WO2014119587A1 - 溶解分離層を備えた薬剤付き支持体 - Google Patents
溶解分離層を備えた薬剤付き支持体 Download PDFInfo
- Publication number
- WO2014119587A1 WO2014119587A1 PCT/JP2014/051893 JP2014051893W WO2014119587A1 WO 2014119587 A1 WO2014119587 A1 WO 2014119587A1 JP 2014051893 W JP2014051893 W JP 2014051893W WO 2014119587 A1 WO2014119587 A1 WO 2014119587A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- drug
- layer
- dissolution
- separation layer
- support
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0048—Eye, e.g. artificial tears
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F9/00—Methods or devices for treatment of the eyes; Devices for putting in contact-lenses; Devices to correct squinting; Apparatus to guide the blind; Protective devices for the eyes, carried on the body or in the hand
- A61F9/0008—Introducing ophthalmic products into the ocular cavity or retaining products therein
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/36—Polysaccharides; Derivatives thereof, e.g. gums, starch, alginate, dextrin, hyaluronic acid, chitosan, inulin, agar or pectin
- A61K47/38—Cellulose; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/70—Web, sheet or filament bases ; Films; Fibres of the matrix type containing drug
- A61K9/7007—Drug-containing films, membranes or sheets
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
Definitions
- the present invention relates to a support with a drug for administering a drug to the eye.
- eye drops are widely known as ophthalmic preparations.
- the drop amount differs depending on the pressing force of the eye drop container, and the intended drop amount may not be administered.
- Patent Document 1 discloses an applicator for administering a drug to a wet biological surface. That is, in this applicator, a matrix layer containing a drug component in a soluble matrix is provided at one end of a disposable applicator having a long and narrow band shape. A separation portion whose thickness is thinner than other portions is formed in the middle of the matrix layer (see FIG. 1 of Patent Document 1). In this applicator, by holding the other end of the applicator opposite to the drug body and bringing the drug body into contact with the living body surface that is the administration site, the thinner separation part dissolves and the drug body. Is separated from the applicator and the drug main body is administered, so that a certain amount of drug can be administered while maintaining the sterilized state when the drug is administered.
- the present invention has been made in view of the above-described background, and an object thereof is to provide a drug-supported support capable of reliably administering a certain amount of drug to the eye.
- a support with a drug for administering a drug to the eye A drug layer comprising a drug and a water-soluble polymer; A dissolution separation layer comprising a water-soluble polymer set to have a higher water solubility than the water-soluble polymer of the drug layer; With a support to be a handle, The drug layer and the support are connected to the opposite side via the dissolution separation layer.
- the form of the drug layer and the dissolution / separation layer is not particularly limited.
- a film-like form is preferable, and the support is a handle of the support with the drug, and the application at the time of drug administration is performed. Can be used as data. Therefore, the drug-supported support of the present invention can administer the drug while maintaining a sterilized state.
- the solubility of the dissolution / separation layer provided between the drug layer and the support is higher than the water solubility of the drug layer. For this reason, when the drug is administered, the dissolution / separation layer dissolves before the drug layer on the surface of the eye, so that the drug layer is separated from the support with the drug and administered to the surface of the eye. As a result, a certain amount of drug (drug layer) can be reliably administered.
- connection strength between the drug layer and the dissolution / separation layer can be increased by increasing the overlap (contact area) between the drug layer and the dissolution / separation layer.
- the dissolution separation layer and the support are partially overlapped.
- the connection strength can be increased with the drug layer, the dissolution / separation layer, and the support as one body.
- the drug layer, the dissolution / separation layer, and the dissolution / separation layer and the support are reliably connected by thermocompression bonding or adhesion.
- the average disintegration time of the dissolution separation layer (disintegration time is the time required for the film-like matrix of a certain size to dissolve in water or physiological saline) is 60 seconds or less, more preferably 50 seconds or less. is there. This is because if the average disintegration time of the dissolved separation layer exceeds 60 seconds, it takes too much time to administer the drug to the eye using the drug-supported support.
- This configuration allows the dissolution / separation layer to dissolve quickly on the ocular surface, so that a certain amount of drug can be quickly administered to the ocular surface.
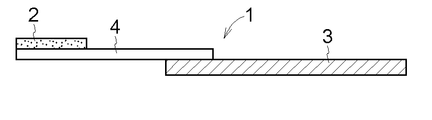
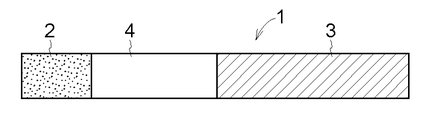
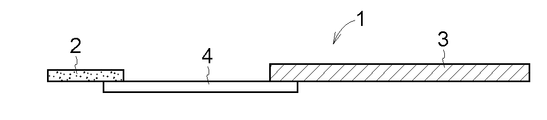
- the drug-supported support 1 is composed of a film-like drug layer 2, a film-like dissolution / separation layer 4, and a support 3 serving as a handle, and the size of the drug layer 2 is not particularly limited.
- the width is preferably about 1 to 4 mm, the length is about 5 to 10 mm, and the thickness is 1 to 300 ⁇ m.
- the size of the dissolution separation layer 4 is not particularly limited, but for example, the width is about 1 to 4 mm and the length is 3 to 3 mm. It is preferably about 10 mm and a thickness of 1 to 300 ⁇ m, and the size of the support 3 is not particularly limited.
- the width is about 1 to 4 mm, the length is about 10 to 60 mm, and the thickness is 50 ⁇ m to 2 mm. Is preferred.
- the support 3 has, for example, a long and thin rectangular shape and serves as a handle for maintaining a sterilized state.
- the material of the support body 3 is not specifically limited, Paper, plastics, aluminum foil, etc. are applicable suitably. Further, the support 3 may be formed by laminating a plurality of these layers.
- each of the drug layer 2 and the dissolution / separation layer 4 is a film-like matrix, and is connected by adhering the entire lower surface of the drug layer 2 and a part of the upper surface of the dissolution / separation layer 4. Then, a part of the lower surface of the dissolution separation layer 4 and a part of the upper surface of the support 3 are connected by thermocompression bonding or adhesion.
- connection strength can be increased by connecting the drug layer 2 and the dissolution / separation layer 4 and the dissolution / separation layer 4 and the support 3 in an overlapping manner and increasing the respective contact areas.
- connection method between the drug layer 2 and the dissolution / separation layer 4 and the connection method between the dissolution / separation layer 4 and the support 3 may be connected by methods other than thermocompression bonding and adhesion.
- the drug layer 2 is composed of a matrix containing a water-soluble polymer and a drug, and an emulsifier such as polysorbate 80 and a plasticizer such as glycerin can be blended in the matrix as necessary. These water-soluble polymers can be mixed, or a matrix having two or more kinds of multilayer structures can be formed.
- the dissolution / separation layer 4 is composed of a matrix containing a water-soluble polymer that does not contain a drug, and an emulsifier and a plasticizer can be blended in the matrix as necessary, and mannitol is used to enhance water solubility.
- the matrix constituting the dissolution / separation layer 4 is set to have higher water solubility than the matrix constituting the drug layer 2. In this way, by making the water solubility of the dissolution / separation layer 4 higher than that of the drug layer 2, the dissolution / separation layer 4 dissolves before the drug layer 2 if it comes into contact with tears during administration.
- the dissolution / separation layer 4 having a higher water solubility than the drug layer 2, there is no problem such as separation from the middle part of the drug layer 2 during administration, and the drug layer 2 is separated from the support 1 with the drug. Thus, it adheres to the ocular surface, so that a certain amount of drug can be reliably administered (see FIGS. 1 to 3).
- the average disintegration time with respect to water or physiological saline when a film-like matrix having a certain size [2 ⁇ 7 mm, film thickness of about 30 ⁇ m] described in detail in the examples can be used.
- the average disintegration time of the matrix constituting the drug layer 2 is not particularly limited, but is preferably 100 minutes or less.
- the average disintegration time of the film-like matrix constituting the dissolution / separation layer 4 is not particularly limited because the dissolution / separation layer 4 is more easily dissolved by tears on the ocular surface as the solubility in water or physiological saline increases. 60 seconds or less, more preferably 50 seconds or less. This is because if the average disintegration time of the dissolved separation layer 4 exceeds 60 seconds, it takes too much time to administer the drug to the eye using the drug-supported support 1. That is, by setting the average disintegration time of the dissolution / separation layer 4 to 60 seconds or less, the dissolution / separation layer 4 quickly dissolves in the tears on the ocular surface, and a certain amount of drug can be quickly administered to the ocular surface. .
- the average disintegration time of the film-like matrix constituting the drug layer 2 is larger than the average disintegration time of the film-like matrix constituting the dissolution / separation layer 4, and the difference is not particularly limited but is preferably 1 second or more. Is 2 seconds or more. That is, by setting the difference in average disintegration time as described above, the dissolution / separation layer 4 dissolves before the drug layer 2 on the surface of the eye at the time of administration, and the drug layer 2 is separated from the drug-supported support 1 to the eye.
- the sum of the average disintegration time of the drug layer 2 and the average disintegration time of the dissolution / separation layer 4 is the average of the dissolution / separation layer 4.
- the dissolution / separation layer 4 can be dissolved on the surface of the eye before the laminate of the drug layer 2 and the dissolution / separation layer 4. .
- the matrix constituting the drug layer 2 is not particularly limited as long as it is a water-soluble matrix.
- cellulose-based water-soluble polymer acrylate-based polymer, polyvinyl pyrrolidone, polyvinyl alcohol, poly (ethylene oxide), polysaccharides Specifically, CMC Daicel 1205 (average disintegration time 10 seconds, manufactured by Daicel Finechem), PLASDONE K-90 (average disintegration time 11 seconds, manufactured by ASHLAND), HPC-L (average disintegration time 27 seconds) , Nippon Soda Co., Ltd.), Gohsenol EG-05 (average decay time 29 seconds, manufactured by Nippon Synthetic Chemical Co., Ltd.), TC-5S (average decay time 34 seconds, manufactured by Shin-Etsu Chemical Co., Ltd.), Metrose 60SH-50 (average decay time 43 Second, manufactured by Shin-Etsu Chemical Co., Ltd.), Metroz SM15 (average decay time 138 seconds, Shin-Ets
- Examples of the matrix constituting the dissolution / separation layer 4 include cellulose-based water-soluble polymers, polyvinyl pyrrolidone, polyvinyl alcohol, and the like.
- CMC Daicel 1205 average disintegration time 10 seconds, manufactured by Daicel Finechem
- PLASDONE K-90 average disintegration time 11 seconds, manufactured by Ashland
- HPC-L average disintegration time 27 seconds, manufactured by Nippon Soda Co., Ltd.
- Gohsenol EG-05 average disintegration time 29 seconds, manufactured by Nippon Synthetic Chemical Co., Ltd.
- a material containing a highly water-soluble polymer such as TC-5S (average disintegration time 34 seconds, manufactured by Shin-Etsu Chemical Co., Ltd.), Metrose 60SH-50 (average disintegration time 43 seconds, manufactured by Shin-Etsu Chemical Co., Ltd.) is preferably used.
- the combination of the matrix constituting the drug layer 2 and the matrix constituting the dissolution / separation layer 4 is not particularly limited.
- the matrix constituting the drug layer 2 include water-soluble polymers such as HPC-L and Metrose SM15.
- the matrix constituting the dissolution / separation layer 4 preferably includes a water-soluble polymer having higher water solubility such as PLASDONE K-90 and CMC Daicel 1205.
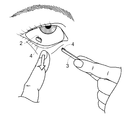
- the user picks the vicinity of the end of the support 3 opposite to the end on the side where the drug layer 2 of the support 1 with the drug is provided, and administers the drug layer 2. Move closer to the site.
- the lower eyelid is pulled down with the finger of one hand to expose the conjunctiva, and the region near the end opposite to the side on which the drug layer 2 is provided of the support 3 with the finger of the other hand
- the drug layer 2 and the dissolution / separation layer 4 are brought close to the eye surface and brought into contact with the exposed conjunctiva.
- the water solubility of the dissolution / separation layer 4 is higher than the water solubility of the drug layer 2, so that the drug separation layer 4 is quickly separated from the support 3 as a result of the dissolution / separation layer 4 being dissolved in tears. Adhere to.
- this support body 1 with a medicine it does not separate in the middle part of the medicine layer 2, and a certain amount of medicine (the medicine layer 2) can be reliably administered.
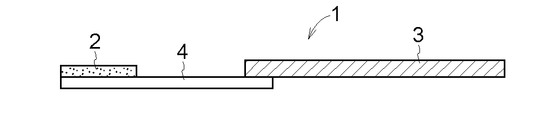
- a part of the lower surface of the drug layer 2 and a part of the upper surface of the dissolution / separation layer 4 are connected, and a part of the lower surface of the dissolution / separation layer 4 and a part of the upper surface of the support 3 are connected.
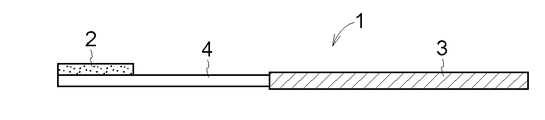
- FIG. 5 the entire lower surface of the drug layer 2 and a part of the upper surface of the dissolution / separation layer 4 are connected, and the upper surface of the dissolution / separation layer 4 and a part of the lower surface of the support 3 are connected. ing.
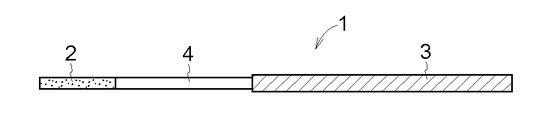
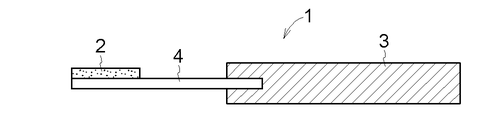
- FIG. 4 Another embodiment of the drug-supported substrate 1 according to the present invention is shown in FIGS.
- FIG. 9 shows a form in which the entire lower surface of the drug layer 2 and a part of the upper surface of the dissolution / separation layer 4 are connected, and the two supports 3 sandwich the dissolution / separation layer 4.
- the support 3 and the dissolution / separation layer 4 do not necessarily require thermocompression bonding or bonding.
- PLASDONE K-90 [Polymer B], HPC-L [Polymer C], Gohsenol EG-05 [Polymer D], TC-5S [Polymer E], Metrolose 60SH-50 are carried out in the same manner as water-soluble polymers.
- [Polymer F] Metrolz SM15 [Polymer G], Metrolz SM100 [Polymer H], Celny M [Polymer I], Kimika Argin IL-2 [Polymer J], and HEC Daicel SE400 [Polymer K] are used as solvents (water, ethanol, water Samples 2 to 11 were prepared as shown in Table 1 by adding glycerin as a plasticizer and polysorbate 80 as an emulsifier as necessary.
- Samples 1 to 11 were coated on a polypropylene film base material using an applicator so as to have a thickness of about 30 ⁇ m, and dried with warm air.
- each of the obtained film-like matrices is chopped into 2 ⁇ 7 mm sizes, immersed in 10 mL of physiological saline at room temperature, and the disintegration time of the film-like matrix [until the matrix completely dissolves and disappears. Time].
- the results are shown in Table 1.
- the average disintegration time of each film matrix obtained from Samples 1 to 11 is an average value of disintegration times when measured three times.
- Comparative Examples 1 and 2 are drug-supported supports in the form of FIG. 4 in which a part of the lower surface of the drug layer 2 and a part of the upper surface of the dissolution / separation layer 4 are connected.
- the drug layer was separated from the support within 10 seconds, and the entire amount could be administered to the rabbit conjunctiva. Therefore, when the drug-supported support of the present invention is used, a certain amount of drug can be reliably administered to the eye.
- the present invention can be applied to a support with a drug for administering a drug to the eye.
