WO2014002344A1 - 耐熱無機繊維 - Google Patents
耐熱無機繊維 Download PDFInfo
- Publication number
- WO2014002344A1 WO2014002344A1 PCT/JP2013/002506 JP2013002506W WO2014002344A1 WO 2014002344 A1 WO2014002344 A1 WO 2014002344A1 JP 2013002506 W JP2013002506 W JP 2013002506W WO 2014002344 A1 WO2014002344 A1 WO 2014002344A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- sio
- weight
- less
- inorganic fiber
- components
- Prior art date
Links
Images
Classifications
-
- D—TEXTILES; PAPER
- D01—NATURAL OR MAN-MADE THREADS OR FIBRES; SPINNING
- D01F—CHEMICAL FEATURES IN THE MANUFACTURE OF ARTIFICIAL FILAMENTS, THREADS, FIBRES, BRISTLES OR RIBBONS; APPARATUS SPECIALLY ADAPTED FOR THE MANUFACTURE OF CARBON FILAMENTS
- D01F9/00—Artificial filaments or the like of other substances; Manufacture thereof; Apparatus specially adapted for the manufacture of carbon filaments
- D01F9/08—Artificial filaments or the like of other substances; Manufacture thereof; Apparatus specially adapted for the manufacture of carbon filaments of inorganic material
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B35/00—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products
- C04B35/622—Forming processes; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products
- C04B35/62227—Forming processes; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products obtaining fibres
- C04B35/62231—Forming processes; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products obtaining fibres based on oxide ceramics
- C04B35/62236—Fibres based on aluminium oxide
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C13/00—Fibre or filament compositions
- C03C13/06—Mineral fibres, e.g. slag wool, mineral wool, rock wool
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C3/00—Glass compositions
- C03C3/04—Glass compositions containing silica
- C03C3/062—Glass compositions containing silica with less than 40% silica by weight
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C3/00—Glass compositions
- C03C3/04—Glass compositions containing silica
- C03C3/076—Glass compositions containing silica with 40% to 90% silica, by weight
- C03C3/083—Glass compositions containing silica with 40% to 90% silica, by weight containing aluminium oxide or an iron compound
- C03C3/085—Glass compositions containing silica with 40% to 90% silica, by weight containing aluminium oxide or an iron compound containing an oxide of a divalent metal
- C03C3/087—Glass compositions containing silica with 40% to 90% silica, by weight containing aluminium oxide or an iron compound containing an oxide of a divalent metal containing calcium oxide, e.g. common sheet or container glass
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B35/00—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products
- C04B35/622—Forming processes; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products
- C04B35/62227—Forming processes; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products obtaining fibres
- C04B35/62231—Forming processes; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products obtaining fibres based on oxide ceramics
- C04B35/6224—Fibres based on silica
- C04B35/62245—Fibres based on silica rich in aluminium oxide
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16L—PIPES; JOINTS OR FITTINGS FOR PIPES; SUPPORTS FOR PIPES, CABLES OR PROTECTIVE TUBING; MEANS FOR THERMAL INSULATION IN GENERAL
- F16L59/00—Thermal insulation in general
- F16L59/02—Shape or form of insulating materials, with or without coverings integral with the insulating materials
- F16L59/028—Composition or method of fixing a thermally insulating material
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C2213/00—Glass fibres or filaments
- C03C2213/02—Biodegradable glass fibres
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/50—Constituents or additives of the starting mixture chosen for their shape or used because of their shape or their physical appearance
- C04B2235/52—Constituents or additives characterised by their shapes
- C04B2235/5208—Fibers
- C04B2235/5264—Fibers characterised by the diameter of the fibers
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/70—Aspects relating to sintered or melt-casted ceramic products
- C04B2235/72—Products characterised by the absence or the low content of specific components, e.g. alkali metal free alumina ceramics
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/70—Aspects relating to sintered or melt-casted ceramic products
- C04B2235/96—Properties of ceramic products, e.g. mechanical properties such as strength, toughness, wear resistance
- C04B2235/9669—Resistance against chemicals, e.g. against molten glass or molten salts
Definitions
- the present invention relates to a biosoluble inorganic fiber having excellent heat resistance.
- conventional inorganic fibers are secondary-processed into shaped products and irregular shaped materials, together with various binders and additives, and joint materials in furnaces such as heat treatment equipment, industrial kilns and incinerators, It is used as a joint material, a sealing material, a packing material, a heat insulating material, and the like for filling gaps such as refractory tiles, heat insulating bricks, iron skin, and mortar refractories. When used, it is often exposed to high temperatures and is required to have heat resistance.
- alumina is often used as a member in the furnace, and there is a problem that the fibers contained in the secondary processed product react with the alumina and the secondary processed product or member adheres or melts. .
- An object of the present invention is to provide a biosoluble inorganic fiber having novel heat resistance.
- the following inorganic fibers and the like are provided.
- the three components are included as a main component
- the two components are included as main components
- Na 2 O and SiO 2 are included but Al 2 O 3 is not included, inorganic fibers containing the two components as main components.
- 3. Inorganic fiber of 3 which has the following compositions. Na 2 O: 5 ⁇ 40 wt% SiO 2 : 0 to 75% by weight Al 2 O 3 : 20 to 95% by weight 5.
- 3. Inorganic fiber of 3 which has the following compositions. Na 2 O: 10 to 40% by weight SiO 2 : 0 to 58% by weight Al 2 O 3 : 32 to 90% by weight 6). 4. Inorganic fiber of 4 which has the following compositions.
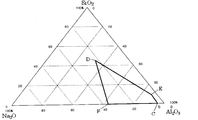
- the phase diagram of Na 2 O—SiO 2 —Al 2 O 3 shown in FIG. 2 it has a composition in a range surrounded by the following points D, F, C, and E.
- Na 2 O, when containing SiO 2 and Al 2 O 3 includes the three components as a main component, When Na 2 O and Al 2 O 3 are included but SiO 2 is not included, the two components are included as main components, Inorganic fiber with Na 2 O + SiO 2 + Al 2 O 3 ⁇ 80% by weight.
- a biosoluble inorganic fiber having novel heat resistance can be provided.
- FIG. 3 is a Na 2 O—SiO 2 —Al 2 O 3 phase diagram showing the preferred fiber composition of the present invention and the fiber compositions of Examples 1-30.
- FIG. 2 is a Na 2 O—SiO 2 —Al 2 O 3 phase diagram showing a preferred fiber composition of the present invention.
- First inorganic fiber of the present invention is mainly composed of three components of Na 2 O, SiO 2, Al 2 O 3.
- the present invention has a biosoluble for containing Na 2 O.
- the main component means that the three components having the highest content (% by weight) among all the components contained in the inorganic fiber are Na 2 O, SiO 2 , and Al 2 O 3 .
- the first inorganic fiber does not react with alumina at least at 600 ° C.
- alumina By not reacting with alumina is meant that the alumina pellets do not adhere to the fleece or blanket made from the fiber as measured by the method described in the Examples.
- the second inorganic fiber of the present invention has a composition in a range surrounded by the following points A, B, and C in the phase diagram of Na 2 O—SiO 2 —Al 2 O 3 shown in FIG.
- Na 2 O, SiO 2 and Al 2 O 3 are contained, Na 2 O, SiO 2 and Al 2 O 3 are contained as main components, and Na 2 O and SiO 2 are contained but Al 2 O 3 is not contained.
- Na 2 O and SiO 2 are contained as main components and Na 2 O and Al 2 O 3 are contained but not SiO 2
- Na 2 O and Al 2 O 3 are contained as main components.
- the main component means three or two components having the highest content (% by weight) among all the components contained in the inorganic fiber.
- the second inorganic fiber is excellent in heat resistance at 1000 ° C. and alumina resistance.
- the third inorganic fiber has the following composition.
- Na 2 O when containing SiO 2 and Al 2 O 3 comprises Na 2 O, SiO 2 and Al 2 O 3 as a main component, when not containing SiO 2 include Na 2 O and Al 2 O 3 is When Na 2 O and Al 2 O 3 are contained as main components and Na 2 O and SiO 2 are contained but Al 2 O 3 is not contained, Na 2 O and SiO 2 are contained as main components.
- the inorganic fiber which has the following compositions is preferred.
- Inorganic fibers having the following composition are more preferable.
- Al 2 O 3 32 to 90% by weight
- Inorganic fibers having the following composition are more preferable.
- the fourth inorganic fiber has a composition in a range surrounded by the following points D, F, C, and E in the phase diagram of Na 2 O—SiO 2 —Al 2 O 3 shown in FIG.
- Na 2 O, SiO 2 and Al 2 O 3 are contained, Na 2 O, SiO 2 and Al 2 O 3 are contained as main components, and Na 2 O and Al 2 O 3 are contained but SiO 2 is not contained.
- Na 2 O and Al 2 O 3 as main components.
- the first to fourth inorganic fibers (hereinafter also referred to as inorganic fibers of the present invention) preferably include three components of Na 2 O, SiO 2 and Al 2 O 3 .
- the blending amount of each component can be appropriately combined.
- SiO 2 may not be contained.
- SiO 2 when SiO 2 is included, for example, 0.1 wt% or more, 0.3 wt% or more, 0.5 wt% or more, 1.0 wt% or more, 5 wt% or more, 10 wt% % Or more and 12% by weight or more.
- it is 5 weight% or more, More preferably, it is 15 weight% or more.
- it can be 50 weight% or less, 45 weight% or less, 42.5 weight% or less, or 25 weight% or less.
- it can be 28.5 wt% to 45.0 wt%, or 28.5 wt% to 42.5 wt%.
- the amount of Na 2 O is preferably 5% by weight or more.
- the amount of Na 2 O is, for example, 10 wt% to 40 wt%, preferably 15 wt% to 30 wt%, more preferably 18 wt% to 30 wt%, 18 wt% to 28 wt%, or 18 wt%. ⁇ 25% by weight.
- Al 2 O 3 may not be contained.
- the inorganic fiber of the present invention contains Al 2 O 3 , it is preferably contained in an amount of 5% by weight or more, more preferably 20% by weight or more, 30% by weight or more, 32% by weight or more, and 35% by weight or more.
- the amount of Al 2 O 3 80 wt% is preferably below 70 wt% and more preferably less, further preferably 67 wt% or less.
- the total of Na 2 O, SiO 2 and Al 2 O 3 is 85% by weight or more, 90% by weight or more, 93% by weight or more, 95% by weight or more, 98% by weight or more, 99% by weight or 100% by weight (however, Inevitable impurities may be included).
- the rest other than the specified components is oxides or impurities of other elements.
- the inorganic fiber of the present invention is Sc, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Y, or a mixture thereof. May or may not be included.
- the amount of these oxides may be 5% by weight or less, 3% by weight or less, 2% by weight or less, 1% by weight or less, or 0.5% by weight or less, respectively.
- Each of alkali metal oxides (K 2 O, Li 2 O, etc.) other than Na 2 O may or may not be included, and each or a total thereof is less than 12 mol%, 5 wt% or less, 3 It can be less than wt%, less than 2 wt%, less than 1 wt%, or less than 0.5 wt%.
- Each of TiO 2 , ZnO, B 2 O 3 , P 2 O 5 , MgO, CaO, SrO, BaO, Cr 2 O 3 , ZrO 2 , Fe 2 O 3 may or may not be included. 20% or less, 18% or less, 15% or less, 10% or less, 7% or less, 5% or less, 3% or less, 2% or less, 1% or less, or 0.5% by weight It can be as follows.
- Each of non-oxide materials such as carbides and nitrides may also be included, each being 20% or less, 18% or less, 15% or less, 10% or less, 7% or less, 5% or less. Hereinafter, it can be 3 wt% or less, 2 wt% or less, 1 wt% or less, or 0.5 wt% or less.
- the atmosphere at the time of melting may be an inert atmosphere or a reducing atmosphere.
- the inorganic fibers of the present invention usually do not contain the following substances, or even if they contain 1.0% or less, 0.5% or less, 0.2% or less, or 0.1% or less, respectively. Germanium oxide, tellurium oxide, vanadium oxide, sulfur oxide, phosphorus compound, tin, cobalt, manganese oxide, fluoride, copper oxide.
- Inorganic fibers can be produced by a known method such as a melting method or a sol-gel method, but the melting method is preferred because of low cost.
- a raw material melt is produced by a normal method, and the melt is made into a fiber.
- it can be manufactured by a spinning method in which a melted raw material is poured onto a wheel rotating at high speed, and a blow method in which the melted raw material is fiberized by applying compressed air.
- the average fiber diameter of the inorganic fiber of the present invention is usually 0.1 to 50 ⁇ m, preferably 0.5 to 20 ⁇ m, more preferably 1 to 10 ⁇ m, and most preferably 1 to 8 ⁇ m.
- the average fiber diameter may be adjusted by a known production method such as the number of rotations, acceleration, compressed air pressure, wind speed, and air volume so as to obtain a desired fiber diameter.
- the fiber may be coated with a known coating material or may not be coated.
- a surface treatment agent such as a surfactant, bundling material, chelating agent, rust inhibitor, water repellent, inorganic component (for example, described in the above paragraphs 0024 to 0026) may be coated.
- the inorganic fiber of this invention does not need to heat-process.
- the temperature may be any temperature that maintains the fiber shape. Since the fiber physical properties change depending on the heating temperature and the heating time, it may be appropriately treated so as to obtain desired performance (creep resistance, shrinkage rate, strength, elasticity).
- the inorganic fiber changes from amorphous to crystalline by the prescribed heat treatment, but it is sufficient that the desired performance is achieved as described above, and it may be in either amorphous or crystalline state.
- the crystalline parts may be mixed together.
- the heating temperature may be, for example, 100 ° C. or more, 300 ° C. or more, preferably 600 ° C. or more, 800 ° C. or more, more preferably 1000 ° C.
- the inorganic fiber of the present invention dissolves in physiological saline having pH 4.5 and pH 7.4 by having the above composition. Furthermore, it has solubility even after heating (after crystallization).
- the solubility in physiological saline at pH 4.5 or 7.4 is preferably 2.0 mg / g or more, more preferably 4.0 mg / g or more, and even more preferably 6.3 mg / g, according to the measurement method of the example. That's it.
- the solubility is 1.0 mg / g or more with respect to physiological saline having a pH of 4.5, it is assumed to have biosolubility.
- the solubility of the fiber can also be measured by the following method.
- the fiber is placed on a membrane filter, and a physiological saline solution having a pH of 4.5 or 7.4 is dropped onto the fiber by a micropump, and the filtrate passing through the fiber and the filter is stored in a container.
- the accumulated filtrate is taken out after 24 and 48 hours, and the eluted components are quantified with an ICP emission spectrometer, and the solubility and dissolution rate constant are calculated.
- the measurement element can be three elements of Al, Si, and Na which are main elements.
- the fiber diameter may be measured and converted to a dissolution rate constant (unit: ng / cm 2 ⁇ h), which is an elution amount per unit surface area / unit time.
- the dissolution rate constant is preferably 10 ng / cm 2 ⁇ h or more, 30 ng / cm 2 ⁇ h or more, 50 ng / cm 2 ⁇ h or more, 100 ng / cm 2 ⁇ h or more, 150 ng / cm 2 ⁇ h or more, 200 ng / cm 2 ⁇ h or more, 300 ng / cm 2 ⁇ h or more, 500 ng / cm 2 ⁇ h or more, 1000 ng / cm 2 ⁇ h or more, 1500 ng / cm 2 ⁇ h or more.
- the inorganic fiber of the present invention preferably has low alumina reactivity.
- Alumina reactivity is preferably no melting, more preferably no melting or adhesion, in the measurement method of the examples.
- the inorganic fibers of the present invention preferably have heat resistance at 600 ° C or higher, 800 ° C or higher, 1000 ° C or higher, 1100 ° C or higher, 1200 ° C or higher, 1300 ° C or higher, 1400 ° C or higher.
- the volumetric shrinkage (%) obtained by heating a cylindrical sample having a diameter of about 7 mm and a height of about 15 mm at a predetermined temperature of 800 to 1400 ° C. for 8 hours is preferably 1400 ° C. for 8 hours.
- the heat shrinkage ratio of the fiber can be measured before and after producing a fleece or a blanket from the fiber and firing it at a predetermined temperature of 600 ° C. to 1600 ° C. for 8 hours. Two or more platinum pins are driven into the surface of each manufactured sample, the distance between the platinum pins is measured before and after heating, and the dimensional change rate is defined as the heat shrinkage rate.
- the heat shrinkage rate is preferably 50% or less, 45% or less, or 40% or less at each temperature (600 ° C, 800 ° C, 1000 ° C, 1100 ° C, 1200 ° C, 1300 ° C, 1400 ° C, 1500 ° C, 1600 ° C). 35% or less, 30% or less, 25% or less, 20% or less, 15% or less, more preferably 10% or less, 8% or less, most preferably 5% or less, 3% or less, 1% or less.
- the inorganic fiber of the present invention can reduce the types of essential components, the number of man-hours for the blending process is reduced and the cost is reduced. In addition, the fact that there are few kinds of components for adjusting delicate blending amounts reduces the difficulty of production.
- a regular product such as a wet felt impregnated with board, mold, paper, felt or colloidal silica is obtained.
- a regular product obtained by treating the regular product with a colloid or the like can be obtained.
- the amorphous material (mastic, a caster, a coating material, etc.) manufactured using solvents, such as water, is also obtained.
- a structure in which the above-mentioned regular product, irregular product and various heating elements are combined can be obtained.
- the fibers of the present invention include heat treatment equipment, joint materials in furnaces such as industrial kilns and incinerators, joint materials for filling gaps such as refractory tiles, heat-insulating bricks, iron skins, mortar refractories, sealing materials, Packing material, cushioning material, heat insulating material, fireproofing material, fireproofing material, heat insulating material, protective material, coating material, filter material, filter material, insulating material, jointing material, filling material, repair material, heat resistant material, noncombustible material, soundproofing material , Sound-absorbing materials, friction materials (for example, brake pad additives), glass plate / steel sheet transport rolls, automobile catalyst carrier holding materials, various fiber reinforced composite materials (for example, fiber reinforced cement, fiber reinforced plastic and other reinforcing fibers, heat resistance Materials, reinforcing fibers such as refractory materials, reinforcing fibers such as adhesives and coating materials) and the like.
- various fiber reinforced composite materials for example, fiber reinforced cement, fiber reinforced plastic and other reinfor
- Examples 1 to 196, Comparative Example 1 Fibers having the compositions shown in Table 1 were produced by a melting method, and heat resistance and alumina reactivity were examined by the following methods. The results are shown in Table 1.
- the compositions of 149, 158, 161, 168, 176, 180 and 190 are shown in FIG.
- the heat shrinkage rate was measured as an evaluation of the heat resistance of the fiber.
- the heat shrinkage rate of the fiber was measured before and after producing a fleece or a blanket (length: 50 mm, thickness: 5 to 50 mm) from the fiber and firing at a predetermined temperature of 600 ° C. to 1400 ° C. for 8 hours.
- Two or more platinum pins were driven into the surface of each manufactured sample, the distance between the platinum pins was measured before and after heating, and the dimensional change rate was defined as the heat shrinkage rate.
- alumina reactivity About 1 g of alumina powder with a purity of 99% or more was press-molded with a 17 mm diameter mold into pellets. This pellet was placed on a fleece-like or blanket sample (50 mm long and 5 mm thick) manufactured from fibers, heated in this state, and the reactivity after heating was confirmed. ⁇ when there is no reaction with the pellet, light adhesion with the sample (the pellet is easily peeled off by hand, the pellet and the sample are not melted), and there is a reaction (the pellet and the sample are melted and adhered) ) was marked with x.
- the biosolubility of the unheated fiber and the biosolubility of the fiber after heating at 1400 ° C. for 8 hours were measured by the following methods.
- the fiber was placed on a membrane filter, and physiological saline of pH 4.5 or pH 7.4 was dropped on the fiber with a micropump, and the filtrate that passed through the fiber and filter was stored in a container.
- the accumulated filtrate was taken out after 24 hours, and the eluted components were quantified with an ICP emission spectrometer, and the solubility was calculated.
- the measurement elements were three elements of Al, Si, and Na which are main elements.
- the average fiber diameter was measured and converted to a dissolution rate constant (unit: ng / cm 2 ⁇ h) which is the amount of elution per unit surface area / unit time.
- the average fiber diameter was measured by the following method. After observing and photographing 400 or more fibers with an electron microscope, the diameter of the photographed fibers was measured, and the average value of all the measured fibers was defined as the average fiber diameter.
- Examples 197 to 216 The fiber composition shown in Table 2 was examined as follows. First, raw materials were mixed so as to have the composition shown in Table 2, and pressed to obtain a molded body. The molded product was melted by heating and rapidly cooled to obtain a sample. Using this sample, the following method was used for evaluation. For comparison, the fiber of Comparative Example 1 was also evaluated in the same manner. The results are shown in Table 2.
- the sample was molded to obtain a cylindrical sample having a diameter of about 7 mm and a thickness of about 5 mm. This cylindrical sample was placed on an alumina plate and heated at 1400 ° C. for 8 hours to observe the presence or absence of adhesion or melting. It was 4 when the cylindrical sample was melted, 3 when it was adhered, 2 when it was not adhered but remained, and 1 when it was not adhered and remained.
- the inorganic fiber of the present invention can be used for various purposes as a heat insulating material or as a substitute for asbestos.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Organic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Manufacturing & Machinery (AREA)
- Ceramic Engineering (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Geochemistry & Mineralogy (AREA)
- Inorganic Chemistry (AREA)
- Structural Engineering (AREA)
- General Engineering & Computer Science (AREA)
- Textile Engineering (AREA)
- General Life Sciences & Earth Sciences (AREA)
- Mechanical Engineering (AREA)
- Inorganic Fibers (AREA)
- Glass Compositions (AREA)
Abstract
Description
1.Na2O、SiO2及びAl2O3を、主成分として含み、600℃でアルミナと反応しない無機繊維。
2.図1に示す、Na2O-SiO2-Al2O3の状態図において、以下の点A、点B、点Cで囲まれる範囲の組成を有し、
Na2O、SiO2及びAl2O3を含むときは、前記3成分を主成分として含み、
Na2O及びSiO2を含みAl2O3は含まないときは、前記2成分を主成分として含み、
Na2O及びAl2O3を含みSiO2は含まないときは、前記2成分を主成分として含み、
Na2O+SiO2+Al2O3≧80重量%である無機繊維。
点A:(Na2O:SiO2:Al2O3=5:95:0)
点B:(Na2O:SiO2:Al2O3=40:0:60)
点C:(Na2O:SiO2:Al2O3=5:0:95)
3.以下の組成を有し、
Na2O、SiO2及びAl2O3を含むときは、前記3成分を主成分として含み、
Na2O及びAl2O3を含みSiO2は含まないときは、前記2成分を主成分として含み、
Na2O及びSiO2を含みAl2O3は含まないときは、前記2成分を主成分として含む
無機繊維。
Na2O:5~50重量%
SiO2:0~95重量%
Al2O3:0~95重量%
Na2O+SiO2+Al2O3≧80重量%
4.以下の組成を有する3記載の無機繊維。
Na2O:5~40重量%
SiO2:0~75重量%
Al2O3:20~95重量%
5.以下の組成を有する3記載の無機繊維。
Na2O:10~40重量%
SiO2:0~58重量%
Al2O3:32~90重量%
6.以下の組成を有する4記載の無機繊維。
Na2O:15~38重量%
SiO2:0~51重量%
Al2O3:34~85重量%
7.図2に示す、Na2O-SiO2-Al2O3の状態図において、以下の点D、点F、点C、点Eで囲まれる範囲の組成を有し、
Na2O、SiO2及びAl2O3を含むときは、前記3成分を主成分として含み、
Na2O及びAl2O3を含みSiO2は含まないときは、前記2成分を主成分として含み、
Na2O+SiO2+Al2O3≧80重量%である無機繊維。
点D:(Na2O:SiO2:Al2O3=23:45:32)
点F:(Na2O:SiO2:Al2O3=38:0:62)
点C:(Na2O:SiO2:Al2O3=5:0:95)
点E:(Na2O:SiO2:Al2O3=5:10:85)
8.SiO2量が25重量%以下である1~7のいずれか記載の無機繊維。
9.SiO2量が28.5重量%~45.0重量%である1~7のいずれか記載の無機繊維。
10.Na2O量が15重量%~30重量%である1~9のいずれか記載の無機繊維。
11.Na2O量が18重量%~28重量%である10記載の無機繊維。
12.Al2O3量が80重量%以下である1~11のいずれか記載の無機繊維。
13.Al2O3量が70重量%以下である12記載の無機繊維。
14.溶融法で製造する1~13のいずれか記載の無機繊維の製造方法。
主成分とは、無機繊維が含む全ての成分のうち最も含有量(重量%)の高い3成分がNa2O、SiO2、Al2O3であることを意味する。
点A:(Na2O:SiO2:Al2O3=5:95:0)
点B:(Na2O:SiO2:Al2O3=40:0:60)
点C:(Na2O:SiO2:Al2O3=5:0:95)
第2の無機繊維は1000℃での耐熱性、耐アルミナ反応性に優れる。
Na2O:5~50重量%
SiO2:0~95重量%
Al2O3:0~95重量%
Na2O+SiO2+Al2O3≧80重量%
Na2O:5~40重量%
SiO2:0~75重量%
Al2O3:20~95重量%
Na2O:10~40重量%
SiO2:0~58重量%
Al2O3:32~90重量%
Na2O:15~38重量%
SiO2:0~51重量%
Al2O3:34~85重量%
点D:(Na2O:SiO2:Al2O3=23:45:32)
点F:(Na2O:SiO2:Al2O3=38:0:62)
点C:(Na2O:SiO2:Al2O3=5:0:95)
点E:(Na2O:SiO2:Al2O3=5:10:85)
特定する成分以外の残りは他の元素の酸化物又は不純物等である。
酸化ゲルマニウム、酸化テルル、酸化バナジウム、酸化イオウ、リン化合物、スズ、コバルト、酸化マンガン、フッ化物、酸化銅。
加熱処理する場合は、繊維形状を維持する温度であればよい。加熱温度、加熱時間により繊維物性が変化するので適宜所望の性能(耐クリープ性、収縮率、強度、弾性)がでるように処理すればよい。
所定の加熱処理により無機繊維は非晶質から結晶質へ変化するが、上記の記載のように所望の性能がでればよく、非晶質、結晶質のどちらの状態でもよく、非晶質、結晶質部分がそれぞれが混在している状態でもよい。
加熱温度は、例えば100℃以上、300℃以上、好ましくは、600℃以上、800℃以上、さらに好ましくは1000℃以上、1200℃以上、1300℃以上、1400℃以上でよく、600℃~1400℃、さらに好ましくは、800℃~1200℃、800℃~1000℃である。
繊維を、メンブレンフィルター上に置き、繊維上にマイクロポンプによりpH4.5又は7.4の生理食塩水を滴下させ、繊維、フィルターを通った濾液を容器内に貯める。貯めた濾液を24、48時間経過後に取り出し、溶出成分をICP発光分析装置により定量し、溶解度及び溶解速度定数を算出する。例えば、測定元素は主要元素であるAl、Si、Naの3元素とすることができる。尚、繊維径を測定して単位表面積・単位時間当たりの溶出量である溶解速度定数(単位:ng/cm2・h)に換算してもよい。
加熱収縮率は、各温度(600℃、800℃、1000℃、1100℃、1200℃、1300℃、1400℃、1500℃、1600℃)において、好ましくは50%以下、45%以下、40%以下、35%以下、30%以下、25%以下、20%以下、15%以下、さらに好ましくは10%以下、8%以下、最も好ましくは5%以下、3%以下、1%以下である。
また、上記定形品、不定形品と各種発熱体を組み合わせた構造体も得られる。
表1に示す組成を有する繊維を溶融法で製造し、以下の方法で耐熱性とアルミナ反応性について検討した。結果を表1に示す。
また、実施例4,11,19,33,40,41,47,51,54,58,59,62,63,76,77,87,93,94,102,107,111,124,143,149,158,161,168,176,180,190の組成を図1に示す。
繊維の耐熱性の評価として加熱収縮率を測定した。
繊維の加熱収縮率は、繊維からフリース又はブランケット(縦横50mm、厚み5~50mm)を製造して、600℃~1400℃の所定の温度で、8時間焼成した前後で測定した。
製造した各サンプル表面に白金ピンを2点以上打ち込み、その白金ピン間の距離を加熱前後で測定し、その寸法変化率を加熱収縮率とした。
純度99%以上のアルミナ粉末約1gを、直径17mmの金型でプレス成形しペレットとした。このペレットを、繊維から製造したフリース状またはブランケット(縦横50mm、厚み5~50mm)のサンプル上に置いて、この状態で加熱し、加熱後の反応性を確認した。ペレットと全く反応していない場合を○、サンプルと軽い付着(簡単に手でペレットがはがせ、外観でペレットとサンプルが溶融していない状態)を△、反応有り(ペレットとサンプルが溶融し付着している状態)を×とした。
以下の方法で、未加熱の繊維の生体溶解性、及び1400℃、8時間での加熱後の繊維の生体溶解性を測定した。
繊維を、メンブレンフィルター上に置き、繊維上にマイクロポンプによりpH4.5又はpH7.4の生理食塩水を滴下させ、繊維、フィルターを通った濾液を容器内に貯めた。貯めた濾液を24時間経過後に取り出し、溶出成分をICP発光分析装置により定量し、溶解度を算出した。測定元素は主要元素であるAl、Si、Naの3元素とした。平均繊維径を測定して単位表面積・単位時間当たりの溶出量である溶解速度定数(単位:ng/cm2・h)に換算した。
平均繊維径は以下の方法で測定した。
400本以上の繊維を、電子顕微鏡で観察・撮影した後、撮影した繊維について、その径を計測し、全計測繊維の平均値を平均繊維径とした。
スピニング法又はブロー法での製繊性について評価した。
いずれかの方法において製繊され吸引チャンバーにて集綿した際の原綿が、フリース状になる場合を◎、フリース状になるが大ショットが多い場合を○、繊維状物質は得られるがフリース状にならない場合を△、繊維状物質が得られない場合を×とした。
上記の評価にて◎及び○のサンプルにてフリース状サンプルの繊維状態を評価した。
フリース状でのハンドリングが良好な場合を◎、ハンドリングは良好であるが大ショットが多い場合を○、ハンドリングするとフリースが部分的に千切れる場合を△、ハンドリングするだけでフリースが崩れる場合を×とした。
表2に示す繊維組成について以下のように検討した。
まず、表2に示す組成となるように原料を混合し、プレス加工して成形体を得た。この成形体を加熱溶融し、急冷して得られた物を粉砕しサンプルを得た。このサンプルを用いて以下の方法で評価した。比較のため比較例1の繊維も同様に評価した。その結果を表2に示す。
サンプル1gを、pH4.5の生理食塩水150mLが入った三角フラスコ(容積300mL)に入れた。このフラスコを、37℃のインキュベーター内に設置して、毎分120回転の水平振動を2.5時間継続した。その後、ろ過により得られた濾液に含有されている各元素の量(mg)をICP発光分析装置により測定し、その合計を溶出量とした(mg/サンプル1g)。
サンプルを成形して、直径約7mm、厚み約5mmの円柱状サンプルを得た。この円柱状サンプルをアルミナ板に載せて、1400℃8時間加熱して、付着や溶融の有無を観察した。円柱状サンプルが溶融したときは4、付着したときは3、付着しないが痕が残ったときは2、付着もせず痕も残らないときは1とした。
この明細書に記載の文献及び本願のパリ優先の基礎となる日本出願明細書及び米国仮出願明細書の内容を全てここに援用する。
Claims (14)
- Na2O、SiO2及びAl2O3を、主成分として含み、600℃でアルミナと反応しない無機繊維。
- 図1に示す、Na2O-SiO2-Al2O3の状態図において、以下の点A、点B、点Cで囲まれる範囲の組成を有し、
Na2O、SiO2及びAl2O3を含むときは、前記3成分を主成分として含み、
Na2O及びSiO2を含みAl2O3は含まないときは、前記2成分を主成分として含み、
Na2O及びAl2O3を含みSiO2は含まないときは、前記2成分を主成分として含み、
Na2O+SiO2+Al2O3≧80重量%である無機繊維。
点A:(Na2O:SiO2:Al2O3=5:95:0)
点B:(Na2O:SiO2:Al2O3=40:0:60)
点C:(Na2O:SiO2:Al2O3=5:0:95) - 以下の組成を有し、
Na2O、SiO2及びAl2O3を含むときは、前記3成分を主成分として含み、
Na2O及びAl2O3を含みSiO2は含まないときは、前記2成分を主成分として含み、
Na2O及びSiO2を含みAl2O3は含まないときは、前記2成分を主成分として含む
無機繊維。
Na2O:5~50重量%
SiO2:0~95重量%
Al2O3:0~95重量%
Na2O+SiO2+Al2O3≧80重量% - 以下の組成を有する請求項3記載の無機繊維。
Na2O:5~40重量%
SiO2:0~75重量%
Al2O3:20~95重量% - 以下の組成を有する請求項3記載の無機繊維。
Na2O:10~40重量%
SiO2:0~58重量%
Al2O3:32~90重量% - 以下の組成を有する請求項4記載の無機繊維。
Na2O:15~38重量%
SiO2:0~51重量%
Al2O3:34~85重量% - 図2に示す、Na2O-SiO2-Al2O3の状態図において、以下の点D、点F、点C、点Eで囲まれる範囲の組成を有し、
Na2O、SiO2及びAl2O3を含むときは、前記3成分を主成分として含み、
Na2O及びAl2O3を含みSiO2は含まないときは、前記2成分を主成分として含み、
Na2O+SiO2+Al2O3≧80重量%である無機繊維。
点D:(Na2O:SiO2:Al2O3=23:45:32)
点F:(Na2O:SiO2:Al2O3=38:0:62)
点C:(Na2O:SiO2:Al2O3=5:0:95)
点E:(Na2O:SiO2:Al2O3=5:10:85) - SiO2量が25重量%以下である請求項1~7のいずれか記載の無機繊維。
- SiO2量が28.5重量%~45.0重量%である請求項1~7のいずれか記載の無機繊維。
- Na2O量が15重量%~30重量%である請求項1~9のいずれか記載の無機繊維。
- Na2O量が18重量%~28重量%である請求項10記載の無機繊維。
- Al2O3量が80重量%以下である請求項1~11のいずれか記載の無機繊維。
- Al2O3量が70重量%以下である請求項12記載の無機繊維。
- 溶融法で製造する請求項1~13のいずれか記載の無機繊維の製造方法。
Priority Applications (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US14/404,311 US20150144830A1 (en) | 2012-06-29 | 2013-04-12 | Heat resistant inorganic fiber |
| CN201380034708.2A CN104395513A (zh) | 2012-06-29 | 2013-04-12 | 耐热无机纤维 |
| EP13808822.4A EP2868784A4 (en) | 2012-06-29 | 2013-04-12 | HEAT-RESISTANT INORGANIC FIBER |
| KR1020147031794A KR20150027049A (ko) | 2012-06-29 | 2013-04-12 | 내열 무기섬유 |
| IN10759DEN2014 IN2014DN10759A (ja) | 2012-06-29 | 2013-04-12 | |
| JP2014522376A JP6212040B2 (ja) | 2012-06-29 | 2013-04-12 | 耐熱無機繊維 |
| AU2013282679A AU2013282679A1 (en) | 2012-06-29 | 2013-04-12 | Heat-resistant inorganic fiber |
| BR112014031000A BR112014031000A2 (pt) | 2012-06-29 | 2013-04-12 | fibra inorgânica resistente ao calor |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2012-147286 | 2012-06-29 | ||
| JP2012147286 | 2012-06-29 | ||
| US201261706348P | 2012-09-27 | 2012-09-27 | |
| US61/706,348 | 2012-09-27 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014002344A1 true WO2014002344A1 (ja) | 2014-01-03 |
Family
ID=49782551
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2013/002506 WO2014002344A1 (ja) | 2012-06-29 | 2013-04-12 | 耐熱無機繊維 |
Country Status (10)
| Country | Link |
|---|---|
| US (1) | US20150144830A1 (ja) |
| EP (1) | EP2868784A4 (ja) |
| JP (1) | JP6212040B2 (ja) |
| KR (1) | KR20150027049A (ja) |
| CN (1) | CN104395513A (ja) |
| AR (1) | AR092341A1 (ja) |
| AU (1) | AU2013282679A1 (ja) |
| BR (1) | BR112014031000A2 (ja) |
| IN (1) | IN2014DN10759A (ja) |
| WO (1) | WO2014002344A1 (ja) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6864693B2 (ja) | 2016-01-15 | 2021-04-28 | サーマル セラミックス ユーケー リミテッド | 溶融形成された無機繊維を形成する装置と方法 |
| JP7264887B2 (ja) * | 2017-10-10 | 2023-04-25 | ユニフラックス アイ エルエルシー | 結晶性シリカを含まない低生体内持続性の無機繊維 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0597426A (ja) * | 1991-10-09 | 1993-04-20 | Mizusawa Ind Chem Ltd | 繊維状アルミノケイ酸塩及びその製法 |
| JP2005514318A (ja) | 2002-01-04 | 2005-05-19 | ザ・モーガン・クルーシブル・カンパニー・ピーエルシー | 生理食塩水に可溶の無機繊維 |
| JP3753416B2 (ja) | 1992-01-17 | 2006-03-08 | ザ・モーガン・クルーシブル・カンパニー・ピーエルシー | 物品の絶縁方法 |
| JP2008534421A (ja) * | 2005-04-01 | 2008-08-28 | サン−ゴバン・イソベール | ミネラルウール、絶縁製品及び製造方法 |
| WO2011017343A2 (en) * | 2009-08-03 | 2011-02-10 | Ppg Industries Ohio, Inc. | Glass compositions and fibers made therefrom |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3239369A (en) * | 1961-07-28 | 1966-03-08 | Owens Corning Fiberglass Corp | Continuous sodium silicate fibers |
| JPS6217041A (ja) * | 1985-07-16 | 1987-01-26 | Nippon Sheet Glass Co Ltd | 繊維用ガラス組成物 |
| DE4418726A1 (de) * | 1994-05-28 | 1995-11-30 | Gruenzweig & Hartmann | Glasfaserzusammensetzungen |
| FR2778401A1 (fr) * | 1998-05-06 | 1999-11-12 | Saint Gobain Isover | Composition de laine minerale |
| WO2004003276A1 (ja) * | 2002-06-28 | 2004-01-08 | Denki Kagaku Kogyo Kabushiki Kaisha | 保持材用無機質短繊維集積体、その製造方法、及び保持材 |
| CN1242105C (zh) * | 2003-11-24 | 2006-02-15 | 山东鲁阳股份有限公司 | 可溶于人体体液的无机硅酸盐纤维纺织品及其制法 |
| FR2883866B1 (fr) * | 2005-04-01 | 2007-05-18 | Saint Gobain Isover Sa | Laine minerale, produit isolant et procede de fabrication |
| JP4932225B2 (ja) * | 2005-04-08 | 2012-05-16 | 旭ファイバーグラス株式会社 | 環状ポリオレフィン樹脂組成物及び成形品 |
| US7189671B1 (en) * | 2005-10-27 | 2007-03-13 | Glass Incorporated | Glass compositions |
| CA2629102C (en) * | 2005-11-10 | 2015-03-31 | The Morgan Crucible Company Plc | High temperature resistant fibres |
| FR2905695B1 (fr) * | 2006-09-13 | 2008-10-24 | Saint Gobain Isover Sa | Compositions pour laines minerales |
| WO2008065363A1 (en) * | 2006-11-28 | 2008-06-05 | The Morgan Crucible Company Plc | Inorganic fibre compositions |
| FR2918053B1 (fr) * | 2007-06-27 | 2011-04-22 | Saint Gobain Vetrotex | Fils de verre aptes a renforcer des matieres organiques et/ou inorganiques. |
-
2013
- 2013-04-12 KR KR1020147031794A patent/KR20150027049A/ko not_active Application Discontinuation
- 2013-04-12 IN IN10759DEN2014 patent/IN2014DN10759A/en unknown
- 2013-04-12 EP EP13808822.4A patent/EP2868784A4/en not_active Withdrawn
- 2013-04-12 JP JP2014522376A patent/JP6212040B2/ja active Active
- 2013-04-12 CN CN201380034708.2A patent/CN104395513A/zh active Pending
- 2013-04-12 US US14/404,311 patent/US20150144830A1/en not_active Abandoned
- 2013-04-12 BR BR112014031000A patent/BR112014031000A2/pt not_active IP Right Cessation
- 2013-04-12 AU AU2013282679A patent/AU2013282679A1/en not_active Abandoned
- 2013-04-12 WO PCT/JP2013/002506 patent/WO2014002344A1/ja active Application Filing
- 2013-06-27 AR ARP130102276A patent/AR092341A1/es unknown
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0597426A (ja) * | 1991-10-09 | 1993-04-20 | Mizusawa Ind Chem Ltd | 繊維状アルミノケイ酸塩及びその製法 |
| JP3753416B2 (ja) | 1992-01-17 | 2006-03-08 | ザ・モーガン・クルーシブル・カンパニー・ピーエルシー | 物品の絶縁方法 |
| JP2005514318A (ja) | 2002-01-04 | 2005-05-19 | ザ・モーガン・クルーシブル・カンパニー・ピーエルシー | 生理食塩水に可溶の無機繊維 |
| JP2008534421A (ja) * | 2005-04-01 | 2008-08-28 | サン−ゴバン・イソベール | ミネラルウール、絶縁製品及び製造方法 |
| WO2011017343A2 (en) * | 2009-08-03 | 2011-02-10 | Ppg Industries Ohio, Inc. | Glass compositions and fibers made therefrom |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP2868784A4 |
Also Published As
| Publication number | Publication date |
|---|---|
| IN2014DN10759A (ja) | 2015-09-04 |
| EP2868784A1 (en) | 2015-05-06 |
| KR20150027049A (ko) | 2015-03-11 |
| EP2868784A4 (en) | 2016-01-27 |
| JP6212040B2 (ja) | 2017-10-11 |
| US20150144830A1 (en) | 2015-05-28 |
| AU2013282679A1 (en) | 2014-11-27 |
| CN104395513A (zh) | 2015-03-04 |
| BR112014031000A2 (pt) | 2017-06-27 |
| AR092341A1 (es) | 2015-04-15 |
| JPWO2014002344A1 (ja) | 2016-05-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5634637B1 (ja) | 生体溶解性無機繊維 | |
| JP6266250B2 (ja) | 耐熱無機繊維 | |
| JP6554269B2 (ja) | 生体溶解性無機繊維の製造方法 | |
| WO2014115550A1 (ja) | 生体溶解性無機繊維及びその組成物 | |
| JP6212040B2 (ja) | 耐熱無機繊維 | |
| JP6348843B2 (ja) | 生体溶解性無機繊維及びその組成物 | |
| WO2014045527A1 (ja) | 耐熱性を有する生体溶解性無機繊維及びその組成物 | |
| JP5945596B2 (ja) | Si−Mg系無機繊維及びその組成物 | |
| JPWO2013132859A1 (ja) | 生理食塩水に可溶なSr/Ba含有無機繊維及びその組成物 | |
| WO2013132858A1 (ja) | 生理食塩水に可溶なLa/Ce含有無機繊維及びその組成物 | |
| JP6513905B2 (ja) | 生体溶解性無機繊維 | |
| WO2013183241A1 (ja) | 生理食塩水に可溶なAl-Ca系無機繊維及びその組成物 | |
| WO2014020842A1 (ja) | Si/Al/Ca含有無機繊維 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 13808822 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2014522376 Country of ref document: JP Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2013808822 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 20147031794 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14404311 Country of ref document: US |
|
| ENP | Entry into the national phase |
Ref document number: 2013282679 Country of ref document: AU Date of ref document: 20130412 Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112014031000 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 112014031000 Country of ref document: BR Kind code of ref document: A2 Effective date: 20141211 |