WO2013145869A1 - Absorbent article - Google Patents
Absorbent article Download PDFInfo
- Publication number
- WO2013145869A1 WO2013145869A1 PCT/JP2013/052628 JP2013052628W WO2013145869A1 WO 2013145869 A1 WO2013145869 A1 WO 2013145869A1 JP 2013052628 W JP2013052628 W JP 2013052628W WO 2013145869 A1 WO2013145869 A1 WO 2013145869A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- acid
- chain hydrocarbon
- sheet
- absorbent article
- hydrocarbon moiety
- Prior art date
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/45—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators characterised by the shape
- A61F13/47—Sanitary towels, incontinence pads or napkins
- A61F13/472—Sanitary towels, incontinence pads or napkins specially adapted for female use
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/45—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators characterised by the shape
- A61F13/47—Sanitary towels, incontinence pads or napkins
- A61F13/4702—Sanitary towels, incontinence pads or napkins having a reinforcing member
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/45—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators characterised by the shape
- A61F13/47—Sanitary towels, incontinence pads or napkins
- A61F13/475—Sanitary towels, incontinence pads or napkins characterised by edge leakage prevention means
- A61F13/4751—Sanitary towels, incontinence pads or napkins characterised by edge leakage prevention means the means preventing fluid flow in a transversal direction
- A61F13/4756—Sanitary towels, incontinence pads or napkins characterised by edge leakage prevention means the means preventing fluid flow in a transversal direction the means consisting of grooves, e.g. channels, depressions or embossments, resulting in a heterogeneous surface level
Definitions
- the present invention relates to absorbent articles such as sanitary napkins, panty liners, incontinence pads, incontinence liners and the like.
- a sanitary napkin having a pair of flaps extending laterally outward beyond the longitudinal side edge of the main part is known in the prior art (e.g., U.S. Pat. No. 5,958,015).
- the flaps are folded under the wearer's underwear.
- the flap is folded along the longitudinal portion of the inner main portion of the proximal end. If the crotch width of the wearer's underwear is wider than the width of the main part, the flap is folded along the hinge of the main part.
- An object of the present invention is to provide an absorbent article which can suppress that the side portion of the absorber hits the inner crotch of the wearer and can suppress the occurrence of large deflection in the side portion of the absorber.
- a liquid-permeable top sheet provided on the skin side having a longitudinal direction and a width direction
- a liquid-impermeable back sheet provided on the clothing side, the top sheet, and the back sheet
- a main body portion provided with a liquid-retaining absorber including a side surface extending in the longitudinal direction on both sides in the width direction, and both sides of the main body portion extending in the width direction from both side edges of the main body portion
- An absorbent article including a wing portion disposed in the body, wherein the main body portion is provided between the absorber and the back sheet, and further includes a bending suppression sheet including longitudinally extending edges on both sides in the width direction The edge on both sides in the width direction of the anti-folding sheet is located outside the side surface of the absorber in the width direction, and the area where the anti-folding sheet is provided is the area where the anti-folding sheet is not provided So that the absorbent article is harder to
- the absorbent While suppressing that the part of a side of a part hits a wearer's inner crotch, it can suppress that a big distortion arises in the part of the side of an absorber.
- FIG. 1 is a partially broken plan view of an embodiment of the absorbent article of the present invention.
- FIG. 2 is a schematic cross-sectional view showing a cross section taken along line AA of FIG.
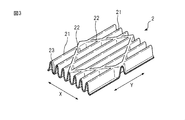
- FIG. 3 shows a first mountain-folded part, a second mountain-folded part, formed in the region on the outer side of both side surfaces in the width direction of the absorber in the top sheet of the main body part according to an embodiment of the present invention It is a figure for demonstrating a part and a valley fold part.
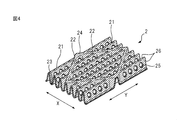
- FIG. 4 is a view for explaining a first mountain fold, a second mountain fold and a valley fold formed on the top sheet of the central part of the main body in the absorbent article according to one embodiment of the present invention. is there.
- FIG. 1 is a partially broken plan view of an embodiment of the absorbent article of the present invention.
- FIG. 2 is a schematic cross-sectional view showing a cross section taken along line AA of FIG.
- FIG. 3 shows a first mountain-folded
- FIG. 5 is a view for explaining an absorbent article according to an embodiment of the present invention attached to the crotch region of the undergarment.
- FIG. 6 is a schematic cross-sectional view showing a cross section taken along the line BB of FIG.
- FIG. 7 is a figure for demonstrating the manufacturing method of the absorbent article of one Embodiment of this invention.
- FIG. 8 is a view for explaining a recess-forming roll used for producing the absorbent article of one embodiment of the present invention.
- FIG. 9 is a view for explaining a region where a recess is formed by the recess forming roll in the top sheet.
- FIG. 10 is a view for explaining an upper stage roll of a drawing gear roll used for manufacturing an absorbent article according to an embodiment of the present invention.
- FIG. 10 is a view for explaining an upper stage roll of a drawing gear roll used for manufacturing an absorbent article according to an embodiment of the present invention.
- FIG. 11 is a view for explaining a lower roll of a drawing gear roll used for manufacturing an absorbent article according to an embodiment of the present invention.
- FIG. 12 is a view for explaining a top sheet sheet stretched by a stretching gear roll.
- FIG. 13 is an electron micrograph of the skin contact surface of the top sheet in the sanitary napkin in which the top sheet contains avian C2L oil fatty acid glyceride.
- FIG. 14 is a photomicrograph of menstrual blood with or without a blood modifying agent.
- FIG. 15 is a diagram for explaining a method of measuring surface tension.
- FIG. 1 is a partially broken plan view of an absorbent article according to an embodiment of the present invention
- FIG. 2 is a schematic cross-sectional view taken along the line AA of FIG.
- the absorbent article 1 has a liquid-permeable top sheet 2 provided on the skin side (skin contact side), a liquid-impermeable back sheet 3 provided on the clothing side (non-skin contact side), and a top sheet
- a liquid-retaining absorber 4 provided between the second sheet 2 and the back sheet 3 and a main body 10 having a bending suppression sheet 5 provided between the absorber 4 and the back sheet 3; It includes a pair of wing portions 6 provided with a top sheet 2 and a back sheet 3 disposed on both sides of the main body portion 10 so as to extend in the width direction from both side edges.
- symbol 61 shows the root of the wing part 6 (boundary between the main-body part 10 and the wing part 6).
- a straight line connecting two points where the width of the absorbent article 1 suddenly increases on both sides in the longitudinal direction of the wing portion 6 can be regarded as the root 61 of the wing portion 6.
- the surface on the opposite side of the surface on the clothing side in the wing portion 6 is configured by the top sheet 2.
- Adhesive portions 7 are provided on the clothes-side surfaces of the main body portion 10 and the wing portion 6 respectively.
- the width direction of the absorbent article 1 is the X direction
- the longitudinal direction is the Y direction.
- the plane direction of the absorbent article 1 is the XY direction.
- the shape of the main body portion 10 is not particularly limited as long as it is a shape that conforms to the shape of a female body and an underwear, such as a rectangular shape, an oval shape, and an hourglass shape.
- the longitudinal dimension in the outer shape of the main body 10 is preferably 100 to 500 mm, more preferably 150 to 350 mm. Further, the dimension in the width direction of the outer shape of the main body 10 is preferably 30 to 200 mm, more preferably 40 to 180 mm.
- the top sheet 2 moves body fluid such as urine and menstrual blood discharged from the wearer to the absorber 4 in the main body portion 10. Further, in the main body portion 10, at least a part of the top sheet 2 has liquid permeability, and has a large number of openings for transmitting body fluid. On the other hand, in the wing portion 6, the top sheet 2 is suitable for the wearer to bend the wing portion 6, and has a suitable rigidity not to give the wearer discomfort after being attached to the undergarment, as described later. Apply to wing 6 together with 3.
- the top sheet 2 is preferably made of a resin film.
- the resin film used as the top sheet 2 is made of a copolymer of an olefin and an acrylic ester, another monomer such as vinyl acetate, polyolefin, polyester, polypropylene, polyethylene, polyethylene terephthalate, polyamide, cellulose acetate or the like.
- the resin film used as the top sheet 2 is preferably a copolymer of an olefin and another monomer, or a polyolefin, in terms of high flexibility and low irritation to the skin.
- the basis weight of the top sheet 2 is preferably 1 g / m 2 or more and 40 g / m 2 or less, more preferably 10 g / m 2 or more and 35 g / m 2 or less.
- the thickness of the resin film which comprises the top sheet 2 becomes like this. Preferably it is 0.01 mm or more and 0.4 mm or less, More preferably, it is 0.1 mm or more and 0.35 mm or less.
- the thickness of the resin film constituting the top sheet 2 is less than 0.01 mm, the hiding property of the top sheet 2 described later may be too small, and the thickness of the resin film constituting the top sheet 2 is 0 When it exceeds .4 mm, the rigidity of the top sheet 2 may be high, and the stimulation of the top sheet 2 to the wearer's skin may be too strong. Since the top sheet 2 has a first mountain fold portion, a second mountain fold portion and a valley fold portion described later, the apparent thickness of the top sheet 2 is greater than the thickness of the resin film constituting the top sheet 2 large.
- the apparent thickness of the top sheet 2 is preferably 0.01 mm or more and 1 mm or less, more preferably 0.1 mm or more and 0.5 mm or less.
- the top sheet 2 has concealability in order to prevent the body fluid absorbed by the absorber 4 from being seen from the outside.
- the hiding property of the top sheet 2 is produced by mixing a filler such as titanium oxide into the resin.
- the filler is titanium oxide
- the content of titanium oxide is preferably 1% to 50%, more preferably 3% to 15%, based on the weight of the resin film.
- the content of the titanium oxide is less than 1% with respect to the weight of the resin film, the effect of hiding the body fluid absorbed by the absorber 4 in the top sheet 2 may be too small.
- sheet forming of the resin containing titanium oxide may be difficult.
- the top sheet 2 extends in the longitudinal direction, and extends in a direction intersecting the first mountain fold 21 and the valley fold 23 alternately arranged in the width direction, and the first mountain fold 21. And a mountain fold portion 22.
- FIG. 3 is a first mountain-folded portion formed on the region 14 outside the side surfaces 41 in the width direction of the absorber 4 in the top sheet 2 of the main body 10 and the top sheet 2 of the wing 6 (see FIG. 1) It is a figure for demonstrating a 2nd mountain fold part and a valley fold part.
- FIG. 4 is a view for explaining a first mountain fold, a second mountain fold, and a valley fold formed on the central portion 12 of the top sheet 2 of the main body 10 (see FIG. 1).
- the first mountain-folded portion, the second mountain-folded portion and the valley-folded portion formed in the central portion 12 of the top sheet 2 of the main body portion 10 are slightly different as described later.
- the top sheet 2 extends in the longitudinal direction (Y direction) in the region 14 and the wing portion 6 outside the side surfaces 41 in the width direction of the absorber 4 in the width direction (X direction)
- the first mountain fold 21 and the valley fold 23 are alternately arranged.
- the shape of the cross section of the first mountain fold 21 and the valley fold 23 is, for example, a substantially U-shape.
- the substantially U-shape includes, in addition to the U-shape, a shape that becomes a U-shape when it is deformed such as rounding corners or changing a straight line into a curve.
- the substantially U-shape also includes V-shape, M-shape, trapezoid and ⁇ -shape.
- the second mountain folds 22 preferably extend in a direction intersecting the first mountain folds 21 extending in the longitudinal direction (Y direction).
- the angle between the direction in which the first mountain fold 21 extends and the direction in which the second mountain fold 22 extends is preferably 10 ° or more and 170 ° or less.
- the shape of the cross section of the second mountain fold 22 when the second mountain fold 22 is cut in a direction orthogonal to the direction in which the second mountain fold 22 extends is, for example, a substantially U-shape.
- the number of first mountain folds 21 per cm in the width direction is preferably 3 or more, and more preferably 5 or more. If the number of first mountain-folded portions 21 per 1 cm in the width direction is 2 or less, the cushioning properties generated on the top sheet 2 by the first mountain-folded portions 21 may be weakened.
- the length in the longitudinal direction (Y direction) of the second mountain-folded portion 22 is preferably 0.3 mm or more and 5 mm or less, more preferably 0.5 mm or more and 3 mm or less.
- the length in the longitudinal direction (Y direction) of the second mountain fold 22 is smaller than 0.3 mm, the effect of making the first mountain fold 21 in the second mountain fold 22 less likely to collapse is reduced There is.
- the length of the second mountain fold 22 in the longitudinal direction (Y direction) is larger than 5 mm, the cushioning property of the top sheet 2 may be weakened by the first mountain fold 21.
- the absorbent article 1 is fixed to the undergarment by providing the top sheet 2 with the first mountain fold 21 and the second mountain fold 22 at least in the area 14 outside the side surfaces 41 in the width direction of the absorbent body 4.
- the first mountain fold 21 and / or the second mountain fold are present in the vicinity of the fold line 31 when the wing 6 is folded (see FIG. 6).
- the first mountain fold 21 and the second mountain fold 22 impart a soft touch to the surface of the absorbent article 1 by providing the top sheet 2 with cushioning properties. Therefore, when this wears the underwear equipped with the absorbent article 1, the touch around the foot of the underwear becomes good.
- the top sheet 2 of the central portion 12 of the main body 10 is the same as the top sheet 2 of the region 14 outside the side surfaces 41 of the main body 10 in the width direction and the wing 6.
- the first mountain fold 21, the second mountain fold 22 and the valley fold 23 of the top sheet 2 of the central portion 12 of the main body 10 have a blood modifying layer 24 described later on the surface on the skin side. Further include.
- the first mountain fold 21 and the valley fold 23 of the top sheet 2 of the central portion 12 of the main body 10 further include an opening 25 described later.
- the body fluid of the wearer discharged to the top sheet 2 of the area 12 where the absorber 4 of the main body 10 is provided passes through the opening 25 and moves to the absorber 4.
- the body fluid of the wearer discharged to the top sheet 2 quickly gathers toward the bottom of the valley fold portion 23 due to the substantially U shape of the cross section (X direction) cross section formed by the valley fold portion 23 .
- the body fluid flowing toward the bottom of the valley fold 23 passes through the opening 25 provided on the side 26 of the first mountain fold 21 and the valley fold 23 before reaching the valley fold 23.
- the body fluid accumulated in the valley fold portion 23 also passes through the openings 25 provided on the side surfaces 26 of the first mountain fold portion 21 and the valley fold portion 23 and flows to the absorber 4. Therefore, the body fluid of the wearer is quickly absorbed by the absorbent body 4 by the openings 25 provided on the side surfaces 26 of the first mountain fold 21 and the valley fold 23. In addition, the amount of body fluid remaining in the top sheet 2 can be reduced by the openings 25 provided on the side faces 26 of the first mountain fold 21 and the valley fold 23.
- a blood modifying agent may be applied to the skin side surface of the top sheet 2 of the central portion 12 of the main body portion 10 to form the blood modifying layer 24 on the skin contact side surface of the top sheet 2.
- the blood modifying agent layer 24 can prevent the body fluid of the wearer, in particular, highly viscous menstrual blood from remaining on the surface of the top sheet 2.
- the blood modifying agent layer 24 will be described in detail later.
- the top sheet 2 Your skin feels good.
- the contact area between the skin of the wearer and the top sheet 2 is reduced, the touch of the top sheet 2 is improved.
- the second mountain fold portion the first mountain fold portion is less likely to be crushed, so the first mountain fold portion, the second mountain fold portion is formed on the top sheet 2 of the central portion 12 of the main body portion 10. It is preferable to provide a section and a valley fold.
- the back sheet 3 shown in FIGS. 1 and 2 prevents the body fluid absorbed by the absorber 4 from leaking out.
- the back sheet 3 is made of a material that is impermeable to body fluid.
- a hydrophobic non-woven fabric an impermeable plastic film such as polyethylene and polypropylene or a laminate sheet of non-woven fabric and an impermeable plastic film, or the like is used.
- a spunbond-meltblown-spunbond (SMS) fibrous nonwoven fabric may be used as the back sheet 3 in which a highly water-resistant meltblown nonwoven fabric is sandwiched by strong spunbond nonwoven fabrics.
- SMS spunbond-meltblown-spunbond
- the absorber 4 absorbs and holds the body fluid. It is preferable that the absorber 4 is bulky, hard to lose its shape, and less in chemical stimulation.
- a composite absorbent made of fluff pulp or air-laid non-woven fabric and super absorbent polymer (SAP) is used as the absorbent 4.
- SAP super absorbent polymer
- the composite absorber may be covered with a liquid permeable material such as a tissue.
- artificial cellulose fibers such as chemical pulp, cellulose fibers, rayon and acetate may be used.
- the basis weight of the absorbent fiber such as pulp in the composite absorbent is preferably 100 g / m 2 or more and 800 g / m 2 or less, and the mass ratio of the superabsorbent polymer in the composite absorbent is the absorbency
- the fiber content is preferably 100% to 10% to 65%.
- the basis weight of a liquid-permeable material such as a tissue covering the composite mixture is preferably 12 g / m 2 or more and 30 g / m 2 or less.
- the air-laid non-woven fabric of the composite mixture for example, a non-woven fabric in which pulp and synthetic fibers are heat-sealed or a non-woven fabric in which pulp and synthetic fibers are fixed with a binder can be used.
- the superabsorbent polymer of the composite absorbent has a three-dimensional network structure in which a water-soluble polymer is appropriately crosslinked.
- the absorbent polymer absorbs 30 to 60 times as much water as the volume of absorbent polymer before absorbing water.
- this absorbable polymer is essentially water insoluble.
- the absorbent polymer does not release the water once absorbed, even if some pressure is applied.
- this absorbent polymer for example, starch-based, acrylic acid-based or amino acid-based particulate or fibrous polymers are used.
- the shape and structure of the absorber 4 can be changed as needed, but the total amount of absorption of the absorber 4 needs to correspond to the designed insertion amount as the absorbent article 1 and the desired application.
- the size, absorption capacity and the like of the absorber 4 change depending on the application.
- Absorber 4 is bonded to top sheet 2 using a hot melt adhesive. Thereby, it can suppress that the top sheet 2 peels from the absorber 4. As shown in FIG.
- the folding prevention sheet 5 folds the wing portion 6 and attaches the absorbent article 1 to the underwear.
- the portion of the side surface 41 (see FIG. 1) of the absorbent body 4 is prevented from hitting the inner crotch of the wearer.
- the folding suppression sheet 5 folds the wing portion 6 to make the absorbent article 1 underwear.
- seat 5 is located in the width direction outer side compared with the side 41 of the width direction of the absorber 4. As shown in FIG. Thereby, when the wing 6 is folded and the absorbent article 1 is attached to the undergarment, it is possible to suppress the wing 6 from being bent so that the portion of the side surface 41 of the absorber 4 has a fold.
- the sheet used as the music suppression sheet 5 is not particularly limited. However, if the rigidity of the absorbent folded sheet 5 is too high, the rigidity of the entire absorbent article 1 becomes too high, and the absorbent article 1 may cause a strong discomfort to the wearer. Therefore, it is preferable that the absorber folded sheet 5 have a rigidity of such a height that a strong discomfort does not occur even when worn by the wearer.
- the bending resistance measured by the cantilever method (JIS L 1096) in the bending suppression sheet 5 is preferably 15 to 120 mm, more preferably 30 to 70 mm.
- seat 5 When the bending resistance by the cantilever method of the bending suppression sheet
- seat 5 is smaller than 15 mm, it may be unable to suppress that the wing part 6 bends so that the part of the side 41 of the absorber 4 may become a crease. That is, when the wearer folds the wing portion 6 in order to attach the absorbent article to the undergarment, there may be a case where the bending suppression sheet 5 is bent at the side 41 of the absorber 4 together with the top sheet 2 and the back sheet 3 is there.
- the sheet used as the bending suppression sheet 5 is preferably a resin film, a perforated resin film, a woven fabric or a non-woven fabric, and more preferably a non-woven fabric.
- a non-woven fabric suitable for the bending suppression sheet 5 is, for example, a non-woven fabric of chemical fibers such as thermoplastic hydrophobic chemical fibers.
- Thermoplastic hydrophobic chemical fibers suitable for the bending suppression sheet 5 include, for example, single fibers such as polyethylene (PE), polypropylene (PP) and polyethylene terephthalate (PET), composite fibers obtained by graft polymerizing PE and PP, There is a composite fiber having a core-sheath structure.
- the web When producing a non-woven fabric used for the bending suppression sheet 5, the web may be formed by a dry method such as a card method, a spun bond method, a meltblown method, an air laid method or a wet method. Moreover, when producing the nonwoven fabric used for the bending suppression sheet 5, you may perform bonding, such as thermal bonding, a needle punch, chemical bonding. For example, an SMS fiber non-woven fabric can be used for the bending suppression sheet 5.
- a dry method such as a card method, a spun bond method, a meltblown method, an air laid method or a wet method.
- bonding such as thermal bonding, a needle punch, chemical bonding.
- an SMS fiber non-woven fabric can be used for the bending suppression sheet 5.
- the basis weight of the non-woven fabric used as the bending suppression sheet 5 is preferably 10 to 40 g / m 2 , more preferably 13 to 20 g / m 2 . If the basis weight of the non-woven fabric is smaller than 10 g / m 2, it may not be possible to suppress bending of the wing portion 6 such that the portion of the side surface 41 of the absorber 4 has a fold. That is, when the wearer folds the wing portion 6 in order to attach the absorbent article to the undergarment, there may be a case where the bending suppression sheet 5 is bent at the side 41 of the absorber 4 together with the top sheet 2 and the back sheet 3 is there. Moreover, when the fabric weight of the nonwoven fabric is larger than 40 g / m 2 , the rigidity of the absorbent article 1 becomes too high, and the absorbent article 1 may cause a strong discomfort to the wearer.
- the sheet used as the bending suppression sheet 5 is preferably a sheet having hydrophobicity or water repellency. Thereby, it can suppress that the bodily fluid which exuded from absorber 4 leaks from the part of the cross direction of absorptive article 1 through bending control sheet 5.
- the bending suppression sheet 5 is joined to the absorber 4 and the back sheet 3 by an adhesive such as a hot melt adhesive. However, the bending suppression sheet 5 is not joined to the top sheet 2. Thereby, the top sheet 2 can have a soft touch in the area
- FIG. 5 is a view for explaining an absorbent article according to an embodiment of the present invention attached to the crotch region of the undergarment.
- FIG. 6 is a schematic cross-sectional view showing a cross section taken along the line BB of FIG. Reference numeral 70 in FIGS.
- FIGS. 5 and 6 denotes the crotch area of the undergarment
- reference numeral 71 denotes the edge of the foot opening of the undergarment. From FIGS. 5 and 6, it can be seen that the width of the crotch region 70 of the wearer's underwear is narrower than the width of the absorbent body 4 provided in the absorbent article 1.
- the wing portion 6 Since the absorbent article 1 is difficult to bend in the area where the folding prevention sheet 5 is provided, when the wearer folds the wing portion 6 and attaches the underwear to the crotch region 70 of the underwear, the wing portion 6 is The folds 31 of the absorbent article 1 produced when folded are substantially coincident with the edge 51 in the width direction of the anti-folding sheet 5. Therefore, as shown in FIG. 5 and FIG. 6, even if the position of the edge 71 of the foot opening of the undergarment overlaps with the position of the absorber 4, when the wing 6 is folded, the side 41 of the absorber 4 absorbs It is suppressed that the sex article 1 bends.
- the thickness of the bending suppression sheet 5 is smaller than the thickness of the absorber 4. Therefore, the sense of incongruity which arises when the edge 51 in the width direction of the bending suppression sheet 5 hits the wearer's inner crotch is weaker than the sense of incongruity which occurs when the portion of the side surface 41 of the absorber 4 hits the wearer's crotch. Further, as described above, the top sheet 2 is provided with the first mountain fold portion 21.
- the first mountain fold 21 acts as a cushion, and the edge 51 in the width direction of the anti-folding sheet 5 is the wearer's It can further reduce the sense of incongruity that occurs when hitting the inner crotch. That is, the first mountain fold 21 imparts cushioning properties to the top sheet 2, and further reduces the discomfort caused when the edge 51 in the width direction of the bending suppression sheet 5 hits the inner crotch of the wearer.
- the protrusion formed on the top sheet 2 is the above-described first mountain-folded portion 21 And it is not limited to the 2nd mountain fold part.
- a cylindrical protrusion, a prismatic protrusion, or the like may be formed on the top sheet 2.
- the wing portion 6 is provided on the absorbent article 1 in order to stably fix the absorbent article 1 to the undergarment.
- the absorbent article 1 can be stably fixed to the undergarment by bending the wing portion 6 to the outer surface side of the undergarment and then sticking it to the crotch region of the undergarment through the adhesive portion 7.
- the adhesive portion 7 is also provided to the main body portion 10, and the main body portion 10 is prevented from shifting and moving in the crotch region of the undergarment.
- the adhesive unit 7 fixes the absorbent article 1 to the crotch area of the undergarment.
- an adhesive which forms the adhesion part 7 what is a styrene-type polymer, a tackifier, and a plasticizer as a main component, for example is used suitably.
- the styrene-based polymer include styrene-ethylene-butylene-styrene block copolymer, styrene-butylene polymer, styrene-butylene-styrene block copolymer, and styrene-isobutylene-styrene copolymer. Or a blend of two or more polymers.
- styrene-ethylene-butylene-styrene block copolymer is preferable in that the thermal stability is good.
- tackifier and the plasticizer those which are solid at normal temperature can be preferably used, and as the tackifier, for example, C5 petroleum resin, C9 petroleum resin, dicyclopentadiene petroleum resin, rosin petroleum resin And polyterpene resins, terpene phenol resins, etc.
- the plasticizer include, in addition to monomer plasticizers such as triflecil phosphate, dibutyl phthalate and dioctyl phthalate, polymer plasticizers such as vinyl polymers and polyesters.
- the top sheet 2 and the absorbent body 4 have compressed grooves 8 extending from the top sheet 2 to the inside of the absorbent body 4 which are formed by being compressed in the thickness direction by embossing.
- the squeeze groove 8 suppresses the diffusion of the body fluid discharged to the central portion of the absorbent article 1 (the portion in contact with the excretory port of the wearer's body fluid) in the width direction (X direction). Moreover, it can suppress that the top sheet 2 peels from the absorber 4 by this.
- the squeeze groove 8 surrounds the central portion of the absorbent article 1 and has a continuous, substantially annular shape.
- the pressing groove 8 surrounding the center part of the absorbent article 1 may be partially disconnected. That is, the pressing groove 8 may have a discontinuous and substantially annular shape.
- the compression bonding of the top sheet 2 to the back sheet 3 by heat embossing forms the seal portions 9 on both sides in the longitudinal direction and both sides in the width direction of the absorbent article 1. Thereby, the top sheet 2 can be prevented from peeling off from the back sheet 3.
- the blood modifying agent of the blood modifying agent layer 24 is a blood modifying agent having an IOB of about 0.00 to about 0.60, a melting point of about 45.degree. C. or less, 100 g of water at 25.degree. It has a water solubility of 00 to about 0.00 to about 0.05 g.
- IOB Inorganic Organic Balance
- IOB value calculated by inorganic value / organic value.
- the IOB is about 0.00 to about 0.60, preferably about 0.00 to about 0.50, and about 0.00 to about 0.40. More preferred is about 0 to about 0.30. This is because the lower the IOB, the higher the organicity and the higher the affinity to blood cells.
- the "melting point” means the peak top temperature of an endothermic peak when changing from solid state to liquid state when measured at a temperature rising rate of 10 ° C./min in a differential scanning calorimeter.
- the melting point can be measured, for example, using a DSC-60 type DSC measurement apparatus manufactured by Shimadzu Corporation.
- the blood modifying agent may be liquid or solid at room temperature as long as it has a melting point of about 45 ° C. or less, ie, even if the melting point is about 25 ° C. or more, or less than about 25 ° C. It may well have a melting point such as, for example, about -5.degree. C., about -20.degree. The reason why the melting point of the blood modifying agent is about 45 ° C. or less will be described later.
- the vapor pressure of the blood modifying agent is preferably about 0.00 to about 0.01 Pa at 1 atm and 25 ° C., more preferably about 0.000 to about 0.001 Pa, and about 0 More preferably, it is from .0000 to about 0.0001 Pa.
- the vapor pressure is preferably about 0.00 to about 0.01 Pa at 1 atm and 40 ° C., and about 0.000 to about 0.01 More preferably, it is about 0.001 Pa, and more preferably, about 0.0000 to about 0.0001 Pa. If the vapor pressure is high, it may be vaporized during storage, which may cause problems such as a decrease in the amount of blood modifying agent and an odor when worn.
- the melting point of the blood modifying agent can be properly used depending on the weather, the length of wearing time, and the like. For example, in areas where the average temperature is less than about 10 ° C, menstrual blood may be excreted and then cooled by the ambient temperature by employing a blood modifying agent having a melting point of less than about 10 ° C. It is believed that blood modifying agents can stably modify blood.
- the melting point of the blood modifying agent is preferably higher in the range of 45 ° C. or less. It is because it is hard to be affected by sweat, friction at the time of wearing, etc., and it is difficult for the blood modifying agent to move even when worn for a long time.
- a water solubility of 0.00 to 0.05 g For a water solubility of 0.00 to 0.05 g, add a 0.05 g sample to 100 g deionized water at 25 ° C., allow to stand for 24 hours, and after 24 hours, lightly stir as needed, Then, it can be measured by visually evaluating whether the sample has dissolved.
- dissolving includes cases where the sample is completely dissolved in deionized water to form a homogeneous mixture and cases where the sample is completely emulsified. "Complete” means that there is no clump of sample in deionized water.
- the surface of the top sheet is coated with a surfactant for the purpose of changing blood surface tension and the like to rapidly absorb the blood.
- the surfactant generally has high water solubility
- the surfactant-coated top sheet is compatible with hydrophilic components (such as plasma) in the blood, rather the blood remains on the top sheet.
- hydrophilic components such as plasma
- solubility in 100 g of water at 25 ° C. may be simply referred to as “water solubility”.
- the weight average molecular weight means a value in terms of polystyrene, which is determined by gel permeation chromatography (GPC).
- GPC measurement conditions include the following. Model: High-performance liquid chromatogram Lachrom Elite manufactured by Hitachi High-Technologies Corporation Column: Showa Denko KK SHODEX KF-801, KF-803 and KF-804 Eluent: THF Flow rate: 1.0 mL / min Implanted volume: 100 ⁇ L Detection: RI (differential refractometer)
- the weight average molecular weight described in the Example of this specification is measured based on the said conditions.
- the above blood modifying agent is preferably selected from the following (i) to (iii), (I) Hydrocarbons, (Ii) from a carbonyl group (-CO-) and an oxy group (-O-) inserted between (ii-1) a hydrocarbon moiety and (ii-2) a C-C single bond of the above-mentioned hydrocarbon moiety
- hydrocarbon means a compound consisting of carbon and hydrogen, and is a chain hydrocarbon, for example, paraffinic hydrocarbon (also referred to as alkane not containing double bond and triple bond) Olefinic hydrocarbons (containing one double bond, also called alkenes), acetylenic hydrocarbons (containing one triple bond, also called alkynes), and a group consisting of double bonds and triple bonds And hydrocarbons containing two or more bonds selected from, as well as cyclic hydrocarbons such as aromatic hydrocarbons and alicyclic hydrocarbons.
- paraffinic hydrocarbon also referred to as alkane not containing double bond and triple bond
- Olefinic hydrocarbons containing one double bond, also called alkenes
- acetylenic hydrocarbons containing one triple bond, also called alkynes
- hydrocarbons containing two or more bonds selected from, as well as cyclic hydrocarbons such as aromatic hydrocarbons and alicyclic hydrocarbons.
- the hydrocarbon is preferably a chain hydrocarbon and an alicyclic hydrocarbon, more preferably a chain hydrocarbon, a paraffin hydrocarbon, an olefin hydrocarbon and two double bonds. It is more preferable that it is the hydrocarbon (it does not contain a triple bond) which contains above, and it is still more preferable that it is a paraffinic hydrocarbon.
- the chained hydrocarbons include straight chained hydrocarbons and branched chained hydrocarbons.
- each oxy group (—O—) is not adjacent. Accordingly, the compounds (ii) and (iii) do not include compounds in which the oxy group is continuous (so-called peroxides).
- At least one hydrogen atom of the hydrocarbon moiety is a hydroxyl group (-) rather than a compound in which at least one hydrogen atom of the hydrocarbon moiety is substituted with a carboxyl group (-COOH).
- Compounds substituted with OH) are preferred.
- Table 1 since the carboxyl group binds to metals and the like in blood, and the inorganic value greatly increases from 150 to 400 or more, the blood modifying agent having a carboxyl group is used at the time of use This is because the IOB value may exceed about 0.60 and the affinity to blood cells may be reduced.
- the above blood modifying agent comprises the following (i ') to (iii'), (I ') hydrocarbons, (Ii ') (ii'-1) a hydrocarbon moiety, and (ii'-2) a carbonyl bond (-CO-), an ester bond (-COO) inserted between a C-C single bond of the above-mentioned hydrocarbon moiety -), A compound having one or more same or different bonds selected from the group consisting of carbonate bond (-OCOO-), and ether bond (-O-), and (iii ') (iii'-) 1) Carbonyl bond (-CO-), ester bond (-COO-), carbonate bond (-OCOO) inserted between the hydrocarbon moiety and the C-C single bond of the above-mentioned hydrocarbon moiety (iii'-2) A carboxyl group (-), and one or more, same or different bond selected from the group consisting of an ether bond (-O-) and (iii'
- the blood modifying agent has about 1.8 or less carbonyl bonds (-CO-) and 2 or less ester bonds (-COO-) per 10 carbon atoms in the hydrocarbon moiety. About 1.5 or less carbonate bond (-OCOO-), about 6 or less ether bond (-O-), about 0.8 or less carboxyl group (-COOH), and / or hydroxyl group (-OH) Or a compound having about 1.2 or less).
- the above blood modifying agent is any of the following (A) to (F), (A) A compound having (A1) a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety, (A2) a chain hydrocarbon moiety, and the above chain Ester with a compound having one carboxyl group replacing the hydrogen atom of the cyclic hydrocarbon moiety, (B) A compound having (B1) a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, and the above chain Ether with a compound having one hydroxyl group replacing the hydrogen atom of the cyclic hydrocarbon moiety, (C) a carboxylic acid, a hydroxy acid, an alkoxy acid or an oxo acid containing a (C1) linear hydrocarbon moiety and 2 to 4 carboxyl groups replacing the hydrogen atom of the linear hydrocarbon moiety; C2) an ester of a compound having a chain hydrocarbon moiety
- a compound having (A1) a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety, (A2) a chain hydrocarbon moiety, and the above chain Ester with a compound having one carboxyl group replacing hydrogen atoms in the cyclic hydrocarbon moiety (hereinafter sometimes referred to as “compound (A)”) has the above-mentioned IOB, melting point and water solubility And all hydroxyl groups may not be esterified.
- (A1) a compound having a chain hydrocarbon portion and 2 to 4 hydroxyl groups replacing the hydrogen atom of the above chain hydrocarbon portion (hereinafter sometimes referred to as “compound (A1)”)
- chain hydrocarbon tetraols such as alkanetetraols such as pentaerythritol
- chain hydrocarbon triols such as alkanetriols such as glycerin
- chain hydrocarbon diols such as alkane diols such as Glycol is mentioned.
- Examples of (A2) a compound having a chain hydrocarbon portion and one carboxyl group replacing the hydrogen atom of the chain hydrocarbon portion include, for example, And compounds in which one hydrogen atom on a hydrocarbon is substituted with one carboxyl group (—COOH), such as fatty acid.
- Examples of the compound (A) include an ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid, an ester of (a 2 ) chain hydrocarbon triol and at least one fatty acid, and (a 3 And esters of linear hydrocarbon diols and at least one fatty acid.
- the ester of the above linear hydrocarbon tetraol and at least one fatty acid may be, for example, the following formula (1): Tetraester of pentaerythritol with fatty acid, the following formula (2): Triester of pentaerythritol with fatty acid, the following formula (3): A diester of pentaerythritol with fatty acid, the following formula (4): And monoesters of fatty acid with pentaerythritol. (Wherein, R 1 to R 4 are each a chain hydrocarbon)
- esters of pentaerythritol and fatty acids have the above IOB, melting point and water solubility
- saturated fatty acids such as C 2 to C 30 saturated fatty acids, for example, acetic acid (C 2 ) (C 2 represents a carbon number, R 1 C, R 2 C, R 3 C or R 4 C, which corresponds to the carbon number of R 2 C, hereinafter the same), propanoic acid (C 3 ), butanoic acid (C 4 ) and isomers thereof, for example, 2-methylpropanoic acid (C 4) ), pentanoic acid (C 5) and isomers thereof such as 2-methylbutanoic acid (C 5), 2,2-dimethyl propanoic acid (C 5), hexanoic acid (C 6), heptanoic acid (C 7) Oct
- the fatty acids can also be unsaturated fatty acids.
- unsaturated fatty acids include C 3 -C 20 unsaturated fatty acids such as monounsaturated fatty acids such as crotonic acid (C 4 ), myristoleic acid (C 14 ), palmitoleic acid (C 16 ), Oleic acid (C 18 ), elaidic acid (C 18 ), vacenic acid (C 18 ), gadeuric acid (C 20 ), eicosenic acid (C 20 ), etc., diunsaturated fatty acids such as linoleic acid (C 18 ), Eicosadienoic acid (C 20 ) and the like, triunsaturated fatty acids such as linolenic acid, for example ⁇ -linolenic acid (C 18 ) and ⁇ -linolenic acid (C 18 ), pinolenic acid (C 18 ), eleostearic acid, For example, ⁇ -eleostearic acid (C 18
- the ester of pentaerythritol and fatty acid is an ester of pentaerythritol and fatty acid derived from saturated fatty acid, that is, an ester of pentaerythritol and saturated fatty acid, in consideration of the possibility of modification by oxidation etc. preferable.
- the ester of pentaerythritol and a fatty acid in order to make IOB small and make it more hydrophobic, it is preferable to be a diester, a triester or a tetraester, and a triester or a tetraester is more preferable. And tetra-esters are more preferred.
- the total carbon number of fatty acids constituting the tetraester of pentaerythritol and fatty acid that is, in the above formula (1), R 1 C, R 2 C, R 3 C and When the total number of carbons in the R 4 C portion is 15, the IOB is 0.60. Therefore, in the case of tetraester of pentaerythritol and fatty acid, IOB satisfies the requirement of about 0.00 to about 0.60 when the total number of carbons is about 15 or more.
- Examples of the tetraester of pentaerythritol and fatty acid include pentaerythritol, hexanoic acid (C 6 ), heptanoic acid (C 7 ), octanoic acid (C 8 ), for example, 2-ethylhexanoic acid (C 8 ), These include tetraesters with nonanoic acid (C 9 ), decanoic acid (C 10 ) and / or dodecanoic acid (C 12 ).
- the total carbon number of the fatty acid constituting the triester of pentaerythritol and fatty acid that is, the R 1 C, R 2 C and R 3 C moieties in the above formula (2)
- the IOB is 0.58 when the sum of the carbon numbers of these is 19. Therefore, in the case of the triester of pentaerythritol and fatty acid, IOB satisfies the requirement of about 0.00 to about 0.60 when the total carbon number of fatty acid is about 19 or more.
- the total carbon number of fatty acids constituting the diester of pentaerythritol and fatty acid that is, the total carbon number of R 1 C and R 2 C in the above formula (3) is In the case of 22, the IOB is 0.59. Accordingly, in the diester of pentaerythritol and fatty acid, IOB satisfies the requirement of about 0.00 to about 0.60 when the total carbon number of fatty acid is about 22 or more.
- esters of pentaerythritol and fatty acid examples include Unistar H-408 BRS, H-2408 BRS-22 (mixed product), etc. (all manufactured by NOF Corporation).
- the ester of the above linear hydrocarbon triol and at least one fatty acid may be, for example, the following formula (5): Triester of glycerin and fatty acid, the following formula (6): A diester of glycerin and a fatty acid, and the following formula (7): (Wherein, each of R 5 to R 7 is a chain hydrocarbon) And monoesters of glycerin and fatty acids.
- fatty acids (R 5 COOH, R 6 COOH and R 7 COOH) constituting the ester of glycerin and fatty acid if the ester of glycerin and fatty acid satisfies the requirements of the above IOB, melting point and water solubility
- the fatty acids listed in the “ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid” are not particularly limited, and examples thereof include saturated fatty acids and unsaturated fatty acids, which are modified by oxidation etc. In consideration of the possibility of doing so, an ester of glycerin and a fatty acid derived from a saturated fatty acid, that is, an ester of glycerin and a saturated fatty acid is preferable.
- ester of glycerol and a fatty acid in order to make IOB small and to make it more hydrophobic, it is preferable that it is diester or triester, and it is more preferable that it is triester.
- triester of glycerin and fatty acid is also referred to as triglyceride, for example, triester of glycerin and octanoic acid (C 8 ), triester of glycerin and decanoic acid (C 10 ), glycerin and dodecanoic acid (C 12 And triesters of glycerin with two or three fatty acids, and mixtures thereof.
- Examples of the triester of the above glycerin and two or more fatty acids include triester of glycerin and octanoic acid (C 8 ) and decanoic acid (C 10 ), glycerin, octanoic acid (C 8 ), decane Acid (C 10 ) and triester with dodecanoic acid (C 12 ), glycerin and octanoic acid (C 8 ), decanoic acid (C 10 ), dodecanoic acid (C 12 ), tetradecanoic acid (C 14 ), hexadecanoic acid Examples thereof include triesters with (C 16 ) and octadecanoic acid (C 18 ).
- the total carbon number of fatty acids constituting the triester of glycerin and fatty acid ie, R 5 C in the formula (5)
- the sum of the carbon numbers of the R 6 C and R 7 C moieties is about 40 or less.
- the total carbon number of fatty acids constituting the triester of glycerin and fatty acid that is, in the formula (5), the R 5 C, R 6 C and R 7 C moieties
- the IOB is 0.60. Therefore, in the above-mentioned triesters of glycerin and fatty acid, the IOB satisfies the requirement of about 0.00 to about 0.60 when the total carbon number of fatty acid is about 12 or more.
- the above-mentioned triester of glycerin and a fatty acid is a so-called fat and is a component that can constitute the human body, and thus is preferable from the viewpoint of safety.
- tricotic oil fatty acid glyceride NA36
- PANACET 800 PANACET 800B
- PANACET 810S avian C2L oil fatty acid glyceride and triCL oil fatty acid glyceride (manufactured by NOF CORPORATION) Etc.).
- the above-mentioned diester of glycerin and fatty acid is also referred to as diglyceride, for example, a diester of glycerin and decanoic acid (C 10 ), a diester of glycerin and dodecanoic acid (C 12 ), and glycerin and hexadecanoic acid (C 16 ) Included are diesters and diesters of glycerin with two fatty acids, and mixtures thereof.
- the total carbon number of fatty acids constituting the diester of glycerin and fatty acid ie, the case where the total carbon number of R 5 C and R 6 C moieties in the formula (6) is 16
- the IOB is 0.58. Therefore, in the case of the diester of glycerin and fatty acid, the IOB satisfies the requirement of about 0.00 to about 0.60 when the total carbon number of fatty acid is about 16 or more.
- the monoester of glycerin and fatty acid is also referred to as monoglyceride, and examples thereof include icosanoic acid (C 20 ) monoester of glycerin, docosanoic acid (C 22 ) monoester of glycerin and the like.
- the carbon number of fatty acid constituting the monoester of glycerin and fatty acid that is, in the formula (7), the IOB is 0.59 when the carbon number of the R 5 C portion is 19 It becomes. Therefore, in the monoester of glycerin and fatty acid, the IOB satisfies the requirement of about 0.00 to about 0.60 when the carbon number of the fatty acid is about 19 or more.
- esters of the above linear hydrocarbon diol and at least one fatty acid include C 2 to C 6 linear hydrocarbon diols, such as C 2 to C 6 glycols, such as ethylene glycol, propylene glycol, butylene And mono- or diesters of glycol, pentylene glycol or hexylene glycol with fatty acids.
- ester of the above linear hydrocarbon diol and at least one fatty acid for example, the following formula (8): R 8 COOC k H 2k OCOR 9 (8) (Wherein k is an integer of 2 to 6 and R 8 and R 9 are each a chain hydrocarbon) A diester of a C 2 -C 6 glycol with a fatty acid, and the following formula (9): R 8 COOC k H 2k OH (9) (Wherein k is an integer of 2 to 6 and R 8 is a chain hydrocarbon) And monoesters of fatty acid with C 2 -C 6 glycol.
- ester of C 2 to C 6 glycol and fatty acid as the fatty acid to be esterified (corresponding to R 8 COOH and R 9 COOH in the formula (8) and the formula (9)), C 2 to C 6 glycol
- ester of fatty acid with the fatty acid provided that it satisfies the requirements of the above IOB, melting point and water solubility, for example, "ester of (a 1 ) chain hydrocarbon tetraol with at least one fatty acid"
- the ester of a C 2 ⁇ C 6 glycols and fatty acid in view of the potential for degradation by oxidation and the like, derived from saturated fatty acids, esters of C 2 ⁇ C 6 glycols and fatty acid, Nachi Suwa, C 2 It is preferably an ester of a -C 6 glycol and a saturated fatty acid.
- ester of C 2 -C 6 glycol and fatty acid an ester of glycol and fatty acid derived from glycol having a large number of carbon atoms, for example, butylene glycol, in order to make IOB small and make it more hydrophobic. It is preferable that it is an ester of a glycol derived from pentylene glycol or hexylene glycol and a fatty acid.
- ester of C 2 -C 6 glycol and fatty acid a diester is preferable in order to make IOB small and to make it more hydrophobic.
- examples of commercial products of the ester of C 2 -C 6 glycol and fatty acid include Commol BL, Commol BS (manufactured by NOF Corporation) and the like.
- (B) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, and Ether with compound having one hydroxyl group replacing hydrogen atom of linear hydrocarbon moiety
- (B) A compound having (B1) a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, and the above chain Ether with a compound having one hydroxyl group replacing hydrogen atoms in the cyclic hydrocarbon moiety (hereinafter sometimes referred to as “compound (B)”) has the above-mentioned IOB, melting point and water solubility And all hydroxyl groups may not be etherified.
- Examples of the compound having (B1) a chain hydrocarbon portion and 2 to 4 hydroxyl groups replacing the hydrogen atoms of the above chain hydrocarbon portion are listed as the compound (A1) in the “compound (A)”.
- Examples of (B2) a compound having a linear hydrocarbon moiety and one hydroxyl group replacing the hydrogen atom of the linear hydrocarbon moiety include, for example, A compound in which one hydrogen atom of hydrocarbon is substituted with one hydroxyl group (—OH), for example, aliphatic monohydric alcohol such as saturated aliphatic monohydric alcohol and unsaturated aliphatic monohydric alcohol Can be mentioned.
- saturated aliphatic monohydric alcohol for example, a C 1 to C 20 saturated aliphatic monohydric alcohol, for example, methyl alcohol (C 1 ) (C 1 represents a carbon number, the same applies hereinafter), ethyl alcohol C 2 ), propyl alcohol (C 3 ) and its isomers, such as isopropyl alcohol (C 3 ), butyl alcohol (C 4 ) and its isomers, such as sec-butyl alcohol (C 4 ) and tert-butyl alcohol (C 4 ), pentyl alcohol (C 5 ), hexyl alcohol (C 6 ), heptyl alcohol (C 7 ), octyl alcohol (C 8 ) and isomers thereof, such as 2-ethylhexyl alcohol (C 8 ), nonyl alcohol (C 9 ), decyl alcohol (C 10 ), dodecyl alcohol (C 12 ), tetradecyl al Call (C 14), hexadect
- an ether of (b 1 ) chain hydrocarbon tetraol and at least one aliphatic monohydric alcohol for example, monoether, diether, triether and tetraether, preferably diether, triether Ethers and tetraethers, more preferably triethers and tetraethers, and even more preferably tetraethers, ethers of (b 2 ) chain hydrocarbon triols and at least one aliphatic monohydric alcohol, such as monoethers, diethers and the like triether, preferably diethers and triethers and more preferably tri-ether, and (b 3) a chain hydrocarbon diol and at least one aliphatic monohydric ether alcohols, for example, mono- and diethers and, Mashiku diethers.
- Examples of the ether of the above linear hydrocarbon tetraol and at least one aliphatic monohydric alcohol include the following formulas (10) to (13): (Wherein, each of R 10 to R 13 is a chain hydrocarbon). And tetraethers of pentaerythritol and aliphatic monohydric alcohols, triethers, diethers and monoethers.
- Examples of the ether of the above linear hydrocarbon triol and at least one aliphatic monohydric alcohol include the following formulas (14) to (16): (Wherein, R 14 to R 16 are each a chain hydrocarbon). And triethers of glycerol and aliphatic monohydric alcohols, diethers and monoethers.
- R 17 OC n H 2n OR 18 (Wherein n is an integer of 2 to 6 and R 17 and R 18 are each a chain hydrocarbon) Diethers of C 2 -C 6 glycols and aliphatic monohydric alcohols, and the following formula (18): R 17 OC n H 2n OH (18) (Wherein n is an integer of 2 to 6 and R 17 is a chain hydrocarbon) And monoethers of C 2 -C 6 glycols and aliphatic monohydric alcohols.
- the total carbon number of aliphatic monohydric alcohol constituting the tetraether of pentaerythritol and aliphatic monohydric alcohol that is, in the above formula (10)
- the IOB is 0.44. Therefore, in the above tetraether of pentaerythritol and aliphatic monohydric alcohol, IOB is required to be about 0.00 to about 0.60 when the total carbon number of aliphatic monohydric alcohol is about 4 or more. Fulfill.
- the total carbon number of aliphatic monohydric alcohol constituting triether of pentaerythritol and aliphatic monohydric alcohol ie, in the above formula (11)
- the IOB is 0.57. Therefore, in the above triether of pentaerythritol and aliphatic monohydric alcohol, IOB is required to be about 0.00 to about 0.60 when the total carbon number of aliphatic monohydric alcohol is about 9 or more. Fulfill.
