KR20190046935A - 엔자스타우린의 활성을 예측하기 위한 방법 및 조성물 - Google Patents
엔자스타우린의 활성을 예측하기 위한 방법 및 조성물 Download PDFInfo
- Publication number
- KR20190046935A KR20190046935A KR1020197009407A KR20197009407A KR20190046935A KR 20190046935 A KR20190046935 A KR 20190046935A KR 1020197009407 A KR1020197009407 A KR 1020197009407A KR 20197009407 A KR20197009407 A KR 20197009407A KR 20190046935 A KR20190046935 A KR 20190046935A
- Authority
- KR
- South Korea
- Prior art keywords
- snps
- treatment
- sequence
- group
- cancer
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 230000000694 effects Effects 0.000 title claims abstract description 36
- 238000000034 method Methods 0.000 title claims description 128
- 239000000203 mixture Substances 0.000 title description 11
- 238000011282 treatment Methods 0.000 claims abstract description 155
- 239000000090 biomarker Substances 0.000 claims abstract description 126
- XOAAWQZATWQOTB-UHFFFAOYSA-N taurine Chemical compound NCCS(O)(=O)=O XOAAWQZATWQOTB-UHFFFAOYSA-N 0.000 claims abstract description 70
- 206010028980 Neoplasm Diseases 0.000 claims abstract description 46
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 43
- 201000010099 disease Diseases 0.000 claims abstract description 36
- 229960003080 taurine Drugs 0.000 claims abstract description 35
- 201000011510 cancer Diseases 0.000 claims abstract description 32
- 238000002405 diagnostic procedure Methods 0.000 claims abstract description 30
- 206010012818 diffuse large B-cell lymphoma Diseases 0.000 claims abstract description 25
- 230000000295 complement effect Effects 0.000 claims description 91
- 108700028369 Alleles Proteins 0.000 claims description 85
- 125000003729 nucleotide group Chemical group 0.000 claims description 65
- 239000002773 nucleotide Substances 0.000 claims description 62
- 108020004414 DNA Proteins 0.000 claims description 60
- 239000003153 chemical reaction reagent Substances 0.000 claims description 57
- 150000007523 nucleic acids Chemical class 0.000 claims description 54
- 108091033319 polynucleotide Proteins 0.000 claims description 54
- 102000040430 polynucleotide Human genes 0.000 claims description 54
- 239000002157 polynucleotide Substances 0.000 claims description 54
- 239000000523 sample Substances 0.000 claims description 52
- 238000012163 sequencing technique Methods 0.000 claims description 52
- 238000004458 analytical method Methods 0.000 claims description 47
- 102000039446 nucleic acids Human genes 0.000 claims description 39
- 108020004707 nucleic acids Proteins 0.000 claims description 39
- 238000002560 therapeutic procedure Methods 0.000 claims description 31
- 238000012300 Sequence Analysis Methods 0.000 claims description 30
- 238000002493 microarray Methods 0.000 claims description 30
- 239000012472 biological sample Substances 0.000 claims description 27
- 230000001225 therapeutic effect Effects 0.000 claims description 27
- 238000004422 calculation algorithm Methods 0.000 claims description 25
- 208000031671 Large B-Cell Diffuse Lymphoma Diseases 0.000 claims description 24
- 229940043355 kinase inhibitor Drugs 0.000 claims description 23
- 208000005017 glioblastoma Diseases 0.000 claims description 21
- 239000000758 substrate Substances 0.000 claims description 21
- 210000002700 urine Anatomy 0.000 claims description 21
- DQYBRTASHMYDJG-UHFFFAOYSA-N Bisindolylmaleimide Chemical compound C1=CC=C2C(C=3C(=O)NC(C=3C=3C4=CC=CC=C4NC=3)=O)=CNC2=C1 DQYBRTASHMYDJG-UHFFFAOYSA-N 0.000 claims description 20
- 238000009396 hybridization Methods 0.000 claims description 20
- 239000003909 protein kinase inhibitor Substances 0.000 claims description 20
- 238000012360 testing method Methods 0.000 claims description 19
- 230000003321 amplification Effects 0.000 claims description 18
- 238000003199 nucleic acid amplification method Methods 0.000 claims description 18
- 108091034117 Oligonucleotide Proteins 0.000 claims description 17
- 102000003923 Protein Kinase C Human genes 0.000 claims description 15
- 108090000315 Protein Kinase C Proteins 0.000 claims description 15
- 102000054765 polymorphisms of proteins Human genes 0.000 claims description 15
- -1 4EBP1 Proteins 0.000 claims description 13
- 108091028043 Nucleic acid sequence Proteins 0.000 claims description 13
- 239000007787 solid Substances 0.000 claims description 12
- 238000001712 DNA sequencing Methods 0.000 claims description 11
- 230000009257 reactivity Effects 0.000 claims description 11
- KIWODJBCHRADND-UHFFFAOYSA-N 3-anilino-4-[1-[3-(1-imidazolyl)propyl]-3-indolyl]pyrrole-2,5-dione Chemical compound O=C1NC(=O)C(C=2C3=CC=CC=C3N(CCCN3C=NC=C3)C=2)=C1NC1=CC=CC=C1 KIWODJBCHRADND-UHFFFAOYSA-N 0.000 claims description 10
- 206010009944 Colon cancer Diseases 0.000 claims description 10
- 102000013530 TOR Serine-Threonine Kinases Human genes 0.000 claims description 10
- 108010065917 TOR Serine-Threonine Kinases Proteins 0.000 claims description 10
- 208000029742 colonic neoplasm Diseases 0.000 claims description 10
- 208000002154 non-small cell lung carcinoma Diseases 0.000 claims description 10
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 claims description 10
- 238000004949 mass spectrometry Methods 0.000 claims description 9
- 229920001184 polypeptide Polymers 0.000 claims description 9
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 9
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 9
- 206010006187 Breast cancer Diseases 0.000 claims description 8
- 208000026310 Breast neoplasm Diseases 0.000 claims description 8
- 206010025323 Lymphomas Diseases 0.000 claims description 8
- 208000034578 Multiple myelomas Diseases 0.000 claims description 8
- 108091032973 (ribonucleotides)n+m Proteins 0.000 claims description 7
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 claims description 7
- 208000032612 Glial tumor Diseases 0.000 claims description 7
- 206010018338 Glioma Diseases 0.000 claims description 7
- 206010058467 Lung neoplasm malignant Diseases 0.000 claims description 7
- 206010035226 Plasma cell myeloma Diseases 0.000 claims description 7
- 206010060862 Prostate cancer Diseases 0.000 claims description 7
- 208000000236 Prostatic Neoplasms Diseases 0.000 claims description 7
- 150000001875 compounds Chemical class 0.000 claims description 7
- 239000003596 drug target Substances 0.000 claims description 7
- 239000003112 inhibitor Substances 0.000 claims description 7
- 201000005202 lung cancer Diseases 0.000 claims description 7
- 208000020816 lung neoplasm Diseases 0.000 claims description 7
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 claims description 6
- 206010061902 Pancreatic neoplasm Diseases 0.000 claims description 6
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 claims description 6
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 claims description 6
- 208000033559 Waldenström macroglobulinemia Diseases 0.000 claims description 6
- 238000003556 assay Methods 0.000 claims description 6
- 238000005251 capillar electrophoresis Methods 0.000 claims description 6
- 210000002889 endothelial cell Anatomy 0.000 claims description 6
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 claims description 6
- 201000002528 pancreatic cancer Diseases 0.000 claims description 6
- 208000008443 pancreatic carcinoma Diseases 0.000 claims description 6
- 238000007894 restriction fragment length polymorphism technique Methods 0.000 claims description 6
- 208000003174 Brain Neoplasms Diseases 0.000 claims description 5
- 102000005636 Cyclic AMP Response Element-Binding Protein Human genes 0.000 claims description 5
- 108010045171 Cyclic AMP Response Element-Binding Protein Proteins 0.000 claims description 5
- 208000008839 Kidney Neoplasms Diseases 0.000 claims description 5
- 238000007397 LAMP assay Methods 0.000 claims description 5
- 206010038389 Renal cancer Diseases 0.000 claims description 5
- 102000003861 Ribosomal protein S6 Human genes 0.000 claims description 5
- 108090000221 Ribosomal protein S6 Proteins 0.000 claims description 5
- 241000700605 Viruses Species 0.000 claims description 5
- 230000002491 angiogenic effect Effects 0.000 claims description 5
- 238000004166 bioassay Methods 0.000 claims description 5
- 230000008236 biological pathway Effects 0.000 claims description 5
- 238000001514 detection method Methods 0.000 claims description 5
- 238000001502 gel electrophoresis Methods 0.000 claims description 5
- 201000010982 kidney cancer Diseases 0.000 claims description 5
- 208000032839 leukemia Diseases 0.000 claims description 5
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 claims description 5
- PEEHTFAAVSWFBL-UHFFFAOYSA-N Maleimide Chemical compound O=C1NC(=O)C=C1 PEEHTFAAVSWFBL-UHFFFAOYSA-N 0.000 claims description 4
- 101100273253 Rhizopus niveus RNAP gene Proteins 0.000 claims description 4
- 208000005718 Stomach Neoplasms Diseases 0.000 claims description 4
- 238000003776 cleavage reaction Methods 0.000 claims description 4
- 238000000840 electrochemical analysis Methods 0.000 claims description 4
- 238000005516 engineering process Methods 0.000 claims description 4
- 206010017758 gastric cancer Diseases 0.000 claims description 4
- 201000010536 head and neck cancer Diseases 0.000 claims description 4
- 208000014829 head and neck neoplasm Diseases 0.000 claims description 4
- 201000007270 liver cancer Diseases 0.000 claims description 4
- 208000014018 liver neoplasm Diseases 0.000 claims description 4
- 235000019689 luncheon sausage Nutrition 0.000 claims description 4
- 238000012544 monitoring process Methods 0.000 claims description 4
- 239000011807 nanoball Substances 0.000 claims description 4
- 230000026731 phosphorylation Effects 0.000 claims description 4
- 238000006366 phosphorylation reaction Methods 0.000 claims description 4
- 238000012175 pyrosequencing Methods 0.000 claims description 4
- 108091008146 restriction endonucleases Proteins 0.000 claims description 4
- 230000007017 scission Effects 0.000 claims description 4
- 230000000638 stimulation Effects 0.000 claims description 4
- 201000011549 stomach cancer Diseases 0.000 claims description 4
- 239000000126 substance Substances 0.000 claims description 4
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 claims description 3
- 206010033128 Ovarian cancer Diseases 0.000 claims description 3
- 206010061535 Ovarian neoplasm Diseases 0.000 claims description 3
- 108020004459 Small interfering RNA Proteins 0.000 claims description 3
- 206010042971 T-cell lymphoma Diseases 0.000 claims description 3
- 208000027585 T-cell non-Hodgkin lymphoma Diseases 0.000 claims description 3
- 238000000376 autoradiography Methods 0.000 claims description 3
- 230000036755 cellular response Effects 0.000 claims description 3
- 229960004397 cyclophosphamide Drugs 0.000 claims description 3
- 238000006073 displacement reaction Methods 0.000 claims description 3
- 229960004679 doxorubicin Drugs 0.000 claims description 3
- 238000011156 evaluation Methods 0.000 claims description 3
- 238000004128 high performance liquid chromatography Methods 0.000 claims description 3
- 238000000386 microscopy Methods 0.000 claims description 3
- 239000008194 pharmaceutical composition Substances 0.000 claims description 3
- 239000004065 semiconductor Substances 0.000 claims description 3
- 238000011301 standard therapy Methods 0.000 claims description 3
- 230000005641 tunneling Effects 0.000 claims description 3
- 229960004528 vincristine Drugs 0.000 claims description 3
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 claims description 3
- 241001474374 Blennius Species 0.000 claims description 2
- 206010008796 Chromaturia Diseases 0.000 claims description 2
- 208000000461 Esophageal Neoplasms Diseases 0.000 claims description 2
- 241000124008 Mammalia Species 0.000 claims description 2
- 206010030155 Oesophageal carcinoma Diseases 0.000 claims description 2
- 208000031673 T-Cell Cutaneous Lymphoma Diseases 0.000 claims description 2
- 201000007241 cutaneous T cell lymphoma Diseases 0.000 claims description 2
- 201000004101 esophageal cancer Diseases 0.000 claims description 2
- 238000012165 high-throughput sequencing Methods 0.000 claims description 2
- 201000005962 mycosis fungoides Diseases 0.000 claims description 2
- 208000025638 primary cutaneous T-cell non-Hodgkin lymphoma Diseases 0.000 claims description 2
- 230000010076 replication Effects 0.000 claims description 2
- 238000012216 screening Methods 0.000 claims description 2
- 238000007834 ligase chain reaction Methods 0.000 claims 3
- AXRCEOKUDYDWLF-UHFFFAOYSA-N 3-(1-methyl-3-indolyl)-4-[1-[1-(2-pyridinylmethyl)-4-piperidinyl]-3-indolyl]pyrrole-2,5-dione Chemical group C12=CC=CC=C2N(C)C=C1C(C(NC1=O)=O)=C1C(C1=CC=CC=C11)=CN1C(CC1)CCN1CC1=CC=CC=N1 AXRCEOKUDYDWLF-UHFFFAOYSA-N 0.000 claims 2
- 229950002189 enzastaurin Drugs 0.000 claims 2
- 238000000211 autoradiogram Methods 0.000 claims 1
- 239000000101 novel biomarker Substances 0.000 claims 1
- 238000011275 oncology therapy Methods 0.000 claims 1
- 239000011800 void material Substances 0.000 claims 1
- 239000003814 drug Substances 0.000 abstract description 88
- 229940079593 drug Drugs 0.000 abstract description 73
- 230000004044 response Effects 0.000 abstract description 39
- 230000008901 benefit Effects 0.000 abstract description 16
- 238000006243 chemical reaction Methods 0.000 abstract description 5
- 239000003550 marker Substances 0.000 description 71
- 108090000623 proteins and genes Proteins 0.000 description 42
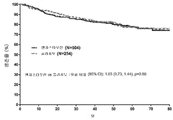
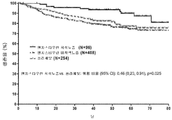
- 230000004083 survival effect Effects 0.000 description 37
- 241000282414 Homo sapiens Species 0.000 description 33
- 210000004027 cell Anatomy 0.000 description 20
- 230000002068 genetic effect Effects 0.000 description 17
- 238000003205 genotyping method Methods 0.000 description 16
- 230000035772 mutation Effects 0.000 description 15
- 230000027455 binding Effects 0.000 description 13
- 229940124597 therapeutic agent Drugs 0.000 description 13
- 210000001519 tissue Anatomy 0.000 description 13
- 102000004169 proteins and genes Human genes 0.000 description 12
- 230000002974 pharmacogenomic effect Effects 0.000 description 11
- 210000004369 blood Anatomy 0.000 description 9
- 239000008280 blood Substances 0.000 description 9
- 239000000902 placebo Substances 0.000 description 9
- 229940068196 placebo Drugs 0.000 description 9
- 230000004043 responsiveness Effects 0.000 description 9
- 108091008611 Protein Kinase B Proteins 0.000 description 8
- 102100033810 RAC-alpha serine/threonine-protein kinase Human genes 0.000 description 8
- 210000000349 chromosome Anatomy 0.000 description 8
- OPTASPLRGRRNAP-UHFFFAOYSA-N cytosine Chemical compound NC=1C=CNC(=O)N=1 OPTASPLRGRRNAP-UHFFFAOYSA-N 0.000 description 8
- 230000018109 developmental process Effects 0.000 description 8
- 230000006870 function Effects 0.000 description 8
- 210000002381 plasma Anatomy 0.000 description 8
- 239000003795 chemical substances by application Substances 0.000 description 7
- 238000011161 development Methods 0.000 description 7
- 208000035475 disorder Diseases 0.000 description 7
- 230000014509 gene expression Effects 0.000 description 7
- 210000004881 tumor cell Anatomy 0.000 description 7
- ZHNUHDYFZUAESO-UHFFFAOYSA-N Formamide Chemical compound NC=O ZHNUHDYFZUAESO-UHFFFAOYSA-N 0.000 description 6
- 208000037065 Subacute sclerosing leukoencephalitis Diseases 0.000 description 6
- 206010042297 Subacute sclerosing panencephalitis Diseases 0.000 description 6
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 6
- 230000000259 anti-tumor effect Effects 0.000 description 6
- 230000003993 interaction Effects 0.000 description 6
- 230000009467 reduction Effects 0.000 description 6
- 239000013074 reference sample Substances 0.000 description 6
- 208000024891 symptom Diseases 0.000 description 6
- 238000011285 therapeutic regimen Methods 0.000 description 6
- 206010071602 Genetic polymorphism Diseases 0.000 description 5
- 101000772560 Homo sapiens Zinc finger transcription factor Trps1 Proteins 0.000 description 5
- 239000000306 component Substances 0.000 description 5
- 210000004602 germ cell Anatomy 0.000 description 5
- 230000008569 process Effects 0.000 description 5
- 102000004190 Enzymes Human genes 0.000 description 4
- 108090000790 Enzymes Proteins 0.000 description 4
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 4
- 230000000875 corresponding effect Effects 0.000 description 4
- 229940104302 cytosine Drugs 0.000 description 4
- 238000003745 diagnosis Methods 0.000 description 4
- 102000054766 genetic haplotypes Human genes 0.000 description 4
- 239000011521 glass Substances 0.000 description 4
- 230000002401 inhibitory effect Effects 0.000 description 4
- 229910052751 metal Inorganic materials 0.000 description 4
- 239000002184 metal Substances 0.000 description 4
- 230000027939 micturition Effects 0.000 description 4
- 230000004048 modification Effects 0.000 description 4
- 238000012986 modification Methods 0.000 description 4
- 230000037361 pathway Effects 0.000 description 4
- 239000003757 phosphotransferase inhibitor Substances 0.000 description 4
- 230000002285 radioactive effect Effects 0.000 description 4
- 230000002829 reductive effect Effects 0.000 description 4
- 235000000346 sugar Nutrition 0.000 description 4
- 231100000419 toxicity Toxicity 0.000 description 4
- 230000001988 toxicity Effects 0.000 description 4
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 3
- 102000015081 Blood Coagulation Factors Human genes 0.000 description 3
- 108010039209 Blood Coagulation Factors Proteins 0.000 description 3
- 108091026890 Coding region Proteins 0.000 description 3
- 102000001267 GSK3 Human genes 0.000 description 3
- 108060006662 GSK3 Proteins 0.000 description 3
- 102100036646 Glutamyl-tRNA(Gln) amidotransferase subunit A, mitochondrial Human genes 0.000 description 3
- 101001072655 Homo sapiens Glutamyl-tRNA(Gln) amidotransferase subunit A, mitochondrial Proteins 0.000 description 3
- 238000009015 Human TaqMan MicroRNA Assay kit Methods 0.000 description 3
- 108091007960 PI3Ks Proteins 0.000 description 3
- 102000038030 PI3Ks Human genes 0.000 description 3
- 108091000080 Phosphotransferase Proteins 0.000 description 3
- 102100024923 Protein kinase C beta type Human genes 0.000 description 3
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 3
- 102100030619 Zinc finger transcription factor Trps1 Human genes 0.000 description 3
- 230000033115 angiogenesis Effects 0.000 description 3
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 3
- 239000003114 blood coagulation factor Substances 0.000 description 3
- 239000000872 buffer Substances 0.000 description 3
- 239000002738 chelating agent Substances 0.000 description 3
- 238000013461 design Methods 0.000 description 3
- 230000005764 inhibitory process Effects 0.000 description 3
- 238000013507 mapping Methods 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- NFVJNJQRWPQVOA-UHFFFAOYSA-N n-[2-chloro-5-(trifluoromethyl)phenyl]-2-[3-(4-ethyl-5-ethylsulfanyl-1,2,4-triazol-3-yl)piperidin-1-yl]acetamide Chemical compound CCN1C(SCC)=NN=C1C1CN(CC(=O)NC=2C(=CC=C(C=2)C(F)(F)F)Cl)CCC1 NFVJNJQRWPQVOA-UHFFFAOYSA-N 0.000 description 3
- 210000000056 organ Anatomy 0.000 description 3
- 102000020233 phosphotransferase Human genes 0.000 description 3
- 229920000642 polymer Polymers 0.000 description 3
- 238000004393 prognosis Methods 0.000 description 3
- 239000000376 reactant Substances 0.000 description 3
- 230000001105 regulatory effect Effects 0.000 description 3
- 230000009870 specific binding Effects 0.000 description 3
- 229940104230 thymidine Drugs 0.000 description 3
- 238000011269 treatment regimen Methods 0.000 description 3
- 238000005406 washing Methods 0.000 description 3
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 2
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- SRBFZHDQGSBBOR-IOVATXLUSA-N D-xylopyranose Chemical compound O[C@@H]1COC(O)[C@H](O)[C@H]1O SRBFZHDQGSBBOR-IOVATXLUSA-N 0.000 description 2
- 230000009946 DNA mutation Effects 0.000 description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- 206010061818 Disease progression Diseases 0.000 description 2
- 101710094033 Protein kinase C beta type Proteins 0.000 description 2
- 206010066901 Treatment failure Diseases 0.000 description 2
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 2
- DZBUGLKDJFMEHC-UHFFFAOYSA-N acridine Chemical compound C1=CC=CC2=CC3=CC=CC=C3N=C21 DZBUGLKDJFMEHC-UHFFFAOYSA-N 0.000 description 2
- 125000002015 acyclic group Chemical group 0.000 description 2
- 125000003275 alpha amino acid group Chemical group 0.000 description 2
- 230000004075 alteration Effects 0.000 description 2
- 125000003277 amino group Chemical group 0.000 description 2
- 239000003146 anticoagulant agent Substances 0.000 description 2
- 229940127219 anticoagulant drug Drugs 0.000 description 2
- 230000006907 apoptotic process Effects 0.000 description 2
- PYMYPHUHKUWMLA-UHFFFAOYSA-N arabinose Natural products OCC(O)C(O)C(O)C=O PYMYPHUHKUWMLA-UHFFFAOYSA-N 0.000 description 2
- 238000003491 array Methods 0.000 description 2
- SRBFZHDQGSBBOR-UHFFFAOYSA-N beta-D-Pyranose-Lyxose Natural products OC1COC(O)C(O)C1O SRBFZHDQGSBBOR-UHFFFAOYSA-N 0.000 description 2
- 210000001124 body fluid Anatomy 0.000 description 2
- 239000010839 body fluid Substances 0.000 description 2
- 229940098773 bovine serum albumin Drugs 0.000 description 2
- 238000004364 calculation method Methods 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 230000004663 cell proliferation Effects 0.000 description 2
- 230000001413 cellular effect Effects 0.000 description 2
- 210000003169 central nervous system Anatomy 0.000 description 2
- 229940121657 clinical drug Drugs 0.000 description 2
- 238000002648 combination therapy Methods 0.000 description 2
- 238000002790 cross-validation Methods 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 239000000539 dimer Substances 0.000 description 2
- 230000005750 disease progression Effects 0.000 description 2
- 238000002651 drug therapy Methods 0.000 description 2
- 210000003722 extracellular fluid Anatomy 0.000 description 2
- 239000007850 fluorescent dye Substances 0.000 description 2
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 2
- 239000010931 gold Substances 0.000 description 2
- 229910052737 gold Inorganic materials 0.000 description 2
- 230000012010 growth Effects 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 238000003780 insertion Methods 0.000 description 2
- 230000037431 insertion Effects 0.000 description 2
- 150000002500 ions Chemical class 0.000 description 2
- 239000003446 ligand Substances 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- 230000002093 peripheral effect Effects 0.000 description 2
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 2
- 229960004618 prednisone Drugs 0.000 description 2
- 230000000069 prophylactic effect Effects 0.000 description 2
- 125000006239 protecting group Chemical group 0.000 description 2
- ZCCUUQDIBDJBTK-UHFFFAOYSA-N psoralen Chemical compound C1=C2OC(=O)C=CC2=CC2=C1OC=C2 ZCCUUQDIBDJBTK-UHFFFAOYSA-N 0.000 description 2
- 230000022983 regulation of cell cycle Effects 0.000 description 2
- 150000003839 salts Chemical class 0.000 description 2
- 230000035945 sensitivity Effects 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
- 230000019491 signal transduction Effects 0.000 description 2
- 230000011664 signaling Effects 0.000 description 2
- 239000000377 silicon dioxide Substances 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 239000001488 sodium phosphate Substances 0.000 description 2
- 229910000162 sodium phosphate Inorganic materials 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 238000012706 support-vector machine Methods 0.000 description 2
- 230000001629 suppression Effects 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 2
- 238000012795 verification Methods 0.000 description 2
- IXDZFGATLNCIOI-NGJCXOISSA-N (3r,4r,5r)-3,4,5,6-tetrahydroxyhexan-2-one Chemical compound CC(=O)[C@H](O)[C@H](O)[C@H](O)CO IXDZFGATLNCIOI-NGJCXOISSA-N 0.000 description 1
- RSDQBPGKMDFRHH-MJVIGCOGSA-N (3s,3as,5ar,9bs)-3,5a,9-trimethyl-3a,4,5,7,8,9b-hexahydro-3h-benzo[g][1]benzofuran-2,6-dione Chemical compound O=C([C@]1(C)CC2)CCC(C)=C1[C@@H]1[C@@H]2[C@H](C)C(=O)O1 RSDQBPGKMDFRHH-MJVIGCOGSA-N 0.000 description 1
- UHDGCWIWMRVCDJ-UHFFFAOYSA-N 1-beta-D-Xylofuranosyl-NH-Cytosine Natural products O=C1N=C(N)C=CN1C1C(O)C(O)C(CO)O1 UHDGCWIWMRVCDJ-UHFFFAOYSA-N 0.000 description 1
- VGONTNSXDCQUGY-RRKCRQDMSA-N 2'-deoxyinosine Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(N=CNC2=O)=C2N=C1 VGONTNSXDCQUGY-RRKCRQDMSA-N 0.000 description 1
- HVCOBJNICQPDBP-UHFFFAOYSA-N 3-[3-[3,5-dihydroxy-6-methyl-4-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxyoxan-2-yl]oxydecanoyloxy]decanoic acid;hydrate Chemical compound O.OC1C(OC(CC(=O)OC(CCCCCCC)CC(O)=O)CCCCCCC)OC(C)C(O)C1OC1C(O)C(O)C(O)C(C)O1 HVCOBJNICQPDBP-UHFFFAOYSA-N 0.000 description 1
- VXGRJERITKFWPL-UHFFFAOYSA-N 4',5'-Dihydropsoralen Natural products C1=C2OC(=O)C=CC2=CC2=C1OCC2 VXGRJERITKFWPL-UHFFFAOYSA-N 0.000 description 1
- 208000035657 Abasia Diseases 0.000 description 1
- 229930024421 Adenine Natural products 0.000 description 1
- GFFGJBXGBJISGV-UHFFFAOYSA-N Adenine Chemical compound NC1=NC=NC2=C1N=CN2 GFFGJBXGBJISGV-UHFFFAOYSA-N 0.000 description 1
- 206010067484 Adverse reaction Diseases 0.000 description 1
- 108090001008 Avidin Proteins 0.000 description 1
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 description 1
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 description 1
- 206010055113 Breast cancer metastatic Diseases 0.000 description 1
- QCMYYKRYFNMIEC-UHFFFAOYSA-N COP(O)=O Chemical class COP(O)=O QCMYYKRYFNMIEC-UHFFFAOYSA-N 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical group [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 208000024172 Cardiovascular disease Diseases 0.000 description 1
- 102000014914 Carrier Proteins Human genes 0.000 description 1
- 206010008342 Cervix carcinoma Diseases 0.000 description 1
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 1
- UHDGCWIWMRVCDJ-PSQAKQOGSA-N Cytidine Natural products O=C1N=C(N)C=CN1[C@@H]1[C@@H](O)[C@@H](O)[C@H](CO)O1 UHDGCWIWMRVCDJ-PSQAKQOGSA-N 0.000 description 1
- HAIWUXASLYEWLM-UHFFFAOYSA-N D-manno-Heptulose Natural products OCC1OC(O)(CO)C(O)C(O)C1O HAIWUXASLYEWLM-UHFFFAOYSA-N 0.000 description 1
- HMFHBZSHGGEWLO-SOOFDHNKSA-N D-ribofuranose Chemical compound OC[C@H]1OC(O)[C@H](O)[C@@H]1O HMFHBZSHGGEWLO-SOOFDHNKSA-N 0.000 description 1
- 102000016928 DNA-directed DNA polymerase Human genes 0.000 description 1
- 108010014303 DNA-directed DNA polymerase Proteins 0.000 description 1
- 108090000626 DNA-directed RNA polymerases Proteins 0.000 description 1
- 102000004163 DNA-directed RNA polymerases Human genes 0.000 description 1
- 238000009007 Diagnostic Kit Methods 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- 239000004593 Epoxy Substances 0.000 description 1
- 102000008946 Fibrinogen Human genes 0.000 description 1
- 108010049003 Fibrinogen Proteins 0.000 description 1
- 229920001917 Ficoll Polymers 0.000 description 1
- 102000038624 GSKs Human genes 0.000 description 1
- 108091007911 GSKs Proteins 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 229930186217 Glycolipid Natural products 0.000 description 1
- 208000017604 Hodgkin disease Diseases 0.000 description 1
- 208000021519 Hodgkin lymphoma Diseases 0.000 description 1
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 1
- 208000026350 Inborn Genetic disease Diseases 0.000 description 1
- HSNZZMHEPUFJNZ-UHFFFAOYSA-N L-galacto-2-Heptulose Natural products OCC(O)C(O)C(O)C(O)C(=O)CO HSNZZMHEPUFJNZ-UHFFFAOYSA-N 0.000 description 1
- 239000002147 L01XE04 - Sunitinib Substances 0.000 description 1
- 208000031422 Lymphocytic Chronic B-Cell Leukemia Diseases 0.000 description 1
- 206010027476 Metastases Diseases 0.000 description 1
- 108091092878 Microsatellite Proteins 0.000 description 1
- 206010048723 Multiple-drug resistance Diseases 0.000 description 1
- 206010028813 Nausea Diseases 0.000 description 1
- 239000000020 Nitrocellulose Substances 0.000 description 1
- 108091092724 Noncoding DNA Proteins 0.000 description 1
- 108700026244 Open Reading Frames Proteins 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 108090000882 Peptidyl-Dipeptidase A Proteins 0.000 description 1
- ABLZXFCXXLZCGV-UHFFFAOYSA-N Phosphorous acid Chemical group OP(O)=O ABLZXFCXXLZCGV-UHFFFAOYSA-N 0.000 description 1
- 108010029485 Protein Isoforms Proteins 0.000 description 1
- 102000001708 Protein Isoforms Human genes 0.000 description 1
- 108010024526 Protein Kinase C beta Proteins 0.000 description 1
- 102000009516 Protein Serine-Threonine Kinases Human genes 0.000 description 1
- 108010009341 Protein Serine-Threonine Kinases Proteins 0.000 description 1
- 108010076504 Protein Sorting Signals Proteins 0.000 description 1
- 208000035977 Rare disease Diseases 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 208000006265 Renal cell carcinoma Diseases 0.000 description 1
- 102000034527 Retinoid X Receptors Human genes 0.000 description 1
- 108010038912 Retinoid X Receptors Proteins 0.000 description 1
- 108091028664 Ribonucleotide Proteins 0.000 description 1
- PYMYPHUHKUWMLA-LMVFSUKVSA-N Ribose Natural products OC[C@@H](O)[C@@H](O)[C@@H](O)C=O PYMYPHUHKUWMLA-LMVFSUKVSA-N 0.000 description 1
- HAIWUXASLYEWLM-AZEWMMITSA-N Sedoheptulose Natural products OC[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@](O)(CO)O1 HAIWUXASLYEWLM-AZEWMMITSA-N 0.000 description 1
- 229940124639 Selective inhibitor Drugs 0.000 description 1
- 240000003705 Senecio vulgaris Species 0.000 description 1
- 208000000453 Skin Neoplasms Diseases 0.000 description 1
- NAVMQTYZDKMPEU-UHFFFAOYSA-N Targretin Chemical compound CC1=CC(C(CCC2(C)C)(C)C)=C2C=C1C(=C)C1=CC=C(C(O)=O)C=C1 NAVMQTYZDKMPEU-UHFFFAOYSA-N 0.000 description 1
- RSDQBPGKMDFRHH-UHFFFAOYSA-N Taurin Natural products C1CC2(C)C(=O)CCC(C)=C2C2C1C(C)C(=O)O2 RSDQBPGKMDFRHH-UHFFFAOYSA-N 0.000 description 1
- BPEGJWRSRHCHSN-UHFFFAOYSA-N Temozolomide Chemical compound O=C1N(C)N=NC2=C(C(N)=O)N=CN21 BPEGJWRSRHCHSN-UHFFFAOYSA-N 0.000 description 1
- RYYWUUFWQRZTIU-UHFFFAOYSA-N Thiophosphoric acid Chemical class OP(O)(S)=O RYYWUUFWQRZTIU-UHFFFAOYSA-N 0.000 description 1
- 108091023040 Transcription factor Proteins 0.000 description 1
- 102000040945 Transcription factor Human genes 0.000 description 1
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 1
- FRTNIYVUDIHXPG-UHFFFAOYSA-N acetic acid;ethane-1,2-diamine Chemical compound CC(O)=O.CC(O)=O.CC(O)=O.CC(O)=O.NCCN FRTNIYVUDIHXPG-UHFFFAOYSA-N 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 229960000643 adenine Drugs 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 230000006838 adverse reaction Effects 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 239000000556 agonist Substances 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 125000003342 alkenyl group Chemical group 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- 239000002168 alkylating agent Substances 0.000 description 1
- HMFHBZSHGGEWLO-UHFFFAOYSA-N alpha-D-Furanose-Ribose Natural products OCC1OC(O)C(O)C1O HMFHBZSHGGEWLO-UHFFFAOYSA-N 0.000 description 1
- SRBFZHDQGSBBOR-STGXQOJASA-N alpha-D-lyxopyranose Chemical compound O[C@@H]1CO[C@H](O)[C@@H](O)[C@H]1O SRBFZHDQGSBBOR-STGXQOJASA-N 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 210000004381 amniotic fluid Anatomy 0.000 description 1
- 210000004102 animal cell Anatomy 0.000 description 1
- 230000003042 antagnostic effect Effects 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 238000011122 anti-angiogenic therapy Methods 0.000 description 1
- 230000000118 anti-neoplastic effect Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 229940045988 antineoplastic drug protein kinase inhibitors Drugs 0.000 description 1
- 238000003782 apoptosis assay Methods 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- PYMYPHUHKUWMLA-WDCZJNDASA-N arabinose Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)C=O PYMYPHUHKUWMLA-WDCZJNDASA-N 0.000 description 1
- 210000003567 ascitic fluid Anatomy 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- SESFRYSPDFLNCH-UHFFFAOYSA-N benzyl benzoate Chemical compound C=1C=CC=CC=1C(=O)OCC1=CC=CC=C1 SESFRYSPDFLNCH-UHFFFAOYSA-N 0.000 description 1
- 229960002938 bexarotene Drugs 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 108091008324 binding proteins Proteins 0.000 description 1
- 239000003124 biologic agent Substances 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000007321 biological mechanism Effects 0.000 description 1
- 238000001574 biopsy Methods 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 210000000601 blood cell Anatomy 0.000 description 1
- 239000012503 blood component Substances 0.000 description 1
- 229910052796 boron Inorganic materials 0.000 description 1
- GXJABQQUPOEUTA-RDJZCZTQSA-N bortezomib Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)B(O)O)NC(=O)C=1N=CC=NC=1)C1=CC=CC=C1 GXJABQQUPOEUTA-RDJZCZTQSA-N 0.000 description 1
- 229960001467 bortezomib Drugs 0.000 description 1
- 150000004657 carbamic acid derivatives Chemical class 0.000 description 1
- 150000001720 carbohydrates Chemical group 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 201000011529 cardiovascular cancer Diseases 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 230000030833 cell death Effects 0.000 description 1
- 210000001175 cerebrospinal fluid Anatomy 0.000 description 1
- 201000010881 cervical cancer Diseases 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 208000032852 chronic lymphocytic leukemia Diseases 0.000 description 1
- 201000002758 colorectal adenoma Diseases 0.000 description 1
- 230000021615 conjugation Effects 0.000 description 1
- 238000011340 continuous therapy Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 210000004748 cultured cell Anatomy 0.000 description 1
- 125000000392 cycloalkenyl group Chemical group 0.000 description 1
- 125000000753 cycloalkyl group Chemical group 0.000 description 1
- UHDGCWIWMRVCDJ-ZAKLUEHWSA-N cytidine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O1 UHDGCWIWMRVCDJ-ZAKLUEHWSA-N 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 239000005547 deoxyribonucleotide Substances 0.000 description 1
- 125000002637 deoxyribonucleotide group Chemical group 0.000 description 1
- VGONTNSXDCQUGY-UHFFFAOYSA-N desoxyinosine Natural products C1C(O)C(CO)OC1N1C(NC=NC2=O)=C2N=C1 VGONTNSXDCQUGY-UHFFFAOYSA-N 0.000 description 1
- 230000009699 differential effect Effects 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 230000008034 disappearance Effects 0.000 description 1
- 208000037765 diseases and disorders Diseases 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- NAGJZTKCGNOGPW-UHFFFAOYSA-N dithiophosphoric acid Chemical class OP(O)(S)=S NAGJZTKCGNOGPW-UHFFFAOYSA-N 0.000 description 1
- 238000001647 drug administration Methods 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 230000036267 drug metabolism Effects 0.000 description 1
- 238000003255 drug test Methods 0.000 description 1
- 125000003700 epoxy group Chemical group 0.000 description 1
- 238000013401 experimental design Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 229940012952 fibrinogen Drugs 0.000 description 1
- 239000000834 fixative Substances 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 230000037406 food intake Effects 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 238000012252 genetic analysis Methods 0.000 description 1
- 208000016361 genetic disease Diseases 0.000 description 1
- 230000007614 genetic variation Effects 0.000 description 1
- 238000011331 genomic analysis Methods 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 230000013595 glycosylation Effects 0.000 description 1
- 238000006206 glycosylation reaction Methods 0.000 description 1
- 230000002962 histologic effect Effects 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 229960000367 inositol Drugs 0.000 description 1
- 239000000138 intercalating agent Substances 0.000 description 1
- 230000035990 intercellular signaling Effects 0.000 description 1
- 238000007917 intracranial administration Methods 0.000 description 1
- 238000011901 isothermal amplification Methods 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 230000003902 lesion Effects 0.000 description 1
- 231100001231 less toxic Toxicity 0.000 description 1
- 238000007477 logistic regression Methods 0.000 description 1
- 201000000564 macroglobulinemia Diseases 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 238000007726 management method Methods 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 108020004999 messenger RNA Proteins 0.000 description 1
- 230000004066 metabolic change Effects 0.000 description 1
- 208000030159 metabolic disease Diseases 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 229910044991 metal oxide Inorganic materials 0.000 description 1
- 150000004706 metal oxides Chemical class 0.000 description 1
- 230000009401 metastasis Effects 0.000 description 1
- 238000010208 microarray analysis Methods 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 210000003470 mitochondria Anatomy 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 230000008693 nausea Effects 0.000 description 1
- 239000002547 new drug Substances 0.000 description 1
- 229920001220 nitrocellulos Polymers 0.000 description 1
- 231100000957 no side effect Toxicity 0.000 description 1
- 108091027963 non-coding RNA Proteins 0.000 description 1
- 102000042567 non-coding RNA Human genes 0.000 description 1
- 238000007899 nucleic acid hybridization Methods 0.000 description 1
- 229940127073 nucleoside analogue Drugs 0.000 description 1
- 210000004940 nucleus Anatomy 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 238000002515 oligonucleotide synthesis Methods 0.000 description 1
- 230000000174 oncolytic effect Effects 0.000 description 1
- 230000002018 overexpression Effects 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 239000013610 patient sample Substances 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 125000002467 phosphate group Chemical group [H]OP(=O)(O[H])O[*] 0.000 description 1
- 229920000729 poly(L-lysine) polymer Polymers 0.000 description 1
- 229920000647 polyepoxide Polymers 0.000 description 1
- 238000003752 polymerase chain reaction Methods 0.000 description 1
- 238000006116 polymerization reaction Methods 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 230000035935 pregnancy Effects 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 230000003449 preventive effect Effects 0.000 description 1
- 239000000092 prognostic biomarker Substances 0.000 description 1
- 230000005522 programmed cell death Effects 0.000 description 1
- 230000002250 progressing effect Effects 0.000 description 1
- 230000035755 proliferation Effects 0.000 description 1
- 238000000159 protein binding assay Methods 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 238000010188 recombinant method Methods 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 238000005215 recombination Methods 0.000 description 1
- 210000005132 reproductive cell Anatomy 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 239000002336 ribonucleotide Substances 0.000 description 1
- 125000002652 ribonucleotide group Chemical group 0.000 description 1
- 238000007480 sanger sequencing Methods 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- HSNZZMHEPUFJNZ-SHUUEZRQSA-N sedoheptulose Chemical compound OC[C@@H](O)[C@@H](O)[C@@H](O)[C@H](O)C(=O)CO HSNZZMHEPUFJNZ-SHUUEZRQSA-N 0.000 description 1
- 238000005204 segregation Methods 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 238000004088 simulation Methods 0.000 description 1
- 201000000849 skin cancer Diseases 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- WINHZLLDWRZWRT-ATVHPVEESA-N sunitinib Chemical compound CCN(CC)CCNC(=O)C1=C(C)NC(\C=C/2C3=CC(F)=CC=C3NC\2=O)=C1C WINHZLLDWRZWRT-ATVHPVEESA-N 0.000 description 1
- 229960001796 sunitinib Drugs 0.000 description 1
- 238000011477 surgical intervention Methods 0.000 description 1
- 230000000946 synaptic effect Effects 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- 239000004557 technical material Substances 0.000 description 1
- 229960004964 temozolomide Drugs 0.000 description 1
- 230000008791 toxic response Effects 0.000 description 1
- 239000003053 toxin Substances 0.000 description 1
- 231100000765 toxin Toxicity 0.000 description 1
- 108700012359 toxins Proteins 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 230000002103 transcriptional effect Effects 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 238000011277 treatment modality Methods 0.000 description 1
- 230000005748 tumor development Effects 0.000 description 1
- 230000004614 tumor growth Effects 0.000 description 1
- 239000000439 tumor marker Substances 0.000 description 1
- 238000011144 upstream manufacturing Methods 0.000 description 1
- 230000002485 urinary effect Effects 0.000 description 1
- 108700026220 vif Genes Proteins 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6876—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes
- C12Q1/6883—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material
- C12Q1/6886—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material for cancer
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/106—Pharmacogenomics, i.e. genetic variability in individual responses to drugs and drug metabolism
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/136—Screening for pharmacological compounds
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/156—Polymorphic or mutational markers
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Zoology (AREA)
- Genetics & Genomics (AREA)
- Pathology (AREA)
- Analytical Chemistry (AREA)
- Wood Science & Technology (AREA)
- Immunology (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Microbiology (AREA)
- Biotechnology (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Physics & Mathematics (AREA)
- Hospice & Palliative Care (AREA)
- Biochemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- Oncology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- General Chemical & Material Sciences (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Apparatus Associated With Microorganisms And Enzymes (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medical Treatment And Welfare Office Work (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662382734P | 2016-09-01 | 2016-09-01 | |
| US62/382,734 | 2016-09-01 | ||
| US201662414601P | 2016-10-28 | 2016-10-28 | |
| US62/414,601 | 2016-10-28 | ||
| PCT/US2017/049747 WO2018045240A1 (en) | 2016-09-01 | 2017-08-31 | Methods and composition for the prediction of the activity of enzastaurin |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20190046935A true KR20190046935A (ko) | 2019-05-07 |
Family
ID=59923555
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197009407A Ceased KR20190046935A (ko) | 2016-09-01 | 2017-08-31 | 엔자스타우린의 활성을 예측하기 위한 방법 및 조성물 |
Country Status (15)
| Country | Link |
|---|---|
| US (2) | US11421280B2 (enExample) |
| EP (1) | EP3507384B1 (enExample) |
| JP (3) | JP7197915B2 (enExample) |
| KR (1) | KR20190046935A (enExample) |
| CN (1) | CN109952383B (enExample) |
| AU (1) | AU2017318669B2 (enExample) |
| BR (1) | BR112019003951A2 (enExample) |
| CA (1) | CA3035386A1 (enExample) |
| JO (1) | JOP20190025A1 (enExample) |
| MX (1) | MX2019002377A (enExample) |
| PH (1) | PH12019500422A1 (enExample) |
| RU (1) | RU2019109011A (enExample) |
| SG (2) | SG11201901762WA (enExample) |
| TW (2) | TW202313973A (enExample) |
| WO (1) | WO2018045240A1 (enExample) |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JOP20190025A1 (ar) | 2016-09-01 | 2019-02-19 | Denovo Biopharma Llc | طرق وتركيبة لتوقع نشاط إنزاستورين |
| CN110496223B (zh) * | 2018-05-17 | 2021-09-10 | 复旦大学附属肿瘤医院 | 一种治疗非霍奇金氏淋巴瘤的药物组合物 |
| US20220193062A1 (en) * | 2018-09-12 | 2022-06-23 | Denovo Biopharma Llc | Combination of enzastaurin and inhibitors of btk and uses thereof |
| CN111549033B (zh) * | 2020-06-11 | 2021-02-12 | 南京市江宁医院 | 慢病毒感染人表皮角质细胞株及其构建方法和应用 |
| US11227690B1 (en) * | 2020-09-14 | 2022-01-18 | Opendna Ltd. | Machine learning prediction of therapy response |
| TWI808838B (zh) * | 2021-07-23 | 2023-07-11 | 高雄醫學大學 | 二代荷爾蒙藥物於治療攝護腺癌療效評估之臨床治療藥物預測暨推薦系統及方法 |
| US20230131677A1 (en) * | 2021-10-21 | 2023-04-27 | Toyota Research Institute, Inc. | Systems and methods for predicting the effect of an intervention via machine learning |
| CN118339312A (zh) * | 2021-11-23 | 2024-07-12 | 索元生物医药(美国)有限公司 | 用于评估多核苷酸递送和癌疗法之功效的组合物和方法 |
| US12474842B2 (en) * | 2021-12-13 | 2025-11-18 | Google Llc | Optimizing data placement based on data temperature and lifetime prediction |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ATE456367T1 (de) | 1993-12-23 | 2010-02-15 | Lilly Co Eli | Proteinkinase c inhibitoren |
| EP1309679A2 (en) * | 2000-08-16 | 2003-05-14 | Chiron Corporation | Human genes and gene expression products |
| US20040072217A1 (en) | 2002-06-17 | 2004-04-15 | Affymetrix, Inc. | Methods of analysis of linkage disequilibrium |
| CA2393720C (en) | 2002-07-12 | 2010-09-14 | Eli Lilly And Company | Crystalline 2,5-dione-3-(1-methyl-1h-indol-3-yl)-4-[1-(pyridin-2-ylmethyl)piperidin-4-yl]-1h-indol-3-yl]-1h-pyrrole mono-hydrochloride |
| WO2007145992A2 (en) | 2006-06-05 | 2007-12-21 | Perlegen Sciences, Inc. | Genetic basis of treatment response in depression patients |
| KR102006417B1 (ko) * | 2011-01-31 | 2019-08-01 | 드노보 바이오마커스 인코포레이티드 | 게놈약학적 바이오마커 동정 방법 |
| EP2718486A4 (en) * | 2011-06-08 | 2014-12-31 | Denovo Biopharma Hangzhou Ltd Co | METHODS AND COMPOSITIONS FOR PREDICTING THE ACTIVITY OF A RETINOID RECEPTOR X MODULATOR |
| JOP20190025A1 (ar) | 2016-09-01 | 2019-02-19 | Denovo Biopharma Llc | طرق وتركيبة لتوقع نشاط إنزاستورين |
-
2017
- 2017-06-16 JO JOP/2019/0025A patent/JOP20190025A1/ar unknown
- 2017-08-31 SG SG11201901762WA patent/SG11201901762WA/en unknown
- 2017-08-31 AU AU2017318669A patent/AU2017318669B2/en not_active Ceased
- 2017-08-31 RU RU2019109011A patent/RU2019109011A/ru unknown
- 2017-08-31 KR KR1020197009407A patent/KR20190046935A/ko not_active Ceased
- 2017-08-31 WO PCT/US2017/049747 patent/WO2018045240A1/en not_active Ceased
- 2017-08-31 US US16/327,788 patent/US11421280B2/en active Active
- 2017-08-31 MX MX2019002377A patent/MX2019002377A/es unknown
- 2017-08-31 SG SG10201912134TA patent/SG10201912134TA/en unknown
- 2017-08-31 CN CN201780066737.5A patent/CN109952383B/zh active Active
- 2017-08-31 TW TW111122709A patent/TW202313973A/zh unknown
- 2017-08-31 CA CA3035386A patent/CA3035386A1/en active Pending
- 2017-08-31 BR BR112019003951A patent/BR112019003951A2/pt not_active IP Right Cessation
- 2017-08-31 TW TW106129838A patent/TWI771317B/zh not_active IP Right Cessation
- 2017-08-31 EP EP17771623.0A patent/EP3507384B1/en active Active
- 2017-08-31 JP JP2019512609A patent/JP7197915B2/ja active Active
-
2019
- 2019-02-27 PH PH12019500422A patent/PH12019500422A1/en unknown
-
2020
- 2020-08-26 JP JP2020142358A patent/JP2020188816A/ja active Pending
-
2022
- 2022-08-18 US US17/820,772 patent/US20230074781A1/en not_active Abandoned
- 2022-09-16 JP JP2022148064A patent/JP2022173308A/ja not_active Withdrawn
Also Published As
| Publication number | Publication date |
|---|---|
| PH12019500422A1 (en) | 2019-05-27 |
| RU2019109011A3 (enExample) | 2021-01-22 |
| AU2017318669A1 (en) | 2019-03-07 |
| MX2019002377A (es) | 2019-09-05 |
| CN109952383B (zh) | 2024-01-05 |
| CN109952383A (zh) | 2019-06-28 |
| JP7197915B2 (ja) | 2022-12-28 |
| BR112019003951A2 (pt) | 2019-06-25 |
| US11421280B2 (en) | 2022-08-23 |
| TW202313973A (zh) | 2023-04-01 |
| SG11201901762WA (en) | 2019-03-28 |
| US20230074781A1 (en) | 2023-03-09 |
| TWI771317B (zh) | 2022-07-21 |
| US20190233902A1 (en) | 2019-08-01 |
| TW201812125A (zh) | 2018-04-01 |
| JP2022173308A (ja) | 2022-11-18 |
| EP3507384B1 (en) | 2023-08-30 |
| JP2019528705A (ja) | 2019-10-17 |
| RU2019109011A (ru) | 2020-10-05 |
| SG10201912134TA (en) | 2020-02-27 |
| AU2017318669B2 (en) | 2023-04-20 |
| CA3035386A1 (en) | 2018-03-08 |
| WO2018045240A1 (en) | 2018-03-08 |
| EP3507384A1 (en) | 2019-07-10 |
| JP2020188816A (ja) | 2020-11-26 |
| JOP20190025A1 (ar) | 2019-02-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20230074781A1 (en) | Methods and composition for the prediction of the activity of enzastaurin | |
| CN102177436B (zh) | 鉴定疾病风险因子的方法 | |
| US20210108266A1 (en) | Method for discovering pharmacogenomic biomarkers | |
| US10954563B2 (en) | Method for predicting the onset of extrapyramidal symptoms (EPS) induced by an antipsychotic-based treatment | |
| US20230193388A1 (en) | Compositions and methods for assessing the efficacy of inhibitors of neurotranmitter transporters | |
| US20240417801A1 (en) | Compositions and methods for assessing the efficacy of polynucleotide delivery and cancer therapy | |
| HK40011257B (en) | Methods and composition for the prediction of the activity of enzastaurin | |
| HK40011257A (en) | Methods and composition for the prediction of the activity of enzastaurin | |
| HK40083504A (en) | Compositions and methods for assessing the efficacy of inhibitors of neurotransmitter transporters |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20190401 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20200825 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20230719 Patent event code: PE09021S01D |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20240131 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20240403 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20240131 Comment text: Notification of reason for refusal Patent event code: PE06011S01I Patent event date: 20230719 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |