WO2013129652A1 - 皮膚洗浄剤組成物 - Google Patents
皮膚洗浄剤組成物 Download PDFInfo
- Publication number
- WO2013129652A1 WO2013129652A1 PCT/JP2013/055686 JP2013055686W WO2013129652A1 WO 2013129652 A1 WO2013129652 A1 WO 2013129652A1 JP 2013055686 W JP2013055686 W JP 2013055686W WO 2013129652 A1 WO2013129652 A1 WO 2013129652A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- mass
- component
- skin
- components
- composition according
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/33—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing oxygen
- A61K8/36—Carboxylic acids; Salts or anhydrides thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/33—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing oxygen
- A61K8/39—Derivatives containing from 2 to 10 oxyalkylene groups
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/40—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing nitrogen
- A61K8/41—Amines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/72—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds
- A61K8/84—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds obtained by reactions otherwise than those involving only carbon-carbon unsaturated bonds
- A61K8/86—Polyethers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
- A61Q19/10—Washing or bathing preparations
Definitions
- the present invention relates to a skin cleanser composition.
- Soap is excellent in foaming and has been used as a skin cleanser for a long time.
- soap has a strong sense of squeak when rinsing, and for consumers, this squeak has a negative effect on the skin, such as "feeling that the oil has been removed” and “feeling that something remains” I was reminded of.
- Alkyl ether carboxylic acid surfactants are known as low-stimulant surfactants for the skin, but they are poor in foaming properties, so they are used in combination with other surfactants such as alkyl ether sulfates.
- Patent Document 1 a detergent composition containing an ether carboxylic acid surfactant having a narrow molecular weight distribution (Patent Document 2, Patent Document 3) and an ether carboxylic acid surfactant having an addition molar distribution of specific ethylene oxide A cleaning composition (Patent Document 4) is also proposed.
- JP 2008-285479 A Japanese Patent Laid-Open No. 61-21199 JP 2001-207189 A Japanese Patent Laid-Open No. 2-175799
- the present invention includes the following components (A) and (B): (A) General formula (1)
- R 1 represents an alkyl group having 4 to 22 carbon atoms, n represents a number of 0 to 20, and M represents a hydrogen atom, an alkali metal, an alkaline earth metal, ammonium or organic ammonium
- the present invention relates to a skin cleanser composition containing
- an Example it is a figure which shows the apparatus and measuring method for measuring the friction behavior at the time of a rinse when using the skin cleansing composition of this invention. It is a figure which shows the friction behavior at the time of a rinse when the skin cleansing composition of Example 1 is used. It is a figure which shows the friction behavior at the time of a rinse when the skin cleansing composition of the comparative example 2 is used. It is a figure which shows the friction behavior at the time of a rinse when the skin cleansing composition of the comparative example 8 is used. It is a figure which shows the friction behavior at the time of a rinse when the skin cleansing composition of the comparative example 6 is used. It is a figure which shows the friction behavior in the case of slimming at the time of a rinse.
- the cleaning compositions of Patent Documents 1 to 4 also have a feeling that the cleaning agent remains on the skin in order to give a feeling that the amount of foam is small and that it is slimy during rinsing.
- the present invention relates to a skin cleanser composition that is excellent in the amount of foam and foam quality, and that provides a good rinsing feeling with reduced residual feeling.
- the present inventors have used a combination of an alkyl ether carboxylic acid having a specific distribution or a salt thereof and a specific fatty acid salt in combination, and has a good rinsing feeling with excellent foam amount and foam quality and reduced residual feeling. It was found that an agent composition was obtained.
- the skin cleanser composition of the present invention is excellent in the amount of foam and the quality of foam, and can provide a good rinsing feeling with reduced residual feeling. More specifically, in the case of rinsing by repeatedly rubbing foam and detergent from the skin with water while rinsing, the touch does not get slimy, and when the hand is rubbing over the skin, it will be caught. It is rare to feel squeaky (squeak is too strong). That is, a characteristic rinsing feeling is obtained in which the hand slips smoothly when rubbing on the skin, but feels a stop and feels a stop when reciprocating.
- the alkyl ether carboxylic acid of component (A) or a salt thereof used in the present invention is represented by the general formula (1).
- R 1 is an alkyl group having 4 to 22 carbon atoms, preferably an alkyl group having 6 to 20 carbon atoms, more preferably an alkyl group having 8 to 18 carbon atoms, and more preferably an alkyl group having 8 to 16 carbon atoms.
- An alkyl group of several tens to 16 is more preferable.
- the alkyl chain of R 1 may be either a straight chain or a branched chain, but a straight chain alkyl group is preferable from the viewpoint of foamability.
- the average carbon number of R 1 is 10.8 to 12.8, preferably 10.8 to 12.5, more preferably 12.1 to 12.4.
- R 1 preferably contains two or more alkyl groups, and the component having the largest alkyl chain length is preferably 55% by mass or more and less than 97% by mass, and 60 to 95% by mass. More preferably, it is more preferably 70 to 95% by mass since the foam amount and foam quality are excellent.
- n represents a number from 0 to 20, and more preferably from 0 to 12.
- n represents the number of moles of ethylene oxidoxide added, and the average number of moles added (average value of n) in the composition of component (A) is 1.5 to 10 from the viewpoint of good foam quality.
- M represents a hydrogen atom; an alkali metal such as sodium or potassium; an alkaline earth metal such as calcium or magnesium; an ammonium; an ammonium derived from an alkanolamine such as monoethanolamine, diethanolamine or triethanolamine; Can be mentioned.
- an alkali metal is preferable and potassium is more preferable from the viewpoint of foamability, low-temperature stability, and lack of coloration over time.
- R 1 is an alkyl group having 4 to 22 carbon atoms, and the average carbon number of R 1 is 10.8 to 12.8.
- the component having a large alkyl chain length is 55% by mass or more and less than 97% by mass
- n represents a number of 0 to 20
- the average value is 1.5 to 10
- the component of n 0
- M is preferably a hydrogen atom, sodium, potassium, or ammonium, and more preferably potassium.
- R 1 is an alkyl group having 6 to 20 carbon atoms, and the average carbon number of R 1 is 10.8 to 12.8.
- the component having a large alkyl chain length is 55% by mass or more and less than 97% by mass
- n represents a number of 0 to 20
- the average value is 2.5 to 4.5
- n 0
- M is preferably a hydrogen atom, sodium, potassium, or ammonium, and more preferably potassium.
- R 1 is an alkyl group having 8 to 18 carbon atoms, and the average carbon number of R 1 is 10.8 to 12.8.
- the component having a large alkyl chain length is 55% by mass or more and less than 97% by mass
- n represents a number of 0 to 20
- the average value is 2.5 to 3.4
- n 0
- M is preferably a hydrogen atom, sodium, potassium, or ammonium, and more preferably potassium.
- R 1 is an alkyl group having 8 to 16 carbon atoms, and the average carbon number of R 1 is 10.8 to 12.5.
- the component having a large alkyl chain length is 60 to 95% by mass
- n represents a number of 0 to 20
- the average value is 2.8 to 3.4
- M is preferably a hydrogen atom, sodium, potassium, or ammonium ammonium, and more preferably potassium. By doing in this way, foam amount and foam quality can be improved.
- R 1 is an alkyl group having 10 to 16 carbon atoms, and the average carbon number of R 1 is 12.1 to 12.4.
- the component having a large alkyl chain length is 60 to 95% by mass
- n represents a number of 0 to 20
- the average value is 2.8 to 3.1
- M is preferably a hydrogen atom, sodium, potassium, or ammonium, and more preferably potassium.
- the mass ratio of the components 3 and 4 is determined as follows by analyzing the alkyl ether carboxylic acid represented by the general formula (1) by gas chromatography.
- the alkyl ether carboxylic acid of component (A) or a salt thereof has the composition as described above, and the content is 0.1% by mass or more in the total composition from the viewpoint of rinsing feeling, 0.5% It is preferably at least mass%, more preferably at least 1 mass%, at most 30 mass%, preferably at most 0.5 mass%, more preferably at most 15 mass%.
- the content of component (A) is from 0.1 to 30% by mass, preferably from 0.5 to 20% by mass, more preferably from 1 to 15% by mass, based on the total composition.
- the fatty acid salt of the component (B) used in the present invention is represented by the general formula (2).
- R 2 is an alkyl group or alkenyl group having 9 to 21 carbon atoms, which may be linear or branched, and is preferably an alkyl group having 11 to 17 carbon atoms.
- Y represents potassium or alkanolamine. Examples of the alkanolamine include monoethanolamine, diethanolamine, and triethanolamine. Of these, potassium is more preferred.
- the component (B) can be used alone or in combination of two or more, and the content is 0.1% by mass or more and 0.5% by mass in the total composition in terms of foamability and foam amount.
- the above is preferable, 1% by mass or more is more preferable, 30% by mass or less, 20% by mass or less is preferable, and 15% by mass or less is more preferable.
- the content of component (B) is 0.1 to 30% by mass, preferably 0.5 to 20% by mass, and more preferably 1 to 15% by mass in the total composition.
- the total content of components (A) and (B) is preferably 0.5% by mass or more, and preferably 1% by mass or more in the total composition from the viewpoint of ease of handling. More preferably, 5% by mass or more is more preferable, 40% by mass or less is preferable, 30% by mass or less is more preferable, and 15% by mass or less is more preferable.
- the total content of components (A) and (B) is preferably 0.5 to 40% by mass, more preferably 1 to 30% by mass, and still more preferably 5 to 15% by mass in the total composition.
- the neutralization degree of the components (A) and (B) is preferably greater than 0.9 equivalent, preferably 1.2 equivalent or less, and more preferably 1 equivalent or less.
- these components (A) and (B) are used in combination, so that the amount of foam and the foam quality are excellent, and when rinsing, the hand slips smoothly when the hand is sliding on the skin. A good rinsing feeling with a reduced residual feeling can be obtained.
- the cleaning composition of the present invention can further contain (C) an amphoteric surfactant and can further improve the foam amount and the foam quality without affecting the rinsing feeling.
- component (C) examples include betaine acetate surfactants such as lauryldimethylaminoacetate betaine, amine oxide surfactants such as lauryldimethylamine oxide, 2-alkyl-N-carboxymethyl-N-hydroxyethylimidazo.
- betaine acetate surfactants such as lauryldimethylaminoacetate betaine
- amine oxide surfactants such as lauryldimethylamine oxide
- 2-alkyl-N-carboxymethyl-N-hydroxyethylimidazo examples include imidazolinium betaine-type surfactants such as linium betaine, amide betaine-type surfactants such as amidepropylbetaine laurate, and sulfobetaine-type surfactants such as lauryl hydroxysulfobetaine.
- lauric acid amidopropyl betaine and lauryl hydroxysulfobetaine are preferable from the viewpoint of foamability.
- Component (C) can be used alone or in combination of two or more, and the content is preferably 0.1% by mass or more, more preferably 0.5% by mass or more in the total composition from the viewpoint of foamability.
- 1% by mass or more is more preferable, 30% by mass or less is preferable, 20% by mass or less is more preferable, and 15% by mass or less is more preferable.
- the content of the component (C) is preferably 0.1 to 30% by mass, more preferably 0.5 to 20% by mass, and further preferably 1 to 15% by mass in the total composition.
- the skin cleansing composition of the present invention can further contain water as a solvent.
- the water content is preferably from 10 to 94.5% by mass, more preferably from 15 to 90% by mass, based on the total composition, and the remainder of the above components and other components constituting the cleaning composition.
- R 1 represents an alkyl group having 4 to 22 carbon atoms, n represents a number of 0 to 20, and M represents a hydrogen atom, an alkali metal, an alkaline earth metal, ammonium or organic ammonium
- R 1 has an average carbon number of 10.8 to 12.8, and n has an average value of 2.5 to 3.4, preferably 2 0.8 to 3.4
- R 1 represents an alkyl group having 4 to 22 carbon atoms, n represents a number of 0 to 20, and M represents a hydrogen atom, an alkali metal, an alkaline earth metal, ammonium or organic ammonium
- the skin cleanser composition of the present invention further comprises components used in ordinary cleansing agents, such as surfactants, moisturizers, oily components, bactericides, anti-inflammatory agents, antiseptics, chelating agents, potentiators other than those described above. It can contain a sticking agent, salts, pearling agent, scrub agent, fragrance, cooling agent, pigment, ultraviolet absorber, antioxidant, plant extract and the like.
- the skin cleansing composition of the present invention is produced by mixing the compounding ingredients by a usual method.
- the resulting detergent composition may be either liquid or solid, but is preferably a liquid aqueous preparation.
- the viscosity is preferably 200 to 80,000 mPa ⁇ s when measured with a B-type viscometer (manufactured by Tokyo Keiki Co., Ltd.) at 25 ° C., and can be adjusted by appropriately selecting the blending components. Can do.
- the pH is preferably 3 to 12, more preferably 5 to 10.5.
- the measurement of pH is a value obtained by diluting each cleaning composition 20 times with ion-exchanged water at 25 ° C.
- the skin cleansing composition of the present invention is suitable as a facial cleanser, body soap, hand soap, and the like.
- the method for washing the skin using the skin cleansing composition of the present invention is, for example, as follows. That is, the skin cleansing composition of the present invention is a method of applying an appropriate amount to the body, that is, the body skin such as the face, limbs, and torso, washing with foaming, and then rinsing using warm water such as a shower. It can also be washed by lathering using a cleaning aid such as a towel, sponge or brush.
- the present invention further discloses the following composition, production method, or application.
- R 1 represents an alkyl group having 4 to 22 carbon atoms, n represents a number of 0 to 20, and M represents a hydrogen atom, an alkali metal, an alkaline earth metal, ammonium or organic ammonium
- a skin cleanser composition comprising:
- R 1 is an alkyl group having 6 to 20 carbon atoms, more preferably an alkyl group having 8 to 18 carbon atoms
- the average carbon number of R 1 is preferably 10.8 to 12.5, and more preferably 12.1 to 12.4 > Or ⁇ 2>.
- the average value of n is preferably 2.8 to 3.4, more preferably 2.8 to 3.1.
- R 1 is an alkyl group having 8 to 18 carbon atoms
- an average value of n is 2.5 to 3.4
- n The skin cleanser composition according to any one of the above ⁇ 1> to ⁇ 6>, comprising 9.9 to 27% by mass of 0 component.
- R 1 is an alkyl group having 8 to 16 carbon atoms
- an average value of n is 2.8 to 3.4
- the skin cleanser composition according to any one of ⁇ 1> to ⁇ 8>, wherein 76 to 3.00: 0.63 to 1.52.
- component (A) preferably, in general formula (1), R 1 contains two or more alkyl groups, and the component having the largest alkyl chain length is 55% by mass or more and 97% by mass.
- the skin cleanser composition according to any one of ⁇ 1> to ⁇ 9>, wherein the composition is less than 1%, more preferably 60 to 95% by mass, and still more preferably 70 to 95% by mass.
- the skin cleansing composition as described.
- the total content of the components (A) and (B) is preferably 0.5 to 40% by mass in the total composition, more preferably 1 to 30% by mass, and 5 to 15% by mass.
- Component (C) amphoteric surfactant is preferably a betaine acetate type surfactant, a network oxide type surfactant, an imidazolinium betaine type surfactant, an amide betaine type surfactant, or a sulfobetaine.
- the content of ⁇ 20> component (C) is preferably 0.1 to 30% by mass in the total composition, more preferably 0.5 to 20% by mass, and further preferably 1 to 15% by mass.
- component (C) is preferably 0.1 to 30% by mass in the total composition, more preferably 0.5 to 20% by mass, and further preferably 1 to 15% by mass.
- ⁇ 22> The skin according to any one of ⁇ 1> to ⁇ 21>, wherein the content of water is preferably 10 to 94.5% by mass in the total composition, and more preferably 15 to 90% by mass Cleaning composition.
- the degree of neutralization of the components (A) and (B) is preferably greater than 0.9 equivalent, preferably 1.2 equivalent or less, and more preferably 1 equivalent or less.
- ⁇ 24> A method for rinsing the skin after applying the skin cleansing composition according to any one of ⁇ 1> to ⁇ 23> above to the skin and washing the skin.
- ⁇ 25> Use of the composition according to any one of ⁇ 1> to ⁇ 23> as a skin cleanser.
- the alkyl composition, EO addition molar distribution, and ratio of each component of the alkyl ether carboxylic acid were measured by gas chromatography (GC) by the following analytical method. Unless otherwise indicated, “%” indicates mass%.
- Example pretreatment method 150 mg of alkyl ether carboxylic acid was dissolved in 50 mL of methanol. Moreover, about the cleaning composition, it extract
- a strong anionic surfactant such as polyoxyethylene alkyl ether sulfate
- the diazomethane-ether solution was prepared by the following procedure using a diazomethane generator (GM-50, manufactured by Miyamoto Riken Kogyo).
- GM-50 manufactured by Miyamoto Riken Kogyo
- the first and second receivers, and the second and third receivers are connected with a silicone rubber stopper and a Teflon (registered trademark) tube.
- a second receiver In a second receiver, 0.8 g of N-methyl-N′-nitro-N-nitrosoguanidine was collected and 2.5 mL of ion exchange water was added.
- a third receiver 10 mL of t-butyl methyl ether was collected. The first, second and third receivers were ice-cooled. Subsequently, a plastic syringe was attached to the second receiver, and 3 mL of a solution in which 20 g of sodium hydroxide was dissolved in 100 mL of ion-exchanged water was placed in this syringe.
- This sodium hydroxide aqueous solution is slowly dropped to produce diazomethane gas, and nitrogen gas is gently blown from the first receiver side, dissolved in t-butyl methyl ether of the third receiver, and diazomethane-ether A solution was obtained.
- the following reagents were used in the sample pretreatment.
