WO2018045911A1 - 二氢嘧啶类化合物及其制备方法和用途 - Google Patents
二氢嘧啶类化合物及其制备方法和用途 Download PDFInfo
- Publication number
- WO2018045911A1 WO2018045911A1 PCT/CN2017/100103 CN2017100103W WO2018045911A1 WO 2018045911 A1 WO2018045911 A1 WO 2018045911A1 CN 2017100103 W CN2017100103 W CN 2017100103W WO 2018045911 A1 WO2018045911 A1 WO 2018045911A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- methyl
- fluorophenyl
- alkyl
- chloro
- group
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 0 CC(C)NC([C@@]1N(CC(*C(c2ncc[s]2)=NC2c(c(Cl)c3)ccc3F)=C2C(OC)=O)CC2(CC2)C1)=O Chemical compound CC(C)NC([C@@]1N(CC(*C(c2ncc[s]2)=NC2c(c(Cl)c3)ccc3F)=C2C(OC)=O)CC2(CC2)C1)=O 0.000 description 2
- RXNKOWTWQSSKCW-LIRRHRJNSA-N COC(C1=C(CN2[C@H](CO)CC3(CC3)C2)NC(c2ncc[s]2)=N[C@H]1c(c(Cl)c1)ccc1F)=O Chemical compound COC(C1=C(CN2[C@H](CO)CC3(CC3)C2)NC(c2ncc[s]2)=N[C@H]1c(c(Cl)c1)ccc1F)=O RXNKOWTWQSSKCW-LIRRHRJNSA-N 0.000 description 2
- QQPCMCRBAHUCCN-RITPCOANSA-N C=C[C@H](C1)CN[C@@H]1C(O)=O Chemical compound C=C[C@H](C1)CN[C@@H]1C(O)=O QQPCMCRBAHUCCN-RITPCOANSA-N 0.000 description 1
- WLZUFMKBOXAIRQ-AIBWNMTMSA-N CCOC(C1=C(CN(CC2(CC2)C2)[C@@H]2C(NS(C2CC2)(=O)=O)=O)NC(c2ncc[s]2)=NC1c(c(Cl)c1)ccc1F)=O Chemical compound CCOC(C1=C(CN(CC2(CC2)C2)[C@@H]2C(NS(C2CC2)(=O)=O)=O)NC(c2ncc[s]2)=NC1c(c(Cl)c1)ccc1F)=O WLZUFMKBOXAIRQ-AIBWNMTMSA-N 0.000 description 1
- ONELXRQCJBCEOV-UHFFFAOYSA-N COC(C1=C(CBr)NC(c2ncc[s]2)=NC1c(c(Cl)c1)ccc1F)=O Chemical compound COC(C1=C(CBr)NC(c2ncc[s]2)=NC1c(c(Cl)c1)ccc1F)=O ONELXRQCJBCEOV-UHFFFAOYSA-N 0.000 description 1
- LBONAQFIKKABCK-UHFFFAOYSA-N COC(C1=C(CBr)NC(c2ncc[s]2)=NC1c1cccc(F)c1Cl)=O Chemical compound COC(C1=C(CBr)NC(c2ncc[s]2)=NC1c1cccc(F)c1Cl)=O LBONAQFIKKABCK-UHFFFAOYSA-N 0.000 description 1
- IBHJQXMHMPEKRW-KKFHFHRHSA-N COC(C1=C(CN(CC2(CC2)C2)[C@@H]2C(NS(C)(=O)=O)=O)NC(c2ncc[s]2)=NC1c(c(Cl)c1)ccc1F)=O Chemical compound COC(C1=C(CN(CC2(CC2)C2)[C@@H]2C(NS(C)(=O)=O)=O)NC(c2ncc[s]2)=NC1c(c(Cl)c1)ccc1F)=O IBHJQXMHMPEKRW-KKFHFHRHSA-N 0.000 description 1
- XTLOHVWJLOAFPG-RXVVDRJESA-N COC(C1=C(CN(CC2(CC2)C2)[C@@H]2C(NS(C2CC2)(=O)=O)=O)NC(c2ncc[s]2)=N[C@H]1c(cccc1F)c1Cl)=O Chemical compound COC(C1=C(CN(CC2(CC2)C2)[C@@H]2C(NS(C2CC2)(=O)=O)=O)NC(c2ncc[s]2)=N[C@H]1c(cccc1F)c1Cl)=O XTLOHVWJLOAFPG-RXVVDRJESA-N 0.000 description 1
- KDFUAVYDWDUXCM-RXVVDRJESA-N COC(C1=C(CN(CC2(CC2)C2)[C@@H]2C(NS(C2CC2)(=O)=O)=O)NC(c2ncc[s]2)=N[C@H]1c1cccc(I)c1Cl)=O Chemical compound COC(C1=C(CN(CC2(CC2)C2)[C@@H]2C(NS(C2CC2)(=O)=O)=O)NC(c2ncc[s]2)=N[C@H]1c1cccc(I)c1Cl)=O KDFUAVYDWDUXCM-RXVVDRJESA-N 0.000 description 1
- ZDAVGKVELQNTIU-FQDNBXNQSA-N COC(C1=C(CN([C@H](C2)C2C2)[C@@H]2C(O)=O)NC(c2ncc[s]2)=N[C@H]1c1cccc(F)c1Cl)=O Chemical compound COC(C1=C(CN([C@H](C2)C2C2)[C@@H]2C(O)=O)NC(c2ncc[s]2)=N[C@H]1c1cccc(F)c1Cl)=O ZDAVGKVELQNTIU-FQDNBXNQSA-N 0.000 description 1
- WMRHLUMWSAZZMJ-LURJTMIESA-N OC[C@H]1NCC2(CC2)C1 Chemical compound OC[C@H]1NCC2(CC2)C1 WMRHLUMWSAZZMJ-LURJTMIESA-N 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/506—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim not condensed and containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/535—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one oxygen as the ring hetero atoms, e.g. 1,2-oxazines
- A61K31/5375—1,4-Oxazines, e.g. morpholine
- A61K31/5377—1,4-Oxazines, e.g. morpholine not condensed and containing further heterocyclic rings, e.g. timolol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/20—Antivirals for DNA viruses
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing three or more hetero rings
Definitions
- the present invention relates to a dihydropyrimidine compound and its use as a medicament.
- Such compounds have the effect of treating and preventing hepatitis B, particularly as a hepatitis B virus (HBV) inhibitor, by treating HBV capsids for the treatment of HBV infection.
- HBV hepatitis B virus
- the invention also relates to a process for the preparation of such compounds.
- Hepatitis B is a disease caused by hepatitis B virus (HBV), which is mainly caused by inflammatory lesions of the liver and can cause damage to multiple organs.
- HBV hepatitis B virus
- Hepatitis B virus referred to as hepatitis B virus
- hepatitis B virus is a DNA virus belonging to the Hepadnavividae family. It can cause acute or persistent/progressive chronic diseases.
- Hepatitis B is widely prevalent in countries around the world, with more than 400 million people sick, especially in the Asia- Pacific region. A few of these patients can be converted to cirrhosis or liver cancer.
- anti-hepatitis B virus nucleoside (acid) drugs on the market include lamivudine, telbivudine, entecavir, tenofovir, clafidine and the like.
- the shortcomings of these drugs are: the treatment is not fixed, prone to virus resistance, and easy to relapse after stopping the drug. These shortcomings prevent patients from being cured.
- heteroaryl ring-substituted dihydropyrimidine (HAP) compounds represented by Bay41-4109 and Bay3905493, which can inhibit HBV replication by preventing the formation of normal nucleocapsids.
- Bay41-4109 showed better drug metabolism parameters in clinical studies (Deres K. et al., Science, 299 (2003), 893-896).
- Studies on its mechanism of action have revealed that the heteroaryl ring-substituted dihydropyrimidine compounds change the angle between the dimers forming the nucleocapsid by acting on the 113-143 amino acid residues of the core protein, resulting in the formation of no Stable expanded nucleocapsid accelerates degradation of core proteins (Biochem. Pharmacol. 66 (2003), 2273-2279).
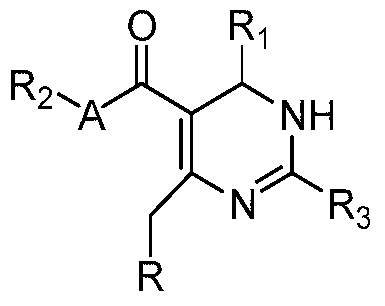
- the present invention provides a compound represented by the formula (I), or a pharmaceutically acceptable salt or tautomer or enantiomer or diastereomer thereof:
- R 1 is phenyl, wherein the phenyl group is optionally further substituted with one or more substituents selected from halogen, C 1-6 alkyl;
- R 2 is selected from hydrogen or C 1-4 alkyl
- A is a bond, -O-, -S- or -N(R 5 )-;
- R 5 is hydrogen or C 1-4 alkyl
- R is a group shown below:
- R 9 is alkyl, alkoxy, aryl, heteroaryl, cycloalkyl, heterocyclyl or arylalkyl;
- R 10 and R 10a are each independently hydrogen, haloalkyl, cycloalkyl, alkyl or hydroxyalkyl, or R 10 and R 10a together with a nitrogen atom attached thereto form a heterocyclic group;
- n are each independently 1, 2, 3, 4;
- n are each independently 0, 1, 2, 3, 4;
- p is each independently 1 or 2.
- R is preferably a substructure of the formula:
- R is preferably selected from:
- R 1 is preferably selected from phenyl, optionally further substituted by one or more halo.
- R 2 is preferably methyl or ethyl.
- A is preferably -O-.
- R 3 is preferably thiazolyl, imidazolyl or pyridyl, wherein said thiazolyl, imidazolyl or pyridyl group is further optionally further selected from one or more selected from the group consisting of halogen, alkyl, Substituted by alkoxy, haloalkyl, alkylsulfonyl or cycloalkyl substituents.
- R 1 is preferably 2 halogen-substituted phenyl;
- R 2 is preferably methyl or ethyl;
- A is preferably -O-;
- n are each independently 0, 1, 2, 3, 4, preferably 0;
- p is each independently 1 or 2.
- the compound of formula (I) according to the invention is selected from the group consisting of:
- a pharmaceutical composition comprising an effective amount of a compound of the formula (I) or a pharmaceutically acceptable salt or tautomer or enantiomer or non-correspondence thereof isomer.
- a further aspect of the invention provides a compound of formula (I), or a pharmaceutically acceptable salt or tautomer or enantiomer or diastereomer thereof, or a pharmaceutical composition thereof, for use in therapy or Application in medicines for preventing diseases of hepatitis B virus infection.
- a further aspect of the invention provides a process for the preparation of a compound of formula (I).
- the method comprises reacting a compound of formula d with an R-H compound or a salt thereof in the presence of a base to give a compound of formula (I):
- R 1 , R 2 , R 3 , R and A are as defined above; wherein the base is preferably an organic base, more preferably triethylamine.
- the compound of the formula d is prepared by the following methods, including:
- Step (1) a compound of the formula a is reacted with a compound of the formula b to give a compound of the formula c.
- Step (2) The compound of formula c is reacted with a brominating reagent to give a compound of formula d:
- the brominating agent in step (2) is preferably N-bromosuccinimide.
- patient may include humans (including adults and children) or other animals. In some embodiments, “patient” refers to a human.
- Alkyl means a straight or branched saturated aliphatic hydrocarbon group.
- the alkyl group in the present application is preferably a C 1-6 alkyl group, that is, a saturated straight or branched alkyl group having 1 to 6 carbon atoms; a particularly preferred alkyl group in the present application is a C 1-4 alkyl group. That is, a saturated linear or branched alkyl group of 1 to 4 carbon atoms such as methyl, ethyl, n-propyl, isopropyl, 1-butyl, 2-butyl and t-butyl groups and the like.
- Alkoxy means a group of (alkyl-O-). Wherein alkyl is as defined above.
- a preferred alkoxy group is a C 1-6 alkoxy group, and a particularly preferred alkoxy group is a C 1-4 alkoxy group.
- the term C 1-6 alkoxy includes methoxy, ethoxy, n-propoxy and isopropoxy and the like.
- cycloalkyl denotes a saturated carbocyclic ring containing from 3 to 12 carbon atoms, especially from 3 to 6 carbon atoms, such as cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl and the like.
- cycloalkyl is cyclopropyl, cyclopentyl or cyclohexyl.
- Haloalkyl or haloalkoxy means that the alkyl or alkoxy group is substituted by one or more of the same or different halogen atoms.
- Preferred alkyl or alkoxy groups are as defined above. Examples include, but are not limited to, trifluoromethyl, trifluoroethyl, trifluoromethoxy.
- Cycloalkylalkyl means that the alkyl group is substituted by one or more cycloalkyl groups. Examples include, but are not limited to, cyclopropylmethyl, cyclopropylethyl, cyclobutylmethyl, cyclopentylmethyl, and the like.
- Aryl means a monocyclic, bicyclic and tricyclic carbocyclic ring system containing from 6 to 14 ring atoms, wherein at least one ring system is aromatic, wherein each ring system comprises from 3 to 7 atoms A ring with one or more attachment points attached to the rest of the molecule. Examples include, but are not limited to, phenyl, naphthyl, anthracene, and the like. Preferably, the aryl group is a carbocyclic ring system of 6-10 or 6-7 ring atoms.
- Heteroaryl means a monocyclic, bicyclic and tricyclic ring system containing from 5 to 14 ring atoms, wherein at least one ring system is aromatic and at least one ring system comprises one or more selected from the group consisting of nitrogen, oxygen, A sulfur heteroatom in which each ring system contains a ring of 5-7 atoms and has one or more attachment points attached to the remainder of the molecule.
- the term “heteroaryl” can be used interchangeably with the terms “heteroaryl ring” or “heteroaromatic compound”. Examples include, but are not limited to, furyl, imidazolyl, 2-pyridyl, 3-pyridyl, thiazolyl, indolyl, quinolyl.
- the heteroaryl is a ring system of 5 to 10 ring atoms.
- Heteroarylalkyl means that a heteroaryl group is attached to the remainder of the molecule through an alkyl group. Examples include, but are not limited to, pyridin-2-ethyl, thiazol-2-methyl, pyrimidine-2-propyl, and the like.
- amino refers to primary (-NH 2 ), secondary (-NH-) or tertiary amino
- halogen means fluoro, chloro, bromo and iodo. In particular, halogen means fluorine, chlorine or bromine.
- cyano refers to the group -CN.
- hydroxy refers to the group -OH.
- sulfonyl refers to the group -S(O) 2- .
- C1-6 alkoxycarbonyl refers to the group C1-6 alkoxy-C(O)-, wherein said " C1-6 alkoxy” is as defined above.
- aminocarbonyl refers to the group amino-C(O)-, wherein the “amino” is as defined above.
- C1-6 alkylsulfonyl refers to the group C1-6 alkyl-S(O) 2- wherein the " C1-6 alkyl” is as defined above.
- aminosulfonyl refers to the group amino-S(O) 2- wherein the “amino” is as defined above.
- heterocyclyl refers to a non-aromatic saturated or partially unsaturated monocyclic, bicyclic or tricyclic ring system comprising from 3 to 12 ring atoms, wherein at least one ring atom is selected from the group consisting of nitrogen, sulfur and oxygen atoms.
- the heterocyclyl group may be optionally substituted by one or more substituents described herein.
- the sulfur atom of the ring can be optionally oxidized to an S-oxide.
- the heterocyclic group is a 3-10, 3-6 ring atom, non-aromatic saturated or partially unsaturated monocyclic, bicyclic or tricyclic system wherein at least one ring atom is selected from nitrogen, Sulfur and oxygen atoms.
- hydroxyalkyl means that the hydroxyalkyl group is attached to the remainder of the molecule through a carbon atom, wherein the alkyl group is as defined above.
- the hydroxyalkyl group is preferably a linear or branched alkyl group having from 1 to 10 carbon atoms and substituted by one or more hydroxyl groups. More preferred hydroxyalkyl groups are lower hydroxyalkyl groups having from 1 to 6 carbon atoms or from 1 to 4 carbon atoms and one or more hydroxyl groups, such as hydroxymethyl (-CH 2 OH), hydroxyethyl (-CH) 2 CH 2 OH) and hydroxypropyl (-CH 2 CH 2 CH 2 OH) and the like.
- tautomer refers to a structural isomer of an organic compound that is readily converted into each other by a chemical reaction known as tautomerization. This reaction usually results in the migration of a hydrogen atom or a proton, accompanied by the conversion of a single bond and an adjacent double bond, such as a compound of formula (I)
- chiral refers to a molecule that has properties that are incapable of overlapping with its mirror image; and “achiral” refers to a molecule that can overlap with its mirror image.
- enantiomer refers to two isomers of a compound that are not superimposable but are mirror images of one another.
- diastereomer refers to a stereoisomer that has two or more centers of chirality and whose molecules are not mirror images of each other. Diastereomers generally have different physical properties such as melting point, boiling point, spectral properties, and reactivity. The mixture of diastereomers can be separated by high resolution analytical procedures such as electrophoresis and chromatography, such as HPLC.
- the compounds of the present invention may be optionally substituted with one or more substituents, such as the compounds of the above formula, specific compounds of the invention, or particular examples, subclasses, as in the examples, And a class of compounds encompassed by the present invention.
