RU2721192C2 - Способ и устройство для экстракорпорального жизнеобеспечения недоношенного плода - Google Patents
Способ и устройство для экстракорпорального жизнеобеспечения недоношенного плода Download PDFInfo
- Publication number
- RU2721192C2 RU2721192C2 RU2018101884A RU2018101884A RU2721192C2 RU 2721192 C2 RU2721192 C2 RU 2721192C2 RU 2018101884 A RU2018101884 A RU 2018101884A RU 2018101884 A RU2018101884 A RU 2018101884A RU 2721192 C2 RU2721192 C2 RU 2721192C2
- Authority
- RU
- Russia
- Prior art keywords
- fetus
- chamber
- amniotic fluid
- fetal
- fetal chamber
- Prior art date
Links
- 210000003754 fetus Anatomy 0.000 title claims abstract description 236
- 230000002028 premature Effects 0.000 title claims abstract description 54
- 238000000034 method Methods 0.000 title abstract description 14
- 238000002618 extracorporeal membrane oxygenation Methods 0.000 title description 52
- 230000001605 fetal effect Effects 0.000 claims abstract description 241
- 239000012530 fluid Substances 0.000 claims abstract description 169
- 210000004381 amniotic fluid Anatomy 0.000 claims abstract description 123
- 230000002093 peripheral effect Effects 0.000 claims abstract 20
- 239000007789 gas Substances 0.000 claims description 61
- 238000006213 oxygenation reaction Methods 0.000 claims description 49
- 238000001802 infusion Methods 0.000 claims description 34
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 27
- 229910052760 oxygen Inorganic materials 0.000 claims description 27
- 239000001301 oxygen Substances 0.000 claims description 27
- 210000004369 blood Anatomy 0.000 claims description 25
- 239000008280 blood Substances 0.000 claims description 25
- 230000007246 mechanism Effects 0.000 claims description 19
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 claims description 18
- 238000010926 purge Methods 0.000 claims description 18
- 229910002092 carbon dioxide Inorganic materials 0.000 claims description 9
- 239000001569 carbon dioxide Substances 0.000 claims description 9
- 238000011049 filling Methods 0.000 claims description 6
- 230000007423 decrease Effects 0.000 claims description 4
- 230000015572 biosynthetic process Effects 0.000 claims description 3
- 238000007599 discharging Methods 0.000 claims description 2
- 239000013536 elastomeric material Substances 0.000 claims description 2
- 238000003780 insertion Methods 0.000 claims description 2
- 230000037431 insertion Effects 0.000 claims description 2
- 238000007789 sealing Methods 0.000 claims description 2
- 230000005070 ripening Effects 0.000 claims 3
- 230000003247 decreasing effect Effects 0.000 claims 1
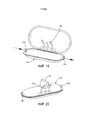
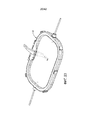
- 210000004291 uterus Anatomy 0.000 abstract description 11
- 230000035935 pregnancy Effects 0.000 abstract description 10
- 206010036590 Premature baby Diseases 0.000 abstract description 8
- 230000012010 growth Effects 0.000 abstract description 7
- 230000035800 maturation Effects 0.000 abstract description 5
- 230000004578 fetal growth Effects 0.000 abstract description 4
- 230000013632 homeostatic process Effects 0.000 abstract description 2
- 239000000126 substance Substances 0.000 abstract description 2
- 238000011038 discontinuous diafiltration by volume reduction Methods 0.000 abstract 1
- 230000000694 effects Effects 0.000 abstract 1
- 238000007654 immersion Methods 0.000 abstract 1
- 230000032633 organ maturation Effects 0.000 abstract 1
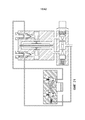
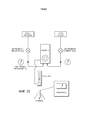
- 230000017531 blood circulation Effects 0.000 description 27
- 238000011161 development Methods 0.000 description 12
- 230000018109 developmental process Effects 0.000 description 10
- 241001465754 Metazoa Species 0.000 description 8
- 241000283903 Ovis aries Species 0.000 description 8
- 210000004700 fetal blood Anatomy 0.000 description 8
- 238000010438 heat treatment Methods 0.000 description 8
- 210000004072 lung Anatomy 0.000 description 8
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 7
- 230000000845 anti-microbial effect Effects 0.000 description 7
- 229960002897 heparin Drugs 0.000 description 7
- 229920000669 heparin Polymers 0.000 description 7
- 230000033001 locomotion Effects 0.000 description 7
- 238000012546 transfer Methods 0.000 description 7
- 235000019687 Lamb Nutrition 0.000 description 6
- UCTWMZQNUQWSLP-UHFFFAOYSA-N adrenaline Chemical compound CNCC(O)C1=CC=C(O)C(O)=C1 UCTWMZQNUQWSLP-UHFFFAOYSA-N 0.000 description 6
- 239000000203 mixture Substances 0.000 description 6
- 230000007704 transition Effects 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- 239000003242 anti bacterial agent Substances 0.000 description 5
- 229940088710 antibiotic agent Drugs 0.000 description 5
- 238000013461 design Methods 0.000 description 5
- 235000013399 edible fruits Nutrition 0.000 description 5
- 210000001161 mammalian embryo Anatomy 0.000 description 5
- 239000012528 membrane Substances 0.000 description 5
- 210000002826 placenta Anatomy 0.000 description 5
- 230000004044 response Effects 0.000 description 5
- 239000000243 solution Substances 0.000 description 5
- 230000001954 sterilising effect Effects 0.000 description 5
- 238000004659 sterilization and disinfection Methods 0.000 description 5
- 238000012384 transportation and delivery Methods 0.000 description 5
- 238000002604 ultrasonography Methods 0.000 description 5
- 239000002699 waste material Substances 0.000 description 5
- 241001494479 Pecora Species 0.000 description 4
- WCUXLLCKKVVCTQ-UHFFFAOYSA-M Potassium chloride Chemical compound [Cl-].[K+] WCUXLLCKKVVCTQ-UHFFFAOYSA-M 0.000 description 4
- 208000005107 Premature Birth Diseases 0.000 description 4
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 4
- 210000001715 carotid artery Anatomy 0.000 description 4
- 210000002257 embryonic structure Anatomy 0.000 description 4
- 210000002458 fetal heart Anatomy 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 230000007040 lung development Effects 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- 238000005086 pumping Methods 0.000 description 4
- 230000004083 survival effect Effects 0.000 description 4
- 230000009885 systemic effect Effects 0.000 description 4
- 210000003954 umbilical cord Anatomy 0.000 description 4
- 210000003606 umbilical vein Anatomy 0.000 description 4
- 210000003462 vein Anatomy 0.000 description 4
- 102000005862 Angiotensin II Human genes 0.000 description 3
- 101800000733 Angiotensin-2 Proteins 0.000 description 3
- 241001631457 Cannula Species 0.000 description 3
- 206010010356 Congenital anomaly Diseases 0.000 description 3
- 208000032589 Diaphragmatic Congenital Hernias Diseases 0.000 description 3
- CZGUSIXMZVURDU-JZXHSEFVSA-N Ile(5)-angiotensin II Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC=1C=CC=CC=1)C([O-])=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)[C@@H]([NH3+])CC([O-])=O)C(C)C)C1=CC=C(O)C=C1 CZGUSIXMZVURDU-JZXHSEFVSA-N 0.000 description 3
- 208000019693 Lung disease Diseases 0.000 description 3
- 239000004712 Metallocene polyethylene (PE-MC) Substances 0.000 description 3
- 208000036830 Normal foetus Diseases 0.000 description 3
- 208000004756 Respiratory Insufficiency Diseases 0.000 description 3
- 229950006323 angiotensin ii Drugs 0.000 description 3
- 238000013459 approach Methods 0.000 description 3
- 230000023555 blood coagulation Effects 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 230000004087 circulation Effects 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- 201000005890 congenital diaphragmatic hernia Diseases 0.000 description 3
- 239000000356 contaminant Substances 0.000 description 3
- 230000037020 contractile activity Effects 0.000 description 3
- 239000003814 drug Substances 0.000 description 3
- 229940079593 drug Drugs 0.000 description 3
- 239000003792 electrolyte Substances 0.000 description 3
- 230000008175 fetal development Effects 0.000 description 3
- 208000015181 infectious disease Diseases 0.000 description 3
- 210000004731 jugular vein Anatomy 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 210000000056 organ Anatomy 0.000 description 3
- 230000000750 progressive effect Effects 0.000 description 3
- 230000002035 prolonged effect Effects 0.000 description 3
- 201000004193 respiratory failure Diseases 0.000 description 3
- 230000029058 respiratory gaseous exchange Effects 0.000 description 3
- 229910052709 silver Inorganic materials 0.000 description 3
- 239000004332 silver Substances 0.000 description 3
- -1 silver cations Chemical class 0.000 description 3
- 230000001225 therapeutic effect Effects 0.000 description 3
- 230000002792 vascular Effects 0.000 description 3
- 230000036266 weeks of gestation Effects 0.000 description 3
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 2
- 206010019280 Heart failures Diseases 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- UIIMBOGNXHQVGW-DEQYMQKBSA-M Sodium bicarbonate-14C Chemical compound [Na+].O[14C]([O-])=O UIIMBOGNXHQVGW-DEQYMQKBSA-M 0.000 description 2
- 208000007536 Thrombosis Diseases 0.000 description 2
- 210000000683 abdominal cavity Anatomy 0.000 description 2
- 239000004599 antimicrobial Substances 0.000 description 2
- 230000036772 blood pressure Effects 0.000 description 2
- 239000001110 calcium chloride Substances 0.000 description 2
- 229910001628 calcium chloride Inorganic materials 0.000 description 2
- 235000011148 calcium chloride Nutrition 0.000 description 2
- 230000002612 cardiopulmonary effect Effects 0.000 description 2
- 230000035606 childbirth Effects 0.000 description 2
- 230000035602 clotting Effects 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 238000010276 construction Methods 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 2
- 238000010494 dissociation reaction Methods 0.000 description 2
- 230000005593 dissociations Effects 0.000 description 2
- 239000012153 distilled water Substances 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 210000002216 heart Anatomy 0.000 description 2
- 230000000004 hemodynamic effect Effects 0.000 description 2
- 239000012510 hollow fiber Substances 0.000 description 2
- 239000004615 ingredient Substances 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 210000001006 meconium Anatomy 0.000 description 2
- 238000012544 monitoring process Methods 0.000 description 2
- XQYZDYMELSJDRZ-UHFFFAOYSA-N papaverine Chemical compound C1=C(OC)C(OC)=CC=C1CC1=NC=CC2=CC(OC)=C(OC)C=C12 XQYZDYMELSJDRZ-UHFFFAOYSA-N 0.000 description 2
- 235000016236 parenteral nutrition Nutrition 0.000 description 2
- 230000010412 perfusion Effects 0.000 description 2
- 230000035479 physiological effects, processes and functions Effects 0.000 description 2
- 230000003169 placental effect Effects 0.000 description 2
- 239000004033 plastic Substances 0.000 description 2
- 229920003023 plastic Polymers 0.000 description 2
- 239000002985 plastic film Substances 0.000 description 2
- 229920001184 polypeptide Polymers 0.000 description 2
- 229920001296 polysiloxane Polymers 0.000 description 2
- 239000001103 potassium chloride Substances 0.000 description 2
- 235000011164 potassium chloride Nutrition 0.000 description 2
- 102000004196 processed proteins & peptides Human genes 0.000 description 2
- 108090000765 processed proteins & peptides Proteins 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 235000002639 sodium chloride Nutrition 0.000 description 2
- 230000009747 swallowing Effects 0.000 description 2
- 238000009423 ventilation Methods 0.000 description 2
- 238000003466 welding Methods 0.000 description 2
- 229930008281 A03AD01 - Papaverine Natural products 0.000 description 1
- 206010000060 Abdominal distension Diseases 0.000 description 1
- 241000272525 Anas platyrhynchos Species 0.000 description 1
- 229930003347 Atropine Natural products 0.000 description 1
- 206010009192 Circulatory collapse Diseases 0.000 description 1
- 208000032170 Congenital Abnormalities Diseases 0.000 description 1
- 208000027205 Congenital disease Diseases 0.000 description 1
- 206010058314 Dysplasia Diseases 0.000 description 1
- JOYRKODLDBILNP-UHFFFAOYSA-N Ethyl urethane Chemical compound CCOC(N)=O JOYRKODLDBILNP-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 241000132456 Haplocarpha Species 0.000 description 1
- 208000010496 Heart Arrest Diseases 0.000 description 1
- 102000001554 Hemoglobins Human genes 0.000 description 1
- 108010054147 Hemoglobins Proteins 0.000 description 1
- RKUNBYITZUJHSG-UHFFFAOYSA-N Hyosciamin-hydrochlorid Natural products CN1C(C2)CCC1CC2OC(=O)C(CO)C1=CC=CC=C1 RKUNBYITZUJHSG-UHFFFAOYSA-N 0.000 description 1
- 206010021143 Hypoxia Diseases 0.000 description 1
- 108010042653 IgA receptor Proteins 0.000 description 1
- 208000032754 Infant Death Diseases 0.000 description 1
- 208000035752 Live birth Diseases 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 208000034702 Multiple pregnancies Diseases 0.000 description 1
- 239000004677 Nylon Substances 0.000 description 1
- 206010030113 Oedema Diseases 0.000 description 1
- 208000001184 Oligohydramnios Diseases 0.000 description 1
- 206010053159 Organ failure Diseases 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 102100034014 Prolyl 3-hydroxylase 3 Human genes 0.000 description 1
- 206010039897 Sedation Diseases 0.000 description 1
- 206010040047 Sepsis Diseases 0.000 description 1
- 206010067726 Shortened cervix Diseases 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 208000005392 Spasm Diseases 0.000 description 1
- 206010047163 Vasospasm Diseases 0.000 description 1
- 210000003815 abdominal wall Anatomy 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 230000002421 anti-septic effect Effects 0.000 description 1
- 210000001367 artery Anatomy 0.000 description 1
- RKUNBYITZUJHSG-SPUOUPEWSA-N atropine Chemical compound O([C@H]1C[C@H]2CC[C@@H](C1)N2C)C(=O)C(CO)C1=CC=CC=C1 RKUNBYITZUJHSG-SPUOUPEWSA-N 0.000 description 1
- 229960000396 atropine Drugs 0.000 description 1
- 230000010455 autoregulation Effects 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000007698 birth defect Effects 0.000 description 1
- 208000024330 bloating Diseases 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 230000036471 bradycardia Effects 0.000 description 1
- 208000006218 bradycardia Diseases 0.000 description 1
- 210000001168 carotid artery common Anatomy 0.000 description 1
- 206010008129 cerebral palsy Diseases 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 238000012864 cross contamination Methods 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 231100000517 death Toxicity 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- 230000035487 diastolic blood pressure Effects 0.000 description 1
- XEYBRNLFEZDVAW-ARSRFYASSA-N dinoprostone Chemical compound CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O XEYBRNLFEZDVAW-ARSRFYASSA-N 0.000 description 1
- 239000013013 elastic material Substances 0.000 description 1
- 238000001839 endoscopy Methods 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 210000003195 fascia Anatomy 0.000 description 1
- 210000003608 fece Anatomy 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 229920002457 flexible plastic Polymers 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 239000003292 glue Substances 0.000 description 1
- 230000002008 hemorrhagic effect Effects 0.000 description 1
- 230000007954 hypoxia Effects 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 230000002458 infectious effect Effects 0.000 description 1
- 230000036512 infertility Effects 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 210000000936 intestine Anatomy 0.000 description 1
- 230000002262 irrigation Effects 0.000 description 1
- 238000003973 irrigation Methods 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 208000018773 low birth weight Diseases 0.000 description 1
- 231100000533 low birth weight Toxicity 0.000 description 1
- 230000004199 lung function Effects 0.000 description 1
- 238000007726 management method Methods 0.000 description 1
- 230000008774 maternal effect Effects 0.000 description 1
- 238000005399 mechanical ventilation Methods 0.000 description 1
- 208000030159 metabolic disease Diseases 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 235000010755 mineral Nutrition 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 235000016709 nutrition Nutrition 0.000 description 1
- 230000035764 nutrition Effects 0.000 description 1
- 229920001778 nylon Polymers 0.000 description 1
- 229960001789 papaverine Drugs 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 230000002572 peristaltic effect Effects 0.000 description 1
- 229920006255 plastic film Polymers 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 229920000306 polymethylpentene Polymers 0.000 description 1
- 239000011116 polymethylpentene Substances 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 238000003825 pressing Methods 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 230000002685 pulmonary effect Effects 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 230000003134 recirculating effect Effects 0.000 description 1
- 210000005245 right atrium Anatomy 0.000 description 1
- 239000000523 sample Substances 0.000 description 1
- 230000036280 sedation Effects 0.000 description 1
- 229940125723 sedative agent Drugs 0.000 description 1
- 239000000932 sedative agent Substances 0.000 description 1
- 206010040560 shock Diseases 0.000 description 1
- 210000000813 small intestine Anatomy 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 230000035488 systolic blood pressure Effects 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 230000003195 tocolytic effect Effects 0.000 description 1
- 239000011573 trace mineral Substances 0.000 description 1
- 235000013619 trace mineral Nutrition 0.000 description 1
- 230000000472 traumatic effect Effects 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- 239000002550 vasoactive agent Substances 0.000 description 1
- 210000001631 vena cava inferior Anatomy 0.000 description 1
- 210000002620 vena cava superior Anatomy 0.000 description 1
- 230000035899 viability Effects 0.000 description 1
- 239000011782 vitamin Substances 0.000 description 1
- 235000013343 vitamin Nutrition 0.000 description 1
- 229940088594 vitamin Drugs 0.000 description 1
- 229930003231 vitamin Natural products 0.000 description 1
- 238000005303 weighing Methods 0.000 description 1
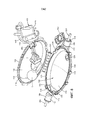
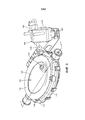
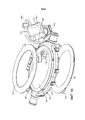
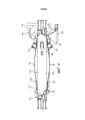
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61G—TRANSPORT, PERSONAL CONVEYANCES, OR ACCOMMODATION SPECIALLY ADAPTED FOR PATIENTS OR DISABLED PERSONS; OPERATING TABLES OR CHAIRS; CHAIRS FOR DENTISTRY; FUNERAL DEVICES
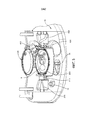
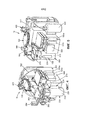
- A61G11/00—Baby-incubators; Couveuses
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N1/00—Preservation of bodies of humans or animals, or parts thereof
- A01N1/10—Preservation of living parts
- A01N1/14—Mechanical aspects of preservation; Apparatus or containers therefor
- A01N1/142—Apparatus
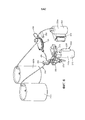
- A01N1/143—Apparatus for organ perfusion
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61G—TRANSPORT, PERSONAL CONVEYANCES, OR ACCOMMODATION SPECIALLY ADAPTED FOR PATIENTS OR DISABLED PERSONS; OPERATING TABLES OR CHAIRS; CHAIRS FOR DENTISTRY; FUNERAL DEVICES
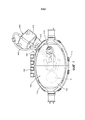
- A61G10/00—Treatment rooms or enclosures for medical purposes
- A61G10/02—Treatment rooms or enclosures for medical purposes with artificial climate; with means to maintain a desired pressure, e.g. for germ-free rooms
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61G—TRANSPORT, PERSONAL CONVEYANCES, OR ACCOMMODATION SPECIALLY ADAPTED FOR PATIENTS OR DISABLED PERSONS; OPERATING TABLES OR CHAIRS; CHAIRS FOR DENTISTRY; FUNERAL DEVICES
- A61G11/00—Baby-incubators; Couveuses
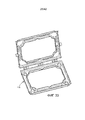
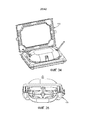
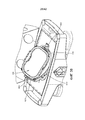
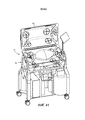
- A61G11/005—Baby-incubators; Couveuses with movable walls, e.g. for accessing the inside, removable walls
- A61G11/006—Baby-incubators; Couveuses with movable walls, e.g. for accessing the inside, removable walls by pivoting
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61G—TRANSPORT, PERSONAL CONVEYANCES, OR ACCOMMODATION SPECIALLY ADAPTED FOR PATIENTS OR DISABLED PERSONS; OPERATING TABLES OR CHAIRS; CHAIRS FOR DENTISTRY; FUNERAL DEVICES
- A61G11/00—Baby-incubators; Couveuses
- A61G11/008—Baby-incubators; Couveuses tiltable about a horizontal axis, e.g. oscillating
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61G—TRANSPORT, PERSONAL CONVEYANCES, OR ACCOMMODATION SPECIALLY ADAPTED FOR PATIENTS OR DISABLED PERSONS; OPERATING TABLES OR CHAIRS; CHAIRS FOR DENTISTRY; FUNERAL DEVICES
- A61G11/00—Baby-incubators; Couveuses
- A61G11/009—Baby-incubators; Couveuses with hand insertion windows, e.g. in the walls
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61G—TRANSPORT, PERSONAL CONVEYANCES, OR ACCOMMODATION SPECIALLY ADAPTED FOR PATIENTS OR DISABLED PERSONS; OPERATING TABLES OR CHAIRS; CHAIRS FOR DENTISTRY; FUNERAL DEVICES
- A61G2203/00—General characteristics of devices
- A61G2203/30—General characteristics of devices characterised by sensor means
- A61G2203/34—General characteristics of devices characterised by sensor means for pressure
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61G—TRANSPORT, PERSONAL CONVEYANCES, OR ACCOMMODATION SPECIALLY ADAPTED FOR PATIENTS OR DISABLED PERSONS; OPERATING TABLES OR CHAIRS; CHAIRS FOR DENTISTRY; FUNERAL DEVICES
- A61G2210/00—Devices for specific treatment or diagnosis
- A61G2210/30—Devices for specific treatment or diagnosis for intensive care
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Pregnancy & Childbirth (AREA)
- Gynecology & Obstetrics (AREA)
- Pediatric Medicine (AREA)
- Pulmonology (AREA)
- Environmental Sciences (AREA)
- Dentistry (AREA)
- Zoology (AREA)
- Engineering & Computer Science (AREA)
- Wood Science & Technology (AREA)
- External Artificial Organs (AREA)
- Accommodation For Nursing Or Treatment Tables (AREA)
- Apparatus Associated With Microorganisms And Enzymes (AREA)
- Endoscopes (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562181861P | 2015-06-19 | 2015-06-19 | |
| US62/181,861 | 2015-06-19 | ||
| US201562260251P | 2015-11-26 | 2015-11-26 | |
| US62/260,251 | 2015-11-26 | ||
| PCT/US2016/038045 WO2016205622A1 (en) | 2015-06-19 | 2016-06-17 | Method and apparatus for extracorporeal support of premature fetus |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2020114672A Division RU2020114672A (ru) | 2015-06-19 | 2016-06-17 | Способ и устройство для экстракорпорального жизнеобеспечения недоношенного плода |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| RU2018101884A RU2018101884A (ru) | 2019-07-19 |
| RU2018101884A3 RU2018101884A3 (enExample) | 2019-10-10 |
| RU2721192C2 true RU2721192C2 (ru) | 2020-05-18 |
Family
ID=57546277
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2018101884A RU2721192C2 (ru) | 2015-06-19 | 2016-06-17 | Способ и устройство для экстракорпорального жизнеобеспечения недоношенного плода |
| RU2020114672A RU2020114672A (ru) | 2015-06-19 | 2016-06-17 | Способ и устройство для экстракорпорального жизнеобеспечения недоношенного плода |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2020114672A RU2020114672A (ru) | 2015-06-19 | 2016-06-17 | Способ и устройство для экстракорпорального жизнеобеспечения недоношенного плода |
Country Status (13)
| Country | Link |
|---|---|
| US (4) | US10751238B2 (enExample) |
| EP (2) | EP3685813B1 (enExample) |
| JP (2) | JP6766081B2 (enExample) |
| KR (1) | KR20180041118A (enExample) |
| AU (3) | AU2016280194B2 (enExample) |
| CA (1) | CA2989857A1 (enExample) |
| DK (1) | DK3310316T3 (enExample) |
| ES (2) | ES2907206T3 (enExample) |
| MX (1) | MX2017016900A (enExample) |
| RS (1) | RS60319B1 (enExample) |
| RU (2) | RU2721192C2 (enExample) |
| WO (1) | WO2016205622A1 (enExample) |
| ZA (1) | ZA201708571B (enExample) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU202283U1 (ru) * | 2020-09-16 | 2021-02-10 | Российская Федерация, от имени которой выступает ФОНД ПЕРСПЕКТИВНЫХ ИССЛЕДОВАНИЙ | Установка для жидкостного дыхания в условиях гипербарии |
| RU2743609C1 (ru) * | 2020-08-06 | 2021-02-20 | Общество с ограниченной ответственностью "Научно-производственное предприятие Биотех-М" | Сдвоенный контейнер для гемокомпонентов и способ его применения |
| RU203446U1 (ru) * | 2020-09-16 | 2021-04-06 | Российская Федерация, от имени которой выступает ФОНД ПЕРСПЕКТИВНЫХ ИССЛЕДОВАНИЙ | Модуль жидкостного дыхания в условиях гипербарии для модельного биообъекта |
| RU209285U1 (ru) * | 2021-10-11 | 2022-03-14 | Федеральное государственное автономное образовательное учреждение высшего образования "Севастопольский государственный университет" | Аппарат жидкостного дыхания |
Families Citing this family (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ES2886598T3 (es) | 2013-03-15 | 2021-12-20 | Childrens Hospital Philadelphia | Sistema de soporte vital extracorpóreo |
| ES2907206T3 (es) | 2015-06-19 | 2022-04-22 | Childrens Hospital Philadelphia | Aparato para soporte extracorpóreo de feto prematuro |
| WO2018111956A1 (en) * | 2016-12-14 | 2018-06-21 | The Children's Hospital Of Philadelphia | System and method configured to provide extracorporeal support for premature fetus |
| WO2018171905A1 (de) * | 2017-03-21 | 2018-09-27 | Universitätsklinikum Halle (Saale) | Künstliches gebärmuttersystem und plazenta |
| DE102018126634A1 (de) * | 2018-10-25 | 2020-04-30 | Prenatal International GmbH Halle | Vorrichtung mit künstlichem Kiemensystem und Verfahren für die Lebenserhaltung eines Neugeborenen |
| EP3946203A1 (en) * | 2019-03-29 | 2022-02-09 | Technische Universiteit Eindhoven | An incubation system for liquid-based incubation of prematurely born infants |
| US12268802B2 (en) | 2019-04-09 | 2025-04-08 | The Children's Hospital Of Philadelphia | Oxygenator for use with extracorporeal support of premature fetus |
| US20220054666A1 (en) * | 2020-08-24 | 2022-02-24 | Covidien Lp | Ventilator filter sterilization systems and methods |
| CA3192675A1 (en) * | 2020-09-15 | 2022-03-24 | Alan W. Flake | Chamber assembly for premature fetus |
| WO2022232468A1 (en) * | 2021-04-28 | 2022-11-03 | The Children's Hospital Of Philadelphia | Transfer tray and priming cart for neonatal cannulation and related methods |
| EP4419113A4 (en) * | 2021-10-20 | 2025-11-26 | Childrens Hospital Philadelphia | PHYSIOLOGICAL SALINE SOLUTION AND ITS PROCESSES OF MANUFACTURING AND USING |
| CA3255419A1 (en) * | 2022-05-20 | 2023-11-23 | Vitara Biomedical Inc | METHOD AND DEVICE FOR MEASURING OXYGEN SATURATION |
| WO2024058775A1 (en) | 2022-09-14 | 2024-03-21 | The Children's Hospital Of Philadelphia | Systems and methods for oxygenating blood, passive oxygenation circuits, and neonatal extracorporeal support systems |
| WO2024059709A2 (en) * | 2022-09-14 | 2024-03-21 | University Of Pittsburgh - Of The Commonwealth System Of Higher Education | Extracorporeal gas exchange systems for use with preterm infants |
| DE102023110564A1 (de) * | 2023-04-25 | 2024-10-31 | Elisabeth Krenkler | Künstliche Gebärmutter |
| EP4663177A1 (en) * | 2024-06-12 | 2025-12-17 | Hospital Sant Joan de Deu | Chamber system for mammalian fetal development and maturation |
| DE102024116589B3 (de) * | 2024-06-13 | 2025-11-20 | Rheinisch-Westfälische Technische Hochschule Aachen, abgekürzt RWTH Aachen, Körperschaft des öffentlichen Rechts | Künstliches Gebärmuttersystem und Verfahren zu dessen Herstellung |
| CN119746195A (zh) * | 2025-01-13 | 2025-04-04 | 北京清瀚医疗科技有限公司 | 一种体外生命支持系统 |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2723660A (en) * | 1954-07-22 | 1955-11-15 | Emanuel M Greenberg | Artificial uterus |
| US4796605A (en) * | 1986-07-11 | 1989-01-10 | Atom Kabushiki Kaisha | Incubator |
| US5063924A (en) * | 1989-09-08 | 1991-11-12 | Comitato Nazionale Per La Ricerca E Per Lo Sviluppo Dell'energia Nucleare E Delle Energie Alternative | Protective device, individual, portable, with total insulation and controlled atmosphere |
| US5218958A (en) * | 1991-02-21 | 1993-06-15 | Cooper William I | Placental chamber - artificial uterus |
| US20040193096A1 (en) * | 2003-03-25 | 2004-09-30 | Cooper William Isaac | Conceptus chamber |
| US20070010005A1 (en) * | 2005-07-08 | 2007-01-11 | James Sitzmann | Neonatal support system and related devices and methods of use |
| WO2014145494A1 (en) * | 2013-03-15 | 2014-09-18 | The Children's Hospital Of Philadelphia | Extracorporeal life support system and methods of use thereof |
Family Cites Families (47)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2600240A (en) | 1948-05-22 | 1952-06-10 | Philadelphia Children Hospital | Construction of incubators for infants |
| GB1082381A (en) | 1964-06-25 | 1967-09-06 | Grant Graham Cameron | Improvements in or relating to infant incubators |
| US4048684A (en) | 1975-12-10 | 1977-09-20 | The Board Of Trustees Of Leland Stanford Junior University | Infant waterbed |
| FR2522963A1 (fr) | 1982-03-12 | 1983-09-16 | Calhene | Installation pour le confinement et le transport en atmosphere sterile d'etres humains, notamment de nouveau-nes |
| CH664892A5 (de) | 1984-05-18 | 1988-04-15 | Ameda Ag | Inkubator. |
| EP0447256A3 (en) | 1990-03-16 | 1992-01-08 | Hitachi, Ltd. | Methods and apparatus for incubation and sterilization |
| US5207639A (en) | 1991-02-21 | 1993-05-04 | Cooper William I | Fetal lung apparatus |
| EP0522674B1 (en) | 1991-07-12 | 1998-11-11 | Mark R. Robinson | Oximeter for reliable clinical determination of blood oxygen saturation in a fetus |
| US5308310A (en) | 1992-08-18 | 1994-05-03 | Vitaltrends Technology, Inc. | Plethysmograph system and air-tight sealing assembly therefor |
| US5624375A (en) | 1995-04-21 | 1997-04-29 | Ohmeda Inc. | Incubator tilt mechanism |
| WO1998039039A1 (en) | 1997-03-05 | 1998-09-11 | Fehrenbacher John W | Aortic cannula |
| US6530902B1 (en) | 1998-01-23 | 2003-03-11 | Medtronic, Inc. | Cannula placement system |
| CN1308549A (zh) | 1998-05-08 | 2001-08-15 | 爱德华兹生命科学公司 | 带有整体热交换器/蓄存器的低灌注膜片充氧器 |
| EP1231883B1 (en) | 1999-11-15 | 2008-09-10 | Draeger Medical Systems, Inc. | Infant care apparatus with movable infant support |
| US20020069877A1 (en) | 2000-12-13 | 2002-06-13 | Villareal Daniel C. | Ventilation transport device |
| US10155082B2 (en) | 2002-04-10 | 2018-12-18 | Baxter International Inc. | Enhanced signal detection for access disconnection systems |
| PE20040074A1 (es) * | 2002-07-12 | 2004-02-25 | Univ Pontificia Catolica Peru | Burbuja artificial neonatal |
| EP1649265A1 (en) | 2003-07-28 | 2006-04-26 | Symyx Technologies, Inc. | Parallel infrared spectroscopy apparatus and method |
| US7108653B2 (en) | 2003-12-04 | 2006-09-19 | Datex-Ohmeda, Inc. | Canopy adjustable mounting system for infant warming apparatus |
| JPWO2005082384A1 (ja) | 2004-02-27 | 2008-03-06 | 株式会社日本トリム | 人工生理的塩類溶液およびその製造方法 |
| US20060270911A1 (en) | 2005-04-08 | 2006-11-30 | Voegele James W | Tissue retraction device |
| EP1886071A1 (en) | 2005-05-27 | 2008-02-13 | Ralph Ellerker (1795) Ltd | Apparatus for use to create a controlled environment |
| US8585968B2 (en) | 2006-04-21 | 2013-11-19 | Scott W. Morley | Method and system for purging moisture from an oxygenator |
| US7763097B2 (en) | 2006-06-08 | 2010-07-27 | University of Pittsburgh—of the Commonwealth System of Higher Education | Devices, systems and methods for reducing the concentration of a chemical entity in fluids |
| US8333686B2 (en) | 2006-08-30 | 2012-12-18 | Circulite, Inc. | Cannula insertion devices, systems, and methods including a compressible member |
| GB2445437B (en) | 2007-07-06 | 2008-11-26 | Applied Medical Technology Ltd | Cannula insertion device |
| US20090149776A1 (en) * | 2007-12-05 | 2009-06-11 | Adams Scott C | Optical sensor for detecting infection and other anomalous conditions associated with catheter systems |
| WO2009077626A1 (es) | 2007-12-19 | 2009-06-25 | Fundacion Para La Investigacion Biomedica Del Hospital Gregorio Marañon | Incubadora para imagen con radiacion no ionizante |
| RU2376969C1 (ru) | 2008-06-11 | 2009-12-27 | Государственное образовательное учреждение высшего профессионального образования "Саратовский государственный медицинский университет Федерального агентства по здравоохранению и социальному развитию" | Устройство для выхаживания недоношенных новорожденных |
| US8329450B2 (en) * | 2008-08-01 | 2012-12-11 | Biomedinnovations, Llc | Methods and apparatus for organ support |
| WO2010078395A2 (en) * | 2008-12-31 | 2010-07-08 | World Medical Technologies, Llc | Modular neonatal intensive care system |
| DE102009037015A1 (de) | 2009-08-07 | 2011-02-17 | Michael Hajek | Vorrichtung und Verfahren zur Eliminierung von bioschädlichen Stoffen aus Körperflüssigkeiten |
| WO2013026148A1 (en) | 2011-08-23 | 2013-02-28 | Mcmaster University | Artificial placenta |
| CA2846590A1 (en) | 2011-08-25 | 2013-02-28 | Breonics, Inc. | Organ chamber for ex vivo warm perfusion |
| JP5912792B2 (ja) | 2012-04-11 | 2016-04-27 | アトムメディカル株式会社 | 保育器 |
| JP5828802B2 (ja) | 2012-05-07 | 2015-12-09 | 英伸 太田 | 早産児用の抱っこ型次世代人工保育器。 |
| CN103381094B (zh) | 2013-05-17 | 2015-01-21 | 王义向 | 胎儿脉搏血氧饱和度监测系统及方法 |
| US9968372B2 (en) | 2013-10-02 | 2018-05-15 | Medical Instrument Development Laboratories, Inc. | Cannula insertion tool |
| DE102013018919B4 (de) | 2013-11-13 | 2018-12-27 | Drägerwerk AG & Co. KGaA | Wärmetherapiegerät für Neu- und Frühgeborene |
| CN203663028U (zh) * | 2014-01-17 | 2014-06-25 | 张洪娟 | 用于产科医疗的新生儿恒温箱 |
| JP6377477B2 (ja) | 2014-09-25 | 2018-08-22 | テルモ株式会社 | クランプ装置 |
| ES2907206T3 (es) | 2015-06-19 | 2022-04-22 | Childrens Hospital Philadelphia | Aparato para soporte extracorpóreo de feto prematuro |
| EP3370676B1 (en) | 2015-11-06 | 2023-12-13 | Amnion Life, LLC | Premature infant amniotic bath incubator |
| CN108471950B (zh) | 2015-12-30 | 2021-01-12 | 曜谛测氧股份有限公司 | 用于进行经腹胎儿血氧饱和度监测的系统、装置及方法 |
| WO2018111956A1 (en) | 2016-12-14 | 2018-06-21 | The Children's Hospital Of Philadelphia | System and method configured to provide extracorporeal support for premature fetus |
| WO2018171905A1 (de) | 2017-03-21 | 2018-09-27 | Universitätsklinikum Halle (Saale) | Künstliches gebärmuttersystem und plazenta |
| CN109528216A (zh) | 2019-01-18 | 2019-03-29 | 京东方科技集团股份有限公司 | 胎儿血氧饱和度的检测方法及装置 |
-
2016
- 2016-06-17 ES ES20163714T patent/ES2907206T3/es active Active
- 2016-06-17 RU RU2018101884A patent/RU2721192C2/ru active
- 2016-06-17 DK DK16812507.8T patent/DK3310316T3/da active
- 2016-06-17 ES ES16812507T patent/ES2791477T3/es active Active
- 2016-06-17 US US15/736,825 patent/US10751238B2/en active Active
- 2016-06-17 KR KR1020187001657A patent/KR20180041118A/ko not_active Withdrawn
- 2016-06-17 JP JP2017565991A patent/JP6766081B2/ja active Active
- 2016-06-17 MX MX2017016900A patent/MX2017016900A/es unknown
- 2016-06-17 WO PCT/US2016/038045 patent/WO2016205622A1/en not_active Ceased
- 2016-06-17 EP EP20163714.7A patent/EP3685813B1/en active Active
- 2016-06-17 CA CA2989857A patent/CA2989857A1/en active Pending
- 2016-06-17 AU AU2016280194A patent/AU2016280194B2/en active Active
- 2016-06-17 RS RS20200605A patent/RS60319B1/sr unknown
- 2016-06-17 RU RU2020114672A patent/RU2020114672A/ru unknown
- 2016-06-17 EP EP16812507.8A patent/EP3310316B1/en active Active
-
2017
- 2017-12-15 ZA ZA2017/08571A patent/ZA201708571B/en unknown
-
2020
- 2020-04-14 US US16/848,085 patent/US10945903B2/en active Active
- 2020-09-16 JP JP2020155293A patent/JP7003199B2/ja active Active
- 2020-12-08 AU AU2020286215A patent/AU2020286215B2/en active Active
-
2021
- 2021-02-10 US US17/172,239 patent/US12083048B2/en active Active
-
2022
- 2022-11-04 AU AU2022263568A patent/AU2022263568B2/en active Active
-
2024
- 2024-07-31 US US18/790,815 patent/US20240390205A1/en active Pending
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2723660A (en) * | 1954-07-22 | 1955-11-15 | Emanuel M Greenberg | Artificial uterus |
| US4796605A (en) * | 1986-07-11 | 1989-01-10 | Atom Kabushiki Kaisha | Incubator |
| US5063924A (en) * | 1989-09-08 | 1991-11-12 | Comitato Nazionale Per La Ricerca E Per Lo Sviluppo Dell'energia Nucleare E Delle Energie Alternative | Protective device, individual, portable, with total insulation and controlled atmosphere |
| US5218958A (en) * | 1991-02-21 | 1993-06-15 | Cooper William I | Placental chamber - artificial uterus |
| US20040193096A1 (en) * | 2003-03-25 | 2004-09-30 | Cooper William Isaac | Conceptus chamber |
| US20070010005A1 (en) * | 2005-07-08 | 2007-01-11 | James Sitzmann | Neonatal support system and related devices and methods of use |
| WO2014145494A1 (en) * | 2013-03-15 | 2014-09-18 | The Children's Hospital Of Philadelphia | Extracorporeal life support system and methods of use thereof |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2743609C1 (ru) * | 2020-08-06 | 2021-02-20 | Общество с ограниченной ответственностью "Научно-производственное предприятие Биотех-М" | Сдвоенный контейнер для гемокомпонентов и способ его применения |
| RU202283U1 (ru) * | 2020-09-16 | 2021-02-10 | Российская Федерация, от имени которой выступает ФОНД ПЕРСПЕКТИВНЫХ ИССЛЕДОВАНИЙ | Установка для жидкостного дыхания в условиях гипербарии |
| RU203446U1 (ru) * | 2020-09-16 | 2021-04-06 | Российская Федерация, от имени которой выступает ФОНД ПЕРСПЕКТИВНЫХ ИССЛЕДОВАНИЙ | Модуль жидкостного дыхания в условиях гипербарии для модельного биообъекта |
| RU209285U1 (ru) * | 2021-10-11 | 2022-03-14 | Федеральное государственное автономное образовательное учреждение высшего образования "Севастопольский государственный университет" | Аппарат жидкостного дыхания |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2721192C2 (ru) | Способ и устройство для экстракорпорального жизнеобеспечения недоношенного плода | |
| US20230000706A1 (en) | System And Method Configured To Provide Extracorporeal Support For Premature Fetus | |
| RU2684470C2 (ru) | Система экстракорпорального жизнеобеспечения и способы ее использования | |
| Bird | Artificial placenta: Analysis of recent progress | |
| JP7495428B2 (ja) | 未熟児の液体ベースのインキュベーションのためのインキュベーションシステム | |
| HK1254674B (en) | Apparatus for extracorporeal support of premature fetus |