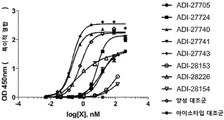
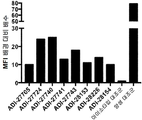
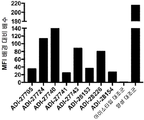
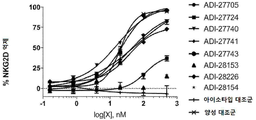
KR20190115469A - Bcma, nkg2d 및 cd16에 결합하는 단백질 - Google Patents
Bcma, nkg2d 및 cd16에 결합하는 단백질 Download PDFInfo
- Publication number
- KR20190115469A KR20190115469A KR1020197026174A KR20197026174A KR20190115469A KR 20190115469 A KR20190115469 A KR 20190115469A KR 1020197026174 A KR1020197026174 A KR 1020197026174A KR 20197026174 A KR20197026174 A KR 20197026174A KR 20190115469 A KR20190115469 A KR 20190115469A
- Authority
- KR
- South Korea
- Prior art keywords
- ser
- gly
- thr
- leu
- gln
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 102000006942 B-Cell Maturation Antigen Human genes 0.000 title claims abstract description 52
- 108010008014 B-Cell Maturation Antigen Proteins 0.000 title claims abstract description 52
- 101000917858 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-A Proteins 0.000 title claims abstract description 30
- 101000917839 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-B Proteins 0.000 title claims abstract description 30
- 102100029185 Low affinity immunoglobulin gamma Fc region receptor III-B Human genes 0.000 title claims abstract description 25
- BGFTWECWAICPDG-UHFFFAOYSA-N 2-[bis(4-chlorophenyl)methyl]-4-n-[3-[bis(4-chlorophenyl)methyl]-4-(dimethylamino)phenyl]-1-n,1-n-dimethylbenzene-1,4-diamine Chemical compound C1=C(C(C=2C=CC(Cl)=CC=2)C=2C=CC(Cl)=CC=2)C(N(C)C)=CC=C1NC(C=1)=CC=C(N(C)C)C=1C(C=1C=CC(Cl)=CC=1)C1=CC=C(Cl)C=C1 BGFTWECWAICPDG-UHFFFAOYSA-N 0.000 title claims abstract 3
- 101001109501 Homo sapiens NKG2-D type II integral membrane protein Proteins 0.000 title claims description 116
- 102100022680 NKG2-D type II integral membrane protein Human genes 0.000 title claims description 108
- 102000004169 proteins and genes Human genes 0.000 title claims description 72
- 108090000623 proteins and genes Proteins 0.000 title claims description 72
- 206010028980 Neoplasm Diseases 0.000 claims abstract description 110
- 201000011510 cancer Diseases 0.000 claims abstract description 58
- 238000000034 method Methods 0.000 claims abstract description 54
- 230000027455 binding Effects 0.000 claims description 209
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 159
- 239000000427 antigen Substances 0.000 claims description 111
- 108091007433 antigens Proteins 0.000 claims description 111
- 102000036639 antigens Human genes 0.000 claims description 111
- 210000004027 cell Anatomy 0.000 claims description 109
- 210000000822 natural killer cell Anatomy 0.000 claims description 93
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 88
- 229920001184 polypeptide Polymers 0.000 claims description 85
- 125000003275 alpha amino acid group Chemical group 0.000 claims description 68
- 241000282414 Homo sapiens Species 0.000 claims description 62
- 239000000203 mixture Substances 0.000 claims description 57
- 238000009472 formulation Methods 0.000 claims description 43
- 210000004881 tumor cell Anatomy 0.000 claims description 20
- 102100035360 Cerebellar degeneration-related antigen 1 Human genes 0.000 claims description 19
- 206010035226 Plasma cell myeloma Diseases 0.000 claims description 17
- 150000001413 amino acids Chemical class 0.000 claims description 17
- 206010025323 Lymphomas Diseases 0.000 claims description 13
- 208000032839 leukemia Diseases 0.000 claims description 11
- 206010042971 T-cell lymphoma Diseases 0.000 claims description 9
- 150000007523 nucleic acids Chemical class 0.000 claims description 9
- 230000001154 acute effect Effects 0.000 claims description 8
- 208000034578 Multiple myelomas Diseases 0.000 claims description 7
- 241000282412 Homo Species 0.000 claims description 6
- 108010003723 Single-Domain Antibodies Proteins 0.000 claims description 5
- 208000027585 T-cell non-Hodgkin lymphoma Diseases 0.000 claims description 5
- 239000012634 fragment Substances 0.000 claims description 5
- 210000001616 monocyte Anatomy 0.000 claims description 5
- 230000003256 osteocytic effect Effects 0.000 claims description 4
- 239000003937 drug carrier Substances 0.000 claims description 3
- 241000283984 Rodentia Species 0.000 claims description 2
- 230000030833 cell death Effects 0.000 claims description 2
- 230000002708 enhancing effect Effects 0.000 claims description 2
- 108020004707 nucleic acids Proteins 0.000 claims description 2
- 102000039446 nucleic acids Human genes 0.000 claims description 2
- 239000001608 potassium adipate Substances 0.000 claims description 2
- 239000001601 sodium adipate Substances 0.000 claims description 2
- 101100112922 Candida albicans CDR3 gene Proteins 0.000 claims 6
- 102100035361 Cerebellar degeneration-related protein 2 Human genes 0.000 claims 6
- 101000737793 Homo sapiens Cerebellar degeneration-related antigen 1 Proteins 0.000 claims 6
- 101000737796 Homo sapiens Cerebellar degeneration-related protein 2 Proteins 0.000 claims 6
- 108091008324 binding proteins Proteins 0.000 abstract description 44
- 230000009870 specific binding Effects 0.000 abstract description 43
- 239000008194 pharmaceutical composition Substances 0.000 abstract description 11
- 108010001657 NK Cell Lectin-Like Receptor Subfamily K Proteins 0.000 abstract description 7
- 102000000812 NK Cell Lectin-Like Receptor Subfamily K Human genes 0.000 abstract description 7
- 102000023732 binding proteins Human genes 0.000 abstract 1
- YOOAQCZYZHGUAZ-KATARQTJSA-N Thr-Leu-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YOOAQCZYZHGUAZ-KATARQTJSA-N 0.000 description 55
- FQPDRTDDEZXCEC-SVSWQMSJSA-N Thr-Ile-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O FQPDRTDDEZXCEC-SVSWQMSJSA-N 0.000 description 48
- 230000037396 body weight Effects 0.000 description 46
- 235000018102 proteins Nutrition 0.000 description 44
- 102000014914 Carrier Proteins Human genes 0.000 description 43
- 241000880493 Leptailurus serval Species 0.000 description 42
- PDIDTSZKKFEDMB-UWVGGRQHSA-N Lys-Pro-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O PDIDTSZKKFEDMB-UWVGGRQHSA-N 0.000 description 35
- 108010051242 phenylalanylserine Proteins 0.000 description 35
- UIGMAMGZOJVTDN-WHFBIAKZSA-N Ser-Gly-Ser Chemical compound OC[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O UIGMAMGZOJVTDN-WHFBIAKZSA-N 0.000 description 34
- ZDILXFDENZVOTL-BPNCWPANSA-N Ala-Val-Tyr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O ZDILXFDENZVOTL-BPNCWPANSA-N 0.000 description 33
- XKUKSGPZAADMRA-UHFFFAOYSA-N glycyl-glycyl-glycine Natural products NCC(=O)NCC(=O)NCC(O)=O XKUKSGPZAADMRA-UHFFFAOYSA-N 0.000 description 33
- PMGDADKJMCOXHX-UHFFFAOYSA-N L-Arginyl-L-glutamin-acetat Natural products NC(=N)NCCCC(N)C(=O)NC(CCC(N)=O)C(O)=O PMGDADKJMCOXHX-UHFFFAOYSA-N 0.000 description 32
- FEHQLKKBVJHSEC-SZMVWBNQSA-N Leu-Glu-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC(C)C)C(O)=O)=CNC2=C1 FEHQLKKBVJHSEC-SZMVWBNQSA-N 0.000 description 32
- 108010008355 arginyl-glutamine Proteins 0.000 description 32
- JXFLPKSDLDEOQK-JHEQGTHGSA-N Gln-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CCC(N)=O JXFLPKSDLDEOQK-JHEQGTHGSA-N 0.000 description 31
- FMDHKPRACUXATF-ACZMJKKPSA-N Ser-Gln-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(O)=O FMDHKPRACUXATF-ACZMJKKPSA-N 0.000 description 31
- 101100505161 Caenorhabditis elegans mel-32 gene Proteins 0.000 description 30
- QOIKZODVIPOPDD-AVGNSLFASA-N Tyr-Cys-Gln Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(O)=O QOIKZODVIPOPDD-AVGNSLFASA-N 0.000 description 30
- 108010003137 tyrosyltyrosine Proteins 0.000 description 30
- SZQCDCKIGWQAQN-FXQIFTODSA-N Cys-Arg-Ala Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(O)=O SZQCDCKIGWQAQN-FXQIFTODSA-N 0.000 description 28
- PDUHNKAFQXQNLH-ZETCQYMHSA-N Gly-Lys-Gly Chemical compound NCCCC[C@H](NC(=O)CN)C(=O)NCC(O)=O PDUHNKAFQXQNLH-ZETCQYMHSA-N 0.000 description 28
- 108010044292 tryptophyltyrosine Proteins 0.000 description 28
- ZFBBMCKQSNJZSN-AUTRQRHGSA-N Gln-Val-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O ZFBBMCKQSNJZSN-AUTRQRHGSA-N 0.000 description 27
- SPSSJSICDYYTQN-HJGDQZAQSA-N Met-Thr-Gln Chemical compound CSCC[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCC(N)=O SPSSJSICDYYTQN-HJGDQZAQSA-N 0.000 description 27
- KLYYKKGCPOGDPE-OEAJRASXSA-N Phe-Thr-Leu Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O KLYYKKGCPOGDPE-OEAJRASXSA-N 0.000 description 27
- QUILOGWWLXMSAT-IHRRRGAJSA-N Tyr-Gln-Gln Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O QUILOGWWLXMSAT-IHRRRGAJSA-N 0.000 description 27
- 108010070409 phenylalanyl-glycyl-glycine Proteins 0.000 description 27
- AOHKLEBWKMKITA-IHRRRGAJSA-N Arg-Phe-Ser Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N AOHKLEBWKMKITA-IHRRRGAJSA-N 0.000 description 26
- GJFYFGOEWLDQGW-GUBZILKMSA-N Ser-Leu-Gln Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CO)N GJFYFGOEWLDQGW-GUBZILKMSA-N 0.000 description 26
- 108010037850 glycylvaline Proteins 0.000 description 26
- BMKNXTJLHFIAAH-CIUDSAMLSA-N Ser-Ser-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O BMKNXTJLHFIAAH-CIUDSAMLSA-N 0.000 description 25
- 108010087846 prolyl-prolyl-glycine Proteins 0.000 description 25
- BTYTYHBSJKQBQA-GCJQMDKQSA-N Ala-Asp-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](C)N)O BTYTYHBSJKQBQA-GCJQMDKQSA-N 0.000 description 24
- ARHJJAAWNWOACN-FXQIFTODSA-N Ala-Ser-Val Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O ARHJJAAWNWOACN-FXQIFTODSA-N 0.000 description 24
- CREYEAPXISDKSB-FQPOAREZSA-N Ala-Thr-Tyr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O CREYEAPXISDKSB-FQPOAREZSA-N 0.000 description 24
- HXWALXSAVBLTPK-NUTKFTJISA-N Leu-Ala-Trp Chemical compound C[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)NC(=O)[C@H](CC(C)C)N HXWALXSAVBLTPK-NUTKFTJISA-N 0.000 description 24
- LINKCQUOMUDLKN-KATARQTJSA-N Leu-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(C)C)N)O LINKCQUOMUDLKN-KATARQTJSA-N 0.000 description 24
- YTJFXEDRUOQGSP-DCAQKATOSA-N Lys-Pro-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O YTJFXEDRUOQGSP-DCAQKATOSA-N 0.000 description 24
- HBOABDXGTMMDSE-GUBZILKMSA-N Ser-Arg-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(O)=O HBOABDXGTMMDSE-GUBZILKMSA-N 0.000 description 24
- 230000004913 activation Effects 0.000 description 24
- 108010072986 threonyl-seryl-lysine Proteins 0.000 description 24
- XYBJLTKSGFBLCS-QXEWZRGKSA-N Asp-Arg-Val Chemical compound NC(N)=NCCC[C@@H](C(=O)N[C@@H](C(C)C)C(O)=O)NC(=O)[C@@H](N)CC(O)=O XYBJLTKSGFBLCS-QXEWZRGKSA-N 0.000 description 23
- WLRYGVYQFXRJDA-DCAQKATOSA-N Gln-Pro-Pro Chemical compound NC(=O)CC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 WLRYGVYQFXRJDA-DCAQKATOSA-N 0.000 description 23
- CQGSYZCULZMEDE-UHFFFAOYSA-N Leu-Gln-Pro Natural products CC(C)CC(N)C(=O)NC(CCC(N)=O)C(=O)N1CCCC1C(O)=O CQGSYZCULZMEDE-UHFFFAOYSA-N 0.000 description 23
- AIRZWUMAHCDDHR-KKUMJFAQSA-N Lys-Leu-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O AIRZWUMAHCDDHR-KKUMJFAQSA-N 0.000 description 23
- AZWNCEBQZXELEZ-FXQIFTODSA-N Ser-Pro-Ser Chemical compound OC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O AZWNCEBQZXELEZ-FXQIFTODSA-N 0.000 description 23
- GXUWHVZYDAHFSV-FLBSBUHZSA-N Thr-Ile-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(O)=O GXUWHVZYDAHFSV-FLBSBUHZSA-N 0.000 description 23
- QRCBQDPRKMYTMB-IHPCNDPISA-N Tyr-Trp-Ser Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CC3=CC=C(C=C3)O)N QRCBQDPRKMYTMB-IHPCNDPISA-N 0.000 description 23
- HTONZBWRYUKUKC-RCWTZXSCSA-N Val-Thr-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O HTONZBWRYUKUKC-RCWTZXSCSA-N 0.000 description 23
- 108010047495 alanylglycine Proteins 0.000 description 23
- 108010077245 asparaginyl-proline Proteins 0.000 description 23
- SGYSTDWPNPKJPP-GUBZILKMSA-N Arg-Ala-Arg Chemical compound NC(=N)NCCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O SGYSTDWPNPKJPP-GUBZILKMSA-N 0.000 description 22
- RTFXPCYMDYBZNQ-SRVKXCTJSA-N Asn-Tyr-Asn Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(N)=O)C(O)=O RTFXPCYMDYBZNQ-SRVKXCTJSA-N 0.000 description 22
- QNFRBNZGVVKBNJ-PEFMBERDSA-N Asp-Ile-Gln Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CC(=O)O)N QNFRBNZGVVKBNJ-PEFMBERDSA-N 0.000 description 22
- UESYBOXFJWJVSB-AVGNSLFASA-N Gln-Phe-Ser Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(O)=O UESYBOXFJWJVSB-AVGNSLFASA-N 0.000 description 22
- RFTVTKBHDXCEEX-WDSKDSINSA-N Glu-Ser-Gly Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)NCC(O)=O RFTVTKBHDXCEEX-WDSKDSINSA-N 0.000 description 22
- ISSDODCYBOWWIP-GJZGRUSLSA-N Gly-Pro-Trp Chemical compound [H]NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O ISSDODCYBOWWIP-GJZGRUSLSA-N 0.000 description 22
- JSLVAHYTAJJEQH-QWRGUYRKSA-N Gly-Ser-Phe Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 JSLVAHYTAJJEQH-QWRGUYRKSA-N 0.000 description 22
- LCRDMSSAKLTKBU-ZDLURKLDSA-N Gly-Ser-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)CN LCRDMSSAKLTKBU-ZDLURKLDSA-N 0.000 description 22
- IXHKPDJKKCUKHS-GARJFASQSA-N Lys-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCCCN)N IXHKPDJKKCUKHS-GARJFASQSA-N 0.000 description 22
- WRODMZBHNNPRLN-SRVKXCTJSA-N Lys-Leu-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O WRODMZBHNNPRLN-SRVKXCTJSA-N 0.000 description 22
- LNICFEXCAHIJOR-DCAQKATOSA-N Pro-Ser-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O LNICFEXCAHIJOR-DCAQKATOSA-N 0.000 description 22
- OQPNSDWGAMFJNU-QWRGUYRKSA-N Ser-Gly-Tyr Chemical compound OC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 OQPNSDWGAMFJNU-QWRGUYRKSA-N 0.000 description 22
- HDBOEVPDIDDEPC-CIUDSAMLSA-N Ser-Lys-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(O)=O HDBOEVPDIDDEPC-CIUDSAMLSA-N 0.000 description 22
- SIEBDTCABMZCLF-XGEHTFHBSA-N Ser-Val-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O SIEBDTCABMZCLF-XGEHTFHBSA-N 0.000 description 22
- GQHAIUPYZPTADF-FDARSICLSA-N Trp-Ile-Arg Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)=CNC2=C1 GQHAIUPYZPTADF-FDARSICLSA-N 0.000 description 22
- SMLCYZYQFRTLCO-UWJYBYFXSA-N Tyr-Cys-Ala Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CS)C(=O)N[C@@H](C)C(O)=O SMLCYZYQFRTLCO-UWJYBYFXSA-N 0.000 description 22
- OVLIFGQSBSNGHY-KKHAAJSZSA-N Val-Asp-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](C(C)C)N)O OVLIFGQSBSNGHY-KKHAAJSZSA-N 0.000 description 22
- SSYBNWFXCFNRFN-GUBZILKMSA-N Val-Pro-Ser Chemical compound CC(C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O SSYBNWFXCFNRFN-GUBZILKMSA-N 0.000 description 22
- 235000001014 amino acid Nutrition 0.000 description 22
- 108010091092 arginyl-glycyl-proline Proteins 0.000 description 22
- 108010093581 aspartyl-proline Proteins 0.000 description 22
- 108010015792 glycyllysine Proteins 0.000 description 22
- 108010034529 leucyl-lysine Proteins 0.000 description 22
- 230000035772 mutation Effects 0.000 description 22
- ICZWAZVKLACMKR-CIUDSAMLSA-N Asp-His-Ser Chemical compound OC(=O)C[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CO)C(O)=O)CC1=CN=CN1 ICZWAZVKLACMKR-CIUDSAMLSA-N 0.000 description 21
- STVHDEHTKFXBJQ-LAEOZQHASA-N Gly-Glu-Ile Chemical compound [H]NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O STVHDEHTKFXBJQ-LAEOZQHASA-N 0.000 description 21
- UHPAZODVFFYEEL-QWRGUYRKSA-N Gly-Leu-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)CN UHPAZODVFFYEEL-QWRGUYRKSA-N 0.000 description 21
- PXKACEXYLPBMAD-JBDRJPRFSA-N Ile-Ser-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)O)N PXKACEXYLPBMAD-JBDRJPRFSA-N 0.000 description 21
- KAFOIVJDVSZUMD-DCAQKATOSA-N Leu-Gln-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O KAFOIVJDVSZUMD-DCAQKATOSA-N 0.000 description 21
- KAFOIVJDVSZUMD-UHFFFAOYSA-N Leu-Gln-Gln Natural products CC(C)CC(N)C(=O)NC(CCC(N)=O)C(=O)NC(CCC(N)=O)C(O)=O KAFOIVJDVSZUMD-UHFFFAOYSA-N 0.000 description 21
- 241000699666 Mus <mouse, genus> Species 0.000 description 21
- DMNANGOFEUVBRV-GJZGRUSLSA-N Pro-Trp-Gly Chemical compound N([C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)NCC(=O)O)C(=O)[C@@H]1CCCN1 DMNANGOFEUVBRV-GJZGRUSLSA-N 0.000 description 21
- UGTZYIPOBYXWRW-SRVKXCTJSA-N Ser-Phe-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(O)=O)C(O)=O UGTZYIPOBYXWRW-SRVKXCTJSA-N 0.000 description 21
- FEZASNVQLJQBHW-CABZTGNLSA-N Trp-Gly-Ala Chemical compound C1=CC=C2C(C[C@H](N)C(=O)NCC(=O)N[C@@H](C)C(O)=O)=CNC2=C1 FEZASNVQLJQBHW-CABZTGNLSA-N 0.000 description 21
- GUIYPEKUEMQBIK-JSGCOSHPSA-N Val-Tyr-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)NCC(O)=O GUIYPEKUEMQBIK-JSGCOSHPSA-N 0.000 description 21
- 239000000833 heterodimer Substances 0.000 description 21
- 108010090333 leucyl-lysyl-proline Proteins 0.000 description 21
- 108010017949 tyrosyl-glycyl-glycine Proteins 0.000 description 21
- 238000006467 substitution reaction Methods 0.000 description 20
- SVFOIXMRMLROHO-SRVKXCTJSA-N Asp-Asp-Phe Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 SVFOIXMRMLROHO-SRVKXCTJSA-N 0.000 description 19
- DBUNZBWUWCIELX-JHEQGTHGSA-N Gly-Thr-Glu Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(O)=O DBUNZBWUWCIELX-JHEQGTHGSA-N 0.000 description 19
- 101001023379 Homo sapiens Lysosome-associated membrane glycoprotein 1 Proteins 0.000 description 19
- 102100035133 Lysosome-associated membrane glycoprotein 1 Human genes 0.000 description 19
- XMBSYZWANAQXEV-UHFFFAOYSA-N N-alpha-L-glutamyl-L-phenylalanine Natural products OC(=O)CCC(N)C(=O)NC(C(O)=O)CC1=CC=CC=C1 XMBSYZWANAQXEV-UHFFFAOYSA-N 0.000 description 19
- JAGGEZACYAAMIL-CQDKDKBSSA-N Tyr-Lys-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC1=CC=C(C=C1)O)N JAGGEZACYAAMIL-CQDKDKBSSA-N 0.000 description 19
- 238000005734 heterodimerization reaction Methods 0.000 description 19
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 19
- XHVONGZZVUUORG-WEDXCCLWSA-N Gly-Thr-Lys Chemical compound NCC(=O)N[C@@H]([C@H](O)C)C(=O)N[C@H](C(O)=O)CCCCN XHVONGZZVUUORG-WEDXCCLWSA-N 0.000 description 18
- 108010017391 lysylvaline Proteins 0.000 description 18
- VCAWFLIWYNMHQP-UKJIMTQDSA-N Val-Glu-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](C(C)C)N VCAWFLIWYNMHQP-UKJIMTQDSA-N 0.000 description 17
- 239000003112 inhibitor Substances 0.000 description 17
- -1 NCR Proteins 0.000 description 16
- 239000002253 acid Substances 0.000 description 16
- 239000012669 liquid formulation Substances 0.000 description 16
- 230000008685 targeting Effects 0.000 description 16
- UQJNXZSSGQIPIQ-FBCQKBJTSA-N Gly-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)CN UQJNXZSSGQIPIQ-FBCQKBJTSA-N 0.000 description 15
- 102000006496 Immunoglobulin Heavy Chains Human genes 0.000 description 15
- 108010019476 Immunoglobulin Heavy Chains Proteins 0.000 description 15
- NAXPHWZXEXNDIW-JTQLQIEISA-N Phe-Gly-Gly Chemical compound OC(=O)CNC(=O)CNC(=O)[C@@H](N)CC1=CC=CC=C1 NAXPHWZXEXNDIW-JTQLQIEISA-N 0.000 description 15
- 108010069205 aspartyl-phenylalanine Proteins 0.000 description 15
- 108010086434 alanyl-seryl-glycine Proteins 0.000 description 14
- 239000000872 buffer Substances 0.000 description 14
- 230000000694 effects Effects 0.000 description 14
- 102000005962 receptors Human genes 0.000 description 14
- 108020003175 receptors Proteins 0.000 description 14
- 102100037850 Interferon gamma Human genes 0.000 description 13
- 108010074328 Interferon-gamma Proteins 0.000 description 13
- BIBYEFRASCNLAA-CDMKHQONSA-N Thr-Phe-Gly Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@H](C(=O)NCC(O)=O)CC1=CC=CC=C1 BIBYEFRASCNLAA-CDMKHQONSA-N 0.000 description 13
- 108010089804 glycyl-threonine Proteins 0.000 description 13
- 150000003839 salts Chemical class 0.000 description 13
- OYTPNWYZORARHL-XHNCKOQMSA-N Gln-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)N)N OYTPNWYZORARHL-XHNCKOQMSA-N 0.000 description 12
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 12
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 12
- PZTZYZUTCPZWJH-FXQIFTODSA-N Val-Ser-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)O)N PZTZYZUTCPZWJH-FXQIFTODSA-N 0.000 description 12
- 239000000243 solution Substances 0.000 description 12
- MNQMTYSEKZHIDF-GCJQMDKQSA-N Asp-Thr-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O MNQMTYSEKZHIDF-GCJQMDKQSA-N 0.000 description 11
- 102000000588 Interleukin-2 Human genes 0.000 description 11
- 108010002350 Interleukin-2 Proteins 0.000 description 11
- OWFGFHQMSBTKLX-UFYCRDLUSA-N Val-Tyr-Tyr Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O)N OWFGFHQMSBTKLX-UFYCRDLUSA-N 0.000 description 11
- 108010069020 alanyl-prolyl-glycine Proteins 0.000 description 11
- 239000003795 chemical substances by application Substances 0.000 description 11
- 238000000684 flow cytometry Methods 0.000 description 11
- 239000013641 positive control Substances 0.000 description 11
- 108050005493 CD3 protein, epsilon/gamma/delta subunit Proteins 0.000 description 10
- BYYNJRSNDARRBX-YFKPBYRVSA-N Gly-Gln-Gly Chemical compound NCC(=O)N[C@@H](CCC(N)=O)C(=O)NCC(O)=O BYYNJRSNDARRBX-YFKPBYRVSA-N 0.000 description 10
- 210000001744 T-lymphocyte Anatomy 0.000 description 10
- MBLJBGZWLHTJBH-SZMVWBNQSA-N Trp-Val-Arg Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)=CNC2=C1 MBLJBGZWLHTJBH-SZMVWBNQSA-N 0.000 description 10
- 229940024606 amino acid Drugs 0.000 description 10
- 108010068265 aspartyltyrosine Proteins 0.000 description 10
- 239000002585 base Substances 0.000 description 10
- 150000001875 compounds Chemical class 0.000 description 10
- VPZXBVLAVMBEQI-UHFFFAOYSA-N glycyl-DL-alpha-alanine Natural products OC(=O)C(C)NC(=O)CN VPZXBVLAVMBEQI-UHFFFAOYSA-N 0.000 description 10
- 108010010147 glycylglutamine Proteins 0.000 description 10
- 238000004519 manufacturing process Methods 0.000 description 10
- 201000000050 myeloid neoplasm Diseases 0.000 description 10
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 10
- 201000009030 Carcinoma Diseases 0.000 description 9
- SOEGEPHNZOISMT-BYPYZUCNSA-N Gly-Ser-Gly Chemical compound NCC(=O)N[C@@H](CO)C(=O)NCC(O)=O SOEGEPHNZOISMT-BYPYZUCNSA-N 0.000 description 9
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 9
- 102000013463 Immunoglobulin Light Chains Human genes 0.000 description 9
- 108010065825 Immunoglobulin Light Chains Proteins 0.000 description 9
- UGCIQUYEJIEHKX-GVXVVHGQSA-N Lys-Val-Glu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O UGCIQUYEJIEHKX-GVXVVHGQSA-N 0.000 description 9
- 229960002685 biotin Drugs 0.000 description 9
- 239000011616 biotin Substances 0.000 description 9
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 9
- 238000001990 intravenous administration Methods 0.000 description 9
- 239000002609 medium Substances 0.000 description 9
- 239000003755 preservative agent Substances 0.000 description 9
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 8
- AVZHGSCDKIQZPQ-CIUDSAMLSA-N Glu-Arg-Ala Chemical compound C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCC(O)=O)C(O)=O AVZHGSCDKIQZPQ-CIUDSAMLSA-N 0.000 description 8
- MIIVFRCYJABHTQ-ONGXEEELSA-N Gly-Leu-Val Chemical compound [H]NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O MIIVFRCYJABHTQ-ONGXEEELSA-N 0.000 description 8
- NVTPVQLIZCOJFK-FOHZUACHSA-N Gly-Thr-Asp Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(O)=O NVTPVQLIZCOJFK-FOHZUACHSA-N 0.000 description 8
- LCNASHSOFMRYFO-WDCWCFNPSA-N Leu-Thr-Gln Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCC(N)=O LCNASHSOFMRYFO-WDCWCFNPSA-N 0.000 description 8
- 229930195725 Mannitol Natural products 0.000 description 8
- 108010079364 N-glycylalanine Proteins 0.000 description 8
- 230000006051 NK cell activation Effects 0.000 description 8
- 230000003213 activating effect Effects 0.000 description 8
- 230000009089 cytolysis Effects 0.000 description 8
- 239000003814 drug Substances 0.000 description 8
- 238000011534 incubation Methods 0.000 description 8
- 239000003446 ligand Substances 0.000 description 8
- 239000000594 mannitol Substances 0.000 description 8
- 235000010355 mannitol Nutrition 0.000 description 8
- 201000001441 melanoma Diseases 0.000 description 8
- 239000008227 sterile water for injection Substances 0.000 description 8
- 108010061238 threonyl-glycine Proteins 0.000 description 8
- YYSWCHMLFJLLBJ-ZLUOBGJFSA-N Ala-Ala-Ser Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O YYSWCHMLFJLLBJ-ZLUOBGJFSA-N 0.000 description 7
- GIVATXIGCXFQQA-FXQIFTODSA-N Arg-Ala-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCN=C(N)N GIVATXIGCXFQQA-FXQIFTODSA-N 0.000 description 7
- MRQQMVZUHXUPEV-IHRRRGAJSA-N Asp-Arg-Phe Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O MRQQMVZUHXUPEV-IHRRRGAJSA-N 0.000 description 7
- USNJAPJZSGTTPX-XVSYOHENSA-N Asp-Phe-Thr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O USNJAPJZSGTTPX-XVSYOHENSA-N 0.000 description 7
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 7
- MNYNCKZAEIAONY-XGEHTFHBSA-N Thr-Val-Ser Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O MNYNCKZAEIAONY-XGEHTFHBSA-N 0.000 description 7
- 150000007513 acids Chemical class 0.000 description 7
- 108010008685 alanyl-glutamyl-aspartic acid Proteins 0.000 description 7
- 238000003556 assay Methods 0.000 description 7
- 238000002648 combination therapy Methods 0.000 description 7
- 108010063718 gamma-glutamylaspartic acid Proteins 0.000 description 7
- 108010050848 glycylleucine Proteins 0.000 description 7
- 239000002953 phosphate buffered saline Substances 0.000 description 7
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 7
- 229920000053 polysorbate 80 Polymers 0.000 description 7
- 108010038745 tryptophylglycine Proteins 0.000 description 7
- JBGSZRYCXBPWGX-BQBZGAKWSA-N Ala-Arg-Gly Chemical compound OC(=O)CNC(=O)[C@@H](NC(=O)[C@@H](N)C)CCCN=C(N)N JBGSZRYCXBPWGX-BQBZGAKWSA-N 0.000 description 6
- VNYMOTCMNHJGTG-JBDRJPRFSA-N Ala-Ile-Ser Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O VNYMOTCMNHJGTG-JBDRJPRFSA-N 0.000 description 6
- MKJBPDLENBUHQU-CIUDSAMLSA-N Asn-Ser-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O MKJBPDLENBUHQU-CIUDSAMLSA-N 0.000 description 6
- SGVGIVDZLSHSEN-RYUDHWBXSA-N Gln-Tyr-Gly Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)NCC(O)=O SGVGIVDZLSHSEN-RYUDHWBXSA-N 0.000 description 6
- INGJLBQKTRJLFO-UKJIMTQDSA-N Glu-Ile-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H](N)CCC(O)=O INGJLBQKTRJLFO-UKJIMTQDSA-N 0.000 description 6
- 101000581981 Homo sapiens Neural cell adhesion molecule 1 Proteins 0.000 description 6
- 108060003951 Immunoglobulin Proteins 0.000 description 6
- 108020003285 Isocitrate lyase Proteins 0.000 description 6
- SBANPBVRHYIMRR-GARJFASQSA-N Leu-Ser-Pro Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CO)C(=O)N1CCC[C@@H]1C(=O)O)N SBANPBVRHYIMRR-GARJFASQSA-N 0.000 description 6
- SBANPBVRHYIMRR-UHFFFAOYSA-N Leu-Ser-Pro Natural products CC(C)CC(N)C(=O)NC(CO)C(=O)N1CCCC1C(O)=O SBANPBVRHYIMRR-UHFFFAOYSA-N 0.000 description 6
- MVJRBCJCRYGCKV-GVXVVHGQSA-N Leu-Val-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O MVJRBCJCRYGCKV-GVXVVHGQSA-N 0.000 description 6
- UWKNTTJNVSYXPC-CIUDSAMLSA-N Lys-Ala-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN UWKNTTJNVSYXPC-CIUDSAMLSA-N 0.000 description 6
- GQZMPWBZQALKJO-UWVGGRQHSA-N Lys-Gly-Arg Chemical compound [H]N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O GQZMPWBZQALKJO-UWVGGRQHSA-N 0.000 description 6
- 102100030301 MHC class I polypeptide-related sequence A Human genes 0.000 description 6
- 101100404853 Mus musculus Klrk1 gene Proteins 0.000 description 6
- 102100027347 Neural cell adhesion molecule 1 Human genes 0.000 description 6
- MCIXMYKSPQUMJG-SRVKXCTJSA-N Phe-Ser-Ser Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O MCIXMYKSPQUMJG-SRVKXCTJSA-N 0.000 description 6
- YMTLKLXDFCSCNX-BYPYZUCNSA-N Ser-Gly-Gly Chemical compound OC[C@H](N)C(=O)NCC(=O)NCC(O)=O YMTLKLXDFCSCNX-BYPYZUCNSA-N 0.000 description 6
- MQQBBLVOUUJKLH-HJPIBITLSA-N Ser-Ile-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O MQQBBLVOUUJKLH-HJPIBITLSA-N 0.000 description 6
- RWDVVSKYZBNDCO-MELADBBJSA-N Ser-Phe-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=CC=C2)NC(=O)[C@H](CO)N)C(=O)O RWDVVSKYZBNDCO-MELADBBJSA-N 0.000 description 6
- XJDMUQCLVSCRSJ-VZFHVOOUSA-N Ser-Thr-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O XJDMUQCLVSCRSJ-VZFHVOOUSA-N 0.000 description 6
- KIEIJCFVGZCUAS-MELADBBJSA-N Ser-Tyr-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=C(C=C2)O)NC(=O)[C@H](CO)N)C(=O)O KIEIJCFVGZCUAS-MELADBBJSA-N 0.000 description 6
- LGIMRDKGABDMBN-DCAQKATOSA-N Ser-Val-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CO)N LGIMRDKGABDMBN-DCAQKATOSA-N 0.000 description 6
- PKZIWSHDJYIPRH-JBACZVJFSA-N Trp-Tyr-Gln Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(O)=O PKZIWSHDJYIPRH-JBACZVJFSA-N 0.000 description 6
- 239000008365 aqueous carrier Substances 0.000 description 6
- 239000008228 bacteriostatic water for injection Substances 0.000 description 6
- 230000001472 cytotoxic effect Effects 0.000 description 6
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 6
- 229940126534 drug product Drugs 0.000 description 6
- 239000001963 growth medium Substances 0.000 description 6
- 102000044042 human KLRK1 Human genes 0.000 description 6
- 102000018358 immunoglobulin Human genes 0.000 description 6
- 230000003993 interaction Effects 0.000 description 6
- 230000001404 mediated effect Effects 0.000 description 6
- 239000010445 mica Substances 0.000 description 6
- 229910052618 mica group Inorganic materials 0.000 description 6
- 239000000825 pharmaceutical preparation Substances 0.000 description 6
- 229920005862 polyol Polymers 0.000 description 6
- 150000003077 polyols Chemical class 0.000 description 6
- KZNICNPSHKQLFF-UHFFFAOYSA-N succinimide Chemical compound O=C1CCC(=O)N1 KZNICNPSHKQLFF-UHFFFAOYSA-N 0.000 description 6
- 230000001225 therapeutic effect Effects 0.000 description 6
- 108010073969 valyllysine Proteins 0.000 description 6
- 101710145634 Antigen 1 Proteins 0.000 description 5
- VKKYFICVTYKFIO-CIUDSAMLSA-N Arg-Ala-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCN=C(N)N VKKYFICVTYKFIO-CIUDSAMLSA-N 0.000 description 5
- KYQNAIMCTRZLNP-QSFUFRPTSA-N Asp-Ile-Val Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C(C)C)C(O)=O KYQNAIMCTRZLNP-QSFUFRPTSA-N 0.000 description 5
- 108010087819 Fc receptors Proteins 0.000 description 5
- 102000009109 Fc receptors Human genes 0.000 description 5
- XZLLTYBONVKGLO-SDDRHHMPSA-N Gln-Lys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(=O)N)N)C(=O)O XZLLTYBONVKGLO-SDDRHHMPSA-N 0.000 description 5
- OTQSTOXRUBVWAP-NRPADANISA-N Gln-Ser-Val Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O OTQSTOXRUBVWAP-NRPADANISA-N 0.000 description 5
- OACQOWPRWGNKTP-AVGNSLFASA-N Gln-Tyr-Asp Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(O)=O)C(O)=O OACQOWPRWGNKTP-AVGNSLFASA-N 0.000 description 5
- PAQUJCSYVIBPLC-AVGNSLFASA-N Glu-Asp-Phe Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 PAQUJCSYVIBPLC-AVGNSLFASA-N 0.000 description 5
- ZYRXTRTUCAVNBQ-GVXVVHGQSA-N Glu-Val-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N ZYRXTRTUCAVNBQ-GVXVVHGQSA-N 0.000 description 5
- WNZOCXUOGVYYBJ-CDMKHQONSA-N Gly-Phe-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)CN)O WNZOCXUOGVYYBJ-CDMKHQONSA-N 0.000 description 5
- LBRCLQMZAHRTLV-ZKWXMUAHSA-N Ile-Gly-Ser Chemical compound CC[C@H](C)[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O LBRCLQMZAHRTLV-ZKWXMUAHSA-N 0.000 description 5
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 5
- AXZGZMGRBDQTEY-SRVKXCTJSA-N Leu-Gln-Met Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCSC)C(O)=O AXZGZMGRBDQTEY-SRVKXCTJSA-N 0.000 description 5
- KIZIOFNVSOSKJI-CIUDSAMLSA-N Leu-Ser-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N KIZIOFNVSOSKJI-CIUDSAMLSA-N 0.000 description 5
- SJDQOYTYNGZZJX-SRVKXCTJSA-N Met-Glu-Leu Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O SJDQOYTYNGZZJX-SRVKXCTJSA-N 0.000 description 5
- FGWUALWGCZJQDJ-URLPEUOOSA-N Phe-Thr-Ile Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O FGWUALWGCZJQDJ-URLPEUOOSA-N 0.000 description 5
- 239000004698 Polyethylene Substances 0.000 description 5
- JMVQDLDPDBXAAX-YUMQZZPRSA-N Pro-Gly-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1 JMVQDLDPDBXAAX-YUMQZZPRSA-N 0.000 description 5
- QEDMOZUJTGEIBF-FXQIFTODSA-N Ser-Arg-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(O)=O QEDMOZUJTGEIBF-FXQIFTODSA-N 0.000 description 5
- UQFYNFTYDHUIMI-WHFBIAKZSA-N Ser-Gly-Ala Chemical compound OC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](N)CO UQFYNFTYDHUIMI-WHFBIAKZSA-N 0.000 description 5
- KDGARKCAKHBEDB-NKWVEPMBSA-N Ser-Gly-Pro Chemical compound C1C[C@@H](N(C1)C(=O)CNC(=O)[C@H](CO)N)C(=O)O KDGARKCAKHBEDB-NKWVEPMBSA-N 0.000 description 5
- SFTZWNJFZYOLBD-ZDLURKLDSA-N Ser-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CO SFTZWNJFZYOLBD-ZDLURKLDSA-N 0.000 description 5
- QYSFWUIXDFJUDW-DCAQKATOSA-N Ser-Leu-Arg Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O QYSFWUIXDFJUDW-DCAQKATOSA-N 0.000 description 5
- UBRMZSHOOIVJPW-SRVKXCTJSA-N Ser-Leu-Lys Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(O)=O UBRMZSHOOIVJPW-SRVKXCTJSA-N 0.000 description 5
- RHAPJNVNWDBFQI-BQBZGAKWSA-N Ser-Pro-Gly Chemical compound OC[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O RHAPJNVNWDBFQI-BQBZGAKWSA-N 0.000 description 5
- 229930006000 Sucrose Natural products 0.000 description 5
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 5
- SVGAWGVHFIYAEE-JSGCOSHPSA-N Trp-Gly-Gln Chemical compound C1=CC=C2C(C[C@H](N)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(O)=O)=CNC2=C1 SVGAWGVHFIYAEE-JSGCOSHPSA-N 0.000 description 5
- VTFWAGGJDRSQFG-MELADBBJSA-N Tyr-Asn-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)N)NC(=O)[C@H](CC2=CC=C(C=C2)O)N)C(=O)O VTFWAGGJDRSQFG-MELADBBJSA-N 0.000 description 5
- JQTYTBPCSOAZHI-FXQIFTODSA-N Val-Ser-Cys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N JQTYTBPCSOAZHI-FXQIFTODSA-N 0.000 description 5
- 108010087924 alanylproline Proteins 0.000 description 5
- 230000006037 cell lysis Effects 0.000 description 5
- 231100000433 cytotoxic Toxicity 0.000 description 5
- 239000003599 detergent Substances 0.000 description 5
- 201000010099 disease Diseases 0.000 description 5
- 238000004090 dissolution Methods 0.000 description 5
- 239000013604 expression vector Substances 0.000 description 5
- 102000037865 fusion proteins Human genes 0.000 description 5
- 108020001507 fusion proteins Proteins 0.000 description 5
- 108010067216 glycyl-glycyl-glycine Proteins 0.000 description 5
- 108010018006 histidylserine Proteins 0.000 description 5
- 108010038320 lysylphenylalanine Proteins 0.000 description 5
- 238000002360 preparation method Methods 0.000 description 5
- 108010020755 prolyl-glycyl-glycine Proteins 0.000 description 5
- 238000000746 purification Methods 0.000 description 5
- 230000000284 resting effect Effects 0.000 description 5
- 239000011780 sodium chloride Substances 0.000 description 5
- 230000000638 stimulation Effects 0.000 description 5
- 239000005720 sucrose Substances 0.000 description 5
- 229940124597 therapeutic agent Drugs 0.000 description 5
- 108010035534 tyrosyl-leucyl-alanine Proteins 0.000 description 5
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N 100676-05-9 Natural products OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1 OWEGMIWEEQEYGQ-UHFFFAOYSA-N 0.000 description 4
- UAIUNKRWKOVEES-UHFFFAOYSA-N 3,3',5,5'-tetramethylbenzidine Chemical compound CC1=C(N)C(C)=CC(C=2C=C(C)C(N)=C(C)C=2)=C1 UAIUNKRWKOVEES-UHFFFAOYSA-N 0.000 description 4
- BUDNAJYVCUHLSV-ZLUOBGJFSA-N Ala-Asp-Ser Chemical compound C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O BUDNAJYVCUHLSV-ZLUOBGJFSA-N 0.000 description 4
- OTOXOKCIIQLMFH-KZVJFYERSA-N Arg-Ala-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCN=C(N)N OTOXOKCIIQLMFH-KZVJFYERSA-N 0.000 description 4
- ISJWBVIYRBAXEB-CIUDSAMLSA-N Arg-Ser-Glu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(O)=O ISJWBVIYRBAXEB-CIUDSAMLSA-N 0.000 description 4
- UGXYFDQFLVCDFC-CIUDSAMLSA-N Asn-Ser-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O UGXYFDQFLVCDFC-CIUDSAMLSA-N 0.000 description 4
- JBDLMLZNDRLDIX-HJGDQZAQSA-N Asn-Thr-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O JBDLMLZNDRLDIX-HJGDQZAQSA-N 0.000 description 4
- QXHVOUSPVAWEMX-ZLUOBGJFSA-N Asp-Asp-Ser Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O QXHVOUSPVAWEMX-ZLUOBGJFSA-N 0.000 description 4
- HTSSXFASOUSJQG-IHPCNDPISA-N Asp-Tyr-Trp Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O HTSSXFASOUSJQG-IHPCNDPISA-N 0.000 description 4
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 4
- 239000004606 Fillers/Extenders Substances 0.000 description 4
- LOJYQMFIIJVETK-WDSKDSINSA-N Gln-Gln Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(O)=O LOJYQMFIIJVETK-WDSKDSINSA-N 0.000 description 4
- ILKYYKRAULNYMS-JYJNAYRXSA-N Gln-Lys-Phe Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O ILKYYKRAULNYMS-JYJNAYRXSA-N 0.000 description 4
- YQPFCZVKMUVZIN-AUTRQRHGSA-N Glu-Val-Gln Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O YQPFCZVKMUVZIN-AUTRQRHGSA-N 0.000 description 4
- CQZDZKRHFWJXDF-WDSKDSINSA-N Gly-Gln-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CN CQZDZKRHFWJXDF-WDSKDSINSA-N 0.000 description 4
- YWAQATDNEKZFFK-BYPYZUCNSA-N Gly-Gly-Ser Chemical compound NCC(=O)NCC(=O)N[C@@H](CO)C(O)=O YWAQATDNEKZFFK-BYPYZUCNSA-N 0.000 description 4
- BHPQOIPBLYJNAW-NGZCFLSTSA-N Gly-Ile-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)CN BHPQOIPBLYJNAW-NGZCFLSTSA-N 0.000 description 4
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 4
- TYYLDKGBCJGJGW-UHFFFAOYSA-N L-tryptophan-L-tyrosine Natural products C=1NC2=CC=CC=C2C=1CC(N)C(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 TYYLDKGBCJGJGW-UHFFFAOYSA-N 0.000 description 4
- LJBVRCDPWOJOEK-PPCPHDFISA-N Leu-Thr-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O LJBVRCDPWOJOEK-PPCPHDFISA-N 0.000 description 4
- QESXLSQLQHHTIX-RHYQMDGZSA-N Leu-Val-Thr Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QESXLSQLQHHTIX-RHYQMDGZSA-N 0.000 description 4
- SKRGVGLIRUGANF-AVGNSLFASA-N Lys-Leu-Glu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O SKRGVGLIRUGANF-AVGNSLFASA-N 0.000 description 4
- GUBGYTABKSRVRQ-PICCSMPSSA-N Maltose Natural products O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-PICCSMPSSA-N 0.000 description 4
- 229930191564 Monensin Natural products 0.000 description 4
- GAOZTHIDHYLHMS-UHFFFAOYSA-N Monensin A Natural products O1C(CC)(C2C(CC(O2)C2C(CC(C)C(O)(CO)O2)C)C)CCC1C(O1)(C)CCC21CC(O)C(C)C(C(C)C(OC)C(C)C(O)=O)O2 GAOZTHIDHYLHMS-UHFFFAOYSA-N 0.000 description 4
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 4
- VCYJKOLZYPYGJV-AVGNSLFASA-N Pro-Arg-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(O)=O VCYJKOLZYPYGJV-AVGNSLFASA-N 0.000 description 4
- MGDFPGCFVJFITQ-CIUDSAMLSA-N Pro-Glu-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O MGDFPGCFVJFITQ-CIUDSAMLSA-N 0.000 description 4
- WDXYVIIVDIDOSX-DCAQKATOSA-N Ser-Arg-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CO)CCCN=C(N)N WDXYVIIVDIDOSX-DCAQKATOSA-N 0.000 description 4
- UIPXCLNLUUAMJU-JBDRJPRFSA-N Ser-Ile-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O UIPXCLNLUUAMJU-JBDRJPRFSA-N 0.000 description 4
- YUJLIIRMIAGMCQ-CIUDSAMLSA-N Ser-Leu-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YUJLIIRMIAGMCQ-CIUDSAMLSA-N 0.000 description 4
- RXUOAOOZIWABBW-XGEHTFHBSA-N Ser-Thr-Arg Chemical compound OC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@H](C(O)=O)CCCN=C(N)N RXUOAOOZIWABBW-XGEHTFHBSA-N 0.000 description 4
- YOPQYBJJNSIQGZ-JNPHEJMOSA-N Thr-Tyr-Tyr Chemical compound C([C@H](NC(=O)[C@@H](N)[C@H](O)C)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=C(O)C=C1 YOPQYBJJNSIQGZ-JNPHEJMOSA-N 0.000 description 4
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 4
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 4
- LMKKMCGTDANZTR-BZSNNMDCSA-N Tyr-Phe-Asp Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC(O)=O)C(O)=O)C1=CC=C(O)C=C1 LMKKMCGTDANZTR-BZSNNMDCSA-N 0.000 description 4
- ZPFLBLFITJCBTP-QWRGUYRKSA-N Tyr-Ser-Gly Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(=O)NCC(O)=O ZPFLBLFITJCBTP-QWRGUYRKSA-N 0.000 description 4
- WUFHZIRMAZZWRS-OSUNSFLBSA-N Val-Thr-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](C(C)C)N WUFHZIRMAZZWRS-OSUNSFLBSA-N 0.000 description 4
- 238000002835 absorbance Methods 0.000 description 4
- 108010081404 acein-2 Proteins 0.000 description 4
- 208000009956 adenocarcinoma Diseases 0.000 description 4
- 108010038633 aspartylglutamate Proteins 0.000 description 4
- 239000011324 bead Substances 0.000 description 4
- GUBGYTABKSRVRQ-QUYVBRFLSA-N beta-maltose Chemical compound OC[C@H]1O[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1O GUBGYTABKSRVRQ-QUYVBRFLSA-N 0.000 description 4
- 229940098773 bovine serum albumin Drugs 0.000 description 4
- KQNZDYYTLMIZCT-KQPMLPITSA-N brefeldin A Chemical compound O[C@@H]1\C=C\C(=O)O[C@@H](C)CCC\C=C\[C@@H]2C[C@H](O)C[C@H]21 KQNZDYYTLMIZCT-KQPMLPITSA-N 0.000 description 4
- 239000007853 buffer solution Substances 0.000 description 4
- 239000000969 carrier Substances 0.000 description 4
- 230000006378 damage Effects 0.000 description 4
- 230000009615 deamination Effects 0.000 description 4
- 238000006481 deamination reaction Methods 0.000 description 4
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 4
- KDQPSPMLNJTZAL-UHFFFAOYSA-L disodium hydrogenphosphate dihydrate Chemical compound O.O.[Na+].[Na+].OP([O-])([O-])=O KDQPSPMLNJTZAL-UHFFFAOYSA-L 0.000 description 4
- 238000002474 experimental method Methods 0.000 description 4
- XBGGUPMXALFZOT-UHFFFAOYSA-N glycyl-L-tyrosine hemihydrate Natural products NCC(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 XBGGUPMXALFZOT-UHFFFAOYSA-N 0.000 description 4
- 239000000710 homodimer Substances 0.000 description 4
- 239000000543 intermediate Substances 0.000 description 4
- 229960005358 monensin Drugs 0.000 description 4
- GAOZTHIDHYLHMS-KEOBGNEYSA-N monensin A Chemical compound C([C@@](O1)(C)[C@H]2CC[C@@](O2)(CC)[C@H]2[C@H](C[C@@H](O2)[C@@H]2[C@H](C[C@@H](C)[C@](O)(CO)O2)C)C)C[C@@]21C[C@H](O)[C@@H](C)[C@@H]([C@@H](C)[C@@H](OC)[C@H](C)C(O)=O)O2 GAOZTHIDHYLHMS-KEOBGNEYSA-N 0.000 description 4
- 230000009871 nonspecific binding Effects 0.000 description 4
- 239000006174 pH buffer Substances 0.000 description 4
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 4
- 229940068968 polysorbate 80 Drugs 0.000 description 4
- 239000000047 product Substances 0.000 description 4
- 239000011734 sodium Substances 0.000 description 4
- 239000001509 sodium citrate Substances 0.000 description 4
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 4
- 239000004094 surface-active agent Substances 0.000 description 4
- 210000001519 tissue Anatomy 0.000 description 4
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 4
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 3
- OOSZCNKVJAVHJI-UHFFFAOYSA-N 1-[(4-fluorophenyl)methyl]piperazine Chemical compound C1=CC(F)=CC=C1CN1CCNCC1 OOSZCNKVJAVHJI-UHFFFAOYSA-N 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- AWAXZRDKUHOPBO-GUBZILKMSA-N Ala-Gln-Lys Chemical compound C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(O)=O AWAXZRDKUHOPBO-GUBZILKMSA-N 0.000 description 3
- SDZRIBWEVVRDQI-CIUDSAMLSA-N Ala-Lys-Asp Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(O)=O SDZRIBWEVVRDQI-CIUDSAMLSA-N 0.000 description 3
- AWNAEZICPNGAJK-FXQIFTODSA-N Ala-Met-Ser Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CO)C(O)=O AWNAEZICPNGAJK-FXQIFTODSA-N 0.000 description 3
- NCQMBSJGJMYKCK-ZLUOBGJFSA-N Ala-Ser-Ser Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O NCQMBSJGJMYKCK-ZLUOBGJFSA-N 0.000 description 3
- HUZGPXBILPMCHM-IHRRRGAJSA-N Asn-Arg-Phe Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O HUZGPXBILPMCHM-IHRRRGAJSA-N 0.000 description 3
- DXVMJJNAOVECBA-WHFBIAKZSA-N Asn-Gly-Asn Chemical compound NC(=O)C[C@H](N)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(O)=O DXVMJJNAOVECBA-WHFBIAKZSA-N 0.000 description 3
- MGSVBZIBCCKGCY-ZLUOBGJFSA-N Asp-Ser-Ser Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O MGSVBZIBCCKGCY-ZLUOBGJFSA-N 0.000 description 3
- CZIVKMOEXPILDK-SRVKXCTJSA-N Asp-Tyr-Ser Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(O)=O CZIVKMOEXPILDK-SRVKXCTJSA-N 0.000 description 3
- 208000003950 B-cell lymphoma Diseases 0.000 description 3
- 235000011299 Brassica oleracea var botrytis Nutrition 0.000 description 3
- 240000003259 Brassica oleracea var. botrytis Species 0.000 description 3
- 102000008203 CTLA-4 Antigen Human genes 0.000 description 3
- 108010021064 CTLA-4 Antigen Proteins 0.000 description 3
- 101100506090 Caenorhabditis elegans hil-2 gene Proteins 0.000 description 3
- 206010008342 Cervix carcinoma Diseases 0.000 description 3
- 108010019670 Chimeric Antigen Receptors Proteins 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 3
- 206010009944 Colon cancer Diseases 0.000 description 3
- NIXHTNJAGGFBAW-CIUDSAMLSA-N Cys-Lys-Ser Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CS)N NIXHTNJAGGFBAW-CIUDSAMLSA-N 0.000 description 3
- 102000004127 Cytokines Human genes 0.000 description 3
- 108090000695 Cytokines Proteins 0.000 description 3
- 238000012286 ELISA Assay Methods 0.000 description 3
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 3
- IKFZXRLDMYWNBU-YUMQZZPRSA-N Gln-Gly-Arg Chemical compound NC(=O)CC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCCN=C(N)N IKFZXRLDMYWNBU-YUMQZZPRSA-N 0.000 description 3
- FQCILXROGNOZON-YUMQZZPRSA-N Gln-Pro-Gly Chemical compound NC(=O)CC[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O FQCILXROGNOZON-YUMQZZPRSA-N 0.000 description 3
- WPJDPEOQUIXXOY-AVGNSLFASA-N Gln-Tyr-Asn Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CCC(=O)N)N)O WPJDPEOQUIXXOY-AVGNSLFASA-N 0.000 description 3
- XTZDZAXYPDISRR-MNXVOIDGSA-N Glu-Ile-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N XTZDZAXYPDISRR-MNXVOIDGSA-N 0.000 description 3
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Chemical compound OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 3
- WTUSRDZLLWGYAT-KCTSRDHCSA-N Gly-Trp-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)NC(=O)CN WTUSRDZLLWGYAT-KCTSRDHCSA-N 0.000 description 3
- FOCSWPCHUDVNLP-PMVMPFDFSA-N His-Trp-Tyr Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC3=CC=C(C=C3)O)C(=O)O)NC(=O)[C@H](CC4=CN=CN4)N FOCSWPCHUDVNLP-PMVMPFDFSA-N 0.000 description 3
- PRTZQMBYUZFSFA-XEGUGMAKSA-N Ile-Tyr-Gly Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)NCC(=O)O)N PRTZQMBYUZFSFA-XEGUGMAKSA-N 0.000 description 3
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 3
- 229940076838 Immune checkpoint inhibitor Drugs 0.000 description 3
- 108010065920 Insulin Lispro Proteins 0.000 description 3
- 102000002698 KIR Receptors Human genes 0.000 description 3
- 108010043610 KIR Receptors Proteins 0.000 description 3
- LZDNBBYBDGBADK-UHFFFAOYSA-N L-valyl-L-tryptophan Natural products C1=CC=C2C(CC(NC(=O)C(N)C(C)C)C(O)=O)=CNC2=C1 LZDNBBYBDGBADK-UHFFFAOYSA-N 0.000 description 3
- ZTLGVASZOIKNIX-DCAQKATOSA-N Leu-Gln-Glu Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N ZTLGVASZOIKNIX-DCAQKATOSA-N 0.000 description 3
- QNBVTHNJGCOVFA-AVGNSLFASA-N Leu-Leu-Glu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CCC(O)=O QNBVTHNJGCOVFA-AVGNSLFASA-N 0.000 description 3
- VUBIPAHVHMZHCM-KKUMJFAQSA-N Leu-Tyr-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CO)C(O)=O)CC1=CC=C(O)C=C1 VUBIPAHVHMZHCM-KKUMJFAQSA-N 0.000 description 3
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 3
- 208000031422 Lymphocytic Chronic B-Cell Leukemia Diseases 0.000 description 3
- PXHCFKXNSBJSTQ-KKUMJFAQSA-N Lys-Asn-Tyr Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CCCCN)N)O PXHCFKXNSBJSTQ-KKUMJFAQSA-N 0.000 description 3
- GILLQRYAWOMHED-DCAQKATOSA-N Lys-Val-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCCCN GILLQRYAWOMHED-DCAQKATOSA-N 0.000 description 3
- WUGMRIBZSVSJNP-UHFFFAOYSA-N N-L-alanyl-L-tryptophan Natural products C1=CC=C2C(CC(NC(=O)C(N)C)C(O)=O)=CNC2=C1 WUGMRIBZSVSJNP-UHFFFAOYSA-N 0.000 description 3
- 108010066427 N-valyltryptophan Proteins 0.000 description 3
- 101100068676 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) gln-1 gene Proteins 0.000 description 3
- 108091028043 Nucleic acid sequence Proteins 0.000 description 3
- YYKZDTVQHTUKDW-RYUDHWBXSA-N Phe-Gly-Gln Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)NCC(=O)N[C@@H](CCC(=O)N)C(=O)O)N YYKZDTVQHTUKDW-RYUDHWBXSA-N 0.000 description 3
- BPCLGWHVPVTTFM-QWRGUYRKSA-N Phe-Ser-Gly Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)NCC(O)=O BPCLGWHVPVTTFM-QWRGUYRKSA-N 0.000 description 3
