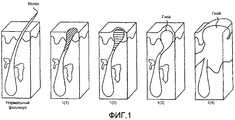
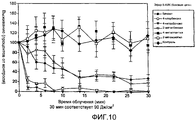
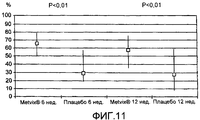
RU2385718C2 - Лечение угрей с использованием производных 5-аминолевулиновой кислоты - Google Patents
Лечение угрей с использованием производных 5-аминолевулиновой кислоты Download PDFInfo
- Publication number
- RU2385718C2 RU2385718C2 RU2007115601/15A RU2007115601A RU2385718C2 RU 2385718 C2 RU2385718 C2 RU 2385718C2 RU 2007115601/15 A RU2007115601/15 A RU 2007115601/15A RU 2007115601 A RU2007115601 A RU 2007115601A RU 2385718 C2 RU2385718 C2 RU 2385718C2
- Authority
- RU
- Russia
- Prior art keywords
- ala
- acne
- photosensitizer
- ester
- ether
- Prior art date
Links
- 206010000496 acne Diseases 0.000 title claims abstract description 114
- 208000002874 Acne Vulgaris Diseases 0.000 title claims abstract description 98
- ZGXJTSGNIOSYLO-UHFFFAOYSA-N 88755TAZ87 Chemical class NCC(=O)CCC(O)=O ZGXJTSGNIOSYLO-UHFFFAOYSA-N 0.000 title claims abstract description 70
- 238000011282 treatment Methods 0.000 title claims abstract description 44
- -1 benzyl ester Chemical class 0.000 claims abstract description 104
- 229960002749 aminolevulinic acid Drugs 0.000 claims abstract description 45
- 150000003839 salts Chemical class 0.000 claims abstract description 27
- 239000003814 drug Substances 0.000 claims abstract description 15
- 150000004702 methyl esters Chemical class 0.000 claims abstract description 14
- 230000002265 prevention Effects 0.000 claims abstract description 8
- 241000186429 Propionibacterium Species 0.000 claims abstract description 3
- 239000003504 photosensitizing agent Substances 0.000 claims description 57
- 238000000034 method Methods 0.000 claims description 39
- 241000186427 Cutibacterium acnes Species 0.000 claims description 15
- 241000024188 Andala Species 0.000 claims description 14
- 241001464975 Cutibacterium granulosum Species 0.000 claims description 13
- 241001464974 Cutibacterium avidum Species 0.000 claims description 12
- 230000000699 topical effect Effects 0.000 claims description 9
- 238000004519 manufacturing process Methods 0.000 claims description 8
- 230000002186 photoactivation Effects 0.000 claims description 7
- 229940055019 propionibacterium acne Drugs 0.000 claims description 5
- 239000002537 cosmetic Substances 0.000 claims description 3
- 241001303601 Rosacea Species 0.000 claims description 2
- 229940055009 propionibacterium avidum Drugs 0.000 claims description 2
- 201000004700 rosacea Diseases 0.000 claims description 2
- 230000000694 effects Effects 0.000 abstract description 21
- 239000000126 substance Substances 0.000 abstract description 6
- 206010015150 Erythema Diseases 0.000 abstract description 4
- 208000002193 Pain Diseases 0.000 abstract description 4
- 230000002411 adverse Effects 0.000 abstract description 3
- 231100000321 erythema Toxicity 0.000 abstract description 3
- 230000007794 irritation Effects 0.000 abstract description 3
- 206010020751 Hypersensitivity Diseases 0.000 abstract description 2
- 208000003251 Pruritus Diseases 0.000 abstract description 2
- 208000005298 acute pain Diseases 0.000 abstract description 2
- 208000026935 allergic disease Diseases 0.000 abstract description 2
- 230000002497 edematous effect Effects 0.000 abstract description 2
- 230000009610 hypersensitivity Effects 0.000 abstract description 2
- 230000007803 itching Effects 0.000 abstract description 2
- 230000002035 prolonged effect Effects 0.000 abstract description 2
- 229940127554 medical product Drugs 0.000 abstract 1
- 150000001875 compounds Chemical class 0.000 description 38
- 238000002428 photodynamic therapy Methods 0.000 description 35
- 125000000217 alkyl group Chemical group 0.000 description 29
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 25
- 239000000203 mixture Substances 0.000 description 25
- 150000002148 esters Chemical class 0.000 description 22
- 241000894006 Bacteria Species 0.000 description 20
- 230000015572 biosynthetic process Effects 0.000 description 20
- 238000011534 incubation Methods 0.000 description 20
- 150000004032 porphyrins Chemical class 0.000 description 19
- 239000003795 chemical substances by application Substances 0.000 description 18
- 125000003118 aryl group Chemical group 0.000 description 17
- ILBBNQMSDGAAPF-UHFFFAOYSA-N 1-(6-hydroxy-6-methylcyclohexa-2,4-dien-1-yl)propan-1-one Chemical compound CCC(=O)C1C=CC=CC1(C)O ILBBNQMSDGAAPF-UHFFFAOYSA-N 0.000 description 14
- 230000004083 survival effect Effects 0.000 description 13
- 239000006071 cream Substances 0.000 description 12
- 230000002757 inflammatory effect Effects 0.000 description 12
- 210000003491 skin Anatomy 0.000 description 12
- 206010037888 Rash pustular Diseases 0.000 description 11
- 230000035515 penetration Effects 0.000 description 11
- 208000029561 pustule Diseases 0.000 description 11
- 231100000419 toxicity Toxicity 0.000 description 11
- 230000001988 toxicity Effects 0.000 description 11
- 206010033733 Papule Diseases 0.000 description 10
- 230000001580 bacterial effect Effects 0.000 description 10
- 230000003902 lesion Effects 0.000 description 10
- 239000002738 chelating agent Substances 0.000 description 9
- 239000000725 suspension Substances 0.000 description 9
- 125000004432 carbon atom Chemical group C* 0.000 description 8
- 210000004027 cell Anatomy 0.000 description 8
- 208000031513 cyst Diseases 0.000 description 8
- 238000002474 experimental method Methods 0.000 description 8
- 230000001737 promoting effect Effects 0.000 description 8
- 241001635598 Enicostema Species 0.000 description 7
- 206010027626 Milia Diseases 0.000 description 7
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 7
- 239000000499 gel Substances 0.000 description 7
- 239000011148 porous material Substances 0.000 description 7
- 210000001732 sebaceous gland Anatomy 0.000 description 7
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 6
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 6
- 230000004907 flux Effects 0.000 description 6
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 5
- 231100000086 high toxicity Toxicity 0.000 description 5
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 5
- 230000001965 increasing effect Effects 0.000 description 5
- YUUAYBAIHCDHHD-UHFFFAOYSA-N methyl 5-aminolevulinate Chemical compound COC(=O)CCC(=O)CN YUUAYBAIHCDHHD-UHFFFAOYSA-N 0.000 description 5