- Methanol manufactured by Kanto Chemical, for high performance liquid chromatography, 25183-1B
- Formic acid manufactured by Wako Pure Chemical Industries, reagent special grade, 066-00461
- Chloroform manufactured by Kanto Chemical, deer grade 1, 07278-01
- N-methyl-N'-nitro-N-nitrosoguanidine manufactured by Kanto Chemical, deer grade 1, 25596-51
- t-Butyl methyl ether manufactured by Kanto Chemical, deer special grade, 04418-00
- Sodium hydroxide (Wako Pure Chemical Industries, special grade, 196-13761)
- the alkyl ether carboxylic acid of component (A) used in the skin cleansing composition of the present invention is obtained by reacting one or more alcohols selected from alcohols having an alkyl group having 4 to 22 carbon atoms with ethylene oxide.
- An alkyl ethoxylate is obtained and further reacted to obtain an alkyl ether carboxylic acid.
- it can be produced as follows. Unless otherwise indicated, “%” indicates mass%.
- M H
- average carbon number is 12.3
- Production Example 4 Following Production Example 1, EO was reacted with decyl alcohol as a raw material to obtain an alkyl ethoxylate having 3.55 moles of EO added. This was subjected to an oxidation reaction as in Example 1, and the resulting alkyl ether carboxylate was treated with hydrochloric acid to obtain an alkyl ether carboxylic acid.
- M H
- R 1 is a decyl group
- n 3.1
- Production Example 5 Following Production Example 1, EO was reacted with lauryl alcohol as a raw material to obtain an alkyl ethoxylate having 3.55 moles of EO added. This was subjected to an oxidation reaction as in Example 1, and the resulting alkyl ether carboxylate was treated with hydrochloric acid to obtain an alkyl ether carboxylic acid.
- M H
- R 1 is a lauryl group
- n 3.1
- Production Example 6 According to Production Example 1, EO was reacted with myristyl alcohol as a raw material to obtain an alkyl ethoxylate having 3.55 moles of EO added. This was subjected to an oxidation reaction as in Example 1, and the resulting alkyl ether carboxylate was treated with hydrochloric acid to obtain an alkyl ether carboxylic acid.
- M H
- R 1 is a myristyl group
- n has an average value of 3.1
- n 0 contains 16% by mass
- Production Example 7 Following Production Example 1, EO was added to a raw material in which lauryl alcohol and cetyl alcohol were mixed at a mass ratio of 20/80 to obtain an alkyl ethoxylate having 3.55 moles of EO added. This was subjected to an oxidation reaction as in Example 1, and the resulting alkyl ether carboxylate was treated with hydrochloric acid to obtain an alkyl ether carboxylic acid.
- M H
- n has an average value of 3.1
- n 0 is 16% by mass.
- Production Example 8 Following Production Example 1, lauryl alcohol was subjected to EO reaction as a raw material to obtain an alkyl ethoxylate having 4.05 moles of EO added. This was subjected to an oxidation reaction as in Production Example 1, and the resulting alkyl ether carboxylate was treated with hydrochloric acid to obtain an alkyl ether carboxylic acid.
- M H
- average carbon number 12.1
- n average value is 3.1
- Examples 1 to 39, Comparative Examples 1 to 14 Skin cleansing compositions having the compositions shown in Tables 3 to 6 were produced, and the amount of foam, foam quality and rinsing feeling were evaluated. The results are also shown in Tables 3-6.
- the structure of the component (A) used in the Examples is as shown in Tables 1 and 2.
- commercially available alkyl ether carboxylic acids AKYPO RLM25 (manufactured by Kao Corporation), AKYPO RLM45 (manufactured by Kao Corporation), AKYPO RLM100NV (manufactured by Kao Corporation), and Viewlite LCA (manufactured by Sanyo Chemical Co., Ltd.) used in Examples and Comparative Examples.
- ECTD-3NEX manufactured by Nikko Chemical Co., Ltd.
- averaged EO addition mole number was based on the catalog value of each company vendor and the value published on the website.
- the obtained solutions of components (A) and (B) and, if necessary, component (C) were mixed so as to have the compositions shown in Tables 3 to 6, and further water was added to adjust to a predetermined concentration. After cooling to 15 ° C. to 35 ° C., a skin cleansing composition was obtained.
- Foam amount After the foaming net (manufactured by Daisansha Hakugen Group) was moistened with water (about 8 g), 1 g of each skin cleansing composition was placed on the foaming net. Wrapped the whipped net with both palms and whipped it like a circle. The palms were rubbed together to draw a circle 40 times and foamed. Bubbles made with a whipping net are collected in a 500 mL beaker (IWAKI, diameter 8.5 cm, height 15 cm), the beaker is shaken to close the bubbles, and the height of the bubbles collected using a ruler is collected. Measure (cm). From the obtained height and the bottom area of the beaker, the volume (cm 3 ) of the collected bubbles was calculated to determine the amount of bubbles.
- Foam quality One expert panelist sensory-evaluated the foam raised in (1) according to the following criteria. A: Foam is very soft and fine. B: Bubbles are soft and fine. C: Foam is hard and difficult to wash. D: Bubbly foam, difficult to clean.
- Rinse feeling The foam made in (1) was spread over the entire forearm, rubbed 10 times, washed with foam, and then rinsed with tap water (about 30 ° C.).
- B A good rinsing feeling that slips smoothly when the hand is sliding on the skin, but feels caught when reciprocating, and does not become slimy and has no residual feeling.
- C Strong squeaky feeling, residual feeling, and poor rinsing feeling.
- D It is slimy, has a residual feeling, and is not easily rinsed.
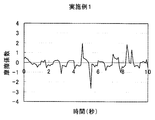
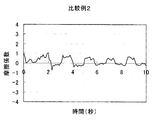
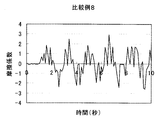
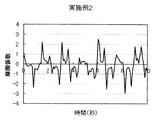
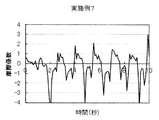
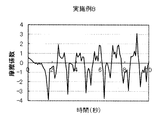
- Test Example 1 (Friction behavior during rinsing) 1 g of each cleaning composition was taken on the forearm wetted with water, washed by reciprocating 10 times with the palm of the other hand, and then rinsed with tap water (about 30 ° C.). The frictional behavior of the wet forearm at that time was measured with a portable tactile meter (manufactured by Shinto Kagaku) as follows (see FIG. 1). (1) Stand perpendicular to the forearm with the probe on its back, (2) Slide the probe from the elbow side of the forearm to the wrist and fold it in front of the wrist. At this time, the speed is adjusted so as to move about 20 cm from the elbow to the wrist in 2 seconds, and further, the pressing pressure is adjusted to about 100 N. (3) (2) was repeated for 10 seconds, and the coefficient of friction was calculated from the resulting frictional force and graphed. The results of Example 1 and Comparative Examples 2, 8 and 6 are shown in FIGS.
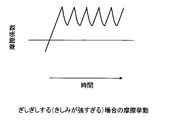
- FIG. 6 shows a schematic diagram of the frictional behavior when slimming during rinsing. In this case, a clear static friction is not seen when sliding begins, and the coefficient of friction becomes almost constant when sliding. If the behavior is close to this, when you are rinsing (rubbing the skin with your hand and flushing with water), you won't feel a sense of stopping all the time, so you can't feel that the cleaning composition has fallen, and the cleaning composition on the skin Reminiscent of the remaining feeling.
- FIG. 7 shows a schematic diagram of the frictional behavior when squeezing during rinsing (squeaking is too strong).
- FIG. 8 shows a schematic diagram of the friction behavior in the case of a good rinsing feeling with reduced residual feeling during rinsing. In this case, a clear static friction is seen when it starts to slide, and furthermore, the coefficient of friction becomes almost constant when it slides. When a behavior close to this is seen, a feeling of stop is felt when rinsing, and further, when the hand is sliding, it does not get caught.
- FIG. 2 shows the friction behavior during rinsing in Example 1.
- A A good rinsing feeling without slippery and slippery when the hand is sliding on the skin, but feels caught when reciprocating.” It was evaluated.
- FIG. 3 shows the friction behavior at the time of rinsing in Comparative Example 2. This result is close to FIG. 6 and no clear static friction is observed.
- sensory evaluation by a specialized panelist was also evaluated as “D; Further, in FIGS.
- Test example 2 (friction behavior during rinsing) In the same manner as in Test Example 1, the frictional behavior during rinsing was measured for the cleaning compositions of Examples 2 to 8. The results are shown in FIGS. From the results of FIGS. 9 to 15, it was confirmed that the cleaning compositions of Examples 2 to 8 can also have a characteristic rinsing feeling (rinsing feeling with reduced residual feeling) as in Example 1. .
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Birds (AREA)
- Epidemiology (AREA)
- Emergency Medicine (AREA)
- Dermatology (AREA)
- Cosmetics (AREA)
- Detergent Compositions (AREA)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP13755570.2A EP2821054B1 (en) | 2012-03-02 | 2013-03-01 | Skin cleansing composition |
| CN201380012254.9A CN104159563B (zh) | 2012-03-02 | 2013-03-01 | 皮肤清洁剂组合物 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2012-047079 | 2012-03-02 | ||
| JP2012047079 | 2012-03-02 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013129652A1 true WO2013129652A1 (ja) | 2013-09-06 |
Family
ID=49082839
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2013/055686 Ceased WO2013129652A1 (ja) | 2012-03-02 | 2013-03-01 | 皮膚洗浄剤組成物 |
Country Status (6)
| Country | Link |
|---|---|
| EP (1) | EP2821054B1 (enExample) |
| JP (1) | JP5399576B2 (enExample) |
| CN (1) | CN104159563B (enExample) |
| MY (1) | MY167793A (enExample) |
| TW (1) | TWI481418B (enExample) |
| WO (1) | WO2013129652A1 (enExample) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2015196713A (ja) * | 2014-03-31 | 2015-11-09 | ミヨシ油脂株式会社 | 透明液体洗浄剤組成物 |
| WO2018190264A1 (ja) * | 2017-04-10 | 2018-10-18 | 花王株式会社 | 皮膚洗浄剤組成物 |
| JP2018177781A (ja) * | 2017-04-10 | 2018-11-15 | 花王株式会社 | 皮膚洗浄剤組成物 |
| JP2018177780A (ja) * | 2017-04-10 | 2018-11-15 | 花王株式会社 | 皮膚洗浄剤組成物 |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN119497606B (zh) * | 2022-07-08 | 2025-11-14 | 三洋化成工业株式会社 | 表面活性剂组合物和清洗剂 |
Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6121199A (ja) | 1984-07-10 | 1986-01-29 | 日本サ−フアクタント工業株式会社 | 化粧用洗浄剤 |
| JPS6197395A (ja) * | 1984-10-12 | 1986-05-15 | ユニリーバー・ナームローゼ・ベンノートシヤープ | 液体洗剤組成物 |
| JPH02175799A (ja) | 1988-04-30 | 1990-07-09 | Sanyo Chem Ind Ltd | 洗浄剤組成物 |
| JPH05112795A (ja) * | 1991-10-22 | 1993-05-07 | Kanebo Ltd | 洗浄剤組成物 |
| JPH0625695A (ja) * | 1991-12-13 | 1994-02-01 | Kanebo Ltd | 洗浄剤組成物 |
| JP2001207189A (ja) | 1999-11-19 | 2001-07-31 | Sanyo Chem Ind Ltd | アニオン性界面活性剤ならびにそれを含有する洗浄剤および水溶性潤滑剤組成物 |
| JP2005112946A (ja) * | 2003-10-06 | 2005-04-28 | Kao Corp | 洗浄剤組成物 |
| JP2008285479A (ja) | 2007-04-20 | 2008-11-27 | Kao Corp | 皮膚洗浄剤組成物 |
| JP2011140485A (ja) * | 2009-12-08 | 2011-07-21 | Kao Corp | 皮膚洗浄剤 |
| JP2011178681A (ja) * | 2010-02-26 | 2011-09-15 | Kao Corp | 皮膚洗浄剤組成物 |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TWI434703B (zh) * | 2007-05-14 | 2014-04-21 | Kao Corp | 皮膚洗淨劑組成物 |
| WO2012029950A1 (ja) * | 2010-09-03 | 2012-03-08 | 花王株式会社 | 洗浄剤組成物 |
| JP5308492B2 (ja) * | 2011-09-02 | 2013-10-09 | 花王株式会社 | 皮膚洗浄剤組成物 |
| MY170616A (en) * | 2011-12-08 | 2019-08-21 | Kao Corp | Skin cleansing composition |
| JP5399577B2 (ja) * | 2012-03-02 | 2014-01-29 | 花王株式会社 | 皮膚洗浄剤組成物 |
-
2013
- 2013-03-01 EP EP13755570.2A patent/EP2821054B1/en not_active Not-in-force
- 2013-03-01 CN CN201380012254.9A patent/CN104159563B/zh active Active
- 2013-03-01 TW TW102107444A patent/TWI481418B/zh active
- 2013-03-01 JP JP2013041028A patent/JP5399576B2/ja active Active
- 2013-03-01 WO PCT/JP2013/055686 patent/WO2013129652A1/ja not_active Ceased
- 2013-03-01 MY MYPI2014001982A patent/MY167793A/en unknown
Patent Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6121199A (ja) | 1984-07-10 | 1986-01-29 | 日本サ−フアクタント工業株式会社 | 化粧用洗浄剤 |
| JPS6197395A (ja) * | 1984-10-12 | 1986-05-15 | ユニリーバー・ナームローゼ・ベンノートシヤープ | 液体洗剤組成物 |
| JPH02175799A (ja) | 1988-04-30 | 1990-07-09 | Sanyo Chem Ind Ltd | 洗浄剤組成物 |
| JPH05112795A (ja) * | 1991-10-22 | 1993-05-07 | Kanebo Ltd | 洗浄剤組成物 |
| JPH0625695A (ja) * | 1991-12-13 | 1994-02-01 | Kanebo Ltd | 洗浄剤組成物 |
| JP2001207189A (ja) | 1999-11-19 | 2001-07-31 | Sanyo Chem Ind Ltd | アニオン性界面活性剤ならびにそれを含有する洗浄剤および水溶性潤滑剤組成物 |
| JP2005112946A (ja) * | 2003-10-06 | 2005-04-28 | Kao Corp | 洗浄剤組成物 |
| JP2008285479A (ja) | 2007-04-20 | 2008-11-27 | Kao Corp | 皮膚洗浄剤組成物 |
| JP2011140485A (ja) * | 2009-12-08 | 2011-07-21 | Kao Corp | 皮膚洗浄剤 |
| JP2011178681A (ja) * | 2010-02-26 | 2011-09-15 | Kao Corp | 皮膚洗浄剤組成物 |
Non-Patent Citations (1)
| Title |
|---|
| NIKKO CHEMICALS CO., LTD. ET AL.: "Keshohin Handbook", 1 November 1996, pages: 163 - 165, XP008174613 * |
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2015196713A (ja) * | 2014-03-31 | 2015-11-09 | ミヨシ油脂株式会社 | 透明液体洗浄剤組成物 |
| WO2018190264A1 (ja) * | 2017-04-10 | 2018-10-18 | 花王株式会社 | 皮膚洗浄剤組成物 |
| JP2018177781A (ja) * | 2017-04-10 | 2018-11-15 | 花王株式会社 | 皮膚洗浄剤組成物 |
| JP2018177779A (ja) * | 2017-04-10 | 2018-11-15 | 花王株式会社 | 皮膚洗浄剤組成物 |
| JP2018177780A (ja) * | 2017-04-10 | 2018-11-15 | 花王株式会社 | 皮膚洗浄剤組成物 |
| US11406849B2 (en) | 2017-04-10 | 2022-08-09 | Kao Corporation | Amino alcohol-containing skin cleansing composition for removing keratotic plugs from skin |
| JP7137338B2 (ja) | 2017-04-10 | 2022-09-14 | 花王株式会社 | 皮膚洗浄剤組成物 |
| JP7137339B2 (ja) | 2017-04-10 | 2022-09-14 | 花王株式会社 | 皮膚洗浄剤組成物 |
| JP7137340B2 (ja) | 2017-04-10 | 2022-09-14 | 花王株式会社 | 皮膚洗浄剤組成物 |
| US11633335B2 (en) | 2017-04-10 | 2023-04-25 | Kao Corporation | Skin cleansing composition |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2013209370A (ja) | 2013-10-10 |
| EP2821054A1 (en) | 2015-01-07 |
| TW201343188A (zh) | 2013-11-01 |
| CN104159563B (zh) | 2016-08-24 |
| EP2821054B1 (en) | 2018-01-31 |
| CN104159563A (zh) | 2014-11-19 |
| EP2821054A4 (en) | 2015-09-16 |
| JP5399576B2 (ja) | 2014-01-29 |
| TWI481418B (zh) | 2015-04-21 |
| MY167793A (en) | 2018-09-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5308492B2 (ja) | 皮膚洗浄剤組成物 | |
| JP6445089B2 (ja) | 洗浄剤組成物 | |
| JP5399577B2 (ja) | 皮膚洗浄剤組成物 | |
| JP5399576B2 (ja) | 皮膚洗浄剤組成物 | |
| JP5308491B2 (ja) | 皮膚洗浄剤組成物 | |
| JP5809517B2 (ja) | 皮膚洗浄剤組成物 | |
| JP5819700B2 (ja) | 皮膚洗浄剤組成物 | |
| JP5917202B2 (ja) | 皮膚洗浄剤組成物 | |
| JP5891074B2 (ja) | 洗浄剤組成物の製造方法 | |
| WO2013133328A1 (ja) | 皮膚洗浄剤組成物 | |
| JP2014091815A (ja) | 洗浄剤組成物 | |
| JP2017149952A (ja) | 洗浄剤組成物 | |
| JP5809519B2 (ja) | 皮膚洗浄剤組成物 | |
| JP5830343B2 (ja) | 皮膚洗浄剤組成物 | |
| JP2015124214A (ja) | 皮膚洗浄剤組成物 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 13755570 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2013755570 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: IDP00201405194 Country of ref document: ID |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |