WO2016002827A1 - Agent thérapeutique contre les maladies démyélinisantes progressives autoimmunes - Google Patents
Agent thérapeutique contre les maladies démyélinisantes progressives autoimmunes Download PDFInfo
- Publication number
- WO2016002827A1 WO2016002827A1 PCT/JP2015/068942 JP2015068942W WO2016002827A1 WO 2016002827 A1 WO2016002827 A1 WO 2016002827A1 JP 2015068942 W JP2015068942 W JP 2015068942W WO 2016002827 A1 WO2016002827 A1 WO 2016002827A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- eomes
- demyelinating disease
- cells
- progressive
- antibody
- Prior art date
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/7105—Natural ribonucleic acids, i.e. containing only riboses attached to adenine, guanine, cytosine or uracil and having 3'-5' phosphodiester links
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/713—Double-stranded nucleic acids or oligonucleotides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
Definitions
- the present invention relates to a therapeutic agent for progressive immune demyelinating disease.
- MS Multiple sclerosis
- RR-MS relapsing-remitting MS
- SP-MS secondary progressive MS
- PR-MS progressive recurrent MS
- DMT disease-modifying therapy
- the present invention has been made in view of these circumstances, and its main object is to provide a therapeutic agent for progressive immune demyelinating disease. Another object of the present invention is to provide a method for diagnosing progressive immune demyelinating disease and to provide a non-human animal model that becomes a model of progressive immune demyelinating disease.
- EAE pathology In the NR4A2-deficient mice in which monophasic experimental autoimmune encephalomyelitis (EAE) was induced, the EAE pathology with normal limb paralysis was not observed in the early stage of induction, whereas the late stage (induction) It was found that EAE pathology (hereinafter also referred to as “late EAE pathology”) was observed about 28 days later) and that late EAE pathology became a model for progressive MS pathology.
- the inventors of the present invention have also found that the late EAE pathology is enhanced by the increased expression of the Eome gene in CD4 + T cells infiltrating the central nervous system (CNS) and the suppression of Eomes gene expression. It was found that it can be suppressed.
- CNS central nervous system
- the present invention provides the following (1) to (22).
- a therapeutic agent for progressive immune demyelinating disease comprising an anti-CD27 antibody or an antigen-binding fragment thereof, or an anti-CD226 antibody or an antigen-binding fragment thereof as an active ingredient.
- a therapeutic agent for progressive immune demyelinating disease comprising an expression inhibitor that suppresses the expression of the Eomes gene as an active ingredient.
- nucleic acid is at least one selected from the group consisting of an antisense oligonucleotide against the Eomes gene, siRNA, shRNA, miRNA and ribozyme.
- a therapeutic agent for progressive immune demyelinating disease comprising an activity inhibitory substance that inhibits the activity of cytotoxic protease as an active ingredient.
- the cytotoxic protease is a cytotoxic protease produced by Eome + CD4 + T cells.
- the therapeutic agent for progressive immune demyelinating disease according to (5) or (6), wherein the activity inhibitor is a protease inhibitor.
- the therapeutic agent for progressive immune demyelinating disease according to (5) or (6), wherein the activity inhibitor is a granzyme B inhibitor.
- a method for diagnosing advanced immune demyelinating disease comprising the steps of detecting Eome + CD4 + T cells in a body fluid containing lymphocytes collected from a human subject, and detected Eomes + CD4 + Determining that the human subject has developed an advanced immune demyelinating disease when the frequency of T cells exceeds a threshold.
- the threshold is set based on the frequency of Eome + CD4 + T cells in the blood of a healthy human subject.
- a data collection method for diagnosis of advanced immune demyelinating disease the method comprising the steps of detecting Eome + CD4 + T cells in a body fluid collected from a human subject, including lymphocytes, Calculating the frequency of Eomes + CD4 + T cells.
- the data collection method according to (14), wherein the body fluid is blood or cerebrospinal fluid.
- (21) Use of an NR4A2 gene knockout non-human animal as a non-human animal model for progressive immune demyelinating disease.
- EAE experimental autoimmune encephalomyelitis
- NR4A2 gene knockout non-human animal is an NR4A2 gene conditional knockout mouse.
- NR4A2 gene knockout non-human animal is a mouse in which the NR4A2 gene is knocked out specifically in CD4 + T cells.
- the present invention it is possible to provide a therapeutic agent for progressive immune demyelinating disease, to provide a diagnostic method for progressive immune demyelinating disease, and to provide a non-human animal model that becomes a progressive immune demyelinating disease model It becomes.
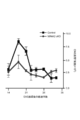
- FIG. 5 is a cytogram showing IL-17, IFN- ⁇ and Eomes expression of CD4 + T cells infiltrating into the CNS in NR4A2-deficient mice and control mice induced in single phase EAE.
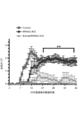
- FIG. 5 is a cytogram showing CD27 and CD11a expression of CD4 + T cells infiltrating into the CNS in NR4A2-deficient mice and control mice in the late phase of EAE that induced monophasic EAE. It is a graph which shows the EAE score of the NR4A2-deficient mouse
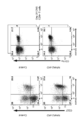
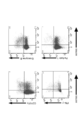
- FIG. 3 is a cytogram showing CD107a, IFN- ⁇ , granzyme B and perforin 1 expression of CD3 + CD4 + PBMC derived from an SP-MS patient.
- FIG. 19A is a cytogram showing CD4 and Eomes expression in CD3 + PBMC from SP-MS patients and CD4 and Eomes expression in CD3 + CSF from SP-MS patients.
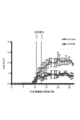
- 19B is a graph plotting the percentage of CD4 + Eome + T cells in CD3 + PBMC and CD3 + CSF from SP-MS patients. It is a graph which shows the change of EAE disease state by administration of granzyme B specific siRNA.
- progressive immune demyelinating disease refers to a disease caused by damage of the myelin sheath caused by an immune reaction and progressing continuously without remission.
- the progressive immune demyelinating disease is preferably a progressive immune demyelinating disease of the central nervous system.
- progressive immune demyelinating diseases include progressive multiple sclerosis such as PP-MS, SP-MS, and PR-MS.
- NR4A2 gene is also called Nurr1 gene, NOT gene, or RNR1 gene, and is a kind of orphan nuclear receptor.