- 230000002165 photosensitisation Effects 0.000 description 5
- 239000000902 placebo Substances 0.000 description 5
- 229940068196 placebo Drugs 0.000 description 5
- 238000002360 preparation method Methods 0.000 description 5
- 125000000547 substituted alkyl group Chemical group 0.000 description 5
- RKTMLPDIXLJDMA-UHFFFAOYSA-N 1-fluoro-2-[(2-fluorophenyl)methoxymethyl]benzene Chemical compound FC1=CC=CC=C1COCC1=CC=CC=C1F RKTMLPDIXLJDMA-UHFFFAOYSA-N 0.000 description 4
- 125000006283 4-chlorobenzyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1Cl)C([H])([H])* 0.000 description 4
- OMPJBNCRMGITSC-UHFFFAOYSA-N Benzoylperoxide Chemical compound C=1C=CC=CC=1C(=O)OOC(=O)C1=CC=CC=C1 OMPJBNCRMGITSC-UHFFFAOYSA-N 0.000 description 4
- UJKPHYRXOLRVJJ-MLSVHJFASA-N CC(O)C1=C(C)/C2=C/C3=N/C(=C\C4=C(CCC(O)=O)C(C)=C(N4)/C=C4\N=C(\C=C\1/N\2)C(C)=C4C(C)O)/C(CCC(O)=O)=C3C Chemical class CC(O)C1=C(C)/C2=C/C3=N/C(=C\C4=C(CCC(O)=O)C(C)=C(N4)/C=C4\N=C(\C=C\1/N\2)C(C)=C4C(C)O)/C(CCC(O)=O)=C3C UJKPHYRXOLRVJJ-MLSVHJFASA-N 0.000 description 4
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 4
- 239000002253 acid Substances 0.000 description 4
- 235000019400 benzoyl peroxide Nutrition 0.000 description 4
- 230000007423 decrease Effects 0.000 description 4
- MHDVGSVTJDSBDK-UHFFFAOYSA-N dibenzyl ether Chemical compound C=1C=CC=CC=1COCC1=CC=CC=C1 MHDVGSVTJDSBDK-UHFFFAOYSA-N 0.000 description 4
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 4
- 125000001072 heteroaryl group Chemical group 0.000 description 4
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 4
- 125000004491 isohexyl group Chemical group C(CCC(C)C)* 0.000 description 4
- 230000002147 killing effect Effects 0.000 description 4
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 4
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 4
- 239000000523 sample Substances 0.000 description 4
- 210000002374 sebum Anatomy 0.000 description 4
- 125000001424 substituent group Chemical group 0.000 description 4
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 description 3
- BPIUIOXAFBGMNB-UHFFFAOYSA-N 1-hexoxyhexane Chemical compound CCCCCCOCCCCCC BPIUIOXAFBGMNB-UHFFFAOYSA-N 0.000 description 3
- LDDKBZYKNBNGSA-UHFFFAOYSA-N 1-nitro-3-[(3-nitrophenyl)methoxymethyl]benzene Chemical compound [O-][N+](=O)C1=CC=CC(COCC=2C=C(C=CC=2)[N+]([O-])=O)=C1 LDDKBZYKNBNGSA-UHFFFAOYSA-N 0.000 description 3
- VJJRVNGOHNKPHB-UHFFFAOYSA-N 1-nitro-4-[(4-nitrophenyl)methoxymethyl]benzene Chemical compound C1=CC([N+](=O)[O-])=CC=C1COCC1=CC=C([N+]([O-])=O)C=C1 VJJRVNGOHNKPHB-UHFFFAOYSA-N 0.000 description 3
- UMJSCPRVCHMLSP-UHFFFAOYSA-N 3-methoxypyridine Chemical compound COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 3
- LXIQJYAYQYNRCS-UHFFFAOYSA-N 4-(4-phenylbutoxy)butylbenzene Chemical compound C=1C=CC=CC=1CCCCOCCCCC1=CC=CC=C1 LXIQJYAYQYNRCS-UHFFFAOYSA-N 0.000 description 3
- 239000004342 Benzoyl peroxide Substances 0.000 description 3
- WVDDGKGOMKODPV-UHFFFAOYSA-N Benzyl alcohol Chemical compound OCC1=CC=CC=C1 WVDDGKGOMKODPV-UHFFFAOYSA-N 0.000 description 3
- 229920000858 Cyclodextrin Polymers 0.000 description 3
- ULGZDMOVFRHVEP-RWJQBGPGSA-N Erythromycin Chemical compound O([C@@H]1[C@@H](C)C(=O)O[C@@H]([C@@]([C@H](O)[C@@H](C)C(=O)[C@H](C)C[C@@](C)(O)[C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C)(C)O)CC)[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1 ULGZDMOVFRHVEP-RWJQBGPGSA-N 0.000 description 3
- SHGAZHPCJJPHSC-NUEINMDLSA-N Isotretinoin Chemical compound OC(=O)C=C(C)/C=C/C=C(C)C=CC1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-NUEINMDLSA-N 0.000 description 3
- 239000007990 PIPES buffer Substances 0.000 description 3
- 238000009825 accumulation Methods 0.000 description 3
- 239000013543 active substance Substances 0.000 description 3
- 125000003545 alkoxy group Chemical group 0.000 description 3
- HUVXQFBFIFIDDU-UHFFFAOYSA-N aluminum phthalocyanine Chemical compound [Al+3].C12=CC=CC=C2C(N=C2[N-]C(C3=CC=CC=C32)=N2)=NC1=NC([C]1C=CC=CC1=1)=NC=1N=C1[C]3C=CC=CC3=C2[N-]1 HUVXQFBFIFIDDU-UHFFFAOYSA-N 0.000 description 3
- 239000003242 anti bacterial agent Substances 0.000 description 3
- 229940088710 antibiotic agent Drugs 0.000 description 3
- 230000009920 chelation Effects 0.000 description 3
- 150000004035 chlorins Chemical class 0.000 description 3
- 229940079593 drug Drugs 0.000 description 3
- 238000000295 emission spectrum Methods 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- 210000002919 epithelial cell Anatomy 0.000 description 3
- 229960003569 hematoporphyrin Drugs 0.000 description 3
- 125000001183 hydrocarbyl group Chemical group 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 229960005280 isotretinoin Drugs 0.000 description 3
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 3
- BDJRBEYXGGNYIS-UHFFFAOYSA-N nonanedioic acid Chemical compound OC(=O)CCCCCCCC(O)=O BDJRBEYXGGNYIS-UHFFFAOYSA-N 0.000 description 3
- 239000002674 ointment Substances 0.000 description 3
- 125000004043 oxo group Chemical group O=* 0.000 description 3
- 125000006503 p-nitrobenzyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1[N+]([O-])=O)C([H])([H])* 0.000 description 3
- 150000002978 peroxides Chemical class 0.000 description 3
- 229940109328 photofrin Drugs 0.000 description 3
- 239000004033 plastic Substances 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 239000002243 precursor Substances 0.000 description 3
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 239000007921 spray Substances 0.000 description 3
- 239000000375 suspending agent Substances 0.000 description 3
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 3
- 238000011200 topical administration Methods 0.000 description 3
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- OYINILBBZAQBEV-UWJYYQICSA-N (17s,18s)-18-(2-carboxyethyl)-20-(carboxymethyl)-12-ethenyl-7-ethyl-3,8,13,17-tetramethyl-17,18,22,23-tetrahydroporphyrin-2-carboxylic acid Chemical compound N1C2=C(C)C(C=C)=C1C=C(N1)C(C)=C(CC)C1=CC(C(C)=C1C(O)=O)=NC1=C(CC(O)=O)C([C@@H](CCC(O)=O)[C@@H]1C)=NC1=C2 OYINILBBZAQBEV-UWJYYQICSA-N 0.000 description 2
- REXQTRHCVRPOMB-UHFFFAOYSA-N 1-chloro-4-[(4-chlorophenyl)methoxymethyl]benzene Chemical compound C1=CC(Cl)=CC=C1COCC1=CC=C(Cl)C=C1 REXQTRHCVRPOMB-UHFFFAOYSA-N 0.000 description 2
- WZKCRJVJFMWNQT-UHFFFAOYSA-N 1-fluoro-3-[(3-fluorophenyl)methoxymethyl]benzene Chemical compound FC1=CC=CC(COCC=2C=C(F)C=CC=2)=C1 WZKCRJVJFMWNQT-UHFFFAOYSA-N 0.000 description 2
- XBXLZNNMEKOYPT-UHFFFAOYSA-N 1-fluoro-4-[(4-fluorophenyl)methoxymethyl]benzene Chemical compound C1=CC(F)=CC=C1COCC1=CC=C(F)C=C1 XBXLZNNMEKOYPT-UHFFFAOYSA-N 0.000 description 2
- SDTORDSXCYSNTD-UHFFFAOYSA-N 1-methoxy-4-[(4-methoxyphenyl)methoxymethyl]benzene Chemical compound C1=CC(OC)=CC=C1COCC1=CC=C(OC)C=C1 SDTORDSXCYSNTD-UHFFFAOYSA-N 0.000 description 2
- LRKLFZPYIDPEHV-UHFFFAOYSA-N 1-methyl-2-[(2-methylphenyl)methoxymethyl]benzene Chemical compound CC1=CC=CC=C1COCC1=CC=CC=C1C LRKLFZPYIDPEHV-UHFFFAOYSA-N 0.000 description 2
- UJIUKTDUCYLQBN-UHFFFAOYSA-N 1-methyl-4-[(4-methylphenyl)methoxymethyl]benzene Chemical compound C1=CC(C)=CC=C1COCC1=CC=C(C)C=C1 UJIUKTDUCYLQBN-UHFFFAOYSA-N 0.000 description 2
- NZJXADCEESMBPW-UHFFFAOYSA-N 1-methylsulfinyldecane Chemical compound CCCCCCCCCCS(C)=O NZJXADCEESMBPW-UHFFFAOYSA-N 0.000 description 2
- OXIQTPVKRUSLOJ-UHFFFAOYSA-N 1-propan-2-yl-4-[(4-propan-2-ylphenyl)methoxymethyl]benzene Chemical compound C1=CC(C(C)C)=CC=C1COCC1=CC=C(C(C)C)C=C1 OXIQTPVKRUSLOJ-UHFFFAOYSA-N 0.000 description 2
- AMOYMEBHYUTMKJ-UHFFFAOYSA-N 2-(2-phenylethoxy)ethylbenzene Chemical compound C=1C=CC=CC=1CCOCCC1=CC=CC=C1 AMOYMEBHYUTMKJ-UHFFFAOYSA-N 0.000 description 2
- 125000006179 2-methyl benzyl group Chemical group [H]C1=C([H])C(=C(C([H])=C1[H])C([H])([H])*)C([H])([H])[H] 0.000 description 2
- MHIITNFQDPFSES-UHFFFAOYSA-N 25,26,27,28-tetrazahexacyclo[16.6.1.13,6.18,11.113,16.019,24]octacosa-1(25),2,4,6,8(27),9,11,13,15,17,19,21,23-tridecaene Chemical class N1C(C=C2C3=CC=CC=C3C(C=C3NC(=C4)C=C3)=N2)=CC=C1C=C1C=CC4=N1 MHIITNFQDPFSES-UHFFFAOYSA-N 0.000 description 2
- 125000002528 4-isopropyl benzyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1C([H])([H])*)C([H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- 125000006181 4-methyl benzyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1C([H])([H])[H])C([H])([H])* 0.000 description 2
- KSFOVUSSGSKXFI-GAQDCDSVSA-N CC1=C/2NC(\C=C3/N=C(/C=C4\N\C(=C/C5=N/C(=C\2)/C(C=C)=C5C)C(C=C)=C4C)C(C)=C3CCC(O)=O)=C1CCC(O)=O Chemical compound CC1=C/2NC(\C=C3/N=C(/C=C4\N\C(=C/C5=N/C(=C\2)/C(C=C)=C5C)C(C=C)=C4C)C(C)=C3CCC(O)=O)=C1CCC(O)=O KSFOVUSSGSKXFI-GAQDCDSVSA-N 0.000 description 2
- 239000004354 Hydroxyethyl cellulose Substances 0.000 description 2
- 229920000663 Hydroxyethyl cellulose Polymers 0.000 description 2
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 2
- QPCDCPDFJACHGM-UHFFFAOYSA-N N,N-bis{2-[bis(carboxymethyl)amino]ethyl}glycine Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(=O)O)CCN(CC(O)=O)CC(O)=O QPCDCPDFJACHGM-UHFFFAOYSA-N 0.000 description 2
- DFPAKSUCGFBDDF-UHFFFAOYSA-N Nicotinamide Chemical compound NC(=O)C1=CC=CN=C1 DFPAKSUCGFBDDF-UHFFFAOYSA-N 0.000 description 2
- 239000004098 Tetracycline Substances 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 230000002378 acidificating effect Effects 0.000 description 2
- 150000007513 acids Chemical class 0.000 description 2
- 239000004480 active ingredient Substances 0.000 description 2
- LZCDAPDGXCYOEH-UHFFFAOYSA-N adapalene Chemical compound C1=C(C(O)=O)C=CC2=CC(C3=CC=C(C(=C3)C34CC5CC(CC(C5)C3)C4)OC)=CC=C21 LZCDAPDGXCYOEH-UHFFFAOYSA-N 0.000 description 2
- 229910052782 aluminium Inorganic materials 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- 230000008952 bacterial invasion Effects 0.000 description 2
- 239000006161 blood agar Substances 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- XMEVHPAGJVLHIG-FMZCEJRJSA-N chembl454950 Chemical compound [Cl-].C1=CC=C2[C@](O)(C)[C@H]3C[C@H]4[C@H]([NH+](C)C)C(O)=C(C(N)=O)C(=O)[C@@]4(O)C(O)=C3C(=O)C2=C1O XMEVHPAGJVLHIG-FMZCEJRJSA-N 0.000 description 2
- 125000001309 chloro group Chemical group Cl* 0.000 description 2
- 230000001332 colony forming effect Effects 0.000 description 2
- 239000013068 control sample Substances 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 125000004122 cyclic group Chemical group 0.000 description 2
- 229940097362 cyclodextrins Drugs 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 208000035475 disorder Diseases 0.000 description 2
- 230000002708 enhancing effect Effects 0.000 description 2
- 125000004494 ethyl ester group Chemical group 0.000 description 2
- 230000005284 excitation Effects 0.000 description 2
- 125000001153 fluoro group Chemical group F* 0.000 description 2
- 150000003278 haem Chemical class 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 235000019447 hydroxyethyl cellulose Nutrition 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- 230000003834 intracellular effect Effects 0.000 description 2
- 229910052742 iron Inorganic materials 0.000 description 2
- 239000006210 lotion Substances 0.000 description 2
- 230000004060 metabolic process Effects 0.000 description 2
- 125000001280 n-hexyl group Chemical group C(CCCCC)* 0.000 description 2
- 239000008194 pharmaceutical composition Substances 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- NTUCQKRAQSWLOP-UHFFFAOYSA-N propan-2-yloxymethylbenzene Chemical compound CC(C)OCC1=CC=CC=C1 NTUCQKRAQSWLOP-UHFFFAOYSA-N 0.000 description 2
- 229950003776 protoporphyrin Drugs 0.000 description 2
- 230000000717 retained effect Effects 0.000 description 2
- 230000003381 solubilizing effect Effects 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 150000003458 sulfonic acid derivatives Chemical class 0.000 description 2
- 150000003462 sulfoxides Chemical class 0.000 description 2
- 229960002180 tetracycline Drugs 0.000 description 2
- 235000019364 tetracycline Nutrition 0.000 description 2
- 229930101283 tetracycline Natural products 0.000 description 2
- 150000003522 tetracyclines Chemical class 0.000 description 2
- 239000002562 thickening agent Substances 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- SGKRLCUYIXIAHR-AKNGSSGZSA-N (4s,4ar,5s,5ar,6r,12ar)-4-(dimethylamino)-1,5,10,11,12a-pentahydroxy-6-methyl-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide Chemical compound C1=CC=C2[C@H](C)[C@@H]([C@H](O)[C@@H]3[C@](C(O)=C(C(N)=O)C(=O)[C@H]3N(C)C)(O)C3=O)C3=C(O)C2=C1O SGKRLCUYIXIAHR-AKNGSSGZSA-N 0.000 description 1
- FFTVPQUHLQBXQZ-KVUCHLLUSA-N (4s,4as,5ar,12ar)-4,7-bis(dimethylamino)-1,10,11,12a-tetrahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide Chemical compound C1C2=C(N(C)C)C=CC(O)=C2C(O)=C2[C@@H]1C[C@H]1[C@H](N(C)C)C(=O)C(C(N)=O)=C(O)[C@@]1(O)C2=O FFTVPQUHLQBXQZ-KVUCHLLUSA-N 0.000 description 1