- substituents such as the compounds of the above formula, specific compounds of the invention, or particular examples, subclasses, as in the examples, And a class of compounds encompassed by the present invention.
- substituents such as the compounds of the above formula, specific compounds of the invention, or particular examples, subclasses, as in the examples, And a class of compounds encompassed by the present invention.
- substituents such as the compounds of the above formula, specific compounds of the invention, or particular examples, subclasses, as in the examples, And a class of compounds encompassed by the present invention.
- substituents such as the compounds of the above formula, specific compounds of the invention, or particular examples, subclasses, as in the examples, And a class of compounds encompassed by the present invention
- substituents may be substituted at the respective positions in the same or different manner.
- pharmaceutically acceptable salt refers to certain salts of the above compounds which retain their original biological activity and are suitable for pharmaceutical use.
- the pharmaceutically acceptable salt of the compound represented by the formula (I) may be a salt formed with a suitable acid, and the suitable acid includes an inorganic acid and an organic acid such as acetic acid, benzenesulfonic acid, and benzoic acid.
- Acid camphorsulfonic acid, citric acid, ethanesulfonic acid, fumaric acid, gluconic acid, glutamic acid, hydrobromic acid, hydrochloric acid, isethionethane, lactic acid, malic acid, maleic acid, mandelic acid, methylsulfonate Acid, nitric acid, phosphoric acid, succinic acid, sulfuric acid, tartaric acid, p-toluenesulfonic acid, and the like. Particularly preferred is hydrochloric acid, phosphoric acid or sulfuric acid.
- the following reactions are generally operated under a positive pressure of nitrogen.
- a suitable rubber stopper is placed on the reaction bottle, and the substrate can be driven through a syringe.
- the glassware is dried.
- the column is a silica gel column.
- Nuclear magnetic resonance data was determined by a Bruker Advance 400 NMR spectrometer using CDCl 3 , d 6 -DMSO or CD 3 OD as a solvent (reported in ppm) with TMS (0 ppm) or chloroform (7.25 ppm) as a reference standard.
- MS mass spectrometry
- the starting materials and reagents of the present invention are all commercially available, and their suppliers are Aldrich Chemical Company, Alfa Chemical Company, Sinopharm Group, Linan Qingshan Chemical Reagent Factory, Jiangsu Huada Chemical Group and Hangzhou Chemical Reagent Co., Ltd. Commercially available starting materials and reagents were used without further purification unless otherwise indicated. For the described embodiments of the invention, all temperatures are in degrees Celsius (° C.) unless otherwise indicated.
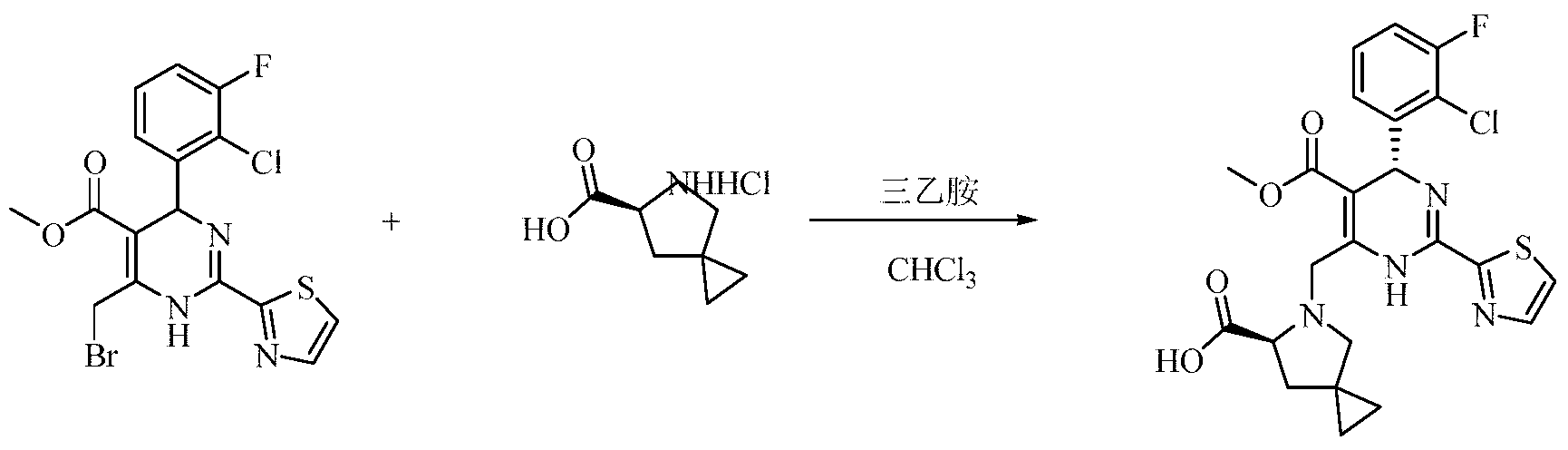
- Example 1 4-(2-Chloro-4-fluorophenyl)-6-(((S)-6-((methylsulfonyl)carbamoyl)-5-azaspiro[2.4]heptane Preparation of methyl 5-amino)methyl)-2-(thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylate
- Step 1) Synthesis of the compound methyl 4-(2-chloro-4-fluorophenyl)-6-methyl-2-(thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylate
- Step 2 Compound 4-(2-chloro-4-fluorophenyl)-6-(bromomethyl)-2-(thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylic acid methyl ester synthesis
- Step 5 Compound 4-(2-chloro-4-fluorophenyl)-6-(((S)-6-((methylsulfonyl)carbamoyl)-5-azaspiro[2.4]heptane Synthesis of methyl 5-(5-yl)methyl)-2-(thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylate
- Example 5 4-(2-Chloro-4-fluorophenyl)-2-(3,5-fluoropyridin-2-yl)-6-(((S)-6-((methylsulfonyl)) Preparation of methyl carbamoyl)-5-azaspiro[2.4]heptane-5-yl)methyl)-1,4-dihydropyrimidine-5-carboxylate
- Example 7 4-(2-Chloro-4-fluorophenyl)-6-(((S)-6-((methylsulfonyl)carbamoyl)-5-azaspiro[2.4]heptane Preparation of ethyl 5-amino)methyl)-2-(thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylate
- Example 8 4-(2-Chloro-4-fluorophenyl)-6-(((S)-6-((cyclopropylsulfonyl)carbamoyl)-5-azaspiro[2.4]g Preparation of ethyl alk-5-yl)methyl)-2-(thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylate
- Example 9 4-(2-Chloro-4-fluorophenyl)-6-(((S)-6-(isopropylcarbamoyl)-5-azaspiro[2.4]heptane-5- Preparation of methyl)methyl)-2-(thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylate
- Example 19 (1S,2S,5R)-3-(((R)-6-(2-chloro-3-fluorophenyl)-5-(methoxycarbonyl)-2-(thiazol-2-yl) Preparation of -3,6-dihydropyrimidin-4-yl)methyl)-3-azabicyclo[3.1.0]hexane-2-carboxylic acid.
- Example 29 (S)-((isopropoxycarbonyl)oxy)methyl 5-((6-(2-chloro-4-fluorophenyl)-5-(methoxycarbonyl)-(thiazole) Preparation of methyl-2-yl)-3,6-dihydropyrimidin-4-yl)methyl)-5-azaspiro[2.4]heptane-6-carboxylate
- qPCR detects the viral DNA content of the cell culture medium and calculates the concentration (EC 50 ) of the compound when the virus is inhibited by half.
- concentration EC 50
- HepG2.2.15 cells were inoculated into 24-well cell culture plates (200,000 cells/well), and cells were treated with cell culture medium containing different concentrations of test compounds on the next day (the highest concentration of compound was 5 ⁇ M, 5 times gradient dilution, 6 Dilution point). On the fifth day, the culture solution containing the drug to be tested was replaced, and on the eighth day, the culture supernatant was collected and centrifuged.
- Quantitative PCR Refer to the Hepatitis B Virus Nucleic Acid Quantification Kit (PCR-Fluorescence Probe Method).
- the nucleic acid release agent was added to the PCR reaction tube, and the diluted standard template was added to each tube (the highest concentration of the standard template was 4 ⁇ 10 7 IU/mL, and the 10-fold dilution was 4 points, and the lowest concentration was 4 ⁇ 10 4 IU/ mL);
- Add the sample template configure the reaction mixture according to the PCR system, add to the reaction tube; cover the PCR reaction tube cover; run the quantitative PCR instrument according to the setting procedure.
- %Inh. [1 - Total amount of compound treated HBV DNA / total amount of control treated HBV DNA] X100.
- EC 50 values calculated for the compound of HBV replication GraphPad Prism5 application software, using the "four parameter logistic equation" calculate EC 50 values.
- Example EC 50 ( ⁇ mol) Example EC 50 ( ⁇ mol) 1 a 11 a 2 a 12 a 4 a 17 a 5 a 19 a 6 a twenty two a 7 a twenty three a 10 a 28 a
- Active interval a (0.001 ⁇ a ⁇ 0.20 ⁇ mol).
- the experimental results show that the compound of the present invention has a strong anti-HBV virus action and is therefore suitable for treating various diseases caused by HBV virus infection.
- the luminescence cell viability assay kit detects the HepG2 cell viability and calculates the concentration (CC 50 ) of the compound to inhibit the HepG2 cell viability by half.
- the specific experimental methods are as follows:
- %Inh. [1-Add compound treatment HepG2 cell viability/control treatment HepG2 cell viability] X100.
- the CC 50 value of the compound for HepG2 cell viability was calculated: using the GraphPad Prism 5 analysis software, the "four-parameter logistic equation" was used to calculate the CC 50 value.
- Example 7 90
- Example 8 110
- Example 10 115
- Example 19 108
- Example 22 70
- Example 23 96
- Example 28 79
- control compound The structural formula of the control compound is shown in the following formula, and the preparation method thereof is described in Example 2 of WO2014037480.
- the toxicity test results show that the compounds of the present invention are less toxic.
- Compound compared to the control (murine LD 50> 600 mg)
- some of the compounds, e.g., compounds of Example 4 (murine LD 50> 1000 mg) embodiment exhibits less toxicity than the control compound, better security.
- Beagle dogs were injected intravenously with 2 mg/kg of test compound through the forelimb.
- Beagle dogs were orally administered with 10 mg/kg of test compound.
- the compounds of the present invention have a strong anti-HBV virus effect; and the compounds of the present invention have more favorable pharmacokinetic test results and good toxicity test results, which will make them more likely to be effective and safe. drug.
- the compound of Example 4 had moderate clearance (4.99 mL/kg/min) and good bioavailability (48%), good toxicity test results (CC 50 of 132 ⁇ mol), which indicates that the compound of Example 4 is The application prospects for anti-HBV viruses are very good.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- Virology (AREA)
- Engineering & Computer Science (AREA)
- Biotechnology (AREA)
- Molecular Biology (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
Abstract
本发明提供一种如式(I)所示的二氢嘧啶类化合物或其可药用盐或互变异构体或对映异构体或非对映异构体,其中R1、R2、R3、R和A如说明书和权利要求所定义。本发明还提供式(I)化合物的制备方法及其用于治疗或者预防乙型肝炎病毒感染的疾病的药物中的用途。
Description
本发明涉及一种二氢嘧啶类化合物及其作为药物的用途。这类化合物具有治疗和预防乙型肝炎的作用,特别是作为乙型肝炎病毒(HBV)抑制剂,通过靶向HBV衣壳治疗HBV感染。本发明还涉及这类化合物的制备方法。
乙型病毒性肝炎是由乙肝病毒(HBV)引起的、以肝脏炎性病变为主,并可引起多器官损害的一种疾病。乙型肝炎病毒简称乙肝病毒,是一种DNA病毒,属于嗜肝DNA病毒科(hepadnavividae)。它可引起急性的或持续/渐进的慢性病。乙肝广泛流行于世界各国,有超过4亿人患病,特别是在亚太地区。其中少数患者可转化为肝硬化或肝癌。目前市场上的抗乙肝病毒核苷(酸)类药物包括拉米夫定、替比夫定、恩替卡韦、替诺福韦酯、克拉夫定等。这类药物的缺点是:疗程不固定、易发生病毒耐药、停药后易复发等的缺点。这些缺点导致患者无法得到根治。
Deres等报道了以Bay41-4109、Bay3905493为代表的杂芳环取代的二氢嘧啶类(HAP)化合物,该类化合物能够通过阻止正常核衣壳的形成起到抑制HBV复制的作用。Bay41-4109在临床研究中表现了较好的药物代谢参数(Deres K.等人,Science,299(2003),893-896)。对其作用机理的研究发现,杂芳环取代的二氢嘧啶类化合物通过与核心蛋白的113-143氨基酸残基作用,改变了形成核衣壳的二聚体之间的夹角,导致形成不稳定的膨胀核衣壳,加速核心蛋白的降解(Biochem.Pharmacol.66(2003),2273-2279)。
