WO2017154998A1 - 生体吸収性のシート又はフィルム - Google Patents
生体吸収性のシート又はフィルム Download PDFInfo
- Publication number
- WO2017154998A1 WO2017154998A1 PCT/JP2017/009320 JP2017009320W WO2017154998A1 WO 2017154998 A1 WO2017154998 A1 WO 2017154998A1 JP 2017009320 W JP2017009320 W JP 2017009320W WO 2017154998 A1 WO2017154998 A1 WO 2017154998A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- sheet
- film
- film according
- phosphorylated pullulan
- pullulan
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/70—Web, sheet or filament bases ; Films; Fibres of the matrix type containing drug
- A61K9/7007—Drug-containing films, membranes or sheets
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/36—Polysaccharides; Derivatives thereof, e.g. gums, starch, alginate, dextrin, hyaluronic acid, chitosan, inulin, agar or pectin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0014—Skin, i.e. galenical aspects of topical compositions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
- A61K9/0024—Solid, semi-solid or solidifying implants, which are implanted or injected in body tissue
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/22—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons containing macromolecular materials
- A61L15/28—Polysaccharides or their derivatives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/42—Use of materials characterised by their function or physical properties
- A61L15/64—Use of materials characterised by their function or physical properties specially adapted to be resorbable inside the body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P41/00—Drugs used in surgical methods, e.g. surgery adjuvants for preventing adhesion or for vitreum substitution
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/60—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a special physical form
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2400/00—Materials characterised by their function or physical properties
- A61L2400/04—Materials for stopping bleeding
Definitions
- the present invention relates to a functional sheet or film for biological sticking that is used by sticking to an organ or tissue in the living body.
- Patent Document 1 includes a first coating layer formed of a biodegradable polymer, and a second coating layer formed of one or more selected from metals, metal oxides, silicon, and silicon oxide.
- An adhesion preventing film is disclosed in which the laminated film is supported by a carrier sheet having air permeability on the second film layer side.
- the laminated film is formed on the second film layer by physical vapor deposition or chemical vapor deposition. It is superimposed on the carrier sheet.
- the laminated film is peeled off from the carrier sheet and pasted so that the surface of the first coating layer is in contact with the surface of the body tissue.
- Patent Document 2 discloses, as a wound healing film for in vivo tissue, ascorbic acid derivatives and specific drugs having a wound healing effect, such as gelatin, starch (starch), sodium alginate, agar, cellulose, polyglutamic acid, A film supported on a sheet made of one or more biodegradable polymers selected from chitin chitosan and konjac is disclosed. Specifically, as a method for forming a film, it is described that a composition obtained by blending an ascorbic acid derivative or a drug with a biodegradable polymer may be prepared and then molded according to a known molding method.
- Patent Document 3 a gelatin film cross-linked by heat treatment under vacuum for a certain period of time retains its form for a certain period of time while being decomposed and absorbed in the living body. Have reported that.
- the problem of the present invention is that it has excellent strength and adhesiveness even under wet conditions, and is excellent in bioabsorbability. Furthermore, when it contains a bioactive agent, it has excellent bioresorbability.
- Another object of the present invention is to provide a bioabsorbable sheet or film having controlled solubility or controlled release of the drug when it contains a bioactive drug. .
- a film obtained using a composition containing phosphorylated pullulan as a main component exhibits high strength and adhesiveness even under wet conditions, and It is also excellent in absorbability, and when it contains a bioactive agent, it has been found that it has excellent release properties, and by using a chemically modified phosphorylated pullulan, solubility, sustained release, etc. can be improved.
- the present inventors have found that it can be controlled and have completed the present invention.
- the present invention relates to a bioabsorbable sheet or film comprising a composition containing phosphorylated pullulan or chemically modified phosphorylated pullulan.
- the bioabsorbable sheet or film of the present invention is excellent in strength and adhesiveness even under wet conditions, is excellent in bioabsorbability, and is excellent in the release of the drug when it contains a bioactive drug. There is an excellent effect. Moreover, the outstanding effect that the reproduction
- the solubility of the phosphorylated pullulan can be controlled by using chemically modified phosphorylated pullulan or by providing another layer, so that the drug can be gradually dissolved. Release can also be controlled.
- FIG. 1 is a graph showing the relationship between the amount of emulsifier added and the film strength.
- FIG. 2 is a graph showing the bioactive agent concentration in the embedded portion of the film of Example 9.
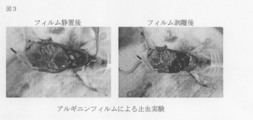
- FIG. 3 is a photograph showing the film after standing and after peeling in the hemostasis experiment of the film of Example 9.
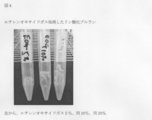
- FIG. 4 is a photograph showing the result of poor solubilization in Example 14.
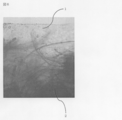
- FIG. 5 is a cross-sectional photograph showing a two-layer structure of Example 15.
- FIG. 6 is a partially enlarged photograph of FIG.
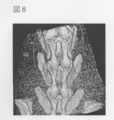
- FIG. 7 is a photograph showing the film resting place in Example 16.
- FIG. 8 is a photograph showing a region where bone formation can be expected in Example 16.
- the bioabsorbable sheet or film of the present invention is composed of a composition containing phosphorylated pullulan (phosphorylated pullulan composition), and has a great feature in using phosphorylated pullulan as a biodegradable polymer.
- the bioabsorbable sheet or film of the present invention is simply referred to as the sheet or film of the present invention.
- [Phosphorylated pullulan composition] [Phosphorylated pullulan] Phosphorylated pullulan not only has a high affinity for living tissue, but its phosphate group forms a chelate bond with the living tissue and exhibits adsorptivity, and also exhibits bioabsorbability.
- tissue fluid also referred to as tissue fluid
- body fluid present on the tissue or organ surface in the living body infiltrates into phosphorylated pullulan, so that the cation in the tissue fluid is changed. Since it forms a cross-linked structure by forming a chelate bond with the phosphate group of phosphorylated pullulan, it becomes possible to exhibit viscosity over a long period of time in addition to the thickening effect by pullulan, and as a result It is thought that the healing power will be improved.
- tissue fluid also referred to as tissue fluid
- Phosphorylated pullulan can be produced by a known method of phosphorylating the hydroxyl group of pullulan. For example, a method of reacting with sodium metaphosphate described in Carbohydrate Research Vol. 302 (1997) pages 27 to 34, reaction with sodium phosphate described in JP-A-2005-330269 and JP-A-2005-330270 And the like. Furthermore, as described in WO 87/07142, a method of obtaining phosphorylated pullulan by reacting phosphorus pentoxide and pullulan is also preferably used. The structure of the obtained phosphorylated pullulan can be confirmed by IR analysis, NMR analysis or the like. The degree of phosphorylation of phosphorylated pullulan can be adjusted by adjusting the amount of raw materials used and reaction conditions according to a known method.
