WO2009093030A1 - Composition - Google Patents
Composition Download PDFInfo
- Publication number
- WO2009093030A1 WO2009093030A1 PCT/GB2009/000177 GB2009000177W WO2009093030A1 WO 2009093030 A1 WO2009093030 A1 WO 2009093030A1 GB 2009000177 W GB2009000177 W GB 2009000177W WO 2009093030 A1 WO2009093030 A1 WO 2009093030A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- cocoa
- nibs
- acid
- red
- purple
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23G—COCOA; COCOA PRODUCTS, e.g. CHOCOLATE; SUBSTITUTES FOR COCOA OR COCOA PRODUCTS; CONFECTIONERY; CHEWING GUM; ICE-CREAM; PREPARATION THEREOF
- A23G1/00—Cocoa; Cocoa products, e.g. chocolate; Substitutes therefor
- A23G1/0003—Processes of manufacture not relating to composition or compounding ingredients
- A23G1/002—Processes for preparing or treating cocoa beans or nibs
-
- A—HUMAN NECESSITIES
- A21—BAKING; EDIBLE DOUGHS
- A21D—TREATMENT, e.g. PRESERVATION, OF FLOUR OR DOUGH, e.g. BY ADDITION OF MATERIALS; BAKING; BAKERY PRODUCTS; PRESERVATION THEREOF
- A21D2/00—Treatment of flour or dough by adding materials thereto before or during baking
- A21D2/08—Treatment of flour or dough by adding materials thereto before or during baking by adding organic substances
- A21D2/36—Vegetable material
- A21D2/364—Nuts, e.g. cocoa
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23C—DAIRY PRODUCTS, e.g. MILK, BUTTER OR CHEESE; MILK OR CHEESE SUBSTITUTES; MAKING THEREOF
- A23C9/00—Milk preparations; Milk powder or milk powder preparations
- A23C9/12—Fermented milk preparations; Treatment using microorganisms or enzymes
- A23C9/13—Fermented milk preparations; Treatment using microorganisms or enzymes using additives
- A23C9/1307—Milk products or derivatives; Fruit or vegetable juices; Sugars, sugar alcohols, sweeteners; Oligosaccharides; Organic acids or salts thereof or acidifying agents; Flavours, dyes or pigments; Inert or aerosol gases; Carbonation methods
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23G—COCOA; COCOA PRODUCTS, e.g. CHOCOLATE; SUBSTITUTES FOR COCOA OR COCOA PRODUCTS; CONFECTIONERY; CHEWING GUM; ICE-CREAM; PREPARATION THEREOF
- A23G1/00—Cocoa; Cocoa products, e.g. chocolate; Substitutes therefor
- A23G1/0003—Processes of manufacture not relating to composition or compounding ingredients
- A23G1/0006—Processes specially adapted for manufacture or treatment of cocoa or cocoa products
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23G—COCOA; COCOA PRODUCTS, e.g. CHOCOLATE; SUBSTITUTES FOR COCOA OR COCOA PRODUCTS; CONFECTIONERY; CHEWING GUM; ICE-CREAM; PREPARATION THEREOF
- A23G1/00—Cocoa; Cocoa products, e.g. chocolate; Substitutes therefor
- A23G1/0003—Processes of manufacture not relating to composition or compounding ingredients
- A23G1/0006—Processes specially adapted for manufacture or treatment of cocoa or cocoa products
- A23G1/0009—Manufacture or treatment of liquid, cream, paste, granule, shred or powder
- A23G1/0016—Transformation of liquid, paste, cream, lump, powder, granule or shred into powder, granule or shred; Manufacture or treatment of powder
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23G—COCOA; COCOA PRODUCTS, e.g. CHOCOLATE; SUBSTITUTES FOR COCOA OR COCOA PRODUCTS; CONFECTIONERY; CHEWING GUM; ICE-CREAM; PREPARATION THEREOF
- A23G1/00—Cocoa; Cocoa products, e.g. chocolate; Substitutes therefor
- A23G1/30—Cocoa products, e.g. chocolate; Substitutes therefor
- A23G1/32—Cocoa products, e.g. chocolate; Substitutes therefor characterised by the composition containing organic or inorganic compounds
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23G—COCOA; COCOA PRODUCTS, e.g. CHOCOLATE; SUBSTITUTES FOR COCOA OR COCOA PRODUCTS; CONFECTIONERY; CHEWING GUM; ICE-CREAM; PREPARATION THEREOF
- A23G3/00—Sweetmeats; Confectionery; Marzipan; Coated or filled products
- A23G3/34—Sweetmeats, confectionery or marzipan; Processes for the preparation thereof
- A23G3/36—Sweetmeats, confectionery or marzipan; Processes for the preparation thereof characterised by the composition containing organic or inorganic compounds
Definitions
- This invention relates to acidified cocoa nibs comprising high levels of polyphenols, as well as refiner or expeller flakes, cocoa liquor, cocoa cake, and cocoa powder obtainable from the nibs and a process for the production of treated cocoa-derived material.
- Cocoa products are often treated with a solution of alkalising agent e.g. a hydroxide or carbonate of sodium or potassium with the object of obtaining a product with a less astringent and richer taste and a deeper and more attractive colour.
- alkalising agent e.g. a hydroxide or carbonate of sodium or potassium
- cocoa products There is a desire amongst some consumers for cocoa products that have a different colour.
- the use of coloured cocoa products can restrict the use of artificial food colourings or allow the use of less colouring material, for example.
- alkalisation of cocoa products such as cocoa seeds, cocoa beans and cocoa nibs has been used as described in, for example, GB 1 243 909, US 2,380,158 US 4,435,436, US 4,704,292, US 4,784,866 and US 5,009,917. Roasting is also described in US 4,704,292 and GB 2 416 106.
- US 5,114,730 discloses a method of making dark cocoa powder from cocoa powder in a water slurry at a temperature below 200°F in less than about 60 minutes and spray drying the final product.
- US 2,957,769 describes the extraction of a fermented unroasted cocoa materia ⁇ and the treatment of both the extract and the residual material.
- US 2,965,490 discloses the hydrolysis of unfermented cocoa to produce chocolate flavour.
- US 2003/0129276 describes a treated cocoa liquor for making chocolate crumb.
- US 2005/0031762 discloses the production of a low fat cocoa extract by adding acetic acid to fresh seeds and/or underfermented seeds.
- US 2,512,663 describes the treatment of roasted cocoa nibs to produce flavour or essence constituents from the cocoa-bearing material.
- US 2007/0254068 discloses a method of producing a cocoa beverage containing beneficial cocoa polyphenols. Such polyphenols are also described in US 7,115,656.
- GB 345,250 discloses the recovery and purification of alkaloids from cocoa products.
- WO 2008/043058 and US 2008/107783 describe a method of producing high-brightness cocoa powder and related components.
- WO 98/09533 discloses cocoa components, edible products having enhanced polyphenol content, methods of making the same and medical uses.
- EP 1946643 A1 discloses food ingredients with reduced sourness at low pH.
- US 5,888,562 describes an enzymatic treatment of cocoa.
- US 2004/0191403 discloses chocolate flavour manipulation.
- cocoa-derived materials which can be produced conveniently and efficiently.
- such materials can provide health benefits and/or have properties suitable for use in a low pH environment, such as for example as colouring in yoghurt.
- Such materials may avoid the disadvantages associated with alkalisation and/or roasting.
- the red or purple cocoa nibs comprise at least 20 mg/g of polyphenols, preferably more than 30 mg/g of polyphenols, most preferably from 40 to 60 mg/g of polyphenols.
- the acidified cocoa nibs are red or purple as defined herein.
- the invention provides red or purple cocoa refiner or expeller flakes obtainable from the nibs according to the first aspect of the invention.
- the refiner or expeller flakes are red or purple.
- the invention provides a red or purple cocoa liquor obtainable from the nibs according to the first aspect of the invention.
- the cocoa liquor is preferably red or purple
- the invention provides a red or purple cocoa cake obtainable from the liquor according to the third aspect of the invention.
- the cake is preferably red or purple.
- the invention provides a red or purple cocoa powder obtainable from the cocoa liquor according to the third aspect of the invention or the cocoa cake according to the fourth aspect of the invention or from the expeller flakes according to the second aspect of the invention.
- the invention provides a cocoa-derived material in the form of a powder and having an L* value of from about 40 to 45, a C* value of from about 28 to 33, an h° value of from about 17 to 25 and optionally an a* to b* ratio of from about 2.2 to 3.1.
- the L* value is from about 40 to 57
- the C* value is from about 18 to 40
- the invention provides a cocoa-derived material in the form of a powder and having an L* value of from about 47 to 57, a C* value of less than about 18, preferably from about 10 to 17, an h° value of from about 20 to about 50, preferably from about 25 to 40 or 25 to 30, and optionally an a* to b* ratio of less than about 2.3, preferably from about 1 to 2.1.
- the cocoa-derived material is preferably acidified and comprises at least 20 mg/g of polyphenols, preferably more than 30 mg/g of polyphenols, most preferably from 40 to 60 mg/g of polyphenols.
- the cocoa-derived material is an optionally defatted material.
- the cocoa-derived material is preferably as defined below.
- the invention provides a food, confectionery, dairy or bakery product comprising the cocoa refiner or expeller flakes according to the second aspect of the invention and/or the cocoa liquor according to the third aspect of the invention and/or the cocoa cake according to the fourth aspect of the invention and/or the red cocoa powder according to the fifth aspect of the invention and/or the cocoa-derived material according to the sixth or seventh aspects of the invention.
- the invention provides a process for producing red or purple cocoa- derived material, comprising the steps of: (i) treating cocoa nibs obtained from cocoa beans or seeds which have a higher polyphenol content than fermented cocoa beans with an acid; and (ii) optionally drying the nibs.
- the invention provides a process for producing red or purple cocoa- derived material, comprising the steps of:
- the cocoa-derived material in the tenth aspect may be as defined below and is preferably selected from cocoa nibs, flakes, such as refiner flakes or expeller flakes, cocoa cakes, cocoa powder, cocoa liquor, more preferably, selected from flakes, cocoa powder and cocoa liquor.
- the cocoa-derived material is preferably red or purple after treating.
- cocoa-derived material includes cocoa nib, refiner flakes, expeller flakes, cocoa cakes, cocoa powder, cocoa liquor and sweetened or unsweetened chocolate, milk chocolate or white chocolate. These are all terms well- known to a person skilled in the art (see Chocolate, Cocoa, and Confectionery: Science and Technology by Bernard W. Minifie Springer; 3 edition (December 15, 1988)).
- nib refers to the cocoa bean without the shell and may comprise 54% fat and 46% non-fat solids on a dry weight basis.
- cocoa liquor refers to ground cocoa nibs and it can be separated into cocoa butter and cocoa solids.
- cocoa flakes refers to cocoa liquor in the form of solid flakes; they typically have a fat content of 54%.
- expeller flakes refers to flakes produced from an expeller press. The fat content of the expeller flakes is typically less than 20 wt.%.
- Cocoa butter is the fat component of chocolate liquor, whereas the remaining part of the chocolate liquor is cocoa solids or cocoa mass. What remains after the removal of the cocoa butter through pressing are cocoa cakes, disks with a thickness of, for example, approximately five centimetres. These cakes can be broken up and ground into a fine cocoa powder.
- cocoa cake refers to the cocoa solids or cocoa mass remaining after extraction of fat by pressing; it can be pulverized to form cocoa powder and so can be considered to be a compressed form of cocoa powder.
- Cocoa powder refers to cocoa solids with, for example, a total of from 0.5 to 26 wt.% of fat where the fat is cocoa butter. Typically, cocoa powders comprise 20 to 22 wt.% fat. Defatted cocoa powders can be produced which comprise reduced (10 to 12 wt.% fat) or substantially no cocoa butter or cocoa fat.
- chocolate is generally obtained by mixing sugar and cocoa butter with cocoa liquor or cocoa nibs, followed by refining, conching and tempering. Milk chocolate is prepared in a similar way but with the addition of milk. White chocolate is prepared in a similar way to milk chocolate but without the addition of cocoa liquor.
- Figure 1 shows the variation in colour parameters L* and h° with the length of time of the process according to the present invention, for a defatted cocoa liquor as powder.
- Figure 2 shows the variation in colour parameters L* and C* with the length of time of the process according to the present invention, for a defatted cocoa liquor as powder.
- Figure 3 shows the variation in colour parameters and polyphenol content with the length of time of the process according to the present invention, for a defatted cocoa liquor as powder.
- Figure 4 shows the variation in colour parameters L* and h° with the length of time of the process according to the present invention, for a cocoa liquor melted and liquid and an external colour at 40°C.
- Figure 5 shows the variation in colour parameters L* and C* with the length of time of the process according to the present invention, for a cocoa liquor melted and liquid and an external colour at 40°C.
- Figure 6 shows the variation in colour parameters and polyphenol content with the length of time of the process according to the present invention, for a cocoa liquor.
- Figure 7 shows a coloured yoghurt produced using a cocoa powder according to the invention.
- Figure 8 shows a suitable mold for a cooked candy according to the invention.
- Figure 9 shows a cooked candy disk for colorimetric analysis.
- Figure 10 shows cooked candies made with acidified cocoa powder (bottom three candies) and non-acidified cocoa powder (top six candies).
- the colours of the candies made with acidified cocoa powder are varying shades of red.
- the colours of the candies made with non-acidified cocoa powder are black to brown in appearance.
- Figure 11 shows the powders produced by the trials in Example 9.
- Figure 12 shows the powders produced by the trials in Example 10.
- Figure 13 shows the powder produced by the scale-up in Example 11.
- Figure 14 shows the cocoa liquor from LB02 in Example 11.
- Figure 15 shows the apparatus used for Example 12.
- Figure 16 shows the powders obtained from Example 12.
- Figures 17 and 18 show a preferred process for producing powders according to the invention.
- the present invention can be considered to recognise that cocoa-based materials having a suitable combination of bacterial content, polyphenols content and colour can be produced.
- the present invention can be considered to relate, at least in part, to the finding that red or purple cocoa-derived materials can be produced from cocoa nibs obtained from cocoa beans or seeds which have a higher polyphenol content than a fermented cocoa bean or seed, using an acid having a suitable pK a .
- the present invention recognises that if the acidic conditions used to produce the red or purple cocoa-derived materials are controlled, in particular if the pH, water content, temperature and length of reaction are controlled, then the level of polyphenols present in a cocoa bean or seed can be preserved to a particular degree in the cocoa-derived material and particular colours can be produced.
- the cocoa beans or seeds used in the present invention may be any variety from Theobroma cacao, for example, Forastero, Criollo, or Trinitario, obtained from any suitable source such as, for example, the Ivory Coast, Brazil, Nigeria, Cameroon, Indonesia and Ghana. It is preferred, however, that the beans or seeds are unfermented and dried, preferably in the sun, or cocoa beans called "lavados” beans, such as from Brazil. These "lavados" beans are beans which are unfermented and washed.
- the colour of cocoa powders can be specified by means of colour coordinates.
- a frequently used system has been developed by R. S. Hunter.
- the colour coordinates are denoted by the characters L*, a* and b*, C* and h°. These colour coordinates are described more fully in Hunter, R. S., The Measurement of Appearance, John Wiley and Sons, New York, 1975.
- the value of the colour coordinates can be determined with an appropriate measuring system.
- the L-, a- and b- values of cocoa powder may be determined, for example, with the Hunterlab Digital Colour Difference Meter, type D 25 D 2 A.
- a method for colour measurement involves suspending cocoa powder at a 2.5% by weight level in an aqueous gelatin solution.
- the solution contains 5.0% gelatin and 0.06% of titanium dioxide which is used as a whitener in order to raise the L value of the suspension to a level where it is easier to differentiate similarly-coloured samples.
- the suspension is placed in a petri dish and quickly cooled to 60°F (15.6°C) to form a solid gelatin disk. Each sample is measured four times through the bottom of the dish using a colorimeter with the well-known Hunter L, a, b scale.
- the colour of the cocoa-derived material is preferably measured as follows.
- the cocoa-derived material preferably cocoa liquor, comprising polyphenols is preferably defatted with, for example, petroleum-ether, followed by washing and centrifugation.
- defatted we preferably mean that less than 5 wt.%, more preferably less than 1 wt.% fat is present, such as about 0 wt.% fat; preferably a powder is formed.
- each sample is placed in a petri dish and measured four times through the bottom of the dish using a colorimeter with the well-known Hunter L*, a*, b* scale.
- the colorimeter used is the Minolta CM-2002 spectrophotometer.
- the conditions for colour measurement are: CIELAB III: D65, Obs: 10°, 3 flashes, mode: SCE and external colour at 20°C.
- red preferably means that the cocoa-derived material, as defined above, when in the form of a powder (optionally after having been defatted), has an L* value of from about 39 to 48, preferably from about 40 to 45, more preferably from about 40 to 43, most preferably from about 40 to 42, an a* to b* ratio of greater than about 1.6, such as greater than about 1.8, more preferably greater than about 2.0, such as from about 2.2 to 3.2, most preferably from about 2.4 to 3.1, a C* value of greater than about 22, such as greater than about 25, preferably from about 25 to 34, more preferably from about 28 to 33, such as from about 30 to 33, and a h° value of from about 16 to 32, preferably from about 17 to 30, more preferably from about 17 to 25, as measured according to the above method.
- the term "red” preferably means that the L* value is from about 40 to 57, preferably from about 42 to 52 more preferably from about 44 to 48, the C* value is from about 18 to 40, preferably from about 25 to 35, more preferably greater than 18 or 30, an h c value of from about 7 to 40, preferably from 10 to 35, more preferably greater than 7, and optionally an a* to b* ratio of from about 1 to 8, preferably from 3 to 6, more preferably from 4 to 5.
- the term “purple”, as defined herein, preferably means that the cocoa-derived material, as defined above, when in the form of a powder (optionally after having been defatted), has an L* value of greater than 46, preferably from about 47 to 57, more preferably from about 48 to 56, most preferably from about 50 to 56, such as from 52 to 56, optionally an a* to b* ratio of less than about 2.3, such as less than about 1.8, more preferably from about 1 to 2.1 , such as from 1.5 to 2.1 , a C* value of less than or about 18, preferably from about 10 to about 17, such as from 11 to 15, and a h° value of from about 20 to about 50, preferably from about 25 to 40 or 25 to 30, as measured according to the above method.
- the terms “red” and “purple” may also be considered to encompass other shades of these colours in substantially the same wavelength, such as pink, mauve, violet and parme.
- the optionally defatted cocoa-derived material such as a defatted cocoa liquor, in the form of a powder, has an L* value of from about 40 to 45, a C* value of from about 28 to 33, an h° value of from about 17 to 25 and optionally an a* to b* ratio of from about 2.2 to 3.1.
- the optionally defatted cocoa-derived material such as a defatted cocoa liquor in the form of a powder, has an L* value of from about 41 to 42, a C* value of from about 32 to 33, an h° value of from about 17 to 19 and optionally an a* to b* ratio of from about 2.8 to 3.1 , as measured according to the above method.
- the term "purple” preferably means an L* value of from about 48 to 56, optionally an a* to b* ratio of from about 1 to 2.1 , such as from 1.5 to 2.1 , a C* value of from about 10 to about 17, such as from 11 to 15, and a h° value of from about 25 to 40 or 25 to 30, as measured according to the above method.
- red or “purple” in relation to cocoa-nibs, refiner or expeller flakes, cocoa liquor and cocoa cake preferably refers to material which can be obtained from, or which produces, cocoa liquor which when defatted has the defined L* values, C* values, h° values and a* to b* ratios, as measured by the specified method above.
- red or purple as used herein preferably refer to cocoa-derived material which has been acidified, or treated with an acid, such as according to the process of the invention as defined in any embodiment herein.
- the acid preferably produces a colour change.
- red and purple may or may not be synonymous with “acidified” or “treated with acid”.
- the external colour of chocolate is measured with spectrocolorimeter optionally after tempering, according to the method of the invention.
- the acidified cocoa-derived materials as defined herein are preferably red or purple, such as defined above.
- Acidified red or purple cocoa nibs are cocoa nibs which were not initially red or purple but which have been subjected to an acid for a sufficient amount of time to become red or purple, preferably as defined above.
- the nibs are preferably dried.
- the moisture or water content of the nibs may be less than 15 wt.%, such as less than 10 wt.%, preferably less than 5 wt.%, for example from 1 to 4 wt.%.
- Polyphenols are a diverse group of compounds (Ferriera et al., "Diversity of Structure and Function in Oligomeric Flavanoids, Tetrahedron, 48:10, 1743-1803, 1992). They occur widely in a variety of plants, some of which enter into the food chain. In some cases they represent an important class of compounds for the human diet.
- polyphenol we mean the well-known group of chemical substances that are found in plants, characterised by the presence more than one phenol group per molecule. Polyphenols are often present as monomers, dimers, trimers and other oligomers. Flavonoids are a subset of polyphenol. Cocoa contains polyphenols such as catechin, epicatechin, gallocatechin, epigallocatechin, epicatechin gallate, epigallocatechin gallate, procyanidins, prodelphinidins, and propelargonidine. Preferred polyphenols include catechin, epicatechin, procyanidins A2, B1 to B5, and C-1. Polyphenols with a molecular weight of less than 3000 are preferred.
- Cocoa has been described as being rich in a particular subgroup of flavonoids named flavanols (flavan-3-ols).
- the flavanols are present as the monomers epicatechin and catechin or as oligomers of epicatechin and/or catechin called procyanidins.
- Epicatechin, catechin and the procyanidins such as, for example, procyanidins B1 , B2 and B3, are the predominant class of polyphenols in cocoa and in any embodiment of the invention, the term "polyphenols" is intended to include or mean these compounds.
- the amount of polyphenols measured as the epicatechin equivalent, can be determined using the Folin-C 10 calteu reagent (Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-C 10 calteu reagent. Meth Enzymol 1999; 99: 152-178).
- the amount of polyphenols can also be determined using high performance liquid chromatography (HPLC).
- HPLC high performance liquid chromatography
- the amount of polyphenols is provided as the epicatechin equivalent in mg/g in the present invention unless stated otherwise.
- the acidified, preferably red or purple cocoa nibs of the invention preferably comprise at least 3 wt.%, preferably about 5 wt.% based on the total weight of the nibs of polyphenols which are naturally present in the untreated, unfermented parent cocoa seeds or cocoa beans obtained directly from the pod.
- the amount of polyphenols as measured by the Folin-C 10 calteu method in epicatechin equivalent, may be from 0 to 15 wt.%, preferably from 2 to 10 wt.%.
- the amount of polyphenols remaining in the cocoa nibs may be varied by controlling the conditions of acidification and can be measured using routine techniques.
- the red or purple cocoa nibs of the invention preferably comprise about 50 mg of polyphenols per gram of nibs.