目前仍然需要有新的能够有效地抗病毒的化合物,尤其是用作治疗和/或预防乙型肝炎的药物。
发明内容
本发明提供了式(I)所表示的化合物,或其可药用盐或互变异构体或对映异构体或非对映异构体:
其中:
R1为苯基,其中所述苯基任选进一步被一个或多个选自卤素、C1-6烷基的取代基所取代;
R2选自氢或C1-4烷基;
A为一个键、-O-、-S-或-N(R5)-;
R5为氢或C1-4烷基;
R3选自杂芳基,优选噻唑基、噁唑基、咪唑基、噻吩基、苯基或吡啶基,所述杂芳基可以进一步被一个或多个选自卤素、亚甲基(=CH2)、氧代(=O)、烷基、烷氧基、氰基、羟基、硝基、烷氨基、巯基、氨基、芳基、卤代烷基、烷基磺酰基、芳基烷基、杂芳基、杂芳基烷基、杂环基、杂环基烷基、环烷基、环烷基烷基、三氟甲基、三氟甲氧基、卤代烷基取代的芳基、卤素取代的芳基或三氟甲磺酰基的取代基所取代;
R为以下所示的基团:
R6各自独立地为-(CR7R7a)m-OH、-(CR7R7a)n-C(=O)O-R8、-(CR7R7a)n-C(=O)-NH-S(O)2-R9、-(CR7R7a)n-C(O)-NR10R10a、-(CR7R7a)n-C(=O)O-(CR7R7a)n-OC(=O)O-R8、-S(=O)pOR8、-(CR7R7a)n-S(=O)pN(R8)2、-(CR7R7a)n-C(=O)O-(CR7R7a)n-OC(=O)-R8、-(CR7R7a)n-C(=O)O-(CR7R7a)n-C(=O)O-R8、-(CR7R7a)n-N(R8)2、或-(CR7R7a)n-C(=O)N(R8)2;
R7和R7a各自独立地为氢、卤素、烷基或卤代烷基,其中所述的烷基、卤代烷基任选进一步被一个或多个选自卤素、亚甲基(=CH2)、氧代(=O)、烷基、烷氧基、氰基、羟基、硝基、烷氨基、巯基、氨基、芳基、芳基烷基、杂芳基、杂芳基烷基、杂环基、杂环基烷基、环烷基、环烷基烷基、三氟甲基、三氟甲氧基、卤代烷基取代的芳基、卤素取代的芳基或三氟甲磺酰基的取代基所取代,或R7和R7a和与之相连的碳原子一起形成环烷基或杂环基;
R8各自独立地为氢、羟基、烷基、烷氧基、烷基-S(=O)p-、芳基、杂芳基、环烷基、杂环基、芳基烷基、杂环基-S(=O)p-、杂芳基-S(=O)p-、环烷基-S(=O)p-或芳基-S(=O)p-,其中所述的烷基、烷氧基、烷基-S(=O)p-、芳基、杂芳基、环烷基、杂环基、芳基烷基、杂环基-S(=O)p-、杂芳基-S(=O)p-、环烷基-S(=O)p-或芳基-S(=O)p-任选进一步被一个或多个选自卤素、亚甲基(=CH2)、氧代(=O)、烷基、烷氧基、氰基、羟基、硝基、烷氨基、巯基、氨基、芳基、芳基烷基、杂芳基、杂芳基烷基、杂环基、杂环基烷基、环烷基、环烷基烷基、三氟甲基、三氟甲氧基、卤代烷基取代的芳基、卤素取代的芳基或三氟甲磺酰基的取代基所取代;
R9为烷基、烷氧基、芳基、杂芳基、环烷基、杂环基或芳基烷基;
R10和R10a各自独立地为氢、卤代烷基、环烷基、烷基或羟烷基,或R10和R10a和与之相连的氮原子一起形成杂环基;
m各自独立地为1、2、3、4;
n各自独立地为0、1、2、3、4;
p各自独立地为1或2。
在本发明的一个实施方案中,R优选为以下所示的子结构式:
在本发明的一个实施方案中,R优选自:
在本发明的一个实施方案中,R1优选自苯基,所述苯基任选进一步被一个或多个卤素所取代。
在本发明的另一个实施方案中,R2优选为甲基或乙基。
在本发明的另一个实施方案中,A优选为-O-。
在本发明的另一个实施方案中,R3优选为噻唑基、咪唑基或吡啶基,其中所述的噻唑基、咪唑基或吡啶基任选进一步被一个或多个选自卤素、烷基、烷氧基、卤代烷基、烷基磺酰基、环烷基的取代基所取代。
在本发明的另一个实施方案中,R1优选为2个卤素取代的苯基;R2优选为甲基或乙基;A优选为-O-;R3优选自噻唑基、咪唑基或吡啶基,其中所述的噻唑基、咪唑基或吡啶基任选进一步被一个或多个选自卤素、亚甲基(=CH2)、氧代(=O)、烷基、烷氧基、氰基、羟基、硝基、烷氨基、巯基、氨基、芳基、卤
代烷基、烷基磺酰基、芳基烷基、杂芳基、杂芳基烷基、杂环基、杂环基烷基、环烷基、环烷基烷基、三氟甲基、三氟甲氧基、卤代烷基取代的芳基、卤素取代的芳基或三氟甲磺酰基的取代基所取代;R优选为以下所示的基团:
R6各自独立地为-(CR7R7a)n-C(=O)O-R8、-(CR7R7a)n-C(=O)O-(CR7R7a)n-OC(=O)-R8或-(CR7R7a)n-C(=O)O-(CR7R7a)n-C(=O)O-R8;
R7和R7a各自独立地为氢、卤素、烷基或卤代烷基,其中所述的烷基、卤代烷基任选进一步被一个或多个选自卤素、亚甲基(=CH2)、氧代(=O)、烷基、烷氧基、氰基、羟基、硝基、烷氨基、巯基、氨基、芳基、芳基烷基、杂芳基、杂芳基烷基、杂环基、杂环基烷基、环烷基、环烷基烷基、三氟甲基、三氟甲氧基、卤代烷基取代的芳基、卤素取代的芳基或三氟甲磺酰基的取代基所取代,或R7和R7a和与之相连的碳原子一起形成环烷基或杂环基;
R8各自独立地为氢、羟基、烷基、烷氧基、烷基-S(=O)p-、芳基、杂芳基、环烷基、杂环基、芳基烷基、杂环基-S(=O)p-、杂芳基-S(=O)p-、环烷基-S(=O)p-或芳基-S(=O)p-,其中所述的烷基、烷氧基、烷基-S(=O)p-、芳基、杂芳基、环烷基、杂环基、芳基烷基、杂环基-S(=O)p-、杂芳基-S(=O)p-、环烷基-S(=O)p-或芳基-S(=O)p-任选进一步被一个或多个选自卤素、亚甲基(=CH2)、氧代(=O)、烷基、烷氧基、氰基、羟基、硝基、烷氨基、巯基、氨基、芳基、芳基烷基、杂芳基、杂芳基烷基、杂环基、杂环基烷基、环烷基、环烷基烷基、三氟甲基、三氟甲氧基、卤代烷基取代的芳基、卤素取代的芳基或三氟甲磺酰基的取代基所取代;
n各自独立地为0、1、2、3、4,优选为0;
p各自独立地为1或2。
更优选地,本发明所述的化合物式(I)选自:
(1)4-(2-氯-4-氟苯基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯;
(2)(S)-5-((6-(2-氯-4-氟苯基)-5-(乙氧羰基)-2-(噻唑-2-基)-1,4-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸;
(3)4-(2-氯-4-氟苯基)-6-(((S)-6-(吗啉-4-羰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯;
(4)(S)-5-(((R)-6-(2-氯-4-氟苯基)-5-(甲氧羰基)-2-(噻唑-2-基)-1,4-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸;
(5)4-(2-氯-4-氟苯基)-2-(3,5-氟吡啶-2-基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-1,4-二氢嘧啶-5-羧酸甲酯;
(6)4-(2-氯-4-氟苯基)-2-(3,5-氟吡啶-2-基)-6-((S)-5-氮杂螺[2.4]庚烷-6-羧酸)-1,4-二氢嘧啶-5-羧酸甲酯;
(7)4-(2-氯-4-氟苯基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸乙酯;
(8)4-(2-氯-4-氟苯基)-6-(((S)-6-((环丙基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸乙酯;
(9)4-(2-氯-4-氟苯基)-6-(((S)-6-(异丙基氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯;
(10)(S)-5-(((R)-6-(2-氯-4-氟苯基)-5-(甲氧羰基)-2-(4-甲基噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸;
(11)(S)-5-(((R)-6-(2-氯-3-氟苯基)-5-(甲氧羰基)-2-(噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸;
(12)(R)-甲基-4-(2-氯-3-氟苯基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯;
(13)(1R,3S,5R)-2-(((R)-6-(2-氯-3-氟苯基)-5-(甲氧羰基)-2-(噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-2-氮杂双环[3.1.0]己烷-3-羧酸;
(14)(1R,3S,5R)-2-(((R)-6-(2-氯-4-氟苯基)-5-(甲氧羰基)-2-(4-甲基噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-2-氮杂双环[3.1.0]己烷-3-羧酸;
(15)(R)-甲基-4-(2-氯-4-氟苯基)-6-(((S)-6-(异丙基氨基甲酰
基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(4-甲基噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯;
(16)(R)-甲基-4-(2-氯-4-氟苯基)-6-(((1R,3S,5R)-3-((甲基磺酰基)氨基甲酰基)-2-氮杂双环[3.1.0]己烷-2-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯;
(17)(S)-5-(((R)-6-(2-氯-4-氟苯基)-5-(甲氧基羰基)-2-(1-甲基-1H-咪唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸;
(18)(R)-甲基4-(2-氯-3-氟苯基)-6-(((S)-6-((环丙基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯;
(19)(1S,2S,5R)-3-(((R)-6-(2-氯-3-氟苯基)-5-(甲氧羰基)-2-(噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-3-氮杂二环[3.1.0]己烷-2-羧酸;
(20)(R)-甲基-4-(2-氯-4-氟苯基)-6-(((S)-6-(羟甲基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻吡啶-2-基)-1,4-二氢嘧啶-5-羧酸甲酯;
(21)(R)-甲基-4-(2-溴-4-氟苯基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(4-甲基噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯;
(22)(S)-5-(((R)-6-(2-溴-4-氟苯基)-5-(甲氧基羰基)-2-(4-甲基噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸;
(23)(1S,2S,5R)-3-(((R)-6-(2-氯-4-氟苯基)-5-(甲氧基羰基)-2-(噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-3-氮杂二环[3.1.0]己烷-2-羧酸;
(24)(R)-甲基-4-(2-氯-4-氟苯基)-2-(1-甲基-1H-咪唑-2-基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-1,4-二氢嘧啶-5-羧酸甲酯;
(25)(R)-甲基-4-(2-溴-4-氟苯基)-2-(4-甲基噻唑-2-基)-6-(((S)-6-((苯基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚-5-基)甲基)-1,4-二氢嘧啶-5-羧酸甲酯;
(26)(R)-甲基-4-(2-氯-4-氟苯基)-6-(((S)-6-(甲基氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯;
(27)(R)-甲基4-(2-氯-3-氟苯基)-6-(((S)-6-(二甲基氨基甲基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻吡啶-2-基)-1,4-二氢嘧啶-5-羧酸甲酯;
(28)(S)-5-(((R)-6-(2-溴-4-氟苯基)-5-(甲氧基羰基)-2-(噻唑-2-基)-3,6-二氢嘧啶-4-基基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸;
(29)(S)-((异丙氧基羰基)氧基)甲基5-((6-(2-氯-4-氟苯基)-5-(甲氧基羰基)-(噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸甲酯;
(30)(S)-(新戊酰氧基)甲基5-((6-(2-氯-4-氟苯基)-5-(甲氧基羰基)-(噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸甲酯。
本发明的第二方面,提供一种药物组合物,所述的药物组合物含有有效剂量的式(I)化合物或其可药用盐或互变异构体或对映异构体或非对应异构体。
本发明的又一方面提供了式(I)化合物或其可药用盐或互变异构体或对映异构体或非对映异构体,或其药物组合物在制备用于治疗或者预防乙型肝炎病毒感染的疾病的药物中的应用。
本发明的又一方面提供了式(I)化合物的制备方法。所述方法包括:式d所示化合物与R-H化合物或其盐在碱存在下反应得到式(I)化合物:
其中,R1、R2、R3、R和A如上所定义;其中所述碱优选为有机碱,更优选为三乙胺。
其中式d化合物通过以下方法进行制备,包括:
步骤(1):式a所示的化合物与式b所示的化合物反应得到式c所示的化合
物:
步骤(2):式c所示的化合物与溴化试剂反应得到式d所示的化合物:
其中,R1、R2、R3、R和A如上所定义;步骤(2)所述溴化试剂优选为N-溴丁二酰亚胺。
现在详细描述本发明的某些实施方案,本发明意图涵盖所有的替代、修改和等同技术方案,它们均包括在如权利要求定义的本发明范围内。本领域技术人员应该认识到,许多与本文所述类似或等同的方法和材料能够用于实现本发明。本发明绝不限于本文所述的方法和材料。在所结合的文献、专利和类似材料的一篇或多篇与本申请不同或相矛盾的情况下(包含但不限于所定义的术语、术语应用、所描述的技术等等),以本发明为准。
如无特别定义,本发明中所使用的术语具有本领域普遍所接受的含义,进一步地,本发明所使用的部分术语定义如下:
术语“包括”为开放式表达,即包括本发明所指明的内容,但并不排除其他方面的内容。
本发明所使用的术语“患者”可以包括人(包括成人和儿童)或者其他动物。在一些实施方案中,“患者”是指人。
“烷基”是指直链或者带有支链的饱和脂肪烃基团。本申请中的烷基优选地为C1-6烷基,即表示包括1至6个碳原子的饱和直链或支链烷基;本申请中特别优
选的烷基是C1-4烷基,即1至4个碳原子的饱和直链或支链烷基,例如甲基、乙基、正丙基、异丙基、1-丁基、2-丁基和叔丁基等。
“烷氧基”是指(烷基-O-)的基团。其中,烷基如上所定义。优选的烷氧基是C1-6烷氧基,特别优选的烷氧基是C1-4烷氧基。术语C1-6烷氧基包括甲氧基、乙氧基、正丙氧基和异丙氧基等。
术语“环烷基”表示包含3至12个碳原子、特别是3至6个碳原子的饱和碳环,例如环丙基、环丁基、环戊基、环己基、环庚基等。特别是,“环烷基”是环丙基、环戊基、环己基。
“卤代烷基”或“卤代烷氧基”表示烷基或烷氧基基团被一个或多个相同或不同的卤素原子所取代。优选的烷基或烷氧基如上面所定义。实例包括,但不限于:三氟甲基、三氟乙基、三氟甲氧气基。
“环烷基烷基”表示烷基基团被一个或多个环烷基基团所取代。实例包括,但不限于:环丙基甲基、环丙基乙基、环丁基甲基、环戊基甲基等。
“芳基”表示含有6-14个环原子的单环、双环和三环的碳环体系、其中,至少一个环体系是芳香族的、其中,每一个环体系包含3-7个原子组成的环,且有一个或多个连接点与分子的其余部分相连。实例包括,但不限于:苯基、奈基、蒽等。优选地,所述芳基为6-10个或6-7个环原子的碳环体系。
“杂芳基”表示含有5-14个环原子的单环、双环和三环体系,其中,至少一个环体系是芳香族的,且至少一个环体系包含一个或多个选自氮、氧、硫的杂原子,其中每一个环体系包含5-7个原子组成的环,且有一个或多个连接点与分子的其余部分相连。术语“杂芳基”可以与术语“杂芳环”或“杂芳族化合物”交换使用。实例包括,但不限于:呋喃基、咪唑基、2-吡啶基、3吡啶基、噻唑基、嘌呤基、喹啉基。优选地,所述杂芳基为5-10个环原子的环体系。
“杂芳基烷基”表示杂芳基基团通过烷基基团与分子的其余部分相连。实例包括,但不限于:吡啶-2-乙基、噻唑-2-甲基、嘧啶-2-丙基等。
术语“羧基“是指基团-COOH。
术语“卤素”是指氟、氯、溴和碘。特别地,卤素是指氟、氯或溴。
术语“氰基”是指基团-CN。
术语“羟基”是指基团-OH。
术语“磺酰基”是指基团-S(O)2-。
术语“羰基”是指基团-C(=O)-。
术语“C1-6烷氧基羰基”是指基团C1-6烷氧基-C(O)-,其中所述“C1-6烷氧基”如上文所定义。
术语“氨基羰基”是指基团氨基-C(O)-,其中所述“氨基”如上文所定义。
术语“C1-6烷基磺酰基”是指基团C1-6烷基-S(O)2-,其中所述“C1-6烷基”如上文所定义。
术语“氨基磺酰基”是指基团氨基-S(O)2-,其中所述“氨基”如上文所定义。
术语“杂环基”是指包括3-12个环原子的,非芳香族的饱和或部分不饱和的单环、双环或三环体系、其中至少一个环原子选自氮、硫和氧原子。其中,所述杂环基基团可以任选地被一个或多个本发明描述的取代基所取代。除非另外说明,杂环基可以是碳基或氮基,且-CH2-基团可以任选地被-C(=O)-替代。环的硫原子可以任选地被氧化成S-氧化物。优选地,所述杂环基为3-10个、3-6个环原子的,非芳香族的饱和或部分不饱和的单环、双环或三环体系、其中至少一个环原子选自氮、硫和氧原子。
术语“羟烷基”是指羟烷基通过碳原子与分子其余部分相连,其中,所述烷基如上所定义。所述羟烷基优选为包括具有1-10个碳原子且被一个或多个羟基取代的直链或支链烷基。更优选的羟基烷基是具有1-6个碳原子或1-4个碳原子和一个或多个羟基的低级羟基烷基,例如羟甲基(-CH2OH)、羟乙基(-CH2CH2OH)和羟丙基(-CH2CH2CH2OH)等。
术语“互变异构体”是指通过称为互变异构化的化学反应容易相互转化的有机化合物的结构异构体。该反应通常导致氢原子或质子的形式迁移,伴随单键和相邻双键的转换,例如通式(I)的化合物
术语“手性”是指具有与其镜像不能重叠性质的分子;而“非手性”是指与其镜像可以重叠的分子。
术语“对映异构体”是指一个化合物的两个不能重叠但互为镜像关系的异构体。
术语“非对映异构体”是指有两个或多个手性中心并且其分子不互为镜像的立体异构体。非对映异构体通常具有不同的物理性质,如熔点、沸点、光谱性质和反应性。非对映异构体混合物可通过高分辨分析操作如电泳和色谱,例如HPLC来分离。
像本发明所描述的,本发明的化合物可以任选地被一个或多个取代基所取代,如上面的通式化合物,本发明的具体化合物,或像实施例里面特殊的例子,子类,和本发明所包含的一类化合物。应了解术语“任选取代的”与术语“取代或非取代的”可以交换使用。一般而言,术语“取代的”,表示所给结构中的一个或多个氢原子被具体取代基所取代。除非其他方面表明,一个任选的取代基团可以在基团的各个可取代的位置进行取代。当所给出的结构式中不知有一个位置能被选自具体基团的一个或多个取代基所取代,那么取代基可以相同或不同地在各个位置取代。其中的取代基可以是,但并不限于:氟、氯、溴、碘、氧代(=O)、亚甲基(=CH2)、烷基、烷氧基、氰基、羟基、硝基、烷氨基、巯基、氨基、芳基、芳基烷基、杂芳基、杂芳基烷基、杂环基、杂环基烷基、环烷基、环烷基烷基、三氟甲基、三氟甲氧基、卤代烷基取代的芳基、卤素取代的芳基或三氟甲磺酰基等。
此外,术语“药学上可接受的盐”是指上述化合物能保持原有生物活性并且适合于医药用途的某些盐类。式(I)所表示的化合物药学上可接受的盐可以为与合适的酸形成的盐,合适的酸包括无机酸和有机酸,例如乙酸、苯磺酸、苯甲
酸、樟脑磺酸、柠檬酸、乙磺酸、富马酸、葡糖酸、谷氨酸、氢溴酸、盐酸、羟乙磺酸、乳酸、苹果酸、马来酸、扁桃酸、甲磺酸、硝酸、磷酸、琥珀酸、硫酸、酒石酸、对甲苯磺酸等。特别优选的是盐酸、磷酸或硫酸。
以下反应一般是在氮气正压下操作的。反应瓶上都塞上合适的橡皮塞,底物可通过注射器打入。玻璃器皿均是经过干燥的。色谱柱是使用硅胶柱。核磁共振数据通过Bruker Advance 400核磁共振仪来测定,以CDCl3,d6-DMSO或CD3OD为溶剂(报导以ppm为单位),用TMS(0ppm)或氯仿(7.25ppm)作为参照标准。当出现多重峰时,使用如下缩写:s(singlet,单峰),s,s(singlet,singlet,单峰,单峰),d(doublet,双峰),t(triplet,三重峰),br(broadened,宽峰),dd(doublet of doublets,四重峰),ddd(doublet of doublet of doublets,双双二重峰),ddt(doublet of doublet of triplets,双双三重峰),dddd(doublet of doublet of doublet of doublets,双双双二重峰),td(triplet of doublets,三双重峰),brs(broadened singlet,宽单峰)。偶合常数,用赫兹(Hz)表示。
低分辨率质谱(MS)数据通过Agilent 1100系列LC-MS的光谱仪来测定的。ESI源应用于LC-MS光谱仪。
化合物纯度是通过Agilent 1100系列高效液相色谱(HPLC)来评价的,其中UV检测在210nm和254nm处,Zorbax SB-C18柱子,规格为2.1X30mm,4μm,10分钟,流速为0.6ml/min,5-95%的(0.1%甲酸乙腈溶液)的(0.1%甲酸水溶液),柱温保持在40℃。
下面简写词的使用贯穿本发明:
DCM或CH2Cl2 二氯甲烷
EtOAc或EA 乙酸乙酯
THF 四氢呋喃
CH3OH或MeOH 甲醇
d6-DMSO 氘代二甲基亚砜
CDCl3 氘代氯仿
CCl4 四氯化碳
Boc 叔丁氧羰基
PE 石油醚
K2CO3 碳酸钾
NaHCO3 碳酸氢钠
Na2SO4 硫酸钠
KOAc 醋酸钾
DIPEA N,N-二异丙基乙胺
NBS N-溴丁二酰亚胺
c 浓度
g 克
v/v或v:v 体积比
mol 摩尔
mmol 毫摩尔
mL 毫升
L 升
h 小时
t1/2 半衰期
AUC 药时曲线下面积
Vss 稳态表观分布容积
CL或clearance 清除率
F,absolute bioavailability 生物利用度
Dose 剂量
Tmax 达峰时间
Cmax 最大浓度
hr*ng/mL 血药浓度*时间
本发明的起始原料和试剂均来自市售,其供应商为Aldrich Chemical Company,Alfa Chemical Company,国药集团,临安青山化工试剂厂,江苏华达化工集团和杭州化学试剂有限公司。除非另有指明,市售原料和试剂均不经进一步纯化直接使用。本发明所描述的实施例,除非另有指明,所有温度为摄氏温度(℃)。
实施例1:4-(2-氯-4-氟苯基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯的制备
步骤1)化合物4-(2-氯-4-氟苯基)-6-甲基-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯的合成
将化合物2-(2-氯-4-氟苄基)-3-氧代丁酸甲酯(5.3g,20.7mmol),2-噻唑甲脒盐酸盐(2.6g,15.9mmol)溶于三氟乙醇(70ml),加入乙酸钾(3.1g,31.8mmol),氮气置换3次,80℃加热回流反应过夜。反应结束后,冷却,抽滤,浓缩反应液,残余物经柱层析分离纯化(正庚烷/乙酯(v/v)=9/1)得到黄色固体4-(2-氯-4-氟苯基)-6-甲基-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯(4.4g,76%)。
MS(ESI,pos.ion)m/z:367[M+H]+;
步骤2)化合物4-(2-氯-4-氟苯基)-6-(溴甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-甲酸甲酯的合成
无水操作下,将4-(2-氯-4-氟苯基)-6-甲基-2-(噻唑-2-基)-1,4-二氢嘧啶
-5-甲酸甲酯(500mg,1.37mmol)溶于CCl4(15ml),加入NBS(244mg,1.37mmol),室温反应1h。反应结束后,浓缩反应液,残余物经柱层析分离纯化(正庚烷/乙酯(v/v)=4/1)得到黄色固体(350mg,57%)。
MS(ESI,pos.ion)m/z:444,446[M+H]+;
步骤3)化合物(S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-甲酸叔丁酯的合成
将化合物(S)-5-(叔丁氧基羰基)-5-氮杂螺[2.4]庚烷-6-羧酸(1.49g,6.18mmol)溶于THF(40ml),加入CDI(2.2g,13.6mmol),室温搅拌反应1h,加入甲基磺酰胺(2.35g,24.7mmol),DBU(2.35g,15.5mmol),70℃加热反应5h。反应结束后,冷却,浓缩反应液,残余物加入DCM(50ml)溶解,分别用1N HCl(50ml×2),饱和食盐水(50ml×2)先后洗涤,浓缩有机相,残余物经柱层析分离纯化(DCM/EA(v/v)=4/1)得到白色固体(S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-甲酸叔丁酯(1.57g,80%)。
MS(ESI,pos.ion)m/z:319[M+H]+;
步骤4)化合物(S)-N-(甲基磺酰基)-5-氮杂螺[2.4]庚烷-6-甲酰胺盐酸盐的合成
冰浴条件下,向THF(50ml)中通入HCl气体(3.6g,98.7mmol),将化合物(S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-甲酸叔丁酯(1.57g,4.94mmol)溶于上述溶液中,室温搅拌反应过夜。反应结束后,浓缩反应液得到白色固体(S)-N-(甲基磺酰基)-5-氮杂螺[2.4]庚烷-6-甲酰胺盐酸盐(0.95g,
88%)。
MS(ESI,pos.ion)m/z:219[M+H-HCl]+;
步骤5)化合物4-(2-氯-4-氟苯基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯的合成
将化合物4-(2-氯-4-氟苯基)-6-(溴甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-甲酸甲酯(240mg,0.539mmol)溶于CHCl3(5ml)中,加入化合物(S)-N-(甲基磺酰基)-5-氮杂螺[2.4]庚烷-6-甲酰胺盐酸盐(176mg,0.809mmol),三乙胺(436mg,4.31mmol),50℃加热反应5h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法分离纯化(EA/MeOH(v/v)=19/1)得到黄色固体4-(2-氯-4-氟苯基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯(70mg,22%)。
MS(ESI,pos.ion)m/z:583[M+H]+;
1H NMR(400MHz,CDCl3):δppm 8.47(br,1H),7.90(s,1H),7.61(s,s,1H),7.34(m,1H),7.21(m,1H),7.16(m,1H),7.04(m,1H),6.16(s,s,1H),4.34(m,2H),3.69(s,3H),3.14(s,s,3H),2.22(m,2H),1.98(m,1H),1.34(m,2H),0.69(m,4H).
实施例2:(S)-5-((6-(2-氯-4-氟苯基)-5-(乙氧羰基)-2-(噻唑-2-基)-1,4-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸的制备
步骤1)化合物4-(2-氯-4-氟苯基)-6-甲基-2-(噻唑-2-基)-1,4-二氢嘧啶-5-甲酸乙酯的合成
将2-(2-氯-4-氟苄基)-3-氧代丁酸乙酯(4.2g,15.8mmol),2-噻唑甲脒盐酸盐(2.0g,12.1mmol)溶于三氟乙醇(40ml),加入乙酸钾(2.4g,24.3mmol),氮气置换3次,80℃加热回流反应过夜。反应结束后,冷却,抽滤,浓缩反应液,残余物经柱层析分离纯化(正庚烷/乙酯(v/v)=9/1)得到黄色固体4-(2-氯-4-氟苯基)-6-甲基-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸乙酯(2.6g,43%)。
MS(ESI,pos.ion)m/z:381[M+H]+
步骤2)化合物4-(2-氯-4-氟苯基)-6-(溴甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-甲酸乙酯的合成
将4-(2-氯-4-氟苯基)-6-甲基-2-(噻唑-2-基)-1,4-二氢嘧啶-5-甲酸乙酯(600mg,1.6mmol)溶于CCl4(5ml),加入NBS(282mg,1.58mmol),在氮气保护下,室温反应1h。反应结束后,浓缩反应液,残余物经柱层析分离纯化(正庚烷/乙酯(v/v)=1/1)得到黄色固体4-(2-氯-4-氟苯基)-6-(溴甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-甲酸乙酯(460mg,63%)。
MS(ESI,pos.ion)m/z:458,460[M+H]+
步骤3)化合物(S)-5-氮杂螺[2.4]庚烷-6-羧酸盐酸盐的合成
将化合物(S)-5-(叔丁氧基羰基)-5-氮杂螺[2.4]庚烷-6-羧酸(5.0g,20.7mmol)溶于50ml的THF中,在冰水浴的条件下,向THF(50ml)中通入HCl气体(8.5g,232.9mmol)室温搅拌反应过夜。反应结束后,浓缩反应液得到白色固体(S)-5-氮杂螺[2.4]庚烷-6-羧酸盐酸盐(3.5g,95%)。
MS(ESI,pos.ion)m/z:142[M+H-HCl]+
步骤4)化合物(S)-5-((6-(2-氯-4-氟苯基)-5-(乙氧羰基)-2-(噻唑-2-基)-1,4-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸的合成
将化合物4-(2-氯-4-氟苯基)-6-(溴甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-甲酸乙酯(100mg,0.218mmol)溶于5ml的CHCl3中,加入化合物(S)-5-氮杂螺[2.4]庚烷-6-羧酸盐酸盐(87mg,0.489mmol),三乙胺(476mg,1.75mmol),40℃加热反应5h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法分离纯化(EA/MeOH(v/v)=9/1)得到黄色固体(S)-5-((6-(2-氯-4-氟苯基)-5-(乙氧羰基)-2-(噻唑-2-基)-1,4-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸(70mg,62%)。
MS(ESI,pos.ion)m/z:520[M+H]+
1H NMR(400MHz,MeOD):δppm8.12(m,2H),7.55(m,1H),7.25(m,1H),7.12(m,1H),6.21(s,s,1H),4.05(m,2H),3.84(m,1H),3.52(m,1H),2.90(m,1H),2.48(m,1H),2.19(m,1H),2.00(m,1H),1.28(m,2H),1.14(m,3H),0.72(m,4H).
实施例3:4-(2-氯-4-氟苯基)-6-(((S)-6-(吗啉-4-羰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯的制备
将化合物4-(2-氯-4-氟苯基)-6-(溴甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-甲酸甲酯(200mg,0.45mmol)溶于CHCl3(5ml)中,加入化合物(S)-吗啉(5-氮杂螺[2.4]庚烷-6-基)乙酮盐酸盐(189mg,0.90mmol),三乙胺(365mg,3.6mmol),45℃加热反应1h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法以EA为展开剂分离纯化得到黄色固体4-(2-氯-4-氟苯基)-6-(((S)-6-(吗啉-4-羰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯(70mg,22%)。
MS(ESI,pos.ion)m/z:575[M+H]+;
1H NMR(400MHz,CDCl3):δppm 10.24(br,1H),7.95(m,1H),7.43(m,2H),7.15(m,1H),6.95(m,1H),6.23(s,s,1H),4.19(m,1H),3.86-3.50(m,12H),2.98-2.70(m,1H),2.22(m,1H),1.93(m,1H),1.52(m,2H),0.69(m,4H).
实施例4:(S)-5-(((R)-6-(2-氯-4-氟苯基)-5-(甲氧羰基)-2-(噻唑-2-基)-1,4-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸的制备
将化合物4-(2-氯-4-氟苯基)-6-(溴甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-甲酸甲酯(70mg,0.157mmol)溶于CHCl3(5ml)中,加入化合物(S)-5-氮杂螺[2.4]庚烷-6-羧酸盐酸盐(42mg,0.236mmol),三乙胺(127mg,1.26mmol),50℃加热反应8h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法分离纯化(EA/MeOH(v/v)=9/1)得到黄色固体(6S)-5-(((R)-6-(2-氯-4-氟苯基)-5-(甲氧羰基)-2-(噻唑-2-基)-1,4-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸(25mg,32%)
MS(ESI,pos.ion)m/z:506[M+H]+;
1H NMR(400MHz,MeOD):δppm 8.19(m,2H),7.47(m,1H),7.28(m,1H),7.14(m,1H),6.20(s,1H),3.68(s,3H),3.58(m,2H),2.90(m,1H),2.54(m,1H),2.22(m,1H),2.00(m,1H),1.40(m,2H),0.78(m,4H).
实施例5:4-(2-氯-4-氟苯基)-2-(3,5-氟吡啶-2-基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-1,4-二氢嘧啶-5-羧酸甲酯的制备
将化合物6-(溴甲基)-4-(2-氯-4-氟苯基)-2-(3,5-二氟吡啶-2-基)-1,4-二氢嘧啶-5-羧酸甲酯(150mg,0.32mmol)溶于CHCl3(5ml)中,加入化合物(S)-N-(甲基磺酰基)-5-氮杂螺[2.4]庚烷-6-甲酰胺盐酸盐(70mg,0.32mmol),三乙胺(259mg,2.56mmol),40℃加热反应4h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法分离纯化(EA/MeOH(v/v)=19/1)得到黄色固体4-(2-氯-4-氟苯基)-2-(3,5-氟吡啶-2-基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-1,4-二氢嘧啶-5-羧酸甲酯(65mg,33%)。
MS(ESI,pos.ion)m/z:613[M+H]+;
1H NMR(400MHz,CDCl3):δppm 9.17(m,1H),8.34(m,1H),7.96(m,1H),7.56(m,1H),7.36(m,1H),7.16(m,1H),6.22(s,s,1H),4.12(m,1H),3.70(m,5H),3.24(s,s,3H),2.83(m,1H),2.42(m,1H),2.05(m,1H),1.29(m,2H),0.69(m,4H).
实施例6:4-(2-氯-4-氟苯基)-2-(3,5-氟吡啶-2-基)-6-((S)-5-氮杂螺[2.4]庚烷-6-羧酸)-1,4-二氢嘧啶-5-羧酸甲酯的制备
将化合物6-(溴甲基)-4-(2-氯-4-氟苯基)-2-(3,5-二氟吡啶-2-基)-1,4-二氢嘧啶-5-羧酸甲酯(100mg,0.21mmol)溶于CHCl3(5ml)中,加入化合物(S)-5-氮杂螺[2.4]庚烷-6-羧酸盐酸盐(49mg,0.27mmol),三乙胺(170mg,1.68mmol),室温搅拌反应5h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法分离纯化(EA/MeOH(v/v)=9/1)得到黄色固体4-(2-氯-4-氟苯基)-2-(3,5-氟吡啶-2-基)-6-((S)-5-氮杂螺[2.4]庚烷-6-羧酸)-1,4-二氢嘧啶-5-羧酸甲酯(90mg,80%)。
MS(ESI,pos.ion)m/z:536[M+H]+;
1H NMR(400MHz,CDCl3):δppm 11.65(br,1H),8.32(m,1H),7.37(m,1H),7.29(m,1H),7.16(m,2H),6.99(m,1H),6.16(s,s,1H),4.12(m,1H),3.80(m,1H),3.63(s,3H),2.42(m,2H),2.02(m,1H),1.32(m,2H),0.69(m,4H).
实施例7:4-(2-氯-4-氟苯基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸乙酯的制备
将化合物4-(2-氯-4-氟苯基)-6-(溴甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸乙酯(100mg,0.22mmol)溶于CHCl3(5ml)中,加入化合物(S)-N-(甲基磺酰基)-5-氮杂螺[2.4]庚烷-6-甲酰胺盐酸盐(62mg,0.28mmol),三乙胺(176mg,1.75mmol),室温搅拌反应6h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法分离纯化(EA/MeOH(v/v)=9/1)得到黄色固体4-(2-氯-4-氟苯基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸乙酯(60mg,46%)。
MS(ESI,pos.ion)m/z:597[M+H]+;
1H NMR(400MHz,CDCl3):δppm 9.17(s,1H),8.51(s,s,1H),7.63(s,s,1H),7.22(m,1H),7.16(m,1H),7.01(m,1H),6.05(s,s,1H),4.14(m,2H),3.67(m,1H),3.48(m,1H),3.16(s,s,3H),2.72(m,1H),2.43(m,1H),2.12(m,1H),1.34(m,2H),1.15(m,3H),0.69(m,4H).
实施例8:4-(2-氯-4-氟苯基)-6-(((S)-6-((环丙基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸乙酯的制备
将化合物4-(2-氯-4-氟苯基)-6-(溴甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸乙酯(100mg,0.22mmol)溶于CHCl3(5ml)中,加入化合物(S)-N-(环丙基磺酰基)-5-氮杂螺[2.4]庚烷-6-甲酰胺盐酸盐(69mg,0.28mmol),三乙胺(176mg,1.75mmol),室温搅拌反应6h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法分离纯化(EA/MeOH(v/v)=19/1)得到黄色固体4-(2-氯-4-氟苯基)-6-(((S)-6-((环丙基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸乙酯(70mg,51%)。
MS(ESI,pos.ion)m/z:623[M+H]+;
1H NMR(400MHz,CDCl3):δppm 9.50(br,1H),8.57(s,s,1H),7.60(s,s,1H),7.27(m,1H),7.14(m,1H),7.03(m,1H),6.07(s,s,1H),4.14(m,2H),3.80-3.12(m,2H),2.80(m,1H),2.43(m,1H),2.20(m,1H),1.32(m,2H),1.21(m,3H),0.95(m,4H),0.71(m,4H).
实施例9:4-(2-氯-4-氟苯基)-6-(((S)-6-(异丙基氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯的制备
将化合物4-(2-氯-4-氟苯基)-6-(溴甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-甲酸甲酯(100mg,0.23mmol)溶于CHCl3(5ml)中,加入化合物(S)-N-异丙基-5-氮杂螺[2.4]庚烷-6-甲酰胺盐酸盐(197mg,0.90mmol),三乙胺(365mg,3.6mmol),45℃加热反应1h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法以EA为展开剂分离纯化得到黄色固体4-(2-氯-4-氟苯基)-6-(((S)-6-(异丙基氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯(30mg,24%)。
MS(ESI,pos.ion)m/z:547[M+H]+;
1H NMR(400MHz,CDCl3):δppm 9.24(br,1H),7.95(m,1H),7.43(m,2H),7.15(m,1H),6.95(m,1H),6.23(s,s,1H),4.39(m,1H),4.13-3.95(m,2H),3.75(s,3H),2.98-2.70(m,1H),2.22(m,1H),1.93(m,1H),1.22(m,6H),1.12(m,2H),0.74(m,4H).
实施例10:(S)-5-(((R)-6-(2-氯-4-氟苯基)-5-(甲氧羰基)-2-(4-甲基噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸的制备
将化合物4-(2-氯-4-氟苯基)-6-(溴甲基)-2-(4-甲基噻唑-2-基)-1,4-二氢嘧啶-5-甲酸甲酯(80mg,0.18mmol)溶于CHCl3(5ml)中,加入化合物(S)-5-氮杂螺[2.4]庚烷-6-羧酸盐酸盐(64mg,0.36mmol),三乙胺(146mg,1.44mmol),45℃加热反应5h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法分离纯化(EA/MeOH(v/v)=9/1)得到黄色固体(20mg,22%)。
MS(ESI,pos.ion)m/z:[M+H]+=520;
1H NMR(400MHz,CDCl3):δppm 11.33(br,1H),7.64(s,1H),7.33(m,1H),7.31(m,1H),7.03(m,1H),6.17(s,1H),5.16(m,1H),4.58(m,1H),3.79(s,3H),3.52(m,1H),2.46(s,3H),2.19(m,1H),2.00(m,1H),1.28(m,2H),0.84(m,4H).
实施例11:(S)-5-(((R)-6-(2-氯-3-氟苯基)-5-(甲氧羰基)-2-(噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸的制备
将化合物6-(溴甲基)-4-(2-氯-3-氟苯基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯(80mg,0.18mmol)溶于CHCl3(15ml)中,加入化合物(S)-5-氮杂螺[2.4]庚烷-6-羧酸盐酸盐(48mg,0.27mmol),三乙胺(146mg,1.44mmol),45℃加热反应1h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法分离纯化(EA:MeOH=9:1)得到黄色固体(S)-5-(((R)-6-(2-氯-3-氟苯基)-5-(甲氧羰基)-2-(噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸(10mg,11%)。
MS(ESI,pos.ion)m/z:[M+H]+=506;
1H NMR(400MHz,CD3OD):δppm 8.17(m,2H),7.37(m,1H),7.29(m,2H),6.31(s,1H),4.56(m,1H),3.87(m,1H),3.69(s,3H),3.52(m,1H),2.90(m,1H),2.51(m,1H),1.28(m,2H),0.79(m,4H)。
实施例12:(R)-甲基4-(2-氯-3-氟苯基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯的制备
将化合物6-(溴甲基)-4-(2-氯-3-氟苯基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯(80mg,0.18mmol)溶于CHCl3(15ml)中,加入化合物(S)-N-(甲基磺酰基)-5-氮杂螺[2.4]庚烷-6-甲酰胺盐酸盐(92mg,0.36mmol),三乙胺(146mg,1.44mmol),45℃加热反应1h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法分离纯化(EA:MeOH=9:1)得到黄色固体(R)-甲基4-(2-氯-3-氟苯基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯(30mg,29%)。
MS(ESI,pos.ion)m/z:[M+H]+=583;
1H NMR(400MHz,CDCl3):δppm 9.46(br,1H),7.99(m,1H),7.64(m,1H),7.26(m,1H),7.15(m,2H),6.34(s,1H),4.88(m,1H),3.80(m,1H),3.63(s,3H),3.02(s,3H),2.76(m,1H),2.41(m,1H),2.23(m,1H),1.24(m,2H),0.79(m,4H).
实施例13:(1R,3S,5R)-2-(((R)-6-(2-氯-3-氟苯基)-5-(甲氧羰基)-2-(噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-2-氮杂双环[3.1.0]己烷-3-羧酸的制备
将化合物6-(溴甲基)-4-(2-氯-3-氟苯基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯(80mg,0.18mmol)溶于CHCl3(5ml)中,加入化合物(1R,3S,5R)-2-氮杂双环[3.1.0]己烷-3-羧酸盐酸盐(44mg,0.27mmol),三乙胺(146mg,1.44mmol),加热45℃搅拌反应5h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法分离纯化(EA/MeOH(v/v)=9/1)得到黄色固体(1R,3S,5R)-2-(((R)-6-(2-氯-3-氟苯基)-5-(甲氧羰基)-2-(噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-2-氮杂双环[3.1.0]己烷-3-羧酸(15mg,17%)。
MS(ESI,pos.ion)m/z:[M+H]+=492;
1H NMR(400MHz,CDCl3):δppm 8.15(br,1H),7.90(m,1H),7.83(m,1H),7.51(m,1H),7.38(m,2H),7.23(m,1H),6.27(s,1H),3.65(s,3H),3.44(m,1H),2.55(m,1H),2.22(m,1H),1.78(m,2H),0.91(m,1H),0.78(m,2H).
实施例14:(1R,3S,5R)-2-(((R)-6-(2-氯-4-氟苯基)-5-(甲氧羰基)-2-(4-甲基噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-2-氮杂双环[3.1.0]己烷-3-羧酸的制备
将化合物4-(2-氯-4-氟苯基)-6-(溴甲基)-2-(4-甲基噻唑-2-基)-1,4-二氢嘧啶-5-甲酸甲酯(80mg,0.18mmol)溶于CHCl3(5ml)中,加入化合物(1R,3S,5R)-2-氮杂双环[3.1.0]己烷-3-羧酸盐酸盐(59mg,0.36mmol),三乙胺(146mg,1.44mmol),加热45℃搅拌反应5h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法分离纯化(EA/MeOH(v/v)=9/1)得到黄色固体A(10mg,11%)。
MS(ESI,pos.ion)m/z:[M+H]+=506;
1H NMR(400MHz,CD3OD):δppm 7.59(s,1H),7.51(m,1H),7.25(m,1H),7.10(m,1H),6.14(s,1H),3.79(m,1H),3.64(s,3H),3.36(m,1H),2.61(s,3H),2.55(m,1H),2.22(m,1H),1.78(m,2H),0.91(m,1H)0.78(m,2H).
实施例15:(R)-甲基-4-(2-氯-4-氟苯基)-6-(((S)-6-(异丙基氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(4-甲基噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯的制备
将化合物4-(2-氯-4-氟苯基)-6-(溴甲基)-2-(4-甲基噻唑-2-基)-1,4-二氢嘧啶-5-甲酸甲酯(80mg,0.18mmol)溶于CHCl3(5ml)中,加入化合物(S)-N-异丙基-5-氮杂螺[2.4]庚烷-6-甲酰胺盐酸盐(197mg,0.90mmol),三乙胺(365mg,3.6mmol),45℃加热反应1h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法以EA为展开剂分离纯化得到黄色固体(R)-甲基-4-(2-氯-4-氟苯基)-6-(((S)-6-(异丙基氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(4-甲基噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯(20mg,24%)。
MS(ESI,pos.ion)m/z:561[M+H]+;
1H NMR(400MHz,CDCl3):δppm 9.20(br,1H),8.02(br,1H),7.43(s,1H),7.15(m,1H),6.95(m,2H),6.12(s,1H),4.39(m,1H),4.13-3.95(m,2H),3.75(s,3H),2.98-2.70(m,1H),2.47(s,3H),2.22(m,1H),1.93(m,1H),1.22(m,6H),1.12(m,2H),0.74(m,4H).
实施例16:(R)-甲基-4-(2-氯-4-氟苯基)-6-(((1R,3S,5R)-3-((甲基磺酰基)氨基甲酰基)-2-氮杂双环[3.1.0]己烷-2-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯的制备
将化合物4-(2-氯-4-氟苯基)-6-(溴甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-甲酸甲酯(180mg,0.41mmol)溶于CHCl3(10ml)中,加入化合物(1R,3S,5R)-N-(甲基磺酰基)-2-氮杂双环[3.1.0]己烷-3-甲酰胺盐酸盐(195mg,0.81mmol),三乙胺(332mg,3.28mmol),加热45℃搅拌反应5h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法以EA为展开剂分离纯化得到黄色固体(R)-甲基-4-(2-氯-4-氟苯基)-6-(((1R,3S,5R)-3-((甲基磺酰基)氨基甲酰基)-2-氮杂双环[3.1.0]己烷-2-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯(37mg,16%)。
MS(ESI,pos.ion)m/z:568[M+H]+=569;
1H NMR(400MHz,CDCl3):δppm 9.70(br,1H),8.22(m,1H),7.73(m,1H),7.34(m,2H),7.02(m,1H),6.11(s,1H),4.64(m,1H),3.66(s,3H),3.22(m,1H),3.05(s,3H),2.48(m,2H),1.43(m,2H),0.96(m,1H),0.87(m,1H),0.50(m,1H).
实施例17:(S)-5-(((R)-6-(2-氯-4-氟苯基)-5-(甲氧基羰基)-2-(1-甲基-1H-咪唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸的制备
将化合物6-(溴甲基)-4-(2-氯-4-氟苯基)-2-(1-甲基-1H-咪唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯(80mg,0.18mmol)溶于CHCl3(5ml)中,加入化合物(S)-5-氮杂螺[2.4]庚烷-6-羧酸盐酸盐(140mg,0.64mmol),三乙胺(200mg,1.98mmol),45℃加热反应5h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法分离纯化(EA:MeOH=9:1)得到黄色固体(20mg,18%)。
MS(ESI,pos.ion)m/z:[M+H]+=503;
1H NMR(400MHz,CD3OD):δppm 7.49(m,1H),7.42(m,1H),7.37(m,1H),7.28(m,1H),7.11(m,1H),6.20(s,1H),4.22(m,1H),3.90(s,3H),3.79(m,1H),3.74(s,3H),3.56(m,1H),2.55(m,1H),2.22(m,1H),1.28(m,2H),0.88(m,4H).
实施例18:(R)-甲基4-(2-氯-3-氟苯基)-6-(((S)-6-((环丙基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯的制备
将化合物6-(溴甲基)-4-(2-氯-3-氟苯基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯(80mg,0.18mmol)溶于CHCl3(15ml)中,加入化合物(S)-N-(环丙基磺酰基)-5-氮杂螺[2.4]庚烷-6-甲酰胺盐酸盐(96mg,0.36mmol),三乙胺(146mg,1.44mmol),45℃加热反应1h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法分离纯化(EA:MeOH=9:1)得到黄色固体(R)-甲基4-(2-氯-3-氟苯基)-6-(((S)-6-((环丙基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯(15mg,15%)。
MS(ESI,pos.ion)m/z:[M+H]+=609;
1H NMR(400MHz,CDCl3):δppm 9.57(br,1H),8.22(m,1H),7.54(m,1H),7.24(m,1H),7.14(m,2H),6.32(s,1H),4.84(m,1H),3.81(m,1H),3.77(s,3H)3.12(m,1H),2.80(m,1H),2.43(m,1H),2.20(m,1H),1.76(m,1H),1.32(m,2H),0.97(m,4H),0.79(m,4H).
实施例19:(1S,2S,5R)-3-(((R)-6-(2-氯-3-氟苯基)-5-(甲氧羰基)-2-(噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-3-氮杂二环[3.1.0]己烷-2-羧酸的制备。
将化合物6-(溴甲基)-4-(2-氯-3-氟苯基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯(70mg,0.16mmol)溶于CHCl3(5ml)中,加入化合物(1S,2S,5R)-3-氮杂二环[3.1.0]己烷-2-羧酸盐酸盐(53mg,0.32mmol),DIPEA(203mg,1.60mmol),50℃加热反应3h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法分离纯化(EA:MeOH=9:1)得到黄色固体(1S,2S,5R)-3-(((R)-6-(2-氯-3-氟苯基)-5-(甲氧羰基)-2-(噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-3-氮杂二环[3.1.0]己烷-2-羧酸(10mg,13%)。
MS(ESI,pos.ion)m/z:[M+H]+=491;
1H NMR(400MHz,CD3OD):δppm 7.96(m,1H),7.82(m,1H),7.34-7.11(m,3H),6.20(s,1H),4.59(m,1H),4.40(m,1H),3.98(m,1H),3.61(s,3H),2.33(m,1H),2.0(m,1H),1.81(m,1H),1.34(m,2H),0.93(m,2H).
实施例20:(R)-甲基-4-(2-氯-4-氟苯基)-6-(((S)-6-(羟甲基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻吡啶-2-基)-1,4-二氢嘧啶-5-羧酸甲酯的制备
将化合物4-(2-氯-4-氟苯基)-6-(溴甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-甲酸甲酯(330mg,0.74mmol)溶于CHCl3(15ml)中,加入化合物(S)-5-氮杂螺[2.4]庚烷-6-基甲醇盐酸盐(242mg,1.48mmol),三乙胺(603mg,5.92mmol),加热50℃搅拌反应3h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法分离纯化(EA)得到黄色固体(R)-甲基-4-(2-氯-4-氟苯基)-6-(((S)-6-(羟甲基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻吡啶-2-基)-1,4-二氢嘧啶-5-羧酸甲酯(45mg,12%)。
MS(ESI,pos.ion)m/z:491[M+H]+;
1H NMR(400MHz,CDCl3):δppm 7.85(m,1H),7.48(s,1H),7.31(m,1H),7.15(m,1H),6.94(m,1H),6.18(s,1H),4.48(m,1H),4.27(m,1H),3.84(m,3H),3.78(s,3H),3.34(m,1H),3.18(m,1H),2.92(m,1H)2.09(m,2H),0.88(m,4H).
实施例21:(R)-甲基-4-(2-溴-4-氟苯基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(4-甲基噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯的制备
将化合物4-(2-溴-4-氟苯基)-6-(溴甲基)-2-(4-甲基噻唑-2-基)-1,4-二氢嘧啶-5-甲酸甲酯(500mg,0.99mmol)溶于CHCl3(25ml)中,加入化合物(S)-N-(甲基磺酰基)-5-氮杂螺[2.4]庚烷-6-甲酰胺盐酸盐(427mg,1.68mmol),三乙胺(800mg,8mmol),加热45℃搅拌反应5h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法分离纯化(EA)得到黄色固体(R)-甲基-4-(2-溴-4-氟苯基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(4-甲基噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯(250mg,39%)。
MS(ESI,pos.ion)m/z:639,641[M+H]+;
1H NMR(400MHz,CDCl3):δppm 7.43(m,3H),7.08(m,1H),6.15(s,1H),4.35(m,1H),4.11(m,1H),3.81(m,1H),3.65(s,3H),3.53(m,1H),3.05(s,3H),2.71(s,3H),2.62(m,2H),2.45(m,1H),1.80(m,1H),0.73(m,4H).
实施例22:(S)-5-(((R)-6-(2-溴-4-氟苯基)-5-(甲氧羰基)-2-(4-甲基噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸的制备
将化合物4-(2-溴-4-氟苯基)-6-(溴甲基)-2-(4-甲基噻唑-2-基)-1,4-二氢嘧啶-5-甲酸甲酯(500mg,0.99mmol)溶于CHCl3(25ml)中,加入化合物(S)-5-氮杂螺[2.4]庚烷-6-羧酸盐酸盐(352mg,2mmol),三乙胺(800mg,8mmol),加热50℃搅拌反应5h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法分离纯化(EA/MeOH(v/v)=19/1)得到黄色固体(S)-5-(((R)-6-(2-溴-4-氟苯基)-5-(甲氧羰基)-2-(4-甲基噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸(120mg,21%)。
MS(ESI,pos.ion)m/z:562,564[M+H]+;
1H NMR(400MHz,CDCl3):δppm 7.77(s,1H),7.49(m,2H),7.21(m,1H),6.25(s,1H),3.74(m,2H),3.67(s,3H),3.61(m,2H),2.87(m,1H),2.69(s,3H),2.53(m,1H),2.02(m,2H),0.90-0.64(m,4H).
实施例23:(1S,2S,5R)-3-(((R)-6-(2-氯-4-氟苯基)-5-(甲氧基羰基)-2-(噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-3-氮杂二环[3.1.0]己烷-2-羧酸的制备
将化合物4-(2-氯-4-氟苯基)-6-(溴甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-甲酸甲酯(100mg,0.22mmol)溶于CHCl3(8ml)中,加入化合物(1S,2S,5R)-3-氮杂二环[3.1.0]己烷-2-羧酸盐酸盐(100mg,0.61mmol),三乙胺(178mg,1.76mmol),加热50℃搅拌反应3h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法分离纯化(EA/MeOH(v/v)=19/1)得到黄色固体(1S,2S,5R)-3-(((R)-6-(2-氯-4-氟苯基)-5-(甲氧基羰基)-2-(噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-3-氮杂二环[3.1.0]己烷-2-羧酸(10mg,9%)。
MS(ESI,pos.ion)m/z:492[M+H]+;
1H NMR(400MHz,CD3OD):δppm 8.17(m,1H),7.25(m,2H),7.12(m,2H),6.25(s,1H),4.63(m,1H),3.98(m,1H),3.61(s,3H),2.33(m,1H),2.0(m,1H),1.81(m,1H),1.34(m,2H),0.93(m,2H).