- the phosphorylated pullulan may be partially or entirely salted, and examples thereof include sodium salts, potassium salts, calcium salts, magnesium salts, ammonium salts, and salts with various amines.
- the salt of phosphorylated pullulan can be prepared according to a known method.
- the number average molecular weight (Mn) of the phosphorylated pullulan is preferably 1000 or more, more preferably 2000 or more, still more preferably 5000 or more, from the viewpoints of adhesion to living tissue, the strength of a sheet or film, production cost, and the like. More preferably, it is 10,000 or more, More preferably, it is 20000 or more. The upper limit value is not particularly limited.
- the weight average molecular weight (Mw) is preferably 10,000 or more, more preferably 20000 or more, still more preferably 50000 or more, from the viewpoints of adhesion to living tissue, sheet or film strength, production cost, and the like. Preferably it is 100,000 or more, More preferably, it is 200,000 or more, and an upper limit is not specifically limited.
- the upper limit of the weight average molecular weight of phosphorylated pullulan is not particularly limited, and includes those exceeding the measurement limit.
- the number average molecular weight (Mn) and the weight average molecular weight (Mw) of phosphorylated pullulan can be measured according to the methods described in the examples described later.
- Phosphorylated pullulan is preferably 0.5 to 15% by number, more preferably 2 to 14% by number, more preferably 2 to 13% by number, and more preferably 2 to 10% of all hydroxyl groups contained in one molecule. Those in which several percent of hydroxyl groups are phosphorylated are desirable.
- the number ratio of the phosphorylated hydroxyl group in phosphorylated pullulan is based on the fact that phosphorous pullulan is subjected to elemental analysis to measure the phosphorus content, and all the measured phosphorus is derived from the phosphorylated hydroxyl group. Can be calculated.
- biodegradable polymer phosphorylated pullulan
- biodegradable polymers may be used as long as the effects of the present invention are not impaired.
- biodegradable polymers are not particularly limited as long as they are known, and include natural biodegradable polymers, modified natural biodegradable polymers, synthetic biodegradable polymers, and the like. Two or more types can be used in combination.
- Natural biodegradable polymers include polysaccharides (eg, alginate, dextran, chitin, chitosan, hyaluronic acid, cellulose, collagen, gelatin, fucoidin, starch, and glycosaminoglycans); proteins (eg, albumin, casein, zein) , Fibroin), and copolymers and blends thereof.
- polysaccharides eg, alginate, dextran, chitin, chitosan, hyaluronic acid, cellulose, collagen, gelatin, fucoidin, starch, and glycosaminoglycans
- proteins eg, albumin, casein, zein
- Fibroin e.g, Fibroin
- modified natural biodegradable polymer examples include natural biodegradable polymers modified by synthesis. Specifically, a chemical derivative of the natural biodegradable polymer (substitution and / or addition of a chemical group (for example, alkyl, alkylene), hydroxylation, oxidation, and combinations thereof) can be used, and a cellulose derivative (For example, alkyl cellulose, hydroxyalkyl cellulose, cellulose ether, nitrocellulose) are exemplified.
- methyl cellulose, ethyl cellulose, hydroxypropyl cellulose, hydroxypropyl methyl cellulose, hydroxybutyl methyl cellulose, cellulose acetate, cellulose propionate, cellulose acetate butyrate, cellulose acetate phthalate, carboxymethyl cellulose, cellulose triacetate, and cellulose sulfate sodium salt are preferred examples.
- methyl cellulose, ethyl cellulose, hydroxypropyl cellulose, hydroxypropyl methyl cellulose, hydroxybutyl methyl cellulose, cellulose acetate, cellulose propionate, cellulose acetate butyrate, cellulose acetate phthalate, carboxymethyl cellulose, cellulose triacetate, and cellulose sulfate sodium salt are preferred examples.
- Synthetic biodegradable polymers include polyhydroxy acids prepared from lactone monomers (eg, glycolide, lactide, caprolactone, ⁇ -caprolactone, valerolactone, and ⁇ -valerolactone); and carbonates (eg, trimethylene carbonate and tetra Methylene carbonate, etc.); dioxanone (eg, 1,4-dioxanone and p-dioxanone); 1, dioxepanone (eg, 1,4-dioxepan-2-one and 1,5-dioxepan-2-one); Combinations are listed.
- lactone monomers eg, glycolide, lactide, caprolactone, ⁇ -caprolactone, valerolactone, and ⁇ -valerolactone
- carbonates eg, trimethylene carbonate and tetra Methylene carbonate, etc.
- dioxanone eg, 1,4-dioxanone and p-dioxanone
- Polymers formed from these include poly (lactic acid); poly (glycolic acid); poly (trimethylene carbonate); poly (dioxanone); poly (hydroxybutyric acid); poly (hydroxyvaleric acid); poly (lactide-co -( ⁇ -caprolactone)); poly (glycolide-co- ( ⁇ -caprolactone)); poly (lactic acid-co-glycolic acid); polycarbonate; poly (pseudo amino acid); poly (amino acid); poly (hydroxyalkanoate) Polyalkylene oxalates; polyoxaesters; polyanhydrides; polyorthoesters; and copolymers, block copolymers, homopolymers, blends, and combinations thereof.
- aliphatic polyesters polyethylene glycol; glycerol; copoly (ether-esters); and copolymers, block copolymers, homopolymers, blends, and combinations thereof may be mentioned.
- the phosphorylated pullulan content is preferably 50% by mass or more, more preferably 60% by mass or more, still more preferably 70% by mass or more, Preferably it is 80 mass% or more, More preferably, it is 90 mass% or more.
- the upper limit is not particularly limited, and the biodegradable polymer may be composed of phosphorylated pullulan.
- seat or a film Preferably it exceeds 30 mass%, More preferably, it is 40 mass% or more, More preferably, it is 50 mass% or more. . Moreover, although an upper limit in particular is not set, it is about 90 mass% normally.
- the sheet or film of the present invention can contain a plasticizer as a component other than phosphorylated pullulan, for example, from the viewpoint of suppressing shrinkage during molding and improving the flexibility of the sheet or film.
- plasticizer examples include glycerin, ethylene glycol, propylene glycol, diethylene glycol, triethylene glycol, dipropylene glycol, sorbitol, polyglycerin, polyethylene glycol, polyglycerin fatty acid ester and the like. These can be used singly or in combination of two or more, but it is preferable to use at least one selected from glycerin and polyethylene glycol from the viewpoint of flexibility of the sheet or film.
- the molecular weight of polyethylene glycol is preferably 100 to 1000, more preferably 200 to 600. It may be a synthetic product or a commercial product.
- the content of the plasticizer is preferably 1 part by mass or more, more preferably 3 parts by mass or more, and further preferably 5 parts by mass with respect to 100 parts by mass of phosphorylated pullulan, from the viewpoint of improving the flexibility of the sheet or film. From the viewpoint of handleability of the sheet or film, it is preferably 30 parts by mass or less, more preferably 25 parts by mass or less, and still more preferably 20 parts by mass or less.
- seat or a film from a viewpoint of improving the softness
- the sheet or film of the present invention Since the sheet or film of the present invention is used by being embedded in a living body, it may be used with a medicinal effect, and may contain a bioactive agent. When the bioactive agent is contained, when the sheet or film is absorbed by the living body due to the interaction between an ionic group such as a phosphate group derived from phosphorylated pullulan and the bioactive agent, the bioactive agent Sequentially released and absorbed. Therefore, the sheet or film of the present invention can function as a substrate for sustained release of the bioactive agent.
- an ionic group such as a phosphate group derived from phosphorylated pullulan
- the bioactive agent Sequentially released and absorbed. Therefore, the sheet or film of the present invention can function as a substrate for sustained release of the bioactive agent.
- Antibacterial drugs Drugs included in the following systems: ⁇ -lactams (penicillins, complex penicillins, ⁇ -lactamase inhibitors combined penicillins, cephem, ⁇ -lactamase inhibitors combined cephem, carbapenems, monobactams, penems) ), Aminoglycoside, lincomycin, fosfomycin, tetracycline, chloramphenicol, macrolide, ketolide, polypeptide, glycopeptide, streptogramin, quinolone, new quinolone, sulfa drugs, oxazolidinone system); Antifungal drugs (drugs in the following systems: polyenes, azoles, allylamines, candins); Antiviral drugs (for example, interferon, anti-herpes drugs, anti-influenza drugs, anti-HIV drugs (including various reverse transcriptase inhibitors and nucleic acid replication inhibitors
- Examples other than the above include, for example, viruses and cells; peptides, polypeptides and proteins, and analogs, muteins, and active fragments thereof; immunoglobulins; cytokines (eg, lymphokines, monokines, chemokines); hematopoietic factors; Leukine (IL-2, IL-3, IL-4, IL-6); erythropoietin; nuclease; tumor necrosis factor; colony stimulating factor (eg, GCSF, MCSF); insulin; tumor suppressor; blood protein ( For example, fibrin, thrombin, fibrinogen, synthetic thrombin, synthetic fibrin, synthetic fibrinogen); gonadotropins (eg, FSH, LH, CG, etc.); hormones and hormone analogs (eg, growth hormone); vaccines (eg, neoplastic) Somatostatin; antigen; growth factor or growth factor (eg, nerve growth factor, insulin-like growth factor); bone morphogenetic protein; T
- bioactive agents include mitoten, halonitrosoureas, anthracyclines, ellipticine, ceftazidime, oxaprozin, valaciclovir, famciclovir, flutamide, enalapril, metformol, metoformol, meformol, , Gabapentin, fosinopril, tramadol, acarbose, lorazepam, lorazepam, omeprazole, ricinopril, tramadol (tramsdol), levofloxacin, zafirlukast, granulocyte stimulating factor, nizatidine, bupropion, min, perindopril, elumin Alendronate (a endronate), alprostadil, betaxolol, bleomycin sulfate, dexfenfluramine, fentanyl, gemcitabine, g
- antimicrobial agents such as triclosan (also known as 2,4,4′-trichloro-2′-hydroxydiphenyl ether) Chlorhexidine and its salts (including chlorhexidine acetate, chlorhexidine gluconate, chlorhexidine hydrochloride, and chlorhexidine sulfate), silver and its salts (silver acetate, silver benzoate, silver carbonate, silver citrate, silver iodate, silver iodide) Silver lactate, silver laurate, silver nitrate, silver oxide, silver palmitate, protein silver, and silver sulfadiazine), polymyxin, tetracycline, aminoglycosides (eg, tobramycin and gentamicin), rifampicin, bacitracin, neomycin, Loramphenicol, miconazole, quinolone (eg, oxophosphate, norf
- antibacterial proteins and peptides eg, lactoferrin and lactoferricin B
- antibacterial polysaccharides eg, fucan and its derivatives
- the content of the bioactive agent cannot be generally determined by the patient to which the sheet or film of the present invention is applied, and can be adjusted as appropriate.
- the bioactive agent may be used by coating with a known coating agent, adsorbing it on a carrier, or encapsulating with a liposome, microcapsule, cyclodextrin or the like.
- seat or film of this invention can contain an emulsifier other than the above.
- An oil-soluble component can be contained by the emulsifier.
- the plasticity of the sheet or film can be controlled.
- absorption into the body can be controlled, for example, by promoting absorption of the bioactive agent into the body by an emulsifier.
- a known emulsifier can be used as the emulsifier.
- phospholipids anionic surfactants, cationic surfactants, nonionic surfactants, and amphoteric surfactants can be used.
- glycerin fatty acid ester sodium lauryl sulfate, sucrose fatty acid ester, sorbitan fatty acid ester, polyoxyethylene hydrogenated castor oil, polyoxyethylene sorbitan fatty acid ester, polyethylene glycol fatty acid ester, polyglycerin fatty acid ester, alkylsulfonic acid, Examples thereof include alkylbenzene sulfonic acid, polyoxyethylene alkyl ether sulfonic acid, soybean-derived lecithin, and salts thereof. These can be used alone or in combination of two or more. It may be a synthetic product or a commercial product.
- the content of the emulsifier is preferably 2 parts by mass or more from the viewpoint of improving the flexibility of the sheet or film with respect to 100 parts by mass of phosphorylated pullulan, or from the viewpoint of dissolving or well dispersing the bioactive agent.
- it is 10 parts by mass or more, more preferably 15 parts by mass or more, and from the viewpoint of handleability of the sheet or film, it is preferably 60 parts by mass or less, more preferably 50 parts by mass or less, still more preferably 40 parts by mass or less. is there.
- the content of the emulsifier in the sheet or film is preferably 5% by mass or more, more preferably 10 from the viewpoint of improving the flexibility of the sheet or film, or from the viewpoint of dissolving or good dispersion of the bioactive agent. From the viewpoint of handleability of the sheet or film, it is preferably 35% by mass or less, more preferably 30% by mass or less, and further preferably 25% by mass or less.
- the sheet or film of the present invention can contain other additives in addition to the above.
- other additives include sweeteners, fragrances, colorants, pigments, preservatives, antioxidants, inorganic fillers, and organic fillers. These contents can be appropriately adjusted within a range that does not impair the effects of the present invention, but are preferably 5% by mass or less, more preferably 1% by mass or less, considering application to the living body. It is desirable.
- the sheet or film of the present invention only needs to be composed of a phosphorylated pullulan composition containing a phosphorylated pullulan.
- the phosphorylated pullulan is dissolved in a solvent, and if necessary, a plasticizer or a bioactive agent.
- various additives may be added and mixed, and then prepared by drying and solidifying.
- the bioactive agent and the emulsifier may be added after being mixed separately.
- the solvent used may be any solvent that can dissolve phosphorylated pullulan, and examples thereof include water, ethanol, acetic acid, and acetone. These can be used alone or in combination of two or more. Of these, water is preferred. Further, the temperature at the time of dissolution is not particularly limited, and examples thereof include 20 to 40 ° C. If necessary, the solution may be defoamed.
- Examples of the method for spreading the phosphorylated pullulan composition include, for example, a method in which a solution of the composition is poured into a mold and solidified, and is applied to a support by a brush or a spatula, a casting method, a printing method, or the like. The method of letting it be mentioned.
- the drying temperature can be appropriately adjusted according to the type of solvent.
- the sheet or film of the present invention may be a laminate of a plurality of phosphorylated pullulan composition layers, a laminate of other known layers, or a combination of these. Good.
- the layers can be prepared by laminating those in which the types, presence / absences, and contents of bioactive agents are adjusted depending on the layer.
- laminating a known layer details will be described in the section of “aspect for providing another layer” described later.
- ethyl cellulose Coating layers containing hypromellose phthalate (HPMCP), hydroxypropyl methylcellulose acetate succinate, kanten, carrageenan, zein, etc. can be used.
- the coating layer is made of a material that is absorbed by a living body and can be prepared according to a known technique.
- the aforementioned raw materials can be dissolved in water and prepared in the same manner as the sheet or film of the present invention.
- the lamination method is not particularly limited.
- the layers may be individually prepared and then bonded and laminated, or may be spread and laminated in order on the already formed layer.
- the sheet or film of the present invention is obtained.
- the thickness of the sheet or film of the present invention the lower limit is about 10 ⁇ m, 20 ⁇ m, 30 ⁇ m, and 50 ⁇ m, and the upper limit is about 2000 ⁇ m, 1500 ⁇ m, 1200 ⁇ m, 800 ⁇ m, 500 ⁇ m, 300 ⁇ m, and 200 ⁇ m. When it is contained, it can be appropriately set according to the release property.
- thickness here is the thickness of a phosphorylated pullulan composition layer, and is a total thickness when a plurality of phosphorylated pullulan composition layers are laminated.
- the “sheet” has a thickness of about 1 to several mm, whereas the “film” is thinner than the sheet (thickness 10 to several 100 ⁇ m) and often refers to a thin film. Both are conventionally distinguished.
- the shape of the sheet or film of the present invention can be arbitrarily determined such as a circle, an ellipse, a square (square, rectangle, rhombus, etc.), a star, a heart, or the like.
- the area cannot be generally set according to the thickness of a sheet
- the sheet or film of the present invention can be used to repair or regenerate injuries to organs and tissues in the living body. Specifically, in vivo organs (heart, lung, liver, stomach, intestine, gallbladder, pancreas, spleen, kidney, bladder, genital organs, etc.), skin, mucous membrane, nervous system tissues (peripheral nerve, brain, spinal cord, etc.) Central nervous system), bone / joint (bone, cartilage, ligament, tendon, etc.), soft tissue (subcutaneous connective tissue, fascia, muscle, adipose tissue, etc.), vascular system tissue (blood vessel, lymphatic vessel), wound part
- the sheet or film of the present invention is applied to the defect part, the weak part, etc., and when the support sheet is included, the support sheet is peeled off, and then the phosphorylated pullulan composition layer is applied directly to the affected part.
- the term “in vivo” in the present invention includes not only the case of being attached to the inside of a living body.
- a sheet or film is affixed to the mucosal surface in the oral cavity, the skin surface, etc.
- the case where one surface is exposed in vivo and the other surface is exposed outside the living body is also included. Therefore, affixing the sheet or film of the present invention to a wound part, a defect part, a fragile part, etc. is intended to repair or regenerate injuries to organs and tissues in the living body in this specification. is there.
- a covering material such as a wound part, a defect part, or a fragile part in an organ or tissue in a living body, including the sheet or film of the present invention.
- a covering material can be used, for example, for wound covering, reinforcing a defect part and a weak part of an organ, reinforcing a difficult-to-sew part, hemostasis (preventing bleeding), preventing air leakage, and the like.
- the sheet or film of the present invention contains a bioactive agent
- a bioactive agent it can be appropriately used for a disease corresponding to the agent.
- the infection is calmed down by applying a film containing anti-infective drugs (antibacterial drugs, antiviral drugs, antifungal drugs, bactericides, etc.) so that they are in direct contact with the infected lesion.
- an antitumor effect can be exerted by applying a film containing an antitumor drug (such as an anticancer drug, an antibody, or a low molecular weight compound) to the tumor site.
- an antitumor drug such as an anticancer drug, an antibody, or a low molecular weight compound
- Tissue repair and regeneration can be achieved by using a film containing a humoral factor such as a growth factor or cytokine for a tissue damaged part or a defect part.
- a film containing an anti-inflammatory drug steroidal or non-steroidal anti-inflammatory drug
- an immunogenic drug is used for inflammation sedation.
- a film containing an anti-inflammatory drug steroidal or non-steroidal anti-inflammatory drug
- an immunogenic drug is used for bleeding.
- a film containing an anticoagulant is applied to the bleeding site.
- a film containing an anesthetic can be applied to the skin or mucous membrane to enable local anesthesia without using a needle.
- the amount of use, the number of times of use, and the period of use of the sheet or film of the present invention vary depending on the type of the bioactive agent contained, and depending on the effective amount of the bioactive agent, the purpose of use and the age of the patient to be applied It is set appropriately depending on body weight and symptoms and is not constant.
- a part or all of the sheet or film of the present invention containing the coating material or bioactive agent is subjected to surface treatment, or other layers other than the sheet or film of the present invention are provided. Also good.
- Examples of the surface treatment include chemical modification of the hydroxyl group portion of phosphorylated pullulan.
- the surface can be hydrophobized or a cross-linked structure can be introduced.
- the chemical modification can be performed according to a known method, and can be anything that reacts with a hydroxyl group.
- the solubility of phosphorylated pullulan can be controlled, and controlled release can be controlled, for example, by providing controlled release to the drug contained in the sheet or film of the present invention. Therefore, it is also an aspect of the present invention to provide a phosphorylated pullulan-containing bioabsorbable sheet or film in which the solubility of phosphorylated pullulan and the sustained release of the drug are controlled.
