WO2012115238A1 - 尿臭発生部の視覚的判定方法 - Google Patents
尿臭発生部の視覚的判定方法 Download PDFInfo
- Publication number
- WO2012115238A1 WO2012115238A1 PCT/JP2012/054609 JP2012054609W WO2012115238A1 WO 2012115238 A1 WO2012115238 A1 WO 2012115238A1 JP 2012054609 W JP2012054609 W JP 2012054609W WO 2012115238 A1 WO2012115238 A1 WO 2012115238A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- urine
- urine odor
- liquid agent
- color
- detection reagent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N21/00—Investigating or analysing materials by the use of optical means, i.e. using sub-millimetre waves, infrared, visible or ultraviolet light
- G01N21/75—Systems in which material is subjected to a chemical reaction, the progress or the result of the reaction being investigated
- G01N21/77—Systems in which material is subjected to a chemical reaction, the progress or the result of the reaction being investigated by observing the effect on a chemical indicator
- G01N21/78—Systems in which material is subjected to a chemical reaction, the progress or the result of the reaction being investigated by observing the effect on a chemical indicator producing a change of colour
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/34—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving hydrolase
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/34—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving hydrolase
- C12Q1/44—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving hydrolase involving esterase
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N21/00—Investigating or analysing materials by the use of optical means, i.e. using sub-millimetre waves, infrared, visible or ultraviolet light
- G01N21/62—Systems in which the material investigated is excited whereby it emits light or causes a change in wavelength of the incident light
- G01N21/63—Systems in which the material investigated is excited whereby it emits light or causes a change in wavelength of the incident light optically excited
- G01N21/64—Fluorescence; Phosphorescence
- G01N21/6428—Measuring fluorescence of fluorescent products of reactions or of fluorochrome labelled reactive substances, e.g. measuring quenching effects, using measuring "optrodes"
Definitions
- the present invention relates to a simple method for determining the urine odor generating part and the urine odor intensity, and further to an absorbent article excellent in urine odor production suppressing effect, a water-absorbing polymer, and a urine odor production inhibitor screening method to which this method is applied.
- the odor derived from urine has a particularly great discomfort given to humans among the bad odors of living environment.
- urine that remains on the outside of the toilet as a droplet may remain on the spot for a long time because it is difficult to visually confirm the presence of the urine, which may be a source of malodor.
- sanitary products such as underwear, diapers, and sanitary products may exist in the living environment for a certain period of time with urine attached, and can be a source of malodor originating from urine.
- the odor of urine immediately after urination is usually very weak, and most of the malodorous components derived from urine are generated with the passage of time due to decomposition of organic matter in urine by microbial enzymes. It is believed that. Deodorization and deodorization technology against such microorganism-derived malodors includes not only deodorants that physically or chemically remove malodorous components or perfumes that mask malodors, but also the generation of malodors from the beginning. It is proposed to use antibacterial agents against odor-causing microorganisms and enzyme inhibitors against causative enzymes (urease, ⁇ -glucuronidase, arylsulfatase, etc.).
- Patent Document 1 discloses sanitary products such as diapers containing an indicator sensitive to odors such as acid-base indicators. .
- Patent Document 2 discloses a technique for detecting the presence of microorganisms by detecting volatile components derived from bacteria by GC analysis or an indicator. According to this, for example, the presence of E. coli is confirmed by exposing cotton dyed with dimethylaminocinnamic aldehyde to a suspension of E. coli and discoloring the indicator by the indole produced by the cells.
- Patent Document 3 proposes a method for detecting bacteria associated with urinary tract infections including Escherichia coli using a medium containing a reagent for detecting substrate-type enzyme activity of ⁇ -glucuronidase or arylsulfatase.
- the specimen is added to the above-mentioned medium and cultured, and microorganisms are detected by visually observing the colony derived from the bacterial cell enzyme and / or the color of the medium.
- the present invention is a first step of applying a liquid agent containing component A to articles, equipment or materials that are subject to urine odor determination; A second step in which an enzymatic reaction is allowed to proceed with an article, facility or material to which the liquid is applied; Having a third step of observing a color change of the article, equipment or material to which the liquid is applied;
- the present invention provides a urine odor generating part that determines a color-changed area as a urine odor generating part, and associates the color change amount with the urine odor intensity, and a visual determination method of the urine odor intensity.
- Component A Enzyme substrate type enzyme activity detection reagent that produces a dye compound or a fluorescent dye compound that is hardly soluble in water by an enzyme reaction
- the present invention provides a urine for selecting an evaluation object with a small change in the color of the evaluation object due to an enzyme reaction by bringing a liquid agent containing component A into contact with a material, member or agent as an evaluation object of the urine odor production inhibitory effect.
- the present invention provides a material, a member, a urine odor generation inhibitor, or a member screening method that is excellent in the effect of suppressing odor generation.
- Component A Enzyme substrate type enzyme activity detection reagent that produces a dye compound or a fluorescent dye compound that is hardly soluble in water by an enzyme reaction
- FIG. 1 is a schematic diagram showing a micro test tube used in Example 1.
- FIG. 3 is a diagram illustrating a coloring state after a test in Example 1. It is a figure which shows the slice of the urine collection pad used in Example 2.
- FIG. It is a graph which shows the correlation of the odor intensity
- 3 is an image of a urine absorption pad section using the water absorbent polymer B in Example 2.
- FIG. It is a figure which shows the image of each sample in Example 3, and an odor intensity
- FIG. 3 is a diagram illustrating a coloring state after a test in Example 1. It is a figure which shows the slice of the urine collection pad used
- the use of various agents such as deodorants, masking agents, antibacterial agents, enzyme inhibitors, etc. can be considered as deodorizing / deodorizing technology for urine odor.
- the position information of the urine odor generating part is very important.