- ZSKJPKFTPQCPIH-RCWTZXSCSA-N Pro-Arg-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O ZSKJPKFTPQCPIH-RCWTZXSCSA-N 0.000 description 3
- 102100040678 Programmed cell death protein 1 Human genes 0.000 description 3
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 3
- 208000015634 Rectal Neoplasms Diseases 0.000 description 3
- 206010039491 Sarcoma Diseases 0.000 description 3
- WTWGOQRNRFHFQD-JBDRJPRFSA-N Ser-Ala-Ile Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O WTWGOQRNRFHFQD-JBDRJPRFSA-N 0.000 description 3
- YQHZVYJAGWMHES-ZLUOBGJFSA-N Ser-Ala-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O YQHZVYJAGWMHES-ZLUOBGJFSA-N 0.000 description 3
- FUMGHWDRRFCKEP-CIUDSAMLSA-N Ser-Leu-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(O)=O FUMGHWDRRFCKEP-CIUDSAMLSA-N 0.000 description 3
- WLJPJRGQRNCIQS-ZLUOBGJFSA-N Ser-Ser-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O WLJPJRGQRNCIQS-ZLUOBGJFSA-N 0.000 description 3
- JURQXQBJKUHGJS-UHFFFAOYSA-N Ser-Ser-Ser-Ser Chemical compound OCC(N)C(=O)NC(CO)C(=O)NC(CO)C(=O)NC(CO)C(O)=O JURQXQBJKUHGJS-UHFFFAOYSA-N 0.000 description 3
- OLKICIBQRVSQMA-SRVKXCTJSA-N Ser-Ser-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O OLKICIBQRVSQMA-SRVKXCTJSA-N 0.000 description 3
- NJEMRSFGDNECGF-GCJQMDKQSA-N Thr-Ala-Asp Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CC(O)=O NJEMRSFGDNECGF-GCJQMDKQSA-N 0.000 description 3
- CAGTXGDOIFXLPC-KZVJFYERSA-N Thr-Arg-Ala Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](C)C(O)=O)CCCN=C(N)N CAGTXGDOIFXLPC-KZVJFYERSA-N 0.000 description 3
- JAWUQFCGNVEDRN-MEYUZBJRSA-N Thr-Tyr-Leu Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC(C)C)C(=O)O)N)O JAWUQFCGNVEDRN-MEYUZBJRSA-N 0.000 description 3
- KZTLZZQTJMCGIP-ZJDVBMNYSA-N Thr-Val-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O KZTLZZQTJMCGIP-ZJDVBMNYSA-N 0.000 description 3
- 102100036922 Tumor necrosis factor ligand superfamily member 13B Human genes 0.000 description 3
- GULIUBBXCYPDJU-CQDKDKBSSA-N Tyr-Leu-Ala Chemical compound [O-]C(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]([NH3+])CC1=CC=C(O)C=C1 GULIUBBXCYPDJU-CQDKDKBSSA-N 0.000 description 3
- NSGZILIDHCIZAM-KKUMJFAQSA-N Tyr-Leu-Ser Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)N NSGZILIDHCIZAM-KKUMJFAQSA-N 0.000 description 3
- ZYVAAYAOTVJBSS-GMVOTWDCSA-N Tyr-Trp-Ala Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](C)C(O)=O ZYVAAYAOTVJBSS-GMVOTWDCSA-N 0.000 description 3
- WYOBRXPIZVKNMF-IRXDYDNUSA-N Tyr-Tyr-Gly Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)NCC(O)=O)C1=CC=C(O)C=C1 WYOBRXPIZVKNMF-IRXDYDNUSA-N 0.000 description 3
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 3
- KOSRFJWDECSPRO-UHFFFAOYSA-N alpha-L-glutamyl-L-glutamic acid Natural products OC(=O)CCC(N)C(=O)NC(CCC(O)=O)C(O)=O KOSRFJWDECSPRO-UHFFFAOYSA-N 0.000 description 3
- 125000000539 amino acid group Chemical group 0.000 description 3
- 108010013835 arginine glutamate Proteins 0.000 description 3
- 108010040443 aspartyl-aspartic acid Proteins 0.000 description 3
- 230000001580 bacterial effect Effects 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- JUMGSHROWPPKFX-UHFFFAOYSA-N brefeldin-A Natural products CC1CCCC=CC2(C)CC(O)CC2(C)C(O)C=CC(=O)O1 JUMGSHROWPPKFX-UHFFFAOYSA-N 0.000 description 3
- 230000036755 cellular response Effects 0.000 description 3
- 201000010881 cervical cancer Diseases 0.000 description 3
- 229960004106 citric acid Drugs 0.000 description 3
- 208000029742 colonic neoplasm Diseases 0.000 description 3
- 230000000295 complement effect Effects 0.000 description 3
- 238000004132 cross linking Methods 0.000 description 3
- 230000003013 cytotoxicity Effects 0.000 description 3
- 231100000135 cytotoxicity Toxicity 0.000 description 3
- 238000000432 density-gradient centrifugation Methods 0.000 description 3
- 238000013461 design Methods 0.000 description 3
- 108010054812 diprotin A Proteins 0.000 description 3
- 150000002016 disaccharides Chemical class 0.000 description 3
- 238000012377 drug delivery Methods 0.000 description 3
- 239000012636 effector Substances 0.000 description 3
- 238000004108 freeze drying Methods 0.000 description 3
- 108010078144 glutaminyl-glycine Proteins 0.000 description 3
- 108010074027 glycyl-seryl-phenylalanine Proteins 0.000 description 3
- 108010087823 glycyltyrosine Proteins 0.000 description 3
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 3
- 108010040030 histidinoalanine Proteins 0.000 description 3
- 230000002209 hydrophobic effect Effects 0.000 description 3
- 239000012274 immune-checkpoint protein inhibitor Substances 0.000 description 3
- 229940072221 immunoglobulins Drugs 0.000 description 3
- 230000002401 inhibitory effect Effects 0.000 description 3
- 238000002372 labelling Methods 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 238000002625 monoclonal antibody therapy Methods 0.000 description 3
- 231100000252 nontoxic Toxicity 0.000 description 3
- 230000003000 nontoxic effect Effects 0.000 description 3
- 102000013415 peroxidase activity proteins Human genes 0.000 description 3
- 108040007629 peroxidase activity proteins Proteins 0.000 description 3
- 230000002974 pharmacogenomic effect Effects 0.000 description 3
- 239000002504 physiological saline solution Substances 0.000 description 3
- 206010038038 rectal cancer Diseases 0.000 description 3
- 201000001275 rectum cancer Diseases 0.000 description 3
- 230000011664 signaling Effects 0.000 description 3
- 229910052708 sodium Inorganic materials 0.000 description 3
- 229940074545 sodium dihydrogen phosphate dihydrate Drugs 0.000 description 3
- 239000003381 stabilizer Substances 0.000 description 3
- 238000007920 subcutaneous administration Methods 0.000 description 3
- 229960002317 succinimide Drugs 0.000 description 3
- 235000000346 sugar Nutrition 0.000 description 3
- 229940121358 tyrosine kinase inhibitor Drugs 0.000 description 3
- 239000005483 tyrosine kinase inhibitor Substances 0.000 description 3
- JNTMAZFVYNDPLB-PEDHHIEDSA-N (2S,3S)-2-[[[(2S)-1-[(2S,3S)-2-amino-3-methyl-1-oxopentyl]-2-pyrrolidinyl]-oxomethyl]amino]-3-methylpentanoic acid Chemical compound CC[C@H](C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(O)=O JNTMAZFVYNDPLB-PEDHHIEDSA-N 0.000 description 2
- 241000251468 Actinopterygii Species 0.000 description 2
- 208000031261 Acute myeloid leukaemia Diseases 0.000 description 2
- XQJAFSDFQZPYCU-UWJYBYFXSA-N Ala-Asn-Tyr Chemical compound C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)N XQJAFSDFQZPYCU-UWJYBYFXSA-N 0.000 description 2
- SIGTYDNEPYEXGK-ZANVPECISA-N Ala-Gly-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)CNC(=O)[C@@H](N)C)C(O)=O)=CNC2=C1 SIGTYDNEPYEXGK-ZANVPECISA-N 0.000 description 2
- MDNAVFBZPROEHO-DCAQKATOSA-N Ala-Lys-Val Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O MDNAVFBZPROEHO-DCAQKATOSA-N 0.000 description 2
- MDNAVFBZPROEHO-UHFFFAOYSA-N Ala-Lys-Val Natural products CC(C)C(C(O)=O)NC(=O)C(NC(=O)C(C)N)CCCCN MDNAVFBZPROEHO-UHFFFAOYSA-N 0.000 description 2
- BHTBAVZSZCQZPT-GUBZILKMSA-N Ala-Pro-Met Chemical compound CSCC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)N BHTBAVZSZCQZPT-GUBZILKMSA-N 0.000 description 2
- OEVCHROQUIVQFZ-YTLHQDLWSA-N Ala-Thr-Ala Chemical compound C[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](C)C(O)=O OEVCHROQUIVQFZ-YTLHQDLWSA-N 0.000 description 2
- WNHNMKOFKCHKKD-BFHQHQDPSA-N Ala-Thr-Gly Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(O)=O WNHNMKOFKCHKKD-BFHQHQDPSA-N 0.000 description 2
- MUGAESARFRGOTQ-IGNZVWTISA-N Ala-Tyr-Tyr Chemical compound C[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O)N MUGAESARFRGOTQ-IGNZVWTISA-N 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-M Aminoacetate Chemical compound NCC([O-])=O DHMQDGOQFOQNFH-UHFFFAOYSA-M 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 2
- YSUVMPICYVWRBX-VEVYYDQMSA-N Arg-Asp-Thr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O YSUVMPICYVWRBX-VEVYYDQMSA-N 0.000 description 2
- IGFJVXOATGZTHD-UHFFFAOYSA-N Arg-Phe-His Natural products NC(CCNC(=N)N)C(=O)NC(Cc1ccccc1)C(=O)NC(Cc2c[nH]cn2)C(=O)O IGFJVXOATGZTHD-UHFFFAOYSA-N 0.000 description 2
- QLSRIZIDQXDQHK-RCWTZXSCSA-N Arg-Val-Thr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QLSRIZIDQXDQHK-RCWTZXSCSA-N 0.000 description 2
- QNJIRRVTOXNGMH-GUBZILKMSA-N Asn-Gln-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CC(N)=O QNJIRRVTOXNGMH-GUBZILKMSA-N 0.000 description 2
- NVWJMQNYLYWVNQ-BYULHYEWSA-N Asn-Ile-Gly Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(O)=O NVWJMQNYLYWVNQ-BYULHYEWSA-N 0.000 description 2
- JPPLRQVZMZFOSX-UWJYBYFXSA-N Asn-Tyr-Ala Chemical compound NC(=O)C[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](C)C(O)=O)CC1=CC=C(O)C=C1 JPPLRQVZMZFOSX-UWJYBYFXSA-N 0.000 description 2
- DBWYWXNMZZYIRY-LPEHRKFASA-N Asp-Arg-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(=O)O)N)C(=O)O DBWYWXNMZZYIRY-LPEHRKFASA-N 0.000 description 2
- ZCKYZTGLXIEOKS-CIUDSAMLSA-N Asp-Asp-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CC(=O)O)N ZCKYZTGLXIEOKS-CIUDSAMLSA-N 0.000 description 2
- JSHWXQIZOCVWIA-ZKWXMUAHSA-N Asp-Ser-Val Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O JSHWXQIZOCVWIA-ZKWXMUAHSA-N 0.000 description 2
- GCACQYDBDHRVGE-LKXGYXEUSA-N Asp-Thr-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H]([C@H](O)C)NC(=O)[C@@H](N)CC(O)=O GCACQYDBDHRVGE-LKXGYXEUSA-N 0.000 description 2
- XWKPSMRPIKKDDU-RCOVLWMOSA-N Asp-Val-Gly Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O XWKPSMRPIKKDDU-RCOVLWMOSA-N 0.000 description 2
- 108010028006 B-Cell Activating Factor Proteins 0.000 description 2
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 description 2
- 206010005003 Bladder cancer Diseases 0.000 description 2
- 101150013553 CD40 gene Proteins 0.000 description 2
- 101100355609 Caenorhabditis elegans rae-1 gene Proteins 0.000 description 2
- 241000282472 Canis lupus familiaris Species 0.000 description 2
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 2
- YASYEJJMZJALEJ-UHFFFAOYSA-N Citric acid monohydrate Chemical compound O.OC(=O)CC(O)(C(O)=O)CC(O)=O YASYEJJMZJALEJ-UHFFFAOYSA-N 0.000 description 2
- PTOAARAWEBMLNO-KVQBGUIXSA-N Cladribine Chemical compound C1=NC=2C(N)=NC(Cl)=NC=2N1[C@H]1C[C@H](O)[C@@H](CO)O1 PTOAARAWEBMLNO-KVQBGUIXSA-N 0.000 description 2
- AMRLSQGGERHDHJ-FXQIFTODSA-N Cys-Ala-Arg Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O AMRLSQGGERHDHJ-FXQIFTODSA-N 0.000 description 2
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 2
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 2
- 108010009540 DNA (Cytosine-5-)-Methyltransferase 1 Proteins 0.000 description 2
- 102100036279 DNA (cytosine-5)-methyltransferase 1 Human genes 0.000 description 2
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 2
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 2
- 229910052693 Europium Inorganic materials 0.000 description 2
- 208000006168 Ewing Sarcoma Diseases 0.000 description 2
- 241000282326 Felis catus Species 0.000 description 2
- NVEASDQHBRZPSU-BQBZGAKWSA-N Gln-Gln-Gly Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)NCC(O)=O NVEASDQHBRZPSU-BQBZGAKWSA-N 0.000 description 2
- GNMQDOGFWYWPNM-LAEOZQHASA-N Gln-Gly-Ile Chemical compound CC[C@H](C)[C@H](NC(=O)CNC(=O)[C@@H](N)CCC(N)=O)C(O)=O GNMQDOGFWYWPNM-LAEOZQHASA-N 0.000 description 2
- ORYMMTRPKVTGSJ-XVKPBYJWSA-N Gln-Gly-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CCC(N)=O ORYMMTRPKVTGSJ-XVKPBYJWSA-N 0.000 description 2
- LGIKBBLQVSWUGK-DCAQKATOSA-N Gln-Leu-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O LGIKBBLQVSWUGK-DCAQKATOSA-N 0.000 description 2
- OSCLNNWLKKIQJM-WDSKDSINSA-N Gln-Ser-Gly Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)NCC(O)=O OSCLNNWLKKIQJM-WDSKDSINSA-N 0.000 description 2
- CMBXOSFZCFGDLE-IHRRRGAJSA-N Gln-Tyr-Gln Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CCC(=O)N)N)O CMBXOSFZCFGDLE-IHRRRGAJSA-N 0.000 description 2
- WIMVKDYAKRAUCG-IHRRRGAJSA-N Gln-Tyr-Glu Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](CCC(=O)N)N)O WIMVKDYAKRAUCG-IHRRRGAJSA-N 0.000 description 2
- BJVBMSTUUWGZKX-JYJNAYRXSA-N Gln-Tyr-His Chemical compound N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O BJVBMSTUUWGZKX-JYJNAYRXSA-N 0.000 description 2
- LKDIBBOKUAASNP-FXQIFTODSA-N Glu-Ala-Glu Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O LKDIBBOKUAASNP-FXQIFTODSA-N 0.000 description 2
- WATXSTJXNBOHKD-LAEOZQHASA-N Glu-Asp-Val Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O WATXSTJXNBOHKD-LAEOZQHASA-N 0.000 description 2
- JWNZHMSRZXXGTM-XKBZYTNZSA-N Glu-Ser-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O JWNZHMSRZXXGTM-XKBZYTNZSA-N 0.000 description 2
- PYTZFYUXZZHOAD-WHFBIAKZSA-N Gly-Ala-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)CN PYTZFYUXZZHOAD-WHFBIAKZSA-N 0.000 description 2
- UGVQELHRNUDMAA-BYPYZUCNSA-N Gly-Ala-Gly Chemical compound [NH3+]CC(=O)N[C@@H](C)C(=O)NCC([O-])=O UGVQELHRNUDMAA-BYPYZUCNSA-N 0.000 description 2
- CUYLIWAAAYJKJH-RYUDHWBXSA-N Gly-Glu-Tyr Chemical compound NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 CUYLIWAAAYJKJH-RYUDHWBXSA-N 0.000 description 2
- KMSGYZQRXPUKGI-BYPYZUCNSA-N Gly-Gly-Asn Chemical compound NCC(=O)NCC(=O)N[C@H](C(O)=O)CC(N)=O KMSGYZQRXPUKGI-BYPYZUCNSA-N 0.000 description 2
- XMPXVJIDADUOQB-RCOVLWMOSA-N Gly-Gly-Ile Chemical compound CC[C@H](C)[C@@H](C([O-])=O)NC(=O)CNC(=O)C[NH3+] XMPXVJIDADUOQB-RCOVLWMOSA-N 0.000 description 2
- INLIXXRWNUKVCF-JTQLQIEISA-N Gly-Gly-Tyr Chemical compound NCC(=O)NCC(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 INLIXXRWNUKVCF-JTQLQIEISA-N 0.000 description 2
- TVUWMSBGMVAHSJ-KBPBESRZSA-N Gly-Leu-Phe Chemical compound NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 TVUWMSBGMVAHSJ-KBPBESRZSA-N 0.000 description 2
- OJNZVYSGVYLQIN-BQBZGAKWSA-N Gly-Met-Asp Chemical compound [H]NCC(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(O)=O OJNZVYSGVYLQIN-BQBZGAKWSA-N 0.000 description 2
- MKIAPEZXQDILRR-YUMQZZPRSA-N Gly-Ser-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)CN MKIAPEZXQDILRR-YUMQZZPRSA-N 0.000 description 2
- ZZWUYQXMIFTIIY-WEDXCCLWSA-N Gly-Thr-Leu Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O ZZWUYQXMIFTIIY-WEDXCCLWSA-N 0.000 description 2
- GBYYQVBXFVDJPJ-WLTAIBSBSA-N Gly-Tyr-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)CN)O GBYYQVBXFVDJPJ-WLTAIBSBSA-N 0.000 description 2
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 2
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 2
- 102100039620 Granulocyte-macrophage colony-stimulating factor Human genes 0.000 description 2
- 206010073073 Hepatobiliary cancer Diseases 0.000 description 2
- LSQHWKPPOFDHHZ-YUMQZZPRSA-N His-Asp-Gly Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)NCC(=O)O)N LSQHWKPPOFDHHZ-YUMQZZPRSA-N 0.000 description 2
- ZHHLTWUOWXHVQJ-YUMQZZPRSA-N His-Ser-Gly Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CO)C(=O)NCC(=O)O)N ZHHLTWUOWXHVQJ-YUMQZZPRSA-N 0.000 description 2
- ASCFJMSGKUIRDU-ZPFDUUQYSA-N Ile-Arg-Gln Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(O)=O ASCFJMSGKUIRDU-ZPFDUUQYSA-N 0.000 description 2
- LVQDUPQUJZWKSU-PYJNHQTQSA-N Ile-Arg-His Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N LVQDUPQUJZWKSU-PYJNHQTQSA-N 0.000 description 2
- IITVUURPOYGCTD-NAKRPEOUSA-N Ile-Pro-Ala Chemical compound CC[C@H](C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O IITVUURPOYGCTD-NAKRPEOUSA-N 0.000 description 2
- FBGXMKUWQFPHFB-JBDRJPRFSA-N Ile-Ser-Cys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N FBGXMKUWQFPHFB-JBDRJPRFSA-N 0.000 description 2
- HXIDVIFHRYRXLZ-NAKRPEOUSA-N Ile-Ser-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)O)N HXIDVIFHRYRXLZ-NAKRPEOUSA-N 0.000 description 2
- DGTOKVBDZXJHNZ-WZLNRYEVSA-N Ile-Thr-Tyr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)N DGTOKVBDZXJHNZ-WZLNRYEVSA-N 0.000 description 2
- NXRNRBOKDBIVKQ-CXTHYWKRSA-N Ile-Tyr-Tyr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O)N NXRNRBOKDBIVKQ-CXTHYWKRSA-N 0.000 description 2
- 102000037984 Inhibitory immune checkpoint proteins Human genes 0.000 description 2
- 108091008026 Inhibitory immune checkpoint proteins Proteins 0.000 description 2
- PWWVAXIEGOYWEE-UHFFFAOYSA-N Isophenergan Chemical compound C1=CC=C2N(CC(C)N(C)C)C3=CC=CC=C3SC2=C1 PWWVAXIEGOYWEE-UHFFFAOYSA-N 0.000 description 2
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 2
- SENJXOPIZNYLHU-UHFFFAOYSA-N L-leucyl-L-arginine Natural products CC(C)CC(N)C(=O)NC(C(O)=O)CCCN=C(N)N SENJXOPIZNYLHU-UHFFFAOYSA-N 0.000 description 2
- USTCFDAQCLDPBD-XIRDDKMYSA-N Leu-Asn-Trp Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)N USTCFDAQCLDPBD-XIRDDKMYSA-N 0.000 description 2
- VQPPIMUZCZCOIL-GUBZILKMSA-N Leu-Gln-Ala Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(O)=O VQPPIMUZCZCOIL-GUBZILKMSA-N 0.000 description 2
- FQZPTCNSNPWHLJ-AVGNSLFASA-N Leu-Gln-Lys Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(O)=O FQZPTCNSNPWHLJ-AVGNSLFASA-N 0.000 description 2
- JRJLGNFWYFSJHB-HOCLYGCPSA-N Leu-Gly-Trp Chemical compound [H]N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O JRJLGNFWYFSJHB-HOCLYGCPSA-N 0.000 description 2
- JFSGIJSCJFQGSZ-MXAVVETBSA-N Leu-Ile-His Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CC(C)C)N JFSGIJSCJFQGSZ-MXAVVETBSA-N 0.000 description 2
- NRFGTHFONZYFNY-MGHWNKPDSA-N Leu-Ile-Tyr Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 NRFGTHFONZYFNY-MGHWNKPDSA-N 0.000 description 2
- VCHVSKNMTXWIIP-SRVKXCTJSA-N Leu-Lys-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O VCHVSKNMTXWIIP-SRVKXCTJSA-N 0.000 description 2
- BRTVHXHCUSXYRI-CIUDSAMLSA-N Leu-Ser-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O BRTVHXHCUSXYRI-CIUDSAMLSA-N 0.000 description 2
- AIMGJYMCTAABEN-GVXVVHGQSA-N Leu-Val-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O AIMGJYMCTAABEN-GVXVVHGQSA-N 0.000 description 2
- LMDVGHQPPPLYAR-IHRRRGAJSA-N Leu-Val-His Chemical compound N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CNC=N1)C(=O)O LMDVGHQPPPLYAR-IHRRRGAJSA-N 0.000 description 2
- YKIRNDPUWONXQN-GUBZILKMSA-N Lys-Asn-Gln Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N YKIRNDPUWONXQN-GUBZILKMSA-N 0.000 description 2
- GQFDWEDHOQRNLC-QWRGUYRKSA-N Lys-Gly-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN GQFDWEDHOQRNLC-QWRGUYRKSA-N 0.000 description 2
- OIQSIMFSVLLWBX-VOAKCMCISA-N Lys-Leu-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OIQSIMFSVLLWBX-VOAKCMCISA-N 0.000 description 2
- DIBZLYZXTSVGLN-CIUDSAMLSA-N Lys-Ser-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O DIBZLYZXTSVGLN-CIUDSAMLSA-N 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- 208000003445 Mouth Neoplasms Diseases 0.000 description 2
- 101100174574 Mus musculus Pikfyve gene Proteins 0.000 description 2
- 241000699670 Mus sp. Species 0.000 description 2
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 2
- 208000027190 Peripheral T-cell lymphomas Diseases 0.000 description 2
- MQVFHOPCKNTHGT-MELADBBJSA-N Phe-Asp-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)O)NC(=O)[C@H](CC2=CC=CC=C2)N)C(=O)O MQVFHOPCKNTHGT-MELADBBJSA-N 0.000 description 2
- CUMXHKAOHNWRFQ-BZSNNMDCSA-N Phe-Asp-Tyr Chemical compound C([C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=CC=C1 CUMXHKAOHNWRFQ-BZSNNMDCSA-N 0.000 description 2
- QPVFUAUFEBPIPT-CDMKHQONSA-N Phe-Gly-Thr Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(O)=O QPVFUAUFEBPIPT-CDMKHQONSA-N 0.000 description 2
- BPIMVBKDLSBKIJ-FCLVOEFKSA-N Phe-Thr-Phe Chemical compound C([C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=CC=C1 BPIMVBKDLSBKIJ-FCLVOEFKSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 2
- JARJPEMLQAWNBR-GUBZILKMSA-N Pro-Asp-Arg Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O JARJPEMLQAWNBR-GUBZILKMSA-N 0.000 description 2
- HJSCRFZVGXAGNG-SRVKXCTJSA-N Pro-Gln-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCN1 HJSCRFZVGXAGNG-SRVKXCTJSA-N 0.000 description 2
- FHZJRBVMLGOHBX-GUBZILKMSA-N Pro-Pro-Asp Chemical compound OC(=O)C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1)C(O)=O FHZJRBVMLGOHBX-GUBZILKMSA-N 0.000 description 2
- LEIKGVHQTKHOLM-IUCAKERBSA-N Pro-Pro-Gly Chemical compound OC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 LEIKGVHQTKHOLM-IUCAKERBSA-N 0.000 description 2
- PCWLNNZTBJTZRN-AVGNSLFASA-N Pro-Pro-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 PCWLNNZTBJTZRN-AVGNSLFASA-N 0.000 description 2
- RCYUBVHMVUHEBM-RCWTZXSCSA-N Pro-Pro-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(O)=O RCYUBVHMVUHEBM-RCWTZXSCSA-N 0.000 description 2
- VBZXFFYOBDLLFE-HSHDSVGOSA-N Pro-Trp-Thr Chemical compound N([C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H]([C@H](O)C)C(O)=O)C(=O)[C@@H]1CCCN1 VBZXFFYOBDLLFE-HSHDSVGOSA-N 0.000 description 2
- RJKFOVLPORLFTN-LEKSSAKUSA-N Progesterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H](C(=O)C)[C@@]1(C)CC2 RJKFOVLPORLFTN-LEKSSAKUSA-N 0.000 description 2
- 102100029986 Receptor tyrosine-protein kinase erbB-3 Human genes 0.000 description 2
- 101710100969 Receptor tyrosine-protein kinase erbB-3 Proteins 0.000 description 2
- 102100029981 Receptor tyrosine-protein kinase erbB-4 Human genes 0.000 description 2
- 101710100963 Receptor tyrosine-protein kinase erbB-4 Proteins 0.000 description 2
- OOKCGAYXSNJBGQ-ZLUOBGJFSA-N Ser-Asn-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O OOKCGAYXSNJBGQ-ZLUOBGJFSA-N 0.000 description 2
- FTVRVZNYIYWJGB-ACZMJKKPSA-N Ser-Asp-Glu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O FTVRVZNYIYWJGB-ACZMJKKPSA-N 0.000 description 2
- XWCYBVBLJRWOFR-WDSKDSINSA-N Ser-Gln-Gly Chemical compound OC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)NCC(O)=O XWCYBVBLJRWOFR-WDSKDSINSA-N 0.000 description 2
- XXXAXOWMBOKTRN-XPUUQOCRSA-N Ser-Gly-Val Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C(C)C)C(O)=O XXXAXOWMBOKTRN-XPUUQOCRSA-N 0.000 description 2
- ZUDXUJSYCCNZQJ-DCAQKATOSA-N Ser-His-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](CO)N ZUDXUJSYCCNZQJ-DCAQKATOSA-N 0.000 description 2
- ZIFYDQAFEMIZII-GUBZILKMSA-N Ser-Leu-Glu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O ZIFYDQAFEMIZII-GUBZILKMSA-N 0.000 description 2
- FPCGZYMRFFIYIH-CIUDSAMLSA-N Ser-Lys-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O FPCGZYMRFFIYIH-CIUDSAMLSA-N 0.000 description 2
- AMRRYKHCILPAKD-FXQIFTODSA-N Ser-Met-Asn Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CO)N AMRRYKHCILPAKD-FXQIFTODSA-N 0.000 description 2
- PJIQEIFXZPCWOJ-FXQIFTODSA-N Ser-Pro-Asp Chemical compound [H]N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(O)=O PJIQEIFXZPCWOJ-FXQIFTODSA-N 0.000 description 2
- CUXJENOFJXOSOZ-BIIVOSGPSA-N Ser-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)[C@H](CO)N)C(=O)O CUXJENOFJXOSOZ-BIIVOSGPSA-N 0.000 description 2
- ZKOKTQPHFMRSJP-YJRXYDGGSA-N Ser-Thr-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O ZKOKTQPHFMRSJP-YJRXYDGGSA-N 0.000 description 2
- VVKVHAOOUGNDPJ-SRVKXCTJSA-N Ser-Tyr-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(O)=O VVKVHAOOUGNDPJ-SRVKXCTJSA-N 0.000 description 2
- OSFZCEQJLWCIBG-BZSNNMDCSA-N Ser-Tyr-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O OSFZCEQJLWCIBG-BZSNNMDCSA-N 0.000 description 2
- JGUWRQWULDWNCM-FXQIFTODSA-N Ser-Val-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O JGUWRQWULDWNCM-FXQIFTODSA-N 0.000 description 2
- 208000000453 Skin Neoplasms Diseases 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 208000031672 T-Cell Peripheral Lymphoma Diseases 0.000 description 2
- NKANXQFJJICGDU-QPLCGJKRSA-N Tamoxifen Chemical compound C=1C=CC=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1 NKANXQFJJICGDU-QPLCGJKRSA-N 0.000 description 2
- GLQFKOVWXPPFTP-VEVYYDQMSA-N Thr-Arg-Asp Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(O)=O GLQFKOVWXPPFTP-VEVYYDQMSA-N 0.000 description 2
- UKBSDLHIKIXJKH-HJGDQZAQSA-N Thr-Arg-Glu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O UKBSDLHIKIXJKH-HJGDQZAQSA-N 0.000 description 2
- PAXANSWUSVPFNK-IUKAMOBKSA-N Thr-Ile-Asn Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H]([C@@H](C)O)N PAXANSWUSVPFNK-IUKAMOBKSA-N 0.000 description 2
- ADPHPKGWVDHWML-PPCPHDFISA-N Thr-Ile-Leu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)O)NC(=O)[C@H]([C@@H](C)O)N ADPHPKGWVDHWML-PPCPHDFISA-N 0.000 description 2
- MECLEFZMPPOEAC-VOAKCMCISA-N Thr-Leu-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)O)N)O MECLEFZMPPOEAC-VOAKCMCISA-N 0.000 description 2
