KR20200122298A - 아르기나제 1 결핍을 치료하기 위한 방법 및 조성물 - Google Patents
아르기나제 1 결핍을 치료하기 위한 방법 및 조성물 Download PDFInfo
- Publication number
- KR20200122298A KR20200122298A KR1020207019283A KR20207019283A KR20200122298A KR 20200122298 A KR20200122298 A KR 20200122298A KR 1020207019283 A KR1020207019283 A KR 1020207019283A KR 20207019283 A KR20207019283 A KR 20207019283A KR 20200122298 A KR20200122298 A KR 20200122298A
- Authority
- KR
- South Korea
- Prior art keywords
- arginase
- subject
- arginine
- plasma level
- gaa
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/43—Enzymes; Proenzymes; Derivatives thereof
- A61K38/46—Hydrolases (3)
- A61K38/50—Hydrolases (3) acting on carbon-nitrogen bonds, other than peptide bonds (3.5), e.g. asparaginase
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/43—Enzymes; Proenzymes; Derivatives thereof
- A61K38/51—Lyases (4)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/56—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule
- A61K47/59—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyureas or polyurethanes
- A61K47/60—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyureas or polyurethanes the organic macromolecular compound being a polyoxyalkylene oligomer, polymer or dendrimer, e.g. PEG, PPG, PEO or polyglycerol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
- A61K48/005—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'active' part of the composition delivered, i.e. the nucleic acid delivered
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/52—Genes encoding for enzymes or proenzymes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/78—Hydrolases (3) acting on carbon to nitrogen bonds other than peptide bonds (3.5)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y305/00—Hydrolases acting on carbon-nitrogen bonds, other than peptide bonds (3.5)
- C12Y305/03—Hydrolases acting on carbon-nitrogen bonds, other than peptide bonds (3.5) in linear amidines (3.5.3)
- C12Y305/03001—Arginase (3.5.3.1)
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Organic Chemistry (AREA)
- Epidemiology (AREA)
- Genetics & Genomics (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Immunology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Gastroenterology & Hepatology (AREA)
- General Engineering & Computer Science (AREA)
- Biochemistry (AREA)
- Biotechnology (AREA)
- Molecular Biology (AREA)
- Biomedical Technology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Obesity (AREA)
- Hematology (AREA)
- Diabetes (AREA)
- Dermatology (AREA)
- Microbiology (AREA)
- Physics & Mathematics (AREA)
- Biophysics (AREA)
- Plant Pathology (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Medicinal Preparation (AREA)
- Enzymes And Modification Thereof (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Applications Claiming Priority (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201762594747P | 2017-12-05 | 2017-12-05 | |
| US62/594,747 | 2017-12-05 | ||
| US201862725612P | 2018-08-31 | 2018-08-31 | |
| US62/725,612 | 2018-08-31 | ||
| US201862745000P | 2018-10-12 | 2018-10-12 | |
| US62/745,000 | 2018-10-12 | ||
| PCT/US2018/063982 WO2019113157A1 (en) | 2017-12-05 | 2018-12-05 | Method and composition for treating arginase 1 deficiency |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20200122298A true KR20200122298A (ko) | 2020-10-27 |
Family
ID=64734275
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020207019283A Ceased KR20200122298A (ko) | 2017-12-05 | 2018-12-05 | 아르기나제 1 결핍을 치료하기 위한 방법 및 조성물 |
Country Status (10)
| Country | Link |
|---|---|
| US (2) | US11717562B2 (enExample) |
| EP (1) | EP3720476A1 (enExample) |
| JP (2) | JP2021505620A (enExample) |
| KR (1) | KR20200122298A (enExample) |
| CN (3) | CN120643682A (enExample) |
| AU (1) | AU2018378485B2 (enExample) |
| CA (1) | CA3084801A1 (enExample) |
| MX (2) | MX2020005933A (enExample) |
| TW (1) | TWI878212B (enExample) |
| WO (1) | WO2019113157A1 (enExample) |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3449935A1 (en) * | 2017-08-31 | 2019-03-06 | Erytech Pharma | Arginine deiminase encapsulated inside erythrocytes and their use in treating cancer and arginase-1 deficiency |
| MX2021004463A (es) * | 2018-10-19 | 2021-08-24 | Aerase Inc | Terapia de disminución de arginina para el tratamiento de la deficiencia de gamt. |
| MX2022002437A (es) | 2019-08-30 | 2022-06-02 | Aeglea Biotherapeutics Inc | Metodos para la produccion de arginasa humana recombinante 1 y sus usos. |
| CN114214353B (zh) * | 2021-11-30 | 2023-07-18 | 新泰市佳禾生物科技有限公司 | 一种发酵生产人重组精氨酸酶i的方法 |
| CN114350721B (zh) * | 2021-11-30 | 2024-05-24 | 新泰市佳禾生物科技有限公司 | 微生物酶法生产l-鸟氨酸的方法 |
| CN114196712B (zh) * | 2021-11-30 | 2024-04-02 | 新泰市佳禾生物科技有限公司 | 一种固定化酶法生产l-鸟氨酸的方法 |
| CN116179452A (zh) * | 2022-07-11 | 2023-05-30 | 山东第一医科大学(山东省医学科学院) | 一种用于治疗精氨酸酶缺乏症的工程益生菌及其构建方法和应用 |
| WO2025171346A1 (en) * | 2024-02-08 | 2025-08-14 | University Of Florida Research Foundation, Incorporated | Indoleamine 2,3-dioxygenase variants |
Family Cites Families (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH02117383A (ja) | 1988-10-26 | 1990-05-01 | Tosoh Corp | ヒトアルギナーゼの製造方法 |
| US5780286A (en) | 1996-03-14 | 1998-07-14 | Smithkline Beecham Corporation | Arginase II |
| EP1499342A2 (en) | 2002-01-25 | 2005-01-26 | Cancer Treatments International | Therapeutic composition for treatment of cancer by arginine depletion |
| HK1053577A2 (en) | 2002-06-20 | 2003-10-10 | Bio-Cancer Treatment International Limited | Pharmaceutical composition and method of treatment of human malignanices with arginine deprivation |
| GB0315248D0 (en) * | 2003-06-30 | 2003-08-06 | Hoffmann La Roche | HCV regulated protein expression |
| FR2873925B1 (fr) | 2004-08-05 | 2006-10-13 | Erytech Pharma Soc Par Actions | Procede et dispositif de lyse-rescellement pour l'incorporation de principe actif notamment asparaginase ou inositol hexaphosphate, dans des erythrocytes |
| CN1745847A (zh) | 2004-09-08 | 2006-03-15 | 康达医药科技有限公司 | 用精氨酸酶治疗肝炎的药物组合物和方法 |
| JP5097714B2 (ja) | 2005-12-12 | 2012-12-12 | カンジ,インコーポレイテッド | E1領域内の発現カセットと不活性化されたe2bポリメラーゼを有するアデノウイルス発現ベクター |
| PT3778885T (pt) * | 2008-10-31 | 2023-05-31 | Aerase Inc | Composições de arginases humanas modificadas e métodos para o tratamento do cancro |
| US9382525B2 (en) | 2009-03-26 | 2016-07-05 | The Hong Kong Polytechnic University | Site-directed pegylation of arginases and the use thereof as anti-cancer and anti-viral agents |
| JP2012531893A (ja) | 2009-06-29 | 2012-12-13 | ボード・オブ・リージエンツ,ザ・ユニバーシテイ・オブ・テキサス・システム | アルギナーゼ製剤および方法 |
| WO2012061015A2 (en) | 2010-11-03 | 2012-05-10 | Scott & White Healthcare | L-citrulline supplementation during arginine depletion therapy with arginase |
| CN103184208B (zh) * | 2011-12-27 | 2015-09-16 | 拜奥生物科技(上海)有限公司 | 人精氨酸酶和定点聚乙二醇化人精氨酸酶及其应用 |
| WO2014093694A1 (en) | 2012-12-12 | 2014-06-19 | The Broad Institute, Inc. | Crispr-cas nickase systems, methods and compositions for sequence manipulation in eukaryotes |
| FR3005420B1 (fr) | 2013-05-07 | 2015-09-18 | Erytech Pharma | Procede de stabilisation de suspensions d'erythrocytes encapsulant un principe actif, suspensions obtenues. |
| CA2938456C (en) | 2014-02-11 | 2022-06-21 | The Regents Of The University Of Colorado, A Body Corporate | Crispr enabled multiplexed genome engineering |
| CA2944903A1 (en) | 2014-04-24 | 2015-10-29 | Dana-Farber Cancer Institute, Inc. | Tumor suppressor and oncogene biomarkers predictive of anti-immune checkpoint inhibitor response |
| WO2015165374A1 (en) * | 2014-04-29 | 2015-11-05 | Bio-Cancer Treatment International Limited | Methods and compositions for modulating the immune system with arginase i |
| EP2982758A1 (en) | 2014-08-04 | 2016-02-10 | Centre Hospitalier Universitaire Vaudois (CHUV) | Genome editing for the treatment of huntington's disease |
| DK3186281T3 (da) | 2014-08-28 | 2019-06-11 | Halozyme Inc | Kombinationsterapi med et hyaluronan-nedbrydende enzym og en immun-checkpoint-inhibitor |
| AU2017221405A1 (en) | 2016-02-16 | 2018-09-20 | Carnegie Mellon University | Compositions for enhancing targeted gene editing and methods of use thereof |
| KR102449167B1 (ko) * | 2016-05-06 | 2022-09-28 | 파세비오 파마수티컬스 인코포레이티드 | 제어된 지속 방출을 위한 elp 융합 단백질 |
-
2018
- 2018-12-05 TW TW107143773A patent/TWI878212B/zh active
- 2018-12-05 KR KR1020207019283A patent/KR20200122298A/ko not_active Ceased
- 2018-12-05 CN CN202510809272.4A patent/CN120643682A/zh active Pending
- 2018-12-05 AU AU2018378485A patent/AU2018378485B2/en active Active
- 2018-12-05 US US16/210,248 patent/US11717562B2/en active Active
- 2018-12-05 MX MX2020005933A patent/MX2020005933A/es unknown
- 2018-12-05 CN CN201880079119.9A patent/CN111787941A/zh active Pending
- 2018-12-05 JP JP2020531164A patent/JP2021505620A/ja active Pending
- 2018-12-05 CA CA3084801A patent/CA3084801A1/en active Pending
- 2018-12-05 WO PCT/US2018/063982 patent/WO2019113157A1/en not_active Ceased
- 2018-12-05 EP EP18821957.0A patent/EP3720476A1/en active Pending
- 2018-12-05 CN CN202510809152.4A patent/CN120661640A/zh active Pending
-
2020
- 2020-07-13 MX MX2025006207A patent/MX2025006207A/es unknown
-
2023
- 2023-06-29 US US18/344,083 patent/US20240009284A1/en active Pending
- 2023-11-27 JP JP2023199616A patent/JP2024028760A/ja active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| MX2025006207A (es) | 2025-07-01 |
| TW201924715A (zh) | 2019-07-01 |
| AU2018378485A1 (en) | 2020-07-23 |
| MX2020005933A (es) | 2021-01-15 |
| CA3084801A1 (en) | 2019-06-13 |
| US20190167770A1 (en) | 2019-06-06 |
| US11717562B2 (en) | 2023-08-08 |
| WO2019113157A1 (en) | 2019-06-13 |
| CN111787941A (zh) | 2020-10-16 |
| TWI878212B (zh) | 2025-04-01 |
| AU2018378485B2 (en) | 2024-06-20 |
| EP3720476A1 (en) | 2020-10-14 |
| CN120643682A (zh) | 2025-09-16 |
| JP2024028760A (ja) | 2024-03-05 |
| JP2021505620A (ja) | 2021-02-18 |
| US20240009284A1 (en) | 2024-01-11 |
| CN120661640A (zh) | 2025-09-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20200122298A (ko) | 아르기나제 1 결핍을 치료하기 위한 방법 및 조성물 | |
| JP4330796B2 (ja) | 改変された毛様体神経栄養因子、それらを作製する方法およびそれらの使用方法 | |
| US10209261B2 (en) | Method for assessing protein identity and stability | |
| KR20220162816A (ko) | 알칼리성 포스파타아제로 근육 약화의 치료 | |
| KR20110016440A (ko) | 연장된 반감기를 가진 제 ⅰⅹ 인자 접합체 | |
| RU2771313C2 (ru) | Композиции и способы для лечения рака с истощающими аргинин и иммуноонкологическими агентами | |
| US20180333464A1 (en) | Methods of using interleukin-10 for treating diseases and disorders | |
| JP2025003988A (ja) | Gamt欠損症の治療のためのアルギニン枯渇療法 | |
| JP2020516322A (ja) | ホモシスチン尿症の治療のための酵素補充療法の最適化 | |
| CN109134664B (zh) | 一种经修饰的生长分化因子及其制备方法和应用 | |
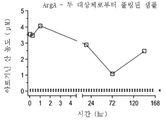
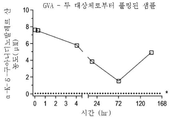
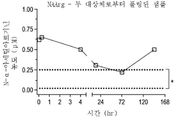
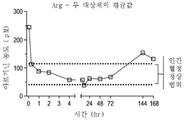
| US20250228921A1 (en) | Treatment of arginase 1 deficiency | |
| Torosantucci et al. | Development of a transgenic mouse model to study the immunogenicity of recombinant human insulin | |
| CN115197923B (zh) | 尿酸酶、其药物组合物及其用途 | |
| HK40039953A (en) | Method and composition for treating arginase 1 deficiency | |
| CN1361865A (zh) | 起催化作用的抗因子ⅷ同种抗体 | |
| EP4547701A1 (en) | Il-12 mutants with reduced toxicity, compositions thereof and methods of using the same | |
| KR20210084539A (ko) | 치료학적 용도를 위한 조작된 영장류 시스틴/시스테인 분해 효소 | |
| HK40016122A (en) | Human-enzyme mediated depletion of homocysteine for treating patients with hyperhomocysteinemia and homocystinuria |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
St.27 status event code: A-0-1-A10-A15-nap-PA0105 |
|
| T11-X000 | Administrative time limit extension requested |
St.27 status event code: U-3-3-T10-T11-oth-X000 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| PG1501 | Laying open of application |
St.27 status event code: A-1-1-Q10-Q12-nap-PG1501 |
|
| E13-X000 | Pre-grant limitation requested |
St.27 status event code: A-2-3-E10-E13-lim-X000 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| PA0201 | Request for examination |
St.27 status event code: A-1-2-D10-D11-exm-PA0201 |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
St.27 status event code: A-1-2-D10-D21-exm-PE0902 |
|
| E13-X000 | Pre-grant limitation requested |
St.27 status event code: A-2-3-E10-E13-lim-X000 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
St.27 status event code: A-1-2-D10-D21-exm-PE0902 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| B15 | Application refused following examination |
Free format text: ST27 STATUS EVENT CODE: N-2-6-B10-B15-EXM-PE0601 (AS PROVIDED BY THE NATIONAL OFFICE) |
|
| PE0601 | Decision on rejection of patent |
St.27 status event code: N-2-6-B10-B15-exm-PE0601 |
|
| PN2301 | Change of applicant |
St.27 status event code: A-3-3-R10-R11-asn-PN2301 |
|
| R18-X000 | Changes to party contact information recorded |
St.27 status event code: A-3-3-R10-R18-oth-X000 |