- deodorizers such as antibacterial agents and enzyme inhibitors may be significantly less effective when the application site of the agent is removed from the odor generating part, and it can be said that application to the odor generating part is essential.
- the method for determining the urine odor generation part and the urine odor intensity is a technique that is strongly desired for optimizing the mixing part and the mixing amount of the deodorant / deodorant.
- the method of detecting the odor component itself with an indicator or GC analysis as in Patent Documents 1 and 2 specifies the odor generation part with high spatial resolution. Is very difficult.
- the method based on the culture in the medium as in Patent Document 3 can specify the odor generating part as the growth site of the microorganism, but it is necessary to finely divide the sample according to the required spatial resolution. Therefore, the operation becomes complicated, and it is difficult to specify the urine odor generating part in the same system as the product. Furthermore, since the observed enzyme activity is also expressed in the medium, there is no speculation about the relationship between the obtained results and the odor intensity.
- the present invention relates to a method for easily specifying the position of a urine odor generating portion that occurs with the passage of time from a subject to which urine has adhered, and has conducted extensive research on a method for visualizing positional information of the urine odor generating portion. As a result, the present invention has been completed.
- the determination method of the present invention is suitably used for visually determining the position of the urine odor generating portion and the urine odor intensity in an article (including its constituent members), equipment, or material that generates urine odor.
- the “article, facility or material generating urine odor” may be an article, facility or material in which urine odor is actually generated, and urine may be absorbed or adhered. A certain article, equipment, or material may be used.
- Such products include urine-absorbing pads, light incontinence products, diapers, sanitary products such as sanitary products, clothing such as underwear, skin, bedding, etc., and pet excrement-related products such as dogs and cats (for pets)
- Facilities include toilet environments such as toilets and toilet floors.
- materials constituting the article or equipment for example, water-absorbing polymer, pulp, nonwoven fabric, paper, cloth and other article materials, ceramics, hard resin, wood, pipes and other metals, or various wall materials, Equipment materials such as flooring are listed.
- the method of the present invention is an absorbent article such as a urine collection pad, a light incontinence article, a diaper, a sanitary article, and a pet urine absorbent sheet that is supposed to absorb and hold urine, and these It can be suitably used for the water-absorbing polymer, pulp, non-woven fabric and paper used in the present invention. More preferably, it can be used for urine absorbing pads, light incontinence products, diapers, urine absorbing sheets for pets, and water-absorbing polymers used in these.
- the water-absorbing polymer that is the subject of the present invention may be any hydrophilic polymer that is blended in the above-described product and plays a role of directly or secondaryly absorbing and retaining urine.
- the composition is not limited.
- a solution containing an enzyme activity detection reagent (component A) is used for the purpose of identifying / visualizing the urine odor generating part and the urine odor intensity.
- the enzyme activity detection reagent (component A) is not a detection indicator for a volatile component with high diffusion, but an enzyme substrate that can directly detect enzyme activity from the viewpoint of specifying a urine odor generating part with good spatial resolution.
- a type of enzyme activity detection reagent is used.
- the enzyme activity detection reagent (component A) used in the present invention generates a dye compound or a fluorescent dye compound by an enzymatic reaction.
- This dye compound or fluorescent dye compound is It is necessary that the diffusibility of the resin is low, that is, it is hardly soluble in water.
- the dye compound or fluorescent dye compound has a solubility in water at 20 ° C. of preferably less than 10 g / L, more preferably less than 6 g / L, even more preferably less than 0.5 g / L, and even more preferably 0.05 g. It is preferably less than / L.
- the enzyme activity detection reagent (component A) used in the present invention is uniformly distributed over the target article. For this reason, the enzyme activity detection reagent (component A) is used in the form of a solution. Therefore, it is preferable that the enzyme activity detection reagent (component A) has a high diffusibility in water before the enzyme reaction, that is, a higher solubility in water than a dye compound produced after the enzyme reaction.
- the compound produced by the enzyme reaction needs to be a compound having absorption in the visible region or a compound emitting fluorescence in the visible region, that is, a dye compound or a fluorescent dye compound.
- a dye compound having absorption in the visible region is more preferable from the viewpoint of easy detection.
- a dye compound having an absorption spectrum at 500 to 800 nm is preferable, and a color compound which exhibits a blue color is more preferable.
- the enzyme to be detected may be an enzyme produced by a microorganism causing urine odor, and examples thereof include glycosidase and sulfatase. From the viewpoint of approximating the intensity and quality of the urine odor actually felt and the time until the urine odor is generated, it is preferable to be able to identify the generation part of the phenolic compound and indole. Therefore, ⁇ -glucuronidase or arylsal Those capable of detecting phatase activity are preferred, and those capable of detecting ⁇ -glucuronidase activity are more preferred.
- the content of the enzyme activity detection reagent (component A) with respect to the total liquid agent is preferably 0.002 to 0.2 w / v% as a converted weight excluding the base, and more Preferably it is 0.005 to 0.05 w / v%, more preferably 0.01 to 0.025 w / v%.
- the glycosidase activity detection reagent used as component A in the present invention is an enzyme substrate-type glycosidase activity detection reagent that generates a dye compound or a fluorescent dye compound that is hardly soluble in water after the enzyme reaction, and can detect the glycosidase activity visually. If it is a thing, it will not specifically limit.
- phenolic acid-base indicators such as phenolphthalein, 5-bromo-4-chloro-3-hydroxyindole, 5 Hydroxylindole analogues such as bromo-6-chloro-3-hydroxyindole, 6-chloro-3-hydroxyindole, 3-hydroxyindole
- umbelliferone analogues such as 4-methylumbelliferone And a glycoside compound as (part
- the glycosidase activity detection reagent used in the present invention is more preferably a glycoside compound in which the above-mentioned aglycone and sugar are bound by a ⁇ -D-glycoside bond.
- hydroxyindole analog glycoside compounds and umbelliferone analog glycoside compounds are preferred, and among these compounds, compounds generated by enzymatic reactions are dye compounds that absorb in the visible region. In view of the fact that it is particularly hardly soluble in water, a hydroxyindole analog glycoside compound is preferred.
- glycosidase activity detection reagents detect various glycosidase activities depending on the sugar moiety, such as glucose, glucuronic acid, mannose, galactose, fructose, fucose, rhamnose, arabinose, and xylose as sugar moieties.
- sugar moiety such as glucose, glucuronic acid, mannose, galactose, fructose, fucose, rhamnose, arabinose, and xylose as sugar moieties.
- glucose, glucuronic acid, and galactose combined with the above-mentioned aglycone are preferable from the viewpoint of detectability of urine odor-related microbial enzymes, and among these, ⁇ - A compound comprising a D-glycoside bond, that is, a ⁇ -glucuronidase activity detection reagent is preferred.
- Such compounds include 5-bromo-4-chloro-3-indolyl- ⁇ -D-glucuronide, 5-bromo-6-chloro-3-indolyl- ⁇ as the hydroxyindole analog glucuronidated compounds.
- -D-glucuronide, 6-chloro-3-indolyl- ⁇ -D-glucuronide, indoxyl- ⁇ -D-glucuronide, and 4-methylumbelliferyl- ⁇ -D as the umbelliferone analog glucuronidation compound -Glucuronide can be mentioned.
- the compound produced by the enzyme reaction is a dye compound having absorption in the visible region, and from the viewpoint that it is particularly hardly soluble in water, a hydroxyindole analog glucuronidated compound, that is, 5-bromo-4 -Chloro-3-indolyl- ⁇ -D-glucuronide, 5-bromo-6-chloro-3-indolyl- ⁇ -D-glucuronide, 6-chloro-3-indolyl- ⁇ -D-glucuronide, indoxyl- ⁇ - D-glucuronide is more preferable, and among these, 5-bromo-4-chloro-3-indolyl- ⁇ -D-glucuronide is preferable from the viewpoint of contrast with the urine color of the coloring compound, stability of color development, and cost.
- a hydroxyindole analog glucuronidated compound that is, 5-bromo-4 -Chloro-3-indolyl- ⁇ -D-glucuronide, 5-bromo-6-ch
- the content of the above-mentioned glycosidase activity detection reagent and ⁇ -glucuronidase activity detection reagent in the total liquid agent is 0.002 to 0.2 w / v% in terms of converted weight excluding the base. More preferably, it is 0.005 to 0.05 w / v%, and still more preferably 0.01 to 0.025 w / v%.
- the sulfatase activity detection reagent used as component A in the present invention is an enzyme substrate type sulfatase activity detection reagent that produces a dye compound or a fluorescent dye compound that is hardly soluble in water after the enzyme reaction, and the sulfatase activity is visually detected. There is no particular limitation as long as it can be detected. In addition, an arylsulfatase activity detection reagent is more preferable in relation to generation of urine odor.
- the arylsulfatase activity detection reagent used in the present invention will be described in detail, but the arylsulfatase activity detection reagent used in the present invention is not particularly limited as long as it has the above-described properties.
- Examples of the arylsulfatase activity detection reagent used in the present invention include a sulfated compound of a hydroxyindole analog and a sulfated compound of an umbelliferone analog.
- 5-bromo-4-chloro-3-indolyl sulfate and indoxyl sulfate are used as sulfated compounds for hydroxyindole analogs
- 4-methylumbellite is used as a sulfated compound for umbelliferone analogs.
- Ferryl sulfate can be suitably used for each.
- the compound produced by the enzymatic reaction is a dye compound having absorption in the visible region, and from the viewpoint that it is particularly hardly soluble in water, a sulfated compound of a hydroxyindole analog, that is, 5-bromo- 4-Chloro-3-indolylsulfate and indoxylsulfate are more preferable, and 5-bromo-4-chloro-3-indolylsulfate from the viewpoint of contrast with the urine color of the coloring compound, stability of color development and price Fate is preferred.
- the content of the above sulfatase activity detection reagent and arylsulfatase activity detection reagent with respect to the whole solution is preferably 0.002 to 0.2 w / v% as a converted weight excluding the base, and more preferably It is 0.005 to 0.05 w / v%, more preferably 0.01 to 0.025 w / v%.
- the liquid used in the first step of the present invention can use water and / or urine as a solvent for the purpose of dissolving or dispersing the above enzyme activity detection reagent (component A). Further, as will be described later, when a method of once drying an article, equipment, or material to which the liquid agent is applied, a volatile organic solvent may be used as a solvent or a dispersion medium.
- the volatile organic solvent used at this time is preferably a water-soluble one such as methanol, ethanol, 1-propanol, 2-propanol, acetonitrile.
- the liquid agent used in the first step of the present invention includes various surfactants, antioxidants, pH adjusters, oil agents, organic solvents, metal salts, metal ions, etc. as additives as necessary. You may contain in the range which does not inhibit the effect of invention.
- the visual determination method and screening method of the present invention are also characterized in that the urine odor generation part and the urine odor intensity can be simulated by coloration of the reagent regardless of whether or not urine is contained.
- the liquid agent containing component A (the liquid agent used in the first step of the present invention) may further contain urine in that it can reproduce a chemical and biological environment close to the actual state of urine odor generation. preferable.
- the content is not limited, but it is preferable that the urine odor is actually generated from the target article, facility, or material from the above viewpoint.
- the target is intended to absorb urine as it is, such as an absorbent article or a water-absorbing polymer
- the urine content is 50 to 100 v / v%, more preferably 80 to 100 v / v, based on the entire liquid. It is good to set it to v%, more preferably 90-100v / v%.
- a solution is prepared using concentrated urine, and converted to 100% v / v% in urine before concentration. A higher concentration may be used.
