-order structure such as a hexatic phase or a crystal phase in X-ray diffraction measurement.
- the Bragg peak is a peak derived from a periodic structure of molecular orientation, and a film having a periodic interval of 3 to 6 mm can be obtained.
- the horizontally aligned liquid crystal cured film B preferably contains a polymer of a polymerizable liquid crystal compound (B) polymerized in a smectic phase from the viewpoint of obtaining higher polarization characteristics.
- the below-mentioned polymerizable group which the polymerizable liquid crystal compound (B) has may be in an unpolymerized state or in a polymerized state in the horizontally aligned liquid crystal cured film B. That is, the horizontal alignment liquid crystal cured film B is in any state of a polymerizable liquid crystal compound (B) (monomer), an oligomer of the polymerizable liquid crystal compound (B), a polymer of the polymerizable liquid crystal compound (B), and a combination thereof. May be included.
- the polymerizable group of the polymerizable liquid crystal compound (B) is preferably in an unpolymerized state in the horizontally aligned liquid crystal cured film B.
- polymerizable liquid crystal compound (B) examples include compounds represented by the following formula (B).
- the said polymerizable liquid crystal may be used independently and may be used in combination of 2 or more type.
- X 1 , X 2 , and X 3 each independently represent a divalent aromatic group or a divalent alicyclic hydrocarbon group, wherein the divalent aromatic group or the divalent fat
- the hydrogen atom contained in the cyclic hydrocarbon group is a halogen atom, an alkyl group having 1 to 4 carbon atoms, a fluoroalkyl group having 1 to 4 carbon atoms, an alkoxy group having 1 to 4 carbon atoms, a cyano group, or a nitro group.
- the carbon atom constituting the divalent aromatic group or the divalent alicyclic hydrocarbon group may be substituted with an oxygen atom, a sulfur atom, or a nitrogen atom.
- at least one of X 1 , X 2 , and X 3 is an optionally substituted 1,4-phenylene group, or an optionally substituted cyclohexane-1,4- Represents a diyl group.
- Y 1 , Y 2 , W 1 and W 2 each independently represent a single bond or a divalent linking group.
- V 1 and V 2 each independently represents an optionally substituted alkanediyl group having 1 to 20 carbon atoms, and —CH 2 — constituting the alkanediyl group represents —O—, — It may be substituted with CO-, -S- or NH-.
- U 1 and U 2 each independently represent a polymerizable group or a hydrogen atom, and at least one of U 1 and U 2 represents a polymerizable group.
- At least one of X 1 , X 2 and X 3 may have a 1,4-phenylene group which may have a substituent, or a substituent.
- X 1 and X 3 preferably represent a cyclohexane-1,4-diyl group which may have a substituent, and the cyclohexane-1,4-diyl group is trans-cyclohexane- More preferably, it represents a 1,4-diyl group.
- Examples of the substituent which the 1,4-phenylene group which may have a substituent or the cyclohexane-1,4-diyl group which may have a substituent optionally have, for example, a methyl group , An alkyl group having 1 to 4 carbon atoms such as an ethyl group and a butyl group, a cyano group, and a halogen atom such as a chlorine atom and a fluorine atom.
- the 1,4-phenylene group which may have a substituent or the cyclohexane-1,4-diyl group which may have a substituent is preferably a 1,4-phenylene group or a cyclohexane.
- Xan-1,4-diyl group when Y 1 and Y 2 have the same structure, it is preferable that at least one of X 1 , X 2 and X 3 is a different structure. When at least one of X 1 , X 2 and X 3 has a different structure, smectic liquid crystallinity tends to be easily exhibited.
- Y 1 and Y 2 are each independently a single bond, —CH 2 CH 2 —, —CH 2 O—, —CH 2 CH 2 O—, —COO—, —OCO—, —N ⁇ N—, — CR a ⁇ CR b —, —C ⁇ C— or CR a ⁇ N— is preferably represented, and R a and R b each independently represent a hydrogen atom or an alkyl group having 1 to 4 carbon atoms. More preferably, Y 1 and Y 2 each independently represent —CH 2 CH 2 —, —COO—, —OCO—, or a single bond.
- Y 1 and Y 2 have different bonding methods.
- Y 1 and Y 2 are different from each other, smectic liquid crystal properties tend to be easily exhibited.
- W 1 and W 2 each independently preferably represents a single bond, —O—, —S—, —COO—, or OCO—, and more preferably each independently represents a single bond or O—. .
- Examples of the alkanediyl group having 1 to 20 carbon atoms represented by V 1 and V 2 include methylene group, ethylene group, propane-1,3-diyl group, butane-1,3-diyl group, butane-1 , 4-diyl group, pentane-1,5-diyl group, hexane-1,6-diyl group, heptane-1,7-diyl group, octane-1,8-diyl group, decane-1,10-diyl group , A tetradecane-1,14-diyl group, and an icosane-1,20-diyl group.
- V 1 and V 2 preferably represent an alkanediyl group having 2 to 12 carbon atoms, more preferably a linear alkanediyl group having 6 to 12 carbon atoms.
- V 1 and V 2 represent a linear alkanediyl group having 6 to 12 carbon atoms
- the orientation of the polymerizable liquid crystal compound (B) is improved, and smectic liquid crystal properties tend to be easily exhibited.
- the substituent that the optionally substituted alkanediyl group having 1 to 20 carbon atoms optionally has include a cyano group and halogen atoms such as a chlorine atom and a fluorine atom.
- the diyl group is preferably unsubstituted, and more preferably an unsubstituted and linear alkanediyl group.
- U 1 and U 2 both preferably represent a polymerizable group, and more preferably both represent a photopolymerizable group. Since the polymerizable liquid crystal compound (B) having a photopolymerizable group can be polymerized under a lower temperature condition than the thermally polymerizable group, it is advantageous in that the liquid crystal can form a polymer with a higher degree of order.
- the polymerizable groups represented by U 1 and U 2 may be the same or different from each other, but are preferably the same.
- the polymerizable group include a vinyl group, vinyloxy group, 1-chlorovinyl group, isopropenyl group, 4-vinylphenyl group, acryloyloxy group, methacryloyloxy group, oxiranyl group, and oxetanyl group.
- an acryloyloxy group, a methacryloyloxy group, a vinyloxy group, an oxiranyl group, and an oxetanyl group are preferable, and a methacryloyloxy group and an acryloyloxy group are more preferable.
- Examples of such a polymerizable liquid crystal compound (B) include polymerizable liquid crystal compounds represented by the following formulas (1-1) to (1-23).
- a polymeric liquid crystal compound may be used individually by 1 type, and may be used in combination of 2 or more type.
- Polymerizable liquid crystal compound (B) is, for example, Lub et al., Recl. Trav. Chim. It can be produced by a known method described in Pays-Bas, 115, 321-328 (1996), or Japanese Patent No. 4719156.
- the composition containing the polymerizable liquid crystal compound (B) may contain other liquid crystal compounds other than the polymerizable liquid crystal compound (B) as long as the effects of the present invention are not impaired.
- the ratio of the polymerizable liquid crystal compound (B) to the total mass of all liquid crystal compounds contained in the composition containing the polymerizable liquid crystal compound (B) is preferably 51% by mass or more, more preferably 70%. It is at least mass%, more preferably at least 90 mass%.
- composition containing the polymerizable liquid crystal compound (B) contains two or more polymerizable liquid crystal compounds (B), at least one of them may be a polymerizable liquid crystal compound (B: exemplary compound). , All of which may be a polymerizable liquid crystal compound (B).
- the content of the polymerizable liquid crystal compound (B) in the composition containing the polymerizable liquid crystal compound (B) is preferably 40 to 99.9 mass with respect to the solid content of the composition containing the polymerizable liquid crystal compound (B). %, More preferably 60 to 99% by mass, still more preferably 70 to 99% by mass.
- solid content means the total amount of the component remove
- a dichroic dye refers to a dye having the property that the absorbance in the major axis direction of a molecule is different from the absorbance in the minor axis direction.
- the dichroic dye preferably has a property of absorbing visible light, and more preferably has a maximum absorption wavelength ( ⁇ MAX ) in a wavelength range of 380 to 680 nm.
- Examples of the dichroic pigment include iodine and dichroic organic dyes.
- Examples of the dichroic organic dye include acridine dyes, oxazine dyes, cyanine dyes, naphthalene dyes, azo dyes, and anthraquinone dyes.
- azo dyes are preferred.
- examples of the azo dye include a monoazo dye, a bisazo dye, a trisazo dye, a tetrakisazo dye, and a stilbene azo dye.
- bisazo dyes and trisazo dyes are preferable.
- the dichroic organic dye may be used alone or in combination of two or more. However, in order to obtain absorption in the entire visible light region, three or more dichroic dyes are used. It is preferable to use in combination, and it is more preferable to use three or more kinds of azo dyes in combination.
- the polyvinyl alcohol-based resin film is preferably subjected to an immersion treatment in water before the dyeing treatment.
- Examples of the azo dye include a compound represented by the formula (I).
- T 1 -A 1 (-N NA 2 )
- p -N NA 3 -T 2 (I)
- a 1 , A 2 , and A 3 each independently have a 1,4-phenylene group, a naphthalene-1,4-diyl group, or a substituent that may have a substituent.
- T 1 and T 2 each independently represent an electron-withdrawing group or an electron-emitting group and have a position substantially 180 ° with respect to the azo bond plane.
- p represents an integer of 0 to 4.
- p represents an integer greater than or equal to 2
- several A2 may mutually be same or different.
- the —N ⁇ N— bond may be replaced with a —C ⁇ C— bond, —COO— bond, —NHCO— bond, or —N ⁇ CH— bond.
- Examples of the substituent that the 1,4-phenylene group, naphthalene-1,4-diyl group, and divalent heterocyclic group represented by A 1 , A 2 , and A 3 optionally have include a methyl group, an ethyl group, and the like.
- an alkyl group having 1 to 4 carbon atoms such as a butyl group; an alkoxy group having 1 to 4 carbon atoms such as a methoxy group, an ethoxy group, and a butoxy group; and an alkyl group having 1 to 4 carbon atoms such as a trifluoromethyl group
- Substituted amino group is -NH 2.
- the alkyl group having 1 to 6 carbon atoms include a methyl group, an ethyl group, and a hexyl group.
- the alkanediyl group having 2 to 8 carbon atoms include ethylene group, propane-1,3-diyl group, butane-1,3-diyl group, butane-1,4-diyl group, pentane-1,5- Examples include a diyl group, a hexane-1,6-diyl group, a heptane-1,7-diyl group, and an octane-1,8-diyl group.
- a 1 , A 2 and A 3 are either 1,4-phenylene groups which are unsubstituted or substituted with a methyl group or a methoxy group, or It preferably represents a divalent heterocyclic group, and p preferably represents an integer of 0-2.
- p is 1 and at least two of the three structures A 1 , A 2 and A 3 are 1,4-phenylene groups in terms of both ease of molecular synthesis and high performance. More preferred.
- Examples of the divalent heterocyclic group include groups obtained by removing two hydrogen atoms from a heterocyclic ring such as quinoline, thiazole, benzothiazole, thienothiazole, imidazole, benzimidazole, oxazole, and benzoxazole.
- a 2 represents a divalent heterocyclic group
- a structure in which the molecular bond angle is substantially 180 ° is preferable.
- a benzoxazole structure is more preferable.
- T 1 and T 2 each independently represents an electron-withdrawing group or electron-emitting group, and preferably represents an electron-withdrawing group or electron-emitting group having a different structure
- T 1 represents an electron-withdrawing group
- T 2 More preferably, it represents an electron emitting group, or T 1 represents an electron emitting group and represents a T 2 electron withdrawing group.
- each of T 1 and T 2 independently represents an alkyl group having 1 to 8 carbon atoms, an alkoxy group having 1 to 8 carbon atoms, a cyano group, a nitro group, or an alkyl group having 1 to 6 carbon atoms.
- a substituted amino group having two or two, an amino group in which the two substituted alkyl groups are bonded to each other to form an alkanediyl group having 2 to 8 carbon atoms, and a trifluoromethyl group are preferable.
- a structure with a smaller excluded volume of molecules so that an alkyl group having 1 to 6 carbon atoms and an alkoxy group having 1 to 6 carbon atoms Group, a cyano group, a substituted amino group having one or two alkyl groups having 1 to 6 carbon atoms, and two amino groups bonded to each other to form an alkanediyl group having 2 to 8 carbon atoms Groups are preferred.
- azo dyes examples include azo dyes represented by the following formulas (2-1) to (2-8).
- B 1 to B 30 are each independently a hydrogen atom, an alkyl group having 1 to 6 carbon atoms, an alkoxy group having 1 to 6 carbon atoms, a cyano group, a nitro group, a substituted or unsubstituted amino group (substituted amino group and As defined above, the unsubstituted amino group represents a chlorine atom or a trifluoromethyl group.
- B 2 , B 6 , B 9 , B 14 , B 18 , B 19 , B 22 , B 23 , B 24 , B 27 , B 28 And B 29 each independently preferably represents a hydrogen atom or a methyl group, and more preferably represents a hydrogen atom.
- n1 to n4 each independently represents an integer of 0 to 2.
- the plurality of B 2 may be the same as or different from each other
- the plurality of B 6 may be the same as or different from each other
- the plurality of B 9 may be the same as or different from each other
- n4 represents 2
- a plurality of B 14 may be the same or may be different from one another.
- the anthraquinone dye is preferably a compound represented by the formula (2-9).
- R 1 to R 8 each independently represents a hydrogen atom, —R x , —NH 2 , —NHR x , —NR x 2 , —SR x , or a halogen atom.
- R x represents an alkyl group having 1 to 4 carbon atoms or an aryl group having 6 to 12 carbon atoms.
- the oxazine dye is preferably a compound represented by the formula (2-10).
- R 9 to R 15 each independently represents a hydrogen atom, —R x , —NH 2 , —NHR x , —NR x 2 , —SR x , or a halogen atom.
- R x represents an alkyl group having 1 to 4 carbon atoms or an aryl group having 6 to 12 carbon atoms.
- the acridine dye is preferably a compound represented by the formula (2-11).
- R 16 to R 23 each independently represents a hydrogen atom, —R x , —NH 2 , —NHR x , —NR x 2 , —SR x , or a halogen atom.
- R x represents an alkyl group having 1 to 4 carbon atoms or an aryl group having 6 to 12 carbon atoms.
- examples of the alkyl group having 1 to 4 carbon atoms represented by R x include a methyl group, an ethyl group, a propyl group, A butyl group, a pentyl group, and a hexyl group are mentioned.
- examples of the aryl group having 6 to 12 carbon atoms represented by R x include a phenyl group, a toluyl group, a xylyl group, And a naphthyl group.
- cyanine dye a compound represented by the formula (2-12) and a compound represented by the formula (2-13) are preferable.
- D 1 and D 2 each independently represent a group represented by any one of formulas (2-12a) to (2-12d).
- n5 represents an integer of 1 to 3.
- D 3 and D 4 each independently represent a group represented by any one of formulas (2-13a) to (2-13h).
- n6 represents an integer of 1 to 3.
- the content of the dichroic organic dye (the total amount in the case of including a plurality of types) is usually 0.1 with respect to 100 parts by mass of the polymerizable liquid crystal compound (B) from the viewpoint of obtaining good light absorption characteristics. -30 parts by mass, preferably 1-20 parts by mass, more preferably 3-15 parts by mass.
- the content of the dichroic organic dye is 0.1 parts by mass or more with respect to 100 parts by mass of the polymerizable liquid crystal compound (B)
- light absorption of the dichroic organic dye is sufficient, and sufficient polarization performance Tends to be obtained.
- the content of the dichroic organic dye is 30 parts by mass or less with respect to 100 parts by mass of the polymerizable liquid crystal compound (B)
- the orientation of the polymerizable liquid crystal compound is hardly inhibited.
- At least one of the stretched films on which the dichroic dye is adsorbed may be provided with a transparent protective film.
- a film containing a stretched film on which a dichroic dye is adsorbed as a polarizer is usually a step of uniaxially stretching a polyvinyl alcohol resin film, and the dichroism is obtained by dyeing the polyvinyl alcohol resin film with a dichroic dye.
- At least one surface of a polarizer produced through a step of adsorbing a dye, a step of treating a polyvinyl alcohol resin film adsorbed with a dichroic dye with an aqueous boric acid solution, and a step of washing with water after the treatment with an aqueous boric acid solution It is produced by sandwiching with a transparent protective film through an adhesive.
- the polyvinyl alcohol resin can be obtained by saponifying a polyvinyl acetate resin.
- a polyvinyl acetate-based resin for example, polyvinyl acetate, which is a homopolymer of vinyl acetate, or a copolymer of vinyl acetate and another monomer copolymerizable therewith is used.
- examples of other monomers copolymerizable with vinyl acetate include unsaturated carboxylic acids, olefins, vinyl ethers, unsaturated sulfonic acids, and acrylamides having an ammonium group.
- the degree of saponification of the polyvinyl alcohol resin is usually about 85 to 100 mol%, preferably 98 mol% or more.
- the polyvinyl alcohol resin may be modified, and for example, polyvinyl formal or polyvinyl acetal modified with aldehydes can also be used.
- the degree of polymerization of the polyvinyl alcohol resin is usually about 1,000 to 10,000, and preferably in the range of 1,500 to 5,000.
- a film obtained by forming such a polyvinyl alcohol resin is used as an original film of a polarizing film.
- the method for forming a polyvinyl alcohol-based resin is not particularly limited, and can be formed by a known method.
- the film thickness of the polyvinyl alcohol-based raw film can be, for example, about 10 to 150 ⁇ m.
- Uniaxial stretching of the polyvinyl alcohol-based resin film can be performed before dyeing with the dichroic dye, simultaneously with dyeing, or after dyeing.
- the uniaxial stretching may be performed before boric acid treatment or during boric acid treatment.
- it is also possible to perform uniaxial stretching in these several steps.
- uniaxial stretching it may be uniaxially stretched between rolls having different peripheral speeds, or may be uniaxially stretched using a hot roll.
- the uniaxial stretching may be dry stretching in which stretching is performed in the air, or may be wet stretching in which stretching is performed in a state where a solvent is used and a polyvinyl alcohol-based resin film is swollen.
- the draw ratio is usually about 3 to 8 times.
- the dyeing of the polyvinyl alcohol resin film with the dichroic dye is performed, for example, by a method of immersing the polyvinyl alcohol resin film in an aqueous solution containing the dichroic dye.
- iodine When iodine is used as the dichroic dye, a method of dyeing a polyvinyl alcohol-based resin film in an aqueous solution containing iodine and potassium iodide is usually employed.
- the iodine content in this aqueous solution is usually about 0.01 to 1 part by mass per 100 parts by mass of water.
- the content of potassium iodide is usually about 0.5 to 20 parts by mass per 100 parts by mass of water.
- the temperature of the aqueous solution used for dyeing is usually about 20 to 40 ° C.
- the immersion time (dyeing time) in this aqueous solution is usually about 20 to 1,800 seconds.
- a method of immersing and dyeing a polyvinyl alcohol-based resin film in an aqueous solution containing a water-soluble dichroic dye is usually employed.
- the content of the dichroic organic dye in this aqueous solution is usually about 1 ⁇ 10 ⁇ 4 to 10 parts by mass, preferably 1 ⁇ 10 ⁇ 3 to 1 part by mass, more preferably 100 parts by mass of water. Is 1 ⁇ 10 ⁇ 3 to 1 ⁇ 10 ⁇ 2 parts by mass.
- This aqueous solution may contain an inorganic salt such as sodium sulfate as a dyeing assistant.
- the temperature of the aqueous dichroic dye solution used for dyeing is usually about 20 to 80 ° C.
- the immersion time (dyeing time) in this aqueous solution is usually about 10 to 1,800 seconds.
- the boric acid treatment after dyeing with a dichroic dye can usually be performed by a method of immersing the dyed polyvinyl alcohol resin film in a boric acid aqueous solution.
- the boric acid content in this aqueous boric acid solution is usually about 2 to 15 parts by mass, preferably 5 to 12 parts by mass, with respect to 100 parts by mass of water.
- this boric acid aqueous solution preferably contains potassium iodide.
- the content of potassium iodide is usually 0.1 to 100 parts by mass of water.
- the amount is about 15 parts by mass, preferably 5 to 12 parts by mass.
- the immersion time in the boric acid aqueous solution is usually about 60 to 1,200 seconds, preferably 150 to 600 seconds, and more preferably 200 to 400 seconds.
- the temperature of boric acid treatment is usually 50 ° C. or higher, preferably 50 to 85 ° C., more preferably 60 to 80 ° C.
- the polyvinyl alcohol resin film after the boric acid treatment is usually washed with water.
- the water washing treatment can be performed, for example, by a method of immersing a boric acid-treated polyvinyl alcohol resin film in water.
- the temperature of water in the water washing treatment is usually about 5 to 40 ° C.
- the immersion time is usually about 1 to 120 seconds.
- a drying process is performed to obtain a polarizer.
- the drying process can be performed using, for example, a hot air dryer or a far infrared heater.
- the temperature for the drying treatment is usually about 30 to 100 ° C., preferably 50 to 80 ° C.
- the drying treatment time is usually about 60 to 600 seconds, preferably 120 to 600 seconds.
- the moisture content of the polarizer is reduced to a practical level.
- the water content is usually about 5 to 20% by weight, preferably 8 to 15% by weight.
- the moisture content is less than 5% by weight, the flexibility of the polarizer is lost, and the polarizer may be damaged or broken after drying.
- the thermal stability of the polarizer may be deteriorated.
- the thickness of the polarizer obtained by subjecting the polyvinyl alcohol resin film to uniaxial stretching, dyeing with a dichroic dye, boric acid treatment, washing with water and drying is preferably 5 to 40 ⁇ m.
- the organic EL display device includes the elliptically polarizing plate.
- a device in which an elliptically polarizing plate is bonded to an organic EL panel through an adhesive is exemplified.
- Liquid crystal compound A was produced according to the method described in JP 2010-31223 A.
- Liquid crystal compound B was produced according to the method described in JP-A-2009-173893. Below, the molecular structure of the liquid crystal compound A and the liquid crystal compound B is shown, respectively.
- Measurement method (Calculation method of ratio of maximum absorption wavelength and maximum absorbance) A 1 mg / 50 mL tetrahydrofuran solution of liquid crystal compound A was prepared and used as a measurement sample. A measurement sample was put in a measurement cell having an optical path length of 1 cm. The measurement sample was set in an ultraviolet-visible spectrophotometer (“UV-2450” manufactured by Shimadzu Corporation), and the absorption spectrum was measured. The reference was only the solvent of the measurement sample. The wavelength at which the maximum absorption was obtained was read from the obtained absorption spectrum, and this was taken as the maximum absorption wavelength ⁇ max .
- UV-2450 ultraviolet-visible spectrophotometer
- the maximum absorption wavelength of the liquid crystal compound A in the region of a wavelength of 260 nm or more and 400 nm or less was read from the obtained absorption spectrum.
- the wavelength having the highest absorbance among the plurality of maximum absorption wavelengths is defined as ⁇ max .
- the obtained maximum absorption wavelength is shown in Table 1.
- the ionic compound (1) was produced according to the method described in Japanese Patent Application No. 2016-514802.
- the ionic compound (2) and the ionic compound (3) were produced according to the method described in JP2013-28586A or JP2013-199509A.
- the structural formulas of the ionic compounds (1) to (3) are shown below.
- Example 1 [Preparation of vertically aligned liquid crystal cured film forming composition (A-1)] Liquid crystal compound A and liquid crystal compound B were mixed at a mass ratio of 90:10 to obtain a mixture. With respect to 100 parts by mass of the resulting mixture, 1.5 parts by mass of a leveling agent (“F-556” manufactured by DIC) and 2-dimethylamino-2-benzyl-1- (4- 6 parts by mass of morpholinophenyl) butan-1-one (“Irgacure (registered trademark) 369 (Irg369)” manufactured by BASF Japan Ltd.) was added.
- a leveling agent F-556” manufactured by DIC
- 2-dimethylamino-2-benzyl-1- (4- 6 parts by mass of morpholinophenyl) butan-1-one (“Irgacure (registered trademark) 369 (Irg369)” manufactured by BASF Japan Ltd.) was added.
- silane compound (1) 3-triethoxysilyl-N- (1,3-dimethyl-butylidene) propylamine (“KBE-9103” manufactured by Shin-Etsu Chemical Co., Ltd.) as a silane compound is adjusted to be 0.5%.
- Compound (1) was further added so as to be 2.0%.
- composition (A-1) N-methyl-2-pyrrolidone (NMP) was added so that the solid concentration was 13%.
- NMP N-methyl-2-pyrrolidone
- A-1 a vertically aligned liquid crystal cured film forming composition
- the silane compound (“KBE-9103” manufactured by Shin-Etsu Chemical Co., Ltd.) reacts with the solvent in the composition (A-1) and moisture in the environment to cause hydrolysis, and the amino group which is a polar group Confirmed to generate.
- phase difference value ZF-14-23 as a substrate is an optically isotropic film having a retardation value of 1 nm or less at a wavelength of 550 nm, and it was confirmed that the measured value of the sample for measuring optical properties is not affected. Subsequently, the phase difference value was measured using a measuring device (“KOBRA-WPR” manufactured by Oji Scientific Co., Ltd.) while changing the incident angle of the light to the optical property measurement sample.
- a measuring device (“KOBRA-WPR” manufactured by Oji Scientific Co., Ltd.
- the average refractive index at wavelengths ⁇ 450 nm and 550 nm was measured using a refractometer (“Multiwave Abbe Refractometer DR-M4” manufactured by Atago Co., Ltd.).
- the orientation of the vertically aligned liquid crystal cured film (A-1) produced from the composition (A-1) was A.
- A very good: The number of alignment defects is 0 or more and 3 or less.
- B very good: The number of alignment defects is 4 or more and 10 or less.
- C good: The number of alignment defects is 11 or more and 50 or less.
- D bad: 51 or more alignment defects or no alignment defect.
- the base material of Example 1, 0.5% of the silane compound, and 2 parts of the ionic compound (2) are the types of base materials described in Table 1, the types and addition amounts of the silane compounds, and the types and additions of ionic compounds.
- the compositions of Examples 2 to 9, Example 20, Example 21, and Comparative Example 1 (A-2) were the same as the preparation method of the composition (A-1) of Example 1 except that the amount was changed. ) To (A-9), (A-20), (A-21), and (B-1) were prepared. The composition (A-1) was changed to compositions (A-2) to (A-9), (A-20), (A-21), and (B-1), and the film thickness of the coating film was further changed.
- Examples 2 to 9 and Examples were performed in the same manner as in the method for producing the vertically aligned liquid crystal cured film (A-1) of Example 1, except that the phase difference values shown in Table 1 were changed. 20, vertically aligned liquid crystal cured films (A-2) to (A-9), (A-20), (A-21), and (B-1) of Example 21 and Comparative Example 1 were produced. . Moreover, the sample for an optical characteristic measurement was produced by the method similar to Example 1, and the phase difference value, the average refractive index, and orientation were evaluated. The results are shown in Table 1.
- Example 10 The substrate was changed from a COP film (“ZF-14-23” manufactured by Nippon Zeon Co., Ltd.) to a polyethylene terephthalate with a protective layer (hereinafter sometimes referred to as “PET with a protective layer”), and when measuring optical properties
- a vertically aligned liquid crystal cured film (A-10) was prepared in the same manner as the method for preparing the vertically aligned liquid crystal cured film (A-1) of Example 1, except that the sample was prepared by peeling the PET substrate. .
- the sample for an optical characteristic measurement was produced by the method similar to Example 1, and the phase difference value, the average refractive index, and orientation were evaluated. The results are shown in Table 1.
- the preparation method of PET with a protective layer is demonstrated.
- the composition for protective layer formation was apply
- the coating film was dried at 50 ° C. for 1 minute to form a dry film.
- a high pressure mercury lamp (“Unicure VB-15201BY-A” manufactured by USHIO INC.)
- the dried coating was irradiated with ultraviolet rays under a nitrogen atmosphere and an integrated light amount of 400 mJ / cm 2 at a wavelength of 365 nm.
- a PET with a protective layer made of an acrylic resin was formed.
- Example 11 Except that the composition of the liquid crystal compound of Example 1 was changed from 90% / 10% of the liquid crystal compound A / liquid crystal compound B to 100% of the liquid crystal compound (A) -2, the composition (A-1) of Example 1 and The composition (A-11) and the vertically aligned liquid crystal cured film (A-11) of Example 11 were prepared in the same manner as the method for preparing the vertical aligned liquid crystal cured film (A-1).
- the liquid crystal compound (A) -2 was prepared with reference to JP-A-2016-81035.
- the liquid crystal compound (A) -2 is represented by the following formula (A) -2.
- Example 2 the sample for a measurement was produced by the method similar to Example 1, and the phase difference value, the average refractive index, and orientation were evaluated. Further, the ratio between the maximum absorption wavelength and the maximum absorbance of the liquid crystal compound (A) -2 was calculated in the same manner as in Example 1. The results are shown in Table 1. ... (A) -2
- Example 2 the sample for an optical characteristic measurement was produced by the method similar to Example 1, and the phase difference value, the average refractive index, and orientation were evaluated. Further, the ratio between the maximum absorption wavelength and the maximum absorbance of the liquid crystal compound (A) -3 was also calculated in the same manner as in Example 1. The results are shown in Table 1. ... (A) -3
- the liquid crystal compound (A) -4 was prepared with reference to JP2011-207765A.
- the liquid crystal compound (A) -4 is represented by the following formula (A) -4.
- Example 2 the sample for an optical characteristic measurement was produced by the method similar to Example 1, and the phase difference value, the average refractive index, and orientation were evaluated. Further, the ratio between the maximum absorption wavelength and the maximum absorbance of the liquid crystal compound (A) -4 was calculated in the same manner as in Example 1. The results are shown in Table 1. ... (A) -4
- the liquid crystal compound (A) -5 was prepared with reference to JP 2010-31223 A.
- the liquid crystal compound (A) -5 is represented by the following formula (A) -5.
- Example 2 the sample for an optical characteristic measurement was produced by the method similar to Example 1, and the phase difference value, the average refractive index, and orientation were evaluated. Further, the ratio between the maximum absorption wavelength and the maximum absorbance of the liquid crystal compound (A) -5 was also calculated in the same manner as in Example 1. The results are shown in Table 1. ... (A) -5
- Example 15 The silane compound of Example 1 was converted from 3-triethoxysilyl-N- (1,3-dimethyl-butylidene) propylamine (“KBE-9103” manufactured by Shin-Etsu Chemical Co., Ltd.) to 3-glycidoxypropyltriethoxysilane ( Except for changing to “KBE-403” manufactured by Shin-Etsu Chemical Co., Ltd., in the same manner as in the preparation method of the composition (A-1) and the vertically aligned liquid crystal cured film (A-1) in Example 1, A composition (A-15) and a vertically aligned liquid crystal cured film (A-15) were prepared in the same manner as in Example 1. A sample for measuring optical properties was prepared in the same manner as in Example 1. The rate and orientation were evaluated and the results are shown in Table 1.
- Example 16> [Production of Polarizing Film A] After immersing a polyvinyl alcohol film (average polymerization degree of about 2,400, saponification degree of 99.9 mol% or more, thickness of 75 ⁇ m) in pure water at 30 ° C., the mass ratio of iodine / potassium iodide / water is 0. Iodine dyeing was carried out by dipping in a 0.02 / 2/100 aqueous solution at 30 ° C. (iodine dyeing step).
- the polyvinyl alcohol film which passed through the iodine dyeing process was immersed in an aqueous solution having a mass ratio of potassium iodide / boric acid / water of 12/5/100 at 56.5 ° C. to perform boric acid treatment (boric acid treatment process). ).
- the polyvinyl alcohol film that had undergone the boric acid treatment step was washed with pure water at 8 ° C. and then dried at 65 ° C. to obtain a polarizer (thickness 27 ⁇ m after stretching) in which iodine was adsorbed and oriented on polyvinyl alcohol. . Under the present circumstances, it extended
- the total draw ratio in such drawing was 5.3 times.
- the obtained polarizer and a saponified triacetyl cellulose film (“KC4UYTAC” 40 ⁇ m manufactured by Konica Minolta) were bonded together with a nip roll through an aqueous adhesive. While maintaining the tension of the obtained bonded product at 430 N / m, it was dried at 60 ° C. for 2 minutes to obtain a polarizing film having a triacetyl cellulose film as a protective film on one side.
- the water-based adhesive is 100 parts of water, 3 parts of carboxyl group-modified polyvinyl alcohol (Kuraray “Kuraray Poval KL318"), water-soluble polyamide epoxy resin (Sumika Chemtex “Smiles Resin 650", solid content concentration 30 parts aqueous solution) and 1.5 parts.
- composition for forming horizontal alignment film A horizontal alignment film was formed by mixing 5 parts of a photoalignable material (weight average molecular weight: 30000) having the following structure and 95 parts of cyclopentanone as a solvent and stirring the resulting mixture at 80 ° C. for 1 hour. A composition was obtained.
- composition for forming horizontally aligned liquid crystal cured film A Liquid crystal compound A and liquid crystal compound B were mixed at a mass ratio of 90:10 to obtain a mixture. For 100 parts by mass of the resulting mixture, 1.0 part of a leveling agent (“F-556” manufactured by DIC) and 2-dimethylamino-2-benzyl-1- (4-morpholinophenyl) as a polymerization initiator ) 6 parts of butan-1-one (“Irgacure (registered trademark) 369 (Irg369)” manufactured by BASF Japan Ltd.) was added. Further, N-methyl-2-pyrrolidone (NMP) was added so that the solid content concentration was 13%.
- the composition for horizontal alignment liquid crystal cured film A formation was obtained by stirring at 80 degreeC for 1 hour.
- the horizontal alignment liquid crystal cured film A formation composition was apply
- the coating film was dried at 120 ° C. for 1 minute to form a dry film.
- a high pressure mercury lamp (“Unicure VB-15201BY-A” manufactured by Ushio Electric Co., Ltd.) under a nitrogen atmosphere and with a cumulative light quantity of 500 mJ / cm 2 at a wavelength of 365 nm
- a horizontal alignment liquid crystal cured film A was formed to obtain a laminate comprising a substrate, a horizontal alignment film and a horizontal alignment liquid crystal cured film A.
- the film thickness of the horizontal alignment liquid crystal cured film A was measured with an ellipsometer, it was 2.3 ⁇ m. Met.
- the laminate (a laminate comprising the horizontally aligned liquid crystal cured film A and the vertically aligned liquid crystal cured film (A-1)) and the polarizing film A prepared by the above method, the absorption axis of the polarizing film A and the horizontally aligned liquid crystal cured film
- the laminate was bonded via an adhesive so that the angle formed with the slow axis of A was 45 °, and the substrate was peeled off to produce an elliptically polarizing plate with an optical compensation function.
- Examples 17 and 18> A retardation value measurement was performed in the same manner as in Example 16 except that the values of RthC (450) and RthC (550) were changed as shown in Table 2 by changing the thickness of the vertically aligned liquid crystal cured film. The oblique reflection hue was confirmed. The results are shown in Tables 2 and 3.
- Example 19 The retardation value of the retardation film A was changed in the same manner as in Example 16 except that the polarizing film A was changed to a polarizing film B containing a horizontally aligned liquid crystal cured film B aligned in the horizontal direction and a dichroic dye prepared by the method shown below. Measurement and evaluation of oblique reflection hue were carried out. The results are shown in Tables 2 and 3.
- composition for forming polarizing film B containing a polymerizable liquid crystal compound (B) and a dichroic dye.
- a dichroic dye an azo dye described in Examples of JP2013-101328A was used.
- the polymerizable liquid crystal compounds represented by the formulas (1-6) and (1-7) as the polymerizable liquid crystal compound (B) are described in lub et al. , Recl. Trav. Chim. It was produced according to the method described in Pays-Bas, 115, 321-328 (1996).
- a polarized UV irradiation device (“SPOT CURE SP-7” manufactured by Ushio Electric Co., Ltd.), a polarized UV exposure is performed on the dried film under the conditions of an integrated light amount of 100 mJ / cm 2 and an axial angle of 90 °, and horizontal alignment is performed. A membrane was obtained. It was 150 nm when the film thickness of the obtained horizontal alignment film was measured with the ellipsometer.
- the polymerizable liquid crystal compound (B) was polymerized by irradiating ultraviolet rays to prepare a polarizing film having a horizontally aligned liquid crystal cured film B containing a dichroic dye.
- the polarization degree and single transmittance of the obtained polarizing film B were measured as follows. Using a device in which the transmittance in the transmission axis direction (T 1 ) and the transmittance in the absorption axis direction (T 2 ) are set in a spectrophotometer (“UV-3150” manufactured by Shimadzu Corporation) with a folder with a polarizer. The measurement was carried out by the double beam method in a wavelength range of 2 nm steps from 380 to 680 nm. Using the following formulas (p) and (q), single transmittance and polarization degree at each wavelength were calculated.
- LC242 (Preparation of composition for forming vertical alignment liquid crystal cured film (B))
- LC242 represented by the following formula (LC242): Paliocolor LC242 (registered trademark of BASF), 0.1 part of a leveling agent (“F-556” manufactured by DIC) and 3 parts of a polymerization initiator Irg369 were added. Then, cyclopentanone was added so that the solid content concentration would be 13%, and these were mixed to obtain a vertically aligned liquid crystal cured film forming composition (B).
- Liquid crystal compound LC242 Paliocolor LC242 (registered trademark of BASF)
- the dry film was irradiated with ultraviolet rays under a nitrogen atmosphere and an integrated light amount of 500 mJ / cm 2 at a wavelength of 365 nm.
- a vertically aligned liquid crystal cured film was formed.
- the film thickness of the obtained vertically aligned liquid crystal cured film was 0.5 ⁇ m.
- the “addition amount” of the column silane compound and the “addition amount” of the ionic compound respectively indicate the addition amount (unit: weight%) to the composition for forming a vertically aligned liquid crystal cured film.
- the number of the ratio of the column liquid crystal compound and the alphabet in parentheses indicate the ratio of the added amount of the added liquid crystal compound.
- 90/10 (A / B) indicates a mass ratio (liquid crystal compound A / liquid crystal compound B) 90/10.
- the vertically aligned liquid crystal cured films (A-1) to (A-15) of Examples 1 to 15 were cured products of the compositions (A-1) to (A-15), respectively.
- the compositions (A-1) to (A-15) contained the silane compound KBE-9103 or KBE-403 and any one of the ionic compounds (1) to (3). Silane compounds KBE-9103 and KBE-403 were nonionic silane compounds.
- the evaluation of the orientation of the vertically aligned liquid crystal cured films (A-1) to (A-15) in Examples 1 to 15 was either A or B.
