KR20200042499A - 지카 바이러스 에피토프에 특이적으로 결합하는 다중특이적 항체 및 이의 용도 - Google Patents
지카 바이러스 에피토프에 특이적으로 결합하는 다중특이적 항체 및 이의 용도 Download PDFInfo
- Publication number
- KR20200042499A KR20200042499A KR1020207007493A KR20207007493A KR20200042499A KR 20200042499 A KR20200042499 A KR 20200042499A KR 1020207007493 A KR1020207007493 A KR 1020207007493A KR 20207007493 A KR20207007493 A KR 20207007493A KR 20200042499 A KR20200042499 A KR 20200042499A
- Authority
- KR
- South Korea
- Prior art keywords
- antibody
- antigen
- binding fragment
- seq
- sequence identity
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/08—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses
- C07K16/10—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses from RNA viruses
- C07K16/1081—Togaviridae, e.g. flavivirus, rubella virus, hog cholera virus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/569—Immunoassay; Biospecific binding assay; Materials therefor for microorganisms, e.g. protozoa, bacteria, viruses
- G01N33/56983—Viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/54—Medicinal preparations containing antigens or antibodies characterised by the route of administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/545—Medicinal preparations containing antigens or antibodies characterised by the dose, timing or administration schedule
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/31—Immunoglobulins specific features characterized by aspects of specificity or valency multispecific
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/33—Crossreactivity, e.g. for species or epitope, or lack of said crossreactivity
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/35—Valency
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/52—Constant or Fc region; Isotype
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/55—Fab or Fab'
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/565—Complementarity determining region [CDR]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/005—Assays involving biological materials from specific organisms or of a specific nature from viruses
- G01N2333/08—RNA viruses
- G01N2333/18—Togaviridae; Flaviviridae
- G01N2333/183—Flaviviridae, e.g. pestivirus, mucosal disease virus, bovine viral diarrhoea virus, classical swine fever virus (hog cholera virus) or border disease virus
- G01N2333/185—Flaviviruses or Group B arboviruses, e.g. yellow fever virus, japanese encephalitis, tick-borne encephalitis, dengue
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/26—Infectious diseases, e.g. generalised sepsis
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/52—Predicting or monitoring the response to treatment, e.g. for selection of therapy based on assay results in personalised medicine; Prognosis
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Virology (AREA)
- Organic Chemistry (AREA)
- Immunology (AREA)
- Molecular Biology (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biomedical Technology (AREA)
- Biotechnology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Animal Behavior & Ethology (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Hematology (AREA)
- Physics & Mathematics (AREA)
- General Engineering & Computer Science (AREA)
- Urology & Nephrology (AREA)
- Microbiology (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Oncology (AREA)
- Communicable Diseases (AREA)
- Analytical Chemistry (AREA)
- Cell Biology (AREA)
- Plant Pathology (AREA)
- Pathology (AREA)
- General Physics & Mathematics (AREA)
- Tropical Medicine & Parasitology (AREA)
- Food Science & Technology (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/EP2017/071891 WO2019042555A1 (en) | 2017-08-31 | 2017-08-31 | MULTISPECIFIC ANTIBODIES SPECIFICALLY BINDING TO ZIKA VIRUS EPITOPES AND USES THEREOF |
| EPPCT/EP2017/071891 | 2017-08-31 | ||
| PCT/EP2018/073489 WO2019043166A1 (en) | 2017-08-31 | 2018-08-31 | MULTISPECIFIC ANTIBODIES SPECIFICALLY BINDING TO ZIKA VIRUS EPITOPES AND USES THEREOF |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20200042499A true KR20200042499A (ko) | 2020-04-23 |
Family
ID=59811299
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020207007493A Abandoned KR20200042499A (ko) | 2017-08-31 | 2018-08-31 | 지카 바이러스 에피토프에 특이적으로 결합하는 다중특이적 항체 및 이의 용도 |
Country Status (21)
| Country | Link |
|---|---|
| US (2) | US11926658B2 (enExample) |
| EP (1) | EP3676290A1 (enExample) |
| JP (2) | JP7244489B2 (enExample) |
| KR (1) | KR20200042499A (enExample) |
| CN (2) | CN116693668A (enExample) |
| AU (1) | AU2018322842A1 (enExample) |
| BR (1) | BR112020000619A2 (enExample) |
| CA (1) | CA3070780A1 (enExample) |
| CL (1) | CL2020000467A1 (enExample) |
| CO (1) | CO2020002060A2 (enExample) |
| CR (1) | CR20200087A (enExample) |
| DO (1) | DOP2020000043A (enExample) |
| EA (1) | EA202090559A1 (enExample) |
| EC (1) | ECSP20014362A (enExample) |
| IL (1) | IL271515A (enExample) |
| MX (1) | MX2020002181A (enExample) |
| MY (1) | MY201774A (enExample) |
| PE (1) | PE20200888A1 (enExample) |
| PH (1) | PH12020550025A1 (enExample) |
| SG (1) | SG11202000206SA (enExample) |
| WO (2) | WO2019042555A1 (enExample) |
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2019042555A1 (en) * | 2017-08-31 | 2019-03-07 | Humabs Biomed Sa | MULTISPECIFIC ANTIBODIES SPECIFICALLY BINDING TO ZIKA VIRUS EPITOPES AND USES THEREOF |
| US11648304B2 (en) | 2017-11-03 | 2023-05-16 | Takeda Vaccines, Inc. | Zika vaccines and immunogenic compositions, and methods of using the same |
| KR20200090893A (ko) | 2017-11-30 | 2020-07-29 | 다케다 백신즈 인코포레이티드 | 지카 바이러스를 비활성화시키기 위한 방법, 및 관련 방법들 |
| MX2022005498A (es) * | 2019-11-07 | 2022-08-11 | Inst Microbiology Cas | Vacuna del zika/dengue y aplicación de la misma. |
| KR102365464B1 (ko) * | 2019-12-24 | 2022-02-22 | 강원대학교산학협력단 | 지카바이러스 재조합 서브유닛 백신의 개발 및 이의 제조방법 |
| WO2021236225A1 (en) * | 2020-05-20 | 2021-11-25 | Takeda Vaccines, Inc. | Method for detection of zika virus specific antibodies |
| EP4153222A1 (en) * | 2020-05-20 | 2023-03-29 | Takeda Vaccines, Inc. | Method for detection of zika virus specific antibodies |
| CN115697392A (zh) * | 2020-06-28 | 2023-02-03 | 神州细胞工程有限公司 | 一种降低病毒ade效应的方法 |
| US20240002479A1 (en) * | 2020-11-23 | 2024-01-04 | The Regents Of The University Of Michigan | Single-chain antibody against flavivirus ns1 protein |