- 125000000008 (C1-C10) alkyl group Chemical group 0.000 description 1
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 description 1
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 1
- NKJOXAZJBOMXID-UHFFFAOYSA-N 1,1'-Oxybisoctane Chemical compound CCCCCCCCOCCCCCCCC NKJOXAZJBOMXID-UHFFFAOYSA-N 0.000 description 1
- JTIUZNMNFPZOKN-UHFFFAOYSA-N 1,2,3,4,5-pentafluoro-6-[(2,3,4,5,6-pentafluorophenyl)methoxymethyl]benzene Chemical compound FC1=C(F)C(F)=C(F)C(F)=C1COCC1=C(F)C(F)=C(F)C(F)=C1F JTIUZNMNFPZOKN-UHFFFAOYSA-N 0.000 description 1
- VDPRSOCKHVPZRS-UHFFFAOYSA-N 1-(2-decylsulfanylethyl)pyrrolidin-2-one Chemical compound CCCCCCCCCCSCCN1CCCC1=O VDPRSOCKHVPZRS-UHFFFAOYSA-N 0.000 description 1
- AYMZQSQRCFSZCR-UHFFFAOYSA-N 1-(3,3-dimethylbutoxy)-3,3-dimethylbutane Chemical compound CC(C)(C)CCOCCC(C)(C)C AYMZQSQRCFSZCR-UHFFFAOYSA-N 0.000 description 1
- DURPTKYDGMDSBL-UHFFFAOYSA-N 1-butoxybutane Chemical compound CCCCOCCCC DURPTKYDGMDSBL-UHFFFAOYSA-N 0.000 description 1
- POEDHWVTLBLWDA-UHFFFAOYSA-N 1-butylindole-2,3-dione Chemical compound C1=CC=C2N(CCCC)C(=O)C(=O)C2=C1 POEDHWVTLBLWDA-UHFFFAOYSA-N 0.000 description 1
- AXTGDCSMTYGJND-UHFFFAOYSA-N 1-dodecylazepan-2-one Chemical compound CCCCCCCCCCCCN1CCCCCC1=O AXTGDCSMTYGJND-UHFFFAOYSA-N 0.000 description 1
- 125000006218 1-ethylbutyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C([H])([H])[H] 0.000 description 1
- DBUJFULDVAZULB-UHFFFAOYSA-N 1-methoxypentane Chemical compound CCCCCOC DBUJFULDVAZULB-UHFFFAOYSA-N 0.000 description 1
- BCAVDSGTTYREGF-UHFFFAOYSA-N 1-methyl-3-[(3-methylphenyl)methoxymethyl]benzene Chemical compound CC1=CC=CC(COCC=2C=C(C)C=CC=2)=C1 BCAVDSGTTYREGF-UHFFFAOYSA-N 0.000 description 1
- BFPYWIDHMRZLRN-UHFFFAOYSA-N 17alpha-ethynyl estradiol Natural products OC1=CC=C2C3CCC(C)(C(CC4)(O)C#C)C4C3CCC2=C1 BFPYWIDHMRZLRN-UHFFFAOYSA-N 0.000 description 1
- RNMCCPMYXUKHAZ-UHFFFAOYSA-N 2-[3,3-diamino-1,2,2-tris(carboxymethyl)cyclohexyl]acetic acid Chemical compound NC1(N)CCCC(CC(O)=O)(CC(O)=O)C1(CC(O)=O)CC(O)=O RNMCCPMYXUKHAZ-UHFFFAOYSA-N 0.000 description 1
- RXYBLXNJYZXINS-UHFFFAOYSA-N 2-methoxyethyl 5-amino-4-oxopentanoate Chemical compound COCCOC(=O)CCC(=O)CN RXYBLXNJYZXINS-UHFFFAOYSA-N 0.000 description 1
- 125000004200 2-methoxyethyl group Chemical group [H]C([H])([H])OC([H])([H])C([H])([H])* 0.000 description 1
- PWRRZALULPEHOC-UHFFFAOYSA-N 2-methyl-1-(2-methylpentoxy)pentane Chemical compound CCCC(C)COCC(C)CCC PWRRZALULPEHOC-UHFFFAOYSA-N 0.000 description 1
- 125000005916 2-methylpentyl group Chemical group 0.000 description 1
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 1
- HYDWALOBQJFOMS-UHFFFAOYSA-N 3,6,9,12,15-pentaoxaheptadecane Chemical compound CCOCCOCCOCCOCCOCC HYDWALOBQJFOMS-UHFFFAOYSA-N 0.000 description 1
- VAJVGAQAYOAJQI-UHFFFAOYSA-N 3-[18-(2-carboxylatoethyl)-3,8,13,17-tetramethyl-22,23-dihydroporphyrin-21,24-diium-2-yl]propanoate Chemical compound N1C(C=C2C(C)=CC(N2)=CC=2C(=C(CCC(O)=O)C(=C3)N=2)C)=CC(C)=C1C=C1C(C)=C(CCC(O)=O)C3=N1 VAJVGAQAYOAJQI-UHFFFAOYSA-N 0.000 description 1
- DDCZIBJTHVPXFT-UHFFFAOYSA-N 3-hexan-3-yloxyhexane Chemical compound CCCC(CC)OC(CC)CCC DDCZIBJTHVPXFT-UHFFFAOYSA-N 0.000 description 1
- 125000005917 3-methylpentyl group Chemical group 0.000 description 1
- RYQOILLJDKPETL-UHFFFAOYSA-N 5-aminolevulinic acid hexyl ester Chemical compound CCCCCCOC(=O)CCC(=O)CN RYQOILLJDKPETL-UHFFFAOYSA-N 0.000 description 1
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical class OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 1
- VTQAGEXLVMJCFP-UHFFFAOYSA-N CC(C)C1=CC=C(C=C1)CC(CC(=O)CN)C(=O)O Chemical compound CC(C)C1=CC=C(C=C1)CC(CC(=O)CN)C(=O)O VTQAGEXLVMJCFP-UHFFFAOYSA-N 0.000 description 1
- SYWOSHKZXSCBKF-UHFFFAOYSA-N CC1=CC=C(CC(C(=O)O)CC(=O)CN)C=C1 Chemical compound CC1=CC=C(CC(C(=O)O)CC(=O)CN)C=C1 SYWOSHKZXSCBKF-UHFFFAOYSA-N 0.000 description 1
- 241001631457 Cannula Species 0.000 description 1
- FCKYPQBAHLOOJQ-UHFFFAOYSA-N Cyclohexane-1,2-diaminetetraacetic acid Chemical compound OC(=O)CN(CC(O)=O)C1CCCCC1N(CC(O)=O)CC(O)=O FCKYPQBAHLOOJQ-UHFFFAOYSA-N 0.000 description 1
- 239000004097 EU approved flavor enhancer Substances 0.000 description 1
- 206010049119 Emotional distress Diseases 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- BFPYWIDHMRZLRN-SLHNCBLASA-N Ethinyl estradiol Chemical compound OC1=CC=C2[C@H]3CC[C@](C)([C@](CC4)(O)C#C)[C@@H]4[C@@H]3CCC2=C1 BFPYWIDHMRZLRN-SLHNCBLASA-N 0.000 description 1
- 102000003875 Ferrochelatase Human genes 0.000 description 1
- 108010057394 Ferrochelatase Proteins 0.000 description 1
- KGHNSNSWRMJVND-UHFFFAOYSA-N Hypocrellin Natural products COC1=CC(=O)C2=C3C4C(C(C(=O)C)C(C)(O)Cc5c(OC)c(O)c6C(=O)C=C(OC)C(=C13)c6c45)C(=C2O)OC KGHNSNSWRMJVND-UHFFFAOYSA-N 0.000 description 1
- 206010058015 Infected cyst Diseases 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 244000062730 Melissa officinalis Species 0.000 description 1
- 235000010654 Melissa officinalis Nutrition 0.000 description 1
- FXHOOIRPVKKKFG-UHFFFAOYSA-N N,N-Dimethylacetamide Chemical compound CN(C)C(C)=O FXHOOIRPVKKKFG-UHFFFAOYSA-N 0.000 description 1
- UBQYURCVBFRUQT-UHFFFAOYSA-N N-benzoyl-Ferrioxamine B Chemical compound CC(=O)N(O)CCCCCNC(=O)CCC(=O)N(O)CCCCCNC(=O)CCC(=O)N(O)CCCCCN UBQYURCVBFRUQT-UHFFFAOYSA-N 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 1
- 239000005642 Oleic acid Substances 0.000 description 1
- 239000004100 Oxytetracycline Substances 0.000 description 1
- SHGAZHPCJJPHSC-UHFFFAOYSA-N Panrexin Chemical compound OC(=O)C=C(C)C=CC=C(C)C=CC1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-UHFFFAOYSA-N 0.000 description 1
- 208000012641 Pigmentation disease Diseases 0.000 description 1
- QOSMNYMQXIVWKY-UHFFFAOYSA-N Propyl levulinate Chemical compound CCCOC(=O)CCC(C)=O QOSMNYMQXIVWKY-UHFFFAOYSA-N 0.000 description 1
- 201000004681 Psoriasis Diseases 0.000 description 1
- 206010039580 Scar Diseases 0.000 description 1
- 241001486234 Sciota Species 0.000 description 1
- 239000000589 Siderophore Substances 0.000 description 1
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 1
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 1
- WDLRUFUQRNWCPK-UHFFFAOYSA-N Tetraxetan Chemical compound OC(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1 WDLRUFUQRNWCPK-UHFFFAOYSA-N 0.000 description 1
- OGQICQVSFDPSEI-UHFFFAOYSA-N Zorac Chemical compound N1=CC(C(=O)OCC)=CC=C1C#CC1=CC=C(SCCC2(C)C)C2=C1 OGQICQVSFDPSEI-UHFFFAOYSA-N 0.000 description 1
- UFUVLHLTWXBHGZ-MGZQPHGTSA-N [(2r,3r,4s,5r,6r)-6-[(1s,2s)-2-chloro-1-[[(2s,4r)-1-methyl-4-propylpyrrolidine-2-carbonyl]amino]propyl]-4,5-dihydroxy-2-methylsulfanyloxan-3-yl] dihydrogen phosphate Chemical compound CN1C[C@H](CCC)C[C@H]1C(=O)N[C@H]([C@H](C)Cl)[C@@H]1[C@H](O)[C@H](O)[C@@H](OP(O)(O)=O)[C@@H](SC)O1 UFUVLHLTWXBHGZ-MGZQPHGTSA-N 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 229960005339 acitretin Drugs 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 125000004423 acyloxy group Chemical group 0.000 description 1
- 229960002916 adapalene Drugs 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 125000003342 alkenyl group Chemical group 0.000 description 1
- 125000005194 alkoxycarbonyloxy group Chemical group 0.000 description 1
- 125000005907 alkyl ester group Chemical group 0.000 description 1
- 125000000304 alkynyl group Chemical group 0.000 description 1
- IHUNBGSDBOWDMA-AQFIFDHZSA-N all-trans-acitretin Chemical compound COC1=CC(C)=C(\C=C\C(\C)=C\C=C\C(\C)=C\C(O)=O)C(C)=C1C IHUNBGSDBOWDMA-AQFIFDHZSA-N 0.000 description 1
- SHGAZHPCJJPHSC-YCNIQYBTSA-N all-trans-retinoic acid Chemical compound OC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-YCNIQYBTSA-N 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 239000003098 androgen Substances 0.000 description 1
- 229940030486 androgens Drugs 0.000 description 1
- 239000000058 anti acne agent Substances 0.000 description 1
- 230000003255 anti-acne Effects 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 229940124340 antiacne agent Drugs 0.000 description 1
- 125000004429 atom Chemical group 0.000 description 1
- 150000004036 bacteriochlorins Chemical class 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- AFFZCQDLQIEQGB-UHFFFAOYSA-N benzyl 5-amino-4-oxopentanoate Chemical compound NCC(=O)CCC(=O)OCC1=CC=CC=C1 AFFZCQDLQIEQGB-UHFFFAOYSA-N 0.000 description 1
- 235000019445 benzyl alcohol Nutrition 0.000 description 1
- 210000000941 bile Anatomy 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 230000006696 biosynthetic metabolic pathway Effects 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 125000001246 bromo group Chemical group Br* 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 239000006285 cell suspension Substances 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- SURLGNKAQXKNSP-DBLYXWCISA-N chlorin Chemical compound C\1=C/2\N/C(=C\C3=N/C(=C\C=4NC(/C=C\5/C=CC/1=N/5)=CC=4)/C=C3)/CC\2 SURLGNKAQXKNSP-DBLYXWCISA-N 0.000 description 1
- 208000037976 chronic inflammation Diseases 0.000 description 1
- 208000037893 chronic inflammatory disorder Diseases 0.000 description 1
- 229960002227 clindamycin Drugs 0.000 description 1
- KDLRVYVGXIQJDK-AWPVFWJPSA-N clindamycin Chemical compound CN1C[C@H](CCC)C[C@H]1C(=O)N[C@H]([C@H](C)Cl)[C@@H]1[C@H](O)[C@H](O)[C@@H](O)[C@@H](SC)O1 KDLRVYVGXIQJDK-AWPVFWJPSA-N 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 239000002872 contrast media Substances 0.000 description 1
- 229960000978 cyproterone acetate Drugs 0.000 description 1
- UWFYSQMTEOIJJG-FDTZYFLXSA-N cyproterone acetate Chemical compound C1=C(Cl)C2=CC(=O)[C@@H]3C[C@@H]3[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(C)=O)(OC(=O)C)[C@@]1(C)CC2 UWFYSQMTEOIJJG-FDTZYFLXSA-N 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 229960000958 deferoxamine Drugs 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 229960003964 deoxycholic acid Drugs 0.000 description 1
- 238000001784 detoxification Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 125000003963 dichloro group Chemical group Cl* 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 229940002658 differin Drugs 0.000 description 1
- SBZXBUIDTXKZTM-UHFFFAOYSA-N diglyme Chemical compound COCCOCCOC SBZXBUIDTXKZTM-UHFFFAOYSA-N 0.000 description 1
- 229960001760 dimethyl sulfoxide Drugs 0.000 description 1
- 229940113088 dimethylacetamide Drugs 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 229960003722 doxycycline Drugs 0.000 description 1
- 239000006196 drop Substances 0.000 description 1
- 230000002500 effect on skin Effects 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 230000001804 emulsifying effect Effects 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 210000002615 epidermis Anatomy 0.000 description 1
- 229960003276 erythromycin Drugs 0.000 description 1
- 229960002568 ethinylestradiol Drugs 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 230000003090 exacerbative effect Effects 0.000 description 1
- 238000004299 exfoliation Methods 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 238000001506 fluorescence spectroscopy Methods 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 235000019264 food flavour enhancer Nutrition 0.000 description 1
- 210000001061 forehead Anatomy 0.000 description 1
- 239000003349 gelling agent Substances 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- 210000004907 gland Anatomy 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 125000001188 haloalkyl group Chemical group 0.000 description 1
- 125000005843 halogen group Chemical group 0.000 description 1
- 229940097293 hexyl 5-aminolevulinate Drugs 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 208000000069 hyperpigmentation Diseases 0.000 description 1
- 230000003810 hyperpigmentation Effects 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 239000000411 inducer Substances 0.000 description 1
- 208000027866 inflammatory disease Diseases 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 125000002346 iodo group Chemical group I* 0.000 description 1
- 230000001678 irradiating effect Effects 0.000 description 1
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 239000000865 liniment Substances 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 231100000053 low toxicity Toxicity 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 238000002595 magnetic resonance imaging Methods 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 1
- UJYSYPVQHFNBML-UHFFFAOYSA-N methyl 5-aminolevulinate hydrochloride Chemical compound [Cl-].COC(=O)CCC(=O)C[NH3+] UJYSYPVQHFNBML-UHFFFAOYSA-N 0.000 description 1
- 229940120732 methyl aminolevulinate hydrochloride Drugs 0.000 description 1
- 229960000282 metronidazole Drugs 0.000 description 1
- VAOCPAMSLUNLGC-UHFFFAOYSA-N metronidazole Chemical compound CC1=NC=C([N+]([O-])=O)N1CCO VAOCPAMSLUNLGC-UHFFFAOYSA-N 0.000 description 1
- 239000004005 microsphere Substances 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 229960004023 minocycline Drugs 0.000 description 1
- 239000004570 mortar (masonry) Substances 0.000 description 1
- 210000004400 mucous membrane Anatomy 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000000740 n-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 125000001624 naphthyl group Chemical group 0.000 description 1
- 229960003966 nicotinamide Drugs 0.000 description 1
- 235000005152 nicotinamide Nutrition 0.000 description 1
- 239000011570 nicotinamide Substances 0.000 description 1
- 239000002353 niosome Substances 0.000 description 1
- 125000006502 nitrobenzyl group Chemical group 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 231100000956 nontoxicity Toxicity 0.000 description 1
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 229960000625 oxytetracycline Drugs 0.000 description 1
- IWVCMVBTMGNXQD-PXOLEDIWSA-N oxytetracycline Chemical compound C1=CC=C2[C@](O)(C)[C@H]3[C@H](O)[C@H]4[C@H](N(C)C)C(O)=C(C(N)=O)C(=O)[C@@]4(O)C(O)=C3C(=O)C2=C1O IWVCMVBTMGNXQD-PXOLEDIWSA-N 0.000 description 1
- 235000019366 oxytetracycline Nutrition 0.000 description 1
- 229940073067 panoxyl Drugs 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 230000005298 paramagnetic effect Effects 0.000 description 1
- 230000000149 penetrating effect Effects 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- IEQIEDJGQAUEQZ-UHFFFAOYSA-N phthalocyanine Chemical class N1C(N=C2C3=CC=CC=C3C(N=C3C4=CC=CC=C4C(=N4)N3)=N2)=C(C=CC=C2)C2=C1N=C1C2=CC=CC=C2C4=N1 IEQIEDJGQAUEQZ-UHFFFAOYSA-N 0.000 description 1
- 230000019612 pigmentation Effects 0.000 description 1
- 150000004033 porphyrin derivatives Chemical class 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000003449 preventive effect Effects 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 239000000651 prodrug Substances 0.000 description 1
- 229940002612 prodrug Drugs 0.000 description 1
- 239000003380 propellant Substances 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 229940002683 retin-a Drugs 0.000 description 1
- 150000004492 retinoid derivatives Chemical class 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 229930195734 saturated hydrocarbon Natural products 0.000 description 1
- 230000037390 scarring Effects 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 208000017520 skin disease Diseases 0.000 description 1
- 239000000344 soap Substances 0.000 description 1
- FHHPUSMSKHSNKW-SMOYURAASA-M sodium deoxycholate Chemical compound [Na+].C([C@H]1CC2)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC([O-])=O)C)[C@@]2(C)[C@@H](O)C1 FHHPUSMSKHSNKW-SMOYURAASA-M 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 239000011550 stock solution Substances 0.000 description 1
- 125000003107 substituted aryl group Chemical group 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 229960002673 sulfacetamide Drugs 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 238000007910 systemic administration Methods 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 229960000565 tazarotene Drugs 0.000 description 1
- LYPFDBRUNKHDGX-UHFFFAOYSA-N temoporfin Chemical compound OC1=CC=CC(C=2C3=CC=C(N3)C(C=3C=C(O)C=CC=3)=C3C=CC(=N3)C(C=3C=C(O)C=CC=3)=C3C=CC(N3)=C(C=3C=C(O)C=CC=3)C=3CCC=2N=3)=C1 LYPFDBRUNKHDGX-UHFFFAOYSA-N 0.000 description 1
- 230000002277 temperature effect Effects 0.000 description 1
- IWVCMVBTMGNXQD-UHFFFAOYSA-N terramycin dehydrate Natural products C1=CC=C2C(O)(C)C3C(O)C4C(N(C)C)C(O)=C(C(N)=O)C(=O)C4(O)C(O)=C3C(=O)C2=C1O IWVCMVBTMGNXQD-UHFFFAOYSA-N 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 231100000820 toxicity test Toxicity 0.000 description 1
- 231100000027 toxicology Toxicity 0.000 description 1
- 238000011277 treatment modality Methods 0.000 description 1
- IEDVJHCEMCRBQM-UHFFFAOYSA-N trimethoprim Chemical compound COC1=C(OC)C(OC)=CC(CC=2C(=NC(N)=NC=2)N)=C1 IEDVJHCEMCRBQM-UHFFFAOYSA-N 0.000 description 1
- 229960001082 trimethoprim Drugs 0.000 description 1
- 125000004417 unsaturated alkyl group Chemical group 0.000 description 1
- 229930195735 unsaturated hydrocarbon Natural products 0.000 description 1
- 231100000925 very toxic Toxicity 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
- 150000002266 vitamin A derivatives Chemical class 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
- A61K31/195—Carboxylic acids, e.g. valproic acid having an amino group
- A61K31/197—Carboxylic acids, e.g. valproic acid having an amino group the amino and the carboxyl groups being attached to the same acyclic carbon chain, e.g. gamma-aminobutyric acid [GABA], beta-alanine, epsilon-aminocaproic acid or pantothenic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N5/00—Radiation therapy
- A61N5/06—Radiation therapy using light
- A61N5/0613—Apparatus adapted for a specific treatment
- A61N5/0616—Skin treatment other than tanning
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K41/00—Medicinal preparations obtained by treating materials with wave energy or particle radiation ; Therapies using these preparations
- A61K41/0057—Photodynamic therapy with a photosensitizer, i.e. agent able to produce reactive oxygen species upon exposure to light or radiation, e.g. UV or visible light; photocleavage of nucleic acids with an agent
- A61K41/0061—5-aminolevulinic acid-based PDT: 5-ALA-PDT involving porphyrins or precursors of protoporphyrins generated in vivo from 5-ALA
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/10—Anti-acne agents
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Biomedical Technology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Dermatology (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Organic Chemistry (AREA)
- Biophysics (AREA)
- Pathology (AREA)
- Radiology & Medical Imaging (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Semiconductor Lasers (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB0424833.2 | 2004-11-10 | ||
| GB0424833A GB0424833D0 (en) | 2004-11-10 | 2004-11-10 | Method |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2007115601A RU2007115601A (ru) | 2008-12-20 |
| RU2385718C2 true RU2385718C2 (ru) | 2010-04-10 |
Family
ID=33523497
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2007115601/15A RU2385718C2 (ru) | 2004-11-10 | 2005-11-04 | Лечение угрей с использованием производных 5-аминолевулиновой кислоты |
Country Status (16)
| Country | Link |
|---|---|
| US (2) | US20080188558A1 (enExample) |
| EP (1) | EP1814534B1 (enExample) |
| JP (1) | JP5134370B2 (enExample) |
| CN (1) | CN101056626B (enExample) |
| AT (1) | ATE469674T1 (enExample) |
| AU (1) | AU2005303565B2 (enExample) |
| CA (1) | CA2586195C (enExample) |
| DE (1) | DE602005021677D1 (enExample) |
| DK (1) | DK1814534T3 (enExample) |
| ES (1) | ES2344986T3 (enExample) |
| GB (1) | GB0424833D0 (enExample) |
| PL (1) | PL1814534T3 (enExample) |
| PT (1) | PT1814534E (enExample) |
| RU (1) | RU2385718C2 (enExample) |
| SI (1) | SI1814534T1 (enExample) |
| WO (1) | WO2006051269A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2635563C2 (ru) * | 2012-08-03 | 2017-11-14 | ФотоКьюэр АСА | Соединения |
Families Citing this family (41)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB0525504D0 (en) | 2005-12-14 | 2006-01-25 | Bristol Myers Squibb Co | Antimicrobial composition |
| US20110224598A1 (en) * | 2010-03-12 | 2011-09-15 | Daniel Barolet | Radiant near infrared light emitting diode exposure as skin preparation |
| GB0609809D0 (en) * | 2006-05-17 | 2006-06-28 | Photocure Asa | Product |
| KR100796450B1 (ko) | 2006-06-29 | 2008-01-22 | 전남대학교산학협력단 | 5-아미노레불린산의 불포화 알킬 에스터 및 약학적으로허용되는 그의 염, 이의 제조방법 및 이의 용도 |
| GB0700580D0 (en) * | 2007-01-11 | 2007-02-21 | Photocure Asa | Use |
| WO2009003173A1 (en) | 2007-06-27 | 2008-12-31 | The General Hospital Corporation | Method and apparatus for optical inhibition of photodymanic therapy |
| JP2011506425A (ja) * | 2007-12-14 | 2011-03-03 | フォトデルマ エスアー | 治療的及び美容的方法に有用な新規化合物 |
| GB0823265D0 (en) * | 2008-12-20 | 2009-01-28 | Convatec Technologies Inc | Antimicrobial Composition |
| GB0823472D0 (en) | 2008-12-23 | 2009-01-28 | Photocure Asa | Product |
| GB0900461D0 (en) | 2009-01-12 | 2009-02-11 | Photocure Asa | Photodynamic therapy device |
| CN102458388B (zh) | 2009-06-11 | 2016-09-28 | 光治疗Asa公司 | 半固体组合物及药物制品 |
| JP2013521251A (ja) | 2010-03-01 | 2013-06-10 | フォトキュア エイエスエイ | 美容組成物 |
| EP2585111A1 (en) | 2010-06-23 | 2013-05-01 | Photocure ASA | Hyperosmotic preparations comprising 5 -amino levulinic acid or derivative as photosensitizing agent |
| BR112013000613A2 (pt) | 2010-07-09 | 2016-06-28 | Photocure Asa | composições secas e dispositivos que contêm tais composições secas |
| US9572880B2 (en) | 2010-08-27 | 2017-02-21 | Sienna Biopharmaceuticals, Inc. | Ultrasound delivery of nanoparticles |
| PL3222266T3 (pl) | 2010-08-27 | 2018-10-31 | Sienna Biopharmaceuticals, Inc. | Kompozycje i sposoby do termomodulacji celowanej |
| GB201020236D0 (en) | 2010-11-30 | 2011-01-12 | Convatec Technologies Inc | A composition for detecting biofilms on viable tissues |
| CN104152530B (zh) | 2010-12-24 | 2017-09-19 | 爱科来株式会社 | 癌细胞的检测方法 |
| KR101308652B1 (ko) * | 2011-05-19 | 2013-09-13 | 제너럴바이오(주) | Mal을 이용한 여드름 치료 조성물 |
| EP2779956A1 (en) | 2011-10-14 | 2014-09-24 | Photocure ASA | Stent |
| US20140276107A1 (en) | 2011-10-14 | 2014-09-18 | Photocure Asa | Photodynamic diagnosis |
| WO2013092505A1 (en) | 2011-12-19 | 2013-06-27 | Photocure Asa | Irradiation apparatus |
| WO2013092936A2 (en) * | 2011-12-20 | 2013-06-27 | Galderma Research & Development | Use of 5-aminolevulinic acid and esters, in combination with a vitamin d derivative or analog in photochemotherapy, and their uses in treating acne |
| CA2881439C (en) * | 2012-08-10 | 2017-08-29 | Dusa Pharmaceuticals, Inc. | Method for the treatment of acne |
| US20140067024A1 (en) | 2012-08-30 | 2014-03-06 | Photocure Asa | Dual panel photodynamic therapy lamp |
| EP2906286B1 (en) | 2012-10-11 | 2017-06-14 | Nanocomposix, Inc. | Silver nanoplate compositions and methods |
| JP2016507663A (ja) | 2012-12-20 | 2016-03-10 | コンバテック・テクノロジーズ・インコーポレイテッドConvatec Technologies Inc | 化学修飾セルロース系繊維の加工 |
| KR101623553B1 (ko) * | 2013-07-23 | 2016-05-23 | 동성제약주식회사 | 여드름 치료, 예방 또는 개선에 유효한 클로린 e6 |
| BR112016014073A2 (pt) | 2013-12-20 | 2017-08-08 | Galderma Res & Dev | Tratamento fotodinâmico de pulso de pele fotodanificada |
| CA2935865A1 (en) | 2014-01-10 | 2015-07-16 | Sebacia, Inc. | Treatment intervals for use of compositions comprising energy absorbing materials for dermatological applications |
| US9956426B2 (en) | 2015-02-26 | 2018-05-01 | Amol Punjabi | Upconverting nanoparticles |
| US20160287615A1 (en) * | 2015-04-03 | 2016-10-06 | BioPharmX, Inc. | Novel encapsulation of fluorescent, photo-sensitive, or oxygen-sensitive active ingredient for topical application |
| US10603508B2 (en) | 2015-10-15 | 2020-03-31 | Dusa Pharmaceuticals, Inc. | Adjustable illuminators and methods for photodynamic therapy and diagnosis |
| EP3851161A1 (en) | 2015-10-15 | 2021-07-21 | DUSA Pharmaceuticals, Inc. | Adjustable illuminator for photodynamic therapy and diagnosis |
| CN106801037B (zh) * | 2017-01-06 | 2021-01-19 | 中国人民解放军南京军区福州总医院 | 体外痤疮炎症模型的建立方法及其应用 |
| US10632324B2 (en) | 2017-04-27 | 2020-04-28 | 9127-4910 Quebec Inc. | Method for the treatment of skin tissues |
| AU2018302103B2 (en) * | 2017-07-17 | 2024-03-07 | Sun Pharmaceutical Industries, Inc. | Photodynamic therapy method for skin disorders |
| US10357567B1 (en) | 2018-01-12 | 2019-07-23 | Dusa Pharmaceuticals, Inc. | Methods for photodynamic therapy |
| KR102171200B1 (ko) * | 2018-11-20 | 2020-10-28 | 제너럴바이오(주) | 5-아미노레불린산 수화염화물과 그 유도체를 유효성분으로 함유하는 여드름성 피부염 개선 또는 그의 조성물 |
| KR102265592B1 (ko) * | 2020-10-29 | 2021-06-17 | 김진왕 | 5-아미노레불린산 수화염화물을 포함하는 조성물 |
| KR20250021825A (ko) | 2023-08-07 | 2025-02-14 | 주식회사 아스트로젠 | 5-아미노레불린산 유도체 및 이를 포함하는 약학 조성물 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1996028412A1 (en) * | 1995-03-10 | 1996-09-19 | Photocure As | Esters of 5-aminolevulinic acid as photosensitizing agents in photochemotherapy |
| RU2128506C1 (ru) * | 1993-12-30 | 1999-04-10 | Л'Ореаль | Композиция против угрей с одновременным лечением поверхностных и глубоких слоев кожи и ее применение |
| WO2002013788A1 (en) * | 2000-08-16 | 2002-02-21 | The General Hospital Corporation D/B/A Massachusetts General Hospital | Topical aminolevulinic acid-photodynamic therapy for acne vulgaris |
Family Cites Families (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5234940A (en) | 1989-07-28 | 1993-08-10 | Queen's University | Photochemotherapeutic method using 5-aminolevulinic acid and precursors thereof |
| US6710066B2 (en) * | 1989-07-28 | 2004-03-23 | Queen's University At Kingston | Photochemotherapeutic method using 5-aminolevulinic acid and other precursors of endogenous porphyrins |
| US5079262A (en) * | 1989-07-28 | 1992-01-07 | Queen's University At Kingston | Method of detection and treatment of malignant and non-malignant lesions utilizing 5-aminolevulinic acid |
| US5955490A (en) * | 1989-07-28 | 1999-09-21 | Queen's University At Kingston | Photochemotherapeutic method using 5-aminolevulinic acid and other precursors of endogenous porphyrins |
| US5422093A (en) * | 1989-07-28 | 1995-06-06 | Queen's University | Photochemotherapeutic method using 5-aminolevulinic acid and precursors thereof |
| US5713845A (en) * | 1991-10-29 | 1998-02-03 | Thermolase Corporation | Laser assisted drug delivery |
| EP0660712B1 (en) | 1992-09-21 | 2001-05-30 | Quadra Logic Technologies Inc. | Transcutaneous in vivo activation of photosensitive agents in blood |
| GB9700396D0 (en) * | 1997-01-10 | 1997-02-26 | Photocure As | Photochemotherapeutic compositions |
| JPH1112197A (ja) | 1997-06-18 | 1999-01-19 | Cosmo Sogo Kenkyusho:Kk | 悪性腫瘍診断剤及び治療剤 |
| US6183773B1 (en) * | 1999-01-04 | 2001-02-06 | The General Hospital Corporation | Targeting of sebaceous follicles as a treatment of sebaceous gland disorders |
| DE10003620A1 (de) * | 2000-01-28 | 2001-08-02 | Asat Ag Applied Science & Tech | 5-Aminolävulinsäure-Formulierung in nichtwässrigen Lösungsmitteln |
| GB0018528D0 (en) * | 2000-07-27 | 2000-09-13 | Photocure Asa | Compounds |
| GB0118251D0 (en) * | 2001-07-26 | 2001-09-19 | Photocure Asa | Method |
| WO2003039597A1 (en) * | 2001-11-09 | 2003-05-15 | Qlt Inc. | Compositions comprising a photosensitizer and a skin-penetration enhancer and their use in photodynamic treatment |
| US20040048842A1 (en) * | 2002-09-10 | 2004-03-11 | Mcmillan Kathleen | Method of treating skin disorders |
| US20050209331A1 (en) * | 2004-03-22 | 2005-09-22 | Syneron Medical Ltd. | Method of treatment of skin |
| US20050209330A1 (en) * | 2004-03-22 | 2005-09-22 | Syneron Medical Ltd. | Method of treatment of skin |
| GB0406917D0 (en) | 2004-03-26 | 2004-04-28 | Photocure Asa | Compounds |
-
2004
- 2004-11-10 GB GB0424833A patent/GB0424833D0/en not_active Ceased
-
2005
- 2005-11-04 SI SI200531079T patent/SI1814534T1/sl unknown
- 2005-11-04 DK DK05800149.6T patent/DK1814534T3/da active
- 2005-11-04 EP EP05800149A patent/EP1814534B1/en not_active Expired - Lifetime
- 2005-11-04 CA CA2586195A patent/CA2586195C/en not_active Expired - Fee Related
- 2005-11-04 JP JP2007540703A patent/JP5134370B2/ja not_active Expired - Fee Related
- 2005-11-04 RU RU2007115601/15A patent/RU2385718C2/ru not_active IP Right Cessation
- 2005-11-04 AT AT05800149T patent/ATE469674T1/de active
- 2005-11-04 ES ES05800149T patent/ES2344986T3/es not_active Expired - Lifetime
- 2005-11-04 WO PCT/GB2005/004253 patent/WO2006051269A1/en not_active Ceased
- 2005-11-04 CN CN2005800384462A patent/CN101056626B/zh not_active Expired - Fee Related
- 2005-11-04 PL PL05800149T patent/PL1814534T3/pl unknown
- 2005-11-04 AU AU2005303565A patent/AU2005303565B2/en not_active Ceased
- 2005-11-04 PT PT05800149T patent/PT1814534E/pt unknown
- 2005-11-04 US US11/667,504 patent/US20080188558A1/en not_active Abandoned
- 2005-11-04 DE DE602005021677T patent/DE602005021677D1/de not_active Expired - Lifetime
-
2012
- 2012-11-09 US US13/673,704 patent/US8546447B2/en not_active Expired - Fee Related
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2128506C1 (ru) * | 1993-12-30 | 1999-04-10 | Л'Ореаль | Композиция против угрей с одновременным лечением поверхностных и глубоких слоев кожи и ее применение |
| WO1996028412A1 (en) * | 1995-03-10 | 1996-09-19 | Photocure As | Esters of 5-aminolevulinic acid as photosensitizing agents in photochemotherapy |
| WO2002013788A1 (en) * | 2000-08-16 | 2002-02-21 | The General Hospital Corporation D/B/A Massachusetts General Hospital | Topical aminolevulinic acid-photodynamic therapy for acne vulgaris |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2635563C2 (ru) * | 2012-08-03 | 2017-11-14 | ФотоКьюэр АСА | Соединения |
Also Published As
| Publication number | Publication date |
|---|---|
| JP5134370B2 (ja) | 2013-01-30 |
| US20130066256A1 (en) | 2013-03-14 |
| CN101056626A (zh) | 2007-10-17 |
| US20080188558A1 (en) | 2008-08-07 |
| RU2007115601A (ru) | 2008-12-20 |
| ATE469674T1 (de) | 2010-06-15 |
| CA2586195C (en) | 2013-12-31 |
| EP1814534A1 (en) | 2007-08-08 |
| AU2005303565A1 (en) | 2006-05-18 |
| ES2344986T3 (es) | 2010-09-13 |
| JP2008519812A (ja) | 2008-06-12 |
| DE602005021677D1 (de) | 2010-07-15 |
| PL1814534T3 (pl) | 2010-11-30 |
| EP1814534B1 (en) | 2010-06-02 |
| SI1814534T1 (sl) | 2010-09-30 |
| PT1814534E (pt) | 2010-06-15 |
| CN101056626B (zh) | 2011-12-14 |
| AU2005303565B2 (en) | 2011-04-14 |
| GB0424833D0 (en) | 2004-12-15 |
| WO2006051269A1 (en) | 2006-05-18 |
| US8546447B2 (en) | 2013-10-01 |
| CA2586195A1 (en) | 2006-05-18 |
| DK1814534T3 (da) | 2010-09-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2385718C2 (ru) | Лечение угрей с использованием производных 5-аминолевулиновой кислоты | |
| US8759396B2 (en) | Use of aminolevulinic acid and derivatives thereof | |
| Sakamoto et al. | Photodynamic therapy for acne vulgaris: a critical review from basics to clinical practice: part II. Understanding parameters for acne treatment with photodynamic therapy | |
| EP3082788B1 (en) | Pulse photodynamic treatment of acne | |
| Monfrecola et al. | A critical reappraisal of off-label use of photodynamic therapy for the treatment of non-neoplastic skin conditions | |
| Piergiacomo et al. | A critical reappraisal of off-label indications for topical photodynamic therapy with aminolevulinic acid and methylaminolevulinate | |
| WO2013092936A2 (en) | Use of 5-aminolevulinic acid and esters, in combination with a vitamin d derivative or analog in photochemotherapy, and their uses in treating acne | |
| Curnow et al. | Enhancing protoporphyrin IX-induced photodynamic therapy with a topical iron chelating agent in a normal skin model | |
| Mikolajewska | Asta Juzeniene |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20181105 |