WO2015072443A1 - 紫外線散乱剤及びその用途 - Google Patents
紫外線散乱剤及びその用途 Download PDFInfo
- Publication number
- WO2015072443A1 WO2015072443A1 PCT/JP2014/079816 JP2014079816W WO2015072443A1 WO 2015072443 A1 WO2015072443 A1 WO 2015072443A1 JP 2014079816 W JP2014079816 W JP 2014079816W WO 2015072443 A1 WO2015072443 A1 WO 2015072443A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- meth
- scattering agent
- particles
- resin
- agent according
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/02—Cosmetics or similar toiletry preparations characterised by special physical form
- A61K8/0241—Containing particulates characterized by their shape and/or structure
- A61K8/0245—Specific shapes or structures not provided for by any of the groups of A61K8/0241
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/02—Cosmetics or similar toiletry preparations characterised by special physical form
- A61K8/0241—Containing particulates characterized by their shape and/or structure
- A61K8/027—Fibers; Fibrils
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/72—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds
- A61K8/81—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds obtained by reactions involving only carbon-to-carbon unsaturated bonds
- A61K8/8105—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers
- A61K8/8117—Homopolymers or copolymers of aromatic olefines, e.g. polystyrene; Compositions of derivatives of such polymers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/72—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds
- A61K8/81—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds obtained by reactions involving only carbon-to-carbon unsaturated bonds
- A61K8/8141—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides or nitriles thereof; Compositions of derivatives of such polymers
- A61K8/8152—Homopolymers or copolymers of esters, e.g. (meth)acrylic acid esters; Compositions of derivatives of such polymers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q1/00—Make-up preparations; Body powders; Preparations for removing make-up
- A61Q1/02—Preparations containing skin colorants, e.g. pigments
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q1/00—Make-up preparations; Body powders; Preparations for removing make-up
- A61Q1/02—Preparations containing skin colorants, e.g. pigments
- A61Q1/10—Preparations containing skin colorants, e.g. pigments for eyes, e.g. eyeliner, mascara
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L101/00—Compositions of unspecified macromolecular compounds
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D11/00—Inks
- C09D11/02—Printing inks
- C09D11/03—Printing inks characterised by features other than the chemical nature of the binder
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D125/00—Coating compositions based on homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by an aromatic carbocyclic ring; Coating compositions based on derivatives of such polymers
- C09D125/02—Homopolymers or copolymers of hydrocarbons
- C09D125/04—Homopolymers or copolymers of styrene
- C09D125/06—Polystyrene
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D125/00—Coating compositions based on homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by an aromatic carbocyclic ring; Coating compositions based on derivatives of such polymers
- C09D125/02—Homopolymers or copolymers of hydrocarbons
- C09D125/04—Homopolymers or copolymers of styrene
- C09D125/08—Copolymers of styrene
- C09D125/14—Copolymers of styrene with unsaturated esters
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D133/00—Coating compositions based on homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides, or nitriles thereof; Coating compositions based on derivatives of such polymers
- C09D133/04—Homopolymers or copolymers of esters
- C09D133/06—Homopolymers or copolymers of esters of esters containing only carbon, hydrogen and oxygen, the oxygen atom being present only as part of the carboxyl radical
- C09D133/10—Homopolymers or copolymers of methacrylic acid esters
- C09D133/12—Homopolymers or copolymers of methyl methacrylate
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H17/00—Non-fibrous material added to the pulp, characterised by its constitution; Paper-impregnating material characterised by its constitution
- D21H17/63—Inorganic compounds
- D21H17/67—Water-insoluble compounds, e.g. fillers, pigments
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/40—Chemical, physico-chemical or functional or structural properties of particular ingredients
- A61K2800/41—Particular ingredients further characterized by their size
- A61K2800/412—Microsized, i.e. having sizes between 0.1 and 100 microns
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q17/00—Barrier preparations; Preparations brought into direct contact with the skin for affording protection against external influences, e.g. sunlight, X-rays or other harmful rays, corrosive materials, bacteria or insect stings
- A61Q17/04—Topical preparations for affording protection against sunlight or other radiation; Topical sun tanning preparations
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q5/00—Preparations for care of the hair
- A61Q5/06—Preparations for styling the hair, e.g. by temporary shaping or colouring
- A61Q5/065—Preparations for temporary colouring the hair, e.g. direct dyes
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D5/00—Coating compositions, e.g. paints, varnishes or lacquers, characterised by their physical nature or the effects produced; Filling pastes
- C09D5/004—Reflecting paints; Signal paints
Definitions
- the present invention relates to an ultraviolet (UV) scattering agent and its use.
- Polymer particles and inorganic particles having a micron size are used as fillers and specimens in various fields such as electronic / electrical materials, optical materials, paints, inks, building materials, biological / pharmaceutical materials, and cosmetics.
- development of fine particles having an irregular shape different from a spherical shape has been actively carried out, and since various properties such as optical properties and tactile sensation are imparted, application development is being promoted every day.
- the present inventors also proceeded with the development of elliptical or acicular polymer particles having a high aspect ratio, and developed particles superior to conventional spherical particles in terms of properties such as concealment, light scattering, and touch. (Patent Documents 1 to 5).
- UV absorbers and UV scattering in order to prevent adverse effects on the human body due to UV and deterioration of molded products and compositions caused by UV, etc. (Diffusion) agents are used.
- UV absorbers In general, the effect of UV absorbers increases as the amount added increases, but the UV shielding mechanism mainly absorbs, so there is a drawback that only UV in a certain wavelength region can be shielded, and the durability is further improved. Problems may arise. In addition, when UV absorbers are used in cosmetics and the like, there is a risk that a person with weak skin may have an effect on the human body such as rough skin and rash.
- UV scattering agents composed of inorganic particles such as zinc oxide and titanium oxide generally have a high UV scattering effect, but it is difficult to maintain dispersion in the state of primary particles, and many of them exist as aggregates. As a result, there is a drawback that the concealment property becomes too high. Further, there is a drawback that the glossiness is impaired even if only a small amount is added.
- Inorganic materials have a higher specific gravity than organic materials, and when used in films, molded products, etc., they are less compatible with resins, which may cause inconveniences in terms of weight reduction and cracking performance. Furthermore, in cosmetics, a large amount of surfactant may have to be used to maintain dispersion, and the use of a large amount of surfactant is not preferable from the viewpoint of rough skin and prevention of skin aging.
- the present invention has been made in view of the above circumstances, and is excellent in lightness, light scattering, tactile sensation, fluidity, etc., and also in safety, and as a UV-cut (shielding) additive, cosmetics, paints, and inks.
- Another object of the present invention is to provide a UV scattering agent that can be used for film sheets and the like.
- this invention provides the following UV scattering agent and its use. 1.
- the average (L AV ) of the major axis (L) of the projection two-dimensional view obtained by irradiating light from the direction orthogonal to the major axis direction is 0.1 to 80 ⁇ m
- the average (D AV ) of the minor axis (D) of the projection two-dimensional drawing obtained by irradiating light from the direction orthogonal to the major axis direction is 0.05 to 40 ⁇ m
- the average (P AV ) of the aspect ratio (L / D) calculated from the diameter and the minor axis (D) is 2 to 30
- a UV scattering agent comprising at least one type of elliptical or acicular polymer particles A.
- Polymer particles A are styrene resin, (meth) acrylic resin, vinyl ester resin, poly-N-vinyl compound resin, polyolefin resin, polydiene resin, polyester resin, silicone resin, polyurethane resin, 1 UV scattering agent comprised from at least 1 sort (s) chosen from polyamide-type resin, polyimide-type resin, epoxy-type resin, polyvinyl butyral-type resin, phenol-type resin, amino-type resin, oxazoline-type resin, and carbodiimide resin. 3.
- the polymer particle A is at least one kind selected from styrenes, (meth) acrylic acid, (meth) acrylic acid esters, vinyl esters, N-vinyl compounds, olefins, fluorinated olefins and conjugated dienes.
- 1 or 2 UV scattering agent which is a (co) polymer consisting of a monomer. 4). Further, (4) any one of 1 to 3 including at least one kind of particles B having a shape different from that of the polymer particles A and having a volume average particle diameter (MV B ) satisfying 1/5 ⁇ D AV ⁇ MV B ⁇ L AV UV scattering agent. 5.
- UV scattering agent in which the particle B is spherical or substantially spherical. 7).