Landscapes
- Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Engineering & Computer Science (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Epidemiology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Ophthalmology & Optometry (AREA)
- Vascular Medicine (AREA)
- Heart & Thoracic Surgery (AREA)
- Biomedical Technology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Medicinal Preparation (AREA)
- Media Introduction/Drainage Providing Device (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013-014670 | 2013-01-29 | ||
| JP2013014670 | 2013-01-29 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014119587A1 true WO2014119587A1 (ja) | 2014-08-07 |
Family
ID=51262300
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2014/051893 Ceased WO2014119587A1 (ja) | 2013-01-29 | 2014-01-29 | 溶解分離層を備えた薬剤付き支持体 |
Country Status (3)
| Country | Link |
|---|---|
| JP (1) | JP6334181B2 (cg-RX-API-DMAC7.html) |
| TW (1) | TW201517919A (cg-RX-API-DMAC7.html) |
| WO (1) | WO2014119587A1 (cg-RX-API-DMAC7.html) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017033967A1 (ja) * | 2015-08-24 | 2017-03-02 | 参天製薬株式会社 | 眼科用フィルム製剤 |
| EP3487480A4 (en) * | 2016-07-20 | 2019-07-24 | Ryan, Edwin | APPLICATOR OF OPHTHALMIC DROPS. |
| KR102467288B1 (ko) * | 2020-06-09 | 2022-11-17 | 동국대학교 산학협력단 | 결막 접촉용 약물 전달기 |
| CN119455236B (zh) * | 2025-01-08 | 2025-05-30 | 湖南省华芯医疗器械有限公司 | 药物释放球囊、药物释放器械及内镜组件 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2004534030A (ja) * | 2001-05-14 | 2004-11-11 | スリーエム イノベイティブ プロパティズ カンパニー | 化粧品および医薬品を送達するためのシステム |
| JP2012045379A (ja) * | 2010-07-29 | 2012-03-08 | Santen Pharmaceut Co Ltd | 薬剤付き支持体及びその製造方法 |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| NZ200429A (en) * | 1981-04-30 | 1984-10-19 | Smith & Nephew Ass | Applicator for placing pharmaceutically active agent in contact with moist surface,e.g.,eye |
| DE10246900A1 (de) * | 2002-10-08 | 2004-04-22 | Horstmann, Michael, Dr. | Am Auge applizierbare Vorrichtung zur sicheren Dosierung systemischer und tropischer Medikation |
-
2014
- 2014-01-29 TW TW103103555A patent/TW201517919A/zh unknown
- 2014-01-29 JP JP2014014461A patent/JP6334181B2/ja not_active Expired - Fee Related
- 2014-01-29 WO PCT/JP2014/051893 patent/WO2014119587A1/ja not_active Ceased
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2004534030A (ja) * | 2001-05-14 | 2004-11-11 | スリーエム イノベイティブ プロパティズ カンパニー | 化粧品および医薬品を送達するためのシステム |
| JP2012045379A (ja) * | 2010-07-29 | 2012-03-08 | Santen Pharmaceut Co Ltd | 薬剤付き支持体及びその製造方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2014166350A (ja) | 2014-09-11 |
| TW201517919A (zh) | 2015-05-16 |
| JP6334181B2 (ja) | 2018-05-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Li et al. | Reversible bioadhesives using tannic acid primed thermally‐responsive polymers | |
| US20250090453A1 (en) | Microstructure array, methods of making, and methods of use | |
| US7758939B2 (en) | Adhesive laminates for rapid wound occlusion | |
| JP6334181B2 (ja) | 溶解分離層を備えた薬剤付き支持体 | |
| CN113332588B (zh) | 用于口腔黏膜给药的尖端载药可溶性微针贴片及其制备方法 | |
| FR2936408A1 (fr) | Pansement pour site de ponction ou de perfusion | |
| KR20090125257A (ko) | 경구 투여제 및 그 제조 방법 | |
| CN110248647A (zh) | 制造基于电纺纤维的双层产品的方法 | |
| CN106061544B (zh) | 微型针薄片 | |
| JP2017104490A (ja) | マイクロ構造体製造方法 | |
| WO2017033967A1 (ja) | 眼科用フィルム製剤 | |
| CN111655242A (zh) | 多层口服薄膜 | |
| CN101422447A (zh) | 含有盐酸去氧肾上腺素的速溶性膜制剂及其制造方法 | |
| CN103189097B (zh) | 带药剂的支承体及其制造方法 | |
| JP7143996B2 (ja) | 口腔内粘膜保護フィルム | |
| JP5227041B2 (ja) | 薬物含有貼付製剤 | |
| US20170340580A1 (en) | Bioadhesive patch | |
| Patel et al. | Design and evaluation of buccal patch containing combination of hydrochlorothiazide and lisinopril | |
| US20170239171A1 (en) | Oromucosal film preparation | |
| CN104415024B (zh) | 含双氯芬酸的巴布剂、及其组合物和制备方法 | |
| CN217091660U (zh) | 一种防脱针的输液器 | |
| Donnelly et al. | Fast-drying multi-laminate bioadhesive films for transdermal and topical drug delivery | |
| JPH04266819A (ja) | 口腔粘膜適用製剤 | |
| JP4790535B2 (ja) | しなやかさ測定方法及び貼付剤 | |
| Paracetamol | BRITISH BIOMEDICAL BULLETIN |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14746271 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 14746271 Country of ref document: EP Kind code of ref document: A1 |