RU2404190C2 - Деамидированный интерферон-бета - Google Patents
Деамидированный интерферон-бета Download PDFInfo
- Publication number
- RU2404190C2 RU2404190C2 RU2007121515/04A RU2007121515A RU2404190C2 RU 2404190 C2 RU2404190 C2 RU 2404190C2 RU 2007121515/04 A RU2007121515/04 A RU 2007121515/04A RU 2007121515 A RU2007121515 A RU 2007121515A RU 2404190 C2 RU2404190 C2 RU 2404190C2
- Authority
- RU
- Russia
- Prior art keywords
- inf
- deamidated
- protein
- interferon
- protein analogue
- Prior art date
Links
- 102000003996 Interferon-beta Human genes 0.000 title claims abstract description 23
- 108090000467 Interferon-beta Proteins 0.000 title claims abstract description 23
- 108090000623 proteins and genes Proteins 0.000 claims abstract description 78
- 235000018102 proteins Nutrition 0.000 claims abstract description 77
- 102000004169 proteins and genes Human genes 0.000 claims abstract description 77
- 238000000034 method Methods 0.000 claims abstract description 36
- 230000004071 biological effect Effects 0.000 claims abstract description 29
- 230000000694 effects Effects 0.000 claims abstract description 23
- 238000004007 reversed phase HPLC Methods 0.000 claims abstract description 21
- 150000001413 amino acids Chemical class 0.000 claims abstract description 20
- 229960001388 interferon-beta Drugs 0.000 claims abstract description 20
- 229940024606 amino acid Drugs 0.000 claims abstract description 15
- 235000001014 amino acid Nutrition 0.000 claims abstract description 15
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 claims abstract description 9
- 230000007935 neutral effect Effects 0.000 claims abstract description 9
- 101001054334 Homo sapiens Interferon beta Proteins 0.000 claims abstract description 8
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 claims abstract description 7
- 229960001230 asparagine Drugs 0.000 claims abstract description 7
- 235000009582 asparagine Nutrition 0.000 claims abstract description 7
- 230000001225 therapeutic effect Effects 0.000 claims abstract description 7
- 235000018417 cysteine Nutrition 0.000 claims abstract description 6
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 claims abstract description 6
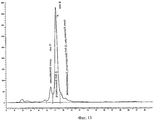
- 238000012510 peptide mapping method Methods 0.000 claims abstract description 6
- 125000000613 asparagine group Chemical group N[C@@H](CC(N)=O)C(=O)* 0.000 claims abstract description 5
- -1 cyclic imide Chemical class 0.000 claims description 39
- 201000006417 multiple sclerosis Diseases 0.000 claims description 17
- 239000000203 mixture Substances 0.000 claims description 15
- PZBFGYYEXUXCOF-UHFFFAOYSA-N TCEP Chemical group OC(=O)CCP(CCC(O)=O)CCC(O)=O PZBFGYYEXUXCOF-UHFFFAOYSA-N 0.000 claims description 13
- 238000011534 incubation Methods 0.000 claims description 9
- 229940009098 aspartate Drugs 0.000 claims description 7
- 238000003776 cleavage reaction Methods 0.000 claims description 7
- 230000007017 scission Effects 0.000 claims description 7
- 239000003638 chemical reducing agent Substances 0.000 claims description 6
- 230000005713 exacerbation Effects 0.000 claims description 6
- 108091006028 deamidated proteins Proteins 0.000 claims description 5
- 238000000746 purification Methods 0.000 claims description 5
- 108010033276 Peptide Fragments Proteins 0.000 claims description 4
- 102000007079 Peptide Fragments Human genes 0.000 claims description 4
- 238000002955 isolation Methods 0.000 claims description 4
- 229930182817 methionine Natural products 0.000 claims description 4
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 claims description 3
- 239000007853 buffer solution Substances 0.000 claims description 3
- 238000004811 liquid chromatography Methods 0.000 claims description 3
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 claims description 3
- CKLJMWTZIZZHCS-REOHCLBHSA-L aspartate group Chemical group N[C@@H](CC(=O)[O-])C(=O)[O-] CKLJMWTZIZZHCS-REOHCLBHSA-L 0.000 claims description 2
- 238000012217 deletion Methods 0.000 claims description 2
- 230000037430 deletion Effects 0.000 claims description 2
- 239000003937 drug carrier Substances 0.000 claims description 2
- 238000000926 separation method Methods 0.000 claims description 2
- 239000003814 drug Substances 0.000 abstract description 24
- 229940079593 drug Drugs 0.000 abstract description 14
- 238000004519 manufacturing process Methods 0.000 abstract description 8
- 238000003908 quality control method Methods 0.000 abstract description 5
- 201000010099 disease Diseases 0.000 abstract description 4
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 abstract description 4
- 230000015556 catabolic process Effects 0.000 abstract description 3
- 230000002255 enzymatic effect Effects 0.000 abstract description 3
- 208000034189 Sclerosis Diseases 0.000 abstract 1
- 238000002483 medication Methods 0.000 abstract 1
- 238000012113 quantitative test Methods 0.000 abstract 1
- 230000006641 stabilisation Effects 0.000 abstract 1
- 239000000126 substance Substances 0.000 abstract 1
- 230000006240 deamidation Effects 0.000 description 38
- 239000000523 sample Substances 0.000 description 30
- 230000000120 cytopathologic effect Effects 0.000 description 21
- 108090000765 processed proteins & peptides Proteins 0.000 description 21
- 230000001965 increasing effect Effects 0.000 description 16
- 239000000047 product Substances 0.000 description 16
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 15
- 210000004027 cell Anatomy 0.000 description 13
- 238000002360 preparation method Methods 0.000 description 12
- 229940126534 drug product Drugs 0.000 description 9
- 230000001028 anti-proliverative effect Effects 0.000 description 8
- 239000000825 pharmaceutical preparation Substances 0.000 description 8
- 229940124597 therapeutic agent Drugs 0.000 description 7
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 6
- 108010005714 Interferon beta-1b Proteins 0.000 description 6
- 230000007423 decrease Effects 0.000 description 6
- 239000012634 fragment Substances 0.000 description 6
- 230000002829 reductive effect Effects 0.000 description 6
- 238000012360 testing method Methods 0.000 description 6
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 5
- 108020004511 Recombinant DNA Proteins 0.000 description 5
- 230000002411 adverse Effects 0.000 description 5
- 150000001875 compounds Chemical class 0.000 description 5
- 238000004128 high performance liquid chromatography Methods 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- 230000004048 modification Effects 0.000 description 5
- 238000012986 modification Methods 0.000 description 5
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical group NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 4
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 4
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 4
- 241000700605 Viruses Species 0.000 description 4
- 235000003704 aspartic acid Nutrition 0.000 description 4
- 238000003556 assay Methods 0.000 description 4
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 239000012149 elution buffer Substances 0.000 description 4
- 229960003161 interferon beta-1b Drugs 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- 238000004458 analytical method Methods 0.000 description 3
- 238000011953 bioanalysis Methods 0.000 description 3
- 230000010261 cell growth Effects 0.000 description 3
- 230000001413 cellular effect Effects 0.000 description 3
- 239000013068 control sample Substances 0.000 description 3