- a resin composition comprising a UV scattering agent according to any one of 1 to 7. 9.1 Dispersion containing any one of UV scattering agents. 10.
- a paint comprising any one of UV scatterers of 10.1 to 7.
- An ink comprising any one of the UV scattering agents of 11.1 to 7.
- a cosmetic composition comprising the UV scattering agent according to any one of 12.1 to 7.
- a method of imparting UV blocking properties by adding any one of UV scattering agents of 1 to 7 to a transparent or translucent resin, water or volatile oil.
- the UV scattering agent of the present invention is a polymer particle, it is excellent in lightness, light scattering property, tactile sensation, fluidity and the like, and is also excellent in safety, and can be suitably used as an additive for UV cut.
- the average (L AV ) of the major axis (L) of the projection two-dimensional view obtained by irradiating light from the direction orthogonal to the major axis direction is 0.1 to 80 ⁇ m
- the average (D AV ) of the minor axis (D) of the projection two-dimensional view obtained by irradiating light from the direction orthogonal to the major axis direction is 0.05 to 40 ⁇ m
- the major axis (L) and the minor axis are short. It contains at least one elliptical or acicular polymer particle A having an average (P AV ) of aspect ratio (L / D) calculated from the diameter (D) of 2 to 30.
- L AV of polymer particles A is 0.1 ⁇ 80 [mu] m, preferably 0.2 ⁇ 50 [mu] m, more preferably 1.0 ⁇ 30 ⁇ m, 2 ⁇ 20 ⁇ m is more preferable.
- L AV exceeds 80 ⁇ m, the UV scattering effect tends to be remarkably reduced as the specific surface area per unit decreases.
- L AV is less than 0.1 ⁇ m, the minor axis becomes thin, so that UV light leakage occurs, and the UV scattering effect may be reduced.
- the D AV of the polymer particles A is 0.05 to 40 ⁇ m, preferably 0.1 to 25 ⁇ m, more preferably 0.5 to 15 ⁇ m, still more preferably 1 to 10 ⁇ m.
- D AV exceeds 40 ⁇ m, the UV scattering effect tends to be remarkably reduced as the specific surface area per unit decreases.
- D AV is less than 0.05 ⁇ m, UV light leakage may occur and the UV scattering effect may be reduced.
- P AV of the polymer particles A is from 2 to 30, preferably from 3 to 25, more preferably from 3.5 to 20, the best is 4 to 18.
- P AV is less than 2, there is only a UV scattering effect as high as that of spherical particles of the same component, and the effect is not superior.
- the volume average particle diameter (MV A ) of the polymer particles A is preferably from 0.06 to 50 ⁇ m, more preferably from 0.1 to 30 ⁇ m, still more preferably from 0.5 to 20 ⁇ m.
- MV A exceeds 50 ⁇ m, the UV scattering effect may be reduced as the specific surface area per unit decreases.
- MV A is less than 0.06 ⁇ m, UV light leakage may occur and the UV scattering effect may be reduced.
- the volume average particle diameter is a value measured by a laser scattering / diffraction method.
- the volume average particle diameter in the case of elliptical or needle-shaped particles or irregularly shaped particles, it means the average particle diameter when the volume is converted to a sphere. To do.
- Polymer particles A are styrene resin, (meth) acrylic resin, vinyl ester resin, poly-N-vinyl compound resin, polyolefin resin, polydiene resin, polyester resin, silicone resin, polyurethane resin, It is preferably composed of at least one selected from polyamide resins, polyimide resins, epoxy resins, polyvinyl butyral resins, phenol resins, amino resins, oxazoline resins and carbodiimide resins. In this case, each of the resins may be a single polymer or a copolymer.
- the “styrene resin” is a resin having styrene as a main structural unit, and includes not only a single polymer of styrene but also a copolymer of styrene or a styrene and another monomer.
- Styrene resins include (co) polymers of styrenes, styrene- (meth) acrylic acid copolymers, styrene- (meth) acrylic acid ester copolymers, acrylonitrile-styrene copolymers, acrylonitrile-chlorinated polyethylene.
- -Styrene copolymer styrene-maleic anhydride copolymer, etc., or modified ones thereof, styrene-butadiene block copolymer (SBR), styrene-butadiene-styrene block copolymer (SBS), hydrogenated styrene- Butadiene-styrene block copolymer (SEBS), styrene-isoprene block copolymer (SIR), styrene-isoprene-styrene block copolymer (SIS), hydrogenated styrene-isoprene-styrene block copolymer (SEPS), etc.
- SBR styrene-butadiene block copolymer
- SBS styrene-butadiene-styrene block copolymer
- SEBS hydrogenated styrene- Butadiene-st
- (Meth) acrylic resins include (meth) acrylic acid (co) polymer, (meth) acrylic acid ester (co) polymer, (meth) acrylic acid- (meth) acrylic acid ester copolymer, vinyl ester -Olefin such as (meth) acrylic acid copolymer, vinyl ester- (meth) acrylic acid ester copolymer, ethylene-acrylic acid copolymer-(meth) acrylic acid copolymer, ethylene-acrylic acid ester copolymer Olefin- (meth) acrylic acid ester copolymer, N-vinyl compound- (meth) acrylic acid copolymer, N-vinyl compound- (meth) acrylic acid ester copolymer, conjugated diene- (meth) Examples thereof include acrylic acid copolymers and conjugated diene- (meth) acrylic acid ester copolymers.
- vinyl ester resins examples include vinyl ester (co) polymers, olefin-vinyl ester copolymers such as ethylene-vinyl acetate copolymers, vinyl ester-conjugated diene copolymers, and the like.
- poly-N-vinyl compound-based resin examples include (co) polymers of N-vinyl compounds, copolymers of olefin-N-vinyl compounds, copolymers of conjugated diene-N-vinyl compounds, and the like.
- polyolefin resin examples include polyolefin, polyfluorinated olefin, olefin and / or fluorinated polyolefin copolymer, and olefin-conjugated diene copolymer.
- polydiene resin examples include (co) polymers of conjugated dienes.
- Resins composed of unsaturated monomers such as styrene resins, (meth) acrylic resins, vinyl ester resins, poly-N-vinyl compound resins, polyolefin resins, polydiene resins, etc. Two or more kinds of copolymers can be prepared according to the use and purpose.
- the polyester resin is not particularly limited.
- terephthalic acid or dimethyl terephthalate is a main acid component, and at least one alkylene glycol selected from ethylene glycol, diethylene glycol, trimethylene glycol, and butylene glycol is a main glycol component.
- Polyester resin polylactic acid and the like. Specifically, polyethylene terephthalate, polyethylene naphthalate, polybutylene terephthalate, polybutylene naphthalate, polytrimethylene terephthalate, polycyclohexylene dimethylene terephthalate, polycyclohexylene dimethylene naphthalate, polybutylene terephthalate, polybutylene naphthalate, Examples include polylactic acid.
- the silicone resin is not particularly limited as long as it contains a silicon-silicon bond, a silicon-carbon bond, a siloxane bond or a silicon-nitrogen bond in the molecular chain.
- Specific examples include polysiloxane, polycarbosilane, and polysilazane.
- polyurethane resin examples include a polyurethane resin obtained by polymerizing a polyol and a polyisocyanate.
- a polyurethane resin obtained by polymerizing a polyol and a polyisocyanate.
- the polyol ethylene glycol, diethylene glycol, propylene glycol, dipropylene glycol, glycerin, 1,1,1-trimethylolpropane, 1,2,5-hexanetriol, 1,3-butanediol, 1,4-butanediol 4,4′-dihydroxyphenylpropane, 4,4′-dihydroxyphenylmethane, pentaerythritol and the like.
- polyisocyanate examples include 4-tolylene diisocyanate, 2,6-tolylene diisocyanate, 4,4′-diphenylmethane diisocyanate, 2,4′-diphenylmethane diisocyanate, p-phenylene diisocyanate, isophorone diisocyanate, xylylene diisocyanate, and the like.
- Polyamide resins include adipic acid, heptanedicarboxylic acid, octanedicarboxylic acid, nonanedicarboxylic acid, undecanedicarboxylic acid, dodecanedicarboxylic acid and other dicarboxylic acids, tetramethylenediamine, hexamethylenediamine, octamethylenediamine, nonamethylenediamine, Examples thereof include polyamide resins obtained by polycondensation with diamines such as undecamethylenediamine and dodecamethylenediamine.