- the main expression site of the NR4A2 gene is the central nervous system, and is particularly strongly expressed in the ventral midbrain, brainstem, and spinal cord.
- NR4A2 is induced in response to prostaglandins, growth factors, inflammatory cytokines, and T cell receptor cross-linking, and directly binds to DNA in a ligand-dependent or ligand-independent manner to control transcription.
- the accession number of NCBI Reference Sequence of the transcription product of human NR4A2 gene is NM_006186.3.
- the Eomes gene also called Eomesodermin or Tbr2
- Tbr2 is a member of the T-box transcription factor family and is a protein involved in vertebrate development and differentiation.
- the Eomes gene is known to be expressed in CD8 + T cells (cytotoxic T cells, CTL) and NK cells. It is also known to directly induce perforin and granzyme B expression.
- the NCBI Reference Sequence accession numbers for the transcripts of the human Eomes gene are NM_001278182.1 (variant 1), NM_005442.3 (variant 2), and NM_001278183.1 (variant 3).
- the therapeutic agent for progressive immune demyelinating disease according to the first embodiment of the present invention contains an anti-CD27 antibody or an antigen-binding fragment thereof, or an anti-CD226 antibody or an antigen-binding fragment thereof as an active ingredient.
- the therapeutic agent can also be used as a progression inhibitor of the pathological condition of progressive immune demyelinating disease.
- the anti-CD27 antibody may be a monoclonal antibody or a polyclonal antibody.
- the anti-CD27 antibody may be any of a mouse antibody, rat antibody, guinea pig antibody, hamster antibody, rabbit antibody, monkey antibody, dog antibody, chimeric antibody, humanized antibody or human antibody.
- the anti-CD27 antibody may be subjected to chemical modification in order to improve physical properties such as retention in blood.
- the anti-CD27 antibody may be one to which a radionuclide, a toxin or the like is bound in order to enhance the therapeutic effect.
- the anti-CD226 antibody may be a monoclonal antibody or a polyclonal antibody. Further, the anti-CD226 antibody may be any of a mouse antibody, rat antibody, guinea pig antibody, hamster antibody, rabbit antibody, monkey antibody, dog antibody, chimeric antibody, humanized antibody or human antibody. The anti-CD226 antibody may be subjected to chemical modification in order to improve physical properties such as retention in blood. The anti-CD226 antibody may be one to which a radionuclide, a toxin or the like is bound in order to enhance the therapeutic effect.
- the antigen-binding fragment may be an antibody fragment containing an antigen-binding site of an antibody, and examples thereof include Fab, Fab ′, F (ab ′) 2 , scFv, and diabody.
- the therapeutic agent for progressive immune demyelinating disease comprises one of the above-described anti-CD27 antibody, antigen-binding fragment of anti-CD27 antibody, anti-CD226 antibody, and antigen-binding fragment of anti-CD226 antibody. It may contain independently and may contain it in combination of 2 or more types.
- the anti-CD27 antibody or antigen-binding fragment thereof, or the anti-CD226 antibody or antigen-binding fragment thereof can be produced according to a conventional method. Moreover, you may use a commercial item.
- the content of the active ingredient (anti-CD27 antibody or antigen-binding fragment thereof, or anti-CD226 antibody or antigen-binding fragment thereof) in the therapeutic agent for progressive immune demyelinating disease according to the first embodiment is particularly limited. For example, it may be 0.001 to 100% by mass based on the total amount of the therapeutic agent for advanced immune demyelinating disease.
- the therapeutic agent for progressive immune demyelinating disease according to the first embodiment may be composed of only the above-mentioned active ingredient, and in addition to the above-mentioned active ingredient, excipients and buffers commonly used in the pharmaceutical technology field And additives such as stabilizers, antioxidants, binders, disintegrants, fillers, emulsifiers and flow control agents.
- the dosage form of the therapeutic agent for progressive immune demyelinating disease according to the first embodiment is, for example, any dosage form such as powder, pill, granule, tablet, syrup, troche, capsule, injection, etc. It may be.
- the therapeutic agent for progressive immune demyelinating disease according to the first embodiment may be administered orally or parenterally.
- a specific dose for example, when administered to a human adult male (body weight 60 kg), the daily dose of the therapeutic agent for progressive immune demyelinating disease is usually 0. 0001 ⁇ g to 10,000 mg / day / person.
- the above first embodiment comprises the step of administering an anti-CD27 antibody or antigen-binding fragment thereof, or an anti-CD226 antibody or antigen-binding fragment thereof to a human subject in need thereof. It can also be referred to as a treatment method of the above or a method of suppressing the progression of the disease state.
- the therapeutic agent for progressive immune demyelinating disease contains an expression inhibitor that suppresses the expression of the Eomes gene as an active ingredient.
- the therapeutic agent can also be used as a progression inhibitor of the pathological condition of progressive immune demyelinating disease.
- the expression suppressing substance that suppresses the expression of the Eomes gene may be any substance that can suppress the function of the Eomes gene as a protein.
- Examples of the expression-suppressing substance include a substance that can suppress the expression of the Eomes gene at the transcription level or the translation level, and a substance that can bind to a functional site of the Eome protein and suppress the functional expression.
- Examples of the substance capable of suppressing the expression of the Eomes gene at the transcription level or the translation level may be, for example, a nucleic acid, a peptide, a sugar or a glycoprotein that suppresses the expression of the Eomes gene, a low molecular compound having a molecular weight of 1000 or less, and the like.
- Examples of the nucleic acid that suppresses Eomes gene expression include at least one selected from the group consisting of antisense oligonucleotides, siRNAs, shRNAs, miRNAs and ribozymes against the Eomes gene.
- substances that can bind to a functional site of the Eomes protein and suppress functional expression include anti-Eomes antibodies or antigen-binding fragments thereof (for example, neutralizing antibodies).
- An expression inhibitor that suppresses the expression of the Eomes gene can be designed and manufactured by a method known in this technical field based on information such as the genome sequence, mRNA sequence, protein sequence, and protein three-dimensional structure of the Eomes gene. .
- the therapeutic agent for progressive immune demyelinating disease according to the second embodiment may contain one kind of expression suppressing substance that suppresses the expression of the above-mentioned Eomes gene alone, or contains two or more kinds in combination. You may do it.
- the content of the active ingredient is not particularly limited. It may be 0.001 to 100% by mass based on the total amount of the therapeutic agent for demyelinating disease.
- the therapeutic agent for progressive immune demyelinating disease according to the second embodiment may be composed of only the above active ingredient, and in addition to the above active ingredient, excipients and buffers commonly used in the pharmaceutical technology field And additives such as stabilizers, antioxidants, binders, disintegrants, fillers, emulsifiers and flow control agents.
- the dosage form of the therapeutic agent for progressive immune demyelinating disease according to the second embodiment is, for example, any dosage form such as powder, pill, granule, tablet, syrup, troche, capsule, injection, etc. It may be.
- the therapeutic agent for progressive immune demyelinating disease according to the second embodiment may be administered orally or parenterally.
- a specific dose for example, when administered to a human adult male (body weight 60 kg), the daily dose of the therapeutic agent for progressive immune demyelinating disease is usually 0. 0001 pg to 10000 mg / day / person.
- the second embodiment includes a method for treating an advanced immune demyelinating disease or a method for inhibiting the progression of a disease state, which comprises the step of administering an expression inhibitor that suppresses the expression of the Eomes gene to a human subject in need thereof. It can also be said.
- the therapeutic agent for progressive immune demyelinating disease contains, as an active ingredient, an activity inhibitor that inhibits the activity of cytotoxic proteases.
- the therapeutic agent can also be used as a progression inhibitor of the pathological condition of progressive immune demyelinating disease.
- the cytotoxic protease may be a cytotoxic protease produced by Eomes + CD4 + T cells.
- cytotoxic protease examples include serine protease, preferably trypsin-like serine protease, and more preferably granzyme.
- granzyme examples include granzyme A, granzyme B, granzyme C, granzyme D, granzyme E, granzyme F, granzyme G and granzyme H, preferably granzyme A or granzyme B.
- the activity inhibitor that inhibits the activity of the cytotoxic protease may be a nucleic acid, peptide, sugar or glycoprotein as long as it can inhibit the activity of the cytotoxic protease, and is a low molecular weight compound having a molecular weight of 2000 or less. May be.
- the substance that inhibits the activity of cytotoxic proteases is preferably a serine protease inhibitor, more preferably a granzyme inhibitor, and more preferably a granzyme A inhibitor or granzyme B inhibitor.
- serine protease inhibitors include tosyl lysine chloromethyl ketone hydrochloride (TLCK hydrochloride), N-tosyl-L-phenylalanine chloromethyl ketone.
- granzyme A inhibitors examples include 3,4-dichloroisocoumarin, nafamostat mesylate, anti-granzyme A antibody or antigen-binding fragment thereof, granzyme A specific siRNA, granzyme A specific shRNA, granzyme A specific.
- a shRNA plasmid is mentioned.
- Examples of the granzyme B inhibitor include benzyloxycarbonyl-isoleucine-glutamic acid-threonine-aspartic acid fluoromethyl ketone (Z-IETDFMK), acetyl-isoleucine-glutamic acid-threonine-aspartic acid-aldehyde (Ac-IETD-CHO), 3,4-dichloroisocoumarin, Ecotin, E .; E.
- coli acetyl-isoleucine-glutamic acid-proline-aspartic acid-aldehyde (Ac-IEPD-CHO), benzyloxycarbonyl-alanine-alanine-aspartic acid, chloromethyl ketone (Z-AAD-CMK), protease inhibitor 9 (J. Immunol 2001; 166: 3218-3225), WO2012 / 076985, anti-granzyme B antibody or antigen-binding fragment thereof, granzyme B-specific siRNA, granzyme B-specific shRNA, granzyme B-specific shRNA plasmid .
- the therapeutic agent for progressive immune demyelinating disease according to the third embodiment may contain one kind of the above-mentioned activity inhibitor that inhibits the activity of the cytotoxic protease, or a combination of two or more kinds. May be contained.
- the content of the active ingredient (activity inhibitor that inhibits the activity of cytotoxic protease) in the therapeutic agent for progressive immune demyelinating disease according to the third embodiment is not particularly limited.
- the amount may be 0.001 to 100% by mass based on the total amount of the therapeutic agent for type immune demyelinating disease.
- the therapeutic agent for progressive immune demyelinating disease according to the third embodiment may be composed of only the above active ingredient, and in addition to the above active ingredient, excipients and buffers commonly used in the pharmaceutical technology field And additives such as stabilizers, antioxidants, binders, disintegrants, fillers, emulsifiers and flow control agents.
- the dosage form of the therapeutic agent for progressive immune demyelinating disease according to the third embodiment is, for example, any dosage form such as powder, pill, granule, tablet, syrup, troche, capsule, injection, etc. It may be.
- the progressive immune demyelinating disease therapeutic agent according to the third embodiment may be administered orally or parenterally.
- a specific dose for example, when administered to a human adult male (body weight 60 kg), the daily dose of the therapeutic agent for progressive immune demyelinating disease is usually 0. 0001 ⁇ g to 10,000 mg / day / person.
- the third embodiment includes a method for treating progressive immune demyelinating disease, or progression of a disease state, comprising administering an activity inhibitor that inhibits the activity of cytotoxic protease to a human subject in need thereof It can also be called a suppression method.
- the therapeutic agent for progressive immune demyelinating disease of the present invention has been described separately from the first embodiment to the third embodiment, the therapeutic agent for progressive immune demyelinating disease of the present invention is the first embodiment.
- the active ingredient in the form, the active ingredient in the second embodiment, and the active ingredient in the third embodiment may be included singly or in combination of two or three kinds. Good.
- the therapeutic agent for progressive immune demyelinating disease of the present invention is preferably used as a therapeutic agent for progressive multiple sclerosis.
- the method for diagnosing progressive immune demyelinating disease comprises a step (detection step) of detecting Eome + CD4 + T cells in a body fluid containing lymphocytes collected from a human subject, and a detected Eomes A step of determining that the human subject has developed an advanced immune demyelinating disease (determination step) when the frequency of + CD4 + T cells exceeds a threshold (determination step).
- Eomes + CD4 + T cells in a body fluid collected from a human subject and containing lymphocytes are detected.
- the body fluid collected from the human subject may be a body fluid containing lymphocytes.
- body fluids collected from human subjects include blood and cerebrospinal fluid.
- the blood may be peripheral blood.
- the detection of Eome + CD4 + T cells can be performed according to a conventional method in this technical field.
- Detection of Eome + CD4 + T cells is not limited to this, for example, a step of separating PBMC from a body fluid sample collected from a human subject according to a conventional method, such as labeled anti-CD3 antibody or Reacting the antigen-binding fragment, the labeled anti-CD4 antibody or the antigen-binding fragment thereof, and the labeled anti-Eomes antibody or the antigen-binding fragment thereof with PBMC and detecting Eome + CD4 + T cells with a flow cytometer. It can be implemented by a method.
- Eome + CD4 + T cells By detecting Eome + CD4 + T cells in the detection step, for example, the concentration of Eome + CD4 + T cells in the body fluid sample (cells / mL), the total number of lymphocytes in the body fluid sample, Eome + CD4 + T cells Indices such as (%), the total number of Eome + CD4 + T cells (%) relative to the total number of CD3 + CD4 + PBMC cells in the body fluid sample can be obtained.
- the determination step when the frequency of detected Eomes + CD4 + T cells exceeds a threshold, it is determined that the human subject has developed progressive immune demyelinating disease.
- Eomes + CD4 + T frequency of cells as long as it reflects the Eomes + CD4 + T cell count in a body fluid sample, specifically, for example, each indicator described above.
- the above frequency is the ratio of Eome + T cells in CD3 + CD4 + human peripheral blood mononuclear cells (Eome to the total number of CD3 + CD4 + PBMC cells in body fluid samples). + Equivalent to the total number of CD4 + T cells).
- the above threshold value is preferably set in advance. Thereby, the dispersion
- the threshold value may be set based on, for example, the frequency of Eomes + CD4 + T cells in the body fluid of a healthy human subject.
- a healthy human subject may be, for example, a human subject not suffering from progressive immune demyelinating disease.
- the above diagnostic method is suitably used as a diagnostic method for progressive multiple sclerosis.
- the present invention also provides, as a modified embodiment of the above diagnostic methods, taken from a human subject, a step (detecting step) of detecting the Eomes + CD4 + T cells in body fluids including lymphocytes, detected Eomes + CD4 +
- the present invention also relates to a data collection method for diagnosing advanced immune demyelinating disease, comprising the step of calculating the frequency of T cells (calculation step).
- diagnosis of advanced immune demyelinating disease may be performed based only on comparison with the above-mentioned threshold.
- diagnosis of advanced immune demyelinating disease may be performed by combining other clinical findings.
- the present invention also provides a reagent A for measuring the amount of transcript or translation product of the Eomes gene, and a reagent B for measuring the amount of transcript or translation product of the CD4 gene, as modified embodiments of the above diagnostic method.
- a diagnostic agent for progressive immune demyelinating disease can also be referred to as a diagnostic kit for progressive immune demyelinating disease.
- the reagent A may be any reagent that can measure the amount of transcription product or translation product of the Eomes gene.
- DNA that can hybridize to the transcription product of the Eomes gene or its complementary strand.
- Nucleic acids such as RNA and peptide nucleic acids (PNA), antibodies capable of binding to the translation product of the Eomes gene (eg, anti-Eomes antibody or antigen-binding fragment thereof), proteins (eg, EOMES, MIXL1, RACHYURY, GOOSECOID, TBX6) , FGF8, SNAI1, SPRY2, SPRY4, WNT3, WNT3A, NODAL, BRachyury, MEOX1, TBX6, KDR, FOXC1, ISL1, PDGFRA, SOX17, CXCR4, LHX1, FOXA1, FOXA2, FOAX3, FOAX3 Eg to a nucleic acid comprising a T-box core motif (TCACACCT), genomic DNA) and the like
- the reagent B may be any reagent that can measure the amount of transcription product or translation product of the CD4 gene. Specifically, for example, DNA that can hybridize to the transcription product of CD4 gene or its complementary strand. , Nucleic acids such as RNA and peptide nucleic acid (PNA), antibodies capable of binding to the translation product of CD4 gene (for example, anti-CD4 antibody or antigen-binding fragment thereof), proteins (for example, MHC class II protein complex) It is done.
- Nucleic acids such as RNA and peptide nucleic acid (PNA)
- PNA peptide nucleic acid
- antibodies capable of binding to the translation product of CD4 gene for example, anti-CD4 antibody or antigen-binding fragment thereof
- proteins for example, MHC class II protein complex
- the diagnostic agent for progressive immune demyelinating disease is an anti-Eomes antibody or an antigen-binding fragment thereof (reagent A), and an anti-CD4 antibody or an antigen-binding fragment thereof (reagent B), for example.
- the advanced immune demyelinating disease can be diagnosed as follows. PBMC are separated from peripheral blood collected from the subject according to a conventional method. Next, the separated PBMC is reacted with the anti-CD4 antibody or antigen-binding fragment thereof, and the anti-Eomes antibody or antigen-binding fragment thereof. In this step, an anti-CD3 antibody or an antigen-binding fragment thereof may be further reacted.
- the ratio of Eomes + CD4 + T cells is measured by analysis using a flow cytometer (for example, Agilent 2100 Bioanalyzer; manufactured by Agilent).
- a flow cytometer for example, Agilent 2100 Bioanalyzer; manufactured by Agilent.
- the reference value may be the same as the threshold value in the diagnostic method for progressive immune demyelinating disease described above.
- the diagnostic agent for progressive immune demyelinating disease may be one in which reagent A and reagent B are immobilized on a base material.
- reagent A and Reagent B are nucleic acids such as DNA that can hybridize to the transcription product of Eomes gene or CD4 gene or its complementary strand
- the DNA or the like is used as a substrate such as a glass substrate or a resin substrate. It may be an immobilized DNA chip or the like.
- Reagent A and Reagent B are antibodies that can bind to the translation product of the Eomes gene or CD4 gene (anti-Eomes antibody or antigen-binding fragment thereof, and anti-CD4 antibody or antigen-binding fragment thereof), the antibody May be an antibody chip immobilized on a substrate such as a glass substrate or a resin substrate.