实施例24:(R)-甲基-4-(2-氯-4-氟苯基)-2-(1-甲基-1H-咪唑-2-基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-1,4-二氢嘧啶-5-羧酸甲酯的制备
将化合物(S)-N-(甲基磺酰基)-5-氮杂螺[2.4]庚烷-6-甲酰胺盐酸盐(36mg,0.12mmol),溶于CHCl3(5ml)中,加入4-(2-氯-4-氟苯基)-6-(溴甲基)-2-(4-甲基噻唑-2-基)-1,4-二氢嘧啶-5-甲酸甲酯(27mg,0.06mmol),三乙胺(49mg,0.48mmol),45℃加热反应5h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法分离纯化(EA:MeOH=9:1)得到黄色固体(R)-甲基-4-(2-氯-4-氟苯基)-2-(1-甲基-1H-咪唑-2-基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-1,4-二氢嘧啶-5-羧酸甲酯(8mg,23.5%)。
MS(ESI,pos.ion)m/z:580[M+H]+;
1H NMR(400MHz,CDCl3):δppm 7.38(m,1H),7.36(m,2H),7.33(m,2H),6.24(s,1H),4.35(m,1H),4.11(m,1H),3.81(m,1H),3.65(s,3H),3.53(m,1H),3.05(s,3H),2.71(s,3H),2.62(m,2H),2.45(m,1H),1.80(m,1H),0.83(m,4H).
实施例25:(R)-甲基-4-(2-溴-4-氟苯基)-2-(4-甲基噻唑-2-基)-6-(((S)-6-((苯基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚-5-基)甲基)-1,4-二氢嘧啶-5-羧酸甲酯的制备
将化合物(S)-N-(苯基磺酰基)-5-氮杂螺[2.4]庚烷-6-甲酰胺盐酸盐(204mg,0.64mmol),溶于CHCl3(10ml)中,加入4-(2溴-4-氟苯基)-6-(溴甲基)-2-(4-甲基噻唑-2-基)-1,4-二氢嘧啶-5-甲酸甲酯(200mg,0.40mmol),三乙胺(324mg,3.2mmol),50℃加热反应5h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法分离纯化(EA/MeOH(v/v)=19/1)得到黄色固体(R)-甲基-4-(2-溴-4-氟苯基)-2-(4-甲基噻唑-2-基)-6-(((S)-6-((苯基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚-5-基)甲基)-1,4-二氢嘧啶-5-羧酸甲酯(90mg,32%)。
MS(ESI,pos.ion)m/z:702,704[M+H]+;
1H NMR(400MHz,CDCl3):δppm 8.90(br,1H),7.77(m,2H),7.45(m,
1H),7.40(m,2H),7.35(s,1H),7.27(m,2H),6.82(m,1H),6.08(s,1H),4.29(m,1H),4.18(m,1H),3.78(m,1H),3.64(s,3H),3.44(m,1H),2.79(s,3H),2.50(m,1H),2.44(m,1H),2.03(m,2H),0.72(m,4H).
实施例26:(R)-甲基-4-(2-氯-4-氟苯基)-6-(((S)-6-(甲基氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯的制备
将化合物(S)-N-(甲基)-5-氮杂螺[2.4]庚烷-6-甲酰胺盐酸盐(380mg,1.99mmol)溶于CHCl3(15ml)中,加入4-(2-氯-4-氟苯基)-6-(溴甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-甲酸甲酯(360mg,0.81mmol),N,N-二异丙基乙胺(1.4ml),45℃加热反应1h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法分离纯化(EA)得到黄色固体(R)-甲基-4-(2-氯-4-氟苯基)-6-(((S)-6-(甲基氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯(125mg,30%)。
MS(ESI,pos.ion)m/z:518[M+H]+;
1H NMR(400MHz,CDCl3):δppm 9.13(br,1H),7.85(m,1H),7.54(m,2H),7.17(m,1H),6.97(m,1H),6.20(s,1H),4.36(m,1H),4.03(m,1H),3.60(s,3H),2.90-2.70(m,5H),2.45(m,2H),1.92(m,2H),0.64(m,4H).
实施例27:(R)-甲基4-(2-氯-3-氟苯基)-6-(((S)-6-(二甲基氨基甲基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻吡啶-2-基)-1,4-二氢嘧啶-5-羧酸甲酯的制备
将化合物(S)-N-(二甲基)-5-氮杂螺[2.4]庚烷-6-甲酰胺盐酸盐(130mg,0.77mmol),溶于CHCl3(6ml)中,加入4-(2-氯-3-氟苯基)-6-(溴甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-甲酸甲酯(115mg,0.26mmol),N,N-二异丙基乙胺(0.45ml),45℃加热反应1h。反应结束后,冷却,洗涤、浓缩反应液,残余物经薄层色谱法分离纯化(EA)得到黄色固体(R)-甲基4-(2-氯-3-氟苯基)-6-(((S)-6-(二甲基氨基甲基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻吡啶-2-基)-1,4-二氢嘧啶-5-羧酸甲酯(42mg,30%)。
MS(ESI,pos.ion)m/z:533[M+H]+;
1H NMR(400MHz,CDCl3):δppm 7.88(m,1H),7.40(m,1H),7.21(m,1H),7.03(m,1H),6.99(m,1H),6.26(s,1H),4.44(m,1H),3.96(m,1H),3.82(m,1H),3.62(s,3H),3.07(s,3H),3.01(s,3H),2.69(m,1H),2.25(m,1H),1.94(m,2H),0.62(m,4H).
实施例28:(S)-5-(((R)-6-(2-溴-4-氟苯基)-5-(甲氧基羰基)-2-(噻唑-2-基)-3,6-二氢嘧啶-4-基基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸的制备
将化合物(S)-5-氮杂螺[2.4]庚烷-6-羧酸盐酸盐(360mg,2.03mmol)溶于CHCl3(20ml)中,加入4-(2-溴-4-氟苯基)-6-(溴甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-甲酸甲酯(496mg,1.01mmol),三乙胺(816mg,8.08mmol),45℃加热反应8h。反应结束后,冷却,浓缩反应液,残余物经薄层色谱法分离纯化(EA/MeOH(v/v)=9/1)得到黄色固体(S)-5-(((R)-6-(2-溴-4-氟苯基)-5-(甲氧基羰基)-2-(噻唑-2-基)-3,6-二氢嘧啶-4-基基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸(150mg,22%)
MS(ESI,pos.ion)m/z:548,550[M+H]+;
1H NMR(400MHz,MeOD):δppm 7.96(m,1H),7.86(m,1H),7.55(m,1H),7.50(m,1H),7.20(m,1H),6.15(s,1H),4.59(m,1H),4.48(m,1H),3.73(m,1H),3.64(s,3H),3.46(m,1H),2.69(m,1H),2.14(m,2H),0.84(m,4H).
实施例29:(S)-((异丙氧基羰基)氧基)甲基5-((6-(2-氯-4-氟苯基)-5-(甲氧基羰基)-(噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸甲酯的制备
将化合物(S)-5-((6-(2-氯-4-氟苯基)-5-(甲氧羰基)-2-(噻唑-2-基)-1,4-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸(1.0g,1.9mmol)溶于乙腈(10mL)中,加入碳酸钾(1.3g,9.4mmol)和催化量的碘化纳,氮气置换3次,室温搅拌30分钟。向反应瓶中加入氯甲基异丙基碳酸酯(1.5g,9.8mmol)升温至55℃反应5个小时。反应结束后,冷却,过滤,浓缩反应液,残余物经柱层析分离纯化(正庚烷/乙酯(v/v)=4/1)得到黄色固体(S)-((异丙氧基羰基)氧基)甲基5-((6-(2-氯-4-氟苯基)-5-(甲氧基羰基)-(噻唑-2-
基)-3,6-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸甲酯(750mg,60.9%)。
MS(ESI,pos.ion)m/z:621[M+H]+;
1H NMR(400MHz,DMSO-d6):δppm 9.75(s,1H),8.15(m,1H),7.92(m,1H),7.41(m,2H),7.18(m,1H),6.01(s,1H),5.75(m,1H),5.67(m,1H),4.80(m,1H),4.30(m,1H),4.10(m,1H),3.96(m,1H),3.50(s,3H),2.95(m,1H),2.76(m,1H),2.30(m,1H),1.98(m,1H),1.22(m,6H),0.60(m,4H).
实施例30:(S)-(新戊酰氧基)甲基5-((6-(2-氯-4-氟苯基)-5-(甲氧基羰基)-(噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸甲酯的制备
将化合物(S)-5-((6-(2-氯-4-氟苯基)-5-(甲氧羰基)-2-(噻唑-2-基)-1,4-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸(0.2g,0.39mmol)溶于乙腈(2mL)中,加入碳酸钾(0.269g,1.95mmol)和催化量的碘化纳,氮气置换3次,室温搅拌30分钟。向反应瓶中加入氯甲基新戊酸酯(0.298g,1.98mmol)升温至55℃反应5个小时。反应结束后,冷却,过滤,浓缩反应液,残余物经柱层析分离纯化(正庚烷/乙酯(v/v)=4/1)得到黄色固体(S)-(新戊酰氧基)甲基5-((6-(2-氯-4-氟苯基)-5-(甲氧基羰基)-(噻唑-2-基)
-3,6-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸甲酯(180mg,73.4%)。
MS(ESI,pos.ion)m/z:619[M+H]+;
1H NMR(400MHz,DMSO-d6):δppm 9.84(s,1H),8.00(m,1H),7.94(m,1H),7.43(m,2H),7.20(m,1H),6.03(s,1H),5.82(m,1H),5.64(m,1H),,4.31(m,1H),4.11(m,1H),3.91(m,1H),3.61(s,3H),2.95(m,1H),2.75(m,1H),2.36(m,1H),1.98(m,1H),1.10(s,9H),0.62(m,4H).
药效学检测实验
用HBV HepG2.2.15细胞株进行体外抗HBV药效活性检测实验
1、实验方法:
qPCR检测细胞培养液病毒DNA含量并计算化合物对病毒抑制一半时的浓度(EC50),具体实验方法如下:
接种HepG2.2.15细胞到24孔细胞培养板(200,000细胞/孔),第二天加入含有不同浓度待测化合物的细胞培养液处理细胞(化合物最高浓度为5μM,5倍梯度稀释,6个稀释点)。第五天更换含待测药物的培养液,第八天收集培养上清,离心。
定量PCR:参照乙型肝炎病毒核酸定量测定试剂盒(PCR-荧光探针法)。在PCR反应管中加入核酸释放剂,再于各管中加入已稀释好的标准品模板(标准品模板最高浓度为4X107IU/mL,十倍稀释4个点,最低浓度为4X104IU/mL);加入样本模板;按照PCR体系配置反应混合液,加入到反应管中;盖上PCR反应管盖;按照设定程序运行定量PCR仪。
化合物对HBV复制抑制百分率计算:%Inh.=【1-加化合物处理HBV DNA总量/对照处理HBV DNA总量】X100。
计算化合物对HBV复制的EC50值:应用GraphPad Prism5分析软件,选用“四参数逻辑斯谛方程”计算出EC50值。
2、实验结果:见表1:
表1:化合物在HBV HepG2.2.15细胞株的抗HBV活性
| 实施例 | EC50(μmol) | 实施例 | EC50(μmol) |
| 1 | a | 11 | a |
| 2 | a | 12 | a |
| 4 | a | 17 | a |
| 5 | a | 19 | a |
| 6 | a | 22 | a |
| 7 | a | 23 | a |
| 10 | a | 28 | a |
活性区间:a(0.001<a≤0.20μmol)。
实验结果显示,本发明化合物有较强的抗HBV病毒的作用,因此适用于治疗因HBV病毒感染引起的各类疾病。
毒性检测实验
用HepG2细胞株进行体外毒性CC50检测实验
1、实验方法:
发光法细胞活力检测试剂盒检测HepG2细胞活率并计算化合物对HepG2细胞活率抑制一半时的浓度(CC50),具体实验方法如下:
接种HepG2细胞到96孔细胞培养板(4,000细胞/孔),第二天加入含有不同浓度待测化合物的细胞培养液处理细胞(化合物最高浓度为200μM,10倍梯度稀释,6个稀释点)。第五天用发光法细胞活力检测试剂盒检测细胞活率。
化合物对HepG2细胞活率抑制百分率计算:%Inh.=【1-加化合物处理HepG2细胞活率/对照处理HepG2细胞活率】X100。
计算化合物对HepG2细胞活率的CC50值:应用GraphPad Prism5分析软件,选用“四参数逻辑斯谛方程”计算出CC50值。
2、实验结果:见表2:
表2:化合物对HepG2细胞的CC50值
| 化合物 | CC50(μmol) |
| 对照化合物 | 82 |
| 实施例1 | 83 |
| 实施例2 | 120 |
| 实施例4 | 132 |
| 实施例7 | 90 |
| 实施例8 | 110 |
| 实施例10 | 115 |
| 实施例19 | 108 |
| 实施例22 | 70 |
| 实施例23 | 96 |
| 实施例28 | 79 |
备注:对照化合物的结构式如下式所示,其制备方法参见WO2014037480实例2。
毒性检测实验结果显示,本发明化合物毒性较小。与对照化合物(鼠LD50>600毫克)相比,有些化合物,例如,实施例4化合物(鼠LD50>1000毫克),显示出比对照化合物更小的毒性,有更好的安全性。
药代动力学检测实验
测试化合物在Beagle犬体内的药代动力学研究
1、实验方法1:
Beagle犬经前肢静脉注射2mg/kg的测试化合物。
给药后按时间点(0.083、0.25、0.5、1、2、4、8、12和24小时)经颈静脉采血,收集于加有EDTA-K2的抗凝管中。血样经过5500rpm离心10min后取得血浆。血浆样品经液液萃取后,在LC-MS/MS上,以多重反应离子监测(MRM)方式进行定量分析。采用WinNonlin6.3软件用非房室模型法计算药动力学参数。
2、数据:见表3:
表3:化合物在Beagle犬体内的药代动力学数据
药代动力学检测实验
测试化合物在Beagle犬体内的药代动力学研究
1、实验方法2:
Beagle犬经口服10mg/kg的测试化合物。
给药后按时间点(0.25、0.5、1、2、4、8、12和24小时)经颈静脉采血,收集于加有EDTA-K2的抗凝管中。血样经过5500rpm离心10min后取得血浆。血浆样品经液液萃取后,在LC-MS/MS上,以多重反应离子监测(MRM)方式进行定量分析。采用WinNonlin6.3软件用非房室模型法计算药动力学参数。
2、数据:见表4:
表4:化合物在Beagle犬体内的药代动力学数据
表3和表4结果显示:Beagle犬IV或PO给药后,与对照化合物相比,实施例4化合物大多数指标如药时曲线下面积(AUC0-t),药时曲线下面积(AUC0-inf),生物利用度(F%)清除率(CL)和稳态表现分部容积(Vss)均远远好于对照化合物的药时曲线下面积(AUC0-t),药时曲线下面积(AUC0-inf),生物利用度(F%)清除率(CL)和稳态表现分部容积(Vss)。
综上所述,本发明化合物有较强的抗HBV病毒的作用;且本发明化合物具有更有利的药代测试结果和良好的毒性检测结果,这将使其更有可能成为有效的和安全的药物。尤其是实施例4化合物具有中等清除率(4.99mL/kg/min)和
较好的生物利用度(48%),良好的毒性检测结果(CC50为132μmol),这预示着实施例4化合物在抗HBV病毒方面的应用前景非常好。
Claims (14)
- 式(I)所表示的化合物,或其可药用盐或互变异构体或对映异构体或非对映异构体:其中:R1为苯基,其中所述苯基任选进一步被一个或多个选自卤素、C1-6烷基的取代基所取代;R2选自氢或C1-4烷基;A为一个键、-O-、-S-或-N(R5)-;R5为氢或C1-4烷基;R3选自杂芳基,优选噻唑基、噁唑基、咪唑基、噻吩基、苯基或吡啶基,所述杂芳基可以进一步被一个或多个选自卤素、亚甲基(=CH2)、氧代(=O)、烷基、烷氧基、氰基、羟基、硝基、烷氨基、巯基、氨基、芳基、卤代烷基、烷基磺酰基、芳基烷基、杂芳基、杂芳基烷基、杂环基、杂环基烷基、环烷基、环烷基烷基、三氟甲基、三氟甲氧基、卤代烷基取代的芳基、卤素取代的芳基或三氟甲磺酰基的取代基所取代;R为以下所示的基团:R6各自独立地为-(CR7R7a)m-OH、-(CR7R7a)n-C(=O)O-R8、-(CR7R7a)n-C(=O)-NH-S(O)2-R9、-(CR7R7a)n-C(=O)-NR10R10a、-(CR7R7a)n-C(=O)O-(CR7R7a)n-OC(=O)O-R8、-S(=O)pOR8、-(CR7R7a)n-S(=O)pN(R8)2、-(CR7R7a)n-C(=O)O- (CR7R7a)n-OC(=O)-R8、-(CR7R7a)n-C(=O)O-(CR7R7a)n-C(=O)O-R8、-(CR7R7a)n-N(R8)2或-(CR7R7a)n-C(=O)N(R8)2;R7和R7a各自独立地为氢、卤素、烷基或卤代烷基,其中所述的烷基、卤代烷基任选进一步被一个或多个选自卤素、亚甲基(=CH2)、氧代(=O)、烷基、烷氧基、氰基、羟基、硝基、烷氨基、巯基、氨基、芳基、芳基烷基、杂芳基、杂芳基烷基、杂环基、杂环基烷基、环烷基、环烷基烷基、三氟甲基、三氟甲氧基、卤代烷基取代的芳基、卤素取代的芳基或三氟甲磺酰基的取代基所取代,或R7和R7a和与之相连的碳原子一起形成环烷基或杂环基;R8各自独立地为氢、羟基、烷基、烷氧基、烷基-S(=O)p-、芳基、杂芳基、环烷基、杂环基、芳基烷基、杂环基-S(=O)p-、杂芳基-S(=O)p-、环烷基-S(=O)p-或芳基-S(=O)p-,其中所述的烷基、烷氧基、烷基-S(=O)p-、芳基、杂芳基、环烷基、杂环基、芳基烷基、杂环基-S(=O)p-、杂芳基-S(=O)p-、环烷基-S(=O)p-或芳基-S(=O)p-任选进一步被一个或多个选自卤素、亚甲基(=CH2)、氧代(=O)、烷基、烷氧基、氰基、羟基、硝基、烷氨基、巯基、氨基、芳基、芳基烷基、杂芳基、杂芳基烷基、杂环基、杂环基烷基、环烷基、环烷基烷基、三氟甲基、三氟甲氧基、卤代烷基取代的芳基、卤素取代的芳基或三氟甲磺酰基的取代基所取代;R9为烷基、烷氧基、芳基、杂芳基、环烷基、杂环基或芳基烷基;R10和R10a各自独立地为氢、卤代烷基、环烷基、烷基或羟烷基,或R10和R10a和与之相连的氮原子一起形成杂环基;m各自独立地为1、2、3、4;n各自独立地为0、1、2、3、4;p各自独立地为1或2。
- 如权利要求1所述的化合物,其中,R1为苯基,其中所述苯基任选进一步被一个或多个选自卤素、C1-6烷基的取代基所取代;R2选自氢或C1-4烷基;A为一个键、-O-、-S-或-N(R5)-;R5为氢或C1-4烷基;R3选自杂芳基,优选噻唑基、噁唑基、咪唑基、噻吩基、苯基或吡啶基,所述杂芳基可以进一步被一个或多个选自卤素、亚甲基(=CH2)、氧代(=O)、烷基、烷氧基、氰基、羟基、硝基、烷氨基、巯基、氨基、芳基、卤代烷基、烷基磺酰基、芳基烷基、杂芳基、杂芳基烷基、杂环基、杂环基烷基、环烷基、环烷基烷基、三氟甲基、三氟甲氧基、卤代烷基取代的芳基、卤素取代的芳基或三氟甲磺酰基的取代基所取代;R为以下所示的基团:R6各自独立地为-(CR7R7a)m-OH、-(CR7R7a)n-C(=O)O-R8、-(CR7R7a)n-C(=O)-NH-S(O)2-R9、-(CR7R7a)n-C(=O)-NR10R10a、-(CR7R7a)n-C(=O)O-(CR7R7a)n-OC(=O)O-R8、-S(=O)pOR8、-(CR7R7a)n-S(=O)pN(R8)2、-(CR7R7a)n-C(=O)O-(CR7R7a)n-OC(=O)-R8、-(CR7R7a)n-C(=O)O-(CR7R7a)n-C(=O)O-R8、-(CR7R7a)n-N(R8)2或-(CR7R7a)n-C(=O)N(R8)2;R7和R7a各自独立地为氢、卤素、烷基或卤代烷基,其中所述的烷基、卤代烷基任选进一步被一个或多个选自卤素、亚甲基(=CH2)、氧代(=O)、烷基、烷氧基、氰基、羟基、硝基、烷氨基、巯基、氨基、芳基、芳基烷基、杂芳基、杂芳基烷基、杂环基、杂环基烷基、环烷基、环烷基烷基、三氟甲基、三氟甲氧基、卤代烷基取代的芳基、卤素取代的芳基或三氟甲磺酰基的取代基所取代,或R7和R7a和与之相连的碳原子一起形成环烷基或杂环基;R8各自独立地为氢、羟基、烷基、烷氧基、烷基-S(=O)p-、芳基、杂芳基、环烷基、杂环基、芳基烷基、杂环基-S(=O)p-、杂芳基-S(=O)p-、环烷基-S(=O)p-或芳基-S(=O)p-,其中所述的烷基、烷氧基、烷基-S(=O)p-、芳基、杂芳基、环烷基、杂环基、芳基烷基、杂环基-S(=O)p-、杂芳基-S(=O)p-、环烷基-S(=O)p-或 芳基-S(=O)p-任选进一步被一个或多个选自卤素、亚甲基(=CH2)、氧代(=O)、烷基、烷氧基、氰基、羟基、硝基、烷氨基、巯基、氨基、芳基、芳基烷基、杂芳基、杂芳基烷基、杂环基、杂环基烷基、环烷基、环烷基烷基、三氟甲基、三氟甲氧基、卤代烷基取代的芳基、卤素取代的芳基或三氟甲磺酰基的取代基所取代;R9为烷基、烷氧基、芳基、杂芳基、环烷基、杂环基或芳基烷基;R10和R10a各自独立地为氢、卤代烷基、环烷基、烷基或羟烷基,或R10和R10a和与之相连的氮原子一起形成杂环基;m各自独立地为1、2、3、4;n各自独立地为0、1、2、3、4;p各自独立地为1或2。
- 如权利要求1或2所述的化合物,其中,R2为甲基或乙基。
- 如权利要求1~3任一项所述的化合物,其中,A为-O-。
- 如权利要求1~4任一项所述的化合物,其中,R3为噻唑基、咪唑基或吡啶基,其中所述的噻唑基、咪唑基或吡啶基任选进一步被一个或多个选自卤素、烷基、烷氧基、卤代烷基、烷基磺酰基、环烷基的取代基所取代。
- 如权利要求1所述的化合物,其中:R1为2个卤素取代的苯基;R2为甲基或乙基;A为-O-;R3选自噻唑基、咪唑基或吡啶基,其中所述的噻唑基、咪唑基或吡啶基任选进一步被一个或多个选自卤素、亚甲基(=CH2)、氧代(=O)、烷基、烷氧基、氰基、羟基、硝基、烷氨基、巯基、氨基、芳基、卤代烷基、烷基磺酰基、芳基烷基、杂芳基、杂芳基烷基、杂环基、杂环基烷基、环烷基、环烷基烷基、三氟甲基、三氟甲氧基、卤代烷基取代的芳基、卤素取代的芳基或三氟甲磺酰基的取代基所取代;R为以下所示的基团:R6各自独立地为-(CR7R7a)n-C(=O)O-R8、-(CR7R7a)n-C(=O)O-(CR7R7a)n-OC(=O)-R8或-(CR7R7a)n-C(=O)O-(CR7R7a)n-C(=O)O-R8;R7和R7a各自独立地为氢、卤素、烷基或卤代烷基,其中所述的烷基、卤代烷基任选进一步被一个或多个选自卤素、亚甲基(=CH2)、氧代(=O)、烷基、烷氧基、氰基、羟基、硝基、烷氨基、巯基、氨基、芳基、芳基烷基、杂芳基、杂芳基烷基、杂环基、杂环基烷基、环烷基、环烷基烷基、三氟甲基、三氟甲氧基、卤代烷基取代的芳基、卤素取代的芳基或三氟甲磺酰基的取代基所取代,或R7和R7a和与之相连的碳原子一起形成环烷基或杂环基;R8各自独立地为氢、羟基、烷基、烷氧基、烷基-S(=O)p-、芳基、杂芳基、环烷基、杂环基、芳基烷基、杂环基-S(=O)p-、杂芳基-S(=O)p-、环烷基-S(=O)p-或芳基-S(=O)p-,其中所述的烷基、烷氧基、烷基-S(=O)p-、芳基、杂芳基、环烷基、杂环基、芳基烷基、杂环基-S(=O)p-、杂芳基-S(=O)p-、环烷基-S(=O)p-或芳基-S(=O)p-任选进一步被一个或多个选自卤素、亚甲基(=CH2)、氧代(=O)、烷基、烷氧基、氰基、羟基、硝基、烷氨基、巯基、氨基、芳基、芳基烷基、杂芳基、杂芳基烷基、杂环基、杂环基烷基、环烷基、环烷基烷基、三氟甲基、三氟甲氧基、卤代烷基取代的芳基、卤素取代的芳基或三氟甲磺酰基的取代基所取代;n各自独立地为0、1、2、3、4,优选为0;p各自独立地为1或2。
- 如权利要求1所述的化合物,其中所述的化合物式(I)选自:(1)4-(2-氯-4-氟苯基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯;(2)(S)-5-((6-(2-氯-4-氟苯基)-5-(乙氧羰基)-2-(噻唑-2-基)-1,4-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸;(3)4-(2-氯-4-氟苯基)-6-(((S)-6-(吗啉-4-羰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯;(4)(S)-5-(((R)-6-(2-氯-4-氟苯基)-5-(甲氧羰基)-2-(噻唑-2-基)-1,4-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸;(5)4-(2-氯-4-氟苯基)-2-(3,5-氟吡啶-2-基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-1,4-二氢嘧啶-5-羧酸甲酯;(6)4-(2-氯-4-氟苯基)-2-(3,5-氟吡啶-2-基)-6-((S)-5-氮杂螺[2.4]庚烷-6-羧酸)-1,4-二氢嘧啶-5-羧酸甲酯;(7)4-(2-氯-4-氟苯基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸乙酯;(8)4-(2-氯-4-氟苯基)-6-(((S)-6-((环丙基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸乙酯;(9)4-(2-氯-4-氟苯基)-6-(((S)-6-(异丙基氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯;(10)(S)-5-(((R)-6-(2-氯-4-氟苯基)-5-(甲氧羰基)-2-(4-甲基噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸;(11)(S)-5-(((R)-6-(2-氯-3-氟苯基)-5-(甲氧羰基)-2-(噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸;(12)(R)-甲基4-(2-氯-3-氟苯基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯;(13)(1R,3S,5R)-2-(((R)-6-(2-氯-3-氟苯基)-5-(甲氧羰基)-2-(噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-2-氮杂双环[3.1.0]己烷-3-羧酸;(14)(1R,3S,5R)-2-(((R)-6-(2-氯-4-氟苯基)-5-(甲氧羰基)-2-(4-甲基噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-2-氮杂双环[3.1.0]己烷-3-羧酸;(15)(R)-甲基-4-(2-氯-4-氟苯基)-6-(((S)-6-(异丙基氨基甲酰 基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(4-甲基噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯;(16)(R)-甲基-4-(2-氯-4-氟苯基)-6-(((1R,3S,5R)-3-((甲基磺酰基)氨基甲酰基)-2-氮杂双环[3.1.0]己烷-2-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯;(17)(S)-5-(((R)-6-(2-氯-4-氟苯基)-5-(甲氧基羰基)-2-(1-甲基-1H-咪唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸;(18)(R)-甲基4-(2-氯-3-氟苯基)-6-(((S)-6-((环丙基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯;(19)(1S,2S,5R)-3-(((R)-6-(2-氯-3-氟苯基)-5-(甲氧羰基)-2-(噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-3-氮杂二环[3.1.0]己烷-2-羧酸;(20)(R)-甲基-4-(2-氯-4-氟苯基)-6-(((S)-6-(羟甲基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻吡啶-2-基)-1,4-二氢嘧啶-5-羧酸甲酯;(21)(R)-甲基-4-(2-溴-4-氟苯基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(4-甲基噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯;(22)(S)-5-(((R)-6-(2-溴-4-氟苯基)-5-(甲氧基羰基)-2-(4-甲基噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸;(23)(1S,2S,5R)-3-(((R)-6-(2-氯-4-氟苯基)-5-(甲氧基羰基)-2-(噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-3-氮杂二环[3.1.0]己烷-2-羧酸;(24)(R)-甲基-4-(2-氯-4-氟苯基)-2-(1-甲基-1H-咪唑-2-基)-6-(((S)-6-((甲基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-1,4-二氢嘧啶-5-羧酸甲酯;(25)(R)-甲基-4-(2-溴-4-氟苯基)-2-(4-甲基噻唑-2-基)-6-(((S)-6-((苯基磺酰基)氨基甲酰基)-5-氮杂螺[2.4]庚-5-基)甲基)-1,4-二氢嘧啶-5-羧酸甲酯;(26)(R)-甲基-4-(2-氯-4-氟苯基)-6-(((S)-6-(甲基氨基甲酰基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻唑-2-基)-1,4-二氢嘧啶-5-羧酸甲酯;(27)(R)-甲基4-(2-氯-3-氟苯基)-6-(((S)-6-(二甲基氨基甲基)-5-氮杂螺[2.4]庚烷-5-基)甲基)-2-(噻吡啶-2-基)-1,4-二氢嘧啶-5-羧酸甲酯;(28)(S)-5-(((R)-6-(2-溴-4-氟苯基)-5-(甲氧基羰基)-2-(噻唑-2-基)-3,6-二氢嘧啶-4-基基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸;(29)(S)-((异丙氧基羰基)氧基)甲基5-((6-(2-氯-4-氟苯基)-5-(甲氧基羰基)-(噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸甲酯;(30)(S)-(新戊酰氧基)甲基5-((6-(2-氯-4-氟苯基)-5-(甲氧基羰基)-(噻唑-2-基)-3,6-二氢嘧啶-4-基)甲基)-5-氮杂螺[2.4]庚烷-6-羧酸甲酯。
- 药物组合物,其中包括有效剂量的权利要求1~8中任一项的式(I)化合物或其可药用盐或互变异构体或对映异构体或非对映异构体。
- 如权利要求1~8中任一项所述的化合物或如权利要求9所述的药物组合物在制备用于治疗或者预防乙型肝炎病毒感染的疾病的药物中的应用。
- 如权利要求11所述的制备方法,其中所述碱为有机碱,优选为三乙胺。
- 如权利要求11所述的制备方法,其中,步骤(2)所述溴化试剂为N-溴丁二酰亚胺。
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201780053025.XA CN109803967B (zh) | 2016-09-09 | 2017-09-01 | 二氢嘧啶类化合物及其制备方法和用途 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201610815367 | 2016-09-09 | ||
| CN201610815367.8 | 2016-09-09 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2018045911A1 true WO2018045911A1 (zh) | 2018-03-15 |
Family
ID=61562685
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/CN2017/100103 Ceased WO2018045911A1 (zh) | 2016-09-09 | 2017-09-01 | 二氢嘧啶类化合物及其制备方法和用途 |
Country Status (3)
| Country | Link |
|---|---|
| CN (1) | CN109803967B (zh) |
| TW (1) | TWI670266B (zh) |
| WO (1) | WO2018045911A1 (zh) |
Cited By (38)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2019165374A1 (en) | 2018-02-26 | 2019-08-29 | Gilead Sciences, Inc. | Substituted pyrrolizine compounds as hbv replication inhibitors |
| WO2019193542A1 (en) | 2018-04-06 | 2019-10-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'3'-cyclic dinucleotides |
| WO2019193543A1 (en) | 2018-04-06 | 2019-10-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3'3'-cyclic dinucleotides |
| WO2019195181A1 (en) | 2018-04-05 | 2019-10-10 | Gilead Sciences, Inc. | Antibodies and fragments thereof that bind hepatitis b virus protein x |
| WO2019193533A1 (en) | 2018-04-06 | 2019-10-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'2'-cyclic dinucleotides |
| WO2019200247A1 (en) | 2018-04-12 | 2019-10-17 | Precision Biosciences, Inc. | Optimized engineered meganucleases having specificity for a recognition sequence in the hepatitis b virus genome |
| WO2019211799A1 (en) | 2018-05-03 | 2019-11-07 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'3'-cyclic dinucleotide analogue comprising a cyclopentanyl modified nucleotide |
| WO2020028097A1 (en) | 2018-08-01 | 2020-02-06 | Gilead Sciences, Inc. | Solid forms of (r)-11-(methoxymethyl)-12-(3-methoxypropoxy)-3,3-dimethyl-8-0x0-2,3,8,13b-tetrahydro-1h-pyrido[2,1-a]pyrrolo[1,2-c] phthalazine-7-c arboxylic acid |
| WO2020092621A1 (en) | 2018-10-31 | 2020-05-07 | Gilead Sciences, Inc. | Substituted 6-azabenzimidazole compounds as hpk1 inhibitors |
| WO2020092528A1 (en) | 2018-10-31 | 2020-05-07 | Gilead Sciences, Inc. | Substituted 6-azabenzimidazole compounds having hpk1 inhibitory activity |
| US10662416B2 (en) | 2016-10-14 | 2020-05-26 | Precision Biosciences, Inc. | Engineered meganucleases specific for recognition sequences in the hepatitis B virus genome |
| WO2020178769A1 (en) | 2019-03-07 | 2020-09-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'3'-cyclic dinucleotides and prodrugs thereof |
| WO2020178768A1 (en) | 2019-03-07 | 2020-09-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3'3'-cyclic dinucleotide analogue comprising a cyclopentanyl modified nucleotide as sting modulator |
| WO2020178770A1 (en) | 2019-03-07 | 2020-09-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3'3'-cyclic dinucleotides and prodrugs thereof |
| WO2020214652A1 (en) | 2019-04-17 | 2020-10-22 | Gilead Sciences, Inc. | Solid forms of a toll-like receptor modulator |
| WO2020214663A1 (en) | 2019-04-17 | 2020-10-22 | Gilead Sciences, Inc. | Solid forms of a toll-like receptor modulator |
| WO2020237025A1 (en) | 2019-05-23 | 2020-11-26 | Gilead Sciences, Inc. | Substituted exo-methylene-oxindoles which are hpk1/map4k1 inhibitors |
| WO2020263830A1 (en) | 2019-06-25 | 2020-12-30 | Gilead Sciences, Inc. | Flt3l-fc fusion proteins and methods of use |
| WO2021034804A1 (en) | 2019-08-19 | 2021-02-25 | Gilead Sciences, Inc. | Pharmaceutical formulations of tenofovir alafenamide |
| US10966999B2 (en) | 2017-12-20 | 2021-04-06 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3′3′ cyclic dinucleotides with phosphonate bond activating the sting adaptor protein |
| WO2021067181A1 (en) | 2019-09-30 | 2021-04-08 | Gilead Sciences, Inc. | Hbv vaccines and methods treating hbv |
| WO2021113765A1 (en) | 2019-12-06 | 2021-06-10 | Precision Biosciences, Inc. | Optimized engineered meganucleases having specificity for a recognition sequence in the hepatitis b virus genome |
| WO2021188959A1 (en) | 2020-03-20 | 2021-09-23 | Gilead Sciences, Inc. | Prodrugs of 4'-c-substituted-2-halo-2'-deoxyadenosine nucleosides and methods of making and using the same |
| US11142527B2 (en) | 2017-06-26 | 2021-10-12 | Sunshine Lake Pharma Co., Ltd. | Dihydropyrimidine compounds and uses thereof in medicine |
| US11203610B2 (en) | 2017-12-20 | 2021-12-21 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2′3′ cyclic dinucleotides with phosphonate bond activating the sting adaptor protein |
| WO2022031894A1 (en) | 2020-08-07 | 2022-02-10 | Gilead Sciences, Inc. | Prodrugs of phosphonamide nucleotide analogues and their pharmaceutical use |
| US11261190B2 (en) | 2017-10-18 | 2022-03-01 | Sunshine Lake Pharma Co., Ltd. | Dihydropyrimidine compounds and uses thereof in medicine |
| WO2022087149A2 (en) | 2020-10-22 | 2022-04-28 | Gilead Sciences, Inc. | Interleukin-2-fc fusion proteins and methods of use |
| WO2022241134A1 (en) | 2021-05-13 | 2022-11-17 | Gilead Sciences, Inc. | COMBINATION OF A TLR8 MODULATING COMPOUND AND ANTI-HBV siRNA THERAPEUTICS |
| WO2022271659A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2022271677A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2022271650A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2022271684A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| US11639350B2 (en) | 2017-06-27 | 2023-05-02 | Janssen Pharmaceutica Nv | Heteroaryldihydropyrimidine derivatives and methods of treating hepatitis B infections |
| WO2025240242A1 (en) | 2024-05-13 | 2025-11-20 | Gilead Sciences, Inc. | Combination therapies with ribavirin |
| WO2025240244A1 (en) | 2024-05-13 | 2025-11-20 | Gilead Sciences, Inc. | Combination therapies comprising bulevirtide and lonafarnib for use in the treatment of hepatitis d virus infection |
| WO2025240243A1 (en) | 2024-05-13 | 2025-11-20 | Gilead Sciences, Inc. | Combination therapies with bulevirtide and an inhibitory nucleic acid targeting hepatitis b virus |
| WO2025240246A1 (en) | 2024-05-13 | 2025-11-20 | Gilead Sciences, Inc. | Combination therapies with ribavirin |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2001068641A1 (de) * | 2000-03-17 | 2001-09-20 | Bayer Aktiengesellschaft | 6-aminoalkyl-dihydroppyrimidine und ihre verwendung als arzneimittel gegen virale erkrankungen |
| CN105209470A (zh) * | 2013-05-17 | 2015-12-30 | 豪夫迈·罗氏有限公司 | 用于治疗和预防乙型肝炎病毒感染的新的6-桥连的杂芳基二氢嘧啶 |
| WO2017011552A1 (en) * | 2015-07-13 | 2017-01-19 | Enanta Pharmaceuticals, Inc. | Hepatitis b antiviral agents |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| MX2015002511A (es) * | 2012-08-24 | 2016-03-08 | Sunshine Lake Pharma Co Ltd | Compuestos de dihidropirimidina y su aplicacion en productos farmaceuticos. |
| CN103664925B (zh) * | 2012-09-07 | 2018-01-23 | 广东东阳光药业有限公司 | 杂芳基取代的二氢嘧啶类化合物及其在药物中的应用 |
| CN103664899B (zh) * | 2012-09-11 | 2017-06-16 | 广东东阳光药业有限公司 | 杂芳基取代的二氢嘧啶类化合物及其在药物中的应用 |
| CN104650069B (zh) * | 2013-11-19 | 2019-04-19 | 广东东阳光药业有限公司 | 4-甲基二氢嘧啶类化合物及其在药物中的应用 |
-
2017
- 2017-09-01 TW TW106130048A patent/TWI670266B/zh not_active IP Right Cessation
- 2017-09-01 WO PCT/CN2017/100103 patent/WO2018045911A1/zh not_active Ceased
- 2017-09-01 CN CN201780053025.XA patent/CN109803967B/zh active Active
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2001068641A1 (de) * | 2000-03-17 | 2001-09-20 | Bayer Aktiengesellschaft | 6-aminoalkyl-dihydroppyrimidine und ihre verwendung als arzneimittel gegen virale erkrankungen |
| CN105209470A (zh) * | 2013-05-17 | 2015-12-30 | 豪夫迈·罗氏有限公司 | 用于治疗和预防乙型肝炎病毒感染的新的6-桥连的杂芳基二氢嘧啶 |
| WO2017011552A1 (en) * | 2015-07-13 | 2017-01-19 | Enanta Pharmaceuticals, Inc. | Hepatitis b antiviral agents |
Cited By (52)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10662416B2 (en) | 2016-10-14 | 2020-05-26 | Precision Biosciences, Inc. | Engineered meganucleases specific for recognition sequences in the hepatitis B virus genome |
| US11274285B2 (en) | 2016-10-14 | 2022-03-15 | Precision Biosciences, Inc. | Engineered meganucleases specific for recognition sequences in the Hepatitis B virus genome |
| US11142527B2 (en) | 2017-06-26 | 2021-10-12 | Sunshine Lake Pharma Co., Ltd. | Dihydropyrimidine compounds and uses thereof in medicine |
| US11639350B2 (en) | 2017-06-27 | 2023-05-02 | Janssen Pharmaceutica Nv | Heteroaryldihydropyrimidine derivatives and methods of treating hepatitis B infections |
| US11261190B2 (en) | 2017-10-18 | 2022-03-01 | Sunshine Lake Pharma Co., Ltd. | Dihydropyrimidine compounds and uses thereof in medicine |
| US11203610B2 (en) | 2017-12-20 | 2021-12-21 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2′3′ cyclic dinucleotides with phosphonate bond activating the sting adaptor protein |
| US10966999B2 (en) | 2017-12-20 | 2021-04-06 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3′3′ cyclic dinucleotides with phosphonate bond activating the sting adaptor protein |
| WO2019165374A1 (en) | 2018-02-26 | 2019-08-29 | Gilead Sciences, Inc. | Substituted pyrrolizine compounds as hbv replication inhibitors |
| WO2019195181A1 (en) | 2018-04-05 | 2019-10-10 | Gilead Sciences, Inc. | Antibodies and fragments thereof that bind hepatitis b virus protein x |
| WO2019193533A1 (en) | 2018-04-06 | 2019-10-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'2'-cyclic dinucleotides |
| US11292812B2 (en) | 2018-04-06 | 2022-04-05 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3′3′-cyclic dinucleotides |
| WO2019193543A1 (en) | 2018-04-06 | 2019-10-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3'3'-cyclic dinucleotides |
| US11149052B2 (en) | 2018-04-06 | 2021-10-19 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2′3′-cyclic dinucleotides |
| WO2019193542A1 (en) | 2018-04-06 | 2019-10-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'3'-cyclic dinucleotides |
| US11788077B2 (en) | 2018-04-12 | 2023-10-17 | Precision Biosciences, Inc. | Polynucleotides encoding optimized engineered meganucleases having specificity for a recognition sequence in the Hepatitis B virus genome |
| WO2019200247A1 (en) | 2018-04-12 | 2019-10-17 | Precision Biosciences, Inc. | Optimized engineered meganucleases having specificity for a recognition sequence in the hepatitis b virus genome |
| US11142750B2 (en) | 2018-04-12 | 2021-10-12 | Precision Biosciences, Inc. | Optimized engineered meganucleases having specificity for a recognition sequence in the Hepatitis B virus genome |
| WO2019211799A1 (en) | 2018-05-03 | 2019-11-07 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'3'-cyclic dinucleotide analogue comprising a cyclopentanyl modified nucleotide |
| WO2020028097A1 (en) | 2018-08-01 | 2020-02-06 | Gilead Sciences, Inc. | Solid forms of (r)-11-(methoxymethyl)-12-(3-methoxypropoxy)-3,3-dimethyl-8-0x0-2,3,8,13b-tetrahydro-1h-pyrido[2,1-a]pyrrolo[1,2-c] phthalazine-7-c arboxylic acid |
| EP4371987A1 (en) | 2018-10-31 | 2024-05-22 | Gilead Sciences, Inc. | Substituted 6-azabenzimidazole compounds as hpk1 inhibitors |
| WO2020092528A1 (en) | 2018-10-31 | 2020-05-07 | Gilead Sciences, Inc. | Substituted 6-azabenzimidazole compounds having hpk1 inhibitory activity |
| WO2020092621A1 (en) | 2018-10-31 | 2020-05-07 | Gilead Sciences, Inc. | Substituted 6-azabenzimidazole compounds as hpk1 inhibitors |
| WO2020178770A1 (en) | 2019-03-07 | 2020-09-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3'3'-cyclic dinucleotides and prodrugs thereof |
| US11766447B2 (en) | 2019-03-07 | 2023-09-26 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3′3′-cyclic dinucleotide analogue comprising a cyclopentanyl modified nucleotide as sting modulator |
| US12318403B2 (en) | 2019-03-07 | 2025-06-03 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2′3′-cyclic dinucleotides and prodrugs thereof |
| WO2020178768A1 (en) | 2019-03-07 | 2020-09-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3'3'-cyclic dinucleotide analogue comprising a cyclopentanyl modified nucleotide as sting modulator |
| WO2020178769A1 (en) | 2019-03-07 | 2020-09-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'3'-cyclic dinucleotides and prodrugs thereof |
| WO2020214663A1 (en) | 2019-04-17 | 2020-10-22 | Gilead Sciences, Inc. | Solid forms of a toll-like receptor modulator |
| WO2020214652A1 (en) | 2019-04-17 | 2020-10-22 | Gilead Sciences, Inc. | Solid forms of a toll-like receptor modulator |
| EP4458416A2 (en) | 2019-04-17 | 2024-11-06 | Gilead Sciences, Inc. | Solid forms of a toll-like receptor modulator |
| WO2020237025A1 (en) | 2019-05-23 | 2020-11-26 | Gilead Sciences, Inc. | Substituted exo-methylene-oxindoles which are hpk1/map4k1 inhibitors |
| WO2020263830A1 (en) | 2019-06-25 | 2020-12-30 | Gilead Sciences, Inc. | Flt3l-fc fusion proteins and methods of use |
| WO2021034804A1 (en) | 2019-08-19 | 2021-02-25 | Gilead Sciences, Inc. | Pharmaceutical formulations of tenofovir alafenamide |
| EP4458975A2 (en) | 2019-09-30 | 2024-11-06 | Gilead Sciences, Inc. | Hbv vaccines and methods treating hbv |
| WO2021067181A1 (en) | 2019-09-30 | 2021-04-08 | Gilead Sciences, Inc. | Hbv vaccines and methods treating hbv |
| EP4567109A2 (en) | 2019-12-06 | 2025-06-11 | Precision Biosciences, Inc. | Optimized engineered meganucleases having specificity for a recognition sequence in the hepatitis b virus genome |
| US12410418B2 (en) | 2019-12-06 | 2025-09-09 | Precision Biosciences, Inc. | Optimized engineered meganucleases having specificity for a recognition sequence in the Hepatitis B virus genome |
| WO2021113765A1 (en) | 2019-12-06 | 2021-06-10 | Precision Biosciences, Inc. | Optimized engineered meganucleases having specificity for a recognition sequence in the hepatitis b virus genome |
| WO2021188959A1 (en) | 2020-03-20 | 2021-09-23 | Gilead Sciences, Inc. | Prodrugs of 4'-c-substituted-2-halo-2'-deoxyadenosine nucleosides and methods of making and using the same |
| EP4667477A2 (en) | 2020-08-07 | 2025-12-24 | Gilead Sciences, Inc. | Prodrugs of phosphonamide nucleotide analogues and their pharmaceutical use |
| WO2022031894A1 (en) | 2020-08-07 | 2022-02-10 | Gilead Sciences, Inc. | Prodrugs of phosphonamide nucleotide analogues and their pharmaceutical use |
| EP4667499A2 (en) | 2020-10-22 | 2025-12-24 | Gilead Sciences, Inc. | Interleukin-2-fc fusion proteins and methods of use |
| WO2022087149A2 (en) | 2020-10-22 | 2022-04-28 | Gilead Sciences, Inc. | Interleukin-2-fc fusion proteins and methods of use |
| WO2022241134A1 (en) | 2021-05-13 | 2022-11-17 | Gilead Sciences, Inc. | COMBINATION OF A TLR8 MODULATING COMPOUND AND ANTI-HBV siRNA THERAPEUTICS |
| WO2022271684A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2022271659A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2022271677A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2022271650A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2025240242A1 (en) | 2024-05-13 | 2025-11-20 | Gilead Sciences, Inc. | Combination therapies with ribavirin |
| WO2025240244A1 (en) | 2024-05-13 | 2025-11-20 | Gilead Sciences, Inc. | Combination therapies comprising bulevirtide and lonafarnib for use in the treatment of hepatitis d virus infection |
| WO2025240243A1 (en) | 2024-05-13 | 2025-11-20 | Gilead Sciences, Inc. | Combination therapies with bulevirtide and an inhibitory nucleic acid targeting hepatitis b virus |
| WO2025240246A1 (en) | 2024-05-13 | 2025-11-20 | Gilead Sciences, Inc. | Combination therapies with ribavirin |
Also Published As
| Publication number | Publication date |
|---|---|
| TW201811770A (zh) | 2018-04-01 |
| TWI670266B (zh) | 2019-09-01 |
| CN109803967A (zh) | 2019-05-24 |
| CN109803967B (zh) | 2022-05-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN109803967B (zh) | 二氢嘧啶类化合物及其制备方法和用途 | |
| US10220030B2 (en) | Amino-quinolines as kinase inhibitors | |
| CN109400625B (zh) | 稠合双环类化合物及其在药物中的应用 | |
| CN110511219B (zh) | 苯基取代的二氢萘啶类化合物及其用途 | |
| TWI649325B (zh) | 用於治療囊狀纖維化之新穎化合物及其醫藥組合物 | |
| CA2718528C (en) | Substituted 4-hydroxypyrimidine-5-carboxamides | |
| JP6453845B2 (ja) | Brd4阻害薬としてのジヒドロキナゾリノン類似体 | |
| CN110475775B (zh) | 4-吡啶酮化合物或其盐、包含4-吡啶酮化合物的药物组合物及剂 | |
| CN103664897B (zh) | 二氢嘧啶类化合物及其在药物中的应用 | |
| CN110582500B (zh) | 双并环类核衣壳抑制剂和其作为药物用于治疗乙型肝炎的用途 | |
| ES2426482T3 (es) | Inhibidor de IGF-1R | |
| TWI833046B (zh) | 吡咯醯胺類化合物及其用途 | |
| WO2018121689A1 (zh) | 磺酰胺-芳基酰胺类化合物及其治疗乙型肝炎的药物用途 | |
| US20240228489A1 (en) | Positive allosteric modulators of the muscarinic acetylcholine receptor m1 | |
| CN112752757B (zh) | 作为rho-激酶抑制剂的酪氨酸酰胺衍生物 | |
| WO2004009556A1 (ja) | 4−置換アリール−5−ヒドロキシイソキノリノン誘導体 | |
| CN116940562B (zh) | 乙酰胺基-苯基苯甲酰胺衍生物及其使用方法 | |
| KR20250116664A (ko) | Tyk2의 저해제 | |
| CN103443107B (zh) | 噻唑并嘧啶化合物 | |
| KR20230141787A (ko) | 피라졸아미드 유도체 | |
| JP7618898B1 (ja) | ヒト呼吸器合胞体ウイルス及びメタニューモウイルスの阻害薬 | |
| WO2024245415A1 (zh) | Polθ抑制剂及其制备方法和用途 | |
| WO2025247379A1 (zh) | 一种苯并咪唑类化合物、其制备方法及应用 | |
| CN115667265A (zh) | Cgas抑制三唑并嘧啶酮衍生物 | |
| HK40010596A (zh) | 4-吡啶酮化合物或其盐、包含4-吡啶酮化合物的药物组合物及剂 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17848084 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 17848084 Country of ref document: EP Kind code of ref document: A1 |