- the urine used in the liquid preparation containing component A is not limited to those derived from humans, and pet excrement-related products such as dogs and cats (pet toilets, urine absorbing sheets, etc.) and absorbent materials used therefor In the case of targeting a water-absorbing polymer, it is preferable to use urine of these animals.
- the collected urine can be used as it is, but when the liquid containing component A contains both the microorganisms and / or enzymes described in detail below, the viewpoint of stabilizing the detection conditions Therefore, it is preferable to use urine from which microorganisms have been removed by a sterilization operation using a filter or the like.
- ⁇ Microorganism> When applying a solution containing component A to an absorbent article with a history of wear or a toilet environment with a history of use, or when the solution containing component A contains urine that has not been sterilized It is possible to carry out the method of the present invention with resident microorganisms, but when the enzyme reaction is desired to be performed with higher reproducibility, or when the target is a new product, material, etc., aseptic or most microorganisms have been removed. In this case, it is more preferable to use the solution by further containing microorganisms.
- the microorganism used at this time may be a strain having an enzyme activity corresponding to the enzyme activity detection reagent used in the solution containing component A. Specifically, from a strain having either glycosidase activity or sulfatase activity. It is preferable to use one or more selected.
- ⁇ -glucuronidase active strain corresponding to the enzyme activity detection reagent (component A) used in the first step of the present invention
- component A ⁇ -glucuronidase active strain
- arylsulfatase activity It is preferable to use a strain, and it is more preferable to use a ⁇ -glucuronidase active strain.
- the microorganisms that are highly related to urine odor.
- the microorganisms are separated from the environment derived from urine such as absorbent articles and the back of the toilet bowl after urine absorption. It is preferable to use different strains.
- the content is not limited, but from the viewpoint of reproducibility, the concentration of the microorganisms contained in the liquid is 10 0 to 10 8 CFU / It is preferably mL, more preferably 10 1 to 10 6 CFU / mL, and still more preferably 10 2 to 10 4 CFU / mL.
- the enzyme used at this time may be an enzyme corresponding to the enzyme activity detection reagent (component A) used in the liquid agent, and specifically, at least one selected from glycosidase and sulfatase is preferably used.
- ⁇ -glucuronidase and arylsulfatase are used corresponding to the enzyme activity detection reagent (component A) contained in the liquid used in the first step because of the relationship between the enzyme and urine odor among these enzymes. It is preferable to use ⁇ -glucuronidase.
- the content varies depending on the target and the assumed situation.
- the range of use of the product is assumed and the range of disposal is assumed.
- disposal it is preferable to adjust so that a sufficient color reaction is observed in a sample (control) that does not contain any urine odor production inhibitor in the range of 30 ° C. and 8 to 120 hours.
- the concentration in the solution is preferably selected within the range of 0.1 to 30000 units / mL according to the target and assumed situation.
- the liquid preparation containing component A may be used with the addition of a nutrient medium for the purpose of promoting the growth of microorganisms and promoting the enzymatic reaction smoothly.
- a nutrient medium for the purpose of promoting the growth of microorganisms and promoting the enzymatic reaction smoothly.
- Any nutrient medium can be used as long as it can contribute to the growth of microorganisms, but it is a component selected from meat extracts, yeast extracts, casein degradation products, amino acids, sugars, polysaccharides, various inorganic salts, pH adjusters, and the like.
- a general-purpose nutrient medium such as SCD medium, Mueller Hinton medium, and LB medium.
- Applying a liquid agent containing component A means contacting, applying, impregnating, spraying, dripping, etc. the liquid agent to an article, facility or material that generates urine odor. From the viewpoint of specifying the urine odor generating part in the actual use environment, when applying to an article, facility or material that generates urine odor, it is preferable to apply it to actual urine, and component A is applied to the absorbent article. In the case of applying a liquid preparation containing urine, it is preferably absorbed so as to be poured from a site where urine is absorbed in an actual use mode.
- the target article to which the liquid preparation containing component A is applied between the first step and the second step is dried and urine is newly added to the dried target article, the urine odor
- the liquid agent containing component A is uniformly absorbed in the absorbent article from the viewpoint of improving the accuracy of generating part specification.
- an absorbent article may be used as it is or a prototype imitating this may be used. Further, if necessary, a section obtained by cutting them into an appropriate size may be used.
- the amount of the liquid agent to be applied is not particularly limited, but from the viewpoint of more clearly determining the urine odor generating part in the actual use environment, when visualizing the urine odor generating part of the water absorbing polymer,
- the weight is preferably 4 to 240 times by weight, more preferably 6 to 120 times by weight, and still more preferably 10 to 60 times by weight.
- a suitable amount of the liquid agent containing component A varies depending on the urine absorption capacity guaranteed by the product, and specifically, 1/4 of the urine absorption capacity.
- the amount is preferably equal to the equivalent amount, more preferably 1/2 amount to the equivalent amount.
- the preferred amount of liquid is the ratio of the urine absorption between the original product and the target prototype or slice. Is preferably calculated to be equal to the ratio of the absorption surface area between the original product and the target prototype or section.
- an enzyme reaction is allowed to proceed with an article, facility or material to which the liquid preparation containing component A is applied.
- the target after application of the liquid agent is preferably maintained at the same temperature as the situation where odor is actually generated, for example, when the odor generated at the time of disposal after the use of the absorbent article is assumed.
- the enzyme reaction may be started immediately, but the reaction may be started after a time.
- another step may be included between the first step and the second step.
- the enzyme reaction is started after a certain period of time, it is preferable that the solution does not contain urine.
- the target article to which the solution containing component A is applied is dried and dried between the first step and the second step.
- the method further includes the step of newly adding urine to the target article.
- a liquid agent containing component A may be applied to the absorbent article itself, or individual members constituting this, that is, a water-absorbing polymer, a non-woven fabric.
- an absorbent article may be produced using the member to which the liquid agent is applied.
