WO2013145869A1 - 吸収性物品 - Google Patents
吸収性物品 Download PDFInfo
- Publication number
- WO2013145869A1 WO2013145869A1 PCT/JP2013/052628 JP2013052628W WO2013145869A1 WO 2013145869 A1 WO2013145869 A1 WO 2013145869A1 JP 2013052628 W JP2013052628 W JP 2013052628W WO 2013145869 A1 WO2013145869 A1 WO 2013145869A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- acid
- chain hydrocarbon
- sheet
- absorbent article
- hydrocarbon moiety
- Prior art date
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/45—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators characterised by the shape
- A61F13/47—Sanitary towels, incontinence pads or napkins
- A61F13/472—Sanitary towels, incontinence pads or napkins specially adapted for female use
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/45—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators characterised by the shape
- A61F13/47—Sanitary towels, incontinence pads or napkins
- A61F13/4702—Sanitary towels, incontinence pads or napkins having a reinforcing member
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/45—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators characterised by the shape
- A61F13/47—Sanitary towels, incontinence pads or napkins
- A61F13/475—Sanitary towels, incontinence pads or napkins characterised by edge leakage prevention means
- A61F13/4751—Sanitary towels, incontinence pads or napkins characterised by edge leakage prevention means the means preventing fluid flow in a transversal direction
- A61F13/4756—Sanitary towels, incontinence pads or napkins characterised by edge leakage prevention means the means preventing fluid flow in a transversal direction the means consisting of grooves, e.g. channels, depressions or embossments, resulting in a heterogeneous surface level
Definitions
- the present invention relates to absorbent articles such as sanitary napkins, panty liners, incontinence pads, incontinence liners and the like.
- a sanitary napkin having a pair of flaps extending laterally outward beyond the longitudinal side edge of the main part is known in the prior art (e.g., U.S. Pat. No. 5,958,015).
- the flaps are folded under the wearer's underwear.
- the flap is folded along the longitudinal portion of the inner main portion of the proximal end. If the crotch width of the wearer's underwear is wider than the width of the main part, the flap is folded along the hinge of the main part.
- An object of the present invention is to provide an absorbent article which can suppress that the side portion of the absorber hits the inner crotch of the wearer and can suppress the occurrence of large deflection in the side portion of the absorber.
- a liquid-permeable top sheet provided on the skin side having a longitudinal direction and a width direction
- a liquid-impermeable back sheet provided on the clothing side, the top sheet, and the back sheet
- a main body portion provided with a liquid-retaining absorber including a side surface extending in the longitudinal direction on both sides in the width direction, and both sides of the main body portion extending in the width direction from both side edges of the main body portion
- An absorbent article including a wing portion disposed in the body, wherein the main body portion is provided between the absorber and the back sheet, and further includes a bending suppression sheet including longitudinally extending edges on both sides in the width direction The edge on both sides in the width direction of the anti-folding sheet is located outside the side surface of the absorber in the width direction, and the area where the anti-folding sheet is provided is the area where the anti-folding sheet is not provided So that the absorbent article is harder to
- the absorbent While suppressing that the part of a side of a part hits a wearer's inner crotch, it can suppress that a big distortion arises in the part of the side of an absorber.
- FIG. 1 is a partially broken plan view of an embodiment of the absorbent article of the present invention.
- FIG. 2 is a schematic cross-sectional view showing a cross section taken along line AA of FIG.
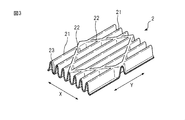
- FIG. 3 shows a first mountain-folded part, a second mountain-folded part, formed in the region on the outer side of both side surfaces in the width direction of the absorber in the top sheet of the main body part according to an embodiment of the present invention It is a figure for demonstrating a part and a valley fold part.
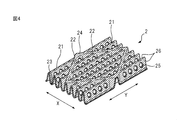
- FIG. 4 is a view for explaining a first mountain fold, a second mountain fold and a valley fold formed on the top sheet of the central part of the main body in the absorbent article according to one embodiment of the present invention. is there.
- FIG. 1 is a partially broken plan view of an embodiment of the absorbent article of the present invention.
- FIG. 2 is a schematic cross-sectional view showing a cross section taken along line AA of FIG.
- FIG. 3 shows a first mountain-folded
- FIG. 5 is a view for explaining an absorbent article according to an embodiment of the present invention attached to the crotch region of the undergarment.
- FIG. 6 is a schematic cross-sectional view showing a cross section taken along the line BB of FIG.
- FIG. 7 is a figure for demonstrating the manufacturing method of the absorbent article of one Embodiment of this invention.
- FIG. 8 is a view for explaining a recess-forming roll used for producing the absorbent article of one embodiment of the present invention.
- FIG. 9 is a view for explaining a region where a recess is formed by the recess forming roll in the top sheet.
- FIG. 10 is a view for explaining an upper stage roll of a drawing gear roll used for manufacturing an absorbent article according to an embodiment of the present invention.
- FIG. 10 is a view for explaining an upper stage roll of a drawing gear roll used for manufacturing an absorbent article according to an embodiment of the present invention.
- FIG. 11 is a view for explaining a lower roll of a drawing gear roll used for manufacturing an absorbent article according to an embodiment of the present invention.
- FIG. 12 is a view for explaining a top sheet sheet stretched by a stretching gear roll.
- FIG. 13 is an electron micrograph of the skin contact surface of the top sheet in the sanitary napkin in which the top sheet contains avian C2L oil fatty acid glyceride.
- FIG. 14 is a photomicrograph of menstrual blood with or without a blood modifying agent.
- FIG. 15 is a diagram for explaining a method of measuring surface tension.
- FIG. 1 is a partially broken plan view of an absorbent article according to an embodiment of the present invention
- FIG. 2 is a schematic cross-sectional view taken along the line AA of FIG.
- the absorbent article 1 has a liquid-permeable top sheet 2 provided on the skin side (skin contact side), a liquid-impermeable back sheet 3 provided on the clothing side (non-skin contact side), and a top sheet
- a liquid-retaining absorber 4 provided between the second sheet 2 and the back sheet 3 and a main body 10 having a bending suppression sheet 5 provided between the absorber 4 and the back sheet 3; It includes a pair of wing portions 6 provided with a top sheet 2 and a back sheet 3 disposed on both sides of the main body portion 10 so as to extend in the width direction from both side edges.
- symbol 61 shows the root of the wing part 6 (boundary between the main-body part 10 and the wing part 6).
- a straight line connecting two points where the width of the absorbent article 1 suddenly increases on both sides in the longitudinal direction of the wing portion 6 can be regarded as the root 61 of the wing portion 6.
- the surface on the opposite side of the surface on the clothing side in the wing portion 6 is configured by the top sheet 2.
- Adhesive portions 7 are provided on the clothes-side surfaces of the main body portion 10 and the wing portion 6 respectively.
- the width direction of the absorbent article 1 is the X direction
- the longitudinal direction is the Y direction.
- the plane direction of the absorbent article 1 is the XY direction.
- the shape of the main body portion 10 is not particularly limited as long as it is a shape that conforms to the shape of a female body and an underwear, such as a rectangular shape, an oval shape, and an hourglass shape.
- the longitudinal dimension in the outer shape of the main body 10 is preferably 100 to 500 mm, more preferably 150 to 350 mm. Further, the dimension in the width direction of the outer shape of the main body 10 is preferably 30 to 200 mm, more preferably 40 to 180 mm.
- the top sheet 2 moves body fluid such as urine and menstrual blood discharged from the wearer to the absorber 4 in the main body portion 10. Further, in the main body portion 10, at least a part of the top sheet 2 has liquid permeability, and has a large number of openings for transmitting body fluid. On the other hand, in the wing portion 6, the top sheet 2 is suitable for the wearer to bend the wing portion 6, and has a suitable rigidity not to give the wearer discomfort after being attached to the undergarment, as described later. Apply to wing 6 together with 3.
- the top sheet 2 is preferably made of a resin film.
- the resin film used as the top sheet 2 is made of a copolymer of an olefin and an acrylic ester, another monomer such as vinyl acetate, polyolefin, polyester, polypropylene, polyethylene, polyethylene terephthalate, polyamide, cellulose acetate or the like.
- the resin film used as the top sheet 2 is preferably a copolymer of an olefin and another monomer, or a polyolefin, in terms of high flexibility and low irritation to the skin.
- the basis weight of the top sheet 2 is preferably 1 g / m 2 or more and 40 g / m 2 or less, more preferably 10 g / m 2 or more and 35 g / m 2 or less.
- the thickness of the resin film which comprises the top sheet 2 becomes like this. Preferably it is 0.01 mm or more and 0.4 mm or less, More preferably, it is 0.1 mm or more and 0.35 mm or less.
- the thickness of the resin film constituting the top sheet 2 is less than 0.01 mm, the hiding property of the top sheet 2 described later may be too small, and the thickness of the resin film constituting the top sheet 2 is 0 When it exceeds .4 mm, the rigidity of the top sheet 2 may be high, and the stimulation of the top sheet 2 to the wearer's skin may be too strong. Since the top sheet 2 has a first mountain fold portion, a second mountain fold portion and a valley fold portion described later, the apparent thickness of the top sheet 2 is greater than the thickness of the resin film constituting the top sheet 2 large.
- the apparent thickness of the top sheet 2 is preferably 0.01 mm or more and 1 mm or less, more preferably 0.1 mm or more and 0.5 mm or less.
- the top sheet 2 has concealability in order to prevent the body fluid absorbed by the absorber 4 from being seen from the outside.
- the hiding property of the top sheet 2 is produced by mixing a filler such as titanium oxide into the resin.
- the filler is titanium oxide
- the content of titanium oxide is preferably 1% to 50%, more preferably 3% to 15%, based on the weight of the resin film.
- the content of the titanium oxide is less than 1% with respect to the weight of the resin film, the effect of hiding the body fluid absorbed by the absorber 4 in the top sheet 2 may be too small.
- sheet forming of the resin containing titanium oxide may be difficult.
- the top sheet 2 extends in the longitudinal direction, and extends in a direction intersecting the first mountain fold 21 and the valley fold 23 alternately arranged in the width direction, and the first mountain fold 21. And a mountain fold portion 22.
- FIG. 3 is a first mountain-folded portion formed on the region 14 outside the side surfaces 41 in the width direction of the absorber 4 in the top sheet 2 of the main body 10 and the top sheet 2 of the wing 6 (see FIG. 1) It is a figure for demonstrating a 2nd mountain fold part and a valley fold part.
- FIG. 4 is a view for explaining a first mountain fold, a second mountain fold, and a valley fold formed on the central portion 12 of the top sheet 2 of the main body 10 (see FIG. 1).
- the first mountain-folded portion, the second mountain-folded portion and the valley-folded portion formed in the central portion 12 of the top sheet 2 of the main body portion 10 are slightly different as described later.
- the top sheet 2 extends in the longitudinal direction (Y direction) in the region 14 and the wing portion 6 outside the side surfaces 41 in the width direction of the absorber 4 in the width direction (X direction)
- the first mountain fold 21 and the valley fold 23 are alternately arranged.
- the shape of the cross section of the first mountain fold 21 and the valley fold 23 is, for example, a substantially U-shape.
- the substantially U-shape includes, in addition to the U-shape, a shape that becomes a U-shape when it is deformed such as rounding corners or changing a straight line into a curve.
- the substantially U-shape also includes V-shape, M-shape, trapezoid and ⁇ -shape.
- the second mountain folds 22 preferably extend in a direction intersecting the first mountain folds 21 extending in the longitudinal direction (Y direction).
- the angle between the direction in which the first mountain fold 21 extends and the direction in which the second mountain fold 22 extends is preferably 10 ° or more and 170 ° or less.