- polyamide resins obtained by ring-opening polymerization of lactams such as ⁇ -pyrrolidone, ⁇ -caprolactam, ⁇ -laurolactam, and ⁇ -enantolactam can be mentioned.
- lactams such as ⁇ -pyrrolidone, ⁇ -caprolactam, ⁇ -laurolactam, and ⁇ -enantolactam
- Specific examples include nylon-6, nylon-11, nylon-12, nylon-6,6, nylon-6, T and the like.
- Polyimide resins include o-phenylenediamine, m-phenylenediamine, p-phenylenediamine, 4,4'-diaminodiphenyl ether, 1,4-bis (aminomethyl) cyclohexane, 1,3-bis (aminomethyl) cyclohexane Diamines such as 1,3-propanediamine, 1,4-butanediamine, 1,5-pentanediamine, 1,6-hexanediamine, and 4,4′-hexafluoropropylidenebisphthalic dianhydride, 4 , 4'-biphthalic anhydride, diphenyl-2,3,3 ', 4'-tetracarboxylic dianhydride, diphenyl-2,2', 3,3'-tetracarboxylic dianhydride, pyromellitic acid Examples thereof include a polyimide resin obtained by polymerizing tetracarboxylic dianhydride such as dianhydride.
- Epoxy resins include polyepoxides, aromatic polyepoxy compounds, glycidyl ethers of polyhydric phenols, glycidyl ester of polyhydric phenols, glycidyl aromatic polyamines, alicyclic polyepoxy compounds, aliphatic polyepoxy compounds. And polyglycidyl ester of polyvalent fatty acid. Of these, aliphatic polyepoxy compounds and aromatic polyepoxy compounds are preferred.
- polyvinyl butyral resin examples include a reaction product of polyvinyl alcohol and butyraldehyde, a product in which molecules are cross-linked by a monobutyral bond, and the like.
- phenolic resins include resins obtained using organic compounds belonging to phenols such as phenol and cresol.
- amino resins examples include urea resins, melamine resins, and guanamine resins.
- the oxazoline-based resin examples include a bisoxazoline compound, a compound having a terminal oxazoline group obtained by reacting two chemical equivalents of a bisoxazoline compound with one chemical equivalent of a carboxyl group of a polybasic carboxylic acid.
- the oxazoline compound may be a polymerized compound having at least two or more oxazoline groups in one molecule obtained from a polymer such as addition polymerization without opening the oxazoline ring. Examples thereof also include a copolymer of an addition-polymerizable oxazoline compound and a copolymerizable monomer that does not react with the oxazoline group.
- Examples of the carbodiimide resin include resins having at least one carbodiimide group obtained using one or more isocyanate compounds as raw materials.
- the polymer particles A are more preferably styrene resins, (meth) acrylic resins, polyolefin resins, vinyl ester resins, poly-N-vinyl compound resins, polydiene resins, and the like.
- the polymer particle A is particularly at least one selected from styrenes, (meth) acrylic acid, (meth) acrylic esters, vinyl esters, N-vinyl compounds, olefins, fluorinated olefins and conjugated dienes.
- a (co) polymer comprising the above monomers is preferred.
- (co) polymers containing, as an essential unit, a repeating unit obtained from at least one monomer selected from styrenes, (meth) acrylic acid, and (meth) acrylic acid esters are preferred.
- the polymer particles A may be a mixture of two or more kinds as long as the above conditions (1) to (3) are satisfied.
- the UV scattering agent of the present invention further comprises (4) at least one particle B having a shape different from that of the polymer particle A and having a volume average particle diameter (MV B ) satisfying 1/5 ⁇ D AV ⁇ MV B ⁇ L AV. It is preferable to include seeds.
- the different shape described here includes elliptical or needle-like particles having a shape or size different from that of the polymer particle A, but is easily oriented, UV scattering effect, light scattering property in the visible light region, light reflection property, etc.
- an arbitrary shape which is preferably different from an elliptical shape or a needle shape is preferable.
- the substantially spherical shape means an ellipse having an aspect ratio of less than 2.
- the polymer particle A has a higher UV scattering effect than conventional spherical or substantially spherical particles having the same composition depending on its shape, but at the time of production and preparation of molded products and compositions.
- the particles are likely to be arranged along the flow direction due to fluidity, and in that case, optical characteristics such as UV scattering effect, light scattering property in the visible light region, and light reflection property may not be stably obtained.
- the anisotropic characteristics can be stably controlled by adding the predetermined particles B to impart steric hindrance. That is, the particle B can maintain a stable UV scattering effect without impairing the characteristics of the polymer particle A.
- the volume average particle diameter (MV B ) of the particle B preferably satisfies 1/3 ⁇ D AV ⁇ MV B ⁇ 0.8 ⁇ L AV, and 1/2 ⁇ D AV ⁇ MV B ⁇ 0.6 ⁇ L. it is more preferable to satisfy the AV, it is most preferable to satisfy D AV ⁇ MV B ⁇ 1/ 2 ⁇ L AV. If MV B is less than 1/5 ⁇ D AV , the stability of steric hindrance may be lacking. If MV B exceeds L AV , the UV scattering effect tends to depend on the volume average particle size, so UV scattering The effect may be reduced.
- Particle B may be inorganic particles or polymer particles.
- the particle B is preferably a polymer particle.
- the polymer constituting the particles preferably contains the same or at least the same component as that of the polymer particles A from the viewpoint of production efficiency. In consideration of improving the diffusion performance in the visible light region, it is effective that the polymer particles A and B have component compositions having different refractive indexes.
- the particle B may be a mixture of two or more kinds as long as the above condition (4) is satisfied. Depending on the application, it may be a mixture of polymer particles and inorganic particles.
- At least one of the polymer particles A and the particles B has at least one of the following characteristics: one having fine irregularities on the particle surface, one having porosity, and one having a relatively large specific surface area. It is preferable that the UV scattering effect and the visible light scattering effect are further improved.
- At least one of the polymer particle A and the particle B can be a composite particle having a core-shell structure or a composite particle obtained by adding other fine particles physically or chemically.
- the composite particles are obtained by (1) incorporating other fine particles during the production of the mother particles, and (2) adding using the polarity of ionic functional groups present on the surface of the mother particles after the production of the mother particles. (3) Chemical methods such as addition polymerization, polycondensation, addition condensation, and seed polymerization.
- the other fine particles are not limited to organic and inorganic substances as long as the particles are smaller than polymer particles A and particles B serving as mother particles.
- the preferred particle diameter of the other fine particles is usually about 0.005 to 50 ⁇ m although it depends on the size of the mother particles.
- organic particles examples include particles made of a polymerizable monomer used for producing polymer particles, curable particles, and organic pigments.
- Inorganic particles include copper powder, iron powder, gold powder, aluminum oxide, titanium oxide, zinc oxide, silicon oxide, tin oxide, copper oxide, iron oxide, magnesium oxide, manganese oxide, calcium carbonate, magnesium hydroxide, aluminum hydroxide And other metals, metal oxides, hydrated metal oxides, inorganic pigments and the like.
- these fine particles may be used as they are, or those that have been surface-modified with a surface treatment agent such as a coupling agent in advance may be used.
- metal oxide fine particles having a particle diameter of 0.005 to 10 ⁇ m particularly titanium oxide and zinc oxide are used for the purpose of controlling the refractive index and improving the UV scattering effect.
- silicon oxide or the like can be added. These can be used alone or in combination of two or more.
- the metal oxide fine particles are mixed with 0.1 to 50% by mass of the fine particles with respect to the whole polymerization component and reacted at the time of production of the polymer particles. It can be taken in by adsorption or bonding. In this way, it is possible to further improve the UV scattering effect while maintaining the glossiness by incorporating inorganic particles in appropriate amounts into polymer fine particles or combining them as a coating.
- the effect as a drug can be imparted by appropriately combining the active substance physically, mechanically or chemically.
- the physical, mechanical, or chemical bond referred to here can be applied by a known technique. For example, if it is a component that dissolves, it can be absorbed / adsorbed inside or on the surface of the particle, a coloring technique such as a dye, A well-known chemical bonding technique that chemically adsorbs the reactive group in the particle or in the surface layer part and the reactive group in the active substance can also be applied.
- Examples of the reactive group herein include ⁇ , ⁇ -unsaturated carbonyl group, ⁇ , ⁇ -unsaturated nitrile group, vinyl halide group, vinylidene halide group, aromatic vinyl group, heterocyclic vinyl group, and conjugated diene.