- the time for performing the enzyme reaction varies depending on the target and the assumed situation, it is not particularly limited.
- the range of use of the product is assumed and the use is assumed.
- it is preferably 1 to 24 hours, more preferably 2 to 16 hours, and further preferably 4 to 8 hours.
- the disposal is assumed, it is preferably 8 to 120 hours, more preferably 16 to 96 hours, and further preferably 24 to 72 hours.
- the color change caused by the enzyme reaction is observed, and the part with the large change is specified as the urine odor generating part.
- the thing with a large area of the part in which the color changed can be specified as an article, equipment, or material with strong urine odor intensity.
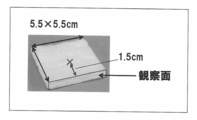
- the color change may be observed directly on the entire article, equipment or material to which the liquid agent is applied, in the evaluation of the absorbent article, the position where it becomes the odor generating part of the absorbent article having a complicated structure is determined. It is preferable to observe the color change occurring on the cut surface from the point that can be made clearer.
- it may be cut at any time before the third step, but before the first step from the viewpoint of accurately identifying the position where it becomes an odor generating part. It is preferred to cut, i.e. to use absorber sections.
- the color change needs to be determined in the dark under ultraviolet irradiation when using an enzyme activity detection reagent that generates a fluorescent dye compound, but the dye has an absorption wavelength in the visible region.
- an enzyme activity detection reagent that generates a fluorescent dye compound when used, no special environmental setting is required, and it can be visually observed.
- it further includes a step of calculating the area where the color change appears after the enzymatic reaction from the image data of the article, equipment or material to be observed and judging the urine odor intensity.
- a method using a digital camera can be used to acquire an image.
- any method can be adopted as long as it is a method that can clarify the colored portion and calculate the increase in the area thereof.
- a liquid agent containing 5-bromo-4-chloro-3-indolyl- ⁇ -D-glucuronide as component A will be described as follows. First, the image data before and after the enzyme reaction is taken into the image editing software. Next, since 5-bromo-4-chloro-3-indolyl- ⁇ -D-glucuronide is a reagent that develops a dark blue color by enzymatic reaction, in order to maximize the contrast of the color change after the enzymatic reaction It is preferable to use an image obtained by selecting only the red channel for analysis. This is converted into two gradations after gray scale conversion, whereby a clear image of the urine odor generation site can be obtained.
- the threshold value used for the two gradations may be the same value in a series of evaluation objects, and more preferably determined so that an image as close as possible to the colored region determined by visual observation is obtained.
- a liquid agent containing component A is brought into contact with a material, member, or agent as an evaluation target of the urine odor generation inhibitory effect, and an evaluation target with a small color change of the evaluation target due to an enzyme reaction is selected. That's fine.
- the color change is observed visually or by image processing, and the color change is more suitable.
- the material, the member, the agent, or the arrangement of the member with a small increase in the colored area can be selected as having excellent urine odor generation suppressing effect.
- Examples of materials to be screened include water-absorbing polymers used in absorbent articles, but the water-absorbing polymers are not limited to polymers that are not specially treated, such as deodorizing, deodorizing, and antibacterial. It may be subjected to chemical or physical treatment for the purpose.
- a first step of applying a liquid agent containing component A to a plurality of types of water-absorbing polymers A second step of performing an enzymatic reaction with a water-absorbing polymer to which the liquid agent is applied; A third step of observing a color change of the water-absorbing polymer to which the liquid agent is applied;
- the fourth step of selecting a water-absorbing polymer with less color change in the third step it is possible to select a water-absorbing polymer having an excellent urine odor production suppressing effect.
- screening can be carried out with the water-absorbing polymer alone, but water absorption with an excellent urine odor generation suppressing effect under the circumstances where the water-absorbing polymer is actually used.
- an adhesive polymer it is preferable to perform screening by allowing the absorbent article to contain a water-absorbing polymer, performing an enzyme reaction, and observing the color of the section of the section.
- a polymer having a smaller color change a polymer having a smaller color density change may be selected, or a polymer having a smaller area of the color change may be selected. It is preferable to select a water-absorbing polymer that does not change in color.
- examples of members to be screened include water-absorbing polymers, non-woven fabrics, pulps, and mounts used as constituent members of absorbent articles.
- the urine odor can also be affected by the types of these constituent members and their arrangement. There may be a case where the site to be generated and the degree of suppression of urine odor generation change. Therefore, by using the method of the present invention, it is also possible to select a component member for obtaining a water-absorbing article having an excellent urine odor production suppressing effect and its optimal arrangement.
- the first step of applying the liquid agent containing the component A to a plurality of absorbent articles having different constituent members A second step of performing an enzyme reaction with the absorbent article to which the liquid agent is applied; A third step of observing a color change of the cut surface of the absorbent article to which the liquid agent is applied;
- the fourth step of selecting the constituent member in the absorbent article with less color change in the third step the constituent member of the absorbent article having an excellent urine odor production suppressing effect can be selected.
- the explanations relating to the respective steps from the first step to the third step of the visual determination method described above are appropriately applied to points that are not particularly described.
- the fourth step of selecting the arrangement of the constituent members in the absorbent article with less color change in the third step the arrangement of the constituent members of the absorbent article excellent in the urine odor generation suppressing effect can be selected.
- the explanations relating to the respective steps from the first step to the third step of the visual determination method described above are appropriately applied to points that are not particularly described.
- the target absorbent article may be cut by using a section for the same reason as described above, and observing the color of the cross section. preferable.
- a suitable urine odor production inhibitor can be selected.
- the urine odor production inhibitor that is the subject of the present invention include inorganic and organic antibacterial agents that suppress or kill the growth and growth of microorganisms related to the generation of urine odor, and the generation of urine odor.
- examples include enzyme inhibitors such as ⁇ -glucuronidase inhibitors and arylsulfatase inhibitors that inhibit the enzymes involved.
- plant extracts, fragrances, oils, surfactants, deodorizers, and the like that have these actions directly or secondaryly can also be targets of the selection method of the present invention.
- the urine odor production inhibitor is not limited thereto, and any urine odor generation inhibitor may be used as long as it is applied to a target and suppresses the generation of urine odor derived from the action of microorganisms.
- the explanations relating to the respective steps from the first step to the third step of the visual determination method described above are appropriately applied to points that are not particularly described.
- the urine odor production inhibitor is a water-absorbing polymer. It is preferable to perform screening after performing operations such as coating, impregnation, and immobilization on absorbent articles.
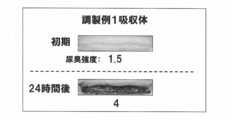
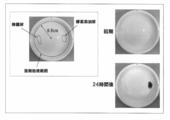
- Example 1 Selection of Enzyme Activity Detection Reagent (Component A) As shown in FIG. 1, 300 ⁇ L of each solution containing the enzyme activity detection reagent or odor color indicator having the composition shown in Table 1 was absorbed in 0.03 g of the water-absorbing polymer. This was further laminated in three layers with a pulp layer sandwiched in a 1.5 mL micro test tube. Only this central layer was added with an enzyme or bacteria as an initiator of odor generation, and the odor was generated in a 37 ° C. constant temperature bath for 72 hours, and the color change was observed. The results are shown in Table 2 and FIG. Table 2 shows the results of discussion by four panels based on the following criteria for color development, contrast to urine color, and specific accuracy of the urine odor generating part.
- Example 2 Visualization of urine odor generation part and urine odor intensity and screening of water-absorbing polymer (1) Collection of ⁇ -glucuronidase active strain 5-bromo-4-chloro-3 from used (urine only) infant paper diaper A ⁇ -glucuronidase active strain was collected and separated by a conventional method using SCDLP agar plate medium (manufactured by Nippon Pharmaceutical Co., Ltd.) supplemented with -indolyl- ⁇ -D-glucuronide. Occurrence of odor was confirmed when these strains were cultured in sterilized urine. Among them, the strain that generated particularly strong odor was used as the ⁇ -glucuronidase active strain in this example. This strain was confirmed to be attributed to E. coli by 16S rDNA partial nucleotide sequence analysis.
- Odor intensity evaluation About each sample of said (5), the odor intensity evaluation of the initial stage, after 8 hours, and 24 hours after was performed.
- the evaluation of odor intensity was carried out in accordance with a 6-step odor intensity display method with an evaluation score of 0 to 5, involving two specialist panels with qualifications for odor judgment.
- the evaluation score is “0” odorless, “1” finally perceived odor, “2” urine odor, but weak odor, “3” odor easily felt urine odor, “4” strong urine odor "5" shows intense urine odor.
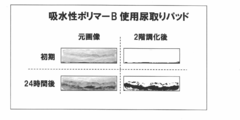
- FIG. 5 shows an image (initial and 24 hours later) of a urine absorption pad section using the water-absorbing polymer B as an example of the image.
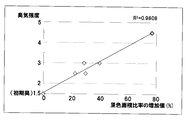
- the increase in the color area ratio reflects the increase in the odor intensity very well, and it is possible to clearly identify the urine odor generating part with this color site, and further visually. From this information, it was shown that the objective evaluation of odor intensity is possible. Further, it can be visually determined that the urine removing pad using the water-absorbing polymer B has the most excellent deodorizing effect, and the water-absorbing polymer excellent in the urine odor generation suppressing effect suitable for the absorbent article by the method of the present invention. It was shown that it becomes possible to select easily.
- Example 3 Screening of antibacterial agent for suppressing urine odor production
- Organic antibacterial agent P, Q or inorganic antibacterial agent R was added as a urine odor generation inhibitor at various concentrations (w / w%) to the water-absorbing polymer.
- the organic antibacterial agent P was a quaternary ammonium salt antibacterial agent
- the organic antibacterial agent Q was a phenolic antibacterial agent
- the inorganic antibacterial agent R was zinc sulfate.
- 0.45 g of each antibacterial agent-added water-absorbing polymer obtained was uniformly placed on the bottom of a container having a bottom of about 5.5 cm square, and 6.5 mL of the liquid prepared in Example 2 (3) was added thereto, and the temperature was kept constant at 30 ° C.
- FIG. 6 shows the image of each sample, and the odor intensity evaluation value obtained by the method (6) of Example 2 is shown below the image.
- Table 4 shows the minimum coloration suppression concentration of each antibacterial agent. From the above results, it was shown that an antibacterial agent for suppressing urine odor generation suitable for a water-absorbing polymer can be easily screened by the method of the present invention.
- Example 4 Screening of enzyme inhibitor for suppressing urine odor production Immediately after collection, 5-bromo-4-chloro-3-indolyl- ⁇ -D- was added to human sterilized urine sterilized by a filter unit manufactured by Nalgen. A solution was prepared by dissolving 1 g / v% of an aqueous solution of ⁇ -glucuronidase Type VII-A (purchased from Sigma) derived from Escherichia coli after dissolving glucuronide at 200 ppm (w / v) and adjusting to 160 units / mL. .
- a water-absorbing polymer to which 1.5 w / w% of ⁇ -glucuronidase inhibitors S and T were added as urine odor production inhibitors was prepared.
- 0.5 g each of the obtained water-absorbing polymer with enzyme inhibitor added and water-absorbing polymer without additive are uniformly arranged on the bottom of a container having a bottom of about 5.5 cm square, and 7.5 mL of the above liquid is added thereto, 30
- the enzymatic reaction was carried out in a constant temperature bath for 48 hours.
- FIG. 7 shows an image of each sample, and an odor intensity evaluation value obtained by the method (6) of Example 2 is shown below the image. From the above results, it was shown that the enzyme inhibitor for suppressing urine odor generation suitable for the water-absorbing polymer can be easily screened by the method of the present invention.
- Preparation Example 1 Preparation of an Absorbent Product whose Urinary Odor Generation Part is Visually Determined 200 ppm (w / v) of 5-bromo-4-chloro-3-indolyl- ⁇ -D-glucuronide is dissolved in ethanol. A solution was prepared by A 5.5 cm square was cut out from a position near the center from a urine collection pad prepared by imitating the structure of a commercially available urine collection pad (for daytime, urine absorption capacity 450 mL). The obtained absorbent slice was uniformly impregnated with 12.5 mL of the present liquid agent and dried in an electric dryer until the weight did not change.
- Example 5 Visualization of urine odor generating part by absorbent article whose urine odor generating part is visually determined
- (1) Preparation of urine added urine ⁇ -glucuronidase active strain described in (2) of Example 2 is SCD agar
- the plate was cultured overnight on a plate medium, and a portion of the obtained colony was scraped off by a sterilization loop, diluted in sterilized physiological saline, and a bacterial solution was prepared so as to be on the order of 10 4 in CFU / mL.
- 1 v / v% of the prepared bacterial solution was added to and mixed with human sterilized urine sterilized using a filter unit manufactured by Nalgen to prepare urine added urine.
- FIG. 8 shows the image of the sample at the initial stage and 24 hours later, and the evaluation value of the odor intensity obtained by the method (6) of Example 2 below the image. From this result, it was shown that the urine odor generating part can be easily visualized in the absorbent article by the method of the present invention.
- Preparation Example 2 Preparation of a hard surface where the urine odor generating part is visually judged By dissolving 2000 ppm (w / v) of 5-bromo-4-chloro-3-indolyl- ⁇ -D-glucuronide in ethanol A solution was prepared. This solution was placed in a spray container, and 3.3 g of the solution was sprayed uniformly several times on the central part (within a radius of 6.5 cm) of a ceramic plate, and dried at room temperature.
- Example 6 Visualization of urine odor generating part by hard surface from which urine odor generating part is visually determined Human sterilized urine sterilized by a filter unit made by Nalgen immediately after collection, and 25400 units for this sterilized urine 1-v / v% of E. coli-derived ⁇ -glucuronidase Type VII-A aqueous solution adjusted to 1 mL / mL was added and mixed to prepare enzyme-added urine so that urine odor was generated continuously. Subsequently, 300 ⁇ L of sterilized urine was dropped on the left side and 300 ⁇ L of enzyme-added urine was dropped on the right side of the ceramic plate described in Preparation Example 4, and the upper surface of the plate was covered with a wrap and allowed to stand at room temperature for 24 hours. FIG. 9 shows images of samples at the initial stage and after 24 hours. From this result, it was shown that the urine odor generating part can be easily visualized on the hard surface by the method of the present invention.
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Physics & Mathematics (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Immunology (AREA)
- Zoology (AREA)
- General Health & Medical Sciences (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biochemistry (AREA)
- Analytical Chemistry (AREA)
- Wood Science & Technology (AREA)
- Pathology (AREA)
- Molecular Biology (AREA)
- Microbiology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- Biotechnology (AREA)
- Genetics & Genomics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Biophysics (AREA)
- General Physics & Mathematics (AREA)
- Plasma & Fusion (AREA)
- Optics & Photonics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Absorbent Articles And Supports Therefor (AREA)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201280010498.9A CN103392007B (zh) | 2011-02-25 | 2012-02-24 | 尿臭产生部的视觉的判定方法 |
| RU2013143302/10A RU2598718C2 (ru) | 2011-02-25 | 2012-02-24 | Способ визуального определения местонахождения источника образования запаха мочи и скрининг ингибиторов образования запаха мочи |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2011-039250 | 2011-02-25 | ||
| JP2011039250 | 2011-02-25 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012115238A1 true WO2012115238A1 (ja) | 2012-08-30 |
Family
ID=46721010
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2012/054609 Ceased WO2012115238A1 (ja) | 2011-02-25 | 2012-02-24 | 尿臭発生部の視覚的判定方法 |
Country Status (4)
| Country | Link |
|---|---|
| JP (1) | JP5398857B2 (enExample) |
| CN (1) | CN103392007B (enExample) |
| RU (1) | RU2598718C2 (enExample) |
| WO (1) | WO2012115238A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2014153127A (ja) * | 2013-02-06 | 2014-08-25 | Railway Technical Research Institute | トイレの尿汚れ判定用の採取器具、トイレの尿汚れ判定キット及びトイレの尿汚れの判定方法 |
| CN105264087A (zh) * | 2013-02-19 | 2016-01-20 | 尼普洛株式会社 | 羟基吲哚硫酸的测定方法 |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6026822B2 (ja) * | 2012-08-28 | 2016-11-16 | 花王株式会社 | 吸収性構造体及び吸収材 |
| CN108432642A (zh) * | 2018-06-28 | 2018-08-24 | 南京凯创协同纳米技术有限公司 | 抗菌除臭宠物尿垫及其制造方法 |
| EP3813753A2 (en) * | 2018-06-29 | 2021-05-05 | International Paper Company | Chemiluminescent wetness indicator for absorbent products |
| JP7557181B2 (ja) * | 2020-06-25 | 2024-09-27 | シヤチハタ株式会社 | 尿成分可視化剤及び尿成分可視化方法 |
| CN112816467B (zh) * | 2021-02-08 | 2023-08-29 | 杭州可靠护理用品股份有限公司 | 一种用于尿液检测的显色剂及其在纸尿裤上的应用 |
| WO2024024648A1 (ja) * | 2022-07-29 | 2024-02-01 | パナソニックIpマネジメント株式会社 | 有機色素組成物及びそれを含むスプレー剤 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009037861A1 (ja) * | 2007-09-20 | 2009-03-26 | Kao Corporation | β-グルクロニダーゼ阻害剤 |
| JP2010220641A (ja) * | 2009-03-19 | 2010-10-07 | Kao Corp | β−グルクロニダーゼ阻害剤 |
| JP2010227130A (ja) * | 2009-03-25 | 2010-10-14 | Kao Corp | β−グルクロニダーゼ阻害剤 |
| JP2010246905A (ja) * | 2009-03-25 | 2010-11-04 | Kao Corp | 尿臭生成抑制用組成物 |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH10257832A (ja) * | 1997-03-18 | 1998-09-29 | Nea Corp:Kk | 健康チェック用発色型トイレ材及び健康チェック用複合トイレ材 |
| RU2385738C2 (ru) * | 2005-10-05 | 2010-04-10 | Ска Хайджин Продактс Аб | Впитывающее изделие, содержащее тонкую пленку, включающую активное вещество |
| JP2009148326A (ja) * | 2007-12-19 | 2009-07-09 | Kao Corp | 尿臭生成抑制剤 |
-
2012
- 2012-02-24 RU RU2013143302/10A patent/RU2598718C2/ru active
- 2012-02-24 JP JP2012038675A patent/JP5398857B2/ja active Active
- 2012-02-24 WO PCT/JP2012/054609 patent/WO2012115238A1/ja not_active Ceased
- 2012-02-24 CN CN201280010498.9A patent/CN103392007B/zh not_active Expired - Fee Related
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009037861A1 (ja) * | 2007-09-20 | 2009-03-26 | Kao Corporation | β-グルクロニダーゼ阻害剤 |
| JP2010220641A (ja) * | 2009-03-19 | 2010-10-07 | Kao Corp | β−グルクロニダーゼ阻害剤 |
| JP2010227130A (ja) * | 2009-03-25 | 2010-10-14 | Kao Corp | β−グルクロニダーゼ阻害剤 |
| JP2010246905A (ja) * | 2009-03-25 | 2010-11-04 | Kao Corp | 尿臭生成抑制用組成物 |
Non-Patent Citations (1)
| Title |
|---|
| MERCK: "X-Gluc Solution", NOVAGEN BUNSHI SEIBUTSUGAKU CATALOG 2005/2006, 10 February 2006 (2006-02-10), pages 8 - 67 * |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2014153127A (ja) * | 2013-02-06 | 2014-08-25 | Railway Technical Research Institute | トイレの尿汚れ判定用の採取器具、トイレの尿汚れ判定キット及びトイレの尿汚れの判定方法 |
| CN105264087A (zh) * | 2013-02-19 | 2016-01-20 | 尼普洛株式会社 | 羟基吲哚硫酸的测定方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| RU2598718C2 (ru) | 2016-09-27 |
| CN103392007A (zh) | 2013-11-13 |
| CN103392007B (zh) | 2015-08-19 |
| JP2012187101A (ja) | 2012-10-04 |
| JP5398857B2 (ja) | 2014-01-29 |
| RU2013143302A (ru) | 2015-03-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5398857B2 (ja) | 尿臭発生部の視覚的判定方法 | |
| JP2004500186A (ja) | 排泄物汚染センサーを有する表面拭き取り使い捨て物品 | |
| CN1325266A (zh) | 含有抗菌蛋白酶抑制剂的一次性的预润湿的擦拭物 | |
| CN104704162A (zh) | 生物类黄酮浸渍的材料 | |
| Kolari | Attachment mechanisms and properties of bacterial biofilms on non-living surfaces | |
| DE69806781T2 (de) | Reduzierung des körpergeruchs | |
| Saleh et al. | In vitro genotoxicity study of the lambda-cyhalothrin insecticide on Sf9 insect cells line using Comet assay. | |
| Schmidt | Amphibian deformities continue to puzzle researchers | |
| Das et al. | Induction of sister chromatid exchanges and chromosome aberrations in vivo in Etroplus suratensis (Bloch) following exposure to organophosphorus pesticides | |
| JP3933788B2 (ja) | 尿検査用試験材並びにこれを用いた健康チェック材及び健康チェック方法 | |
| Smoot et al. | Periphyton growth on submerged artificial substrate as a predictor of phytoplankton response to nutrient enrichment | |
| JP6026822B2 (ja) | 吸収性構造体及び吸収材 | |
| JP7488709B2 (ja) | 猫の排泄行動阻害抑制剤 | |
| Birke et al. | Scent-marking behaviour in response to conspecific odours by the rat, Rattus norvegicus | |
| JP6307110B2 (ja) | 吸水性ポリマー及び尿臭発生部の可視化方法 | |
| Jacobs et al. | An in vitro model for detecting skin irritants: methyl green-pyronine staining of human skin explant cultures | |
| Yangaza et al. | Prevalence of urogenital Schistosomiasis and risk factors for transmission among primary school children in an endemic urban area of Kinondoni municipality in Dar es Salaam, Tanzania | |
| CN108289973B (zh) | 尿臭抑制剂 | |
| Richter et al. | Decontamination efficacy of common liquid disinfectants against non‐spore‐forming biological agents in soil matrices | |
| JP2010220641A (ja) | β−グルクロニダーゼ阻害剤 | |
| Hakkinen | Seveso disaster, and the Seveso and Seveso II directives | |
| JP2022018279A (ja) | 猫用猫尿臭抑制剤 | |
| HEGNA | An examination of the effect of three phenolic disinfectants on Mycobacterium tuberculosis | |
| JPH09275975A (ja) | バイオ消臭剤 | |
| Dixit et al. | Biofilm Formation on Different Fabrics in the Presence of Sweat |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12750086 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2013143302 Country of ref document: RU Kind code of ref document: A |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 12750086 Country of ref document: EP Kind code of ref document: A1 |