- the shape of the cross section of the second mountain fold 22 when the second mountain fold 22 is cut in a direction orthogonal to the direction in which the second mountain fold 22 extends is, for example, a substantially U-shape.
- the number of first mountain folds 21 per cm in the width direction is preferably 3 or more, and more preferably 5 or more. If the number of first mountain-folded portions 21 per 1 cm in the width direction is 2 or less, the cushioning properties generated on the top sheet 2 by the first mountain-folded portions 21 may be weakened.
- the length in the longitudinal direction (Y direction) of the second mountain-folded portion 22 is preferably 0.3 mm or more and 5 mm or less, more preferably 0.5 mm or more and 3 mm or less.
- the length in the longitudinal direction (Y direction) of the second mountain fold 22 is smaller than 0.3 mm, the effect of making the first mountain fold 21 in the second mountain fold 22 less likely to collapse is reduced There is.
- the length of the second mountain fold 22 in the longitudinal direction (Y direction) is larger than 5 mm, the cushioning property of the top sheet 2 may be weakened by the first mountain fold 21.
- the absorbent article 1 is fixed to the undergarment by providing the top sheet 2 with the first mountain fold 21 and the second mountain fold 22 at least in the area 14 outside the side surfaces 41 in the width direction of the absorbent body 4.
- the first mountain fold 21 and / or the second mountain fold are present in the vicinity of the fold line 31 when the wing 6 is folded (see FIG. 6).
- the first mountain fold 21 and the second mountain fold 22 impart a soft touch to the surface of the absorbent article 1 by providing the top sheet 2 with cushioning properties. Therefore, when this wears the underwear equipped with the absorbent article 1, the touch around the foot of the underwear becomes good.
- the top sheet 2 of the central portion 12 of the main body 10 is the same as the top sheet 2 of the region 14 outside the side surfaces 41 of the main body 10 in the width direction and the wing 6.
- the first mountain fold 21, the second mountain fold 22 and the valley fold 23 of the top sheet 2 of the central portion 12 of the main body 10 have a blood modifying layer 24 described later on the surface on the skin side. Further include.
- the first mountain fold 21 and the valley fold 23 of the top sheet 2 of the central portion 12 of the main body 10 further include an opening 25 described later.
- the body fluid of the wearer discharged to the top sheet 2 of the area 12 where the absorber 4 of the main body 10 is provided passes through the opening 25 and moves to the absorber 4.
- the body fluid of the wearer discharged to the top sheet 2 quickly gathers toward the bottom of the valley fold portion 23 due to the substantially U shape of the cross section (X direction) cross section formed by the valley fold portion 23 .
- the body fluid flowing toward the bottom of the valley fold 23 passes through the opening 25 provided on the side 26 of the first mountain fold 21 and the valley fold 23 before reaching the valley fold 23.
- the body fluid accumulated in the valley fold portion 23 also passes through the openings 25 provided on the side surfaces 26 of the first mountain fold portion 21 and the valley fold portion 23 and flows to the absorber 4. Therefore, the body fluid of the wearer is quickly absorbed by the absorbent body 4 by the openings 25 provided on the side surfaces 26 of the first mountain fold 21 and the valley fold 23. In addition, the amount of body fluid remaining in the top sheet 2 can be reduced by the openings 25 provided on the side faces 26 of the first mountain fold 21 and the valley fold 23.
- a blood modifying agent may be applied to the skin side surface of the top sheet 2 of the central portion 12 of the main body portion 10 to form the blood modifying layer 24 on the skin contact side surface of the top sheet 2.
- the blood modifying agent layer 24 can prevent the body fluid of the wearer, in particular, highly viscous menstrual blood from remaining on the surface of the top sheet 2.
- the blood modifying agent layer 24 will be described in detail later.
- the top sheet 2 Your skin feels good.
- the contact area between the skin of the wearer and the top sheet 2 is reduced, the touch of the top sheet 2 is improved.
- the second mountain fold portion the first mountain fold portion is less likely to be crushed, so the first mountain fold portion, the second mountain fold portion is formed on the top sheet 2 of the central portion 12 of the main body portion 10. It is preferable to provide a section and a valley fold.
- the back sheet 3 shown in FIGS. 1 and 2 prevents the body fluid absorbed by the absorber 4 from leaking out.
- the back sheet 3 is made of a material that is impermeable to body fluid.
- a hydrophobic non-woven fabric an impermeable plastic film such as polyethylene and polypropylene or a laminate sheet of non-woven fabric and an impermeable plastic film, or the like is used.
- a spunbond-meltblown-spunbond (SMS) fibrous nonwoven fabric may be used as the back sheet 3 in which a highly water-resistant meltblown nonwoven fabric is sandwiched by strong spunbond nonwoven fabrics.
- SMS spunbond-meltblown-spunbond
- the absorber 4 absorbs and holds the body fluid. It is preferable that the absorber 4 is bulky, hard to lose its shape, and less in chemical stimulation.
- a composite absorbent made of fluff pulp or air-laid non-woven fabric and super absorbent polymer (SAP) is used as the absorbent 4.
- SAP super absorbent polymer
- the composite absorber may be covered with a liquid permeable material such as a tissue.
- artificial cellulose fibers such as chemical pulp, cellulose fibers, rayon and acetate may be used.
- the basis weight of the absorbent fiber such as pulp in the composite absorbent is preferably 100 g / m 2 or more and 800 g / m 2 or less, and the mass ratio of the superabsorbent polymer in the composite absorbent is the absorbency
- the fiber content is preferably 100% to 10% to 65%.
- the basis weight of a liquid-permeable material such as a tissue covering the composite mixture is preferably 12 g / m 2 or more and 30 g / m 2 or less.
- the air-laid non-woven fabric of the composite mixture for example, a non-woven fabric in which pulp and synthetic fibers are heat-sealed or a non-woven fabric in which pulp and synthetic fibers are fixed with a binder can be used.
- the superabsorbent polymer of the composite absorbent has a three-dimensional network structure in which a water-soluble polymer is appropriately crosslinked.
- the absorbent polymer absorbs 30 to 60 times as much water as the volume of absorbent polymer before absorbing water.
- this absorbable polymer is essentially water insoluble.
- the absorbent polymer does not release the water once absorbed, even if some pressure is applied.
- this absorbent polymer for example, starch-based, acrylic acid-based or amino acid-based particulate or fibrous polymers are used.
- the shape and structure of the absorber 4 can be changed as needed, but the total amount of absorption of the absorber 4 needs to correspond to the designed insertion amount as the absorbent article 1 and the desired application.
- the size, absorption capacity and the like of the absorber 4 change depending on the application.
- Absorber 4 is bonded to top sheet 2 using a hot melt adhesive. Thereby, it can suppress that the top sheet 2 peels from the absorber 4. As shown in FIG.
- the folding prevention sheet 5 folds the wing portion 6 and attaches the absorbent article 1 to the underwear.
- the portion of the side surface 41 (see FIG. 1) of the absorbent body 4 is prevented from hitting the inner crotch of the wearer.
- the folding suppression sheet 5 folds the wing portion 6 to make the absorbent article 1 underwear.
- seat 5 is located in the width direction outer side compared with the side 41 of the width direction of the absorber 4. As shown in FIG. Thereby, when the wing 6 is folded and the absorbent article 1 is attached to the undergarment, it is possible to suppress the wing 6 from being bent so that the portion of the side surface 41 of the absorber 4 has a fold.
- the sheet used as the music suppression sheet 5 is not particularly limited. However, if the rigidity of the absorbent folded sheet 5 is too high, the rigidity of the entire absorbent article 1 becomes too high, and the absorbent article 1 may cause a strong discomfort to the wearer. Therefore, it is preferable that the absorber folded sheet 5 have a rigidity of such a height that a strong discomfort does not occur even when worn by the wearer.
- the bending resistance measured by the cantilever method (JIS L 1096) in the bending suppression sheet 5 is preferably 15 to 120 mm, more preferably 30 to 70 mm.
- seat 5 When the bending resistance by the cantilever method of the bending suppression sheet
- seat 5 is smaller than 15 mm, it may be unable to suppress that the wing part 6 bends so that the part of the side 41 of the absorber 4 may become a crease. That is, when the wearer folds the wing portion 6 in order to attach the absorbent article to the undergarment, there may be a case where the bending suppression sheet 5 is bent at the side 41 of the absorber 4 together with the top sheet 2 and the back sheet 3 is there.
- the sheet used as the bending suppression sheet 5 is preferably a resin film, a perforated resin film, a woven fabric or a non-woven fabric, and more preferably a non-woven fabric.
- a non-woven fabric suitable for the bending suppression sheet 5 is, for example, a non-woven fabric of chemical fibers such as thermoplastic hydrophobic chemical fibers.
- Thermoplastic hydrophobic chemical fibers suitable for the bending suppression sheet 5 include, for example, single fibers such as polyethylene (PE), polypropylene (PP) and polyethylene terephthalate (PET), composite fibers obtained by graft polymerizing PE and PP, There is a composite fiber having a core-sheath structure.
- the web When producing a non-woven fabric used for the bending suppression sheet 5, the web may be formed by a dry method such as a card method, a spun bond method, a meltblown method, an air laid method or a wet method. Moreover, when producing the nonwoven fabric used for the bending suppression sheet 5, you may perform bonding, such as thermal bonding, a needle punch, chemical bonding. For example, an SMS fiber non-woven fabric can be used for the bending suppression sheet 5.
- a dry method such as a card method, a spun bond method, a meltblown method, an air laid method or a wet method.
- bonding such as thermal bonding, a needle punch, chemical bonding.
- an SMS fiber non-woven fabric can be used for the bending suppression sheet 5.
- the basis weight of the non-woven fabric used as the bending suppression sheet 5 is preferably 10 to 40 g / m 2 , more preferably 13 to 20 g / m 2 . If the basis weight of the non-woven fabric is smaller than 10 g / m 2, it may not be possible to suppress bending of the wing portion 6 such that the portion of the side surface 41 of the absorber 4 has a fold. That is, when the wearer folds the wing portion 6 in order to attach the absorbent article to the undergarment, there may be a case where the bending suppression sheet 5 is bent at the side 41 of the absorber 4 together with the top sheet 2 and the back sheet 3 is there. Moreover, when the fabric weight of the nonwoven fabric is larger than 40 g / m 2 , the rigidity of the absorbent article 1 becomes too high, and the absorbent article 1 may cause a strong discomfort to the wearer.
- the sheet used as the bending suppression sheet 5 is preferably a sheet having hydrophobicity or water repellency. Thereby, it can suppress that the bodily fluid which exuded from absorber 4 leaks from the part of the cross direction of absorptive article 1 through bending control sheet 5.
- the bending suppression sheet 5 is joined to the absorber 4 and the back sheet 3 by an adhesive such as a hot melt adhesive. However, the bending suppression sheet 5 is not joined to the top sheet 2. Thereby, the top sheet 2 can have a soft touch in the area
- FIG. 5 is a view for explaining an absorbent article according to an embodiment of the present invention attached to the crotch region of the undergarment.
- FIG. 6 is a schematic cross-sectional view showing a cross section taken along the line BB of FIG. Reference numeral 70 in FIGS.
- FIGS. 5 and 6 denotes the crotch area of the undergarment
- reference numeral 71 denotes the edge of the foot opening of the undergarment. From FIGS. 5 and 6, it can be seen that the width of the crotch region 70 of the wearer's underwear is narrower than the width of the absorbent body 4 provided in the absorbent article 1.