- 238000010586 diagram Methods 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- 230000006862 enzymatic digestion Effects 0.000 description 3
- 150000002500 ions Chemical class 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 230000035772 mutation Effects 0.000 description 3
- 230000004044 response Effects 0.000 description 3
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 2
- ATRRKUHOCOJYRX-UHFFFAOYSA-N Ammonium bicarbonate Chemical compound [NH4+].OC([O-])=O ATRRKUHOCOJYRX-UHFFFAOYSA-N 0.000 description 2
- 241000710188 Encephalomyocarditis virus Species 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 239000004471 Glycine Chemical group 0.000 description 2
- 102000003839 Human Proteins Human genes 0.000 description 2
- 108090000144 Human Proteins Proteins 0.000 description 2
- 101001018085 Lysobacter enzymogenes Lysyl endopeptidase Proteins 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 239000001099 ammonium carbonate Substances 0.000 description 2
- 229940021459 betaseron Drugs 0.000 description 2
- 238000004166 bioassay Methods 0.000 description 2
- 238000005277 cation exchange chromatography Methods 0.000 description 2
- 238000007385 chemical modification Methods 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 2
- 238000004587 chromatography analysis Methods 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 230000029087 digestion Effects 0.000 description 2
- VHJLVAABSRFDPM-QWWZWVQMSA-N dithiothreitol Chemical compound SC[C@@H](O)[C@H](O)CS VHJLVAABSRFDPM-QWWZWVQMSA-N 0.000 description 2
- 231100000673 dose–response relationship Toxicity 0.000 description 2
- 229940088679 drug related substance Drugs 0.000 description 2
- 238000010353 genetic engineering Methods 0.000 description 2
- 230000028993 immune response Effects 0.000 description 2
- 238000011031 large-scale manufacturing process Methods 0.000 description 2
- 210000004962 mammalian cell Anatomy 0.000 description 2
- 238000004949 mass spectrometry Methods 0.000 description 2
- 239000002609 medium Substances 0.000 description 2
- 238000002703 mutagenesis Methods 0.000 description 2
- 231100000350 mutagenesis Toxicity 0.000 description 2
- 238000006386 neutralization reaction Methods 0.000 description 2
- 230000003647 oxidation Effects 0.000 description 2
- 238000007254 oxidation reaction Methods 0.000 description 2
- 230000035484 reaction time Effects 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 238000013207 serial dilution Methods 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- CWERGRDVMFNCDR-UHFFFAOYSA-N thioglycolic acid Chemical compound OC(=O)CS CWERGRDVMFNCDR-UHFFFAOYSA-N 0.000 description 2
- DGVVWUTYPXICAM-UHFFFAOYSA-N β‐Mercaptoethanol Chemical compound OCCS DGVVWUTYPXICAM-UHFFFAOYSA-N 0.000 description 2
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 1
- QZTKDVCDBIDYMD-UHFFFAOYSA-N 2,2'-[(2-amino-2-oxoethyl)imino]diacetic acid Chemical compound NC(=O)CN(CC(O)=O)CC(O)=O QZTKDVCDBIDYMD-UHFFFAOYSA-N 0.000 description 1
- IHPYMWDTONKSCO-UHFFFAOYSA-N 2,2'-piperazine-1,4-diylbisethanesulfonic acid Chemical compound OS(=O)(=O)CCN1CCN(CCS(O)(=O)=O)CC1 IHPYMWDTONKSCO-UHFFFAOYSA-N 0.000 description 1
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 1
- AJTVSSFTXWNIRG-UHFFFAOYSA-N 2-[bis(2-hydroxyethyl)amino]ethanesulfonic acid Chemical compound OCC[NH+](CCO)CCS([O-])(=O)=O AJTVSSFTXWNIRG-UHFFFAOYSA-N 0.000 description 1
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 1
- LVQFQZZGTZFUNF-UHFFFAOYSA-N 2-hydroxy-3-[4-(2-hydroxy-3-sulfonatopropyl)piperazine-1,4-diium-1-yl]propane-1-sulfonate Chemical compound OS(=O)(=O)CC(O)CN1CCN(CC(O)CS(O)(=O)=O)CC1 LVQFQZZGTZFUNF-UHFFFAOYSA-N 0.000 description 1
- DVLFYONBTKHTER-UHFFFAOYSA-N 3-(N-morpholino)propanesulfonic acid Chemical compound OS(=O)(=O)CCCN1CCOCC1 DVLFYONBTKHTER-UHFFFAOYSA-N 0.000 description 1
- NUFBIAUZAMHTSP-UHFFFAOYSA-N 3-(n-morpholino)-2-hydroxypropanesulfonic acid Chemical compound OS(=O)(=O)CC(O)CN1CCOCC1 NUFBIAUZAMHTSP-UHFFFAOYSA-N 0.000 description 1
- RZQXOGQSPBYUKH-UHFFFAOYSA-N 3-[[1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl]azaniumyl]-2-hydroxypropane-1-sulfonate Chemical compound OCC(CO)(CO)NCC(O)CS(O)(=O)=O RZQXOGQSPBYUKH-UHFFFAOYSA-N 0.000 description 1
- XCBLFURAFHFFJF-UHFFFAOYSA-N 3-[bis(2-hydroxyethyl)azaniumyl]-2-hydroxypropane-1-sulfonate Chemical compound OCCN(CCO)CC(O)CS(O)(=O)=O XCBLFURAFHFFJF-UHFFFAOYSA-N 0.000 description 1
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 1
- 239000007991 ACES buffer Substances 0.000 description 1
- 239000007988 ADA buffer Substances 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- USFZMSVCRYTOJT-UHFFFAOYSA-N Ammonium acetate Chemical compound N.CC(O)=O USFZMSVCRYTOJT-UHFFFAOYSA-N 0.000 description 1
- 239000005695 Ammonium acetate Substances 0.000 description 1
- 229910000013 Ammonium bicarbonate Inorganic materials 0.000 description 1
- 108700016232 Arg(2)-Sar(4)- dermorphin (1-4) Proteins 0.000 description 1
- KLKHFFMNGWULBN-VKHMYHEASA-N Asn-Gly Chemical class NC(=O)C[C@H](N)C(=O)NCC(O)=O KLKHFFMNGWULBN-VKHMYHEASA-N 0.000 description 1
- 239000007992 BES buffer Substances 0.000 description 1
- 239000007989 BIS-Tris Propane buffer Substances 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical compound OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- BDAGIHXWWSANSR-UHFFFAOYSA-M Formate Chemical compound [O-]C=O BDAGIHXWWSANSR-UHFFFAOYSA-M 0.000 description 1
- 108010024636 Glutathione Proteins 0.000 description 1
- 239000007995 HEPES buffer Substances 0.000 description 1
- OWXMKDGYPWMGEB-UHFFFAOYSA-N HEPPS Chemical compound OCCN1CCN(CCCS(O)(=O)=O)CC1 OWXMKDGYPWMGEB-UHFFFAOYSA-N 0.000 description 1
- GIZQLVPDAOBAFN-UHFFFAOYSA-N HEPPSO Chemical compound OCCN1CCN(CC(O)CS(O)(=O)=O)CC1 GIZQLVPDAOBAFN-UHFFFAOYSA-N 0.000 description 1
- 102000008100 Human Serum Albumin Human genes 0.000 description 1
- 108091006905 Human Serum Albumin Proteins 0.000 description 1
- 108010005716 Interferon beta-1a Proteins 0.000 description 1
- 102000014150 Interferons Human genes 0.000 description 1
- 108010050904 Interferons Proteins 0.000 description 1
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 1
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical group C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical group OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical group CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 1
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical group CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 1
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical group OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 1
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical group C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical group C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical group OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical group CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Chemical group CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- 108010053229 Lysyl endopeptidase Proteins 0.000 description 1
- 239000007987 MES buffer Substances 0.000 description 1
- 239000007993 MOPS buffer Substances 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- DBXNUXBLKRLWFA-UHFFFAOYSA-N N-(2-acetamido)-2-aminoethanesulfonic acid Chemical compound NC(=O)CNCCS(O)(=O)=O DBXNUXBLKRLWFA-UHFFFAOYSA-N 0.000 description 1
- JOCBASBOOFNAJA-UHFFFAOYSA-N N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid Chemical compound OCC(CO)(CO)NCCS(O)(=O)=O JOCBASBOOFNAJA-UHFFFAOYSA-N 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 239000007990 PIPES buffer Substances 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 1
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 1
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 1
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Chemical group CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 1
- 239000004473 Threonine Chemical group 0.000 description 1
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 1
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Chemical group C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Chemical group CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 1
- 239000008186 active pharmaceutical agent Substances 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 235000004279 alanine Nutrition 0.000 description 1
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 1
- 125000000539 amino acid group Chemical group 0.000 description 1
- 229940043376 ammonium acetate Drugs 0.000 description 1
- 235000019257 ammonium acetate Nutrition 0.000 description 1
- 235000012538 ammonium bicarbonate Nutrition 0.000 description 1
- 235000012501 ammonium carbonate Nutrition 0.000 description 1
- 230000000840 anti-viral effect Effects 0.000 description 1
- 229940003504 avonex Drugs 0.000 description 1
- OWMVSZAMULFTJU-UHFFFAOYSA-N bis-tris Chemical compound OCCN(CCO)C(CO)(CO)CO OWMVSZAMULFTJU-UHFFFAOYSA-N 0.000 description 1
- HHKZCCWKTZRCCL-UHFFFAOYSA-N bis-tris propane Chemical compound OCC(CO)(CO)NCCCNC(CO)(CO)CO HHKZCCWKTZRCCL-UHFFFAOYSA-N 0.000 description 1
- 239000010836 blood and blood product Substances 0.000 description 1
- 229940125691 blood product Drugs 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 238000005341 cation exchange Methods 0.000 description 1
- 208000015114 central nervous system disease Diseases 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000013375 chromatographic separation Methods 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- UFULAYFCSOUIOV-UHFFFAOYSA-N cysteamine Chemical compound NCCS UFULAYFCSOUIOV-UHFFFAOYSA-N 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 239000000539 dimer Substances 0.000 description 1
- 238000002330 electrospray ionisation mass spectrometry Methods 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000000284 extract Substances 0.000 description 1
- 239000012467 final product Substances 0.000 description 1
- RWSXRVCMGQZWBV-WDSKDSINSA-N glutathione Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@@H](CS)C(=O)NCC(O)=O RWSXRVCMGQZWBV-WDSKDSINSA-N 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Chemical group OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 238000009776 industrial production Methods 0.000 description 1
- 229940079322 interferon Drugs 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Chemical group CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 201000005296 lung carcinoma Diseases 0.000 description 1
- 238000013507 mapping Methods 0.000 description 1
- 229940126601 medicinal product Drugs 0.000 description 1
- 201000001441 melanoma Diseases 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 229960003151 mercaptamine Drugs 0.000 description 1
- 125000001360 methionine group Chemical group N[C@@H](CCSC)C(=O)* 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 238000013048 microbiological method Methods 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 230000000869 mutational effect Effects 0.000 description 1
- 210000000822 natural killer cell Anatomy 0.000 description 1
- 210000004126 nerve fiber Anatomy 0.000 description 1
- 238000000424 optical density measurement Methods 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Chemical group OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 229940124272 protein stabilizer Drugs 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 238000010405 reoxidation reaction Methods 0.000 description 1
- 230000010076 replication Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 238000012163 sequencing technique Methods 0.000 description 1
- 238000002741 site-directed mutagenesis Methods 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000001488 sodium phosphate Substances 0.000 description 1
- 229910000162 sodium phosphate Inorganic materials 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 238000004611 spectroscopical analysis Methods 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 239000003104 tissue culture media Substances 0.000 description 1
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Chemical group OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 239000004474 valine Chemical group 0.000 description 1
- 230000003612 virological effect Effects 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/52—Cytokines; Lymphokines; Interferons
- C07K14/555—Interferons [IFN]
- C07K14/565—IFN-beta
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Gastroenterology & Hepatology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Molecular Biology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Genetics & Genomics (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Toxicology (AREA)
- Zoology (AREA)
- General Chemical & Material Sciences (AREA)
- Psychiatry (AREA)
- Hospice & Palliative Care (AREA)
- Virology (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US62683704P | 2004-11-10 | 2004-11-10 | |
| US60/626,837 | 2004-11-10 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2007121515A RU2007121515A (ru) | 2008-12-20 |
| RU2404190C2 true RU2404190C2 (ru) | 2010-11-20 |
Family
ID=36337216
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2007121515/04A RU2404190C2 (ru) | 2004-11-10 | 2005-11-10 | Деамидированный интерферон-бета |
Country Status (16)
| Country | Link |
|---|---|
| US (2) | US7595040B2 (enExample) |
| EP (1) | EP1809662B1 (enExample) |
| JP (1) | JP4891250B2 (enExample) |
| KR (1) | KR101330626B1 (enExample) |
| CN (1) | CN101056890B (enExample) |
| AT (1) | ATE415421T1 (enExample) |
| AU (1) | AU2005304486B2 (enExample) |
| BR (1) | BRPI0517697A (enExample) |
| CA (1) | CA2587061C (enExample) |
| DE (1) | DE602005011321D1 (enExample) |
| ES (1) | ES2318575T3 (enExample) |
| MX (1) | MX2007004990A (enExample) |
| PL (1) | PL1809662T3 (enExample) |
| PT (1) | PT1809662E (enExample) |
| RU (1) | RU2404190C2 (enExample) |
| WO (1) | WO2006053134A2 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
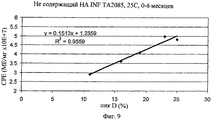
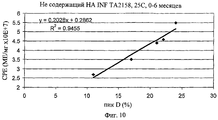
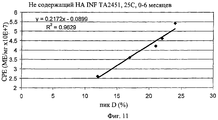
| RU2739261C1 (ru) * | 2019-12-31 | 2020-12-22 | Федеральное государственное бюджетное учреждение науки институт биоорганической химии им. академиков М.М. Шемякина и Ю.А. Овчинникова Российской академии наук (ИБХ РАН) | Способ количественного определения антипролиферативной активности интерферона-бета человека |
| RU2812047C1 (ru) * | 2020-04-29 | 2024-01-22 | Абион Инк. | Вариант интерферона-бета человека с двойной мутацией и способ улучшения стабильности варианта интерферона-бета человека |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030190307A1 (en) | 1996-12-24 | 2003-10-09 | Biogen, Inc. | Stable liquid interferon formulations |
| EP2059528A2 (en) * | 2006-08-08 | 2009-05-20 | Novartis Ag | Recombinant interferon-beta with enhanced biological activity |
| WO2008137471A2 (en) | 2007-05-02 | 2008-11-13 | Ambrx, Inc. | Modified interferon beta polypeptides and their uses |
| WO2009045553A1 (en) * | 2007-10-05 | 2009-04-09 | Barofold, Inc. | High pressure treatment of aggregated interferons |
| WO2015150468A2 (en) * | 2014-04-04 | 2015-10-08 | Ares Trading S.A. | Novel ifn beta protein analogs |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2216345C1 (ru) * | 2002-03-21 | 2003-11-20 | Закрытое Акционерное Общество "Биокад" | Средство, обладающее иммуномодулирующим, противовирусным, противобактериальным, регенерирующим, репаративным, мембрано- и гепатопротекторным действием |
| WO2004087753A1 (en) * | 2003-03-31 | 2004-10-14 | Samsung Fine Chemicals Co., Ltd. | Mutein of human interferon-beta and its preparation method |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4737462A (en) | 1982-10-19 | 1988-04-12 | Cetus Corporation | Structural genes, plasmids and transformed cells for producing cysteine depleted muteins of interferon-β |
| US4588585A (en) | 1982-10-19 | 1986-05-13 | Cetus Corporation | Human recombinant cysteine depleted interferon-β muteins |
| US4959314A (en) | 1984-11-09 | 1990-09-25 | Cetus Corporation | Cysteine-depleted muteins of biologically active proteins |
| GB9111902D0 (en) | 1991-06-03 | 1991-07-24 | Glaxo Group Ltd | Chemical compounds |
| US20030190307A1 (en) | 1996-12-24 | 2003-10-09 | Biogen, Inc. | Stable liquid interferon formulations |
| TR199901968T2 (xx) | 1996-12-24 | 1999-12-21 | Biogen, Inc. | Sabit s�v� interferon form�lleri. |
| US6696423B1 (en) | 1997-08-29 | 2004-02-24 | Biogen, Inc. | Methods and compositions for therapies using genes encoding secreted proteins such as interferon-beta |
| CA2304808C (en) * | 1997-09-23 | 2011-03-22 | Michael Tschope | Liquid interferon-.beta. formulations |
| DE60045247D1 (de) | 1999-07-28 | 2010-12-30 | Genentech Inc | Zusammensetzungen und verfahren zur behandlung von tumoren |
| ES2180416B1 (es) | 2001-03-12 | 2004-06-01 | BIOTOOLS BIOTECHNOLOGICAL & MEDICAL LABORATORIES, S.A. | Procedimiento para la preparacion de mezclas de reaccion estabilizadas, total o parcialmente desecadas, que comprenden, al menos, una enzima, mezclas de reaccion y kits que las contienen. |
| AR034749A1 (es) * | 2001-07-09 | 2004-03-17 | Schering Ag | Formulaciones de interferon beta humano |
-
2005
- 2005-11-10 AU AU2005304486A patent/AU2005304486B2/en not_active Ceased
- 2005-11-10 BR BRPI0517697-2A patent/BRPI0517697A/pt not_active IP Right Cessation
- 2005-11-10 EP EP05818235A patent/EP1809662B1/en not_active Expired - Lifetime
- 2005-11-10 WO PCT/US2005/040758 patent/WO2006053134A2/en not_active Ceased
- 2005-11-10 MX MX2007004990A patent/MX2007004990A/es active IP Right Grant
- 2005-11-10 RU RU2007121515/04A patent/RU2404190C2/ru not_active IP Right Cessation
- 2005-11-10 CA CA2587061A patent/CA2587061C/en not_active Expired - Fee Related
- 2005-11-10 AT AT05818235T patent/ATE415421T1/de active
- 2005-11-10 CN CN2005800381182A patent/CN101056890B/zh not_active Expired - Fee Related
- 2005-11-10 DE DE602005011321T patent/DE602005011321D1/de not_active Expired - Lifetime
- 2005-11-10 PT PT05818235T patent/PT1809662E/pt unknown
- 2005-11-10 JP JP2007540208A patent/JP4891250B2/ja not_active Expired - Fee Related
- 2005-11-10 PL PL05818235T patent/PL1809662T3/pl unknown
- 2005-11-10 ES ES05818235T patent/ES2318575T3/es not_active Expired - Lifetime
- 2005-11-10 US US11/271,516 patent/US7595040B2/en not_active Expired - Fee Related
- 2005-11-10 KR KR1020077011869A patent/KR101330626B1/ko not_active Expired - Fee Related
-
2009
- 2009-03-25 US US12/410,540 patent/US20090263355A1/en not_active Abandoned
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2216345C1 (ru) * | 2002-03-21 | 2003-11-20 | Закрытое Акционерное Общество "Биокад" | Средство, обладающее иммуномодулирующим, противовирусным, противобактериальным, регенерирующим, репаративным, мембрано- и гепатопротекторным действием |
| WO2004087753A1 (en) * | 2003-03-31 | 2004-10-14 | Samsung Fine Chemicals Co., Ltd. | Mutein of human interferon-beta and its preparation method |
Non-Patent Citations (1)
| Title |
|---|
| KAWASAKI HIROAKI ET AL: "A protein with antimicrobial activity in the skin of Schlegel's green tree frog Rhacophorus schlegelii (Rhacophoridae) identified as histone H2B." BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS, 2003, vol. 312, no.4, pages 1082-1086. * |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2739261C1 (ru) * | 2019-12-31 | 2020-12-22 | Федеральное государственное бюджетное учреждение науки институт биоорганической химии им. академиков М.М. Шемякина и Ю.А. Овчинникова Российской академии наук (ИБХ РАН) | Способ количественного определения антипролиферативной активности интерферона-бета человека |
| RU2812047C1 (ru) * | 2020-04-29 | 2024-01-22 | Абион Инк. | Вариант интерферона-бета человека с двойной мутацией и способ улучшения стабильности варианта интерферона-бета человека |
Also Published As
| Publication number | Publication date |
|---|---|
| BRPI0517697A (pt) | 2008-10-14 |
| CN101056890B (zh) | 2012-05-09 |
| PT1809662E (pt) | 2009-02-26 |
| WO2006053134A3 (en) | 2006-08-31 |
| CA2587061C (en) | 2011-08-16 |
| US7595040B2 (en) | 2009-09-29 |
| PL1809662T3 (pl) | 2009-07-31 |
| KR101330626B1 (ko) | 2013-11-18 |
| JP4891250B2 (ja) | 2012-03-07 |
| US20090263355A1 (en) | 2009-10-22 |
| AU2005304486B2 (en) | 2011-08-11 |
| DE602005011321D1 (de) | 2009-01-08 |
| CA2587061A1 (en) | 2006-05-18 |
| US20060120998A1 (en) | 2006-06-08 |
| JP2008519769A (ja) | 2008-06-12 |
| ATE415421T1 (de) | 2008-12-15 |
| RU2007121515A (ru) | 2008-12-20 |
| AU2005304486A1 (en) | 2006-05-18 |
| MX2007004990A (es) | 2007-06-14 |
| EP1809662A2 (en) | 2007-07-25 |
| WO2006053134A2 (en) | 2006-05-18 |
| ES2318575T3 (es) | 2009-05-01 |
| CN101056890A (zh) | 2007-10-17 |
| KR20070084559A (ko) | 2007-08-24 |
| EP1809662B1 (en) | 2008-11-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR0184235B1 (ko) | 거핵구 형성 인자 | |
| Ferguson et al. | Starch-gel electrophoresis of anterior pituitary hormones | |
| JPH06502987A (ja) | O―グリコシル化ifn―アルファ | |
| BRPI0707529A2 (pt) | métodos para modulação do teor de manose de proteìnas recombinantes | |
| JP7493540B2 (ja) | Fc含有タンパク質の発現 | |
| DE69533359T2 (de) | Veränderte menschliche c3-proteine | |
| US20090263355A1 (en) | Deamidated interferon-beta | |
| BG107844A (bg) | Методи за пречистване и регенерация на протеин | |
| KR20160052537A (ko) | 조직 손상과 연관된 질환 및 장애를 예방 및 치료하기 위한 조직 보호 펩티드 및 펩티드 유사체 | |
| BG107214A (bg) | Пептид, модулиращ тромбопоетиновия рецептор | |
| JP2011083292A (ja) | 空間構造によるタンパク質の機能の調節 | |
| Parks et al. | The cyclic nucleotide-dependent phosphorylation of aortic smooth muscle membrane proteins | |
| US20090311216A1 (en) | Recombinant interferon-beta with enhanced biological activity | |
| EP1803465A1 (en) | Pharmaceutical composition and method of treating hepatitis with arginases | |
| US20220265765A1 (en) | C3 fusion protein and methods of making and using thereof | |
| US5059418A (en) | Synergistic effect of human recombinant interferon-beta on halogenated pyrimidines | |
| US20020173475A1 (en) | Methods to inhibit viral replication | |
| Warfield et al. | Targeting ER α-glucosidase I with a single-dose iminosugar treatment protects against lethal influenza and dengue virus infections | |
| Mulvey et al. | The cellular U-particle, whose synthesis is induced by mengovirus infection, is homologous to apoferritin | |
| EP0399777A2 (en) | Hematopoietic growth factor derived from T lymphocytes and methods of use therefor | |
| RU2607527C2 (ru) | Ковалентный моноконъюгат полиэтиленгликоля с тимозином бета 4, устойчивый к деградации в токе крови, и способ его получения | |
| CA2256368A1 (en) | An interleukin-5 antagonist |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20151111 |