- the vertically aligned liquid crystal cured film (A-20) of Example 20 was a cured product of the composition (A-20).
- Composition (A-20) contained an ionic compound.
- the vertically aligned liquid crystal cured film (A-21) of Example 21 was a cured product of the composition (A-21).
- the composition (A-21) contained a nonionic silane compound.
- the orientation evaluations of the vertically aligned liquid crystal cured film (A-20) of Example 20 and the vertically aligned liquid crystal cured film (A-21) of Example 21 were both C.
- the alignment liquid crystal cured film (B-1) of Comparative Example 1 was a cured product of the composition (B-1).
- the composition (B-1) did not contain a nonionic silane compound and an ionic compound.
- the evaluation of the orientation of the vertically aligned liquid crystal cured film (B-1) of Comparative Example 1 was D.
- the vertically aligned liquid crystal cured films (A-1) to (A-15), (A-20), and (A-21) of Examples 1 to 15 are the aligned liquid crystal cured films (B It is clear that the orientation is excellent compared to -1).
- the elliptically polarizing plates of Examples 16 to 19 included a vertically aligned liquid crystal cured film prepared using the composition (A-1), a horizontal aligned film, a horizontal aligned liquid crystal cured film A, and a polarizing film. .
- the oblique reflection hues of the elliptically polarizing plates of Examples 16 to 19 were all A.
- the elliptically polarizing plate of Comparative Example 2 contained a vertically aligned liquid crystal cured film prepared using the composition (B-2), a horizontal aligned film, a horizontally aligned liquid crystal cured film A, and a polarizing film.
- the composition (B-2) contained a liquid crystal compound LC242.
- Ar in the liquid crystal compound is a divalent group having one ring structure and was not included in the liquid crystal compound represented by the formula (I) -1.
- the oblique reflection hue of the elliptically polarizing plate of Comparative Example 2 was B.
Landscapes
- Physics & Mathematics (AREA)
- Chemical & Material Sciences (AREA)
- General Physics & Mathematics (AREA)
- Optics & Photonics (AREA)
- Crystallography & Structural Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Organic Chemistry (AREA)
- Theoretical Computer Science (AREA)
- Liquid Crystal (AREA)
- Polarising Elements (AREA)
- Electroluminescent Light Sources (AREA)
- Devices For Indicating Variable Information By Combining Individual Elements (AREA)
- Laminated Bodies (AREA)
- Silicon Polymers (AREA)
- Manufacture Of Macromolecular Shaped Articles (AREA)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020247040356A KR20250002767A (ko) | 2018-02-14 | 2019-02-12 | 수직 배향 액정 경화막 |
| CN201980011832.4A CN111684327B (zh) | 2018-02-14 | 2019-02-12 | 垂直取向液晶固化膜 |
| KR1020207024523A KR102740705B1 (ko) | 2018-02-14 | 2019-02-12 | 수직 배향 액정 경화막 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2018024565A JP7059035B2 (ja) | 2018-02-14 | 2018-02-14 | 垂直配向液晶硬化膜 |
| JP2018-024565 | 2018-02-14 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2019159887A1 true WO2019159887A1 (ja) | 2019-08-22 |
Family
ID=67618708
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2019/004844 Ceased WO2019159887A1 (ja) | 2018-02-14 | 2019-02-12 | 垂直配向液晶硬化膜 |
Country Status (5)
| Country | Link |
|---|---|
| JP (2) | JP7059035B2 (enExample) |
| KR (2) | KR102740705B1 (enExample) |
| CN (1) | CN111684327B (enExample) |
| TW (3) | TWI800602B (enExample) |
| WO (1) | WO2019159887A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2021047245A (ja) * | 2019-09-17 | 2021-03-25 | 住友化学株式会社 | 積層体およびこれを含む楕円偏光板 |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2022149377A1 (ja) * | 2021-01-05 | 2022-07-14 | 東レ株式会社 | 積層フィルムおよびその製造方法 |
| TWI878135B (zh) * | 2024-05-31 | 2025-03-21 | 瀚宇彩晶股份有限公司 | 反射式顯示面板 |
Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007225912A (ja) * | 2006-02-23 | 2007-09-06 | Fujifilm Corp | 液晶表示装置 |
| JP2007264403A (ja) * | 2006-03-29 | 2007-10-11 | Fujifilm Corp | 位相差板、偏光板、輝度向上フィルム、及び液晶表示装置 |
| JP2008020904A (ja) * | 2006-06-13 | 2008-01-31 | Dainippon Printing Co Ltd | 液晶組成物、カラーフィルタ及び液晶表示装置 |
| JP2009040984A (ja) * | 2007-08-13 | 2009-02-26 | Dainippon Printing Co Ltd | 液晶組成物、該液晶組成物を用いた位相差制御部材、及び、液晶表示装置 |
| JP2010084032A (ja) * | 2008-09-30 | 2010-04-15 | Fujifilm Corp | 液晶化合物、光学異方性膜、位相差板及び偏光板 |
| WO2014054769A1 (ja) * | 2012-10-04 | 2014-04-10 | 富士フイルム株式会社 | 円偏光板およびその製造方法、光学積層体 |
| JP2015043073A (ja) * | 2013-07-25 | 2015-03-05 | 富士フイルム株式会社 | 位相差フィルム、偏光板および液晶表示装置 |
| JP2016206239A (ja) * | 2015-04-15 | 2016-12-08 | 大日本印刷株式会社 | 光学フィルム |
| JP2017014381A (ja) * | 2015-06-30 | 2017-01-19 | Jnc株式会社 | 重合性液晶化合物、組成物およびその重合体 |