- the wing portion 6 Since the absorbent article 1 is difficult to bend in the area where the folding prevention sheet 5 is provided, when the wearer folds the wing portion 6 and attaches the underwear to the crotch region 70 of the underwear, the wing portion 6 is The folds 31 of the absorbent article 1 produced when folded are substantially coincident with the edge 51 in the width direction of the anti-folding sheet 5. Therefore, as shown in FIG. 5 and FIG. 6, even if the position of the edge 71 of the foot opening of the undergarment overlaps with the position of the absorber 4, when the wing 6 is folded, the side 41 of the absorber 4 absorbs It is suppressed that the sex article 1 bends.
- the thickness of the bending suppression sheet 5 is smaller than the thickness of the absorber 4. Therefore, the sense of incongruity which arises when the edge 51 in the width direction of the bending suppression sheet 5 hits the wearer's inner crotch is weaker than the sense of incongruity which occurs when the portion of the side surface 41 of the absorber 4 hits the wearer's crotch. Further, as described above, the top sheet 2 is provided with the first mountain fold portion 21.
- the first mountain fold 21 acts as a cushion, and the edge 51 in the width direction of the anti-folding sheet 5 is the wearer's It can further reduce the sense of incongruity that occurs when hitting the inner crotch. That is, the first mountain fold 21 imparts cushioning properties to the top sheet 2, and further reduces the discomfort caused when the edge 51 in the width direction of the bending suppression sheet 5 hits the inner crotch of the wearer.
- the protrusion formed on the top sheet 2 is the above-described first mountain-folded portion 21 And it is not limited to the 2nd mountain fold part.
- a cylindrical protrusion, a prismatic protrusion, or the like may be formed on the top sheet 2.
- the wing portion 6 is provided on the absorbent article 1 in order to stably fix the absorbent article 1 to the undergarment.
- the absorbent article 1 can be stably fixed to the undergarment by bending the wing portion 6 to the outer surface side of the undergarment and then sticking it to the crotch region of the undergarment through the adhesive portion 7.
- the adhesive portion 7 is also provided to the main body portion 10, and the main body portion 10 is prevented from shifting and moving in the crotch region of the undergarment.
- the adhesive unit 7 fixes the absorbent article 1 to the crotch area of the undergarment.
- an adhesive which forms the adhesion part 7 what is a styrene-type polymer, a tackifier, and a plasticizer as a main component, for example is used suitably.
- the styrene-based polymer include styrene-ethylene-butylene-styrene block copolymer, styrene-butylene polymer, styrene-butylene-styrene block copolymer, and styrene-isobutylene-styrene copolymer. Or a blend of two or more polymers.
- styrene-ethylene-butylene-styrene block copolymer is preferable in that the thermal stability is good.
- tackifier and the plasticizer those which are solid at normal temperature can be preferably used, and as the tackifier, for example, C5 petroleum resin, C9 petroleum resin, dicyclopentadiene petroleum resin, rosin petroleum resin And polyterpene resins, terpene phenol resins, etc.
- the plasticizer include, in addition to monomer plasticizers such as triflecil phosphate, dibutyl phthalate and dioctyl phthalate, polymer plasticizers such as vinyl polymers and polyesters.
- the top sheet 2 and the absorbent body 4 have compressed grooves 8 extending from the top sheet 2 to the inside of the absorbent body 4 which are formed by being compressed in the thickness direction by embossing.
- the squeeze groove 8 suppresses the diffusion of the body fluid discharged to the central portion of the absorbent article 1 (the portion in contact with the excretory port of the wearer's body fluid) in the width direction (X direction). Moreover, it can suppress that the top sheet 2 peels from the absorber 4 by this.
- the squeeze groove 8 surrounds the central portion of the absorbent article 1 and has a continuous, substantially annular shape.
- the pressing groove 8 surrounding the center part of the absorbent article 1 may be partially disconnected. That is, the pressing groove 8 may have a discontinuous and substantially annular shape.
- the compression bonding of the top sheet 2 to the back sheet 3 by heat embossing forms the seal portions 9 on both sides in the longitudinal direction and both sides in the width direction of the absorbent article 1. Thereby, the top sheet 2 can be prevented from peeling off from the back sheet 3.
- the blood modifying agent of the blood modifying agent layer 24 is a blood modifying agent having an IOB of about 0.00 to about 0.60, a melting point of about 45.degree. C. or less, 100 g of water at 25.degree. It has a water solubility of 00 to about 0.00 to about 0.05 g.
- IOB Inorganic Organic Balance
- IOB value calculated by inorganic value / organic value.
- the IOB is about 0.00 to about 0.60, preferably about 0.00 to about 0.50, and about 0.00 to about 0.40. More preferred is about 0 to about 0.30. This is because the lower the IOB, the higher the organicity and the higher the affinity to blood cells.
- the "melting point” means the peak top temperature of an endothermic peak when changing from solid state to liquid state when measured at a temperature rising rate of 10 ° C./min in a differential scanning calorimeter.
- the melting point can be measured, for example, using a DSC-60 type DSC measurement apparatus manufactured by Shimadzu Corporation.
- the blood modifying agent may be liquid or solid at room temperature as long as it has a melting point of about 45 ° C. or less, ie, even if the melting point is about 25 ° C. or more, or less than about 25 ° C. It may well have a melting point such as, for example, about -5.degree. C., about -20.degree. The reason why the melting point of the blood modifying agent is about 45 ° C. or less will be described later.
- the vapor pressure of the blood modifying agent is preferably about 0.00 to about 0.01 Pa at 1 atm and 25 ° C., more preferably about 0.000 to about 0.001 Pa, and about 0 More preferably, it is from .0000 to about 0.0001 Pa.
- the vapor pressure is preferably about 0.00 to about 0.01 Pa at 1 atm and 40 ° C., and about 0.000 to about 0.01 More preferably, it is about 0.001 Pa, and more preferably, about 0.0000 to about 0.0001 Pa. If the vapor pressure is high, it may be vaporized during storage, which may cause problems such as a decrease in the amount of blood modifying agent and an odor when worn.
- the melting point of the blood modifying agent can be properly used depending on the weather, the length of wearing time, and the like. For example, in areas where the average temperature is less than about 10 ° C, menstrual blood may be excreted and then cooled by the ambient temperature by employing a blood modifying agent having a melting point of less than about 10 ° C. It is believed that blood modifying agents can stably modify blood.
- the melting point of the blood modifying agent is preferably higher in the range of 45 ° C. or less. It is because it is hard to be affected by sweat, friction at the time of wearing, etc., and it is difficult for the blood modifying agent to move even when worn for a long time.
- a water solubility of 0.00 to 0.05 g For a water solubility of 0.00 to 0.05 g, add a 0.05 g sample to 100 g deionized water at 25 ° C., allow to stand for 24 hours, and after 24 hours, lightly stir as needed, Then, it can be measured by visually evaluating whether the sample has dissolved.
- dissolving includes cases where the sample is completely dissolved in deionized water to form a homogeneous mixture and cases where the sample is completely emulsified. "Complete” means that there is no clump of sample in deionized water.
- the surface of the top sheet is coated with a surfactant for the purpose of changing blood surface tension and the like to rapidly absorb the blood.
- the surfactant generally has high water solubility
- the surfactant-coated top sheet is compatible with hydrophilic components (such as plasma) in the blood, rather the blood remains on the top sheet.
- hydrophilic components such as plasma
- solubility in 100 g of water at 25 ° C. may be simply referred to as “water solubility”.
- the weight average molecular weight means a value in terms of polystyrene, which is determined by gel permeation chromatography (GPC).
- GPC measurement conditions include the following. Model: High-performance liquid chromatogram Lachrom Elite manufactured by Hitachi High-Technologies Corporation Column: Showa Denko KK SHODEX KF-801, KF-803 and KF-804 Eluent: THF Flow rate: 1.0 mL / min Implanted volume: 100 ⁇ L Detection: RI (differential refractometer)
- the weight average molecular weight described in the Example of this specification is measured based on the said conditions.
- the above blood modifying agent is preferably selected from the following (i) to (iii), (I) Hydrocarbons, (Ii) from a carbonyl group (-CO-) and an oxy group (-O-) inserted between (ii-1) a hydrocarbon moiety and (ii-2) a C-C single bond of the above-mentioned hydrocarbon moiety
- hydrocarbon means a compound consisting of carbon and hydrogen, and is a chain hydrocarbon, for example, paraffinic hydrocarbon (also referred to as alkane not containing double bond and triple bond) Olefinic hydrocarbons (containing one double bond, also called alkenes), acetylenic hydrocarbons (containing one triple bond, also called alkynes), and a group consisting of double bonds and triple bonds And hydrocarbons containing two or more bonds selected from, as well as cyclic hydrocarbons such as aromatic hydrocarbons and alicyclic hydrocarbons.
- paraffinic hydrocarbon also referred to as alkane not containing double bond and triple bond
- Olefinic hydrocarbons containing one double bond, also called alkenes
- acetylenic hydrocarbons containing one triple bond, also called alkynes
- hydrocarbons containing two or more bonds selected from, as well as cyclic hydrocarbons such as aromatic hydrocarbons and alicyclic hydrocarbons.
- the hydrocarbon is preferably a chain hydrocarbon and an alicyclic hydrocarbon, more preferably a chain hydrocarbon, a paraffin hydrocarbon, an olefin hydrocarbon and two double bonds. It is more preferable that it is the hydrocarbon (it does not contain a triple bond) which contains above, and it is still more preferable that it is a paraffinic hydrocarbon.
- the chained hydrocarbons include straight chained hydrocarbons and branched chained hydrocarbons.
- each oxy group (—O—) is not adjacent. Accordingly, the compounds (ii) and (iii) do not include compounds in which the oxy group is continuous (so-called peroxides).
- At least one hydrogen atom of the hydrocarbon moiety is a hydroxyl group (-) rather than a compound in which at least one hydrogen atom of the hydrocarbon moiety is substituted with a carboxyl group (-COOH).
- Compounds substituted with OH) are preferred.
- Table 1 since the carboxyl group binds to metals and the like in blood, and the inorganic value greatly increases from 150 to 400 or more, the blood modifying agent having a carboxyl group is used at the time of use This is because the IOB value may exceed about 0.60 and the affinity to blood cells may be reduced.
- the above blood modifying agent comprises the following (i ') to (iii'), (I ') hydrocarbons, (Ii ') (ii'-1) a hydrocarbon moiety, and (ii'-2) a carbonyl bond (-CO-), an ester bond (-COO) inserted between a C-C single bond of the above-mentioned hydrocarbon moiety -), A compound having one or more same or different bonds selected from the group consisting of carbonate bond (-OCOO-), and ether bond (-O-), and (iii ') (iii'-) 1) Carbonyl bond (-CO-), ester bond (-COO-), carbonate bond (-OCOO) inserted between the hydrocarbon moiety and the C-C single bond of the above-mentioned hydrocarbon moiety (iii'-2) A carboxyl group (-), and one or more, same or different bond selected from the group consisting of an ether bond (-O-) and (iii'
- the blood modifying agent has about 1.8 or less carbonyl bonds (-CO-) and 2 or less ester bonds (-COO-) per 10 carbon atoms in the hydrocarbon moiety. About 1.5 or less carbonate bond (-OCOO-), about 6 or less ether bond (-O-), about 0.8 or less carboxyl group (-COOH), and / or hydroxyl group (-OH) Or a compound having about 1.2 or less).
- the above blood modifying agent is any of the following (A) to (F), (A) A compound having (A1) a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety, (A2) a chain hydrocarbon moiety, and the above chain Ester with a compound having one carboxyl group replacing the hydrogen atom of the cyclic hydrocarbon moiety, (B) A compound having (B1) a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, and the above chain Ether with a compound having one hydroxyl group replacing the hydrogen atom of the cyclic hydrocarbon moiety, (C) a carboxylic acid, a hydroxy acid, an alkoxy acid or an oxo acid containing a (C1) linear hydrocarbon moiety and 2 to 4 carboxyl groups replacing the hydrogen atom of the linear hydrocarbon moiety; C2) an ester of a compound having a chain hydrocarbon moiety
- a compound having (A1) a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety, (A2) a chain hydrocarbon moiety, and the above chain Ester with a compound having one carboxyl group replacing hydrogen atoms in the cyclic hydrocarbon moiety (hereinafter sometimes referred to as “compound (A)”) has the above-mentioned IOB, melting point and water solubility And all hydroxyl groups may not be esterified.
- (A1) a compound having a chain hydrocarbon portion and 2 to 4 hydroxyl groups replacing the hydrogen atom of the above chain hydrocarbon portion (hereinafter sometimes referred to as “compound (A1)”)
- chain hydrocarbon tetraols such as alkanetetraols such as pentaerythritol
- chain hydrocarbon triols such as alkanetriols such as glycerin
- chain hydrocarbon diols such as alkane diols such as Glycol is mentioned.
- Examples of (A2) a compound having a chain hydrocarbon portion and one carboxyl group replacing the hydrogen atom of the chain hydrocarbon portion include, for example, And compounds in which one hydrogen atom on a hydrocarbon is substituted with one carboxyl group (—COOH), such as fatty acid.
- Examples of the compound (A) include an ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid, an ester of (a 2 ) chain hydrocarbon triol and at least one fatty acid, and (a 3 And esters of linear hydrocarbon diols and at least one fatty acid.
- the ester of the above linear hydrocarbon tetraol and at least one fatty acid may be, for example, the following formula (1): Tetraester of pentaerythritol with fatty acid, the following formula (2): Triester of pentaerythritol with fatty acid, the following formula (3): A diester of pentaerythritol with fatty acid, the following formula (4): And monoesters of fatty acid with pentaerythritol. (Wherein, R 1 to R 4 are each a chain hydrocarbon)
- esters of pentaerythritol and fatty acids have the above IOB, melting point and water solubility
- saturated fatty acids such as C 2 to C 30 saturated fatty acids, for example, acetic acid (C 2 ) (C 2 represents a carbon number, R 1 C, R 2 C, R 3 C or R 4 C, which corresponds to the carbon number of R 2 C, hereinafter the same), propanoic acid (C 3 ), butanoic acid (C 4 ) and isomers thereof, for example, 2-methylpropanoic acid (C 4) ), pentanoic acid (C 5) and isomers thereof such as 2-methylbutanoic acid (C 5), 2,2-dimethyl propanoic acid (C 5), hexanoic acid (C 6), heptanoic acid (C 7) Oct
- the fatty acids can also be unsaturated fatty acids.
- unsaturated fatty acids include C 3 -C 20 unsaturated fatty acids such as monounsaturated fatty acids such as crotonic acid (C 4 ), myristoleic acid (C 14 ), palmitoleic acid (C 16 ), Oleic acid (C 18 ), elaidic acid (C 18 ), vacenic acid (C 18 ), gadeuric acid (C 20 ), eicosenic acid (C 20 ), etc., diunsaturated fatty acids such as linoleic acid (C 18 ), Eicosadienoic acid (C 20 ) and the like, triunsaturated fatty acids such as linolenic acid, for example ⁇ -linolenic acid (C 18 ) and ⁇ -linolenic acid (C 18 ), pinolenic acid (C 18 ), eleostearic acid, For example, ⁇ -eleostearic acid (C 18
- the ester of pentaerythritol and fatty acid is an ester of pentaerythritol and fatty acid derived from saturated fatty acid, that is, an ester of pentaerythritol and saturated fatty acid, in consideration of the possibility of modification by oxidation etc. preferable.
- the ester of pentaerythritol and a fatty acid in order to make IOB small and make it more hydrophobic, it is preferable to be a diester, a triester or a tetraester, and a triester or a tetraester is more preferable. And tetra-esters are more preferred.
- the total carbon number of fatty acids constituting the tetraester of pentaerythritol and fatty acid that is, in the above formula (1), R 1 C, R 2 C, R 3 C and When the total number of carbons in the R 4 C portion is 15, the IOB is 0.60. Therefore, in the case of tetraester of pentaerythritol and fatty acid, IOB satisfies the requirement of about 0.00 to about 0.60 when the total number of carbons is about 15 or more.
- Examples of the tetraester of pentaerythritol and fatty acid include pentaerythritol, hexanoic acid (C 6 ), heptanoic acid (C 7 ), octanoic acid (C 8 ), for example, 2-ethylhexanoic acid (C 8 ), These include tetraesters with nonanoic acid (C 9 ), decanoic acid (C 10 ) and / or dodecanoic acid (C 12 ).
- the total carbon number of the fatty acid constituting the triester of pentaerythritol and fatty acid that is, the R 1 C, R 2 C and R 3 C moieties in the above formula (2)
- the IOB is 0.58 when the sum of the carbon numbers of these is 19. Therefore, in the case of the triester of pentaerythritol and fatty acid, IOB satisfies the requirement of about 0.00 to about 0.60 when the total carbon number of fatty acid is about 19 or more.
- the total carbon number of fatty acids constituting the diester of pentaerythritol and fatty acid that is, the total carbon number of R 1 C and R 2 C in the above formula (3) is In the case of 22, the IOB is 0.59. Accordingly, in the diester of pentaerythritol and fatty acid, IOB satisfies the requirement of about 0.00 to about 0.60 when the total carbon number of fatty acid is about 22 or more.
- esters of pentaerythritol and fatty acid examples include Unistar H-408 BRS, H-2408 BRS-22 (mixed product), etc. (all manufactured by NOF Corporation).
- the ester of the above linear hydrocarbon triol and at least one fatty acid may be, for example, the following formula (5): Triester of glycerin and fatty acid, the following formula (6): A diester of glycerin and a fatty acid, and the following formula (7): (Wherein, each of R 5 to R 7 is a chain hydrocarbon) And monoesters of glycerin and fatty acids.
- fatty acids (R 5 COOH, R 6 COOH and R 7 COOH) constituting the ester of glycerin and fatty acid if the ester of glycerin and fatty acid satisfies the requirements of the above IOB, melting point and water solubility
- the fatty acids listed in the “ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid” are not particularly limited, and examples thereof include saturated fatty acids and unsaturated fatty acids, which are modified by oxidation etc. In consideration of the possibility of doing so, an ester of glycerin and a fatty acid derived from a saturated fatty acid, that is, an ester of glycerin and a saturated fatty acid is preferable.
- ester of glycerol and a fatty acid in order to make IOB small and to make it more hydrophobic, it is preferable that it is diester or triester, and it is more preferable that it is triester.
- triester of glycerin and fatty acid is also referred to as triglyceride, for example, triester of glycerin and octanoic acid (C 8 ), triester of glycerin and decanoic acid (C 10 ), glycerin and dodecanoic acid (C 12 And triesters of glycerin with two or three fatty acids, and mixtures thereof.
- Examples of the triester of the above glycerin and two or more fatty acids include triester of glycerin and octanoic acid (C 8 ) and decanoic acid (C 10 ), glycerin, octanoic acid (C 8 ), decane Acid (C 10 ) and triester with dodecanoic acid (C 12 ), glycerin and octanoic acid (C 8 ), decanoic acid (C 10 ), dodecanoic acid (C 12 ), tetradecanoic acid (C 14 ), hexadecanoic acid Examples thereof include triesters with (C 16 ) and octadecanoic acid (C 18 ).
- the total carbon number of fatty acids constituting the triester of glycerin and fatty acid ie, R 5 C in the formula (5)
- the sum of the carbon numbers of the R 6 C and R 7 C moieties is about 40 or less.
- the total carbon number of fatty acids constituting the triester of glycerin and fatty acid that is, in the formula (5), the R 5 C, R 6 C and R 7 C moieties
- the IOB is 0.60. Therefore, in the above-mentioned triesters of glycerin and fatty acid, the IOB satisfies the requirement of about 0.00 to about 0.60 when the total carbon number of fatty acid is about 12 or more.
- the above-mentioned triester of glycerin and a fatty acid is a so-called fat and is a component that can constitute the human body, and thus is preferable from the viewpoint of safety.
- tricotic oil fatty acid glyceride NA36
- PANACET 800 PANACET 800B
- PANACET 810S avian C2L oil fatty acid glyceride and triCL oil fatty acid glyceride (manufactured by NOF CORPORATION) Etc.).
- the above-mentioned diester of glycerin and fatty acid is also referred to as diglyceride, for example, a diester of glycerin and decanoic acid (C 10 ), a diester of glycerin and dodecanoic acid (C 12 ), and glycerin and hexadecanoic acid (C 16 ) Included are diesters and diesters of glycerin with two fatty acids, and mixtures thereof.
- the total carbon number of fatty acids constituting the diester of glycerin and fatty acid ie, the case where the total carbon number of R 5 C and R 6 C moieties in the formula (6) is 16
- the IOB is 0.58. Therefore, in the case of the diester of glycerin and fatty acid, the IOB satisfies the requirement of about 0.00 to about 0.60 when the total carbon number of fatty acid is about 16 or more.
- the monoester of glycerin and fatty acid is also referred to as monoglyceride, and examples thereof include icosanoic acid (C 20 ) monoester of glycerin, docosanoic acid (C 22 ) monoester of glycerin and the like.
- the carbon number of fatty acid constituting the monoester of glycerin and fatty acid that is, in the formula (7), the IOB is 0.59 when the carbon number of the R 5 C portion is 19 It becomes. Therefore, in the monoester of glycerin and fatty acid, the IOB satisfies the requirement of about 0.00 to about 0.60 when the carbon number of the fatty acid is about 19 or more.
- esters of the above linear hydrocarbon diol and at least one fatty acid include C 2 to C 6 linear hydrocarbon diols, such as C 2 to C 6 glycols, such as ethylene glycol, propylene glycol, butylene And mono- or diesters of glycol, pentylene glycol or hexylene glycol with fatty acids.
- ester of the above linear hydrocarbon diol and at least one fatty acid for example, the following formula (8): R 8 COOC k H 2k OCOR 9 (8) (Wherein k is an integer of 2 to 6 and R 8 and R 9 are each a chain hydrocarbon) A diester of a C 2 -C 6 glycol with a fatty acid, and the following formula (9): R 8 COOC k H 2k OH (9) (Wherein k is an integer of 2 to 6 and R 8 is a chain hydrocarbon) And monoesters of fatty acid with C 2 -C 6 glycol.
- ester of C 2 to C 6 glycol and fatty acid as the fatty acid to be esterified (corresponding to R 8 COOH and R 9 COOH in the formula (8) and the formula (9)), C 2 to C 6 glycol
- ester of fatty acid with the fatty acid provided that it satisfies the requirements of the above IOB, melting point and water solubility, for example, "ester of (a 1 ) chain hydrocarbon tetraol with at least one fatty acid"
- the ester of a C 2 ⁇ C 6 glycols and fatty acid in view of the potential for degradation by oxidation and the like, derived from saturated fatty acids, esters of C 2 ⁇ C 6 glycols and fatty acid, Nachi Suwa, C 2 It is preferably an ester of a -C 6 glycol and a saturated fatty acid.
- ester of C 2 -C 6 glycol and fatty acid an ester of glycol and fatty acid derived from glycol having a large number of carbon atoms, for example, butylene glycol, in order to make IOB small and make it more hydrophobic. It is preferable that it is an ester of a glycol derived from pentylene glycol or hexylene glycol and a fatty acid.
- ester of C 2 -C 6 glycol and fatty acid a diester is preferable in order to make IOB small and to make it more hydrophobic.
- examples of commercial products of the ester of C 2 -C 6 glycol and fatty acid include Commol BL, Commol BS (manufactured by NOF Corporation) and the like.
- (B) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, and Ether with compound having one hydroxyl group replacing hydrogen atom of linear hydrocarbon moiety
- (B) A compound having (B1) a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, and the above chain Ether with a compound having one hydroxyl group replacing hydrogen atoms in the cyclic hydrocarbon moiety (hereinafter sometimes referred to as “compound (B)”) has the above-mentioned IOB, melting point and water solubility And all hydroxyl groups may not be etherified.
- Examples of the compound having (B1) a chain hydrocarbon portion and 2 to 4 hydroxyl groups replacing the hydrogen atoms of the above chain hydrocarbon portion are listed as the compound (A1) in the “compound (A)”.
- Examples of (B2) a compound having a linear hydrocarbon moiety and one hydroxyl group replacing the hydrogen atom of the linear hydrocarbon moiety include, for example, A compound in which one hydrogen atom of hydrocarbon is substituted with one hydroxyl group (—OH), for example, aliphatic monohydric alcohol such as saturated aliphatic monohydric alcohol and unsaturated aliphatic monohydric alcohol Can be mentioned.
- saturated aliphatic monohydric alcohol for example, a C 1 to C 20 saturated aliphatic monohydric alcohol, for example, methyl alcohol (C 1 ) (C 1 represents a carbon number, the same applies hereinafter), ethyl alcohol C 2 ), propyl alcohol (C 3 ) and its isomers, such as isopropyl alcohol (C 3 ), butyl alcohol (C 4 ) and its isomers, such as sec-butyl alcohol (C 4 ) and tert-butyl alcohol (C 4 ), pentyl alcohol (C 5 ), hexyl alcohol (C 6 ), heptyl alcohol (C 7 ), octyl alcohol (C 8 ) and isomers thereof, such as 2-ethylhexyl alcohol (C 8 ), nonyl alcohol (C 9 ), decyl alcohol (C 10 ), dodecyl alcohol (C 12 ), tetradecyl al Call (C 14), hexadect
- an ether of (b 1 ) chain hydrocarbon tetraol and at least one aliphatic monohydric alcohol for example, monoether, diether, triether and tetraether, preferably diether, triether Ethers and tetraethers, more preferably triethers and tetraethers, and even more preferably tetraethers, ethers of (b 2 ) chain hydrocarbon triols and at least one aliphatic monohydric alcohol, such as monoethers, diethers and the like triether, preferably diethers and triethers and more preferably tri-ether, and (b 3) a chain hydrocarbon diol and at least one aliphatic monohydric ether alcohols, for example, mono- and diethers and, Mashiku diethers.
- Examples of the ether of the above linear hydrocarbon tetraol and at least one aliphatic monohydric alcohol include the following formulas (10) to (13): (Wherein, each of R 10 to R 13 is a chain hydrocarbon). And tetraethers of pentaerythritol and aliphatic monohydric alcohols, triethers, diethers and monoethers.
- Examples of the ether of the above linear hydrocarbon triol and at least one aliphatic monohydric alcohol include the following formulas (14) to (16): (Wherein, R 14 to R 16 are each a chain hydrocarbon). And triethers of glycerol and aliphatic monohydric alcohols, diethers and monoethers.
- R 17 OC n H 2n OR 18 (Wherein n is an integer of 2 to 6 and R 17 and R 18 are each a chain hydrocarbon) Diethers of C 2 -C 6 glycols and aliphatic monohydric alcohols, and the following formula (18): R 17 OC n H 2n OH (18) (Wherein n is an integer of 2 to 6 and R 17 is a chain hydrocarbon) And monoethers of C 2 -C 6 glycols and aliphatic monohydric alcohols.
- the total carbon number of aliphatic monohydric alcohol constituting the tetraether of pentaerythritol and aliphatic monohydric alcohol that is, in the above formula (10)
- the IOB is 0.44. Therefore, in the above tetraether of pentaerythritol and aliphatic monohydric alcohol, IOB is required to be about 0.00 to about 0.60 when the total carbon number of aliphatic monohydric alcohol is about 4 or more. Fulfill.
- the total carbon number of aliphatic monohydric alcohol constituting triether of pentaerythritol and aliphatic monohydric alcohol ie, in the above formula (11)
- the IOB is 0.57. Therefore, in the above triether of pentaerythritol and aliphatic monohydric alcohol, IOB is required to be about 0.00 to about 0.60 when the total carbon number of aliphatic monohydric alcohol is about 9 or more. Fulfill.
- the total carbon number of aliphatic monohydric alcohols constituting the diether of pentaerythritol and aliphatic monohydric alcohol that is, R 10 in the above formula (12)
- the IOB is 0.60 when the sum of the carbon numbers of the and R 11 moieties is 15. Therefore, in the diether of pentaerythritol and aliphatic monohydric alcohol, IOB satisfies the requirement of about 0.00 to about 0.60 when the total carbon number of aliphatic monohydric alcohol is about 15 or more. .
- the carbon number of the aliphatic monohydric alcohol constituting the monoether of pentaerythritol and aliphatic monohydric alcohol that is, R 10 in the above formula (13)
- the IOB is 0.59. Therefore, in the monoether of pentaerythritol and aliphatic monohydric alcohol, the IOB satisfies the requirement of about 0.00 to about 0.60 when the carbon number of the aliphatic monohydric alcohol is about 22 or more.
- the total carbon number of aliphatic monohydric alcohol constituting triether of glycerin and aliphatic monohydric alcohol that is, R in the formula (14)
- the IOB is 0.50. Therefore, in the above triether of glycerin and aliphatic monohydric alcohol, IOB satisfies the requirement of about 0.00 to about 0.60 when the total carbon number of aliphatic monohydric alcohol is about 3 or more. .
- the total carbon number of aliphatic monohydric alcohols constituting the diether of glycerin and an aliphatic monohydric alcohol that is, in the formula (15), R 14 and R 15
- the IOB is 0.58. Therefore, in the diether of glycerin and an aliphatic monohydric alcohol, the IOB satisfies the requirement of about 0.00 to about 0.60 when the total carbon number of the aliphatic monohydric alcohol is about 9 or more.
- the carbon number of the aliphatic monohydric alcohol constituting the monoether of glycerin and aliphatic monohydric alcohol that is, the carbon of R 14 in the formula (16)
- the IOB is 0.58. Therefore, in the monoether of glycerin and aliphatic monohydric alcohol, the IOB satisfies the requirement of about 0.00 to about 0.60 when the carbon number of the aliphatic monohydric alcohol is about 16 or more.
- the compound (B) can be produced by dehydration condensation of the compound (B1) and the compound (B2) in the presence of an acid catalyst.
- compound C1 a linear hydrocarbon moiety and a carboxylic acid, a hydroxy acid, an alkoxy acid or an oxo acid containing 2 to 4 carboxyl groups replacing the hydrogen atom of the linear hydrocarbon moiety
- compound C1 may be, for example, a linear hydrocarbon carboxylic acid having 2 to 4 carboxyl groups, such as a linear hydrocarbon dicarboxylic acid, such as an alkane dicarboxylic acid, such as ethanedioic acid.
- a linear hydrocarbon hydroxy acid having 2 to 4 carboxyl groups for example, a linear chain having 2 to 4 carboxyl groups, such as malic acid, tartaric acid, citric acid, isocitric acid, etc.
- Hydrocarbon alkoxy acids such as O-acetyl citric acid, and linear hydrocarbon oxo acids having 2 to 4 carboxyl groups are included.
- Examples of the compound having a (C2) linear hydrocarbon moiety and one hydroxyl group replacing the hydrogen atom of the linear hydrocarbon moiety include those listed in the “compound (B)”, for example, Aliphatic monohydric alcohols are mentioned.
- an ester of a linear hydrocarbon tetracarboxylic acid having 4 carboxyl groups (c 1 ), a hydroxy acid, an alkoxy acid or an oxo acid, and at least one aliphatic monohydric alcohol for example, Mono-, di-, tri- and tetra-esters, preferably diesters, tri- and tetra-esters, more preferably tri- and tetra-esters, and still more preferably tetra-esters, chained with 3 (c 2 ) carboxyl groups
- Esters of hydrocarbon tricarboxylic acids, hydroxy acids, alkoxy acids or oxo acids with at least one aliphatic monohydric alcohol such as monoesters, diesters and triesters, preferably diesters and triesters, and more preferably triesters Ester
- aliphatic monohydric alcohol constituting the above ether corresponding to R 19 OH and R 20 OH in the formula (19)
- the above ether satisfies the requirements of the above IOB, melting point and water solubility It is not particularly limited, and examples thereof include aliphatic monohydric alcohols listed in the “compound (B)” section.
- the total carbon number of the aliphatic monohydric alcohol constituting the ether that is, the carbon number of the R 19 and R 20 moieties in the above formula (19) Since the IOB is 0.50 when the sum of the two is 2, the requirement of the above IOB is satisfied if the total carbon number is about 2 or more. However, when the total carbon number is about 6, the water solubility is as high as about 2 g, and there is also a problem from the viewpoint of the vapor pressure. In order to satisfy the requirement of water solubility of about 0.00 to about 0.05 g, the total carbon number is preferably about 8 or more.
- dialkyl ketone [(D 2 ) dialkyl ketone]
- dialkyl ketone following Formula (20): R 21 COR 22 (20) (Wherein, each of R 21 and R 22 is an alkyl group) And compounds having the formula:
- the total carbon number is preferably about 8 or more. Also, in consideration of the vapor pressure, the carbon number is preferably about 10 or more, and preferably about 12 or more.
- the melting point is about ⁇ 50 ° C.
- the vapor pressure is about 230 Pa at 20 ° C.
- the above-mentioned dialkyl ketone is commercially available and can be obtained by a known method, for example, oxidation of a secondary alcohol with chromic acid or the like.
- Examples of the fatty acid (corresponding to R 23 COOH in the formula (21)) constituting the above-mentioned ester are listed, for example, in “ester of (a 1 ) chain hydrocarbon tetraol with at least one fatty acid”.
- a fatty acid that is, a saturated fatty acid or an unsaturated fatty acid is mentioned, and considering the possibility of modification by oxidation etc., a saturated fatty acid is preferred.
- the aliphatic monohydric alcohol (corresponding to R 24 OH in the formula (21)) constituting the above-mentioned ester include, for example, aliphatic monohydric alcohols listed in the “compound (B)” section.
- the sum of carbon number of fatty acid and aliphatic monohydric alcohol that is, the sum of carbon number of R 23 C and R 24 in the formula (21) is 5
- the IOB is 0.60
- the requirements of the above IOB are satisfied when the total carbon number of the R 23 C and R 24 moieties is about 5 or more.
- the vapor pressure is as high as 2,000 Pa or more. Therefore, in consideration of the vapor pressure, the total carbon number is preferably about 12 or more. If the total carbon number is about 11 or more, the water solubility can satisfy the requirement of about 0.00 to about 0.05 g.
- esters of the above fatty acids with aliphatic monohydric alcohols include, for example, esters of dodecanoic acid (C 12 ), dodecyl alcohol (C 12 ), tetradecanoic acid (C 14 ), and dodecyl alcohol (C 12 )
- esters of fatty acids and aliphatic monohydric alcohols include Electol WE 20 and Electol WE 40 (all manufactured by NOF Corporation).
- the total carbon number of R 25 and R 26 is preferably about 7 or more, and more preferably about 9 or more.
- the above dialkyl carbonate is commercially available, and can be synthesized by the reaction of phosgene with alcohol, the reaction of formic acid chloride ester with alcohol or alcoholate, and the reaction of silver carbonate with alkyl iodide.
- Examples of the above-mentioned polyoxy C 2 -C 6 alkylene glycol or ester or ether thereof include (e 1 ) polyoxy C 2 -C 6 alkylene glycol, (e 2 ) polyoxy C An ester of a 2 to C 6 alkylene glycol with at least one fatty acid, an ether of (e 3 ) polyoxy C 2 to C 6 alkylene glycol and at least one aliphatic monohydric alcohol, (e 4 ) polyoxy C 2 to C 6 An ester of an alkylene glycol with a linear hydrocarbon tetracarboxylic acid, a linear hydrocarbon tricarboxylic acid, or a linear hydrocarbon dicarboxylic acid, and (e 5 ) polyoxy C 2 -C 6 alkylene glycol, with a linear hydrocarbon tetra And ethers with linear hydro
- oxy C 2 ⁇ C 6 alkylene backbone from the viewpoint of lowering the polyoxy C 2 ⁇ C 6 IOB of alkylene glycols, polyoxypropylene skeleton, oxybutylene skeleton, it is oxypentylene skeleton, or an oxy hexylene skeleton preferably More preferably, they are an oxybutylene skeleton, an oxypentylene skeleton, or an oxyhexylene skeleton.
- the above polyoxy C 2 -C 6 alkylene glycol has the following formula (23): HO- (C m H 2m O) n -H (23) (In the formula, m is an integer of 2 to 6) Can be represented by
- homopolymers of formula (23) may include homopolymers of propylene glycol, butylene glycol, pentylene glycol or hexylene glycol. From the above, in the formula (23), m is about 3 to about 6, and more preferably about 4 to about 6, and n is 2 or more.
- n the poly C 2 -C 6 alkylene glycol has an IOB of about 0.00 to about 0.60, a melting point of about 45 ° C. or less, and 100 g of water at 25 ° C. It is a value that has a water solubility of about 0.00 to about 0.05 g.
- the weight average molecular weight of the polyoxy C 2 -C 6 alkylene glycol is preferably about 200 to about 10,000, more preferably about 250 to about 8,000, and more preferably It is in the range of about 250 to about 5,000.
- the weight average molecular weight of the poly C 3 alkylene glycol, ie, polypropylene glycol is preferably about 1,000 to about 10,000, more preferably about 3,000 to about 8 And more preferably in the range of about 4,000 to about 5,000.
- the weight average molecular weight is less than about 1,000, the water solubility does not satisfy the requirements, and the larger the weight average molecular weight, the more the absorber transfer rate and the top sheet whiteness tend to be improved.
- Examples of commercial products of polyoxy C 2 ⁇ C 6 alkylene glycol e.g., UNIOL (TM) D-1000, D-1200 , D-2000, D-3000, D-4000, PB-500, PB-700, PB -1000 and PB-2000 (manufactured by NOF Corporation).
- UNIOL TM
- D-1000, D-1200 , D-2000, D-3000, D-4000, PB-500, PB-700, PB -1000 and PB-2000 manufactured by NOF Corporation.
- ester of (e 2 ) polyoxy C 2 -C 6 alkylene glycol with at least one fatty acid is the OH terminal of the polyoxy C 2 to C 6 alkylene glycol described in the section “(e 1 ) polyoxy C 2 to C 6 alkylene glycol”.
- fatty acids ie, monoesters and diesters.
- the fatty acid to be esterified is, for example, listed in “ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid”.
- Fatty acids that is, saturated fatty acids or unsaturated fatty acids, and in view of the possibility of modification by oxidation etc., saturated fatty acids are preferred.
- Examples of commercially available esters of the polyoxy C 2 -C 6 alkylene glycol and fatty acid include Wilbright cp 9 (manufactured by NOF Corporation).
- aliphatic monohydric alcohols to be etherified in the ether of polyoxy C 2 -C 6 alkylene glycol and at least one aliphatic monohydric alcohol include, for example, aliphatics listed in the “compound (B)” section. Monohydric alcohol is mentioned.
- esters of the above polyoxy C 2 -C 6 alkylene glycol with chain hydrocarbon tetracarboxylic acid, chain hydrocarbon tricarboxylic acid, or chain hydrocarbon dicarboxylic acid are commercially available, and chain hydrocarbon tetracarbons are also commercially available. It can be produced by polycondensation of C 2 -C 6 alkylene glycol with an acid, linear hydrocarbon tricarboxylic acid or linear hydrocarbon dicarboxylic acid under known conditions.
- chain hydrocarbon tetraol to be etherified the chain hydrocarbon triol, and the chain hydrocarbon diol, those described in the “compound (A)” section, for example, pentaerythritol, glycerin and glycol Can be mentioned.
- ethers of the above polyoxy C 2 -C 6 alkylene glycol and chain hydrocarbon tetraol, chain hydrocarbon triol, or chain hydrocarbon diol are, for example, UnilobeTM 5TP-300 KB, and Uniol (trademark) TG-3000 and TG-4000 (made by NOF Corporation) can be mentioned.
- Unilube (TM) 5TP-300KB is a compound obtained by polycondensation of 65 moles of propylene glycol and 5 moles of ethylene glycol with 1 mole of pentaerythritol, and its IOB is 0.39, and the melting point is less than 45 ° C. And the water solubility was less than 0.05 g.
- Uniol (TM) TG-3000 is a compound obtained by polycondensing 50 moles of propylene glycol with 1 mole of glycerin, its IOB is 0.42, its melting point is less than 45 ° C, and its water solubility is 0.05 g And the weight average molecular weight was about 3,000.
- Uniol (TM) TG-4000 is a compound obtained by polycondensing 70 moles of propylene glycol with 1 mole of glycerin, its IOB is 0.40, melting point is less than 45 ° C., and water solubility is 0.05 g And the weight average molecular weight was about 4,000.
- the ether of the polyoxy C 2 -C 6 alkylene glycol and the chain hydrocarbon tetraol, chain hydrocarbon triol or chain hydrocarbon diol is also a chain hydrocarbon tetraol, chain hydrocarbon triol or It can be produced by adding a C 2 -C 6 alkylene oxide to a linear hydrocarbon diol under known conditions.
- the chain hydrocarbon has an IOB of 0.00 and an aqueous solubility of almost 0 g because the inorganic value is 0, and the blood has a melting point of about 45 ° C. or less. It may be included in the modifier.
- Examples of the chain hydrocarbon include (f 1 ) chain alkanes such as straight chain alkanes and branched chain alkanes, and in the case of straight chain alkanes, for example, the melting point is about 45 ° C. or less In general, those containing 22 or less carbon atoms are included. Also, in consideration of the vapor pressure, those having 13 or more carbon atoms are generally included.
- the blood modifying agent has been found to at least have the effect of reducing blood viscosity and surface tension. Since the menstrual blood to be absorbed by the absorbent article contains proteins such as the endometrial wall as compared with normal blood, they act to connect the blood cells to each other, and the blood cells are in a continuous state. Cheap. Therefore, the menstrual blood to be absorbed by the absorbent article tends to have a high viscosity, and when the top sheet is a non-woven fabric or a woven fabric, menstrual blood tends to be clogged between fibers, and the wearer feels sticky. And it spreads and leaks on the surface of the top sheet.
- a blood modifying agent having an IOB of about 0.00 to about 0.60 is highly organic and easily enters between blood cells, thereby stabilizing the blood cells and making it difficult for the blood cells to form a continuous structure. It is thought that can be done. It is considered that the absorber makes it easy to absorb menstrual blood by stabilizing the blood cells and making the blood cells less likely to form a continuous structure.
- the so-called SAP when menstrual blood is absorbed, blood cells that have undergone continuous change cover the SAP surface, making it difficult for the SAP to exhibit its absorption performance.
- SAP By stabilizing blood cells, it is considered that SAP can more easily exhibit absorption performance.
- the blood modifying agent having high affinity for red blood cells protects the red blood cell membrane, so that the red blood cells are less likely to be destroyed.
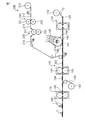
- FIG. 7 is a figure for demonstrating the absorbent article manufacturing apparatus 100 used for the manufacturing method of the absorbent article 1 in one Embodiment of this invention.
- a process of forming a folding suppression sheet, a process of forming an absorber, a process of preparing a sheet for top sheet, a process of forming compressed grooves in a laminate, and a sheet for backsheet a process of forming a folding suppression sheet, a process of forming an absorber, a process of preparing a sheet for top sheet, a process of forming compressed grooves in a laminate, and a sheet for backsheet , Cutting the continuum of the absorbent article and applying a blood modifying agent to the absorbent article.
- the top sheet sheet used in the step of preparing the top sheet sheet includes a process of preparing the resin film sheet, a process of forming the recess in the resin film sheet, and a process of gear stretching the resin film sheet. It is manufactured by the manufacturing method of the seat for sheets.
- the bending suppression sheet sheet 112 supplied from the bending suppression sheet sheet roll 110 is placed on the belt 120. Then, after applying a hot melt adhesive to the sheet 112 for bending suppression sheet with a coating device (not shown), the cutter 130 is used to cut the sheet 112 for bending suppression sheet into a predetermined shape and the bending suppression sheet Make
- the absorber 148 is formed on a bending suppression sheet.
- Pulverized pulp 142 is supplied to pattern drum 140 from a pulverized pulp supply device (not shown).
- a recess 144 is formed on the outer peripheral portion of the pattern drum 140 as a mold for packing crushed pulp.
- the inside of the pattern drum 140 is suctioned 146, and the crushed pulp 142 supplied to the pattern drum 140 is sucked into the recess 144 and compressed. Then, the pulp 142 compressed in the recess 144 is formed into the absorber 148.
- the absorber 148 is placed on the bending suppression sheet.
- a hot melt adhesive is applied to the absorbent body 148 with a coating device (not shown), and then the top sheet sheet 216 manufactured by the top sheet sheet manufacturing method described later is used as the absorbent body 148. And the top sheet sheet 216 is adhered to the absorber 148.
- the pressing device is used to form the pressing groove in the top sheet sheet 216, the absorber 148 and the laminate 262 of the bending suppression sheet.
- the laminate 262 passes between the upper roll 151 and the lower roll 152 of the embossing device 150.
- a convex portion (not shown) having a shape corresponding to the pressing groove 8 of the absorbent article 1 shown in FIG. 1 is provided.
- the lower roll 152 is a plain roll whose outer peripheral surface is smooth.
- the portion of the laminate 262 corresponding to the compressed grooves 8 of the absorbent article 1 shown in FIG. 1 is in the thickness direction And compressed grooves are formed in the laminate 262.
- an adhesive is applied to the backsheet sheet 162 supplied from the backsheet sheet roll 160 using a coating device (not shown), and then lamination is performed in which compressed grooves are formed. It is overlapped and adhered to the surface on the side of the bending suppression sheet of the body 154 to form a continuum 164 of the absorbent article.
- the cutter 170 is used to cut the continuum 164 of the absorbent article into the shape of the absorbent article to produce the absorbent article.
- the above-described blood modifying agent 181 is applied to the central region of the absorbent article using the modifier application spray 180 to apply blood to the surface of the top sheet. Form a modifier layer.
- the blood modifying agent 181 applied to the central region of the absorbent article may be applied at least to a portion that abuts on the excretory port of the wearer's body fluid.
- the length in the lengthwise direction is preferably 50 mm or more, more preferably 100 mm or more
- the length in the width direction is preferably 10 mm or more, more preferably 30 mm or more, centering on the center of the absorbent article.
- a blood modifying agent may be applied to an area.
- the blood modifying agent was applied after the step of cutting the continuum of the absorbent article, the blood modifying agent may be applied in the step of producing the top sheet described later.
- the downstream stage of the manufacturing process of the absorbent article for example, the blood just before packaging the absorbent article It is preferred to apply the modifier to the absorbent article.
- the method of manufacturing the absorbent article 1 includes a step of forming an adhesive portion on the continuous body 164 of the absorbent article, a step of forming a seal portion on the continuous body 164 of the absorbent article, and the like.
- the resin film sheet 212 supplied from the roll 210 of the resin film sheet is supplied to the recess forming roll 220.
- the resin film sheet 212 is made to pass the recessed part formation roll 220, and the resin film sheet 214 (refer FIG. 9) in which the recessed part 2141 was formed is produced.
- the recess forming roll 220 comprises a knurling roll 221 and a preheating roll 222 having a smooth surface.
- FIGS. 8A and 8B show an example of the knurled roll 221.
- FIG. FIG. 8A is a view showing the entire knurled roll 221
- FIG. 8B is an enlarged view of a portion 223 having unevenness on the outer peripheral surface of the knurled roll 221.
- FIG. 8C shows an example of the preheating roll 222 having a smooth surface.
- a grid-like convex portion 224 is provided on the surface 223 of the knurled roll 221.
- a rhombus recess 225 is formed on the surface of the knurled roll 221.
- the shape of the recessed part 225 of the knurl roll 221 is not limited to a rhombus, You may be shapes, such as a square, a rectangle, a parallelogram, a trapezoid, a triangle, and a hexagon.
- the center line spacing of the convex parts 224 arranged in parallel in the lattice-like convex parts 224 is preferably 0.2 mm or more and 10 mm or less, more preferably 0.4 mm or more and 2 mm It is below.
- the pitch of the grid-like convex portions 224 is less than 0.2 mm or greater than 10 mm, the concave portion may not be formed in the resin film.
- the width of the grid-like convex portion 224 is preferably 0.01 mm or more and 1 mm or less, and more preferably 0.03 mm or more and 0.1 or less.
- the length of one side of the rhombic recess 225 is preferably 0.1 mm or more and 5 mm or less, and more preferably 0.2 mm or more and 1 mm or less.
- the width of the grid-like convex part 224 is less than 0.01 mm or more than 1 mm, or when the length of one side of the rhombus concave part 225 is less than 0.1 mm or more than 5 mm, the concave part may not be formed in the resin film. .
- the preheated roll 222 having a smooth surface is maintained at a temperature of 70 ° C. or more and 100 ° C. or less, and heats the supplied resin film sheet 212. Thereby, the resin film sheet 212 becomes soft and easy to form.
- the resin film sheet 212 passes between the knurled roll 221 and the roll 222 having a smooth surface, the resin film sheet 212 receives a strong pressure in the thickness direction in a portion in contact with the grid-like convex portion 224. Thereby, as shown in FIG. 9, the fine recessed part 2141 is formed in the resin film sheet 214. As shown in FIG. In fact, the number of recesses 2141 formed in the resin film sheet 212 is smaller than that shown in FIG. 9, and the number of recesses 2141 per unit area is also much larger than that shown in FIG.
- the concave portion 2141 is formed only in a range 2143 corresponding to the central portion 12 (see FIG. 1) of the main body portion 10 in the resin film sheet 214.
- the range corresponding to the absorbent article 1 in the resin film sheet 214 is the range indicated by the dotted line 2142.
- the first mountain fold portion, the second mountain fold portion, and the second mountain fold portion of the top sheet 2 are caused to pass through the resin film sheet 214 having the concave portion formed in the stretching gear roll 230 shown in FIG.
- the valley folds are formed in the resin film sheet 214.
- symbol 216 is a resin film sheet which formed the 1st mountain fold part, the 2nd mountain fold part, and the valley fold part.
- the drawing gear roll 230 includes an upper roll 231 and a lower roll 232.
- 10 (a) is a view for explaining the upper roll 231 of the drawing gear roll 230
- FIG. 10 (b) is a view for explaining the gear teeth 233 arranged on the outer peripheral surface of the upper roll 231.
- FIG. 10 (c) is a cross-sectional view taken along the line CC of FIG. 10 (b).
- the gear teeth 233 extend discontinuously in the circumferential direction of the upper roll 231. That is, the gear teeth 233 extending in the circumferential direction of the upper roll 231 are interrupted at multiple points along the way.
- a second mountain-folded portion is formed in the resin film sheet 214 by the portion 234 where the gear teeth 233 are disconnected.
- the width of gear teeth 233 is, for example, 0.3 mm or more and 0.5 mm or less, and the distance between the centers of adjacent gear teeth 233 is, for example, 1.0 mm or more and 1.2 mm or less.
- FIG. 11 (a) is a view for explaining the lower roll 232 of the drawing gear roll 230
- FIG. 11 (b) is a view for explaining the gear teeth 235 disposed on the outer peripheral surface of the lower roll 232
- 11 (c) is a cross-sectional view taken along the line DD of FIG. 11 (b).
- the gear teeth 235 extend in the circumferential direction of the lower roll 232.
- the lower roll 232 is not interrupted at multiple points along the way.
- the width of the gear teeth 235 is, for example, equal to the width of the gear teeth 233 of the upper roll 231, and the distance between the centers of adjacent gear teeth 235 is, for example, equal to the distance between the centers of the gear teeth 233 of the upper roll 231.
- the radial length of the upper roll 231 at the portion where the gear teeth 233 of the upper roll 231 and the gear teeth 235 of the lower roll 232 are engaged, that is, the biting depth is, for example, 1.25 mm.
- the gap between the gear teeth 233 of the upper roll 231 and the gear teeth 235 of the lower roll 232 when the gear teeth 233 of the upper roll 231 and the gear teeth 235 of the lower roll 232 are engaged is, for example, 0.25 mm or more , 0.45 mm or less.
- the resin film sheet 214 passes through the drawing gear roll 230, the resin film 214 is bent in a substantially wave shape, and the resin film 214 forms the first mountain fold 21 of the top sheet 2 and the second mountain fold. Portions 22 and valley folds 23 are formed. Further, an opening 25 (see FIG. 4) of the top sheet 2 is further formed in the region 2143 (see FIG. 9) in which the recess 2141 of the resin film sheet 214 is formed.
- the resin film sheet 214 is largely drawn at a portion 236 where the gear teeth 233 of the upper roll 231 and the gear teeth 235 of the lower roll 232 are engaged.
- the resin film sheet 214 is thin at the part where the concave part 2141 (see FIG. 9) is formed in the above-mentioned concave part forming step, and the part where the scratch is given by the grid-like convex part 224 of the knurling roll 221 As it is present, the strength is weak, and the recess 2141 of the resin film sheet 214 breaks when it is stretched.
- the concave portion 2141 of the resin film sheet 214 is torn, the torn portion of the resin film sheet 214 is spread, and the opening portion is to the torn portion of the resin film sheet 214. It is formed.
- the resin film sheet 214 is not stretched much at the portions 237 and 238 where the gear teeth 233 of the upper roll 231 and the gear teeth 235 of the lower roll 232 are not engaged. For this reason, in the portions 237 and 238 in which the gear teeth 233 of the upper roll 231 and the gear teeth 235 of the lower roll 232 in the resin film sheet 241 are not engaged, the resin film sheet 214 described above The recess 2141 formed in the recess forming step is not broken and does not become an opening.
- the blood modifying agent since the blood modifying agent has a mechanism to lower the viscosity and surface tension of the blood, the body fluid is transferred to the absorber 4 without remaining on the top sheet 2 by the blood modifying layer 24 and absorbed. 4 can be absorbed.
- the following examples confirmed that the blood modifying agent has a mechanism to lower the viscosity and surface tension of blood. This check was performed using a non-woven fabric in which the body fluid tends to remain as compared to the resin film.
- the sanitary napkin comprises a top sheet formed of an air through non-woven fabric (composite fiber of polyester and polyethylene terephthalate, basis weight: 35 g / m 2 ) treated with a hydrophilic agent, and a composite of air through non-woven fabric (polyester and polyethylene terephthalate) Fiber, second sheet formed of basis weight: 30 g / m 2 ), pulp (basis weight: 150 to 450 g / m 2 , more in the central part), acrylic high absorption polymer (basis weight: 15 g / m 2 ) And, it was formed from an absorbent including a tissue as a core wrap, a water repellent treated side sheet, and a back sheet made of polyethylene film.
- an absorbent including a tissue as a core wrap, a water repellent treated side sheet, and a back sheet made of polyethylene film.
- Triethylene CL oil fatty acid glycerides, manufactured by NOF Corporation C 8 fatty acid: fatty acid of C 12 is approximately included in a weight ratio of 44:56, triesters of glycerin and fatty acid, the weight average molecular weight: about 570
- -Panaceto 800 B manufactured by NOF Corporation Triester of glycerin and fatty acid in which all fatty acids are 2-ethylhexanoic acid (C 8 ), weight average molecular weight: about 470 NA36, manufactured by NOF Corporation C 16 fatty acids: C 18 fatty acids: C 20 fatty acids (including both saturated fatty acids and unsaturated fatty acids) in a weight ratio of approximately 5: 92: 3, Triester of glycerin and fatty acid, weight average molecular weight: about 880
- Tricot oil fatty acid glyceride manufactured by NOF Corporation C 8 fatty acid: C 10 fatty acid: C 12 fatty acid: C 14 fatty acid: C 16 fatty acid (including both saturated fatty acid and unsaturated fatty acid) is approximately 4 Triester of glycerin and fatty acid, contained in a weight ratio of 8: 60: 25: 3, weight average molecular weight: 670 ⁇ Caprylic diglyceride, manufactured by NOF Corporation Diester of glycerin and fatty acid wherein fatty acid is octanoic acid, weight average molecular weight: 340
- Uniol D-4000 polypropylene glycol manufactured by NOF Corporation, weight average molecular weight: about 4,000 Uniol PB500, polybutylene glycol manufactured by NOF Corporation, weight average molecular weight: about 500 Uniol PB700, manufactured by NOF Corporation, polyoxybutylene polyoxypropylene glycol, weight average molecular weight: about 700
- Uniol PB 1000 R polybutylene glycol manufactured by NOF Corporation, weight average molecular weight: about 1000 [Ester of (e 2 ) polyoxy C 2 -C 6 alkylene glycol with at least one fatty acid] ⁇ Wilbright cp 9, a compound in which OH groups at both ends of polybutylene glycol manufactured by NOF Corporation were esterified with hexadecanoic acid (C 16 ), weight average molecular weight: about 1,150
- UNIOL TG-3000 glyceryl ether of polypropylene glycol manufactured by NOF Corporation, about 16 repeating units, weight average molecular weight: about 3,000 ⁇ UNIOL TG-4000, glyceryl ether of polypropylene glycol manufactured by NOF Corporation, about 16 repeating units, weight average molecular weight: about 4,000
- -PEG 1500 polyethylene glycol manufactured by NOF Corporation, weight average molecular weight: about 1,500 to about 1,600 Nonion S-6, manufactured by NOF Corporation, polyoxyethylene monostearate, repeating unit of about 7 weight average molecular weight: about 880 Will Bright s 753, manufactured by NOF Corporation polyoxyethylene polyoxypropylene polyoxybutylene glycerin, weight average molecular weight: about 960
- TG-330 a glyceryl ether of polypropylene glycol manufactured by NOF Corporation, about 6 repeating units, weight average molecular weight: about 330 ⁇ UNIOL TG-1000, glyceryl ether of polypropylene glycol manufactured by NOF Corporation, about 16 repeating units, weight average molecular weight: about 1,000
- Unirube DGP-700 a diglyceryl ether of polypropylene glycol manufactured by NOF Corporation, about 9 repeating units, weight average molecular weight: about 700 -Uniox HC60, manufactured by NOF Co., Ltd. polyoxyethylene hydrogenated castor oil, weight average molecular weight: about 3,570 ⁇ Vaseline, Cognis Japan Ltd. Petroleum derived hydrocarbon, semi-solid
- the IOB, melting point and water solubility of the above sample are shown in Table 2 below.
- the water solubility was measured according to the above-mentioned method, but 20.0 g was added to 100 g of demineralized water, and a sample dissolved after 24 hours was evaluated as "20 g ⁇ ", and 100 g of demineralized water was used. A sample in which 0.05 g was dissolved but not 1.00 g was evaluated as 0.05 to 1.00 g.
- fusing point " ⁇ 45" means that melting
- the skin contact surface of the top sheet of the above-mentioned sanitary napkin was coated with the above-mentioned blood modifier. Heat each blood modifier to its melting point + 20 ° C if the blood modifier is liquid at room temperature, and if the blood modifier is solid at room temperature, then control seam HMA gun Each blood modifying agent was atomized and applied to the entire skin contact surface of the top sheet so that the basis weight was approximately 5 g / m 2 .
- FIG. 13 is an electron micrograph of the skin contact surface of the top sheet in the sanitary napkin (No. 2-5) in which the top sheet contains avian C2L oil fatty acid glyceride. As apparent from FIG. 13, the tri-C2L oil fatty acid glyceride is in the form of fine particles and adheres to the surface of the fiber. Rewet rates and absorber transfer rates were measured according to the procedure described above. The results are shown in Table 2 below.
- Rewet rate (%) 100 ⁇ (weight of filter paper after test ⁇ weight of original filter paper) / 6
- absorber transfer speed which is the time for blood to transfer from the top sheet to the absorber after the second drop of blood.
- absorber transfer rate means the time from when blood is introduced into the top sheet to when the red color of blood is not seen on the surface and inside of the top sheet.
- the rewet rate was 22.7% and the absorber transfer rate was over 60 seconds, but both the glycerin and fatty acid triesters had rewet rates From the fact that it is 7.0% or less and the absorber transfer rate is 8 seconds or less, it can be seen that the absorption performance is greatly improved. However, among triesters of glycerin and fatty acid, NA50 of which the melting point exceeds 45 ° C. shows no significant improvement in the absorption performance.
- a blood modifying agent having an IOB of about 0.00 to about 0.60, a melting point of about 45 ° C. or less, and an aqueous solubility of about 0.00 to about 0.05 g per 100 g of water at 25 ° C. It was found that the absorption performance was greatly improved.
- sanitary napkins were worn by a plurality of volunteer subjects.
- sanitary napkins containing blood modifiers 2-1 to 2-3 there was no tackiness on the top sheet even after menstrual blood absorption, and it was answered that the top sheet was smooth. .
- Defibrillation blood after blood collection, stirred for about 5 minutes in an Erlenmeyer flask together with glass beads
- EDTA blood Addition of 0.5 mL of 12% EDTA ⁇ 2K saline to 65 mL of venous blood
- Serum or plasma Supernatant after centrifuging defibrillated blood or EDTA blood, respectively, at about 1900 G at room temperature for 10 minutes
- Blood cells Remove the serum from the blood and remove the residual phosphate buffered saline (PBS) ) Washed twice and then added with phosphate buffered saline for the removed serum
- An absorbent article was produced in the same manner as in Example 2 except that avian C2L oil fatty acid glyceride was applied so as to give a basis weight of approximately 5 g / m 2, and the rewet rate was evaluated for the above various blood. . Three measurements were taken for each blood and the mean value was taken. The results are shown in Table 3 below.
- Example 2 The same tendency as equine EDTA blood obtained in Example 2 was also obtained in human and sheep blood. The same tendency was also observed in defibrinated blood and EDTA blood.
- the top sheet containing the blood modifying agent has low blood retention and can be rapidly transferred to the absorber after absorbing blood.
- Example 4 [Viscosity of blood containing blood modifying agent] The viscosity of the blood containing the blood modifying agent was measured using Rheometric Expansion System ARES (Rheometric Scientific, Inc). 2% by weight of Panaceto 810s was added to equine defibrinated blood, the mixture was lightly stirred to form a sample, the sample was loaded on a parallel plate of 50 mm in diameter, the gap was made 100 ⁇ m, and the viscosity was measured at 37 ⁇ 0.5 ° C. . Because of the parallel plate, the sample was not subjected to a uniform shear rate, but the average shear rate displayed on the instrument was 10 s ⁇ 1 .
- the viscosity of horse-defibrillated blood containing 2% by mass of Panaceto 810s was 5.9 mPa ⁇ s, while the viscosity of horse-defibrillated blood containing no blood modifying agent was 50.4 mPa ⁇ s.
- equine defibrinated blood containing 2% by weight of Panaceto 810s reduces the viscosity by about 90% as compared to the case without blood modifying agent.
- blood contains components such as blood cells and is known to have thixotropy properties, it is considered that the blood modifying agent of the present disclosure can lower the viscosity of blood in a low viscosity region. By reducing the viscosity of blood, it is thought that absorbed menstrual blood can be rapidly transferred from the top sheet to the absorber.
- Example 5 [Micrograph of blood containing blood modifier] A healthy volunteer's menstrual blood is collected on Saran wrap (registered trademark), and in part of it, Panaceto 810s dispersed in 10 times mass of phosphate buffered saline, and the concentration of Panaceto 810s is 1% by mass As it was added. The menstrual blood was applied to a slide glass, covered with a cover glass, and the condition of red blood cells was observed with a light microscope. A photomicrograph of menstrual blood containing no blood modifying agent is shown in FIG. 14 (a), and a photomicrograph of menstrual blood containing PANACET 810s is shown in FIG. 14 (b).
- red blood cells form a lump of rhomsen, etc., but in menstrual blood containing PANACET 810s, the red blood cells are dispersed stably. I understand. Therefore, it is suggested that the blood modifying agent works to stabilize red blood cells in the blood.
- Example 6 [Surface tension of blood containing blood modifier] The surface tension of blood containing a blood modifying agent was measured by a pendant drop method using a contact angle meter Drop Master 500 manufactured by Kyowa Interface Science Co., Ltd. The surface tension was measured after adding a predetermined amount of blood modifying agent to sheep defibrinated blood and shaking sufficiently. The measurement is automatically performed by the device, but the surface tension ⁇ is obtained by the following equation (see FIG. 15).
- the density ⁇ is the density test method and density / mass / volume conversion table in JIS K 2249-1995, 5. It was measured at the temperature shown in Table 5 below according to the vibrational density test method. For measurement, DA-505 of Kyoto Electronics Industries Ltd. was used. The results are shown in Table 5.
- the blood modifying agent has a water solubility of about 0.00 to about 0.05 g per 100 g of water at 25 ° C., but its solubility in water is very low. It can be seen that the surface tension of the blood can be reduced. By lowering the surface tension of the blood, it is considered that the absorbed blood can be rapidly transferred to the absorber without being held between the fibers of the top sheet.
Landscapes
- Health & Medical Sciences (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Physics & Mathematics (AREA)
- Fluid Mechanics (AREA)
- Absorbent Articles And Supports Therefor (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2012082184A JP5762346B2 (ja) | 2012-03-30 | 2012-03-30 | 吸収性物品 |
| JP2012-082184 | 2012-03-30 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013145869A1 true WO2013145869A1 (ja) | 2013-10-03 |
Family
ID=49259154
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2013/052628 WO2013145869A1 (ja) | 2012-03-30 | 2013-02-05 | 吸収性物品 |
Country Status (4)
| Country | Link |
|---|---|
| JP (1) | JP5762346B2 (zh) |
| AR (1) | AR090518A1 (zh) |
| TW (1) | TWI573573B (zh) |
| WO (1) | WO2013145869A1 (zh) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6585785B2 (ja) * | 2018-08-10 | 2019-10-02 | 大王製紙株式会社 | 吸収性物品 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050137556A1 (en) * | 2003-12-04 | 2005-06-23 | Henri Brisebois | Sanitary absorbent article having a reinforced structure |
| JP2006510456A (ja) * | 2002-12-18 | 2006-03-30 | ザ プロクター アンド ギャンブル カンパニー | 身体清潔効果のための生理用ナプキン |
| JP2008519672A (ja) * | 2004-11-30 | 2008-06-12 | ザ プロクター アンド ギャンブル カンパニー | 一対の後部サイドフラップを含む吸収性物品 |
| JP2010136833A (ja) * | 2008-12-10 | 2010-06-24 | Kao Corp | 吸収性物品 |
| JP5122007B1 (ja) * | 2011-03-31 | 2013-01-16 | ユニ・チャーム株式会社 | 吸収性物品 |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4812614B2 (ja) * | 2006-12-27 | 2011-11-09 | 花王株式会社 | 吸収性物品 |
-
2012
- 2012-03-30 JP JP2012082184A patent/JP5762346B2/ja not_active Expired - Fee Related
-
2013
- 2013-02-05 WO PCT/JP2013/052628 patent/WO2013145869A1/ja active Application Filing
- 2013-03-27 AR ARP130100997A patent/AR090518A1/es unknown
- 2013-03-28 TW TW102111223A patent/TWI573573B/zh not_active IP Right Cessation
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006510456A (ja) * | 2002-12-18 | 2006-03-30 | ザ プロクター アンド ギャンブル カンパニー | 身体清潔効果のための生理用ナプキン |
| US20050137556A1 (en) * | 2003-12-04 | 2005-06-23 | Henri Brisebois | Sanitary absorbent article having a reinforced structure |
| JP2008519672A (ja) * | 2004-11-30 | 2008-06-12 | ザ プロクター アンド ギャンブル カンパニー | 一対の後部サイドフラップを含む吸収性物品 |
| JP2010136833A (ja) * | 2008-12-10 | 2010-06-24 | Kao Corp | 吸収性物品 |
| JP5122007B1 (ja) * | 2011-03-31 | 2013-01-16 | ユニ・チャーム株式会社 | 吸収性物品 |
Also Published As
| Publication number | Publication date |
|---|---|
| TWI573573B (zh) | 2017-03-11 |
| AR090518A1 (es) | 2014-11-19 |
| JP5762346B2 (ja) | 2015-08-12 |
| TW201404372A (zh) | 2014-02-01 |
| JP2013208381A (ja) | 2013-10-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5925015B2 (ja) | 吸収性物品 | |
| JP5713951B2 (ja) | 吸収性物品 | |
| JP5847055B2 (ja) | 吸収性物品 | |
| WO2013129327A1 (ja) | 吸収性物品 | |
| JP5843740B2 (ja) | 吸収性物品 | |
| JP6037606B2 (ja) | 吸収性物品、吸収性物品のトップシートおよびそのトップシートの製造方法 | |
| JP5963639B2 (ja) | 吸収性物品 | |
| JP5709788B2 (ja) | 吸収性物品 | |
| JP2013075099A (ja) | 吸収性物品 | |
| JP6041473B2 (ja) | 吸収性物品 | |
| JP5745377B2 (ja) | 吸収性物品 | |
| JP5769655B2 (ja) | 吸収性物品 | |
| JP5762346B2 (ja) | 吸収性物品 | |
| JP6041472B2 (ja) | 吸収性物品、吸収性物品のトップシートおよびそのトップシートの製造方法 | |
| JP5726123B2 (ja) | 吸収性物品 | |
| JP5709789B2 (ja) | 吸収性物品 | |
| JP5713950B2 (ja) | 吸収性物品 | |
| WO2013129332A1 (ja) | 吸収性物品 | |
| WO2013129326A1 (ja) | 吸収性物品 | |
| WO2013129325A1 (ja) | 吸収性物品 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 13767419 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 13767419 Country of ref document: EP Kind code of ref document: A1 |