- DXPURPNJDFCKKO-RHYQMDGZSA-N Thr-Lys-Val Chemical compound CC(C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)[C@@H](C)O)C(O)=O DXPURPNJDFCKKO-RHYQMDGZSA-N 0.000 description 2
- GUHLYMZJVXUIPO-RCWTZXSCSA-N Thr-Met-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C(C)C)C(O)=O GUHLYMZJVXUIPO-RCWTZXSCSA-N 0.000 description 2
- VTMGKRABARCZAX-OSUNSFLBSA-N Thr-Pro-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)[C@@H](C)O VTMGKRABARCZAX-OSUNSFLBSA-N 0.000 description 2
- DEGCBBCMYWNJNA-RHYQMDGZSA-N Thr-Pro-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)[C@@H](C)O DEGCBBCMYWNJNA-RHYQMDGZSA-N 0.000 description 2
- YRXXUYPYPHRJPB-RXVVDRJESA-N Trp-Gly-Trp Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)NCC(=O)N[C@@H](CC3=CNC4=CC=CC=C43)C(=O)O)N YRXXUYPYPHRJPB-RXVVDRJESA-N 0.000 description 2
- AZBIIKDSDLVJAK-VHWLVUOQSA-N Trp-Ile-Asn Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)N AZBIIKDSDLVJAK-VHWLVUOQSA-N 0.000 description 2
- KIMOCKLJBXHFIN-YLVFBTJISA-N Trp-Ile-Gly Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(O)=O)=CNC2=C1 KIMOCKLJBXHFIN-YLVFBTJISA-N 0.000 description 2
- YXSSXUIBUJGHJY-SFJXLCSZSA-N Trp-Thr-Phe Chemical compound C([C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CC=1C2=CC=CC=C2NC=1)[C@H](O)C)C(O)=O)C1=CC=CC=C1 YXSSXUIBUJGHJY-SFJXLCSZSA-N 0.000 description 2
- 102100040245 Tumor necrosis factor receptor superfamily member 5 Human genes 0.000 description 2
- VCXWRWYFJLXITF-AUTRQRHGSA-N Tyr-Ala-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 VCXWRWYFJLXITF-AUTRQRHGSA-N 0.000 description 2
- VFJIWSJKZJTQII-SRVKXCTJSA-N Tyr-Asp-Ser Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O VFJIWSJKZJTQII-SRVKXCTJSA-N 0.000 description 2
- MNMYOSZWCKYEDI-JRQIVUDYSA-N Tyr-Asp-Thr Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O MNMYOSZWCKYEDI-JRQIVUDYSA-N 0.000 description 2
- NMKJPMCEKQHRPD-IRXDYDNUSA-N Tyr-Gly-Tyr Chemical compound C([C@H](N)C(=O)NCC(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=C(O)C=C1 NMKJPMCEKQHRPD-IRXDYDNUSA-N 0.000 description 2
- XYNFFTNEQDWZNY-ULQDDVLXSA-N Tyr-Met-His Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CC2=CC=C(C=C2)O)N XYNFFTNEQDWZNY-ULQDDVLXSA-N 0.000 description 2
- VXFXIBCCVLJCJT-JYJNAYRXSA-N Tyr-Pro-Pro Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(O)=O VXFXIBCCVLJCJT-JYJNAYRXSA-N 0.000 description 2
- MWUYSCVVPVITMW-IGNZVWTISA-N Tyr-Tyr-Ala Chemical compound C([C@@H](C(=O)N[C@@H](C)C(O)=O)NC(=O)[C@@H](N)CC=1C=CC(O)=CC=1)C1=CC=C(O)C=C1 MWUYSCVVPVITMW-IGNZVWTISA-N 0.000 description 2
- AGDDLOQMXUQPDY-BZSNNMDCSA-N Tyr-Tyr-Ser Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(O)=O AGDDLOQMXUQPDY-BZSNNMDCSA-N 0.000 description 2
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 2
- CKTMJBPRVQWPHU-JSGCOSHPSA-N Val-Phe-Gly Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)NCC(=O)O)N CKTMJBPRVQWPHU-JSGCOSHPSA-N 0.000 description 2
- VHIZXDZMTDVFGX-DCAQKATOSA-N Val-Ser-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](C(C)C)N VHIZXDZMTDVFGX-DCAQKATOSA-N 0.000 description 2
- CEKSLIVSNNGOKH-KZVJFYERSA-N Val-Thr-Ala Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](C)C(=O)O)NC(=O)[C@H](C(C)C)N)O CEKSLIVSNNGOKH-KZVJFYERSA-N 0.000 description 2
- USXYVSTVPHELAF-RCWTZXSCSA-N Val-Thr-Met Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCSC)C(=O)O)NC(=O)[C@H](C(C)C)N)O USXYVSTVPHELAF-RCWTZXSCSA-N 0.000 description 2
- DVLWZWNAQUBZBC-ZNSHCXBVSA-N Val-Thr-Pro Chemical compound C[C@H]([C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](C(C)C)N)O DVLWZWNAQUBZBC-ZNSHCXBVSA-N 0.000 description 2
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 2
- 239000004480 active ingredient Substances 0.000 description 2
- 230000002776 aggregation Effects 0.000 description 2
- 238000004220 aggregation Methods 0.000 description 2
- 108010045350 alanyl-tyrosyl-alanine Proteins 0.000 description 2
- 108010044940 alanylglutamine Proteins 0.000 description 2
- 150000001412 amines Chemical class 0.000 description 2
- 239000013011 aqueous formulation Substances 0.000 description 2
- 108010089975 arginyl-glycyl-aspartyl-serine Proteins 0.000 description 2
- 108010069926 arginyl-glycyl-serine Proteins 0.000 description 2
- 210000003719 b-lymphocyte Anatomy 0.000 description 2
- SRSXLGNVWSONIS-UHFFFAOYSA-N benzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-N 0.000 description 2
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 210000004369 blood Anatomy 0.000 description 2
- 239000008280 blood Substances 0.000 description 2
- 210000001185 bone marrow Anatomy 0.000 description 2
- 238000002619 cancer immunotherapy Methods 0.000 description 2
- 150000001720 carbohydrates Chemical class 0.000 description 2
- 210000000845 cartilage Anatomy 0.000 description 2
- 210000003169 central nervous system Anatomy 0.000 description 2
- 238000005119 centrifugation Methods 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 208000006990 cholangiocarcinoma Diseases 0.000 description 2
- 239000007979 citrate buffer Substances 0.000 description 2
- 229960002303 citric acid monohydrate Drugs 0.000 description 2
- 238000003501 co-culture Methods 0.000 description 2
- 239000012228 culture supernatant Substances 0.000 description 2
- 208000035250 cutaneous malignant susceptibility to 1 melanoma Diseases 0.000 description 2
- 230000016396 cytokine production Effects 0.000 description 2
- 206010052015 cytokine release syndrome Diseases 0.000 description 2
- 230000002939 deleterious effect Effects 0.000 description 2
- 238000010790 dilution Methods 0.000 description 2
- 239000012895 dilution Substances 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 229920001971 elastomer Polymers 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 2
- OGPBJKLSAFTDLK-UHFFFAOYSA-N europium atom Chemical compound [Eu] OGPBJKLSAFTDLK-UHFFFAOYSA-N 0.000 description 2
- 238000005558 fluorometry Methods 0.000 description 2
- 210000004907 gland Anatomy 0.000 description 2
- 108010055341 glutamyl-glutamic acid Proteins 0.000 description 2
- 108010090037 glycyl-alanyl-isoleucine Proteins 0.000 description 2
- 108010010096 glycyl-glycyl-tyrosine Proteins 0.000 description 2
- 108010028188 glycyl-histidyl-serine Proteins 0.000 description 2
- 108010036413 histidylglycine Proteins 0.000 description 2
- 230000007062 hydrolysis Effects 0.000 description 2
- 238000006460 hydrolysis reaction Methods 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-M hydroxide Chemical compound [OH-] XLYOFNOQVPJJNP-UHFFFAOYSA-M 0.000 description 2
- 210000002865 immune cell Anatomy 0.000 description 2
- 230000001976 improved effect Effects 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 230000003834 intracellular effect Effects 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- 108010044374 isoleucyl-tyrosine Proteins 0.000 description 2
- 230000002147 killing effect Effects 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 108010000761 leucylarginine Proteins 0.000 description 2
- 210000000265 leukocyte Anatomy 0.000 description 2
- 208000012987 lip and oral cavity carcinoma Diseases 0.000 description 2
- 239000012931 lyophilized formulation Substances 0.000 description 2
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 2
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 2
- 108010005942 methionylglycine Proteins 0.000 description 2
- 150000007524 organic acids Chemical class 0.000 description 2
- 201000002528 pancreatic cancer Diseases 0.000 description 2
- 208000008443 pancreatic carcinoma Diseases 0.000 description 2
- VLTRZXGMWDSKGL-UHFFFAOYSA-N perchloric acid Chemical compound OCl(=O)(=O)=O VLTRZXGMWDSKGL-UHFFFAOYSA-N 0.000 description 2
- 210000005259 peripheral blood Anatomy 0.000 description 2
- 239000011886 peripheral blood Substances 0.000 description 2
- 108010089198 phenylalanyl-prolyl-arginine Proteins 0.000 description 2
- 239000008363 phosphate buffer Substances 0.000 description 2
- 229920000136 polysorbate Polymers 0.000 description 2
- 229950008882 polysorbate Drugs 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- 239000011591 potassium Substances 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 108010079317 prolyl-tyrosine Proteins 0.000 description 2
- 108010029020 prolylglycine Proteins 0.000 description 2
- 230000001177 retroviral effect Effects 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 2
- 238000012216 screening Methods 0.000 description 2
- 230000035945 sensitivity Effects 0.000 description 2
- 201000000849 skin cancer Diseases 0.000 description 2
- 208000000649 small cell carcinoma Diseases 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 239000000600 sorbitol Substances 0.000 description 2
- 206010041823 squamous cell carcinoma Diseases 0.000 description 2
- 230000006641 stabilisation Effects 0.000 description 2
- 238000011105 stabilization Methods 0.000 description 2
- 238000010186 staining Methods 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 2
- 150000008163 sugars Chemical class 0.000 description 2
- 230000002195 synergetic effect Effects 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 2
- 238000001890 transfection Methods 0.000 description 2
- 108010080629 tryptophan-leucine Proteins 0.000 description 2
- 108010084932 tryptophyl-proline Proteins 0.000 description 2
- 230000004614 tumor growth Effects 0.000 description 2
- 108010071635 tyrosyl-prolyl-arginine Proteins 0.000 description 2
- 201000005112 urinary bladder cancer Diseases 0.000 description 2
- 238000005406 washing Methods 0.000 description 2
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 1
- LSPHULWDVZXLIL-UHFFFAOYSA-N (+/-)-Camphoric acid Chemical class CC1(C)C(C(O)=O)CCC1(C)C(O)=O LSPHULWDVZXLIL-UHFFFAOYSA-N 0.000 description 1
- WDQLRUYAYXDIFW-RWKIJVEZSA-N (2r,3r,4s,5r,6r)-4-[(2s,3r,4s,5r,6r)-3,5-dihydroxy-4-[(2r,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-6-[[(2r,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxymethyl]oxan-2-yl]oxy-6-(hydroxymethyl)oxane-2,3,5-triol Chemical compound O[C@@H]1[C@@H](CO)O[C@@H](O)[C@H](O)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O2)O)[C@H](O)[C@@H](CO[C@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O2)O)O1 WDQLRUYAYXDIFW-RWKIJVEZSA-N 0.000 description 1
- SSOORFWOBGFTHL-OTEJMHTDSA-N (4S)-5-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[2-[(2S)-2-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-1-[[(2S)-1-[[(2S,3S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-5-amino-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-6-amino-1-[[(2S)-1-[[(2S)-1-[[(2S)-5-amino-1-[[(2S)-5-carbamimidamido-1-[[(2S)-5-carbamimidamido-1-[[(1S)-4-carbamimidamido-1-carboxybutyl]amino]-1-oxopentan-2-yl]amino]-1-oxopentan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-1-oxohexan-2-yl]amino]-1-oxohexan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]amino]-1-oxohexan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-3-methyl-1-oxopentan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-1-oxohexan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-1-oxopropan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]carbamoyl]pyrrolidin-1-yl]-2-oxoethyl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-1-oxohexan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-3-(1H-imidazol-4-yl)-1-oxopropan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-4-[[(2S)-2-[[(2S)-2-[[(2S)-2,6-diaminohexanoyl]amino]-3-methylbutanoyl]amino]propanoyl]amino]-5-oxopentanoic acid Chemical compound CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H](N)CCCCN)C(C)C)C(C)C)C(C)C)C(C)C)C(C)C)C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O SSOORFWOBGFTHL-OTEJMHTDSA-N 0.000 description 1
- FPVKHBSQESCIEP-UHFFFAOYSA-N (8S)-3-(2-deoxy-beta-D-erythro-pentofuranosyl)-3,6,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol Natural products C1C(O)C(CO)OC1N1C(NC=NCC2O)=C2N=C1 FPVKHBSQESCIEP-UHFFFAOYSA-N 0.000 description 1
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 description 1
- LKJPYSCBVHEWIU-KRWDZBQOSA-N (R)-bicalutamide Chemical compound C([C@@](O)(C)C(=O)NC=1C=C(C(C#N)=CC=1)C(F)(F)F)S(=O)(=O)C1=CC=C(F)C=C1 LKJPYSCBVHEWIU-KRWDZBQOSA-N 0.000 description 1
- ZUQUTHURQVDNKF-KEWYIRBNSA-N 1-[(3R,4R,5S,6R)-3-amino-2,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]ethanone Chemical compound CC(=O)C1(O)O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1N ZUQUTHURQVDNKF-KEWYIRBNSA-N 0.000 description 1
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical compound COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 description 1
- BOMZMNZEXMAQQW-UHFFFAOYSA-N 2,5,11-trimethyl-6h-pyrido[4,3-b]carbazol-2-ium-9-ol;acetate Chemical compound CC([O-])=O.C[N+]1=CC=C2C(C)=C(NC=3C4=CC(O)=CC=3)C4=C(C)C2=C1 BOMZMNZEXMAQQW-UHFFFAOYSA-N 0.000 description 1
- 229940080296 2-naphthalenesulfonate Drugs 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 1
- ZHSKUOZOLHMKEA-UHFFFAOYSA-N 4-[5-[bis(2-chloroethyl)amino]-1-methylbenzimidazol-2-yl]butanoic acid;hydron;chloride Chemical compound Cl.ClCCN(CCCl)C1=CC=C2N(C)C(CCCC(O)=O)=NC2=C1 ZHSKUOZOLHMKEA-UHFFFAOYSA-N 0.000 description 1
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 1
- 102100031585 ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 Human genes 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 description 1
- 208000014697 Acute lymphocytic leukaemia Diseases 0.000 description 1
- 208000003200 Adenoma Diseases 0.000 description 1
- 206010001233 Adenoma benign Diseases 0.000 description 1
- 206010068873 Adenosquamous cell carcinoma Diseases 0.000 description 1
- 108010029445 Agammaglobulinaemia Tyrosine Kinase Proteins 0.000 description 1
- KQFRUSHJPKXBMB-BHDSKKPTSA-N Ala-Ala-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](N)C)C(O)=O)=CNC2=C1 KQFRUSHJPKXBMB-BHDSKKPTSA-N 0.000 description 1
- STACJSVFHSEZJV-GHCJXIJMSA-N Ala-Asn-Ile Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O STACJSVFHSEZJV-GHCJXIJMSA-N 0.000 description 1
- MKZCBYZBCINNJN-DLOVCJGASA-N Ala-Asp-Phe Chemical compound C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 MKZCBYZBCINNJN-DLOVCJGASA-N 0.000 description 1
- NJIFPLAJSVUQOZ-JBDRJPRFSA-N Ala-Cys-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](C)N NJIFPLAJSVUQOZ-JBDRJPRFSA-N 0.000 description 1
- XYTNPQNAZREREP-XQXXSGGOSA-N Ala-Glu-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O XYTNPQNAZREREP-XQXXSGGOSA-N 0.000 description 1
- QQACQIHVWCVBBR-GVARAGBVSA-N Ala-Ile-Tyr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O QQACQIHVWCVBBR-GVARAGBVSA-N 0.000 description 1
- LBYMZCVBOKYZNS-CIUDSAMLSA-N Ala-Leu-Asp Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O LBYMZCVBOKYZNS-CIUDSAMLSA-N 0.000 description 1
- SOBIAADAMRHGKH-CIUDSAMLSA-N Ala-Leu-Ser Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O SOBIAADAMRHGKH-CIUDSAMLSA-N 0.000 description 1
- REAQAWSENITKJL-DDWPSWQVSA-N Ala-Met-Asp-Tyr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O REAQAWSENITKJL-DDWPSWQVSA-N 0.000 description 1
- DWYROCSXOOMOEU-CIUDSAMLSA-N Ala-Met-Glu Chemical compound C[C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N DWYROCSXOOMOEU-CIUDSAMLSA-N 0.000 description 1
- OMDNCNKNEGFOMM-BQBZGAKWSA-N Ala-Met-Gly Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCSC)C(=O)NCC(O)=O OMDNCNKNEGFOMM-BQBZGAKWSA-N 0.000 description 1
- RUXQNKVQSKOOBS-JURCDPSOSA-N Ala-Phe-Ile Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O RUXQNKVQSKOOBS-JURCDPSOSA-N 0.000 description 1
- YHBDGLZYNIARKJ-GUBZILKMSA-N Ala-Pro-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)N YHBDGLZYNIARKJ-GUBZILKMSA-N 0.000 description 1
- KLALXKYLOMZDQT-ZLUOBGJFSA-N Ala-Ser-Asn Chemical compound C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC(N)=O KLALXKYLOMZDQT-ZLUOBGJFSA-N 0.000 description 1
- HOVPGJUNRLMIOZ-CIUDSAMLSA-N Ala-Ser-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](C)N HOVPGJUNRLMIOZ-CIUDSAMLSA-N 0.000 description 1
- WQKAQKZRDIZYNV-VZFHVOOUSA-N Ala-Ser-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O WQKAQKZRDIZYNV-VZFHVOOUSA-N 0.000 description 1
- LSMDIAAALJJLRO-XQXXSGGOSA-N Ala-Thr-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(O)=O LSMDIAAALJJLRO-XQXXSGGOSA-N 0.000 description 1
- AENHOIXXHKNIQL-AUTRQRHGSA-N Ala-Tyr-Ala Chemical compound [O-]C(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H]([NH3+])C)CC1=CC=C(O)C=C1 AENHOIXXHKNIQL-AUTRQRHGSA-N 0.000 description 1
- YCTIYBUTCKNOTI-UWJYBYFXSA-N Ala-Tyr-Asp Chemical compound C[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC(=O)O)C(=O)O)N YCTIYBUTCKNOTI-UWJYBYFXSA-N 0.000 description 1
- XSLGWYYNOSUMRM-ZKWXMUAHSA-N Ala-Val-Asn Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O XSLGWYYNOSUMRM-ZKWXMUAHSA-N 0.000 description 1
- DHONNEYAZPNGSG-UBHSHLNASA-N Ala-Val-Phe Chemical compound C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 DHONNEYAZPNGSG-UBHSHLNASA-N 0.000 description 1
- 206010061424 Anal cancer Diseases 0.000 description 1
- 206010073478 Anaplastic large-cell lymphoma Diseases 0.000 description 1
- 201000003076 Angiosarcoma Diseases 0.000 description 1
- 208000007860 Anus Neoplasms Diseases 0.000 description 1
- DBKNLHKEVPZVQC-LPEHRKFASA-N Arg-Ala-Pro Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](C)C(=O)N1CCC[C@@H]1C(O)=O DBKNLHKEVPZVQC-LPEHRKFASA-N 0.000 description 1
- IASNWHAGGYTEKX-IUCAKERBSA-N Arg-Arg-Gly Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)NCC(O)=O IASNWHAGGYTEKX-IUCAKERBSA-N 0.000 description 1
- HPSVTWMFWCHKFN-GARJFASQSA-N Arg-Glu-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CCCN=C(N)N)N)C(=O)O HPSVTWMFWCHKFN-GARJFASQSA-N 0.000 description 1
- HQIZDMIGUJOSNI-IUCAKERBSA-N Arg-Gly-Arg Chemical compound N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O HQIZDMIGUJOSNI-IUCAKERBSA-N 0.000 description 1
- QKSAZKCRVQYYGS-UWVGGRQHSA-N Arg-Gly-His Chemical compound N[C@@H](CCCN=C(N)N)C(=O)NCC(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O QKSAZKCRVQYYGS-UWVGGRQHSA-N 0.000 description 1
- OQCWXQJLCDPRHV-UWVGGRQHSA-N Arg-Gly-Leu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CC(C)C)C(O)=O OQCWXQJLCDPRHV-UWVGGRQHSA-N 0.000 description 1
- OTZMRMHZCMZOJZ-SRVKXCTJSA-N Arg-Leu-Glu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O OTZMRMHZCMZOJZ-SRVKXCTJSA-N 0.000 description 1
- YVTHEZNOKSAWRW-DCAQKATOSA-N Arg-Lys-Ala Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O YVTHEZNOKSAWRW-DCAQKATOSA-N 0.000 description 1
- BNYNOWJESJJIOI-XUXIUFHCSA-N Arg-Lys-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCN=C(N)N)N BNYNOWJESJJIOI-XUXIUFHCSA-N 0.000 description 1
- FIQKRDXFTANIEJ-ULQDDVLXSA-N Arg-Phe-His Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC2=CN=CN2)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N FIQKRDXFTANIEJ-ULQDDVLXSA-N 0.000 description 1
- ADPACBMPYWJJCE-FXQIFTODSA-N Arg-Ser-Asp Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O ADPACBMPYWJJCE-FXQIFTODSA-N 0.000 description 1
- YNSUUAOAFCVINY-OSUNSFLBSA-N Arg-Thr-Ile Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O YNSUUAOAFCVINY-OSUNSFLBSA-N 0.000 description 1
- AOJYORNRFWWEIV-IHRRRGAJSA-N Arg-Tyr-Asp Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CC(O)=O)C(O)=O)CC1=CC=C(O)C=C1 AOJYORNRFWWEIV-IHRRRGAJSA-N 0.000 description 1
- BFDDUDQCPJWQRQ-IHRRRGAJSA-N Arg-Tyr-Cys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N)O BFDDUDQCPJWQRQ-IHRRRGAJSA-N 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- SLKLLQWZQHXYSV-CIUDSAMLSA-N Asn-Ala-Lys Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(O)=O SLKLLQWZQHXYSV-CIUDSAMLSA-N 0.000 description 1
- XWGJDUSDTRPQRK-ZLUOBGJFSA-N Asn-Ala-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC(N)=O XWGJDUSDTRPQRK-ZLUOBGJFSA-N 0.000 description 1
- VDCIPFYVCICPEC-FXQIFTODSA-N Asn-Arg-Ala Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(O)=O VDCIPFYVCICPEC-FXQIFTODSA-N 0.000 description 1
- DAPLJWATMAXPPZ-CIUDSAMLSA-N Asn-Asn-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CC(N)=O DAPLJWATMAXPPZ-CIUDSAMLSA-N 0.000 description 1
- GJFYPBDMUGGLFR-NKWVEPMBSA-N Asn-Gly-Pro Chemical compound C1C[C@@H](N(C1)C(=O)CNC(=O)[C@H](CC(=O)N)N)C(=O)O GJFYPBDMUGGLFR-NKWVEPMBSA-N 0.000 description 1
- UDSVWSUXKYXSTR-QWRGUYRKSA-N Asn-Gly-Tyr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O UDSVWSUXKYXSTR-QWRGUYRKSA-N 0.000 description 1
- GOKCTAJWRPSCHP-VHWLVUOQSA-N Asn-Ile-Trp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)NC(=O)[C@H](CC(=O)N)N GOKCTAJWRPSCHP-VHWLVUOQSA-N 0.000 description 1
- MYCSPQIARXTUTP-SRVKXCTJSA-N Asn-Leu-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CC(=O)N)N MYCSPQIARXTUTP-SRVKXCTJSA-N 0.000 description 1
- JTXVXGXTRXMOFJ-FXQIFTODSA-N Asn-Pro-Asn Chemical compound NC(=O)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(O)=O JTXVXGXTRXMOFJ-FXQIFTODSA-N 0.000 description 1
- GZXOUBTUAUAVHD-ACZMJKKPSA-N Asn-Ser-Glu Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(O)=O GZXOUBTUAUAVHD-ACZMJKKPSA-N 0.000 description 1
- HPBNLFLSSQDFQW-WHFBIAKZSA-N Asn-Ser-Gly Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O HPBNLFLSSQDFQW-WHFBIAKZSA-N 0.000 description 1
- WLVLIYYBPPONRJ-GCJQMDKQSA-N Asn-Thr-Ala Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O WLVLIYYBPPONRJ-GCJQMDKQSA-N 0.000 description 1
- ZUFPUBYQYWCMDB-NUMRIWBASA-N Asn-Thr-Glu Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O ZUFPUBYQYWCMDB-NUMRIWBASA-N 0.000 description 1
- BIGRHVNFFJTHEB-UBHSHLNASA-N Asn-Trp-Asp Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC(O)=O)C(O)=O BIGRHVNFFJTHEB-UBHSHLNASA-N 0.000 description 1
- CGYKCTPUGXFPMG-IHPCNDPISA-N Asn-Tyr-Trp Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O CGYKCTPUGXFPMG-IHPCNDPISA-N 0.000 description 1
- LTDGPJKGJDIBQD-LAEOZQHASA-N Asn-Val-Gln Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O LTDGPJKGJDIBQD-LAEOZQHASA-N 0.000 description 1
- NECWUSYTYSIFNC-DLOVCJGASA-N Asp-Ala-Phe Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 NECWUSYTYSIFNC-DLOVCJGASA-N 0.000 description 1
- NJIKKGUVGUBICV-ZLUOBGJFSA-N Asp-Ala-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC(O)=O NJIKKGUVGUBICV-ZLUOBGJFSA-N 0.000 description 1
- ICTXFVKYAGQURS-UBHSHLNASA-N Asp-Asn-Trp Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O ICTXFVKYAGQURS-UBHSHLNASA-N 0.000 description 1
- SBHUBSDEZQFJHJ-CIUDSAMLSA-N Asp-Asp-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(O)=O SBHUBSDEZQFJHJ-CIUDSAMLSA-N 0.000 description 1
- VAWNQIGQPUOPQW-ACZMJKKPSA-N Asp-Glu-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O VAWNQIGQPUOPQW-ACZMJKKPSA-N 0.000 description 1
- OVPHVTCDVYYTHN-AVGNSLFASA-N Asp-Glu-Phe Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 OVPHVTCDVYYTHN-AVGNSLFASA-N 0.000 description 1
- SVABRQFIHCSNCI-FOHZUACHSA-N Asp-Gly-Thr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(O)=O SVABRQFIHCSNCI-FOHZUACHSA-N 0.000 description 1
- CMCIMCAQIULNDJ-CIUDSAMLSA-N Asp-His-Cys Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(=O)O)N CMCIMCAQIULNDJ-CIUDSAMLSA-N 0.000 description 1
- SPKCGKRUYKMDHP-GUDRVLHUSA-N Asp-Ile-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CC(=O)O)N SPKCGKRUYKMDHP-GUDRVLHUSA-N 0.000 description 1
- DWOGMPWRQQWPPF-GUBZILKMSA-N Asp-Leu-Glu Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O DWOGMPWRQQWPPF-GUBZILKMSA-N 0.000 description 1
- OZBXOELNJBSJOA-UBHSHLNASA-N Asp-Ser-Trp Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(=O)O)N OZBXOELNJBSJOA-UBHSHLNASA-N 0.000 description 1
- KCOPOPKJRHVGPE-AQZXSJQPSA-N Asp-Thr-Trp Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O KCOPOPKJRHVGPE-AQZXSJQPSA-N 0.000 description 1
- GHAHOJDCBRXAKC-IHPCNDPISA-N Asp-Trp-Tyr Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC3=CC=C(C=C3)O)C(=O)O)NC(=O)[C@H](CC(=O)O)N GHAHOJDCBRXAKC-IHPCNDPISA-N 0.000 description 1
- ALMIMUZAWTUNIO-BZSNNMDCSA-N Asp-Tyr-Tyr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O ALMIMUZAWTUNIO-BZSNNMDCSA-N 0.000 description 1
- CPMKYMGGYUFOHS-FSPLSTOPSA-N Asp-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@@H](N)CC(O)=O CPMKYMGGYUFOHS-FSPLSTOPSA-N 0.000 description 1
- GIKOVDMXBAFXDF-NHCYSSNCSA-N Asp-Val-Leu Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O GIKOVDMXBAFXDF-NHCYSSNCSA-N 0.000 description 1
- GYNUXDMCDILYIQ-QRTARXTBSA-N Asp-Val-Trp Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)NC(=O)[C@H](CC(=O)O)N GYNUXDMCDILYIQ-QRTARXTBSA-N 0.000 description 1
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 1
