RU2696497C2 - Композиция с факторами роста для применения в интраназальном лечении нейродегенеративного заболевания или других заболеваний центральной нервной системы и способ ее получения - Google Patents
Композиция с факторами роста для применения в интраназальном лечении нейродегенеративного заболевания или других заболеваний центральной нервной системы и способ ее получения Download PDFInfo
- Publication number
- RU2696497C2 RU2696497C2 RU2015104076A RU2015104076A RU2696497C2 RU 2696497 C2 RU2696497 C2 RU 2696497C2 RU 2015104076 A RU2015104076 A RU 2015104076A RU 2015104076 A RU2015104076 A RU 2015104076A RU 2696497 C2 RU2696497 C2 RU 2696497C2
- Authority
- RU
- Russia
- Prior art keywords
- composition
- growth factor
- blood
- nervous system
- central nervous
- Prior art date
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 47
- 239000003102 growth factor Substances 0.000 title claims abstract description 46
- 230000004770 neurodegeneration Effects 0.000 title claims abstract description 19
- 208000015122 neurodegenerative disease Diseases 0.000 title claims abstract description 19
- 210000003169 central nervous system Anatomy 0.000 title description 20
- 201000010099 disease Diseases 0.000 title description 18
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 title description 18
- 238000004519 manufacturing process Methods 0.000 title description 6
- 239000012503 blood component Substances 0.000 claims abstract description 23
- 239000006228 supernatant Substances 0.000 claims abstract description 17
- 239000003814 drug Substances 0.000 abstract description 28
- 239000000126 substance Substances 0.000 abstract description 20
- 210000004369 blood Anatomy 0.000 abstract description 10
- 239000008280 blood Substances 0.000 abstract description 10
- 239000000306 component Substances 0.000 abstract description 10
- 230000000694 effects Effects 0.000 abstract description 4
- 239000012530 fluid Substances 0.000 abstract description 2
- 241001465754 Metazoa Species 0.000 description 25
- 210000002381 plasma Anatomy 0.000 description 25
- 229940124597 therapeutic agent Drugs 0.000 description 23
- 238000000034 method Methods 0.000 description 15
- 210000004027 cell Anatomy 0.000 description 12
- 241000699660 Mus musculus Species 0.000 description 11
- 238000011830 transgenic mouse model Methods 0.000 description 11
- 210000004556 brain Anatomy 0.000 description 9
- 108010090849 Amyloid beta-Peptides Proteins 0.000 description 8
- 102000013455 Amyloid beta-Peptides Human genes 0.000 description 8
- 230000015572 biosynthetic process Effects 0.000 description 6
- 238000005755 formation reaction Methods 0.000 description 6
- 210000002569 neuron Anatomy 0.000 description 6
- 238000012360 testing method Methods 0.000 description 6
- 208000024827 Alzheimer disease Diseases 0.000 description 5
- 210000001320 hippocampus Anatomy 0.000 description 5
- 206010018341 Gliosis Diseases 0.000 description 4
- 210000001130 astrocyte Anatomy 0.000 description 4
- 208000037875 astrocytosis Diseases 0.000 description 4
- 230000007341 astrogliosis Effects 0.000 description 4
- 210000001772 blood platelet Anatomy 0.000 description 4
- 210000001175 cerebrospinal fluid Anatomy 0.000 description 4
- 239000003795 chemical substances by application Substances 0.000 description 4
- 238000002474 experimental method Methods 0.000 description 4
- 210000004379 membrane Anatomy 0.000 description 4
- 239000012528 membrane Substances 0.000 description 4
- 210000003928 nasal cavity Anatomy 0.000 description 4
- 210000000278 spinal cord Anatomy 0.000 description 4
- 238000010186 staining Methods 0.000 description 4
- 230000001225 therapeutic effect Effects 0.000 description 4
- 108010073929 Vascular Endothelial Growth Factor A Proteins 0.000 description 3
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 3
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 3
- 108010064539 amyloid beta-protein (1-42) Proteins 0.000 description 3
- 238000009227 behaviour therapy Methods 0.000 description 3
- 230000004071 biological effect Effects 0.000 description 3
- 230000005540 biological transmission Effects 0.000 description 3
- 230000006378 damage Effects 0.000 description 3
- 230000012010 growth Effects 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 238000012544 monitoring process Methods 0.000 description 3
- 230000004766 neurogenesis Effects 0.000 description 3
- 210000000196 olfactory nerve Anatomy 0.000 description 3
- 108090000765 processed proteins & peptides Proteins 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 208000037259 Amyloid Plaque Diseases 0.000 description 2
- 206010002942 Apathy Diseases 0.000 description 2
- 101001092197 Homo sapiens RNA binding protein fox-1 homolog 3 Proteins 0.000 description 2
- 108090000723 Insulin-Like Growth Factor I Proteins 0.000 description 2
- 108010025020 Nerve Growth Factor Proteins 0.000 description 2
- 102000015336 Nerve Growth Factor Human genes 0.000 description 2
- 102100035530 RNA binding protein fox-1 homolog 3 Human genes 0.000 description 2
- 208000027418 Wounds and injury Diseases 0.000 description 2
- 108010064397 amyloid beta-protein (1-40) Proteins 0.000 description 2
- 230000002424 anti-apoptotic effect Effects 0.000 description 2
- 230000004888 barrier function Effects 0.000 description 2
- 230000006399 behavior Effects 0.000 description 2
- 230000008499 blood brain barrier function Effects 0.000 description 2
- 210000004204 blood vessel Anatomy 0.000 description 2
- 210000001218 blood-brain barrier Anatomy 0.000 description 2
- 210000000988 bone and bone Anatomy 0.000 description 2
- 210000003710 cerebral cortex Anatomy 0.000 description 2
- 210000004720 cerebrum Anatomy 0.000 description 2
- 230000015271 coagulation Effects 0.000 description 2
- 238000005345 coagulation Methods 0.000 description 2
- 208000010877 cognitive disease Diseases 0.000 description 2
- 210000001787 dendrite Anatomy 0.000 description 2
- 210000001947 dentate gyrus Anatomy 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 208000014674 injury Diseases 0.000 description 2
- 210000003097 mucus Anatomy 0.000 description 2
- 230000017074 necrotic cell death Effects 0.000 description 2
- 229940053128 nerve growth factor Drugs 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- 210000003446 pia mater Anatomy 0.000 description 2
- 108010088880 plasmagel Proteins 0.000 description 2
- 210000004623 platelet-rich plasma Anatomy 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 230000008929 regeneration Effects 0.000 description 2
- 238000011069 regeneration method Methods 0.000 description 2
- 230000007723 transport mechanism Effects 0.000 description 2
- 210000003901 trigeminal nerve Anatomy 0.000 description 2
- WOVKYSAHUYNSMH-RRKCRQDMSA-N 5-bromodeoxyuridine Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(Br)=C1 WOVKYSAHUYNSMH-RRKCRQDMSA-N 0.000 description 1
- 101710137189 Amyloid-beta A4 protein Proteins 0.000 description 1
- 101710151993 Amyloid-beta precursor protein Proteins 0.000 description 1
- 102100022704 Amyloid-beta precursor protein Human genes 0.000 description 1
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 1
- 208000028698 Cognitive impairment Diseases 0.000 description 1
- 208000020406 Creutzfeldt Jacob disease Diseases 0.000 description 1
- 208000003407 Creutzfeldt-Jakob Syndrome Diseases 0.000 description 1
- 208000010859 Creutzfeldt-Jakob disease Diseases 0.000 description 1
- 208000023105 Huntington disease Diseases 0.000 description 1
- 102000004218 Insulin-Like Growth Factor I Human genes 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 206010029113 Neovascularisation Diseases 0.000 description 1
- 208000018737 Parkinson disease Diseases 0.000 description 1
- 102000013275 Somatomedins Human genes 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- DZHSAHHDTRWUTF-SIQRNXPUSA-N amyloid-beta polypeptide 42 Chemical compound C([C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(O)=O)[C@@H](C)CC)C(C)C)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(C)C)C1=CC=CC=C1 DZHSAHHDTRWUTF-SIQRNXPUSA-N 0.000 description 1
- 210000003484 anatomy Anatomy 0.000 description 1
- 230000002491 angiogenic effect Effects 0.000 description 1
- 210000000576 arachnoid Anatomy 0.000 description 1
- 210000003050 axon Anatomy 0.000 description 1
- 230000028600 axonogenesis Effects 0.000 description 1
- 230000003542 behavioural effect Effects 0.000 description 1
- 210000000601 blood cell Anatomy 0.000 description 1
- 230000017531 blood circulation Effects 0.000 description 1
- 210000000133 brain stem Anatomy 0.000 description 1
- 210000005013 brain tissue Anatomy 0.000 description 1
- 229910001628 calcium chloride Inorganic materials 0.000 description 1
- 239000001110 calcium chloride Substances 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 230000001149 cognitive effect Effects 0.000 description 1
- 230000003920 cognitive function Effects 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 230000007850 degeneration Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 210000001951 dura mater Anatomy 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 210000004955 epithelial membrane Anatomy 0.000 description 1
- 210000003743 erythrocyte Anatomy 0.000 description 1
- 210000003722 extracellular fluid Anatomy 0.000 description 1
- 239000003889 eye drop Substances 0.000 description 1
- 229940012356 eye drops Drugs 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 210000004884 grey matter Anatomy 0.000 description 1
- 244000144993 groups of animals Species 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 238000011532 immunohistochemical staining Methods 0.000 description 1
- 239000000411 inducer Substances 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 230000006662 intracellular pathway Effects 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 210000001503 joint Anatomy 0.000 description 1
- 230000003902 lesion Effects 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 239000007791 liquid phase Substances 0.000 description 1
- 238000013017 mechanical damping Methods 0.000 description 1
- 230000008655 medium-term memory Effects 0.000 description 1
- 210000000214 mouth Anatomy 0.000 description 1
- 201000006417 multiple sclerosis Diseases 0.000 description 1
- 210000003205 muscle Anatomy 0.000 description 1
- 210000002850 nasal mucosa Anatomy 0.000 description 1
- 210000005036 nerve Anatomy 0.000 description 1
- 210000001640 nerve ending Anatomy 0.000 description 1
- 210000000653 nervous system Anatomy 0.000 description 1
- 230000001537 neural effect Effects 0.000 description 1
- 210000004498 neuroglial cell Anatomy 0.000 description 1
- 230000003961 neuronal insult Effects 0.000 description 1
- 230000000508 neurotrophic effect Effects 0.000 description 1
- 210000000956 olfactory bulb Anatomy 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 230000001717 pathogenic effect Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 230000008884 pinocytosis Effects 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 210000001533 respiratory mucosa Anatomy 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 230000035807 sensation Effects 0.000 description 1
- 230000001953 sensory effect Effects 0.000 description 1
- 230000006403 short-term memory Effects 0.000 description 1
- 210000003625 skull Anatomy 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 210000000225 synapse Anatomy 0.000 description 1
- 210000002435 tendon Anatomy 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 230000017423 tissue regeneration Effects 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000001228 trophic effect Effects 0.000 description 1
- VBEQCZHXXJYVRD-GACYYNSASA-N uroanthelone Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(C)C)[C@@H](C)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)CNC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CS)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O)C(C)C)[C@@H](C)CC)C1=CC=C(O)C=C1 VBEQCZHXXJYVRD-GACYYNSASA-N 0.000 description 1
- 210000004885 white matter Anatomy 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/14—Blood; Artificial blood
- A61K35/16—Blood plasma; Blood serum
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/14—Blood; Artificial blood
- A61K35/19—Platelets; Megacaryocytes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/18—Growth factors; Growth regulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0043—Nose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- Hematology (AREA)
- Biomedical Technology (AREA)
- Immunology (AREA)
- Zoology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Cell Biology (AREA)
- Virology (AREA)
- Developmental Biology & Embryology (AREA)
- Biotechnology (AREA)
- Neurosurgery (AREA)
- Neurology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Gastroenterology & Hepatology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Otolaryngology (AREA)
- Psychiatry (AREA)
- Hospice & Palliative Care (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Medicinal Preparation (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| ES201200810A ES2442242B1 (es) | 2012-08-09 | 2012-08-09 | Composición con factores de crecimiento destinada al tratamiento intranasal de una enfermedad neurodegenerativa u otra patología del sistema nervioso central, y su método de fabricación. |
| ESP201200810 | 2012-08-09 | ||
| PCT/ES2013/000176 WO2014023860A1 (es) | 2012-08-09 | 2013-07-19 | Composición con factores de crecimiento obtenidos a partir del plasma sanguíneo destinada al tratamiento de enfermedades neurodegenerativas |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2015104076A RU2015104076A (ru) | 2016-09-27 |
| RU2696497C2 true RU2696497C2 (ru) | 2019-08-02 |
Family
ID=49111229
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2015104076A RU2696497C2 (ru) | 2012-08-09 | 2013-07-19 | Композиция с факторами роста для применения в интраназальном лечении нейродегенеративного заболевания или других заболеваний центральной нервной системы и способ ее получения |
Country Status (17)
| Country | Link |
|---|---|
| US (2) | US20140044795A1 (enExample) |
| EP (1) | EP2883569B1 (enExample) |
| JP (1) | JP6339568B2 (enExample) |
| KR (1) | KR102149162B1 (enExample) |
| CN (1) | CN104519961A (enExample) |
| AR (1) | AR092086A1 (enExample) |
| BR (1) | BR112015002760A2 (enExample) |
| CA (1) | CA2881075A1 (enExample) |
| CL (1) | CL2014003510A1 (enExample) |
| CO (1) | CO7170177A2 (enExample) |
| ES (2) | ES2442242B1 (enExample) |
| IN (1) | IN2014MN02675A (enExample) |
| MX (1) | MX357828B (enExample) |
| PE (1) | PE20150616A1 (enExample) |
| RU (1) | RU2696497C2 (enExample) |
| TW (1) | TWI649084B (enExample) |
| WO (1) | WO2014023860A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2784952C2 (ru) * | 2020-12-24 | 2022-12-01 | Федеральное государственное бюджетное учреждение "Национальный медицинский исследовательский центр травматологии и ортопедии имени Н.Н. Приорова" Министерства здравоохранения Российской Федерации (ФГБУ "НМИЦ ТО им. Н.Н. Приорова" Минздрава России) | In vivo биореактор для восстановления целостности проводящих путей спинного мозга после анатомического перерыва и стимуляции нейрорегенерации при травматической болезни спинного мозга |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CL2016003282A1 (es) | 2016-12-21 | 2017-08-18 | Univ Chile | Virus aav/igf2, método de tratamiento genético y su uso en enfermedades relacionadas con mal plegamiento de proteínas tal como la enfermedad de huntington |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1066838A1 (en) * | 1999-01-26 | 2001-01-10 | Eduardo Anitua Aldecoa | Bone tissue regenerating composition |
| ES2369945A1 (es) * | 2011-07-29 | 2011-12-09 | Eduardo Anitua Aldecoa | Procedimiento de obtención de una composición que contiene factores de crecimiento a partir de un compuesto sanguíneo, y composición obtenible por dicho procedimiento. |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ES2252993T3 (es) * | 1998-12-09 | 2006-05-16 | Chiron Corporation | Administracion de agentes neurotroficos al sistema nervioso central. |
| US7608258B2 (en) * | 2002-04-13 | 2009-10-27 | Allan Mishra | Method for treatment of tendinosis using platelet rich plasma |
| US6811777B2 (en) * | 2002-04-13 | 2004-11-02 | Allan Mishra | Compositions and minimally invasive methods for treating incomplete connective tissue repair |
| ES2221770B2 (es) | 2002-04-19 | 2006-07-16 | Eduardo Anitua Aldecoa | Metodo de preparacion de un compuesto para la regeneracion de tejidos. |
| US20060004189A1 (en) * | 2004-07-02 | 2006-01-05 | James Gandy | Compositions for treating wounds and processes for their preparation |
| WO2007025249A2 (en) * | 2005-08-26 | 2007-03-01 | The Board Of Trustees Of The Leland Stanford Junior University | Methods for treatment of headaches by administration of oxytocin |
| JP2011504163A (ja) * | 2007-06-08 | 2011-02-03 | ヘルスパートナーズ リサーチ ファウンデーション | 中枢神経系への治療化合物の標的化を高めるための薬学的組成物および方法 |
| ES2333498B1 (es) * | 2007-08-02 | 2011-01-10 | Biotechnology Institute, I Mas D, S.L. | Metodo y compuesto para el tratamiento de enfermedades o dolencias articulares o para el tratamiento de la piel con fines esteticos u otros, y el metodo de preparacion del compuesto. |
| BRPI0816784A8 (pt) * | 2007-09-14 | 2016-01-19 | Scil Tech Gmbh | Fatores neuro-endócrinos para o tratamento de doenças degenerativas |
| PE20120317A1 (es) * | 2009-05-14 | 2012-04-16 | Biotechnology Inst I Mas D Sl | Metodo para la preparacion de al menos un compuesto a partir de sangre |
| KR101114712B1 (ko) * | 2009-10-23 | 2012-02-29 | 세원셀론텍(주) | 염화칼슘용액과 제1형 콜라겐으로 혈소판풍부혈장(prp)을 활성화하여 조직재생을 유도하는 조성물의 제조방법 |
-
2012
- 2012-08-09 ES ES201200810A patent/ES2442242B1/es not_active Expired - Fee Related
-
2013
- 2013-07-19 TW TW102125858A patent/TWI649084B/zh not_active IP Right Cessation
- 2013-07-19 CN CN201380041280.4A patent/CN104519961A/zh active Pending
- 2013-07-19 BR BR112015002760A patent/BR112015002760A2/pt not_active Application Discontinuation
- 2013-07-19 EP EP13756518.0A patent/EP2883569B1/en not_active Not-in-force
- 2013-07-19 PE PE2015000116A patent/PE20150616A1/es not_active Application Discontinuation
- 2013-07-19 CA CA2881075A patent/CA2881075A1/en not_active Abandoned
- 2013-07-19 RU RU2015104076A patent/RU2696497C2/ru active
- 2013-07-19 JP JP2015525915A patent/JP6339568B2/ja not_active Expired - Fee Related
- 2013-07-19 KR KR1020157001551A patent/KR102149162B1/ko not_active Expired - Fee Related
- 2013-07-19 WO PCT/ES2013/000176 patent/WO2014023860A1/es not_active Ceased
- 2013-07-19 ES ES13756518.0T patent/ES2662385T3/es active Active
- 2013-07-19 MX MX2015001677A patent/MX357828B/es active IP Right Grant
- 2013-08-08 AR ARP130102835A patent/AR092086A1/es unknown
- 2013-08-08 US US13/962,340 patent/US20140044795A1/en not_active Abandoned
-
2014
- 2014-12-23 CL CL2014003510A patent/CL2014003510A1/es unknown
- 2014-12-31 IN IN2675MUN2014 patent/IN2014MN02675A/en unknown
-
2015
- 2015-01-20 CO CO15010369A patent/CO7170177A2/es unknown
-
2016
- 2016-03-08 US US15/063,969 patent/US20160184360A1/en not_active Abandoned
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1066838A1 (en) * | 1999-01-26 | 2001-01-10 | Eduardo Anitua Aldecoa | Bone tissue regenerating composition |
| ES2369945A1 (es) * | 2011-07-29 | 2011-12-09 | Eduardo Anitua Aldecoa | Procedimiento de obtención de una composición que contiene factores de crecimiento a partir de un compuesto sanguíneo, y composición obtenible por dicho procedimiento. |
Non-Patent Citations (3)
| Title |
|---|
| DE ROSA R., et al., Intranasal administration of nerve growth factor (NGF) rescues recognition memory deficits in AD11 anti-NGF transgenic mice. Proc Natl Acad Sci US A. 2005 Mar 8;102(10):3811-6. Epub 200 * |
| HARGUINDEY S., et al., Growth and trophic factors, pH and the Na+/H+ exchanger in Alzheimer's disease, other neurodegenerative diseases and cancer: new therapeutic possibilities and potential dangers. Curr Alzheimer Res. 2007 Feb;4(1):53-65. 5 Feb 23. * |
| HARGUINDEY S., et al., Growth and trophic factors, pH and the Na+/H+ exchanger in Alzheimer's disease, other neurodegenerative diseases and cancer: new therapeutic possibilities and potential dangers. Curr Alzheimer Res. 2007 Feb;4(1):53-65. DE ROSA R., et al., Intranasal administration of nerve growth factor (NGF) rescues recognition memory deficits in AD11 anti-NGF transgenic mice. Proc Natl Acad Sci US A. 2005 Mar 8;102(10):3811-6. Epub 2005 Feb 23. * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2784952C2 (ru) * | 2020-12-24 | 2022-12-01 | Федеральное государственное бюджетное учреждение "Национальный медицинский исследовательский центр травматологии и ортопедии имени Н.Н. Приорова" Министерства здравоохранения Российской Федерации (ФГБУ "НМИЦ ТО им. Н.Н. Приорова" Минздрава России) | In vivo биореактор для восстановления целостности проводящих путей спинного мозга после анатомического перерыва и стимуляции нейрорегенерации при травматической болезни спинного мозга |
Also Published As
| Publication number | Publication date |
|---|---|
| TW201408310A (zh) | 2014-03-01 |
| MX2015001677A (es) | 2015-04-10 |
| KR102149162B1 (ko) | 2020-08-31 |
| WO2014023860A1 (es) | 2014-02-13 |
| ES2662385T3 (es) | 2018-04-06 |
| ES2442242A1 (es) | 2014-02-10 |
| JP6339568B2 (ja) | 2018-06-06 |
| US20160184360A1 (en) | 2016-06-30 |
| IN2014MN02675A (enExample) | 2015-08-28 |
| AR092086A1 (es) | 2015-03-25 |
| CO7170177A2 (es) | 2015-01-28 |
| KR20150040860A (ko) | 2015-04-15 |
| ES2442242B1 (es) | 2014-11-25 |
| EP2883569B1 (en) | 2017-12-20 |
| CL2014003510A1 (es) | 2015-05-04 |
| TWI649084B (zh) | 2019-02-01 |
| RU2015104076A (ru) | 2016-09-27 |
| MX357828B (es) | 2018-07-25 |
| JP2015524464A (ja) | 2015-08-24 |
| CN104519961A (zh) | 2015-04-15 |
| EP2883569A1 (en) | 2015-06-17 |
| US20140044795A1 (en) | 2014-02-13 |
| CA2881075A1 (en) | 2014-02-13 |
| BR112015002760A2 (pt) | 2020-04-22 |
| PE20150616A1 (es) | 2015-05-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Silva et al. | From basics to clinical: a comprehensive review on spinal cord injury | |
| Ruehle et al. | Effects of BMP‐2 dose and delivery of microvascular fragments on healing of bone defects with concomitant volumetric muscle loss | |
| Yao et al. | Tetrahedral framework nucleic acids facilitate neurorestoration of facial nerves by activating the NGF/PI3K/AKT pathway | |
| Lekic et al. | Evaluation of the hematoma consequences, neurobehavioral profiles, and histopathology in a rat model of pontine hemorrhage | |
| Fadeev et al. | Combination of epidural electrical stimulation with ex vivo triple gene therapy for spinal cord injury: a proof of principle study | |
| Sheykhhasan et al. | Plasma-rich in growth factor and its clinical application | |
| Lee et al. | Functional recovery after injury of motor cortex in rats: effects of rehabilitation and stem cell transplantation in a traumatic brain injury model of cortical resection | |
| Wu et al. | Effects of electroacupuncture on the cortical extracellular signal regulated kinase pathway in rats with cerebral ischaemia/reperfusion | |
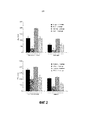
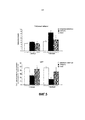
| RU2696497C2 (ru) | Композиция с факторами роста для применения в интраназальном лечении нейродегенеративного заболевания или других заболеваний центральной нервной системы и способ ее получения | |
| Xie et al. | Therapeutic effect of transplantation of human bone marrow‑derived mesenchymal stem cells on neuron regeneration in a rat model of middle cerebral artery occlusion | |
| TWI327473B (en) | Composition for treating a cerebrovascular disease and a method for increasing expression of erythropoietin | |
| Wang et al. | The promotion of neural regeneration in a rat facial nerve crush injury model using collagen-binding NT-3 | |
| WO2012087180A1 (ru) | Способ лечения острого нарушения мозгового и спинального кровообращения ишемического и геморрагического характера | |
| Attia et al. | Autologous platelet rich plasma enhances satellite cells expression of MyoD and exerts angiogenic and antifibrotic effects in experimental rat model of traumatic skeletal muscle injury | |
| Lee et al. | Effect of arecoline on regeneration of injured peripheral nerves | |
| Huang et al. | Paeoniae alba radix promotes peripheral nerve regeneration | |
| Markosyan et al. | Model of small-focal ischemic cerebral infarction as a basis for the development of new methods of stroke therapy | |
| Ilhanli et al. | Is platelet-rich plasma a promising treatment in severe knee osteoarthritis | |
| Hesse | 11 Surgical Treatments | |
| Liu et al. | Tannic acid as building block constructing injectable hydrogel and regulating microglial phenotype to enhance neuroplasticity for post-stroke rehabilitation | |
| Gomazkov et al. | Current concepts of neurocytoprotective therapy | |
| FENG et al. | Effects of butylphthalide combined with ozagrel sodium on National Institutes of Health Stroke Scale score, activities of daily living score, and coagulation function in patients with acute cerebral infarction | |
| Kim et al. | Chirality-dependent anti-inflammatory effect of glutathione after spinal cord injury | |
| Bellák | Transplanted neuroectodermal and induced pluripotent stem cells improve the outcome of spinal cord contusion injury: modulation of the lesion microenvironment | |
| Tas et al. | Investigation of various events occurring in the brain tissue after calvarial defects in rats |