- Acidified cocoa-derived materials other than cocoa nibs, preferably comprise the amount of polyphenols set out above for cocoa nibs (by wt.% or gram of material).
- the acidified cocoa nibs of the invention are preferably acidified cocoa nibs from cocoa seeds or cocoa beans that have a higher polyphenol content than a fermented cocoa bean.
- the fermented cocoa bean for comparison may be the parent cocoa bean or seed.
- the cocoa nibs which have the higher polyphenol content are preferably underfermented or unfermented cocoa beans or seeds. Unfermented and underfermented cocoa beans or seeds have a higher polyphenol content than fermented beans. Preferably, the cocoa beans or seeds are unfermented.
- the unfermented cocoa beans or seeds are beans or seeds which are obtained directly from cocoa pods and which have not been subjected to processing other than separation from the pulp.
- the unfermented cocoa beans or seeds are cocoa beans or seeds which have been depodded and have not been subjected to processing other than washing with, for example, water and optionally drying in the sun.
- Cocoa beans or seeds which have been fermented can be distinguished from unfermented cocoa beans on the basis of their colour. Thus, fully fermented cocoa beans are predominantly brown. Unfermented cocoa beans or seeds are predominantly slaty and may have blue, purple or violet parts on their surface. It will be understood by a person skilled in the art that the cocoa materials derived from these beans will not be coloured, that is products obtained from the beans will not be coloured in the absence of the process of the invention.
- Underfermented beans are beans which have been fermented for up to 3 days. These beans are usually purple, blue and/or violet and may also be slaty, but not predominantly. It will be understood by a person skilled in the art that the cocoa materials derived from these beans will not be coloured, that is products obtained from the beans will not be coloured in the absence of the process of the invention.
- fertilizers it is intended to mean beans which have been fermented for more than 3 days, such as between 3 and 7 days.
- the term “fermented beans” also includes beans which have been overfermented i.e., fermented for more than 7 days, for example up to 15 days.
- cocoa-derived materials to be produced according to the invention are preferably derived from the cocoa beans or seeds described above.
- cocoa powder or cocoa liquor derived from unfermented or underfermented cocoa beans or seeds i.e. unfermented or underfermented cocoa powder or cocoa liquor
- an acid as described herein.
- red or purple cocoa liquor is produced.
- the acid treated cocoa liquor may then be used in the production of chocolate, in particular red or purple chocolate.
- the acidified nibs of the invention are preferably further processed to produce red or purple cocoa refiner flakes or expeller flakes.
- the refiner or expeller flakes may be produced from the nibs using any means which does not substantially affect the polyphenol content of the flakes. It is preferred, for example, to avoid heating during the production of flakes, for example as in a pin mill.
- the flakes of the invention may, for example, be produced using a 3 roll refiner or other equivalent means or expeller, such as an expeller press well known in the art.
- a red or purple cocoa liquor is preferably obtained from the acidified cocoa nibs of the invention or by direct treatment of cocoa liquor with acid as defined herein.
- the cocoa liquor is typically a viscous pasty substance which can be used as the basic ingredient for chocolate.
- the red or purple cocoa liquor is obtained by grinding the acidified cocoa nibs at a low temperature using, for example, a triple stone mill, or a 3, 4 or 5 rolls refiner.
- the temperature of the mill or refiner is preferably from 10 to 60°C, more preferably from 20 to 40°C, measured as the temperature set on the refiner or mill.
- the red or purple cocoa liquor may be obtained by gentle melting of the refiner flakes or expeller flakes of the invention.
- the refiner or expeller flakes may be melted at a temperature of from 40 to 60°C, preferably from 42 to 50°C.
- cocoa liquor or a composition comprising cocoa liquor such as chocolate, obtained from beans or seeds which have a higher polyphenol content than fermented cocoa beans (such as unfermented or underfermented cocoa beans or seeds) may be acidified or treated with an acid as described herein.
- the cocoa liquor may be acidified directly in the conche.
- the cocoa liquor is red or purple, preferably as defined herein.
- the red or purple cocoa liquor can be further processed by extraction and/or pressing or expelling to separate the cocoa butter and the cocoa powder.
- red or purple cocoa cake and cocoa powder can be obtained from the acidified cocoa nibs of the invention.
- the pressing is carried out at a temperature of from about 70 to 100°C, such as about 80°C.
- the red or purple cocoa cake or expeller flakes obtained by pressing or expelling can be ground to produce the cocoa powder according to known means.
- Defatted cocoa powder may be prepared by a defatting treatment with a supercritical fluid.
- the supercritical fluid may comprise any solvent which will not leave a toxic residue. While cocoa powders defatted with solvents other than CO 2 may be used, such as hexane and propane, CO 2 is preferred. The latter is a substance present in ambient air, as well as body tissues and fluids. Hence it is ideal for food processing.
- the red or purple refiner flakes, red or purple cocoa liquor and red or purple cocoa powder of the invention are acidified i.e., they are obtained or derived from acidified cocoa nibs or other suitable cocoa-derived material.
- the red or purple cocoa powder of the invention preferably has a pH within the range of 2 to 8. More preferably, the pH of the cocoa powder is less than 7, such as, for example, from 2 to 5.
- the red or purple cocoa powder of the invention may therefore be compatible with an acidic environment such as that found in yoghurt.
- the cocoa refiner or expeller flakes and/or the cocoa liquor and/or the cocoa cake and/or the cocoa powder, which are preferably red or purple, according to the invention may be incorporated into any one of food products, such as confectionery products, bakery products and dairy products in place of and/or in addition to traditional flakes, liquor, cake or powder.
- the amount of flakes, liquor, cake or cocoa powder according to the invention, incorporated into the food, confectionery, bakery or dairy products may, for example, be from 1 wt.% to 50 wt.% based on the total weight of the product, such as from 5 to 30 wt.%, more preferably from 10 to 20 wt.%.
- the amount of flakes, liquor, cake or cocoa powder used is sufficient to impart a red or purple colour to the product.
- the food product is a liquid e.g., a beverage or a solid which is packaged or labelled for use as a foodstuff.
- the food product may be savoury i.e., comprise meat and/or fish and/or vegetables and/or eggs and/or dairy products and/or be sweet i.e., comprise sugar and/or butter and/or fruit.
- the confectionery product is selected from the group consisting of cooked candies, chocolate, chocolate-like products, fat continuous fillings and water-based fillings.
- the chocolate or chocolate-like product is preferably red or purple.
- Chocolate-like products are materials in which at least a part of the cocoa butter in chocolate is replaced by another fat, such as butterfat or a vegetable fat such as a cocoa butter equivalent (CBE).
- another fat such as butterfat or a vegetable fat such as a cocoa butter equivalent (CBE).
- a preferred process for producing a chocolate or chocolate-like product comprises the steps of:
- cocoa butter replacements include, cocoa butter equivalents, butter fat or fractions thereof, palm oil or fractions thereof, coconut or fractions thereof, palm kernel oil or fractions thereof, liquid vegetable oils, interesterified mixtures of the above fats or fractions or hardened components thereof, or mixtures thereof
- the chocolate or chocolate-like product is preferably red or purple.
- the cocoa liquor may also be heated, if necessary, to produce a paste.
- the paste/cocoa liquor may be fatted or defatted.
- the paste/cocoa liquor is combined with sugar or a sugar substitute.
- sugar substitutes include sweeteners, fructooligosaccharides and polyols, such as, for example, fructose, lactose and dextrose.
- the weight ratio of paste/cocoa liquor to sugar or sugar substitute is preferably from 3:1 to 1 :3, more preferably from 2:1 to 1 :2, such as about 1 :1.
- the mixture of paste/cocoa liquor and sugar or sugar substitute may be refined, preferably after combination with the cocoa butter or replacement, using any of the known techniques in the art. It is preferred that the refining conditions are selected such that any red or purple colour is maintained.
- the cocoa butter which may be obtained from any source, but preferably is cocoa butter obtained from red or purple cocoa liquor according to the invention, may be added to the mixture in order to liquefy it and achieve a total fat content of, for example, less than 60 wt.%, preferably from 30 to 40 wt.%, based on the total weight of the chocolate or chocolate-like product.
- Flavourings may optionally be added to the chocolate or chocolate-like material. Suitable flavourings include natural vanilla or those indicated below.
- the process for producing chocolate preferably also comprises the steps of conching, tempering and optionally molding.
- the chocolate may be produced according to the well-known methods in the art.
- Suitable dairy products include, for example, milk.
- the milk is preferably obtained from cows.
- the milk may alternatively be soya milk.
- the milk may be low-fat, skimmed or powdered milk.
- Other dairy products include ice-cream, such as low-fat and low-sugar ice-cream, cream, yoghurt, and dessert.
- Bakery products include, for example, breads, cakes and biscuits.
- the confectionery product comprises a fat continuous filling. This filling typically comprises solid particles (preferably in the form of fine particles) dispersed in a fat phase.
- the filling may be a reduced fat and/or a reduced sucrose filling.
- the filling comprises one or more cocoa based materials.
- the cocoa based materials may be selected from the group consisting of cocoa powder (preferably defatted cocoa powder), chocolate powder, cocoa mass, cocoa liquor and mixtures thereof produced according to the invention.
- the filling comprises from 5 to 40% by weight of cocoa powder (preferably defatted cocoa powder), more preferably from 10 to 30% by weight or from 12 to 23% by weight, most preferably from 15 to 20% by weight of cocoa powder (preferably defatted cocoa powder).
- the filling may constitute from 30 to 85% by weight of the confectionery product, preferably from 45 to 80% by weight, such as from 55 to 75% by weight of the confectionery product.
- the confectionery product such as chocolate, optionally comprises one or more flavourings.
- suitable flavourings include, but are not limited to, fruit, nut, and vanilla flavourings, fruit powder and pieces, nuts, vanilla, herbs, herb flavourings, caramel and caramel flavourings, spices and extracts from flowers, such as rose.
- Those skilled in the art are familiar with numerous flavourings that can be selected for use in this invention.
- the red or purple cocoa powder according to the invention can also be used as a natural colouring or flavouring agent, such as, for example, in food, confectionery, baking or dairy products and so reduce the need for artificial colourings.
- the powder may be used as a colouring or flavouring agent in the domestic or industrial kitchen.
- the powder may be sprinkled over foods, confectionery, baking or dairy products or be used in beverages.
- the powder may be packaged in sachets, for multiple or single use, for example.
- the confectionery products may each take any suitable form. For example, they may each (separately) be packaged and sold as a block or bar.
- the confectionery product may take any suitable form.
- the confectionery product is chocolate.
- the confectionery products are preferably bite-sized and generally weigh from 2 to 40g, such as from 3 to 2Og.
- the confectionery products are typically packaged and sold in a box, generally comprising more than one confectionery product.
- the confectionery product of the invention may comprise one or more food additives such as biscuit, nuts (whole or pieces), crispies, sponge, wafer or fruit, such as cherries, ginger and raisins or other dried fruit. These are typically embedded in the product.
- the confectionery product is dusted with additives (as above) or flavourings such as cocoa powder and/or sugar.
- the present invention provides a process for producing red or purple cocoa-derived material, comprising the steps of:
- the nibs may be dried such that the moisture or water content of the nibs is preferably less than 15 wt.%, such as less than 10 wt.%, preferably less than 5 wt.%, for example from 1 to 4 wt.%.
- the treated nibs are preferably dried by being extruded so as to reduce the moisture level and optionally microbial content.
- the temperature in the extruder may suitably be from 50 to 200°C, preferably from 70 to 150°C, such as from 90 to 130°C.
- the temperature within the extruder may also vary within these ranges. It has been found that such conditions can provide dried flakes which are coloured and have an acceptable microbial count and polyphenol content.
- the treatment in step (i) may, for example, comprise soaking or immersion of the nibs and/or spraying of the nibs with an acid/acidic solution and/or washing the nibs with an acid/acidic solution.
- the nibs may be added to an acid/acidic solution or vice versa.
- the red or purple cocoa nibs produced according to the above process preferably comprise at least 3 wt.%, preferably about 5 wt.% based on the total weight of the nibs of polyphenols which are naturally present in the untreated, unfermented parent cocoa seeds or cocoa beans obtained directly from the pod.
- the amount of polyphenols as measured by the Folin-C 10 calteu method in epicatechin equivalent, may be from 0 to 15 wt.%, preferably from 2 to 10 wt.%.
- the amount of polyphenols remaining in the cocoa nibs may be varied by controlling the conditions of acidification and can be measured using routine techniques. The acidity can be measured using routine techniques, for example with a pH meter.
- cocoa nibs used in the process of the invention or obtainable by the process of the invention are not obtained from fermented cocoa beans, nor are they already red or purple.
- the cocoa nibs or other cocoa-derived materials used in the process of present invention or obtainable by the process of the invention are not roasted or treated with alkali prior to acid treatment.
- the cocoa nibs used to obtain red or purple cocoa nibs are untreated, other than by washing with water, drying, size reduction, or limited fermentation, prior to treatment with acid.
- the process of the present invention preferably does not comprise any step of alkalisation or roasting i.e., before and after acidification.
- the cocoa nibs are obtained from cocoa beans or seeds that have a higher polyphenol content than a fermented cocoa bean.
- the fermented cocoa bean for comparison may be the parent cocoa bean or seed.
- the cocoa nibs which have the higher polyphenol content are preferably obtained from underfermented and/or unfermented cocoa beans. Unfermented and underfermented cocoa beans, and their nibs, have a higher polyphenol content than fermented beans, and their nibs, and can be distinguished from fermented beans as explained above.
- the invention provides a process for producing red or purple cocoa- derived material, comprising the steps of:
- cocoa-derived material obtained from beans or seeds which have a higher polyphenol content than fermented cocoa beans with an acid to form red or purple cocoa-derived material, optionally having a polyphenol content as described above;
- the cocoa-derived material produced is preferably chocolate or chocolate-like product or a precursor thereof, and the treated material is preferably cocoa liquor or a composition comprising cocoa liquor, preferably unfermented or underfermented, optionally acidified directly in the conche which optionally comprises other components.
- the cocoa liquor may be acticoaTM cocoa liquor.
- the term "conch” or “conche” preferably refers to a composition comprising cocoa liquor.
- the conch may also comprise other components such as those typically used for making chocolate.
- the conch has been, is being or will be treated with acid or has been, is being or will be subjected to the process of conching.
- the conching process or process of or comprising conching is continuous or batchwise, preferably continuous.
- the cocoa liquor or conch is treated with acid during conching.
- the amount of polyphenols in the treated cocoa-derived material may be as described above for cocoa nibs.
- the acid treatment may be the same as for cocoa nibs.
- cocoa-derived material is defined above and includes cocoa nibs, refiner or expeller flakes, cocoa liquor, cocoa cake, cocoa powder and chocolate. Clearly, where refiner or expeller flakes, cocoa liquor and chocolate are produced, additional steps, other than those defined above will be involved in order to obtain the specific product.
- the cocoa-derived material is refiner or expeller flakes and the process preferably further comprises the step of:
- the cocoa-derived material is cocoa liquor and the process preferably further comprises the step of:
- the cocoa-derived material is red or purple chocolate or chocolate-like material and the process preferably further comprises the steps of: (iv) treating the nibs to form a cocoa liquor preferably as defined above for obtaining cocoa liquor; and (v) combining the cocoa liquor with cocoa butter or a replacement fat to form a red or purple chocolate product or chocolate-like product.
- process for producing red or purple chocolate may comprise the steps of conching, tempering and molding.
- the process of the present invention comprises acidification or treatment with an acid of a cocoa liquor, which has not previously been treated with an acid, preferably directly or when present in the conche.
- the cocoa liquor is preferably unfermented or underfermented.
- the cocoa liquor is acticoaTM cocoa liquor.
- the cocoa liquor, or a composition comprising the cocoa liquor such as chocolate or chocolate-like product is treated with acid, preferably after the step of refining, and preferably before, during or after the process of conching, such as during conching.
- the acid may be added at any stage of the conching process.
- the treatment with acid may be as defined in any of the embodiments herein.
- the acid is citric acid, optionally in the form of citric acid such as powder or crystals.
- the conching time is preferably from 1 to 5 hours, such as from 2 to 4 hours.
- the temperature of the conching may be carried out from 40 to 80°C, such as from 50 to 70°C.
- the acid such as citric acid, may be added in an amount of from 0.1 to 2 wt.% based on the weight of the composition to be treated.
- the composition to be treated may comprise other components typically used to produce chocolate, in addition to cocoa liquor. Water may optionally be added in an amount of 0.1 to 5 wt.%, preferably 1 to 2 wt.% based on the weight of the composition.
- the process may produce red or purple chocolate.
- the chocolate preferably produced according to the process, without defatting, preferably comprises an L* value of from about 20 to 25, a C* value of from about 10 to 20, such as from 12 to 18, an h° value of less than about 1 , preferably from about 0.5 to 0.8, and optionally an a* to b* ratio of from 0.5 to 2.0, such as from 0.9 to 1.6.
- the red or purple cocoa nibs preferably produced according to the process of the invention may comprise at least 20 mg/g of polyphenols, preferably more than 30 mg/g of polyphenols, most preferably from 40 to 60 mg/g of polyphenols, measured as the epicatechin equivalent.
- the acid for use in treating the cocoa nibs, or other cocoa-derived material such as cocoa powder and cocoa liquor may be any acid which has a suitable pK s to produce the red or purple cocoa nibs or other treated cocoa-derived material.
- the acid may be a mineral acid, such as, for example, hydrochloric acid, phosphoric acid or sulphuric acid, or may be an organic acid, such as, for example, one or more of citric acid, lactic acid, tartaric acid, ascorbic acid and acetic acid.
- the acid is a food-grade acceptable acid.
- the acid may be added to the cocoa-derived material, such as cocoa nibs, cocoa powder or cocoa liquor in the form of a solid, such as, for example, a powder.
- a powder such as, for example, citric acid or tartaric acid.
- the acid used in the process is preferably a mineral acid, more preferably a food-grade mineral acid, such as phosphoric acid.
- the acid comprises one or more of phosphoric acid, lactic acid, citric acid, ascorbic acid or acetic acid.
- the acid is phosphoric acid.
- the acid is in the form of an acidic aqueous solution, obtained by combining the acid and water, for example, and this is used for treating the cocoa nibs.
- the acid/acidic solution preferably does not comprise an alcohol such as ethanol.
- the acid/acidic solution may preferably comprise from 0.5 wt.% to 20 wt.% acid, more preferably from 1 to 10 wt.%, most preferably from 2 to 5 wt.% acid based on the weight of the solution.
- the cocoa nibs are soaked or treated in an acidic solution.
- the nibs are treated, preferably in an aqueous solution, preferably at a pH of less than 6, more preferably at a pH of from about 1 to 4, such as from 2 to 3. Not all of the nibs may be submerged in the solution, but substantially all is preferred (such as, for example, greater than 70 wt.%, 80 wt.% or 90 wt.%).
- the nibs may be treated with the acid/acidic solution under agitation, for example by a magnetic stirrer or rod.
- the cocoa nibs are obtained from cocoa beans or seeds, such as Lavados beans, which are substantially unfermented.
- the beans are preferably not roasted since this can reduce the colouring potential.
- the preferred pH range for the acid treatment is from 2 to 4 and the particularly preferred acid is citric acid.
- the treated nibs are preferably dried by being extruded so as to reduce the moisture level and microbial content.
- the temperature in the extruder may suitably be from 50 to 200°C, preferably from 70 to 150°C, such as from 90 to 130°C. It has been found that such conditions can provide dried flakes which are coloured, contain levels of polyphenols as defined herein and have an acceptable microbial count.
- the production of red cocoa-derived material (preferably comprising a C* value of greater than about 18) may be obtained using a pH of less than 4, such as from 2 to 3.5, more preferably from 2 to 3.
- the production of purple cocoa-derived material (preferably comprising a C* value of less than about 18) may be obtained using a pH of greater than 4, such as from 4.2 to 6.6, more preferably from 4.5 to 6.0, particularly from 4.5 to 5.6.
- the amount of water, such as in the aqueous solution is preferably from about 1 to 1000 wt.% based on the weight of the cocoa-derived material, such as cocoa nibs, cocoa powder or cocoa liquor, more preferably from about 25 to 500 wt.%, particularly from about 100 to 300 wt.%.
- the nibs or other cocoa-derived material are treated with the acid for a period of up to about 24 hours, preferably up to 12 hours.
- the nibs may be treated with the acid for a period of from about 2 to 8 hours, preferably from about 3 to 6 hours. A period of from about 4 to 5 hours is particularly preferred.
- the soaking time may, however, be a few minutes, for example at least 5 minutes. Other preferred reaction times are from 20 to 60 minutes.
- reaction between the acid and the polyphenols in the cocoa nibs produces the redness or purpleness.
- the reaction can be instantaneous, although leaving the reaction mixture for longer can produce changes in the colour of the nib, such that the red deepens eventually to russet/bordeaux.
- the nibs are preferably treated at a temperature of less than or about 50°C, most preferably at a temperature of from 5 to 30°C.
- the cocoa nibs or other cocoa-derived material are treated with an acidic aqueous solution, preferably having a pH of from 1 to 3, for a period of from about 3 to 6 hours at a temperature of from 5 to 30 °C. It has been unexpectedly found that controlling the reaction conditions in this way produces red cocoa nibs from nibs obtained from unfermented or underfermented cocoa beans and can also operate so as to substantially preserve the level of polyphenols present in the cocoa nibs.
- the drying of the red or purple nibs in (ii) is carried out at a temperature such that the level of polyphenols in the nibs is substantially conserved (for example, greater than 80 wt.%, such as greater than 90 wt.% remains).
- the drying is preferably carried out at an ambient air temperature of less than about 115°C, more preferably at a temperature of from 40 to 100 °C, such as from 60 to 80°C.
- the drying may be carried out using an infra-red heater such as that available from Micronizing Company, UK., or using an extruder device for drying and reducing the microbial content.
- the nibs may be dried in a tornado drier, preferably at about 105°C for about 5 minutes.
- the cocoa nibs are not roasted.
- the term "roasted” will be understood by a person skilled in the art.
- the process is a non-enzymatic process, that is the process does not comprise the addition of an enzyme.
- the treating of the nibs with an acid is carried out when the nibs are in an expeller press.
- the temperature of the nibs is preferably below 40°C.
- Expeller presses are well known for extracting oil from raw materials.
- the process of the invention comprises treating the nibs with an acid, wherein the nibs are in an expeller press.
- the process may be a continuous or batch process. Preferably, it is a continuous process.
- the nibs are preferably red or purple.
- the nibs may be treated with the acid, as defined herein, in any part of an expeller apparatus, such as before flakes are formed.
- the nibs may be acidified in a heating zone, such as a mixing heater, before pressing and preferably before inserting into a worm press.
- the amount of acid, such as citric acid is preferably from about 1 to 20 wt.%, more preferably from 1.5 to 10 wt.% based on the weight of the nibs.
- the amount of water is preferably from about 1 to 20 wt.% based on the weight of the nibs, more preferably from about 2 to 10 wt.%.
- the nibs are treated with a 1 to 10%, such as from 3 to 7%, solution of acid, such as citric acid, in water.
- the nibs may be reacted with the acid for up to 24 hours, more preferably from 1 to 12 hours, such as from 2 to 4 hours.
- the acid is in an aqueous solution at a concentration of acid of 2 to 20 %.
- the pH of the solution may be less than 5, such as less than 4 and preferably from 3 to 4, for red flakes (a C* value of greater than about 18) and above 4 for purple flakes (a C* value of less than about 18).
- the nibs may be fed into a press such as a worm press.
- the heater/feeding screw flow is preferably from 30 to 200 kg nibs/h, more preferably from 40 to 110 kg nibs/h.
- the nibs can be separated into expeller flakes and cocoa butter.
- the pressure in the expeller may be from 0 to 100 bar, more preferably from about 20 to 80 bar, such as about 75 bar.
- the temperature in the press is preferably below the melting range for flakes and is preferably less than or about 40°C.
- the speed of the press is preferably from 10 to 100 kg flakes/h, more preferably from 20 to 80 kg flakes/h.
- the expeller flakes may have a fat content of less than 20 wt.%, preferably less than 18 wt.%, such as less than 10 wt%.
- the moisture content of the expeller flake may be less than 15 wt.%, such as less than 11 wt.%, for example, from 5 to 11 wt.%.
- the cocoa butter obtained may be further processed, for example by filtration and/or cetrifugation and/or sedimentation.
- the invention relates to the coloured, preferably red or purple, flakes produced according to the process.
- the flakes have preferably been extruded and optionally sterilized.
- sterilized we mean that the microbial content of the flakes after extrusion is less than the microbial content before extrusion.
- the microbial content may be reduced by greater than 90%, such as greater than 95%, for example, up to 99.99% following extrusion.
- the moisture content of the flakes may also be reduced by greater than 90%, such as greater than 95%, for example, up to 99.99% following extrusion.
- the flakes obtained according to the invention may also be further processed.
- the flakes are preferably subjected to nibbling and/or extruding such as defined above.
- the extruded flakes may then be formed into red or purple cocoa powder by, for example, milling to form the powder and optionally sieving.
- the powder may be packaged and/or palettized to form packed red or purple cocoa powder.
- the flow in the extruder is from 100 to 300 kg flakes, optionally nibbled/h more preferably from 150 to 250 kg/h.
- the extruder may contain 10 to 20%, such as 15%, water.
- the temperature may vary within the extruder. Thus, in an initial stage the temperature may be lower, such as less than 100°C than one or more later stages, which may be greater than 100°C i.e. the temperature may be ramped.
- drying of the nibs as described in any of the above embodiments of the invention is preferably carried out for a period of at least 2 hours, more preferably for at least 4 hours, most preferably for a period of about 12 hours. In the case of extrusion, drying may only be required for a few minutes, for example, from 5 to 20 minutes.
- the cocoa nibs used in the process of the invention are obtained from washed unfermented cocoa seeds, such as those processed in Brazil and known as "lavados" beans.
- the process of the invention may further comprising predrying and/or heating of cocoa beans or seeds, prior to acid treatment, to produce the cocoa nibs.
- the conditions of the predrying and/or heating are preferably controlled in order to avoid damaging the natural polyphenols.
- the heating and/or predrying may assist in winnowing i.e., removing the shells from the cocoa beans.
- an additional step is carried out which comprises reducing the size of the cocoa nibs by mechanical means before treatment with the acid or acidic solution.
- the nibs may be ground using a three roll refiner in order to expedite reaction and/or drying of the nibs following reaction.
- the temperature of the cooling jacket of the refiner is preferably set to 10 to 20°C.
- Unfermented cocoa seeds from Brazil were washed after depodding and dried.
- the depodded beans were then treated by predrying/heating (the conditions were such as to avoid damage of the natural pool of polyphenols in the beans) to ease removal of the shells.
- the nibs were soaked in the acidic solution for a period of up to 24 hours at ambient temperature.
- the colour of the nibs changed with the degree of soaking. Up to 24 hours the nibs have a reddish colour, comparable to raspberry red. If the reaction is allowed to continue, then the colour can become bordeaux / russet / red claret.
- the nibs were then separated from the soaking solution by sieving and dried in a heating cabinet at a temperature of 100 to 110°C for several hours. During the drying step, the colour of the nibs changed from reddish to russet.
- the nibs were then broken up on a three roll refiner (to avoid heating as in a pin mill).
- the end result was refiner flakes, not a liquor.
- the refiner flakes can be used to make chocolate, as set out below.
- the refiner flakes produced according to Example 1 were melted gently at 45°C to obtain a more or less liquid paste i.e., red cocoa liquor.
- the cocoa liquor was mixed with sugar in a 50:50 weight ratio. This mixture was refined and liquefied by the addition of cocoa butter in order to increase the total fat content to 35% and produce red chocolate.
- Natural vanilla was then added to the chocolate in order to mask the bitter taste.
- the result of the taste test was: very fruity and pleasant.
- the Folin-C 10 calteu reagent is a mixture of phosphotungstic acid (H 3 PW 13 O 40 ) and phosphomolybdic acid (H 3 PmO 12 O 40 ). Polyphenols are reduced by oxido-reduction to give a mix of blue oxides of tungsten (W 8 O 23 ) and molybdenum (MoO 23 ). The intensity of the coloration is measured at 760nm.
- the standards used for the Folin-C 10 calteu method are: Solution at 100mg/L and 5 daughter solutions at 0, 5, 10, 15 and 20 mg/L.
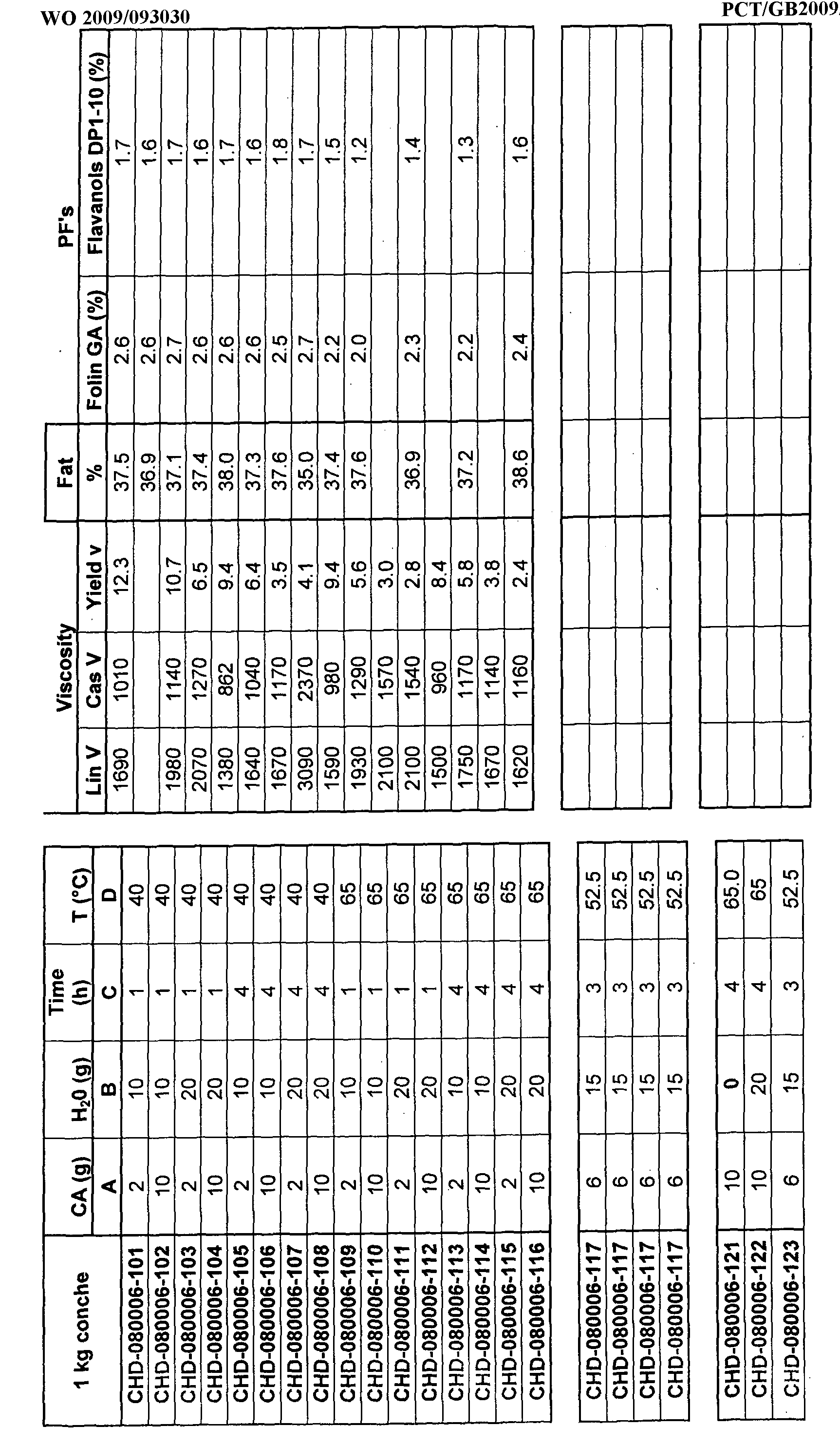
- the polyphenol content of cocoa-derived material produced according to the process of the invention is provided in Table 1.
- the majority of the polyphenols contained in cocoa beans are from the flavonoid family: C6-C3-C6.
- the monomers that are commonly present include (-)-epicatechin and (+)- catechin.
- the polyphenols contained in cocoa seeds include:
- the cocoa seed is a reservoir of epicatechin (it constitutes about 35% of the total polyphenol content).
- Epicatechin, catechin and the Procyanidins B1 , B2, B3 can be analysed by HPLC using a fluorescence detector.
- epicatechin, catechin and the procyanidins are extracted from the cocoa with a solution of acidified water/actonitrile.
- Filter syringe RC regenerated Cellulose
- fatty product was weighed.
- the fatty material in the product was extracted by adding hexane (to determine MG: the percentage of fatty material in the sample) to the cocoa followed by centrifugation and dephasing.
- the defatted extract was then sieved. 200 mg of the sieved extract was weighed into a 50 ml flask.
- the extract was dissolved in the extraction solvent in the ultrasonic bath for about 10 minutes and the extraction solvent used to make up the volume.
- the solution was filtered with a 0.45 ⁇ m filter syringe and placed in a vial.
- Aet is the area of the epicatechin standard
- Aech is the area of the epicatechin sample
- A is the concentration of epicatechin in the standard in mg/l
- B is the concentration of epicatechin in the sample in mg/l m is the weight of the defatted sieved extract
- MG is the percentage of the fatty material in the product
- cocoa-derived material A cocoa liquor obtained according to the invention, as indicated below, comprising polyphenols was defatted with petroleum-ether followed by three washing steps and centrifugation. After drying at room temperature, each powder sample was placed in a petri dish and measured four times through the bottom of the dish using a colorimeter with the well-known Hunter L*, a*, b* scale. The colorimeter used was the Minolta CM- 2002 spectrophotometer. The conditions for colour measurement were: CIELAB III: D65, Obs : 10°, 3 flashes, mode: SCE and external colour at 20°C.
- the progress of a process carried out according to the invention is shown in Table 2 in terms of colour parameters and polyphenol content for a fully defatted cocoa liquor in the form of a powder, as measured according to the above method.
- the reaction was carried out using a mixer to acidify the nibs and was carried out on lab scale (1/2kgs) except for the process mixer (pilot plant, 15kgs).
- a coloured yoghurt product was made by adding 1wt. %, based on the total weight of the yoghurt, of the cocoa powder obtained from nibs soaked for 4 hours under the conditions described in Example 1 to a commercially available natural yoghurt (i.e., which is uncoloured).
- a red cooked candy can be produced from:
- the citric acid was dissolved in the mixture of ethanol and water. In order to limit loss of the product it was preferred to weigh the mixture of ethanol and water in a beaker containing citric acid. The acidified cocoa powder was then dissolved in the mixture of citric acid, ethanol and water to form a homogeneous solution with stirring (Mixture 1).
- the isomaltol was heated in a pan at thermostat 2 for 1 minute 30 seconds, thermostat 3 for 3 minutes and thermostat 4 for 4 minutes. The isomalt was completely melted and colourless at this stage. This was followed by further heating at thermostat 1 for about 6 minutes. The isomalt was approaching crystallization. At this moment Mixture 1 was added to prevent evaporation of the alcohol and spraying of the powder around the vessel.
- Mixture 1 was stirred with the heated isomalt to form a homogeneous mixture.
- Aroma was then added - 40 drops of aroma with a pasteur pipette.
- the mixture containing the aroma was heated at thermostat 3 for a further 1 minute to make the mixture as homogenous as possible.
- Figure 10 shows the different colours for candies that it is possible to obtain using different cocoa powders.
- the following powders were used to produce the cooked candies in Figure 10, according to the method set out above.
- H (factor 1) 1 ⁇ 4 (- A + B - C + D)
- H (factor 2) 1 ⁇ 4 (- A - B + C + D)
- n number of trials (center point not included)
- t value for a risk of 5% and the degree of freedom is N-1 , where N is the number of centre trials (see Table below) 2- Interpretation of the effects
- the parameter When the value of the effect is negative, the parameter has a negative influence on the measured value. If we increase the value of this parameter, the measured value, such as pH and colour will decrease.
- the parameter When the value of the effect is positive, the parameter has a positive influence on the measured value. If we increase the value of this parameter, the measured value, such as pH and colour will increase.
- Polyphenol contents are provided in % of epicatechin on dried and defatted cocoa according to Folin's method (see Example 4).
- the samples obtained are shown in Figure 11.
- the trials with a C* value less than 18 are preferably considered to be non-limiting examples of purple.
- the trials with a C* value greater than 18 are preferably considered to be non-limiting examples of red.
- the quantity of acid has a positive influence on the ash content.
- the more acid we use the more ashes we will find in the final product.
- Parameter with two signs has more influence (positive or negative) than parameter with only one sign.
- Raw material Lavados brazil cocoa beans
- Acid citric acid or tartaric acid Water. 25% Time: 20 min Temperature: 50°C
- the colours and polyphenol content are provided for dried and defatted cocoa.
- the polyphenol is provided in % of epicatechin Folin's method.
- citric and tartaric acids are organic acids, we do not "find" them in the ashes anymore.
- citric acid is suitable for use in the acidification process of the invention. It is more interesting for low supply in ashes and easy manipulation. Also it seems that citric acid, which is weaker than phosphoric acid, is particularly useful for preserving the polyphenol content.
- Example 11 citric acid, which is weaker than phosphoric acid, is particularly useful for preserving the polyphenol content.
- a steam flow temperature of 70°C was used to regulate the double jacket on the pilot line. The flow was cut off when the jacket was at 70°C.
- the mixing time was slightly modified. To have a good moisture before drying, warm air was injected during the mixing step (5min only mixing and the rest of time mixing and air injection).
- the cooling system and the door of the Tornado were abraded slightly by the acid, because these parts are not made from stainless steel.
- This process enables a correct colour and an acceptable polyphenol content to be obtained with a reaction time of 40min.
- the moisture of the nibs after the drying is about 2-3%.
- the cut grinding step is more difficult than "Ball Milling" step.
- the height of a bag containing cocoa nibs (1 ) is raised over a conveying screw.
- the nibs are carried to a mixing heater (2), wherein the nibs are acidified, heated (50°C) and mixed.
- the red/purple nibs are inserted to a worm press (3).
- the screw is bound by a cage, that only lets the butter (4) go through.
- a screwplate (5) enables the flakes (6) to be expellled.
- the expeller flakes are picked up in a bag (7) and the butter is picked up in a box (8).
- This equipment enables a product called "Flakes" to be obtained from cocoa nibs. In the end the fat content of the flakes is about 10%.
- the colour is measured on dried and defatted cocoa.
- the microbiological results show that the level of moisture can be reduced significantly following extrusion, while also reducing the microbial level.
- the extruder therefore provides a convenient method for drying and sterilizing the flakes.
- the polyphenol content of the powder obtained from the flakes is as follows.
- ORAC hydr o reflects water- soluble antioxidant capacity.
- Trolox a water-soluble Vitamin E analog, is used as the calibration standard and the ORAC result is expressed as micromole Trolox equivalent (TE) per gram.
- the tannin content of the powder is provided in the following table (referring to Red Deep Purple CP).
- composition used in the experiments was as follows. All of the experiments were carried out using 1kg of the composition (referred to as "conche” in the tables below).
- composition used was:
- Acticoa® is a registered trademark of the Barry Callebaut Group.
- the polyphenol and fat content of the purple chocolate is as defined below.
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Food Science & Technology (AREA)
- Polymers & Plastics (AREA)
- Inorganic Chemistry (AREA)
- Microbiology (AREA)
- Confectionery (AREA)
Abstract
Description
Claims
Priority Applications (9)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2010543561A JP5323862B2 (en) | 2008-01-22 | 2009-01-22 | Composition |
| CA2712597A CA2712597C (en) | 2008-01-22 | 2009-01-22 | Process for producing acidified cocoa material comprising at least 20mg/g polyphenols |
| PL09704776T PL2237677T3 (en) | 2008-01-22 | 2009-01-22 | Process for producing cocoa-derived material |
| EP09704776A EP2237677B9 (en) | 2008-01-22 | 2009-01-22 | Process for producing cocoa-derived material |
| CN200980102736.7A CN101925306B (en) | 2008-01-22 | 2009-01-22 | Composition |
| ES09704776T ES2395151T3 (en) | 2008-01-22 | 2009-01-22 | Procedure for the production of cocoa derived material |
| MX2010008007A MX2010008007A (en) | 2008-01-22 | 2009-01-22 | Composition. |
| US12/456,971 US8460739B2 (en) | 2008-01-22 | 2009-06-25 | Process for making red or purple cocoa material |
| US13/898,217 US9107430B2 (en) | 2008-01-22 | 2013-05-20 | Process for producing red or purple cocoa-derived material |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB0801119.9 | 2008-01-22 | ||
| GBGB0801119.9A GB0801119D0 (en) | 2008-01-22 | 2008-01-22 | Composition |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/456,971 Continuation US8460739B2 (en) | 2008-01-22 | 2009-06-25 | Process for making red or purple cocoa material |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2009093030A1 true WO2009093030A1 (en) | 2009-07-30 |
Family
ID=39166141
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/GB2009/000177 WO2009093030A1 (en) | 2008-01-22 | 2009-01-22 | Composition |
Country Status (11)
| Country | Link |
|---|---|
| US (2) | US8460739B2 (en) |
| EP (1) | EP2237677B9 (en) |
| JP (1) | JP5323862B2 (en) |
| CN (1) | CN101925306B (en) |
| CA (1) | CA2712597C (en) |
| ES (1) | ES2395151T3 (en) |
| GB (1) | GB0801119D0 (en) |
| MX (1) | MX2010008007A (en) |
| MY (1) | MY150996A (en) |
| PL (1) | PL2237677T3 (en) |
| WO (1) | WO2009093030A1 (en) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20100151087A1 (en) * | 2008-01-22 | 2010-06-17 | Arnaud Dumarche | Composition |
| EP2222182A4 (en) * | 2007-11-19 | 2010-11-17 | Hershey Co | Process for preparing red cocoa ingredients, red chocolate, and food products |
| WO2011012680A3 (en) * | 2009-07-29 | 2011-03-31 | Barry Callebaut Ag | Microbial composition for the fermentation of cocoa material |
| US20150017302A1 (en) * | 2011-04-08 | 2015-01-15 | Harrold Glenn Anijs | Fruity flavored cocoa products and processes for producing such cocoa products |
| US10327453B2 (en) | 2013-06-25 | 2019-06-25 | Olam International Limited | Processes for producing dark red and dark brown natural cocoa |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20100173042A1 (en) * | 2008-12-12 | 2010-07-08 | Andrew McShea | Chocolate extract, process of making, and uses thereof |
| US20110236560A1 (en) * | 2010-02-11 | 2011-09-29 | Daniel Perlman | Food supplementation with phenolic antioxidants in vinegar |
| MX365153B (en) | 2013-06-25 | 2019-05-24 | Olam International Ltd | Process for producing dark brown natural cocoa. |
| GB2522007B (en) * | 2013-11-01 | 2018-04-11 | Tate & Lyle Custom Ingredients Llc | Method of preparing an alkalized carob |
| US10463057B2 (en) * | 2015-01-16 | 2019-11-05 | CocoTerra Company | Chocolate processing system and method |
| WO2018031569A1 (en) * | 2016-08-09 | 2018-02-15 | Cargill, Incorporated | Compositions comprising cocoa butter |
| EP4309509A3 (en) | 2016-12-29 | 2024-03-13 | Mars Incorporated | Systems and methods for reduced refining during cocoa liquor processing |
| WO2020010060A1 (en) * | 2018-07-02 | 2020-01-09 | Mars, Incorporated | Chocolate confection |
| US11470853B2 (en) | 2019-03-15 | 2022-10-18 | CocoTerra Company | Interface and application for designing a chocolate-making experience |
| US11547123B2 (en) * | 2019-08-16 | 2023-01-10 | The Folger Coffee Company | Methods for reducing negative flavor attributes in coffee and compositions therefrom |
| JPWO2022255350A1 (en) | 2021-05-31 | 2022-12-08 | ||
| JPWO2023032108A1 (en) * | 2021-09-01 | 2023-03-09 | ||
| DE102022109209A1 (en) | 2022-04-14 | 2023-10-19 | Heiko Langer | Oral administration agent with pumpkin seed oil |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2835585A (en) | 1953-07-10 | 1958-05-20 | Gen Foods Corp | Chocolate product and process therefor |
| US2957769A (en) * | 1958-04-03 | 1960-10-25 | Gen Foods Corp | Chocolate product and process therefor |
| US4784866A (en) * | 1985-11-07 | 1988-11-15 | Nestec S. A. | Process of alkalization of cocoa in aqueous phase |
| US5888562A (en) * | 1995-06-20 | 1999-03-30 | Nestec S.A. | Enzymatic treatment of cocoa |
| US20010007693A1 (en) | 1996-09-06 | 2001-07-12 | Mars, Incorporated | Food products having enhanced cocoa polyphenol content and processes for producing the same |
| WO2002063974A1 (en) | 2001-02-14 | 2002-08-22 | Societe Des Produits Nestle S.A. | Enzymatically hydrolysed cocoa in chocolate flavour reactions |
| WO2009067533A1 (en) | 2007-11-19 | 2009-05-28 | The Hershey Company | Process for preparing red cocoa ingredients, red chocolate, and food products |
Family Cites Families (98)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB14624A (en) | 1900-01-01 | |||
| CA840382A (en) | 1970-04-28 | M. Weber Meyer | Method of making a cocoa extract and cocoa flavored chocolate beverage | |
| GB189714624A (en) | 1897-06-16 | 1897-07-31 | Hans Christensen | A New or Improved Extract of Cocoa Husks, and a Method of Preparing the same. |
| US1892449A (en) | 1929-06-05 | 1932-12-27 | Lloyd M Brown | Process of treating chocolate liquor containing proteins, starches and fats |
| GB345250A (en) | 1929-10-07 | 1931-03-09 | Monsanto Chemical Works | Improvements in and relating to the recovery and purification of alkaloids from cocoa products |
| GB341000A (en) | 1929-10-07 | 1931-01-07 | Monsanto Chemical Works | Process for recovering valuable products from cocoa by-products |
| US2275835A (en) | 1940-10-03 | 1942-03-10 | Citro Chemical Co | Extraction of theobromine |
| GB562123A (en) | 1942-10-21 | 1944-06-19 | Inredeco Inc | Improvements in method of preparing a cocoa extract |
| US2380158A (en) | 1942-10-21 | 1945-07-10 | Inredeco Inc | Method of preparing a cocoa extract |
| US2397402A (en) | 1943-08-27 | 1946-03-26 | United Rexall Drug Company | Cocoa manufacture |
| US2512663A (en) | 1947-04-25 | 1950-06-27 | Choco Essence Inc | Chocolate essence or extract |
| GB653240A (en) | 1948-06-22 | 1951-05-09 | Gen Foods Corp | Improvements in or relating to the treatment of raw cacao and the product thereof |
| GB751121A (en) | 1953-07-10 | 1956-06-27 | Gen Foods Corp | Improvements in or relating to chocolate flavoring material |
| US2812663A (en) | 1955-07-20 | 1957-11-12 | Gilbert & Barker Mfg Co | Apparatus for sampling or injecting fluids |
| US2965490A (en) | 1959-04-02 | 1960-12-20 | Gen Foods Corp | Flavor product and process |
| IT954041B (en) | 1969-09-27 | 1973-08-30 | Carle E Montanari Spa | PROCEDURE FOR THE NEUTRALIZATION TREATMENT SOLUBILIZATION AND COLOR ENHANCEMENT OF COCOA POWDER WITH ALKALINE SOLUTIONS |
| DE2055030C3 (en) | 1970-11-09 | 1978-06-22 | Dragoco Gerberding & Co Gmbh, 3450 Holzminden | Process for the production of a cocoa extract |
| US4331692A (en) | 1971-07-23 | 1982-05-25 | Ulla Drevici | Cocoa fruits and products |
| SU646254A1 (en) | 1977-08-05 | 1979-02-05 | Предприятие П/Я А-3715 | Method of evaluating the fermentation degree of cacao-seeds |
| CH634726A5 (en) | 1978-09-29 | 1983-02-28 | Nestle Sa | PROCESS FOR THE PREPARATION OF ANTIOXYGEN SUBSTANCES. |
| US4318305A (en) | 1979-04-04 | 1982-03-09 | Deere & Company | Synchronized transmission |
| GB2059243B (en) | 1979-09-24 | 1983-06-08 | Anvar | Process for the fermentation of cocoa beans |
| NL8102377A (en) | 1981-05-14 | 1982-12-01 | Zaan Cacaofab Bv | ALKALIZED COCOA POWDER AND FOODSTUFFS INCLUDING SUCH POWDER. |
| JPS57206391A (en) | 1981-06-12 | 1982-12-17 | Morinaga & Co Ltd | Preparation of flavonoid natural pigment by enzymic treatment |
| NL8403748A (en) | 1984-12-10 | 1986-07-01 | Zaan Cacaofab Bv | COCOA POWDER. |
| JPS61173745A (en) | 1985-01-28 | 1986-08-05 | Miyoshi Oil & Fat Co Ltd | Chocolate-like food |
| JPS61239872A (en) | 1985-04-13 | 1986-10-25 | Naozo Kishida | Production of cocoa for drink |
| FR2609395B1 (en) | 1988-02-23 | 1992-01-10 | Serobiologiques Lab Sa | USE OF XANTHIC BASES AS ACTIVE INGREDIENT OF A PHARMACEUTICAL COMPOSITION, ESPECIALLY DERMATOLOGICAL, OR COSMETIC, COMPOSITION ESPECIALLY USEFUL AS BASE MATERIAL FOR THE PREPARATION OF PHARMACEUTICAL OR COSMETIC COMPOSITION AND PHARMACEUTICAL OR COSMETIC COMPOSITION THUS PREPARED |
| JP2527788B2 (en) | 1988-05-31 | 1996-08-28 | 株式会社ロッテ | Oral composition |
| US4970090A (en) | 1988-09-26 | 1990-11-13 | Mccormick & Company, Inc. | Enhanced cocoa extract flavorings |
| KR910004494B1 (en) | 1988-10-21 | 1991-07-05 | 롯데제과 주식회사 | Process for preparing chewing-gum |
| GB8901837D0 (en) | 1989-01-27 | 1989-03-15 | Beecham Group Plc | Novel compositions |
| DE3912819A1 (en) | 1989-04-19 | 1990-11-08 | Jacobs Suchard Ag | PROCESS FOR THE PRODUCTION OF COCOA EXTRACT |
| US5026482A (en) | 1989-09-26 | 1991-06-25 | Air Products And Chemicals, Inc. | Separation of liquid mixtures by concentration swing adsorption |
| FR2654935B1 (en) | 1989-11-28 | 1994-07-01 | Lvmh Rech | USE OF XANTHINES, WHICH MAY BE INCORPORATED IN LIPOSOMES, TO PROMOTE PIGMENTATION OF THE SKIN OR HAIR. |
| US5009917A (en) | 1989-12-18 | 1991-04-23 | Kraft General Foods, Inc. | Method for producing deep red and black cocoa |
| TW226324B (en) | 1990-02-12 | 1994-07-11 | Nestle Sa | |
| US5114730A (en) | 1990-11-21 | 1992-05-19 | Consolidated Flavor Corporation | Process for making dark cocoa |
| JPH0538255A (en) | 1991-08-05 | 1993-02-19 | Saneigen F F I Kk | Method for coloring chocolate to red color |
| JP2631805B2 (en) | 1992-03-24 | 1997-07-16 | 江崎グリコ株式会社 | Method for producing white cocoa nibs and food using the same |
| JPH06128164A (en) | 1992-10-16 | 1994-05-10 | Hitoshi Ito | Oral administration agent, beverage and food having anti-cancer activity |
| JP3095605B2 (en) | 1994-02-04 | 2000-10-10 | 明治製菓株式会社 | Health foods and beverages containing antioxidants and methods for producing antioxidants |
| JP3063818B2 (en) | 1994-04-01 | 2000-07-12 | 明治製菓株式会社 | Food and drink for gastric ulcer prevention |
| GB9419715D0 (en) | 1994-09-30 | 1994-11-16 | Boots Co Plc | Hair stimulant composition |
| US5554645A (en) | 1994-10-03 | 1996-09-10 | Mars, Incorporated | Antineoplastic cocoa extracts and methods for making and using the same |
| JPH08291018A (en) | 1995-04-18 | 1996-11-05 | Kanebo Ltd | Humectant for cosmetic |
| ES2099676B1 (en) | 1995-07-19 | 1997-11-16 | Moner Y Llacuna S A | NEW PRODUCT OF COCOA FIBER BASED ON ROASTED COCOA SHELL. |
| JPH09224606A (en) | 1996-02-19 | 1997-09-02 | Meiji Seika Kaisha Ltd | Health food and drink containing carcinostatics |
| JPH09234018A (en) | 1996-03-01 | 1997-09-09 | Meiji Seika Kaisha Ltd | Beverage and food product having preventing action on diabetic complication |
| GB9605076D0 (en) | 1996-03-11 | 1996-05-08 | Mars Uk Ltd | Drying method |
| US6261565B1 (en) | 1996-03-13 | 2001-07-17 | Archer Daniels Midland Company | Method of preparing and using isoflavones |
| US6297273B1 (en) | 1996-04-02 | 2001-10-02 | Mars, Inc. | Use of cocoa solids having high cocoa polyphenol content in tabletting compositions and capsule filling compositions |
| PL329325A1 (en) | 1996-04-02 | 1999-03-29 | Mars Inc | Cocoa extract compounds, method of obtaining them and their application |
| AU5711696A (en) | 1996-04-02 | 1997-10-22 | Mars, Incorporated | Cocoa extract compounds and methods for making and using the same |
| US6905715B1 (en) | 1996-09-06 | 2005-06-14 | Mars, Incorporated | Cocoa solids having a high cocoa procyanidin content |
| US7968140B2 (en) | 1997-09-08 | 2011-06-28 | Mars, Incorporated | Chocolates and chocolate liquor having an enhanced polyphenol content |
| US6015913A (en) | 1996-09-06 | 2000-01-18 | Mars, Incorporated | Method for producing fat and/or solids from cocoa beans |
| DE59712694D1 (en) | 1997-04-18 | 2006-08-24 | Fritz Stanislaus | STABILIZED MEDICAMENT CONTAINING CYSTEINYL DERIVATIVES |
| FR2768927B1 (en) | 1997-10-01 | 2000-01-21 | Lvmh Rech | USE OF ELLAGIC ACID, ITS SALTS, ITS METAL COMPLEXES, ITS MONO- OR POLY-ETHERS, MONO- OR POLY-ACYL DERIVATIVES IN THE FIELD OF COSMETICS AND PHARMACY, ESPECIALLY DERMATOLOGY |
| US8507018B2 (en) | 1998-03-12 | 2013-08-13 | Mars, Incorporated | Products containing polyphenol(s) and L-arginine and methods of use thereof |
| ATE242795T1 (en) | 1998-03-13 | 2003-06-15 | Frische Gmbh | METHOD FOR SOLVENT EXTRACTION OF HYDROPHOBIC COMPOUNDS |
| JPH11308978A (en) | 1998-04-28 | 1999-11-09 | Meiji Seika Kaisha Ltd | Hypercholesterolemia preventive beverage and food |
| CA2333926A1 (en) | 1998-06-01 | 1999-12-09 | Kaneka Corporation | Method to control atom transfer radical polymerization |
| ATE405169T1 (en) | 1998-06-18 | 2008-09-15 | Meiji Seika Kaisha | COCOA POWDER RICH IN POLYPHENOLS, METHOD FOR PRODUCING THE SAME AND MODIFIED COCOA CONTAINING THE SAME |
| DE19860754B4 (en) | 1998-06-24 | 2004-10-28 | Coty B.V. | Cosmetic preparation |
| CA2336682C (en) | 1998-07-07 | 2006-10-10 | Transdermal Technologies, Inc. | Compositions for rapid and non-irritating transdermal delivery of pharmaceutically active agents and methods for formulating such compositions and delivery thereof |
| EP1121020B1 (en) | 1998-10-15 | 2004-07-07 | Kraft Foods R&D, Inc. | Low-flavor cocoa, a method of its production and a use thereof |
| EP1026164A1 (en) | 1999-02-02 | 2000-08-09 | ADM Cocoa B.V. | Process for extracting polyphenolic antioxidants from purine-containing plants |
| US6381618B1 (en) | 1999-06-17 | 2002-04-30 | International Business Machines Corporation | Method and apparatus for autosynchronizing distributed versions of documents |
| US6660322B2 (en) | 1999-12-21 | 2003-12-09 | Loretta Zapp | Method for enhancing post-processing content of beneficial compounds in foodstuffs made with cocoa beans |
| IL152175A (en) | 2000-04-14 | 2005-08-31 | Mars Inc | Compositions and methods for improving vascular health |
| FR2808691B1 (en) | 2000-05-12 | 2005-06-24 | Coletica | CYCLODEXTRINS SUBSTITUTED PREFERENTIALLY ON THEIR PRIMARY SURFACE BY ACID OR AMINE FUNCTIONS |
| US6627232B1 (en) | 2000-06-09 | 2003-09-30 | Mars Incorporated | Method for extracting cocoa procyanidins |
| US6403133B1 (en) | 2000-06-20 | 2002-06-11 | Nestec S.A. | Cocoa product preparation |
| US20020022040A1 (en) | 2000-07-10 | 2002-02-21 | The Proctor & Gamble Company | Methods of enhancing delivery of oil-soluble skin care actives |
| FR2812873B1 (en) | 2000-08-11 | 2003-08-01 | Barry Callebaut France | PROCESS FOR PRODUCING POLYPHENOLS FROM COCOA BEANS |
| PT102509A (en) | 2000-08-16 | 2002-02-28 | Fundacao Essprit Icarus | PHARMACEUTICAL, COSMETIC AND DERMO-COSMETIC COMPOSITIONS, OF HYGIENE, FOOD AND FODDER, BASED ON FOCUS OIL, AND THEIR USE |
| US20020106388A1 (en) | 2000-11-24 | 2002-08-08 | Pugliese Peter T. | Formulation of flavones and isoflavones for treatment of cellulite |
| FR2818095B1 (en) | 2000-12-20 | 2003-04-25 | M3C | PROCESS FOR THE MANUFACTURE OF COLORED COCOA POWDER |
| EP1308094A3 (en) | 2001-10-30 | 2004-02-25 | Societe Des Produits Nestle S.A. | Chocolate crumb flavour manipulation |
| CN1635837A (en) | 2001-10-30 | 2005-07-06 | 雀巢产品有限公司 | Chocolate flavor manipulation |
| AU2003214218A1 (en) | 2002-03-19 | 2003-10-08 | North Carolina State University | Chocolate coated beverage creamer |
| ATE361671T1 (en) | 2002-03-20 | 2007-06-15 | Nestle Sa | LOW FAT COCOA EXTRACT |
| JP4050222B2 (en) | 2003-11-28 | 2008-02-20 | 江崎グリコ株式会社 | Combination chocolate |
| GB2416106A (en) | 2004-07-15 | 2006-01-18 | Cargill Inc | Roasting cocoa |
| JP2009542206A (en) * | 2005-06-29 | 2009-12-03 | マース インコーポレーテッド | Polyphenol-containing products |
| EP2325295A3 (en) | 2005-09-12 | 2013-05-29 | Vrije Universiteit Brussel | Cacao starter cultures and fermentation method |
| ES2280141B1 (en) * | 2006-02-27 | 2008-10-01 | Natraceutical, S.A. | "PROCESS FOR OBTAINING COCOA POWDER RICH IN POLYPHENOLS AND UNDER CONTENT IN FAT MATTER AND COCOA POWDER OBTAINED". |
| WO2007106473A2 (en) * | 2006-03-13 | 2007-09-20 | The Hershey Company | Steeped cocoa compositions and functional cocoa beverages made from them |
| MX2008015330A (en) | 2006-06-02 | 2009-04-22 | Toms Gruppen As | A method of producing cocoa mass, and chocolate and other cocoa containing products produced from the cocoa mass. |
| US20080107783A1 (en) | 2006-10-05 | 2008-05-08 | Anijs Harrold G | Method of producing high-brightness cocoa powder and related compositions |
| US20080145497A1 (en) | 2006-12-15 | 2008-06-19 | Jimbay Peter Loh | Food Ingredients With Reduced Sourness At Low pH |
| US20080268097A1 (en) | 2007-04-26 | 2008-10-30 | Hurst W Jeffrey | Cocoa ingredients having enhanced levels of stilbene compounds and methods of producing them |
| US9114114B2 (en) * | 2007-06-21 | 2015-08-25 | Mars, Inc. | Edible products having a high cocoa polyphenol content and improved flavor and the milled cocoa extracts used therein |
| GB0724716D0 (en) * | 2007-12-19 | 2008-01-30 | Barry Callebaut Ag | Process |
| GB0801119D0 (en) * | 2008-01-22 | 2008-02-27 | Barry Callebaut Ag | Composition |
| US20110123675A1 (en) | 2008-05-15 | 2011-05-26 | Herwig Bernaert | Method for processing cocoa beans |
| ES2551862T3 (en) | 2009-07-29 | 2015-11-24 | Barry Callebaut Ag | Microbial composition for the fermentation of cocoa material |
-
2008
- 2008-01-22 GB GBGB0801119.9A patent/GB0801119D0/en not_active Ceased
-
2009
- 2009-01-22 ES ES09704776T patent/ES2395151T3/en active Active
- 2009-01-22 WO PCT/GB2009/000177 patent/WO2009093030A1/en active Application Filing
- 2009-01-22 CA CA2712597A patent/CA2712597C/en active Active
- 2009-01-22 JP JP2010543561A patent/JP5323862B2/en active Active
- 2009-01-22 PL PL09704776T patent/PL2237677T3/en unknown
- 2009-01-22 MX MX2010008007A patent/MX2010008007A/en unknown
- 2009-01-22 EP EP09704776A patent/EP2237677B9/en active Active
- 2009-01-22 MY MYPI20103461 patent/MY150996A/en unknown
- 2009-01-22 CN CN200980102736.7A patent/CN101925306B/en active Active
- 2009-06-25 US US12/456,971 patent/US8460739B2/en active Active
-
2013
- 2013-05-20 US US13/898,217 patent/US9107430B2/en active Active
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2835585A (en) | 1953-07-10 | 1958-05-20 | Gen Foods Corp | Chocolate product and process therefor |
| US2957769A (en) * | 1958-04-03 | 1960-10-25 | Gen Foods Corp | Chocolate product and process therefor |
| US4784866A (en) * | 1985-11-07 | 1988-11-15 | Nestec S. A. | Process of alkalization of cocoa in aqueous phase |
| US5888562A (en) * | 1995-06-20 | 1999-03-30 | Nestec S.A. | Enzymatic treatment of cocoa |
| US20010007693A1 (en) | 1996-09-06 | 2001-07-12 | Mars, Incorporated | Food products having enhanced cocoa polyphenol content and processes for producing the same |
| WO2002063974A1 (en) | 2001-02-14 | 2002-08-22 | Societe Des Produits Nestle S.A. | Enzymatically hydrolysed cocoa in chocolate flavour reactions |
| WO2009067533A1 (en) | 2007-11-19 | 2009-05-28 | The Hershey Company | Process for preparing red cocoa ingredients, red chocolate, and food products |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2222182A4 (en) * | 2007-11-19 | 2010-11-17 | Hershey Co | Process for preparing red cocoa ingredients, red chocolate, and food products |
| US8709524B2 (en) | 2007-11-19 | 2014-04-29 | The Hersey Company | Process for preparing red cocoa ingredients, red chocolate, and food products |
| US20100151087A1 (en) * | 2008-01-22 | 2010-06-17 | Arnaud Dumarche | Composition |
| US8460739B2 (en) * | 2008-01-22 | 2013-06-11 | Barry Callebaut Ag | Process for making red or purple cocoa material |
| US9107430B2 (en) | 2008-01-22 | 2015-08-18 | Barry Callebaut Ag | Process for producing red or purple cocoa-derived material |
| WO2011012680A3 (en) * | 2009-07-29 | 2011-03-31 | Barry Callebaut Ag | Microbial composition for the fermentation of cocoa material |
| US9701986B2 (en) | 2009-07-29 | 2017-07-11 | Barry Callebaut Ag | Microbial composition for the fermentation of cocoa material |
| US20150017302A1 (en) * | 2011-04-08 | 2015-01-15 | Harrold Glenn Anijs | Fruity flavored cocoa products and processes for producing such cocoa products |
| US10327453B2 (en) | 2013-06-25 | 2019-06-25 | Olam International Limited | Processes for producing dark red and dark brown natural cocoa |
Also Published As
| Publication number | Publication date |
|---|---|
| MX2010008007A (en) | 2010-08-10 |
| US20100151087A1 (en) | 2010-06-17 |
| CN101925306B (en) | 2014-02-19 |
| CA2712597C (en) | 2016-09-13 |
| ES2395151T9 (en) | 2013-05-07 |
| GB0801119D0 (en) | 2008-02-27 |
| PL2237677T3 (en) | 2012-11-30 |
| CA2712597A1 (en) | 2009-07-30 |
| EP2237677A1 (en) | 2010-10-13 |
| MY150996A (en) | 2014-03-31 |
| CN101925306A (en) | 2010-12-22 |
| EP2237677B1 (en) | 2012-06-13 |
| ES2395151T3 (en) | 2013-02-08 |
| JP2011509681A (en) | 2011-03-31 |
| EP2237677B9 (en) | 2013-04-03 |
| US8460739B2 (en) | 2013-06-11 |
| US20130251858A1 (en) | 2013-09-26 |
| JP5323862B2 (en) | 2013-10-23 |
| US9107430B2 (en) | 2015-08-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8460739B2 (en) | Process for making red or purple cocoa material | |
| US6558713B2 (en) | Health of a mammal by administering a composition containing at least one cocoa polyphenol ingredient | |
| US6312753B1 (en) | Cocoa components, edible products having enriched polyphenol content, methods of making same and medical uses | |
| US8541045B2 (en) | Process for extracting cocoa polyphenols from cocoa beans | |
| US8709524B2 (en) | Process for preparing red cocoa ingredients, red chocolate, and food products | |
| WO1998009533A1 (en) | Cocoa components, edible products having enhanced polyphenol content, methods of making same and medical uses | |
| WO2021066119A1 (en) | Composition and method for producing same | |
| Habibzadeh et al. | Evaluation of physicochemical, rheological and sensory properties of wafer cream by replacing cocoa powder with carob pod and chicory root powders | |
| Šeremet et al. | Ruby chocolate–Bioactive potential and sensory quality characteristics compared with dark, milk and white chocolate | |
| US6054129A (en) | Flavor-improved extract from Cassia mimosoides L. var. nomame Makino and method of preparing the same | |
| WO2022215733A1 (en) | Frozen confectionery containing cacao composition | |
| WO2022215732A1 (en) | Baked confectionary containing cacao composition | |
| Nur Fitriani et al. | Evaluation of Physicochemical Properties and Sensory Products of Cocoa Liquor and Dark Chocolate High Polyphenols and Flavanoids | |
| GANACHE | PHYSICOCHEMICAL, TOTAL PHENOLIC CONTENT, ANTIOXIDANT ACTIVITY, AND SENSORY ACCEPTABILITY OF MILK AND DARK CHOCOLATES FILLED WITH SACHA | |
| AU2007203454A1 (en) | Cocoa components, edible products having enhanced polyphenol content, methods of making same and medical uses |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 200980102736.7 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 09704776 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE2 | Request for preliminary examination filed before expiration of 19th month from priority date (pct application filed from 20040101) | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2712597 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2010543561 Country of ref document: JP Ref document number: MX/A/2010/008007 Country of ref document: MX |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2009704776 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: PI 2010003461 Country of ref document: MY |