- the total carbon number of aliphatic monohydric alcohols constituting the diether of pentaerythritol and aliphatic monohydric alcohol that is, R 10 in the above formula (12)
- the IOB is 0.60 when the sum of the carbon numbers of the and R 11 moieties is 15. Therefore, in the diether of pentaerythritol and aliphatic monohydric alcohol, IOB satisfies the requirement of about 0.00 to about 0.60 when the total carbon number of aliphatic monohydric alcohol is about 15 or more. .
- the carbon number of the aliphatic monohydric alcohol constituting the monoether of pentaerythritol and aliphatic monohydric alcohol that is, R 10 in the above formula (13)
- the IOB is 0.59. Therefore, in the monoether of pentaerythritol and aliphatic monohydric alcohol, the IOB satisfies the requirement of about 0.00 to about 0.60 when the carbon number of the aliphatic monohydric alcohol is about 22 or more.
- the total carbon number of aliphatic monohydric alcohol constituting triether of glycerin and aliphatic monohydric alcohol that is, R in the formula (14)
- the IOB is 0.50. Therefore, in the above triether of glycerin and aliphatic monohydric alcohol, IOB satisfies the requirement of about 0.00 to about 0.60 when the total carbon number of aliphatic monohydric alcohol is about 3 or more. .
- the total carbon number of aliphatic monohydric alcohols constituting the diether of glycerin and an aliphatic monohydric alcohol that is, in the formula (15), R 14 and R 15
- the IOB is 0.58. Therefore, in the diether of glycerin and an aliphatic monohydric alcohol, the IOB satisfies the requirement of about 0.00 to about 0.60 when the total carbon number of the aliphatic monohydric alcohol is about 9 or more.
- the carbon number of the aliphatic monohydric alcohol constituting the monoether of glycerin and aliphatic monohydric alcohol that is, the carbon of R 14 in the formula (16)
- the IOB is 0.58. Therefore, in the monoether of glycerin and aliphatic monohydric alcohol, the IOB satisfies the requirement of about 0.00 to about 0.60 when the carbon number of the aliphatic monohydric alcohol is about 16 or more.
- the compound (B) can be produced by dehydration condensation of the compound (B1) and the compound (B2) in the presence of an acid catalyst.
- compound C1 a linear hydrocarbon moiety and a carboxylic acid, a hydroxy acid, an alkoxy acid or an oxo acid containing 2 to 4 carboxyl groups replacing the hydrogen atom of the linear hydrocarbon moiety
- compound C1 may be, for example, a linear hydrocarbon carboxylic acid having 2 to 4 carboxyl groups, such as a linear hydrocarbon dicarboxylic acid, such as an alkane dicarboxylic acid, such as ethanedioic acid.
- a linear hydrocarbon hydroxy acid having 2 to 4 carboxyl groups for example, a linear chain having 2 to 4 carboxyl groups, such as malic acid, tartaric acid, citric acid, isocitric acid, etc.
- Hydrocarbon alkoxy acids such as O-acetyl citric acid, and linear hydrocarbon oxo acids having 2 to 4 carboxyl groups are included.
- Examples of the compound having a (C2) linear hydrocarbon moiety and one hydroxyl group replacing the hydrogen atom of the linear hydrocarbon moiety include those listed in the “compound (B)”, for example, Aliphatic monohydric alcohols are mentioned.
- an ester of a linear hydrocarbon tetracarboxylic acid having 4 carboxyl groups (c 1 ), a hydroxy acid, an alkoxy acid or an oxo acid, and at least one aliphatic monohydric alcohol for example, Mono-, di-, tri- and tetra-esters, preferably diesters, tri- and tetra-esters, more preferably tri- and tetra-esters, and still more preferably tetra-esters, chained with 3 (c 2 ) carboxyl groups
- Esters of hydrocarbon tricarboxylic acids, hydroxy acids, alkoxy acids or oxo acids with at least one aliphatic monohydric alcohol such as monoesters, diesters and triesters, preferably diesters and triesters, and more preferably triesters Ester
- aliphatic monohydric alcohol constituting the above ether corresponding to R 19 OH and R 20 OH in the formula (19)
- the above ether satisfies the requirements of the above IOB, melting point and water solubility It is not particularly limited, and examples thereof include aliphatic monohydric alcohols listed in the “compound (B)” section.
- the total carbon number of the aliphatic monohydric alcohol constituting the ether that is, the carbon number of the R 19 and R 20 moieties in the above formula (19) Since the IOB is 0.50 when the sum of the two is 2, the requirement of the above IOB is satisfied if the total carbon number is about 2 or more. However, when the total carbon number is about 6, the water solubility is as high as about 2 g, and there is also a problem from the viewpoint of the vapor pressure. In order to satisfy the requirement of water solubility of about 0.00 to about 0.05 g, the total carbon number is preferably about 8 or more.
- dialkyl ketone [(D 2 ) dialkyl ketone]
- dialkyl ketone following Formula (20): R 21 COR 22 (20) (Wherein, each of R 21 and R 22 is an alkyl group) And compounds having the formula:
- the total carbon number is preferably about 8 or more. Also, in consideration of the vapor pressure, the carbon number is preferably about 10 or more, and preferably about 12 or more.
- the melting point is about ⁇ 50 ° C.
- the vapor pressure is about 230 Pa at 20 ° C.
- the above-mentioned dialkyl ketone is commercially available and can be obtained by a known method, for example, oxidation of a secondary alcohol with chromic acid or the like.
- Examples of the fatty acid (corresponding to R 23 COOH in the formula (21)) constituting the above-mentioned ester are listed, for example, in “ester of (a 1 ) chain hydrocarbon tetraol with at least one fatty acid”.
- a fatty acid that is, a saturated fatty acid or an unsaturated fatty acid is mentioned, and considering the possibility of modification by oxidation etc., a saturated fatty acid is preferred.
- the aliphatic monohydric alcohol (corresponding to R 24 OH in the formula (21)) constituting the above-mentioned ester include, for example, aliphatic monohydric alcohols listed in the “compound (B)” section.
- the sum of carbon number of fatty acid and aliphatic monohydric alcohol that is, the sum of carbon number of R 23 C and R 24 in the formula (21) is 5
- the IOB is 0.60
- the requirements of the above IOB are satisfied when the total carbon number of the R 23 C and R 24 moieties is about 5 or more.
- the vapor pressure is as high as 2,000 Pa or more. Therefore, in consideration of the vapor pressure, the total carbon number is preferably about 12 or more. If the total carbon number is about 11 or more, the water solubility can satisfy the requirement of about 0.00 to about 0.05 g.
- esters of the above fatty acids with aliphatic monohydric alcohols include, for example, esters of dodecanoic acid (C 12 ), dodecyl alcohol (C 12 ), tetradecanoic acid (C 14 ), and dodecyl alcohol (C 12 )
- esters of fatty acids and aliphatic monohydric alcohols include Electol WE 20 and Electol WE 40 (all manufactured by NOF Corporation).
- the total carbon number of R 25 and R 26 is preferably about 7 or more, and more preferably about 9 or more.
- the above dialkyl carbonate is commercially available, and can be synthesized by the reaction of phosgene with alcohol, the reaction of formic acid chloride ester with alcohol or alcoholate, and the reaction of silver carbonate with alkyl iodide.
- Examples of the above-mentioned polyoxy C 2 -C 6 alkylene glycol or ester or ether thereof include (e 1 ) polyoxy C 2 -C 6 alkylene glycol, (e 2 ) polyoxy C An ester of a 2 to C 6 alkylene glycol with at least one fatty acid, an ether of (e 3 ) polyoxy C 2 to C 6 alkylene glycol and at least one aliphatic monohydric alcohol, (e 4 ) polyoxy C 2 to C 6 An ester of an alkylene glycol with a linear hydrocarbon tetracarboxylic acid, a linear hydrocarbon tricarboxylic acid, or a linear hydrocarbon dicarboxylic acid, and (e 5 ) polyoxy C 2 -C 6 alkylene glycol, with a linear hydrocarbon tetra And ethers with linear hydro
- oxy C 2 ⁇ C 6 alkylene backbone from the viewpoint of lowering the polyoxy C 2 ⁇ C 6 IOB of alkylene glycols, polyoxypropylene skeleton, oxybutylene skeleton, it is oxypentylene skeleton, or an oxy hexylene skeleton preferably More preferably, they are an oxybutylene skeleton, an oxypentylene skeleton, or an oxyhexylene skeleton.
- the above polyoxy C 2 -C 6 alkylene glycol has the following formula (23): HO- (C m H 2m O) n -H (23) (In the formula, m is an integer of 2 to 6) Can be represented by
- homopolymers of formula (23) may include homopolymers of propylene glycol, butylene glycol, pentylene glycol or hexylene glycol. From the above, in the formula (23), m is about 3 to about 6, and more preferably about 4 to about 6, and n is 2 or more.
- n the poly C 2 -C 6 alkylene glycol has an IOB of about 0.00 to about 0.60, a melting point of about 45 ° C. or less, and 100 g of water at 25 ° C. It is a value that has a water solubility of about 0.00 to about 0.05 g.
- the weight average molecular weight of the polyoxy C 2 -C 6 alkylene glycol is preferably about 200 to about 10,000, more preferably about 250 to about 8,000, and more preferably It is in the range of about 250 to about 5,000.
- the weight average molecular weight of the poly C 3 alkylene glycol, ie, polypropylene glycol is preferably about 1,000 to about 10,000, more preferably about 3,000 to about 8 And more preferably in the range of about 4,000 to about 5,000.
- the weight average molecular weight is less than about 1,000, the water solubility does not satisfy the requirements, and the larger the weight average molecular weight, the more the absorber transfer rate and the top sheet whiteness tend to be improved.
- Examples of commercial products of polyoxy C 2 ⁇ C 6 alkylene glycol e.g., UNIOL (TM) D-1000, D-1200 , D-2000, D-3000, D-4000, PB-500, PB-700, PB -1000 and PB-2000 (manufactured by NOF Corporation).
- UNIOL TM
- D-1000, D-1200 , D-2000, D-3000, D-4000, PB-500, PB-700, PB -1000 and PB-2000 manufactured by NOF Corporation.
- ester of (e 2 ) polyoxy C 2 -C 6 alkylene glycol with at least one fatty acid is the OH terminal of the polyoxy C 2 to C 6 alkylene glycol described in the section “(e 1 ) polyoxy C 2 to C 6 alkylene glycol”.
- fatty acids ie, monoesters and diesters.
- the fatty acid to be esterified is, for example, listed in “ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid”.
- Fatty acids that is, saturated fatty acids or unsaturated fatty acids, and in view of the possibility of modification by oxidation etc., saturated fatty acids are preferred.
- Examples of commercially available esters of the polyoxy C 2 -C 6 alkylene glycol and fatty acid include Wilbright cp 9 (manufactured by NOF Corporation).
- aliphatic monohydric alcohols to be etherified in the ether of polyoxy C 2 -C 6 alkylene glycol and at least one aliphatic monohydric alcohol include, for example, aliphatics listed in the “compound (B)” section. Monohydric alcohol is mentioned.
- esters of the above polyoxy C 2 -C 6 alkylene glycol with chain hydrocarbon tetracarboxylic acid, chain hydrocarbon tricarboxylic acid, or chain hydrocarbon dicarboxylic acid are commercially available, and chain hydrocarbon tetracarbons are also commercially available. It can be produced by polycondensation of C 2 -C 6 alkylene glycol with an acid, linear hydrocarbon tricarboxylic acid or linear hydrocarbon dicarboxylic acid under known conditions.
- chain hydrocarbon tetraol to be etherified the chain hydrocarbon triol, and the chain hydrocarbon diol, those described in the “compound (A)” section, for example, pentaerythritol, glycerin and glycol Can be mentioned.
- ethers of the above polyoxy C 2 -C 6 alkylene glycol and chain hydrocarbon tetraol, chain hydrocarbon triol, or chain hydrocarbon diol are, for example, UnilobeTM 5TP-300 KB, and Uniol (trademark) TG-3000 and TG-4000 (made by NOF Corporation) can be mentioned.
- Unilube (TM) 5TP-300KB is a compound obtained by polycondensation of 65 moles of propylene glycol and 5 moles of ethylene glycol with 1 mole of pentaerythritol, and its IOB is 0.39, and the melting point is less than 45 ° C. And the water solubility was less than 0.05 g.
- Uniol (TM) TG-3000 is a compound obtained by polycondensing 50 moles of propylene glycol with 1 mole of glycerin, its IOB is 0.42, its melting point is less than 45 ° C, and its water solubility is 0.05 g And the weight average molecular weight was about 3,000.
- Uniol (TM) TG-4000 is a compound obtained by polycondensing 70 moles of propylene glycol with 1 mole of glycerin, its IOB is 0.40, melting point is less than 45 ° C., and water solubility is 0.05 g And the weight average molecular weight was about 4,000.
- the ether of the polyoxy C 2 -C 6 alkylene glycol and the chain hydrocarbon tetraol, chain hydrocarbon triol or chain hydrocarbon diol is also a chain hydrocarbon tetraol, chain hydrocarbon triol or It can be produced by adding a C 2 -C 6 alkylene oxide to a linear hydrocarbon diol under known conditions.
- the chain hydrocarbon has an IOB of 0.00 and an aqueous solubility of almost 0 g because the inorganic value is 0, and the blood has a melting point of about 45 ° C. or less. It may be included in the modifier.
- Examples of the chain hydrocarbon include (f 1 ) chain alkanes such as straight chain alkanes and branched chain alkanes, and in the case of straight chain alkanes, for example, the melting point is about 45 ° C. or less In general, those containing 22 or less carbon atoms are included. Also, in consideration of the vapor pressure, those having 13 or more carbon atoms are generally included.
- the blood modifying agent has been found to at least have the effect of reducing blood viscosity and surface tension. Since the menstrual blood to be absorbed by the absorbent article contains proteins such as the endometrial wall as compared with normal blood, they act to connect the blood cells to each other, and the blood cells are in a continuous state. Cheap. Therefore, the menstrual blood to be absorbed by the absorbent article tends to have a high viscosity, and when the top sheet is a non-woven fabric or a woven fabric, menstrual blood tends to be clogged between fibers, and the wearer feels sticky. And it spreads and leaks on the surface of the top sheet.
- a blood modifying agent having an IOB of about 0.00 to about 0.60 is highly organic and easily enters between blood cells, thereby stabilizing the blood cells and making it difficult for the blood cells to form a continuous structure. It is thought that can be done. It is considered that the absorber makes it easy to absorb menstrual blood by stabilizing the blood cells and making the blood cells less likely to form a continuous structure.
- the so-called SAP when menstrual blood is absorbed, blood cells that have undergone continuous change cover the SAP surface, making it difficult for the SAP to exhibit its absorption performance.
- SAP By stabilizing blood cells, it is considered that SAP can more easily exhibit absorption performance.
- the blood modifying agent having high affinity for red blood cells protects the red blood cell membrane, so that the red blood cells are less likely to be destroyed.
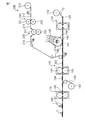
- FIG. 7 is a figure for demonstrating the absorbent article manufacturing apparatus 100 used for the manufacturing method of the absorbent article 1 in one Embodiment of this invention.
- a process of forming a folding suppression sheet, a process of forming an absorber, a process of preparing a sheet for top sheet, a process of forming compressed grooves in a laminate, and a sheet for backsheet a process of forming a folding suppression sheet, a process of forming an absorber, a process of preparing a sheet for top sheet, a process of forming compressed grooves in a laminate, and a sheet for backsheet , Cutting the continuum of the absorbent article and applying a blood modifying agent to the absorbent article.
- the top sheet sheet used in the step of preparing the top sheet sheet includes a process of preparing the resin film sheet, a process of forming the recess in the resin film sheet, and a process of gear stretching the resin film sheet. It is manufactured by the manufacturing method of the seat for sheets.
- the bending suppression sheet sheet 112 supplied from the bending suppression sheet sheet roll 110 is placed on the belt 120. Then, after applying a hot melt adhesive to the sheet 112 for bending suppression sheet with a coating device (not shown), the cutter 130 is used to cut the sheet 112 for bending suppression sheet into a predetermined shape and the bending suppression sheet Make
- the absorber 148 is formed on a bending suppression sheet.
- Pulverized pulp 142 is supplied to pattern drum 140 from a pulverized pulp supply device (not shown).
- a recess 144 is formed on the outer peripheral portion of the pattern drum 140 as a mold for packing crushed pulp.
- the inside of the pattern drum 140 is suctioned 146, and the crushed pulp 142 supplied to the pattern drum 140 is sucked into the recess 144 and compressed. Then, the pulp 142 compressed in the recess 144 is formed into the absorber 148.
- the absorber 148 is placed on the bending suppression sheet.
- a hot melt adhesive is applied to the absorbent body 148 with a coating device (not shown), and then the top sheet sheet 216 manufactured by the top sheet sheet manufacturing method described later is used as the absorbent body 148. And the top sheet sheet 216 is adhered to the absorber 148.
- the pressing device is used to form the pressing groove in the top sheet sheet 216, the absorber 148 and the laminate 262 of the bending suppression sheet.
- the laminate 262 passes between the upper roll 151 and the lower roll 152 of the embossing device 150.
- a convex portion (not shown) having a shape corresponding to the pressing groove 8 of the absorbent article 1 shown in FIG. 1 is provided.
- the lower roll 152 is a plain roll whose outer peripheral surface is smooth.
- the portion of the laminate 262 corresponding to the compressed grooves 8 of the absorbent article 1 shown in FIG. 1 is in the thickness direction And compressed grooves are formed in the laminate 262.
- an adhesive is applied to the backsheet sheet 162 supplied from the backsheet sheet roll 160 using a coating device (not shown), and then lamination is performed in which compressed grooves are formed. It is overlapped and adhered to the surface on the side of the bending suppression sheet of the body 154 to form a continuum 164 of the absorbent article.
- the cutter 170 is used to cut the continuum 164 of the absorbent article into the shape of the absorbent article to produce the absorbent article.
- the above-described blood modifying agent 181 is applied to the central region of the absorbent article using the modifier application spray 180 to apply blood to the surface of the top sheet. Form a modifier layer.
- the blood modifying agent 181 applied to the central region of the absorbent article may be applied at least to a portion that abuts on the excretory port of the wearer's body fluid.
- the length in the lengthwise direction is preferably 50 mm or more, more preferably 100 mm or more
- the length in the width direction is preferably 10 mm or more, more preferably 30 mm or more, centering on the center of the absorbent article.
- a blood modifying agent may be applied to an area.
- the blood modifying agent was applied after the step of cutting the continuum of the absorbent article, the blood modifying agent may be applied in the step of producing the top sheet described later.
- the downstream stage of the manufacturing process of the absorbent article for example, the blood just before packaging the absorbent article It is preferred to apply the modifier to the absorbent article.
- the method of manufacturing the absorbent article 1 includes a step of forming an adhesive portion on the continuous body 164 of the absorbent article, a step of forming a seal portion on the continuous body 164 of the absorbent article, and the like.
- the resin film sheet 212 supplied from the roll 210 of the resin film sheet is supplied to the recess forming roll 220.
- the resin film sheet 212 is made to pass the recessed part formation roll 220, and the resin film sheet 214 (refer FIG. 9) in which the recessed part 2141 was formed is produced.
- the recess forming roll 220 comprises a knurling roll 221 and a preheating roll 222 having a smooth surface.
- FIGS. 8A and 8B show an example of the knurled roll 221.
- FIG. FIG. 8A is a view showing the entire knurled roll 221
- FIG. 8B is an enlarged view of a portion 223 having unevenness on the outer peripheral surface of the knurled roll 221.
- FIG. 8C shows an example of the preheating roll 222 having a smooth surface.
- a grid-like convex portion 224 is provided on the surface 223 of the knurled roll 221.
- a rhombus recess 225 is formed on the surface of the knurled roll 221.
- the shape of the recessed part 225 of the knurl roll 221 is not limited to a rhombus, You may be shapes, such as a square, a rectangle, a parallelogram, a trapezoid, a triangle, and a hexagon.
- the center line spacing of the convex parts 224 arranged in parallel in the lattice-like convex parts 224 is preferably 0.2 mm or more and 10 mm or less, more preferably 0.4 mm or more and 2 mm It is below.
- the pitch of the grid-like convex portions 224 is less than 0.2 mm or greater than 10 mm, the concave portion may not be formed in the resin film.
- the width of the grid-like convex portion 224 is preferably 0.01 mm or more and 1 mm or less, and more preferably 0.03 mm or more and 0.1 or less.
- the length of one side of the rhombic recess 225 is preferably 0.1 mm or more and 5 mm or less, and more preferably 0.2 mm or more and 1 mm or less.
- the width of the grid-like convex part 224 is less than 0.01 mm or more than 1 mm, or when the length of one side of the rhombus concave part 225 is less than 0.1 mm or more than 5 mm, the concave part may not be formed in the resin film. .
- the preheated roll 222 having a smooth surface is maintained at a temperature of 70 ° C. or more and 100 ° C. or less, and heats the supplied resin film sheet 212. Thereby, the resin film sheet 212 becomes soft and easy to form.
- the resin film sheet 212 passes between the knurled roll 221 and the roll 222 having a smooth surface, the resin film sheet 212 receives a strong pressure in the thickness direction in a portion in contact with the grid-like convex portion 224. Thereby, as shown in FIG. 9, the fine recessed part 2141 is formed in the resin film sheet 214. As shown in FIG. In fact, the number of recesses 2141 formed in the resin film sheet 212 is smaller than that shown in FIG. 9, and the number of recesses 2141 per unit area is also much larger than that shown in FIG.
- the concave portion 2141 is formed only in a range 2143 corresponding to the central portion 12 (see FIG. 1) of the main body portion 10 in the resin film sheet 214.
- the range corresponding to the absorbent article 1 in the resin film sheet 214 is the range indicated by the dotted line 2142.
- the first mountain fold portion, the second mountain fold portion, and the second mountain fold portion of the top sheet 2 are caused to pass through the resin film sheet 214 having the concave portion formed in the stretching gear roll 230 shown in FIG.
- the valley folds are formed in the resin film sheet 214.
- symbol 216 is a resin film sheet which formed the 1st mountain fold part, the 2nd mountain fold part, and the valley fold part.
- the drawing gear roll 230 includes an upper roll 231 and a lower roll 232.
- 10 (a) is a view for explaining the upper roll 231 of the drawing gear roll 230
- FIG. 10 (b) is a view for explaining the gear teeth 233 arranged on the outer peripheral surface of the upper roll 231.
- FIG. 10 (c) is a cross-sectional view taken along the line CC of FIG. 10 (b).
- the gear teeth 233 extend discontinuously in the circumferential direction of the upper roll 231. That is, the gear teeth 233 extending in the circumferential direction of the upper roll 231 are interrupted at multiple points along the way.
- a second mountain-folded portion is formed in the resin film sheet 214 by the portion 234 where the gear teeth 233 are disconnected.
- the width of gear teeth 233 is, for example, 0.3 mm or more and 0.5 mm or less, and the distance between the centers of adjacent gear teeth 233 is, for example, 1.0 mm or more and 1.2 mm or less.
- FIG. 11 (a) is a view for explaining the lower roll 232 of the drawing gear roll 230
- FIG. 11 (b) is a view for explaining the gear teeth 235 disposed on the outer peripheral surface of the lower roll 232
- 11 (c) is a cross-sectional view taken along the line DD of FIG. 11 (b).
- the gear teeth 235 extend in the circumferential direction of the lower roll 232.
- the lower roll 232 is not interrupted at multiple points along the way.
- the width of the gear teeth 235 is, for example, equal to the width of the gear teeth 233 of the upper roll 231, and the distance between the centers of adjacent gear teeth 235 is, for example, equal to the distance between the centers of the gear teeth 233 of the upper roll 231.
- the radial length of the upper roll 231 at the portion where the gear teeth 233 of the upper roll 231 and the gear teeth 235 of the lower roll 232 are engaged, that is, the biting depth is, for example, 1.25 mm.
- the gap between the gear teeth 233 of the upper roll 231 and the gear teeth 235 of the lower roll 232 when the gear teeth 233 of the upper roll 231 and the gear teeth 235 of the lower roll 232 are engaged is, for example, 0.25 mm or more , 0.45 mm or less.
- the resin film sheet 214 passes through the drawing gear roll 230, the resin film 214 is bent in a substantially wave shape, and the resin film 214 forms the first mountain fold 21 of the top sheet 2 and the second mountain fold. Portions 22 and valley folds 23 are formed. Further, an opening 25 (see FIG. 4) of the top sheet 2 is further formed in the region 2143 (see FIG. 9) in which the recess 2141 of the resin film sheet 214 is formed.
- the resin film sheet 214 is largely drawn at a portion 236 where the gear teeth 233 of the upper roll 231 and the gear teeth 235 of the lower roll 232 are engaged.
- the resin film sheet 214 is thin at the part where the concave part 2141 (see FIG. 9) is formed in the above-mentioned concave part forming step, and the part where the scratch is given by the grid-like convex part 224 of the knurling roll 221 As it is present, the strength is weak, and the recess 2141 of the resin film sheet 214 breaks when it is stretched.
- the concave portion 2141 of the resin film sheet 214 is torn, the torn portion of the resin film sheet 214 is spread, and the opening portion is to the torn portion of the resin film sheet 214. It is formed.
- the resin film sheet 214 is not stretched much at the portions 237 and 238 where the gear teeth 233 of the upper roll 231 and the gear teeth 235 of the lower roll 232 are not engaged. For this reason, in the portions 237 and 238 in which the gear teeth 233 of the upper roll 231 and the gear teeth 235 of the lower roll 232 in the resin film sheet 241 are not engaged, the resin film sheet 214 described above The recess 2141 formed in the recess forming step is not broken and does not become an opening.
- the blood modifying agent since the blood modifying agent has a mechanism to lower the viscosity and surface tension of the blood, the body fluid is transferred to the absorber 4 without remaining on the top sheet 2 by the blood modifying layer 24 and absorbed. 4 can be absorbed.
- the following examples confirmed that the blood modifying agent has a mechanism to lower the viscosity and surface tension of blood. This check was performed using a non-woven fabric in which the body fluid tends to remain as compared to the resin film.
- the sanitary napkin comprises a top sheet formed of an air through non-woven fabric (composite fiber of polyester and polyethylene terephthalate, basis weight: 35 g / m 2 ) treated with a hydrophilic agent, and a composite of air through non-woven fabric (polyester and polyethylene terephthalate) Fiber, second sheet formed of basis weight: 30 g / m 2 ), pulp (basis weight: 150 to 450 g / m 2 , more in the central part), acrylic high absorption polymer (basis weight: 15 g / m 2 ) And, it was formed from an absorbent including a tissue as a core wrap, a water repellent treated side sheet, and a back sheet made of polyethylene film.
- an absorbent including a tissue as a core wrap, a water repellent treated side sheet, and a back sheet made of polyethylene film.
- Triethylene CL oil fatty acid glycerides, manufactured by NOF Corporation C 8 fatty acid: fatty acid of C 12 is approximately included in a weight ratio of 44:56, triesters of glycerin and fatty acid, the weight average molecular weight: about 570
- -Panaceto 800 B manufactured by NOF Corporation Triester of glycerin and fatty acid in which all fatty acids are 2-ethylhexanoic acid (C 8 ), weight average molecular weight: about 470 NA36, manufactured by NOF Corporation C 16 fatty acids: C 18 fatty acids: C 20 fatty acids (including both saturated fatty acids and unsaturated fatty acids) in a weight ratio of approximately 5: 92: 3, Triester of glycerin and fatty acid, weight average molecular weight: about 880
- Tricot oil fatty acid glyceride manufactured by NOF Corporation C 8 fatty acid: C 10 fatty acid: C 12 fatty acid: C 14 fatty acid: C 16 fatty acid (including both saturated fatty acid and unsaturated fatty acid) is approximately 4 Triester of glycerin and fatty acid, contained in a weight ratio of 8: 60: 25: 3, weight average molecular weight: 670 ⁇ Caprylic diglyceride, manufactured by NOF Corporation Diester of glycerin and fatty acid wherein fatty acid is octanoic acid, weight average molecular weight: 340
- Uniol D-4000 polypropylene glycol manufactured by NOF Corporation, weight average molecular weight: about 4,000 Uniol PB500, polybutylene glycol manufactured by NOF Corporation, weight average molecular weight: about 500 Uniol PB700, manufactured by NOF Corporation, polyoxybutylene polyoxypropylene glycol, weight average molecular weight: about 700
- Uniol PB 1000 R polybutylene glycol manufactured by NOF Corporation, weight average molecular weight: about 1000 [Ester of (e 2 ) polyoxy C 2 -C 6 alkylene glycol with at least one fatty acid] ⁇ Wilbright cp 9, a compound in which OH groups at both ends of polybutylene glycol manufactured by NOF Corporation were esterified with hexadecanoic acid (C 16 ), weight average molecular weight: about 1,150
- UNIOL TG-3000 glyceryl ether of polypropylene glycol manufactured by NOF Corporation, about 16 repeating units, weight average molecular weight: about 3,000 ⁇ UNIOL TG-4000, glyceryl ether of polypropylene glycol manufactured by NOF Corporation, about 16 repeating units, weight average molecular weight: about 4,000
- -PEG 1500 polyethylene glycol manufactured by NOF Corporation, weight average molecular weight: about 1,500 to about 1,600 Nonion S-6, manufactured by NOF Corporation, polyoxyethylene monostearate, repeating unit of about 7 weight average molecular weight: about 880 Will Bright s 753, manufactured by NOF Corporation polyoxyethylene polyoxypropylene polyoxybutylene glycerin, weight average molecular weight: about 960
- TG-330 a glyceryl ether of polypropylene glycol manufactured by NOF Corporation, about 6 repeating units, weight average molecular weight: about 330 ⁇ UNIOL TG-1000, glyceryl ether of polypropylene glycol manufactured by NOF Corporation, about 16 repeating units, weight average molecular weight: about 1,000
- Unirube DGP-700 a diglyceryl ether of polypropylene glycol manufactured by NOF Corporation, about 9 repeating units, weight average molecular weight: about 700 -Uniox HC60, manufactured by NOF Co., Ltd. polyoxyethylene hydrogenated castor oil, weight average molecular weight: about 3,570 ⁇ Vaseline, Cognis Japan Ltd. Petroleum derived hydrocarbon, semi-solid
- the IOB, melting point and water solubility of the above sample are shown in Table 2 below.
- the water solubility was measured according to the above-mentioned method, but 20.0 g was added to 100 g of demineralized water, and a sample dissolved after 24 hours was evaluated as "20 g ⁇ ", and 100 g of demineralized water was used. A sample in which 0.05 g was dissolved but not 1.00 g was evaluated as 0.05 to 1.00 g.
- fusing point " ⁇ 45" means that melting
- the skin contact surface of the top sheet of the above-mentioned sanitary napkin was coated with the above-mentioned blood modifier. Heat each blood modifier to its melting point + 20 ° C if the blood modifier is liquid at room temperature, and if the blood modifier is solid at room temperature, then control seam HMA gun Each blood modifying agent was atomized and applied to the entire skin contact surface of the top sheet so that the basis weight was approximately 5 g / m 2 .
- FIG. 13 is an electron micrograph of the skin contact surface of the top sheet in the sanitary napkin (No. 2-5) in which the top sheet contains avian C2L oil fatty acid glyceride. As apparent from FIG. 13, the tri-C2L oil fatty acid glyceride is in the form of fine particles and adheres to the surface of the fiber. Rewet rates and absorber transfer rates were measured according to the procedure described above. The results are shown in Table 2 below.
- Rewet rate (%) 100 ⁇ (weight of filter paper after test ⁇ weight of original filter paper) / 6
- absorber transfer speed which is the time for blood to transfer from the top sheet to the absorber after the second drop of blood.
- absorber transfer rate means the time from when blood is introduced into the top sheet to when the red color of blood is not seen on the surface and inside of the top sheet.
- the rewet rate was 22.7% and the absorber transfer rate was over 60 seconds, but both the glycerin and fatty acid triesters had rewet rates From the fact that it is 7.0% or less and the absorber transfer rate is 8 seconds or less, it can be seen that the absorption performance is greatly improved. However, among triesters of glycerin and fatty acid, NA50 of which the melting point exceeds 45 ° C. shows no significant improvement in the absorption performance.
- a blood modifying agent having an IOB of about 0.00 to about 0.60, a melting point of about 45 ° C. or less, and an aqueous solubility of about 0.00 to about 0.05 g per 100 g of water at 25 ° C. It was found that the absorption performance was greatly improved.
- sanitary napkins were worn by a plurality of volunteer subjects.
- sanitary napkins containing blood modifiers 2-1 to 2-3 there was no tackiness on the top sheet even after menstrual blood absorption, and it was answered that the top sheet was smooth. .
- Defibrillation blood after blood collection, stirred for about 5 minutes in an Erlenmeyer flask together with glass beads
- EDTA blood Addition of 0.5 mL of 12% EDTA ⁇ 2K saline to 65 mL of venous blood
- Serum or plasma Supernatant after centrifuging defibrillated blood or EDTA blood, respectively, at about 1900 G at room temperature for 10 minutes
- Blood cells Remove the serum from the blood and remove the residual phosphate buffered saline (PBS) ) Washed twice and then added with phosphate buffered saline for the removed serum
- An absorbent article was produced in the same manner as in Example 2 except that avian C2L oil fatty acid glyceride was applied so as to give a basis weight of approximately 5 g / m 2, and the rewet rate was evaluated for the above various blood. . Three measurements were taken for each blood and the mean value was taken. The results are shown in Table 3 below.
- Example 2 The same tendency as equine EDTA blood obtained in Example 2 was also obtained in human and sheep blood. The same tendency was also observed in defibrinated blood and EDTA blood.
- the top sheet containing the blood modifying agent has low blood retention and can be rapidly transferred to the absorber after absorbing blood.
- Example 4 [Viscosity of blood containing blood modifying agent] The viscosity of the blood containing the blood modifying agent was measured using Rheometric Expansion System ARES (Rheometric Scientific, Inc). 2% by weight of Panaceto 810s was added to equine defibrinated blood, the mixture was lightly stirred to form a sample, the sample was loaded on a parallel plate of 50 mm in diameter, the gap was made 100 ⁇ m, and the viscosity was measured at 37 ⁇ 0.5 ° C. . Because of the parallel plate, the sample was not subjected to a uniform shear rate, but the average shear rate displayed on the instrument was 10 s ⁇ 1 .
- the viscosity of horse-defibrillated blood containing 2% by mass of Panaceto 810s was 5.9 mPa ⁇ s, while the viscosity of horse-defibrillated blood containing no blood modifying agent was 50.4 mPa ⁇ s.
- equine defibrinated blood containing 2% by weight of Panaceto 810s reduces the viscosity by about 90% as compared to the case without blood modifying agent.
- blood contains components such as blood cells and is known to have thixotropy properties, it is considered that the blood modifying agent of the present disclosure can lower the viscosity of blood in a low viscosity region. By reducing the viscosity of blood, it is thought that absorbed menstrual blood can be rapidly transferred from the top sheet to the absorber.
- Example 5 [Micrograph of blood containing blood modifier] A healthy volunteer's menstrual blood is collected on Saran wrap (registered trademark), and in part of it, Panaceto 810s dispersed in 10 times mass of phosphate buffered saline, and the concentration of Panaceto 810s is 1% by mass As it was added. The menstrual blood was applied to a slide glass, covered with a cover glass, and the condition of red blood cells was observed with a light microscope. A photomicrograph of menstrual blood containing no blood modifying agent is shown in FIG. 14 (a), and a photomicrograph of menstrual blood containing PANACET 810s is shown in FIG. 14 (b).
- red blood cells form a lump of rhomsen, etc., but in menstrual blood containing PANACET 810s, the red blood cells are dispersed stably. I understand. Therefore, it is suggested that the blood modifying agent works to stabilize red blood cells in the blood.
- Example 6 [Surface tension of blood containing blood modifier] The surface tension of blood containing a blood modifying agent was measured by a pendant drop method using a contact angle meter Drop Master 500 manufactured by Kyowa Interface Science Co., Ltd. The surface tension was measured after adding a predetermined amount of blood modifying agent to sheep defibrinated blood and shaking sufficiently. The measurement is automatically performed by the device, but the surface tension ⁇ is obtained by the following equation (see FIG. 15).
- the density ⁇ is the density test method and density / mass / volume conversion table in JIS K 2249-1995, 5. It was measured at the temperature shown in Table 5 below according to the vibrational density test method. For measurement, DA-505 of Kyoto Electronics Industries Ltd. was used. The results are shown in Table 5.
- the blood modifying agent has a water solubility of about 0.00 to about 0.05 g per 100 g of water at 25 ° C., but its solubility in water is very low. It can be seen that the surface tension of the blood can be reduced. By lowering the surface tension of the blood, it is considered that the absorbed blood can be rapidly transferred to the absorber without being held between the fibers of the top sheet.
Abstract
Provided is an absorbent article (1) that minimizes contact of a section of the side surfaces (41, 41) of an absorbent body (4) with the inner thigh of a wearer when wing sections (6, 6) have been folded and attached to underwear, and that minimizes the occurrence of large twists in the aforementioned section of the side surfaces (41, 41) of the absorbent body (4). The present invention is an absorbent article (1) comprising: a main body section (10) provided with a top sheet (2), a back sheet (3), and an absorbent body (4); and wing sections (6, 6) that are arranged on both sides of the main body section (10). The main body section (10) also comprises a bend-inhibiting sheet (5) that is provided between the absorbent body (4) and the back sheet (3). The edges (51, 51) on both sides of the bend-inhibiting sheet (5) in the width direction are positioned farther outward in the width direction than the side surfaces (41, 41) of the absorbent body. The bend-inhibiting sheet (5) imparts rigidity to the absorbent article (1) such that the absorbent article (1) is harder to bend in the area in which the bend-inhibiting sheet (5) is provided than in the area in which the bend-inhibiting sheet (5) is not provided.
Description
本発明は、生理用ナプキン、パンティライナ、失禁パッド、失禁ライナなどの吸収性物品に関する。
The present invention relates to absorbent articles such as sanitary napkins, panty liners, incontinence pads, incontinence liners and the like.
主体部分の縦サイドエッジを越えて横に外側に伸びる一対のフラップを具備した生理用ナプキンが従来技術として知られている(たとえば、特許文献1)。生理用ナプキンが着用されるとき、フラップは着用者の下着の下に折られる。この生理用ナプキンでは、着用者の下着のクロッチの幅が主体部分の幅より狭い場合、フラップは、近位端の内側の主体部分の縦部分に沿って折られる。着用者の下着のクロッチの幅が主体部分の幅より広い場合、フラップは、主体部分のヒンジに沿って折られる。
A sanitary napkin having a pair of flaps extending laterally outward beyond the longitudinal side edge of the main part is known in the prior art (e.g., U.S. Pat. No. 5,958,015). When the sanitary napkin is worn, the flaps are folded under the wearer's underwear. In this sanitary napkin, when the width of the crotch of the wearer's underwear is smaller than the width of the main portion, the flap is folded along the longitudinal portion of the inner main portion of the proximal end. If the crotch width of the wearer's underwear is wider than the width of the main part, the flap is folded along the hinge of the main part.
しかしながら、特許文献1に記載されているような従来の吸収性物品では、着用者の下着のクロッチ域の幅が吸収性物品に設けられている吸収体の幅より狭いとき、ウイング部は吸収体の側面に沿って折られる場合がある。この場合、吸収体の側面の部分が着用者の内股に当たり、着用者の内股に強い違和感が生ずる場合がある。また、吸収体の側面の部分に大きなヨレが生じ、着用者の体液が吸収性物品の幅方向へ漏れてしまう場合がある。
However, in the conventional absorbent article as described in Patent Document 1, when the width of the crotch area of the wearer's underwear is narrower than the width of the absorbent provided in the absorbent article, the wing portion is the absorbent May be broken along the sides of the In this case, the side portion of the absorber may hit the wearer's inner crotch, causing a strong discomfort in the wearer's inner crotch. In addition, large deflection may occur in the side portion of the absorber, and the body fluid of the wearer may leak in the width direction of the absorbent article.
本発明は、着用者の下着のクロッチ域の幅が吸収性物品に設けられている吸収体の幅より狭い場合であっても、ウイング部を折り畳んで吸収性物品を下着に取り付けたときに、吸収体の側面の部分が着用者の内股に当たることを抑制するとともに、吸収体の側面の部分に大きなヨレが生じることを抑制できる吸収性物品を提供することを目的とする。
According to the present invention, even when the width of the crotch area of the wearer's underwear is narrower than the width of the absorbent provided in the absorbent article, when the wing is folded and the absorbent article is attached to the underwear, An object of the present invention is to provide an absorbent article which can suppress that the side portion of the absorber hits the inner crotch of the wearer and can suppress the occurrence of large deflection in the side portion of the absorber.
本発明は、上記課題を解決するため、以下の構成を採用した。
すなわち、本発明は、長手方向および幅方向を有し、肌側に設けられた液透過性のトップシート、着衣側に設けられた液不透過性のバックシートおよび該トップシートと該バックシートとの間に設けられ幅方向の両側に長手方向に延びる側面を含む液保持性の吸収体を備えた本体部と、該本体部の両側縁から幅方向に延出するように該本体部の両側に配置されたウイング部とを含む吸収性物品であって、本体部は、吸収体とバックシートとの間に設けられ、幅方向の両側に長手方向に延びる縁を含む折曲抑制シートをさらに備え、折曲抑制シートの幅方向の両側の縁は、吸収体の側面よりも幅方向外側に位置し、折曲抑制シートが設けられている領域が、折曲抑制シートが設けられていない領域に比べて吸収性物品が折り曲げにくくなるように、折曲抑制シートは吸収性物品に剛性を付与する。 The present invention adopts the following configuration in order to solve the above-mentioned problems.
That is, according to the present invention, a liquid-permeable top sheet provided on the skin side, having a longitudinal direction and a width direction, a liquid-impermeable back sheet provided on the clothing side, the top sheet, and the back sheet A main body portion provided with a liquid-retaining absorber including a side surface extending in the longitudinal direction on both sides in the width direction, and both sides of the main body portion extending in the width direction from both side edges of the main body portion An absorbent article including a wing portion disposed in the body, wherein the main body portion is provided between the absorber and the back sheet, and further includes a bending suppression sheet including longitudinally extending edges on both sides in the width direction The edge on both sides in the width direction of the anti-folding sheet is located outside the side surface of the absorber in the width direction, and the area where the anti-folding sheet is provided is the area where the anti-folding sheet is not provided So that the absorbent article is harder to bend than , Folding suppression sheet imparts rigidity to the absorbent article.
すなわち、本発明は、長手方向および幅方向を有し、肌側に設けられた液透過性のトップシート、着衣側に設けられた液不透過性のバックシートおよび該トップシートと該バックシートとの間に設けられ幅方向の両側に長手方向に延びる側面を含む液保持性の吸収体を備えた本体部と、該本体部の両側縁から幅方向に延出するように該本体部の両側に配置されたウイング部とを含む吸収性物品であって、本体部は、吸収体とバックシートとの間に設けられ、幅方向の両側に長手方向に延びる縁を含む折曲抑制シートをさらに備え、折曲抑制シートの幅方向の両側の縁は、吸収体の側面よりも幅方向外側に位置し、折曲抑制シートが設けられている領域が、折曲抑制シートが設けられていない領域に比べて吸収性物品が折り曲げにくくなるように、折曲抑制シートは吸収性物品に剛性を付与する。 The present invention adopts the following configuration in order to solve the above-mentioned problems.
That is, according to the present invention, a liquid-permeable top sheet provided on the skin side, having a longitudinal direction and a width direction, a liquid-impermeable back sheet provided on the clothing side, the top sheet, and the back sheet A main body portion provided with a liquid-retaining absorber including a side surface extending in the longitudinal direction on both sides in the width direction, and both sides of the main body portion extending in the width direction from both side edges of the main body portion An absorbent article including a wing portion disposed in the body, wherein the main body portion is provided between the absorber and the back sheet, and further includes a bending suppression sheet including longitudinally extending edges on both sides in the width direction The edge on both sides in the width direction of the anti-folding sheet is located outside the side surface of the absorber in the width direction, and the area where the anti-folding sheet is provided is the area where the anti-folding sheet is not provided So that the absorbent article is harder to bend than , Folding suppression sheet imparts rigidity to the absorbent article.
本発明によれば、着用者の下着のクロッチ域の幅が吸収性物品に設けられている吸収体の幅より狭い場合であっても、ウイング部を折り畳んで下着に取り付けたときに、吸収体の側面の部分が着用者の内股に当たることを抑制するとともに、吸収体の側面の部分に大きなヨレが生じることを抑制できる。
According to the present invention, even when the width of the crotch area of the wearer's underwear is narrower than the width of the absorbent provided in the absorbent article, when the wing is folded and attached to the underwear, the absorbent While suppressing that the part of a side of a part hits a wearer's inner crotch, it can suppress that a big distortion arises in the part of the side of an absorber.
以下、図面を参照して、本発明を説明するが、本発明は図面に記載されたものに限定されるものではない。
Hereinafter, the present invention will be described with reference to the drawings, but the present invention is not limited to those described in the drawings.
図1は本発明の一実施形態の吸収性物品の部分破断平面図であり、図2は図1のA-A線断面を示す概略断面図である。吸収性物品1は、肌側(肌当接側)に設けられた液透過性のトップシート2、着衣側(非肌当接側)に設けられた液不透過性のバックシート3、トップシート2とバックシート3との間に設けられた液保持性の吸収体4および吸収体4とバックシート3との間に設けられた折曲抑制シート5を備える本体部10と、本体部10の両側縁から幅方向に延出するように本体部10の両側に配置された、トップシート2およびバックシート3を備えた一対のウイング部6とを含む。符号61はウイング部6の付け根(本体部10とウイング部6との間の境界)を示す。たとえば、ウイング部6の長手方向両側において、吸収性物品1の幅が急に大きくなる2つの点を結んだ直線がウイング部6の付け根61と見なすことができる。ウイング部6における着衣側の面の反対側の面は、トップシート2で構成されている。本体部10およびウイング部6の着衣側の面には、粘着部7がそれぞれ設けられている。なお、図1において、吸収性物品1の幅方向はX方向であり、長手方向はY方向である。また、吸収性物品1の平面方向は、XY方向である。
FIG. 1 is a partially broken plan view of an absorbent article according to an embodiment of the present invention, and FIG. 2 is a schematic cross-sectional view taken along the line AA of FIG. The absorbent article 1 has a liquid-permeable top sheet 2 provided on the skin side (skin contact side), a liquid-impermeable back sheet 3 provided on the clothing side (non-skin contact side), and a top sheet A liquid-retaining absorber 4 provided between the second sheet 2 and the back sheet 3 and a main body 10 having a bending suppression sheet 5 provided between the absorber 4 and the back sheet 3; It includes a pair of wing portions 6 provided with a top sheet 2 and a back sheet 3 disposed on both sides of the main body portion 10 so as to extend in the width direction from both side edges. The code | symbol 61 shows the root of the wing part 6 (boundary between the main-body part 10 and the wing part 6). For example, a straight line connecting two points where the width of the absorbent article 1 suddenly increases on both sides in the longitudinal direction of the wing portion 6 can be regarded as the root 61 of the wing portion 6. The surface on the opposite side of the surface on the clothing side in the wing portion 6 is configured by the top sheet 2. Adhesive portions 7 are provided on the clothes-side surfaces of the main body portion 10 and the wing portion 6 respectively. In addition, in FIG. 1, the width direction of the absorbent article 1 is the X direction, and the longitudinal direction is the Y direction. Moreover, the plane direction of the absorbent article 1 is the XY direction.
本体部10の形状は、長方形、楕円型、砂時計型など、女性の身体および下着の形状に適合する形状であればとくに限定されない。本体部10の外形における長手方向の延べ寸法は、好ましくは100~500mmであり、より好ましくは150~350mmである。また、本体部10の外形における幅方向の延べ寸法は、好ましくは30~200mmであり、より好ましくは40~180mmである。
The shape of the main body portion 10 is not particularly limited as long as it is a shape that conforms to the shape of a female body and an underwear, such as a rectangular shape, an oval shape, and an hourglass shape. The longitudinal dimension in the outer shape of the main body 10 is preferably 100 to 500 mm, more preferably 150 to 350 mm. Further, the dimension in the width direction of the outer shape of the main body 10 is preferably 30 to 200 mm, more preferably 40 to 180 mm.
トップシート2は、本体部10では、着用者から排出された尿、経血などの体液を、吸収体4へ移動させる。また、本体部10では、トップシート2の少なくとも一部は液透過性を有し、体液を透過するための多数の開口部を有する。一方、ウイング部6では、トップシート2は、着用者がウイング部6を折り曲げるのに適するとともに、下着に取り付けられた後も着用者に違和感を与えないような適度な剛性を、後述のバックシート3と一緒になってウイング部6に付与する。
The top sheet 2 moves body fluid such as urine and menstrual blood discharged from the wearer to the absorber 4 in the main body portion 10. Further, in the main body portion 10, at least a part of the top sheet 2 has liquid permeability, and has a large number of openings for transmitting body fluid. On the other hand, in the wing portion 6, the top sheet 2 is suitable for the wearer to bend the wing portion 6, and has a suitable rigidity not to give the wearer discomfort after being attached to the undergarment, as described later. Apply to wing 6 together with 3.
トップシート2は、好ましくは樹脂フィルムから作製される。トップシート2として使用される樹脂フィルムは、オレフィンとアクリル酸エステル、酢酸ビニルなどの他モノマーとの共重合体、ポリオレフィン、ポリエステル、ポリプロピレン、ポリエチレン、ポリエチレンテレフタレート、ポリアミド、酢酸セルロースなどから作製される。柔軟性が高く肌に対する刺激が少ない点から、トップシート2として使用される樹脂フィルムは、好ましくはオレフィンと他モノマーとの共重合体、またはポリオレフィンである。
The top sheet 2 is preferably made of a resin film. The resin film used as the top sheet 2 is made of a copolymer of an olefin and an acrylic ester, another monomer such as vinyl acetate, polyolefin, polyester, polypropylene, polyethylene, polyethylene terephthalate, polyamide, cellulose acetate or the like. The resin film used as the top sheet 2 is preferably a copolymer of an olefin and another monomer, or a polyolefin, in terms of high flexibility and low irritation to the skin.