- alkyl halides such as methyl chloride and ethyl chloride
- dialkyl carbonates such as dimethyl carbonate and diethyl carbonate
- dialkyl sulfates such as dimethyl sulfate and diethyl sulfate
- alkylene oxides such as ethylene oxide and propylene oxide
- ethylene oxide gas is preferably used.
- it is not limited to alkyl etherification, Etherification by benzyl bromide etc. are also preferable.
- esterifying agents include carboxylic acids, acid anhydrides, carboxylic acid halides, ketene, diketene, etc., which may contain heteroatoms, and acetic acid, propionic acid, butyric acid, acrylic acid, methacrylic acid and derivatives thereof are used.
- acetic anhydride is preferable.
- a phosphorylated pullulan film may be dipped in an acetic anhydride solution or treated with acetic anhydride vapor.
- phosphoryl chloride etc. can be used as a phosphoric acid esterifying agent.
- Examples of functional groups introduced by chemical modification include acetyl, methacryloyl, propanoyl, butanoyl, iso-butanoyl, pentanoyl, hexanoyl, heptanoyl, octanoyl, methyl, ethyl, and propyl groups. Iso-propyl group, butyl group, iso-butyl group, tert-butyl group, pentyl group, hexyl group, heptyl group, octyl group and the like.
- An acetalizing agent includes aldehyde.
- the aldehyde is not particularly limited as long as it can acetalize a hydroxyl group.
- formaldehyde, acetaldehyde, propionaldehyde, normal butyl aldehyde and the like are preferable, and two or more kinds may be combined.
- isocyanate examples include methyl isocyanate, ethyl isocyanate, isopropyl isocyanate, isobutyl isocyanate, trichloromethyl ethyl isocyanate, chloroethyl isocyanate, isopropyl isothiocyanate, isobutyl isothiocyanate, diphenylmethane isocyanate, hexamethylene diisocyanate, toluene diisocyanate, and isophorone diisocyanate. .
- isothiocyanate examples include methyl isothiocyanate, ethyl isothiocyanate, isopropyl isothiocyanate, isobutyl isothiocyanate, chloroethyl isothiocyanate, n-octyl isothiocyanate, cyclohexyl isothiocyanate, phenyl isothiocyanate, 1,4-phenylene diisothiocyanate, and the like. Can be mentioned.
- carbamoyl chloride examples include dimethylcarbamoyl chloride, diethylcarbamoyl chloride, diphenylcarbamoyl chloride, bis (2-chloroethyl) carbamoyl chloride, N-methoxy-N-methylcarbamoyl chloride, 4-morpholinylcarbamoyl chloride and the like.
- thiocarbamoyl chloride examples include dimethylthiocarbamoyl chloride, diethylcarbamoyl chloride, diphenylcarbamoyl chloride and the like.
- silane coupling agents include silazanes such as vinylsilazane, hexamethyldisilazane, tetramethyldisilazane, and diphenyltetramethyldisilazane; chlorosilanes such as trimethylchlorosilane, dimethyldichlorosilane, methyltrichlorosilane, and vinyltrichlorosilane; trimethyl Methoxysilane, dimethyldimethoxysilane, methyltrimethoxysilane, vinyltrimethoxysilane, n-butoxytrimethylsilane, tert-butoxytrimethylsilane, sec-butoxytrimethylsilane, isobutoxytrimethylsilane, ethoxytriethylsilane, octyldimethylethoxysilane or cyclohexyl Alkoxysilanes such as oxytrimethylsilane; alkoxy such as butoxypolydimethyls
- sulfonyl chloride examples include methanesulfonyl chloride, ethanesulfonyl chloride, chloromethylsulfonyl chloride, methanedisulfonyl dichloride, p-toluenesulfonyl chloride and the like.
- sulfonic acid anhydride examples include benzenesulfonic acid anhydride, trifluoromethanesulfonic acid anhydride, p-toluenesulfonic acid anhydride, and the like.
- amphiphilic polymer can be used as the surface modification using a polymer or the like.
- the amphiphilic polymer is a polymer having both a hydrophilic component and a lipophilic component in a polymer unit, and has a property of being dissolved and dispersed in both an organic solvent and water. It may be a homopolymer composed of a single monomer having both a hydrophilic component and a lipophilic component in the side chain, or a copolymer soot polymer obtained by copolymerizing a hydrophilic component monomer and a lipophilic component monomer. Examples of a single monomer having both a hydrophilic component and a lipophilic component include (polyoxyalkylene) acrylate and methacrylate.
- an amphiphilic polymer may be prepared by copolymerizing a hydrophilic component monomer and a lipophilic component monomer.
- an acrylic resin that is easy to polymerize and has a wide variety of monomers is preferred.
- the acrylic resin may be a homopolymer based only on the acrylic monomer, or may be copolymerized with other monomers based on the acrylic monomer. May be.
- Examples of the acrylic monomer include general acrylic acid, methacrylic acid, methyl acrylate, methyl methacrylate, acrylic chloride, methacrylic chloride, and acrylic acid anhydride.
- a layer containing a biocompatible polymer or other general materials can be used.
- Biocompatible polymers include polylactic acid (PLA), polyglycolic acid (PGA), polycaprolactone (PCL), and poly (esters) based on their copolymers, PHB-PHV poly (hydroxy) Natural polymers such as alkanoic acids), other poly (esters), starch, cellulose, chitin, chitosan, gelatin, chondroitin sulfate and salts thereof, hyaluronic acid and salts thereof, alginic acid and salts thereof, dextran, dextrin and collagen , Polycarbonate, polyurethane, polypeptide, polyethylene oxide (PEO), multiblock copolymer of polyethylene oxide (PEO) and poly (butylene terephthalate) (PBT), and ethylene-vinyl acetate copolymer.
- PHA polylactic acid
- PGA polyglycolic acid
- PCL polycaprolactone
- PHB-PHV poly (hydroxy) Natural polymers such as alkanoic acids
- PE polyethylene
- PVC polyvinyl chloride
- PS polyvinylidene chloride
- PVAc polyvinyl acetate
- Teflon Teflon (registered trademark) (polytetrafluoroethylene, PTFE)
- ABS resin acrylonitrile butadiene styrene resin
- AS resin acrylic resin (PMMA), polyacrylonitrile, ethylene-vinyl alcohol copolymer
- PA polyamide
- Nylon polyacetal
- POM modified polyphenylene ether
- PPE modified PPE
- PPO polybutylene terephthalate
- PET polyethylene terephthalate
- GFP glass fiber reinforced polyethylene Terephthalate
- COP polyphenylene sulfide
- PPS polytetrafluoroethylene
- PTFE polytetrafluoroethylene
- the method for laminating other layers on the sheet or film of the present invention is not particularly limited, and it can be laminated by a known method such as dry lamination, extrusion lamination, or wet lamination.
- the sheet or film of the present invention when the sheet or film of the present invention is applied to an organ or tissue in a living body, a regeneration or reconstruction material of the in vivo tissue or a bioactive agent is previously applied to the site, and phosphorylation is performed thereon.
- the pullulan composition layer can be applied and used so as to cover the material or the bioactive agent.
- a material for regenerating or reconstructing a living tissue or a material for preventing leakage or diffusion of the material or a fixing material can be used as an aspect of the sheet or film of the present invention.
- another embodiment includes a material for preventing leakage or diffusion of bioactive agents.
- the sheet or film of the present invention can be used as an artificial dura mater, an adhesion prevention film, a periodontal tissue regeneration film, a scaffold, a bone fixing material, an adhesive material, and the like.
- a bone filling material artificial bone (paste type, granule type) or the like can be used as a material for regenerating or reconstructing in vivo tissues.
- the bioactive agent those described above can be used. Since the sheet or film of the present invention is excellent in strength and adhesiveness under wet conditions, the form of a material for regenerating or reconstructing in vivo tissue or a bioactive agent is not particularly limited, and is a paste type, powder type, granule type, Various forms such as sponge type and liquid form can be used.
- the precipitate was dissolved in distilled water (400 mL), and the solution was added little by little over 5 minutes to 99.5% ethanol (2000 mL) with stirring.
- the deposited precipitate was collected by filtration with a Kiriyama funnel (3G), washed with 99.5% ethanol (500 mL), and then dried under reduced pressure at 60 ° C. for 12 hours to obtain 28.5 g of a slightly brownish white solid. .
- 25 g of this white solid was dissolved in distilled water, and this solution was applied to a small tabletop electrodialyzer (Micro Acylizer S3, manufactured by San Actis) to obtain 13 g of phosphorylated pullulan as a transparent light brown solid. It was.
- Test example 1 The strength of the film under wet conditions was examined by the type of biodegradable polymer.
- the amount of raw material shown in Table 1 is dissolved in 19 mL of distilled water, and the resulting solution is decompressed and defoamed with an aspirator, spread by hand, and dried with a dryer (about 55 ° C.). As a result, a film having a thickness of 70 ⁇ m was obtained.
- the phosphorylated pullulan used was manufactured in Production Example 1, the pullulan used was a pharmacopoeia, and the collagen used was manufactured by Nitta Gelatin.
- pullulan has a low shear adhesive force under wet conditions
- the phosphorylated pullulan film is a new bioabsorbable film that exhibits higher adhesiveness under wet conditions than conventional collagen films.
- Test example 2 The emulsifier is an ingredient that can be expected to promote absorption of the active ingredient into the body, but since it affects the physical properties such as the strength of the film in addition to the dissolution or dispersion of the active ingredient, the amount added was examined.
- the film was dissolved in the order of 1), and the obtained solution was decompressed and defoamed with an aspirator, spread by hand, and dried (about 55 ° C.) with a dryer to obtain a film having a thickness of 70 ⁇ m.
- Dissolution test A film cut to 16.5 ⁇ 22 mm is floated on a petri dish containing water, the state of dissolution in a stationary state is observed, and the time of complete dissolution is measured. Four samples are used, and the average value is used for evaluation. The longer the dissolution time is, the higher the solubility of the film is. However, if it is about 30 to 45 seconds, there is no problem for biological use.
- the film strength tends to decrease as the amount of the emulsifier added increases, and saturates at about 8 MPa.
- the standard of the film strength is about 2 MPa, suggesting that the strength can be sufficiently maintained even when an emulsifier is added.
- dissolution in about 40 seconds was observed regardless of the amount of emulsifier added.
- Test example 3 In the case where the film contains a bioactive agent, it was examined whether the film could be formed.
- the active agent is mixed, and further, phosphorylated pullulan (Production Example 1) is added and dissolved, and the resulting solution is decompressed and defoamed with an aspirator, spread by hand, and dried with a dryer (about 55). And a film having a thickness of 70 ⁇ m was obtained.
- Bioactive agents include calcium chloride (Tonda Pharmaceutical), estradiol (ZHEJIANGZXIANJU PHARMACEUTICAL CO., LTD.), Raloxifene (LKT Laboratories, ⁇ ⁇ Inc.), arginine (Ajinomoto), gentamicin ( ⁇ ⁇ ⁇ ⁇ ) (Manufactured by Shaku Pharmaceutical Co., Ltd.)
- the addition of the bioactive agent decreased the tensile strength compared to the placebo (Example 3), and the degree of decrease of estradiol (Example 7) and raloxifene (Example 8) was greater than others.
- this is considered to be because the bioactive agent is blended in a dispersed state, and since the strength of any film is sufficiently higher than the standard (about 2 MPa), the strength can be obtained even if the bioactive agent is added. It became clear that sufficient film could be obtained.
- dissolution in about 40 seconds was observed regardless of the addition of the bioactive agent. It was found that the adhesive strength was a result that almost satisfied the target value (about 2.0 N / 15 mm width) in all formulations.
- Test example 4 When the film contained a bioactive agent, the effect of emulsification of the bioactive agent was examined.
- a film was obtained in the same manner as in Test Example 3 (thickness: 70 ⁇ m) except that the bioactive agent was premixed with an emulsifier and emulsified and then mixed.
- Test Example 5 The film of Example 10 (10 ⁇ 10 mm) was implanted subcutaneously on the back of the mouse (C57BL / 6) and on the dorsum of the lower back, and the mouse was sacrificed 24 hours later, and the tissue of the implant was extracted. Gentamicin drug concentration in the tissue was measured by ELISA (one day after implantation). For reference, the film of Example 10 was left overnight in 1 mL of PBS, and the supernatant was collected the next day, and the gentamicin drug concentration was measured in the same manner (film content). The results are shown in FIG. In addition, at the time of film implantation, it was confirmed that the film was immediately adapted to the tissue and showed tackiness when pinched with tweezers.
- the drug concentration of gentamicin dissolved in PBS was 33126 ng / mL
- the concentration in the tissue one day after implantation was 13 ng / mL
- Test Example 6 Hemostasis experiment
- the hemostatic effect of the arginine film obtained in Example 9 was examined (FIG. 3).
- the mouse liver surface was rubbed and damaged, and an arginine film was allowed to stand there. There was a tendency that bleeding was reduced macroscopically by standing the film. Even if the film was peeled off immediately after that, bleeding from the liver surface could not be confirmed.
- Example 11 A 30 L reactor was charged with 26 L of ultrapure water and 350 g (preparation standard) of food additive pullulan (Pulllan / Hayashibara). 581 mL of 50 w / v% sodium hydroxide aqueous solution was added at an internal temperature of 20 to 25 ° C., and the mixture was stirred for 19 hours in the same temperature range. The internal temperature was cooled to 0 ° C., and 229 g of phosphoryl chloride was added at 0 to 10 ° C. After stirring in the same temperature range for 1 hour, the internal temperature was adjusted to 20 to 25 ° C. and stirred for 15 hours.
- food additive pullulan Pulllan / Hayashibara
- Membrane filtration concentration was performed using a UF membrane, and after concentration until the distillate was reduced, ultrapure water was added to obtain a phosphorylated pullulan aqueous solution.
- the obtained phosphorylated pullulan aqueous solution was pulverized with a spray dryer to obtain 280 g of phosphorylated pullulan.
- the weight average molecular weight could not be measured.
- Example 12 In a 1000 LGL reactor, 508 L of ion-exchanged water and 7 kg of food additive pullulan (Pullan / Hayashibara) (preparation standard) were charged. A sodium hydroxide aqueous solution (5.81 kg of sodium hydroxide and 18 kg of ion-exchanged water) prepared in advance was added at an internal temperature of 20 to 25 ° C., stirred for 5 hours in the same temperature range, and phosphoryl chloride 4 at an internal temperature of 0 to 10 ° C. .56 kg was added dropwise. After stirring for 1 hour in the same temperature range, the internal temperature was adjusted to 20-25 ° C. Further aqueous sodium hydroxide solution was added and stirred overnight.
- a sodium hydroxide aqueous solution (5.81 kg of sodium hydroxide and 18 kg of ion-exchanged water) prepared in advance was added at an internal temperature of 20 to 25 ° C., stirred for 5 hours in the same temperature range, and phosphoryl chloride 4 at an internal
- Membrane filtration concentration was performed using a UF membrane, and after concentration until the distillate was reduced, ultrapure water was added to obtain a phosphorylated pullulan aqueous solution.
- the obtained phosphorylated pullulan aqueous solution was pulverized with a spray dryer to obtain 3.5 kg of phosphorylated pullulan (weight average molecular weight 210110).
- Example 12 The weight average molecular weight of Example 12 is measured under the following conditions. ⁇ Measurement conditions> Measuring instrument: High-performance liquid chromatograph column: TSKgel GMPWXL (7.8 mm ID ⁇ 390 mm) ⁇ 2 linked mobile phase: 200 mM sodium nitrate aqueous solution Flow rate: 1 mL / min Detector: Suggested refraction detector Column temperature: 40 ° C Injection volume: 100 ⁇ L Sample solution: 2 mg / mL (After adding 5 mL of mobile phase to 10 mg of sample, shake to dissolve) Measurement: Create calibration curve with pullulan standard and calculate weight average molecular weight
- Example 13 (Solubility Control Example 1) 0.0236 g of the phosphorylated pullulan film prepared in Example 6 was treated with acetic anhydride vapor heated to 120 ° C. for 5 minutes. The untreated phosphorylated pullulan film swelled and dissolved when immersed in ultrapure water for 2 hours. On the other hand, phosphorylated pullulan treated with acetic anhydride swelled to 0.0916 g and dried to 0.0176 g. When this was immersed in water, the emulsifier and the like were eluted, but the flexibility was slightly lost as a phosphorylated pullulan film, but the film-like form was maintained.
- Example 14 (solubility control example 2)
- the phosphorylated pullulan powder was sterilized with ethylene oxide gas.
- the amount of reaction with ethylene oxide gas was changed by changing the exposure time to the gas, and the change was observed.
- the phosphorylated pullulan was modified as the ethylene oxide gas exposure time progressed, and the obtained phosphorylated pullulan became less soluble in water. It was found that pullulan can be hardly soluble in water.
- the results are shown in FIG. 5%, 10%, and 20% in the figure indicate the concentration of ethylene oxide gas.
- Example 15 Example of production of a two-layer structure
- a polyglycolic acid nonwoven fabric product name: Neobale 0.3 mm thickness
- a phosphorylated pullulan film prepared in the same manner as in Example 5 by a known wet laminating method, and a phosphorylated pullulan layer and a polyglycolic acid nonwoven fabric 2 A layer structure sheet was prepared. The results are shown in FIGS.
- FIG. 7 shows a phosphorylated pullulan film (5 mm square) containing BMP-2, which was prepared in the same manner as that except that the rat spinal transverse process was cut with a drill and BMP-2 was mixed at 1 ⁇ g / cm 2 at the same site. Left in position. The film immediately adsorbed to the tissue and was difficult to remove. Bone formation can be expected at a site surrounded by a circle in FIG.
- the bioabsorbable sheet or film of the present invention can be suitably used in the medical field, for example, as a medical adhesive or the like to be affixed to a body tissue such as an organ or a blood vessel during surgery.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Dermatology (AREA)
- Hematology (AREA)
- Materials Engineering (AREA)
- Inorganic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Biomedical Technology (AREA)
- Neurosurgery (AREA)
- General Chemical & Material Sciences (AREA)
- Surgery (AREA)
- Materials For Medical Uses (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2018504565A JP6619870B2 (ja) | 2016-03-09 | 2017-03-08 | 生体吸収性のシート又はフィルム |
| EP17763338.5A EP3427762A4 (en) | 2016-03-09 | 2017-03-08 | BIO-ABSORBABLE SHEET OR FILM |
| CN201780028979.5A CN109310795A (zh) | 2016-03-09 | 2017-03-08 | 生物体吸收性薄片或薄膜 |
| US16/083,071 US20190091166A1 (en) | 2016-03-09 | 2017-03-08 | Bioabsorbable sheet or film |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2016045567 | 2016-03-09 | ||
| JP2016-045567 | 2016-03-09 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2017154998A1 true WO2017154998A1 (ja) | 2017-09-14 |
Family
ID=59790617
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2017/009320 Ceased WO2017154998A1 (ja) | 2016-03-09 | 2017-03-08 | 生体吸収性のシート又はフィルム |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US20190091166A1 (enExample) |
| EP (1) | EP3427762A4 (enExample) |
| JP (2) | JP6619870B2 (enExample) |
| CN (1) | CN109310795A (enExample) |
| WO (1) | WO2017154998A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2019126667A (ja) * | 2018-01-26 | 2019-08-01 | 国立大学法人 岡山大学 | 徐放性デバイス |
| JP2023037097A (ja) * | 2021-09-03 | 2023-03-15 | 日本Mdbソリューションズ株式会社 | 注入材 |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2015066819A1 (en) | 2013-11-08 | 2015-05-14 | Carlos Filipe | Method of stabilizing molecules without refrigeration using water soluble polymers and applications thereof in performing chemical reactions |
| CN114432490B (zh) * | 2021-11-10 | 2023-01-06 | 北京大学口腔医学院 | 3d打印材料及其制备方法和应用 |
| CN115607309B (zh) * | 2022-10-21 | 2024-06-21 | 华中科技大学 | 一种3d打印peek材料引导性骨再生膜及其制备方法 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS63109865A (ja) * | 1986-06-23 | 1988-05-14 | 東洋製薬化成株式会社 | 薬物を有効成分として含有するプルランフイルム製剤 |
| WO2008010517A1 (fr) * | 2006-07-20 | 2008-01-24 | National University Corporation Okayama University | Composition orale pour applications dentaires |
| WO2011102530A1 (ja) * | 2010-02-22 | 2011-08-25 | 国立大学法人岡山大学 | 生体硬組織接着用キット |
| WO2015133439A1 (ja) * | 2014-03-07 | 2015-09-11 | 日本全薬工業株式会社 | プルランゲルならびにその製造方法および利用 |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1580229A1 (en) * | 2004-03-22 | 2005-09-28 | Warner-Lambert Company Llc | Biopolymer compositons and products thereof |
| CN1768859A (zh) * | 2005-10-24 | 2006-05-10 | 天津大学 | 基于醛基的微粒表面多重生物功能因子组装方法 |
| KR100888219B1 (ko) * | 2007-06-01 | 2009-03-12 | (주) 태웅메디칼 | 약물 방출 스텐트용 코팅제, 그의 제조방법 및 이 코팅제로코팅된 약물 방출 스텐트 |
| US9636362B2 (en) * | 2010-03-04 | 2017-05-02 | The Board Of Trustees Of The Leland Stanford Junior University | Pullulan regenerative matrix |
| CN102600493B (zh) * | 2012-03-06 | 2013-12-18 | 四川大学 | 天然普鲁兰多糖水凝胶伤口敷料及其制备方法 |
| WO2014141983A1 (ja) * | 2013-03-15 | 2014-09-18 | 東レ株式会社 | ポリ乳酸系樹脂を用いた積層フィルム |
-
2017
- 2017-03-08 CN CN201780028979.5A patent/CN109310795A/zh active Pending
- 2017-03-08 WO PCT/JP2017/009320 patent/WO2017154998A1/ja not_active Ceased
- 2017-03-08 EP EP17763338.5A patent/EP3427762A4/en not_active Withdrawn
- 2017-03-08 JP JP2018504565A patent/JP6619870B2/ja active Active
- 2017-03-08 US US16/083,071 patent/US20190091166A1/en not_active Abandoned
-
2019
- 2019-11-15 JP JP2019207022A patent/JP6964312B2/ja active Active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS63109865A (ja) * | 1986-06-23 | 1988-05-14 | 東洋製薬化成株式会社 | 薬物を有効成分として含有するプルランフイルム製剤 |
| WO2008010517A1 (fr) * | 2006-07-20 | 2008-01-24 | National University Corporation Okayama University | Composition orale pour applications dentaires |
| WO2011102530A1 (ja) * | 2010-02-22 | 2011-08-25 | 国立大学法人岡山大学 | 生体硬組織接着用キット |
| WO2015133439A1 (ja) * | 2014-03-07 | 2015-09-11 | 日本全薬工業株式会社 | プルランゲルならびにその製造方法および利用 |
Non-Patent Citations (2)
| Title |
|---|
| NAOKO NANBA: "Yokaigosha Muke Koku Care-zai no Kaihatsu", JAPAN SCIENCE AND TECHNOLOGY AGENCY (JST) 'WAKATE KENKYUSHA VENTURE SOSHUTSU SUISHIN JIGYO' HEISEI 21 NENDO SHINKI KADAI SAITAKU, 25 September 2009 (2009-09-25), XP055579746, Retrieved from the Internet <URL:http://www.okayama- u.ac.jp/jp/press/data/210925/press-090925-1- 1.pdf>, <URL: http://www.okayama-u.ac.jp/tp/ profile/press_info_21.html> [retrieved on 20170424] * |
| See also references of EP3427762A4 * |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2019126667A (ja) * | 2018-01-26 | 2019-08-01 | 国立大学法人 岡山大学 | 徐放性デバイス |
| JP2023037097A (ja) * | 2021-09-03 | 2023-03-15 | 日本Mdbソリューションズ株式会社 | 注入材 |
| JP7740691B2 (ja) | 2021-09-03 | 2025-09-17 | 国立大学法人 岡山大学 | 注入材 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20190091166A1 (en) | 2019-03-28 |
| JP2020022861A (ja) | 2020-02-13 |
| JPWO2017154998A1 (ja) | 2019-02-21 |
| CN109310795A (zh) | 2019-02-05 |
| JP6964312B2 (ja) | 2021-11-10 |
| EP3427762A1 (en) | 2019-01-16 |
| JP6619870B2 (ja) | 2019-12-11 |
| EP3427762A4 (en) | 2019-10-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6964312B2 (ja) | 生体吸収性のシート又はフィルム | |
| US8795713B2 (en) | Mucosal tissue dressing and method of use | |
| ES2593841T3 (es) | Artículos adhesivos | |
| AU2009225332B2 (en) | Hemostatic implant | |
| CN101378791B (zh) | 组织粘合材料 | |
| JP2007503462A (ja) | 瘢痕組織形成を減少させるための組成物および方法 | |
| EP3061471B1 (en) | Medical devices with sealing properties | |
| AU2011202275A1 (en) | Biodegradable polymer encapsulated microsphere particulate film and method of making thereof | |
| JP2007526793A (ja) | 生分解性を有する薬剤溶出ステント | |
| FR2949688A1 (fr) | Tissu avec picots revetu d’une couche microporeuse bioresorbable | |
| Mojsiewicz‐Pieńkowska | Review of current pharmaceutical applications of polysiloxanes (Silicones) | |
| CN109310799A (zh) | 组织粘合材料 | |
| JP2012148075A (ja) | 細孔内フィルムを備える医療デバイス | |
| CN102343110A (zh) | 消炎促愈合复合功能防粘连载药敷料 | |
| JP7709145B2 (ja) | 薬物送達デバイス | |
| HK40000424A (en) | Bioabsorbable sheet or film | |
| US20220241204A1 (en) | Topical time release delivery using layered biopolymer | |
| Bansal et al. | Global patent and technological status of biodegradable polymers in drug delivery and tissue engineering | |
| TW202448544A (zh) | 用於插入醫療裝置之插入系統及方法 | |
| WO2024181920A1 (en) | A bioactive poly(lactic-co-glycolic acid) (plga) material, related printed structure and related methods thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 2018504565 Country of ref document: JP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2017763338 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2017763338 Country of ref document: EP Effective date: 20181009 |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17763338 Country of ref document: EP Kind code of ref document: A1 |