RU2337108C2 - Полипептиды для индукции защитного иммунного ответа против staphylococcus aureus - Google Patents
Полипептиды для индукции защитного иммунного ответа против staphylococcus aureus Download PDFInfo
- Publication number
- RU2337108C2 RU2337108C2 RU2006105498/13A RU2006105498A RU2337108C2 RU 2337108 C2 RU2337108 C2 RU 2337108C2 RU 2006105498/13 A RU2006105498/13 A RU 2006105498/13A RU 2006105498 A RU2006105498 A RU 2006105498A RU 2337108 C2 RU2337108 C2 RU 2337108C2
- Authority
- RU
- Russia
- Prior art keywords
- seq
- polypeptide
- paragraph
- pct
- amino acid
- Prior art date
Links
- 108090000765 processed proteins & peptides Proteins 0.000 title claims abstract description 240
- 229920001184 polypeptide Polymers 0.000 title claims abstract description 235
- 102000004196 processed proteins & peptides Human genes 0.000 title claims abstract description 235
- 230000001681 protective effect Effects 0.000 title claims abstract description 60
- 230000028993 immune response Effects 0.000 title claims abstract description 32
- 241000191967 Staphylococcus aureus Species 0.000 title claims abstract description 13
- 230000006698 induction Effects 0.000 title claims description 14
- 230000014509 gene expression Effects 0.000 claims abstract description 100
- 239000000203 mixture Substances 0.000 claims abstract description 11
- 125000003275 alpha amino acid group Chemical group 0.000 claims abstract 14
- 108090000623 proteins and genes Proteins 0.000 claims description 170
- 102000004169 proteins and genes Human genes 0.000 claims description 126
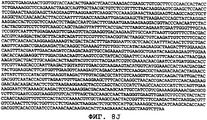
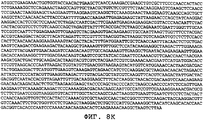
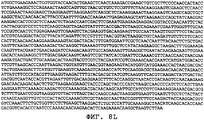
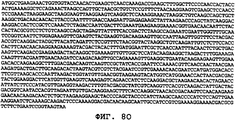
- 150000001413 amino acids Chemical class 0.000 claims description 103
- 108020004705 Codon Proteins 0.000 claims description 102
- 240000004808 Saccharomyces cerevisiae Species 0.000 claims description 102
- 235000014680 Saccharomyces cerevisiae Nutrition 0.000 claims description 98
- 210000004027 cell Anatomy 0.000 claims description 70
- 150000007523 nucleic acids Chemical class 0.000 claims description 58
- 238000000034 method Methods 0.000 claims description 55
- 239000012634 fragment Substances 0.000 claims description 44
- 230000036039 immunity Effects 0.000 claims description 44
- 208000015181 infectious disease Diseases 0.000 claims description 43
- 108020004707 nucleic acids Proteins 0.000 claims description 40
- 102000039446 nucleic acids Human genes 0.000 claims description 40
- 238000004519 manufacturing process Methods 0.000 claims description 29
- 108010076504 Protein Sorting Signals Proteins 0.000 claims description 27
- 230000002163 immunogen Effects 0.000 claims description 27
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims description 24
- 210000002421 cell wall Anatomy 0.000 claims description 24
- 108091028043 Nucleic acid sequence Proteins 0.000 claims description 21
- 238000006243 chemical reaction Methods 0.000 claims description 20
- 210000005253 yeast cell Anatomy 0.000 claims description 15
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 claims description 14
- 239000002671 adjuvant Substances 0.000 claims description 14
- 230000001939 inductive effect Effects 0.000 claims description 14
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 claims description 14
- 239000002773 nucleotide Substances 0.000 claims description 12
- 125000003729 nucleotide group Chemical group 0.000 claims description 12
- 238000000746 purification Methods 0.000 claims description 12
- 239000013604 expression vector Substances 0.000 claims description 11
- 210000004899 c-terminal region Anatomy 0.000 claims description 9
- 241000191940 Staphylococcus Species 0.000 claims description 6
- 239000003937 drug carrier Substances 0.000 claims description 4
- 238000012258 culturing Methods 0.000 claims description 3
- 230000002349 favourable effect Effects 0.000 claims description 3
- 230000002265 prevention Effects 0.000 claims description 2
- 230000003449 preventive effect Effects 0.000 claims description 2
- 230000008707 rearrangement Effects 0.000 claims 9
- 230000000694 effects Effects 0.000 abstract description 7
- 239000000126 substance Substances 0.000 abstract description 4
- 239000003814 drug Substances 0.000 abstract description 3
- 235000018102 proteins Nutrition 0.000 description 121
- 229940024606 amino acid Drugs 0.000 description 74
- 235000001014 amino acid Nutrition 0.000 description 74
- 239000002609 medium Substances 0.000 description 65
- 241000588724 Escherichia coli Species 0.000 description 63
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 43
- 239000000499 gel Substances 0.000 description 43
- 239000013592 cell lysate Substances 0.000 description 38
- 239000013598 vector Substances 0.000 description 35
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 33
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 32
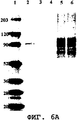
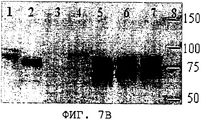
- 238000001262 western blot Methods 0.000 description 32
- 108020004414 DNA Proteins 0.000 description 26
- 239000013612 plasmid Substances 0.000 description 25
- 229960004452 methionine Drugs 0.000 description 21
- 241000699670 Mus sp. Species 0.000 description 20
- 239000000047 product Substances 0.000 description 20
- 229930182817 methionine Natural products 0.000 description 19
- 206010041925 Staphylococcal infections Diseases 0.000 description 18
- 238000000855 fermentation Methods 0.000 description 18
- 230000004151 fermentation Effects 0.000 description 18
- 208000015688 methicillin-resistant staphylococcus aureus infectious disease Diseases 0.000 description 18
- 238000001962 electrophoresis Methods 0.000 description 17
- 239000006166 lysate Substances 0.000 description 16
- 230000004048 modification Effects 0.000 description 16
- 238000012986 modification Methods 0.000 description 16
- 229930027917 kanamycin Natural products 0.000 description 15
- 229960000318 kanamycin Drugs 0.000 description 15
- SBUJHOSQTJFQJX-NOAMYHISSA-N kanamycin Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N SBUJHOSQTJFQJX-NOAMYHISSA-N 0.000 description 15
- 229930182823 kanamycin A Natural products 0.000 description 15
- 238000010186 staining Methods 0.000 description 15
- 101150009006 HIS3 gene Proteins 0.000 description 14
- 210000004369 blood Anatomy 0.000 description 14
- 239000008280 blood Substances 0.000 description 14
- 230000000692 anti-sense effect Effects 0.000 description 13
- 230000001965 increasing effect Effects 0.000 description 13
- 229960005486 vaccine Drugs 0.000 description 13
- 230000003834 intracellular effect Effects 0.000 description 12
- 238000011282 treatment Methods 0.000 description 12
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 11
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 11
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 11
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 11
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 11
- 229940047712 aluminum hydroxyphosphate Drugs 0.000 description 11
- 238000002474 experimental method Methods 0.000 description 11
- 239000008103 glucose Substances 0.000 description 11
- 235000011187 glycerol Nutrition 0.000 description 11
- 239000002054 inoculum Substances 0.000 description 11
- 125000001360 methionine group Chemical group N[C@@H](CCSC)C(=O)* 0.000 description 11
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 11
- 239000000600 sorbitol Substances 0.000 description 11
- 229920001817 Agar Polymers 0.000 description 10
- 102000004190 Enzymes Human genes 0.000 description 10
- 108090000790 Enzymes Proteins 0.000 description 10
- ISAKRJDGNUQOIC-UHFFFAOYSA-N Uracil Chemical compound O=C1C=CNC(=O)N1 ISAKRJDGNUQOIC-UHFFFAOYSA-N 0.000 description 10
- 239000008272 agar Substances 0.000 description 10
- 238000010171 animal model Methods 0.000 description 10
- 229940088598 enzyme Drugs 0.000 description 10
- 239000008188 pellet Substances 0.000 description 10
- 239000000523 sample Substances 0.000 description 10
- 239000006228 supernatant Substances 0.000 description 10
- 108091026890 Coding region Proteins 0.000 description 9
- 239000003153 chemical reaction reagent Substances 0.000 description 9
- 239000002158 endotoxin Substances 0.000 description 9
- 238000005457 optimization Methods 0.000 description 9
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 8
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 8
- 101150075484 MNN9 gene Proteins 0.000 description 8
- 241001465754 Metazoa Species 0.000 description 8
- 101100394989 Rhodopseudomonas palustris (strain ATCC BAA-98 / CGA009) hisI gene Proteins 0.000 description 8
- 238000004458 analytical method Methods 0.000 description 8
- 229930182830 galactose Natural products 0.000 description 8
- 230000003053 immunization Effects 0.000 description 8
- 238000002649 immunization Methods 0.000 description 8
- 239000012452 mother liquor Substances 0.000 description 8
- 230000001105 regulatory effect Effects 0.000 description 8
- 230000004044 response Effects 0.000 description 8
- 230000003248 secreting effect Effects 0.000 description 8
- AXAVXPMQTGXXJZ-UHFFFAOYSA-N 2-aminoacetic acid;2-amino-2-(hydroxymethyl)propane-1,3-diol Chemical compound NCC(O)=O.OCC(N)(CO)CO AXAVXPMQTGXXJZ-UHFFFAOYSA-N 0.000 description 7
- 241000283707 Capra Species 0.000 description 7
- 239000004471 Glycine Substances 0.000 description 7
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 7
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 7
- 238000007792 addition Methods 0.000 description 7
- 239000000872 buffer Substances 0.000 description 7
- 238000004925 denaturation Methods 0.000 description 7
- 230000036425 denaturation Effects 0.000 description 7
- 229960002885 histidine Drugs 0.000 description 7
- 239000012528 membrane Substances 0.000 description 7
- 239000007787 solid Substances 0.000 description 7
- 239000000243 solution Substances 0.000 description 7
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 6
- 101150006914 TRP1 gene Proteins 0.000 description 6
- 206010052428 Wound Diseases 0.000 description 6
- 208000027418 Wounds and injury Diseases 0.000 description 6
- 238000010367 cloning Methods 0.000 description 6
- 230000034994 death Effects 0.000 description 6
- 201000010099 disease Diseases 0.000 description 6
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 6
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 6
- NHBKXEKEPDILRR-UHFFFAOYSA-N 2,3-bis(butanoylsulfanyl)propyl butanoate Chemical compound CCCC(=O)OCC(SC(=O)CCC)CSC(=O)CCC NHBKXEKEPDILRR-UHFFFAOYSA-N 0.000 description 5
- 108010014303 DNA-directed DNA polymerase Proteins 0.000 description 5
- 102000016928 DNA-directed DNA polymerase Human genes 0.000 description 5
- 102100028501 Galanin peptides Human genes 0.000 description 5
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 5
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 5
- 239000006137 Luria-Bertani broth Substances 0.000 description 5
- 101150101314 PRB1 gene Proteins 0.000 description 5
- 241000295644 Staphylococcaceae Species 0.000 description 5
- 230000002068 genetic effect Effects 0.000 description 5
- 239000001963 growth medium Substances 0.000 description 5
- 230000000977 initiatory effect Effects 0.000 description 5
- 230000035772 mutation Effects 0.000 description 5
- 238000002360 preparation method Methods 0.000 description 5
- 238000012216 screening Methods 0.000 description 5
- 239000011780 sodium chloride Substances 0.000 description 5
- 239000000725 suspension Substances 0.000 description 5
- 238000012360 testing method Methods 0.000 description 5
- 230000009466 transformation Effects 0.000 description 5
- 229940035893 uracil Drugs 0.000 description 5
- 238000002255 vaccination Methods 0.000 description 5
- 239000004475 Arginine Substances 0.000 description 4
- 241000894006 Bacteria Species 0.000 description 4
- 208000035473 Communicable disease Diseases 0.000 description 4
- 102100039556 Galectin-4 Human genes 0.000 description 4
- 101000608765 Homo sapiens Galectin-4 Proteins 0.000 description 4
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 4
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 4
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 4
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 4
- 239000004472 Lysine Substances 0.000 description 4
- 241000282560 Macaca mulatta Species 0.000 description 4
- 239000002033 PVDF binder Substances 0.000 description 4
- 108091005804 Peptidases Proteins 0.000 description 4
- 239000004365 Protease Substances 0.000 description 4
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 description 4
- 238000002105 Southern blotting Methods 0.000 description 4
- 108091023040 Transcription factor Proteins 0.000 description 4
- 102000040945 Transcription factor Human genes 0.000 description 4
- BFNBIHQBYMNNAN-UHFFFAOYSA-N ammonium sulfate Chemical compound N.N.OS(O)(=O)=O BFNBIHQBYMNNAN-UHFFFAOYSA-N 0.000 description 4
- 229910052921 ammonium sulfate Inorganic materials 0.000 description 4
- 235000011130 ammonium sulphate Nutrition 0.000 description 4
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 4
- 235000009697 arginine Nutrition 0.000 description 4
- 238000005119 centrifugation Methods 0.000 description 4
- 238000010276 construction Methods 0.000 description 4
- 238000011156 evaluation Methods 0.000 description 4
- 238000001502 gel electrophoresis Methods 0.000 description 4
- 150000004676 glycans Chemical class 0.000 description 4
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 239000012139 lysis buffer Substances 0.000 description 4
- 244000005700 microbiome Species 0.000 description 4
- 230000003287 optical effect Effects 0.000 description 4
- 230000037361 pathway Effects 0.000 description 4
- 239000002953 phosphate buffered saline Substances 0.000 description 4
- 229920001282 polysaccharide Polymers 0.000 description 4
- 239000005017 polysaccharide Substances 0.000 description 4
- 229920000136 polysorbate Polymers 0.000 description 4
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 4
- 235000019419 proteases Nutrition 0.000 description 4
- 238000000751 protein extraction Methods 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- 238000010561 standard procedure Methods 0.000 description 4
- KDYFGRWQOYBRFD-UHFFFAOYSA-N succinic acid Chemical compound OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 4
- 238000004448 titration Methods 0.000 description 4