- the progressive immune demyelinating disease can be diagnosed as follows. PBMC are separated from peripheral blood collected from the subject according to a conventional method. Next, CD3 + CD4 + cells are separated from the separated PBMC using anti-CD3 antibody and anti-CD4 antibody (this step may or may not be performed). Total RNA or mRNA is extracted from the separated PBMC or CD3 + CD4 + cells according to a conventional method. Using the extracted total RNA or mRNA as a sample, the expression levels of the Eomes gene and the CD4 gene are analyzed with a DNA chip according to a conventional method. When the expression levels of the Eomes gene and the CD4 gene exceed the reference values, it is determined that the subject has developed (or may have developed) an advanced immune demyelinating disease. The reference value can be determined from, for example, measured values in healthy human subjects.
- Eome + CD4 + T cells can be detected in a body fluid collected from a human subject and containing lymphocytes.
- the diagnostic agent may further contain an anti-CD3 antibody or an antigen-binding fragment thereof. This also makes it possible to detect the proportion of Eome + T cells occupying CD3 + CD4 + human peripheral blood mononuclear cells.
- Non-human animal model of advanced immune demyelinating disease The present invention further provides the use of NR4A2 gene knockout non-human animals as non-human animal models for advanced immune demyelinating diseases.
- the NR4A2 gene knockout non-human animal is not particularly limited as long as it is a non-human animal deficient in the NR4A2 gene (including a case where it is deficient only in a specific tissue or cell).
- non-human animals include mice, rats, guinea pigs, hamsters, rabbits, monkeys, and dogs.
- EAE experimental autoimmune encephalomyelitis
- NR4A2 gene knockout non-human animals can cause a pathological condition of progressive immune demyelinating disease (eg, progressive MS). Therefore, it can be suitably used as a non-human animal model of progressive immune demyelinating disease for elucidating the disease mechanism and screening for effective therapeutic agents.
- progressive MS progressive immune demyelinating disease
- the NR4A2 gene knockout non-human animal is preferably an NR4A2 gene conditional knockout mouse.
- conditional knockout means that a target gene is deleted in a specific tissue or a specific cell of a non-human animal, unlike the case where all genes are deleted. Examples thereof include a mouse (NR4A2cKO mouse) in which the NR4A2 gene is specifically deleted in CD4 + T cells.
- NR4A2cKO mice can be established, for example, by Cre-loxP site-specific recombination techniques. Specifically, in the NR4A2cKO mouse, the target cell is expressed so that the Cre enzyme is expressed in the mouse in which two loxP genes are introduced so as to sandwich the NR4A2 gene, which is the target gene to be deleted, and in the cell in which the target gene is to be deleted. It can be produced by mating with a mouse into which the cre gene has been introduced downstream of the promoter region. A tissue or cell in which a gene of interest is deficient can be selected by those skilled in the art according to its purpose. For example, if the target cell is a CD4 + T cell, a mouse deficient in the NR4A2 gene can be produced only in the CD4 + T cell.
- EAE autoimmune encephalomyelitis
- the EAE model inoculates myelin proteins such as spinal cord crush fluid, purified myelin, myelin basic protein (MBP), PLP, MOG, and peptides obtained from these proteins as protein antigens derived from central nervous tissue.
- myelin proteins such as spinal cord crush fluid, purified myelin, myelin basic protein (MBP), PLP, MOG, and peptides obtained from these proteins as protein antigens derived from central nervous tissue.
- MBP myelin basic protein
- MOG protein antigens derived from central nervous tissue.
- a chronic persistent EAE model is usually created as follows. First, an equivalent amount of a peptide corresponding to MOG 35-55 residues (hereinafter also referred to as “MOG peptide”) and Freund's incomplete adjuvant plus Mycobacterium tuberculosis H37Ra is mixed to prepare an emulsion.
- the MOG peptide is preferably 95% or more pure.
- PBS phosphate buffer
- Pertussis toxin in PBS (phosphate buffer) is administered 0 and 2 days after injection. Pertussis toxin may be administered intravenously or intraperitoneally.
- the administration method is preferably intraperitoneal administration.
- a chronic relapsing EAE model is usually made as follows. First, an equivalent amount of a peptide corresponding to PLP 139-151 residues (hereinafter also referred to as “PLP peptide”) and Freund's incomplete adjuvant plus Mycobacterium tuberculosis H37Ra is mixed to prepare an emulsion.
- the PLP peptide is preferably 95% or more pure.
- PLP peptide When producing an emulsion, you may stir by hand and you may stir using a homogenizer or a sonicator.
- the resulting emulsion is then injected subcutaneously at 1-2 locations on the back of SJL / J mice.
- Pertussis toxin in PBS is administered at 0 and 2 days after injection. Pertussis toxin may be administered intravenously or intraperitoneally. The administration method is preferably intraperitoneal administration.
- EAE pathology may be observed without administration of pertussis toxin.
- the passive EAE model is used to collect an EAE model by collecting CD4 + T cells reactive with central nerve protein in an animal that has been induced with EAE by the chronic persistent EAE model or the chronic recurrent EAE model. It can be made by transferring to an animal. As a method of transfer, intravenous injection may be used.
- the NR4A2 gene knockout non-human animal in which EAE has been induced can be used as a pathological model of advanced immune demyelinating disease, particularly as an animal model showing the late stage (advanced stage) of advanced MS.
- EAE experimental autoimmune encephalomyelitis
- the step of inducing experimental autoimmune encephalomyelitis (EAE) in a non-human animal can be performed according to the general conditions for inducing EAE.
- the NR4A2 gene knockout non-human animal is preferably an NR4A2cKO mouse.
- the NR4A2cKO mouse can be used as an appropriate animal model depending on the pathological state of progressive MS.
- the pathological condition of progressive immune demyelinating disease can be analyzed in more detail.
- NR4A2cKO mice EAE analysis of NR4A2cKO mice
- NR4A2 fl / fl mice were established using a targeting vector with the NR4A2 gene sandwiched between loxp sequences. That is, the NR4A2 transgene sandwiched between loxp sequences was introduced into C57BL / 6 embryonic stem cells by microinjection. The established strain was crossed with C57BL / 6 FLPe mice (RIKEN BioResource Center) and the strains from which the neomycin cassette was removed were crossed to produce homozygous NR4A2 fl / fl C57BL / 6 mice.
- mice were crossed with C57BL / 6 CD4-Cre mice (Taconic) to establish CD4-specific NR4A2cKO C57BL / 6 mice (C57BL / 6 Cre-CD4 / NR4A2 fl ⁇ fl mice).
