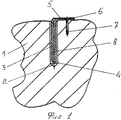
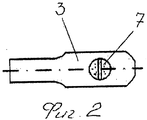
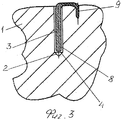
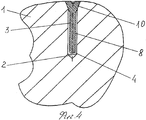
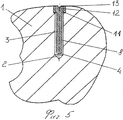
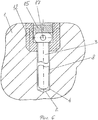
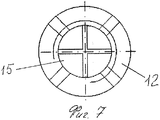
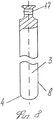
RU2563107C2 - Способ лечения артрозов, остеонекрозов и других видов артропатий и устройство для его осуществления - Google Patents
Способ лечения артрозов, остеонекрозов и других видов артропатий и устройство для его осуществления Download PDFInfo
- Publication number
- RU2563107C2 RU2563107C2 RU2013151988/14A RU2013151988A RU2563107C2 RU 2563107 C2 RU2563107 C2 RU 2563107C2 RU 2013151988/14 A RU2013151988/14 A RU 2013151988/14A RU 2013151988 A RU2013151988 A RU 2013151988A RU 2563107 C2 RU2563107 C2 RU 2563107C2
- Authority
- RU
- Russia
- Prior art keywords
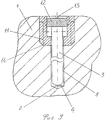
- bone
- holder
- rod
- hole
- holder according
- Prior art date
Links
- 238000000034 method Methods 0.000 title claims abstract description 44
- 201000008482 osteoarthritis Diseases 0.000 title claims abstract description 8
- 208000036487 Arthropathies Diseases 0.000 title claims abstract 3
- 208000012659 Joint disease Diseases 0.000 title claims abstract 3
- 206010031264 Osteonecrosis Diseases 0.000 title claims 2
- 210000000988 bone and bone Anatomy 0.000 claims abstract description 97
- 230000008719 thickening Effects 0.000 claims description 6
- 229910052751 metal Inorganic materials 0.000 claims description 4
- 239000002184 metal Substances 0.000 claims description 4
- BPUBBGLMJRNUCC-UHFFFAOYSA-N oxygen(2-);tantalum(5+) Chemical compound [O-2].[O-2].[O-2].[O-2].[O-2].[Ta+5].[Ta+5] BPUBBGLMJRNUCC-UHFFFAOYSA-N 0.000 claims description 3
- -1 polytetrafluoroethylene Polymers 0.000 claims description 3
- PBCFLUZVCVVTBY-UHFFFAOYSA-N tantalum pentoxide Inorganic materials O=[Ta](=O)O[Ta](=O)=O PBCFLUZVCVVTBY-UHFFFAOYSA-N 0.000 claims description 3
- 229920001577 copolymer Polymers 0.000 claims description 2
- 229910052755 nonmetal Inorganic materials 0.000 claims description 2
- 229920001343 polytetrafluoroethylene Polymers 0.000 claims description 2
- 239000004810 polytetrafluoroethylene Substances 0.000 claims description 2
- 239000007787 solid Substances 0.000 claims description 2
- 230000036407 pain Effects 0.000 abstract description 6
- 238000009434 installation Methods 0.000 abstract description 5
- 239000003814 drug Substances 0.000 abstract description 4
- 230000000694 effects Effects 0.000 abstract description 4
- 208000011580 syndromic disease Diseases 0.000 abstract description 2
- 206010061818 Disease progression Diseases 0.000 abstract 1
- 230000005750 disease progression Effects 0.000 abstract 1
- 239000000126 substance Substances 0.000 abstract 1
- 230000008569 process Effects 0.000 description 10
- 230000005686 electrostatic field Effects 0.000 description 8
- 210000001519 tissue Anatomy 0.000 description 7
- 230000001225 therapeutic effect Effects 0.000 description 6
- 210000004394 hip joint Anatomy 0.000 description 5
- 210000001503 joint Anatomy 0.000 description 5
- 229910052715 tantalum Inorganic materials 0.000 description 5
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 description 5
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 4
- 230000005684 electric field Effects 0.000 description 4
- 230000001575 pathological effect Effects 0.000 description 4
- 210000000689 upper leg Anatomy 0.000 description 4
- 208000037273 Pathologic Processes Diseases 0.000 description 3
- 238000003745 diagnosis Methods 0.000 description 3
- 201000010099 disease Diseases 0.000 description 3
- 239000007943 implant Substances 0.000 description 3
- 230000009054 pathological process Effects 0.000 description 3
- 230000007170 pathology Effects 0.000 description 3
- 238000000576 coating method Methods 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 210000001624 hip Anatomy 0.000 description 2
- 210000001981 hip bone Anatomy 0.000 description 2
- 210000000629 knee joint Anatomy 0.000 description 2
- 230000003902 lesion Effects 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- 230000000399 orthopedic effect Effects 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 230000003068 static effect Effects 0.000 description 2
- 229910000851 Alloy steel Inorganic materials 0.000 description 1
- 241000309551 Arthraxon hispidus Species 0.000 description 1
- 208000027205 Congenital disease Diseases 0.000 description 1
- 208000007353 Hip Osteoarthritis Diseases 0.000 description 1
- 206010028851 Necrosis Diseases 0.000 description 1
- 206010036030 Polyarthritis Diseases 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- QCWXUUIWCKQGHC-UHFFFAOYSA-N Zirconium Chemical compound [Zr] QCWXUUIWCKQGHC-UHFFFAOYSA-N 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000002730 additional effect Effects 0.000 description 1
- 229910045601 alloy Inorganic materials 0.000 description 1
- 239000000956 alloy Substances 0.000 description 1
- 210000001264 anterior cruciate ligament Anatomy 0.000 description 1
- 206010003246 arthritis Diseases 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 230000031018 biological processes and functions Effects 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- 231100000481 chemical toxicant Toxicity 0.000 description 1
- 230000009693 chronic damage Effects 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 230000006837 decompression Effects 0.000 description 1
- 230000007123 defense Effects 0.000 description 1
- 230000003412 degenerative effect Effects 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 230000004821 effect on bone Effects 0.000 description 1
- 230000005611 electricity Effects 0.000 description 1
- 238000001827 electrotherapy Methods 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 210000003414 extremity Anatomy 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 238000002513 implantation Methods 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 230000008407 joint function Effects 0.000 description 1
- 210000003127 knee Anatomy 0.000 description 1
- 210000002414 leg Anatomy 0.000 description 1
- 210000003041 ligament Anatomy 0.000 description 1
- 238000002690 local anesthesia Methods 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 230000005499 meniscus Effects 0.000 description 1
- 210000002346 musculoskeletal system Anatomy 0.000 description 1
- 230000017074 necrotic cell death Effects 0.000 description 1
- 210000001640 nerve ending Anatomy 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 229940124583 pain medication Drugs 0.000 description 1
- 210000003049 pelvic bone Anatomy 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 210000004560 pineal gland Anatomy 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 208000030428 polyarticular arthritis Diseases 0.000 description 1
- 230000002980 postoperative effect Effects 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- 210000002474 sphenoid bone Anatomy 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 238000011477 surgical intervention Methods 0.000 description 1
- 210000001694 thigh bone Anatomy 0.000 description 1
- 210000002303 tibia Anatomy 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 239000003440 toxic substance Substances 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- 229910052726 zirconium Inorganic materials 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/10—Applying static electricity
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/28—Materials for coating prostheses
- A61L27/30—Inorganic materials
- A61L27/306—Other specific inorganic materials not covered by A61L27/303 - A61L27/32
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/28—Materials for coating prostheses
- A61L27/34—Macromolecular materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/08—Materials for coatings
- A61L31/082—Inorganic materials
- A61L31/088—Other specific inorganic materials not covered by A61L31/084 or A61L31/086
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/08—Materials for coatings
- A61L31/10—Macromolecular materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/14—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/20—Applying electric currents by contact electrodes continuous direct currents
- A61N1/205—Applying electric currents by contact electrodes continuous direct currents for promoting a biological process
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/326—Applying electric currents by contact electrodes alternating or intermittent currents for promoting growth of cells, e.g. bone cells
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L27/00—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a halogen; Compositions of derivatives of such polymers
- C08L27/02—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a halogen; Compositions of derivatives of such polymers not modified by chemical after-treatment
- C08L27/12—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a halogen; Compositions of derivatives of such polymers not modified by chemical after-treatment containing fluorine atoms
- C08L27/18—Homopolymers or copolymers or tetrafluoroethene
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2400/00—Materials characterised by their function or physical properties
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2430/00—Materials or treatment for tissue regeneration
- A61L2430/02—Materials or treatment for tissue regeneration for reconstruction of bones; weight-bearing implants
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical & Material Sciences (AREA)
- Epidemiology (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Radiology & Medical Imaging (AREA)
- Biomedical Technology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Dermatology (AREA)
- Heart & Thoracic Surgery (AREA)
- Surgery (AREA)
- Vascular Medicine (AREA)
- Inorganic Chemistry (AREA)
- Molecular Biology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Cell Biology (AREA)
- Prostheses (AREA)
- Electrotherapy Devices (AREA)
- Surgical Instruments (AREA)
Priority Applications (14)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| RU2013151988/14A RU2563107C2 (ru) | 2013-11-21 | 2013-11-21 | Способ лечения артрозов, остеонекрозов и других видов артропатий и устройство для его осуществления |
| PCT/RU2014/000511 WO2015076698A1 (en) | 2013-11-21 | 2014-07-11 | Method for treatment of arthrosis, electret implant, bushing for its placing and removal from bone |
| PL14783914T PL3030312T3 (pl) | 2013-11-21 | 2014-07-11 | Implant elektretowy do leczenia artrozy, tuleja do jego umieszczania i usuwania z kości |
| KR1020167011245A KR102221145B1 (ko) | 2013-11-21 | 2014-07-11 | 일렉트렛 임플란트 |
| ES14783914.6T ES2651850T3 (es) | 2013-11-21 | 2014-07-11 | Implante de electreto para tratamiento de artrosis, casquillo para su colocación y extracción del hueso |
| US15/034,220 US9757559B2 (en) | 2013-11-21 | 2014-07-11 | Electret implant for treatment of arthrosis |
| AU2014353618A AU2014353618B2 (en) | 2013-11-21 | 2014-07-11 | Method for treatment of arthrosis, electret implant, bushing for its placing and removal from bone |
| BR112016011458-2A BR112016011458B1 (pt) | 2013-11-21 | 2014-07-11 | Implante de eletreto para o tratamento de artrose |
| EP14783914.6A EP3030312B8 (en) | 2013-11-21 | 2014-07-11 | Electret implant for treatment of arthrosis, bushing for its placing and removal from bone |
| CN201480063792.5A CN105764565B (zh) | 2013-11-21 | 2014-07-11 | 治疗关节病的方法、驻极体植入物以及用于设置驻极体植入物及将其从骨骼上移除的套管 |
| JP2016555441A JP6370396B2 (ja) | 2013-11-21 | 2014-07-11 | 関節症の治療用のエレクトレット・インプラント |
| CA2928449A CA2928449C (en) | 2013-11-21 | 2014-07-11 | Method for treatment of arthrosis, electret implant, bushing for its placing and removal from bone |
| EA201400852A EA025289B1 (ru) | 2013-11-21 | 2014-08-29 | Способ лечения артроза, электретный имплантат и втулка для установки и извлечения его из кости |
| IL245750A IL245750B (en) | 2013-11-21 | 2016-05-19 | A method of treating arthrosis, an electrical implant, a prosthesis, its placement and removal from a bone |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| RU2013151988/14A RU2563107C2 (ru) | 2013-11-21 | 2013-11-21 | Способ лечения артрозов, остеонекрозов и других видов артропатий и устройство для его осуществления |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2013151988A RU2013151988A (ru) | 2015-05-27 |
| RU2563107C2 true RU2563107C2 (ru) | 2015-09-20 |
Family
ID=51691123
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2013151988/14A RU2563107C2 (ru) | 2013-11-21 | 2013-11-21 | Способ лечения артрозов, остеонекрозов и других видов артропатий и устройство для его осуществления |
Country Status (14)
| Country | Link |
|---|---|
| US (1) | US9757559B2 (enExample) |
| EP (1) | EP3030312B8 (enExample) |
| JP (1) | JP6370396B2 (enExample) |
| KR (1) | KR102221145B1 (enExample) |
| CN (1) | CN105764565B (enExample) |
| AU (1) | AU2014353618B2 (enExample) |
| BR (1) | BR112016011458B1 (enExample) |
| CA (1) | CA2928449C (enExample) |
| EA (1) | EA025289B1 (enExample) |
| ES (1) | ES2651850T3 (enExample) |
| IL (1) | IL245750B (enExample) |
| PL (1) | PL3030312T3 (enExample) |
| RU (1) | RU2563107C2 (enExample) |
| WO (1) | WO2015076698A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU216301U1 (ru) * | 2022-06-29 | 2023-01-27 | Общество с ограниченной ответственностью "ЭЛЕКТРЕТНЫЕ НАНОТЕХНОЛОГИИ" | Имплантат с электретным биоактивным покрытием для лечения повреждений и заболеваний костей и суставов |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ES2939164T3 (es) * | 2016-08-22 | 2023-04-19 | Link Waldemar Gmbh Co | Recubrimiento para un implante |
| EP3773280A1 (en) * | 2018-04-10 | 2021-02-17 | DePuy Synthes Products, Inc. | Bipolar bone anchor with connection for electrostimulation |
| US11305112B2 (en) | 2018-05-16 | 2022-04-19 | DePuy Synthes Products, Inc. | Electrical stimulation implants |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| SU1657171A1 (ru) * | 1988-04-06 | 1991-06-23 | Makishev Otan M | Устройство дл остеосинтеза переломов шейки бедра |
| US5384337A (en) * | 1993-02-05 | 1995-01-24 | Budinger; William D. | Poromeric material having uniformly distributed electrets for maintaining an electrostatic charge |
| RU2422113C1 (ru) * | 2009-11-19 | 2011-06-27 | Государственное образовательное учреждение высшего профессионального образования "Российский государственный медицинский университет Федерального агентства по здравоохранению и социальному развитию" (ГОУ ВПО РГМУ Росздрава) | Устройство для проведения спиц и способ его использования при остеосинтезе переломов дистального метаэпифиза лучевой кости |
Family Cites Families (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3968790A (en) | 1975-02-26 | 1976-07-13 | Rikagaku Kenkyusho | Electret method of promoting callus formation in regeneration of bones |
| JPS60188167A (ja) * | 1984-03-07 | 1985-09-25 | 理化学研究所 | 高分子エレクトレツトフイルムから成る仮骨形成材 |
| WO1995019796A1 (en) | 1994-01-21 | 1995-07-27 | Brown University Research Foundation | Biocompatible implants |
| RU2225181C2 (ru) * | 2002-03-21 | 2004-03-10 | Шагивалеев Наиль Анварович | Фиксатор для остеосинтеза шейки бедренной кости |
| DE10236691B4 (de) * | 2002-08-09 | 2005-12-01 | Biedermann Motech Gmbh | Dynamische Stabilisierungseinrichtung für Knochen, insbesondere für Wirbel |
| US8070785B2 (en) * | 2003-09-16 | 2011-12-06 | Spineco, Inc. | Bone anchor prosthesis and system |
| US7431734B2 (en) | 2005-02-04 | 2008-10-07 | Intellistem Orthopaedic Innovations, Inc. | Implanted prosthetic device |
| US8145319B1 (en) | 2005-10-11 | 2012-03-27 | Ebi, Llc | Methods and devices for treatment of osteonecrosis of the femoral head with core decompression |
| US20090048675A1 (en) * | 2007-04-25 | 2009-02-19 | Bhatnagar Mohit K | Spinal Fusion Implants with Selectively Applied Bone Growth Promoting Agent |
| US8257395B2 (en) * | 2007-09-21 | 2012-09-04 | Jmea Corporation | Spinal fixation with selectively applied bone growth promoting agent |
| JP2008100112A (ja) * | 2008-01-17 | 2008-05-01 | Senko Medical Instr Mfg Co Ltd | 人工関節用中遠位チップ |
| EP2249914A4 (en) * | 2008-02-21 | 2011-03-30 | Integrity Intellect Inc | IMPLANT EQUIPPED FOR NERVE LOCATION AND METHOD OF USE |
| US20100318140A1 (en) * | 2009-06-16 | 2010-12-16 | Medtronic, Inc. | Volumetric energy density electrodes |
| WO2011146930A2 (en) * | 2010-05-21 | 2011-11-24 | Revent Medical, Inc. | Systems and methods for treatment of sleep apnea |
| US8882806B2 (en) * | 2011-04-25 | 2014-11-11 | Said ELSHIHABI | Spine stabilization system with self-cutting rod |
-
2013
- 2013-11-21 RU RU2013151988/14A patent/RU2563107C2/ru active
-
2014
- 2014-07-11 ES ES14783914.6T patent/ES2651850T3/es active Active
- 2014-07-11 KR KR1020167011245A patent/KR102221145B1/ko active Active
- 2014-07-11 JP JP2016555441A patent/JP6370396B2/ja active Active
- 2014-07-11 CA CA2928449A patent/CA2928449C/en active Active
- 2014-07-11 AU AU2014353618A patent/AU2014353618B2/en active Active
- 2014-07-11 EP EP14783914.6A patent/EP3030312B8/en active Active
- 2014-07-11 US US15/034,220 patent/US9757559B2/en active Active
- 2014-07-11 BR BR112016011458-2A patent/BR112016011458B1/pt active IP Right Grant
- 2014-07-11 CN CN201480063792.5A patent/CN105764565B/zh active Active
- 2014-07-11 WO PCT/RU2014/000511 patent/WO2015076698A1/en not_active Ceased
- 2014-07-11 PL PL14783914T patent/PL3030312T3/pl unknown
- 2014-08-29 EA EA201400852A patent/EA025289B1/ru unknown
-
2016
- 2016-05-19 IL IL245750A patent/IL245750B/en active IP Right Grant
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| SU1657171A1 (ru) * | 1988-04-06 | 1991-06-23 | Makishev Otan M | Устройство дл остеосинтеза переломов шейки бедра |
| US5384337A (en) * | 1993-02-05 | 1995-01-24 | Budinger; William D. | Poromeric material having uniformly distributed electrets for maintaining an electrostatic charge |
| RU2422113C1 (ru) * | 2009-11-19 | 2011-06-27 | Государственное образовательное учреждение высшего профессионального образования "Российский государственный медицинский университет Федерального агентства по здравоохранению и социальному развитию" (ГОУ ВПО РГМУ Росздрава) | Устройство для проведения спиц и способ его использования при остеосинтезе переломов дистального метаэпифиза лучевой кости |
Non-Patent Citations (2)
| Title |
|---|
| NAKAMURA M. et al. Endothelial cell migration and morphogenesis on silk fibroin scaffolds containing hydroxyapatite electret. J Biomed Mater Res A. 2012 Apr;100(4):969-77. doi: 10.1002/jbm.a.34046. Epub 2012 Jan 24 (Abstract) * |
| АРТЕМЬЕВ А.А. и др. Влияние электретов на остеорепарацию при интрамедуллярном остеосинтезе. Ортопедия, травматология и протезирование. 1990, N 7, с.26-30. * |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2810013C2 (ru) * | 2021-05-04 | 2023-12-21 | Общество с ограниченной ответственностью "Медэл" (ООО "Медэл") | Способ замещения костных дефектов и электретный имплантат для его осуществления |
| RU216301U1 (ru) * | 2022-06-29 | 2023-01-27 | Общество с ограниченной ответственностью "ЭЛЕКТРЕТНЫЕ НАНОТЕХНОЛОГИИ" | Имплантат с электретным биоактивным покрытием для лечения повреждений и заболеваний костей и суставов |
| RU2802152C1 (ru) * | 2022-11-21 | 2023-08-22 | федеральное государственное бюджетное учреждение "Санкт-Петербургский научно-исследовательский институт фтизиопульмонологии" Министерства здравоохранения Российской Федерации | Способ хирургического лечения остеоартрита коленного сустава |
Also Published As
| Publication number | Publication date |
|---|---|
| US20160367798A1 (en) | 2016-12-22 |
| EP3030312B1 (en) | 2017-11-22 |
| IL245750B (en) | 2019-02-28 |
| BR112016011458A2 (enExample) | 2017-08-08 |
| CA2928449A1 (en) | 2015-05-28 |
| JP6370396B2 (ja) | 2018-08-08 |
| CA2928449C (en) | 2022-07-19 |
| KR102221145B1 (ko) | 2021-02-25 |
| BR112016011458B1 (pt) | 2021-10-19 |
| EP3030312B8 (en) | 2018-01-03 |
| IL245750A0 (en) | 2016-07-31 |
| ES2651850T3 (es) | 2018-01-30 |
| JP2016538103A (ja) | 2016-12-08 |
| RU2013151988A (ru) | 2015-05-27 |
| CN105764565A (zh) | 2016-07-13 |
| AU2014353618B2 (en) | 2019-07-04 |
| EA025289B1 (ru) | 2016-12-30 |
| US9757559B2 (en) | 2017-09-12 |
| CN105764565B (zh) | 2018-09-04 |
| KR20160088296A (ko) | 2016-07-25 |
| EA201400852A1 (ru) | 2015-05-29 |
| EP3030312A1 (en) | 2016-06-15 |
| WO2015076698A1 (en) | 2015-05-28 |
| PL3030312T3 (pl) | 2018-04-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10531897B2 (en) | Implantable device for relieving ankle pain | |
| US9440069B2 (en) | Percutaneous continual electro-acupuncture stimulation for in vivo and in situ tissue engineering | |
| MONTICELLI et al. | Distraction epiphysiolysis as a method of limb lengthening: III. Clinical applications | |
| CN106456970A (zh) | 用于刺激骨生长、组织痊愈和/或疼痛控制的系统及使用方法 | |
| RU2563107C2 (ru) | Способ лечения артрозов, остеонекрозов и других видов артропатий и устройство для его осуществления | |
| WO2010036440A1 (en) | A device for facilitating the healing of bone including olecranan | |
| AU2014353618A1 (en) | Method for treatment of arthrosis, electret implant, bushing for its placing and removal from bone | |
| Carraro | Modulation of trophism and fiber type expression of denervated muscle by different patterns of electrical stimulation | |
| RU2284785C1 (ru) | Способ лечения юношеского эпифизеолиза головки бедренной кости у детей | |
| HK1226346A1 (en) | Method for treatment of arthrosis, electret implant, bushing for its placing and removal from bone | |
| Al Muderis | Osseointegration for Amputees: Past, Present and Future: Basic Science, Innovations in Surgical Technique, Implant Design and Rehabilitation Strategies | |
| HK1226346B (zh) | 治疗关节病的方法、驻极体植入物以及用於设置驻极体植入物及将其从骨骼上移除的套管 | |
| RU2802152C1 (ru) | Способ хирургического лечения остеоартрита коленного сустава | |
| Kim et al. | The effect of Micro-current electrical stimulation on muscle atrophy caused by sciatic nerve compression | |
| RU2361531C1 (ru) | Способ лечения внутрисуставных переломов дистального отдела плеча | |
| RU2336046C1 (ru) | Имплантат для биомедицинского применения и способ стимуляции регенеративного процесса в области повреждения кости | |
| Barone | Physical activity and osseointegration:" step forward" to improve the quality of life of a trans-femoral amputee person | |
| Krishnan | Bioengineers are close to finding a cure for arthritis. | |
| RU56166U1 (ru) | Устройство для комбинированного остеосинтеза | |
| Schneider et al. | Case 58: Below knee amputation stump lengthening | |
| RU2635441C2 (ru) | Способ лечения врожденной варусной деформации шейки бедренной кости тяжелой степени | |
| Katz | A proposal to study the effect of electrical stimulation on osteogenesis in non-united fractures with a thorough review of the related literature and information of commercial units available | |
| RU2310413C1 (ru) | Способ и устройство для комбинированного остеосинтеза | |
| Noble | Evaluation of a Press Fit, Percutaneous, Skeletally Anchored Endoprosthesis for Prosthetic Limb Attachment: Bone Response and the Effect of Low Intensity Vibration | |
| RU2526956C1 (ru) | Способ лечения больных с парапротезной инфекцией тазобедренного сустава |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| QB4A | Licence on use of patent |
Free format text: PLEDGE FORMERLY AGREED ON 20191024 Effective date: 20191024 |
|
| QB4A | Licence on use of patent |
Free format text: SUBSEQUENT PLEDGE FORMERLY AGREED ON 20200121 Effective date: 20200121 |
|
| QC41 | Official registration of the termination of the licence agreement or other agreements on the disposal of an exclusive right |
Free format text: SUBSEQUENT PLEDGE FORMERLY AGREED ON 20200121 Effective date: 20200729 |
|
| QC41 | Official registration of the termination of the licence agreement or other agreements on the disposal of an exclusive right |
Free format text: PLEDGE FORMERLY AGREED ON 20191024 Effective date: 20200826 |