- 238000001712 DNA sequencing Methods 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- 108010001496 Galectin 2 Proteins 0.000 description 3
- 102100021735 Galectin-2 Human genes 0.000 description 3
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 3
- ODKSFYDXXFIFQN-BYPYZUCNSA-N L-arginine Chemical compound OC(=O)[C@@H](N)CCCN=C(N)N ODKSFYDXXFIFQN-BYPYZUCNSA-N 0.000 description 3
- FSBIGDSBMBYOPN-VKHMYHEASA-N L-canavanine Chemical compound OC(=O)[C@@H](N)CCONC(N)=N FSBIGDSBMBYOPN-VKHMYHEASA-N 0.000 description 3
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 3
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 3
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 3
- 239000007993 MOPS buffer Substances 0.000 description 3
- FSBIGDSBMBYOPN-UHFFFAOYSA-N O-guanidino-DL-homoserine Natural products OC(=O)C(N)CCON=C(N)N FSBIGDSBMBYOPN-UHFFFAOYSA-N 0.000 description 3
- 229930006000 Sucrose Natural products 0.000 description 3
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 3
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 3
- 239000004473 Threonine Substances 0.000 description 3
- 101150050575 URA3 gene Proteins 0.000 description 3
- 235000004279 alanine Nutrition 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 229910052799 carbon Inorganic materials 0.000 description 3
- 230000015556 catabolic process Effects 0.000 description 3
- 238000004140 cleaning Methods 0.000 description 3
- 238000006731 degradation reaction Methods 0.000 description 3
- 238000001514 detection method Methods 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- 239000000284 extract Substances 0.000 description 3
- 238000001914 filtration Methods 0.000 description 3
- 230000001900 immune effect Effects 0.000 description 3
- 230000005847 immunogenicity Effects 0.000 description 3
- 238000011534 incubation Methods 0.000 description 3
- 101150057984 isdB gene Proteins 0.000 description 3
- 229960000310 isoleucine Drugs 0.000 description 3
- 229960003085 meticillin Drugs 0.000 description 3
- 230000002018 overexpression Effects 0.000 description 3
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 3
- 229960005190 phenylalanine Drugs 0.000 description 3
- 230000004481 post-translational protein modification Effects 0.000 description 3
- 238000012545 processing Methods 0.000 description 3
- 238000003259 recombinant expression Methods 0.000 description 3
- 238000011084 recovery Methods 0.000 description 3
- 230000002441 reversible effect Effects 0.000 description 3
- 230000000638 stimulation Effects 0.000 description 3
- 238000006467 substitution reaction Methods 0.000 description 3
- 239000005720 sucrose Substances 0.000 description 3
- 230000004083 survival effect Effects 0.000 description 3
- 239000006150 trypticase soy agar Substances 0.000 description 3
- 229960004799 tryptophan Drugs 0.000 description 3
- 229960004441 tyrosine Drugs 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- WQZGKKKJIJFFOK-SVZMEOIVSA-N (+)-Galactose Chemical compound OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-SVZMEOIVSA-N 0.000 description 2
- DGZSVBBLLGZHSF-UHFFFAOYSA-N 4,4-diethylpiperidine Chemical compound CCC1(CC)CCNCC1 DGZSVBBLLGZHSF-UHFFFAOYSA-N 0.000 description 2
- QFVHZQCOUORWEI-UHFFFAOYSA-N 4-[(4-anilino-5-sulfonaphthalen-1-yl)diazenyl]-5-hydroxynaphthalene-2,7-disulfonic acid Chemical compound C=12C(O)=CC(S(O)(=O)=O)=CC2=CC(S(O)(=O)=O)=CC=1N=NC(C1=CC=CC(=C11)S(O)(=O)=O)=CC=C1NC1=CC=CC=C1 QFVHZQCOUORWEI-UHFFFAOYSA-N 0.000 description 2
- 101710163881 5,6-dihydroxyindole-2-carboxylic acid oxidase Proteins 0.000 description 2
- 102100030310 5,6-dihydroxyindole-2-carboxylic acid oxidase Human genes 0.000 description 2
- 229930024421 Adenine Natural products 0.000 description 2
- GFFGJBXGBJISGV-UHFFFAOYSA-N Adenine Chemical compound NC1=NC=NC2=C1N=CN2 GFFGJBXGBJISGV-UHFFFAOYSA-N 0.000 description 2
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 2
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 2
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 2
- 208000035143 Bacterial infection Diseases 0.000 description 2
- 102100029516 Basic salivary proline-rich protein 1 Human genes 0.000 description 2
- 101100327917 Caenorhabditis elegans chup-1 gene Proteins 0.000 description 2
- 241000282693 Cercopithecidae Species 0.000 description 2
- 108090000695 Cytokines Proteins 0.000 description 2
- 102000004127 Cytokines Human genes 0.000 description 2
- 238000002965 ELISA Methods 0.000 description 2
- 101150094690 GAL1 gene Proteins 0.000 description 2
- 101150038242 GAL10 gene Proteins 0.000 description 2
- 102100024637 Galectin-10 Human genes 0.000 description 2
- 241000238631 Hexapoda Species 0.000 description 2
- 101001125486 Homo sapiens Basic salivary proline-rich protein 1 Proteins 0.000 description 2
- 206010061598 Immunodeficiency Diseases 0.000 description 2
- 108010002350 Interleukin-2 Proteins 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 2
- 235000019766 L-Lysine Nutrition 0.000 description 2
- FFEARJCKVFRZRR-UHFFFAOYSA-N L-Methionine Natural products CSCCC(N)C(O)=O FFEARJCKVFRZRR-UHFFFAOYSA-N 0.000 description 2
- 229930064664 L-arginine Natural products 0.000 description 2
- 235000014852 L-arginine Nutrition 0.000 description 2
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 2
- 229930182844 L-isoleucine Natural products 0.000 description 2
- 229930195722 L-methionine Natural products 0.000 description 2
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 2
- 108010052285 Membrane Proteins Proteins 0.000 description 2
- 102000018697 Membrane Proteins Human genes 0.000 description 2
- RJQXTJLFIWVMTO-TYNCELHUSA-N Methicillin Chemical compound COC1=CC=CC(OC)=C1C(=O)N[C@@H]1C(=O)N2[C@@H](C(O)=O)C(C)(C)S[C@@H]21 RJQXTJLFIWVMTO-TYNCELHUSA-N 0.000 description 2
- 101100494726 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) pep-4 gene Proteins 0.000 description 2
- 239000000020 Nitrocellulose Substances 0.000 description 2
- 230000004989 O-glycosylation Effects 0.000 description 2
- 241000235648 Pichia Species 0.000 description 2
- 239000012506 Sephacryl® Substances 0.000 description 2
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 2
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 2
- IXKSXJFAGXLQOQ-XISFHERQSA-N WHWLQLKPGQPMY Chemical compound C([C@@H](C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)NC(=O)[C@@H](N)CC=1C2=CC=CC=C2NC=1)C1=CNC=N1 IXKSXJFAGXLQOQ-XISFHERQSA-N 0.000 description 2
- 229960000643 adenine Drugs 0.000 description 2
- 239000011543 agarose gel Substances 0.000 description 2
- 239000000427 antigen Substances 0.000 description 2
- 210000000612 antigen-presenting cell Anatomy 0.000 description 2
- 108091007433 antigens Proteins 0.000 description 2
- 102000036639 antigens Human genes 0.000 description 2
- 235000009582 asparagine Nutrition 0.000 description 2
- 229960001230 asparagine Drugs 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- 208000022362 bacterial infectious disease Diseases 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 229940041514 candida albicans extract Drugs 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- 230000001413 cellular effect Effects 0.000 description 2
- 229920002301 cellulose acetate Polymers 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 239000007857 degradation product Substances 0.000 description 2
- 238000012217 deletion Methods 0.000 description 2
- 230000037430 deletion Effects 0.000 description 2
- 239000008121 dextrose Substances 0.000 description 2
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 2
- 229940042399 direct acting antivirals protease inhibitors Drugs 0.000 description 2
- 239000012153 distilled water Substances 0.000 description 2
- 239000000975 dye Substances 0.000 description 2
- 210000003527 eukaryotic cell Anatomy 0.000 description 2
- 230000005713 exacerbation Effects 0.000 description 2
- 125000000291 glutamic acid group Chemical group N[C@@H](CCC(O)=O)C(=O)* 0.000 description 2
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 2
- RWSXRVCMGQZWBV-WDSKDSINSA-N glutathione Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@@H](CS)C(=O)NCC(O)=O RWSXRVCMGQZWBV-WDSKDSINSA-N 0.000 description 2
- 125000003630 glycyl group Chemical group [H]N([H])C([H])([H])C(*)=O 0.000 description 2
- 239000012510 hollow fiber Substances 0.000 description 2
- 230000008348 humoral response Effects 0.000 description 2
- 230000007062 hydrolysis Effects 0.000 description 2
- 238000006460 hydrolysis reaction Methods 0.000 description 2
- 238000003119 immunoblot Methods 0.000 description 2
- 239000007943 implant Substances 0.000 description 2
- 230000002458 infectious effect Effects 0.000 description 2
- 238000011081 inoculation Methods 0.000 description 2
- 230000010354 integration Effects 0.000 description 2
- 238000007918 intramuscular administration Methods 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- XIXADJRWDQXREU-UHFFFAOYSA-M lithium acetate Chemical compound [Li+].CC([O-])=O XIXADJRWDQXREU-UHFFFAOYSA-M 0.000 description 2
- 230000004807 localization Effects 0.000 description 2
- 235000018977 lysine Nutrition 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 229920001220 nitrocellulos Polymers 0.000 description 2
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 2
- 239000012071 phase Substances 0.000 description 2
- YBYRMVIVWMBXKQ-UHFFFAOYSA-N phenylmethanesulfonyl fluoride Chemical compound FS(=O)(=O)CC1=CC=CC=C1 YBYRMVIVWMBXKQ-UHFFFAOYSA-N 0.000 description 2
- 125000006239 protecting group Chemical group 0.000 description 2
- 238000011218 seed culture Methods 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
- 230000028070 sporulation Effects 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 239000001384 succinic acid Substances 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- 238000013518 transcription Methods 0.000 description 2
- 230000035897 transcription Effects 0.000 description 2
- 238000013519 translation Methods 0.000 description 2
- 125000001493 tyrosinyl group Chemical group [H]OC1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 2
- 239000004474 valine Substances 0.000 description 2
- 230000002792 vascular Effects 0.000 description 2
- 230000003313 weakening effect Effects 0.000 description 2
- 239000012138 yeast extract Substances 0.000 description 2
- DIGQNXIGRZPYDK-WKSCXVIASA-N (2R)-6-amino-2-[[2-[[(2S)-2-[[2-[[(2R)-2-[[(2S)-2-[[(2R,3S)-2-[[2-[[(2S)-2-[[2-[[(2S)-2-[[(2S)-2-[[(2R)-2-[[(2S,3S)-2-[[(2R)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[2-[[(2S)-2-[[(2R)-2-[[2-[[2-[[2-[(2-amino-1-hydroxyethylidene)amino]-3-carboxy-1-hydroxypropylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1-hydroxyethylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1,3-dihydroxypropylidene]amino]-1-hydroxyethylidene]amino]-1-hydroxypropylidene]amino]-1,3-dihydroxypropylidene]amino]-1,3-dihydroxypropylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1,3-dihydroxybutylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1-hydroxypropylidene]amino]-1,3-dihydroxypropylidene]amino]-1-hydroxyethylidene]amino]-1,5-dihydroxy-5-iminopentylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1,3-dihydroxybutylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1,3-dihydroxypropylidene]amino]-1-hydroxyethylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1-hydroxyethylidene]amino]hexanoic acid Chemical compound C[C@@H]([C@@H](C(=N[C@@H](CS)C(=N[C@@H](C)C(=N[C@@H](CO)C(=NCC(=N[C@@H](CCC(=N)O)C(=NC(CS)C(=N[C@H]([C@H](C)O)C(=N[C@H](CS)C(=N[C@H](CO)C(=NCC(=N[C@H](CS)C(=NCC(=N[C@H](CCCCN)C(=O)O)O)O)O)O)O)O)O)O)O)O)O)O)O)N=C([C@H](CS)N=C([C@H](CO)N=C([C@H](CO)N=C([C@H](C)N=C(CN=C([C@H](CO)N=C([C@H](CS)N=C(CN=C(C(CS)N=C(C(CC(=O)O)N=C(CN)O)O)O)O)O)O)O)O)O)O)O)O DIGQNXIGRZPYDK-WKSCXVIASA-N 0.000 description 1
- GZCWLCBFPRFLKL-UHFFFAOYSA-N 1-prop-2-ynoxypropan-2-ol Chemical compound CC(O)COCC#C GZCWLCBFPRFLKL-UHFFFAOYSA-N 0.000 description 1
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N 100676-05-9 Natural products OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1 OWEGMIWEEQEYGQ-UHFFFAOYSA-N 0.000 description 1
- 125000004080 3-carboxypropanoyl group Chemical group O=C([*])C([H])([H])C([H])([H])C(O[H])=O 0.000 description 1
- JLLYLQLDYORLBB-UHFFFAOYSA-N 5-bromo-n-methylthiophene-2-sulfonamide Chemical group CNS(=O)(=O)C1=CC=C(Br)S1 JLLYLQLDYORLBB-UHFFFAOYSA-N 0.000 description 1
- 108010042708 Acetylmuramyl-Alanyl-Isoglutamine Proteins 0.000 description 1
- 102000013563 Acid Phosphatase Human genes 0.000 description 1
- 108010051457 Acid Phosphatase Proteins 0.000 description 1
- 229920000936 Agarose Polymers 0.000 description 1
- 102000007698 Alcohol dehydrogenase Human genes 0.000 description 1
- 108010021809 Alcohol dehydrogenase Proteins 0.000 description 1
- 102100034044 All-trans-retinol dehydrogenase [NAD(+)] ADH1B Human genes 0.000 description 1
- 101710193111 All-trans-retinol dehydrogenase [NAD(+)] ADH4 Proteins 0.000 description 1
- 241000193830 Bacillus <bacterium> Species 0.000 description 1
- 235000014469 Bacillus subtilis Nutrition 0.000 description 1
- 208000031729 Bacteremia Diseases 0.000 description 1
- 102000015081 Blood Coagulation Factors Human genes 0.000 description 1
- 108010039209 Blood Coagulation Factors Proteins 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 206010006563 Bullous impetigo Diseases 0.000 description 1
- 101100275473 Caenorhabditis elegans ctc-3 gene Proteins 0.000 description 1
- 101001007681 Candida albicans (strain WO-1) Kexin Proteins 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 206010007247 Carbuncle Diseases 0.000 description 1
- 208000035484 Cellulite Diseases 0.000 description 1
- 208000014912 Central Nervous System Infections Diseases 0.000 description 1
- 108010049048 Cholera Toxin Proteins 0.000 description 1
- 102000009016 Cholera Toxin Human genes 0.000 description 1
- 108010035532 Collagen Proteins 0.000 description 1
- 102000008186 Collagen Human genes 0.000 description 1
- 238000011537 Coomassie blue staining Methods 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 230000008836 DNA modification Effects 0.000 description 1
- 238000007900 DNA-DNA hybridization Methods 0.000 description 1
- 101100390736 Danio rerio fign gene Proteins 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 241000620209 Escherichia coli DH5[alpha] Species 0.000 description 1
- 241000206602 Eukaryota Species 0.000 description 1
- 108700024394 Exon Proteins 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 206010016936 Folliculitis Diseases 0.000 description 1
- 108010058643 Fungal Proteins Proteins 0.000 description 1
- 230000005526 G1 to G0 transition Effects 0.000 description 1
- 102100039555 Galectin-7 Human genes 0.000 description 1
- 108010024636 Glutathione Proteins 0.000 description 1
- 101100246753 Halobacterium salinarum (strain ATCC 700922 / JCM 11081 / NRC-1) pyrF gene Proteins 0.000 description 1
- 241000125500 Hedypnois rhagadioloides Species 0.000 description 1
- 102100024594 Histone-lysine N-methyltransferase PRDM16 Human genes 0.000 description 1
- 101100121078 Homo sapiens GAL gene Proteins 0.000 description 1
- 101000608772 Homo sapiens Galectin-7 Proteins 0.000 description 1
- 101000686942 Homo sapiens Histone-lysine N-methyltransferase PRDM16 Proteins 0.000 description 1
- 101000687323 Homo sapiens Rabenosyn-5 Proteins 0.000 description 1
- 101000632319 Homo sapiens Septin-7 Proteins 0.000 description 1
- 101000637726 Homo sapiens Toll/interleukin-1 receptor domain-containing adapter protein Proteins 0.000 description 1
- 101150017040 I gene Proteins 0.000 description 1
- -1 IFNγ Proteins 0.000 description 1
- 108060003951 Immunoglobulin Proteins 0.000 description 1
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 1
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 1
- 206010021531 Impetigo Diseases 0.000 description 1
- 241000235649 Kluyveromyces Species 0.000 description 1
- 244000285963 Kluyveromyces fragilis Species 0.000 description 1
- 241001138401 Kluyveromyces lactis Species 0.000 description 1
- 241000235058 Komagataella pastoris Species 0.000 description 1
- 241000186660 Lactobacillus Species 0.000 description 1
- 240000006024 Lactobacillus plantarum Species 0.000 description 1
- 241000194036 Lactococcus Species 0.000 description 1
- 208000018982 Leg injury Diseases 0.000 description 1
- 101100533567 Leptosphaeria maculans sirH gene Proteins 0.000 description 1
- 102000002704 Leucyl aminopeptidase Human genes 0.000 description 1
- 206010061225 Limb injury Diseases 0.000 description 1
- NPBGTPKLVJEOBE-IUCAKERBSA-N Lys-Arg Chemical group NCCCC[C@H](N)C(=O)N[C@H](C(O)=O)CCCNC(N)=N NPBGTPKLVJEOBE-IUCAKERBSA-N 0.000 description 1
- 108090000988 Lysostaphin Proteins 0.000 description 1
- GUBGYTABKSRVRQ-PICCSMPSSA-N Maltose Natural products O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-PICCSMPSSA-N 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 102000003792 Metallothionein Human genes 0.000 description 1
- 108090000157 Metallothionein Proteins 0.000 description 1
- 102000016943 Muramidase Human genes 0.000 description 1
- 108010014251 Muramidase Proteins 0.000 description 1
- MSFSPUZXLOGKHJ-UHFFFAOYSA-N Muraminsaeure Natural products OC(=O)C(C)OC1C(N)C(O)OC(CO)C1O MSFSPUZXLOGKHJ-UHFFFAOYSA-N 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- 101100390738 Mus musculus Fign gene Proteins 0.000 description 1
- 108010062010 N-Acetylmuramoyl-L-alanine Amidase Proteins 0.000 description 1
- 230000004988 N-glycosylation Effects 0.000 description 1
- 125000001429 N-terminal alpha-amino-acid group Chemical group 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 206010031252 Osteomyelitis Diseases 0.000 description 1
- 206010049752 Peau d'orange Diseases 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 108010013639 Peptidoglycan Proteins 0.000 description 1
- 108010081690 Pertussis Toxin Proteins 0.000 description 1
- 108010002747 Pfu DNA polymerase Proteins 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 1
- 229940124158 Protease/peptidase inhibitor Drugs 0.000 description 1
- 101100408135 Pseudomonas aeruginosa (strain ATCC 15692 / DSM 22644 / CIP 104116 / JCM 14847 / LMG 12228 / 1C / PRS 101 / PAO1) phnA gene Proteins 0.000 description 1
- 102100024910 Rabenosyn-5 Human genes 0.000 description 1
- 206010057190 Respiratory tract infections Diseases 0.000 description 1
- 241000220010 Rhode Species 0.000 description 1
- 101150014136 SUC2 gene Proteins 0.000 description 1
- 241000235070 Saccharomyces Species 0.000 description 1
- 241000235346 Schizosaccharomyces Species 0.000 description 1
- 206010040070 Septic Shock Diseases 0.000 description 1
- 102100027981 Septin-7 Human genes 0.000 description 1
- 238000012300 Sequence Analysis Methods 0.000 description 1
- 108010034546 Serratia marcescens nuclease Proteins 0.000 description 1
- 241000344863 Staphylococcus aureus subsp. aureus COL Species 0.000 description 1
- 241000191963 Staphylococcus epidermidis Species 0.000 description 1
- 241000191984 Staphylococcus haemolyticus Species 0.000 description 1
- 241000192086 Staphylococcus warneri Species 0.000 description 1
- 108091081024 Start codon Proteins 0.000 description 1
- 244000057717 Streptococcus lactis Species 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- 208000002847 Surgical Wound Diseases 0.000 description 1
- 108091008874 T cell receptors Proteins 0.000 description 1
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 1
- 206010044248 Toxic shock syndrome Diseases 0.000 description 1
- 231100000650 Toxic shock syndrome Toxicity 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 description 1
- 230000021736 acetylation Effects 0.000 description 1
- 238000006640 acetylation reaction Methods 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 230000002730 additional effect Effects 0.000 description 1
- 238000000246 agarose gel electrophoresis Methods 0.000 description 1
- AZDRQVAHHNSJOQ-UHFFFAOYSA-N alumane Chemical class [AlH3] AZDRQVAHHNSJOQ-UHFFFAOYSA-N 0.000 description 1
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 1
- ILRRQNADMUWWFW-UHFFFAOYSA-K aluminium phosphate Chemical compound O1[Al]2OP1(=O)O2 ILRRQNADMUWWFW-UHFFFAOYSA-K 0.000 description 1
- 150000001408 amides Chemical group 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 230000003698 anagen phase Effects 0.000 description 1
- 238000000137 annealing Methods 0.000 description 1
- 239000008346 aqueous phase Substances 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 125000001584 benzyloxycarbonyl group Chemical group C(=O)(OCC1=CC=CC=C1)* 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 229920001400 block copolymer Polymers 0.000 description 1
- 239000003114 blood coagulation factor Substances 0.000 description 1
- 229940019700 blood coagulation factors Drugs 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- QXJJQWWVWRCVQT-UHFFFAOYSA-K calcium;sodium;phosphate Chemical compound [Na+].[Ca+2].[O-]P([O-])([O-])=O QXJJQWWVWRCVQT-UHFFFAOYSA-K 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 230000006037 cell lysis Effects 0.000 description 1
- 230000036232 cellulite Effects 0.000 description 1
- 208000025222 central nervous system infectious disease Diseases 0.000 description 1
- MYPYJXKWCTUITO-KIIOPKALSA-N chembl3301825 Chemical compound O([C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1OC1=C2C=C3C=C1OC1=CC=C(C=C1Cl)[C@@H](O)[C@H](C(N[C@@H](CC(N)=O)C(=O)N[C@H]3C(=O)N[C@H]1C(=O)N[C@H](C(N[C@H](C3=CC(O)=CC(O)=C3C=3C(O)=CC=C1C=3)C(O)=O)=O)[C@H](O)C1=CC=C(C(=C1)Cl)O2)=O)NC(=O)[C@@H](CC(C)C)NC)[C@H]1C[C@](C)(N)C(O)[C@H](C)O1 MYPYJXKWCTUITO-KIIOPKALSA-N 0.000 description 1
- ZYWFEOZQIUMEGL-UHFFFAOYSA-N chloroform;3-methylbutan-1-ol;phenol Chemical compound ClC(Cl)Cl.CC(C)CCO.OC1=CC=CC=C1 ZYWFEOZQIUMEGL-UHFFFAOYSA-N 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 229920001436 collagen Polymers 0.000 description 1
- 230000001332 colony forming effect Effects 0.000 description 1
- 229940001442 combination vaccine Drugs 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000004590 computer program Methods 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 238000009295 crossflow filtration Methods 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- CTMZLDSMFCVUNX-VMIOUTBZSA-N cytidylyl-(3'->5')-guanosine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@H](O)[C@H](OP(O)(=O)OC[C@@H]2[C@H]([C@@H](O)[C@@H](O2)N2C3=C(C(N=C(N)N3)=O)N=C2)O)[C@@H](CO)O1 CTMZLDSMFCVUNX-VMIOUTBZSA-N 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 238000000326 densiometry Methods 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 230000007717 exclusion Effects 0.000 description 1
- 238000002270 exclusion chromatography Methods 0.000 description 1
- 239000013613 expression plasmid Substances 0.000 description 1
- 210000003414 extremity Anatomy 0.000 description 1
- 208000030533 eye disease Diseases 0.000 description 1
- 239000004744 fabric Substances 0.000 description 1
- 102000025748 fibrinogen binding proteins Human genes 0.000 description 1
- 108091009104 fibrinogen binding proteins Proteins 0.000 description 1
- 210000000245 forearm Anatomy 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 108091008053 gene clusters Proteins 0.000 description 1
- 238000003167 genetic complementation Methods 0.000 description 1
- 238000012268 genome sequencing Methods 0.000 description 1
- 239000003365 glass fiber Substances 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- 229960003180 glutathione Drugs 0.000 description 1
- 125000003147 glycosyl group Chemical group 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 229960004198 guanidine Drugs 0.000 description 1
- PJJJBBJSCAKJQF-UHFFFAOYSA-N guanidinium chloride Chemical compound [Cl-].NC(N)=[NH2+] PJJJBBJSCAKJQF-UHFFFAOYSA-N 0.000 description 1
- 108010037896 heparin-binding hemagglutinin Proteins 0.000 description 1
- 230000002519 immonomodulatory effect Effects 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 102000018358 immunoglobulin Human genes 0.000 description 1
- 229940072221 immunoglobulins Drugs 0.000 description 1
- 230000003308 immunostimulating effect Effects 0.000 description 1
- 201000007119 infective endocarditis Diseases 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 230000015788 innate immune response Effects 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- 125000000741 isoleucyl group Chemical group [H]N([H])C(C(C([H])([H])[H])C([H])([H])C([H])([H])[H])C(=O)O* 0.000 description 1
- 210000001503 joint Anatomy 0.000 description 1
- 229940039696 lactobacillus Drugs 0.000 description 1
- 231100000518 lethal Toxicity 0.000 description 1
- 230000001665 lethal effect Effects 0.000 description 1
- 125000001909 leucine group Chemical group [H]N(*)C(C(*)=O)C([H])([H])C(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 244000144972 livestock Species 0.000 description 1
- 239000012160 loading buffer Substances 0.000 description 1
- 229960000274 lysozyme Drugs 0.000 description 1
- 239000004325 lysozyme Substances 0.000 description 1
- 235000010335 lysozyme Nutrition 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 101150110704 melC2 gene Proteins 0.000 description 1
- MGJXBDMLVWIYOQ-UHFFFAOYSA-N methylazanide Chemical group [NH-]C MGJXBDMLVWIYOQ-UHFFFAOYSA-N 0.000 description 1
- 239000004005 microsphere Substances 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 229940035032 monophosphoryl lipid a Drugs 0.000 description 1
- 229940126619 mouse monoclonal antibody Drugs 0.000 description 1
- 210000004877 mucosa Anatomy 0.000 description 1
- BSOQXXWZTUDTEL-ZUYCGGNHSA-N muramyl dipeptide Chemical compound OC(=O)CC[C@H](C(N)=O)NC(=O)[C@H](C)NC(=O)[C@@H](C)O[C@H]1[C@H](O)[C@@H](CO)O[C@@H](O)[C@@H]1NC(C)=O BSOQXXWZTUDTEL-ZUYCGGNHSA-N 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 229940046166 oligodeoxynucleotide Drugs 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 230000001717 pathogenic effect Effects 0.000 description 1
- 239000012466 permeate Substances 0.000 description 1
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 1
- UYWQUFXKFGHYNT-UHFFFAOYSA-N phenylmethyl ester of formic acid Chemical group O=COCC1=CC=CC=C1 UYWQUFXKFGHYNT-UHFFFAOYSA-N 0.000 description 1
- 238000002264 polyacrylamide gel electrophoresis Methods 0.000 description 1
- 230000008488 polyadenylation Effects 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 229920002704 polyhistidine Polymers 0.000 description 1
- 108091033319 polynucleotide Proteins 0.000 description 1
- 102000040430 polynucleotide Human genes 0.000 description 1
- 239000002157 polynucleotide Substances 0.000 description 1
- 229950008882 polysorbate Drugs 0.000 description 1
- 230000025572 positive regulation of antigen processing and presentation Effects 0.000 description 1
- 230000001323 posttranslational effect Effects 0.000 description 1
- 238000011045 prefiltration Methods 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 210000001236 prokaryotic cell Anatomy 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000011546 protein dye Substances 0.000 description 1
- 230000017854 proteolysis Effects 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 230000010076 replication Effects 0.000 description 1
- 208000020029 respiratory tract infectious disease Diseases 0.000 description 1
- 108091008146 restriction endonucleases Proteins 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 229930182490 saponin Natural products 0.000 description 1
- 150000007949 saponins Chemical class 0.000 description 1
- 235000017709 saponins Nutrition 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 239000006152 selective media Substances 0.000 description 1
- 238000012163 sequencing technique Methods 0.000 description 1
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 description 1
- 230000035939 shock Effects 0.000 description 1
- 238000002741 site-directed mutagenesis Methods 0.000 description 1
- 239000012064 sodium phosphate buffer Substances 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 239000008227 sterile water for injection Substances 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 239000011550 stock solution Substances 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 208000011580 syndromic disease Diseases 0.000 description 1
- 230000009897 systematic effect Effects 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- 125000005931 tert-butyloxycarbonyl group Chemical group [H]C([H])([H])C(OC(*)=O)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- RTKIYNMVFMVABJ-UHFFFAOYSA-L thimerosal Chemical compound [Na+].CC[Hg]SC1=CC=CC=C1C([O-])=O RTKIYNMVFMVABJ-UHFFFAOYSA-L 0.000 description 1
- 229940033663 thimerosal Drugs 0.000 description 1
- 125000000341 threoninyl group Chemical group [H]OC([H])(C([H])([H])[H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- 239000003053 toxin Substances 0.000 description 1
- 231100000765 toxin Toxicity 0.000 description 1
- 230000005030 transcription termination Effects 0.000 description 1
- 230000002103 transcriptional effect Effects 0.000 description 1
- 238000000844 transformation Methods 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- 101150044170 trpE gene Proteins 0.000 description 1
- 239000001974 tryptic soy broth Substances 0.000 description 1
- 108010050327 trypticase-soy broth Proteins 0.000 description 1
- 125000000430 tryptophan group Chemical group [H]N([H])C(C(=O)O*)C([H])([H])C1=C([H])N([H])C2=C([H])C([H])=C([H])C([H])=C12 0.000 description 1
- 108010087967 type I signal peptidase Proteins 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 239000011534 wash buffer Substances 0.000 description 1
- DGVVWUTYPXICAM-UHFFFAOYSA-N β‐Mercaptoethanol Chemical compound OCCS DGVVWUTYPXICAM-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/02—Bacterial antigens
- A61K39/085—Staphylococcus
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2/00—Peptides of undefined number of amino acids; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/195—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from bacteria
- C07K14/305—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from bacteria from Micrococcaceae (F)
- C07K14/31—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from bacteria from Micrococcaceae (F) from Staphylococcus (G)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/35—Fusion polypeptide containing a fusion for enhanced stability/folding during expression, e.g. fusions with chaperones or thioredoxin
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Immunology (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Molecular Biology (AREA)
- Genetics & Genomics (AREA)
- Mycology (AREA)
- Communicable Diseases (AREA)
- Epidemiology (AREA)
- Oncology (AREA)
- Microbiology (AREA)
- Physical Education & Sports Medicine (AREA)
- Rheumatology (AREA)
- Gastroenterology & Hepatology (AREA)
- Dermatology (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
- Neurosurgery (AREA)
- Pain & Pain Management (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Pulmonology (AREA)
- Ophthalmology & Optometry (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US48984003P | 2003-07-24 | 2003-07-24 | |
| US60/489,840 | 2003-07-24 | ||
| US52011503P | 2003-11-14 | 2003-11-14 | |
| US60/520,115 | 2003-11-14 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2006105498A RU2006105498A (ru) | 2006-09-10 |
| RU2337108C2 true RU2337108C2 (ru) | 2008-10-27 |
Family
ID=34107815
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2006105498/13A RU2337108C2 (ru) | 2003-07-24 | 2004-07-22 | Полипептиды для индукции защитного иммунного ответа против staphylococcus aureus |
Country Status (23)
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2639287C2 (ru) * | 2012-06-27 | 2017-12-20 | Ф. Хоффманн-Ля Рош Аг | Способ отбора и получения высокоселективных и мультиспецифичных нацеливающих групп с заданными свойствами, включающих по меньшей мере две различные связывающие группировки, и их применения |
| US11421022B2 (en) | 2012-06-27 | 2022-08-23 | Hoffmann-La Roche Inc. | Method for making antibody Fc-region conjugates comprising at least one binding entity that specifically binds to a target and uses thereof |
Families Citing this family (29)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AT410798B (de) | 2001-01-26 | 2003-07-25 | Cistem Biotechnologies Gmbh | Verfahren zur identifizierung, isolierung und herstellung von antigenen gegen ein spezifisches pathogen |
| EP1725575B1 (en) * | 2004-02-18 | 2012-11-28 | Merck Sharp & Dohme Corp. | Polypeptides for inducing a protective immune response against staphylococcus aureus |
| WO2006059247A2 (en) * | 2004-10-25 | 2006-06-08 | The University Of Western Ontario | VACCINES, COMPOSITIONS AND METHODS BASED ON STAPHYLOCOCCUS AUREUS IRON-REGULATED SURFACE DETERMINANTS IsdA, IsdB, and IsdC |
| GB0526038D0 (en) * | 2005-12-21 | 2006-02-01 | Glaxosmithkline Biolog Sa | Immunogenic composition |
| JP2009524428A (ja) * | 2006-01-27 | 2009-07-02 | メルク エンド カムパニー インコーポレーテッド | スタヒロコッカス・アウレウスorf0657nを標的とする抗原結合タンパク質 |
| AU2008251982A1 (en) | 2007-01-24 | 2008-11-20 | Merck Sharp & Dohme Corp. | Polypeptides for inducing a protective immune response against Staphylococcus epidermidis |
| ATE537839T1 (de) * | 2007-05-04 | 2012-01-15 | Martin Kroenke | Impfstoff zum schutz gegen staphylococcus aureus auf der basis zellwandassoziierter proteine |
| MX2009012891A (es) * | 2007-05-31 | 2009-12-10 | Merck & Co Inc | Proteinas de union a antigeno dirigidas a staphylococcus aureus orf0657n. |
| WO2009022236A2 (en) | 2007-08-16 | 2009-02-19 | Tripep Ab | Immunogen platform |
| US9181329B2 (en) | 2007-08-31 | 2015-11-10 | The University Of Chicago | Methods and compositions related to immunizing against Staphylococcal lung diseases and conditions |
| AU2008292897B2 (en) | 2007-08-31 | 2015-01-22 | University Of Chicago | Methods and compositions related to immunizing against staphylococcal lung diseases and conditions |
| WO2010014304A1 (en) | 2008-07-29 | 2010-02-04 | University Of Chicago | Compositions and methods related to staphylococcal bacterium proteins |
| AU2009319947A1 (en) | 2008-11-26 | 2010-06-03 | Merck Sharp & Dohme Corp. | Polypeptides for inducing a protective immune response against Staphylococcus aureus |
| SI3281947T1 (sl) | 2009-04-03 | 2020-07-31 | The University Of Chicago | Sestave in metode v zvezi z variantami proteina A (SPA) |
| DK2510947T3 (en) | 2009-04-14 | 2016-03-21 | Glaxosmithkline Biolog Sa | Compositions for Immunization against Staphylococcus aureus. |
| GB0913680D0 (en) | 2009-08-05 | 2009-09-16 | Glaxosmithkline Biolog Sa | Immunogenic composition |
| GB0913681D0 (en) | 2009-08-05 | 2009-09-16 | Glaxosmithkline Biolog Sa | Immunogenic composition |
| MX373250B (es) | 2009-09-30 | 2020-05-04 | Glaxosmithkline Biologicals S A Star | Conjugación de polisacáridos capsulares de tipo 5 y de tipo 8 de staphylococcus aureus. |
| EP3199177A1 (en) | 2009-10-30 | 2017-08-02 | GlaxoSmithKline Biologicals S.A. | Purification of staphylococcus aureus type 5 and type 8 capsular saccharides |
| GB0919690D0 (en) | 2009-11-10 | 2009-12-23 | Guy S And St Thomas S Nhs Foun | compositions for immunising against staphylococcus aureus |
| EP2544709A4 (en) * | 2010-03-12 | 2014-01-08 | Univ Pennsylvania | METHOD FOR PREVENTING AND TREATING THE COLONIZATION OF STAPHYLOCOCCUS AUREUS AND THEIR INFECTIOUS DISEASES AND DISEASES |
| CA2803298C (en) | 2010-07-02 | 2020-07-14 | The University Of Chicago | Compositions and methods related to protein a (spa) variants |
| US8747858B2 (en) | 2010-07-13 | 2014-06-10 | Merck Sharp & Dohme Corp. | Staphylococcus aureus surface protein SA1789 and protective vaccine based thereon |
| WO2012065034A1 (en) | 2010-11-12 | 2012-05-18 | Merck Sharp & Dohme Corp. | Enolase peptide conjugate vaccines against staphylococcus aureus |
| US8945588B2 (en) | 2011-05-06 | 2015-02-03 | The University Of Chicago | Methods and compositions involving protective staphylococcal antigens, such as EBH polypeptides |
| US20140363461A1 (en) | 2011-09-01 | 2014-12-11 | Fabio Bagnoli | Adjuvanted formulations of staphylococcus aureus antigens |
| EP2773370A4 (en) | 2011-10-31 | 2016-09-28 | Merck Sharp & Dohme | PROTECTIVE VACCINE BASED ON THE STAPHYLOCOCCUS AUREUS PROTEIN SA2451 |
| EP2872173A4 (en) | 2012-07-10 | 2016-03-23 | Merck Sharp & Dohme | PROTECTIVE VACCINE BASED ON THE STAPHYLOCOCCUS AUREUS PROTEIN SA2493 |
| US10738338B2 (en) | 2016-10-18 | 2020-08-11 | The Research Foundation for the State University | Method and composition for biocatalytic protein-oligonucleotide conjugation and protein-oligonucleotide conjugate |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2000071585A1 (en) * | 1999-05-03 | 2000-11-30 | Medarex, Inc. | Human antibodies to staphylococcus aureus |
| WO2003053462A3 (en) * | 2001-12-11 | 2003-09-04 | Merck & Co Inc | Staphylococcus aureus exopolysaccharide and process |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6248329B1 (en) * | 1998-06-01 | 2001-06-19 | Ramaswamy Chandrashekar | Parasitic helminth cuticlin nucleic acid molecules and uses thereof |
| GB0014907D0 (en) * | 2000-06-20 | 2000-08-09 | Univ Sheffield | Antigenic polypeptides |
| AT410798B (de) * | 2001-01-26 | 2003-07-25 | Cistem Biotechnologies Gmbh | Verfahren zur identifizierung, isolierung und herstellung von antigenen gegen ein spezifisches pathogen |
| EP2339344A1 (en) * | 2001-06-15 | 2011-06-29 | Inhibitex, Inc. | Cross-reactive monoclonal and polyclonal antibodies which recognize surface proteins from coagulase-negative staphylococci and staphylococcus aureus |
| ES2322338T3 (es) * | 2001-11-12 | 2009-06-19 | Novo Nordisk A/S | Purificacion de peptidos por medio de cromatografia de afinidad con iones metalicos. |
| US20080050361A1 (en) * | 2004-10-25 | 2008-02-28 | Heinrichs David E | Staphylococcus aureas specific anti-infectives |
| EP2368570A3 (en) * | 2006-01-18 | 2012-05-02 | University Of Chicago | Compositions and methods related to staphylococcal bacterium proteins |
-
2004
- 2004-07-22 EP EP04778847A patent/EP1651166B8/en not_active Expired - Lifetime
- 2004-07-22 US US10/564,458 patent/US20060177462A1/en not_active Abandoned
- 2004-07-22 DK DK04778847.6T patent/DK1651166T3/da active
- 2004-07-22 KR KR1020067001610A patent/KR20060065643A/ko not_active Ceased
- 2004-07-22 HR HR20100240T patent/HRP20100240T1/hr unknown
- 2004-07-22 JP JP2006521226A patent/JP2007502101A/ja active Pending
- 2004-07-22 RU RU2006105498/13A patent/RU2337108C2/ru not_active IP Right Cessation
- 2004-07-22 PL PL04778847T patent/PL1651166T3/pl unknown
- 2004-07-22 BR BRPI0412799-4A patent/BRPI0412799A/pt not_active Application Discontinuation
- 2004-07-22 NZ NZ570750A patent/NZ570750A/en not_active IP Right Cessation
- 2004-07-22 CA CA002532370A patent/CA2532370A1/en not_active Abandoned
- 2004-07-22 MX MXPA06000854A patent/MXPA06000854A/es active IP Right Grant
- 2004-07-22 SI SI200431389T patent/SI1651166T1/sl unknown
- 2004-07-22 NZ NZ544542A patent/NZ544542A/en not_active IP Right Cessation
- 2004-07-22 PT PT04778847T patent/PT1651166E/pt unknown
- 2004-07-22 AU AU2004258979A patent/AU2004258979B2/en not_active Ceased
- 2004-07-22 WO PCT/US2004/023523 patent/WO2005009379A2/en not_active Ceased
- 2004-07-22 ES ES04778847T patent/ES2342778T3/es not_active Expired - Lifetime
- 2004-07-22 DE DE602004025579T patent/DE602004025579D1/de not_active Expired - Lifetime
- 2004-07-22 AT AT04778847T patent/ATE457737T1/de active
-
2005
- 2005-12-30 IS IS8213A patent/IS8213A/is unknown
-
2006
- 2006-01-19 IL IL173253A patent/IL173253A0/en unknown
- 2006-02-23 NO NO20060898A patent/NO20060898L/no not_active Application Discontinuation
-
2010
- 2010-04-30 US US12/771,345 patent/US20100247561A1/en not_active Abandoned
- 2010-05-14 CY CY20101100419T patent/CY1110028T1/el unknown
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2000071585A1 (en) * | 1999-05-03 | 2000-11-30 | Medarex, Inc. | Human antibodies to staphylococcus aureus |
| WO2003053462A3 (en) * | 2001-12-11 | 2003-09-04 | Merck & Co Inc | Staphylococcus aureus exopolysaccharide and process |
Non-Patent Citations (1)
| Title |
|---|
| РОЙТ А. и др. Иммунология, М., Изд. Мир, 2000 г., стр.237-238. * |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2639287C2 (ru) * | 2012-06-27 | 2017-12-20 | Ф. Хоффманн-Ля Рош Аг | Способ отбора и получения высокоселективных и мультиспецифичных нацеливающих групп с заданными свойствами, включающих по меньшей мере две различные связывающие группировки, и их применения |
| US10106612B2 (en) | 2012-06-27 | 2018-10-23 | Hoffmann-La Roche Inc. | Method for selection and production of tailor-made highly selective and multi-specific targeting entities containing at least two different binding entities and uses thereof |
| US11407836B2 (en) | 2012-06-27 | 2022-08-09 | Hoffmann-La Roche Inc. | Method for selection and production of tailor-made highly selective and multi-specific targeting entities containing at least two different binding entities and uses thereof |
| US11421022B2 (en) | 2012-06-27 | 2022-08-23 | Hoffmann-La Roche Inc. | Method for making antibody Fc-region conjugates comprising at least one binding entity that specifically binds to a target and uses thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| IS8213A (is) | 2005-12-30 |
| WO2005009379A3 (en) | 2006-11-30 |
| JP2007502101A (ja) | 2007-02-08 |
| CA2532370A1 (en) | 2005-02-03 |
| ATE457737T1 (de) | 2010-03-15 |
| SI1651166T1 (sl) | 2010-06-30 |
| AU2004258979B2 (en) | 2007-12-06 |
| IL173253A0 (en) | 2006-06-11 |
| BRPI0412799A (pt) | 2006-09-26 |
| US20060177462A1 (en) | 2006-08-10 |
| NZ570750A (en) | 2009-12-24 |
| ES2342778T3 (es) | 2010-07-14 |
| NO20060898L (no) | 2006-04-24 |
| EP1651166A2 (en) | 2006-05-03 |
| DK1651166T3 (da) | 2010-05-31 |
| PL1651166T3 (pl) | 2010-08-31 |
| HRP20100240T1 (hr) | 2010-09-30 |
| EP1651166A4 (en) | 2008-03-05 |
| US20100247561A1 (en) | 2010-09-30 |
| NZ544542A (en) | 2009-01-31 |
| DE602004025579D1 (de) | 2010-04-01 |
| WO2005009379A2 (en) | 2005-02-03 |
| EP1651166B1 (en) | 2010-02-17 |
| MXPA06000854A (es) | 2006-03-30 |
| KR20060065643A (ko) | 2006-06-14 |
| PT1651166E (pt) | 2010-04-23 |
| CY1110028T1 (el) | 2015-01-14 |
| RU2006105498A (ru) | 2006-09-10 |
| AU2004258979A1 (en) | 2005-02-03 |
| EP1651166B8 (en) | 2010-05-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2337108C2 (ru) | Полипептиды для индукции защитного иммунного ответа против staphylococcus aureus | |
| ZA200600247B (en) | Polypeptides for inducing a protective immune response against staphylococcus aureus | |
| EP1725575B1 (en) | Polypeptides for inducing a protective immune response against staphylococcus aureus | |
| US20100203588A1 (en) | Polypeptides for inducing a protective immune response against staphylococcus aureus | |
| US20070243205A1 (en) | Polypeptides for Inducing a Protective Immune Response Against Staphylococcus Aureus | |
| US20070264278A1 (en) | Polypeptides for Inducing a Protective Immune Response Against Staphylococcus Aureus | |
| US20080095792A1 (en) | Polypeptides For Inducing A Protective Immune Response Against Staphylococcus Aureus | |
| EP2376111A1 (en) | Polypeptides for inducing a protective immune response against staphylococcus aureus | |
| US8747858B2 (en) | Staphylococcus aureus surface protein SA1789 and protective vaccine based thereon | |
| AU2008251982A1 (en) | Polypeptides for inducing a protective immune response against Staphylococcus epidermidis | |
| WO2006121664A2 (en) | Polypeptides for inducing a protective immune response against staphylococcus aureus |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PD4A | Correction of name of patent owner | ||
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20120723 |