トップシート2の坪量は、好ましくは1g/m2以上、40g/m2以下であり、より好ましくは10g/m2以上、35g/m2以下である。また、トップシート2を構成する樹脂フィルムの厚さは、好ましくは0.01mm以上、0.4mm以下であり、より好ましくは0.1mm以上、0.35mm以下である。トップシート2を構成する樹脂フィルムの厚さが0.01mm未満の場合には、トップシート2の後述の隠ぺい性が小さくなりすぎる場合があり、トップシート2を構成する樹脂フィルムの厚さが0.4mmを超える場合には、トップシート2の剛性が高くなり、着用者の肌に対するトップシート2の刺激が強くなりすぎる場合がある。トップシート2は後述する第1の山折り部、第2の山折り部および谷折り部を有するので、トップシート2の見かけ上の厚さは、トップシート2を構成する樹脂フィルムの厚さよりも大きい。トップシート2の見かけ上の厚さは、好ましくは0.01mm以上、1mm以下であり、より好ましくは0.1mm以上、0.5mm以下である。
The basis weight of the top sheet 2 is preferably 1 g / m 2 or more and 40 g / m 2 or less, more preferably 10 g / m 2 or more and 35 g / m 2 or less. Moreover, the thickness of the resin film which comprises the top sheet 2 becomes like this. Preferably it is 0.01 mm or more and 0.4 mm or less, More preferably, it is 0.1 mm or more and 0.35 mm or less. When the thickness of the resin film constituting the top sheet 2 is less than 0.01 mm, the hiding property of the top sheet 2 described later may be too small, and the thickness of the resin film constituting the top sheet 2 is 0 When it exceeds .4 mm, the rigidity of the top sheet 2 may be high, and the stimulation of the top sheet 2 to the wearer's skin may be too strong. Since the top sheet 2 has a first mountain fold portion, a second mountain fold portion and a valley fold portion described later, the apparent thickness of the top sheet 2 is greater than the thickness of the resin film constituting the top sheet 2 large. The apparent thickness of the top sheet 2 is preferably 0.01 mm or more and 1 mm or less, more preferably 0.1 mm or more and 0.5 mm or less.
トップシート2は、吸収体4に吸収された体液が外部から見えないようにするため、隠ぺい性を有する。トップシート2の隠ぺい性は、酸化チタンなどのフィラーを樹脂に混ぜることによって生ずる。フィラーが酸化チタンの場合、酸化チタンの含有量は、樹脂フィルムの重量に対して、好ましくは1%以上、50%以下であり、より好ましくは3%以上、15%以下である。酸化チタンの含有量が樹脂フィルムの重量に対して1%未満であると、トップシート2における吸収体4に吸収された体液を隠ぺいする効果が小さすぎる場合がある。酸化チタンの含有量が樹脂フィルムの重量に対して50%を超えると、酸化チタンを含有した樹脂のシート成形が困難になる場合がある。
The top sheet 2 has concealability in order to prevent the body fluid absorbed by the absorber 4 from being seen from the outside. The hiding property of the top sheet 2 is produced by mixing a filler such as titanium oxide into the resin. When the filler is titanium oxide, the content of titanium oxide is preferably 1% to 50%, more preferably 3% to 15%, based on the weight of the resin film. When the content of the titanium oxide is less than 1% with respect to the weight of the resin film, the effect of hiding the body fluid absorbed by the absorber 4 in the top sheet 2 may be too small. When the content of titanium oxide exceeds 50% with respect to the weight of the resin film, sheet forming of the resin containing titanium oxide may be difficult.
トップシート2は、長手方向に延在し、幅方向に交互に並ぶ第1の山折り部21および谷折り部23と、第1の山折り部21に交差する方向に延在する第2の山折り部22とを有する。
The top sheet 2 extends in the longitudinal direction, and extends in a direction intersecting the first mountain fold 21 and the valley fold 23 alternately arranged in the width direction, and the first mountain fold 21. And a mountain fold portion 22.
図3および図4を参照して、トップシート2に設けられた第1の山折り部21、第2の山折り部22および谷折り部23を詳細に説明する。図3は、本体部10のトップシート2における吸収体4の幅方向の両側面41の外側の領域14およびウイング部6のトップシート2に形成した(図1参照)第1の山折り部、第2の山折り部および谷折り部を説明するための図である。図4は、本体部10のトップシート2における中央部分12に形成した(図1参照)第1の山折り部、第2の山折り部および谷折り部を説明するための図である。本体部10のトップシート2における吸収体4の幅方向の両側面41の外側の領域14およびウイング部6のトップシート2に形成した第1の山折り部、第2の山折り部および谷折り部と、本体部10のトップシート2における中央部分12に形成した第1の山折り部、第2の山折り部および谷折り部とは、後述するように少し異なる。
The first mountain fold 21, the second mountain fold 22 and the valley fold 23 provided in the top sheet 2 will be described in detail with reference to FIGS. 3 and 4. FIG. 3 is a first mountain-folded portion formed on the region 14 outside the side surfaces 41 in the width direction of the absorber 4 in the top sheet 2 of the main body 10 and the top sheet 2 of the wing 6 (see FIG. 1) It is a figure for demonstrating a 2nd mountain fold part and a valley fold part. FIG. 4 is a view for explaining a first mountain fold, a second mountain fold, and a valley fold formed on the central portion 12 of the top sheet 2 of the main body 10 (see FIG. 1). A first mountain fold, a second mountain fold, and a valley fold formed on the region 14 outside the both side surfaces 41 in the width direction of the absorber 4 in the top sheet 2 of the main body 10 and the top sheet 2 of the wing 6 The first mountain-folded portion, the second mountain-folded portion and the valley-folded portion formed in the central portion 12 of the top sheet 2 of the main body portion 10 are slightly different as described later.
図3に示すように、吸収体4の幅方向の両側面41の外側の領域14およびウイング部6において、トップシート2は、長手方向(Y方向)に延びており、幅方向(X方向)に交互に並ぶ第1の山折り部21および谷折り部23を含む。第1の山折り部21および谷折り部23の断面の形状は、たとえば、略U字形状である。略U字形状には、U字形状の他に、角を丸めたり、直線を曲線に変えたりするなどの変形を加えるとU字形状になる形状も含まれる。たとえば、略U字形状には、V字形状、M字形状、台形およびΩ形状も含まれる。
As shown in FIG. 3, the top sheet 2 extends in the longitudinal direction (Y direction) in the region 14 and the wing portion 6 outside the side surfaces 41 in the width direction of the absorber 4 in the width direction (X direction) The first mountain fold 21 and the valley fold 23 are alternately arranged. The shape of the cross section of the first mountain fold 21 and the valley fold 23 is, for example, a substantially U-shape. The substantially U-shape includes, in addition to the U-shape, a shape that becomes a U-shape when it is deformed such as rounding corners or changing a straight line into a curve. For example, the substantially U-shape also includes V-shape, M-shape, trapezoid and Ω-shape.
第2の山折り部22は、長手方向(Y方向)に延びる第1の山折り部21に対して交差する方向に延びていることが好ましい。たとえば、第1の山折り部21が延びる方向と第2の山折り部22が延びる方向との間のなす角度は、10°以上、170°以下であることが好ましい。第2の山折り部22が延びる方向に対して直交する方向で第2の山折り部22を切断したときの第2の山折り部22の断面の形状は、たとえば略U字形状である。
The second mountain folds 22 preferably extend in a direction intersecting the first mountain folds 21 extending in the longitudinal direction (Y direction). For example, the angle between the direction in which the first mountain fold 21 extends and the direction in which the second mountain fold 22 extends is preferably 10 ° or more and 170 ° or less. The shape of the cross section of the second mountain fold 22 when the second mountain fold 22 is cut in a direction orthogonal to the direction in which the second mountain fold 22 extends is, for example, a substantially U-shape.
幅方向における1cm当たりの第1の山折り部21の数は、好ましくは3以上であり、より好ましくは、5以上である。幅方向における1cm当たりの第1の山折り部21の数が2以下であると、第1の山折り部21によってトップシート2に生じるクッション性が弱くなる場合がある。
The number of first mountain folds 21 per cm in the width direction is preferably 3 or more, and more preferably 5 or more. If the number of first mountain-folded portions 21 per 1 cm in the width direction is 2 or less, the cushioning properties generated on the top sheet 2 by the first mountain-folded portions 21 may be weakened.
第2の山折り部22の長手方向(Y方向)の長さは、好ましくは0.3mm以上、5mm以下であり、より好ましくは0.5mm以上、3mm以下である。第2の山折り部22の長手方向(Y方向)の長さが0.3mmよりも小さい場合、第2の山折り部22における第1の山折り部21をつぶれにくくする効果が小さくなる場合がある。また、第2の山折り部22の長手方向(Y方向)の長さが5mmよりも大きい場合、第1の山折り部21によってトップシート2に生じるクッション性が弱くなる場合がある。
The length in the longitudinal direction (Y direction) of the second mountain-folded portion 22 is preferably 0.3 mm or more and 5 mm or less, more preferably 0.5 mm or more and 3 mm or less. When the length in the longitudinal direction (Y direction) of the second mountain fold 22 is smaller than 0.3 mm, the effect of making the first mountain fold 21 in the second mountain fold 22 less likely to collapse is reduced There is. When the length of the second mountain fold 22 in the longitudinal direction (Y direction) is larger than 5 mm, the cushioning property of the top sheet 2 may be weakened by the first mountain fold 21.
少なくとも吸収体4の幅方向の両側面41の外側の領域14においてトップシート2に第1の山折り部21および第2の山折り部22を設けることによって、吸収性物品1を下着に固定するためにウイング部6を折り曲げたときの折り目31付近に第1の山折り部21および/または第2の山折り部が存在することになる(図6参照)。第1の山折り部21および第2の山折り部22は、トップシート2にクッション性を付与することによって軟らかい肌触りを吸収性物品1の表面に付与する。したがって、これにより、吸収性物品1を装着した下着を履いたときの下着の足ぐり周りの肌触りが良好になる。
The absorbent article 1 is fixed to the undergarment by providing the top sheet 2 with the first mountain fold 21 and the second mountain fold 22 at least in the area 14 outside the side surfaces 41 in the width direction of the absorbent body 4. For this purpose, the first mountain fold 21 and / or the second mountain fold are present in the vicinity of the fold line 31 when the wing 6 is folded (see FIG. 6). The first mountain fold 21 and the second mountain fold 22 impart a soft touch to the surface of the absorbent article 1 by providing the top sheet 2 with cushioning properties. Therefore, when this wears the underwear equipped with the absorbent article 1, the touch around the foot of the underwear becomes good.
図4に示すように、本体部10の中央部分12のトップシート2は、本体部10の吸収体4の幅方向の両側面41の外側の領域14およびウイング部6におけるトップシート2と同様に、第1の山折り部21、第2の山折り部22および谷折り部23を含む。しかし、本体部10の中央部分12のトップシート2の第1の山折り部21、第2の山折り部22および谷折り部23は、肌側の表面に後述の血液改質剤層24をさらに含む。また、本体部10の中央部分12のトップシート2の第1の山折り部21および谷折り部23は、後述の開口部25をさらに含む。
As shown in FIG. 4, the top sheet 2 of the central portion 12 of the main body 10 is the same as the top sheet 2 of the region 14 outside the side surfaces 41 of the main body 10 in the width direction and the wing 6. , A first mountain fold 21, a second mountain fold 22 and a valley fold 23. However, the first mountain fold 21, the second mountain fold 22 and the valley fold 23 of the top sheet 2 of the central portion 12 of the main body 10 have a blood modifying layer 24 described later on the surface on the skin side. Further include. Further, the first mountain fold 21 and the valley fold 23 of the top sheet 2 of the central portion 12 of the main body 10 further include an opening 25 described later.
本体部10の吸収体4が設けられている領域12のトップシート2の第1の山折り部21および谷折り部23の両側面26に、長手方向(Y方向)に並ぶ複数の開口部25が設けられている。本体部10の吸収体4が設けられている領域12のトップシート2に排出された着用者の体液は、開口部25を通過して吸収体4へ移動する。また、谷折り部23によって形成される幅方向(X方向)断面の略U字形状によって、トップシート2に排出された着用者の体液は、谷折り部23の底の方に向かって素早く集まる。谷折り部23の底の方に向かって流れる体液は、谷折り部23に到達する前に、第1の山折り部21および谷折り部23の側面26に設けられた開口部25を通過し、吸収体4に流れる。また、谷折り部23に溜まった体液も第1の山折り部21および谷折り部23の側面26に設けられた開口部25を通過し、吸収体4に流れる。したがって、第1の山折り部21および谷折り部23の側面26に設けられた開口部25によって、着用者の体液は、吸収体4に素早く吸収される。また、第1の山折り部21および谷折り部23の側面26に設けられた開口部25によって、トップシート2に残留する体液の量を減少させることができる。
A plurality of openings 25 aligned in the longitudinal direction (Y direction) on both side surfaces 26 of first mountain fold 21 and valley fold 23 of top sheet 2 of region 12 of main body 10 in which absorber 4 is provided. Is provided. The body fluid of the wearer discharged to the top sheet 2 of the area 12 where the absorber 4 of the main body 10 is provided passes through the opening 25 and moves to the absorber 4. In addition, the body fluid of the wearer discharged to the top sheet 2 quickly gathers toward the bottom of the valley fold portion 23 due to the substantially U shape of the cross section (X direction) cross section formed by the valley fold portion 23 . The body fluid flowing toward the bottom of the valley fold 23 passes through the opening 25 provided on the side 26 of the first mountain fold 21 and the valley fold 23 before reaching the valley fold 23. , Flows to the absorber 4. Further, the body fluid accumulated in the valley fold portion 23 also passes through the openings 25 provided on the side surfaces 26 of the first mountain fold portion 21 and the valley fold portion 23 and flows to the absorber 4. Therefore, the body fluid of the wearer is quickly absorbed by the absorbent body 4 by the openings 25 provided on the side surfaces 26 of the first mountain fold 21 and the valley fold 23. In addition, the amount of body fluid remaining in the top sheet 2 can be reduced by the openings 25 provided on the side faces 26 of the first mountain fold 21 and the valley fold 23.
本体部10の中央部分12のトップシート2の肌側の表面に血液改質剤を塗布して、トップシート2の肌当接側の表面に血液改質剤層24を形成してもよい。この血液改質剤層24によって、トップシート2の表面に、着用者の体液、とくに粘度の高い経血が残存することを抑制できる。この血液改質剤層24は、後で詳細に説明する。
A blood modifying agent may be applied to the skin side surface of the top sheet 2 of the central portion 12 of the main body portion 10 to form the blood modifying layer 24 on the skin contact side surface of the top sheet 2. The blood modifying agent layer 24 can prevent the body fluid of the wearer, in particular, highly viscous menstrual blood from remaining on the surface of the top sheet 2. The blood modifying agent layer 24 will be described in detail later.
本体部10の中央部分12のトップシート2に設けられた第1の山折り部21および谷折り部23によって着用者の肌とトップシート2との間の接触面積が小さくなるので、トップシート2の肌触りが良好になる。なお、本体部10の中央部分12のトップシート2に第1の山折り部および谷折り部のみを形成し、第2の山折り部を形成しなくてもよい。この場合も着用者の肌とトップシート2との間の接触面積が小さくなるので、トップシート2の肌触りが良好になる。しかし、第2の山折り部を設けることによって、第1の山折り部はつぶれにくくなるので、本体部10の中央部分12のトップシート2に、第1の山折り部、第2の山折り部および谷折り部を設けることが好ましい。
Since the contact area between the skin of the wearer and the top sheet 2 is reduced by the first mountain fold 21 and the valley fold 23 provided on the top sheet 2 of the central portion 12 of the main body 10, the top sheet 2 Your skin feels good. In addition, it is not necessary to form only a 1st mountain fold part and a valley fold part in the top sheet 2 of the center part 12 of the main-body part 10, and to form a 2nd mountain fold part. Also in this case, since the contact area between the skin of the wearer and the top sheet 2 is reduced, the touch of the top sheet 2 is improved. However, by providing the second mountain fold portion, the first mountain fold portion is less likely to be crushed, so the first mountain fold portion, the second mountain fold portion is formed on the top sheet 2 of the central portion 12 of the main body portion 10. It is preferable to provide a section and a valley fold.
図1および図2に示すバックシート3は、吸収体4に吸収された体液が外へ漏れ出すのを防止する。バックシート3には、体液を透過しない材料が使用される。たとえば、バックシート3として、疎水性の不織布、ポリエチレンおよびポリプロピレンなどの不透水性のプラスチックフィルムまたは不織布と不透水性プラスチックフィルムとのラミネートシートなどが使用される。また、耐水性の高いメルトブローン不織布を強度の強いスパンボンド不織布で挟んだスパンボンド・メルトブロー・スパンボンド(SMS)繊維不織布をバックシート3として使用してもよい。体液は通さない通気性を有する材料をバックシート3として使用することにより、着用時のムレを低減させることができる。バックシート3はホットメルト接着剤などの接着剤を使用してトップシート2の谷折り部23の底と接合している。
The back sheet 3 shown in FIGS. 1 and 2 prevents the body fluid absorbed by the absorber 4 from leaking out. The back sheet 3 is made of a material that is impermeable to body fluid. For example, as the back sheet 3, a hydrophobic non-woven fabric, an impermeable plastic film such as polyethylene and polypropylene or a laminate sheet of non-woven fabric and an impermeable plastic film, or the like is used. Alternatively, a spunbond-meltblown-spunbond (SMS) fibrous nonwoven fabric may be used as the back sheet 3 in which a highly water-resistant meltblown nonwoven fabric is sandwiched by strong spunbond nonwoven fabrics. By using a material having air permeability that does not pass body fluid as the back sheet 3, it is possible to reduce stuffiness when worn. The back sheet 3 is joined to the bottom of the valley fold 23 of the top sheet 2 using an adhesive such as a hot melt adhesive.
吸収体4は体液を吸収して保持する。吸収体4は、嵩高であり、型崩れし難く、化学的刺激が少ないものであることが好ましい。たとえば、吸収体4として、フラッフ状パルプもしくはエアレイド不織布と高吸収性ポリマー(SAP)とからなる複合吸収体が使用される。この複合吸収体は、ティッシュなどの液透過性の材料で覆われていてもよい。
The absorber 4 absorbs and holds the body fluid. It is preferable that the absorber 4 is bulky, hard to lose its shape, and less in chemical stimulation. For example, a composite absorbent made of fluff pulp or air-laid non-woven fabric and super absorbent polymer (SAP) is used as the absorbent 4. The composite absorber may be covered with a liquid permeable material such as a tissue.
また、上記複合吸収体のフラッフ状パルプの代わりに、たとえば、化学パルプ、セルロース繊維、レーヨンおよびアセテートなどの人工セルロース繊維を使用してもよい。上記複合吸収体中のパルプなどの吸収性繊維の坪量は、好ましくは100g/m2以上、800g/m2以下であり、上記複合吸収体中の高吸収性ポリマーの質量比は、吸収性繊維を100%として好ましくは10%以上、65%以下である。上記複合混合体を覆うティッシュなど液透過性の材料の坪量は、好ましくは12g/m2以上、30g/m2以下である。
Also, instead of the fluff pulp of the composite absorbent, artificial cellulose fibers such as chemical pulp, cellulose fibers, rayon and acetate may be used. The basis weight of the absorbent fiber such as pulp in the composite absorbent is preferably 100 g / m 2 or more and 800 g / m 2 or less, and the mass ratio of the superabsorbent polymer in the composite absorbent is the absorbency The fiber content is preferably 100% to 10% to 65%. The basis weight of a liquid-permeable material such as a tissue covering the composite mixture is preferably 12 g / m 2 or more and 30 g / m 2 or less.
上記複合混合体のエアレイド不織布として、たとえば、パルプと合成繊維とを熱融着させた不織布またはパルプと合成繊維とをバインダーで固着させた不織布を使用することができる。
As the air-laid non-woven fabric of the composite mixture, for example, a non-woven fabric in which pulp and synthetic fibers are heat-sealed or a non-woven fabric in which pulp and synthetic fibers are fixed with a binder can be used.
上記複合吸収体の高吸収性ポリマーは、水溶性高分子が適度に架橋した三次元網目構造を有する。この吸収性ポリマーは、水を吸収する前の吸収性ポリマーの体積に対して30~60倍の水を吸収する。しかし、この吸収性ポリマーは本質的に水不溶性である。また、この吸収性ポリマーは、多少の圧力を加えられても、一旦吸収された水を離水しない。この吸収性ポリマーとして、たとえば、デンプン系、アクリル酸系またはアミノ酸系の粒子状または繊維状のポリマーが使用される。
The superabsorbent polymer of the composite absorbent has a three-dimensional network structure in which a water-soluble polymer is appropriately crosslinked. The absorbent polymer absorbs 30 to 60 times as much water as the volume of absorbent polymer before absorbing water. However, this absorbable polymer is essentially water insoluble. Also, the absorbent polymer does not release the water once absorbed, even if some pressure is applied. As this absorbent polymer, for example, starch-based, acrylic acid-based or amino acid-based particulate or fibrous polymers are used.
吸収体4の形状および構造は必要に応じて変えることができるが、吸収体4の全吸収量は、吸収性物品1としての設計挿入量および所望の用途に対応させる必要がある。また、吸収体4のサイズや吸収能力などは用途に応じて変わる。
The shape and structure of the absorber 4 can be changed as needed, but the total amount of absorption of the absorber 4 needs to correspond to the designed insertion amount as the absorbent article 1 and the desired application. In addition, the size, absorption capacity and the like of the absorber 4 change depending on the application.
ホットメルト接着剤を使用して吸収体4はトップシート2と接着している。これによりトップシート2が吸収体4から剥がれることを抑制できる。
Absorber 4 is bonded to top sheet 2 using a hot melt adhesive. Thereby, it can suppress that the top sheet 2 peels from the absorber 4. As shown in FIG.
折曲抑制シート5は、下着のクロッチ域の幅が吸収性物品1に設けられている吸収体4の幅より狭い場合に、ウイング部6を折り畳んで吸収性物品1を下着に取り付けたときに、吸収体4の側面41(図1参照)の部分が着用者の内股に当たることを抑制する。また、折曲抑制シート5は、着用者の下着のクロッチ域の幅が吸収性物品1に設けられている吸収体4の幅より狭い場合に、ウイング部6を折り畳んで吸収性物品1を下着に取り付けるときに、吸収体4の側面41の部分に大きなヨレが生じることを抑制できる。
When the width of the crotch region of the undergarment is narrower than the width of the absorbent body 4 provided in the absorbent article 1, the folding prevention sheet 5 folds the wing portion 6 and attaches the absorbent article 1 to the underwear. The portion of the side surface 41 (see FIG. 1) of the absorbent body 4 is prevented from hitting the inner crotch of the wearer. In addition, when the width of the crotch region of the wearer's underwear is narrower than the width of the absorbent body 4 provided in the absorbent article 1, the folding suppression sheet 5 folds the wing portion 6 to make the absorbent article 1 underwear. When attached to the case, it is possible to suppress the occurrence of a large deflection in the portion of the side surface 41 of the absorber 4.
図1および図2に示すように、折曲抑制シート5の幅方向の縁51は、吸収体4の幅方向の側面41に比べて幅方向外側に位置する。これにより、ウイング部6を折り畳んで吸収性物品1を下着に取り付けたときに、吸収体4の側面41の部分が折り目になるようにウイング部6が折り曲がることを抑制できる。
As shown to FIG. 1 and FIG. 2, the edge 51 of the width direction of the bending suppression sheet | seat 5 is located in the width direction outer side compared with the side 41 of the width direction of the absorber 4. As shown in FIG. Thereby, when the wing 6 is folded and the absorbent article 1 is attached to the undergarment, it is possible to suppress the wing 6 from being bent so that the portion of the side surface 41 of the absorber 4 has a fold.
折曲抑制シート5が設けられている領域が、折曲抑制シート5が設けられていない領域に比べて、吸収性物品1が折り曲げにくくなるように、吸収性物品1に剛性を付与できれば、折曲抑制シート5として用いるシートはとくに限定されない。しかし、吸収体折曲シート5の剛性が高すぎると、吸収性物品1全体の剛性が高くなり過ぎるため、吸収性物品1が着用者に対して強い違和感を生じさせる場合がある。したがって、吸収体折曲シート5は、着用者が着用しても強い違和感が生じない程度の高さの剛性を有していることが好ましい。
If rigidity can be imparted to the absorbent article 1 so that the absorbent article 1 becomes difficult to bend compared to the area where the bending suppression sheet 5 is provided, compared to the area where the bending suppression sheet 5 is not provided, folding The sheet used as the music suppression sheet 5 is not particularly limited. However, if the rigidity of the absorbent folded sheet 5 is too high, the rigidity of the entire absorbent article 1 becomes too high, and the absorbent article 1 may cause a strong discomfort to the wearer. Therefore, it is preferable that the absorber folded sheet 5 have a rigidity of such a height that a strong discomfort does not occur even when worn by the wearer.
たとえば、折曲抑制シート5におけるカンチレバー法(JIS L 1096)によって測定される剛軟度は、好ましくは15~120mmであり、より好ましくは30~70mmである。折曲抑制シート5のカンチレバー法による剛軟度が15mmよりも小さい場合、吸収体4の側面41の部分が折り目になるようにウイング部6が折り曲がることを抑制できない場合がある。すなわち、着用者が吸収性物品を下着に取り付けるためにウイング部6を折り畳むとき、トップシート2およびバックシート3と一緒に折曲抑制シート5が吸収体4の側面41の部分で折り曲がる場合がある。また、折曲抑制シート5のカンチレバー法による剛軟度が120mmよりも大きい場合、吸収性物品1の剛性が高くなり過ぎてしまい、吸収性物品1は着用者に対して強い違和感を生じさせる場合がある。
For example, the bending resistance measured by the cantilever method (JIS L 1096) in the bending suppression sheet 5 is preferably 15 to 120 mm, more preferably 30 to 70 mm. When the bending resistance by the cantilever method of the bending suppression sheet | seat 5 is smaller than 15 mm, it may be unable to suppress that the wing part 6 bends so that the part of the side 41 of the absorber 4 may become a crease. That is, when the wearer folds the wing portion 6 in order to attach the absorbent article to the undergarment, there may be a case where the bending suppression sheet 5 is bent at the side 41 of the absorber 4 together with the top sheet 2 and the back sheet 3 is there. Moreover, when the bending resistance by the cantilever method of the bending suppression sheet | seat 5 is larger than 120 mm, the rigidity of the absorbent article 1 becomes high too much, and the absorbent article 1 produces strong discomfort with respect to a wearer. There is.
折曲抑制シート5として用いるシートは、好ましくは樹脂フィルム、開孔樹脂フィルム、織布または不織布であり、より好ましくは不織布である。折曲抑制シート5に好適な不織布は、たとえば、熱可塑性疎水性化学繊維などの化学繊維の不織布である。折曲抑制シート5に好適な熱可塑性疎水性化学繊維には、たとえば、ポリエチレン(PE)、ポリプロピレン(PP)、ポリエチレンテレフタレート(PET)などの単繊維、PEとPPとをグラフト重合した複合繊維、芯鞘構造を有する複合繊維などがある。
The sheet used as the bending suppression sheet 5 is preferably a resin film, a perforated resin film, a woven fabric or a non-woven fabric, and more preferably a non-woven fabric. A non-woven fabric suitable for the bending suppression sheet 5 is, for example, a non-woven fabric of chemical fibers such as thermoplastic hydrophobic chemical fibers. Thermoplastic hydrophobic chemical fibers suitable for the bending suppression sheet 5 include, for example, single fibers such as polyethylene (PE), polypropylene (PP) and polyethylene terephthalate (PET), composite fibers obtained by graft polymerizing PE and PP, There is a composite fiber having a core-sheath structure.
折曲抑制シート5に用いる不織布を作製するとき、カード法、スパンボンド法、メルトブローン法、エアレイド法などの乾式法または湿式法でウエブを形成してもよい。また、折曲抑制シート5に用いる不織布を作製するとき、サーマルボンディング、ニードルパンチ、ケミカルボンディングなどのボンディングを行ってもよい。折曲抑制シート5には、たとえば、SMS繊維不織布を使用することができる。
When producing a non-woven fabric used for the bending suppression sheet 5, the web may be formed by a dry method such as a card method, a spun bond method, a meltblown method, an air laid method or a wet method. Moreover, when producing the nonwoven fabric used for the bending suppression sheet 5, you may perform bonding, such as thermal bonding, a needle punch, chemical bonding. For example, an SMS fiber non-woven fabric can be used for the bending suppression sheet 5.
折曲抑制シート5として使用する不織布の目付けは、好ましくは10~40g/m2であり、より好ましくは13~20g/m2である。その不織布の目付けが10g/m2よりも小さい場合、吸収体4の側面41の部分が折り目になるようにウイング部6が折り曲がることを抑制できない場合がある。すなわち、着用者が吸収性物品を下着に取り付けるためにウイング部6を折り畳むとき、トップシート2およびバックシート3と一緒に折曲抑制シート5が吸収体4の側面41の部分で折り曲がる場合がある。また、その不織布の目付けが40g/m2よりも大きい場合、吸収性物品1の剛性が高くなり過ぎてしまい、吸収性物品1は着用者に対して強い違和感を生じさせる場合がある。
The basis weight of the non-woven fabric used as the bending suppression sheet 5 is preferably 10 to 40 g / m 2 , more preferably 13 to 20 g / m 2 . If the basis weight of the non-woven fabric is smaller than 10 g / m 2, it may not be possible to suppress bending of the wing portion 6 such that the portion of the side surface 41 of the absorber 4 has a fold. That is, when the wearer folds the wing portion 6 in order to attach the absorbent article to the undergarment, there may be a case where the bending suppression sheet 5 is bent at the side 41 of the absorber 4 together with the top sheet 2 and the back sheet 3 is there. Moreover, when the fabric weight of the nonwoven fabric is larger than 40 g / m 2 , the rigidity of the absorbent article 1 becomes too high, and the absorbent article 1 may cause a strong discomfort to the wearer.
折曲抑制シート5として用いるシートは、疎水性または撥水性を有するシートであることが好ましい。これにより、吸収体4からしみ出した体液が折曲抑制シート5を通って吸収性物品1の幅方向の部分から漏れることを抑制できる。
The sheet used as the bending suppression sheet 5 is preferably a sheet having hydrophobicity or water repellency. Thereby, it can suppress that the bodily fluid which exuded from absorber 4 leaks from the part of the cross direction of absorptive article 1 through bending control sheet 5.
折曲抑制シート5は、ホットメルト接着剤などの接着剤で吸収体4およびバックシート3と接合している。しかし、折曲抑制シート5は、トップシート2とは接合していない。これにより、トップシート2は、折曲抑制シート5の影響を受けずに、吸収体4の側面41の幅方向外側の領域において柔らかな肌触り有することができる。
The bending suppression sheet 5 is joined to the absorber 4 and the back sheet 3 by an adhesive such as a hot melt adhesive. However, the bending suppression sheet 5 is not joined to the top sheet 2. Thereby, the top sheet 2 can have a soft touch in the area | region of the width direction outer side of the side surface 41 of the absorber 4, without receiving to the influence of the bending suppression sheet | seat 5. FIG.
図5および図6を参照して、折曲抑制シート5が、着用者の下着のクロッチ域の幅が吸収性物品1に設けられている吸収体4の幅より狭い場合に、ウイング部6を折り畳んで吸収性物品1を下着に取り付けたときに、吸収体4の側面41の部分が着用者の内股に当たることを抑制する原理を説明する。図5は、下着のクロッチ域に装着した本発明の一実施形態の吸収性物品を説明するための図である。図6は、図1のB-B線断面を示す概略断面図である。図5および6の符号70は、下着のクロッチ域を示し、符号71は、下着の足開口部の縁を示す。これより、図5および図6において、着用者の下着のクロッチ域70の幅が吸収性物品1に設けられている吸収体4の幅より狭いことがわかる。
With reference to FIGS. 5 and 6, when the width of the crotch area of the wearer's underwear is narrower than the width of the absorbent body 4 provided in the absorbent article 1, the folding suppression sheet 5 is used. When it folds and attaches the absorbent article 1 to underwear, the principle which suppresses that the part of the side surface 41 of the absorber 4 hits a wearer's inner crotch is demonstrated. FIG. 5 is a view for explaining an absorbent article according to an embodiment of the present invention attached to the crotch region of the undergarment. FIG. 6 is a schematic cross-sectional view showing a cross section taken along the line BB of FIG. Reference numeral 70 in FIGS. 5 and 6 denotes the crotch area of the undergarment, and reference numeral 71 denotes the edge of the foot opening of the undergarment. From FIGS. 5 and 6, it can be seen that the width of the crotch region 70 of the wearer's underwear is narrower than the width of the absorbent body 4 provided in the absorbent article 1.
折曲抑制シート5が設けられている領域では、吸収性物品1は折り曲げづらくなっているため、着用者がウイング部6を折り畳んで下着のクロッチ域70に下着を取り付けた場合、ウイング部6を折り畳んだときに生じる吸収性物品1の折り目31は、折曲抑制シート5の幅方向の縁51とほぼ一致するようになる。したがって、図5および図6に示すように、下着の足開口の縁71の位置が吸収体4の位置と重なっても、ウイング部6を折り畳んだときに吸収体4の側面41の位置で吸収性物品1が折れ曲がることが抑制される。これにより、吸収体40の側面41が幅方向外側に突出することがなくなるので、吸収体4の側面41の部分が着用者の内股に当たることが抑制される。また、ウイング部6を折り畳んだとき、吸収体40の側面41の部分に大きなヨレが生じることが抑制されるので、着用者の体液が吸収性物品1から幅方向へ漏れることを抑制できる。
Since the absorbent article 1 is difficult to bend in the area where the folding prevention sheet 5 is provided, when the wearer folds the wing portion 6 and attaches the underwear to the crotch region 70 of the underwear, the wing portion 6 is The folds 31 of the absorbent article 1 produced when folded are substantially coincident with the edge 51 in the width direction of the anti-folding sheet 5. Therefore, as shown in FIG. 5 and FIG. 6, even if the position of the edge 71 of the foot opening of the undergarment overlaps with the position of the absorber 4, when the wing 6 is folded, the side 41 of the absorber 4 absorbs It is suppressed that the sex article 1 bends. Since this prevents the side surface 41 of the absorber 40 from protruding outward in the width direction, it is possible to suppress that the portion of the side surface 41 of the absorber 4 hits the inner crotch of the wearer. Further, when the wing portion 6 is folded, generation of a large deflection at the side surface 41 of the absorber 40 is suppressed, so that leakage of the body fluid of the wearer from the absorbent article 1 in the width direction can be suppressed.
折曲抑制シート5は着用者の体液を吸収するために設けられたものではないので、折曲抑制シート5の厚さは吸収体4の厚さよりも小さい。したがって、折曲抑制シート5の幅方向の縁51が着用者の内股に当たるときに生じる違和感は、吸収体4の側面41の部分が着用者の内股に当たるときに生じる違和感に比べて弱い。また、上述したように、トップシート2には、第1の山折り部21が形成されている。折曲抑制シート5の幅方向の縁51が着用者の内股に当たるときに、第1の山折り部21が、クッションの役割を果たし、折曲抑制シート5の幅方向の縁51が着用者の内股に当たるときに生じる違和感をさらに弱めることができる。すなわち、第1の山折り部21は、トップシート2にクッション性を付与し、折曲抑制シート5の幅方向の縁51が着用者の内股に当たるときに生じる違和感をさらに弱める。
Since the bending suppression sheet 5 is not provided to absorb the body fluid of the wearer, the thickness of the bending suppression sheet 5 is smaller than the thickness of the absorber 4. Therefore, the sense of incongruity which arises when the edge 51 in the width direction of the bending suppression sheet 5 hits the wearer's inner crotch is weaker than the sense of incongruity which occurs when the portion of the side surface 41 of the absorber 4 hits the wearer's crotch. Further, as described above, the top sheet 2 is provided with the first mountain fold portion 21. When the edge 51 in the width direction of the anti-folding sheet 5 hits the inner crotch of the wearer, the first mountain fold 21 acts as a cushion, and the edge 51 in the width direction of the anti-folding sheet 5 is the wearer's It can further reduce the sense of incongruity that occurs when hitting the inner crotch. That is, the first mountain fold 21 imparts cushioning properties to the top sheet 2, and further reduces the discomfort caused when the edge 51 in the width direction of the bending suppression sheet 5 hits the inner crotch of the wearer.
なお、折曲抑制シート5の幅方向の縁51が着用者の内股に当たるときに、クッションの役割を果たすことができれば、トップシート2に形成される突部は上述の第1の山折り部21および第2の山折り部に限定されない。たとえば、円柱状の突部、角柱状の突部などをトップシート2に形成してもよい。また、トップシート2にクッション性を付与する突部は、トップシート全体に設ける必要はなく、少なくとも前記吸収体の幅方向の両側面の外側において設けられていればよい。
In addition, when the edge 51 in the width direction of the bending suppression sheet 5 can act as a cushion when hitting the inner crotch of the wearer, the protrusion formed on the top sheet 2 is the above-described first mountain-folded portion 21 And it is not limited to the 2nd mountain fold part. For example, a cylindrical protrusion, a prismatic protrusion, or the like may be formed on the top sheet 2. Moreover, it is not necessary to provide the protrusion which gives cushioning properties to the top sheet 2 in the whole top sheet, and should just be provided in the outer side of the both sides of the width direction of the said absorber at least.
上述したように、ウイング部6は、吸収性物品1を下着に安定して固定するために吸収性物品1に設けられている。ウイング部6を下着の外面側に折り曲げた後、粘着部7を介して下着のクロッチ域に張り付けることによって、吸収性物品1を下着に安定して固定することができる。また、本体部10にも粘着部7が設けられており、下着のクロッチ域において本体部10がズレて動くことを防止する。
As described above, the wing portion 6 is provided on the absorbent article 1 in order to stably fix the absorbent article 1 to the undergarment. The absorbent article 1 can be stably fixed to the undergarment by bending the wing portion 6 to the outer surface side of the undergarment and then sticking it to the crotch region of the undergarment through the adhesive portion 7. Further, the adhesive portion 7 is also provided to the main body portion 10, and the main body portion 10 is prevented from shifting and moving in the crotch region of the undergarment.
粘着部7は、吸収性物品1を下着のクロッチ域に固定する。粘着部7を形成する粘着剤としては、たとえばスチレン系ポリマー、粘着付与剤、可塑剤のいずれかが主成分であるものが好適に使用される。前記スチレン系ポリマーとしては、スチレン-エチレン-ブチレン-スチレンブロック共重合体、スチレン-ブチレン重合体、スチレン-ブチレン-スチレンブロック共重合体、スチレン-イソブチレン-スチレン共重合体などが挙げられるが、これらのうち1種のみを使用しても、二種以上のポリマーブレンドであってもよい。この中でも熱安定性が良好であるという点で、スチレン-エチレン-ブチレン-スチレンブロック共重合体が好ましい。
The adhesive unit 7 fixes the absorbent article 1 to the crotch area of the undergarment. As an adhesive which forms the adhesion part 7, what is a styrene-type polymer, a tackifier, and a plasticizer as a main component, for example is used suitably. Examples of the styrene-based polymer include styrene-ethylene-butylene-styrene block copolymer, styrene-butylene polymer, styrene-butylene-styrene block copolymer, and styrene-isobutylene-styrene copolymer. Or a blend of two or more polymers. Among these, styrene-ethylene-butylene-styrene block copolymer is preferable in that the thermal stability is good.
また、前記粘着付与剤および可塑剤としては、常温で固体のものを好ましく用いることができ、粘着付与剤ではたとえばC5系石油樹脂、C9系石油樹脂、ジシクロペンタジエン系石油樹脂、ロジン系石油樹脂、ポリテルペン樹脂、テルペンフェノール樹脂などが挙げられ、前記可塑剤ではたとえば、リン酸トリフレシル、フタル酸ジブチル、フタル酸ジオクチルなどのモノマー可塑剤の他、ビニル重合体やポリエステルのようなポリマー可塑剤が挙げられる。
Further, as the tackifier and the plasticizer, those which are solid at normal temperature can be preferably used, and as the tackifier, for example, C5 petroleum resin, C9 petroleum resin, dicyclopentadiene petroleum resin, rosin petroleum resin And polyterpene resins, terpene phenol resins, etc., and examples of the plasticizer include, in addition to monomer plasticizers such as triflecil phosphate, dibutyl phthalate and dioctyl phthalate, polymer plasticizers such as vinyl polymers and polyesters. Be
図1および図2に示すように、トップシート2および吸収体4は、エンボス加工により厚み方向に圧縮して形成された、トップシート2から吸収体4の内部に至る圧搾溝8を有する。圧搾溝8は、吸収性物品1の中心部分(着用者の体液の排泄口に当接する部分)に排出された体液が幅方向(X方向)に拡散するのを抑制する。また、これにより吸収体4からトップシート2が剥がれることを抑制できる。圧搾溝8は、吸収性物品1の中心部分を囲み、連続的な略環状の形状を有する。なお、吸収性物品1の中央部を囲む圧搾溝8は、部分的に途切れていてもよい。すなわち、圧搾溝8は、非連続的な略環状の形状を有していてもよい。
As shown in FIGS. 1 and 2, the top sheet 2 and the absorbent body 4 have compressed grooves 8 extending from the top sheet 2 to the inside of the absorbent body 4 which are formed by being compressed in the thickness direction by embossing. The squeeze groove 8 suppresses the diffusion of the body fluid discharged to the central portion of the absorbent article 1 (the portion in contact with the excretory port of the wearer's body fluid) in the width direction (X direction). Moreover, it can suppress that the top sheet 2 peels from the absorber 4 by this. The squeeze groove 8 surrounds the central portion of the absorbent article 1 and has a continuous, substantially annular shape. In addition, the pressing groove 8 surrounding the center part of the absorbent article 1 may be partially disconnected. That is, the pressing groove 8 may have a discontinuous and substantially annular shape.
また、ヒートエンボス加工によりトップシート2をバックシート3に圧縮接合させることによって、吸収性物品1の長手方向両側および幅方向両側にシール部9が形成される。これにより、トップシート2がバックシート3から剥がれないようにできる。
Further, the compression bonding of the top sheet 2 to the back sheet 3 by heat embossing forms the seal portions 9 on both sides in the longitudinal direction and both sides in the width direction of the absorbent article 1. Thereby, the top sheet 2 can be prevented from peeling off from the back sheet 3.
次に、上述の血液改質剤層24を詳細に説明する。血液改質剤層24の血液改質剤は、血液改質剤は、約0.00~約0.60のIOBと、約45℃以下の融点と、25℃の水100gに対する、約0.00~約0.00~約0.05gの水溶解度とを有する。
Next, the above-mentioned blood modifying agent layer 24 will be described in detail. The blood modifying agent of the blood modifying agent layer 24 is a blood modifying agent having an IOB of about 0.00 to about 0.60, a melting point of about 45.degree. C. or less, 100 g of water at 25.degree. It has a water solubility of 00 to about 0.00 to about 0.05 g.
IOB(Inorganic Organic Balance)は、親水性および親油性のバランスを示す指標であり、本明細書では、小田らによる次式:
IOB=無機性値/有機性値
により算出される値を意味する。 IOB (Inorganic Organic Balance) is an index showing the balance of hydrophilicity and lipophilicity, and in the present specification, the following equation by Oda et al .:
IOB = value calculated by inorganic value / organic value.
IOB=無機性値/有機性値
により算出される値を意味する。 IOB (Inorganic Organic Balance) is an index showing the balance of hydrophilicity and lipophilicity, and in the present specification, the following equation by Oda et al .:
IOB = value calculated by inorganic value / organic value.
上記無機性値と、有機性値とは、藤田穆「有機化合物の予測と有機概念図」化学の領域Vol.11,No.10(1957)p.719-725)に記載される有機概念図に基づく。藤田氏による、主要な基の有機性値および無機性値を、下記表1にまとめる。
The above inorganic values and organic values are described in Satoshi Fujita, "Presence of Organic Compounds and Organic Conceptual Diagram", Chemistry Vol. 11, No. 10 (1957) p. Based on the organic conceptual diagram described in 719-725). The organic and inorganic values of the major groups by Mr. Fujita are summarized in Table 1 below.
たとえば、炭素数14のテトラデカン酸と、炭素数12のドデシルアルコールとのエステルの場合には、有機性値が520(CH2,20×26個)、無機性値が60(-COOR,60×1個)となるため、IOB=0.12となる。
For example, in the case of an ester of C14 tetradecanoic acid and C12 dodecyl alcohol, the organic value is 520 (CH 2 , 20 × 26) and the inorganic value is 60 (-COOR, 60 ×). Therefore, IOB = 0.12.
上記血液改質剤において、IOBは、約0.00~約0.60であり、約0.00~約0.50であることが好ましく、約0.00~約0.40であることがより好ましく、そして約0~約0.30であることがさらに好ましい。IOBが低いほど、有機性が高く、血球との親和性が高くなると考えられるからである。
In the above blood modifying agent, the IOB is about 0.00 to about 0.60, preferably about 0.00 to about 0.50, and about 0.00 to about 0.40. More preferred is about 0 to about 0.30. This is because the lower the IOB, the higher the organicity and the higher the affinity to blood cells.
本明細書において、「融点」は、示差走査熱量分析計において、昇温速度10℃/分で測定した場合の、固形状から液状に変化する際の吸熱ピークのピークトップ温度を意味する。上記融点は、たとえば、島津製作所社製のDSC-60型DSC測定装置を用いて測定することができる。
In the present specification, the "melting point" means the peak top temperature of an endothermic peak when changing from solid state to liquid state when measured at a temperature rising rate of 10 ° C./min in a differential scanning calorimeter. The melting point can be measured, for example, using a DSC-60 type DSC measurement apparatus manufactured by Shimadzu Corporation.
上記血液改質剤は、約45℃以下の融点を有すれば、室温で液体であっても、または固体であってもよい、すなわち、融点が約25℃以上でも、または約25℃未満でもよく、そしてたとえば、約-5℃、約-20℃等の融点を有することができる。上記血液改質剤の融点が約45℃以下である根拠は、後述する。
The blood modifying agent may be liquid or solid at room temperature as long as it has a melting point of about 45 ° C. or less, ie, even if the melting point is about 25 ° C. or more, or less than about 25 ° C. It may well have a melting point such as, for example, about -5.degree. C., about -20.degree. The reason why the melting point of the blood modifying agent is about 45 ° C. or less will be described later.
上記血液改質剤は、その融点に下限は存在しないが、その蒸気圧が低いことが好ましい。上記血液改質剤の蒸気圧は、1気圧および25℃で約0.00~約0.01Paであることが好ましく、約0.000~約0.001Paであることがより好ましく、そして約0.0000~約0.0001Paであることがさらに好ましい。本開示の吸収性物品が、人体に接して用いられることを考慮すると、上記蒸気圧は、1気圧および40℃で約0.00~約0.01Paであることが好ましく、約0.000~約0.001Paであることがより好ましく、そして約0.0000~約0.0001Paであることがさらに好ましい。蒸気圧が高いと、保存中に気化し、血液改質剤の量の減少、着用時の臭気等の問題が発生する場合があるからである。
Although there is no lower limit to the melting point of the blood modifying agent, it is preferable that its vapor pressure is low. The vapor pressure of the blood modifying agent is preferably about 0.00 to about 0.01 Pa at 1 atm and 25 ° C., more preferably about 0.000 to about 0.001 Pa, and about 0 More preferably, it is from .0000 to about 0.0001 Pa. Considering that the absorbent article of the present disclosure is used in contact with the human body, the vapor pressure is preferably about 0.00 to about 0.01 Pa at 1 atm and 40 ° C., and about 0.000 to about 0.01 More preferably, it is about 0.001 Pa, and more preferably, about 0.0000 to about 0.0001 Pa. If the vapor pressure is high, it may be vaporized during storage, which may cause problems such as a decrease in the amount of blood modifying agent and an odor when worn.
また、血液改質剤の融点を、気候、着用時間の長さ等に応じて、使い分けることができる。たとえば、平均気温が約10℃以下の地域では、約10℃以下の融点を有する血液改質剤を採用することにより、経血が排泄された後、周囲温度によって冷却された場合であっても、血液改質剤が、安定して血液を改質することができると考えられる。また、吸収性物品が長時間にわたって使用される場合には、血液改質剤の融点は、45℃以下の範囲で高い方が好ましい。汗、着用時の摩擦等の影響を受けにくく、長時間着用した場合であっても、血液改質剤が移動しにくいからである。
In addition, the melting point of the blood modifying agent can be properly used depending on the weather, the length of wearing time, and the like. For example, in areas where the average temperature is less than about 10 ° C, menstrual blood may be excreted and then cooled by the ambient temperature by employing a blood modifying agent having a melting point of less than about 10 ° C. It is believed that blood modifying agents can stably modify blood. When the absorbent article is used for a long time, the melting point of the blood modifying agent is preferably higher in the range of 45 ° C. or less. It is because it is hard to be affected by sweat, friction at the time of wearing, etc., and it is difficult for the blood modifying agent to move even when worn for a long time.
0.00~0.05gの水溶解度は、25℃において、100gの脱イオン水に、0.05gの試料を添加し、24時間静置し、24時間後に、必要に応じて軽く攪拌し、次いで、試料が溶解したか否か目視で評価することにより測定することができる。なお、本明細書では、水溶解度に関して、「溶解」には、試料が脱イオン水に完全に溶解し、均一混合物を形成した場合と、試料が完全にエマルション化した場合とが含まれる。なお、「完全」とは、脱イオン水に、試料の塊が存在しないことを意味する。
For a water solubility of 0.00 to 0.05 g, add a 0.05 g sample to 100 g deionized water at 25 ° C., allow to stand for 24 hours, and after 24 hours, lightly stir as needed, Then, it can be measured by visually evaluating whether the sample has dissolved. As used herein, with regard to water solubility, "dissolving" includes cases where the sample is completely dissolved in deionized water to form a homogeneous mixture and cases where the sample is completely emulsified. "Complete" means that there is no clump of sample in deionized water.
当技術分野では、血液の表面張力等を変化させ、血液を迅速に吸収することを目的として、トップシートの表面を、界面活性剤でコーティングすることが行われている。しかし、界面活性剤は、一般に水溶解度が高いため、界面活性剤がコーティングされたトップシートは、血液中の親水性成分(血漿等)となじみがよく、むしろ血液をトップシートに残存させるようにはたらく傾向がある。上記血液改質剤は、水溶解度が低いため、従来公知の界面活性剤と異なり、血液をトップシートに残存させず、迅速に吸収体に移行させることができると考えられる。
In the art, the surface of the top sheet is coated with a surfactant for the purpose of changing blood surface tension and the like to rapidly absorb the blood. However, since the surfactant generally has high water solubility, the surfactant-coated top sheet is compatible with hydrophilic components (such as plasma) in the blood, rather the blood remains on the top sheet. There is a tendency to work. Since the above-mentioned blood modifying agent has low water solubility, unlike the conventionally known surfactants, it is considered that blood can be rapidly transferred to the absorber without remaining on the top sheet.
本明細書において、25℃における、100gの水に対する溶解度を、単に、「水溶解度」と称する場合がある。
In the present specification, the solubility in 100 g of water at 25 ° C. may be simply referred to as “water solubility”.
本明細書において、「重量平均分子量」は、多分散系の化合物(たとえば、逐次重合により製造された化合物、複数の脂肪酸と、複数の脂肪族1価アルコールとから生成されたエステル)と、単一化合物(たとえば、1種の脂肪酸と、1種の脂肪族1価アルコールから生成されたエステル)とを含む概念であり、Ni個の分子量Miの分子(i=1、またはi=1,2・・・)からなる系において、次の式:
Mw=ΣNiMi 2/ΣNiMi
により求められるMwを意味する。 In the present specification, “weight-average molecular weight” refers to a polydispersed compound (for example, a compound produced by sequential polymerization, an ester produced from a plurality of fatty acids and a plurality of aliphatic monohydric alcohols), and It is a concept that includes one compound (for example, one fatty acid and an ester formed from one aliphatic monohydric alcohol), and N i molecular weight M i molecules (i = 1 or i = 1) , 2...) In the system:
M w = ΣN i M i 2 / ΣN i M i
Means M w determined by
Mw=ΣNiMi 2/ΣNiMi
により求められるMwを意味する。 In the present specification, “weight-average molecular weight” refers to a polydispersed compound (for example, a compound produced by sequential polymerization, an ester produced from a plurality of fatty acids and a plurality of aliphatic monohydric alcohols), and It is a concept that includes one compound (for example, one fatty acid and an ester formed from one aliphatic monohydric alcohol), and N i molecular weight M i molecules (i = 1 or i = 1) , 2...) In the system:
M w = ΣN i M i 2 / ΣN i M i
Means M w determined by
本明細書において、重量平均分子量は、ゲルパーミエーションクロマトグラフィー(GPC)により求められる、ポリスチレン換算の値を意味する。GPCの測定条件としては、たとえば、以下が挙げられる。
機種:(株)日立ハイテクノロジーズ製 高速液体クロマトグラム Lachrom Elite
カラム:昭和電工(株)製 SHODEX KF-801、KF-803およびKF-804
溶離液:THF
流量 :1.0mL/分
打込み量:100μL
検出:RI(示差屈折計)
なお、本明細書の実施例に記載される重量平均分子量は、上記条件により測定したものである。 In the present specification, the weight average molecular weight means a value in terms of polystyrene, which is determined by gel permeation chromatography (GPC). Examples of GPC measurement conditions include the following.
Model: High-performance liquid chromatogram Lachrom Elite manufactured by Hitachi High-Technologies Corporation
Column: Showa Denko KK SHODEX KF-801, KF-803 and KF-804
Eluent: THF
Flow rate: 1.0 mL / min Implanted volume: 100 μL
Detection: RI (differential refractometer)
In addition, the weight average molecular weight described in the Example of this specification is measured based on the said conditions.
機種:(株)日立ハイテクノロジーズ製 高速液体クロマトグラム Lachrom Elite
カラム:昭和電工(株)製 SHODEX KF-801、KF-803およびKF-804
溶離液:THF
流量 :1.0mL/分
打込み量:100μL
検出:RI(示差屈折計)
なお、本明細書の実施例に記載される重量平均分子量は、上記条件により測定したものである。 In the present specification, the weight average molecular weight means a value in terms of polystyrene, which is determined by gel permeation chromatography (GPC). Examples of GPC measurement conditions include the following.
Model: High-performance liquid chromatogram Lachrom Elite manufactured by Hitachi High-Technologies Corporation
Column: Showa Denko KK SHODEX KF-801, KF-803 and KF-804
Eluent: THF
Flow rate: 1.0 mL / min Implanted volume: 100 μL
Detection: RI (differential refractometer)
In addition, the weight average molecular weight described in the Example of this specification is measured based on the said conditions.
上記血液改質剤は、好ましくは、次の(i)~(iii)、
(i)炭化水素、
(ii) (ii-1)炭化水素部分と、(ii-2)上記炭化水素部分のC-C単結合間に挿入された、カルボニル基(-CO-)およびオキシ基(-O-)から成る群から選択される、一または複数の、同一または異なる基とを有する化合物、および
(iii) (iii-1)炭化水素部分と、(iii-2)上記炭化水素部分のC-C単結合間に挿入された、カルボニル基(-CO-)およびオキシ基(-O-)から成る群から選択される、一または複数の、同一または異なる基と、(iii-3)上記炭化水素部分の水素原子を置換する、カルボキシル基(-COOH)およびヒドロキシル基(-OH)から成る群から選択される、一または複数の、同一または異なる基とを有する化合物、
ならびにそれらの任意の組み合わせから成る群から選択される。 The above blood modifying agent is preferably selected from the following (i) to (iii),
(I) Hydrocarbons,
(Ii) from a carbonyl group (-CO-) and an oxy group (-O-) inserted between (ii-1) a hydrocarbon moiety and (ii-2) a C-C single bond of the above-mentioned hydrocarbon moiety A compound having one or more same or different groups selected from the group consisting of: (iii) (iii-1) a hydrocarbon moiety, and (iii-2) a C—C single bond of the above-mentioned hydrocarbon moiety (Iii-3) one or more, same or different group selected from the group consisting of carbonyl group (-CO-) and oxy group (-O-) inserted between (iii-3) A compound having one or more and the same or different groups selected from the group consisting of a carboxyl group (—COOH) and a hydroxyl group (—OH), which substitutes a hydrogen atom,
And any combination thereof.
(i)炭化水素、
(ii) (ii-1)炭化水素部分と、(ii-2)上記炭化水素部分のC-C単結合間に挿入された、カルボニル基(-CO-)およびオキシ基(-O-)から成る群から選択される、一または複数の、同一または異なる基とを有する化合物、および
(iii) (iii-1)炭化水素部分と、(iii-2)上記炭化水素部分のC-C単結合間に挿入された、カルボニル基(-CO-)およびオキシ基(-O-)から成る群から選択される、一または複数の、同一または異なる基と、(iii-3)上記炭化水素部分の水素原子を置換する、カルボキシル基(-COOH)およびヒドロキシル基(-OH)から成る群から選択される、一または複数の、同一または異なる基とを有する化合物、
ならびにそれらの任意の組み合わせから成る群から選択される。 The above blood modifying agent is preferably selected from the following (i) to (iii),
(I) Hydrocarbons,
(Ii) from a carbonyl group (-CO-) and an oxy group (-O-) inserted between (ii-1) a hydrocarbon moiety and (ii-2) a C-C single bond of the above-mentioned hydrocarbon moiety A compound having one or more same or different groups selected from the group consisting of: (iii) (iii-1) a hydrocarbon moiety, and (iii-2) a C—C single bond of the above-mentioned hydrocarbon moiety (Iii-3) one or more, same or different group selected from the group consisting of carbonyl group (-CO-) and oxy group (-O-) inserted between (iii-3) A compound having one or more and the same or different groups selected from the group consisting of a carboxyl group (—COOH) and a hydroxyl group (—OH), which substitutes a hydrogen atom,
And any combination thereof.
本明細書において、「炭化水素」は、炭素と水素とから成る化合物を意味し、鎖状炭化水素、たとえば、パラフィン系炭化水素(二重結合および三重結合を含まない、アルカンとも称される)、オレフィン系炭化水素(二重結合を1つ含む、アルケンとも称される)、アセチレン系炭化水素(三重結合を1つ含む、アルキンとも称される)、および二重結合および三重結合から成る群から選択される結合を2つ以上含む炭化水素、ならびに環状炭化水素、たとえば、芳香族炭化水素、脂環式炭化水素が挙げられる。
In the present specification, "hydrocarbon" means a compound consisting of carbon and hydrogen, and is a chain hydrocarbon, for example, paraffinic hydrocarbon (also referred to as alkane not containing double bond and triple bond) Olefinic hydrocarbons (containing one double bond, also called alkenes), acetylenic hydrocarbons (containing one triple bond, also called alkynes), and a group consisting of double bonds and triple bonds And hydrocarbons containing two or more bonds selected from, as well as cyclic hydrocarbons such as aromatic hydrocarbons and alicyclic hydrocarbons.
上記炭化水素としては、鎖状炭化水素および脂環式炭化水素であることが好ましく、鎖状炭化水素であることがより好ましく、パラフィン系炭化水素、オレフィン系炭化水素、および二重結合を2つ以上含む炭化水素(三重結合を含まない)であることがさらに好ましく、そしてパラフィン系炭化水素であることがさらに好ましい。上記鎖状炭化水素には、直鎖状炭化水素および分岐鎖状炭化水素が含まれる。
The hydrocarbon is preferably a chain hydrocarbon and an alicyclic hydrocarbon, more preferably a chain hydrocarbon, a paraffin hydrocarbon, an olefin hydrocarbon and two double bonds. It is more preferable that it is the hydrocarbon (it does not contain a triple bond) which contains above, and it is still more preferable that it is a paraffinic hydrocarbon. The chained hydrocarbons include straight chained hydrocarbons and branched chained hydrocarbons.
上記(ii)および(iii)の化合物において、オキシ基(-O-)が2つ以上挿入されている場合には、各オキシ基(-O-)は隣接していない。したがって、上記(ii)および(iii)の化合物には、オキシ基が連続する化合物(いわゆる、過酸化物)は含まれない。
In the compounds (ii) and (iii), when two or more oxy groups (—O—) are inserted, each oxy group (—O—) is not adjacent. Accordingly, the compounds (ii) and (iii) do not include compounds in which the oxy group is continuous (so-called peroxides).
また、上記(iii)の化合物では、炭化水素部分の少なくとも1つの水素原子が、カルボキシル基(-COOH)で置換された化合物よりも、炭化水素部分の少なくとも1つの水素原子が、ヒドロキシル基(-OH)で置換された化合物の方が好ましい。表1に示すように、カルボキシル基は、経血中の金属等と結合し、無機性値が150から、400以上へと大幅に上昇するため、カルボキシル基を有する血液改質剤は、使用時にIOBの値が約0.60を上回り、血球との親和性が低下する可能性があるからである。
Further, in the compound of (iii) above, at least one hydrogen atom of the hydrocarbon moiety is a hydroxyl group (-) rather than a compound in which at least one hydrogen atom of the hydrocarbon moiety is substituted with a carboxyl group (-COOH). Compounds substituted with OH) are preferred. As shown in Table 1, since the carboxyl group binds to metals and the like in blood, and the inorganic value greatly increases from 150 to 400 or more, the blood modifying agent having a carboxyl group is used at the time of use This is because the IOB value may exceed about 0.60 and the affinity to blood cells may be reduced.
上記血液改質剤は、より好ましくは、次の(i’)~(iii’)、
(i’)炭化水素、
(ii’) (ii’-1)炭化水素部分と、(ii’-2)上記炭化水素部分のC-C単結合間に挿入された、カルボニル結合(-CO-)、エステル結合(-COO-)、カーボネート結合(-OCOO-)、およびエーテル結合(-O-)から成る群から選択される、一または複数の、同一または異なる結合とを有する化合物、および
(iii’) (iii’-1)炭化水素部分と、(iii’-2)上記炭化水素部分のC-C単結合間に挿入された、カルボニル結合(-CO-)、エステル結合(-COO-)、カーボネート結合(-OCOO-)、およびエーテル結合(-O-)から成る群から選択される、一または複数の、同一または異なる結合と、(iii’-3)上記炭化水素部分の水素原子を置換する、カルボキシル基(-COOH)およびヒドロキシル基(-OH)から成る群から選択される、一または複数の、同一または異なる基とを有する化合物、
ならびにそれらの任意の組み合わせから成る群から選択される。 More preferably, the above blood modifying agent comprises the following (i ') to (iii'),
(I ') hydrocarbons,
(Ii ') (ii'-1) a hydrocarbon moiety, and (ii'-2) a carbonyl bond (-CO-), an ester bond (-COO) inserted between a C-C single bond of the above-mentioned hydrocarbon moiety -), A compound having one or more same or different bonds selected from the group consisting of carbonate bond (-OCOO-), and ether bond (-O-), and (iii ') (iii'-) 1) Carbonyl bond (-CO-), ester bond (-COO-), carbonate bond (-OCOO) inserted between the hydrocarbon moiety and the C-C single bond of the above-mentioned hydrocarbon moiety (iii'-2) A carboxyl group (-), and one or more, same or different bond selected from the group consisting of an ether bond (-O-) and (iii'-3) a hydrogen atom of the above-mentioned hydrocarbon moiety -COOH Compounds having one or more same or different groups selected from the group consisting of) and hydroxyl groups (—OH),
And any combination thereof.
(i’)炭化水素、
(ii’) (ii’-1)炭化水素部分と、(ii’-2)上記炭化水素部分のC-C単結合間に挿入された、カルボニル結合(-CO-)、エステル結合(-COO-)、カーボネート結合(-OCOO-)、およびエーテル結合(-O-)から成る群から選択される、一または複数の、同一または異なる結合とを有する化合物、および
(iii’) (iii’-1)炭化水素部分と、(iii’-2)上記炭化水素部分のC-C単結合間に挿入された、カルボニル結合(-CO-)、エステル結合(-COO-)、カーボネート結合(-OCOO-)、およびエーテル結合(-O-)から成る群から選択される、一または複数の、同一または異なる結合と、(iii’-3)上記炭化水素部分の水素原子を置換する、カルボキシル基(-COOH)およびヒドロキシル基(-OH)から成る群から選択される、一または複数の、同一または異なる基とを有する化合物、
ならびにそれらの任意の組み合わせから成る群から選択される。 More preferably, the above blood modifying agent comprises the following (i ') to (iii'),
(I ') hydrocarbons,
(Ii ') (ii'-1) a hydrocarbon moiety, and (ii'-2) a carbonyl bond (-CO-), an ester bond (-COO) inserted between a C-C single bond of the above-mentioned hydrocarbon moiety -), A compound having one or more same or different bonds selected from the group consisting of carbonate bond (-OCOO-), and ether bond (-O-), and (iii ') (iii'-) 1) Carbonyl bond (-CO-), ester bond (-COO-), carbonate bond (-OCOO) inserted between the hydrocarbon moiety and the C-C single bond of the above-mentioned hydrocarbon moiety (iii'-2) A carboxyl group (-), and one or more, same or different bond selected from the group consisting of an ether bond (-O-) and (iii'-3) a hydrogen atom of the above-mentioned hydrocarbon moiety -COOH Compounds having one or more same or different groups selected from the group consisting of) and hydroxyl groups (—OH),
And any combination thereof.
上記(ii’)および(iii’)の化合物において、2以上の同一または異なる結合が挿入されている場合、すなわち、カルボニル結合(-CO-)、エステル結合(-COO-)、カーボネート結合(-OCOO-)およびエーテル結合(-O-)から選択される2以上の同一または異なる結合が挿入されている場合には、各結合は隣接しておらず、各結合の間には、少なくとも、炭素原子が1つ介在する。
In the compounds of (ii ′) and (iii ′) above, two or more identical or different bonds are inserted, ie, a carbonyl bond (—CO—), an ester bond (—COO—), a carbonate bond (— When two or more identical or different bonds selected from OCOO-) and an ether bond (-O-) are inserted, each bond is not adjacent, and at least carbon is separated between each bond. One atom intervenes.
上記血液改質剤は、さらに好ましくは、炭化水素部分中に、炭素原子10個当たり、カルボニル結合(-CO-)を約1.8個以下、エステル結合(-COO-)を2個以下、カーボネート結合(-OCOO-)を約1.5個以下、エーテル結合(-O-)を約6個以下、カルボキシル基(-COOH)を約0.8個以下、そして/またはヒドロキシル基(-OH)を約1.2個以下有する化合物であることができる。
More preferably, the blood modifying agent has about 1.8 or less carbonyl bonds (-CO-) and 2 or less ester bonds (-COO-) per 10 carbon atoms in the hydrocarbon moiety. About 1.5 or less carbonate bond (-OCOO-), about 6 or less ether bond (-O-), about 0.8 or less carboxyl group (-COOH), and / or hydroxyl group (-OH) Or a compound having about 1.2 or less).
上記血液改質剤は、さらに好ましくは、次の(A)~(F)、
(A) (A1)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(A2)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する1個のカルボキシル基とを有する化合物とのエステル、
(B) (B1)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(B2)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエーテル、
(C) (C1)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する、2~4個のカルボキシル基とを含むカルボン酸、ヒドロキシ酸、アルコキシ酸またはオキソ酸と、(C2)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエステル、
(D)鎖状炭化水素部分と、上記鎖状炭化水素部分のC-C単結合間に挿入された、エーテル結合(-O-)、カルボニル結合(-CO-)、エステル結合(-COO-)、およびカーボネート結合(-OCOO-)から成る群から選択されるいずれか1つの結合とを有する化合物、
(E)ポリオキシC2~C6アルキレングリコール、またはそのアルキルエステルもしくはアルキルエーテル、および
(F)鎖状炭化水素、
ならびにそれらの任意の組み合わせから成る群から選択される。以下、(A)~(F)に従う血液改質剤について詳細に説明する。 More preferably, the above blood modifying agent is any of the following (A) to (F),
(A) A compound having (A1) a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety, (A2) a chain hydrocarbon moiety, and the above chain Ester with a compound having one carboxyl group replacing the hydrogen atom of the cyclic hydrocarbon moiety,
(B) A compound having (B1) a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, and the above chain Ether with a compound having one hydroxyl group replacing the hydrogen atom of the cyclic hydrocarbon moiety,
(C) a carboxylic acid, a hydroxy acid, an alkoxy acid or an oxo acid containing a (C1) linear hydrocarbon moiety and 2 to 4 carboxyl groups replacing the hydrogen atom of the linear hydrocarbon moiety; C2) an ester of a compound having a chain hydrocarbon moiety and one hydroxyl group replacing the hydrogen atom of the chain hydrocarbon moiety,
(D) A chain hydrocarbon moiety and an ether bond (-O-), a carbonyl bond (-CO-), an ester bond (-COO-) inserted between the C-C single bond of the chain hydrocarbon moiety and the chain hydrocarbon moiety ), And a compound having any one bond selected from the group consisting of carbonate bonds (—OCOO—),
(E) polyoxy C 2 -C 6 alkylene glycol or its alkyl ester or alkyl ether, and (F) chain hydrocarbon
And any combination thereof. Hereinafter, the blood modifying agent according to (A) to (F) will be described in detail.
(A) (A1)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(A2)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する1個のカルボキシル基とを有する化合物とのエステル、
(B) (B1)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(B2)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエーテル、
(C) (C1)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する、2~4個のカルボキシル基とを含むカルボン酸、ヒドロキシ酸、アルコキシ酸またはオキソ酸と、(C2)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエステル、
(D)鎖状炭化水素部分と、上記鎖状炭化水素部分のC-C単結合間に挿入された、エーテル結合(-O-)、カルボニル結合(-CO-)、エステル結合(-COO-)、およびカーボネート結合(-OCOO-)から成る群から選択されるいずれか1つの結合とを有する化合物、
(E)ポリオキシC2~C6アルキレングリコール、またはそのアルキルエステルもしくはアルキルエーテル、および
(F)鎖状炭化水素、
ならびにそれらの任意の組み合わせから成る群から選択される。以下、(A)~(F)に従う血液改質剤について詳細に説明する。 More preferably, the above blood modifying agent is any of the following (A) to (F),
(A) A compound having (A1) a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety, (A2) a chain hydrocarbon moiety, and the above chain Ester with a compound having one carboxyl group replacing the hydrogen atom of the cyclic hydrocarbon moiety,
(B) A compound having (B1) a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, and the above chain Ether with a compound having one hydroxyl group replacing the hydrogen atom of the cyclic hydrocarbon moiety,
(C) a carboxylic acid, a hydroxy acid, an alkoxy acid or an oxo acid containing a (C1) linear hydrocarbon moiety and 2 to 4 carboxyl groups replacing the hydrogen atom of the linear hydrocarbon moiety; C2) an ester of a compound having a chain hydrocarbon moiety and one hydroxyl group replacing the hydrogen atom of the chain hydrocarbon moiety,
(D) A chain hydrocarbon moiety and an ether bond (-O-), a carbonyl bond (-CO-), an ester bond (-COO-) inserted between the C-C single bond of the chain hydrocarbon moiety and the chain hydrocarbon moiety ), And a compound having any one bond selected from the group consisting of carbonate bonds (—OCOO—),
(E) polyoxy C 2 -C 6 alkylene glycol or its alkyl ester or alkyl ether, and (F) chain hydrocarbon
And any combination thereof. Hereinafter, the blood modifying agent according to (A) to (F) will be described in detail.
[(A) (A1)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(A2)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する1個のカルボキシル基とを有する化合物とのエステル]
(A) (A1)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(A2)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する1個のカルボキシル基とを有する化合物とのエステル(以下、「化合物(A)」と称する場合がある)は、上述のIOB、融点および水溶解度を有する限り、全てのヒドロキシル基がエステル化されていなくともよい。 [(A) (A1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety, (A2) a chain hydrocarbon moiety, and Ester with a compound having one carboxyl group replacing a hydrogen atom of a linear hydrocarbon moiety]
(A) A compound having (A1) a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety, (A2) a chain hydrocarbon moiety, and the above chain Ester with a compound having one carboxyl group replacing hydrogen atoms in the cyclic hydrocarbon moiety (hereinafter sometimes referred to as “compound (A)”) has the above-mentioned IOB, melting point and water solubility And all hydroxyl groups may not be esterified.
(A) (A1)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(A2)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する1個のカルボキシル基とを有する化合物とのエステル(以下、「化合物(A)」と称する場合がある)は、上述のIOB、融点および水溶解度を有する限り、全てのヒドロキシル基がエステル化されていなくともよい。 [(A) (A1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety, (A2) a chain hydrocarbon moiety, and Ester with a compound having one carboxyl group replacing a hydrogen atom of a linear hydrocarbon moiety]
(A) A compound having (A1) a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety, (A2) a chain hydrocarbon moiety, and the above chain Ester with a compound having one carboxyl group replacing hydrogen atoms in the cyclic hydrocarbon moiety (hereinafter sometimes referred to as “compound (A)”) has the above-mentioned IOB, melting point and water solubility And all hydroxyl groups may not be esterified.
(A1)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物(以下、「化合物(A1)」と称する場合がある)としては、たとえば、鎖状炭化水素テトラオール、たとえば、アルカンテトラオール、たとえば、ペンタエリトリトール、鎖状炭化水素トリオール、たとえば、アルカントリオール、たとえば、グリセリン、および鎖状炭化水素ジオール、たとえば、アルカンジオール、たとえば、グリコールが挙げられる。
(A2)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する1個のカルボキシル基とを有する化合物(以下、「化合物(A2)」と称する場合がある)としては、たとえば、炭化水素上の1つの水素原子が、1つのカルボキシル基(-COOH)で置換された化合物、たとえば、脂肪酸が挙げられる。
化合物(A)としては、たとえば、(a1)鎖状炭化水素テトラオールと少なくとも1の脂肪酸とのエステル、(a2)鎖状炭化水素トリオールと少なくとも1の脂肪酸とのエステル、および(a3)鎖状炭化水素ジオールと少なくとも1の脂肪酸とのエステルが挙げられる。 (A1) a compound having a chain hydrocarbon portion and 2 to 4 hydroxyl groups replacing the hydrogen atom of the above chain hydrocarbon portion (hereinafter sometimes referred to as “compound (A1)”) For example, chain hydrocarbon tetraols such as alkanetetraols such as pentaerythritol, chain hydrocarbon triols such as alkanetriols such as glycerin, and chain hydrocarbon diols such as alkane diols such as Glycol is mentioned.
Examples of (A2) a compound having a chain hydrocarbon portion and one carboxyl group replacing the hydrogen atom of the chain hydrocarbon portion (hereinafter sometimes referred to as “compound (A2)”) include, for example, And compounds in which one hydrogen atom on a hydrocarbon is substituted with one carboxyl group (—COOH), such as fatty acid.
Examples of the compound (A) include an ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid, an ester of (a 2 ) chain hydrocarbon triol and at least one fatty acid, and (a 3 And esters of linear hydrocarbon diols and at least one fatty acid.
(A2)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する1個のカルボキシル基とを有する化合物(以下、「化合物(A2)」と称する場合がある)としては、たとえば、炭化水素上の1つの水素原子が、1つのカルボキシル基(-COOH)で置換された化合物、たとえば、脂肪酸が挙げられる。
化合物(A)としては、たとえば、(a1)鎖状炭化水素テトラオールと少なくとも1の脂肪酸とのエステル、(a2)鎖状炭化水素トリオールと少なくとも1の脂肪酸とのエステル、および(a3)鎖状炭化水素ジオールと少なくとも1の脂肪酸とのエステルが挙げられる。 (A1) a compound having a chain hydrocarbon portion and 2 to 4 hydroxyl groups replacing the hydrogen atom of the above chain hydrocarbon portion (hereinafter sometimes referred to as “compound (A1)”) For example, chain hydrocarbon tetraols such as alkanetetraols such as pentaerythritol, chain hydrocarbon triols such as alkanetriols such as glycerin, and chain hydrocarbon diols such as alkane diols such as Glycol is mentioned.
Examples of (A2) a compound having a chain hydrocarbon portion and one carboxyl group replacing the hydrogen atom of the chain hydrocarbon portion (hereinafter sometimes referred to as “compound (A2)”) include, for example, And compounds in which one hydrogen atom on a hydrocarbon is substituted with one carboxyl group (—COOH), such as fatty acid.
Examples of the compound (A) include an ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid, an ester of (a 2 ) chain hydrocarbon triol and at least one fatty acid, and (a 3 And esters of linear hydrocarbon diols and at least one fatty acid.
[(a1)鎖状炭化水素テトラオールと少なくとも1の脂肪酸とのエステル]
上記鎖状炭化水素テトラオールと少なくとも1の脂肪酸とのエステルとしては、たとえば、次の式(1):
のペンタエリトリトールと脂肪酸とのテトラエステル、次の式(2):
のペンタエリトリトールと脂肪酸とのトリエステル、次の式(3):
のペンタエリトリトールと脂肪酸とのジエステル、次の式(4):
のペンタエリトリトールと脂肪酸とのモノエステルが挙げられる。
(式中、R1~R4は、それぞれ、鎖状炭化水素である) [Ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid]
The ester of the above linear hydrocarbon tetraol and at least one fatty acid may be, for example, the following formula (1):
Tetraester of pentaerythritol with fatty acid, the following formula (2):
Triester of pentaerythritol with fatty acid, the following formula (3):
A diester of pentaerythritol with fatty acid, the following formula (4):
And monoesters of fatty acid with pentaerythritol.
(Wherein, R 1 to R 4 are each a chain hydrocarbon)
上記鎖状炭化水素テトラオールと少なくとも1の脂肪酸とのエステルとしては、たとえば、次の式(1):
(式中、R1~R4は、それぞれ、鎖状炭化水素である) [Ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid]
The ester of the above linear hydrocarbon tetraol and at least one fatty acid may be, for example, the following formula (1):
(Wherein, R 1 to R 4 are each a chain hydrocarbon)
上記ペンタエリトリトールと脂肪酸とのエステルを構成する脂肪酸(R1COOH、R2COOH,R3COOH,およびR4COOH)としては、ペンタエリトリトールと脂肪酸とのエステルが、上記IOB、融点および水溶解度の要件を満たすものであれば、特に制限されないが、たとえば、飽和脂肪酸、たとえば、C2~C30の飽和脂肪酸、たとえば、酢酸(C2)(C2は、炭素数を示し、R1C、R2C,R3CまたはR4Cの炭素数に相当する、以下同じ)、プロパン酸(C3)、ブタン酸(C4)およびその異性体、たとえば、2-メチルプロパン酸(C4)、ペンタン酸(C5)およびその異性体、たとえば、2-メチルブタン酸(C5)、2,2-ジメチルプロパン酸(C5)、ヘキサン酸(C6)、ヘプタン酸(C7)、オクタン酸(C8)およびその異性体、たとえば、2-エチルヘキサン酸(C8)、ノナン酸(C9)、デカン酸(C10)、ドデカン酸(C12)、テトラデカン酸(C14)、ヘキサデカン酸(C16)、ヘプタデカン酸(C17)、オクタデカン酸(C18)、エイコサン酸(C20)、ドコサン酸(C22)、テトラコサン酸(C24)、ヘキサコサン酸(C26)、オクタコサン酸(C28)、トリアコンタン酸(C30)等、ならびにこれらの異性体(上述のものを除く)が挙げられる。
As fatty acids (R 1 COOH, R 2 COOH, R 3 COOH, and R 4 COOH) constituting esters of pentaerythritol and fatty acids, esters of pentaerythritol and fatty acids have the above IOB, melting point and water solubility For example, saturated fatty acids such as C 2 to C 30 saturated fatty acids, for example, acetic acid (C 2 ) (C 2 represents a carbon number, R 1 C, R 2 C, R 3 C or R 4 C, which corresponds to the carbon number of R 2 C, hereinafter the same), propanoic acid (C 3 ), butanoic acid (C 4 ) and isomers thereof, for example, 2-methylpropanoic acid (C 4) ), pentanoic acid (C 5) and isomers thereof such as 2-methylbutanoic acid (C 5), 2,2-dimethyl propanoic acid (C 5), hexanoic acid (C 6), heptanoic acid (C 7) Octanoic acid (C 8) and isomers thereof, e.g., 2-ethylhexanoic acid (C 8), nonanoic acid (C 9), decanoic acid (C 10), dodecanoic acid (C 12), tetradecanoic acid (C 14) , Hexadecanoic acid (C 16 ), heptadecanoic acid (C 17 ), octadecanoic acid (C 18 ), eicosanoic acid (C 20 ), docosanoic acid (C 22 ), tetracosanoic acid (C 24 ), hexacosanoic acid (C 26 ), Examples include octacosanoic acid (C 28 ), triacontanic acid (C 30 ) and the like, as well as their isomers (except those mentioned above).
上記脂肪酸はまた、不飽和脂肪酸であることができる。上記不飽和脂肪酸としては、たとえば、C3~C20の不飽和脂肪酸、たとえば、モノ不飽和脂肪酸、たとえば、クロトン酸(C4)、ミリストレイン酸(C14)、パルミトレイン酸(C16)、オレイン酸(C18)、エライジン酸(C18)、バクセン酸(C18)、ガドレイン酸(C20)、エイコセン酸(C20)等、ジ不飽和脂肪酸、たとえば、リノール酸(C18)、エイコサジエン酸(C20)等、トリ不飽和脂肪酸、たとえば、リノレン酸、たとえば、α-リノレン酸(C18)およびγ-リノレン酸(C18)、ピノレン酸(C18)、エレオステアリン酸、たとえば、α-エレオステアリン酸(C18)およびβ-エレオステアリン酸(C18)、ミード酸(C20)、ジホモ-γ-リノレン酸(C20)、エイコサトリエン酸(C20)等、テトラ不飽和脂肪酸、たとえば、ステアリドン酸(C20)、アラキドン酸(C20)、エイコサテトラエン酸(C20)等、ペンタ不飽和脂肪酸、たとえば、ボセオペンタエン酸(C18)、エイコサペンタエン酸(C20)等、ならびにこれらの部分水素付加物が挙げられる。
The fatty acids can also be unsaturated fatty acids. Examples of the unsaturated fatty acids include C 3 -C 20 unsaturated fatty acids such as monounsaturated fatty acids such as crotonic acid (C 4 ), myristoleic acid (C 14 ), palmitoleic acid (C 16 ), Oleic acid (C 18 ), elaidic acid (C 18 ), vacenic acid (C 18 ), gadeuric acid (C 20 ), eicosenic acid (C 20 ), etc., diunsaturated fatty acids such as linoleic acid (C 18 ), Eicosadienoic acid (C 20 ) and the like, triunsaturated fatty acids such as linolenic acid, for example α-linolenic acid (C 18 ) and γ-linolenic acid (C 18 ), pinolenic acid (C 18 ), eleostearic acid, For example, α-eleostearic acid (C 18 ) and β-eleostearic acid (C 18 ), meade acid (C 20 ), dihomo-γ-linolenic acid (C 20 ), eicosatrienoic acid (C 20 ) Etc, tetra-unsaturated Fatty acid, e.g., stearidonic acid (C 20), arachidonic acid (C 20), eicosatetraenoic acid (C 20), etc., penta-unsaturated fatty acids, for example, bosseopentaenoic acid (C 18), eicosapentaenoic acid (C 20 And the like, as well as partial hydrogenated adducts thereof.
上記ペンタエリトリトールと脂肪酸とのエステルとしては、酸化等により変性する可能性を考慮すると、飽和脂肪酸に由来する、ペンタエリトリトールと脂肪酸とのエステル、すなわち、ペンタエリトリトールと飽和脂肪酸とのエステルであることが好ましい。また、上記ペンタエリトリトールと脂肪酸とのエステルとしては、IOBを小さくし、より疎水性とするために、ジエステル、トリエステルまたはテトラエステルであることが好ましく、トリエステルまたはテトラエステルであることがより好ましく、そしてテトラエステルであることがさらに好ましい。
The ester of pentaerythritol and fatty acid is an ester of pentaerythritol and fatty acid derived from saturated fatty acid, that is, an ester of pentaerythritol and saturated fatty acid, in consideration of the possibility of modification by oxidation etc. preferable. Further, as the ester of pentaerythritol and a fatty acid, in order to make IOB small and make it more hydrophobic, it is preferable to be a diester, a triester or a tetraester, and a triester or a tetraester is more preferable. And tetra-esters are more preferred.
上記ペンタエリトリトールと脂肪酸とのテトラエステルでは、ペンタエリトリトールと脂肪酸とのテトラエステルを構成する脂肪酸の炭素数の合計、すなわち、上記式(1)において、R1C、R2C、R3CおよびR4C部分の炭素数の合計が15の場合にIOBが0.60となる。したがって、上記ペンタエリトリトールと脂肪酸とのテトラエステルでは、上記炭素数の合計が約15以上である場合に、IOBが約0.00~約0.60の要件を満たす。上記ペンタエリトリトールと脂肪酸とのテトラエステルでは、たとえば、ペンタエリトリトールと、ヘキサン酸(C6)、ヘプタン酸(C7)、オクタン酸(C8)、たとえば、2-エチルヘキサン酸(C8)、ノナン酸(C9)、デカン酸(C10)および/またはドデカン酸(C12)とのテトラエステルが挙げられる。
In the tetraester of pentaerythritol and fatty acid, the total carbon number of fatty acids constituting the tetraester of pentaerythritol and fatty acid, that is, in the above formula (1), R 1 C, R 2 C, R 3 C and When the total number of carbons in the R 4 C portion is 15, the IOB is 0.60. Therefore, in the case of tetraester of pentaerythritol and fatty acid, IOB satisfies the requirement of about 0.00 to about 0.60 when the total number of carbons is about 15 or more. Examples of the tetraester of pentaerythritol and fatty acid include pentaerythritol, hexanoic acid (C 6 ), heptanoic acid (C 7 ), octanoic acid (C 8 ), for example, 2-ethylhexanoic acid (C 8 ), These include tetraesters with nonanoic acid (C 9 ), decanoic acid (C 10 ) and / or dodecanoic acid (C 12 ).
上記ペンタエリトリトールと脂肪酸とのトリエステルでは、ペンタエリトリトールと脂肪酸とのトリエステルを構成する脂肪酸の炭素数の合計、すなわち、上記式(2)において、R1C、R2CおよびR3C部分の炭素数の合計が19の場合にIOBが0.58となる。したがって、上記ペンタエリトリトールと脂肪酸とのトリエステルでは、脂肪酸の炭素数の合計が約19以上である場合に、IOBが約0.00~約0.60の要件を満たす。
In the above-described triester of pentaerythritol and fatty acid, the total carbon number of the fatty acid constituting the triester of pentaerythritol and fatty acid, that is, the R 1 C, R 2 C and R 3 C moieties in the above formula (2) The IOB is 0.58 when the sum of the carbon numbers of these is 19. Therefore, in the case of the triester of pentaerythritol and fatty acid, IOB satisfies the requirement of about 0.00 to about 0.60 when the total carbon number of fatty acid is about 19 or more.
上記ペンタエリトリトールと脂肪酸とのジエステルでは、ペンタエリトリトールと脂肪酸とのジエステルを構成する脂肪酸の炭素数の合計、すなわち、上記式(3)において、R1CおよびR2C部分の炭素数の合計が22の場合にIOBが0.59となる。したがって、上記ペンタエリトリトールと脂肪酸とのジエステルでは、脂肪酸の炭素数の合計が約22以上である場合に、IOBが約0.00~約0.60の要件を満たす。
In the diester of pentaerythritol and fatty acid, the total carbon number of fatty acids constituting the diester of pentaerythritol and fatty acid, that is, the total carbon number of R 1 C and R 2 C in the above formula (3) is In the case of 22, the IOB is 0.59. Accordingly, in the diester of pentaerythritol and fatty acid, IOB satisfies the requirement of about 0.00 to about 0.60 when the total carbon number of fatty acid is about 22 or more.
上記ペンタエリトリトールと脂肪酸とのモノエステルでは、ペンタエリトリトールと脂肪酸とのモノエステルを構成する脂肪酸の炭素数、すなわち、上記式(4)において、R1C部分の炭素数が25の場合にIOBが0.60となる。したがって、上記ペンタエリトリトールと脂肪酸とのモノエステルでは、脂肪酸の炭素数が約25以上である場合に、IOBが約0.00~約0.60の要件を満たす。なお、上記計算に当たっては、二重結合、三重結合、iso分岐、およびtert分岐の影響は、考慮していない。
In the monoester of pentaerythritol and fatty acid, when the carbon number of the fatty acid constituting the monoester of pentaerythritol and fatty acid, that is, the carbon number of R 1 C portion in the above formula (4) is 25, IOB is It will be 0.60. Therefore, in the monoester of pentaerythritol and fatty acid, the IOB satisfies the requirement of about 0.00 to about 0.60 when the carbon number of the fatty acid is about 25 or more. In the above calculation, the effects of double bond, triple bond, iso branch and tert branch are not taken into consideration.
上記ペンタエリトリトールと脂肪酸とのエステルの市販品としては、ユニスター H-408BRS、H-2408BRS-22(混合品)等(以上、日油株式会社製)が挙げられる。
Examples of commercially available esters of pentaerythritol and fatty acid include Unistar H-408 BRS, H-2408 BRS-22 (mixed product), etc. (all manufactured by NOF Corporation).
[(a2)鎖状炭化水素トリオールと少なくとも1の脂肪酸とのエステル]
上記鎖状炭化水素トリオールと少なくとも1の脂肪酸とのエステルとしては、たとえば、次の式(5):
のグリセリンと脂肪酸とのトリエステル、次の式(6):
のグリセリンと脂肪酸とのジエステル、および次の式(7):
(式中、R5~R7は、それぞれ、鎖状炭化水素である)
のグリセリンと脂肪酸とのモノエステルが挙げられる。 [Ester of (a 2 ) chain hydrocarbon triol and at least one fatty acid]
The ester of the above linear hydrocarbon triol and at least one fatty acid may be, for example, the following formula (5):
Triester of glycerin and fatty acid, the following formula (6):
A diester of glycerin and a fatty acid, and the following formula (7):
(Wherein, each of R 5 to R 7 is a chain hydrocarbon)
And monoesters of glycerin and fatty acids.
上記鎖状炭化水素トリオールと少なくとも1の脂肪酸とのエステルとしては、たとえば、次の式(5):
のグリセリンと脂肪酸とのモノエステルが挙げられる。 [Ester of (a 2 ) chain hydrocarbon triol and at least one fatty acid]
The ester of the above linear hydrocarbon triol and at least one fatty acid may be, for example, the following formula (5):
And monoesters of glycerin and fatty acids.
上記グリセリンと脂肪酸とのエステルを構成する脂肪酸(R5COOH、R6COOHおよびR7COOH)としては、グリセリンと脂肪酸とのエステルが、上記IOB、融点および水溶解度の要件を満たすものであれば、特に制限されず、たとえば、「(a1)鎖状炭化水素テトラオールと少なくとも1の脂肪酸とのエステル」において列挙される脂肪酸、すなわち、飽和脂肪酸および不飽和脂肪酸が挙げられ、酸化等により変性する可能性を考慮すると、飽和脂肪酸に由来する、グリセリンと脂肪酸とのエステル、すなわち、グリセリンと飽和脂肪酸とのエステルであることが好ましい。
As fatty acids (R 5 COOH, R 6 COOH and R 7 COOH) constituting the ester of glycerin and fatty acid, if the ester of glycerin and fatty acid satisfies the requirements of the above IOB, melting point and water solubility The fatty acids listed in the “ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid” are not particularly limited, and examples thereof include saturated fatty acids and unsaturated fatty acids, which are modified by oxidation etc. In consideration of the possibility of doing so, an ester of glycerin and a fatty acid derived from a saturated fatty acid, that is, an ester of glycerin and a saturated fatty acid is preferable.
また、上記グリセリンと脂肪酸とのエステルとしては、IOBを小さくし、より疎水性とするために、ジエステルまたはトリエステルであることが好ましく、そしてトリエステルであることがより好ましい。
Moreover, as ester of glycerol and a fatty acid, in order to make IOB small and to make it more hydrophobic, it is preferable that it is diester or triester, and it is more preferable that it is triester.
上記グリセリンと脂肪酸とのトリエステルは、トリグリセリドとも称され、たとえば、グリセリンとオクタン酸(C8)とのトリエステル、グリセリンとデカン酸(C10)とのトリエステル、グリセリンとドデカン酸(C12)とのトリエステル、およびグリセリンと、2種または3種の脂肪酸とのトリエステル、ならびにこれらの混合物が挙げられる。
The above-mentioned triester of glycerin and fatty acid is also referred to as triglyceride, for example, triester of glycerin and octanoic acid (C 8 ), triester of glycerin and decanoic acid (C 10 ), glycerin and dodecanoic acid (C 12 And triesters of glycerin with two or three fatty acids, and mixtures thereof.
上記グリセリンと、2種以上の脂肪酸とのトリエステルとしては、たとえば、グリセリンと、オクタン酸(C8)およびデカン酸(C10)とのトリエステル、グリセリンと、オクタン酸(C8)、デカン酸(C10)およびドデカン酸(C12)とのトリエステル、グリセリンと、オクタン酸(C8)、デカン酸(C10)、ドデカン酸(C12)、テトラデカン酸(C14)、ヘキサデカン酸(C16)およびオクタデカン酸(C18)とのトリエステル等が挙げられる。
Examples of the triester of the above glycerin and two or more fatty acids include triester of glycerin and octanoic acid (C 8 ) and decanoic acid (C 10 ), glycerin, octanoic acid (C 8 ), decane Acid (C 10 ) and triester with dodecanoic acid (C 12 ), glycerin and octanoic acid (C 8 ), decanoic acid (C 10 ), dodecanoic acid (C 12 ), tetradecanoic acid (C 14 ), hexadecanoic acid Examples thereof include triesters with (C 16 ) and octadecanoic acid (C 18 ).
上記グリセリンと脂肪酸とのトリエステルとしては、融点を約45℃以下とするために、グリセリンと脂肪酸とのトリエステルを構成する脂肪酸の炭素数の合計、すなわち、式(5)において、R5C、R6CおよびR7C部分の炭素数の合計が、約40以下であることが好ましい。
As the above-mentioned triester of glycerin and fatty acid, in order to set the melting point to about 45 ° C. or less, the total carbon number of fatty acids constituting the triester of glycerin and fatty acid, ie, R 5 C in the formula (5) Preferably, the sum of the carbon numbers of the R 6 C and R 7 C moieties is about 40 or less.
また、上記グリセリンと脂肪酸とのトリエステルでは、グリセリンと脂肪酸とのトリエステルを構成する脂肪酸の炭素数の合計、すなわち、式(5)において、R5C、R6CおよびR7C部分の炭素数の合計が12の場合にIOBが0.60となる。したがって、上記グリセリンと脂肪酸とのトリエステルでは、脂肪酸の炭素数の合計が約12以上である場合に、IOBが約0.00~約0.60の要件を満たす。上記グリセリンと脂肪酸とのトリエステルは、いわゆる、脂肪であり、人体を構成しうる成分であるため、安全性の観点から好ましい。
Further, in the above-mentioned triester of glycerin and fatty acid, the total carbon number of fatty acids constituting the triester of glycerin and fatty acid, that is, in the formula (5), the R 5 C, R 6 C and R 7 C moieties When the total carbon number is 12, the IOB is 0.60. Therefore, in the above-mentioned triesters of glycerin and fatty acid, the IOB satisfies the requirement of about 0.00 to about 0.60 when the total carbon number of fatty acid is about 12 or more. The above-mentioned triester of glycerin and a fatty acid is a so-called fat and is a component that can constitute the human body, and thus is preferable from the viewpoint of safety.
上記グリセリンと脂肪酸とのトリエステルの市販品としては、トリヤシ油脂肪酸グリセリド、NA36、パナセート800、パナセート800Bおよびパナセート810S、ならびにトリC2L油脂肪酸グリセリドおよびトリCL油脂肪酸グリセリド(以上、日油株式会社製)等が挙げられる。
Commercial products of the above triester of glycerin and fatty acid include tricotic oil fatty acid glyceride, NA36, PANACET 800, PANACET 800B and PANACET 810S, and avian C2L oil fatty acid glyceride and triCL oil fatty acid glyceride (manufactured by NOF CORPORATION) Etc.).
上記グリセリンと脂肪酸とのジエステルは、ジグリセリドとも称され、たとえば、グリセリンとデカン酸(C10)とのジエステル、グリセリンとドデカン酸(C12)とのジエステル、グリセリンとヘキサデカン酸(C16)とのジエステル、およびグリセリンと、2種の脂肪酸とのジエステル、ならびにこれらの混合物が挙げられる。
The above-mentioned diester of glycerin and fatty acid is also referred to as diglyceride, for example, a diester of glycerin and decanoic acid (C 10 ), a diester of glycerin and dodecanoic acid (C 12 ), and glycerin and hexadecanoic acid (C 16 ) Included are diesters and diesters of glycerin with two fatty acids, and mixtures thereof.
上記グリセリンと脂肪酸とのジエステルでは、グリセリンと脂肪酸とのジエステルを構成する脂肪酸の炭素数の合計、すなわち、式(6)において、R5CおよびR6C部分の炭素数の合計が16の場合にIOBが0.58となる。したがって、上記グリセリンと脂肪酸とのジエステルでは、脂肪酸の炭素数の合計が約16以上である場合に、IOBが約0.00~約0.60の要件を満たす。
In the case of a diester of glycerin and a fatty acid, the total carbon number of fatty acids constituting the diester of glycerin and fatty acid, ie, the case where the total carbon number of R 5 C and R 6 C moieties in the formula (6) is 16 The IOB is 0.58. Therefore, in the case of the diester of glycerin and fatty acid, the IOB satisfies the requirement of about 0.00 to about 0.60 when the total carbon number of fatty acid is about 16 or more.
上記グリセリンと脂肪酸とのモノエステルは、モノグリセリドとも称され、たとえば、グリセリンのイコサン酸(C20)モノエステル、グリセリンのドコサン酸(C22)モノエステル等が挙げられる。上記グリセリンと脂肪酸とのモノエステルでは、グリセリンと脂肪酸とのモノエステルを構成する脂肪酸の炭素数、すなわち、式(7)において、R5C部分の炭素数が19の場合にIOBが0.59となる。したがって、上記グリセリンと脂肪酸とのモノエステルでは、脂肪酸の炭素数が約19以上である場合に、IOBが約0.00~約0.60の要件を満たす。
The monoester of glycerin and fatty acid is also referred to as monoglyceride, and examples thereof include icosanoic acid (C 20 ) monoester of glycerin, docosanoic acid (C 22 ) monoester of glycerin and the like. In the monoester of glycerin and fatty acid, the carbon number of fatty acid constituting the monoester of glycerin and fatty acid, that is, in the formula (7), the IOB is 0.59 when the carbon number of the R 5 C portion is 19 It becomes. Therefore, in the monoester of glycerin and fatty acid, the IOB satisfies the requirement of about 0.00 to about 0.60 when the carbon number of the fatty acid is about 19 or more.
[(a3)鎖状炭化水素ジオールと少なくとも1の脂肪酸とのエステル]
上記鎖状炭化水素ジオールと少なくとも1の脂肪酸とのエステルとしては、たとえば、C2~C6の鎖状炭化水素ジオール、たとえば、C2~C6のグリコール、たとえば、エチレングリコール、プロピレングリコール、ブチレングリコール、ペンチレングリコールまたはヘキシレングリコールと、脂肪酸とのモノエステルまたはジエステルが挙げられる。 [Ester of (a 3 ) chain hydrocarbon diol and at least one fatty acid]
Examples of esters of the above linear hydrocarbon diol and at least one fatty acid include C 2 to C 6 linear hydrocarbon diols, such as C 2 to C 6 glycols, such as ethylene glycol, propylene glycol, butylene And mono- or diesters of glycol, pentylene glycol or hexylene glycol with fatty acids.
上記鎖状炭化水素ジオールと少なくとも1の脂肪酸とのエステルとしては、たとえば、C2~C6の鎖状炭化水素ジオール、たとえば、C2~C6のグリコール、たとえば、エチレングリコール、プロピレングリコール、ブチレングリコール、ペンチレングリコールまたはヘキシレングリコールと、脂肪酸とのモノエステルまたはジエステルが挙げられる。 [Ester of (a 3 ) chain hydrocarbon diol and at least one fatty acid]
Examples of esters of the above linear hydrocarbon diol and at least one fatty acid include C 2 to C 6 linear hydrocarbon diols, such as C 2 to C 6 glycols, such as ethylene glycol, propylene glycol, butylene And mono- or diesters of glycol, pentylene glycol or hexylene glycol with fatty acids.
具体的には、上記鎖状炭化水素ジオールと少なくとも1の脂肪酸とのエステルとしては、たとえば、次の式(8):
R8COOCkH2kOCOR9 (8)
(式中、kは、2~6の整数であり、そしてR8およびR9は、それぞれ、鎖状炭化水素である)
のC2~C6グリコールと脂肪酸とのジエステル、および次の式(9):
R8COOCkH2kOH (9)
(式中、kは、2~6の整数であり、そしてR8は、鎖状炭化水素である)
のC2~C6グリコールと脂肪酸とのモノエステルが挙げられる。 Specifically, as the ester of the above linear hydrocarbon diol and at least one fatty acid, for example, the following formula (8):
R 8 COOC k H 2k OCOR 9 (8)
(Wherein k is an integer of 2 to 6 and R 8 and R 9 are each a chain hydrocarbon)
A diester of a C 2 -C 6 glycol with a fatty acid, and the following formula (9):
R 8 COOC k H 2k OH (9)
(Wherein k is an integer of 2 to 6 and R 8 is a chain hydrocarbon)
And monoesters of fatty acid with C 2 -C 6 glycol.
R8COOCkH2kOCOR9 (8)
(式中、kは、2~6の整数であり、そしてR8およびR9は、それぞれ、鎖状炭化水素である)
のC2~C6グリコールと脂肪酸とのジエステル、および次の式(9):
R8COOCkH2kOH (9)
(式中、kは、2~6の整数であり、そしてR8は、鎖状炭化水素である)
のC2~C6グリコールと脂肪酸とのモノエステルが挙げられる。 Specifically, as the ester of the above linear hydrocarbon diol and at least one fatty acid, for example, the following formula (8):
R 8 COOC k H 2k OCOR 9 (8)
(Wherein k is an integer of 2 to 6 and R 8 and R 9 are each a chain hydrocarbon)
A diester of a C 2 -C 6 glycol with a fatty acid, and the following formula (9):
R 8 COOC k H 2k OH (9)
(Wherein k is an integer of 2 to 6 and R 8 is a chain hydrocarbon)
And monoesters of fatty acid with C 2 -C 6 glycol.
上記C2~C6グリコールと脂肪酸とのエステルにおいて、エステル化すべき脂肪酸(式(8)および式(9)において、R8COOHおよびR9COOHに相当する)としては、C2~C6グリコールと脂肪酸とのエステルが、上記IOB、融点および水溶解度の要件を満たすものであれば、特に制限されず、たとえば、「(a1)鎖状炭化水素テトラオールと少なくとも1の脂肪酸とのエステル」において列挙されている脂肪酸、すなわち、飽和脂肪酸および不飽和脂肪酸が挙げられ、酸化等により変性する可能性を考慮すると、飽和脂肪酸が好ましい。
In the ester of C 2 to C 6 glycol and fatty acid, as the fatty acid to be esterified (corresponding to R 8 COOH and R 9 COOH in the formula (8) and the formula (9)), C 2 to C 6 glycol No particular limitation is imposed on the ester of fatty acid with the fatty acid, provided that it satisfies the requirements of the above IOB, melting point and water solubility, for example, "ester of (a 1 ) chain hydrocarbon tetraol with at least one fatty acid" The fatty acids listed in the above, ie, saturated fatty acids and unsaturated fatty acids, may be mentioned, and in view of the possibility of modification by oxidation etc., saturated fatty acids are preferred.
式(8)に示されるブチレングリコール(k=4)と脂肪酸とのジエステルでは、R8CおよびR9C部分の炭素数の合計が6の場合に、IOBが、0.60となる。したがって、式(8)に示されるブチレングリコール(k=4)と脂肪酸とのジエステルでは、上記炭素数の合計が約6以上の場合に、IOBが約0.00~約0.60の要件を満たす。また、式(9)に示されるエチレングリコール(k=2)と脂肪酸とのモノエステルでは、R8C部分の炭素数が12の場合に、IOBが0.57となる。したがって、式(9)に示されるエチレングリコール(k=2)と脂肪酸とのモノエステルでは、脂肪酸の炭素数が約12以上である場合に、IOBが約0.00~約0.60の要件を満たす。
In the case of the diester of butylene glycol (k = 4) and a fatty acid shown in the formula (8), the IOB is 0.60 when the total carbon number of the R 8 C and R 9 C moieties is six. Therefore, in the case of the diester of butylene glycol (k = 4) and fatty acid shown in the formula (8), the IOB is about 0.00 to about 0.60 when the total carbon number is about 6 or more. Fulfill. Further, the monoester of a fatty acid ethylene glycol (k = 2) represented by formula (9), when the number of carbons of the R 8 C portion is 12, IOB is 0.57. Therefore, in the monoester of ethylene glycol (k = 2) shown in the formula (9) and a fatty acid, the requirement of an IOB of about 0.00 to about 0.60 when the carbon number of the fatty acid is about 12 or more Meet.
上記C2~C6グリコールと脂肪酸とのエステルとしては、酸化等により変性する可能性を考慮すると、飽和脂肪酸に由来する、C2~C6グリコールと脂肪酸とのエステル、すわなち、C2~C6グリコールと飽和脂肪酸とのエステルであることが好ましい。
The ester of a C 2 ~ C 6 glycols and fatty acid, in view of the potential for degradation by oxidation and the like, derived from saturated fatty acids, esters of C 2 ~ C 6 glycols and fatty acid, Nachi Suwa, C 2 It is preferably an ester of a -C 6 glycol and a saturated fatty acid.
また、上記C2~C6グリコールと脂肪酸とのエステルとしては、IOBを小さくし、より疎水性とするために、炭素数の大きいグリコールに由来する、グリコールと脂肪酸とのエステル、たとえば、ブチレングリコール、ペンチレングリコールまたはヘキシレングリコールに由来するグリコールと脂肪酸とのエステルであることが好ましい。
Also, as the ester of C 2 -C 6 glycol and fatty acid, an ester of glycol and fatty acid derived from glycol having a large number of carbon atoms, for example, butylene glycol, in order to make IOB small and make it more hydrophobic. It is preferable that it is an ester of a glycol derived from pentylene glycol or hexylene glycol and a fatty acid.
さらに、上記C2~C6グリコールと脂肪酸とのエステルとしては、IOBを小さくし、より疎水性とするために、ジエステルであることが好ましい。上記C2~C6グリコールと脂肪酸とのエステルの市販品としては、たとえば、コムポールBL、コムポールBS(以上、日油株式会社製)等が挙げられる。
Furthermore, as the ester of C 2 -C 6 glycol and fatty acid, a diester is preferable in order to make IOB small and to make it more hydrophobic. Examples of commercial products of the ester of C 2 -C 6 glycol and fatty acid include Commol BL, Commol BS (manufactured by NOF Corporation) and the like.
[(B) (B1)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(B2)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエーテル]
(B) (B1)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(B2)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエーテル(以下、「化合物(B)」と称する場合がある)は、上述のIOB、融点および水溶解度を有する限り、全てのヒドロキシル基がエーテル化されていなくともよい。 [(B) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, and Ether with compound having one hydroxyl group replacing hydrogen atom of linear hydrocarbon moiety]
(B) A compound having (B1) a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, and the above chain Ether with a compound having one hydroxyl group replacing hydrogen atoms in the cyclic hydrocarbon moiety (hereinafter sometimes referred to as “compound (B)”) has the above-mentioned IOB, melting point and water solubility And all hydroxyl groups may not be etherified.
(B) (B1)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(B2)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエーテル(以下、「化合物(B)」と称する場合がある)は、上述のIOB、融点および水溶解度を有する限り、全てのヒドロキシル基がエーテル化されていなくともよい。 [(B) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, and Ether with compound having one hydroxyl group replacing hydrogen atom of linear hydrocarbon moiety]
(B) A compound having (B1) a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, and the above chain Ether with a compound having one hydroxyl group replacing hydrogen atoms in the cyclic hydrocarbon moiety (hereinafter sometimes referred to as “compound (B)”) has the above-mentioned IOB, melting point and water solubility And all hydroxyl groups may not be etherified.
(B1)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物としては、「化合物(A)」において化合物(A1)として列挙されるもの、たとえば、ペンタエリトリトール、グリセリン、およびグリコールが挙げられる。
(B2)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物(以下、「化合物(B2)」と称する場合がある)としては、たとえば、炭化水素の1個の水素原子が、1個のヒドロキシル基(-OH)で置換された化合物、たとえば、脂肪族1価アルコール、たとえば、飽和脂肪族1価アルコールおよび不飽和脂肪族1価アルコールが挙げられる。 Examples of the compound having (B1) a chain hydrocarbon portion and 2 to 4 hydroxyl groups replacing the hydrogen atoms of the above chain hydrocarbon portion are listed as the compound (A1) in the “compound (A)”. For example, pentaerythritol, glycerin and glycol.
Examples of (B2) a compound having a linear hydrocarbon moiety and one hydroxyl group replacing the hydrogen atom of the linear hydrocarbon moiety (hereinafter may be referred to as “compound (B2)”) include, for example, A compound in which one hydrogen atom of hydrocarbon is substituted with one hydroxyl group (—OH), for example, aliphatic monohydric alcohol such as saturated aliphatic monohydric alcohol and unsaturated aliphatic monohydric alcohol Can be mentioned.
(B2)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物(以下、「化合物(B2)」と称する場合がある)としては、たとえば、炭化水素の1個の水素原子が、1個のヒドロキシル基(-OH)で置換された化合物、たとえば、脂肪族1価アルコール、たとえば、飽和脂肪族1価アルコールおよび不飽和脂肪族1価アルコールが挙げられる。 Examples of the compound having (B1) a chain hydrocarbon portion and 2 to 4 hydroxyl groups replacing the hydrogen atoms of the above chain hydrocarbon portion are listed as the compound (A1) in the “compound (A)”. For example, pentaerythritol, glycerin and glycol.
Examples of (B2) a compound having a linear hydrocarbon moiety and one hydroxyl group replacing the hydrogen atom of the linear hydrocarbon moiety (hereinafter may be referred to as “compound (B2)”) include, for example, A compound in which one hydrogen atom of hydrocarbon is substituted with one hydroxyl group (—OH), for example, aliphatic monohydric alcohol such as saturated aliphatic monohydric alcohol and unsaturated aliphatic monohydric alcohol Can be mentioned.
上記飽和脂肪族1価アルコールとしては、たとえば、C1~C20の飽和脂肪族1価アルコール、たとえば、メチルアルコール(C1)(C1は、炭素数を示す、以下同じ)、エチルアルコール(C2)、プロピルアルコール(C3)およびその異性体、たとえば、イソプロピルアルコール(C3)、ブチルアルコール(C4)およびその異性体、たとえば、sec-ブチルアルコール(C4)およびtert-ブチルアルコール(C4)、ペンチルアルコール(C5)、ヘキシルアルコール(C6)、ヘプチルアルコール(C7)、オクチルアルコール(C8)およびその異性体、たとえば、2-エチルヘキシルアルコール(C8)、ノニルアルコール(C9)、デシルアルコール(C10)、ドデシルアルコール(C12)、テトラデシルアルコール(C14)、ヘキサデシルアルコール(C16)、へプラデシルアルコール(C17)、オクタデシルアルコール(C18)、およびエイコシルアルコール(C20)、ならびにこれらの列挙されていない異性体が挙げられる。
As the saturated aliphatic monohydric alcohol, for example, a C 1 to C 20 saturated aliphatic monohydric alcohol, for example, methyl alcohol (C 1 ) (C 1 represents a carbon number, the same applies hereinafter), ethyl alcohol C 2 ), propyl alcohol (C 3 ) and its isomers, such as isopropyl alcohol (C 3 ), butyl alcohol (C 4 ) and its isomers, such as sec-butyl alcohol (C 4 ) and tert-butyl alcohol (C 4 ), pentyl alcohol (C 5 ), hexyl alcohol (C 6 ), heptyl alcohol (C 7 ), octyl alcohol (C 8 ) and isomers thereof, such as 2-ethylhexyl alcohol (C 8 ), nonyl alcohol (C 9 ), decyl alcohol (C 10 ), dodecyl alcohol (C 12 ), tetradecyl al Call (C 14), hexadecyl alcohol (C 16), to heptadecyl alcohol (C 17), octadecyl alcohol (C 18), and eicosyl alcohol (C 20), and isomers thereof that are not listed in these Be
上記不飽和脂肪族1価アルコールとしては、上記飽和脂肪族1価アルコールのC-C単結合の1つを、C=C二重結合で置換したもの、たとえば、オレイルアルコールが挙げられ、たとえば、新日本理化株式会社から、リカコールシリーズおよびアンジェコオールシリーズの名称で市販されている。
Examples of the unsaturated aliphatic monohydric alcohol include those obtained by replacing one of the C—C single bonds of the above saturated aliphatic monohydric alcohol with a C = C double bond, such as oleyl alcohol. It is commercially available from Shin Nippon Rika Co., Ltd. under the names of Rikacoll series and Angekoall series.
化合物(B)としては、たとえば、(b1)鎖状炭化水素テトラオールと少なくとも1の脂肪族1価アルコールとのエーテル、たとえば、モノエーテル、ジエーテル、トリエーテルおよびテトラエーテル、好ましくはジエーテル、トリエーテルおよびテトラエーテル、より好ましくはトリエーテルおよびテトラエーテル、そしてさらに好ましくはテトラエーテル、(b2)鎖状炭化水素トリオールと少なくとも1の脂肪族1価アルコールとのエーテル、たとえば、モノエーテル、ジエーテルおよびトリエーテル、好ましくはジエーテルおよびトリエーテル、そしてより好ましくはトリエーテル、ならびに(b3)鎖状炭化水素ジオールと少なくとも1の脂肪族1価アルコールとのエーテル、たとえば、モノエーテルおよびジエーテル、そして好ましくはジエーテルが挙げられる。
As compound (B), for example, an ether of (b 1 ) chain hydrocarbon tetraol and at least one aliphatic monohydric alcohol, for example, monoether, diether, triether and tetraether, preferably diether, triether Ethers and tetraethers, more preferably triethers and tetraethers, and even more preferably tetraethers, ethers of (b 2 ) chain hydrocarbon triols and at least one aliphatic monohydric alcohol, such as monoethers, diethers and the like triether, preferably diethers and triethers and more preferably tri-ether, and (b 3) a chain hydrocarbon diol and at least one aliphatic monohydric ether alcohols, for example, mono- and diethers and, Mashiku diethers.
上記鎖状炭化水素テトラオールと少なくとも1の脂肪族1価アルコールとのエーテルとしては、たとえば、次の式(10)~(13):
(式中、R10~R13は、それぞれ、鎖状炭化水素である。)
の、ペンタエリトリトールと脂肪族1価アルコールとのテトラエーテル、トリエーテル、ジエーテルおよびモノエーテルが挙げられる。 Examples of the ether of the above linear hydrocarbon tetraol and at least one aliphatic monohydric alcohol include the following formulas (10) to (13):
(Wherein, each of R 10 to R 13 is a chain hydrocarbon).
And tetraethers of pentaerythritol and aliphatic monohydric alcohols, triethers, diethers and monoethers.
の、ペンタエリトリトールと脂肪族1価アルコールとのテトラエーテル、トリエーテル、ジエーテルおよびモノエーテルが挙げられる。 Examples of the ether of the above linear hydrocarbon tetraol and at least one aliphatic monohydric alcohol include the following formulas (10) to (13):
And tetraethers of pentaerythritol and aliphatic monohydric alcohols, triethers, diethers and monoethers.
上記鎖状炭化水素トリオールと少なくとも1の脂肪族1価アルコールとのエーテルとしては、たとえば、次の式(14)~(16):
(式中、R14~R16は、それぞれ、鎖状炭化水素である。)
の、グリセリンと脂肪族1価アルコールとのトリエーテル、ジエーテルおよびモノエーテルが挙げられる。 Examples of the ether of the above linear hydrocarbon triol and at least one aliphatic monohydric alcohol include the following formulas (14) to (16):
(Wherein, R 14 to R 16 are each a chain hydrocarbon).
And triethers of glycerol and aliphatic monohydric alcohols, diethers and monoethers.
の、グリセリンと脂肪族1価アルコールとのトリエーテル、ジエーテルおよびモノエーテルが挙げられる。 Examples of the ether of the above linear hydrocarbon triol and at least one aliphatic monohydric alcohol include the following formulas (14) to (16):
And triethers of glycerol and aliphatic monohydric alcohols, diethers and monoethers.
上記鎖状炭化水素ジオールと少なくとも1の脂肪族1価アルコールとのエーテルとしては、次の式(17):
R17OCnH2nOR18 (17)
(式中、nは、2~6の整数であり、そしてR17およびR18は、それぞれ、鎖状炭化水素である)
のC2~C6グリコールと脂肪族1価アルコールとのジエーテル、および次の式(18):
R17OCnH2nOH (18)
(式中、nは、2~6の整数であり、そしてR17は、鎖状炭化水素である)
のC2~C6グリコールと脂肪族1価アルコールとのモノエーテルが挙げられる。 As the ether of the above linear hydrocarbon diol and at least one aliphatic monohydric alcohol, the following formula (17):
R 17 OC n H 2n OR 18 (17)
(Wherein n is an integer of 2 to 6 and R 17 and R 18 are each a chain hydrocarbon)
Diethers of C 2 -C 6 glycols and aliphatic monohydric alcohols, and the following formula (18):
R 17 OC n H 2n OH (18)
(Wherein n is an integer of 2 to 6 and R 17 is a chain hydrocarbon)
And monoethers of C 2 -C 6 glycols and aliphatic monohydric alcohols.
R17OCnH2nOR18 (17)
(式中、nは、2~6の整数であり、そしてR17およびR18は、それぞれ、鎖状炭化水素である)
のC2~C6グリコールと脂肪族1価アルコールとのジエーテル、および次の式(18):
R17OCnH2nOH (18)
(式中、nは、2~6の整数であり、そしてR17は、鎖状炭化水素である)
のC2~C6グリコールと脂肪族1価アルコールとのモノエーテルが挙げられる。 As the ether of the above linear hydrocarbon diol and at least one aliphatic monohydric alcohol, the following formula (17):
R 17 OC n H 2n OR 18 (17)
(Wherein n is an integer of 2 to 6 and R 17 and R 18 are each a chain hydrocarbon)
Diethers of C 2 -C 6 glycols and aliphatic monohydric alcohols, and the following formula (18):
R 17 OC n H 2n OH (18)
(Wherein n is an integer of 2 to 6 and R 17 is a chain hydrocarbon)
And monoethers of C 2 -C 6 glycols and aliphatic monohydric alcohols.
上記ペンタエリトリトールと脂肪族1価アルコールとのテトラエーテルでは、ペンタエリトリトールと脂肪族1価アルコールとのテトラエーテルを構成する脂肪族1価アルコールの炭素数の合計、すなわち、上記式(10)において、R10、R11、R12およびR13部分の炭素数の合計が4の場合にIOBが0.44となる。したがって、上記ペンタエリトリトールと脂肪族1価アルコールとのテトラエーテルでは、脂肪族1価アルコールの炭素数の合計が約4以上である場合に、IOBが約0.00~約0.60の要件を満たす。
In the tetraether of pentaerythritol and aliphatic monohydric alcohol, the total carbon number of aliphatic monohydric alcohol constituting the tetraether of pentaerythritol and aliphatic monohydric alcohol, that is, in the above formula (10), When the total carbon number of the R 10 , R 11 , R 12 and R 13 moieties is 4, the IOB is 0.44. Therefore, in the above tetraether of pentaerythritol and aliphatic monohydric alcohol, IOB is required to be about 0.00 to about 0.60 when the total carbon number of aliphatic monohydric alcohol is about 4 or more. Fulfill.
上記ペンタエリトリトールと脂肪族1価アルコールとのトリエーテルでは、ペンタエリトリトールと脂肪族1価アルコールとのトリエーテルを構成する脂肪族1価アルコールの炭素数の合計、すなわち、上記式(11)において、R10、R11およびR12部分の炭素数の合計が9の場合にIOBが0.57となる。したがって、上記ペンタエリトリトールと脂肪族1価アルコールとのトリエーテルでは、脂肪族1価アルコールの炭素数の合計が約9以上である場合に、IOBが約0.00~約0.60の要件を満たす。
In the triether of pentaerythritol and aliphatic monohydric alcohol, the total carbon number of aliphatic monohydric alcohol constituting triether of pentaerythritol and aliphatic monohydric alcohol, ie, in the above formula (11), When the sum of the carbon numbers of the R 10 , R 11 and R 12 moieties is 9, the IOB is 0.57. Therefore, in the above triether of pentaerythritol and aliphatic monohydric alcohol, IOB is required to be about 0.00 to about 0.60 when the total carbon number of aliphatic monohydric alcohol is about 9 or more. Fulfill.
上記ペンタエリトリトールと脂肪族1価アルコールとのジエーテルでは、ペンタエリトリトールと脂肪族1価アルコールとのジエーテルを構成する脂肪族1価アルコールの炭素数の合計、すなわち、上記式(12)において、R10およびR11部分の炭素数の合計が15の場合にIOBが0.60となる。したがって、上記ペンタエリトリトールと脂肪族1価アルコールとのジエーテルでは、脂肪族1価アルコールの炭素数の合計が約15以上である場合に、IOBが約0.00~約0.60の要件を満たす。
In the diether of pentaerythritol and aliphatic monohydric alcohol, the total carbon number of aliphatic monohydric alcohols constituting the diether of pentaerythritol and aliphatic monohydric alcohol, that is, R 10 in the above formula (12) The IOB is 0.60 when the sum of the carbon numbers of the and R 11 moieties is 15. Therefore, in the diether of pentaerythritol and aliphatic monohydric alcohol, IOB satisfies the requirement of about 0.00 to about 0.60 when the total carbon number of aliphatic monohydric alcohol is about 15 or more. .
上記ペンタエリトリトールと脂肪族1価アルコールとのモノエーテルでは、ペンタエリトリトールと脂肪族1価アルコールとのモノエーテルを構成する脂肪族1価アルコールの炭素数、すなわち、上記式(13)において、R10部分の炭素数が22の場合にIOBが0.59となる。したがって、上記ペンタエリトリトールと脂肪族1価アルコールとのモノエーテルでは、脂肪族1価アルコールの炭素数が約22以上である場合に、IOBが約0.00~約0.60の要件を満たす。
In the monoether of pentaerythritol and aliphatic monohydric alcohol, the carbon number of the aliphatic monohydric alcohol constituting the monoether of pentaerythritol and aliphatic monohydric alcohol, that is, R 10 in the above formula (13) When the carbon number of the portion is 22, the IOB is 0.59. Therefore, in the monoether of pentaerythritol and aliphatic monohydric alcohol, the IOB satisfies the requirement of about 0.00 to about 0.60 when the carbon number of the aliphatic monohydric alcohol is about 22 or more.
また、上記グリセリンと脂肪族1価アルコールとのトリエーテルでは、グリセリンと脂肪族1価アルコールとのトリエーテルを構成する脂肪族1価アルコールの炭素数の合計、すなわち、式(14)において、R14、R15およびR16部分の炭素数の合計が3の場合にIOBが0.50となる。したがって、上記グリセリンと脂肪族1価アルコールとのトリエーテルでは、脂肪族1価アルコールの炭素数の合計が約3以上である場合に、IOBが約0.00~約0.60の要件を満たす。
In the above triether of glycerin and aliphatic monohydric alcohol, the total carbon number of aliphatic monohydric alcohol constituting triether of glycerin and aliphatic monohydric alcohol, that is, R in the formula (14) When the sum of the carbon numbers of 14 , R 15 and R 16 is 3, the IOB is 0.50. Therefore, in the above triether of glycerin and aliphatic monohydric alcohol, IOB satisfies the requirement of about 0.00 to about 0.60 when the total carbon number of aliphatic monohydric alcohol is about 3 or more. .
上記グリセリンと脂肪族1価アルコールとのジエーテルでは、グリセリンと脂肪族1価アルコールとのジエーテルを構成する脂肪族1価アルコールの炭素数の合計、すなわち、式(15)において、R14およびR15部分の炭素数の合計が9の場合にIOBが0.58となる。したがって、上記グリセリンと脂肪族1価アルコールとのジエーテルでは、脂肪族1価アルコールの炭素数の合計が約9以上である場合に、IOBが約0.00~約0.60の要件を満たす。
In the diether of glycerin and an aliphatic monohydric alcohol, the total carbon number of aliphatic monohydric alcohols constituting the diether of glycerin and an aliphatic monohydric alcohol, that is, in the formula (15), R 14 and R 15 When the total number of carbon atoms in the portion is 9, the IOB is 0.58. Therefore, in the diether of glycerin and an aliphatic monohydric alcohol, the IOB satisfies the requirement of about 0.00 to about 0.60 when the total carbon number of the aliphatic monohydric alcohol is about 9 or more.
上記グリセリンと脂肪族1価アルコールとのモノエーテルでは、グリセリンと脂肪族1価アルコールとのモノエーテルを構成する脂肪族1価アルコールの炭素数、すなわち、式(16)において、R14部分の炭素数が16の場合にIOBが0.58となる。したがって、上記グリセリンと脂肪族1価アルコールとのモノエーテルでは、脂肪族1価アルコールの炭素数が約16以上である場合に、IOBが約0.00~約0.60の要件を満たす。
In the monoether of glycerin and aliphatic monohydric alcohol, the carbon number of the aliphatic monohydric alcohol constituting the monoether of glycerin and aliphatic monohydric alcohol, that is, the carbon of R 14 in the formula (16) When the number is 16, the IOB is 0.58. Therefore, in the monoether of glycerin and aliphatic monohydric alcohol, the IOB satisfies the requirement of about 0.00 to about 0.60 when the carbon number of the aliphatic monohydric alcohol is about 16 or more.
式(17)に示されるブチレングリコール(n=4)と脂肪族1価アルコールとのジエーテルでは、R17およびR18部分の炭素数の合計が2の場合に、IOBが、0.33となる。したがって、式(17)に示されるブチレングリコール(n=4)と脂肪族1価アルコールとのジエーテルでは、脂肪族1価アルコールの炭素数の合計が2以上の場合に、IOBが約0.00~約0.60の要件を満たす。また、式(18)に示されるエチレングリコール(n=2)と脂肪族1価アルコールとのモノエーテルでは、R17部分の炭素数が8の場合に、IOBが0.60となる。したがって、式(18)に示されるエチレングリコール(n=2)と脂肪族1価アルコールとのモノエーテルでは、脂肪族1価アルコールの炭素数が約8以上である場合に、IOBが約0.00~約0.60の要件を満たす。
In the diether of butylene glycol (n = 4) represented by the formula (17) and an aliphatic monohydric alcohol, the IOB is 0.33 when the total carbon number of the R 17 and R 18 moieties is two. . Therefore, in the diether of butylene glycol (n = 4) shown in the formula (17) and an aliphatic monohydric alcohol, the IOB is about 0.00 when the total carbon number of the aliphatic monohydric alcohol is 2 or more. Meet the requirements of ~ 0.60. Further, in the monoether of ethylene glycol (n = 2) and aliphatic monohydric alcohol represented by the formula (18), the IOB is 0.60 when the carbon number of the R 17 portion is eight. Therefore, in the monoether of ethylene glycol (n = 2) and aliphatic monohydric alcohol shown in the formula (18), when the carbon number of the aliphatic monohydric alcohol is about 8 or more, IOB is about 0. Meet the requirements of 00 to about 0.60.
化合物(B)としては、化合物(B1)と、化合物(B2)とを、酸触媒の存在下で、脱水縮合することにより生成することができる。
The compound (B) can be produced by dehydration condensation of the compound (B1) and the compound (B2) in the presence of an acid catalyst.
[(C) (C1)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する、2~4個のカルボキシル基とを含むカルボン酸、ヒドロキシ酸、アルコキシ酸またはオキソ酸と、(C2)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエステル]
(C) (C1)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する、2~4個のカルボキシル基とを含むカルボン酸、ヒドロキシ酸、アルコキシ酸またはオキソ酸と、(C2)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエステル(以下、「化合物(C)」と称する場合がある)は、上述のIOB、融点および水溶解度を有する限り、全てのカルボキシル基がエステル化されていなくともよい。 [(C) (C1) a chain hydrocarbon moiety and a carboxylic acid, hydroxy acid, alkoxy acid or oxo acid containing 2 to 4 carboxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety; (C2) Ester of a compound having a linear hydrocarbon moiety and one hydroxyl group replacing the hydrogen atom of the linear hydrocarbon moiety]
(C) a carboxylic acid, a hydroxy acid, an alkoxy acid or an oxo acid containing a (C1) linear hydrocarbon moiety and 2 to 4 carboxyl groups replacing the hydrogen atom of the linear hydrocarbon moiety; C2) An ester of a chain hydrocarbon moiety and a compound having one hydroxyl group replacing the hydrogen atom of the chain hydrocarbon moiety (hereinafter sometimes referred to as “compound (C)”) is All carboxyl groups may not be esterified as long as they have the above IOB, melting point and water solubility.
(C) (C1)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する、2~4個のカルボキシル基とを含むカルボン酸、ヒドロキシ酸、アルコキシ酸またはオキソ酸と、(C2)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエステル(以下、「化合物(C)」と称する場合がある)は、上述のIOB、融点および水溶解度を有する限り、全てのカルボキシル基がエステル化されていなくともよい。 [(C) (C1) a chain hydrocarbon moiety and a carboxylic acid, hydroxy acid, alkoxy acid or oxo acid containing 2 to 4 carboxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety; (C2) Ester of a compound having a linear hydrocarbon moiety and one hydroxyl group replacing the hydrogen atom of the linear hydrocarbon moiety]
(C) a carboxylic acid, a hydroxy acid, an alkoxy acid or an oxo acid containing a (C1) linear hydrocarbon moiety and 2 to 4 carboxyl groups replacing the hydrogen atom of the linear hydrocarbon moiety; C2) An ester of a chain hydrocarbon moiety and a compound having one hydroxyl group replacing the hydrogen atom of the chain hydrocarbon moiety (hereinafter sometimes referred to as “compound (C)”) is All carboxyl groups may not be esterified as long as they have the above IOB, melting point and water solubility.
(C1)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する、2~4個のカルボキシル基とを含むカルボン酸、ヒドロキシ酸、アルコキシ酸またはオキソ酸(以下、「化合物(C1)」と称する場合がある)としては、たとえば、2~4個のカルボキシル基を有する鎖状炭化水素カルボン酸、たとえば、鎖状炭化水素ジカルボン酸、たとえば、アルカンジカルボン酸、たとえば、エタン二酸、プロパン二酸、ブタン二酸、ペンタン二酸、ヘキサン二酸、ヘプタン二酸、オクタン二酸、ノナン二酸およびデカン二酸、鎖状炭化水素トリカルボン酸、たとえば、アルカントリカルボン酸、たとえば、プロパン三酸、ブタン三酸、ペンタン三酸、ヘキサン三酸、ヘプタン三酸、オクタン三酸、ノナン三酸およびデカン三酸、ならびに鎖状炭化水素テトラカルボン酸、たとえば、アルカンテトラカルボン酸、たとえば、ブタン四酸、ペンタン四酸、ヘキサン四酸、ヘプタン四酸、オクタン四酸、ノナン四酸およびデカン四酸が挙げられる。
(C1) a linear hydrocarbon moiety and a carboxylic acid, a hydroxy acid, an alkoxy acid or an oxo acid containing 2 to 4 carboxyl groups replacing the hydrogen atom of the linear hydrocarbon moiety (hereinafter referred to as “compound C1) may be, for example, a linear hydrocarbon carboxylic acid having 2 to 4 carboxyl groups, such as a linear hydrocarbon dicarboxylic acid, such as an alkane dicarboxylic acid, such as ethanedioic acid. Propanedioic acid, butanedioic acid, pentanedioic acid, hexanedioic acid, heptanedioic acid, octanedioic acid, nonanedioic acid and decanedioic acid, linear hydrocarbon tricarboxylic acids such as alkanetricarboxylic acids such as propane tricarboxylic acid Acid, butane triacid, pentane triacid, hexane triacid, heptane triacid, octane triacid, nonane triacid and decane triacid, and Chain hydrocarbon tetracarboxylic acids such as alkane tetracarboxylic acids such as butane tetra acid, pentane tetra acid, hexane tetra acid, heptane tetra acid, octane tetra acid, octane tetra acid, nonane tetra acid and decane tetra acid.
また、化合物(C1)には、2~4個のカルボキシル基を有する鎖状炭化水素ヒドロキシ酸、たとえば、リンゴ酸、酒石酸、クエン酸、イソクエン酸等、2~4個のカルボキシル基を有する鎖状炭化水素アルコキシ酸、たとえば、O-アセチルクエン酸、および2~4個のカルボキシル基を有する鎖状炭化水素オキソ酸が含まれる。(C2)鎖状炭化水素部分と、上記鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物としては、「化合物(B)」の項で列挙されるもの、たとえば、脂肪族1価アルコールが挙げられる。
Further, in the compound (C1), a linear hydrocarbon hydroxy acid having 2 to 4 carboxyl groups, for example, a linear chain having 2 to 4 carboxyl groups, such as malic acid, tartaric acid, citric acid, isocitric acid, etc. Hydrocarbon alkoxy acids, such as O-acetyl citric acid, and linear hydrocarbon oxo acids having 2 to 4 carboxyl groups are included. Examples of the compound having a (C2) linear hydrocarbon moiety and one hydroxyl group replacing the hydrogen atom of the linear hydrocarbon moiety include those listed in the “compound (B)”, for example, Aliphatic monohydric alcohols are mentioned.
化合物(C)としては、(c1)4個のカルボキシル基を有する鎖状炭化水素テトラカルボン酸、ヒドロキシ酸、アルコキシ酸またはオキソ酸と、少なくとも1の脂肪族1価アルコールとのエステル、たとえば、モノエステル、ジエステル、トリエステルおよびテトラエステル、好ましくはジエステル、トリエステルおよびテトラエステル、より好ましくはトリエステルおよびテトラエステル、そしてさらに好ましくはテトラエステル、(c2)3個のカルボキシル基を有する鎖状炭化水素トリカルボン酸、ヒドロキシ酸、アルコキシ酸またはオキソ酸と、少なくとも1の脂肪族1価アルコールとのエステル、たとえば、モノエステル、ジエステルおよびトリエステル、好ましくはジエステルおよびトリエステル、そしてより好ましくはトリエステル、ならびに(c3)2個のカルボキシル基を有する鎖状炭化水素ジカルボン酸、ヒドロキシ酸、アルコキシ酸またはオキソ酸と、少なくとも1の脂肪族1価アルコールとのエステル、たとえば、モノエステルおよびジエステル、好ましくはジエステルが挙げられる。化合物(C)の例としては、アジピン酸ジオクチル、O-アセチルクエン酸トリブチル等が挙げられ、そして市販されている。
As the compound (C), an ester of a linear hydrocarbon tetracarboxylic acid having 4 carboxyl groups (c 1 ), a hydroxy acid, an alkoxy acid or an oxo acid, and at least one aliphatic monohydric alcohol, for example, Mono-, di-, tri- and tetra-esters, preferably diesters, tri- and tetra-esters, more preferably tri- and tetra-esters, and still more preferably tetra-esters, chained with 3 (c 2 ) carboxyl groups Esters of hydrocarbon tricarboxylic acids, hydroxy acids, alkoxy acids or oxo acids with at least one aliphatic monohydric alcohol, such as monoesters, diesters and triesters, preferably diesters and triesters, and more preferably triesters Ester, and (c 3) a chain hydrocarbon dicarboxylic acids having two carboxyl groups, hydroxy acids, esters of alkoxy acids or oxoacids, and at least one aliphatic monohydric alcohol, for example, mono- and diesters, Preferably, diester is mentioned. Examples of the compound (C) include dioctyl adipate, tributyl O-acetyl citrate and the like, and are commercially available.
[(D)鎖状炭化水素部分と、上記鎖状炭化水素部分のC-C単結合間に挿入された、エーテル結合(-O-)、カルボニル結合(-CO-)、エステル結合(-COO-)、およびカーボネート結合(-OCOO-)から成る群から選択されるいずれか1つの結合とを有する化合物]
(D)鎖状炭化水素部分と、上記鎖状炭化水素部分のC-C単結合間に挿入された、エーテル結合(-O-)、カルボニル結合(-CO-)、エステル結合(-COO-)、およびカーボネート結合(-OCOO-)から成る群から選択されるいずれか1つの結合とを有する化合物(以下、「化合物(D)」と称する場合がある)としては、(d1)脂肪族1価アルコールと脂肪族1価アルコールとのエーテル、(d2)ジアルキルケトン、(d3)脂肪酸と脂肪族1価アルコールとのエステル、および(d4)ジアルキルカーボネートが挙げられる。 [(D) a linear hydrocarbon moiety and an ether bond (-O-), a carbonyl bond (-CO-), an ester bond (-COO) inserted between the C—C single bond of the above linear hydrocarbon moiety A compound having one or more bonds selected from the group consisting of-), and a carbonate bond (-OCOO-)]
(D) A chain hydrocarbon moiety and an ether bond (-O-), a carbonyl bond (-CO-), an ester bond (-COO-) inserted between the C-C single bond of the chain hydrocarbon moiety and the chain hydrocarbon moiety And (d 1 ) aliphatic as a compound having a bond selected from the group consisting of carbonate bond (—OCOO—) and any one bond (hereinafter may be referred to as “compound (D)”) Ethers of monohydric alcohols and aliphatic monohydric alcohols, (d 2 ) dialkyl ketones, esters of (d 3 ) fatty acids and aliphatic monohydric alcohols, and (d 4 ) dialkyl carbonates can be mentioned.
(D)鎖状炭化水素部分と、上記鎖状炭化水素部分のC-C単結合間に挿入された、エーテル結合(-O-)、カルボニル結合(-CO-)、エステル結合(-COO-)、およびカーボネート結合(-OCOO-)から成る群から選択されるいずれか1つの結合とを有する化合物(以下、「化合物(D)」と称する場合がある)としては、(d1)脂肪族1価アルコールと脂肪族1価アルコールとのエーテル、(d2)ジアルキルケトン、(d3)脂肪酸と脂肪族1価アルコールとのエステル、および(d4)ジアルキルカーボネートが挙げられる。 [(D) a linear hydrocarbon moiety and an ether bond (-O-), a carbonyl bond (-CO-), an ester bond (-COO) inserted between the C—C single bond of the above linear hydrocarbon moiety A compound having one or more bonds selected from the group consisting of-), and a carbonate bond (-OCOO-)]
(D) A chain hydrocarbon moiety and an ether bond (-O-), a carbonyl bond (-CO-), an ester bond (-COO-) inserted between the C-C single bond of the chain hydrocarbon moiety and the chain hydrocarbon moiety And (d 1 ) aliphatic as a compound having a bond selected from the group consisting of carbonate bond (—OCOO—) and any one bond (hereinafter may be referred to as “compound (D)”) Ethers of monohydric alcohols and aliphatic monohydric alcohols, (d 2 ) dialkyl ketones, esters of (d 3 ) fatty acids and aliphatic monohydric alcohols, and (d 4 ) dialkyl carbonates can be mentioned.
[(d1)脂肪族1価アルコールと脂肪族1価アルコールとのエーテル]
上記脂肪族1価アルコールと脂肪族1価アルコールとのエーテルとしては、次の式(19):
R19OR20 (19)
(式中、R19およびR20は、それぞれ、鎖状炭化水素である)
を有する化合物が挙げられる。 [(D 1 ) ether of aliphatic monohydric alcohol and aliphatic monohydric alcohol]
As the ether of the above aliphatic monohydric alcohol and aliphatic monohydric alcohol, the following formula (19):
R 19 OR 20 (19)
(Wherein, R 19 and R 20 are each a chain hydrocarbon)
And compounds having the formula:
上記脂肪族1価アルコールと脂肪族1価アルコールとのエーテルとしては、次の式(19):
R19OR20 (19)
(式中、R19およびR20は、それぞれ、鎖状炭化水素である)
を有する化合物が挙げられる。 [(D 1 ) ether of aliphatic monohydric alcohol and aliphatic monohydric alcohol]
As the ether of the above aliphatic monohydric alcohol and aliphatic monohydric alcohol, the following formula (19):
R 19 OR 20 (19)
(Wherein, R 19 and R 20 are each a chain hydrocarbon)
And compounds having the formula:
上記エーテルを構成する脂肪族1価アルコール(式(19)において、R19OHおよびR20OHに相当する)としては、上記エーテルが、上記IOB、融点および水溶解度の要件を満たすものであれば、特に制限されず、たとえば、「化合物(B)」の項で列挙される脂肪族1価アルコールが挙げられる。
As the aliphatic monohydric alcohol constituting the above ether (corresponding to R 19 OH and R 20 OH in the formula (19)), if the above ether satisfies the requirements of the above IOB, melting point and water solubility It is not particularly limited, and examples thereof include aliphatic monohydric alcohols listed in the “compound (B)” section.
脂肪族1価アルコールと脂肪族1価アルコールとのエーテルでは、当該エーテルを構成する脂肪族1価アルコールの炭素数の合計、すなわち、上記式(19)において、R19およびR20部分の炭素数の合計が2の場合にIOBが0.50となるため、当該炭素数の合計が約2以上であれば、上記IOBの要件を満たす。しかし、上記炭素数の合計が6程度では、水溶解度が約2gと高く、蒸気圧の観点からも問題がある。水溶解度が約0.00~約0.05gの要件を満たすためには、上記炭素数の合計が約8以上であることが好ましい。
In the ether of an aliphatic monohydric alcohol and an aliphatic monohydric alcohol, the total carbon number of the aliphatic monohydric alcohol constituting the ether, that is, the carbon number of the R 19 and R 20 moieties in the above formula (19) Since the IOB is 0.50 when the sum of the two is 2, the requirement of the above IOB is satisfied if the total carbon number is about 2 or more. However, when the total carbon number is about 6, the water solubility is as high as about 2 g, and there is also a problem from the viewpoint of the vapor pressure. In order to satisfy the requirement of water solubility of about 0.00 to about 0.05 g, the total carbon number is preferably about 8 or more.
[(d2)ジアルキルケトン]
上記ジアルキルケトンとしては、次の式(20):
R21COR22 (20)
(式中、R21およびR22は、それぞれ、アルキル基である)
を有する化合物が挙げられる。 [(D 2 ) dialkyl ketone]
As said dialkyl ketone, following Formula (20):
R 21 COR 22 (20)
(Wherein, each of R 21 and R 22 is an alkyl group)
And compounds having the formula:
上記ジアルキルケトンとしては、次の式(20):
R21COR22 (20)
(式中、R21およびR22は、それぞれ、アルキル基である)
を有する化合物が挙げられる。 [(D 2 ) dialkyl ketone]
As said dialkyl ketone, following Formula (20):
R 21 COR 22 (20)
(Wherein, each of R 21 and R 22 is an alkyl group)
And compounds having the formula:
上記ジアルキルケトンでは、R21およびR22の炭素数の合計が5の場合にIOBが0.54となるため、当該炭素数の合計が約5以上であれば、上記IOBの要件を満たす。しかし、上記炭素数の合計が5程度では、水溶解度が約2gと高い。したがって、水溶解度が約0.00~約0.05gの要件を満たすためには、上記炭素数の合計が約8以上であることが好ましい。また、蒸気圧を考慮すると、上記炭素数は、約10以上であることが好ましく、そして約12以上であることが好ましい。なお、上記炭素数の合計が約8の場合、たとえば、5-ノナノンでは、融点は約-50℃であり、蒸気圧は20℃で約230Paである。上記ジアルキルケトンは、市販されている他、公知の方法、たとえば、第二級アルコールを、クロム酸等で酸化することにより得ることができる。
In the above-mentioned dialkyl ketone, since the IOB is 0.54 when the total carbon number of R 21 and R 22 is 5, the requirement of the above IOB is satisfied if the total carbon number is about 5 or more. However, when the total carbon number is about 5, the water solubility is as high as about 2 g. Therefore, in order to satisfy the requirement of water solubility of about 0.00 to about 0.05 g, the total carbon number is preferably about 8 or more. Also, in consideration of the vapor pressure, the carbon number is preferably about 10 or more, and preferably about 12 or more. When the total carbon number is about 8, for example, in the case of 5-nonanone, the melting point is about −50 ° C., and the vapor pressure is about 230 Pa at 20 ° C. The above-mentioned dialkyl ketone is commercially available and can be obtained by a known method, for example, oxidation of a secondary alcohol with chromic acid or the like.
[(d3)脂肪酸と脂肪族1価アルコールとのエステル]
上記脂肪酸と脂肪族1価アルコールとのエステルとしては、たとえば、次の式(21):
R23COOR24 (21)
(式中、R23およびR24は、それぞれ、鎖状炭化水素である)
を有する化合物が挙げられる。 [(D 3 ) ester of fatty acid and aliphatic monohydric alcohol]
As ester of the said fatty acid and aliphatic monohydric alcohol, following Formula (21):
R 23 COOR 24 (21)
(Wherein, R 23 and R 24 are each a chain hydrocarbon)
And compounds having the formula:
上記脂肪酸と脂肪族1価アルコールとのエステルとしては、たとえば、次の式(21):
R23COOR24 (21)
(式中、R23およびR24は、それぞれ、鎖状炭化水素である)
を有する化合物が挙げられる。 [(D 3 ) ester of fatty acid and aliphatic monohydric alcohol]
As ester of the said fatty acid and aliphatic monohydric alcohol, following Formula (21):
R 23 COOR 24 (21)
(Wherein, R 23 and R 24 are each a chain hydrocarbon)
And compounds having the formula:
上記エステルを構成する脂肪酸(式(21)において、R23COOHに相当する)としては、たとえば、「(a1)鎖状炭化水素テトラオールと少なくとも1の脂肪酸とのエステル」において列挙されている脂肪酸、すなわち、飽和脂肪酸または不飽和脂肪酸が挙げられ、酸化等により変性する可能性を考慮すると、飽和脂肪酸が好ましい。上記エステルを構成する脂肪族1価アルコール(式(21)において、R24OHに相当する)としては、たとえば、「化合物(B)」の項で列挙される脂肪族1価アルコールが挙げられる。
Examples of the fatty acid (corresponding to R 23 COOH in the formula (21)) constituting the above-mentioned ester are listed, for example, in “ester of (a 1 ) chain hydrocarbon tetraol with at least one fatty acid”. A fatty acid, that is, a saturated fatty acid or an unsaturated fatty acid is mentioned, and considering the possibility of modification by oxidation etc., a saturated fatty acid is preferred. Examples of the aliphatic monohydric alcohol (corresponding to R 24 OH in the formula (21)) constituting the above-mentioned ester include, for example, aliphatic monohydric alcohols listed in the “compound (B)” section.
なお、上記脂肪酸と脂肪族1価アルコールとのエステルでは、脂肪酸および脂肪族1価アルコールの炭素数の合計、すなわち、式(21)において、R23CおよびR24部分の炭素数の合計が5の場合にIOBが0.60となるため、R23CおよびR24部分の炭素数の合計が約5以上である場合に、上記IOBの要件を満たす。しかし、たとえば、上記炭素数の合計が6の酢酸ブチルでは、蒸気圧が2,000Pa超と高い。したがって、蒸気圧を考慮すると、上記炭素数の合計が約12以上であることが好ましい。なお、上記炭素数の合計が約11以上であれば、水溶解度が約0.00~約0.05gの要件を満たすことができる。
In the ester of fatty acid and aliphatic monohydric alcohol, the sum of carbon number of fatty acid and aliphatic monohydric alcohol, that is, the sum of carbon number of R 23 C and R 24 in the formula (21) is 5 In this case, since the IOB is 0.60, the requirements of the above IOB are satisfied when the total carbon number of the R 23 C and R 24 moieties is about 5 or more. However, for example, with butyl acetate having a total of 6 carbon atoms, the vapor pressure is as high as 2,000 Pa or more. Therefore, in consideration of the vapor pressure, the total carbon number is preferably about 12 or more. If the total carbon number is about 11 or more, the water solubility can satisfy the requirement of about 0.00 to about 0.05 g.
上記脂肪酸と脂肪族1価アルコールとのエステルの例としては、たとえば、ドデカン酸(C12)と、ドデシルアルコール(C12)とのエステル、テトラデカン酸(C14)と、ドデシルアルコール(C12)とのエステル等が挙げられ、上記脂肪酸と脂肪族1価アルコールとのエステルの市販品としては、たとえば、エレクトールWE20、およびエレクトールWE40(以上、日油株式会社製)が挙げられる。
Examples of esters of the above fatty acids with aliphatic monohydric alcohols include, for example, esters of dodecanoic acid (C 12 ), dodecyl alcohol (C 12 ), tetradecanoic acid (C 14 ), and dodecyl alcohol (C 12 ) Examples of commercially available esters of fatty acids and aliphatic monohydric alcohols include Electol WE 20 and Electol WE 40 (all manufactured by NOF Corporation).
[(d4)ジアルキルカーボネート]
上記ジアルキルカーボネートとしては、次の式(22):
R25OC(=O)OR26 (22)
(式中、R25およびR26は、それぞれ、アルキル基である)
を有する化合物が挙げられる。 [(D 4 ) dialkyl carbonate]
As said dialkyl carbonate, following Formula (22):
R 25 OC (= O) OR 26 (22)
(Wherein, R 25 and R 26 are each an alkyl group)
And compounds having the formula:
上記ジアルキルカーボネートとしては、次の式(22):
R25OC(=O)OR26 (22)
(式中、R25およびR26は、それぞれ、アルキル基である)
を有する化合物が挙げられる。 [(D 4 ) dialkyl carbonate]
As said dialkyl carbonate, following Formula (22):
R 25 OC (= O) OR 26 (22)
(Wherein, R 25 and R 26 are each an alkyl group)
And compounds having the formula:
上記ジアルキルカーボネートでは、R25およびR26の炭素数の合計が6の場合にIOBが0.57となるため、R25およびR26の炭素数の合計が、約6以上であれば、IOBの要件を満たす。水溶解度を考慮すると、R25およびR26の炭素数の合計が約7以上であることが好ましく、そして約9以上であることがより好ましい。上記ジアルキルカーボネートは、市販されている他、ホスゲンとアルコールとの反応、塩化ギ酸エステルとアルコールまたはアルコラートとの反応、および炭酸銀とヨウ化アルキルとの反応により合成することができる。
In the above dialkyl carbonate, since the IOB is 0.57 when the total carbon number of R 25 and R 26 is 6, if the total carbon number of R 25 and R 26 is about 6 or more, Meet the requirements. In view of water solubility, the total carbon number of R 25 and R 26 is preferably about 7 or more, and more preferably about 9 or more. The above dialkyl carbonate is commercially available, and can be synthesized by the reaction of phosgene with alcohol, the reaction of formic acid chloride ester with alcohol or alcoholate, and the reaction of silver carbonate with alkyl iodide.