- C57BL / 6 CD4-Cre mice Teconic mice
- CD4-specific NR4A2cKO C57BL / 6 mice C57BL / 6 Cre-CD4 / NR4A2 fl ⁇ fl mice.
- SJL / J mice female, Charles River Japan Co., Ltd.
- NR4A2 fl / fl SJL mice and CD4-specific NR4A2cKO SJL Mice SJL / J Cre-CD4 / NR4A2 fl ⁇ fl mice
- Eomes fl / fl mice purchased from Jackson Laboratories were crossed with C57BL / 6 CD4-Cre mice to obtain Cre-CD4 Eomes fl / fl C57BL / 6 mice. Further, by mating Eomes fl / fl mice and Cre-CD4 / NR4A2 fl / fl C57BL / 6 mice to obtain Cre-CD4 / NR4A2 fl / fl Eomes fl / fl C57BL / 6 mice.
- EAE induction single phase EAE 100 ⁇ g of peptide corresponding to MOG 35-55 residue (synthesized at Toray Research Center, Tokyo, Japan, hereinafter also referred to as “MOG peptide”) and 1 mg of Mycobacterium tuberculosis H37Ra (Difco, Kansas, USA) Emulsified with complete Freund's adjuvant was mixed in an equal amount and emulsified with a homogenizer to prepare a MOG emulsion.
- the resulting MOG emulsion was applied subcutaneously to the back of CD4-specific NR4A2cKO C57BL / 6 mice (Cre-CD4 / NR4A2 fl / fl C57BL / 6 mice, NR4A2cKO) and NR4A2 fl / fl C57BL / 6 mice (Control) as controls.
- 200 ⁇ L of a 200 ng pertussis toxin (List Biological Laboratories, USA) PBS solution was injected into the abdominal cavity of mice on the 0th and 2nd days after immunization. After injection, the EAE pathology of the mice was evaluated daily according to the EAE evaluation criteria shown below.
- the obtained tissue homogenate was passed through a 70 ⁇ m cell strainer (manufactured by GE Healthcare Science), and white blood cells were concentrated using a discontinuous Percoll density gradient centrifugation method (37% / 80%). The number of T cells infiltrating into the CNS was measured by FACS ARIA II (manufactured by BD Cytometry Systems). At that time, an anti-CD3 antibody (manufactured by Biolegend) was used for detection of T cells.
- the obtained T cells were stimulated with the MOG peptide, and the IL-17 content in the culture supernatant was measured using a Flow Cytomix cytometric bead array (manufactured by eBioscience) and a Bio-Plex suspension array system (BioRad). It was measured. The amount of IL-17 obtained was divided by the number of CNS infiltrating T cells, and the amount of IL-17 production per CNS infiltrating T cell was calculated.
- EAE induction (relapsing-remitting EAE) 100 ⁇ g of a peptide corresponding to PLP 139-151 residues (hereinafter also referred to as “PLP peptide”) and 1 mg of Mycobacterium tuberculosis H37Ra killed in incomplete Freund's adjuvant are mixed together, and a homogenizer is used. Emulsified to prepare a PLP emulsion. The obtained PLP emulsion was injected subcutaneously into the back of CD4-specific NR4A2cKO SJL mice (NR4A2cKO SJL mice) and NR4A2 fl / fl SJL mice (Control SJL mice) as a control to give immunity.
- mice were evaluated daily for EAE pathology according to the EAE criteria described above.
- NR4A2-deficient mice have suppressed relapsing-remitting EAE pathology and Th17 cell response, while NR4A2-deficient mice have a single pathological EAE that has a new pathological condition (late stage) while the initial pathology is suppressed.
- EAE pathology appears, T cells infiltrate the central nervous system even in late EAE pathology, but there is no Th17 cell response, and monophasic EAE pathology has two different pathologies with different NR4A2 dependence It became clear that it consisted of (early pathological condition and late pathological condition).
- cDNA was synthesized using a first strand cDNA synthesis kit (manufactured by Takara).
- the Light Cycler apparatus was used with the Light Cycler-FastStart DNA Master SYBR Green I kit (Roche Diagnostics) or the ABI 7300 real-time PCR instrument with the Power SYBR Green Master Mix (Applied Bios). Quantitative real-time PCR was performed using commercially available primers (QuantiTect Primer Assay, QT01074332, manufactured by Qiagen).
- the Eomes gene expression level was corrected based on the expression level of the GAPDH housekeeping gene.
- the antibodies used in the analysis were an anti-CD3 antibody (manufactured by Biolegend), an anti-CD4 antibody (manufactured by Biolegend), an anti-IL-17 antibody (manufactured by eBioscience), and an anti-IFN- ⁇ antibody (manufactured by eBioscience). is there.
- Eomes + CD4 + T surface antigens the surface antigen expressed on the cell surface of Eomes + CD4 + T cells were analyzed using a flow cytometer.
- the antibodies used in the analysis were an anti-CD27 antibody (manufactured by eBioscience), an anti-CD11a antibody (manufactured by Biolegend), and an anti-CD226 antibody (manufactured by Biolegend).
- Eome + CD4 + T cells expressed CD27 and highly expressed CD11a. Although data are not shown, Eomes + CD4 + T cells expressed CD226.
- the antibodies used for the separation were an anti-CD3 antibody (manufactured by Biolegend), an anti-CD4 antibody (manufactured by Biolegend), and an anti-CD27 antibody (manufactured by eBioscience).
- the isolated CD4 + CD27 + T cells were restimulated with anti-CD3 and anti-CD28 antibodies, and 10,000 cells were treated as described above in 1.
- Single-phase EAE was induced in the same manner as in (2), and transferred to NR4A2cKO mice and control mice 10 days after induction of single-phase EAE by intravenous injection. After injection, the mice were evaluated daily for EAE pathology according to the EAE criteria described above.
- EAE could be induced by transferring CNS-derived CD4 + CD27 + T cells.
- control siRNA Negative control, manufactured by Koken Co., Ltd.
- a specific siRNA SEQ ID NO: 1: 5′-ggcucuuuauucucauucauUU-3 ′
- the control siRNA is commercially available as a control siRNA containing a sequence that does not match any gene of human, mouse, or rat.
- EAE pathology of NR4A2 / Eomes deficient mice In the same manner as (2), Eomes fl / fl mouse (Control eomes fl / fl ), Cre-CD4 Eomes fl / fl C57BL / 6 mouse (Cre-CD4 eomes fl / fl ), NR4A2 fl / fl Efls fl Monophasic EAE was induced in C57BL / 6 mice (Control), CD4-specific NR4A2cKO mice (NR4A2cKO) and Cre-CD4 / NR4A2 fl / fl Eomes fl / fl C57BL / 6 mice (Eomes / NR4A2cKO). Thereafter, the EAE pathology of the mice was evaluated daily according to the EAE evaluation criteria.
- FIGS. As shown in FIG. 10, Eome-deficient mice in which the cre gene was introduced under the CD4 gene promoter and the eomes gene was sandwiched between loxP genes (Cre-CD4 eomes fl / fl ) were sandwiched between the eomes genes with the loxP gene. Compared to control mice (Control eomes fl / fl ), the EAE score was slightly improved.
- NR4A2-deficient mice NR4A2cKO
- EAE score increased in late EAE pathology
- NR4A2 / Eomes-deficient mice NR4A2 / eomes cKO
- late EAE score did not increase and late EAE pathology did not develop. It was.
- the antibodies used for the separation were an anti-CD3 antibody (manufactured by Biolegend), an anti-CD4 antibody (manufactured by Biolegend), and an anti-CD27 antibody (manufactured by eBioscience).
- RNA was extracted from the obtained cells, and the expression levels of perforin 1 and granzyme B were measured with the LightCycler device using the Light Cycler-FastStart DNA Master SYBR Green I kit (Roche Diagnostics) or ABI 7300 real-time PCR.
- FIGS. 12 and 13 The results are shown in FIGS. As shown in FIGS. 12 and 13, in CD4 + CD27 + T cells derived from NR4A2 deficient mice, no significant difference was observed in the expression level of perforin 1 compared to CD4 + CD27 ⁇ T cells, but granzyme B The expression level of was increased. Moreover, as shown in FIG. 14, the expression level of CD107a was increased by stimulating Eomes + CD4 + T cells with an anti-CD3 antibody. That is, Eome + CD4 + T cells are considered to degranulate upon stimulation with anti-CD3 antibody.
- the chronic persistent EAE model can be classified into two types of early EAE pathology and late EAE pathology. Specifically, the early EAE pathology is dependent on the NR4A2 gene and involves Th17 cells, and the late EAE pathology is dependent on the Eomes gene and involves pathological cytotoxic CD4 + T cells. It is believed that there is.
- the antibodies used in the analysis were anti-CD3 antibody (manufactured by Biolegend), anti-CD4 antibody (manufactured by Biolegend), anti-CD11a antibody (manufactured by Biolegend), anti-CD27 antibody (manufactured by eBioscience), and anti-Eomes antibody ( eBioscience).
- FIGS. Figure 16 is a cytogram showing the CD4 and Eomes expression in CD3 + PBMC, a cytogram showing the CD27 and Eomes expression in the cytogram, and CD3 + CD4 + PBMC showing the CD11a and Eomes expression in CD3 + CD4 + PBMC.
- FIG. 17 is a plot of the proportion of Eomes + cells in CD3 + CD4 + PBMC for healthy adult volunteers, RR-MS patients and SP-MS patients. As shown in FIGS. 16 and 17, Eome + CD4 + T cells were selectively enhanced in SP-MS patients.
- CD3 + CD4 + PBMC derived from SP-MS patients highly express cytotoxic factors (IFN- ⁇ , perforin 1, granzyme B), and when stimulated with anti-CD3 antibody, The expression level of CD107a increased. Therefore, Eome + CD4 + T cells are thought to degranulate upon stimulation.
- the expression levels of Eomes and CD4 in CD3 + PBMC and CD3 + CSF were analyzed using a flow cytometer.
- the antibodies used in the analysis were anti-CD3 antibody (manufactured by Biolegend), anti-CD4 antibody (manufactured by Biolegend), and anti-Eomes antibody (manufactured by eBioscience).
- FIG. 19A is a cytogram showing CD4 and Eomes expression in CD3 + PBMC, and a cytogram showing CD4 and Eomes expression in CD3 + CSF.
- FIG. 19B is a graph plotting the percentage of CD4 + Eomes + T cells in CD3 + PBMC and CD3 + CSF. As shown in FIG. 19B, in 5 SP-MS patients, the proportion of CD4 + Eomes + T cells in CD3 + CSF was higher than the proportion of CD4 + Eomes + T cells in CD3 + PBMC.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Molecular Biology (AREA)
- Engineering & Computer Science (AREA)
- Biochemistry (AREA)
- Genetics & Genomics (AREA)
- Immunology (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Biotechnology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Abstract
L'invention concerne un agent thérapeutique contre les maladies démyélinisantes progressives autoimmunes contenant, en tant que composant actif, un anticorps anti-CD27, un fragment de liaison à l'antigène de celui-ci, un anticorps anti-CD226, ou un fragment de celui-ci se liant à l'antigène ; un agent thérapeutique contre les maladies démyélinisantes progressives autoimmunes contenant, en tant que composant actif, un agent inhibiteur de l'expression inhibant l'expression du gène Eomes ; ou un agent thérapeutique contre les maladies démyélinisantes progressives autoimmunes contenant, en tant que composant actif, une substance inhibitrice qui inhibe l'activité d'une protéase cytotoxique.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2016531412A JPWO2016002827A1 (ja) | 2014-07-01 | 2015-07-01 | 進行型免疫性脱髄疾患治療剤 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2014-135630 | 2014-07-01 | ||
| JP2014135630 | 2014-07-01 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2016002827A1 true WO2016002827A1 (fr) | 2016-01-07 |
Family
ID=55019357
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2015/068942 WO2016002827A1 (fr) | 2014-07-01 | 2015-07-01 | Agent thérapeutique contre les maladies démyélinisantes progressives autoimmunes |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JPWO2016002827A1 (fr) |
| WO (1) | WO2016002827A1 (fr) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016114386A1 (fr) * | 2015-01-15 | 2016-07-21 | 国立研究開発法人国立精神・神経医療研究センター | Agent thérapeutique contre les maladies de démyélinisation immunologiques de type progressives |
| WO2018101261A1 (fr) | 2016-11-29 | 2018-06-07 | 国立研究開発法人国立精神・神経医療研究センター | Agent prophylactique, agent de suppression de l'apparition de la maladie ou agent thérapeutique contre des maladies démyélinisantes progressives d'origine immunologique |
| CN111363042A (zh) * | 2020-03-30 | 2020-07-03 | 中国人民解放军第四军医大学 | 一种高特异性抗小鼠cd226单克隆抗体及其应用 |
| KR20230104903A (ko) | 2020-11-06 | 2023-07-11 | 고쿠리쯔겡뀨가이하쯔호징 고꾸리쯔 세이신ㆍ신께이 이료겡뀨센따 | Eomes 양성 CD4 양성 T 세포의 증가에 기인하는 진행형 질환 치료제 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2013531970A (ja) * | 2010-04-13 | 2013-08-15 | セルデックス・セラピューティクス・インコーポレイテッド | ヒトcd27に結合する抗体およびその使用 |
-
2015
- 2015-07-01 WO PCT/JP2015/068942 patent/WO2016002827A1/fr active Application Filing
- 2015-07-01 JP JP2016531412A patent/JPWO2016002827A1/ja active Pending
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2013531970A (ja) * | 2010-04-13 | 2013-08-15 | セルデックス・セラピューティクス・インコーポレイテッド | ヒトcd27に結合する抗体およびその使用 |
Non-Patent Citations (3)
| Title |
|---|
| CONSTANTINESCU, C.S. ET AL.: "Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS", BRITISH JOURNAL OF PHARMACOLOGY, vol. 164, 2011, pages 1079 - 1106, XP055216575 * |
| DARDALHON, V. ET AL.: "CD 226 is specifically expressed on the surface of Th1 cells and regulates their expansion and effector functions", THE JOURNAL OF IMMUNOLOGY, vol. 175, 2005, pages 1558 - 1565, XP003018252 * |
| NAKAJIMA, A. ET AL.: "Involvement of CD 70- CD 27 interactions in the induction of experimental autoimmune encephalomyelitis", JOURNAL OF NEUROIMMUNOLOGY, vol. 109, 2000, pages 188 - 196, XP002423716 * |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016114386A1 (fr) * | 2015-01-15 | 2016-07-21 | 国立研究開発法人国立精神・神経医療研究センター | Agent thérapeutique contre les maladies de démyélinisation immunologiques de type progressives |
| WO2018101261A1 (fr) | 2016-11-29 | 2018-06-07 | 国立研究開発法人国立精神・神経医療研究センター | Agent prophylactique, agent de suppression de l'apparition de la maladie ou agent thérapeutique contre des maladies démyélinisantes progressives d'origine immunologique |
| CN111363042A (zh) * | 2020-03-30 | 2020-07-03 | 中国人民解放军第四军医大学 | 一种高特异性抗小鼠cd226单克隆抗体及其应用 |
| CN111363042B (zh) * | 2020-03-30 | 2022-02-11 | 中国人民解放军第四军医大学 | 一种高特异性抗小鼠cd226单克隆抗体及其应用 |
| KR20230104903A (ko) | 2020-11-06 | 2023-07-11 | 고쿠리쯔겡뀨가이하쯔호징 고꾸리쯔 세이신ㆍ신께이 이료겡뀨센따 | Eomes 양성 CD4 양성 T 세포의 증가에 기인하는 진행형 질환 치료제 |
Also Published As
| Publication number | Publication date |
|---|---|
| JPWO2016002827A1 (ja) | 2017-04-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Pereira et al. | Epidemiological, clinical, and immunological characteristics of neuromyelitis optica: a review | |
| US8053197B2 (en) | Methods for detecting and treating autoimmune disorders | |
| JP6999417B2 (ja) | Ibdにおける治療標的及びバイオマーカー | |
| EP2510948B1 (fr) | Inhibiteur d'activation des lymphocytes t, composition pharmaceutique contenant celui-ci, et procédé de criblage pour une substance inhibant l'activation des lymphocytes t | |
| US8815526B2 (en) | Methods and reagents for identifying/isolating T regulatory (Treg) cells and for treating individuals | |
| WO2016002827A1 (fr) | Agent thérapeutique contre les maladies démyélinisantes progressives autoimmunes | |
| US8629108B2 (en) | Rheumatoid arthritis T cell vaccine | |
| Djikić et al. | Age-related changes in spleen of Dark Agouti rats immunized for experimental autoimmune encephalomyelitis | |
| US20200371087A1 (en) | Methods for identifying and separating pro-allergic specific t cells | |
| Erlandsson et al. | IGF1R signalling is a guardian of self-tolerance restricting autoantibody production | |
| US20210230282A1 (en) | Prophylactic agent, onset-suppressing agent or therapeutic agent for progressive immune demyelinating diseases | |
| JP2021521121A (ja) | 自己免疫疾患を処置するための方法 | |
| JP2010004750A (ja) | 自己免疫疾患による組織傷害の発症または発症可能性の診断方法、およびその利用 | |
| JP2020100582A (ja) | 多発性硬化症抑制剤 | |
| WO2023232826A1 (fr) | Biomarqueurs de l'activité du modulateur de l'il7r | |
| Lee | B Cell Responses in an Animal Model of Neuroinflammation: Implications for Multiple Sclerosis | |
| Salvador | Evolution of the B cell repertoire in a spontaneous model of experimental autoimmune encephalomyelitis (EAE) | |
| WO2010150819A1 (fr) | Agent thérapeutique destiné aux troubles cérébrovasculaires ischémiques | |
| WO2017061125A1 (fr) | Thérapie, diagnostic, et criblage utilisant card14 | |
| Falk et al. | T. 33. In vitro Blockade of GSK3-β Selectively Reduces the Proinflammatory Phenotype of Lymphocytes from Chronic Inflamed Intestinal Tissue Claudia Hofmann, Nadja Dunger, Juergen Schoelmerich | |
| Krishnaswami et al. | Hepatic Steatosis in Rheumatoid Arthritis: Associations with Disease Characteristics, Pharmacotherapies, and Atherosclerosis. Jon T. Giles | |
| Altintas et al. | BOARD MEMBERS |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15814726 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2016531412 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 15814726 Country of ref document: EP Kind code of ref document: A1 |