| JP2017068005A (ja) * | 2015-09-30 | 2017-04-06 | 富士フイルム株式会社 | 光学異方性層の製造方法および偏光板の製造方法 |
| JP2018022152A (ja) * | 2016-07-21 | 2018-02-08 | 住友化学株式会社 | 楕円偏光板 |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2005266696A (ja) * | 2004-03-22 | 2005-09-29 | Nitto Denko Corp | 円偏光板、光学フィルムおよび画像表示装置 |
| JP4548727B2 (ja) * | 2005-04-28 | 2010-09-22 | 大日本印刷株式会社 | 液晶分子をホメオトロピック配向させた光学素子並びにこれを用いた液晶表示装置及び液晶表示装置 |
| JP2007332290A (ja) | 2006-06-15 | 2007-12-27 | Dainippon Printing Co Ltd | 液晶組成物、カラーフィルタおよび液晶表示装置 |
| JP5533257B2 (ja) | 2010-05-25 | 2014-06-25 | Jnc株式会社 | 重合性液晶化合物、組成物およびその重合体 |
| JP5924877B2 (ja) * | 2011-06-09 | 2016-05-25 | 林テレンプ株式会社 | 光学フィルム積層体 |
| KR102129135B1 (ko) * | 2012-02-28 | 2020-07-01 | 스미또모 가가꾸 가부시키가이샤 | 편광막, 원편광판 및 이들의 제조 방법 |
| KR102237396B1 (ko) * | 2013-04-11 | 2021-04-07 | 스미또모 가가꾸 가부시키가이샤 | 광학 이방성 필름용 배향층 |
| CN104339796B (zh) | 2013-08-09 | 2018-03-02 | 住友化学株式会社 | 层叠体 |
| JP6287371B2 (ja) | 2014-03-10 | 2018-03-07 | 大日本印刷株式会社 | 光学フィルム、光学フィルム用転写体、及び画像表示装置 |
| JPWO2016136231A1 (ja) * | 2015-02-25 | 2017-12-07 | 富士フイルム株式会社 | 積層体及び光学フィルム |
| CN106371163B (zh) * | 2015-07-24 | 2020-08-25 | 住友化学株式会社 | 液晶固化膜、包含液晶固化膜的光学膜及显示装置 |
-
2018
- 2018-02-14 JP JP2018024565A patent/JP7059035B2/ja active Active
-
2019
- 2019-02-12 KR KR1020207024523A patent/KR102740705B1/ko active Active
- 2019-02-12 TW TW108104556A patent/TWI800602B/zh active
- 2019-02-12 KR KR1020247040356A patent/KR20250002767A/ko active Pending
- 2019-02-12 TW TW112113003A patent/TW202330657A/zh unknown
- 2019-02-12 WO PCT/JP2019/004844 patent/WO2019159887A1/ja not_active Ceased
- 2019-02-12 TW TW114106385A patent/TW202523655A/zh unknown
- 2019-02-12 CN CN201980011832.4A patent/CN111684327B/zh active Active
-
2022
- 2022-04-13 JP JP2022066051A patent/JP7443413B2/ja active Active
Patent Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007225912A (ja) * | 2006-02-23 | 2007-09-06 | Fujifilm Corp | 液晶表示装置 |
| JP2007264403A (ja) * | 2006-03-29 | 2007-10-11 | Fujifilm Corp | 位相差板、偏光板、輝度向上フィルム、及び液晶表示装置 |
| JP2008020904A (ja) * | 2006-06-13 | 2008-01-31 | Dainippon Printing Co Ltd | 液晶組成物、カラーフィルタ及び液晶表示装置 |
| JP2009040984A (ja) * | 2007-08-13 | 2009-02-26 | Dainippon Printing Co Ltd | 液晶組成物、該液晶組成物を用いた位相差制御部材、及び、液晶表示装置 |
| JP2010084032A (ja) * | 2008-09-30 | 2010-04-15 | Fujifilm Corp | 液晶化合物、光学異方性膜、位相差板及び偏光板 |
| WO2014054769A1 (ja) * | 2012-10-04 | 2014-04-10 | 富士フイルム株式会社 | 円偏光板およびその製造方法、光学積層体 |
| JP2015043073A (ja) * | 2013-07-25 | 2015-03-05 | 富士フイルム株式会社 | 位相差フィルム、偏光板および液晶表示装置 |
| JP2016206239A (ja) * | 2015-04-15 | 2016-12-08 | 大日本印刷株式会社 | 光学フィルム |
| JP2017014381A (ja) * | 2015-06-30 | 2017-01-19 | Jnc株式会社 | 重合性液晶化合物、組成物およびその重合体 |
| JP2017068005A (ja) * | 2015-09-30 | 2017-04-06 | 富士フイルム株式会社 | 光学異方性層の製造方法および偏光板の製造方法 |
| JP2018022152A (ja) * | 2016-07-21 | 2018-02-08 | 住友化学株式会社 | 楕円偏光板 |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2021047245A (ja) * | 2019-09-17 | 2021-03-25 | 住友化学株式会社 | 積層体およびこれを含む楕円偏光板 |
| WO2021054031A1 (ja) * | 2019-09-17 | 2021-03-25 | 住友化学株式会社 | 積層体およびこれを含む楕円偏光板 |
| CN114375418A (zh) * | 2019-09-17 | 2022-04-19 | 住友化学株式会社 | 层叠体及包含该层叠体的椭圆偏振板 |
| CN114375418B (zh) * | 2019-09-17 | 2024-03-12 | 住友化学株式会社 | 层叠体及包含该层叠体的椭圆偏振板 |
| JP7461122B2 (ja) | 2019-09-17 | 2024-04-03 | 住友化学株式会社 | 積層体およびこれを含む楕円偏光板 |
Also Published As
| Publication number | Publication date |
|---|---|
| TW202523655A (zh) | 2025-06-16 |
| JP2019139168A (ja) | 2019-08-22 |
| JP7443413B2 (ja) | 2024-03-05 |
| KR102740705B1 (ko) | 2024-12-09 |
| TW201936661A (zh) | 2019-09-16 |
| TW202330657A (zh) | 2023-08-01 |
| CN111684327B (zh) | 2022-10-14 |
| TWI800602B (zh) | 2023-05-01 |
| KR20200118822A (ko) | 2020-10-16 |
| JP7059035B2 (ja) | 2022-04-25 |
| JP2022093389A (ja) | 2022-06-23 |
| CN111684327A (zh) | 2020-09-18 |
| KR20250002767A (ko) | 2025-01-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US12110439B2 (en) | Perpendicularly aligned liquid crystal cured film and laminate including same | |
| CN108508519B (zh) | 光学膜及其制造方法 | |
| JP7398868B2 (ja) | 組成物 | |
| KR102890509B1 (ko) | 적층체 및 수직 배향 액정 경화막 형성용 조성물 | |
| KR20200039731A (ko) | 광학 보상 기능 부착 위상차판 | |
| JP7443413B2 (ja) | 垂直配向液晶硬化膜 | |
| WO2021006068A1 (ja) | 長尺フィルム | |
| WO2020149357A1 (ja) | 積層体、楕円偏光板および重合性液晶組成物 | |
| KR20200036928A (ko) | 플렉시블 디스플레이용 광학 보상 기능 부착 위상차판 | |
| WO2022196632A1 (ja) | 光学積層体 | |
| WO2019159886A1 (ja) | 組成物 | |
| WO2019159889A1 (ja) | 積層体およびその製造方法 | |
| JP2022145604A (ja) | 光学積層体 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 19753806 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20207024523 Country of ref document: KR Kind code of ref document: A |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 19753806 Country of ref document: EP Kind code of ref document: A1 |