[(E)ポリオキシC2~C6アルキレングリコール、またはそのエステルもしくはエーテル]
上記ポリオキシC2~C6アルキレングリコール、またはそのエステルもしくはエーテル(以下、化合物(E)と称する場合がある)としては、(e1)ポリオキシC2~C6アルキレングリコール、(e2)ポリオキシC2~C6アルキレングリコールと少なくとも1の脂肪酸とのエステル、(e3)ポリオキシC2~C6アルキレングリコールと少なくとも1の脂肪族1価アルコールとのエーテル、(e4)ポリオキシC2~C6アルキレングリコールと、鎖状炭化水素テトラカルボン酸、鎖状炭化水素トリカルボン酸、または鎖状炭化水素ジカルボン酸とのエステル、および(e5)ポリオキシC2~C6アルキレングリコールと、鎖状炭化水素テトラオール、鎖状炭化水素トリオール、または鎖状炭化水素ジオールとのエーテルが挙げられる。以下、説明する。 [(E) Polyoxy C 2 -C 6 alkylene glycol, or ester or ether thereof]
Examples of the above-mentioned polyoxy C 2 -C 6 alkylene glycol or ester or ether thereof (hereinafter sometimes referred to as compound (E)) include (e 1 ) polyoxy C 2 -C 6 alkylene glycol, (e 2 ) polyoxy C An ester of a 2 to C 6 alkylene glycol with at least one fatty acid, an ether of (e 3 ) polyoxy C 2 to C 6 alkylene glycol and at least one aliphatic monohydric alcohol, (e 4 ) polyoxy C 2 to C 6 An ester of an alkylene glycol with a linear hydrocarbon tetracarboxylic acid, a linear hydrocarbon tricarboxylic acid, or a linear hydrocarbon dicarboxylic acid, and (e 5 ) polyoxy C 2 -C 6 alkylene glycol, with a linear hydrocarbon tetra And ethers with linear hydrocarbon triols or linear hydrocarbon diols. That. This will be described below.
上記ポリオキシC2~C6アルキレングリコール、またはそのエステルもしくはエーテル(以下、化合物(E)と称する場合がある)としては、(e1)ポリオキシC2~C6アルキレングリコール、(e2)ポリオキシC2~C6アルキレングリコールと少なくとも1の脂肪酸とのエステル、(e3)ポリオキシC2~C6アルキレングリコールと少なくとも1の脂肪族1価アルコールとのエーテル、(e4)ポリオキシC2~C6アルキレングリコールと、鎖状炭化水素テトラカルボン酸、鎖状炭化水素トリカルボン酸、または鎖状炭化水素ジカルボン酸とのエステル、および(e5)ポリオキシC2~C6アルキレングリコールと、鎖状炭化水素テトラオール、鎖状炭化水素トリオール、または鎖状炭化水素ジオールとのエーテルが挙げられる。以下、説明する。 [(E) Polyoxy C 2 -C 6 alkylene glycol, or ester or ether thereof]
Examples of the above-mentioned polyoxy C 2 -C 6 alkylene glycol or ester or ether thereof (hereinafter sometimes referred to as compound (E)) include (e 1 ) polyoxy C 2 -C 6 alkylene glycol, (e 2 ) polyoxy C An ester of a 2 to C 6 alkylene glycol with at least one fatty acid, an ether of (e 3 ) polyoxy C 2 to C 6 alkylene glycol and at least one aliphatic monohydric alcohol, (e 4 ) polyoxy C 2 to C 6 An ester of an alkylene glycol with a linear hydrocarbon tetracarboxylic acid, a linear hydrocarbon tricarboxylic acid, or a linear hydrocarbon dicarboxylic acid, and (e 5 ) polyoxy C 2 -C 6 alkylene glycol, with a linear hydrocarbon tetra And ethers with linear hydrocarbon triols or linear hydrocarbon diols. That. This will be described below.
[(e1)ポリオキシC2~C6アルキレングリコール]
上記ポリオキシC2~C6アルキレングリコールは、i)オキシC2~C6アルキレン骨格、すなわち、オキシエチレン骨格、オキシプロピレン骨格、オキシブチレン骨格、オキシペンチレン骨格、およびオキシヘキシレン骨格からなる群から選択されるいずれか1種の骨格を有しかつ両末端にヒドロキシ基を有するホモポリマー、ii)上記群から選択される2種以上の骨格を有しかつ両末端にヒドロキシ基を有するブロックコポリマー、またはiii)上記群から選択される2種以上の骨格を有しかつ両末端にヒドロキシ基を有するランダムコポリマーを意味する。 [(E 1 ) polyoxy C 2 -C 6 alkylene glycol]
Polyoxy C 2 ~ C 6 alkylene glycol, i) oxy C 2 ~ C 6 alkylene backbone, i.e., polyoxyethylene backbone, oxypropylene backbone, oxybutylene skeleton, from the group consisting of oxypentylene skeleton, and oxy hexylene backbone A homopolymer having any one selected skeleton and having hydroxy groups at both ends, ii) a block copolymer having two or more kinds of backbones selected from the above group and having hydroxy groups at both ends, Or iii) means a random copolymer having two or more skeletons selected from the above group and having hydroxy groups at both ends.
上記ポリオキシC2~C6アルキレングリコールは、i)オキシC2~C6アルキレン骨格、すなわち、オキシエチレン骨格、オキシプロピレン骨格、オキシブチレン骨格、オキシペンチレン骨格、およびオキシヘキシレン骨格からなる群から選択されるいずれか1種の骨格を有しかつ両末端にヒドロキシ基を有するホモポリマー、ii)上記群から選択される2種以上の骨格を有しかつ両末端にヒドロキシ基を有するブロックコポリマー、またはiii)上記群から選択される2種以上の骨格を有しかつ両末端にヒドロキシ基を有するランダムコポリマーを意味する。 [(E 1 ) polyoxy C 2 -C 6 alkylene glycol]
Polyoxy C 2 ~ C 6 alkylene glycol, i) oxy C 2 ~ C 6 alkylene backbone, i.e., polyoxyethylene backbone, oxypropylene backbone, oxybutylene skeleton, from the group consisting of oxypentylene skeleton, and oxy hexylene backbone A homopolymer having any one selected skeleton and having hydroxy groups at both ends, ii) a block copolymer having two or more kinds of backbones selected from the above group and having hydroxy groups at both ends, Or iii) means a random copolymer having two or more skeletons selected from the above group and having hydroxy groups at both ends.
上記オキシC2~C6アルキレン骨格は、ポリオキシC2~C6アルキレングリコールのIOBを低くする観点から、オキシプロピレン骨格、オキシブチレン骨格、オキシペンチレン骨格、又はオキシヘキシレン骨格であることが好ましく、オキシブチレン骨格、オキシペンチレン骨格、又はオキシヘキシレン骨格であることがより好ましい。
It said oxy C 2 ~ C 6 alkylene backbone, from the viewpoint of lowering the polyoxy C 2 ~ C 6 IOB of alkylene glycols, polyoxypropylene skeleton, oxybutylene skeleton, it is oxypentylene skeleton, or an oxy hexylene skeleton preferably More preferably, they are an oxybutylene skeleton, an oxypentylene skeleton, or an oxyhexylene skeleton.
上記ポリオキシC2~C6アルキレングリコールは、次の式(23):
HO-(CmH2mO)n-H (23)
(式中、mは2~6の整数である)
により表わされうる。 The above polyoxy C 2 -C 6 alkylene glycol has the following formula (23):
HO- (C m H 2m O) n -H (23)
(In the formula, m is an integer of 2 to 6)
Can be represented by
HO-(CmH2mO)n-H (23)
(式中、mは2~6の整数である)
により表わされうる。 The above polyoxy C 2 -C 6 alkylene glycol has the following formula (23):
HO- (C m H 2m O) n -H (23)
(In the formula, m is an integer of 2 to 6)
Can be represented by
なお、本発明者が確認したところ、ポリエチレングリコール(式(23)において、m=2のホモポリマーに相当する)は、n≧45(重量平均分子量約2,000超)の場合に、約0.00~約0.60のIOBの要件を満たすものの、重量平均分子量が約4,000を超えた場合であっても、水溶解度の要件を満たさなかった。したがって、(e1)ポリオキシC2~C6アルキレングリコールには、エチレングリコールのホモポリマーは含まれないと考えられ、エチレングリコールは、他のグリコールとのブロックコポリマー又はランダムコポリマーとして、(e1)ポリオキシC2~C6アルキレングリコールに含まれるべきである。
As confirmed by the present inventor, polyethylene glycol (corresponding to a homopolymer of m = 2 in the formula (23)) is about 0 when n045 (weight average molecular weight is more than about 2,000). Even though the weight average molecular weight exceeded about 4,000, it did not meet the requirement of water solubility, although it met the requirement of an IOB of .00 to about 0.60. Therefore, (e 1 ) polyoxy C 2 -C 6 alkylene glycol is considered not to include homopolymers of ethylene glycol, and ethylene glycol (e 1 ) as a block copolymer or random copolymer with other glycols It should be included in the polyoxy C 2 -C 6 alkylene glycol.
したがって、式(23)のホモポリマーには、プロピレングリコール、ブチレングリコール、ペンチレングリコール又はヘキシレングリコールのホモポリマーが含まれうる。以上より、式(23)において、mは、約3~約6であり、そして約4~約6であることがより好ましく、そしてnは2以上である。
Thus, homopolymers of formula (23) may include homopolymers of propylene glycol, butylene glycol, pentylene glycol or hexylene glycol. From the above, in the formula (23), m is about 3 to about 6, and more preferably about 4 to about 6, and n is 2 or more.
上記式(23)において、nの値は、ポリC2~C6アルキレングリコールが、約0.00~約0.60のIOBと、約45℃以下の融点と、25℃の水100gに対する、約0.00~約0.05gの水溶解度とを有するような値である。たとえば、式(23)がポリプロピレングリコール(m=3のホモポリマー)である場合には、n=12の場合に、IOBが0.58となる。したがって、式(23)がポリプロピレングリコール(m=3のホモポリマー)である場合には、m≧約12の場合に、上記IOBの要件を満たす。また、式(21)がポリブチレングリコール(m=4のホモポリマー)である場合には、n=7の場合に、IOBが0.57となる。したがって、式(23)がポリブチレングリコール(m=4のホモポリマー)である場合には、n≧約7の場合に、上記IOBの要件を満たす。
In the above formula (23), the value of n is that the poly C 2 -C 6 alkylene glycol has an IOB of about 0.00 to about 0.60, a melting point of about 45 ° C. or less, and 100 g of water at 25 ° C. It is a value that has a water solubility of about 0.00 to about 0.05 g. For example, when formula (23) is polypropylene glycol (m = 3 homopolymer), IOB is 0.58 when n = 12. Therefore, when the formula (23) is polypropylene glycol (m = 3 homopolymer), the above IOB requirement is satisfied when m ≧ about 12. In the case where the formula (21) is polybutylene glycol (m = 4 homopolymer), the IOB is 0.57 when n = 7. Therefore, when the formula (23) is polybutylene glycol (m = 4 homopolymer), the above IOB requirement is satisfied when n ≧ about 7.
IOB、融点および水溶解度の観点から、ポリオキシC2~C6アルキレングリコールの重量平均分子量は、好ましくは約200~約10,000、より好ましくは約250~約8,000、そしてさらに好ましくは、約250~約5,000の範囲にある。また、IOB、融点および水溶解度の観点から、ポリC3アルキレングリコール、すなわち、ポリプロピレングリコールの重量平均分子量は、好ましくは約1,000~約10,000、より好ましくは約3,000~約8,000、そしてさらに好ましくは、約4,000~約5,000の範囲にある。上記重量平均分子量が約1,000未満では、水溶解度が要件を満たさず、そして重量平均分子量が大きいほど、特に、吸収体移行速度およびトップシートの白さが向上する傾向があるからである。
From the viewpoint of IOB, melting point and water solubility, the weight average molecular weight of the polyoxy C 2 -C 6 alkylene glycol is preferably about 200 to about 10,000, more preferably about 250 to about 8,000, and more preferably It is in the range of about 250 to about 5,000. Also, in view of IOB, melting point and water solubility, the weight average molecular weight of the poly C 3 alkylene glycol, ie, polypropylene glycol, is preferably about 1,000 to about 10,000, more preferably about 3,000 to about 8 And more preferably in the range of about 4,000 to about 5,000. When the weight average molecular weight is less than about 1,000, the water solubility does not satisfy the requirements, and the larger the weight average molecular weight, the more the absorber transfer rate and the top sheet whiteness tend to be improved.
上記ポリオキシC2~C6アルキレングリコールの市販品としては、たとえば、ユニオール(商標)D-1000,D-1200,D-2000,D-3000,D-4000,PB-500,PB-700,PB-1000およびPB-2000(以上、日油株式会社製)が挙げられる。
Examples of commercial products of polyoxy C 2 ~ C 6 alkylene glycol, e.g., UNIOL (TM) D-1000, D-1200 , D-2000, D-3000, D-4000, PB-500, PB-700, PB -1000 and PB-2000 (manufactured by NOF Corporation).
[(e2)ポリオキシC2~C6アルキレングリコールと少なくとも1の脂肪酸とのエステル]
上記ポリオキシC2~C6アルキレングリコールと少なくとも1の脂肪酸とのエステルとしては、「(e1)ポリオキシC2~C6アルキレングリコール」の項で説明したポリオキシC2~C6アルキレングリコールのOH末端の一方または両方が、脂肪酸によりエステル化されているもの、すなわち、モノエステルおよびジエステルが挙げられる。 [Ester of (e 2 ) polyoxy C 2 -C 6 alkylene glycol with at least one fatty acid]
The ester of the polyoxy C 2 to C 6 alkylene glycol and at least one fatty acid is the OH terminal of the polyoxy C 2 to C 6 alkylene glycol described in the section “(e 1 ) polyoxy C 2 to C 6 alkylene glycol”. One or both of which are esterified with fatty acids, ie, monoesters and diesters.
上記ポリオキシC2~C6アルキレングリコールと少なくとも1の脂肪酸とのエステルとしては、「(e1)ポリオキシC2~C6アルキレングリコール」の項で説明したポリオキシC2~C6アルキレングリコールのOH末端の一方または両方が、脂肪酸によりエステル化されているもの、すなわち、モノエステルおよびジエステルが挙げられる。 [Ester of (e 2 ) polyoxy C 2 -C 6 alkylene glycol with at least one fatty acid]
The ester of the polyoxy C 2 to C 6 alkylene glycol and at least one fatty acid is the OH terminal of the polyoxy C 2 to C 6 alkylene glycol described in the section “(e 1 ) polyoxy C 2 to C 6 alkylene glycol”. One or both of which are esterified with fatty acids, ie, monoesters and diesters.
ポリオキシC2~C6アルキレングリコールと少なくとも1の脂肪酸とのエステルにおいて、エステル化すべき脂肪酸としては、たとえば、「(a1)鎖状炭化水素テトラオールと少なくとも1の脂肪酸とのエステル」において列挙されている脂肪酸、すなわち、飽和脂肪酸または不飽和脂肪酸が挙げられ、酸化等により変性する可能性を考慮すると、飽和脂肪酸が好ましい。上記ポリオキシC2~C6アルキレングリコールと脂肪酸とのエステルの市販品としては、たとえば、ウィルブライトcp9(日油株式会社製)が挙げられる。
In the ester of polyoxy C 2 -C 6 alkylene glycol and at least one fatty acid, the fatty acid to be esterified is, for example, listed in “ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid”. Fatty acids, that is, saturated fatty acids or unsaturated fatty acids, and in view of the possibility of modification by oxidation etc., saturated fatty acids are preferred. Examples of commercially available esters of the polyoxy C 2 -C 6 alkylene glycol and fatty acid include Wilbright cp 9 (manufactured by NOF Corporation).
[(e3)ポリオキシC2~C6アルキレングリコールと少なくとも1の脂肪族1価アルコールとのエーテル]
上記ポリオキシC2~C6アルキレングリコールと少なくとも1の脂肪族1価アルコールとのエーテルとしては、「(e1)ポリオキシC2~C6アルキレングリコール」の項で説明したポリオキシC2~C6アルキレングリコールのOH末端の一方または両方が、脂肪族1価アルコールによりエーテル化されているもの、すなわち、モノエーテルおよびジエーテルが挙げられる。ポリオキシC2~C6アルキレングリコールと少なくとも1の脂肪族1価アルコールとのエーテルにおいて、エーテル化すべき脂肪族1価アルコールとしては、たとえば、「化合物(B)」の項で列挙されている脂肪族1価アルコールが挙げられる。 [(E 3 ) Ether of polyoxy C 2 -C 6 alkylene glycol and at least one aliphatic monohydric alcohol]
The ether and at least one aliphatic monohydric alcohol and polyoxy C 2 ~ C 6 alkylene glycol, "(e 1) polyoxy C 2 ~ C 6 alkylene glycol" polyoxy C 2 ~ C 6 alkylene which is described in the Those in which one or both of the OH ends of the glycol are etherified with an aliphatic monohydric alcohol, that is, monoethers and diethers are mentioned. Examples of aliphatic monohydric alcohols to be etherified in the ether of polyoxy C 2 -C 6 alkylene glycol and at least one aliphatic monohydric alcohol include, for example, aliphatics listed in the “compound (B)” section. Monohydric alcohol is mentioned.
上記ポリオキシC2~C6アルキレングリコールと少なくとも1の脂肪族1価アルコールとのエーテルとしては、「(e1)ポリオキシC2~C6アルキレングリコール」の項で説明したポリオキシC2~C6アルキレングリコールのOH末端の一方または両方が、脂肪族1価アルコールによりエーテル化されているもの、すなわち、モノエーテルおよびジエーテルが挙げられる。ポリオキシC2~C6アルキレングリコールと少なくとも1の脂肪族1価アルコールとのエーテルにおいて、エーテル化すべき脂肪族1価アルコールとしては、たとえば、「化合物(B)」の項で列挙されている脂肪族1価アルコールが挙げられる。 [(E 3 ) Ether of polyoxy C 2 -C 6 alkylene glycol and at least one aliphatic monohydric alcohol]
The ether and at least one aliphatic monohydric alcohol and polyoxy C 2 ~ C 6 alkylene glycol, "(e 1) polyoxy C 2 ~ C 6 alkylene glycol" polyoxy C 2 ~ C 6 alkylene which is described in the Those in which one or both of the OH ends of the glycol are etherified with an aliphatic monohydric alcohol, that is, monoethers and diethers are mentioned. Examples of aliphatic monohydric alcohols to be etherified in the ether of polyoxy C 2 -C 6 alkylene glycol and at least one aliphatic monohydric alcohol include, for example, aliphatics listed in the “compound (B)” section. Monohydric alcohol is mentioned.
[(e4)ポリオキシC2~C6アルキレングリコールと、鎖状炭化水素テトラカルボン酸、鎖状炭化水素トリカルボン酸、または鎖状炭化水素ジカルボン酸とのエステル]
上記ポリオキシC2~C6アルキレングリコールと、鎖状炭化水素テトラカルボン酸、鎖状炭化水素トリカルボン酸、または鎖状炭化水素ジカルボン酸とのエステルにおいて、エステル化すべきポリオキシC2~C6アルキレングリコールとしては、「(e1)ポリオキシC2~C6アルキレングリコール」の項で説明したポリオキシC2~C6アルキレングリコールが挙げられる。また、エステル化すべき鎖状炭化水素テトラカルボン酸、鎖状炭化水素トリカルボン酸、および鎖状炭化水素ジカルボン酸としては、「化合物(C)」の項で説明されるものが挙げられる。 [Ester of (e 4 ) polyoxy C 2 -C 6 alkylene glycol with chain hydrocarbon tetracarboxylic acid, chain hydrocarbon tricarboxylic acid, or chain hydrocarbon dicarboxylic acid]
And polyoxy C 2 ~ C 6 alkylene glycol and a chain hydrocarbon tetracarboxylic acid, in an ester of a chain hydrocarbon tricarboxylic acid or chain hydrocarbon dicarboxylic acids, as the polyoxy C 2 ~ C 6 alkylene glycol to be esterified It is polyoxy C 2 ~ C 6 alkylene glycol described in "(e 1) polyoxy C 2 ~ C 6 alkylene glycol" section and the like. Further, as the chain hydrocarbon tetracarboxylic acid to be esterified, the chain hydrocarbon tricarboxylic acid, and the chain hydrocarbon dicarboxylic acid, those described in the “compound (C)” can be mentioned.
上記ポリオキシC2~C6アルキレングリコールと、鎖状炭化水素テトラカルボン酸、鎖状炭化水素トリカルボン酸、または鎖状炭化水素ジカルボン酸とのエステルにおいて、エステル化すべきポリオキシC2~C6アルキレングリコールとしては、「(e1)ポリオキシC2~C6アルキレングリコール」の項で説明したポリオキシC2~C6アルキレングリコールが挙げられる。また、エステル化すべき鎖状炭化水素テトラカルボン酸、鎖状炭化水素トリカルボン酸、および鎖状炭化水素ジカルボン酸としては、「化合物(C)」の項で説明されるものが挙げられる。 [Ester of (e 4 ) polyoxy C 2 -C 6 alkylene glycol with chain hydrocarbon tetracarboxylic acid, chain hydrocarbon tricarboxylic acid, or chain hydrocarbon dicarboxylic acid]
And polyoxy C 2 ~ C 6 alkylene glycol and a chain hydrocarbon tetracarboxylic acid, in an ester of a chain hydrocarbon tricarboxylic acid or chain hydrocarbon dicarboxylic acids, as the polyoxy C 2 ~ C 6 alkylene glycol to be esterified It is polyoxy C 2 ~ C 6 alkylene glycol described in "(e 1) polyoxy C 2 ~ C 6 alkylene glycol" section and the like. Further, as the chain hydrocarbon tetracarboxylic acid to be esterified, the chain hydrocarbon tricarboxylic acid, and the chain hydrocarbon dicarboxylic acid, those described in the “compound (C)” can be mentioned.
上記ポリオキシC2~C6アルキレングリコールと、鎖状炭化水素テトラカルボン酸、鎖状炭化水素トリカルボン酸、または鎖状炭化水素ジカルボン酸とのエステルは、市販されているほか、鎖状炭化水素テトラカルボン酸、鎖状炭化水素トリカルボン酸、または鎖状炭化水素ジカルボン酸に、C2~C6アルキレングリコールを、公知の条件で重縮合させることにより製造することができる。
The esters of the above polyoxy C 2 -C 6 alkylene glycol with chain hydrocarbon tetracarboxylic acid, chain hydrocarbon tricarboxylic acid, or chain hydrocarbon dicarboxylic acid are commercially available, and chain hydrocarbon tetracarbons are also commercially available. It can be produced by polycondensation of C 2 -C 6 alkylene glycol with an acid, linear hydrocarbon tricarboxylic acid or linear hydrocarbon dicarboxylic acid under known conditions.
[(e5)ポリオキシC2~C6アルキレングリコールと、鎖状炭化水素テトラオール、鎖状炭化水素トリオール、または鎖状炭化水素ジオールとのエーテル]
上記ポリオキシC2~C6アルキレングリコールと、鎖状炭化水素テトラオール、鎖状炭化水素トリオール、または鎖状炭化水素ジオールとのエーテルにおいて、エーテル化すべきポリオキシC2~C6アルキレングリコールとしては、「(e1)ポリオキシC2~C6アルキレングリコール」の項で説明したポリオキシC2~C6アルキレングリコールが挙げられる。また、エーテル化すべき鎖状炭化水素テトラオール、鎖状炭化水素トリオール、および鎖状炭化水素ジオールとしては、「化合物(A)」の項で説明されるもの、たとえば、ペンタエリトリトール、グリセリン、およびグリコールが挙げられる。 [(E 5 ) Ether of polyoxy C 2 -C 6 alkylene glycol and chain hydrocarbon tetraol, chain hydrocarbon triol, or chain hydrocarbon diol]
And polyoxy C 2 ~ C 6 alkylene glycol and a chain hydrocarbon tetraol, chain hydrocarbon triol or in an ether of a chain hydrocarbon diol, as the polyoxy C 2 ~ C 6 alkylene glycol to be etherified, " (e 1) polyoxy C 2 ~ polyoxy C 2 ~ C 6 alkylene glycol is described in the section of C 6 alkylene glycol "may be mentioned. Moreover, as the chain hydrocarbon tetraol to be etherified, the chain hydrocarbon triol, and the chain hydrocarbon diol, those described in the “compound (A)” section, for example, pentaerythritol, glycerin and glycol Can be mentioned.
上記ポリオキシC2~C6アルキレングリコールと、鎖状炭化水素テトラオール、鎖状炭化水素トリオール、または鎖状炭化水素ジオールとのエーテルにおいて、エーテル化すべきポリオキシC2~C6アルキレングリコールとしては、「(e1)ポリオキシC2~C6アルキレングリコール」の項で説明したポリオキシC2~C6アルキレングリコールが挙げられる。また、エーテル化すべき鎖状炭化水素テトラオール、鎖状炭化水素トリオール、および鎖状炭化水素ジオールとしては、「化合物(A)」の項で説明されるもの、たとえば、ペンタエリトリトール、グリセリン、およびグリコールが挙げられる。 [(E 5 ) Ether of polyoxy C 2 -C 6 alkylene glycol and chain hydrocarbon tetraol, chain hydrocarbon triol, or chain hydrocarbon diol]
And polyoxy C 2 ~ C 6 alkylene glycol and a chain hydrocarbon tetraol, chain hydrocarbon triol or in an ether of a chain hydrocarbon diol, as the polyoxy C 2 ~ C 6 alkylene glycol to be etherified, " (e 1) polyoxy C 2 ~ polyoxy C 2 ~ C 6 alkylene glycol is described in the section of C 6 alkylene glycol "may be mentioned. Moreover, as the chain hydrocarbon tetraol to be etherified, the chain hydrocarbon triol, and the chain hydrocarbon diol, those described in the “compound (A)” section, for example, pentaerythritol, glycerin and glycol Can be mentioned.
上記ポリオキシC2~C6アルキレングリコールと、鎖状炭化水素テトラオール、鎖状炭化水素トリオール、または鎖状炭化水素ジオールとのエーテルの市販品としては、たとえば、ユニルーブ(商標)5TP-300KB,ならびにユニオール(商標)TG-3000およびTG-4000(日油株式会社製)が挙げられる。ユニルーブ(商標)5TP-300KBは、ペンタエリトリトール1モルに、プロピレングリコール65モルと、エチレングリコール5モルとを重縮合させた化合物であり、そのIOBは0.39であり、融点は45℃未満であり、そして水溶解度は0.05g未満であった。
Examples of commercially available ethers of the above polyoxy C 2 -C 6 alkylene glycol and chain hydrocarbon tetraol, chain hydrocarbon triol, or chain hydrocarbon diol are, for example, UnilobeTM 5TP-300 KB, and Uniol (trademark) TG-3000 and TG-4000 (made by NOF Corporation) can be mentioned. Unilube (TM) 5TP-300KB is a compound obtained by polycondensation of 65 moles of propylene glycol and 5 moles of ethylene glycol with 1 mole of pentaerythritol, and its IOB is 0.39, and the melting point is less than 45 ° C. And the water solubility was less than 0.05 g.
ユニオール(商標)TG-3000は、グリセリン1モルに、プロピレングリコール50モルを重縮合させた化合物であり、そのIOBは0.42であり、融点は45℃未満であり、水溶解度は0.05g未満であり、そして重量平均分子量は約3,000であった。ユニオール(商標)TG-4000は、グリセリン1モルに、プロピレングリコール70モルを重縮合させた化合物であり、そのIOBは0.40であり、融点は45℃未満であり、水溶解度は0.05g未満であり、そして重量平均分子量は約4,000であった。
Uniol (TM) TG-3000 is a compound obtained by polycondensing 50 moles of propylene glycol with 1 mole of glycerin, its IOB is 0.42, its melting point is less than 45 ° C, and its water solubility is 0.05 g And the weight average molecular weight was about 3,000. Uniol (TM) TG-4000 is a compound obtained by polycondensing 70 moles of propylene glycol with 1 mole of glycerin, its IOB is 0.40, melting point is less than 45 ° C., and water solubility is 0.05 g And the weight average molecular weight was about 4,000.
上記ポリオキシC2~C6アルキレングリコールと、鎖状炭化水素テトラオール、鎖状炭化水素トリオール、または鎖状炭化水素ジオールとのエーテルはまた、鎖状炭化水素テトラオール、鎖状炭化水素トリオール、または鎖状炭化水素ジオールに、C2~C6アルキレンオキシドを、公知の条件で付加させることにより製造することができる。
The ether of the polyoxy C 2 -C 6 alkylene glycol and the chain hydrocarbon tetraol, chain hydrocarbon triol or chain hydrocarbon diol is also a chain hydrocarbon tetraol, chain hydrocarbon triol or It can be produced by adding a C 2 -C 6 alkylene oxide to a linear hydrocarbon diol under known conditions.
[(F)鎖状炭化水素]
上記鎖状炭化水素は、上記無機性値が0であることから、IOBが0.00であり、そして水溶解度がほぼ0gであるので、融点が約45℃以下のものであれば、上記血液改質剤に含まれうる。上記鎖状炭化水素としては、たとえば、(f1)鎖状アルカン、たとえば、直鎖アルカンおよび分岐鎖アルカンが挙げられ、たとえば、直鎖アルカンの場合には、融点が約45℃以下であることを考慮すると、おおむね、炭素数が22以下のものが含まれる。また、蒸気圧を考慮すると、おおむね、炭素数が13以上のものが含まれる。分岐鎖アルカンの場合には、直鎖アルカンよりも、同一炭素数において、融点が低くなる場合があるため、炭素数が22以上のものも含まれうる。上記炭化水素の市販品としては、たとえば、パールリーム6(日油株式会社)が挙げられる。 [(F) chain hydrocarbon]
The chain hydrocarbon has an IOB of 0.00 and an aqueous solubility of almost 0 g because the inorganic value is 0, and the blood has a melting point of about 45 ° C. or less. It may be included in the modifier. Examples of the chain hydrocarbon include (f 1 ) chain alkanes such as straight chain alkanes and branched chain alkanes, and in the case of straight chain alkanes, for example, the melting point is about 45 ° C. or less In general, those containing 22 or less carbon atoms are included. Also, in consideration of the vapor pressure, those having 13 or more carbon atoms are generally included. In the case of a branched alkane, since the melting point may be lower at the same carbon number than a linear alkane, one having a carbon number of 22 or more may also be included. As a commercial item of the above-mentioned hydrocarbon, Pearl Reem 6 (NOF Corporation) is mentioned, for example.
上記鎖状炭化水素は、上記無機性値が0であることから、IOBが0.00であり、そして水溶解度がほぼ0gであるので、融点が約45℃以下のものであれば、上記血液改質剤に含まれうる。上記鎖状炭化水素としては、たとえば、(f1)鎖状アルカン、たとえば、直鎖アルカンおよび分岐鎖アルカンが挙げられ、たとえば、直鎖アルカンの場合には、融点が約45℃以下であることを考慮すると、おおむね、炭素数が22以下のものが含まれる。また、蒸気圧を考慮すると、おおむね、炭素数が13以上のものが含まれる。分岐鎖アルカンの場合には、直鎖アルカンよりも、同一炭素数において、融点が低くなる場合があるため、炭素数が22以上のものも含まれうる。上記炭化水素の市販品としては、たとえば、パールリーム6(日油株式会社)が挙げられる。 [(F) chain hydrocarbon]
The chain hydrocarbon has an IOB of 0.00 and an aqueous solubility of almost 0 g because the inorganic value is 0, and the blood has a melting point of about 45 ° C. or less. It may be included in the modifier. Examples of the chain hydrocarbon include (f 1 ) chain alkanes such as straight chain alkanes and branched chain alkanes, and in the case of straight chain alkanes, for example, the melting point is about 45 ° C. or less In general, those containing 22 or less carbon atoms are included. Also, in consideration of the vapor pressure, those having 13 or more carbon atoms are generally included. In the case of a branched alkane, since the melting point may be lower at the same carbon number than a linear alkane, one having a carbon number of 22 or more may also be included. As a commercial item of the above-mentioned hydrocarbon, Pearl Reem 6 (NOF Corporation) is mentioned, for example.
上記血液改質剤は、実施例と共に詳細に考察するが、血液の粘度および表面張力を下げる作用を少なくとも有することが見いだされた。吸収性物品が吸収すべき経血は、通常の血液と比較して、子宮内膜壁等のタンパク質を含むため、それらが血球同士を繋ぐように作用して、血球が連銭した状態をとりやすい。そのため、吸収性物品が吸収すべき経血は、高粘度となりやすく、トップシートが不織布または織布である場合には、経血が繊維の間に目詰まりしやすく、着用者はベタつき感を覚えやすく、そしてトップシートの表面で経血が拡散し、漏れやすくなる。
The blood modifying agent, as discussed in detail in conjunction with the examples, has been found to at least have the effect of reducing blood viscosity and surface tension. Since the menstrual blood to be absorbed by the absorbent article contains proteins such as the endometrial wall as compared with normal blood, they act to connect the blood cells to each other, and the blood cells are in a continuous state. Cheap. Therefore, the menstrual blood to be absorbed by the absorbent article tends to have a high viscosity, and when the top sheet is a non-woven fabric or a woven fabric, menstrual blood tends to be clogged between fibers, and the wearer feels sticky. And it spreads and leaks on the surface of the top sheet.
また、IOBが約0.00~約0.60である血液改質剤は、有機性が高く、血球の間に入り込みやすいので、血球を安定化させ、血球に連銭構造を形成しにくくすることができると考えられる。上記改質剤が、血球を安定化させ、血球に連銭構造を形成しにくくすることにより、吸収体が経血を吸収しやすくなると考えられる。たとえば、アクリル系高吸収ポリマー、いわゆる、SAPを含む吸収性物品では、経血を吸収すると、連銭した血球がSAP表面を覆い、SAPが吸収性能を発揮しにくくなることが知られているが、血球を安定化することにより、SAPが吸収性能を発揮しやすくなると考えられる。また、赤血球と親和性の高い血液改質剤が、赤血球膜を保護するため、赤血球が破壊されにくくなると考えられる。
In addition, a blood modifying agent having an IOB of about 0.00 to about 0.60 is highly organic and easily enters between blood cells, thereby stabilizing the blood cells and making it difficult for the blood cells to form a continuous structure. It is thought that can be done. It is considered that the absorber makes it easy to absorb menstrual blood by stabilizing the blood cells and making the blood cells less likely to form a continuous structure. For example, it is known that in absorbent articles containing an acrylic superabsorbent polymer, the so-called SAP, when menstrual blood is absorbed, blood cells that have undergone continuous change cover the SAP surface, making it difficult for the SAP to exhibit its absorption performance. By stabilizing blood cells, it is considered that SAP can more easily exhibit absorption performance. In addition, it is considered that the blood modifying agent having high affinity for red blood cells protects the red blood cell membrane, so that the red blood cells are less likely to be destroyed.
次に、図7を参照して、本発明の一実施形態における吸収性物品1の製造方法を説明する。図7は、本発明の一実施形態における吸収性物品1の製造方法に使用する吸収性物品製造装置100を説明するための図である。吸収性物品1の製造方法は、折曲抑制シートを形成する工程、吸収体を形成する工程、トップシート用シートを用意する工程、積層体に圧搾溝を形成する工程、バックシート用シートを用意する工程、吸収性物品の連続体を切断する工程および吸収性物品に血液改質剤を塗布する工程を含む。また、トップシート用シートを用意する工程で使用されるトップシート用シートは、樹脂フィルムシートを用意する工程、樹脂フィルムシートに凹部を形成する工程および樹脂フィルムシートにギア延伸する工程を含むトップシート用シートの製造方法によって製造される。
Next, with reference to FIG. 7, the manufacturing method of the absorbent article 1 in one embodiment of this invention is demonstrated. FIG. 7 is a figure for demonstrating the absorbent article manufacturing apparatus 100 used for the manufacturing method of the absorbent article 1 in one Embodiment of this invention. In the method of manufacturing the absorbent article 1, a process of forming a folding suppression sheet, a process of forming an absorber, a process of preparing a sheet for top sheet, a process of forming compressed grooves in a laminate, and a sheet for backsheet , Cutting the continuum of the absorbent article and applying a blood modifying agent to the absorbent article. In addition, the top sheet sheet used in the step of preparing the top sheet sheet includes a process of preparing the resin film sheet, a process of forming the recess in the resin film sheet, and a process of gear stretching the resin film sheet. It is manufactured by the manufacturing method of the seat for sheets.
折曲抑制シートを形成する工程では、折曲抑制シート用シートロール110から供給された折曲抑制シート用シート112を、ベルト120に載置する。そして、不図示の塗布装置でホットメルト接着剤を折曲抑制シート用シート112に塗布した後、カッタ130を使用して折曲抑制シート用シート112を所定の形状に切断して折曲抑制シートを作製する。
In the step of forming the bending suppression sheet, the bending suppression sheet sheet 112 supplied from the bending suppression sheet sheet roll 110 is placed on the belt 120. Then, after applying a hot melt adhesive to the sheet 112 for bending suppression sheet with a coating device (not shown), the cutter 130 is used to cut the sheet 112 for bending suppression sheet into a predetermined shape and the bending suppression sheet Make
吸収体を形成する工程では、吸収体148を折曲抑制シート上に形成する。不図示の粉砕パルプ供給装置から粉砕パルプ142をパターンドラム140に供給する。パターンドラム140の外周部には粉砕パルプを詰める型として凹部144が形成されている。パターンドラム140の内部は吸引146されており、パターンドラム140に供給された粉砕パルプ142は、凹部144の中に吸い込まれ、圧縮される。そして、凹部144の中で圧縮されたパルプ142は吸収体148に成形される。吸収体148は折曲抑制シート上に載置される。
At the process of forming an absorber, the absorber 148 is formed on a bending suppression sheet. Pulverized pulp 142 is supplied to pattern drum 140 from a pulverized pulp supply device (not shown). A recess 144 is formed on the outer peripheral portion of the pattern drum 140 as a mold for packing crushed pulp. The inside of the pattern drum 140 is suctioned 146, and the crushed pulp 142 supplied to the pattern drum 140 is sucked into the recess 144 and compressed. Then, the pulp 142 compressed in the recess 144 is formed into the absorber 148. The absorber 148 is placed on the bending suppression sheet.
トップシート用シートを用意する工程では、不図示の塗布装置でホットメルト接着剤を吸収体148に塗布した後、後述するトップシート用シートの製造方法によって製造したトップシート用シート216を吸収体148の上に配置して、トップシート用シート216を吸収体148に接着する。
In the step of preparing the top sheet sheet, a hot melt adhesive is applied to the absorbent body 148 with a coating device (not shown), and then the top sheet sheet 216 manufactured by the top sheet sheet manufacturing method described later is used as the absorbent body 148. And the top sheet sheet 216 is adhered to the absorber 148.
積層体に圧搾溝を形成する工程では、エンボス加工装置150を使用して、トップシート用シート216、吸収体148および折曲抑制シートの積層体262に圧搾溝を形成する。積層体262は、エンボス加工装置150の上段ロール151と下段ロール152との間を通過する。上段ロール151の外周の表面には、図1に示す吸収性物品1の圧搾溝8に対応する形状の凸部(不図示)が設けられている。下段ロール152は、外周の表面が平滑であるプレーンロールである。エンボス加工装置150の上段ロール151と下段ロール152との間に積層体262を通過させることによって、積層体262における、図1に示す吸収性物品1の圧搾溝8に対応する部分が厚さ方向に圧縮され、圧搾溝が積層体262に形成される。
In the step of forming the pressing groove in the laminate, the pressing device is used to form the pressing groove in the top sheet sheet 216, the absorber 148 and the laminate 262 of the bending suppression sheet. The laminate 262 passes between the upper roll 151 and the lower roll 152 of the embossing device 150. On the surface of the outer periphery of the upper roll 151, a convex portion (not shown) having a shape corresponding to the pressing groove 8 of the absorbent article 1 shown in FIG. 1 is provided. The lower roll 152 is a plain roll whose outer peripheral surface is smooth. By passing the laminate 262 between the upper roll 151 and the lower roll 152 of the embossing device 150, the portion of the laminate 262 corresponding to the compressed grooves 8 of the absorbent article 1 shown in FIG. 1 is in the thickness direction And compressed grooves are formed in the laminate 262.
バックシート用シートを用意する工程では、バックシート用シートロール160から供給されたバックシート用シート162を、不図示の塗布装置を使用して接着剤を塗布した後、圧搾溝が形成された積層体154の折曲抑制シート側の面に重ねて接着し、吸収性物品の連続体164を形成する。
In the step of preparing the backsheet sheet, an adhesive is applied to the backsheet sheet 162 supplied from the backsheet sheet roll 160 using a coating device (not shown), and then lamination is performed in which compressed grooves are formed. It is overlapped and adhered to the surface on the side of the bending suppression sheet of the body 154 to form a continuum 164 of the absorbent article.
吸収性物品の連続体を切断する工程では、カッタ170を使用して吸収性物品の連続体164を吸収性物品の形状となるように切断して吸収性物品を作製する。
In the step of cutting the continuum of the absorbent article, the cutter 170 is used to cut the continuum 164 of the absorbent article into the shape of the absorbent article to produce the absorbent article.
吸収性物品に血液改質剤を塗布する工程では、改質剤塗布スプレー180を使用して吸収性物品の中央の領域に上述の血液改質剤181を塗布して、トップシートの表面に血液改質剤層を形成する。
In the step of applying the blood modifying agent to the absorbent article, the above-described blood modifying agent 181 is applied to the central region of the absorbent article using the modifier application spray 180 to apply blood to the surface of the top sheet. Form a modifier layer.
吸収性物品の中央の領域に塗布される血液改質剤181は、少なくとも着用者の体液の排泄口に当接する部分に塗布されていればよい。たとえば、吸収性物品の中心を中心として、長さ方向の長さが、好ましくは50mm以上、より好ましくは100mm以上であり、幅方向の長さが、好ましくは10mm以上、より好ましくは30mm以上である領域に血液改質剤が塗布されてもよい。
The blood modifying agent 181 applied to the central region of the absorbent article may be applied at least to a portion that abuts on the excretory port of the wearer's body fluid. For example, the length in the lengthwise direction is preferably 50 mm or more, more preferably 100 mm or more, and the length in the width direction is preferably 10 mm or more, more preferably 30 mm or more, centering on the center of the absorbent article. A blood modifying agent may be applied to an area.
吸収性物品の連続体を切断する工程の後で、血液改質剤を塗布したが、後述のトップシート用シートの製造工程で血液改質剤を塗布してもよい。吸収性物品を製造する途中に、塗布した血液改質剤が吸収性物品から落ちることを抑制するために、吸収性物品の製造工程の川下の段階、たとえば、吸収性物品を包装する直前に血液改質剤を吸収性物品に塗布することが好ましい。
Although the blood modifying agent was applied after the step of cutting the continuum of the absorbent article, the blood modifying agent may be applied in the step of producing the top sheet described later. In the process of manufacturing the absorbent article, in order to prevent the applied blood modifying agent from falling off the absorbent article, the downstream stage of the manufacturing process of the absorbent article, for example, the blood just before packaging the absorbent article It is preferred to apply the modifier to the absorbent article.
吸収性物品1の製造方法には、他に、吸収性物品の連続体164に粘着部を形成する工程および吸収性物品の連続体164にシール部を形成する工程などを含む。
In addition, the method of manufacturing the absorbent article 1 includes a step of forming an adhesive portion on the continuous body 164 of the absorbent article, a step of forming a seal portion on the continuous body 164 of the absorbent article, and the like.
次に、トップシート用シートの製造方法を説明する。
Next, a method of manufacturing a top sheet will be described.
樹脂フィルムシートを用意する工程では、図7に示すように、樹脂フィルムシートのロール210から供給された樹脂フィルムシート212を凹部形成ロール220に供給する。
In the step of preparing the resin film sheet, as shown in FIG. 7, the resin film sheet 212 supplied from the roll 210 of the resin film sheet is supplied to the recess forming roll 220.
樹脂フィルムシートに凹部を形成する工程では、樹脂フィルムシート212を凹部形成ロール220に通過させて、凹部2141が形成された樹脂フィルムシート214(図9参照)を作製する。凹部形成ロール220は、ローレットロール221と平滑な表面を有する予熱ロール222とからなる。
At the process of forming a recessed part in a resin film sheet, the resin film sheet 212 is made to pass the recessed part formation roll 220, and the resin film sheet 214 (refer FIG. 9) in which the recessed part 2141 was formed is produced. The recess forming roll 220 comprises a knurling roll 221 and a preheating roll 222 having a smooth surface.
図8(a)および(b)は、ローレットロール221の一例を示す図である。図8(a)は、ローレットロール221の全体を示す図であり、図8(b)は、ローレットロール221の外周表面の凹凸を有する部分223を拡大した図である。図8(c)は、平滑な表面を有する予熱ロール222の一例を示す図である。ローレットロール221の表面223には、格子状の凸部224が設けられている。これにより、ローレットロール221の表面に菱形の凹部225が形成される。なお、ローレットロール221の凹部225の形状は菱形に限定されず、正方形、長方形、平行四辺形、台形、三角形、六角形などの形状であってもよい。
FIGS. 8A and 8B show an example of the knurled roll 221. FIG. FIG. 8A is a view showing the entire knurled roll 221, and FIG. 8B is an enlarged view of a portion 223 having unevenness on the outer peripheral surface of the knurled roll 221. As shown in FIG. FIG. 8C shows an example of the preheating roll 222 having a smooth surface. A grid-like convex portion 224 is provided on the surface 223 of the knurled roll 221. As a result, a rhombus recess 225 is formed on the surface of the knurled roll 221. In addition, the shape of the recessed part 225 of the knurl roll 221 is not limited to a rhombus, You may be shapes, such as a square, a rectangle, a parallelogram, a trapezoid, a triangle, and a hexagon.
格子状の凸部224において平行に並ぶ凸部224の中心線間隔、すなわち格子状の凸部224のピッチは、好ましくは0.2mm以上、10mm以下であり、より好ましくは0.4mm以上、2mm以下である。格子状の凸部224のピッチが0.2mm未満、または10mmより大きい場合、樹脂フィルムに凹部が形成されない場合がある。また、格子状の凸部224の幅は、好ましくは0.01mm以上、1mm以下であり、より好ましくは0.03mm以上、0.1以下である。また、菱形の凹部225の一辺の長さは、好ましくは0.1mm以上、5mm以下であり、より好ましくは0.2mm以上、1mm以下である。格子状の凸部224の幅が0.01mm未満もしくは1mmより大きい場合、または菱形の凹部225の一辺の長さが0.1mm未満もしくは5mmより大きい場合、樹脂フィルムに凹部が形成されない場合がある。
The center line spacing of the convex parts 224 arranged in parallel in the lattice-like convex parts 224, that is, the pitch of the lattice-like convex parts 224 is preferably 0.2 mm or more and 10 mm or less, more preferably 0.4 mm or more and 2 mm It is below. When the pitch of the grid-like convex portions 224 is less than 0.2 mm or greater than 10 mm, the concave portion may not be formed in the resin film. In addition, the width of the grid-like convex portion 224 is preferably 0.01 mm or more and 1 mm or less, and more preferably 0.03 mm or more and 0.1 or less. Further, the length of one side of the rhombic recess 225 is preferably 0.1 mm or more and 5 mm or less, and more preferably 0.2 mm or more and 1 mm or less. When the width of the grid-like convex part 224 is less than 0.01 mm or more than 1 mm, or when the length of one side of the rhombus concave part 225 is less than 0.1 mm or more than 5 mm, the concave part may not be formed in the resin film. .
平滑な表面を有する予熱ロール222は、70℃以上、100℃以下の温度に保持されており、供給された樹脂フィルムシート212を加熱する。これにより、樹脂フィルムシート212は柔らかくなり、成形しやすくなる。
The preheated roll 222 having a smooth surface is maintained at a temperature of 70 ° C. or more and 100 ° C. or less, and heats the supplied resin film sheet 212. Thereby, the resin film sheet 212 becomes soft and easy to form.
樹脂フィルムシート212が、ローレットロール221と平滑な表面を有するロール222との間を通過するとき、格子状の凸部224と接する部分では、樹脂フィルムシート212は厚さ方向に強い圧力を受ける。これにより、図9に示すように、樹脂フィルムシート214に細かい凹部2141が形成される。なお、実際に、樹脂フィルムシート212に形成される凹部2141は、図9に示されているよりも小さく、単位面積当たりの凹部2141の数も図9に示されているよりもずっと多い。凹部2141は、樹脂フィルムシート214における、本体部10の中央部分12(図1参照)に対応する範囲2143のみに形成される。ここで、樹脂フィルムシート214における、吸収性物品1に対応する範囲は、符号2142の点線で示される範囲である。
When the resin film sheet 212 passes between the knurled roll 221 and the roll 222 having a smooth surface, the resin film sheet 212 receives a strong pressure in the thickness direction in a portion in contact with the grid-like convex portion 224. Thereby, as shown in FIG. 9, the fine recessed part 2141 is formed in the resin film sheet 214. As shown in FIG. In fact, the number of recesses 2141 formed in the resin film sheet 212 is smaller than that shown in FIG. 9, and the number of recesses 2141 per unit area is also much larger than that shown in FIG. The concave portion 2141 is formed only in a range 2143 corresponding to the central portion 12 (see FIG. 1) of the main body portion 10 in the resin film sheet 214. Here, the range corresponding to the absorbent article 1 in the resin film sheet 214 is the range indicated by the dotted line 2142.
樹脂フィルムシートにギア延伸する工程では、図7に示す延伸ギアロール230に凹部を形成した樹脂フィルムシート214を通過させることによって、トップシート2の第1の山折り部、第2の山折り部および谷折り部を樹脂フィルムシート214に形成する。符号216は、第1の山折り部、第2の山折り部および谷折り部を形成した樹脂フィルムシートである。
In the step of gear-drawing the resin film sheet, the first mountain fold portion, the second mountain fold portion, and the second mountain fold portion of the top sheet 2 are caused to pass through the resin film sheet 214 having the concave portion formed in the stretching gear roll 230 shown in FIG. The valley folds are formed in the resin film sheet 214. The code | symbol 216 is a resin film sheet which formed the 1st mountain fold part, the 2nd mountain fold part, and the valley fold part.
延伸ギアロール230は、上段ロール231と下段ロール232とを含む。図10(a)は、延伸ギアロール230の上段ロール231を説明するための図であり、図10(b)は、上段ロール231の外周面上に配置されたギア歯233を説明するための図であり、図10(c)は、図10(b)のC-C線断面図である。ギア歯233は、上段ロール231の円周方向に非連続的に延びている。すなわち、上段ロール231の円周方向に延びているギア歯233は、途中で複数箇所途切れている。このギア歯233が途切れている箇所234により、樹脂フィルムシート214に第2の山折り部が形成される。
The drawing gear roll 230 includes an upper roll 231 and a lower roll 232. 10 (a) is a view for explaining the upper roll 231 of the drawing gear roll 230, and FIG. 10 (b) is a view for explaining the gear teeth 233 arranged on the outer peripheral surface of the upper roll 231. FIG. 10 (c) is a cross-sectional view taken along the line CC of FIG. 10 (b). The gear teeth 233 extend discontinuously in the circumferential direction of the upper roll 231. That is, the gear teeth 233 extending in the circumferential direction of the upper roll 231 are interrupted at multiple points along the way. A second mountain-folded portion is formed in the resin film sheet 214 by the portion 234 where the gear teeth 233 are disconnected.
ギア歯233の幅は、たとえば0.3mm以上、0.5mm以下であり、隣接するギア歯233の中心間の距離は、たとえば1.0mm以上、1.2mm以下である。
The width of gear teeth 233 is, for example, 0.3 mm or more and 0.5 mm or less, and the distance between the centers of adjacent gear teeth 233 is, for example, 1.0 mm or more and 1.2 mm or less.
図11(a)は、延伸ギアロール230の下段ロール232を説明するための図であり、図11(b)は、下段ロール232の外周面上に配置されたギア歯235を説明するための図であり、図11(c)は、図11(b)のD-D線断面図である。ギア歯235は、下段ロール232の円周方向に延びている。下段ロール232は、上段ロール231のように途中で複数箇所途切れていない。ギア歯235の幅は、たとえば上段ロール231のギア歯233の幅と等しく、隣接するギア歯235の中心間の距離は、たとえば上段ロール231のギア歯233の中心間の距離と等しい。
11 (a) is a view for explaining the lower roll 232 of the drawing gear roll 230, and FIG. 11 (b) is a view for explaining the gear teeth 235 disposed on the outer peripheral surface of the lower roll 232. 11 (c) is a cross-sectional view taken along the line DD of FIG. 11 (b). The gear teeth 235 extend in the circumferential direction of the lower roll 232. Like the upper roll 231, the lower roll 232 is not interrupted at multiple points along the way. The width of the gear teeth 235 is, for example, equal to the width of the gear teeth 233 of the upper roll 231, and the distance between the centers of adjacent gear teeth 235 is, for example, equal to the distance between the centers of the gear teeth 233 of the upper roll 231.
上段ロール231のギア歯233と下段ロール232のギア歯235とが噛み合っている部分における上段ロール231の径方向の長さ、すなわち噛み込み深さは、たとえば1.25mmである。上段ロール231のギア歯233と下段ロール232のギア歯235とが噛み合っているときの上段ロール231のギア歯233と下段ロール232のギア歯235との間の隙間は、たとえば、0.25mm以上、0.45mm以下である。
The radial length of the upper roll 231 at the portion where the gear teeth 233 of the upper roll 231 and the gear teeth 235 of the lower roll 232 are engaged, that is, the biting depth is, for example, 1.25 mm. The gap between the gear teeth 233 of the upper roll 231 and the gear teeth 235 of the lower roll 232 when the gear teeth 233 of the upper roll 231 and the gear teeth 235 of the lower roll 232 are engaged is, for example, 0.25 mm or more , 0.45 mm or less.
図7に示すように樹脂フィルムシート214が延伸ギアロール230を通過するとき、樹脂フィルム214は略波状に折り曲げられ、樹脂フィルム214にトップシート2の第1の山折り部21、第2の山折り部22および谷折り部23が形成される。また、樹脂フィルムシート214の凹部2141が形成された領域2143(図9参照)には、トップシート2の開口部25(図4参照)がさらに形成される。
As shown in FIG. 7, when the resin film sheet 214 passes through the drawing gear roll 230, the resin film 214 is bent in a substantially wave shape, and the resin film 214 forms the first mountain fold 21 of the top sheet 2 and the second mountain fold. Portions 22 and valley folds 23 are formed. Further, an opening 25 (see FIG. 4) of the top sheet 2 is further formed in the region 2143 (see FIG. 9) in which the recess 2141 of the resin film sheet 214 is formed.
次に、図12を参照して、延伸ギアロール230に樹脂フィルムシート214を通過させたとき、樹脂フィルムシート214に開口部が形成される原理を説明する。なお、この原理は、本発明を限定するものではない。
Next, with reference to FIG. 12, the principle of forming an opening in the resin film sheet 214 when the resin film sheet 214 passes through the drawing gear roll 230 will be described. Note that this principle does not limit the present invention.
樹脂フィルムシート214は、上段ロール231のギア歯233と下段ロール232のギア歯235とが噛み合っている部分236で大きく延伸される。上述の凹部形成工程で凹部2141(図9参照)が形成された部分は、樹脂フィルムシート214が薄くなっており、さらにローレットロール221の格子状の凸部224により傷が付与されている部分であるので、強度が弱く、樹脂フィルムシート214の凹部2141は、延伸を受けると破れる。このため、樹脂フィルムシート214の延伸を受けた部分236では、樹脂フィルムシート214の凹部2141が破れて、樹脂フィルムシート214の破れた部分が広がり、樹脂フィルムシート214の破れた部分に開口部が形成される。
The resin film sheet 214 is largely drawn at a portion 236 where the gear teeth 233 of the upper roll 231 and the gear teeth 235 of the lower roll 232 are engaged. The resin film sheet 214 is thin at the part where the concave part 2141 (see FIG. 9) is formed in the above-mentioned concave part forming step, and the part where the scratch is given by the grid-like convex part 224 of the knurling roll 221 As it is present, the strength is weak, and the recess 2141 of the resin film sheet 214 breaks when it is stretched. Therefore, in the stretched portion 236 of the resin film sheet 214, the concave portion 2141 of the resin film sheet 214 is torn, the torn portion of the resin film sheet 214 is spread, and the opening portion is to the torn portion of the resin film sheet 214. It is formed.
樹脂フィルムシート214は、上段ロール231のギア歯233と下段ロール232のギア歯235とが噛み合っていない部分237,238ではあまり延伸されない。このため、樹脂フィルムシート241における上段ロール231のギア歯233と下段ロール232のギア歯235とが噛み合っていない部分237,238では、樹脂フィルムシート214が延伸ギアロール230を通過しても、上述の凹部形成工程で形成された凹部2141は、破れず開口部にならない。
The resin film sheet 214 is not stretched much at the portions 237 and 238 where the gear teeth 233 of the upper roll 231 and the gear teeth 235 of the lower roll 232 are not engaged. For this reason, in the portions 237 and 238 in which the gear teeth 233 of the upper roll 231 and the gear teeth 235 of the lower roll 232 in the resin film sheet 241 are not engaged, the resin film sheet 214 described above The recess 2141 formed in the recess forming step is not broken and does not become an opening.
樹脂フィルムシート214の凹部2141が形成されていない領域では、上段ロール231のギア歯233と下段ロール232のギア歯235とが噛み合っている部分236で樹脂フィルムシート214が大きく延伸されても樹脂フィルムシート214は破れない。このため、樹脂フィルムシート214における、本体部10の吸収体4の幅方向の両側面41の外側の領域14およびウイング部6にそれぞれ対応する領域には開口部は形成されない。
In the region where the concave portion 2141 of the resin film sheet 214 is not formed, even if the resin film sheet 214 is greatly stretched in the portion 236 where the gear teeth 233 of the upper roll 231 and the gear teeth 235 of the lower roll 232 are engaged The sheet 214 is not torn. For this reason, in the resin film sheet 214, the opening is not formed in the region 14 and the wing 6 respectively corresponding to the outer side 14 and the wing 6 of the both sides 41 in the width direction of the absorber 4 of the main body 10.
本発明では、血液改質剤が、血液の粘度および表面張力を下げるメカニズムを有するために、体液が、血液改質剤層24によってトップシート2に残存せずに吸収体4へ移動し吸収体4に吸収されることができる。以下の実施例によって、血液改質剤が、血液の粘度および表面張力を下げるメカニズムを有することを確認した。この確認は、樹脂フィルムに比べて体液が残存しやすい不織布を用いて行った。
In the present invention, since the blood modifying agent has a mechanism to lower the viscosity and surface tension of the blood, the body fluid is transferred to the absorber 4 without remaining on the top sheet 2 by the blood modifying layer 24 and absorbed. 4 can be absorbed. The following examples confirmed that the blood modifying agent has a mechanism to lower the viscosity and surface tension of blood. This check was performed using a non-woven fabric in which the body fluid tends to remain as compared to the resin film.
[例1]
[リウェット率および吸収体移行速度の評価]
[血液改質剤のデータ]
市販の生理用ナプキンを準備した。当該生理用ナプキンは、親水剤で処理されたエアスルー不織布(ポリエステルおよびポリエチレンテレフタレートから成る複合繊維、坪量:35g/m2)から形成されたトップシートと、エアスルー不織布(ポリエステルおよびポリエチレンテレフタレートから成る複合繊維、坪量:30g/m2)から形成されたセカンドシートと、パルプ(坪量:150~450g/m2、中央部ほど多い)、アクリル系高吸収ポリマー(坪量:15g/m2)およびコアラップとしてのティッシュを含む吸収体と、撥水剤処理されたサイドシートと、ポリエチレンフィルムから成るバックシートとから形成されていた。 [Example 1]
[Evaluation of rewet rate and absorber transfer speed]
[Blood modifier data]
A commercially available sanitary napkin was prepared. The sanitary napkin comprises a top sheet formed of an air through non-woven fabric (composite fiber of polyester and polyethylene terephthalate, basis weight: 35 g / m 2 ) treated with a hydrophilic agent, and a composite of air through non-woven fabric (polyester and polyethylene terephthalate) Fiber, second sheet formed of basis weight: 30 g / m 2 ), pulp (basis weight: 150 to 450 g / m 2 , more in the central part), acrylic high absorption polymer (basis weight: 15 g / m 2 ) And, it was formed from an absorbent including a tissue as a core wrap, a water repellent treated side sheet, and a back sheet made of polyethylene film.
[リウェット率および吸収体移行速度の評価]
[血液改質剤のデータ]
市販の生理用ナプキンを準備した。当該生理用ナプキンは、親水剤で処理されたエアスルー不織布(ポリエステルおよびポリエチレンテレフタレートから成る複合繊維、坪量:35g/m2)から形成されたトップシートと、エアスルー不織布(ポリエステルおよびポリエチレンテレフタレートから成る複合繊維、坪量:30g/m2)から形成されたセカンドシートと、パルプ(坪量:150~450g/m2、中央部ほど多い)、アクリル系高吸収ポリマー(坪量:15g/m2)およびコアラップとしてのティッシュを含む吸収体と、撥水剤処理されたサイドシートと、ポリエチレンフィルムから成るバックシートとから形成されていた。 [Example 1]
[Evaluation of rewet rate and absorber transfer speed]
[Blood modifier data]
A commercially available sanitary napkin was prepared. The sanitary napkin comprises a top sheet formed of an air through non-woven fabric (composite fiber of polyester and polyethylene terephthalate, basis weight: 35 g / m 2 ) treated with a hydrophilic agent, and a composite of air through non-woven fabric (polyester and polyethylene terephthalate) Fiber, second sheet formed of basis weight: 30 g / m 2 ), pulp (basis weight: 150 to 450 g / m 2 , more in the central part), acrylic high absorption polymer (basis weight: 15 g / m 2 ) And, it was formed from an absorbent including a tissue as a core wrap, a water repellent treated side sheet, and a back sheet made of polyethylene film.
以下に、実験に用いられた血液改質剤を列挙する。
[(a1)鎖状炭化水素テトラオールと少なくとも1の脂肪酸とのエステル]
・ユニスター H-408BRS,日油株式会社製
テトラ2-エチルヘキサン酸ペンタエリトリトール,重量平均分子量:約640
・ユニスター H-2408BRS-22,日油株式会社製
テトラ2-エチルヘキサン酸ペンタエリトリトールと、ジ2-エチルヘキサン酸ネオペンチルグリコールとの混合物(58:42,重量比),重量平均分子量:約520 The blood modifiers used in the experiments are listed below.
[Ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid]
-Unistar H-408 BRS, manufactured by NOF Corporation, pentaerythritol tetra 2-ethylhexanoate, weight average molecular weight: about 640
Unister H-2408 BRS-22, a mixture of NOF Corporation pentaerythritol tetra-2-ethylhexanoate and neopentyl glycol di-2-ethylhexanoate (58:42, weight ratio), weight average molecular weight: about 520
[(a1)鎖状炭化水素テトラオールと少なくとも1の脂肪酸とのエステル]
・ユニスター H-408BRS,日油株式会社製
テトラ2-エチルヘキサン酸ペンタエリトリトール,重量平均分子量:約640
・ユニスター H-2408BRS-22,日油株式会社製
テトラ2-エチルヘキサン酸ペンタエリトリトールと、ジ2-エチルヘキサン酸ネオペンチルグリコールとの混合物(58:42,重量比),重量平均分子量:約520 The blood modifiers used in the experiments are listed below.
[Ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid]
-Unistar H-408 BRS, manufactured by NOF Corporation, pentaerythritol tetra 2-ethylhexanoate, weight average molecular weight: about 640
Unister H-2408 BRS-22, a mixture of NOF Corporation pentaerythritol tetra-2-ethylhexanoate and neopentyl glycol di-2-ethylhexanoate (58:42, weight ratio), weight average molecular weight: about 520
[(a2)鎖状炭化水素トリオールと少なくとも1の脂肪酸とのエステル]
・Cetiol SB45DEO,コグニスジャパン株式会社製
脂肪酸が、オレイン酸またはステアリル酸である、グリセリンと脂肪酸とのトリエステル
・SOY42,日油株式会社製
C14の脂肪酸:C16の脂肪酸:C18の脂肪酸:C20の脂肪酸(飽和脂肪酸および不飽和脂肪酸の両方を含む)がおおよそ0.2:11:88:0.8の重量比で含まれている、グリセリンと脂肪酸とのトリエステル,重量平均分子量:880 [Ester of (a 2 ) chain hydrocarbon triol and at least one fatty acid]
· Cetiol SB 45 DEO, manufactured by Cognis Japan Ltd. Triester of glycerin and fatty acid in which the fatty acid is oleic acid or stearyl acid · SOY 42 manufactured by NOF Corporation C 14 fatty acid: C 16 fatty acid: C 18 fatty acid: C 20 fatty acids (including both saturated and unsaturated fatty acids) is approximately 0.2: 11: 88: 0.8 are contained in a weight ratio of triesters of glycerol with fatty acids, the weight average molecular weight: 880
・Cetiol SB45DEO,コグニスジャパン株式会社製
脂肪酸が、オレイン酸またはステアリル酸である、グリセリンと脂肪酸とのトリエステル
・SOY42,日油株式会社製
C14の脂肪酸:C16の脂肪酸:C18の脂肪酸:C20の脂肪酸(飽和脂肪酸および不飽和脂肪酸の両方を含む)がおおよそ0.2:11:88:0.8の重量比で含まれている、グリセリンと脂肪酸とのトリエステル,重量平均分子量:880 [Ester of (a 2 ) chain hydrocarbon triol and at least one fatty acid]
· Cetiol SB 45 DEO, manufactured by Cognis Japan Ltd. Triester of glycerin and fatty acid in which the fatty acid is oleic acid or stearyl acid · SOY 42 manufactured by NOF Corporation C 14 fatty acid: C 16 fatty acid: C 18 fatty acid: C 20 fatty acids (including both saturated and unsaturated fatty acids) is approximately 0.2: 11: 88: 0.8 are contained in a weight ratio of triesters of glycerol with fatty acids, the weight average molecular weight: 880
・トリC2L油脂肪酸グリセリド,日油株式会社製
C8の脂肪酸:C10の脂肪酸:C12の脂肪酸がおおよそ37:7:56の重量比で含まれている、グリセリンと脂肪酸とのトリエステル,重量平均分子量:約570
・トリCL油脂肪酸グリセリド,日油株式会社製
C8の脂肪酸:C12の脂肪酸がおおよそ44:56の重量比で含まれている、グリセリンと脂肪酸とのトリエステル,重量平均分子量:約570 Tri C2 L oil fatty acid glyceride, manufactured by NOF Corporation C 8 fatty acid: C 10 fatty acid: C 12 fatty acid is contained in a weight ratio of approximately 37: 7: 56, triester of glycerin and fatty acid, Weight average molecular weight: about 570
Triethylene CL oil fatty acid glycerides, manufactured by NOF Corporation C 8 fatty acid: fatty acid of C 12 is approximately included in a weight ratio of 44:56, triesters of glycerin and fatty acid, the weight average molecular weight: about 570
C8の脂肪酸:C10の脂肪酸:C12の脂肪酸がおおよそ37:7:56の重量比で含まれている、グリセリンと脂肪酸とのトリエステル,重量平均分子量:約570
・トリCL油脂肪酸グリセリド,日油株式会社製
C8の脂肪酸:C12の脂肪酸がおおよそ44:56の重量比で含まれている、グリセリンと脂肪酸とのトリエステル,重量平均分子量:約570 Tri C2 L oil fatty acid glyceride, manufactured by NOF Corporation C 8 fatty acid: C 10 fatty acid: C 12 fatty acid is contained in a weight ratio of approximately 37: 7: 56, triester of glycerin and fatty acid, Weight average molecular weight: about 570
Triethylene CL oil fatty acid glycerides, manufactured by NOF Corporation C 8 fatty acid: fatty acid of C 12 is approximately included in a weight ratio of 44:56, triesters of glycerin and fatty acid, the weight average molecular weight: about 570
・パナセート810s,日油株式会社製
C8の脂肪酸:C10の脂肪酸がおおよそ85:15の重量比で含まれている、グリセリンと脂肪酸とのトリエステル,重量平均分子量:約480
・パナセート800,日油株式会社製
脂肪酸が全てオクタン酸(C8)である、グリセリンと脂肪酸とのトリエステル,重量平均分子量:約470 Panaceto 810s, manufactured by NOF Corporation C 8 fatty acid: Triester of glycerin and fatty acid containing C 10 fatty acid at a weight ratio of approximately 85: 15, weight average molecular weight: about 480
-Panaceto 800, manufactured by NOF Corporation Triester of glycerin and fatty acid in which all fatty acids are octanoic acid (C 8 ), weight average molecular weight: about 470
C8の脂肪酸:C10の脂肪酸がおおよそ85:15の重量比で含まれている、グリセリンと脂肪酸とのトリエステル,重量平均分子量:約480
・パナセート800,日油株式会社製
脂肪酸が全てオクタン酸(C8)である、グリセリンと脂肪酸とのトリエステル,重量平均分子量:約470 Panaceto 810s, manufactured by NOF Corporation C 8 fatty acid: Triester of glycerin and fatty acid containing C 10 fatty acid at a weight ratio of approximately 85: 15, weight average molecular weight: about 480
-Panaceto 800, manufactured by NOF Corporation Triester of glycerin and fatty acid in which all fatty acids are octanoic acid (C 8 ), weight average molecular weight: about 470
・パナセート800B,日油株式会社製
脂肪酸が全て2-エチルヘキサン酸(C8)である、グリセリンと脂肪酸とのトリエステル,重量平均分子量:約470
・NA36,日油株式会社製
C16の脂肪酸:C18の脂肪酸:C20の脂肪酸(飽和脂肪酸および不飽和脂肪酸の両方を含む)がおおよそ5:92:3の重量比で含まれている、グリセリンと脂肪酸とのトリエステル,重量平均分子量:約880 -Panaceto 800 B, manufactured by NOF Corporation Triester of glycerin and fatty acid in which all fatty acids are 2-ethylhexanoic acid (C 8 ), weight average molecular weight: about 470
NA36, manufactured by NOF Corporation C 16 fatty acids: C 18 fatty acids: C 20 fatty acids (including both saturated fatty acids and unsaturated fatty acids) in a weight ratio of approximately 5: 92: 3, Triester of glycerin and fatty acid, weight average molecular weight: about 880
脂肪酸が全て2-エチルヘキサン酸(C8)である、グリセリンと脂肪酸とのトリエステル,重量平均分子量:約470
・NA36,日油株式会社製
C16の脂肪酸:C18の脂肪酸:C20の脂肪酸(飽和脂肪酸および不飽和脂肪酸の両方を含む)がおおよそ5:92:3の重量比で含まれている、グリセリンと脂肪酸とのトリエステル,重量平均分子量:約880 -Panaceto 800 B, manufactured by NOF Corporation Triester of glycerin and fatty acid in which all fatty acids are 2-ethylhexanoic acid (C 8 ), weight average molecular weight: about 470
NA36, manufactured by NOF Corporation C 16 fatty acids: C 18 fatty acids: C 20 fatty acids (including both saturated fatty acids and unsaturated fatty acids) in a weight ratio of approximately 5: 92: 3, Triester of glycerin and fatty acid, weight average molecular weight: about 880
・トリヤシ油脂肪酸グリセリド,日油株式会社製
C8の脂肪酸:C10の脂肪酸:C12の脂肪酸:C14の脂肪酸:C16の脂肪酸(飽和脂肪酸および不飽和脂肪酸の両方を含む)がおおよそ4:8:60:25:3の重量比で含まれている、グリセリンと脂肪酸とのトリエステル,重量平均分子量:670
・カプリル酸ジグリセリド,日油株式会社製
脂肪酸がオクタン酸である、グリセリンと脂肪酸とのジエステル,重量平均分子量:340 · Tricot oil fatty acid glyceride, manufactured by NOF Corporation C 8 fatty acid: C 10 fatty acid: C 12 fatty acid: C 14 fatty acid: C 16 fatty acid (including both saturated fatty acid and unsaturated fatty acid) is approximately 4 Triester of glycerin and fatty acid, contained in a weight ratio of 8: 60: 25: 3, weight average molecular weight: 670
・ Caprylic diglyceride, manufactured by NOF Corporation Diester of glycerin and fatty acid wherein fatty acid is octanoic acid, weight average molecular weight: 340
C8の脂肪酸:C10の脂肪酸:C12の脂肪酸:C14の脂肪酸:C16の脂肪酸(飽和脂肪酸および不飽和脂肪酸の両方を含む)がおおよそ4:8:60:25:3の重量比で含まれている、グリセリンと脂肪酸とのトリエステル,重量平均分子量:670
・カプリル酸ジグリセリド,日油株式会社製
脂肪酸がオクタン酸である、グリセリンと脂肪酸とのジエステル,重量平均分子量:340 · Tricot oil fatty acid glyceride, manufactured by NOF Corporation C 8 fatty acid: C 10 fatty acid: C 12 fatty acid: C 14 fatty acid: C 16 fatty acid (including both saturated fatty acid and unsaturated fatty acid) is approximately 4 Triester of glycerin and fatty acid, contained in a weight ratio of 8: 60: 25: 3, weight average molecular weight: 670
・ Caprylic diglyceride, manufactured by NOF Corporation Diester of glycerin and fatty acid wherein fatty acid is octanoic acid, weight average molecular weight: 340
[(a3)鎖状炭化水素ジオールと少なくとも1の脂肪酸とのエステル]
・コムポールBL,日油株式会社製
ブチレングリコールのドデカン酸(C12)モノエステル,重量平均分子量:約270
・コムポールBS,日油株式会社製
ブチレングリコールのオクタデカン酸(C18)モノエステル,重量平均分子量:約350
・ユニスター H-208BRS,日油株式会社製
ジ2-エチルヘキサン酸ネオペンチルグリコール,重量平均分子量:約360 [Ester of (a 3 ) chain hydrocarbon diol and at least one fatty acid]
-Commol BL, manufactured by NOF Corporation, dodecanoic acid (C 12 ) monoester of butylene glycol, weight average molecular weight: about 270
・ Compol BS, manufactured by NOF Corporation Octadecanoic acid (C 18 ) monoester of butylene glycol, weight average molecular weight: about 350
-Unistar H-208 BRS, manufactured by NOF Corporation Dipentyl 2-ethylhexanoate neopentyl glycol, weight average molecular weight: about 360
・コムポールBL,日油株式会社製
ブチレングリコールのドデカン酸(C12)モノエステル,重量平均分子量:約270
・コムポールBS,日油株式会社製
ブチレングリコールのオクタデカン酸(C18)モノエステル,重量平均分子量:約350
・ユニスター H-208BRS,日油株式会社製
ジ2-エチルヘキサン酸ネオペンチルグリコール,重量平均分子量:約360 [Ester of (a 3 ) chain hydrocarbon diol and at least one fatty acid]
-Commol BL, manufactured by NOF Corporation, dodecanoic acid (C 12 ) monoester of butylene glycol, weight average molecular weight: about 270
・ Compol BS, manufactured by NOF Corporation Octadecanoic acid (C 18 ) monoester of butylene glycol, weight average molecular weight: about 350
-Unistar H-208 BRS, manufactured by NOF Corporation Dipentyl 2-ethylhexanoate neopentyl glycol, weight average molecular weight: about 360
[(c2)3個のカルボキシル基を有する鎖状炭化水素トリカルボン酸、ヒドロキシ酸、アルコキシ酸またはオキソ酸と、少なくとも1の脂肪族1価アルコールとのエステル]
・O-アセチルクエン酸トリブチル,東京化成工業株式会社製
重量平均分子量:約400 [(C 2 ) ester of linear hydrocarbon tricarboxylic acid having 3 carboxyl groups, hydroxy acid, alkoxy acid or oxo acid, and at least one aliphatic monohydric alcohol]
-Tributyl O-acetyl citrate, manufactured by Tokyo Chemical Industry Co., Ltd. Weight average molecular weight: about 400
・O-アセチルクエン酸トリブチル,東京化成工業株式会社製
重量平均分子量:約400 [(C 2 ) ester of linear hydrocarbon tricarboxylic acid having 3 carboxyl groups, hydroxy acid, alkoxy acid or oxo acid, and at least one aliphatic monohydric alcohol]
-Tributyl O-acetyl citrate, manufactured by Tokyo Chemical Industry Co., Ltd. Weight average molecular weight: about 400
[(c3)2個のカルボキシル基を有する鎖状炭化水素ジカルボン酸、ヒドロキシ酸、アルコキシ酸またはオキソ酸と、少なくとも1の脂肪族1価アルコールとのエステル]
・アジピン酸ジオクチル,和光純薬工業製
重量平均分子量:約380 [(C 3 ) ester of linear hydrocarbon dicarboxylic acid having 2 carboxyl groups, hydroxy acid, alkoxy acid or oxo acid, and at least one aliphatic monohydric alcohol]
・ Dioctyl adipate, manufactured by Wako Pure Chemical Industries, Ltd. Weight average molecular weight: about 380
・アジピン酸ジオクチル,和光純薬工業製
重量平均分子量:約380 [(C 3 ) ester of linear hydrocarbon dicarboxylic acid having 2 carboxyl groups, hydroxy acid, alkoxy acid or oxo acid, and at least one aliphatic monohydric alcohol]
・ Dioctyl adipate, manufactured by Wako Pure Chemical Industries, Ltd. Weight average molecular weight: about 380
[(d3)脂肪酸と脂肪族1価アルコールとのエステル]
・エレクトールWE20,日油株式会社製
ドデカン酸(C12)と、ドデシルアルコール(C12)とのエステル,重量平均分子量:約360
・エレクトールWE40,日油株式会社製
テトラデカン酸(C14)と、ドデシルアルコール(C12)とのエステル,重量平均分子量:約390 [(D 3 ) ester of fatty acid and aliphatic monohydric alcohol]
Electol WE20, an oil-in-oil company ester of dodecanoic acid (C 12 ) and dodecyl alcohol (C 12 ), weight average molecular weight: about 360
Electol WE40, an ester of tetradecanoic acid (C 14 ) manufactured by NOF Corporation, and dodecyl alcohol (C 12 ), weight average molecular weight: about 390
・エレクトールWE20,日油株式会社製
ドデカン酸(C12)と、ドデシルアルコール(C12)とのエステル,重量平均分子量:約360
・エレクトールWE40,日油株式会社製
テトラデカン酸(C14)と、ドデシルアルコール(C12)とのエステル,重量平均分子量:約390 [(D 3 ) ester of fatty acid and aliphatic monohydric alcohol]
Electol WE20, an oil-in-oil company ester of dodecanoic acid (C 12 ) and dodecyl alcohol (C 12 ), weight average molecular weight: about 360
Electol WE40, an ester of tetradecanoic acid (C 14 ) manufactured by NOF Corporation, and dodecyl alcohol (C 12 ), weight average molecular weight: about 390
[(e1)ポリオキシC2~C6アルキレングリコール]
・ユニオールD-1000,日油株式会社製
ポリプロピレングリコール,重量平均分子量:約1,000
・ユニオールD-1200,日油株式会社製
ポリプロピレングリコール,重量平均分子量:約1,200
・ユニオールD-3000,日油株式会社製
ポリプロピレングリコール,重量平均分子量:約3,000 [(E 1 ) polyoxy C 2 -C 6 alkylene glycol]
Uniol D-1000, a polypropylene glycol manufactured by NOF Corporation, weight average molecular weight: about 1,000
Uniol D-1200, polypropylene glycol manufactured by NOF Corporation, weight average molecular weight: about 1,200
Uniol D-3000, a polypropylene glycol manufactured by NOF Corporation, weight average molecular weight: about 3,000
・ユニオールD-1000,日油株式会社製
ポリプロピレングリコール,重量平均分子量:約1,000
・ユニオールD-1200,日油株式会社製
ポリプロピレングリコール,重量平均分子量:約1,200
・ユニオールD-3000,日油株式会社製
ポリプロピレングリコール,重量平均分子量:約3,000 [(E 1 ) polyoxy C 2 -C 6 alkylene glycol]
Uniol D-1000, a polypropylene glycol manufactured by NOF Corporation, weight average molecular weight: about 1,000
Uniol D-1200, polypropylene glycol manufactured by NOF Corporation, weight average molecular weight: about 1,200
Uniol D-3000, a polypropylene glycol manufactured by NOF Corporation, weight average molecular weight: about 3,000
・ユニオールD-4000,日油株式会社製
ポリプロピレングリコール,重量平均分子量:約4,000
・ユニオールPB500,日油株式会社製
ポリブチレングリコール,重量平均分子量:約500
・ユニオールPB700,日油株式会社製
ポリオキシブチレンポリオキシプロピレングリコール,重量平均分子量:約700 Uniol D-4000, polypropylene glycol manufactured by NOF Corporation, weight average molecular weight: about 4,000
Uniol PB500, polybutylene glycol manufactured by NOF Corporation, weight average molecular weight: about 500
Uniol PB700, manufactured by NOF Corporation, polyoxybutylene polyoxypropylene glycol, weight average molecular weight: about 700
ポリプロピレングリコール,重量平均分子量:約4,000
・ユニオールPB500,日油株式会社製
ポリブチレングリコール,重量平均分子量:約500
・ユニオールPB700,日油株式会社製
ポリオキシブチレンポリオキシプロピレングリコール,重量平均分子量:約700 Uniol D-4000, polypropylene glycol manufactured by NOF Corporation, weight average molecular weight: about 4,000
Uniol PB500, polybutylene glycol manufactured by NOF Corporation, weight average molecular weight: about 500
Uniol PB700, manufactured by NOF Corporation, polyoxybutylene polyoxypropylene glycol, weight average molecular weight: about 700
・ユニオールPB1000R,日油株式会社製
ポリブチレングリコール,重量平均分子量:約1000
[(e2)ポリオキシC2~C6アルキレングリコールと少なくとも1の脂肪酸とのエステル]
・ウィルブライトcp9,日油株式会社製
ポリブチレングリコールの両末端のOH基が、ヘキサデカン酸(C16)によりエステル化された化合物,重量平均分子量:約1,150 Uniol PB 1000 R, polybutylene glycol manufactured by NOF Corporation, weight average molecular weight: about 1000
[Ester of (e 2 ) polyoxy C 2 -C 6 alkylene glycol with at least one fatty acid]
·Wilbright cp 9, a compound in which OH groups at both ends of polybutylene glycol manufactured by NOF Corporation were esterified with hexadecanoic acid (C 16 ), weight average molecular weight: about 1,150
ポリブチレングリコール,重量平均分子量:約1000
[(e2)ポリオキシC2~C6アルキレングリコールと少なくとも1の脂肪酸とのエステル]
・ウィルブライトcp9,日油株式会社製
ポリブチレングリコールの両末端のOH基が、ヘキサデカン酸(C16)によりエステル化された化合物,重量平均分子量:約1,150 Uniol PB 1000 R, polybutylene glycol manufactured by NOF Corporation, weight average molecular weight: about 1000
[Ester of (e 2 ) polyoxy C 2 -C 6 alkylene glycol with at least one fatty acid]
·
[(e3)ポリオキシC2~C6アルキレングリコールと少なくとも1の脂肪酸とのエーテル]
・ユニルーブMS-70K,日油株式会社製
ポリプロピレングリコールのステアリルエーテル,約15の繰返し単位,重量平均分子量:約1,140 [(E 3 ) Ether of polyoxy C 2 -C 6 alkylene glycol and at least one fatty acid]
・ Unileub MS-70K, stearyl ether of polypropylene glycol manufactured by NOF Corporation, about 15 repeating units, weight average molecular weight: about 1,140
・ユニルーブMS-70K,日油株式会社製
ポリプロピレングリコールのステアリルエーテル,約15の繰返し単位,重量平均分子量:約1,140 [(E 3 ) Ether of polyoxy C 2 -C 6 alkylene glycol and at least one fatty acid]
・ Unileub MS-70K, stearyl ether of polypropylene glycol manufactured by NOF Corporation, about 15 repeating units, weight average molecular weight: about 1,140
[(e5)ポリオキシC2~C6アルキレングリコールと、鎖状炭化水素テトラオール、鎖状炭化水素トリオール、または鎖状炭化水素ジオールとのエーテル]
・ユニルーブ5TP-300KB
ペンタエリトリトール1モルに、エチレンオキシド5モルと、プロピレンオキシド65モルとを付加させることにより生成した、ポリオキシエチレンポリオキシプロピレンペンタエリスリトールエーテル,重量平均分子量:4,130 [(E 5 ) Ether of polyoxy C 2 -C 6 alkylene glycol and chain hydrocarbon tetraol, chain hydrocarbon triol, or chain hydrocarbon diol]
・ Unileve 5TP-300KB
Polyoxyethylene polyoxypropylene pentaerythritol ether, weight average molecular weight: 4,130, produced by adding 5 moles of ethylene oxide and 65 moles of propylene oxide to 1 mole of pentaerythritol.
・ユニルーブ5TP-300KB
ペンタエリトリトール1モルに、エチレンオキシド5モルと、プロピレンオキシド65モルとを付加させることにより生成した、ポリオキシエチレンポリオキシプロピレンペンタエリスリトールエーテル,重量平均分子量:4,130 [(E 5 ) Ether of polyoxy C 2 -C 6 alkylene glycol and chain hydrocarbon tetraol, chain hydrocarbon triol, or chain hydrocarbon diol]
・ Unileve 5TP-300KB
Polyoxyethylene polyoxypropylene pentaerythritol ether, weight average molecular weight: 4,130, produced by adding 5 moles of ethylene oxide and 65 moles of propylene oxide to 1 mole of pentaerythritol.
・ユニオール TG-3000,日油株式会社製
ポリプロピレングリコールのグリセリルエーテル,約16の繰返し単位,重量平均分子量:約3,000
・ユニオール TG-4000,日油株式会社製
ポリプロピレングリコールのグリセリルエーテル,約16の繰返し単位,重量平均分子量:約4,000 ・ UNIOL TG-3000, glyceryl ether of polypropylene glycol manufactured by NOF Corporation, about 16 repeating units, weight average molecular weight: about 3,000
・ UNIOL TG-4000, glyceryl ether of polypropylene glycol manufactured by NOF Corporation, about 16 repeating units, weight average molecular weight: about 4,000
ポリプロピレングリコールのグリセリルエーテル,約16の繰返し単位,重量平均分子量:約3,000
・ユニオール TG-4000,日油株式会社製
ポリプロピレングリコールのグリセリルエーテル,約16の繰返し単位,重量平均分子量:約4,000 ・ UNIOL TG-3000, glyceryl ether of polypropylene glycol manufactured by NOF Corporation, about 16 repeating units, weight average molecular weight: about 3,000
・ UNIOL TG-4000, glyceryl ether of polypropylene glycol manufactured by NOF Corporation, about 16 repeating units, weight average molecular weight: about 4,000
[(f1)鎖状アルカン]
・パールリーム6,日油株式会社製
流動イソパラフィン、イソブテンおよびn-ブテンを共重合し、次いで水素を付加することにより生成された分岐鎖炭化水素、重合度:約5~約10,重量平均分子量:約330 [(F 1 ) chain alkane]
Pearl Reem 6, manufactured by NOF Corporation, branched chain hydrocarbon produced by copolymerizing liquid isoparaffin, isobutene and n-butene, and then adding hydrogen, degree of polymerization: about 5 to about 10, weight average molecular weight : About 330
・パールリーム6,日油株式会社製
流動イソパラフィン、イソブテンおよびn-ブテンを共重合し、次いで水素を付加することにより生成された分岐鎖炭化水素、重合度:約5~約10,重量平均分子量:約330 [(F 1 ) chain alkane]
[その他の材料]
・NA50,日油株式会社製
NA36に水素を付加し、原料である不飽和脂肪酸に由来する二重結合の比率を下げたグリセリンと脂肪酸とのトリエステル,重量平均分子量:約880
・(カプリル酸/カプリン酸)モノグリセリド,日油株式会社製
オクタン酸(C8)およびデカン酸(C10)がおおよそ85:15の重量比で含まれている、グリセリンと脂肪酸とのモノエステル,重量平均分子量:約220
・Monomuls 90-L2ラウリン酸モノグリセリド,コグニスジャパン株式会社製 [Other materials]
· NA50, a triester of glycerin and fatty acid in which hydrogen is added to NA36 manufactured by NOF Corporation and the ratio of double bonds derived from unsaturated fatty acid as a raw material is reduced, weight average molecular weight: about 880
・ (Caprylic acid / capric acid) monoglyceride, monoester of glycerin and fatty acid, which contains octanoic acid (C 8 ) and decanoic acid (C 10 ) manufactured by NOF Corporation at a weight ratio of about 85:15, Weight average molecular weight: about 220
Monomuls 90-L2 lauric acid monoglyceride, manufactured by Cognis Japan Ltd.
・NA50,日油株式会社製
NA36に水素を付加し、原料である不飽和脂肪酸に由来する二重結合の比率を下げたグリセリンと脂肪酸とのトリエステル,重量平均分子量:約880
・(カプリル酸/カプリン酸)モノグリセリド,日油株式会社製
オクタン酸(C8)およびデカン酸(C10)がおおよそ85:15の重量比で含まれている、グリセリンと脂肪酸とのモノエステル,重量平均分子量:約220
・Monomuls 90-L2ラウリン酸モノグリセリド,コグニスジャパン株式会社製 [Other materials]
· NA50, a triester of glycerin and fatty acid in which hydrogen is added to NA36 manufactured by NOF Corporation and the ratio of double bonds derived from unsaturated fatty acid as a raw material is reduced, weight average molecular weight: about 880
・ (Caprylic acid / capric acid) monoglyceride, monoester of glycerin and fatty acid, which contains octanoic acid (C 8 ) and decanoic acid (C 10 ) manufactured by NOF Corporation at a weight ratio of about 85:15, Weight average molecular weight: about 220
Monomuls 90-L2 lauric acid monoglyceride, manufactured by Cognis Japan Ltd.
・クエン酸イソプロピル,東京化成工業株式会社製
重量平均分子量:約230
・リンゴ酸ジイソステアリル
重量平均分子量:約640
・ユニオールD-400,日油株式会社製
ポリプロピレングリコール,重量平均分子量:約400 Isopropyl citrate, manufactured by Tokyo Chemical Industry Co., Ltd. Weight average molecular weight: about 230
・ Diisostearyl malate Weight average molecular weight: about 640
Uniol D-400, a polypropylene glycol manufactured by NOF Corporation, weight average molecular weight: about 400
重量平均分子量:約230
・リンゴ酸ジイソステアリル
重量平均分子量:約640
・ユニオールD-400,日油株式会社製
ポリプロピレングリコール,重量平均分子量:約400 Isopropyl citrate, manufactured by Tokyo Chemical Industry Co., Ltd. Weight average molecular weight: about 230
・ Diisostearyl malate Weight average molecular weight: about 640
Uniol D-400, a polypropylene glycol manufactured by NOF Corporation, weight average molecular weight: about 400
・PEG1500,日油株式会社製
ポリエチレングリコール,重量平均分子量:約1,500~約1,600
・ノニオンS-6,日油株式会社製
ポリオキシエチレンモノステアレート、約7の繰返し単位、重量平均分子量:約880
・ウィルブライトs753,日油株式会社製
ポリオキシエチレンポリオキシプロピレンポリオキシブチレングリセリン,重量平均分子量:約960 -PEG 1500, polyethylene glycol manufactured by NOF Corporation, weight average molecular weight: about 1,500 to about 1,600
Nonion S-6, manufactured by NOF Corporation, polyoxyethylene monostearate, repeating unit of about 7 weight average molecular weight: about 880
Will Bright s 753, manufactured by NOF Corporation polyoxyethylene polyoxypropylene polyoxybutylene glycerin, weight average molecular weight: about 960
ポリエチレングリコール,重量平均分子量:約1,500~約1,600
・ノニオンS-6,日油株式会社製
ポリオキシエチレンモノステアレート、約7の繰返し単位、重量平均分子量:約880
・ウィルブライトs753,日油株式会社製
ポリオキシエチレンポリオキシプロピレンポリオキシブチレングリセリン,重量平均分子量:約960 -PEG 1500, polyethylene glycol manufactured by NOF Corporation, weight average molecular weight: about 1,500 to about 1,600
Nonion S-6, manufactured by NOF Corporation, polyoxyethylene monostearate, repeating unit of about 7 weight average molecular weight: about 880
Will Bright s 753, manufactured by NOF Corporation polyoxyethylene polyoxypropylene polyoxybutylene glycerin, weight average molecular weight: about 960
・ユニオール TG-330,日油株式会社製
ポリプロピレングリコールのグリセリルエーテル,約6の繰返し単位,重量平均分子量:約330
・ユニオール TG-1000,日油株式会社製
ポリプロピレングリコールのグリセリルエーテル,約16の繰返し単位,重量平均分子量:約1,000 -Uniol TG-330, a glyceryl ether of polypropylene glycol manufactured by NOF Corporation, about 6 repeating units, weight average molecular weight: about 330
・ UNIOL TG-1000, glyceryl ether of polypropylene glycol manufactured by NOF Corporation, about 16 repeating units, weight average molecular weight: about 1,000
ポリプロピレングリコールのグリセリルエーテル,約6の繰返し単位,重量平均分子量:約330
・ユニオール TG-1000,日油株式会社製
ポリプロピレングリコールのグリセリルエーテル,約16の繰返し単位,重量平均分子量:約1,000 -Uniol TG-330, a glyceryl ether of polypropylene glycol manufactured by NOF Corporation, about 6 repeating units, weight average molecular weight: about 330
・ UNIOL TG-1000, glyceryl ether of polypropylene glycol manufactured by NOF Corporation, about 16 repeating units, weight average molecular weight: about 1,000
・ユニルーブ DGP-700,日油株式会社製
ポリプロピレングリコールのジグリセリルエーテル,約9の繰返し単位,重量平均分子量:約700
・ユニオックスHC60,日油株式会社製
ポリオキシエチレン硬化ヒマシ油,重量平均分子量:約3,570
・ワセリン,コグニスジャパン株式会社製
石油に由来する炭化水素、半固形 ・ Unirube DGP-700, a diglyceryl ether of polypropylene glycol manufactured by NOF Corporation, about 9 repeating units, weight average molecular weight: about 700
-Uniox HC60, manufactured by NOF Co., Ltd. polyoxyethylene hydrogenated castor oil, weight average molecular weight: about 3,570
・ Vaseline, Cognis Japan Ltd. Petroleum derived hydrocarbon, semi-solid
ポリプロピレングリコールのジグリセリルエーテル,約9の繰返し単位,重量平均分子量:約700
・ユニオックスHC60,日油株式会社製
ポリオキシエチレン硬化ヒマシ油,重量平均分子量:約3,570
・ワセリン,コグニスジャパン株式会社製
石油に由来する炭化水素、半固形 ・ Unirube DGP-700, a diglyceryl ether of polypropylene glycol manufactured by NOF Corporation, about 9 repeating units, weight average molecular weight: about 700
-Uniox HC60, manufactured by NOF Co., Ltd. polyoxyethylene hydrogenated castor oil, weight average molecular weight: about 3,570
・ Vaseline, Cognis Japan Ltd. Petroleum derived hydrocarbon, semi-solid
上記試料の、IOB,融点および水溶解度を、下記表2に示す。なお、水溶解度は、上述の方法にしたがって測定したが、100gの脱塩水に、20.0gを添加し、24時間後に溶解した試料は、「20g<」と評価し、そして100gの脱塩水に、0.05gは溶解したが、1.00gは溶解しなかった試料は、0.05~1.00gと評価した。また、融点に関し、「<45」は、融点が45℃未満であることを意味する。
The IOB, melting point and water solubility of the above sample are shown in Table 2 below. The water solubility was measured according to the above-mentioned method, but 20.0 g was added to 100 g of demineralized water, and a sample dissolved after 24 hours was evaluated as "20 g <", and 100 g of demineralized water was used. A sample in which 0.05 g was dissolved but not 1.00 g was evaluated as 0.05 to 1.00 g. Moreover, regarding melting | fusing point, "<45" means that melting | fusing point is less than 45 degreeC.
上記生理用ナプキンのトップシートの肌当接面を、上述の血液改質剤で塗工した。各血液改質剤を、血液改質剤が室温で液体である場合にはそのまま、そして血液改質剤が室温で固体である場合には、融点+20℃まで加熱し、次いで、コントロールシームHMAガンを用いて、各血液改質剤を微粒化し、トップシートの肌当接面の全体に、坪量がおおよそ5g/m2となるように塗布した。
The skin contact surface of the top sheet of the above-mentioned sanitary napkin was coated with the above-mentioned blood modifier. Heat each blood modifier to its melting point + 20 ° C if the blood modifier is liquid at room temperature, and if the blood modifier is solid at room temperature, then control seam HMA gun Each blood modifying agent was atomized and applied to the entire skin contact surface of the top sheet so that the basis weight was approximately 5 g / m 2 .
図13は、トップシートがトリC2L油脂肪酸グリセリドを含む生理用ナプキン(No.2-5)における、トップシートの肌当接面の電子顕微鏡写真である。図13から明らかなように、トリC2L油脂肪酸グリセリドは、微粒子状で、繊維の表面に付着している。
上述の手順にしたがって、リウェット率と、吸収体移行速度とを測定した。結果を、下記表2に示す。 FIG. 13 is an electron micrograph of the skin contact surface of the top sheet in the sanitary napkin (No. 2-5) in which the top sheet contains avian C2L oil fatty acid glyceride. As apparent from FIG. 13, the tri-C2L oil fatty acid glyceride is in the form of fine particles and adheres to the surface of the fiber.
Rewet rates and absorber transfer rates were measured according to the procedure described above. The results are shown in Table 2 below.
上述の手順にしたがって、リウェット率と、吸収体移行速度とを測定した。結果を、下記表2に示す。 FIG. 13 is an electron micrograph of the skin contact surface of the top sheet in the sanitary napkin (No. 2-5) in which the top sheet contains avian C2L oil fatty acid glyceride. As apparent from FIG. 13, the tri-C2L oil fatty acid glyceride is in the form of fine particles and adheres to the surface of the fiber.
Rewet rates and absorber transfer rates were measured according to the procedure described above. The results are shown in Table 2 below.
[試験方法]
各血液改質剤を含むトップシートの上に、穴の開いたアクリル板(200mm×100mm,125g,中央に、40mm×10mmの穴が開いている)を置き、上記穴から、37±1℃のウマEDTA血(ウマの血液に、凝結防止のため、エチレンジアミン四酢酸(以下、「EDTA」と称する)が添加されたもの)3gを、ピペットを用いて滴下(1回目)し、1分後、37±1℃のウマEDTA血3gを、アクリル板の穴から、ピペットで再度滴下した(2回目)。 [Test method]
Place a perforated acrylic plate (200 mm x 100 mm, 125 g, with a 40 mm x 10 mm hole in the center) on the top sheet containing each blood modifying agent, and from the holes, 37 ± 1 ° C 3g of equine EDTA blood (into the blood of the horse, ethylenediaminetetraacetic acid (hereinafter referred to as "EDTA") added to prevent coagulation) was dropped (first time) using a pipette, and one minute later Then, 3 g of equine EDTA blood at 37 ± 1 ° C. was pipetted again from the hole of the acrylic plate (second time).
各血液改質剤を含むトップシートの上に、穴の開いたアクリル板(200mm×100mm,125g,中央に、40mm×10mmの穴が開いている)を置き、上記穴から、37±1℃のウマEDTA血(ウマの血液に、凝結防止のため、エチレンジアミン四酢酸(以下、「EDTA」と称する)が添加されたもの)3gを、ピペットを用いて滴下(1回目)し、1分後、37±1℃のウマEDTA血3gを、アクリル板の穴から、ピペットで再度滴下した(2回目)。 [Test method]
Place a perforated acrylic plate (200 mm x 100 mm, 125 g, with a 40 mm x 10 mm hole in the center) on the top sheet containing each blood modifying agent, and from the holes, 37 ± 1 ° C 3g of equine EDTA blood (into the blood of the horse, ethylenediaminetetraacetic acid (hereinafter referred to as "EDTA") added to prevent coagulation) was dropped (first time) using a pipette, and one minute later Then, 3 g of equine EDTA blood at 37 ± 1 ° C. was pipetted again from the hole of the acrylic plate (second time).
2回目の血液の滴下後、直ちに上記アクリル板を外し、血液を滴下した場所に、ろ紙(アドバンテック東洋株式会社 定性濾紙 No.2,50mm×35mm)10枚を置き、その上から、圧力が30g/cm2となるようにおもりを置いた。1分後、上記ろ紙を取出し、以下の式に従って、「リウェット率」を算出した。
リウェット率(%)=100×(試験後のろ紙質量-当初のろ紙質量)/6 After the second drop of blood, immediately remove the above acrylic plate andplace 10 sheets of filter paper (Advantec Toyo Co., Ltd. Qualitative filter paper No. 2, 50 mm x 35 mm) in the place where the blood is dropped, and 30 g of pressure from above The weight was placed to be / cm 2 . One minute later, the filter paper was taken out, and the "rewet rate" was calculated according to the following equation.
Rewet rate (%) = 100 × (weight of filter paper after test−weight of original filter paper) / 6
リウェット率(%)=100×(試験後のろ紙質量-当初のろ紙質量)/6 After the second drop of blood, immediately remove the above acrylic plate and
Rewet rate (%) = 100 × (weight of filter paper after test−weight of original filter paper) / 6
また、リウェット率の評価とは別に、2回目の血液の滴下後、血液がトップシートから吸収体に移行する時間である「吸収体移行速度」を測定した。上記吸収体移行速度は、トップシートに血液を投入してから、トップシートの表面及び内部に、血液の赤さが見られなくなるまでの時間を意味する。
リウェット率と、吸収体移行速度の結果を、以下の表2に示す。 In addition to the evaluation of the rewet rate, “absorber transfer speed”, which is the time for blood to transfer from the top sheet to the absorber after the second drop of blood, was measured. The above-mentioned absorber transfer rate means the time from when blood is introduced into the top sheet to when the red color of blood is not seen on the surface and inside of the top sheet.
The results of the rewet rate and the absorber transfer rate are shown in Table 2 below.
リウェット率と、吸収体移行速度の結果を、以下の表2に示す。 In addition to the evaluation of the rewet rate, “absorber transfer speed”, which is the time for blood to transfer from the top sheet to the absorber after the second drop of blood, was measured. The above-mentioned absorber transfer rate means the time from when blood is introduced into the top sheet to when the red color of blood is not seen on the surface and inside of the top sheet.
The results of the rewet rate and the absorber transfer rate are shown in Table 2 below.
次いで、吸収体移行速度の試験後のトップシートの肌当接面の白さを、以下の基準にしたがって、目視で評価した。
◎:血液の赤さがほとんど残っておらず、血液が存在した場所と、存在していない場所の区別がつかない
○:血液の赤さが若干残っているが、血液の存在した場所と、存在していない場所の区別がつきいにくい
△:血液の赤さが若干残っており、血液が存在した場所が分かる
×:血液の赤さがそのまま残っている
結果を、併せて下記表2に示す。 Next, the whiteness of the skin contact surface of the top sheet after the test of the absorber transfer speed was visually evaluated according to the following criteria.
◎: Almost no blood red remains, and there is no distinction between where blood was present and where it did not exist ○: Some blood red remains, but where blood was present, It is difficult to distinguish where it does not exist :: Some redness of the blood remains, and the place where the blood exists is known ×: The redness of the blood remains The results are also shown in Table 2 below. Show.
◎:血液の赤さがほとんど残っておらず、血液が存在した場所と、存在していない場所の区別がつかない
○:血液の赤さが若干残っているが、血液の存在した場所と、存在していない場所の区別がつきいにくい
△:血液の赤さが若干残っており、血液が存在した場所が分かる
×:血液の赤さがそのまま残っている
結果を、併せて下記表2に示す。 Next, the whiteness of the skin contact surface of the top sheet after the test of the absorber transfer speed was visually evaluated according to the following criteria.
◎: Almost no blood red remains, and there is no distinction between where blood was present and where it did not exist ○: Some blood red remains, but where blood was present, It is difficult to distinguish where it does not exist :: Some redness of the blood remains, and the place where the blood exists is known ×: The redness of the blood remains The results are also shown in Table 2 below. Show.
血液改質剤を有しない場合には、リウェット率は22.7%であり、そして吸収体移行速度は60秒超であったが、グリセリンと脂肪酸とのトリエステルは、いずれも、リウェット率が7.0%以下であり、そして吸収体移行速度が8秒以下であることから、吸収性能が大幅に改善されていることが分かる。しかし、グリセリンと脂肪酸とのトリエステルのうち、融点が45℃を超えるNA50では、吸収性能に大きな改善はみられなかった。
In the absence of a blood modifying agent, the rewet rate was 22.7% and the absorber transfer rate was over 60 seconds, but both the glycerin and fatty acid triesters had rewet rates From the fact that it is 7.0% or less and the absorber transfer rate is 8 seconds or less, it can be seen that the absorption performance is greatly improved. However, among triesters of glycerin and fatty acid, NA50 of which the melting point exceeds 45 ° C. shows no significant improvement in the absorption performance.
同様に、約0.00~約0.60のIOBと、約45℃以下の融点と、25℃の水100gに対する、約0.00~約0.05gの水溶解度を有する血液改質剤では、吸収性能が大きく改善されることが分かった。
Similarly, a blood modifying agent having an IOB of about 0.00 to about 0.60, a melting point of about 45 ° C. or less, and an aqueous solubility of about 0.00 to about 0.05 g per 100 g of water at 25 ° C. It was found that the absorption performance was greatly improved.
次に、No.2-1~2-47の生理用ナプキンを、複数のボランティアの被験者に着用してもらったところ、No.2-1~2-32の血液改質剤を含む生理用ナプキンでは、経血を吸収した後であってもトップシートにべたつき感がなく、トップシートがサラサラしているとの回答を得た。
Next, No. No. 2 to No. 2-2-47 sanitary napkins were worn by a plurality of volunteer subjects. With sanitary napkins containing blood modifiers 2-1 to 2-3, there was no tackiness on the top sheet even after menstrual blood absorption, and it was answered that the top sheet was smooth. .
また、No.2-1~No.2-32の生理用ナプキンでは、そして特に、No.2-1~11,15~19および32の血液改質剤を含む生理用ナプキンでは、経血を吸収後のトップシートの肌当接面が、血液で赤く染まっておらず、不快感が少ないとの回答を得た。
Also, no. 2-1 to No. No. 2-32 sanitary napkins, and in particular. In the sanitary napkins containing blood modifiers 2-1 to 11, 15 to 19 and 32, the skin contact surface of the top sheet after absorption of menstrual blood is not stained red with blood and there is less discomfort I got an answer with.
[例2]
動物の各種血液に関して、上述の手順にしたがって、リウェット率を評価した。実験に用いられた血液は、以下の通りである。
[動物種]
(1)ヒト
(2)ウマ
(3)ヒツジ [Example 2]
Rewet rates were assessed for the various blood of the animals according to the procedure described above. The blood used for the experiment is as follows.
[Animal species]
(1) human (2) horse (3) sheep
動物の各種血液に関して、上述の手順にしたがって、リウェット率を評価した。実験に用いられた血液は、以下の通りである。
[動物種]
(1)ヒト
(2)ウマ
(3)ヒツジ [Example 2]
Rewet rates were assessed for the various blood of the animals according to the procedure described above. The blood used for the experiment is as follows.
[Animal species]
(1) human (2) horse (3) sheep
[血液種]
・脱繊維血:血液を採取後、ガラスビーズと共に、三角フラスコ内で約5分間撹拌したもの
・EDTA血:静脈血65mLに、12%EDTA・2K生理食塩液0.5mLを添加したもの [Blood type]
Defibrillation blood: after blood collection, stirred for about 5 minutes in an Erlenmeyer flask together with glass beads EDTA blood: Addition of 0.5 mL of 12% EDTA · 2K saline to 65 mL of venous blood
・脱繊維血:血液を採取後、ガラスビーズと共に、三角フラスコ内で約5分間撹拌したもの
・EDTA血:静脈血65mLに、12%EDTA・2K生理食塩液0.5mLを添加したもの [Blood type]
Defibrillation blood: after blood collection, stirred for about 5 minutes in an Erlenmeyer flask together with glass beads EDTA blood: Addition of 0.5 mL of 12% EDTA · 2K saline to 65 mL of venous blood
[分画]
血清または血漿:それぞれ、脱繊維血またはEDTA血を、室温下で、約1900Gで10分間遠心分離した後の上清
血球:血液から血清を除去し、残差をリン酸緩衝生理食塩液(PBS)で2回洗浄し、次いで除去した血清分のリン酸緩衝生理食塩液を加えたもの [Fraction]
Serum or plasma: Supernatant after centrifuging defibrillated blood or EDTA blood, respectively, at about 1900 G at room temperature for 10 minutes Blood cells: Remove the serum from the blood and remove the residual phosphate buffered saline (PBS) ) Washed twice and then added with phosphate buffered saline for the removed serum
血清または血漿:それぞれ、脱繊維血またはEDTA血を、室温下で、約1900Gで10分間遠心分離した後の上清
血球:血液から血清を除去し、残差をリン酸緩衝生理食塩液(PBS)で2回洗浄し、次いで除去した血清分のリン酸緩衝生理食塩液を加えたもの [Fraction]
Serum or plasma: Supernatant after centrifuging defibrillated blood or EDTA blood, respectively, at about 1900 G at room temperature for 10 minutes Blood cells: Remove the serum from the blood and remove the residual phosphate buffered saline (PBS) ) Washed twice and then added with phosphate buffered saline for the removed serum
トリC2L油脂肪酸グリセリドが、坪量がおおよそ5g/m2となるように塗布されている以外は、例2と同様にして吸収性物品を製造し、上述の各種血液に関して、リウェット率を評価した。各血液に関して測定を3回行い、その平均値を採用した。
結果を、下記表3に示す。 An absorbent article was produced in the same manner as in Example 2 except that avian C2L oil fatty acid glyceride was applied so as to give a basis weight of approximately 5 g / m 2, and the rewet rate was evaluated for the above various blood. . Three measurements were taken for each blood and the mean value was taken.
The results are shown in Table 3 below.
結果を、下記表3に示す。 An absorbent article was produced in the same manner as in Example 2 except that avian C2L oil fatty acid glyceride was applied so as to give a basis weight of approximately 5 g / m 2, and the rewet rate was evaluated for the above various blood. . Three measurements were taken for each blood and the mean value was taken.
The results are shown in Table 3 below.
例2で得られた、ウマEDTA血と同様の傾向が、ヒトおよびヒツジの血液でも得られた。また、脱繊維血およびEDTA血においても、同様の傾向が観察された。
The same tendency as equine EDTA blood obtained in Example 2 was also obtained in human and sheep blood. The same tendency was also observed in defibrinated blood and EDTA blood.
[例3]
[血液保持性の評価]
血液改質剤を含むトップシートと、血液改質剤を含まないトップシートとにおける血液保持性を評価した。 [Example 3]
[Evaluation of blood retention]
The blood retention of the top sheet containing the blood modifying agent and the top sheet not containing the blood modifying agent was evaluated.
[血液保持性の評価]
血液改質剤を含むトップシートと、血液改質剤を含まないトップシートとにおける血液保持性を評価した。 [Example 3]
[Evaluation of blood retention]
The blood retention of the top sheet containing the blood modifying agent and the top sheet not containing the blood modifying agent was evaluated.
[試験方法]
(1)エアスルー不織布(ポリエステルおよびポリエチレンテレフタレートから成る複合繊維、坪量:35g/m2)から形成されたトップシートの肌当接面に、トリC2L油脂肪酸グリセリドを、コントロールシームHMAガンを用いて微粒化し、坪量がおおよそ5g/m2となるように塗布する。また、比較のため、トリC2L油脂肪酸グリセリドを塗布していないものも準備する。次いで、トリC2L油脂肪酸グリセリドが塗布されているトップシートと、塗布されていないトップシートとの両方を、0.2gの大きさにカットし、セルストレイナー+トップシートの質量(a)を正確に測定する。 [Test method]
(1) Using a control seam HMA gun with tri-C2L oil fatty acid glyceride on the skin contact surface of the top sheet formed of air-through non-woven fabric (composite fiber consisting of polyester and polyethylene terephthalate, basis weight: 35 g / m 2 ) It is atomized and applied so that the basis weight is approximately 5 g / m 2 . Also, for comparison, those not coated with tri-C2L oil fatty acid glyceride are also prepared. Then, both the top sheet coated with avian C2L oil fatty acid glyceride and the top sheet not coated are cut to a size of 0.2 g, and the mass of the cell strainer + top sheet (a) is accurately determined taking measurement.
(1)エアスルー不織布(ポリエステルおよびポリエチレンテレフタレートから成る複合繊維、坪量:35g/m2)から形成されたトップシートの肌当接面に、トリC2L油脂肪酸グリセリドを、コントロールシームHMAガンを用いて微粒化し、坪量がおおよそ5g/m2となるように塗布する。また、比較のため、トリC2L油脂肪酸グリセリドを塗布していないものも準備する。次いで、トリC2L油脂肪酸グリセリドが塗布されているトップシートと、塗布されていないトップシートとの両方を、0.2gの大きさにカットし、セルストレイナー+トップシートの質量(a)を正確に測定する。 [Test method]
(1) Using a control seam HMA gun with tri-C2L oil fatty acid glyceride on the skin contact surface of the top sheet formed of air-through non-woven fabric (composite fiber consisting of polyester and polyethylene terephthalate, basis weight: 35 g / m 2 ) It is atomized and applied so that the basis weight is approximately 5 g / m 2 . Also, for comparison, those not coated with tri-C2L oil fatty acid glyceride are also prepared. Then, both the top sheet coated with avian C2L oil fatty acid glyceride and the top sheet not coated are cut to a size of 0.2 g, and the mass of the cell strainer + top sheet (a) is accurately determined taking measurement.
(2)ウマEDTA血約2mLを、肌当接面側から添加し、1分間静置する。
(3)セルストレイナーを、遠心管にセットし、スピンダウンして、余剰のウマEDTA血を取り除く。
(4)セルストレイナー+ウマEDTA血を含むトップシートの重量(b)を測定する。
(5)下式にしたがって、トップシート1g当たりの当初吸収量(g)を算出する。
当初吸収量=[重量(b)-重量(a)]/0.2
(6)セルストレイナーを、遠心管に再セットし、室温下、約1200Gで1分間遠心分離する。 (2) Add approximately 2 mL of horse EDTA blood from the skin contact side, and let it stand for 1 minute.
(3) Place a cell strainer in a centrifuge tube and spin down to remove excess equine EDTA blood.
(4) Measure the weight (b) of the top sheet containing Cell Trainer + Horse EDTA Blood.
(5) Calculate the initial absorption amount (g) per 1 g of top sheet according to the following formula.
Initial absorption amount = [weight (b)-weight (a)] / 0.2
(6) Re-set the cell strainer into a centrifuge tube and centrifuge at about 1200 G for 1 minute at room temperature.
(3)セルストレイナーを、遠心管にセットし、スピンダウンして、余剰のウマEDTA血を取り除く。
(4)セルストレイナー+ウマEDTA血を含むトップシートの重量(b)を測定する。
(5)下式にしたがって、トップシート1g当たりの当初吸収量(g)を算出する。
当初吸収量=[重量(b)-重量(a)]/0.2
(6)セルストレイナーを、遠心管に再セットし、室温下、約1200Gで1分間遠心分離する。 (2) Add approximately 2 mL of horse EDTA blood from the skin contact side, and let it stand for 1 minute.
(3) Place a cell strainer in a centrifuge tube and spin down to remove excess equine EDTA blood.
(4) Measure the weight (b) of the top sheet containing Cell Trainer + Horse EDTA Blood.
(5) Calculate the initial absorption amount (g) per 1 g of top sheet according to the following formula.
Initial absorption amount = [weight (b)-weight (a)] / 0.2
(6) Re-set the cell strainer into a centrifuge tube and centrifuge at about 1200 G for 1 minute at room temperature.
(7)セルストレイナー+ウマEDTA血を含むトップシートの重量(c)を測定する。
(8)下式にしたがって、トップシート1g当たりの試験後吸収量(g)を算出する。
試験後吸収量=[重量(c)-重量(a)]/0.2
(9)下式にしたがって血液保持率(%)を算出した。
血液保持率(%)=100×試験後吸収量/当初吸収量
なお、測定は3回行い、その平均値を採用した。結果を、下記表4に示す。 (7) Measure the weight (c) of the top sheet containing Cell Trainer + Horse EDTA Blood.
(8) The absorbed amount after test (g) per 1 g of top sheet is calculated according to the following equation.
Absorption after test = [Weight (c)-Weight (a)] / 0.2
(9) The blood retention rate (%) was calculated according to the following equation.
Blood retention rate (%) = 100 × absorption after test / initial absorption The measurement was performed three times, and the average value was adopted. The results are shown in Table 4 below.
(8)下式にしたがって、トップシート1g当たりの試験後吸収量(g)を算出する。
試験後吸収量=[重量(c)-重量(a)]/0.2
(9)下式にしたがって血液保持率(%)を算出した。
血液保持率(%)=100×試験後吸収量/当初吸収量
なお、測定は3回行い、その平均値を採用した。結果を、下記表4に示す。 (7) Measure the weight (c) of the top sheet containing Cell Trainer + Horse EDTA Blood.
(8) The absorbed amount after test (g) per 1 g of top sheet is calculated according to the following equation.
Absorption after test = [Weight (c)-Weight (a)] / 0.2
(9) The blood retention rate (%) was calculated according to the following equation.
Blood retention rate (%) = 100 × absorption after test / initial absorption The measurement was performed three times, and the average value was adopted. The results are shown in Table 4 below.
血液改質剤を含むトップシートは、血液保持性が低く、血液を吸収後、迅速に吸収体に移行させることができることが示唆される。
It is suggested that the top sheet containing the blood modifying agent has low blood retention and can be rapidly transferred to the absorber after absorbing blood.
[例4]
[血液改質剤を含む血液の粘性]
血液改質剤を含む血液の粘性を、Rheometric Expansion System ARES(Rheometric Scientific,Inc)を用いて測定した。ウマ脱繊維血に、パナセート810sを2質量%添加し、軽く撹拌して試料を形成し、直径50mmのパラレルプレートに試料を載せ、ギャップを100μmとし、37±0.5℃で粘度を測定した。パラレルプレートゆえ、試料に均一なせん断速度はかかっていないが、機器に表示された平均せん断速度は、10s-1であった。 [Example 4]
[Viscosity of blood containing blood modifying agent]
The viscosity of the blood containing the blood modifying agent was measured using Rheometric Expansion System ARES (Rheometric Scientific, Inc). 2% by weight of Panaceto 810s was added to equine defibrinated blood, the mixture was lightly stirred to form a sample, the sample was loaded on a parallel plate of 50 mm in diameter, the gap was made 100 μm, and the viscosity was measured at 37 ± 0.5 ° C. . Because of the parallel plate, the sample was not subjected to a uniform shear rate, but the average shear rate displayed on the instrument was 10 s −1 .
[血液改質剤を含む血液の粘性]
血液改質剤を含む血液の粘性を、Rheometric Expansion System ARES(Rheometric Scientific,Inc)を用いて測定した。ウマ脱繊維血に、パナセート810sを2質量%添加し、軽く撹拌して試料を形成し、直径50mmのパラレルプレートに試料を載せ、ギャップを100μmとし、37±0.5℃で粘度を測定した。パラレルプレートゆえ、試料に均一なせん断速度はかかっていないが、機器に表示された平均せん断速度は、10s-1であった。 [Example 4]
[Viscosity of blood containing blood modifying agent]
The viscosity of the blood containing the blood modifying agent was measured using Rheometric Expansion System ARES (Rheometric Scientific, Inc). 2% by weight of Panaceto 810s was added to equine defibrinated blood, the mixture was lightly stirred to form a sample, the sample was loaded on a parallel plate of 50 mm in diameter, the gap was made 100 μm, and the viscosity was measured at 37 ± 0.5 ° C. . Because of the parallel plate, the sample was not subjected to a uniform shear rate, but the average shear rate displayed on the instrument was 10 s −1 .
パナセート810sを2質量%含むウマ脱繊維血の粘度は、5.9mPa・sであり、一方、血液改質剤を含まないウマ脱繊維血の粘度は、50.4mPa・sであった。したがって、パナセート810sを2質量%含むウマ脱繊維血は、血液改質剤を含まない場合と比較して、約90%粘度を下げることが分かる。血液は、血球等の成分を含み、チキソトロピーの性質を有することが知られているが、本開示の血液改質剤は、低粘度域で、血液の粘度を下げることができると考えられる。血液の粘度を下げることにより、吸収した経血を、トップシートから吸収体に速やかに移行させることができると考えられる。
The viscosity of horse-defibrillated blood containing 2% by mass of Panaceto 810s was 5.9 mPa · s, while the viscosity of horse-defibrillated blood containing no blood modifying agent was 50.4 mPa · s. Thus, it can be seen that equine defibrinated blood containing 2% by weight of Panaceto 810s reduces the viscosity by about 90% as compared to the case without blood modifying agent. Although blood contains components such as blood cells and is known to have thixotropy properties, it is considered that the blood modifying agent of the present disclosure can lower the viscosity of blood in a low viscosity region. By reducing the viscosity of blood, it is thought that absorbed menstrual blood can be rapidly transferred from the top sheet to the absorber.
[例5]
[血液改質剤を含む血液の顕微鏡写真]
健常ボランティアの経血をサランラップ(登録商標)上に採取し、その一部に、10倍の質量のリン酸緩衝生理食塩水中に分散されたパナセート810sを、パナセート810sの濃度が1質量%となるように添加した。経血を、スライドグラスに適下し、カバーグラスをかけ、光学顕微鏡にて、赤血球の状態を観察した。血液改質剤を含まない経血の顕微鏡写真を図14(a)に、そしてパナセート810sを含む経血の顕微鏡写真を図14(b)に示す。 [Example 5]
[Micrograph of blood containing blood modifier]
A healthy volunteer's menstrual blood is collected on Saran wrap (registered trademark), and in part of it, Panaceto 810s dispersed in 10 times mass of phosphate buffered saline, and the concentration of Panaceto 810s is 1% by mass As it was added. The menstrual blood was applied to a slide glass, covered with a cover glass, and the condition of red blood cells was observed with a light microscope. A photomicrograph of menstrual blood containing no blood modifying agent is shown in FIG. 14 (a), and a photomicrograph of menstrual blood containing PANACET 810s is shown in FIG. 14 (b).
[血液改質剤を含む血液の顕微鏡写真]
健常ボランティアの経血をサランラップ(登録商標)上に採取し、その一部に、10倍の質量のリン酸緩衝生理食塩水中に分散されたパナセート810sを、パナセート810sの濃度が1質量%となるように添加した。経血を、スライドグラスに適下し、カバーグラスをかけ、光学顕微鏡にて、赤血球の状態を観察した。血液改質剤を含まない経血の顕微鏡写真を図14(a)に、そしてパナセート810sを含む経血の顕微鏡写真を図14(b)に示す。 [Example 5]
[Micrograph of blood containing blood modifier]
A healthy volunteer's menstrual blood is collected on Saran wrap (registered trademark), and in part of it, Panaceto 810s dispersed in 10 times mass of phosphate buffered saline, and the concentration of Panaceto 810s is 1% by mass As it was added. The menstrual blood was applied to a slide glass, covered with a cover glass, and the condition of red blood cells was observed with a light microscope. A photomicrograph of menstrual blood containing no blood modifying agent is shown in FIG. 14 (a), and a photomicrograph of menstrual blood containing PANACET 810s is shown in FIG. 14 (b).
図14から、血液改質剤を含まない経血では、赤血球が連銭等の集合塊を形成しているが、パナセート810sを含む経血では、赤血球が、それぞれ、安定に分散していることが分かる。したがって、血液改質剤は、血液の中で、赤血球を安定化させる働きをしていることが示唆される。
From FIG. 14, in the menstrual blood containing no blood modifying agent, red blood cells form a lump of rhomsen, etc., but in menstrual blood containing PANACET 810s, the red blood cells are dispersed stably. I understand. Therefore, it is suggested that the blood modifying agent works to stabilize red blood cells in the blood.
[例6]
[血液改質剤を含む血液の表面張力]
血液改質剤を含む血液の表面張力を、協和界面科学社製接触角計 Drop Master500を用い、ペンダントドロップ法にて測定した。表面張力は、ヒツジ脱繊維血に、所定の量の血液改質剤を添加し、十分振とうした後に測定した。測定は、機器が自動で行うが、表面張力γは、以下の式により求められる(図15を参照)。 [Example 6]
[Surface tension of blood containing blood modifier]
The surface tension of blood containing a blood modifying agent was measured by a pendant drop method using a contact angle meter Drop Master 500 manufactured by Kyowa Interface Science Co., Ltd. The surface tension was measured after adding a predetermined amount of blood modifying agent to sheep defibrinated blood and shaking sufficiently. The measurement is automatically performed by the device, but the surface tension γ is obtained by the following equation (see FIG. 15).
[血液改質剤を含む血液の表面張力]
血液改質剤を含む血液の表面張力を、協和界面科学社製接触角計 Drop Master500を用い、ペンダントドロップ法にて測定した。表面張力は、ヒツジ脱繊維血に、所定の量の血液改質剤を添加し、十分振とうした後に測定した。測定は、機器が自動で行うが、表面張力γは、以下の式により求められる(図15を参照)。 [Example 6]
[Surface tension of blood containing blood modifier]
The surface tension of blood containing a blood modifying agent was measured by a pendant drop method using a contact angle meter Drop Master 500 manufactured by Kyowa Interface Science Co., Ltd. The surface tension was measured after adding a predetermined amount of blood modifying agent to sheep defibrinated blood and shaking sufficiently. The measurement is automatically performed by the device, but the surface tension γ is obtained by the following equation (see FIG. 15).
γ=g×ρ×(de)2×1/H
g:重力定数
1/H:ds/deから求められる補正項
ρ:密度
de:最大直径
ds:滴下端よりdeだけ上がった位置での径 γ = g × ρ × (de) 2 × 1 / H
g: Gravitational constant 1 / H: correction term obtained from ds / de ρ: density de: maximum diameter ds: diameter at a position de up from the dropping end
g:重力定数
1/H:ds/deから求められる補正項
ρ:密度
de:最大直径
ds:滴下端よりdeだけ上がった位置での径 γ = g × ρ × (de) 2 × 1 / H
g: Gravitational constant 1 / H: correction term obtained from ds / de ρ: density de: maximum diameter ds: diameter at a position de up from the dropping end
密度ρは、JIS K 2249-1995の「密度試験方法および密度・質量・容量換算表」の5.振動式密度試験方法に準拠し、下記表5に示される温度で測定した。測定には、京都電子工業株式会社のDA-505を用いた。結果を、表5に示す。
The density ρ is the density test method and density / mass / volume conversion table in JIS K 2249-1995, 5. It was measured at the temperature shown in Table 5 below according to the vibrational density test method. For measurement, DA-505 of Kyoto Electronics Industries Ltd. was used. The results are shown in Table 5.
表5から、血液改質剤は、25℃の水100gに対する、約0.00~約0.05gの水溶解度を有することからも明らかなように、水への溶解性が非常に低いが、血液の表面張力を下げることができることが分かる。血液の表面張力を下げることにより、吸収した血液をトップシートの繊維間に保持せず、速やかに吸収体に移行させることができると考えられる。
From Table 5, it is apparent from the fact that the blood modifying agent has a water solubility of about 0.00 to about 0.05 g per 100 g of water at 25 ° C., but its solubility in water is very low. It can be seen that the surface tension of the blood can be reduced. By lowering the surface tension of the blood, it is considered that the absorbed blood can be rapidly transferred to the absorber without being held between the fibers of the top sheet.
以上の説明はあくまで一例であり、発明は、上記の実施形態に何ら限定されるものではない。
The above description is merely an example, and the invention is not limited to the above embodiment.
1 吸収性物品
2 トップシート
3 バックシート
4 吸収体
5 折曲抑制シート
6 ウイング部
7 粘着部
8 圧搾溝
9 シール部
10 本体部
21 第1の山折り部
22 第2の山折り部
23 谷折り部
24 血液改質剤層
25 開口部
26 第1の山折り部および谷折り部の側面
100 不織布製造装置
110 折曲抑制シート用シートロール
130,170 カッタ
140 パターンドラム
150 エンボス加工装置
160 バックシート用ロール
180 改質剤塗布スプレー
210 樹脂フィルムシートのロール
212,214,216 樹脂フィルムシート
220 凹部形成ロール
230 延伸ギアロール
231 上段ロール
232 下段ロール
233,235 ギア歯
234 ギア歯が途切れている箇所 DESCRIPTION OF SYMBOLS 1Absorbent article 2 top sheet 3 back sheet 4 absorber 5 bending suppression sheet 6 wing part 7 adhesive part 8 pressing groove 9 seal part 10 main body part 21 1st mountain fold part 22 2nd mountain fold part 23 valley fold Part 24 Blood modifier layer 25 Opening 26 Side of first mountain fold and valley fold 100 Non-woven fabric manufacturing device 110 Sheet roll for bending sheet 130, 170 Cutter 140 Pattern drum 150 Embossing device 160 for back sheet Roll 180 Modifier application spray 210 Roll of resin film sheet 212, 214, 216 Resin film sheet 220 Recess forming roll 230 Stretching gear roll 231 Upper roll 232 Lower roll 233, 235 Gear teeth 234 Gear teeth are broken
2 トップシート
3 バックシート
4 吸収体
5 折曲抑制シート
6 ウイング部
7 粘着部
8 圧搾溝
9 シール部
10 本体部
21 第1の山折り部
22 第2の山折り部
23 谷折り部
24 血液改質剤層
25 開口部
26 第1の山折り部および谷折り部の側面
100 不織布製造装置
110 折曲抑制シート用シートロール
130,170 カッタ
140 パターンドラム
150 エンボス加工装置
160 バックシート用ロール
180 改質剤塗布スプレー
210 樹脂フィルムシートのロール
212,214,216 樹脂フィルムシート
220 凹部形成ロール
230 延伸ギアロール
231 上段ロール
232 下段ロール
233,235 ギア歯
234 ギア歯が途切れている箇所 DESCRIPTION OF SYMBOLS 1
Claims (12)
- 長手方向および幅方向を有し、肌側に設けられた液透過性のトップシート、着衣側に設けられた液不透過性のバックシートおよび該トップシートと該バックシートとの間に設けられ幅方向の両側に長手方向に延びる側面を含む液保持性の吸収体を備えた本体部と、該本体部の両側縁から幅方向に延出するように該本体部の両側に配置されたウイング部とを含む吸収性物品であって、
前記本体部は、前記吸収体と前記バックシートとの間に設けられ、幅方向の両側に長手方向に延びる縁を含む折曲抑制シートをさらに備え、
前記折曲抑制シートの前記幅方向の両側の縁は、前記吸収体の前記側面よりも幅方向外側に位置し、
前記折曲抑制シートが設けられている領域が、前記折曲抑制シートが設けられていない領域に比べて前記吸収性物品が折り曲げにくくなるように、前記折曲抑制シートは前記吸収性物品に剛性を付与する吸収性物品。 A liquid-permeable top sheet provided on the skin side, having a longitudinal direction and a width direction, a liquid-impermeable back sheet provided on the clothing side, and a width provided between the top sheet and the back sheet Body portion provided with a liquid-retaining absorber including longitudinally extending side surfaces on both sides of the direction, and wing portions disposed on both sides of the body portion so as to extend in the width direction from both side edges of the body portion An absorbent article comprising
The main body portion further includes a bending suppression sheet provided between the absorber and the back sheet and including longitudinally extending edges on both sides in the width direction,
Edges on both sides in the width direction of the bending suppression sheet are positioned on the outer side in the width direction than the side surface of the absorber,
The bending suppression sheet is rigid to the absorbent article such that the area in which the bending suppression sheet is provided is less likely to bend than the area in which the bending suppression sheet is not provided. An absorbent article to impart. - 前記折曲抑制シートのカンチレバー法による剛軟度は15~120mmである、請求項1に記載の吸収性物品。 The absorbent article according to claim 1, wherein the bending resistance of the bending suppression sheet according to a cantilever method is 15 to 120 mm.
- 前記折曲抑制シートは10~40g/m2の目付を有する不織布である、請求項1に記載の吸収性物品。 The absorbent article according to claim 1, wherein the anti-folding sheet is a non-woven fabric having a basis weight of 10 to 40 g / m 2 .
- 前記折曲抑制シートは、疎水性または撥水性を有する、請求項1~3のいずれか1項に記載の吸収性物品。 The absorbent article according to any one of claims 1 to 3, wherein the anti-folding sheet has hydrophobicity or water repellency.
- 前記トップシートは、少なくとも前記吸収体の幅方向の両側面の外側において、前記トップシートにクッション性を付与する突部を有する、請求項1~4のいずれか1項に記載の吸収性物品。 The absorbent article according to any one of claims 1 to 4, wherein the top sheet has a protrusion for providing a cushioning property to the top sheet at least outside of both side surfaces in the width direction of the absorber.
- 少なくとも前記吸収体の幅方向の両側面の外側において、前記トップシートは、長手方向に延在し、幅方向に交互に並ぶ第1の山折り部および谷折り部と、該第1の山折り部に交差する方向に延在する第2の山折り部とを含む、請求項1~4のいずれか1項に記載の吸収性物品。 At least outside of both side surfaces in the width direction of the absorber, the top sheet extends in the longitudinal direction, and includes first mountain folds and valley folds alternately arranged in the width direction, and the first mountain folds. The absorbent article according to any one of claims 1 to 4, comprising a second mountain fold portion extending in a direction intersecting the portion.
- 前記折曲抑制シートは、前記トップシートと接合していない、請求項1~6のいずれか1項に記載の吸収性物品。 The absorbent article according to any one of claims 1 to 6, wherein the bending suppression sheet is not joined to the top sheet.
- 前記本体部の前記トップシートは、前記第1の山折り部および前記谷折り部を含み、
前記第1の山折り部および前記谷折り部の側面には、長手方向に並ぶ複数の開口部が設けられ、
前記本体部の前記トップシートは、肌側の表面に血液改質剤層をさらに含み、
前記血液改質剤層の血液改質剤が、0.00~0.60のIOBと、45℃以下の融点と、25℃の水100gに対する、0.00~0.05gの水溶解度とを有する、請求項1~7のいずれか1項に記載の吸収性物品。 The top sheet of the body portion includes the first mountain fold and the valley fold,
A plurality of openings aligned in the longitudinal direction are provided on side surfaces of the first mountain fold and the valley fold,
The top sheet of the body further includes a blood modifier layer on the skin side surface,
The blood modifying agent layer has an IOB of 0.00 to 0.60, a melting point of 45 ° C. or less, and an aqueous solubility of 0.00 to 0.05 g in 100 g of water at 25 ° C. The absorbent article according to any one of claims 1 to 7, which has. - 前記血液改質剤が、次の(i)~(iii)、
(i)炭化水素、
(ii) (ii-1)炭化水素部分と、(ii-2)前記炭化水素部分のC-C単結合間に挿入された、カルボニル基(-CO-)およびオキシ基(-O-)から成る群から選択される、一または複数の、同一または異なる基とを有する化合物、および
(iii) (iii-1)炭化水素部分と、(iii-2)前記炭化水素部分のC-C単結合間に挿入された、カルボニル基(-CO-)およびオキシ基(-O-)から成る群から選択される、一または複数の、同一または異なる基と、(iii-3)前記炭化水素部分の水素原子を置換する、カルボキシル基(-COOH)およびヒドロキシル基(-OH)から成る群から選択される、一または複数の、同一または異なる基とを有する化合物、
ならびにそれらの任意の組み合わせから成る群から選択され、
ここで、(ii)または(iii)の化合物において、オキシ基が2つ以上挿入されている場合には、各オキシ基は隣接していない、請求項8に記載の吸収性物品。 The blood modifying agent comprises the following (i) to (iii),
(I) Hydrocarbons,
(Ii) from a carbonyl group (-CO-) and an oxy group (-O-) inserted between (ii-1) a hydrocarbon moiety and (ii-2) a C-C single bond of the hydrocarbon moiety A compound having one or more same or different groups selected from the group consisting of: (iii) (iii-1) a hydrocarbon moiety, and (iii-2) a C—C single bond of said hydrocarbon moiety (Iii-3) one or more same or different groups selected from the group consisting of carbonyl group (—CO—) and oxy group (—O—) inserted between A compound having one or more and the same or different groups selected from the group consisting of a carboxyl group (—COOH) and a hydroxyl group (—OH), which substitutes a hydrogen atom,
And selected from the group consisting of any combination thereof,
The absorbent article according to claim 8, wherein, in the compound (ii) or (iii), when two or more oxy groups are inserted, each oxy group is not adjacent. - 前記血液改質剤が、次の(i’)~(iii’)、
(i’)炭化水素、
(ii’) (ii’-1)炭化水素部分と、(ii’-2)前記炭化水素部分のC-C単結合間に挿入された、カルボニル結合(-CO-)、エステル結合(-COO-)、カーボネート結合(-OCOO-)、およびエーテル結合(-O-)から成る群から選択される、一または複数の、同一または異なる結合とを有する化合物、および
(iii’) (iii’-1)炭化水素部分と、(iii’-2)前記炭化水素部分のC-C単結合間に挿入された、カルボニル結合(-CO-)、エステル結合(-COO-)、カーボネート結合(-OCOO-)、およびエーテル結合(-O-)から成る群から選択される、一または複数の、同一または異なる結合と、(iii’-3)前記炭化水素部分の水素原子を置換する、カルボキシル基(-COOH)およびヒドロキシル基(-OH)から成る群から選択される、一または複数の、同一または異なる基とを有する化合物、
ならびにそれらの任意の組み合わせから成る群から選択され、
ここで、(ii’)または(iii’)の化合物において、2以上の同一または異なる結合が挿入されている場合には、各結合は隣接していない、請求項8または9に記載の吸収性物品。 The blood modifying agent comprises the following (i ') to (iii'),
(I ') hydrocarbons,
(Ii ') (ii'-1) a hydrocarbon moiety, and (ii'-2) a carbonyl bond (-CO-), an ester bond (-COO) inserted between a C-C single bond of the hydrocarbon moiety -), A compound having one or more same or different bonds selected from the group consisting of carbonate bond (-OCOO-), and ether bond (-O-), and (iii ') (iii'-) 1) A carbonyl bond (-CO-), an ester bond (-COO-), a carbonate bond (-OCOO) inserted between a hydrocarbon moiety and (iii'-2) a C-C single bond of the hydrocarbon moiety A carboxyl group (-), and one or more, same or different bond selected from the group consisting of an ether bond (-O-) and (iii'-3) a hydrogen atom of the hydrocarbon moiety -COOH Compounds having one or more same or different groups selected from the group consisting of) and hydroxyl groups (—OH),
And selected from the group consisting of any combination thereof,
Here, in the compound of (ii ′) or (iii ′), in the case where two or more identical or different bonds are inserted, each bond is not adjacent. Goods. - 前記血液改質剤が、次の(A)~(F)、
(A) (A1)鎖状炭化水素部分と、前記鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(A2)鎖状炭化水素部分と、前記鎖状炭化水素部分の水素原子を置換する1個のカルボキシル基とを有する化合物とのエステル、
(B) (B1)鎖状炭化水素部分と、前記鎖状炭化水素部分の水素原子を置換する2~4個のヒドロキシル基とを有する化合物と、(B2)鎖状炭化水素部分と、前記鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエーテル、
(C) (C1)鎖状炭化水素部分と、前記鎖状炭化水素部分の水素原子を置換する、2~4個のカルボキシル基とを含むカルボン酸、ヒドロキシ酸、アルコキシ酸またはオキソ酸と、(C2)鎖状炭化水素部分と、前記鎖状炭化水素部分の水素原子を置換する1個のヒドロキシル基とを有する化合物とのエステル、
(D)鎖状炭化水素部分と、前記鎖状炭化水素部分のC-C単結合間に挿入された、エーテル結合(-O-)、カルボニル結合(-CO-)、エステル結合(-COO-)、およびカーボネート結合(-OCOO-)から成る群から選択されるいずれか1つの結合とを有する化合物、
(E)ポリオキシC2~C6アルキレングリコール、またはそのアルキルエステルもしくはアルキルエーテル、および
(F)鎖状炭化水素、
ならびにそれらの任意の組み合わせから成る群から選択される、請求項8~10のいずれか1項に記載の吸収性物品。 The blood modifying agent comprises the following (A) to (F),
(A) A compound having (A1) a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing hydrogen atoms of the chain hydrocarbon moiety, (A2) a chain hydrocarbon moiety, and the chain Ester with a compound having one carboxyl group replacing the hydrogen atom of the cyclic hydrocarbon moiety,
(B) a compound having (B1) a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing hydrogen atoms of the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, and the chain Ether with a compound having one hydroxyl group replacing the hydrogen atom of the cyclic hydrocarbon moiety,
(C) a carboxylic acid, a hydroxy acid, an alkoxy acid or an oxo acid containing a (C1) chain hydrocarbon moiety and 2 to 4 carboxyl groups replacing the hydrogen atom of the chain hydrocarbon moiety; C2) an ester of a compound having a chain hydrocarbon moiety and one hydroxyl group replacing a hydrogen atom of the chain hydrocarbon moiety,
(D) an ether bond (-O-), a carbonyl bond (-CO-), an ester bond (-COO-) inserted between a chain hydrocarbon moiety and a C-C single bond of the chain hydrocarbon moiety ), And a compound having any one bond selected from the group consisting of carbonate bonds (—OCOO—),
(E) polyoxy C 2 -C 6 alkylene glycol or its alkyl ester or alkyl ether, and (F) chain hydrocarbon
The absorbent article according to any one of claims 8 to 10, selected from the group consisting of and any combination thereof. - 前記血液改質剤が、(a1)鎖状炭化水素テトラオールと少なくとも1の脂肪酸とのエステル、(a2)鎖状炭化水素トリオールと少なくとも1の脂肪酸とのエステル、(a3)鎖状炭化水素ジオールと少なくとも1の脂肪酸とのエステル、(b1)鎖状炭化水素テトラオールと少なくとも1の脂肪族1価アルコールとのエーテル、(b2)鎖状炭化水素トリオールと少なくとも1の脂肪族1価アルコールとのエーテル、(b3)鎖状炭化水素ジオールと少なくとも1の脂肪族1価アルコールとのエーテル、(c1)4個のカルボキシル基を有する鎖状炭化水素テトラカルボン酸、ヒドロキシ酸、アルコキシ酸またはオキソ酸と、少なくとも1の脂肪族1価アルコールとのエステル、(c2)3個のカルボキシル基を有する鎖状炭化水素トリカルボン酸、ヒドロキシ酸、アルコキシ酸またはオキソ酸と、少なくとも1の脂肪族1価アルコールとのエステル、(c3)2個のカルボキシル基を有する鎖状炭化水素ジカルボン酸、ヒドロキシ酸、アルコキシ酸またはオキソ酸と、少なくとも1の脂肪族1価アルコールとのエステル、(d1)脂肪族1価アルコールと脂肪族1価アルコールとのエーテル、(d2)ジアルキルケトン、(d3)脂肪酸と脂肪族1価アルコールとのエステル、(d4)ジアルキルカーボネート、(e1)ポリオキシC2~C6アルキレングリコール、(e2)ポリオキシC2~C6アルキレングリコールと少なくとも1の脂肪酸とのエステル、(e3)ポリオキシC2~C6アルキレングリコールと少なくとも1の脂肪族1価アルコールとのエーテル、(e4)ポリオキシC2~C6アルキレングリコールと、鎖状炭化水素テトラカルボン酸、鎖状炭化水素トリカルボン酸、または鎖状炭化水素ジカルボン酸とのエステル、(e5)ポリオキシC2~C6アルキレングリコールと、鎖状炭化水素テトラオール、鎖状炭化水素トリオール、または鎖状炭化水素ジオールとのエーテル、および(f1)鎖状アルカン、ならびにそれらの任意の組み合わせから成る群から選択される、請求項8~11のいずれか1項に記載の吸収性物品。 The blood modifying agent is an ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid, an ester of (a 2 ) chain hydrocarbon triol and at least one fatty acid, (a 3 ) chain An ester of a hydrocarbon diol and at least one fatty acid, an ether of (b 1 ) a chain hydrocarbon tetraol and at least one aliphatic monohydric alcohol, (b 2 ) a chain hydrocarbon triol and at least one aliphatic Ether with monohydric alcohol, Ether of (b 3 ) chain hydrocarbon diol and at least one aliphatic monohydric alcohol, Chain hydrocarbon tetracarboxylic acid having (c 1 ) 4 carboxyl groups, hydroxy acid , alkoxy acid or oxo acid, at least one ester of an aliphatic monohydric alcohol, (c 2) a chain hydrocarbon birds with 3 carboxyl groups Carboxylic acid, hydroxy acids, esters of alkoxy acids or oxoacids, and at least one aliphatic monohydric alcohol, (c 3) a chain hydrocarbon dicarboxylic acids having two carboxyl groups, hydroxy acid, alkoxy acid or oxo An ester of an acid and at least one aliphatic monohydric alcohol, (d 1 ) an ether of an aliphatic monohydric alcohol and an aliphatic monohydric alcohol, (d 2 ) a dialkyl ketone, (d 3 ) a fatty acid and an aliphatic 1 (E 4 ) dialkyl carbonate, (e 1 ) polyoxy C 2 -C 6 alkylene glycol, (e 2 ) ester of polyoxy C 2 -C 6 alkylene glycol with at least one fatty acid, (e 3 ) polyoxy C 2 ~ C 6 alkylene glycol and at least one aliphatic monohydric ether alcohols, (e 4 Polyoxy C 2 ~ C 6 alkylene glycol, a chain hydrocarbon tetracarboxylic acid, esters of chain hydrocarbon tricarboxylic acid or chain hydrocarbon dicarboxylic acid,, (e 5) polyoxy C 2 ~ C 6 alkylene glycol, 9. A chain hydrocarbon tetraol, a chain hydrocarbon triol, or an ether with a chain hydrocarbon diol, and (f 1 ) a chain alkane, and any combination thereof, and selected from the group consisting of The absorbent article of any one of 11.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2012-082184 | 2012-03-30 | ||
| JP2012082184A JP5762346B2 (en) | 2012-03-30 | 2012-03-30 | Absorbent articles |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013145869A1 true WO2013145869A1 (en) | 2013-10-03 |
Family
ID=49259154
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2013/052628 WO2013145869A1 (en) | 2012-03-30 | 2013-02-05 | Absorbent article |
Country Status (4)
| Country | Link |
|---|---|
| JP (1) | JP5762346B2 (en) |
| AR (1) | AR090518A1 (en) |
| TW (1) | TWI573573B (en) |
| WO (1) | WO2013145869A1 (en) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6585785B2 (en) * | 2018-08-10 | 2019-10-02 | 大王製紙株式会社 | Absorbent articles |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050137556A1 (en) * | 2003-12-04 | 2005-06-23 | Henri Brisebois | Sanitary absorbent article having a reinforced structure |
| JP2006510456A (en) * | 2002-12-18 | 2006-03-30 | ザ プロクター アンド ギャンブル カンパニー | Sanitary napkin for body cleansing effect |
| JP2008519672A (en) * | 2004-11-30 | 2008-06-12 | ザ プロクター アンド ギャンブル カンパニー | Absorbent article including a pair of rear side flaps |
| JP2010136833A (en) * | 2008-12-10 | 2010-06-24 | Kao Corp | Absorbent article |
| JP5122007B1 (en) * | 2011-03-31 | 2013-01-16 | ユニ・チャーム株式会社 | Absorbent articles |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4812614B2 (en) * | 2006-12-27 | 2011-11-09 | 花王株式会社 | Absorbent articles |
-
2012
- 2012-03-30 JP JP2012082184A patent/JP5762346B2/en not_active Expired - Fee Related
-
2013
- 2013-02-05 WO PCT/JP2013/052628 patent/WO2013145869A1/en active Application Filing
- 2013-03-27 AR ARP130100997A patent/AR090518A1/en unknown
- 2013-03-28 TW TW102111223A patent/TWI573573B/en not_active IP Right Cessation
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006510456A (en) * | 2002-12-18 | 2006-03-30 | ザ プロクター アンド ギャンブル カンパニー | Sanitary napkin for body cleansing effect |
| US20050137556A1 (en) * | 2003-12-04 | 2005-06-23 | Henri Brisebois | Sanitary absorbent article having a reinforced structure |
| JP2008519672A (en) * | 2004-11-30 | 2008-06-12 | ザ プロクター アンド ギャンブル カンパニー | Absorbent article including a pair of rear side flaps |
| JP2010136833A (en) * | 2008-12-10 | 2010-06-24 | Kao Corp | Absorbent article |
| JP5122007B1 (en) * | 2011-03-31 | 2013-01-16 | ユニ・チャーム株式会社 | Absorbent articles |
Also Published As
| Publication number | Publication date |
|---|---|
| AR090518A1 (en) | 2014-11-19 |
| JP2013208381A (en) | 2013-10-10 |
| JP5762346B2 (en) | 2015-08-12 |
| TWI573573B (en) | 2017-03-11 |
| TW201404372A (en) | 2014-02-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5925015B2 (en) | Absorbent articles | |
| JP5713951B2 (en) | Absorbent articles | |
| JP5847055B2 (en) | Absorbent articles | |
| WO2013129327A1 (en) | Absorbent article | |
| JP5843740B2 (en) | Absorbent articles | |
| JP6037606B2 (en) | Absorbent article, top sheet of absorbent article, and method for producing the top sheet | |
| JP5963639B2 (en) | Absorbent articles | |
| JP5709788B2 (en) | Absorbent articles | |
| JP2013075099A (en) | Absorbent article | |
| JP6041473B2 (en) | Absorbent articles | |
| JP5745377B2 (en) | Absorbent articles | |
| JP5769655B2 (en) | Absorbent articles | |
| JP5762346B2 (en) | Absorbent articles | |
| JP6041472B2 (en) | Absorbent article, top sheet of absorbent article, and method for producing the top sheet | |
| JP5726123B2 (en) | Absorbent articles | |
| JP5709789B2 (en) | Absorbent articles | |
| JP5713950B2 (en) | Absorbent articles | |
| WO2013129332A1 (en) | Absorbent article | |
| WO2013129326A1 (en) | Absorbent article | |
| WO2013129325A1 (en) | Absorbent article |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 13767419 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 13767419 Country of ref document: EP Kind code of ref document: A1 |