- Polymerizable unsaturated bond-containing groups such as carboxylic acid vinyl ester, carboxyl group, carbonyl group, epoxy group, isocyanate group, hydroxy group, amide group, cyano group, amino group, epoxy group, chloromethyl group, glycidyl ether group, Lithio group, ester group, formyl group, nitrile group, nitro group, carbodiimide group, oxazoline group and the like can be mentioned.
- the mixing ratio is preferably 99: 1 to 10:90, more preferably 98: 2 to 30:70 97: 3 to 50:50 is more preferable. Most preferably, it is 95: 5 to 80:20.
- the mixing ratio of the particles B exceeds 90% by mass, the optical characteristics in the visible light region of the polymer particles A may be greatly reduced simultaneously with the UV scattering effect. If the mixing ratio of the particles B is less than 1% by mass, there may be a problem in the stability of optical properties in the visible light region of the polymer particles A at the same time as the UV scattering effect.
- the method for producing the polymer particles is not particularly limited as long as the method can obtain the shape described above.
- the particle B when it is a polymer particle, it can be obtained by pulverization or solution polymerization.
- the polymer particles A can be obtained, for example, by the methods described in Patent Documents 1 to 5 and the like. Specifically, the particle diameter obtained by using a monomer having a general unsaturated double bond can be easily controlled. It is preferable to produce by solution polymerization.
- Solution polymerization includes (1) emulsion or suspension polymerization performed in an aqueous solution, (2) dispersion polymerization performed in the presence of a dispersant in water, an organic solvent, or a mixed solvent of water and an organic solvent, (3) The method etc. which combine (1) or (2) and a seed method are mentioned.
- the mixed solution polymerization of (1) and (2) can be suitably used.
- the particle B is a polymer particle and the component is the same as that of the polymer particle A
- the target mixed particles can be easily prepared as appropriate by changing the amount ratio.
- Examples of the polymerizable monomer that is a raw material for the polymer particles used in the present invention include: (I) Styrene, o-methylstyrene, m-methylstyrene, p-methylstyrene, ⁇ -methylstyrene, o-ethylstyrene, m-ethylstyrene, p-ethylstyrene, 2,4-dimethylstyrene, pn -Butyl styrene, pt-butyl styrene, pn-hexyl styrene, pn-octyl styrene, pn-nonyl styrene, pn-decyl styrene, pn-dodecyl styrene, p-methoxy styrene , Styrenes such as
- (Meth) acrylic monomer 2-methy (ethyl) acrylate, N-propylaminoethyl (meth) acrylate, N-ethylaminopropyl (meth) acrylate, N-phenylamino (meth) acrylate
- Amino group-containing (meth) acrylic monomers such as ethyl and (meth) acrylic acid N-cyclohexylaminoethyl; 3- (meth) acryloyloxypropyltrimethoxysilane, 3- (meth) acryloyloxypropyldimethoxymethylsilane, etc.
- Silicon-containing (meth) acrylic monomers (poly) ethylene glycol mono (meth) acrylate, 2-methacrylic acid (meth) acrylate And (meth) acrylic monomers such as 3-methoxybutyl (meth) acrylate; (poly) alkylene glycol (meth) acrylic monomers such as (poly) propylene glycol mono (meth) acrylate Alkoxy (poly) alkylene glycol (meth) acrylic monomers such as methoxy (poly) ethylene glycol mono (meth) acrylate and methoxy (poly) propylene glycol mono (meth) acrylate; 2-mercaptoethyl (meth) acrylate (Meth) acrylic monomers containing a mercapto group such as 2-mercapto-1-carboxyethyl (meth) acrylate; (meth) acrylic monomers such as 2-chloroethyl (meth) acrylate and methyl ⁇ -chloro (meth)
- styrenes (meth) acrylic acid, (meth) acrylic acid esters, vinyl esters and the like as polymerizable monomers, and by using these, polymer particles having the shape described above. Can be easily obtained at low cost.
- an unsaturated monomer having a reactive functional group such as a hydrophilic functional group or an active hydrogen group
- reactive functional groups include amino groups, carboxyl groups, hydroxy groups, thiol groups, carbonyl groups, ether groups, cyano groups, amide groups, alkylene oxide groups, epoxy groups, and ionic functional groups.
- the functional group may be present alone or in combination of two or more.
- Introducing reactive functional groups such as hydrophilic functional groups and active hydrogen groups into the interior of the particle or the surface layer portion not only improves the functions of hydrophilicity and oil resistance, but also the inorganic particles and other polymer fine particles described above.
- Application as auxiliary functional groups with various functions such as formation of cross-linked structures by reaction of functional groups, surface treatment and surface modification by binding of reactive compounds, addition of active substances, etc. Can do.
- Examples of the unsaturated monomer having such a reactive functional group include those shown below.
- C n means that the number of carbon atoms is n.
- Amino group-containing monomers Allylamine derivatives such as allylamine and N-methylallylamine; Amino group-containing styrene derivatives such as p-aminostyrene; Triazine derivatives such as 2-vinyl-4,6-diamino-S-triazine Etc. Of these, compounds having a primary or secondary amino group are preferred.
- Carboxyl group-containing monomers Unsaturated carboxylic acids such as crotonic acid, cinnamic acid, itaconic acid, maleic acid and fumaric acid; itaconic acid mono C 1 -C 8 alkyl esters such as monobutyl itaconic acid; monobutyl maleate And maleic acid mono C 1 -C 8 alkyl esters; vinyl group-containing aromatic carboxylic acids such as vinyl benzoic acid; and salts thereof.
- Unsaturated carboxylic acids such as crotonic acid, cinnamic acid, itaconic acid, maleic acid and fumaric acid
- itaconic acid mono C 1 -C 8 alkyl esters such as monobutyl itaconic acid
- monobutyl maleate And maleic acid mono C 1 -C 8 alkyl esters vinyl group-containing aromatic carboxylic acids such as vinyl benzoic acid; and salts thereof.
- Hydroxy group-containing monomers Hydroxyalkyl vinyl ether monomers such as hydroxyethyl vinyl ether and hydroxybutyl vinyl ether; and hydroxy group-containing allyl monomers such as allyl alcohol and 2-hydroxyethyl allyl ether.
- Vinyl ketones such as vinyl methyl ketone, vinyl hexyl ketone, and methyl isopropenyl ketone are listed.
- Vinyl ether monomers such as vinyl methyl ether, vinyl ethyl ether, and vinyl isobutyl ether are listed.
- Cyano group-containing monomer examples include acrylonitrile, methacrylonitrile, hexene nitrile, 4-pentenenitrile, and p-cyanostyrene.
- Epoxy group-containing monomer examples include allyl glycidyl ether, 3,4-epoxyvinylcyclohexane, di ( ⁇ -methyl) glycidyl malate, and di ( ⁇ -methyl) glycidyl fumarate.
- the ionic functional group may be either an anionic functional group or a cationic functional group.
- anionic functional group include a carboxyl group, a sulfonic acid group, a phosphoric acid group, a phenolic hydroxy group, and salts thereof.
- cationic functional groups include amino groups, imidazole groups, pyridine groups, amidino groups, and salts thereof.
- anionic functional groups are preferred because there are many general-purpose products, a wide variety of types, and in the case of elliptical or acicular polymer particles, the size, shape, etc. can be controlled efficiently. Furthermore, since introduction into the molecule is easy and stability and safety are excellent, among them, one or more functional groups selected from carboxylic acid groups, sulfonic acid groups, phosphoric acid groups, and derivatives thereof It is preferably a group.
- Examples of compounds that can be counterions of these ionic functional groups include metal cations, ammonium cations, pyridinium cations, phosphonium cations, etc. for anionic functional groups, and chloride ions for cationic functional groups. And halide ions such as bromide ions and iodide ions.
- a metal cation is particularly preferable as a counter ion in consideration of production cost and abundant types and efficient control of the accuracy, size, shape, etc. of elliptical or acicular polymer particles. It is.
- metal cation examples include alkali metal cations such as lithium, sodium, rubidium and cesium; alkaline earth metal cations such as magnesium, calcium, strontium and barium; other non-transition metal cations such as aluminum; zinc, copper, manganese and nickel , Transition metal cations such as cobalt, iron, and chromium.
- alkali metal cations such as lithium, sodium, rubidium and cesium
- alkaline earth metal cations such as magnesium, calcium, strontium and barium
- other non-transition metal cations such as aluminum
- zinc, copper, manganese and nickel Transition metal cations such as cobalt, iron, and chromium.
- Examples of the monomer having an anionic functional group include a monocarboxylic acid monomer, a dicarboxylic acid monomer, a sulfonic acid monomer, a sulfate ester monomer, a phenolic hydroxy group-containing monomer, and a phosphoric acid monomer.
- Monocarboxylic acid monomers include (meth) acrylic acid, crotonic acid, cinnamic acid, maleic acid mono C 1 -C 8 alkyl ester, itaconic acid mono C 1 -C 8 alkyl ester, vinyl benzoic acid and salts thereof Etc.
- Examples of the dicarboxylic acid monomer include (anhydrous) maleic acid, ⁇ -methyl (anhydride) maleic acid, ⁇ -phenyl (anhydride) maleic acid, fumaric acid, itaconic acid and salts thereof.
- sulfonic acid monomer examples include alkene sulfonic acids such as ethylene sulfonic acid, vinyl sulfonic acid, and (meth) allyl sulfonic acid; aromatic (styrene sulfonic acid) such as styrene sulfonic acid and ⁇ -methylstyrene sulfonic acid; C 1 ⁇ C 10 alkyl (meth) allyl sulfosuccinate, sulfo C 2 ⁇ C 6 alkyl (meth) such as sulfopropyl (meth) acrylate acrylate; methyl vinyl sulfonate, 2-hydroxy-3- (meth) acryloxy propyl sulfonic acid 2- (meth) acryloylamino-2,2-dimethylethanesulfonic acid, 3- (meth) acryloyloxyethanesulfonic acid, 3- (meth) acryloyloxy-2-hydroxy
- sulfate ester-based monomer examples include (meth) acryloyl polyoxyalkylene (polymerization degree: 2 to 15) sulfate such as polyoxypropylene monomethacrylate sulfate ester and salts thereof.
- phenolic hydroxy group-containing monomer examples include hydroxystyrene, bisphenol A monoallyl ether, bisphenol A mono (meth) acrylic ester, and salts thereof.
- Examples of phosphoric acid monomers include (meth) acrylic acid hydroxyalkyl phosphate monoesters such as 2-hydroxyethyl (meth) acryloyl phosphate and phenyl-2-acryloyloxyethyl phosphate, and vinyl phosphate.
- examples of the salt include alkali metal salts such as sodium salt and potassium salt; amine salts such as triethanolamine; quaternary ammonium salts such as tetra C 4 to C 18 alkyl ammonium salts and the like.
- the monomer having a cationic functional group includes a primary amino group-containing monomer, a secondary amino group-containing monomer, a tertiary amino group-containing monomer, a quaternary ammonium base-containing monomer, a heterocyclic ring-containing monomer, and a phosphonium group-containing monomer.
- Sulfonium group-containing monomers, sulfonic acid group-containing polymerizable unsaturated monomers, and the like are examples of Sulfonium group-containing monomers, sulfonic acid group-containing polymerizable unsaturated monomers, and the like.
- Primary amino group-containing monomers include C 3 -C 6 alkenylamines such as allylamine and crotylamine; amino C 2 -C 6 alkyl (meth) acrylates such as aminoethyl (meth) acrylate; vinylaniline, p-aminostyrene, etc.
- a monomer having an aromatic ring and a primary amino group ethylenediamine; polyalkylenepolyamine and the like.
- the secondary amino group-containing monomer t- butyl aminoethyl (meth) acrylate, C 1 ⁇ C 6 alkylamino C 2 ⁇ C 6 alkyl such as methyl aminoethyl (meth) acrylate (meth) acrylate; di (meth) dialkenylamines amine C 6 ⁇ C 12 of allylamine; ethyleneimine; diallylamine, and the like.
- Tertiary amino group-containing monomers include N, N-dimethylaminoethyl (meth) acrylate, N, N-diethylaminoethyl (meth) acrylate, N, N-dimethylaminopropyl (meth) acrylate, N, N-diethylaminopropyl Di-C 1 -C 4 alkylamino such as (meth) acrylate, N, N-dibutylaminoethyl (meth) acrylate, Nt-butylaminoethyl (meth) acrylate, N, N-dimethylaminobutyl (meth) acrylate, etc.
- C 2 -C 6 alkyl (meth) acrylate di-C 1 -C 4 alkylamino C 2 -C 6 alkyl such as N, N-dimethylaminoethyl (meth) acrylamide and N, N-dimethylaminopropyl (meth) acrylamide
- Method) acrylamide N, N-dimethylaminostyrene and other monomers having an aromatic ring and a tertiary amino group It is.
- Examples of the quaternary ammonium base-containing monomer include those obtained by quaternizing a tertiary amine using a quaternizing agent such as C 1 to C 12 alkyl chloride, dialkyl sulfuric acid, dialkyl carbonate, benzyl chloride and the like.
- a quaternizing agent such as C 1 to C 12 alkyl chloride, dialkyl sulfuric acid, dialkyl carbonate, benzyl chloride and the like.
- Heterocycle-containing monomers include N-vinylcarbazole, N-vinylimidazole, N-vinyl-2,3-dimethylimidazoline, N-methyl-2-vinylimidazoline, 2-vinylpyridine, 4-vinylpyridine, N-methyl Examples thereof include vinyl pyridine and oxyethyl-1-methylene pyridine.
- Examples of the phosphonium group-containing monomer include glycidyl tributyl phosphone.
- sulfonium group-containing monomer examples include 2-acryloxyethyldimethylsulfone, glycidylmethylsulfonium, and the like.
- sulfonic acid group-containing polymerizable unsaturated monomer examples include (meth) acrylamide-alkanesulfonic acid such as 2-acrylamido-2-methylpropanesulfonic acid, and sulfoalkyl (meth) acrylate such as 2-sulfoethyl (meth) acrylate. Can be mentioned.
- the monomer having a cationic functional group may be used as an inorganic acid salt such as hydrochloride or phosphate; or an organic acid salt such as formate or acetate.
- the unsaturated monomer which has the reactive functional group mentioned above can be used individually by 1 type or in combination of 2 or more types.
- monomers having a hydroxy group, a carboxyl group, an amino group, an amide group, an alkylene oxide group or an ionic functional group are preferable, and in particular, a hydroxy group , A monomer having a carboxyl group, an ethylene oxide group or an ionic functional group is more preferable.
- the hydrophilicity becomes stronger and the repulsion between particles obtained in the solution becomes stronger, so that the stability of the dispersion system becomes higher and the monodispersibility can be further improved, so that It is possible to reduce deterioration of the particle size accuracy due to aggregation, and to obtain polymer particles excellent in chemical resistance, reactivity, solution dispersibility and powder dispersibility, mechanical properties and the like.
- the unsaturated monomer having a reactive functional group is preferably a water-soluble compound.
- a water-soluble monomer it is possible to further improve the monodispersity.
- the polymer particles obtained can be easily dispersed in water or an aqueous medium.
- crosslinking agent in the polymerization reaction, may be blended in an appropriate amount of 0.01 to 80% by mass with respect to the total mass of the polymerization components depending on the heat resistance and chemical resistance applications of the particles obtained. You can also.
- Crosslinking agents include aromatic divinyl compounds such as divinylbenzene and divinylnaphthalene; ethylene glycol diacrylate, ethylene glycol dimethacrylate, triethylene glycol dimethacrylate, tetraethylene glycol dimethacrylate, 1,3-butylene glycol dimethacrylate, trimethylol Propane triacrylate, trimethylolpropane trimethacrylate, 1,4-butanediol diacrylate, neopentyl glycol diacrylate, 1,6-hexanediol diacrylate, pentaerythritol triacrylate, pentaerythritol tetraacrylate, pentaerythritol dimethacrylate, pen
- polymerization initiator used in conducting the polymerization reaction various known polymerization initiators can be used.
- these polymerization initiators can be used singly or in combination of two or more.
- the amount of the radical polymerization initiator is preferably 0.1 to 50 parts by mass with
- the solvent for synthesis is not particularly limited, and an appropriate solvent may be selected from common solvents according to the raw materials used.
- Usable solvents include, for example, water and, as hydrophilic organic solvents, methanol, ethanol, 1-propanol, 2-propanol, ethylene glycol, propylene glycol, methyl cellosolve, ethyl cellosolve, propyl cellosolve, methyl cellosolve acetate, ethyl Examples include cellosolve acetate, methyl carbitol, ethyl carbitol, butyl carbitol, ethyl carbitol acetate, acetone, tetrahydrofuran, dimethylformamide, N-methyl-2-pyrrolidone, and acetonitrile. These can be used individually by 1 type or in mixture of 2 or more types.
- the hydrophilic organic solvent means that the liquid mixture with water maintains a uniform appearance
- the hydrophobic organic solvent gently means the same volume of pure water at a temperature of 20 ° C. at 1 atmosphere. It means that the mixed liquid cannot maintain a uniform appearance after stirring and flow stop.
- Hydrophobic organic solvents can also be used. Specific examples thereof include 1-butanol, 2-butanol, isobutanol, tert-butanol, 1-pentanol, 2-pentanol, 3-pentanol, 2-methyl-1- Butanol, isopentyl alcohol, tert-pentyl alcohol, 1-hexanol, 2-methyl-1-pentanol, 4-methyl-2-pentanol, 2-ethylbutanol, 1-heptanol, 2-heptanol, 3-heptanol, Higher alcohols such as 2-octanol, 2-ethyl-1-hexanol, benzyl alcohol, cyclohexanol; ether alcohols such as butyl cellosolve; ketones such as methyl ethyl ketone, methyl isobutyl ketone, cyclohexanone; ethyl acetate, butyl acetate, pro
- a mixed solvent of water and a hydrophilic organic solvent or a hydrophobic organic solvent may be used. It is preferable to use together. Thereby, the particle surface and the inside can be appropriately modified.
- the mixing ratio of the solvent is arbitrary and may be appropriately adjusted according to the monomer to be used.
- 10 : 90 to 80:20, particularly 30:70 to 70:30 10 : 90 to 80:20, particularly 30:70 to 70:30.
- the fuzzy state is a state in which both the part in which the monomer is dissolved and the part in which the monomer is dispersed are obtained by arbitrarily selecting the mixing medium, that is, at least the emulsified part and the dissolved part. It is a state having both. By making such a fuzzy state, it is possible to efficiently modify the surface of the particles obtained after polymerization, reduce the diameter, adjust the aspect ratio, and the like (Patent Document 5).
- the solvent composition as described above, it is possible to control the particle size and aspect ratio of the polymer particles, the size of the fine irregularities on the surface, and the porosity, thereby improving the UV scattering effect.
- Various properties such as optical properties, water absorption, oil absorption, etc. can be controlled in a well-balanced manner.
- the content of the raw material monomer in the reaction solution is preferably 1 to 80% by mass in the total reaction solution, more preferably 5 to 50% by mass, and still more preferably 10 to 30% by mass.
- the content of the raw material monomer exceeds 80% by mass, it becomes difficult to obtain polymer particles having the above physical properties in a high yield in a monodispersed state.
- it is less than 1% by mass, it takes a long time to complete the reaction, and it is not practical from an industrial viewpoint.
- the reaction temperature at the time of polymerization varies depending on the type of solvent used, and cannot be generally specified, but is usually about 10 to 200 ° C, preferably 30 to 130 ° C, more preferably 40 to 90 ° C. It is.
- reaction time is not particularly limited as long as it is a time required for the target reaction to be almost completed, and greatly depends on the monomer species and the amount of the monomer, the viscosity and concentration of the solution, the target particle size, and the like.
- 40 to 90 ° C. it is 1 to 72 hours, preferably 2 to 24 hours.
- Dispersants and stabilizers include polyhydroxystyrene, polystyrene sulfonic acid, hydroxystyrene- (meth) acrylic acid ester copolymer, styrene- (meth) acrylic acid ester copolymer, styrene-hydroxystyrene- (meth) acrylic Polystyrene derivatives such as acid ester copolymers; poly (meth) acrylic acid derivatives such as poly (meth) acrylic acid, poly (meth) acrylamide, polyacrylonitrile, polyethyl (meth) acrylate, polybutyl (meth) acrylate; polymethyl vinyl ether , Polyvinyl alkyl ether derivatives such as polyethyl vinyl ether, polybutyl vinyl ether, polyisobutyl vinyl ether; cellulose, methyl cellulose, cellulose acetate, cellulose nitrate, hydroxymethyl cellulose, hydroxyethyl cellulose Cellulose derivatives such
- emulsifiers include alkyl sulfate esters such as sodium dodecyl sulfate, alkyl benzene sulfonates such as sodium dodecyl benzene sulfonate, alkyl naphthalene sulfonates, fatty acid salts, alkyl phosphates, and alkyl sulfosuccinates.
- Anionic emulsifiers such as alkylamine salts, quaternary ammonium salts, alkylbetaines, amine oxides; polyoxyethylene alkyl ethers, polyoxyethylene alkyl allyl ethers, polyoxyethylene alkyl phenyl ethers, sorbitan fatty acid esters, Nonionic emulsifiers such as glycerin fatty acid ester and polyoxyethylene fatty acid ester are listed. These can be used alone or in combination of two or more.
- a catalyst reaction accelerator
- the blending amount can be an appropriate amount that does not adversely affect the physical properties of the particles, for example, 0.01 to 20% by mass relative to the total mass of the polymerization components.
- the catalyst is not particularly limited as long as it is a positive catalyst, and can be appropriately selected from known ones.
- Specific examples include tertiary amines such as benzyldimethylamine, triethylamine, tributylamine, pyridine and triphenylamine; quaternary ammonium compounds such as triethylbenzylammonium chloride and tetramethylammonium chloride; triphenylphosphine, tricyclo Phosphines such as phosphine; Phosphonium compounds such as benzyltrimethylphosphonium chloride; Imidazole compounds such as 2-methylimidazole and 2-methyl-4-ethylimidazole; Alkali metals such as potassium hydroxide, sodium hydroxide and lithium hydroxide Hydroxides; alkali metal carbonates such as sodium carbonate and lithium carbonate; alkali metal salts of organic acids; Lewis acidity such as boron trichloride, boron trifluoride, t
- the blending amount can be an appropriate amount that does not adversely affect the physical properties of the particles, for example, 0.01 to 80% by mass with respect to the total mass of the polymerization components.
- the UV scattering agent of the present invention is suitable as an additive for UV cut.
- the composition containing the UV scattering agent of the present invention is excellent in production suitability when kneaded into a polymer or dispersed in a medium, does not cause bleed out due to long-term use, has excellent UV scattering performance, and The scattering performance is maintained for a long time, and the light resistance (UV fastness) is excellent. Moreover, it is easy to handle because it is not a structure with skin irritation.
- the UV scattering agent of the present invention has excellent light resistance, it can be used for polymer molded products such as plastics, containers, paints, coating films, fibers, and building materials. In order to protect UV-sensitive contents, etc., it can be used for, for example, filters, packaging materials, containers, paints, coatings, inks, fibers, building materials, recording media, image display devices, solar cell covers, It is also possible to suppress the decomposition of highly unstable compounds.
- the UV scattering agent of the present invention can be dispersed in water, a hydrophilic organic solvent, a hydrophobic organic solvent or a mixed solvent thereof and used as a dispersion.
- a hydrophilic organic solvent and a hydrophobic organic solvent the thing similar to what was illustrated in the manufacturing method of a polymer particle is mentioned.
- the transmittance of UV at a wavelength of 360 nm is preferably less than 8%, more preferably less than 5%, still more preferably 3%. Is less than.
- the UV scattering agent preferably has a UV-A and UV-B transmittance of 1 ⁇ 2 or less when compared with a spherical polymer having the same composition as the polymer particles A and the same volume converted diameter.
- UV-A, UV-B, and UV-C are UVs having wavelengths of 315 to 400 nm, 280 to 315 nm, and 200 to 280 nm, respectively.
- the UV scattering agent of the present invention can be used as an additive for molded products such as liquids, coating films, films, board materials, and paper.
- the UV scattering agent-containing composition of the present invention comprises a light scattering agent, an optical filter material, a colorant, a cosmetic, an absorbent, an adsorbent, an ink, an electromagnetic wave shielding material, a fluorescent sensor, a biomarker, a recording material, a recording element, and a polarizing material. It can be widely used for drug carriers for drug delivery systems (DDS), biosensors, DNA chips, test drugs and the like.
- DDS drug delivery systems
- shielding UV that is incident on the interior of the vehicle and the interior of the vehicle with interior products such as window glass products, curtains, and wall materials not only prevents sunburn and adverse effects on the human body, but also decorates the interior and interior of the vehicle. This is also useful in that deterioration of the product or the like can be prevented.
- the UV scattering agent of the present invention is suitable as a cosmetic additive.
- Elliptical or acicular polymer particles A Eliminate or reduce the use of UV absorbers or other UV scattering agents while maintaining the original light weight, light scattering, tactile properties, flow characteristics, solution dispersibility, etc. Because it can be done.
- the fact that the UV scattering agent of the present invention has no skin irritation is also a useful point as a cosmetic additive.
- the UV scattering agent of the present invention has an adhesive force different from a general spherical shape due to its unique shape, and has an effect of improving the fixing force of a molded body such as a foundation and the holding force after application.
- the skin can be brightened by the optical characteristics, and the covering power can be improved by the blurring effect.
- the slipperiness unique to the shape makes it easy to spread on the skin, and by filling the textured grooves finely, wrinkles and pores can be made inconspicuous and the flowability of the entire product can be freely controlled. it can.
- the amount of polymer added to the entire product can be increased by utilizing the adhesive force and holding force, and an unprecedented cosmetic effect can be found.
- a preferred addition amount is 0.1 to 50% by mass, preferably 0.5 to 30% by mass, based on the amount of product blended.
- Light scattering properties such as UV scattering effect and blurring effect, fluidity, moldability, adhesion improvement, finish feeling, etc. can be appropriately adjusted according to the application / purpose.
- 1 to 20% by mass is particularly preferable as a cosmetic additive.
- grains suitably.
- cosmetics that are highly effective in preventing UV-cutting and UV deterioration may be skin care products, hair products, antiperspirant products, makeup products, UV protection products, fragrance products, and the like.
- basic cosmetics such as milky lotion, cream, lotion, calamine lotion, sunscreen agent, makeup base material, suntan agent, after shave lotion, pre-shave lotion, pack fee, cleansing agent, face wash, anti-acne cosmetics, essence, etc.
- foundation Makeup cosmetics such as white powder, mascara, eye shadow, eyeliner, eyebrow, teak, nail color, lip balm, lipstick, shampoo, rinse, conditioner, hair color, hair tonic, set agent, body powder, hair restorer, Deodorant, hair remover, soap, body shampoo, bath agent, hand soap, perfume and the like.
- the form of the product is not particularly limited, and it may be liquid, emulsion, cream, solid, paste, gel, powder, multilayer, mousse, spray or the like. A useful effect can be expected as an additive for these cosmetics.
- the UV scattering agent of the present invention is an additive for printing ink used for screen printing, offset printing, process printing, gravure printing, tampo printing, coater, inkjet, etc., for marking pen, for ballpoint pen, for fountain pen, for brush pen, It can be used as an additive for writing instrument inks such as magic, and for stationery items such as crayons, paints, and erasers.
- the UV scattering agent of the present invention is suitable as an additive for coating materials used for brush coating, spray coating, electrostatic coating, electrodeposition coating, flow coating, roller coating, dip coating and the like.
- transportation equipment such as automobiles, trains, helicopters, boats, bicycles, snow vehicles, ropeways, lifts, fovercrafts, motorcycles, sashes, shutters, water tanks, doors, balconies, building skin panels, roofing materials Building materials such as stairs, skylights, concrete fences, exterior and interior walls inside and outside buildings, guardrails, pedestrian bridges, soundproof walls, signs, highway side walls, railway viaducts, road members such as bridges, tanks, pipes, towers, Plant components such as chimneys, greenhouses, greenhouses, silos, agricultural equipment such as agricultural seats, communication equipment such as utility poles, power transmission towers, parabolic antennas, electrical wiring boxes, lighting equipment, air conditioner outdoor units, washing machines, refrigerators , Electric devices such as microwave ovens, and their covers, monuments, tombstones, pavement materials, windshield sheets, waterproof sheets, building cu
- a water-dispersed paint, a non-water-dispersed paint, a powder paint, an electrodeposition paint, etc. can be appropriately selected as required in addition to the solvent-type paint.
- Aqueous phase 1280.0 g Polyvinylpyrrolidone (K-15) 8.0g 4.8g ammonium persulfate Oil phase Toluene 80.0g Polystyrene 16.0g Methyl methacrylate 160.0 g (Polystyrene: Sigma-Aldrich, weight average molecular weight of about 45,000)
- this particle solution was vacuum-dried by repeating washing and filtration five times with methanol using a known suction filtration equipment to obtain polymer particles A1.
- Synthesis Example 5 Polystyrene polymer particles A5 were obtained in the same manner as in Synthesis Example 1 except that methyl methacrylate was changed to styrene (manufactured by Wako Pure Chemical Industries, Ltd.).
- this particle solution was vacuum-dried by repeating washing-filtration 5 times with methanol using a known suction filtration equipment to obtain polymer particles A6.
- Synthesis Example 7 A styrene-2-hydroxyethyl methacrylate copolymer is produced in the same manner as in Synthesis Example 6 except that the copolymer components are styrene and 2-hydroxyethyl methacrylate and the composition ratio is 3: 7 (mass ratio). Particle A7 was obtained.
- Synthesis Example 11 The particles obtained in Synthesis Example 1 are dissolved in toluene, made into a sheet, re-dried, then applied to a pulverizer, and subjected to a classification operation to pulverize a single polymethyl methacrylate having an average particle diameter of 5 ⁇ m (an irregular shape). Polymer particle B4 was produced.
- Table 1 shows a summary of the MV, L AV , D AV , P AV , particle component, and shape of the particles obtained in Synthesis Examples 1 to 11.
- Dispersions 1 to 20 were each injected into the attached quartz cell, and UV transmission light was used when dispersing particles at wavelengths of 320 nm, 360 nm, and 400 nm using an ultraviolet-visible spectrophotometer (UV-2450 manufactured by JASCO Corporation). Analysis was carried out. The results are shown in Table 3.
- the additive containing the elliptical or acicular polymer particles of the present invention has a clearly high UV scattering effect because the transmitted light is reduced in the UV region (particularly UV-A). did. Moreover, since the scattering effect was high also in the visible light region, it was confirmed that the concealability was also high.
- the additive containing the elliptical or acicular polymer particles of the present invention is mixed with the base material or attached to the surface layer of the base material, etc., in the UV region (UV-A, UV-B, UV In -C), it was confirmed that there was a UV scattering effect, and that it was possible to suppress the adverse effects on UV degradation, discoloration, health damage, etc. of cosmetics, inks, paints, coloring materials, and the like.
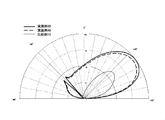
- Example 45 and 46, Comparative Example 15 The particles blended in Example 19 were uniformly applied to black synthetic leather (5 cm ⁇ 8 cm) while putting with a cosmetic puff (0.24 mg / cm 2 ) to prepare an evaluation sheet. Next, using an automatic goniophotometer (Gonio Photometer GP-200 manufactured by Murakami Color Research Laboratory Co., Ltd.), the evaluation sheet is irradiated with a certain amount of light at an incident angle of 45 °, and the light scattering distribution of the reflected light is measured. (Example 45). Moreover, the light-scattering distribution of reflected light was measured by the same method using the particles blended in Example 24 and Comparative Example 6 instead of the particles blended in Example 19 (Example 46 and Comparative Example 15). The results are shown in FIG.
- Example 47 to 60, Comparative Examples 16 to 19 The particles blended in Examples 17 to 30 and Comparative Examples 6 to 9 were evaluated by the methods shown below. The results are shown in Table 7.
- Evaluation item and touch Evaluated by the feeling when each particle was stretched on the skin.
- -Sliding property 1 g of each particle was placed on a black synthetic leather, and the length when stretched with a finger was evaluated.
- ⁇ Particle adhesion force 1g of each particle is placed on the black synthetic leather, spread evenly with a puff, then the synthetic leather is struck three times, and the residual amount of the particles is observed and evaluated with a digital microscope (VHX200, manufactured by Keyence Corporation). did.
- the UV scattering agent of the present invention is also effective as a light scattering agent for hair care products, particularly hair dyeing.
- the UV scattering agent of the present invention was useful as an additive (material) for general cosmetics such as makeup and skin care while maintaining the UV scattering characteristics. Moreover, since it is effective for both UV scattering and visible light scattering, it is also effective as a light scattering agent for hair care products, particularly hair dyeing.
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Birds (AREA)
- Epidemiology (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Wood Science & Technology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Physics & Mathematics (AREA)
- Geometry (AREA)
- Medicinal Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Polymers & Plastics (AREA)
- General Chemical & Material Sciences (AREA)
- Dermatology (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Paper (AREA)
- Cosmetics (AREA)
- Polymerisation Methods In General (AREA)
- Paints Or Removers (AREA)
- Inks, Pencil-Leads, Or Crayons (AREA)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP14862260.8A EP3070138B1 (en) | 2013-11-14 | 2014-11-11 | Ultraviolet scattering agent and application therefor |
| CN201480062130.6A CN105722940B (zh) | 2013-11-14 | 2014-11-11 | 紫外线散射剂及其用途 |
| US15/035,964 US20160262988A1 (en) | 2013-11-14 | 2014-11-11 | Ultraviolet scattering agent and application therefor |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013-235873 | 2013-11-14 | ||
| JP2013235873A JP5780285B2 (ja) | 2013-11-14 | 2013-11-14 | 紫外線散乱剤及びその用途 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015072443A1 true WO2015072443A1 (ja) | 2015-05-21 |
Family
ID=53057377
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2014/079816 Ceased WO2015072443A1 (ja) | 2013-11-14 | 2014-11-11 | 紫外線散乱剤及びその用途 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US20160262988A1 (enExample) |
| EP (1) | EP3070138B1 (enExample) |
| JP (1) | JP5780285B2 (enExample) |
| CN (1) | CN105722940B (enExample) |
| WO (1) | WO2015072443A1 (enExample) |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN107614542B (zh) * | 2015-05-08 | 2020-03-03 | 日清纺控股株式会社 | 椭圆状、针状或棒状聚合物粒子的制造方法 |
| EP3296325A4 (en) * | 2015-05-08 | 2018-12-19 | Nisshinbo Holdings Inc. | Elliptical, needle-shaped, or rod-shaped crosslinked polymer particles, and use thereof |
| US10058502B2 (en) | 2015-12-31 | 2018-08-28 | L'oreal | Nail polish compositions |
| JP6613904B2 (ja) * | 2016-01-12 | 2019-12-04 | 株式会社リコー | インクジェット用インク及びインクジェット記録方法 |
| US20190254938A1 (en) * | 2016-11-07 | 2019-08-22 | Nisshinbo Holdings Inc. | Skin cosmetics |
| JP6907586B2 (ja) | 2017-02-22 | 2021-07-21 | 日清紡ホールディングス株式会社 | 皮膚化粧料 |
| EP3729995A4 (en) * | 2017-12-21 | 2021-01-20 | Panasonic Intellectual Property Management Co., Ltd. | COSMETIC SHEET AND ITS MANUFACTURING PROCESS, INK FOR COSMETIC PRODUCT, INKJET PRINTING INK AND DEVICE FOR MANUFACTURING COSMETIC SHEET |
| US12202986B2 (en) * | 2018-10-03 | 2025-01-21 | Northwestern University | Two-dimensional insulator based printable ion-conductive and viscosity-tunable inks, fabricating methods and applications of same |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH11180827A (ja) * | 1997-12-22 | 1999-07-06 | Kao Corp | 化粧料 |
| JP2004115342A (ja) * | 2002-09-27 | 2004-04-15 | Ishihara Sangyo Kaisha Ltd | 針状二酸化チタン微粒子及びその製造方法 |
| JP2005247979A (ja) | 2004-03-03 | 2005-09-15 | Nisshinbo Ind Inc | 楕円球状有機ポリマー粒子およびその製造方法 |
| JP2006104401A (ja) | 2004-10-08 | 2006-04-20 | Nisshinbo Ind Inc | 針状または楕円球状有機ポリマー粒子の製造方法 |
| JP2007070372A (ja) | 2005-09-02 | 2007-03-22 | Nisshinbo Ind Inc | 楕円球状有機ポリマー粒子およびその製造方法 |
| JP2009001759A (ja) * | 2007-05-21 | 2009-01-08 | Kri Inc | 偏平粒子及びその製造方法 |
| JP2009235353A (ja) | 2008-03-28 | 2009-10-15 | Nisshinbo Holdings Inc | 微粒子含有楕円状または針状ポリマー粒子およびその製造方法 |
| JP2009235355A (ja) | 2008-03-28 | 2009-10-15 | Nisshinbo Holdings Inc | 楕円状または針状ポリマー粒子およびその製造方法 |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2850866B1 (fr) * | 2003-02-06 | 2007-04-20 | Seppic Sa | Melange de latex inverse auto-inversible et d'une poudre a usage cosmetique ou pharmaceutique; utilisation comme agent de texture |
| JP2012523378A (ja) * | 2009-03-20 | 2012-10-04 | ザ プロクター アンド ギャンブル カンパニー | 油溶性固体日焼け止め剤を含むパーソナルケア組成物 |
-
2013
- 2013-11-14 JP JP2013235873A patent/JP5780285B2/ja active Active
-
2014
- 2014-11-11 EP EP14862260.8A patent/EP3070138B1/en active Active
- 2014-11-11 CN CN201480062130.6A patent/CN105722940B/zh active Active
- 2014-11-11 WO PCT/JP2014/079816 patent/WO2015072443A1/ja not_active Ceased
- 2014-11-11 US US15/035,964 patent/US20160262988A1/en not_active Abandoned
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH11180827A (ja) * | 1997-12-22 | 1999-07-06 | Kao Corp | 化粧料 |
| JP2004115342A (ja) * | 2002-09-27 | 2004-04-15 | Ishihara Sangyo Kaisha Ltd | 針状二酸化チタン微粒子及びその製造方法 |
| JP2005247979A (ja) | 2004-03-03 | 2005-09-15 | Nisshinbo Ind Inc | 楕円球状有機ポリマー粒子およびその製造方法 |
| JP2006104401A (ja) | 2004-10-08 | 2006-04-20 | Nisshinbo Ind Inc | 針状または楕円球状有機ポリマー粒子の製造方法 |
| JP2007070372A (ja) | 2005-09-02 | 2007-03-22 | Nisshinbo Ind Inc | 楕円球状有機ポリマー粒子およびその製造方法 |
| JP2009001759A (ja) * | 2007-05-21 | 2009-01-08 | Kri Inc | 偏平粒子及びその製造方法 |
| JP2009235353A (ja) | 2008-03-28 | 2009-10-15 | Nisshinbo Holdings Inc | 微粒子含有楕円状または針状ポリマー粒子およびその製造方法 |
| JP2009235355A (ja) | 2008-03-28 | 2009-10-15 | Nisshinbo Holdings Inc | 楕円状または針状ポリマー粒子およびその製造方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3070138B1 (en) | 2019-01-02 |
| US20160262988A1 (en) | 2016-09-15 |
| EP3070138A4 (en) | 2017-06-28 |
| EP3070138A1 (en) | 2016-09-21 |
| JP2015093973A (ja) | 2015-05-18 |
| JP5780285B2 (ja) | 2015-09-16 |
| CN105722940B (zh) | 2018-04-03 |
| CN105722940A (zh) | 2016-06-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5780285B2 (ja) | 紫外線散乱剤及びその用途 | |
| JP6168239B2 (ja) | 扁平楕円状ポリマー粒子及びその用途 | |
| Billon et al. | Tailoring highly ordered honeycomb films based on ionomer macromolecules by the bottom-up approach | |
| JP6164376B2 (ja) | 架橋ポリマー粒子及びその用途 | |
| JP6763375B2 (ja) | 楕円状、針状又は棒状ポリマー粒子の製造方法 | |
| EP3176216A1 (en) | Hydrophilic material comprising sulfonate copolymer and amino resin | |
| Yang et al. | Synthesis of polystyrene/polysilsesquioxane core/shell composite particles via emulsion polymerization in the existence of poly (γ-methacryloxypropyl trimethoxysilane) sol | |
| JP6617789B2 (ja) | 円盤状ポリマー粒子群の製造方法 | |
| Xu et al. | Nanocomposite polymer colloids prepared via emulsion polymerization and stabilized using polydopamine-coated silica particles | |
| TW201311741A (zh) | 樹脂粒子及其用途 | |
| Chen et al. | Well-defined PMMA brush on silica particles fabricated by surface-initiated photopolymerization (SIPP) | |
| Joshi | Synthesis of Hybrid Inorganic-Organic Microparticles |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14862260 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15035964 Country of ref document: US |
|
| REEP | Request for entry into the european phase |
Ref document number: 2014862260 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2014862260 Country of ref document: EP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |