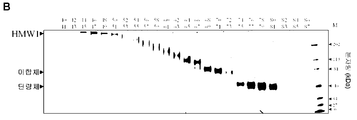
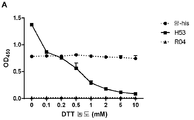
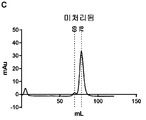
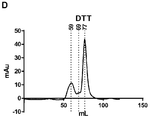
KR20190078574A - 조립된 당단백질 - Google Patents
조립된 당단백질 Download PDFInfo
- Publication number
- KR20190078574A KR20190078574A KR1020197011934A KR20197011934A KR20190078574A KR 20190078574 A KR20190078574 A KR 20190078574A KR 1020197011934 A KR1020197011934 A KR 1020197011934A KR 20197011934 A KR20197011934 A KR 20197011934A KR 20190078574 A KR20190078574 A KR 20190078574A
- Authority
- KR
- South Korea
- Prior art keywords
- gly
- leu
- thr
- ala
- ser
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 0 CC[C@@](C)[C@@](*CCC*(C)O[C@@](C)(C([C@](C)NC(C(**OC)O)O)I)NC)N Chemical compound CC[C@@](C)[C@@](*CCC*(C)O[C@@](C)(C([C@](C)NC(C(**OC)O)O)I)NC)N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K1/00—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length
- C07K1/107—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length by chemical modification of precursor peptides
- C07K1/113—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length by chemical modification of precursor peptides without change of the primary structure
- C07K1/1133—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length by chemical modification of precursor peptides without change of the primary structure by redox-reactions involving cystein/cystin side chains
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
- A61K39/29—Hepatitis virus
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K1/00—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length
- C07K1/107—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length by chemical modification of precursor peptides
- C07K1/113—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length by chemical modification of precursor peptides without change of the primary structure
- C07K1/1136—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length by chemical modification of precursor peptides without change of the primary structure by reversible modification of the secondary, tertiary or quarternary structure, e.g. using denaturating or stabilising agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K1/00—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length
- C07K1/13—Labelling of peptides
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K1/00—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length
- C07K1/14—Extraction; Separation; Purification
- C07K1/16—Extraction; Separation; Purification by chromatography
- C07K1/22—Affinity chromatography or related techniques based upon selective absorption processes
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/005—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from viruses
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/005—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from viruses
- C07K14/08—RNA viruses
- C07K14/18—Togaviridae; Flaviviridae
- C07K14/1816—Flaviviridae, e.g. pestivirus, mucosal disease virus, bovine viral diarrhoea virus, classical swine fever virus (hog cholera virus), border disease virus
- C07K14/1833—Hepatitis C; Hepatitis NANB
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/08—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses
- C07K16/10—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses from RNA viruses
- C07K16/1081—Togaviridae, e.g. flavivirus, rubella virus, hog cholera virus
- C07K16/109—Hepatitis C virus; Hepatitis G virus
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/5005—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells
- G01N33/5008—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics
- G01N33/5044—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics involving specific cell types
- G01N33/5047—Cells of the immune system
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/5005—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells
- G01N33/5008—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics
- G01N33/5044—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics involving specific cell types
- G01N33/5047—Cells of the immune system
- G01N33/5052—Cells of the immune system involving B-cells
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/569—Immunoassay; Biospecific binding assay; Materials therefor for microorganisms, e.g. protozoa, bacteria, viruses
- G01N33/56966—Animal cells
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/576—Immunoassay; Biospecific binding assay; Materials therefor for hepatitis
- G01N33/5767—Immunoassay; Biospecific binding assay; Materials therefor for hepatitis non-A, non-B hepatitis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/64—Medicinal preparations containing antigens or antibodies characterised by the architecture of the carrier-antigen complex, e.g. repetition of carrier-antigen units
- A61K2039/645—Dendrimers; Multiple antigen peptides
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/33—Crossreactivity, e.g. for species or epitope, or lack of said crossreactivity
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2770/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses positive-sense
- C12N2770/00011—Details
- C12N2770/24011—Flaviviridae
- C12N2770/24211—Hepacivirus, e.g. hepatitis C virus, hepatitis G virus
- C12N2770/24222—New viral proteins or individual genes, new structural or functional aspects of known viral proteins or genes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2770/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses positive-sense
- C12N2770/00011—Details
- C12N2770/24011—Flaviviridae
- C12N2770/24211—Hepacivirus, e.g. hepatitis C virus, hepatitis G virus
- C12N2770/24234—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Molecular Biology (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Biomedical Technology (AREA)
- Biochemistry (AREA)
- Analytical Chemistry (AREA)
- Hematology (AREA)
- Urology & Nephrology (AREA)
- Virology (AREA)
- Cell Biology (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Microbiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Pathology (AREA)
- General Physics & Mathematics (AREA)
- Biotechnology (AREA)
- Food Science & Technology (AREA)
- Physics & Mathematics (AREA)
- Communicable Diseases (AREA)
- Veterinary Medicine (AREA)
- General Chemical & Material Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Tropical Medicine & Parasitology (AREA)
- Toxicology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Mycology (AREA)
- Gastroenterology & Hepatology (AREA)
- Epidemiology (AREA)
- Oncology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020247025714A KR20240122578A (ko) | 2016-09-29 | 2017-09-22 | 조립된 당단백질 |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2016903961 | 2016-09-29 | ||
| AU2016903961A AU2016903961A0 (en) | 2016-09-29 | Refolded glycoproteins for vaccine production | |
| PCT/AU2017/051037 WO2018058177A1 (en) | 2016-09-29 | 2017-09-22 | Assembled glycoproteins |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020247025714A Division KR20240122578A (ko) | 2016-09-29 | 2017-09-22 | 조립된 당단백질 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20190078574A true KR20190078574A (ko) | 2019-07-04 |
Family
ID=61762303
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197011934A Pending KR20190078574A (ko) | 2016-09-29 | 2017-09-22 | 조립된 당단백질 |
| KR1020247025714A Pending KR20240122578A (ko) | 2016-09-29 | 2017-09-22 | 조립된 당단백질 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020247025714A Pending KR20240122578A (ko) | 2016-09-29 | 2017-09-22 | 조립된 당단백질 |
Country Status (8)
| Country | Link |
|---|---|
| US (2) | US12162906B2 (enExample) |
| EP (1) | EP3519424A4 (enExample) |
| JP (1) | JP7068284B2 (enExample) |
| KR (2) | KR20190078574A (enExample) |
| CN (1) | CN110049993A (enExample) |
| AU (1) | AU2017334263B2 (enExample) |
| IL (1) | IL265524B2 (enExample) |
| WO (1) | WO2018058177A1 (enExample) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| BR112022023101A2 (pt) * | 2020-05-11 | 2023-01-17 | Macfarlane Burnet Institute For Medical Res And Public Health Limited | Composição compreendendo uma molécula de ácido nucleico que codifica um polipeptídeo e2 excluído de domínio variável de hcv para uso no tratamento ou prevenção de infecção por hcv, uso de uma molécula de ácido nucleico que codifica um polipeptídeo e2 excluído de domínio variável de hcv, e método de tratamento ou prevenção de infecção por hcv |
| CN112285361B (zh) * | 2020-09-27 | 2023-12-05 | 中国人民解放军空军军医大学 | 排除抗-cd38单克隆抗体药物对抗人球蛋白检测干扰的试剂 |
| CN115389681B (zh) * | 2022-11-01 | 2023-01-20 | 常州百瑞吉生物医药有限公司 | 一种巯基化透明质酸衍生物中二硫苏糖醇残留的检测方法 |
| WO2025231336A1 (en) * | 2024-05-02 | 2025-11-06 | Avista Therapeutics, Inc. | Adeno-associated virus vectors |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| SG71728A1 (en) * | 1994-07-29 | 2000-04-18 | Innogenetics Nv | Purified hepatitis c virus envelope proteins for diagnostic and therapeutic use |
| AU2002360673A1 (en) * | 2001-12-17 | 2003-06-30 | The Government Of The Usa As Represented By The Secretary Of The Dept. Of Health And Human Services | Gp41 inhibitor |
| CN1622828A (zh) | 2001-12-18 | 2005-06-01 | 基因创新有限公司 | 用于诊断和治疗用途的纯化的丙型肝炎病毒外被蛋白 |
| US8535686B2 (en) | 2006-08-25 | 2013-09-17 | The Macfarlane Burnet Institute For Medical Research And Public Health Limited | Recombinant HCV E2 glycoprotein |
| AU2011286168B2 (en) * | 2010-08-04 | 2015-05-28 | The Macfarlane Burnet Institute For Medical Research And Public Health Ltd | Modified hepatitis C virus proteins |
| JP5897024B2 (ja) | 2010-11-26 | 2016-03-30 | ザ マクファーレーン バーネット インスティテュート フォー メディカル リサーチ アンド パブリック ヘルス リミテッドThe Macfarlane Burnet Institute for Medical Research and Public Health Ltd | 組成物および方法 |
-
2017
- 2017-09-22 KR KR1020197011934A patent/KR20190078574A/ko active Pending
- 2017-09-22 US US16/337,900 patent/US12162906B2/en active Active
- 2017-09-22 JP JP2019517034A patent/JP7068284B2/ja active Active
- 2017-09-22 EP EP17854239.5A patent/EP3519424A4/en active Pending
- 2017-09-22 KR KR1020247025714A patent/KR20240122578A/ko active Pending
- 2017-09-22 AU AU2017334263A patent/AU2017334263B2/en active Active
- 2017-09-22 CN CN201780063166.XA patent/CN110049993A/zh active Pending
- 2017-09-22 WO PCT/AU2017/051037 patent/WO2018058177A1/en not_active Ceased
-
2019
- 2019-03-20 IL IL265524A patent/IL265524B2/en unknown
-
2024
- 2024-12-09 US US18/974,404 patent/US20250215048A1/en active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| JP7068284B2 (ja) | 2022-05-16 |
| JP2020500837A (ja) | 2020-01-16 |
| KR20240122578A (ko) | 2024-08-12 |
| AU2017334263B2 (en) | 2024-11-28 |
| AU2017334263A1 (en) | 2019-04-04 |
| EP3519424A1 (en) | 2019-08-07 |
| US20250215048A1 (en) | 2025-07-03 |
| CN110049993A (zh) | 2019-07-23 |
| EP3519424A4 (en) | 2020-06-03 |
| IL265524B (en) | 2022-10-01 |
| US12162906B2 (en) | 2024-12-10 |
| IL265524B2 (en) | 2023-02-01 |
| IL265524A (en) | 2019-05-30 |
| US20190284230A1 (en) | 2019-09-19 |
| WO2018058177A1 (en) | 2018-04-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
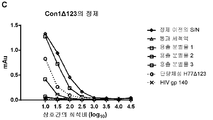
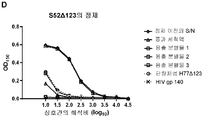
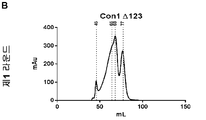
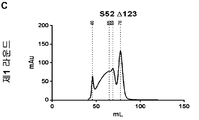
| RU2247729C2 (ru) | Олигомерная частица, индуцирующая иммунитет против вируса гепатита с, способ получения олигомерной частицы, композиция, специфическое антитело, набор (варианты), иммунологический анализ и вакцина против вируса гепатита с | |
| JP4173741B2 (ja) | コアグリコシル化されたhcvエンベロープタンパク質 | |
| US20250215048A1 (en) | Assembled glycoproteins | |
| US20210188917A1 (en) | Nucleic acids encoding zika virus-like particles and their use in zika virus vaccines and diagnostic assays | |
| JP2018533934A (ja) | C型肝炎ウイルスe1/e2ヘテロダイマー及びそれを生成する方法 | |
| CN103476788A (zh) | 免疫原性屈曲病毒肽 | |
| US11179460B2 (en) | Virus-like particles comprising zika antigen | |
| US20030021805A1 (en) | Generation of HCV-like particles and chimeric HCV virus | |
| CN101397335A (zh) | Hcv包膜蛋白颗粒:用于疫苗接种的用途 | |
| CZ20004802A3 (cs) | Částice obsahující obalové proteiny HCV a jejich použití pro očkování | |
| HK40029257A (en) | Virus-like particles comprising zika antigen | |
| HK1201451A1 (en) | Compositions and methods | |
| HK1037639B (en) | Particles of hcv envelope proteins: use for vaccination | |
| CN107648601A (zh) | 一种丙型肝炎病毒三价亚单位疫苗的制备及应用 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
St.27 status event code: A-0-1-A10-A15-nap-PA0105 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| PG1501 | Laying open of application |
St.27 status event code: A-1-1-Q10-Q12-nap-PG1501 |
|
| A201 | Request for examination | ||
| AMND | Amendment | ||
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| PA0201 | Request for examination |
St.27 status event code: A-1-2-D10-D11-exm-PA0201 |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
St.27 status event code: A-1-2-D10-D21-exm-PE0902 |
|
| T11-X000 | Administrative time limit extension requested |
St.27 status event code: U-3-3-T10-T11-oth-X000 |
|
| AMND | Amendment | ||
| E13-X000 | Pre-grant limitation requested |
St.27 status event code: A-2-3-E10-E13-lim-X000 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
St.27 status event code: N-2-6-B10-B15-exm-PE0601 |
|
| AMND | Amendment | ||
| E13-X000 | Pre-grant limitation requested |
St.27 status event code: A-2-3-E10-E13-lim-X000 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| PX0901 | Re-examination |
St.27 status event code: A-2-3-E10-E12-rex-PX0901 |
|
| PX0601 | Decision of rejection after re-examination |
St.27 status event code: N-2-6-B10-B17-rex-PX0601 |
|
| X601 | Decision of rejection after re-examination | ||
| T11-X000 | Administrative time limit extension requested |
St.27 status event code: U-3-3-T10-T11-oth-X000 |
|
| T13-X000 | Administrative time limit extension granted |
St.27 status event code: U-3-3-T10-T13-oth-X000 |
|
| T13-X000 | Administrative time limit extension granted |
St.27 status event code: U-3-3-T10-T13-oth-X000 |
|
| A107 | Divisional application of patent | ||
| J201 | Request for trial against refusal decision | ||
| PA0104 | Divisional application for international application |
St.27 status event code: A-0-1-A10-A18-div-PA0104 St.27 status event code: A-0-1-A10-A16-div-PA0104 |
|
| PJ0201 | Trial against decision of rejection |
St.27 status event code: A-3-3-V10-V11-apl-PJ0201 |
|
| PJ1301 | Trial decision |
St.27 status event code: A-3-3-V10-V15-crt-PJ1301 Decision date: 20251031 Appeal event data comment text: Appeal Kind Category : Appeal against decision to decline refusal, Appeal Ground Text : 2019 7011934 Appeal request date: 20240730 Appellate body name: Patent Examination Board Decision authority category: Office appeal board Decision identifier: 2024101001688 |
|
| V15 | Decision substituted |
Free format text: ST27 STATUS EVENT CODE: A-3-3-V10-V15-CRT-PJ1301 (AS PROVIDED BY THE NATIONAL OFFICE); DECISION IDENTIFIER: 2024101001688; DECISION AUTHORITY: OFFICE APPEAL BOARD, APPEAL KIND CATEGORY : APPEAL AGAINST DECISION TO DECLINE REFUSAL |
|
| E12 | Pre-grant re-examination requested |
Free format text: ST27 STATUS EVENT CODE: A-6-3-E10-E12-REX-PS0901 (AS PROVIDED BY THE NATIONAL OFFICE) |
|
| PS0901 | Examination by remand of revocation |
St.27 status event code: A-6-3-E10-E12-rex-PS0901 |
|
| PE0902 | Notice of grounds for rejection |
St.27 status event code: A-1-2-D10-D21-exm-PE0902 |