| CN114958747B (zh) * | 2022-06-08 | 2024-08-20 | 中国科学院动物研究所 | 一种多能干细胞诱导产生兴奋性和抑制性神经元的方法 |
| WO2025107050A1 (pt) * | 2023-11-23 | 2025-05-30 | Imunotera Soluções Terapêuticas Ltda | Anticorpo monoclonal, vetor de expressão, uso, composição farmacêutica, processo para produção, método de prevenção e/ou tratamento de infecções por flavivírus e kit para a detecção de vírus da zika e da dengue |
| CN119119256B (zh) * | 2024-09-28 | 2025-07-18 | 宁波大学 | 一种乙型脑炎病毒和寨卡病毒的单克隆抗体及其应用 |
Family Cites Families (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| NL154600B (nl) | 1971-02-10 | 1977-09-15 | Organon Nv | Werkwijze voor het aantonen en bepalen van specifiek bindende eiwitten en hun corresponderende bindbare stoffen. |
| US3817837A (en) | 1971-05-14 | 1974-06-18 | Syva Corp | Enzyme amplification assay |
| US3766162A (en) | 1971-08-24 | 1973-10-16 | Hoffmann La Roche | Barbituric acid antigens and antibodies specific therefor |
| US4179337A (en) | 1973-07-20 | 1979-12-18 | Davis Frank F | Non-immunogenic polypeptides |
| US4233402A (en) | 1978-04-05 | 1980-11-11 | Syva Company | Reagents and method employing channeling |
| JPS5896026A (ja) | 1981-10-30 | 1983-06-07 | Nippon Chemiphar Co Ltd | 新規ウロキナ−ゼ誘導体およびその製造法ならびにそれを含有する血栓溶解剤 |
| US4609546A (en) | 1982-06-24 | 1986-09-02 | Japan Chemical Research Co., Ltd. | Long-acting composition |
| US4766106A (en) | 1985-06-26 | 1988-08-23 | Cetus Corporation | Solubilization of proteins for pharmaceutical compositions using polymer conjugation |
| US4676980A (en) | 1985-09-23 | 1987-06-30 | The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Target specific cross-linked heteroantibodies |
| US4831175A (en) | 1986-09-05 | 1989-05-16 | The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Backbone polysubstituted chelates for forming a metal chelate-protein conjugate |
| US5595721A (en) | 1993-09-16 | 1997-01-21 | Coulter Pharmaceutical, Inc. | Radioimmunotherapy of lymphoma using anti-CD20 |
| MY133346A (en) | 1999-03-01 | 2007-11-30 | Biogen Inc | Kit for radiolabeling ligands with yttrium-90 |
| US20020102208A1 (en) | 1999-03-01 | 2002-08-01 | Paul Chinn | Radiolabeling kit and binding assay |
| WO2005000898A2 (en) | 2003-06-27 | 2005-01-06 | Biogen Idec Ma Inc. | Use of hydrophobic-interaction-chromatography or hinge-region modifications for the production of homogeneous antibody-solutions |
| BRPI0811857A2 (pt) | 2007-05-14 | 2014-10-21 | Biogen Idec Inc | Regiões fc (scfc) de cadeia simples, polipeptídeos de aglutinação que as compreendem e métodos relacionados. |
| ES2923641T3 (es) * | 2013-12-30 | 2022-09-29 | Epimab Biotherapeutics Inc | Inmunoglobulina con Fabs en tándem y usos de la misma |
| EP2980099A1 (en) * | 2014-07-31 | 2016-02-03 | Bernhard-Nocht-Institut für Tropenmedizin | Compositions and methods for detecting flaviviridae infections |
| MY187459A (en) | 2015-07-16 | 2021-09-23 | Bharat Biotech Int Ltd | Vaccine compositions |
| KR20180127397A (ko) | 2016-03-11 | 2018-11-28 | 더 유나이티드 스테이츠 오브 어메리카, 애즈 리프리젠티드 바이 더 세크러테리, 디파트먼트 오브 헬쓰 앤드 휴먼 서비씨즈 | 생약독화된 지카 바이러스 백신 |
| CN105954512A (zh) | 2016-06-01 | 2016-09-21 | 卢氏实验室公司 | 一种快速免疫层析法检测塞卡病毒Zika virus的检测条 |
| WO2018010789A1 (en) | 2016-07-13 | 2018-01-18 | Humabs Biomed Sa | Novel antibodies specifically binding to zika virus epitopes and uses thereof |
| TW201815821A (zh) | 2016-07-18 | 2018-05-01 | 美商再生元醫藥公司 | 抗茲卡病毒抗體及使用方法 |
| CN110066333B (zh) | 2016-08-10 | 2020-09-04 | 中国科学院微生物研究所 | 一种寨卡病毒特异性中和抗体及其应用 |
| US20190324040A1 (en) * | 2016-10-29 | 2019-10-24 | University Of Miami | Zika virus antibodies |
| US11021533B2 (en) * | 2016-11-02 | 2021-06-01 | Vanderbilt University | Human Zika virus antibodies and methods of use therefor |
| CN106841601A (zh) | 2016-12-30 | 2017-06-13 | 广州市达瑞生物技术股份有限公司 | 寨卡病毒多片段融合蛋白的制备及IgG/IgM抗体检测试剂盒 |
| CN106885903B (zh) * | 2017-03-08 | 2018-12-28 | 中国人民解放军军事医学科学院基础医学研究所 | 一种寨卡病毒e抗原及其在检测抗寨卡病毒抗体中的应用 |
| WO2019042555A1 (en) * | 2017-08-31 | 2019-03-07 | Humabs Biomed Sa | MULTISPECIFIC ANTIBODIES SPECIFICALLY BINDING TO ZIKA VIRUS EPITOPES AND USES THEREOF |
-
2017
- 2017-08-31 WO PCT/EP2017/071891 patent/WO2019042555A1/en not_active Ceased
-
2018
- 2018-08-31 CN CN202310134988.XA patent/CN116693668A/zh active Pending
- 2018-08-31 EP EP18762834.2A patent/EP3676290A1/en active Pending
- 2018-08-31 WO PCT/EP2018/073489 patent/WO2019043166A1/en not_active Ceased
- 2018-08-31 SG SG11202000206SA patent/SG11202000206SA/en unknown
- 2018-08-31 US US16/640,622 patent/US11926658B2/en active Active
- 2018-08-31 MX MX2020002181A patent/MX2020002181A/es unknown
- 2018-08-31 KR KR1020207007493A patent/KR20200042499A/ko not_active Abandoned
- 2018-08-31 BR BR112020000619-0A patent/BR112020000619A2/pt not_active IP Right Cessation
- 2018-08-31 CA CA3070780A patent/CA3070780A1/en active Pending
- 2018-08-31 JP JP2020507007A patent/JP7244489B2/ja active Active
- 2018-08-31 MY MYPI2019007495A patent/MY201774A/en unknown
- 2018-08-31 EA EA202090559A patent/EA202090559A1/ru unknown
- 2018-08-31 PE PE2020000287A patent/PE20200888A1/es unknown
- 2018-08-31 CR CR20200087A patent/CR20200087A/es unknown
- 2018-08-31 AU AU2018322842A patent/AU2018322842A1/en not_active Abandoned
- 2018-08-31 CN CN201880057824.9A patent/CN111344302B/zh active Active
-
2019
- 2019-12-17 IL IL271515A patent/IL271515A/en unknown
-
2020
- 2020-01-16 PH PH12020550025A patent/PH12020550025A1/en unknown
- 2020-02-26 DO DO2020000043A patent/DOP2020000043A/es unknown
- 2020-02-26 CO CONC2020/0002060A patent/CO2020002060A2/es unknown
- 2020-02-26 CL CL2020000467A patent/CL2020000467A1/es unknown
- 2020-02-27 EC ECSENADI202014362A patent/ECSP20014362A/es unknown
-
2023
- 2023-03-09 JP JP2023036084A patent/JP2023071956A/ja not_active Withdrawn
-
2024
- 2024-02-22 US US18/584,654 patent/US20240254207A1/en active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| PH12020550025A1 (en) | 2021-02-15 |
| US20200317755A1 (en) | 2020-10-08 |
| ECSP20014362A (es) | 2020-04-22 |
| US11926658B2 (en) | 2024-03-12 |
| WO2019042555A1 (en) | 2019-03-07 |
| AU2018322842A1 (en) | 2020-01-16 |
| CL2020000467A1 (es) | 2020-06-19 |
| BR112020000619A2 (pt) | 2020-07-14 |
| CA3070780A1 (en) | 2019-03-07 |
| PE20200888A1 (es) | 2020-09-03 |
| JP2023071956A (ja) | 2023-05-23 |
| CN116693668A (zh) | 2023-09-05 |
| JP2020531000A (ja) | 2020-11-05 |
| EP3676290A1 (en) | 2020-07-08 |
| SG11202000206SA (en) | 2020-03-30 |
| DOP2020000043A (es) | 2020-09-15 |
| MY201774A (en) | 2024-03-16 |
| CO2020002060A2 (es) | 2020-06-09 |
| WO2019043166A1 (en) | 2019-03-07 |
| CN111344302B (zh) | 2023-03-10 |
| JP7244489B2 (ja) | 2023-03-22 |
| CR20200087A (es) | 2020-08-07 |
| IL271515A (en) | 2020-02-27 |
| MX2020002181A (es) | 2020-07-20 |
| CN111344302A (zh) | 2020-06-26 |
| EA202090559A1 (ru) | 2020-06-17 |
| US20240254207A1 (en) | 2024-08-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
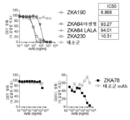
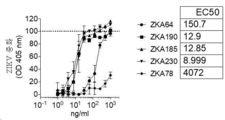
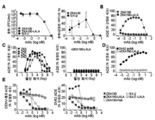
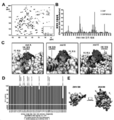
| CN109563157B (zh) | 特异性地结合至寨卡病毒表位的新型抗体和其用途 | |
| US20240254207A1 (en) | Multispecific antibodies specifically binding to zika virus epitopes and uses thereof | |
| KR20140012131A (ko) | 인플루엔자의 치료 및 진단을 위한 조성물 및 방법 | |
| EA043940B1 (ru) | Мультиспецифические антитела, специфичные в отношении эпитопов вируса зика, и их применение | |
| HK40006233B (en) | Novel antibodies specifically binding to zika virus epitopes and uses thereof | |
| HK40006233A (en) | Novel antibodies specifically binding to zika virus epitopes and uses thereof | |
| HK40025147A (en) | Multispecific antibodies specifically binding to zika virus epitopes and uses thereof | |
| EA046175B1 (ru) | Выделенное антитело или его антигенсвязывающий фрагмент, которые специфически связываются с эпитопом вируса зика, их получение и применения | |
| HK40002097A (en) | Novel antibodies specifically binding to zika virus epitopes and uses thereof | |
| HK40002097B (en) | Novel antibodies specifically binding to zika virus epitopes and uses thereof | |
| HK40025147B (en) | Multispecific antibodies specifically binding to zika virus epitopes and uses thereof | |
| BR122024006890A2 (pt) | Anticorpo isolado, ou um fragmento de ligação ao antígeno do mesmo, molécula de ácido nucleico, vetor, célula, composição farmacêutica, uso dos mesmos, kit de partes, bem como ensaio de bloqueio de ligação para diagóstico in vitro de infecção por zika vírus | |
| EA039682B1 (ru) | Антитела, нейтрализующие rsv, mpv и pvm, и их применения |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
St.27 status event code: A-0-1-A10-A15-nap-PA0105 |
|
| PG1501 | Laying open of application |
St.27 status event code: A-1-1-Q10-Q12-nap-PG1501 |
|
| A201 | Request for examination | ||
| E13-X000 | Pre-grant limitation requested |
St.27 status event code: A-2-3-E10-E13-lim-X000 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| PA0201 | Request for examination |
St.27 status event code: A-1-2-D10-D11-exm-PA0201 |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
St.27 status event code: A-1-2-D10-D21-exm-PE0902 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| E701 | Decision to grant or registration of patent right | ||
| PE0701 | Decision of registration |
St.27 status event code: A-1-2-D10-D22-exm-PE0701 |
|
| PC1904 | Unpaid initial registration fee |
St.27 status event code: A-2-2-U10-U14-oth-PC1904 St.27 status event code: N-2-6-B10-B12-nap-PC1904 |