WO2007083904A1 - C-kit activation inhibitor, skin whitening compound and composition for skin whitening containing the same - Google Patents
C-kit activation inhibitor, skin whitening compound and composition for skin whitening containing the same Download PDFInfo
- Publication number
- WO2007083904A1 WO2007083904A1 PCT/KR2007/000227 KR2007000227W WO2007083904A1 WO 2007083904 A1 WO2007083904 A1 WO 2007083904A1 KR 2007000227 W KR2007000227 W KR 2007000227W WO 2007083904 A1 WO2007083904 A1 WO 2007083904A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- skin whitening
- formula
- composition
- kit
- skin
- Prior art date
Links
- RIRAMOVDGJDPRU-UHFFFAOYSA-N COc(cc1)ccc1C(Oc(c(C1=O)c2)ccc2OC)=C1O Chemical compound COc(cc1)ccc1C(Oc(c(C1=O)c2)ccc2OC)=C1O RIRAMOVDGJDPRU-UHFFFAOYSA-N 0.000 description 1
- XZQLSABETMKIGG-UHFFFAOYSA-N COc(cc12)ccc1OC(c1ccccc1)=CC2=O Chemical compound COc(cc12)ccc1OC(c1ccccc1)=CC2=O XZQLSABETMKIGG-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
- A61Q19/02—Preparations for care of the skin for chemically bleaching or whitening the skin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/49—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds
- A61K8/4973—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds with oxygen as the only hetero atom
- A61K8/498—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds with oxygen as the only hetero atom having 6-membered rings or their condensed derivatives, e.g. coumarin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/96—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution
- A61K8/97—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution from algae, fungi, lichens or plants; from derivatives thereof
- A61K8/9783—Angiosperms [Magnoliophyta]
- A61K8/9789—Magnoliopsida [dicotyledons]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Definitions
- the present invention relates to an activation inhibitor of c-Kit associated with
- composition for skin whitening comprising the skin whitening compound as an active
- c-Kit which is a receptor belonging to a class III receptor
- tyrosine kinase is associated with survival, proliferation and differentiation of
- the number of the melanocyte is increased in skins when the skins are
- SCFs stem cell factors
- mice have white hair and white skin if the mice are treated with ACK2 which is
- STI-571 has been widely known as an anti-cancer drug for treating leukemia, which targets Bcr-Abl kinase.
- Bcr-Abl kinase targets Bcr-Abl kinase.
- melanotic conditions such as UVB-melanosis, lentigo senilis, dermatofibroma and Caffe
- c-Kit If c-Kit is activated, the activated c-Kit activates MAP kinase, and the activated MAP kinase sequentially phosphorylates a microphthalmia-associated transcription factor (Mitf), which is a helix-loop-helix/leucine zipper protein, into an activated state.
- Mitf microphthalmia-associated transcription factor
- the activated Mitf stimulates transcription of melanin-synthesizing enzymes such as
- keratinocyte when exposed to UV-rays, stimulates differentiation of precursor melanocyte into mature melanocyte harboring c-Kit, and also stimulates synthesis of
- melanin pigment by stimulating transcription of enzymes associated with the melanin
- the c-Kit is mainly associated with a signal transduction system which stimulates synthesis of melanin by UV-rays. Accordingly, if it is
- a skin tone may be brightened to thereby whiten skin, and cutaneous
- hyperpigmentation such as melasma, freckles, lentigo senilis, dermatofibroma, Caffe
- kojic acid and glutathione which have an inhibitory activity on tyrosinase, were mixed with skin-applicable compositions such as ointment or cosmetics, and then the resultant
- compositions were used in the art to whiten skin, for example to treat melasma and
- ascorbic acid has a problem that smells and colors of an ascorbic acid-containing
- cosmetic composition are easily changed due to its susceptibility to oxidation.
- thiol compounds such as glutathione, cysteine, etc. have an inherent
- one object of the present invention is to provide a c-Kit activation inhibitor capable of inhibiting activity of c-Kit associated with melanin synthesis, anti-cancer activity, etc.
- Another object of the present invention is to provide a skin whitening
- the present invention provides a c-Kit
- the present invention provides a skin whitening compound, one
- flavone derivative selected from the group consisting of compounds represented by the
- Formulas 1 to 10 and a composition for skin whitening comprising any of the
- these flavone derivatives can be added to various formulations such as skin, lotion, cream, foundation, essence, gel, pack, foam cleansing, soap, ointment, etc.
- the present invention provides a c-Kit inhibitor, one flavone derivative, selected from the group consisting of 6-methoxyflavone
- diosmetin of Formula 10 is a compound that has been reported
- Pedalium murex Petroselinum crispum, Rosmarinus officinalis, Salvia candidissima, Salvia nutans, Salvia reptans, Soroseris hookeriana, Stemodia viscose, Tanacetum vulgare, Toddalia floribunda, Thymbra capitata, Thymus hirtus, Thymus vulgaris,
- Verbena bipinnatifida Valeriana cardamines, Valeriana eriophylla, Valeriana fedtschenkoi, Valeriana laxiflora, Vicia truncatula, Xanthorrhoea hastile sou and
- Xanthorrhoea hastila. It has been known that the diosmetin has an anti-bacterial effect
- the inventors have studied about compounds that can inhibit the activity of the
- skin whitening effect is generally intended
- Chrysanthemum morifolium commercially available as a medicinal herb
- the purified active ingredient was confirmed to be diosmetin using
- NMR nuclear magnetic resonance
- Mass mass spectrometry
- the above-mentioned pure flavone derivatives of Formulas 1 to 10 or the diosmetin extracts can be added in an effective
- compositions such as an ointment and cosmetic products including a skin, a lotion, a cream, a foundation, an essence, a gel, a pack, a foam cleansing, a soap,
- flavone derivatives or the diosmetin extracts can be used alone or in combination thereof.
- the added flavone derivatives preferably ranges from 0.000001 to 10 % by weight
- composition more preferably from 0.0001 to 1 % by weight, based on the total weight of the composition.
- FIG. 1 is a graph showing a H 1 -NMR spectrum of diosmetin extracted
- FIG. 2 is a graph showing a C 13 NMR spectrum of diosmetin extracted
- FIG. 3 is a graph showing a mass spectrometry spectrum of diosmetin extracted
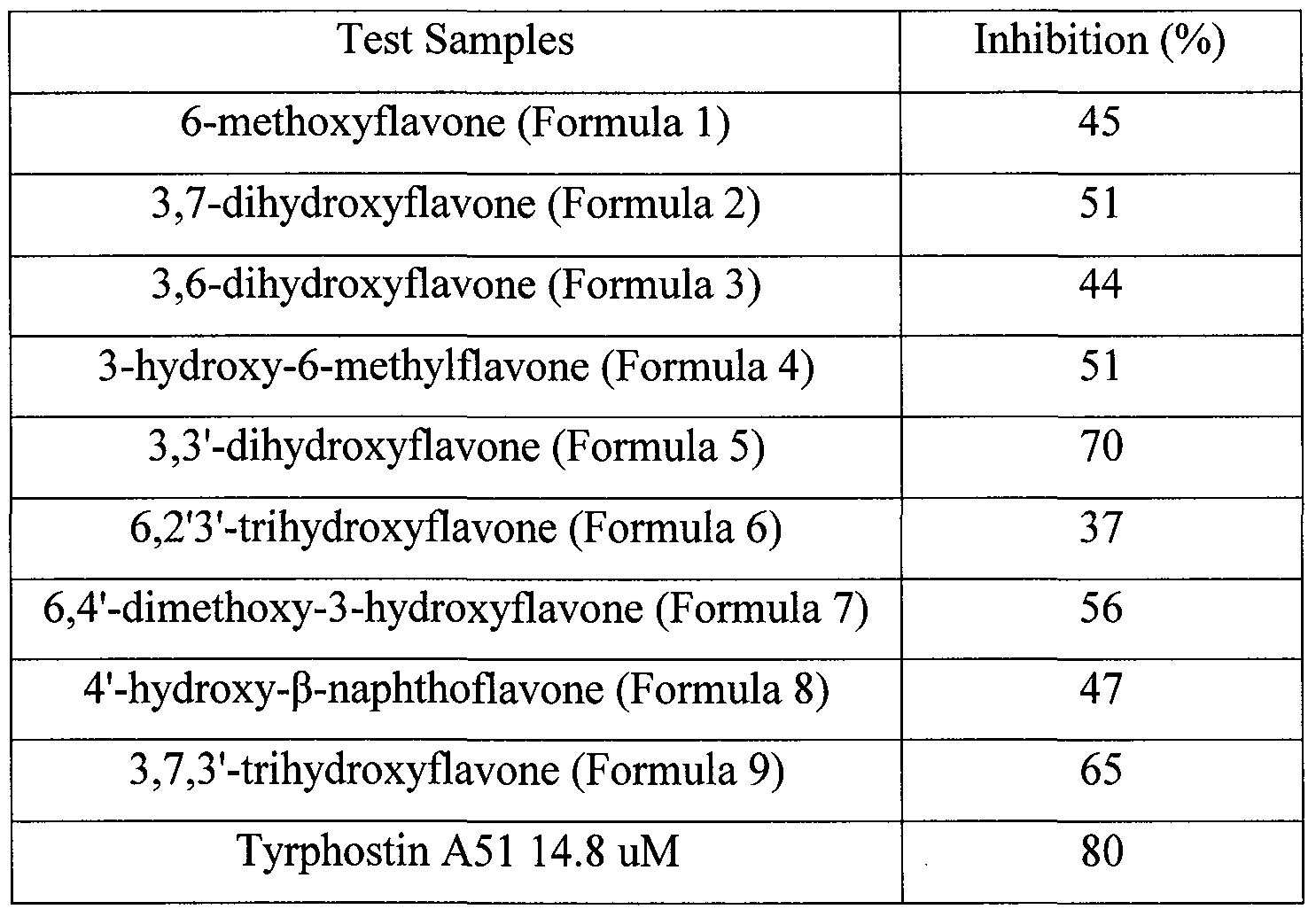
- test samples and the enzyme instead of the test samples and the enzyme as a positive control, respectively.
- Equation 1 Inhibition (%) (Mean Value of Test Samples - Mean Value of Negative Control) / (Mean Value of Positive Control - Mean Value of Negative Control) X 100
- Formulas 1 to 9 exhibit a potent inhibitory activity on the c-Kit in a lower concentration
- compositions of the preparative examples were evaluated in two level, namely to be
- creams of Preparative examples 1 -3 have a whitening effect but no side effect in at least
- Example 2 was subject to silica gel column chromatography using mixed
- Example 4 which is the purified diosmetin, has an excellent
- Preparative example 4 has a whitening effect but no side effect on skin in 14 of the 20
- the flavone derivatives of the present invention are effective as described above.
- active ingredients can be highly effectively used to treat melasma and freckles, improve
- cutaneous hyperpigmentations such as lentigo senilis, dermatofibroma, Caffe aure
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- Birds (AREA)
- Dermatology (AREA)
- Medicinal Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Chemical & Material Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Biotechnology (AREA)
- Botany (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Cosmetics (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Pyrane Compounds (AREA)
- Medicines Containing Plant Substances (AREA)
Abstract
Disclosed are a c-Kit activation inhibitor, a skin whitening compound and a composition for skin whitening comprising the skin whitening compound as an active ingredient. The c-Kit activation inhibitor of the present invention is a flavone derivative selected from the group consisting of the compounds represented by Formulas 1 to 10. The flavone derivative inhibits activity of the c-Kit associated with melanin synthesis, melanocyte differentiation and maturity, etc. Accordingly, the above-mentioned flavone derivatives are useful as skin whitening compound, and the cosmetic compositions comprising the flavone derivatives as an active ingredient can be highly effectively used for skin whitening, for example treating melasma, freckles, etc.
Description
C-KIT ACTIVATION INHIBITOR, SKIN WHITENING COMPOUND AND
COMPOSITION FOR SKIN WHITENING CONTAINING THE SAME
TECHNICAL FIELD
The present invention relates to an activation inhibitor of c-Kit associated with
melanin synthesis, melanocyte differentiation and maturity, etc., a skin whitening
compound having skin whitening effect such as treatment of melasma or freckles, and a
composition for skin whitening comprising the skin whitening compound as an active
ingredient.
BACKGROUND ART
It has been known that c-Kit, which is a receptor belonging to a class III receptor
tyrosine kinase (RTK), is associated with survival, proliferation and differentiation of
melanocyte. The number of the melanocyte is increased in skins when the skins are
exposed to UV rays. In this reaction, the c-Kit plays an important role. It was
confirmed that increased stem cell factors (SCFs) in a hair follicle 'niche,' which
generates and differentiates melanocyte, grow and differentiate melanocyte to migrate
out of the niche in the case of a Kl 4 (keratin promoter)-steel factor transgenic mouse,
and that mice have white hair and white skin if the mice are treated with ACK2 which is
an antibody against c-Kit in an embryonic step (Nature 416, 854-860, 2002).
Form the beginning, there have been many more attempts to study c-Kit as a target of an anti-cancer drug than as a whitening compound. Imatinib (Gleevec,
STI-571, Novatis, East Hanover, NJ, USA) has been widely known as an anti-cancer
drug for treating leukemia, which targets Bcr-Abl kinase. However, it was revealed
that a level of melanin pigment is decreased in skins of 6 patients who receive a
prescription of imatinib, indicating that the imatinib inhibits activity of c-Kit in addition
to the Bcr-Abl. Accordingly, it might be seen that SCF/c-Kit plays an essential role in
growing and sustaining human melanocyte (Cancer 98, 2483-7, 2003).
Meanwhile, it was reported that SCF, which is a ligand of c-kit, is more
excessively expressed in a lesion region of melasma than a normal skin region (Korean J.
Dermatol. 2005; 43 (8): 1046-1052), and that SCF is excessively expressed in excessive
melanotic conditions such as UVB-melanosis, lentigo senilis, dermatofibroma and Caffe
aure macule (Pigment Cell Research 17:96- 110. 2004).
If c-Kit is activated, the activated c-Kit activates MAP kinase, and the activated MAP kinase sequentially phosphorylates a microphthalmia-associated transcription factor (Mitf), which is a helix-loop-helix/leucine zipper protein, into an activated state.
The activated Mitf stimulates transcription of melanin-synthesizing enzymes such as
tyrosinase, Tyrp-2, etc. to synthesize melanin pigment. SCF, secreted from
keratinocyte when exposed to UV-rays, stimulates differentiation of precursor melanocyte into mature melanocyte harboring c-Kit, and also stimulates synthesis of
melanin pigment by stimulating transcription of enzymes associated with the melanin
synthesis.
As described above, the c-Kit is mainly associated with a signal transduction system which stimulates synthesis of melanin by UV-rays. Accordingly, if it is
possible to inhibit the c-Kit activity, differentiation and maturity of the melanocyte may
be inhibited, in addition to the anti-cancer effect.
Generally, many persons hope to have a white and soft skin, but if melanin is
excessively synthesized in their skins, they have a dark skin tone, and also have
melasma, freckles, etc. Accordingly, if the synthesis of melanin pigment in skin is
inhibited, a skin tone may be brightened to thereby whiten skin, and cutaneous
hyperpigmentation such as melasma, freckles, lentigo senilis, dermatofibroma, Caffe
aure macule etc., which are caused by UV-rays, hormones and other genetic factors, may
be treated to thereby whiten skin.
Accordingly, conventional compounds such as hydroquinone, ascorbic acid,
kojic acid and glutathione, which have an inhibitory activity on tyrosinase, were mixed with skin-applicable compositions such as ointment or cosmetics, and then the resultant
compositions were used in the art to whiten skin, for example to treat melasma and
freckles. However, it was confirmed that the hydroquinone has some whitening effect,
but it is used in an extremely low amount due to severe irritation to skin. Also, the
ascorbic acid has a problem that smells and colors of an ascorbic acid-containing
cosmetic composition are easily changed due to its susceptibility to oxidation.
Meanwhile, thiol compounds such as glutathione, cysteine, etc. have an inherent
disgusted smell and a transdermal delivery-related problem, and their glycosides and derivatives are also difficult to use as mixing ingredients due to its high polarity.
DISCLOSURE OF INVENTION [Technical Problem]
Therefore, one object of the present invention is to provide a c-Kit activation inhibitor capable of inhibiting activity of c-Kit associated with melanin synthesis,
anti-cancer activity, etc.
In addition, another object of the present invention is to provide a skin whitening
compound and a composition for skin whitening being able to be safely used without
any side effect on skin and having an excellent inhibitory effect on pigmentation.
[Technical Solution]
In order to accomplish the first object, the present invention provides a c-Kit
activation inhibitor which is a flavone derivative selected from the group consisting of
compounds represented by the following Formulas 1 to 10.
[Formula 1 ]
6-methoxyflavone
[Formula 2]
3,7-dihydroxyflavone
[Formula 3 ]
3,6-dihydroxyflavone
[Formula 4]
3-hydroxy-6-m ethylf lavone
[Formula 5]
3, 3'-dihydroxyf lavone
[Formula 6]
6,2',3'-trihydroxyflavone
[Formula 7]
6,4'-dimethoxy-3-hydroxyflavone
[Formula 8]
4 '-hydroxy-beta-naphthof lavone
[Formula 9]
3,7,3'-trihydroxyflavone
[Formula 10]
In addition, the present invention provides a skin whitening compound, one
flavone derivative, selected from the group consisting of compounds represented by the
Formulas 1 to 10, and a composition for skin whitening comprising any of the
compounds as an active ingredient.
In the composition for skin whitening, a content of the flavone derivatives
preferably ranges from 0.000001 to 10 % by weight, based on the total weight of the composition, and these flavone derivatives can be added to various formulations such as skin, lotion, cream, foundation, essence, gel, pack, foam cleansing, soap, ointment, etc.
to give a skin whitening effect.
Hereinafter, the present invention will be described in more detail.
The present invention provides a c-Kit inhibitor, one flavone derivative, selected from the group consisting of 6-methoxyflavone
(6-methoxy-2-phenyl-4H-chromen-4-one, C16H12O3, CAS No. 26964-24-9) of Formula 1,
3,7-dihydroxyflavone (CI5HI0O4, CAS NO. 492-00-2) of Formula 2,
3,6-Dihydroxyflavone (C15H10O4, CAS No. 08238-41-1) of Formula 3,
3-hydroxy-6-methylflavone (6-methyl-3-hydroxyflavone, C16H12O3, CAS No.
6971-18-2) of Formula 4, 3,3'-dihydroxyflavone (C15H10O4, CAS No. 55977-09-8) of
Formula 5, 6,2',3'-trihydroxyflavone (C15Hi0O5, CAS No. 108238-47-7) of Formula 6,
6,4'-dimethoxy-3-hydroxyfiavone (C17H14O5, CAS No. 93176-02-4) of Formula 7,
4'-hydroxy-β -naphthoflavone (C19H12O3, CAS No. 98166-72-4) of Formula 8,
3,7,3'-trihydroxyflavone (C15H10Os) of Formula 9 and diosmetin (5,7-dihydroxy-2-
(3-hydroxy-4-methoxy-phenyl)-chromen-4-one, C16H12O6, CAS No. 520-34-3) of
Formula 10.
In particular, the diosmetin of Formula 10 is a compound that has been reported
as an ingredient of plants including Acacia farnesiana, Achillea asiatica, Arnica
longifolia, Artemisia rutifolia, Artemisia vulgaris, Caleriana chionophila, Capsella
bursa-pastoris, Citrus limon, Cnidium monnieri, Cyperus alopecuroides, Gentiana
barbata, Hieracium compositum, Lnaria macroura, Mentha spicata, Origaganum vulgare,
Pedalium murex, Petroselinum crispum, Rosmarinus officinalis, Salvia candidissima, Salvia nutans, Salvia reptans, Soroseris hookeriana, Stemodia viscose, Tanacetum vulgare, Toddalia floribunda, Thymbra capitata, Thymus hirtus, Thymus vulgaris,
Verbena bipinnatifida, Valeriana cardamines, Valeriana eriophylla, Valeriana
fedtschenkoi, Valeriana laxiflora, Vicia truncatula, Xanthorrhoea hastile durch and
Xanthorrhoea hastila. It has been known that the diosmetin has an anti-bacterial effect
(Planta Medica, 70 (6), 2004, 509-514), and anti-allergic effect (Bioorg. Med. Chem, 10
(10), 2002, 3123-3128), anti-inflammation effect (J. haram. Pharmacol., 50 (9), 1998,
1069-1074), etc.
The inventors have studied about compounds that can inhibit the activity of the
c-KIT associated with melanin synthesis, melanocyte differentiation and maturity, etc.,
and have found that the above-mentioned flavone derivatives show a very potent
inhibitory effect on the c-Kit, on which the present invention is based. That is to say, the above-mentioned flavone derivatives inhibit the activity of the
c-Kit, and therefore they can be used as multifunctional skin whitening compounds that
inhibit synthesis of enzymes associated with melanocyte differentiation and maturity
and melanin synthesis, as well as an anti-cancer effect. Particularly, the c-Kit
activation inhibitor such as the above-mentioned flavone derivatives can be useful to
attain an excellent skin whitening effect even if it is used in a small amount since it acts
at the beginning of signal transduction for the melanin synthesis.
In the present invention, the term "skin whitening effect " is generally intended
to include effects of improving undesirable conditions associated with the skin colors,
for example treating melasma and freckles, improving lentigo senilis, dermatofibroma,
and cutaneous hyperpigmentation, as well as an effect of making skin whiter.
The above-mentioned flavone derivatives of Formulas 1 to 10 are commercially available. However, it is more preferable to use an extract of Chrysanthemum
morifolium {Chrysanthemum morifolium petals) as the diosmetin of Formula 10. A
method for extracting diosmetin from Chrysanthemum morifolium will be illustrated as
described below, but the scope of the present invention is not limited to the subject to be
extracted or the extraction methods as described in the present invention.
Chrysanthemum morifolium, commercially available as a medicinal herb, was
purchased, ground into small pieces, and the ground substance was extracted with 5-20
times volume of methanol at 50-100 °C for 1-5 hours in an extractor having a reflux
cooler. The residue was extracted through a filter cloth using the same method, and
then the remaining extract was filtered once or more. The resultant extracts were
collected, concentrated under a reduced pressure, and then freeze-dried or spray-dried to
obtain a dry extract. In order to confirm the presence of an active ingredient in the
methanol extract of the Chrysanthemum morifolium, the methanol extract was
suspended in 10 times volume of water, and fractionated with an equivalent amount of
hexane to remove non-polar compounds, and the remaining aqueous layer was then
fractionated with an equivalent amount of butanol solvent to obtain a butanol fraction of
the Chrysanthemum morifolium. An active ingredient was separated from the resultant
butanol fraction of the Chrysanthemum morifolium with a silica gel column
chromatography using mixed chloroform/methanol solvent mixture as an eluent, and the
obtained active ingredient was purified using a preparative HPLC under the same
conditions. The purified active ingredient was confirmed to be diosmetin using
nuclear magnetic resonance (NMR) and mass spectrometry (Mass).
In order to give a skin whitening effect, the above-mentioned pure flavone derivatives of Formulas 1 to 10 or the diosmetin extracts can be added in an effective
amount to various compositions such as an ointment and cosmetic products including a
skin, a lotion, a cream, a foundation, an essence, a gel, a pack, a foam cleansing, a soap,
etc., and the flavone derivatives or the diosmetin extracts can be used alone or in combination thereof.
In consideration of a skin whitening effect and economical efficiency, an amount
of the added flavone derivatives preferably ranges from 0.000001 to 10 % by weight,
more preferably from 0.0001 to 1 % by weight, based on the total weight of the composition.
BRIEF DESCRIPTION OF THE DRAWINGS FIG. 1 is a graph showing a H1 -NMR spectrum of diosmetin extracted and
purified according to one embodiment of the present invention.
FIG. 2 is a graph showing a C13 NMR spectrum of diosmetin extracted and
purified according to one embodiment of the present invention.
FIG. 3 is a graph showing a mass spectrometry spectrum of diosmetin extracted
and purified according to one embodiment of the present invention.
BEST MODES FOR CARRYING OUT THE INVENTION
Hereinafter, preferred embodiments of the present invention will be described in
detail referring to the accompanying drawings. However, the description proposed
herein is just a preferable example for the purpose of illustrations only, not intended to limit the scope of the invention, so it should be understood that other equivalents and modifications could be made thereto without departing from the spirit and scope of the
invention. The preferred embodiments of the present invention will be described in
detail for the purpose of better understandings, as apparent to those skilled in the art.
Evaluation of Flavone Derivatives of Formulas 1 to 9
The commercially available flavone derivative compounds of Formulas 1 to 9
were purchased and used. Each of the flavone derivative compounds was adjusted to a
final concentration of 1 uM and added to each well of a 384-well plate, and c-Kit RTK
and ATP were also added to each well of the 384-well plate, and then subject to a
primary reaction at a room temperature. Then, biotinylated-poly[Glu:Tyr] (4:1) as a
substrate was added, and then subject to a secondary enzyme reaction.
A capture buffer containing donor beads coated with streptavidin and acceptor
beads to which antibody (P-Tyr-100) binds was added, and then subject to a tertiary
reaction to bind to the substrate. Phosphorylation levels of the substrates were
determined by measuring AlphaScreen signal using a Fusion™ microplate analyzer.
Also, their inhibitory effects were compared using Tyrphostin A51 known as c-Kit
inhibitor in the prior art.
In order to measure the AlphaScreen signal to an accurate level, each test sample
was tested three times. Here, in order to determine inhibition by c-Kit, DMSO was
used as a positive control instead of the test samples, and DMSO and a buffer were used
instead of the test samples and the enzyme as a positive control, respectively.
The inhibition (%) on c-Kit was calculated from the detected signal according to the following Equation 1. The results are listed in the following Table 1.
Equation 1 Inhibition (%) = (Mean Value of Test Samples - Mean Value of Negative Control) /
(Mean Value of Positive Control - Mean Value of Negative Control) X 100
Table 1
As listed in Table 1, it was revealed that the flavone derivative compounds of
Formulas 1 to 9 exhibit a potent inhibitory activity on the c-Kit in a lower concentration
than the Tyrphostin A51 known as the c-Kit activation inhibitor.
On the basis of compositions and their contents as listed in the following Table 2,
some of the above-mentioned flavone derivative compounds were added to a cream, and
then evaluated for a whitening effect.
Table 2
In order to inspect skin whitening effects of the creams produced on the basis of
the above-mentioned compositions, tests on inhibition of pigmentation and treatment of
melasma were carried out, as follows.
Test of Inhibitory Effect on Pigmentation
20 healthy men and women were selected, and aluminum foil having two
grooved lines, each having 6 holes with a diameter of 7 mm, was attached to both
forearms of their arms. Then, the forearms were irradiated with a radiation intensity of
60 mJ/cm2 using a IOOOW ORIEL solar simulator located at a distance of 10 cm from
the arms. Before the irradiation, sites to be irradiated were washed thoroughly with an aqueous 70 % ethanol solution. The base compositions prepared according to
Preparative example 1-3 and Comparative example 1 were applied in pairs to the same
grooved lines two times daily during a period from 3 days before the irradiation to 3 weeks after the irradiation.
Pigmentation rates on the base compositions of the preparative examples and the
comparative examples were evaluated with the naked eye, and then the base
compositions of the preparative examples were evaluated in two level, namely to be
effective and not to be effective, compared to the base compositions of the comparative
examples. The results are listed in the following Table 3.
Table 3
As listed in Table 3, it was revealed that the flavone derivative-containing
creams of Preparative examples 1 -3 have a whitening effect but no side effect in at least
10 of the 20 subjects.
Test of Effect on Treatment of Melasma
10 healthy women having melasma were selected, and then the base composition
prepared according to Preparative example 1 was applied to their faces with melasma
two times daily for three weeks.
After the experiment was completed, medical specialists valuated pigmentations
with the naked eye, and the subjects to be tested evaluated treatment of melasma on the basis of their subjective judgments, and therefore the cream of Preparative example 1 was evaluated in two level, namely to be effective and not to be effective on the
As listed in Table 4, it was revealed that the 6-Methoxyflavone-containing cream
prepared according to Preparative examples 1 has an effect on the treatment of melasma
but no side effect in at least 6 of the 10 subjects.
Preparation and Evaluation of Diosmetin Extract of Formula 10
Example 1
Dry Chrysanthemum morifolium commercially available as a medicinal herb was
purchased and ground into small pieces, and 10 kg of the ground substance was heated
to 70 °C for 3 hours in an extractor provided with a reflux cooler and extracted with
150L of methanol. The resultant extract was filtered through a filter cloth, and the
remaining extract was then filtered once or more. The resultant extracts were collected
and concentrated under a reduced pressure to obtain 1.2 kg of a dry extract.
Example 2
1 kg of the Chrysanthemum morifolium methanol extract prepared in Example 1
was suspended in 1OL of water and fractionated with 1OL of hexane solvent three times to remove a hexane fraction. Then, the remaining aqueous layer was fractionated with
1OL of butanol solvent three times to obtain a butanol fraction. The butanol fraction was concentrated under a reduced pressure to obtain 125 g of a dry substance.
Example 3
1 kg of the Chrysanthemum morifolium methanol extract prepared in Example 1
was suspended in 1OL of water and fractionated with 1OL of ethylacetate solvent three
times to obtain an ethylacetate fraction. Then, the ethylacetate fraction was
concentrated under a reduced pressure to obtain 230 g of a dry substance.
Example 4
100 g of the soluble Chrysanthemum morifolium butanol fraction prepared in
Example 2 was subject to silica gel column chromatography using mixed
chloroform/methanol solvent as an eluent to obtain an active fraction. Then, the
resultant active fraction was purified with preparative HPLC to obtain 1.2 g of an active ingredient. The purified active ingredient was confirmed to be diosmetin, represented
by Formula 10, through nuclear magnetic resonance (NMR) and mass spectrometry
(Mass) (see FIGs. 1 to 3), as follows.
Molecular Formula: C16H12O6
Molecular Weight: 300.27
Melting Point: 260-264 °C
UV ( λ ax, nm) (MeOH): 272, 342
IH-NMR (DMSO-d6) σ : 3H (6.69,s), 6H (6.13,s), 8H (6.39,s), 2Η (7.41, S),
51H (7.06,d,8.6Hz), 6'H (7.51,d,8.6Hz), 5-OH (12.89,br), 7-OH (lO.O.br), 4'-OMe
(3.85,s)
Inhibitory Effect on c-Kit Activity
Each concentrations of the methanol extract of Example 1 and the soluble
butanol fraction of Example 2 were adjusted to 20 ug/ml, and a final concentration of
the diosmetin of Example 4 were adjusted to 0.3 uM, and then evaluated in the same
manner as the evaluation method of the above-mentioned flavone derivative compounds
of Formulas 1 to 9. The results are listed in the following Table 5.
Table 5
As listed in Table 5, it was revealed that the test samples of Examples 1 and 2,
which are the diosmetin-containing crude extracts, have a high inhibitory effect on c-Kit,
and the test sample of Example 4, which is the purified diosmetin, has an excellent
inhibitory effect on c-Kit activity, which is comparable to that of Tyrphostin A51 known
as the c-Kit activation inhibitor.
Evaluation of Whitening Effect
In order to evaluate a whitening effect of the diosmetin-containing cosmetics,
creams were prepared on the basis of compositions and their contents listed in the following Table 2.
Table 6
The creams prepared as listed in Table 6 were evaluated for an inhibitory effect
on pigmentation in the same manner as in the above-mentioned Preparative examples
1-3. The results are listed in the following Table 7.
Table 7
As listed in Table 7, it was revealed that the diosmetin-containing cream of
Preparative example 4 has a whitening effect but no side effect on skin in 14 of the 20
subjects.
Effect on Treatment of Melasma
10 healthy women having melasma were selected, and then the base composition
prepared according to Preparative example 4 was applied to their faces with melasma
two times daily for three weeks.
After the experiment was completed, medical specialists valuated pigmentations with the naked eye, and the subjects to be tested evaluated treatment of melasma on the basis of their subjective judgments, and therefore the cream of Preparative example 4
was evaluated in two level, namely to be effective and not to be effective on the
treatment of melasma. The results are listed in the following Table 8.
Table 8
As shown in the Table 8, it was revealed that the diosmetin-containing cream
prepared according to the preparative example 4 shows an effect on the treatment of
melasma in 7 of 10 subjects.
INDUSTRIAL APPLICABILITY
As described above, the flavone derivatives of the present invention are effective
c-Kit activation inhibitors. Particularly, the above-mentioned flavone derivatives have
no side effect on skin, and inhibit melanin synthesis, and differentiation and maturity of melanocyte, and therefore the composition containing the flavone derivatives the as
active ingredients can be highly effectively used to treat melasma and freckles, improve
cutaneous hyperpigmentations such as lentigo senilis, dermatofibroma, Caffe aure
macule, etc. and attain a skin whitening effect.
Claims
1. A c-Kit activation inhibitor which is a flavone derivative selected from
the group consisting of the following Formulas 1 to 10.
Formula 1
6-methoxyflavone
Formula 2
3 , 7-dihydroxyf lavone
Formula 3
3, 6-dihydroxyf lavone
3-hydroxy-6-methylflavone
Formula 5
3 , 3 '-dihydroxyf lavone
Formula 6
6 , 2 ' , 3 '-trihydroxyf lavone
6,4'-dimethoxy-3-hydroxyflavone
Formula 8
4 '-hydroxy-beta-naphthof lavone
Formula 9
3,7,3'-trihydroxyflavone
Formula 10
2. A composition for skin whitening comprising, as an active ingredient, at
least one flavone derivative selected from the group consisting of the compounds
represented by the Formulas 1 to 10 as defined in Claim 1.
3. A composition for skin whitening comprising a compound of the
following Formula 10 as an active ingredient.
Formula 10
4. A composition for skin whitening comprising a Chrysanthemum
morifolium {Chrysanthemum morifolium petals) extract as an active ingredient.
5. The composition for skin whitening according to any one of claims 2 to 4,
wherein the skin whitening is the treatment of melasma or cutaneous
hyperpigmentation.
6. The composition for skin whitening according to any one of claims 2 to 4,
wherein a content of the flavone derivative ranges from 0.000001 to 10 % by weight,
based on the total weight of the composition.
7. The composition for skin whitening according to any one of claims 2 to 4,
wherein the composition for skin whitening is one formulation selected from the group consisting of skin, lotion, cream, foundation, essence, gel, pack, foam cleansing, soap
and ointment.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN2007800026809A CN101370470B (en) | 2006-01-18 | 2007-01-12 | C-KIT activation inhibitor, skin whitening compound and composition for skin whitening containing the same |
| JP2008551174A JP5583346B2 (en) | 2006-01-18 | 2007-01-12 | c-Kit activity inhibitor and skin lightening agent |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020060005369A KR100798252B1 (en) | 2006-01-18 | 2006-01-18 | C-Kit activation inhibitor, skin whitening compound and composition for skin whitening containing the same |
| KR10-2006-0005369 | 2006-01-18 | ||
| KR10-2006-0026683 | 2006-03-23 | ||
| KR1020060026683A KR100817662B1 (en) | 2006-03-23 | 2006-03-23 | c-Kit activation inhibitor, skin whitening compound and composition for skin whitening containing the same |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2007083904A1 true WO2007083904A1 (en) | 2007-07-26 |
Family
ID=38287817
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/KR2007/000227 WO2007083904A1 (en) | 2006-01-18 | 2007-01-12 | C-kit activation inhibitor, skin whitening compound and composition for skin whitening containing the same |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JP5583346B2 (en) |
| WO (1) | WO2007083904A1 (en) |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2112145A1 (en) * | 2008-04-24 | 2009-10-28 | AxoGlia Therapeutics S.A. | Chromenone derivatives useful for the treatment of neurodegenerative diseases |
| WO2010072805A3 (en) * | 2008-12-24 | 2011-04-28 | Unilever Plc | Method and composition for skin color modulation |
| JP2012527224A (en) * | 2009-05-18 | 2012-11-08 | ネステク ソシエテ アノニム | Opioid receptor stimulating compounds (thymoquinone, Nigella sativa) and food allergies |
| US20150335558A1 (en) * | 2012-10-22 | 2015-11-26 | Hoyu Co., Ltd. | Nerve growth inhibitor, cutaneous-sensory-irritation inhibitor, and marker for cutaneous-sensory-irritation detection |
| US20170080041A1 (en) * | 2012-03-28 | 2017-03-23 | Hoyu Co., Ltd. | HDC Activation Inhibitor, HDC Activation Inhibition Composition, Antipruritic Agent, and Antipruritic Agent Composition |
| US9931132B2 (en) | 2008-06-12 | 2018-04-03 | Atricure, Inc. | Dissecting cannula and methods of use thereof |
| CN114129495A (en) * | 2021-12-10 | 2022-03-04 | 北京安德普泰医疗科技有限公司 | Anti-inflammatory and allergy-relieving repairing composition and preparation method and application thereof |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9161894B2 (en) * | 2011-03-01 | 2015-10-20 | Npharmakon, Llc | Use of N-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-3-amine and related compounds |
| JP6031334B2 (en) * | 2012-11-14 | 2016-11-24 | 日本メナード化粧品株式会社 | Melanocyte differentiation induction inhibitor and method of using the same |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5735506A (en) * | 1980-08-11 | 1982-02-26 | Sansho Seiyaku Kk | Bleaching cosmetic |
| KR20050001899A (en) * | 2003-06-26 | 2005-01-07 | 한국과학기술연구원 | Compound showing anti-oxidant and anti-viral activity and extract of Chrysanthemum indicum comprising the same |
| KR20050030821A (en) * | 2003-09-26 | 2005-03-31 | 한불화장품주식회사 | Cosmetic composition containing 2',4'-dihydroxyflavone or its derivatives |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS55111411A (en) * | 1979-02-19 | 1980-08-28 | Sansho Seiyaku Kk | Cosmetic for making fair skin |
| JP3231908B2 (en) * | 1993-07-13 | 2001-11-26 | 同和鉱業株式会社 | Whitening cosmetics |
| JP3993936B2 (en) * | 1998-05-22 | 2007-10-17 | 一丸ファルコス株式会社 | Melanin production inhibitor and cosmetic composition |
| US20020192738A1 (en) * | 2001-04-27 | 2002-12-19 | Janice Brissette | Tyrosinase assay |
| DE602004017623D1 (en) * | 2003-08-21 | 2008-12-18 | Osi Pharm Inc | N-SUBSTITUTED PYRAZOLYL-AMIDYL-BENZIMIDAZOLYL C-KIT INHIBITORS |
| CA2536954C (en) * | 2003-08-29 | 2012-11-27 | Exelixis, Inc. | C-kit modulators and methods of use |
| FR2865132A1 (en) * | 2004-07-06 | 2005-07-22 | Oreal | Use of diosmetin and its derivatives for cosmetic or therapeutic pigmentation of skin and hair, e.g. for treating vitiligo, acts by stimulating melanogenesis |
-
2007
- 2007-01-12 JP JP2008551174A patent/JP5583346B2/en not_active Expired - Fee Related
- 2007-01-12 WO PCT/KR2007/000227 patent/WO2007083904A1/en active Application Filing
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5735506A (en) * | 1980-08-11 | 1982-02-26 | Sansho Seiyaku Kk | Bleaching cosmetic |
| KR20050001899A (en) * | 2003-06-26 | 2005-01-07 | 한국과학기술연구원 | Compound showing anti-oxidant and anti-viral activity and extract of Chrysanthemum indicum comprising the same |
| KR20050030821A (en) * | 2003-09-26 | 2005-03-31 | 한불화장품주식회사 | Cosmetic composition containing 2',4'-dihydroxyflavone or its derivatives |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2112145A1 (en) * | 2008-04-24 | 2009-10-28 | AxoGlia Therapeutics S.A. | Chromenone derivatives useful for the treatment of neurodegenerative diseases |
| US9931132B2 (en) | 2008-06-12 | 2018-04-03 | Atricure, Inc. | Dissecting cannula and methods of use thereof |
| WO2010072805A3 (en) * | 2008-12-24 | 2011-04-28 | Unilever Plc | Method and composition for skin color modulation |
| JP2012527224A (en) * | 2009-05-18 | 2012-11-08 | ネステク ソシエテ アノニム | Opioid receptor stimulating compounds (thymoquinone, Nigella sativa) and food allergies |
| US20170080041A1 (en) * | 2012-03-28 | 2017-03-23 | Hoyu Co., Ltd. | HDC Activation Inhibitor, HDC Activation Inhibition Composition, Antipruritic Agent, and Antipruritic Agent Composition |
| US20150335558A1 (en) * | 2012-10-22 | 2015-11-26 | Hoyu Co., Ltd. | Nerve growth inhibitor, cutaneous-sensory-irritation inhibitor, and marker for cutaneous-sensory-irritation detection |
| CN114129495A (en) * | 2021-12-10 | 2022-03-04 | 北京安德普泰医疗科技有限公司 | Anti-inflammatory and allergy-relieving repairing composition and preparation method and application thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| JP5583346B2 (en) | 2014-09-03 |
| JP2009523786A (en) | 2009-06-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2007083904A1 (en) | C-kit activation inhibitor, skin whitening compound and composition for skin whitening containing the same | |
| KR100844516B1 (en) | Cosmetic composition containing astragalus root extract | |
| JP4233734B2 (en) | Skin external preparation for whitening | |
| KR101230644B1 (en) | Composition useful for human skin whitening comprising vaccinium bracteatum thunb extract | |
| KR100777554B1 (en) | Composition for skin whitening containing Sitosterol | |
| JP2007063191A (en) | New bleaching agent | |
| KR100798252B1 (en) | C-Kit activation inhibitor, skin whitening compound and composition for skin whitening containing the same | |
| KR100190993B1 (en) | Water soluble fraction extracts of asparagus cochinchinensis having improved skin whitening defects by removing darkening components and a composition for improving freckles and skin whitening containing thereof | |
| KR100640246B1 (en) | Fermented product, new compound obtained therefrom, and skin whitening products containing the same | |
| KR101126818B1 (en) | c-Kit activation inhibitor, skin whitening compound and composition for skin whitening containing the same | |
| WO2017035775A1 (en) | Product containing rhodiola rosea extract and ginkgo biloba extract, preparation method therefor, and use thereof | |
| KR101034010B1 (en) | Skin whitening composition comprising patchouli alcohol | |
| KR100867202B1 (en) | A composition for external application for skinwhitening containing extract of seeds of cassia tora or emodin isolated therefrom | |
| KR20030061167A (en) | Composition for skin whitening containing veratramine | |
| KR100757130B1 (en) | Cosmetic for skin whitening containing verazine and epi-verazine with inhibitory activity of melanin formation | |
| Sonka | Exploring anti-tyrosinase bioactive compounds from the Cape flora | |
| KR100482695B1 (en) | Composition for skin whitening containing extract from Melia azedarach or β-carboline alkaloids | |
| KR20060110578A (en) | C-kit activation inhibitor, skin whitening compound and composition for skin whitening containing the same | |
| KR101358718B1 (en) | Cosmetic composition which has ricinus communis l. extract and sophora japonica flower extract as active components, and improves cellulite | |
| KR100190989B1 (en) | Water soluble fraction extracts of pinelliae tuber having improved skin whitening effects by removing darkening components and a composition for improvining freckles and skin whitening containing thereof | |
| Hakim et al. | Potential of The Tyrosinase Enzyme Inhibition by Standardized Ethanol Extract And Ethyl Acetate Fraction of Bengkoang Peel (Pachyrhizus erosus L.) | |
| KR20100016815A (en) | Cosmetic compositions for skin whitening containing sedum sarmentosum extract and arbutin | |
| CN110300584A (en) | The method for treating hyperpigmentation illness | |
| KR102295904B1 (en) | Cosmetic composition for skin whitening comprising Astilboides tabularis flower extracts or fraction thereof as an active ingredient | |
| CN109943422A (en) | Natural soap composition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2008551174 Country of ref document: JP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 200780002680.9 Country of ref document: CN |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 07700954 Country of ref document: EP Kind code of ref document: A1 |