- 206010003571 Astrocytoma Diseases 0.000 description 1
- 208000036170 B-Cell Marginal Zone Lymphoma Diseases 0.000 description 1
- 102100024222 B-lymphocyte antigen CD19 Human genes 0.000 description 1
- 102100022005 B-lymphocyte antigen CD20 Human genes 0.000 description 1
- 229940124290 BCR-ABL tyrosine kinase inhibitor Drugs 0.000 description 1
- 208000032791 BCR-ABL1 positive chronic myelogenous leukemia Diseases 0.000 description 1
- 208000005440 Basal Cell Neoplasms Diseases 0.000 description 1
- 206010004146 Basal cell carcinoma Diseases 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- 102100021277 Beta-secretase 2 Human genes 0.000 description 1
- 101710150190 Beta-secretase 2 Proteins 0.000 description 1
- 206010005949 Bone cancer Diseases 0.000 description 1
- 208000018084 Bone neoplasm Diseases 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 208000003174 Brain Neoplasms Diseases 0.000 description 1
- 206010006187 Breast cancer Diseases 0.000 description 1
- 208000026310 Breast neoplasm Diseases 0.000 description 1
- 208000011691 Burkitt lymphomas Diseases 0.000 description 1
- 102100024217 CAMPATH-1 antigen Human genes 0.000 description 1
- 102100038077 CD226 antigen Human genes 0.000 description 1
- 102100027207 CD27 antigen Human genes 0.000 description 1
- 102000017420 CD3 protein, epsilon/gamma/delta subunit Human genes 0.000 description 1
- 108010065524 CD52 Antigen Proteins 0.000 description 1
- 102100025221 CD70 antigen Human genes 0.000 description 1
- 239000012275 CTLA-4 inhibitor Substances 0.000 description 1
- 229940045513 CTLA4 antagonist Drugs 0.000 description 1
- 241000282832 Camelidae Species 0.000 description 1
- 241000282836 Camelus dromedarius Species 0.000 description 1
- 102100034744 Cell division cycle 7-related protein kinase Human genes 0.000 description 1
- 102000019034 Chemokines Human genes 0.000 description 1
- 108010012236 Chemokines Proteins 0.000 description 1
- 201000005262 Chondroma Diseases 0.000 description 1
- 208000005243 Chondrosarcoma Diseases 0.000 description 1
- 208000004378 Choroid plexus papilloma Diseases 0.000 description 1
- 208000010833 Chronic myeloid leukaemia Diseases 0.000 description 1
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 1
- GRNOCLDFUNCIDW-ACZMJKKPSA-N Cys-Ala-Glu Chemical compound C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](CS)N GRNOCLDFUNCIDW-ACZMJKKPSA-N 0.000 description 1
- BYALSSDCQYHKMY-XGEHTFHBSA-N Cys-Arg-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CS)N)O BYALSSDCQYHKMY-XGEHTFHBSA-N 0.000 description 1
- SFRQEQGPRTVDPO-NRPADANISA-N Cys-Gln-Val Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O SFRQEQGPRTVDPO-NRPADANISA-N 0.000 description 1
- ZEXHDOQQYZKOIB-ACZMJKKPSA-N Cys-Glu-Ser Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O ZEXHDOQQYZKOIB-ACZMJKKPSA-N 0.000 description 1
- CMYVIUWVYHOLRD-ZLUOBGJFSA-N Cys-Ser-Ala Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O CMYVIUWVYHOLRD-ZLUOBGJFSA-N 0.000 description 1
- ZGERHCJBLPQPGV-ACZMJKKPSA-N Cys-Ser-Gln Chemical compound C(CC(=O)N)[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CS)N ZGERHCJBLPQPGV-ACZMJKKPSA-N 0.000 description 1
- 201000005171 Cystadenoma Diseases 0.000 description 1
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 1
- RGHNJXZEOKUKBD-SQOUGZDYSA-M D-gluconate Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O RGHNJXZEOKUKBD-SQOUGZDYSA-M 0.000 description 1
- 108010049207 Death Domain Receptors Proteins 0.000 description 1
- 102000009058 Death Domain Receptors Human genes 0.000 description 1
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 1
- 108010052167 Dihydroorotate Dehydrogenase Proteins 0.000 description 1
- 102100032823 Dihydroorotate dehydrogenase (quinone), mitochondrial Human genes 0.000 description 1
- 206010061825 Duodenal neoplasm Diseases 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 102000001301 EGF receptor Human genes 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 208000001976 Endocrine Gland Neoplasms Diseases 0.000 description 1
- 206010014733 Endometrial cancer Diseases 0.000 description 1
- 206010014759 Endometrial neoplasm Diseases 0.000 description 1
- OBMLHUPNRURLOK-XGRAFVIBSA-N Epitiostanol Chemical compound C1[C@@H]2S[C@@H]2C[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CC[C@H]21 OBMLHUPNRURLOK-XGRAFVIBSA-N 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 241000283073 Equus caballus Species 0.000 description 1
- 208000000461 Esophageal Neoplasms Diseases 0.000 description 1
- CTKXFMQHOOWWEB-UHFFFAOYSA-N Ethylene oxide/propylene oxide copolymer Chemical compound CCCOC(C)COCCO CTKXFMQHOOWWEB-UHFFFAOYSA-N 0.000 description 1
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 1
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 1
- 208000004057 Focal Nodular Hyperplasia Diseases 0.000 description 1
- 208000022072 Gallbladder Neoplasms Diseases 0.000 description 1
- 206010017993 Gastrointestinal neoplasms Diseases 0.000 description 1
- 208000032612 Glial tumor Diseases 0.000 description 1
- 206010018338 Glioma Diseases 0.000 description 1
- PHZYLYASFWHLHJ-FXQIFTODSA-N Gln-Asn-Glu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O PHZYLYASFWHLHJ-FXQIFTODSA-N 0.000 description 1
- ULXXDWZMMSQBDC-ACZMJKKPSA-N Gln-Asp-Asp Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC(=O)O)C(=O)O)N ULXXDWZMMSQBDC-ACZMJKKPSA-N 0.000 description 1
- CRRFJBGUGNNOCS-PEFMBERDSA-N Gln-Asp-Ile Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O CRRFJBGUGNNOCS-PEFMBERDSA-N 0.000 description 1
- MFLMFRZBAJSGHK-ACZMJKKPSA-N Gln-Cys-Ser Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)O)N MFLMFRZBAJSGHK-ACZMJKKPSA-N 0.000 description 1
- QYKBTDOAMKORGL-FXQIFTODSA-N Gln-Gln-Asp Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC(=O)O)C(=O)O)N QYKBTDOAMKORGL-FXQIFTODSA-N 0.000 description 1
- LVNILKSSFHCSJZ-IHRRRGAJSA-N Gln-Gln-Phe Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CCC(=O)N)N LVNILKSSFHCSJZ-IHRRRGAJSA-N 0.000 description 1
- IVCOYUURLWQDJQ-LPEHRKFASA-N Gln-Gln-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CCC(=O)N)N)C(=O)O IVCOYUURLWQDJQ-LPEHRKFASA-N 0.000 description 1
- GQZDDFRXSDGUNG-YVNDNENWSA-N Gln-Ile-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(O)=O GQZDDFRXSDGUNG-YVNDNENWSA-N 0.000 description 1
- ZBKUIQNCRIYVGH-SDDRHHMPSA-N Gln-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)N)N ZBKUIQNCRIYVGH-SDDRHHMPSA-N 0.000 description 1
- SWDSRANUCKNBLA-AVGNSLFASA-N Gln-Phe-Asp Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H](CCC(=O)N)N SWDSRANUCKNBLA-AVGNSLFASA-N 0.000 description 1
- FNAJNWPDTIXYJN-CIUDSAMLSA-N Gln-Pro-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCC(N)=O FNAJNWPDTIXYJN-CIUDSAMLSA-N 0.000 description 1
- KUBFPYIMAGXGBT-ACZMJKKPSA-N Gln-Ser-Ala Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O KUBFPYIMAGXGBT-ACZMJKKPSA-N 0.000 description 1
- NYCVMJGIJYQWDO-CIUDSAMLSA-N Gln-Ser-Arg Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O NYCVMJGIJYQWDO-CIUDSAMLSA-N 0.000 description 1
- OKARHJKJTKFQBM-ACZMJKKPSA-N Gln-Ser-Asn Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(=O)N)C(=O)O)N OKARHJKJTKFQBM-ACZMJKKPSA-N 0.000 description 1
- LPIKVBWNNVFHCQ-GUBZILKMSA-N Gln-Ser-Leu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O LPIKVBWNNVFHCQ-GUBZILKMSA-N 0.000 description 1
- KPNWAJMEMRCLAL-GUBZILKMSA-N Gln-Ser-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(=O)N)N KPNWAJMEMRCLAL-GUBZILKMSA-N 0.000 description 1
- GHAXJVNBAKGWEJ-AVGNSLFASA-N Gln-Ser-Tyr Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O GHAXJVNBAKGWEJ-AVGNSLFASA-N 0.000 description 1
- GTBXHETZPUURJE-KKUMJFAQSA-N Gln-Tyr-Arg Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O GTBXHETZPUURJE-KKUMJFAQSA-N 0.000 description 1
- UBRQJXFDVZNYJP-AVGNSLFASA-N Gln-Tyr-Ser Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCC(=O)N)N)O UBRQJXFDVZNYJP-AVGNSLFASA-N 0.000 description 1
- QZQYITIKPAUDGN-GVXVVHGQSA-N Gln-Val-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CCC(=O)N)N QZQYITIKPAUDGN-GVXVVHGQSA-N 0.000 description 1
- FTMLQFPULNGION-ZVZYQTTQSA-N Gln-Val-Trp Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O FTMLQFPULNGION-ZVZYQTTQSA-N 0.000 description 1
- WZZSKAJIHTUUSG-ACZMJKKPSA-N Glu-Ala-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCC(O)=O WZZSKAJIHTUUSG-ACZMJKKPSA-N 0.000 description 1
- JPHYJQHPILOKHC-ACZMJKKPSA-N Glu-Asp-Asp Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O JPHYJQHPILOKHC-ACZMJKKPSA-N 0.000 description 1
- NADWTMLCUDMDQI-ACZMJKKPSA-N Glu-Asp-Cys Chemical compound C(CC(=O)O)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CS)C(=O)O)N NADWTMLCUDMDQI-ACZMJKKPSA-N 0.000 description 1
- JRCUFCXYZLPSDZ-ACZMJKKPSA-N Glu-Asp-Ser Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O JRCUFCXYZLPSDZ-ACZMJKKPSA-N 0.000 description 1
- UENPHLAAKDPZQY-XKBZYTNZSA-N Glu-Cys-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](CCC(=O)O)N)O UENPHLAAKDPZQY-XKBZYTNZSA-N 0.000 description 1
- AIGROOHQXCACHL-WDSKDSINSA-N Glu-Gly-Ala Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](C)C(O)=O AIGROOHQXCACHL-WDSKDSINSA-N 0.000 description 1
- LGYCLOCORAEQSZ-PEFMBERDSA-N Glu-Ile-Asp Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(O)=O)C(O)=O LGYCLOCORAEQSZ-PEFMBERDSA-N 0.000 description 1
- DNPCBMNFQVTHMA-DCAQKATOSA-N Glu-Leu-Gln Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O DNPCBMNFQVTHMA-DCAQKATOSA-N 0.000 description 1
- IVGJYOOGJLFKQE-AVGNSLFASA-N Glu-Leu-Lys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N IVGJYOOGJLFKQE-AVGNSLFASA-N 0.000 description 1
- QJVZSVUYZFYLFQ-CIUDSAMLSA-N Glu-Pro-Ala Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O QJVZSVUYZFYLFQ-CIUDSAMLSA-N 0.000 description 1
- DMYACXMQUABZIQ-NRPADANISA-N Glu-Ser-Val Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O DMYACXMQUABZIQ-NRPADANISA-N 0.000 description 1
- UCZXXMREFIETQW-AVGNSLFASA-N Glu-Tyr-Asn Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(N)=O)C(O)=O UCZXXMREFIETQW-AVGNSLFASA-N 0.000 description 1
- RMWAOBGCZZSJHE-UMNHJUIQSA-N Glu-Val-Pro Chemical compound CC(C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)O)N RMWAOBGCZZSJHE-UMNHJUIQSA-N 0.000 description 1
- 102400000321 Glucagon Human genes 0.000 description 1
- 108060003199 Glucagon Proteins 0.000 description 1
- YMUFWNJHVPQNQD-ZKWXMUAHSA-N Gly-Ala-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)CN YMUFWNJHVPQNQD-ZKWXMUAHSA-N 0.000 description 1
- LJPIRKICOISLKN-WHFBIAKZSA-N Gly-Ala-Ser Chemical compound NCC(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O LJPIRKICOISLKN-WHFBIAKZSA-N 0.000 description 1
- QSDKBRMVXSWAQE-BFHQHQDPSA-N Gly-Ala-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)CN QSDKBRMVXSWAQE-BFHQHQDPSA-N 0.000 description 1
- WJZLEENECIOOSA-WDSKDSINSA-N Gly-Asn-Gln Chemical compound NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)O WJZLEENECIOOSA-WDSKDSINSA-N 0.000 description 1
- XCLCVBYNGXEVDU-WHFBIAKZSA-N Gly-Asn-Ser Chemical compound NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O XCLCVBYNGXEVDU-WHFBIAKZSA-N 0.000 description 1
- PABFFPWEJMEVEC-JGVFFNPUSA-N Gly-Gln-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCC(=O)N)NC(=O)CN)C(=O)O PABFFPWEJMEVEC-JGVFFNPUSA-N 0.000 description 1
- HHSOPSCKAZKQHQ-PEXQALLHSA-N Gly-His-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)CN HHSOPSCKAZKQHQ-PEXQALLHSA-N 0.000 description 1
- AAHSHTLISQUZJL-QSFUFRPTSA-N Gly-Ile-Ile Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O AAHSHTLISQUZJL-QSFUFRPTSA-N 0.000 description 1
- TWTPDFFBLQEBOE-IUCAKERBSA-N Gly-Leu-Gln Chemical compound [H]NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O TWTPDFFBLQEBOE-IUCAKERBSA-N 0.000 description 1
- NNCSJUBVFBDDLC-YUMQZZPRSA-N Gly-Leu-Ser Chemical compound NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O NNCSJUBVFBDDLC-YUMQZZPRSA-N 0.000 description 1
- VBOBNHSVQKKTOT-YUMQZZPRSA-N Gly-Lys-Ala Chemical compound [H]NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O VBOBNHSVQKKTOT-YUMQZZPRSA-N 0.000 description 1
- RVGMVLVBDRQVKB-UWVGGRQHSA-N Gly-Met-His Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)CN RVGMVLVBDRQVKB-UWVGGRQHSA-N 0.000 description 1
- GAFKBWKVXNERFA-QWRGUYRKSA-N Gly-Phe-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)CN)CC1=CC=CC=C1 GAFKBWKVXNERFA-QWRGUYRKSA-N 0.000 description 1
- IRJWAYCXIYUHQE-WHFBIAKZSA-N Gly-Ser-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)CN IRJWAYCXIYUHQE-WHFBIAKZSA-N 0.000 description 1
- IALQAMYQJBZNSK-WHFBIAKZSA-N Gly-Ser-Asn Chemical compound [H]NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O IALQAMYQJBZNSK-WHFBIAKZSA-N 0.000 description 1
- OHUKZZYSJBKFRR-WHFBIAKZSA-N Gly-Ser-Asp Chemical compound [H]NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O OHUKZZYSJBKFRR-WHFBIAKZSA-N 0.000 description 1
- VNNRLUNBJSWZPF-ZKWXMUAHSA-N Gly-Ser-Ile Chemical compound [H]NCC(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O VNNRLUNBJSWZPF-ZKWXMUAHSA-N 0.000 description 1
- POJJAZJHBGXEGM-YUMQZZPRSA-N Gly-Ser-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)CN POJJAZJHBGXEGM-YUMQZZPRSA-N 0.000 description 1
- WCORRBXVISTKQL-WHFBIAKZSA-N Gly-Ser-Ser Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O WCORRBXVISTKQL-WHFBIAKZSA-N 0.000 description 1
- FFJQHWKSGAWSTJ-BFHQHQDPSA-N Gly-Thr-Ala Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O FFJQHWKSGAWSTJ-BFHQHQDPSA-N 0.000 description 1
- LLWQVJNHMYBLLK-CDMKHQONSA-N Gly-Thr-Phe Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O LLWQVJNHMYBLLK-CDMKHQONSA-N 0.000 description 1
- FFALDIDGPLUDKV-ZDLURKLDSA-N Gly-Thr-Ser Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O FFALDIDGPLUDKV-ZDLURKLDSA-N 0.000 description 1
- TVTZEOHWHUVYCG-KYNKHSRBSA-N Gly-Thr-Thr Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O TVTZEOHWHUVYCG-KYNKHSRBSA-N 0.000 description 1
- DNAZKGFYFRGZIH-QWRGUYRKSA-N Gly-Tyr-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)CN)CC1=CC=C(O)C=C1 DNAZKGFYFRGZIH-QWRGUYRKSA-N 0.000 description 1
- YGHSQRJSHKYUJY-SCZZXKLOSA-N Gly-Val-Pro Chemical compound CC(C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)CN YGHSQRJSHKYUJY-SCZZXKLOSA-N 0.000 description 1
- SBVMXEZQJVUARN-XPUUQOCRSA-N Gly-Val-Ser Chemical compound NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O SBVMXEZQJVUARN-XPUUQOCRSA-N 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 108010017080 Granulocyte Colony-Stimulating Factor Proteins 0.000 description 1
- 102000004269 Granulocyte Colony-Stimulating Factor Human genes 0.000 description 1
- 102000001398 Granzyme Human genes 0.000 description 1
- 108060005986 Granzyme Proteins 0.000 description 1
- 208000002125 Hemangioendothelioma Diseases 0.000 description 1
- 208000001258 Hemangiosarcoma Diseases 0.000 description 1
- 102100034458 Hepatitis A virus cellular receptor 2 Human genes 0.000 description 1
- 229920000209 Hexadimethrine bromide Polymers 0.000 description 1
- STGQSBKUYSPPIG-CIUDSAMLSA-N His-Ser-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CN=CN1 STGQSBKUYSPPIG-CIUDSAMLSA-N 0.000 description 1
- 208000017604 Hodgkin disease Diseases 0.000 description 1
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 1
- 241001272567 Hominoidea Species 0.000 description 1
- 101000777636 Homo sapiens ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 Proteins 0.000 description 1
- 101000980825 Homo sapiens B-lymphocyte antigen CD19 Proteins 0.000 description 1
- 101000897405 Homo sapiens B-lymphocyte antigen CD20 Proteins 0.000 description 1
- 101000884298 Homo sapiens CD226 antigen Proteins 0.000 description 1
- 101000914511 Homo sapiens CD27 antigen Proteins 0.000 description 1
- 101000934356 Homo sapiens CD70 antigen Proteins 0.000 description 1
- 101000945740 Homo sapiens Cell division cycle 7-related protein kinase Proteins 0.000 description 1
- 101000851181 Homo sapiens Epidermal growth factor receptor Proteins 0.000 description 1
- 101001068133 Homo sapiens Hepatitis A virus cellular receptor 2 Proteins 0.000 description 1
- 101001034652 Homo sapiens Insulin-like growth factor 1 receptor Proteins 0.000 description 1
- 101001137987 Homo sapiens Lymphocyte activation gene 3 protein Proteins 0.000 description 1
- 101000991061 Homo sapiens MHC class I polypeptide-related sequence B Proteins 0.000 description 1
- 101000950695 Homo sapiens Mitogen-activated protein kinase 8 Proteins 0.000 description 1
- 101001133056 Homo sapiens Mucin-1 Proteins 0.000 description 1
- 101000744394 Homo sapiens Oxidized purine nucleoside triphosphate hydrolase Proteins 0.000 description 1
- 101001117317 Homo sapiens Programmed cell death 1 ligand 1 Proteins 0.000 description 1
- 101001136592 Homo sapiens Prostate stem cell antigen Proteins 0.000 description 1
- 101100101727 Homo sapiens RAET1L gene Proteins 0.000 description 1
- 101000633784 Homo sapiens SLAM family member 7 Proteins 0.000 description 1
- 101000777293 Homo sapiens Serine/threonine-protein kinase Chk1 Proteins 0.000 description 1
- 101000934346 Homo sapiens T-cell surface antigen CD2 Proteins 0.000 description 1
- 101000610605 Homo sapiens Tumor necrosis factor receptor superfamily member 10A Proteins 0.000 description 1
- 101000610604 Homo sapiens Tumor necrosis factor receptor superfamily member 10B Proteins 0.000 description 1
- 101000801234 Homo sapiens Tumor necrosis factor receptor superfamily member 18 Proteins 0.000 description 1
- 101000679903 Homo sapiens Tumor necrosis factor receptor superfamily member 25 Proteins 0.000 description 1
- 101000851376 Homo sapiens Tumor necrosis factor receptor superfamily member 8 Proteins 0.000 description 1
- 101000851370 Homo sapiens Tumor necrosis factor receptor superfamily member 9 Proteins 0.000 description 1
- 101000621390 Homo sapiens Wee1-like protein kinase Proteins 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 1
- DCQMJRSOGCYKTR-GHCJXIJMSA-N Ile-Asp-Ser Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O DCQMJRSOGCYKTR-GHCJXIJMSA-N 0.000 description 1
- KUHFPGIVBOCRMV-MNXVOIDGSA-N Ile-Gln-Leu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC(C)C)C(=O)O)N KUHFPGIVBOCRMV-MNXVOIDGSA-N 0.000 description 1
- DFJJAVZIHDFOGQ-MNXVOIDGSA-N Ile-Glu-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CCCCN)C(=O)O)N DFJJAVZIHDFOGQ-MNXVOIDGSA-N 0.000 description 1
- SLQVFYWBGNNOTK-BYULHYEWSA-N Ile-Gly-Asn Chemical compound CC[C@H](C)[C@@H](C(=O)NCC(=O)N[C@@H](CC(=O)N)C(=O)O)N SLQVFYWBGNNOTK-BYULHYEWSA-N 0.000 description 1
- KEKTTYCXKGBAAL-VGDYDELISA-N Ile-His-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)N[C@@H](CO)C(=O)O)N KEKTTYCXKGBAAL-VGDYDELISA-N 0.000 description 1
- TWPSALMCEHCIOY-YTFOTSKYSA-N Ile-Ile-Leu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(=O)O)N TWPSALMCEHCIOY-YTFOTSKYSA-N 0.000 description 1
- PFPUFNLHBXKPHY-HTFCKZLJSA-N Ile-Ile-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)O)N PFPUFNLHBXKPHY-HTFCKZLJSA-N 0.000 description 1
- RQQCJTLBSJMVCR-DSYPUSFNSA-N Ile-Leu-Trp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)N RQQCJTLBSJMVCR-DSYPUSFNSA-N 0.000 description 1
- NZGTYCMLUGYMCV-XUXIUFHCSA-N Ile-Lys-Arg Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N NZGTYCMLUGYMCV-XUXIUFHCSA-N 0.000 description 1
- AKOYRLRUFBZOSP-BJDJZHNGSA-N Ile-Lys-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)O)N AKOYRLRUFBZOSP-BJDJZHNGSA-N 0.000 description 1
- JHNJNTMTZHEDLJ-NAKRPEOUSA-N Ile-Ser-Arg Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O JHNJNTMTZHEDLJ-NAKRPEOUSA-N 0.000 description 1
- JODPUDMBQBIWCK-GHCJXIJMSA-N Ile-Ser-Asn Chemical compound [H]N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O JODPUDMBQBIWCK-GHCJXIJMSA-N 0.000 description 1
- RKQAYOWLSFLJEE-SVSWQMSJSA-N Ile-Thr-Cys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)O)N RKQAYOWLSFLJEE-SVSWQMSJSA-N 0.000 description 1
- WXLYNEHOGRYNFU-URLPEUOOSA-N Ile-Thr-Phe Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)N WXLYNEHOGRYNFU-URLPEUOOSA-N 0.000 description 1
- NSPNUMNLZNOPAQ-SJWGOKEGSA-N Ile-Tyr-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N2CCC[C@@H]2C(=O)O)N NSPNUMNLZNOPAQ-SJWGOKEGSA-N 0.000 description 1
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 1
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 1
- 102100039688 Insulin-like growth factor 1 receptor Human genes 0.000 description 1
- 102000006992 Interferon-alpha Human genes 0.000 description 1
- 108010047761 Interferon-alpha Proteins 0.000 description 1
- 102000003996 Interferon-beta Human genes 0.000 description 1
- 108090000467 Interferon-beta Proteins 0.000 description 1
- 102000013462 Interleukin-12 Human genes 0.000 description 1
- 108010065805 Interleukin-12 Proteins 0.000 description 1
- 102000003812 Interleukin-15 Human genes 0.000 description 1
- 108090000172 Interleukin-15 Proteins 0.000 description 1
- SHGAZHPCJJPHSC-NUEINMDLSA-N Isotretinoin Chemical compound OC(=O)C=C(C)/C=C/C=C(C)C=CC1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-NUEINMDLSA-N 0.000 description 1
- 108010055717 JNK Mitogen-Activated Protein Kinases Proteins 0.000 description 1
- 102000019145 JUN kinase activity proteins Human genes 0.000 description 1
- 229940122245 Janus kinase inhibitor Drugs 0.000 description 1
- 101150069255 KLRC1 gene Proteins 0.000 description 1
- 208000007766 Kaposi sarcoma Diseases 0.000 description 1
- 208000008839 Kidney Neoplasms Diseases 0.000 description 1
- HGCNKOLVKRAVHD-UHFFFAOYSA-N L-Met-L-Phe Natural products CSCCC(N)C(=O)NC(C(O)=O)CC1=CC=CC=C1 HGCNKOLVKRAVHD-UHFFFAOYSA-N 0.000 description 1
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- LHSGPCFBGJHPCY-UHFFFAOYSA-N L-leucine-L-tyrosine Natural products CC(C)CC(N)C(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 LHSGPCFBGJHPCY-UHFFFAOYSA-N 0.000 description 1
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 1
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 1
- 102000017578 LAG3 Human genes 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 1
- 208000031671 Large B-Cell Diffuse Lymphoma Diseases 0.000 description 1
- 208000032004 Large-Cell Anaplastic Lymphoma Diseases 0.000 description 1
- 206010023825 Laryngeal cancer Diseases 0.000 description 1
- 208000018142 Leiomyosarcoma Diseases 0.000 description 1
- BQSLGJHIAGOZCD-CIUDSAMLSA-N Leu-Ala-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O BQSLGJHIAGOZCD-CIUDSAMLSA-N 0.000 description 1
- CNNQBZRGQATKNY-DCAQKATOSA-N Leu-Arg-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CS)C(=O)O)N CNNQBZRGQATKNY-DCAQKATOSA-N 0.000 description 1
- ZURHXHNAEJJRNU-CIUDSAMLSA-N Leu-Asp-Asn Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O ZURHXHNAEJJRNU-CIUDSAMLSA-N 0.000 description 1
- RSFGIMMPWAXNML-MNXVOIDGSA-N Leu-Gln-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O RSFGIMMPWAXNML-MNXVOIDGSA-N 0.000 description 1
- GPICTNQYKHHHTH-GUBZILKMSA-N Leu-Gln-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(O)=O GPICTNQYKHHHTH-GUBZILKMSA-N 0.000 description 1
- HPBCTWSUJOGJSH-MNXVOIDGSA-N Leu-Glu-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O HPBCTWSUJOGJSH-MNXVOIDGSA-N 0.000 description 1
- LLBQJYDYOLIQAI-JYJNAYRXSA-N Leu-Glu-Tyr Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O LLBQJYDYOLIQAI-JYJNAYRXSA-N 0.000 description 1
- QPXBPQUGXHURGP-UWVGGRQHSA-N Leu-Gly-Met Chemical compound CC(C)C[C@@H](C(=O)NCC(=O)N[C@@H](CCSC)C(=O)O)N QPXBPQUGXHURGP-UWVGGRQHSA-N 0.000 description 1
- IAJFFZORSWOZPQ-SRVKXCTJSA-N Leu-Leu-Asn Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O IAJFFZORSWOZPQ-SRVKXCTJSA-N 0.000 description 1
- KYIIALJHAOIAHF-KKUMJFAQSA-N Leu-Leu-His Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CC1=CN=CN1 KYIIALJHAOIAHF-KKUMJFAQSA-N 0.000 description 1
- FAELBUXXFQLUAX-AJNGGQMLSA-N Leu-Leu-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC(C)C FAELBUXXFQLUAX-AJNGGQMLSA-N 0.000 description 1
- XVZCXCTYGHPNEM-UHFFFAOYSA-N Leu-Leu-Pro Natural products CC(C)CC(N)C(=O)NC(CC(C)C)C(=O)N1CCCC1C(O)=O XVZCXCTYGHPNEM-UHFFFAOYSA-N 0.000 description 1
- FIICHHJDINDXKG-IHPCNDPISA-N Leu-Lys-Trp Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O FIICHHJDINDXKG-IHPCNDPISA-N 0.000 description 1
- CHJKEDSZNSONPS-DCAQKATOSA-N Leu-Pro-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O CHJKEDSZNSONPS-DCAQKATOSA-N 0.000 description 1
- JLYUZRKPDKHUTC-WDSOQIARSA-N Leu-Pro-Trp Chemical compound [H]N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O JLYUZRKPDKHUTC-WDSOQIARSA-N 0.000 description 1
- RNYLNYTYMXACRI-VFAJRCTISA-N Leu-Thr-Trp Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O RNYLNYTYMXACRI-VFAJRCTISA-N 0.000 description 1
- ISSAURVGLGAPDK-KKUMJFAQSA-N Leu-Tyr-Asp Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(O)=O)C(O)=O ISSAURVGLGAPDK-KKUMJFAQSA-N 0.000 description 1
- HLFSDGLLUJUHTE-SNVBAGLBSA-N Levamisole Chemical compound C1([C@H]2CN3CCSC3=N2)=CC=CC=C1 HLFSDGLLUJUHTE-SNVBAGLBSA-N 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- WXJKFRMKJORORD-DCAQKATOSA-N Lys-Arg-Ala Chemical compound NC(=N)NCCC[C@@H](C(=O)N[C@@H](C)C(O)=O)NC(=O)[C@@H](N)CCCCN WXJKFRMKJORORD-DCAQKATOSA-N 0.000 description 1
- QUCDKEKDPYISNX-HJGDQZAQSA-N Lys-Asn-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QUCDKEKDPYISNX-HJGDQZAQSA-N 0.000 description 1
- YPLVCBKEPJPBDQ-MELADBBJSA-N Lys-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCCCN)N YPLVCBKEPJPBDQ-MELADBBJSA-N 0.000 description 1
- LUTDBHBIHHREDC-IHRRRGAJSA-N Lys-Pro-Lys Chemical compound NCCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O LUTDBHBIHHREDC-IHRRRGAJSA-N 0.000 description 1
- WQDKIVRHTQYJSN-DCAQKATOSA-N Lys-Ser-Arg Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N WQDKIVRHTQYJSN-DCAQKATOSA-N 0.000 description 1
- MIFFFXHMAHFACR-KATARQTJSA-N Lys-Ser-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CCCCN MIFFFXHMAHFACR-KATARQTJSA-N 0.000 description 1
- XYLSGAWRCZECIQ-JYJNAYRXSA-N Lys-Tyr-Glu Chemical compound NCCCC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CCC(O)=O)C(O)=O)CC1=CC=C(O)C=C1 XYLSGAWRCZECIQ-JYJNAYRXSA-N 0.000 description 1
- FPQMQEOVSKMVMA-ACRUOGEOSA-N Lys-Tyr-Tyr Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O)NC(=O)[C@H](CCCCN)N)O FPQMQEOVSKMVMA-ACRUOGEOSA-N 0.000 description 1
- 229940124787 MELK inhibitor Drugs 0.000 description 1
- 102100030300 MHC class I polypeptide-related sequence B Human genes 0.000 description 1
- 241000282567 Macaca fascicularis Species 0.000 description 1
- 101100404845 Macaca mulatta NKG2A gene Proteins 0.000 description 1
- 108010046938 Macrophage Colony-Stimulating Factor Proteins 0.000 description 1
- 102100028123 Macrophage colony-stimulating factor 1 Human genes 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 208000032271 Malignant tumor of penis Diseases 0.000 description 1
- 208000025205 Mantle-Cell Lymphoma Diseases 0.000 description 1
- 208000000172 Medulloblastoma Diseases 0.000 description 1
- 108010061593 Member 14 Tumor Necrosis Factor Receptors Proteins 0.000 description 1
- IVDYZAAPOLNZKG-KWHRADDSSA-N Mepitiostane Chemical compound O([C@@H]1[C@]2(CC[C@@H]3[C@@]4(C)C[C@H]5S[C@H]5C[C@@H]4CC[C@H]3[C@@H]2CC1)C)C1(OC)CCCC1 IVDYZAAPOLNZKG-KWHRADDSSA-N 0.000 description 1
- 206010027406 Mesothelioma Diseases 0.000 description 1
- GAELMDJMQDUDLJ-BQBZGAKWSA-N Met-Ala-Gly Chemical compound CSCC[C@H](N)C(=O)N[C@@H](C)C(=O)NCC(O)=O GAELMDJMQDUDLJ-BQBZGAKWSA-N 0.000 description 1
- FVKRQMQQFGBXHV-QXEWZRGKSA-N Met-Asp-Val Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O FVKRQMQQFGBXHV-QXEWZRGKSA-N 0.000 description 1
- ZIIMORLEZLVRIP-SRVKXCTJSA-N Met-Leu-Gln Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O ZIIMORLEZLVRIP-SRVKXCTJSA-N 0.000 description 1
- HLZORBMOISUNIV-DCAQKATOSA-N Met-Ser-Leu Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC(C)C HLZORBMOISUNIV-DCAQKATOSA-N 0.000 description 1
- 206010027476 Metastases Diseases 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 1
- VFKZTMPDYBFSTM-KVTDHHQDSA-N Mitobronitol Chemical compound BrC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CBr VFKZTMPDYBFSTM-KVTDHHQDSA-N 0.000 description 1
- 102100037808 Mitogen-activated protein kinase 8 Human genes 0.000 description 1
- 229930192392 Mitomycin Natural products 0.000 description 1
- 102100034256 Mucin-1 Human genes 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- 208000002231 Muscle Neoplasms Diseases 0.000 description 1
- 208000033761 Myelogenous Chronic BCR-ABL Positive Leukemia Diseases 0.000 description 1
- 208000033776 Myeloid Acute Leukemia Diseases 0.000 description 1
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 1
- YBAFDPFAUTYYRW-UHFFFAOYSA-N N-L-alpha-glutamyl-L-leucine Natural products CC(C)CC(C(O)=O)NC(=O)C(N)CCC(O)=O YBAFDPFAUTYYRW-UHFFFAOYSA-N 0.000 description 1
- XZFYRXDAULDNFX-UHFFFAOYSA-N N-L-cysteinyl-L-phenylalanine Natural products SCC(N)C(=O)NC(C(O)=O)CC1=CC=CC=C1 XZFYRXDAULDNFX-UHFFFAOYSA-N 0.000 description 1
- AJHCSUXXECOXOY-UHFFFAOYSA-N N-glycyl-L-tryptophan Natural products C1=CC=C2C(CC(NC(=O)CN)C(O)=O)=CNC2=C1 AJHCSUXXECOXOY-UHFFFAOYSA-N 0.000 description 1
- 108010002311 N-glycylglutamic acid Proteins 0.000 description 1
- 108010057466 NF-kappa B Proteins 0.000 description 1
- 102100022682 NKG2-A/NKG2-B type II integral membrane protein Human genes 0.000 description 1
- 206010061309 Neoplasm progression Diseases 0.000 description 1
- 206010029260 Neuroblastoma Diseases 0.000 description 1
- 101100342977 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) leu-1 gene Proteins 0.000 description 1
- PVNIIMVLHYAWGP-UHFFFAOYSA-N Niacin Chemical compound OC(=O)C1=CC=CN=C1 PVNIIMVLHYAWGP-UHFFFAOYSA-N 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- 206010029488 Nodular melanoma Diseases 0.000 description 1
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 description 1
- 102100023050 Nuclear factor NF-kappa-B p105 subunit Human genes 0.000 description 1
- 206010030155 Oesophageal carcinoma Diseases 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 206010033128 Ovarian cancer Diseases 0.000 description 1
- 206010061535 Ovarian neoplasm Diseases 0.000 description 1
- 102100039792 Oxidized purine nucleoside triphosphate hydrolase Human genes 0.000 description 1
- 239000012661 PARP inhibitor Substances 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 208000037064 Papilloma of choroid plexus Diseases 0.000 description 1
- 208000004091 Parotid Neoplasms Diseases 0.000 description 1
- 206010061336 Pelvic neoplasm Diseases 0.000 description 1
- 208000002471 Penile Neoplasms Diseases 0.000 description 1
- 206010034299 Penile cancer Diseases 0.000 description 1
- KHGNFPUMBJSZSM-UHFFFAOYSA-N Perforine Natural products COC1=C2CCC(O)C(CCC(C)(C)O)(OC)C2=NC2=C1C=CO2 KHGNFPUMBJSZSM-UHFFFAOYSA-N 0.000 description 1
- 206010057249 Phagocytosis Diseases 0.000 description 1
- 208000009565 Pharyngeal Neoplasms Diseases 0.000 description 1
- 206010034811 Pharyngeal cancer Diseases 0.000 description 1
- LBSARGIQACMGDF-WBAXXEDZSA-N Phe-Ala-Phe Chemical compound C([C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=CC=C1 LBSARGIQACMGDF-WBAXXEDZSA-N 0.000 description 1
- UHRNIXJAGGLKHP-DLOVCJGASA-N Phe-Ala-Ser Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O UHRNIXJAGGLKHP-DLOVCJGASA-N 0.000 description 1
- FRPVPGRXUKFEQE-YDHLFZDLSA-N Phe-Asp-Val Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O FRPVPGRXUKFEQE-YDHLFZDLSA-N 0.000 description 1
- BEEVXUYVEHXWRQ-YESZJQIVSA-N Phe-His-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CN=CN2)NC(=O)[C@H](CC3=CC=CC=C3)N)C(=O)O BEEVXUYVEHXWRQ-YESZJQIVSA-N 0.000 description 1
- YTILBRIUASDGBL-BZSNNMDCSA-N Phe-Leu-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC1=CC=CC=C1 YTILBRIUASDGBL-BZSNNMDCSA-N 0.000 description 1
- AAERWTUHZKLDLC-IHRRRGAJSA-N Phe-Pro-Asp Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(O)=O AAERWTUHZKLDLC-IHRRRGAJSA-N 0.000 description 1
- YMIZSYUAZJSOFL-SRVKXCTJSA-N Phe-Ser-Asn Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O YMIZSYUAZJSOFL-SRVKXCTJSA-N 0.000 description 1
- XDMMOISUAHXXFD-SRVKXCTJSA-N Phe-Ser-Asp Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O XDMMOISUAHXXFD-SRVKXCTJSA-N 0.000 description 1
- RAGOJJCBGXARPO-XVSYOHENSA-N Phe-Thr-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H]([C@H](O)C)NC(=O)[C@@H](N)CC1=CC=CC=C1 RAGOJJCBGXARPO-XVSYOHENSA-N 0.000 description 1
- BSKMOCNNLNDIMU-CDMKHQONSA-N Phe-Thr-Gly Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(O)=O BSKMOCNNLNDIMU-CDMKHQONSA-N 0.000 description 1
- KIQUCMUULDXTAZ-HJOGWXRNSA-N Phe-Tyr-Tyr Chemical compound N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O KIQUCMUULDXTAZ-HJOGWXRNSA-N 0.000 description 1
- 208000007452 Plasmacytoma Diseases 0.000 description 1
- RVGRUAULSDPKGF-UHFFFAOYSA-N Poloxamer Chemical compound C1CO1.CC1CO1 RVGRUAULSDPKGF-UHFFFAOYSA-N 0.000 description 1
- 108010064218 Poly (ADP-Ribose) Polymerase-1 Proteins 0.000 description 1
- 229940121906 Poly ADP ribose polymerase inhibitor Drugs 0.000 description 1
- 102100023712 Poly [ADP-ribose] polymerase 1 Human genes 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 229920001213 Polysorbate 20 Polymers 0.000 description 1
- 208000006664 Precursor Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 description 1
- LCRSGSIRKLXZMZ-BPNCWPANSA-N Pro-Ala-Tyr Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O LCRSGSIRKLXZMZ-BPNCWPANSA-N 0.000 description 1
- BNBBNGZZKQUWCD-IUCAKERBSA-N Pro-Arg-Gly Chemical compound NC(N)=NCCC[C@@H](C(=O)NCC(O)=O)NC(=O)[C@@H]1CCCN1 BNBBNGZZKQUWCD-IUCAKERBSA-N 0.000 description 1
- FUVBEZJCRMHWEM-FXQIFTODSA-N Pro-Asn-Ser Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O FUVBEZJCRMHWEM-FXQIFTODSA-N 0.000 description 1
- DIZLUAZLNDFDPR-CIUDSAMLSA-N Pro-Cys-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CS)NC(=O)[C@@H]1CCCN1 DIZLUAZLNDFDPR-CIUDSAMLSA-N 0.000 description 1
- PULPZRAHVFBVTO-DCAQKATOSA-N Pro-Glu-Arg Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O PULPZRAHVFBVTO-DCAQKATOSA-N 0.000 description 1
- BCNRNJWSRFDPTQ-HJWJTTGWSA-N Pro-Ile-Phe Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O BCNRNJWSRFDPTQ-HJWJTTGWSA-N 0.000 description 1
- BRJGUPWVFXKBQI-XUXIUFHCSA-N Pro-Leu-Ile Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O BRJGUPWVFXKBQI-XUXIUFHCSA-N 0.000 description 1
- MRYUJHGPZQNOAD-IHRRRGAJSA-N Pro-Leu-Lys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@@H]1CCCN1 MRYUJHGPZQNOAD-IHRRRGAJSA-N 0.000 description 1
- MCWHYUWXVNRXFV-RWMBFGLXSA-N Pro-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@@H]2CCCN2 MCWHYUWXVNRXFV-RWMBFGLXSA-N 0.000 description 1
- RMODQFBNDDENCP-IHRRRGAJSA-N Pro-Lys-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O RMODQFBNDDENCP-IHRRRGAJSA-N 0.000 description 1
- CGSOWZUPLOKYOR-AVGNSLFASA-N Pro-Pro-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 CGSOWZUPLOKYOR-AVGNSLFASA-N 0.000 description 1
- FDMKYQQYJKYCLV-GUBZILKMSA-N Pro-Pro-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 FDMKYQQYJKYCLV-GUBZILKMSA-N 0.000 description 1
- DYJTXTCEXMCPBF-UFYCRDLUSA-N Pro-Tyr-Phe Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)N[C@@H](CC3=CC=CC=C3)C(=O)O DYJTXTCEXMCPBF-UFYCRDLUSA-N 0.000 description 1
- IMNVAOPEMFDAQD-NHCYSSNCSA-N Pro-Val-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O IMNVAOPEMFDAQD-NHCYSSNCSA-N 0.000 description 1
- 102100024216 Programmed cell death 1 ligand 1 Human genes 0.000 description 1
- 101710089372 Programmed cell death protein 1 Proteins 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 102100036735 Prostate stem cell antigen Human genes 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- 229940079156 Proteasome inhibitor Drugs 0.000 description 1
- 201000008183 Pulmonary blastoma Diseases 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 206010038389 Renal cancer Diseases 0.000 description 1
- 208000006265 Renal cell carcinoma Diseases 0.000 description 1
- 201000000582 Retinoblastoma Diseases 0.000 description 1
- BKRGVLQUQGGVSM-KBXCAEBGSA-N Revanil Chemical compound C1=CC(C=2[C@H](N(C)C[C@H](C=2)NC(=O)N(CC)CC)C2)=C3C2=CNC3=C1 BKRGVLQUQGGVSM-KBXCAEBGSA-N 0.000 description 1
- 102100029198 SLAM family member 7 Human genes 0.000 description 1
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 1
- 229920002305 Schizophyllan Polymers 0.000 description 1
- 206010070834 Sensitisation Diseases 0.000 description 1
- IDQFQFVEWMWRQQ-DLOVCJGASA-N Ser-Ala-Phe Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O IDQFQFVEWMWRQQ-DLOVCJGASA-N 0.000 description 1
- OYEDZGNMSBZCIM-XGEHTFHBSA-N Ser-Arg-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OYEDZGNMSBZCIM-XGEHTFHBSA-N 0.000 description 1
- WXUBSIDKNMFAGS-IHRRRGAJSA-N Ser-Arg-Tyr Chemical compound NC(N)=NCCC[C@H](NC(=O)[C@H](CO)N)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 WXUBSIDKNMFAGS-IHRRRGAJSA-N 0.000 description 1
- OBXVZEAMXFSGPU-FXQIFTODSA-N Ser-Asn-Arg Chemical compound C(C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CO)N)CN=C(N)N OBXVZEAMXFSGPU-FXQIFTODSA-N 0.000 description 1
- FIDMVVBUOCMMJG-CIUDSAMLSA-N Ser-Asn-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CO FIDMVVBUOCMMJG-CIUDSAMLSA-N 0.000 description 1
- ICHZYBVODUVUKN-SRVKXCTJSA-N Ser-Asn-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O ICHZYBVODUVUKN-SRVKXCTJSA-N 0.000 description 1
- CNIIKZQXBBQHCX-FXQIFTODSA-N Ser-Asp-Arg Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O CNIIKZQXBBQHCX-FXQIFTODSA-N 0.000 description 1
- SFZKGGOGCNQPJY-CIUDSAMLSA-N Ser-Asp-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CO)N SFZKGGOGCNQPJY-CIUDSAMLSA-N 0.000 description 1
- BTPAWKABYQMKKN-LKXGYXEUSA-N Ser-Asp-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O BTPAWKABYQMKKN-LKXGYXEUSA-N 0.000 description 1
- ULVMNZOKDBHKKI-ACZMJKKPSA-N Ser-Gln-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O ULVMNZOKDBHKKI-ACZMJKKPSA-N 0.000 description 1
- HJEBZBMOTCQYDN-ACZMJKKPSA-N Ser-Glu-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O HJEBZBMOTCQYDN-ACZMJKKPSA-N 0.000 description 1
- VQBCMLMPEWPUTB-ACZMJKKPSA-N Ser-Glu-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O VQBCMLMPEWPUTB-ACZMJKKPSA-N 0.000 description 1
- WBINSDOPZHQPPM-AVGNSLFASA-N Ser-Glu-Tyr Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CO)N)O WBINSDOPZHQPPM-AVGNSLFASA-N 0.000 description 1
- BPMRXBZYPGYPJN-WHFBIAKZSA-N Ser-Gly-Asn Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(O)=O BPMRXBZYPGYPJN-WHFBIAKZSA-N 0.000 description 1
- IOVHBRCQOGWAQH-ZKWXMUAHSA-N Ser-Gly-Ile Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H]([C@@H](C)CC)C(O)=O IOVHBRCQOGWAQH-ZKWXMUAHSA-N 0.000 description 1
- LQESNKGTTNHZPZ-GHCJXIJMSA-N Ser-Ile-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(O)=O LQESNKGTTNHZPZ-GHCJXIJMSA-N 0.000 description 1
- ZOPISOXXPQNOCO-SVSWQMSJSA-N Ser-Ile-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)O)NC(=O)[C@H](CO)N ZOPISOXXPQNOCO-SVSWQMSJSA-N 0.000 description 1
- KCGIREHVWRXNDH-GARJFASQSA-N Ser-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CO)N KCGIREHVWRXNDH-GARJFASQSA-N 0.000 description 1
- KZPRPBLHYMZIMH-MXAVVETBSA-N Ser-Phe-Ile Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O KZPRPBLHYMZIMH-MXAVVETBSA-N 0.000 description 1
- MQUZANJDFOQOBX-SRVKXCTJSA-N Ser-Phe-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(O)=O MQUZANJDFOQOBX-SRVKXCTJSA-N 0.000 description 1
- ZKBKUWQVDWWSRI-BZSNNMDCSA-N Ser-Phe-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O ZKBKUWQVDWWSRI-BZSNNMDCSA-N 0.000 description 1
- ADJDNJCSPNFFPI-FXQIFTODSA-N Ser-Pro-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CO ADJDNJCSPNFFPI-FXQIFTODSA-N 0.000 description 1
- DINQYZRMXGWWTG-GUBZILKMSA-N Ser-Pro-Pro Chemical compound OC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 DINQYZRMXGWWTG-GUBZILKMSA-N 0.000 description 1
- PPCZVWHJWJFTFN-ZLUOBGJFSA-N Ser-Ser-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O PPCZVWHJWJFTFN-ZLUOBGJFSA-N 0.000 description 1
- JCLAFVNDBJMLBC-JBDRJPRFSA-N Ser-Ser-Ile Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O JCLAFVNDBJMLBC-JBDRJPRFSA-N 0.000 description 1
- XQJCEKXQUJQNNK-ZLUOBGJFSA-N Ser-Ser-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O XQJCEKXQUJQNNK-ZLUOBGJFSA-N 0.000 description 1
- DKGRNFUXVTYRAS-UBHSHLNASA-N Ser-Ser-Trp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O DKGRNFUXVTYRAS-UBHSHLNASA-N 0.000 description 1
- SQHKXWODKJDZRC-LKXGYXEUSA-N Ser-Thr-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(O)=O SQHKXWODKJDZRC-LKXGYXEUSA-N 0.000 description 1
- UBTNVMGPMYDYIU-HJPIBITLSA-N Ser-Tyr-Ile Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O UBTNVMGPMYDYIU-HJPIBITLSA-N 0.000 description 1
- ZVBCMFDJIMUELU-BZSNNMDCSA-N Ser-Tyr-Phe Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)O)NC(=O)[C@H](CC2=CC=C(C=C2)O)NC(=O)[C@H](CO)N ZVBCMFDJIMUELU-BZSNNMDCSA-N 0.000 description 1
- HAYADTTXNZFUDM-IHRRRGAJSA-N Ser-Tyr-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C(C)C)C(O)=O HAYADTTXNZFUDM-IHRRRGAJSA-N 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- 102100031081 Serine/threonine-protein kinase Chk1 Human genes 0.000 description 1
- 206010054184 Small intestine carcinoma Diseases 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- 208000032383 Soft tissue cancer Diseases 0.000 description 1
- 102000005157 Somatostatin Human genes 0.000 description 1
- 108010056088 Somatostatin Proteins 0.000 description 1
- 208000005718 Stomach Neoplasms Diseases 0.000 description 1
- 108010090804 Streptavidin Proteins 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- 230000005867 T cell response Effects 0.000 description 1
- 208000031673 T-Cell Cutaneous Lymphoma Diseases 0.000 description 1
- 208000000389 T-cell leukemia Diseases 0.000 description 1
- 208000028530 T-cell lymphoblastic leukemia/lymphoma Diseases 0.000 description 1
- 102100025237 T-cell surface antigen CD2 Human genes 0.000 description 1
- 108010065917 TOR Serine-Threonine Kinases Proteins 0.000 description 1
- 102000013530 TOR Serine-Threonine Kinases Human genes 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 208000024313 Testicular Neoplasms Diseases 0.000 description 1
- 206010057644 Testis cancer Diseases 0.000 description 1
- ZMZDMBWJUHKJPS-UHFFFAOYSA-M Thiocyanate anion Chemical compound [S-]C#N ZMZDMBWJUHKJPS-UHFFFAOYSA-M 0.000 description 1
- MQCPGOZXFSYJPS-KZVJFYERSA-N Thr-Ala-Arg Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O MQCPGOZXFSYJPS-KZVJFYERSA-N 0.000 description 1
- SWIKDOUVROTZCW-GCJQMDKQSA-N Thr-Asn-Ala Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](C)C(=O)O)N)O SWIKDOUVROTZCW-GCJQMDKQSA-N 0.000 description 1
- YLXAMFZYJTZXFH-OLHMAJIHSA-N Thr-Asn-Asp Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC(=O)O)C(=O)O)N)O YLXAMFZYJTZXFH-OLHMAJIHSA-N 0.000 description 1
- JVTHIXKSVYEWNI-JRQIVUDYSA-N Thr-Asn-Tyr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O JVTHIXKSVYEWNI-JRQIVUDYSA-N 0.000 description 1
- KRPKYGOFYUNIGM-XVSYOHENSA-N Thr-Asp-Phe Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)N)O KRPKYGOFYUNIGM-XVSYOHENSA-N 0.000 description 1
- UTCFSBBXPWKLTG-XKBZYTNZSA-N Thr-Cys-Gln Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N)O UTCFSBBXPWKLTG-XKBZYTNZSA-N 0.000 description 1
- ASJDFGOPDCVXTG-KATARQTJSA-N Thr-Cys-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(O)=O ASJDFGOPDCVXTG-KATARQTJSA-N 0.000 description 1
- DIPIPFHFLPTCLK-LOKLDPHHSA-N Thr-Gln-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N1CCC[C@@H]1C(=O)O)N)O DIPIPFHFLPTCLK-LOKLDPHHSA-N 0.000 description 1
- XOTBWOCSLMBGMF-SUSMZKCASA-N Thr-Glu-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O XOTBWOCSLMBGMF-SUSMZKCASA-N 0.000 description 1
- KCRQEJSKXAIULJ-FJXKBIBVSA-N Thr-Gly-Arg Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O KCRQEJSKXAIULJ-FJXKBIBVSA-N 0.000 description 1
- NIEWSKWFURSECR-FOHZUACHSA-N Thr-Gly-Asp Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(O)=O NIEWSKWFURSECR-FOHZUACHSA-N 0.000 description 1
- JQAWYCUUFIMTHE-WLTAIBSBSA-N Thr-Gly-Tyr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O JQAWYCUUFIMTHE-WLTAIBSBSA-N 0.000 description 1
- YJCVECXVYHZOBK-KNZXXDILSA-N Thr-Ile-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H]([C@@H](C)O)N YJCVECXVYHZOBK-KNZXXDILSA-N 0.000 description 1
- MEJHFIOYJHTWMK-VOAKCMCISA-N Thr-Leu-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)[C@@H](C)O MEJHFIOYJHTWMK-VOAKCMCISA-N 0.000 description 1
- VRUFCJZQDACGLH-UVOCVTCTSA-N Thr-Leu-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O VRUFCJZQDACGLH-UVOCVTCTSA-N 0.000 description 1
- KZSYAEWQMJEGRZ-RHYQMDGZSA-N Thr-Leu-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O KZSYAEWQMJEGRZ-RHYQMDGZSA-N 0.000 description 1
- SPVHQURZJCUDQC-VOAKCMCISA-N Thr-Lys-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O SPVHQURZJCUDQC-VOAKCMCISA-N 0.000 description 1
- HSQXHRIRJSFDOH-URLPEUOOSA-N Thr-Phe-Ile Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O HSQXHRIRJSFDOH-URLPEUOOSA-N 0.000 description 1
- FWTFAZKJORVTIR-VZFHVOOUSA-N Thr-Ser-Ala Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O FWTFAZKJORVTIR-VZFHVOOUSA-N 0.000 description 1
- NQQMWWVVGIXUOX-SVSWQMSJSA-N Thr-Ser-Ile Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O NQQMWWVVGIXUOX-SVSWQMSJSA-N 0.000 description 1
- WPSKTVVMQCXPRO-BWBBJGPYSA-N Thr-Ser-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O WPSKTVVMQCXPRO-BWBBJGPYSA-N 0.000 description 1
- COYHRQWNJDJCNA-NUJDXYNKSA-N Thr-Thr-Thr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O COYHRQWNJDJCNA-NUJDXYNKSA-N 0.000 description 1
- VGNKUXWYFFDWDH-BEMMVCDISA-N Thr-Trp-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N3CCC[C@@H]3C(=O)O)N)O VGNKUXWYFFDWDH-BEMMVCDISA-N 0.000 description 1
- XVHAUVJXBFGUPC-RPTUDFQQSA-N Thr-Tyr-Phe Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O XVHAUVJXBFGUPC-RPTUDFQQSA-N 0.000 description 1
- KPMIQCXJDVKWKO-IFFSRLJSSA-N Thr-Val-Glu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O KPMIQCXJDVKWKO-IFFSRLJSSA-N 0.000 description 1
- PWONLXBUSVIZPH-RHYQMDGZSA-N Thr-Val-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)O)N)O PWONLXBUSVIZPH-RHYQMDGZSA-N 0.000 description 1
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 1
- 239000004473 Threonine Substances 0.000 description 1
- 208000024770 Thyroid neoplasm Diseases 0.000 description 1
- IVTVGDXNLFLDRM-HNNXBMFYSA-N Tomudex Chemical compound C=1C=C2NC(C)=NC(=O)C2=CC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)S1 IVTVGDXNLFLDRM-HNNXBMFYSA-N 0.000 description 1
- 206010062129 Tongue neoplasm Diseases 0.000 description 1
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 1
- AVYVKJMBNLPWRX-WFBYXXMGSA-N Trp-Ala-Ser Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O)=CNC2=C1 AVYVKJMBNLPWRX-WFBYXXMGSA-N 0.000 description 1
- LTLBNCDNXQCOLB-UBHSHLNASA-N Trp-Asp-Ser Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O)=CNC2=C1 LTLBNCDNXQCOLB-UBHSHLNASA-N 0.000 description 1
- WNZRNOGHEONFMS-PXDAIIFMSA-N Trp-Ile-Tyr Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O WNZRNOGHEONFMS-PXDAIIFMSA-N 0.000 description 1
- KCZGSXPFPNKGLE-WDSOQIARSA-N Trp-Met-His Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CC2=CNC3=CC=CC=C32)N KCZGSXPFPNKGLE-WDSOQIARSA-N 0.000 description 1
- UOXPLPBMEPLZBW-WDSOQIARSA-N Trp-Val-Lys Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(O)=O)=CNC2=C1 UOXPLPBMEPLZBW-WDSOQIARSA-N 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- 108060008683 Tumor Necrosis Factor Receptor Proteins 0.000 description 1
- 102100040113 Tumor necrosis factor receptor superfamily member 10A Human genes 0.000 description 1
- 102100040112 Tumor necrosis factor receptor superfamily member 10B Human genes 0.000 description 1
- 102100028785 Tumor necrosis factor receptor superfamily member 14 Human genes 0.000 description 1
- 102100033728 Tumor necrosis factor receptor superfamily member 18 Human genes 0.000 description 1
- 102100022203 Tumor necrosis factor receptor superfamily member 25 Human genes 0.000 description 1
- 102100022153 Tumor necrosis factor receptor superfamily member 4 Human genes 0.000 description 1
- 101710165473 Tumor necrosis factor receptor superfamily member 4 Proteins 0.000 description 1
- 102100036857 Tumor necrosis factor receptor superfamily member 8 Human genes 0.000 description 1
- 102100036856 Tumor necrosis factor receptor superfamily member 9 Human genes 0.000 description 1
- BURPTJBFWIOHEY-UWJYBYFXSA-N Tyr-Ala-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 BURPTJBFWIOHEY-UWJYBYFXSA-N 0.000 description 1
- CDRYEAWHKJSGAF-BPNCWPANSA-N Tyr-Ala-Met Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCSC)C(O)=O CDRYEAWHKJSGAF-BPNCWPANSA-N 0.000 description 1
- AKXBNSZMYAOGLS-STQMWFEESA-N Tyr-Arg-Gly Chemical compound NC(N)=NCCC[C@@H](C(=O)NCC(O)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 AKXBNSZMYAOGLS-STQMWFEESA-N 0.000 description 1
- GFZQWWDXJVGEMW-ULQDDVLXSA-N Tyr-Arg-Lys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)O)N)O GFZQWWDXJVGEMW-ULQDDVLXSA-N 0.000 description 1
- GFHYISDTIWZUSU-QWRGUYRKSA-N Tyr-Asn-Gly Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O GFHYISDTIWZUSU-QWRGUYRKSA-N 0.000 description 1
- GAYLGYUVTDMLKC-UWJYBYFXSA-N Tyr-Asp-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 GAYLGYUVTDMLKC-UWJYBYFXSA-N 0.000 description 1
- WPVGRKLNHJJCEN-BZSNNMDCSA-N Tyr-Asp-Phe Chemical compound C([C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=C(O)C=C1 WPVGRKLNHJJCEN-BZSNNMDCSA-N 0.000 description 1
- FGJWNBBFAUHBEP-IHPCNDPISA-N Tyr-Asp-Trp Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CC3=CC=C(C=C3)O)N FGJWNBBFAUHBEP-IHPCNDPISA-N 0.000 description 1
- TZXFLDNBYYGLKA-BZSNNMDCSA-N Tyr-Asp-Tyr Chemical compound C([C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=C(O)C=C1 TZXFLDNBYYGLKA-BZSNNMDCSA-N 0.000 description 1
- UABYBEBXFFNCIR-YDHLFZDLSA-N Tyr-Asp-Val Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O UABYBEBXFFNCIR-YDHLFZDLSA-N 0.000 description 1
- MOCXXGZHHSPNEJ-AVGNSLFASA-N Tyr-Cys-Glu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(O)=O)C(O)=O MOCXXGZHHSPNEJ-AVGNSLFASA-N 0.000 description 1
- YLRLHDFMMWDYTK-KKUMJFAQSA-N Tyr-Cys-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CS)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 YLRLHDFMMWDYTK-KKUMJFAQSA-N 0.000 description 1
- FFCRCJZJARTYCG-KKUMJFAQSA-N Tyr-Cys-Lys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CCCCN)C(=O)O)N)O FFCRCJZJARTYCG-KKUMJFAQSA-N 0.000 description 1
- CNLKDWSAORJEMW-KWQFWETISA-N Tyr-Gly-Ala Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)NCC(=O)N[C@@H](C)C(O)=O CNLKDWSAORJEMW-KWQFWETISA-N 0.000 description 1
- NXRGXTBPMOGFID-CFMVVWHZSA-N Tyr-Ile-Asn Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(O)=O NXRGXTBPMOGFID-CFMVVWHZSA-N 0.000 description 1
- YMUQBRQQCPQEQN-CXTHYWKRSA-N Tyr-Ile-Tyr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)NC(=O)[C@H](CC2=CC=C(C=C2)O)N YMUQBRQQCPQEQN-CXTHYWKRSA-N 0.000 description 1
- WTTRJMAZPDHPGS-KKXDTOCCSA-N Tyr-Phe-Ala Chemical compound C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O WTTRJMAZPDHPGS-KKXDTOCCSA-N 0.000 description 1
- PLXQRTXVLZUNMU-RNXOBYDBSA-N Tyr-Phe-Trp Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC2=CNC3=CC=CC=C32)C(=O)O)NC(=O)[C@H](CC4=CC=C(C=C4)O)N PLXQRTXVLZUNMU-RNXOBYDBSA-N 0.000 description 1
- VPEFOFYNHBWFNQ-UFYCRDLUSA-N Tyr-Pro-Tyr Chemical compound C([C@H](N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=C(O)C=C1 VPEFOFYNHBWFNQ-UFYCRDLUSA-N 0.000 description 1
- MDXLPNRXCFOBTL-BZSNNMDCSA-N Tyr-Ser-Tyr Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O MDXLPNRXCFOBTL-BZSNNMDCSA-N 0.000 description 1
- LDKDSFQSEUOCOO-RPTUDFQQSA-N Tyr-Thr-Phe Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O LDKDSFQSEUOCOO-RPTUDFQQSA-N 0.000 description 1
- WQOHKVRQDLNDIL-YJRXYDGGSA-N Tyr-Thr-Ser Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O WQOHKVRQDLNDIL-YJRXYDGGSA-N 0.000 description 1
- NUQZCPSZHGIYTA-HKUYNNGSSA-N Tyr-Trp-Gly Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)NCC(=O)O)NC(=O)[C@H](CC3=CC=C(C=C3)O)N NUQZCPSZHGIYTA-HKUYNNGSSA-N 0.000 description 1
- LYPKCSYAKLTBHJ-ILWGZMRPSA-N Tyr-Trp-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CNC3=CC=CC=C32)NC(=O)[C@H](CC4=CC=C(C=C4)O)N)C(=O)O LYPKCSYAKLTBHJ-ILWGZMRPSA-N 0.000 description 1
- GZWPQZDVTBZVEP-BZSNNMDCSA-N Tyr-Tyr-Asn Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(N)=O)C(O)=O GZWPQZDVTBZVEP-BZSNNMDCSA-N 0.000 description 1
- ANHVRCNNGJMJNG-BZSNNMDCSA-N Tyr-Tyr-Cys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)N[C@@H](CS)C(=O)O)N)O ANHVRCNNGJMJNG-BZSNNMDCSA-N 0.000 description 1
- KSGKJSFPWSMJHK-JNPHEJMOSA-N Tyr-Tyr-Thr Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O KSGKJSFPWSMJHK-JNPHEJMOSA-N 0.000 description 1
- FZADUTOCSFDBRV-RNXOBYDBSA-N Tyr-Tyr-Trp Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(O)=O)C1=CC=C(O)C=C1 FZADUTOCSFDBRV-RNXOBYDBSA-N 0.000 description 1
- 102100029823 Tyrosine-protein kinase BTK Human genes 0.000 description 1
- 102100040013 UL16-binding protein 6 Human genes 0.000 description 1
- 101150013568 US16 gene Proteins 0.000 description 1
- 208000023915 Ureteral Neoplasms Diseases 0.000 description 1
- 206010046392 Ureteric cancer Diseases 0.000 description 1
- 206010046431 Urethral cancer Diseases 0.000 description 1
- 206010046458 Urethral neoplasms Diseases 0.000 description 1
- 208000006593 Urologic Neoplasms Diseases 0.000 description 1
- 208000002495 Uterine Neoplasms Diseases 0.000 description 1
- 201000005969 Uveal melanoma Diseases 0.000 description 1
- 108091008605 VEGF receptors Proteins 0.000 description 1
- YODDULVCGFQRFZ-ZKWXMUAHSA-N Val-Asp-Ser Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O YODDULVCGFQRFZ-ZKWXMUAHSA-N 0.000 description 1
- NYTKXWLZSNRILS-IFFSRLJSSA-N Val-Gln-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](C(C)C)N)O NYTKXWLZSNRILS-IFFSRLJSSA-N 0.000 description 1
- XGJLNBNZNMVJRS-NRPADANISA-N Val-Glu-Ala Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O XGJLNBNZNMVJRS-NRPADANISA-N 0.000 description 1
- HQYVQDRYODWONX-DCAQKATOSA-N Val-His-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)N[C@@H](CO)C(=O)O)N HQYVQDRYODWONX-DCAQKATOSA-N 0.000 description 1
- BZOSBRIDWSSTFN-AVGNSLFASA-N Val-Leu-Met Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCSC)C(=O)O)NC(=O)[C@H](C(C)C)N BZOSBRIDWSSTFN-AVGNSLFASA-N 0.000 description 1
- DIOSYUIWOQCXNR-ONGXEEELSA-N Val-Lys-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O DIOSYUIWOQCXNR-ONGXEEELSA-N 0.000 description 1
- ZXYPHBKIZLAQTL-QXEWZRGKSA-N Val-Pro-Asp Chemical compound CC(C)[C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(=O)O)C(=O)O)N ZXYPHBKIZLAQTL-QXEWZRGKSA-N 0.000 description 1
- AJNUKMZFHXUBMK-GUBZILKMSA-N Val-Ser-Arg Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N AJNUKMZFHXUBMK-GUBZILKMSA-N 0.000 description 1
- LTTQCQRTSHJPPL-ZKWXMUAHSA-N Val-Ser-Asp Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(=O)O)C(=O)O)N LTTQCQRTSHJPPL-ZKWXMUAHSA-N 0.000 description 1
- QZKVWWIUSQGWMY-IHRRRGAJSA-N Val-Ser-Phe Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 QZKVWWIUSQGWMY-IHRRRGAJSA-N 0.000 description 1
- HWNYVQMOLCYHEA-IHRRRGAJSA-N Val-Ser-Tyr Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)N HWNYVQMOLCYHEA-IHRRRGAJSA-N 0.000 description 1
- NZYNRRGJJVSSTJ-GUBZILKMSA-N Val-Ser-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O NZYNRRGJJVSSTJ-GUBZILKMSA-N 0.000 description 1
- PQSNETRGCRUOGP-KKHAAJSZSA-N Val-Thr-Asn Chemical compound CC(C)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CC(N)=O PQSNETRGCRUOGP-KKHAAJSZSA-N 0.000 description 1
- JAIZPWVHPQRYOU-ZJDVBMNYSA-N Val-Thr-Thr Chemical compound C[C@H]([C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)O)NC(=O)[C@H](C(C)C)N)O JAIZPWVHPQRYOU-ZJDVBMNYSA-N 0.000 description 1
- QHSSPPHOHJSTML-HOCLYGCPSA-N Val-Trp-Gly Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)NCC(=O)O)N QHSSPPHOHJSTML-HOCLYGCPSA-N 0.000 description 1
- PGBMPFKFKXYROZ-UFYCRDLUSA-N Val-Tyr-Phe Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC2=CC=CC=C2)C(=O)O)N PGBMPFKFKXYROZ-UFYCRDLUSA-N 0.000 description 1
- 102100033177 Vascular endothelial growth factor receptor 2 Human genes 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 206010047741 Vulval cancer Diseases 0.000 description 1
- 208000004354 Vulvar Neoplasms Diseases 0.000 description 1
- 102100023037 Wee1-like protein kinase Human genes 0.000 description 1
- 208000008383 Wilms tumor Diseases 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 150000001242 acetic acid derivatives Chemical class 0.000 description 1
- 208000009621 actinic keratosis Diseases 0.000 description 1
- 239000008186 active pharmaceutical agent Substances 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 208000002517 adenoid cystic carcinoma Diseases 0.000 description 1
- 201000008395 adenosquamous carcinoma Diseases 0.000 description 1
- WNLRTRBMVRJNCN-UHFFFAOYSA-N adipic acid Chemical class OC(=O)CCCCC(O)=O WNLRTRBMVRJNCN-UHFFFAOYSA-N 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 238000001261 affinity purification Methods 0.000 description 1
- 235000004279 alanine Nutrition 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 150000001340 alkali metals Chemical class 0.000 description 1
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 1
- 150000001342 alkaline earth metals Chemical class 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- SHGAZHPCJJPHSC-YCNIQYBTSA-N all-trans-retinoic acid Chemical compound OC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-YCNIQYBTSA-N 0.000 description 1
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 1
- AWUCVROLDVIAJX-UHFFFAOYSA-N alpha-glycerophosphate Natural products OCC(O)COP(O)(O)=O AWUCVROLDVIAJX-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 239000004178 amaranth Substances 0.000 description 1
- 229960003437 aminoglutethimide Drugs 0.000 description 1
- ROBVIMPUHSLWNV-UHFFFAOYSA-N aminoglutethimide Chemical compound C=1C=C(N)C=CC=1C1(CC)CCC(=O)NC1=O ROBVIMPUHSLWNV-UHFFFAOYSA-N 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 235000019270 ammonium chloride Nutrition 0.000 description 1
- 229960001220 amsacrine Drugs 0.000 description 1
- XCPGHVQEEXUHNC-UHFFFAOYSA-N amsacrine Chemical compound COC1=CC(NS(C)(=O)=O)=CC=C1NC1=C(C=CC=C2)C2=NC2=CC=CC=C12 XCPGHVQEEXUHNC-UHFFFAOYSA-N 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- 230000010056 antibody-dependent cellular cytotoxicity Effects 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 230000005975 antitumor immune response Effects 0.000 description 1
- 201000011165 anus cancer Diseases 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 108010084758 arginyl-tyrosyl-aspartic acid Proteins 0.000 description 1
- 108010062796 arginyllysine Proteins 0.000 description 1
- 235000009582 asparagine Nutrition 0.000 description 1
- 229960001230 asparagine Drugs 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-L aspartate group Chemical class N[C@@H](CC(=O)[O-])C(=O)[O-] CKLJMWTZIZZHCS-REOHCLBHSA-L 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 108010092854 aspartyllysine Proteins 0.000 description 1
- 230000000712 assembly Effects 0.000 description 1
- 238000000429 assembly Methods 0.000 description 1
- 230000006472 autoimmune response Effects 0.000 description 1
- KLNFSAOEKUDMFA-UHFFFAOYSA-N azanide;2-hydroxyacetic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OCC(O)=O KLNFSAOEKUDMFA-UHFFFAOYSA-N 0.000 description 1
- VSRXQHXAPYXROS-UHFFFAOYSA-N azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OC(=O)C1(C(O)=O)CCC1 VSRXQHXAPYXROS-UHFFFAOYSA-N 0.000 description 1
- 230000033590 base-excision repair Effects 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 229940092714 benzenesulfonic acid Drugs 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 150000001558 benzoic acid derivatives Chemical class 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 229960000997 bicalutamide Drugs 0.000 description 1
- 230000002146 bilateral effect Effects 0.000 description 1
- 210000000941 bile Anatomy 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-M bisulphate group Chemical group S([O-])(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-M 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 210000001754 blood buffy coat Anatomy 0.000 description 1
- 230000036765 blood level Effects 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- 239000010839 body fluid Substances 0.000 description 1
- 201000006491 bone marrow cancer Diseases 0.000 description 1
- 201000010983 breast ductal carcinoma Diseases 0.000 description 1
- 239000004067 bulking agent Substances 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 150000004648 butanoic acid derivatives Chemical class 0.000 description 1
- MIOPJNTWMNEORI-UHFFFAOYSA-N camphorsulfonic acid Chemical class C1CC2(CS(O)(=O)=O)C(=O)CC1C2(C)C MIOPJNTWMNEORI-UHFFFAOYSA-N 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 229960004562 carboplatin Drugs 0.000 description 1
- 208000002458 carcinoid tumor Diseases 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 238000000423 cell based assay Methods 0.000 description 1
- 230000011712 cell development Effects 0.000 description 1
- 239000006285 cell suspension Substances 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 208000006571 choroid plexus carcinoma Diseases 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 208000032852 chronic lymphocytic leukemia Diseases 0.000 description 1
- 229960002436 cladribine Drugs 0.000 description 1
- 208000009060 clear cell adenocarcinoma Diseases 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 201000010918 connective tissue cancer Diseases 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 230000009260 cross reactivity Effects 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 239000002875 cyclin dependent kinase inhibitor Substances 0.000 description 1
- 229940043378 cyclin-dependent kinase inhibitor Drugs 0.000 description 1
- 108010016616 cysteinylglycine Proteins 0.000 description 1
- 229960000684 cytarabine Drugs 0.000 description 1
- 229940127089 cytotoxic agent Drugs 0.000 description 1
- 239000002254 cytotoxic agent Substances 0.000 description 1
- 238000002784 cytotoxicity assay Methods 0.000 description 1
- 231100000263 cytotoxicity test Toxicity 0.000 description 1
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 1
- 229960000975 daunorubicin Drugs 0.000 description 1
- 230000001934 delay Effects 0.000 description 1
- 210000004443 dendritic cell Anatomy 0.000 description 1
- 238000011118 depth filtration Methods 0.000 description 1
- 206010012818 diffuse large B-cell lymphoma Diseases 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 239000003534 dna topoisomerase inhibitor Substances 0.000 description 1
- MOTZDAYCYVMXPC-UHFFFAOYSA-N dodecyl hydrogen sulfate Chemical compound CCCCCCCCCCCCOS(O)(=O)=O MOTZDAYCYVMXPC-UHFFFAOYSA-N 0.000 description 1
- 229940043264 dodecyl sulfate Drugs 0.000 description 1
- 231100000371 dose-limiting toxicity Toxicity 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 229960004679 doxorubicin Drugs 0.000 description 1
- 239000013583 drug formulation Substances 0.000 description 1
- 229940088679 drug related substance Drugs 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 201000000312 duodenum cancer Diseases 0.000 description 1
- 239000000806 elastomer Substances 0.000 description 1
- 229950000549 elliptinium acetate Drugs 0.000 description 1
- 210000001900 endoderm Anatomy 0.000 description 1
- 201000003908 endometrial adenocarcinoma Diseases 0.000 description 1
- 230000002357 endometrial effect Effects 0.000 description 1
- 201000006828 endometrial hyperplasia Diseases 0.000 description 1
- 208000029382 endometrium adenocarcinoma Diseases 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 210000003979 eosinophil Anatomy 0.000 description 1
- 102000052116 epidermal growth factor receptor activity proteins Human genes 0.000 description 1
- 108700015053 epidermal growth factor receptor activity proteins Proteins 0.000 description 1
- 210000002919 epithelial cell Anatomy 0.000 description 1
- 229950002973 epitiostanol Drugs 0.000 description 1
- 210000003743 erythrocyte Anatomy 0.000 description 1
- 201000004101 esophageal cancer Diseases 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 1
- 229960005420 etoposide Drugs 0.000 description 1
- HQMNCQVAMBCHCO-DJRRULDNSA-N etretinate Chemical compound CCOC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)C=C(OC)C(C)=C1C HQMNCQVAMBCHCO-DJRRULDNSA-N 0.000 description 1
- 229960002199 etretinate Drugs 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 230000005284 excitation Effects 0.000 description 1
- 210000002744 extracellular matrix Anatomy 0.000 description 1
- 208000024519 eye neoplasm Diseases 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 238000000855 fermentation Methods 0.000 description 1
- 230000004151 fermentation Effects 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 1
- 229960002074 flutamide Drugs 0.000 description 1
- MKXKFYHWDHIYRV-UHFFFAOYSA-N flutamide Chemical compound CC(C)C(=O)NC1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1 MKXKFYHWDHIYRV-UHFFFAOYSA-N 0.000 description 1
- 235000019253 formic acid Nutrition 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 230000005714 functional activity Effects 0.000 description 1
- 201000010175 gallbladder cancer Diseases 0.000 description 1
- 150000002270 gangliosides Chemical class 0.000 description 1
- 206010017758 gastric cancer Diseases 0.000 description 1
- 208000010749 gastric carcinoma Diseases 0.000 description 1
- 201000011555 gastric fundus cancer Diseases 0.000 description 1
- 208000015419 gastrin-producing neuroendocrine tumor Diseases 0.000 description 1
- 201000000052 gastrinoma Diseases 0.000 description 1
- 238000002523 gelfiltration Methods 0.000 description 1
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 1
- 229960005277 gemcitabine Drugs 0.000 description 1
- 210000004392 genitalia Anatomy 0.000 description 1
- 208000005017 glioblastoma Diseases 0.000 description 1
- MASNOZXLGMXCHN-ZLPAWPGGSA-N glucagon Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)C(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)O)C1=CC=CC=C1 MASNOZXLGMXCHN-ZLPAWPGGSA-N 0.000 description 1
- 229960004666 glucagon Drugs 0.000 description 1
- 229940050410 gluconate Drugs 0.000 description 1
- 108010079413 glycyl-prolyl-glutamic acid Proteins 0.000 description 1
- 108010082286 glycyl-seryl-alanine Proteins 0.000 description 1
- XLXSAKCOAKORKW-AQJXLSMYSA-N gonadorelin Chemical compound C([C@@H](C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1[C@@H](CCC1)C(=O)NCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)C1=CC=C(O)C=C1 XLXSAKCOAKORKW-AQJXLSMYSA-N 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 230000003394 haemopoietic effect Effects 0.000 description 1
- 201000009277 hairy cell leukemia Diseases 0.000 description 1
- 230000005802 health problem Effects 0.000 description 1
- 201000010235 heart cancer Diseases 0.000 description 1
- 208000024348 heart neoplasm Diseases 0.000 description 1
- 201000002222 hemangioblastoma Diseases 0.000 description 1
- 201000011066 hemangioma Diseases 0.000 description 1
- 231100000844 hepatocellular carcinoma Toxicity 0.000 description 1
- MNWFXJYAOYHMED-UHFFFAOYSA-N heptanoic acid Chemical compound CCCCCCC(O)=O MNWFXJYAOYHMED-UHFFFAOYSA-N 0.000 description 1
- 229940022353 herceptin Drugs 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-N hexanoic acid Chemical compound CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 1
- 210000003630 histaminocyte Anatomy 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 108010025306 histidylleucine Proteins 0.000 description 1
- 239000003276 histone deacetylase inhibitor Substances 0.000 description 1
- 238000000265 homogenisation Methods 0.000 description 1
- 239000003906 humectant Substances 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- ZMZDMBWJUHKJPS-UHFFFAOYSA-N hydrogen thiocyanate Natural products SC#N ZMZDMBWJUHKJPS-UHFFFAOYSA-N 0.000 description 1
- HOMGKSMUEGBAAB-UHFFFAOYSA-N ifosfamide Chemical compound ClCCNP1(=O)OCCCN1CCCl HOMGKSMUEGBAAB-UHFFFAOYSA-N 0.000 description 1
- 229960001101 ifosfamide Drugs 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 238000009169 immunotherapy Methods 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 108091008042 inhibitory receptors Proteins 0.000 description 1
- 239000003999 initiator Substances 0.000 description 1
- 210000005007 innate immune system Anatomy 0.000 description 1
- 206010022498 insulinoma Diseases 0.000 description 1
- 229960001388 interferon-beta Drugs 0.000 description 1
- 238000001361 intraarterial administration Methods 0.000 description 1
- 238000010212 intracellular staining Methods 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 238000004255 ion exchange chromatography Methods 0.000 description 1
- 229960005386 ipilimumab Drugs 0.000 description 1
- SUMDYPCJJOFFON-UHFFFAOYSA-N isethionic acid Chemical compound OCCS(O)(=O)=O SUMDYPCJJOFFON-UHFFFAOYSA-N 0.000 description 1
- 229960005280 isotretinoin Drugs 0.000 description 1
- FPCCSQOGAWCVBH-UHFFFAOYSA-N ketanserin Chemical compound C1=CC(F)=CC=C1C(=O)C1CCN(CCN2C(C3=CC=CC=C3NC2=O)=O)CC1 FPCCSQOGAWCVBH-UHFFFAOYSA-N 0.000 description 1
- 201000010982 kidney cancer Diseases 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 208000003849 large cell carcinoma Diseases 0.000 description 1
- 206010023841 laryngeal neoplasm Diseases 0.000 description 1
- HPJKCIUCZWXJDR-UHFFFAOYSA-N letrozole Chemical compound C1=CC(C#N)=CC=C1C(N1N=CN=C1)C1=CC=C(C#N)C=C1 HPJKCIUCZWXJDR-UHFFFAOYSA-N 0.000 description 1
- 229960003881 letrozole Drugs 0.000 description 1
- 108010047926 leucyl-lysyl-tyrosine Proteins 0.000 description 1
- 108010057821 leucylproline Proteins 0.000 description 1
- 108010012058 leucyltyrosine Proteins 0.000 description 1
- 229960001614 levamisole Drugs 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 229960003587 lisuride Drugs 0.000 description 1
- 201000007270 liver cancer Diseases 0.000 description 1
- 208000014018 liver neoplasm Diseases 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 208000020816 lung neoplasm Diseases 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 239000008176 lyophilized powder Substances 0.000 description 1
- 239000012139 lysis buffer Substances 0.000 description 1
- 108010003700 lysyl aspartic acid Proteins 0.000 description 1
- 108010057952 lysyl-phenylalanyl-lysine Proteins 0.000 description 1
- 108010009298 lysylglutamic acid Proteins 0.000 description 1
- 230000002101 lytic effect Effects 0.000 description 1
- 229940124302 mTOR inhibitor Drugs 0.000 description 1
- 210000002540 macrophage Anatomy 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 201000005831 male reproductive organ cancer Diseases 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- 208000029559 malignant endocrine neoplasm Diseases 0.000 description 1
- 208000006178 malignant mesothelioma Diseases 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 239000003628 mammalian target of rapamycin inhibitor Substances 0.000 description 1
- 201000007924 marginal zone B-cell lymphoma Diseases 0.000 description 1
- 208000021937 marginal zone lymphoma Diseases 0.000 description 1
- 208000020968 mature T-cell and NK-cell non-Hodgkin lymphoma Diseases 0.000 description 1
- 210000002418 meninge Anatomy 0.000 description 1
- 229950009246 mepitiostane Drugs 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- 230000009401 metastasis Effects 0.000 description 1
- 208000011645 metastatic carcinoma Diseases 0.000 description 1
- 206010061289 metastatic neoplasm Diseases 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- 238000000386 microscopy Methods 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 229960005485 mitobronitol Drugs 0.000 description 1
- 239000002829 mitogen activated protein kinase inhibitor Substances 0.000 description 1
- VFKZTMPDYBFSTM-GUCUJZIJSA-N mitolactol Chemical compound BrC[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)CBr VFKZTMPDYBFSTM-GUCUJZIJSA-N 0.000 description 1
- 229950010913 mitolactol Drugs 0.000 description 1
- 229960004857 mitomycin Drugs 0.000 description 1
- 238000012434 mixed-mode chromatography Methods 0.000 description 1
- 238000001565 modulated differential scanning calorimetry Methods 0.000 description 1
- 150000002772 monosaccharides Chemical class 0.000 description 1
- 201000002077 muscle cancer Diseases 0.000 description 1
- 210000004985 myeloid-derived suppressor cell Anatomy 0.000 description 1
- YOHYSYJDKVYCJI-UHFFFAOYSA-N n-[3-[[6-[3-(trifluoromethyl)anilino]pyrimidin-4-yl]amino]phenyl]cyclopropanecarboxamide Chemical compound FC(F)(F)C1=CC=CC(NC=2N=CN=C(NC=3C=C(NC(=O)C4CC4)C=CC=3)C=2)=C1 YOHYSYJDKVYCJI-UHFFFAOYSA-N 0.000 description 1
- KVBGVZZKJNLNJU-UHFFFAOYSA-M naphthalene-2-sulfonate Chemical compound C1=CC=CC2=CC(S(=O)(=O)[O-])=CC=C21 KVBGVZZKJNLNJU-UHFFFAOYSA-M 0.000 description 1
- KVBGVZZKJNLNJU-UHFFFAOYSA-N naphthalene-2-sulfonic acid Chemical compound C1=CC=CC2=CC(S(=O)(=O)O)=CC=C21 KVBGVZZKJNLNJU-UHFFFAOYSA-N 0.000 description 1
- 229950007221 nedaplatin Drugs 0.000 description 1
- 201000008026 nephroblastoma Diseases 0.000 description 1
- 230000000926 neurological effect Effects 0.000 description 1
- 210000000440 neutrophil Anatomy 0.000 description 1
- 235000001968 nicotinic acid Nutrition 0.000 description 1
- 239000011664 nicotinic acid Substances 0.000 description 1
- VFEDRRNHLBGPNN-UHFFFAOYSA-N nimustine Chemical compound CC1=NC=C(CNC(=O)N(CCCl)N=O)C(N)=N1 VFEDRRNHLBGPNN-UHFFFAOYSA-N 0.000 description 1
- 229960001420 nimustine Drugs 0.000 description 1
- YMVWGSQGCWCDGW-UHFFFAOYSA-N nitracrine Chemical compound C1=CC([N+]([O-])=O)=C2C(NCCCN(C)C)=C(C=CC=C3)C3=NC2=C1 YMVWGSQGCWCDGW-UHFFFAOYSA-N 0.000 description 1
- 229950008607 nitracrine Drugs 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 201000000032 nodular malignant melanoma Diseases 0.000 description 1
- 231100000065 noncytotoxic Toxicity 0.000 description 1
- 230000002020 noncytotoxic effect Effects 0.000 description 1
- 229920002113 octoxynol Polymers 0.000 description 1
- 201000008106 ocular cancer Diseases 0.000 description 1
- 201000000890 orbital cancer Diseases 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 201000008968 osteosarcoma Diseases 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- ICMWWNHDUZJFDW-UHFFFAOYSA-N oxymetholone Natural products C1CC2CC(=O)C(=CO)CC2(C)C2C1C1CCC(C)(O)C1(C)CC2 ICMWWNHDUZJFDW-UHFFFAOYSA-N 0.000 description 1
- ICMWWNHDUZJFDW-DHODBPELSA-N oxymetholone Chemical compound C([C@@H]1CC2)C(=O)\C(=C/O)C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@](C)(O)[C@@]2(C)CC1 ICMWWNHDUZJFDW-DHODBPELSA-N 0.000 description 1
- 229960005244 oxymetholone Drugs 0.000 description 1
- 208000021255 pancreatic insulinoma Diseases 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 201000005163 papillary serous adenocarcinoma Diseases 0.000 description 1
- 208000024641 papillary serous cystadenocarcinoma Diseases 0.000 description 1
- 201000001219 parotid gland cancer Diseases 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 238000005453 pelletization Methods 0.000 description 1
- 229960002340 pentostatin Drugs 0.000 description 1
- FPVKHBSQESCIEP-JQCXWYLXSA-N pentostatin Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(N=CNC[C@H]2O)=C2N=C1 FPVKHBSQESCIEP-JQCXWYLXSA-N 0.000 description 1
- 229930192851 perforin Natural products 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- JRKICGRDRMAZLK-UHFFFAOYSA-L peroxydisulfate Chemical compound [O-]S(=O)(=O)OOS([O-])(=O)=O JRKICGRDRMAZLK-UHFFFAOYSA-L 0.000 description 1
- 238000002823 phage display Methods 0.000 description 1
- 230000008782 phagocytosis Effects 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- DYUMLJSJISTVPV-UHFFFAOYSA-N phenyl propanoate Chemical compound CCC(=O)OC1=CC=CC=C1 DYUMLJSJISTVPV-UHFFFAOYSA-N 0.000 description 1
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 1
- 108010083476 phenylalanyltryptophan Proteins 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 239000002935 phosphatidylinositol 3 kinase inhibitor Substances 0.000 description 1
- 229940043441 phosphoinositide 3-kinase inhibitor Drugs 0.000 description 1
- 229940075930 picrate Drugs 0.000 description 1
- OXNIZHLAWKMVMX-UHFFFAOYSA-M picrate anion Chemical compound [O-]C1=C([N+]([O-])=O)C=C([N+]([O-])=O)C=C1[N+]([O-])=O OXNIZHLAWKMVMX-UHFFFAOYSA-M 0.000 description 1
- 208000010916 pituitary tumor Diseases 0.000 description 1
- 229950010765 pivalate Drugs 0.000 description 1
- IUGYQRQAERSCNH-UHFFFAOYSA-N pivalic acid Chemical compound CC(C)(C)C(O)=O IUGYQRQAERSCNH-UHFFFAOYSA-N 0.000 description 1
- 229960000502 poloxamer Drugs 0.000 description 1
- 229920001983 poloxamer Polymers 0.000 description 1
- 229920001993 poloxamer 188 Polymers 0.000 description 1
- 229940044519 poloxamer 188 Drugs 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 1
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 1
- 229940068977 polysorbate 20 Drugs 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 235000015497 potassium bicarbonate Nutrition 0.000 description 1
- 229910000028 potassium bicarbonate Inorganic materials 0.000 description 1
- 239000011736 potassium bicarbonate Substances 0.000 description 1
- TYJJADVDDVDEDZ-UHFFFAOYSA-M potassium hydrogencarbonate Chemical compound [K+].OC([O-])=O TYJJADVDDVDEDZ-UHFFFAOYSA-M 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 201000006037 primary mediastinal B-cell lymphoma Diseases 0.000 description 1
- 239000000186 progesterone Substances 0.000 description 1
- 229960003387 progesterone Drugs 0.000 description 1
- 230000035755 proliferation Effects 0.000 description 1
- 108010077112 prolyl-proline Proteins 0.000 description 1
- 108010031719 prolyl-serine Proteins 0.000 description 1
- 108010004914 prolylarginine Proteins 0.000 description 1
- 108010015796 prolylisoleucine Proteins 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 239000003207 proteasome inhibitor Substances 0.000 description 1
- 210000004203 pyloric antrum Anatomy 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 229960004432 raltitrexed Drugs 0.000 description 1
- 230000007115 recruitment Effects 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 238000007634 remodeling Methods 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000000241 respiratory effect Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 201000009410 rhabdomyosarcoma Diseases 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- 201000007416 salivary gland adenoid cystic carcinoma Diseases 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 238000013341 scale-up Methods 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 230000008313 sensitization Effects 0.000 description 1
- 208000004548 serous cystadenocarcinoma Diseases 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 108010026333 seryl-proline Proteins 0.000 description 1
- 208000037968 sinus cancer Diseases 0.000 description 1
- 201000002314 small intestine cancer Diseases 0.000 description 1
- 201000000267 smooth muscle cancer Diseases 0.000 description 1
- 230000008410 smoothened signaling pathway Effects 0.000 description 1
- AWUCVROLDVIAJX-GSVOUGTGSA-N sn-glycerol 3-phosphate Chemical compound OC[C@@H](O)COP(O)(O)=O AWUCVROLDVIAJX-GSVOUGTGSA-N 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- 229960002668 sodium chloride Drugs 0.000 description 1
- 239000008354 sodium chloride injection Substances 0.000 description 1
- 229940074404 sodium succinate Drugs 0.000 description 1
- ZDQYSKICYIVCPN-UHFFFAOYSA-L sodium succinate (anhydrous) Chemical compound [Na+].[Na+].[O-]C(=O)CCC([O-])=O ZDQYSKICYIVCPN-UHFFFAOYSA-L 0.000 description 1
- VBJGJHBYWREJQD-UHFFFAOYSA-M sodium;dihydrogen phosphate;dihydrate Chemical compound O.O.[Na+].OP(O)([O-])=O VBJGJHBYWREJQD-UHFFFAOYSA-M 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- NHXLMOGPVYXJNR-ATOGVRKGSA-N somatostatin Chemical compound C([C@H]1C(=O)N[C@H](C(N[C@@H](CO)C(=O)N[C@@H](CSSC[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CC=2C3=CC=CC=C3NC=2)C(=O)N[C@@H](CCCCN)C(=O)N[C@H](C(=O)N1)[C@@H](C)O)NC(=O)CNC(=O)[C@H](C)N)C(O)=O)=O)[C@H](O)C)C1=CC=CC=C1 NHXLMOGPVYXJNR-ATOGVRKGSA-N 0.000 description 1
- 229960000553 somatostatin Drugs 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 201000011096 spinal cancer Diseases 0.000 description 1
- 208000014618 spinal cord cancer Diseases 0.000 description 1
- 210000000952 spleen Anatomy 0.000 description 1
- 210000004989 spleen cell Anatomy 0.000 description 1
- 230000003393 splenic effect Effects 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 208000017572 squamous cell neoplasm Diseases 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 230000001954 sterilising effect Effects 0.000 description 1
- 238000004659 sterilization and disinfection Methods 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 201000011549 stomach cancer Diseases 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- ZSJLQEPLLKMAKR-GKHCUFPYSA-N streptozocin Chemical compound O=NN(C)C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O ZSJLQEPLLKMAKR-GKHCUFPYSA-N 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 229960001603 tamoxifen Drugs 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- 201000003120 testicular cancer Diseases 0.000 description 1
- 238000011287 therapeutic dose Methods 0.000 description 1
- 230000004797 therapeutic response Effects 0.000 description 1
- 108010033670 threonyl-aspartyl-tyrosine Proteins 0.000 description 1
- 201000002510 thyroid cancer Diseases 0.000 description 1
- 201000006134 tongue cancer Diseases 0.000 description 1
- 230000001256 tonic effect Effects 0.000 description 1
- 229940044693 topoisomerase inhibitor Drugs 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000014616 translation Effects 0.000 description 1
- 102000035160 transmembrane proteins Human genes 0.000 description 1
- 108091005703 transmembrane proteins Proteins 0.000 description 1
- 238000002054 transplantation Methods 0.000 description 1
- 230000032258 transport Effects 0.000 description 1
- 229960001727 tretinoin Drugs 0.000 description 1
- 230000005909 tumor killing Effects 0.000 description 1
- 230000006433 tumor necrosis factor production Effects 0.000 description 1
- 102000003298 tumor necrosis factor receptor Human genes 0.000 description 1
- 230000005751 tumor progression Effects 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- ZDPHROOEEOARMN-UHFFFAOYSA-N undecanoic acid Chemical compound CCCCCCCCCCC(O)=O ZDPHROOEEOARMN-UHFFFAOYSA-N 0.000 description 1
- 208000010576 undifferentiated carcinoma Diseases 0.000 description 1
- 201000011294 ureter cancer Diseases 0.000 description 1
- 206010046766 uterine cancer Diseases 0.000 description 1
- 206010046885 vaginal cancer Diseases 0.000 description 1
- 208000013139 vaginal neoplasm Diseases 0.000 description 1
- 239000004474 valine Substances 0.000 description 1
- 108010072644 valyl-alanyl-prolyl-glycine Proteins 0.000 description 1
- 206010055031 vascular neoplasm Diseases 0.000 description 1
- 239000013598 vector Substances 0.000 description 1
- 230000002861 ventricular Effects 0.000 description 1
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 1
- 229960004528 vincristine Drugs 0.000 description 1
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 1
- GBABOYUKABKIAF-GHYRFKGUSA-N vinorelbine Chemical compound C1N(CC=2C3=CC=CC=C3NC=22)CC(CC)=C[C@H]1C[C@]2(C(=O)OC)C1=CC([C@]23[C@H]([C@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC GBABOYUKABKIAF-GHYRFKGUSA-N 0.000 description 1
- 229960002066 vinorelbine Drugs 0.000 description 1
- 201000005102 vulva cancer Diseases 0.000 description 1
- 239000008215 water for injection Substances 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
- 108010027345 wheylin-1 peptide Proteins 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/283—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against Fc-receptors, e.g. CD16, CD32, CD64
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2851—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the lectin superfamily, e.g. CD23, CD72
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2878—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the NGF-receptor/TNF-receptor superfamily, e.g. CD27, CD30, CD40, CD95
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/31—Immunoglobulins specific features characterized by aspects of specificity or valency multispecific
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/33—Crossreactivity, e.g. for species or epitope, or lack of said crossreactivity
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/52—Constant or Fc region; Isotype
- C07K2317/526—CH3 domain
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/55—Fab or Fab'
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/62—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising only variable region components
- C07K2317/622—Single chain antibody (scFv)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/62—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising only variable region components
- C07K2317/624—Disulfide-stabilized antibody (dsFv)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/64—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising a combination of variable region and constant region components
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/66—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising a swap of domains, e.g. CH3-CH2, VH-CL or VL-CH1
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/94—Stability, e.g. half-life, pH, temperature or enzyme-resistance
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/01—Fusion polypeptide containing a localisation/targetting motif
- C07K2319/03—Fusion polypeptide containing a localisation/targetting motif containing a transmembrane segment
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/30—Non-immunoglobulin-derived peptide or protein having an immunoglobulin constant or Fc region, or a fragment thereof, attached thereto
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/32—Fusion polypeptide fusions with soluble part of a cell surface receptor, "decoy receptors"
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/33—Fusion polypeptide fusions for targeting to specific cell types, e.g. tissue specific targeting, targeting of a bacterial subspecies
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Veterinary Medicine (AREA)
- General Chemical & Material Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020247004861A KR20240078657A (ko) | 2017-02-10 | 2018-02-09 | Bcma, nkg2d 및 cd16에 결합하는 단백질 |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201762457780P | 2017-02-10 | 2017-02-10 | |
| US62/457,780 | 2017-02-10 | ||
| PCT/US2018/017653 WO2018148566A1 (en) | 2017-02-10 | 2018-02-09 | Proteins binding bcma, nkg2d and cd16 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020247004861A Division KR20240078657A (ko) | 2017-02-10 | 2018-02-09 | Bcma, nkg2d 및 cd16에 결합하는 단백질 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20190115469A true KR20190115469A (ko) | 2019-10-11 |
Family
ID=63107848
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020247004861A Ceased KR20240078657A (ko) | 2017-02-10 | 2018-02-09 | Bcma, nkg2d 및 cd16에 결합하는 단백질 |
| KR1020197026174A Ceased KR20190115469A (ko) | 2017-02-10 | 2018-02-09 | Bcma, nkg2d 및 cd16에 결합하는 단백질 |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020247004861A Ceased KR20240078657A (ko) | 2017-02-10 | 2018-02-09 | Bcma, nkg2d 및 cd16에 결합하는 단백질 |
Country Status (14)
| Country | Link |
|---|---|
| US (1) | US20190375838A1 (cg-RX-API-DMAC7.html) |
| EP (1) | EP3579876A4 (cg-RX-API-DMAC7.html) |
| JP (2) | JP7257323B2 (cg-RX-API-DMAC7.html) |
| KR (2) | KR20240078657A (cg-RX-API-DMAC7.html) |
| CN (1) | CN110461361A (cg-RX-API-DMAC7.html) |
| AU (1) | AU2018219348A1 (cg-RX-API-DMAC7.html) |
| BR (1) | BR112019016424A2 (cg-RX-API-DMAC7.html) |
| CA (1) | CA3054642A1 (cg-RX-API-DMAC7.html) |
| IL (1) | IL268567A (cg-RX-API-DMAC7.html) |
| MA (1) | MA47465A (cg-RX-API-DMAC7.html) |
| MX (1) | MX2019009566A (cg-RX-API-DMAC7.html) |
| MY (1) | MY205850A (cg-RX-API-DMAC7.html) |
| SG (1) | SG11201907253VA (cg-RX-API-DMAC7.html) |
| WO (1) | WO2018148566A1 (cg-RX-API-DMAC7.html) |
Families Citing this family (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6698546B2 (ja) * | 2014-04-14 | 2020-05-27 | セレクティスCellectis | 癌免疫療法のためのbcma(cd269)特異的キメラ抗原受容体 |
| KR20250089564A (ko) | 2017-02-08 | 2025-06-18 | 드래곤플라이 쎄라퓨틱스, 인크. | 천연 킬러 세포의 활성화를 위한 다중-특이적 결합 단백질 및 암 치료에서의 그의 치료적 용도 |
| SG11201907253VA (en) * | 2017-02-10 | 2019-09-27 | Dragonfly Therapeutics Inc | Proteins binding bcma, nkg2d and cd16 |
| KR20190120782A (ko) | 2017-02-20 | 2019-10-24 | 드래곤플라이 쎄라퓨틱스, 인크. | Her2, nkg2d 및 cd16에 결합하는 단백질 |
| MX2020002036A (es) * | 2017-08-23 | 2020-03-24 | Dragonfly Therapeutics Inc | Proteinas de union a nkg2d, cd16 y un antigeno asociado al tumor. |
| CA3090244A1 (en) * | 2018-02-08 | 2019-08-15 | Dragonfly Therapeutics, Inc. | Antibody variable domains targeting the nkg2d receptor |
| PE20210375A1 (es) | 2018-02-08 | 2021-03-02 | Dragonfly Therapeutics Inc | Terapia de combinacion del cancer que incluye proteinas de union multiespecificas que activan a las celulas asesinas naturales |
| CN112119093A (zh) | 2018-02-20 | 2020-12-22 | 蜻蜓治疗公司 | 结合cd33、nkg2d和cd16的多特异性结合蛋白和使用方法 |
| EA202091977A1 (ru) * | 2018-05-28 | 2021-02-09 | Драгонфлай Терапьютикс, Инк. | Мультиспецифические связывающие белки, которые связывают cd33, nkg2d и cd16, и способы применения |
| BR112021002276A2 (pt) | 2018-08-08 | 2021-05-04 | Dragonfly Therapeutics, Inc. | proteínas de ligação a nkg2d, cd16 e um antígeno associado ao tumor |
| CA3108427A1 (en) * | 2018-08-08 | 2020-02-13 | Dragonfly Therapeutics, Inc. | Multi-specific binding proteins that bind bcma, nkg2d and cd16, and methods of use |
| EA202091888A1 (ru) | 2018-08-08 | 2020-10-23 | Драгонфлай Терапьютикс, Инк. | Вариабельные домены антител, нацеленные на рецептор nkg2d |
| AU2020279230A1 (en) * | 2019-05-20 | 2021-12-02 | Les Laboratoires Servier | Mcl-1 inhibitor antibody-drug conjugates and methods of use |
| US12157771B2 (en) | 2020-05-06 | 2024-12-03 | Dragonfly Therapeutics, Inc. | Proteins binding NKG2D, CD16 and CLEC12A |
| MX2022015374A (es) | 2020-06-05 | 2023-01-16 | Eisai R&D Man Co Ltd | Conjugados de anticuerpo anti antigeno de maduracion de linfocitos b (bcma)-farmaco y metodos de uso. |
| CN112029001B (zh) * | 2020-09-02 | 2022-09-06 | 南京北恒生物科技有限公司 | 靶向nk激活性受体的嵌合抗原受体 |
| JP2023545099A (ja) * | 2020-10-08 | 2023-10-26 | アフィメド ゲーエムベーハー | 三重特異性バインダー |
| CA3210650A1 (en) | 2021-03-03 | 2022-09-09 | Winfried Wels | Bispecific antibodies enhancing cell mediated immune responses |
| WO2022187539A1 (en) | 2021-03-03 | 2022-09-09 | Dragonfly Therapeutics, Inc. | Methods of treating cancer using multi-specific binding proteins that bind nkg2d, cd16 and a tumor-associated antigen |
| JP2024512135A (ja) * | 2021-03-31 | 2024-03-18 | ヤンセン バイオテツク,インコーポレーテツド | 免疫エフェクター細胞再指向のための材料及び方法 |
| WO2024020577A2 (en) * | 2022-07-22 | 2024-01-25 | Fred Hutchinson Cancer Center | Antibodies against sars-cov-2 |
Family Cites Families (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20070071759A1 (en) * | 2005-06-29 | 2007-03-29 | University Of Miami | Antibody-immune cell ligand fusion protein for cancer therapy |
| MX2008015524A (es) * | 2006-06-12 | 2009-01-13 | Trubion Pharmaceuticals Inc | Proteinas de union multivalentes monocatenarias con funcion efectora. |
| DK2235064T3 (en) * | 2008-01-07 | 2016-01-11 | Amgen Inc | A process for the preparation of heterodimeric Fc molecules using electrostatic control effects |
| UY32808A (es) * | 2009-07-29 | 2011-02-28 | Abbott Lab | Inmunoglobulinas como dominio variable dual y usos de las mismas |
| EP2306234A3 (de) * | 2009-10-02 | 2011-06-22 | Fraunhofer-Gesellschaft zur Förderung der Angewandten Forschung e.V. | Optischer Aliasfilter, Pixelsensoranordnung und digitale Aufnahmevorrichtung |
| SG188638A1 (en) * | 2011-03-25 | 2013-05-31 | Glenmark Pharmaceuticals Sa | Hetero-dimeric immunoglobulins |
| TWI679212B (zh) * | 2011-11-15 | 2019-12-11 | 美商安進股份有限公司 | 針對bcma之e3以及cd3的結合分子 |
| TWI617578B (zh) * | 2012-05-30 | 2018-03-11 | Chugai Pharmaceutical Co Ltd | 標的組織專一的抗原結合分子 |
| RU2639287C2 (ru) * | 2012-06-27 | 2017-12-20 | Ф. Хоффманн-Ля Рош Аг | Способ отбора и получения высокоселективных и мультиспецифичных нацеливающих групп с заданными свойствами, включающих по меньшей мере две различные связывающие группировки, и их применения |
| EP3620468A1 (en) * | 2013-02-05 | 2020-03-11 | EngMab Sàrl | Method for the selection of antibodies against bcma |
| US10156574B2 (en) * | 2013-04-29 | 2018-12-18 | Adimab, Llc | Polyspecificity reagents, methods for their preparation and use |
| WO2015197582A1 (en) * | 2014-06-27 | 2015-12-30 | Innate Pharma | Monomeric multispecific antigen binding proteins |
| KR102612313B1 (ko) * | 2014-07-21 | 2023-12-12 | 노파르티스 아게 | 인간화 항-bcma 키메라 항원 수용체를 사용한 암의 치료 |
| CN107530424A (zh) * | 2015-02-20 | 2018-01-02 | 俄亥俄州国家创新基金会 | 针对nkg2d和肿瘤相关抗原的二价抗体 |
| PH12017501834B1 (en) * | 2015-04-06 | 2024-01-17 | Arcellx Inc | De novo binding domain containing polypeptides and uses thereof |
| JP7082604B2 (ja) * | 2016-03-21 | 2022-06-08 | マレンゴ・セラピューティクス,インコーポレーテッド | 多重特異性および多機能性分子ならびにその使用 |
| KR20250089564A (ko) * | 2017-02-08 | 2025-06-18 | 드래곤플라이 쎄라퓨틱스, 인크. | 천연 킬러 세포의 활성화를 위한 다중-특이적 결합 단백질 및 암 치료에서의 그의 치료적 용도 |
| US20200095327A1 (en) * | 2017-02-08 | 2020-03-26 | Dragonfly Therapeutics, Inc. | Antibody heavy chain variable domains targeting the nkg2d receptor |
| SG11201907253VA (en) * | 2017-02-10 | 2019-09-27 | Dragonfly Therapeutics Inc | Proteins binding bcma, nkg2d and cd16 |
| EP3615068A1 (en) * | 2017-04-28 | 2020-03-04 | Novartis AG | Bcma-targeting agent, and combination therapy with a gamma secretase inhibitor |
-
2018
- 2018-02-09 SG SG11201907253VA patent/SG11201907253VA/en unknown
- 2018-02-09 CN CN201880021558.4A patent/CN110461361A/zh active Pending
- 2018-02-09 WO PCT/US2018/017653 patent/WO2018148566A1/en not_active Ceased
- 2018-02-09 MA MA047465A patent/MA47465A/fr unknown
- 2018-02-09 JP JP2019543761A patent/JP7257323B2/ja active Active
- 2018-02-09 MY MYPI2019004552A patent/MY205850A/en unknown
- 2018-02-09 BR BR112019016424A patent/BR112019016424A2/pt not_active Application Discontinuation
- 2018-02-09 EP EP18750616.7A patent/EP3579876A4/en active Pending
- 2018-02-09 US US16/484,936 patent/US20190375838A1/en active Pending
- 2018-02-09 KR KR1020247004861A patent/KR20240078657A/ko not_active Ceased
- 2018-02-09 CA CA3054642A patent/CA3054642A1/en active Pending
- 2018-02-09 AU AU2018219348A patent/AU2018219348A1/en not_active Abandoned
- 2018-02-09 MX MX2019009566A patent/MX2019009566A/es unknown
- 2018-02-09 KR KR1020197026174A patent/KR20190115469A/ko not_active Ceased
-
2019
- 2019-08-07 IL IL268567A patent/IL268567A/en unknown
-
2022
- 2022-12-08 JP JP2022196214A patent/JP2023029988A/ja active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| KR20240078657A (ko) | 2024-06-04 |
| SG11201907253VA (en) | 2019-09-27 |
| RU2019127910A3 (cg-RX-API-DMAC7.html) | 2021-06-15 |
| JP2023029988A (ja) | 2023-03-07 |
| WO2018148566A1 (en) | 2018-08-16 |
| AU2018219348A1 (en) | 2019-08-29 |
| CA3054642A1 (en) | 2018-08-16 |
| JP7257323B2 (ja) | 2023-04-13 |
| BR112019016424A2 (pt) | 2020-04-07 |
| CN110461361A (zh) | 2019-11-15 |
| EP3579876A4 (en) | 2020-11-18 |
| EP3579876A1 (en) | 2019-12-18 |
| MX2019009566A (es) | 2020-01-20 |
| US20190375838A1 (en) | 2019-12-12 |
| RU2019127910A (ru) | 2021-03-10 |
| MY205850A (en) | 2024-11-15 |
| JP2020507328A (ja) | 2020-03-12 |
| MA47465A (fr) | 2019-12-18 |
| IL268567A (en) | 2019-09-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20230357409A1 (en) | Proteins binding nkg2d, cd16 and nectin4 | |
| US20210261668A1 (en) | Proteins binding nkg2d, cd16, and egfr, ccr4, or pd-l1 | |
| US11884732B2 (en) | Proteins binding HER2, NKG2D and CD16 | |
| JP7257323B2 (ja) | Bcma、nkg2d及びcd16と結合するタンパク質 | |
| US20240018266A1 (en) | Proteins binding cd123, nkg2d and cd16 | |
| US20200277384A1 (en) | Proteins binding nkg2d, cd16, and c-type lectin-like molecule-1 (cll-1) | |
| KR102835308B1 (ko) | Nkg2d, cd16 및 종양 관련 항원에 결합하는 단백질 | |
| KR20190120775A (ko) | Cd33, nkg2d 및 cd16에 결합하는 단백질 | |
| KR20190123299A (ko) | Caix, ano1, 메소텔린, trop2, cea, 또는 클라우딘-18.2를 표적화하는 다중특이적 결합 단백질 | |
| KR20200010428A (ko) | Nkg2d, cd16, 및 ror1 또는 ror2에 결합하는 단백질 | |
| US20200024353A1 (en) | Proteins binding psma, nkg2d and cd16 | |
| CA3070986A1 (en) | Proteins binding nkg2d, cd16 and flt3 | |
| KR20200010430A (ko) | Nkg2d, cd16 및 종양-연관 항원에 결합하는 단백질 | |
| RU2816716C2 (ru) | Белки, связывающие NKG2D, CD16 и опухолеассоциированный антиген | |
| HK40062610A (en) | Proteins binding nkg2d, cd16, and egfr, ccr4, or pd-l1 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20190905 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20210208 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20230215 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20230908 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20230215 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |