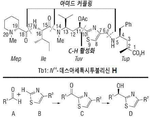
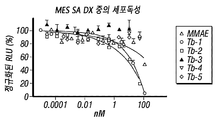
KR20170137725A - 데스아세톡시투불리신 h 및 이의 유사체 - Google Patents
데스아세톡시투불리신 h 및 이의 유사체 Download PDFInfo
- Publication number
- KR20170137725A KR20170137725A KR1020177027116A KR20177027116A KR20170137725A KR 20170137725 A KR20170137725 A KR 20170137725A KR 1020177027116 A KR1020177027116 A KR 1020177027116A KR 20177027116 A KR20177027116 A KR 20177027116A KR 20170137725 A KR20170137725 A KR 20170137725A
- Authority
- KR
- South Korea
- Prior art keywords
- substituted
- cycloalkyl
- alkyl
- groups
- alkanediyl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 150000001875 compounds Chemical class 0.000 claims abstract description 181
- 238000000034 method Methods 0.000 claims abstract description 92
- 238000004519 manufacturing process Methods 0.000 claims abstract description 10
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 6
- -1 amino, hydroxy Chemical group 0.000 claims description 290
- 229910052739 hydrogen Inorganic materials 0.000 claims description 139
- 125000000753 cycloalkyl group Chemical group 0.000 claims description 138
- 239000001257 hydrogen Substances 0.000 claims description 138
- 150000002431 hydrogen Chemical class 0.000 claims description 105
- 125000000217 alkyl group Chemical group 0.000 claims description 104
- 125000004663 dialkyl amino group Chemical group 0.000 claims description 99
- 125000003118 aryl group Chemical group 0.000 claims description 82
- 206010028980 Neoplasm Diseases 0.000 claims description 80
- 125000003282 alkyl amino group Chemical group 0.000 claims description 74
- 125000003545 alkoxy group Chemical group 0.000 claims description 72
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical group N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 claims description 71
- 125000000547 substituted alkyl group Chemical group 0.000 claims description 71
- 239000000203 mixture Substances 0.000 claims description 65
- 125000003368 amide group Chemical group 0.000 claims description 64
- 125000001072 heteroaryl group Chemical group 0.000 claims description 64
- 125000000592 heterocycloalkyl group Chemical group 0.000 claims description 64
- 125000003710 aryl alkyl group Chemical group 0.000 claims description 63
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 58
- 229910052760 oxygen Inorganic materials 0.000 claims description 58
- 239000001301 oxygen Substances 0.000 claims description 58
- 229910052757 nitrogen Inorganic materials 0.000 claims description 54
- 201000011510 cancer Diseases 0.000 claims description 47
- 125000005346 substituted cycloalkyl group Chemical group 0.000 claims description 46
- 125000004475 heteroaralkyl group Chemical group 0.000 claims description 43
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 claims description 42
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 37
- 238000011282 treatment Methods 0.000 claims description 37
- 125000003277 amino group Chemical group 0.000 claims description 35
- 150000003839 salts Chemical class 0.000 claims description 35
- 125000004104 aryloxy group Chemical group 0.000 claims description 34
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 33
- 125000005415 substituted alkoxy group Chemical group 0.000 claims description 33
- 125000004423 acyloxy group Chemical group 0.000 claims description 27
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 26
- 125000001769 aryl amino group Chemical group 0.000 claims description 19
- 125000006310 cycloalkyl amino group Chemical group 0.000 claims description 19
- 238000001959 radiotherapy Methods 0.000 claims description 19
- 238000002560 therapeutic procedure Methods 0.000 claims description 19
- 201000009030 Carcinoma Diseases 0.000 claims description 18
- 125000001691 aryl alkyl amino group Chemical group 0.000 claims description 18
- 201000010099 disease Diseases 0.000 claims description 18
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical group C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 claims description 17
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 claims description 15
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 claims description 15
- 239000003814 drug Substances 0.000 claims description 15
- 125000006239 protecting group Chemical group 0.000 claims description 14
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 13
- 125000002252 acyl group Chemical group 0.000 claims description 12
- 230000004927 fusion Effects 0.000 claims description 12
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 claims description 12
- 238000009169 immunotherapy Methods 0.000 claims description 11
- 206010039491 Sarcoma Diseases 0.000 claims description 9
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 claims description 9
- 238000002512 chemotherapy Methods 0.000 claims description 9
- 201000001441 melanoma Diseases 0.000 claims description 9
- 238000001356 surgical procedure Methods 0.000 claims description 9
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 claims description 8
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 claims description 8
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 claims description 8
- 208000035475 disorder Diseases 0.000 claims description 8
- 208000032839 leukemia Diseases 0.000 claims description 8
- 125000005647 linker group Chemical group 0.000 claims description 8
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 claims description 8
- 206010025323 Lymphomas Diseases 0.000 claims description 7
- 230000000699 topical effect Effects 0.000 claims description 7
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 claims description 6
- 239000005977 Ethylene Substances 0.000 claims description 6
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 6
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 6
- 241000124008 Mammalia Species 0.000 claims description 5
- 210000000481 breast Anatomy 0.000 claims description 5
- 238000001990 intravenous administration Methods 0.000 claims description 5
- 150000002513 isocyanates Chemical class 0.000 claims description 5
- 210000002784 stomach Anatomy 0.000 claims description 5
- 239000000611 antibody drug conjugate Substances 0.000 claims description 4
- 229940049595 antibody-drug conjugate Drugs 0.000 claims description 4
- 210000004369 blood Anatomy 0.000 claims description 4
- 239000008280 blood Substances 0.000 claims description 4
- 210000001072 colon Anatomy 0.000 claims description 4
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 claims description 4
- 210000001035 gastrointestinal tract Anatomy 0.000 claims description 4
- 125000004029 hydroxymethyl group Chemical group [H]OC([H])([H])* 0.000 claims description 4
- 238000007918 intramuscular administration Methods 0.000 claims description 4
- 239000012948 isocyanate Substances 0.000 claims description 4
- 210000003491 skin Anatomy 0.000 claims description 4
- 206010027406 Mesothelioma Diseases 0.000 claims description 3
- 208000034578 Multiple myelomas Diseases 0.000 claims description 3
- 206010035226 Plasma cell myeloma Diseases 0.000 claims description 3
- 210000000988 bone and bone Anatomy 0.000 claims description 3
- 210000004556 brain Anatomy 0.000 claims description 3
- 210000003679 cervix uteri Anatomy 0.000 claims description 3
- 210000003238 esophagus Anatomy 0.000 claims description 3
- 238000007912 intraperitoneal administration Methods 0.000 claims description 3
- 125000003566 oxetanyl group Chemical group 0.000 claims description 3
- 210000000496 pancreas Anatomy 0.000 claims description 3
- 241000894007 species Species 0.000 claims description 3
- 210000001550 testis Anatomy 0.000 claims description 3
- 210000003932 urinary bladder Anatomy 0.000 claims description 3
- 125000004206 2,2,2-trifluoroethyl group Chemical group [H]C([H])(*)C(F)(F)F 0.000 claims description 2
- 125000001255 4-fluorophenyl group Chemical group [H]C1=C([H])C(*)=C([H])C([H])=C1F 0.000 claims description 2
- 239000012190 activator Substances 0.000 claims description 2
- 230000001588 bifunctional effect Effects 0.000 claims description 2
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 claims description 2
- 210000003169 central nervous system Anatomy 0.000 claims description 2
- 238000010511 deprotection reaction Methods 0.000 claims description 2
- 210000004696 endometrium Anatomy 0.000 claims description 2
- 210000000232 gallbladder Anatomy 0.000 claims description 2
- 210000004392 genitalia Anatomy 0.000 claims description 2
- 210000004072 lung Anatomy 0.000 claims description 2
- 210000001672 ovary Anatomy 0.000 claims description 2
- 238000011321 prophylaxis Methods 0.000 claims description 2
- 210000002307 prostate Anatomy 0.000 claims description 2
- 210000000813 small intestine Anatomy 0.000 claims description 2
- 210000000952 spleen Anatomy 0.000 claims description 2
- 125000003107 substituted aryl group Chemical group 0.000 claims description 2
- 125000002053 thietanyl group Chemical group 0.000 claims description 2
- 210000001685 thyroid gland Anatomy 0.000 claims description 2
- 230000037317 transdermal delivery Effects 0.000 claims 8
- 125000004199 4-trifluoromethylphenyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1*)C(F)(F)F 0.000 claims 1
- 150000001728 carbonyl compounds Chemical class 0.000 claims 1
- HCDGVLDPFQMKDK-UHFFFAOYSA-N hexafluoropropylene Chemical group FC(F)=C(F)C(F)(F)F HCDGVLDPFQMKDK-UHFFFAOYSA-N 0.000 claims 1
- 238000001361 intraarterial administration Methods 0.000 claims 1
- 238000007917 intracranial administration Methods 0.000 claims 1
- 210000002200 mouth mucosa Anatomy 0.000 claims 1
- 210000003205 muscle Anatomy 0.000 claims 1
- 210000002850 nasal mucosa Anatomy 0.000 claims 1
- 210000003739 neck Anatomy 0.000 claims 1
- 125000000246 pyrimidin-2-yl group Chemical group [H]C1=NC(*)=NC([H])=C1[H] 0.000 claims 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 abstract description 10
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 abstract description 3
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 222
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 158
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 139
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 127
- 238000005481 NMR spectroscopy Methods 0.000 description 108
- 239000000243 solution Substances 0.000 description 100
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 92
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 88
- 239000000741 silica gel Substances 0.000 description 83
- 229910002027 silica gel Inorganic materials 0.000 description 83
- 235000019439 ethyl acetate Nutrition 0.000 description 75
- 210000004027 cell Anatomy 0.000 description 72
- 239000011541 reaction mixture Substances 0.000 description 71
- 239000011734 sodium Substances 0.000 description 57
- 230000002829 reductive effect Effects 0.000 description 56
- 239000007821 HATU Substances 0.000 description 54
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 51
- 239000002253 acid Substances 0.000 description 48
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 46
- 150000001412 amines Chemical class 0.000 description 44
- 239000003607 modifier Substances 0.000 description 44
- 238000006243 chemical reaction Methods 0.000 description 42
- 229910052799 carbon Inorganic materials 0.000 description 40
- 238000003818 flash chromatography Methods 0.000 description 40
- 238000005033 Fourier transform infrared spectroscopy Methods 0.000 description 39
- 230000015572 biosynthetic process Effects 0.000 description 36
- HMVYYTRDXNKRBQ-UHFFFAOYSA-N 1,3-thiazole-4-carboxylic acid Chemical compound OC(=O)C1=CSC=N1 HMVYYTRDXNKRBQ-UHFFFAOYSA-N 0.000 description 35
- 206010057190 Respiratory tract infections Diseases 0.000 description 35
- 150000001299 aldehydes Chemical class 0.000 description 35
- 238000003786 synthesis reaction Methods 0.000 description 35
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 33
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical compound C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 description 32
- 230000003211 malignant effect Effects 0.000 description 31
- 239000002904 solvent Substances 0.000 description 31
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 30
- 230000008878 coupling Effects 0.000 description 28
- 238000010168 coupling process Methods 0.000 description 28
- 238000005859 coupling reaction Methods 0.000 description 28
- 238000003756 stirring Methods 0.000 description 28
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 27
- JNWGVJCGMNPJHV-UHFFFAOYSA-N bis(1,3-thiazol-2-yl)methanone Chemical compound N=1C=CSC=1C(=O)C1=NC=CS1 JNWGVJCGMNPJHV-UHFFFAOYSA-N 0.000 description 27
- 125000004429 atom Chemical group 0.000 description 26
- KXDHJXZQYSOELW-UHFFFAOYSA-M Carbamate Chemical compound NC([O-])=O KXDHJXZQYSOELW-UHFFFAOYSA-M 0.000 description 24
- 230000000670 limiting effect Effects 0.000 description 24
- 125000003088 (fluoren-9-ylmethoxy)carbonyl group Chemical group 0.000 description 23
- 125000004432 carbon atom Chemical group C* 0.000 description 23
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 23
- 239000007787 solid Substances 0.000 description 23
- 239000012230 colorless oil Substances 0.000 description 21
- ZVQXQPNJHRNGID-UHFFFAOYSA-N tetramethylsuccinonitrile Chemical compound N#CC(C)(C)C(C)(C)C#N ZVQXQPNJHRNGID-UHFFFAOYSA-N 0.000 description 21
- PEZNEXFPRSOYPL-UHFFFAOYSA-N (bis(trifluoroacetoxy)iodo)benzene Chemical compound FC(F)(F)C(=O)OI(OC(=O)C(F)(F)F)C1=CC=CC=C1 PEZNEXFPRSOYPL-UHFFFAOYSA-N 0.000 description 20
- OAYLNYINCPYISS-UHFFFAOYSA-N ethyl acetate;hexane Chemical compound CCCCCC.CCOC(C)=O OAYLNYINCPYISS-UHFFFAOYSA-N 0.000 description 20
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 20
- 239000012267 brine Substances 0.000 description 19
- 239000003153 chemical reaction reagent Substances 0.000 description 19
- 239000003795 chemical substances by application Substances 0.000 description 19
- 125000004122 cyclic group Chemical group 0.000 description 19
- 229920006395 saturated elastomer Polymers 0.000 description 19
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 19
- KRHYYFGTRYWZRS-UHFFFAOYSA-M Fluoride anion Chemical compound [F-] KRHYYFGTRYWZRS-UHFFFAOYSA-M 0.000 description 18
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 18
- 230000008685 targeting Effects 0.000 description 18
- 150000001408 amides Chemical class 0.000 description 17
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 17
- ORQVYLFICNWZHI-CMPLNLGQSA-N methyl (2s,4r)-4-amino-2-methyl-5-phenylpentanoate Chemical compound COC(=O)[C@@H](C)C[C@@H](N)CC1=CC=CC=C1 ORQVYLFICNWZHI-CMPLNLGQSA-N 0.000 description 17
- 150000004702 methyl esters Chemical class 0.000 description 17
- BKOOMYPCSUNDGP-UHFFFAOYSA-N 2-methylbut-2-ene Chemical compound CC=C(C)C BKOOMYPCSUNDGP-UHFFFAOYSA-N 0.000 description 16
- NVOZDFQRRUJDTA-DJJJIMSYSA-N 9h-fluoren-9-ylmethyl n-[(2s,3s)-1-fluoro-3-methyl-1-oxopentan-2-yl]carbamate Chemical compound C1=CC=C2C(COC(=O)N[C@@H]([C@@H](C)CC)C(F)=O)C3=CC=CC=C3C2=C1 NVOZDFQRRUJDTA-DJJJIMSYSA-N 0.000 description 16
- 125000004093 cyano group Chemical group *C#N 0.000 description 16
- 108090000765 processed proteins & peptides Proteins 0.000 description 16
- WSLDOOZREJYCGB-UHFFFAOYSA-N 1,2-Dichloroethane Chemical compound ClCCCl WSLDOOZREJYCGB-UHFFFAOYSA-N 0.000 description 15
- 125000001246 bromo group Chemical group Br* 0.000 description 15
- 150000002576 ketones Chemical class 0.000 description 15
- 239000012044 organic layer Substances 0.000 description 15
- 238000010992 reflux Methods 0.000 description 15
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 14
- 125000001931 aliphatic group Chemical group 0.000 description 14
- 238000009472 formulation Methods 0.000 description 14
- 239000010410 layer Substances 0.000 description 14
- SUKJFIGYRHOWBL-UHFFFAOYSA-N sodium hypochlorite Chemical compound [Na+].Cl[O-] SUKJFIGYRHOWBL-UHFFFAOYSA-N 0.000 description 14
- 208000009956 adenocarcinoma Diseases 0.000 description 13
- FUZZWVXGSFPDMH-UHFFFAOYSA-N hexanoic acid Chemical compound CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 13
- 108090000623 proteins and genes Proteins 0.000 description 13
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 12
- 239000004480 active ingredient Substances 0.000 description 12
- 229940079593 drug Drugs 0.000 description 12
- 230000005855 radiation Effects 0.000 description 12
- 108010016626 Dipeptides Proteins 0.000 description 11
- 102000004169 proteins and genes Human genes 0.000 description 11
- 238000000746 purification Methods 0.000 description 11
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 10
- 239000000427 antigen Substances 0.000 description 10
- 108091007433 antigens Proteins 0.000 description 10
- 102000036639 antigens Human genes 0.000 description 10
- OEYIOHPDSNJKLS-UHFFFAOYSA-N choline Chemical compound C[N+](C)(C)CCO OEYIOHPDSNJKLS-UHFFFAOYSA-N 0.000 description 10
- 229960001231 choline Drugs 0.000 description 10
- 125000004433 nitrogen atom Chemical class N* 0.000 description 10
- FPGGTKZVZWFYPV-UHFFFAOYSA-M tetrabutylammonium fluoride Chemical compound [F-].CCCC[N+](CCCC)(CCCC)CCCC FPGGTKZVZWFYPV-UHFFFAOYSA-M 0.000 description 10
- 210000001519 tissue Anatomy 0.000 description 10
- QCHPKSFMDHPSNR-UHFFFAOYSA-N 3-aminoisobutyric acid Chemical compound NCC(C)C(O)=O QCHPKSFMDHPSNR-UHFFFAOYSA-N 0.000 description 9
- 102000004127 Cytokines Human genes 0.000 description 9
- 108090000695 Cytokines Proteins 0.000 description 9
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 9
- 239000007864 aqueous solution Substances 0.000 description 9
- 150000001721 carbon Chemical class 0.000 description 9
- CSJLBAMHHLJAAS-UHFFFAOYSA-N diethylaminosulfur trifluoride Chemical compound CCN(CC)S(F)(F)F CSJLBAMHHLJAAS-UHFFFAOYSA-N 0.000 description 9
- 239000000284 extract Substances 0.000 description 9
- 238000002360 preparation method Methods 0.000 description 9
- 230000001225 therapeutic effect Effects 0.000 description 9
- 210000004881 tumor cell Anatomy 0.000 description 9
- FPIRBHDGWMWJEP-UHFFFAOYSA-N 1-hydroxy-7-azabenzotriazole Chemical compound C1=CN=C2N(O)N=NC2=C1 FPIRBHDGWMWJEP-UHFFFAOYSA-N 0.000 description 8
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 8
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 8
- 108010002350 Interleukin-2 Proteins 0.000 description 8
- 102000000588 Interleukin-2 Human genes 0.000 description 8
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 8
- 239000003242 anti bacterial agent Substances 0.000 description 8
- 239000002585 base Substances 0.000 description 8
- 239000006185 dispersion Substances 0.000 description 8
- 230000003463 hyperproliferative effect Effects 0.000 description 8
- 239000003112 inhibitor Substances 0.000 description 8
- 229910052751 metal Inorganic materials 0.000 description 8
- 239000002184 metal Substances 0.000 description 8
- GDOPTJXRTPNYNR-UHFFFAOYSA-N methyl-cyclopentane Natural products CC1CCCC1 GDOPTJXRTPNYNR-UHFFFAOYSA-N 0.000 description 8
- 229940002612 prodrug Drugs 0.000 description 8
- 239000000651 prodrug Substances 0.000 description 8
- 229910052717 sulfur Inorganic materials 0.000 description 8
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 7
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 7
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 7
- FFDGPVCHZBVARC-UHFFFAOYSA-N N,N-dimethylglycine Chemical compound CN(C)CC(O)=O FFDGPVCHZBVARC-UHFFFAOYSA-N 0.000 description 7
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 7
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 7
- 150000001336 alkenes Chemical class 0.000 description 7
- 125000003342 alkenyl group Chemical group 0.000 description 7
- 125000000304 alkynyl group Chemical group 0.000 description 7
- 125000000539 amino acid group Chemical group 0.000 description 7
- 150000001413 amino acids Chemical class 0.000 description 7
- 125000001584 benzyloxycarbonyl group Chemical group C(=O)(OCC1=CC=CC=C1)* 0.000 description 7
- 230000006378 damage Effects 0.000 description 7
- 230000000694 effects Effects 0.000 description 7
- 229960000310 isoleucine Drugs 0.000 description 7
- 239000007788 liquid Substances 0.000 description 7
- 239000003550 marker Substances 0.000 description 7
- 239000012071 phase Substances 0.000 description 7
- 230000008569 process Effects 0.000 description 7
- 230000009467 reduction Effects 0.000 description 7
- 125000006413 ring segment Chemical group 0.000 description 7
- 239000011593 sulfur Substances 0.000 description 7
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 7
- 238000004809 thin layer chromatography Methods 0.000 description 7
- WFDIJRYMOXRFFG-UHFFFAOYSA-N Acetic anhydride Chemical compound CC(=O)OC(C)=O WFDIJRYMOXRFFG-UHFFFAOYSA-N 0.000 description 6
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 6
- XFXPMWWXUTWYJX-UHFFFAOYSA-N Cyanide Chemical compound N#[C-] XFXPMWWXUTWYJX-UHFFFAOYSA-N 0.000 description 6
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 6
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 6
- 108010017080 Granulocyte Colony-Stimulating Factor Proteins 0.000 description 6
- 102000004269 Granulocyte Colony-Stimulating Factor Human genes 0.000 description 6
- SJRJJKPEHAURKC-UHFFFAOYSA-N N-Methylmorpholine Chemical compound CN1CCOCC1 SJRJJKPEHAURKC-UHFFFAOYSA-N 0.000 description 6
- 150000001335 aliphatic alkanes Chemical class 0.000 description 6
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 6
- 238000003776 cleavage reaction Methods 0.000 description 6
- 238000000576 coating method Methods 0.000 description 6
- 150000002148 esters Chemical class 0.000 description 6
- 239000012634 fragment Substances 0.000 description 6
- 125000000524 functional group Chemical group 0.000 description 6
- 230000001965 increasing effect Effects 0.000 description 6
- SNHMUERNLJLMHN-UHFFFAOYSA-N iodobenzene Chemical compound IC1=CC=CC=C1 SNHMUERNLJLMHN-UHFFFAOYSA-N 0.000 description 6
- 230000003647 oxidation Effects 0.000 description 6
- 238000007254 oxidation reaction Methods 0.000 description 6
- 102000004196 processed proteins & peptides Human genes 0.000 description 6
- 230000007017 scission Effects 0.000 description 6
- 125000003396 thiol group Chemical class [H]S* 0.000 description 6
- GVNVAWHJIKLAGL-UHFFFAOYSA-N 2-(cyclohexen-1-yl)cyclohexan-1-one Chemical compound O=C1CCCCC1C1=CCCCC1 GVNVAWHJIKLAGL-UHFFFAOYSA-N 0.000 description 5
- BYHDDXPKOZIZRV-UHFFFAOYSA-N 5-phenylpentanoic acid Chemical compound OC(=O)CCCCC1=CC=CC=C1 BYHDDXPKOZIZRV-UHFFFAOYSA-N 0.000 description 5
- 108010017987 CD30 Ligand Proteins 0.000 description 5
- 101150065749 Churc1 gene Proteins 0.000 description 5
- 108090000978 Interleukin-4 Proteins 0.000 description 5
- 102000004388 Interleukin-4 Human genes 0.000 description 5
- WMFOQBRAJBCJND-UHFFFAOYSA-M Lithium hydroxide Chemical compound [Li+].[OH-] WMFOQBRAJBCJND-UHFFFAOYSA-M 0.000 description 5
- 102100038239 Protein Churchill Human genes 0.000 description 5
- 102100032100 Tumor necrosis factor ligand superfamily member 8 Human genes 0.000 description 5
- 238000006640 acetylation reaction Methods 0.000 description 5
- 230000009471 action Effects 0.000 description 5
- 230000004913 activation Effects 0.000 description 5
- 125000006242 amine protecting group Chemical group 0.000 description 5
- 229940024606 amino acid Drugs 0.000 description 5
- 238000004458 analytical method Methods 0.000 description 5
- 229940088710 antibiotic agent Drugs 0.000 description 5
- 239000002246 antineoplastic agent Substances 0.000 description 5
- 239000000562 conjugate Substances 0.000 description 5
- 150000001924 cycloalkanes Chemical class 0.000 description 5
- 125000000392 cycloalkenyl group Chemical group 0.000 description 5
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- GTCAXTIRRLKXRU-UHFFFAOYSA-N methyl carbamate Chemical compound COC(N)=O GTCAXTIRRLKXRU-UHFFFAOYSA-N 0.000 description 5
- 238000011275 oncology therapy Methods 0.000 description 5
- 239000000047 product Substances 0.000 description 5
- 125000000472 sulfonyl group Chemical group *S(*)(=O)=O 0.000 description 5
- 150000004992 toluidines Chemical class 0.000 description 5
- 238000006257 total synthesis reaction Methods 0.000 description 5
- 125000000026 trimethylsilyl group Chemical group [H]C([H])([H])[Si]([*])(C([H])([H])[H])C([H])([H])[H] 0.000 description 5
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 4
- XZMCDFZZKTWFGF-UHFFFAOYSA-N Cyanamide Chemical compound NC#N XZMCDFZZKTWFGF-UHFFFAOYSA-N 0.000 description 4
- 108020004414 DNA Proteins 0.000 description 4
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 4
- RRSNDVCODIMOFX-MPKOGUQCSA-N Fc1c(Cl)cccc1[C@H]1[C@@H](NC2(CCCCC2)[C@@]11C(=O)Nc2cc(Cl)ccc12)C(=O)Nc1ccc(cc1)C(=O)NCCCCCc1cccc2C(=O)N(Cc12)C1CCC(=O)NC1=O Chemical compound Fc1c(Cl)cccc1[C@H]1[C@@H](NC2(CCCCC2)[C@@]11C(=O)Nc2cc(Cl)ccc12)C(=O)Nc1ccc(cc1)C(=O)NCCCCCc1cccc2C(=O)N(Cc12)C1CCC(=O)NC1=O RRSNDVCODIMOFX-MPKOGUQCSA-N 0.000 description 4
- 206010029260 Neuroblastoma Diseases 0.000 description 4
- DTQVDTLACAAQTR-UHFFFAOYSA-M Trifluoroacetate Chemical compound [O-]C(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-M 0.000 description 4
- 238000010521 absorption reaction Methods 0.000 description 4
- 230000021736 acetylation Effects 0.000 description 4
- 230000006907 apoptotic process Effects 0.000 description 4
- 230000001580 bacterial effect Effects 0.000 description 4
- 230000008901 benefit Effects 0.000 description 4
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 4
- HQCVEGNPQFRFPC-UHFFFAOYSA-N carboxy acetate Chemical compound CC(=O)OC(O)=O HQCVEGNPQFRFPC-UHFFFAOYSA-N 0.000 description 4
- 239000011248 coating agent Substances 0.000 description 4
- 229940127089 cytotoxic agent Drugs 0.000 description 4
- 150000002009 diols Chemical class 0.000 description 4
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N diphenyl Chemical compound C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 4
- 239000002612 dispersion medium Substances 0.000 description 4
- OVBPIULPVIDEAO-LBPRGKRZSA-N folic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-LBPRGKRZSA-N 0.000 description 4
- 230000012010 growth Effects 0.000 description 4
- 150000002440 hydroxy compounds Chemical class 0.000 description 4
- 229940121354 immunomodulator Drugs 0.000 description 4
- 238000002347 injection Methods 0.000 description 4
- 239000007924 injection Substances 0.000 description 4
- 125000001160 methoxycarbonyl group Chemical group [H]C([H])([H])OC(*)=O 0.000 description 4
- KUWWRNNYEYGSBQ-UHFFFAOYSA-N methyl 1,3-thiazole-4-carboxylate Chemical compound COC(=O)C1=CSC=N1 KUWWRNNYEYGSBQ-UHFFFAOYSA-N 0.000 description 4
- 150000002894 organic compounds Chemical class 0.000 description 4
- SIOXPEMLGUPBBT-UHFFFAOYSA-N picolinic acid Chemical compound OC(=O)C1=CC=CC=N1 SIOXPEMLGUPBBT-UHFFFAOYSA-N 0.000 description 4
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 4
- 229920001223 polyethylene glycol Polymers 0.000 description 4
- 229920001184 polypeptide Polymers 0.000 description 4
- 239000011780 sodium chloride Substances 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- 125000004495 thiazol-4-yl group Chemical group S1C=NC(=C1)* 0.000 description 4
- 125000000335 thiazolyl group Chemical group 0.000 description 4
- WYWHKKSPHMUBEB-UHFFFAOYSA-N tioguanine Chemical compound N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 4
- UFDULEKOJAEIRI-UHFFFAOYSA-N (2-acetyloxy-3-iodophenyl) acetate Chemical group CC(=O)OC1=CC=CC(I)=C1OC(C)=O UFDULEKOJAEIRI-UHFFFAOYSA-N 0.000 description 3
- YQTCQNIPQMJNTI-UHFFFAOYSA-N 2,2-dimethylpropan-1-one Chemical group CC(C)(C)[C]=O YQTCQNIPQMJNTI-UHFFFAOYSA-N 0.000 description 3
- 125000000022 2-aminoethyl group Chemical group [H]C([*])([H])C([H])([H])N([H])[H] 0.000 description 3
- LPTQVFNYTBMJED-UHFFFAOYSA-N 3-[methyl(phenylmethoxycarbonyl)amino]bicyclo[1.1.1]pentane-1-carboxylic acid Chemical compound C(C1=CC=CC=C1)OC(=O)N(C12CC(C1)(C2)C(=O)O)C LPTQVFNYTBMJED-UHFFFAOYSA-N 0.000 description 3
- OOVJVOUDUZUOGM-UHFFFAOYSA-N 3-[methyl-[(2-methylpropan-2-yl)oxycarbonyl]amino]bicyclo[1.1.1]pentane-1-carboxylic acid Chemical compound C(C)(C)(C)OC(=O)N(C12CC(C1)(C2)C(=O)O)C OOVJVOUDUZUOGM-UHFFFAOYSA-N 0.000 description 3
- 125000002672 4-bromobenzoyl group Chemical group BrC1=CC=C(C(=O)*)C=C1 0.000 description 3
- 125000000242 4-chlorobenzoyl group Chemical group ClC1=CC=C(C(=O)*)C=C1 0.000 description 3
- QMHIMXFNBOYPND-UHFFFAOYSA-N 4-methylthiazole Chemical compound CC1=CSC=N1 QMHIMXFNBOYPND-UHFFFAOYSA-N 0.000 description 3
- 102100031585 ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 Human genes 0.000 description 3
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- 206010003571 Astrocytoma Diseases 0.000 description 3
- WVDDGKGOMKODPV-UHFFFAOYSA-N Benzyl alcohol Chemical compound OCC1=CC=CC=C1 WVDDGKGOMKODPV-UHFFFAOYSA-N 0.000 description 3
- 201000000274 Carcinosarcoma Diseases 0.000 description 3
- 102000000844 Cell Surface Receptors Human genes 0.000 description 3
- 108010001857 Cell Surface Receptors Proteins 0.000 description 3
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 3
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 3
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 3
- 101000777636 Homo sapiens ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 Proteins 0.000 description 3
- 206010020843 Hyperthermia Diseases 0.000 description 3
- 102000000589 Interleukin-1 Human genes 0.000 description 3
- 108010002352 Interleukin-1 Proteins 0.000 description 3
- 108090001005 Interleukin-6 Proteins 0.000 description 3
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 3
- 102000004058 Leukemia inhibitory factor Human genes 0.000 description 3
- 108090000581 Leukemia inhibitory factor Proteins 0.000 description 3
- 241001465754 Metazoa Species 0.000 description 3
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 3
- 101710151805 Mitochondrial intermediate peptidase 1 Proteins 0.000 description 3
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 3
- 229930012538 Paclitaxel Natural products 0.000 description 3
- 239000002202 Polyethylene glycol Substances 0.000 description 3
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 3
- 229920002472 Starch Polymers 0.000 description 3
- FOCVUCIESVLUNU-UHFFFAOYSA-N Thiotepa Chemical compound C1CN1P(N1CC1)(=S)N1CC1 FOCVUCIESVLUNU-UHFFFAOYSA-N 0.000 description 3
- SORGEQQSQGNZFI-UHFFFAOYSA-N [azido(phenoxy)phosphoryl]oxybenzene Chemical compound C=1C=CC=CC=1OP(=O)(N=[N+]=[N-])OC1=CC=CC=C1 SORGEQQSQGNZFI-UHFFFAOYSA-N 0.000 description 3
- 125000002015 acyclic group Chemical group 0.000 description 3
- 125000005076 adamantyloxycarbonyl group Chemical group C12(CC3CC(CC(C1)C3)C2)OC(=O)* 0.000 description 3
- 235000010443 alginic acid Nutrition 0.000 description 3
- 229920000615 alginic acid Polymers 0.000 description 3
- 150000004703 alkoxides Chemical class 0.000 description 3
- 150000003862 amino acid derivatives Chemical class 0.000 description 3
- 230000000259 anti-tumor effect Effects 0.000 description 3
- 150000004945 aromatic hydrocarbons Chemical class 0.000 description 3
- 125000003236 benzoyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C(*)=O 0.000 description 3
- ORCXPYPDHLVQFP-UHFFFAOYSA-N bicyclo[1.1.1]pentane-3-carboxylic acid Chemical compound C1C2CC1(C(=O)O)C2 ORCXPYPDHLVQFP-UHFFFAOYSA-N 0.000 description 3
- 239000011230 binding agent Substances 0.000 description 3
- 239000000872 buffer Substances 0.000 description 3
- CREMABGTGYGIQB-UHFFFAOYSA-N carbon carbon Chemical compound C.C CREMABGTGYGIQB-UHFFFAOYSA-N 0.000 description 3
- 230000021164 cell adhesion Effects 0.000 description 3
- 230000004663 cell proliferation Effects 0.000 description 3
- 208000035250 cutaneous malignant susceptibility to 1 melanoma Diseases 0.000 description 3
- NZNMSOFKMUBTKW-UHFFFAOYSA-N cyclohexanecarboxylic acid Chemical compound OC(=O)C1CCCCC1 NZNMSOFKMUBTKW-UHFFFAOYSA-N 0.000 description 3
- 229960004397 cyclophosphamide Drugs 0.000 description 3
- 238000002784 cytotoxicity assay Methods 0.000 description 3
- 231100000263 cytotoxicity test Toxicity 0.000 description 3
- 230000006196 deacetylation Effects 0.000 description 3
- 238000003381 deacetylation reaction Methods 0.000 description 3
- 230000034994 death Effects 0.000 description 3
- 238000005828 desilylation reaction Methods 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 230000018109 developmental process Effects 0.000 description 3
- GLUUGHFHXGJENI-UHFFFAOYSA-N diethylenediamine Natural products C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 3
- 108700003601 dimethylglycine Proteins 0.000 description 3
- 239000002552 dosage form Substances 0.000 description 3
- 239000003937 drug carrier Substances 0.000 description 3
- 239000012636 effector Substances 0.000 description 3
- 238000000132 electrospray ionisation Methods 0.000 description 3
- 125000003754 ethoxycarbonyl group Chemical group C(=O)(OCC)* 0.000 description 3
- 229960002949 fluorouracil Drugs 0.000 description 3
- 125000002485 formyl group Chemical group [H]C(*)=O 0.000 description 3
- 238000001415 gene therapy Methods 0.000 description 3
- 208000005017 glioblastoma Diseases 0.000 description 3
- 235000011187 glycerol Nutrition 0.000 description 3
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 3
- 125000005549 heteroarylene group Chemical group 0.000 description 3
- 239000005556 hormone Substances 0.000 description 3
- 229940088597 hormone Drugs 0.000 description 3
- 150000002429 hydrazines Chemical class 0.000 description 3
- 230000036031 hyperthermia Effects 0.000 description 3
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 3
- 239000002955 immunomodulating agent Substances 0.000 description 3
- 238000001802 infusion Methods 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 125000000741 isoleucyl group Chemical group [H]N([H])C(C(C([H])([H])[H])C([H])([H])C([H])([H])[H])C(=O)O* 0.000 description 3
- 239000007951 isotonicity adjuster Substances 0.000 description 3
- 210000004698 lymphocyte Anatomy 0.000 description 3
- 230000036210 malignancy Effects 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 229960000485 methotrexate Drugs 0.000 description 3
- 239000008108 microcrystalline cellulose Substances 0.000 description 3
- 229940016286 microcrystalline cellulose Drugs 0.000 description 3
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 3
- 239000002480 mineral oil Substances 0.000 description 3
- 235000010446 mineral oil Nutrition 0.000 description 3
- 229940078490 n,n-dimethylglycine Drugs 0.000 description 3
- QAPTWHXHEYAIKG-RCOXNQKVSA-N n-[(1r,2s,5r)-5-(tert-butylamino)-2-[(3s)-2-oxo-3-[[6-(trifluoromethyl)quinazolin-4-yl]amino]pyrrolidin-1-yl]cyclohexyl]acetamide Chemical compound CC(=O)N[C@@H]1C[C@H](NC(C)(C)C)CC[C@@H]1N1C(=O)[C@@H](NC=2C3=CC(=CC=C3N=CN=2)C(F)(F)F)CC1 QAPTWHXHEYAIKG-RCOXNQKVSA-N 0.000 description 3
- 125000006574 non-aromatic ring group Chemical group 0.000 description 3
- 239000003921 oil Substances 0.000 description 3
- 235000019198 oils Nutrition 0.000 description 3
- 201000008968 osteosarcoma Diseases 0.000 description 3
- 125000004430 oxygen atom Chemical group O* 0.000 description 3
- 125000003232 p-nitrobenzoyl group Chemical group [N+](=O)([O-])C1=CC=C(C(=O)*)C=C1 0.000 description 3
- 229960001592 paclitaxel Drugs 0.000 description 3
- 208000004019 papillary adenocarcinoma Diseases 0.000 description 3
- 125000006678 phenoxycarbonyl group Chemical group 0.000 description 3
- UYWQUFXKFGHYNT-UHFFFAOYSA-N phenylmethyl ester of formic acid Natural products O=COCC1=CC=CC=C1 UYWQUFXKFGHYNT-UHFFFAOYSA-N 0.000 description 3
- 125000003170 phenylsulfonyl group Chemical group C1(=CC=CC=C1)S(=O)(=O)* 0.000 description 3
- 125000003367 polycyclic group Chemical group 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 150000003138 primary alcohols Chemical class 0.000 description 3
- 125000001501 propionyl group Chemical group O=C([*])C([H])([H])C([H])([H])[H] 0.000 description 3
- 239000002510 pyrogen Substances 0.000 description 3
- 230000009257 reactivity Effects 0.000 description 3
- HXCHCVDVKSCDHU-PJKCJEBCSA-N s-[(2r,3s,4s,6s)-6-[[(2r,3s,4s,5r,6r)-5-[(2s,4s,5s)-5-(ethylamino)-4-methoxyoxan-2-yl]oxy-4-hydroxy-6-[[(2s,5z,9r,13e)-9-hydroxy-12-(methoxycarbonylamino)-13-[2-(methyltrisulfanyl)ethylidene]-11-oxo-2-bicyclo[7.3.1]trideca-1(12),5-dien-3,7-diynyl]oxy]-2-m Chemical compound C1[C@H](OC)[C@@H](NCC)CO[C@H]1O[C@H]1[C@H](O[C@@H]2C\3=C(NC(=O)OC)C(=O)C[C@@](C/3=C/CSSSC)(O)C#C\C=C/C#C2)O[C@H](C)[C@@H](NO[C@@H]2O[C@H](C)[C@@H](SC(=O)C=3C(=C(OC)C(O[C@H]4[C@@H]([C@H](OC)[C@@H](O)[C@H](C)O4)O)=C(I)C=3C)OC)[C@@H](O)C2)[C@@H]1O HXCHCVDVKSCDHU-PJKCJEBCSA-N 0.000 description 3
- 150000003333 secondary alcohols Chemical class 0.000 description 3
- 125000003808 silyl group Chemical group [H][Si]([H])([H])[*] 0.000 description 3
- MNWBNISUBARLIT-UHFFFAOYSA-N sodium cyanide Chemical compound [Na+].N#[C-] MNWBNISUBARLIT-UHFFFAOYSA-N 0.000 description 3
- 239000012453 solvate Substances 0.000 description 3
- 239000008107 starch Substances 0.000 description 3
- 235000019698 starch Nutrition 0.000 description 3
- 238000007920 subcutaneous administration Methods 0.000 description 3
- 235000000346 sugar Nutrition 0.000 description 3
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 3
- 125000005931 tert-butyloxycarbonyl group Chemical group [H]C([H])([H])C(OC(*)=O)(C([H])([H])[H])C([H])([H])[H] 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- 239000000606 toothpaste Substances 0.000 description 3
- 239000003053 toxin Substances 0.000 description 3
- 231100000765 toxin Toxicity 0.000 description 3
- 108700012359 toxins Proteins 0.000 description 3
- 125000002221 trityl group Chemical group [H]C1=C([H])C([H])=C([H])C([H])=C1C([*])(C1=C(C(=C(C(=C1[H])[H])[H])[H])[H])C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 description 3
- 239000003039 volatile agent Substances 0.000 description 3
- GHYOCDFICYLMRF-UTIIJYGPSA-N (2S,3R)-N-[(2S)-3-(cyclopenten-1-yl)-1-[(2R)-2-methyloxiran-2-yl]-1-oxopropan-2-yl]-3-hydroxy-3-(4-methoxyphenyl)-2-[[(2S)-2-[(2-morpholin-4-ylacetyl)amino]propanoyl]amino]propanamide Chemical compound C1(=CCCC1)C[C@@H](C(=O)[C@@]1(OC1)C)NC([C@H]([C@@H](C1=CC=C(C=C1)OC)O)NC([C@H](C)NC(CN1CCOCC1)=O)=O)=O GHYOCDFICYLMRF-UTIIJYGPSA-N 0.000 description 2
- LNAZSHAWQACDHT-XIYTZBAFSA-N (2r,3r,4s,5r,6s)-4,5-dimethoxy-2-(methoxymethyl)-3-[(2s,3r,4s,5r,6r)-3,4,5-trimethoxy-6-(methoxymethyl)oxan-2-yl]oxy-6-[(2r,3r,4s,5r,6r)-4,5,6-trimethoxy-2-(methoxymethyl)oxan-3-yl]oxyoxane Chemical compound CO[C@@H]1[C@@H](OC)[C@H](OC)[C@@H](COC)O[C@H]1O[C@H]1[C@H](OC)[C@@H](OC)[C@H](O[C@H]2[C@@H]([C@@H](OC)[C@H](OC)O[C@@H]2COC)OC)O[C@@H]1COC LNAZSHAWQACDHT-XIYTZBAFSA-N 0.000 description 2
- SZXBQTSZISFIAO-ZETCQYMHSA-N (2s)-3-methyl-2-[(2-methylpropan-2-yl)oxycarbonylamino]butanoic acid Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)OC(C)(C)C SZXBQTSZISFIAO-ZETCQYMHSA-N 0.000 description 2
- LXRCSRYYCAEDRJ-UHFFFAOYSA-N (6-bromopyridin-2-yl)methoxy-tert-butyl-dimethylsilane Chemical compound CC(C)(C)[Si](C)(C)OCC1=CC=CC(Br)=N1 LXRCSRYYCAEDRJ-UHFFFAOYSA-N 0.000 description 2
- QRDAPCMJAOQZSU-KQQUZDAGSA-N (e)-3-[4-[(e)-3-(3-fluorophenyl)-3-oxoprop-1-enyl]-1-methylpyrrol-2-yl]-n-hydroxyprop-2-enamide Chemical compound C1=C(\C=C\C(=O)NO)N(C)C=C1\C=C\C(=O)C1=CC=CC(F)=C1 QRDAPCMJAOQZSU-KQQUZDAGSA-N 0.000 description 2
- WQVOUANKVCYEIQ-UHFFFAOYSA-N 1,3-thiazol-4-ylmethanol Chemical compound OCC1=CSC=N1 WQVOUANKVCYEIQ-UHFFFAOYSA-N 0.000 description 2
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 2
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 2
- UTQNKKSJPHTPBS-UHFFFAOYSA-N 2,2,2-trichloroethanone Chemical group ClC(Cl)(Cl)[C]=O UTQNKKSJPHTPBS-UHFFFAOYSA-N 0.000 description 2
- GQHTUMJGOHRCHB-UHFFFAOYSA-N 2,3,4,6,7,8,9,10-octahydropyrimido[1,2-a]azepine Chemical compound C1CCCCN2CCCN=C21 GQHTUMJGOHRCHB-UHFFFAOYSA-N 0.000 description 2
- IZHVBANLECCAGF-UHFFFAOYSA-N 2-hydroxy-3-(octadecanoyloxy)propyl octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)COC(=O)CCCCCCCCCCCCCCCCC IZHVBANLECCAGF-UHFFFAOYSA-N 0.000 description 2
- LPUUYZVKCMCHLO-UHFFFAOYSA-N 4,5,6,7-tetrachloroisoindole-1,3-dione Chemical compound ClC1=C(Cl)C(Cl)=C2C(=O)NC(=O)C2=C1Cl LPUUYZVKCMCHLO-UHFFFAOYSA-N 0.000 description 2
- YMDNAUSNGZAGNY-UHFFFAOYSA-N 4-(methoxymethoxymethyl)-1,3-thiazole Chemical compound COCOCC=1N=CSC=1 YMDNAUSNGZAGNY-UHFFFAOYSA-N 0.000 description 2
- JSEIUBRZXQHQKH-UHFFFAOYSA-N 4-(oxan-2-yloxymethyl)-1,3-thiazole Chemical compound O1C(CCCC1)OCC=1N=CSC=1 JSEIUBRZXQHQKH-UHFFFAOYSA-N 0.000 description 2
- GREAZUDNBWZNCK-UHFFFAOYSA-N 4-(phenylmethoxymethyl)-1,3-thiazole Chemical compound C(C1=CC=CC=C1)OCC=1N=CSC=1 GREAZUDNBWZNCK-UHFFFAOYSA-N 0.000 description 2
- VHYFNPMBLIVWCW-UHFFFAOYSA-N 4-Dimethylaminopyridine Chemical compound CN(C)C1=CC=NC=C1 VHYFNPMBLIVWCW-UHFFFAOYSA-N 0.000 description 2
- 201000003076 Angiosarcoma Diseases 0.000 description 2
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 2
- 108010046080 CD27 Ligand Proteins 0.000 description 2
- 101150013553 CD40 gene Proteins 0.000 description 2
- 102100025221 CD70 antigen Human genes 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- KLWPJMFMVPTNCC-UHFFFAOYSA-N Camptothecin Natural products CCC1(O)C(=O)OCC2=C1C=C3C4Nc5ccccc5C=C4CN3C2=O KLWPJMFMVPTNCC-UHFFFAOYSA-N 0.000 description 2
- 208000005243 Chondrosarcoma Diseases 0.000 description 2
- RGSFGYAAUTVSQA-UHFFFAOYSA-N Cyclopentane Chemical compound C1CCCC1 RGSFGYAAUTVSQA-UHFFFAOYSA-N 0.000 description 2
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 2
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 2
- 108010092160 Dactinomycin Proteins 0.000 description 2
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 2
- 102000003951 Erythropoietin Human genes 0.000 description 2
- 108090000394 Erythropoietin Proteins 0.000 description 2
- 239000001856 Ethyl cellulose Substances 0.000 description 2
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 2
- JOYRKODLDBILNP-UHFFFAOYSA-N Ethyl urethane Chemical compound CCOC(N)=O JOYRKODLDBILNP-UHFFFAOYSA-N 0.000 description 2
- 208000006168 Ewing Sarcoma Diseases 0.000 description 2
- 201000008808 Fibrosarcoma Diseases 0.000 description 2
- 108010010803 Gelatin Proteins 0.000 description 2
- 206010018338 Glioma Diseases 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- 208000005234 Granulosa Cell Tumor Diseases 0.000 description 2
- 208000001258 Hemangiosarcoma Diseases 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 101000914484 Homo sapiens T-lymphocyte activation antigen CD80 Proteins 0.000 description 2
- 101000800116 Homo sapiens Thy-1 membrane glycoprotein Proteins 0.000 description 2
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 2
- 108060003951 Immunoglobulin Proteins 0.000 description 2
- 108090000467 Interferon-beta Proteins 0.000 description 2
- 102000014150 Interferons Human genes 0.000 description 2
- 108010050904 Interferons Proteins 0.000 description 2
- 102000013462 Interleukin-12 Human genes 0.000 description 2
- 108010065805 Interleukin-12 Proteins 0.000 description 2
- 108090000172 Interleukin-15 Proteins 0.000 description 2
- 102000003812 Interleukin-15 Human genes 0.000 description 2
- 102000003810 Interleukin-18 Human genes 0.000 description 2
- 108090000171 Interleukin-18 Proteins 0.000 description 2
- 108010002616 Interleukin-5 Proteins 0.000 description 2
- 102100039897 Interleukin-5 Human genes 0.000 description 2
- 102000004889 Interleukin-6 Human genes 0.000 description 2
- 108090001007 Interleukin-8 Proteins 0.000 description 2
- 229930182844 L-isoleucine Natural products 0.000 description 2
- 208000018142 Leiomyosarcoma Diseases 0.000 description 2
- ZOKVLMBYDSIDKG-CSMHCCOUSA-N Lys-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@@H](N)CCCCN ZOKVLMBYDSIDKG-CSMHCCOUSA-N 0.000 description 2
- XUMBMVFBXHLACL-UHFFFAOYSA-N Melanin Chemical compound O=C1C(=O)C(C2=CNC3=C(C(C(=O)C4=C32)=O)C)=C2C4=CNC2=C1C XUMBMVFBXHLACL-UHFFFAOYSA-N 0.000 description 2
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 2
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 2
- OVBPIULPVIDEAO-UHFFFAOYSA-N N-Pteroyl-L-glutaminsaeure Natural products C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)NC(CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-UHFFFAOYSA-N 0.000 description 2
- ZDZOTLJHXYCWBA-VCVYQWHSSA-N N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol Chemical compound O([C@H]1[C@H]2[C@@](C([C@H](O)C3=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=4C=CC=CC=4)C[C@]1(O)C3(C)C)=O)(C)[C@@H](O)C[C@H]1OC[C@]12OC(=O)C)C(=O)C1=CC=CC=C1 ZDZOTLJHXYCWBA-VCVYQWHSSA-N 0.000 description 2
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 description 2
- 108090000630 Oncostatin M Proteins 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 2
- SMWDFEZZVXVKRB-UHFFFAOYSA-N Quinoline Chemical compound N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 description 2
- 108010003723 Single-Domain Antibodies Proteins 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- 210000001744 T-lymphocyte Anatomy 0.000 description 2
- 102100027222 T-lymphocyte activation antigen CD80 Human genes 0.000 description 2
- QYTDEUPAUMOIOP-UHFFFAOYSA-N TEMPO Chemical group CC1(C)CCCC(C)(C)N1[O] QYTDEUPAUMOIOP-UHFFFAOYSA-N 0.000 description 2
- 108700012411 TNFSF10 Proteins 0.000 description 2
- NKANXQFJJICGDU-QPLCGJKRSA-N Tamoxifen Chemical compound C=1C=CC=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1 NKANXQFJJICGDU-QPLCGJKRSA-N 0.000 description 2
- 102100033523 Thy-1 membrane glycoprotein Human genes 0.000 description 2
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 2
- 102100040247 Tumor necrosis factor Human genes 0.000 description 2
- 102100024598 Tumor necrosis factor ligand superfamily member 10 Human genes 0.000 description 2
- 102100040245 Tumor necrosis factor receptor superfamily member 5 Human genes 0.000 description 2
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 2
- JJSCUXAFAJEQGB-QMMMGPOBSA-N [(1s)-1-isocyanatoethyl]benzene Chemical compound O=C=N[C@@H](C)C1=CC=CC=C1 JJSCUXAFAJEQGB-QMMMGPOBSA-N 0.000 description 2
- 239000003070 absorption delaying agent Substances 0.000 description 2
- 235000011054 acetic acid Nutrition 0.000 description 2
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 2
- RJURFGZVJUQBHK-UHFFFAOYSA-N actinomycin D Natural products CC1OC(=O)C(C(C)C)N(C)C(=O)CN(C)C(=O)C2CCCN2C(=O)C(C(C)C)NC(=O)C1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)NC4C(=O)NC(C(N5CCCC5C(=O)N(C)CC(=O)N(C)C(C(C)C)C(=O)OC4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-UHFFFAOYSA-N 0.000 description 2
- 239000000783 alginic acid Substances 0.000 description 2
- 229960001126 alginic acid Drugs 0.000 description 2
- 150000004781 alginic acids Chemical class 0.000 description 2
- 150000007824 aliphatic compounds Chemical class 0.000 description 2
- 150000001345 alkine derivatives Chemical class 0.000 description 2
- 229940100198 alkylating agent Drugs 0.000 description 2
- 239000002168 alkylating agent Substances 0.000 description 2
- 125000001118 alkylidene group Chemical group 0.000 description 2
- 150000003863 ammonium salts Chemical class 0.000 description 2
- MWPLVEDNUUSJAV-UHFFFAOYSA-N anthracene Chemical compound C1=CC=CC2=CC3=CC=CC=C3C=C21 MWPLVEDNUUSJAV-UHFFFAOYSA-N 0.000 description 2
- 230000000844 anti-bacterial effect Effects 0.000 description 2
- 230000002236 anti-hypertrophic effect Effects 0.000 description 2
- 230000000340 anti-metabolite Effects 0.000 description 2
- 239000003429 antifungal agent Substances 0.000 description 2
- 229940121375 antifungal agent Drugs 0.000 description 2
- 229940100197 antimetabolite Drugs 0.000 description 2
- 239000002256 antimetabolite Substances 0.000 description 2
- 230000001640 apoptogenic effect Effects 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 239000012736 aqueous medium Substances 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- 208000027697 autoimmune lymphoproliferative syndrome due to CTLA4 haploinsuffiency Diseases 0.000 description 2
- VSRXQHXAPYXROS-UHFFFAOYSA-N azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OC(=O)C1(C(O)=O)CCC1 VSRXQHXAPYXROS-UHFFFAOYSA-N 0.000 description 2
- 210000003719 b-lymphocyte Anatomy 0.000 description 2
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 2
- 125000002619 bicyclic group Chemical group 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 201000000053 blastoma Diseases 0.000 description 2
- 229910021538 borax Inorganic materials 0.000 description 2
- 230000031709 bromination Effects 0.000 description 2
- 238000005893 bromination reaction Methods 0.000 description 2
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 2
- 229930195731 calicheamicin Natural products 0.000 description 2
- VSJKWCGYPAHWDS-FQEVSTJZSA-N camptothecin Chemical compound C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-FQEVSTJZSA-N 0.000 description 2
- 229940127093 camptothecin Drugs 0.000 description 2
- 229960004562 carboplatin Drugs 0.000 description 2
- 230000010261 cell growth Effects 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 125000003636 chemical group Chemical group 0.000 description 2
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 2
- 208000006990 cholangiocarcinoma Diseases 0.000 description 2
- 238000011097 chromatography purification Methods 0.000 description 2
- 238000002648 combination therapy Methods 0.000 description 2
- 229940125797 compound 12 Drugs 0.000 description 2
- 238000013270 controlled release Methods 0.000 description 2
- PSNOPSMXOBPNNV-VVCTWANISA-N cryptophycin 1 Chemical compound C1=C(Cl)C(OC)=CC=C1C[C@@H]1C(=O)NC[C@@H](C)C(=O)O[C@@H](CC(C)C)C(=O)O[C@H]([C@H](C)[C@@H]2[C@H](O2)C=2C=CC=CC=2)C/C=C/C(=O)N1 PSNOPSMXOBPNNV-VVCTWANISA-N 0.000 description 2
- PSNOPSMXOBPNNV-UHFFFAOYSA-N cryptophycin-327 Natural products C1=C(Cl)C(OC)=CC=C1CC1C(=O)NCC(C)C(=O)OC(CC(C)C)C(=O)OC(C(C)C2C(O2)C=2C=CC=CC=2)CC=CC(=O)N1 PSNOPSMXOBPNNV-UHFFFAOYSA-N 0.000 description 2
- TXWRERCHRDBNLG-UHFFFAOYSA-N cubane Chemical compound C12C3C4C1C1C4C3C12 TXWRERCHRDBNLG-UHFFFAOYSA-N 0.000 description 2
- 238000005520 cutting process Methods 0.000 description 2
- 125000000000 cycloalkoxy group Chemical group 0.000 description 2
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 2
- 125000001887 cyclopentyloxy group Chemical group C1(CCCC1)O* 0.000 description 2
- 230000001472 cytotoxic effect Effects 0.000 description 2
- 231100000135 cytotoxicity Toxicity 0.000 description 2
- 230000003013 cytotoxicity Effects 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- VSJKWCGYPAHWDS-UHFFFAOYSA-N dl-camptothecin Natural products C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)C5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-UHFFFAOYSA-N 0.000 description 2
- 229960003668 docetaxel Drugs 0.000 description 2
- POULHZVOKOAJMA-UHFFFAOYSA-N dodecanoic acid Chemical compound CCCCCCCCCCCC(O)=O POULHZVOKOAJMA-UHFFFAOYSA-N 0.000 description 2
- 229960004679 doxorubicin Drugs 0.000 description 2
- 230000009977 dual effect Effects 0.000 description 2
- 201000008184 embryoma Diseases 0.000 description 2
- 210000000981 epithelium Anatomy 0.000 description 2
- 229940105423 erythropoietin Drugs 0.000 description 2
- 235000019325 ethyl cellulose Nutrition 0.000 description 2
- 229920001249 ethyl cellulose Polymers 0.000 description 2
- IRXSLJNXXZKURP-UHFFFAOYSA-N fluorenylmethyloxycarbonyl chloride Chemical compound C1=CC=C2C(COC(=O)Cl)C3=CC=CC=C3C2=C1 IRXSLJNXXZKURP-UHFFFAOYSA-N 0.000 description 2
- 125000005519 fluorenylmethyloxycarbonyl group Chemical group 0.000 description 2
- 229910052731 fluorine Inorganic materials 0.000 description 2
- 125000003709 fluoroalkyl group Chemical group 0.000 description 2
- 235000019152 folic acid Nutrition 0.000 description 2
- 239000011724 folic acid Substances 0.000 description 2
- 229960000304 folic acid Drugs 0.000 description 2
- 150000002224 folic acids Chemical class 0.000 description 2
- 239000003205 fragrance Substances 0.000 description 2
- CHPZKNULDCNCBW-UHFFFAOYSA-N gallium nitrate Chemical compound [Ga+3].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O CHPZKNULDCNCBW-UHFFFAOYSA-N 0.000 description 2
- 210000003976 gap junction Anatomy 0.000 description 2
- 239000008273 gelatin Substances 0.000 description 2
- 229920000159 gelatin Polymers 0.000 description 2
- 229940014259 gelatin Drugs 0.000 description 2
- 235000019322 gelatine Nutrition 0.000 description 2
- 235000011852 gelatine desserts Nutrition 0.000 description 2
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 2
- 229960005277 gemcitabine Drugs 0.000 description 2
- 239000008103 glucose Substances 0.000 description 2
- 239000003102 growth factor Substances 0.000 description 2
- 125000001188 haloalkyl group Chemical group 0.000 description 2
- 125000005843 halogen group Chemical group 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 230000002489 hematologic effect Effects 0.000 description 2
- 231100000844 hepatocellular carcinoma Toxicity 0.000 description 2
- 150000002390 heteroarenes Chemical class 0.000 description 2
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 2
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 2
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 2
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 2
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 2
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 2
- HOMGKSMUEGBAAB-UHFFFAOYSA-N ifosfamide Chemical compound ClCCNP1(=O)OCCCN1CCCl HOMGKSMUEGBAAB-UHFFFAOYSA-N 0.000 description 2
- 229960001101 ifosfamide Drugs 0.000 description 2
- 239000012216 imaging agent Substances 0.000 description 2
- 125000002883 imidazolyl group Chemical group 0.000 description 2
- 239000012642 immune effector Substances 0.000 description 2
- 102000018358 immunoglobulin Human genes 0.000 description 2
- 230000003308 immunostimulating effect Effects 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- 230000036512 infertility Effects 0.000 description 2
- 229940047124 interferons Drugs 0.000 description 2
- INQOMBQAUSQDDS-UHFFFAOYSA-N iodomethane Chemical compound IC INQOMBQAUSQDDS-UHFFFAOYSA-N 0.000 description 2
- 230000005865 ionizing radiation Effects 0.000 description 2
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 2
- 125000005928 isopropyloxycarbonyl group Chemical group [H]C([H])([H])C([H])(OC(*)=O)C([H])([H])[H] 0.000 description 2
- 210000003734 kidney Anatomy 0.000 description 2
- 239000003446 ligand Substances 0.000 description 2
- 239000000314 lubricant Substances 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 238000012423 maintenance Methods 0.000 description 2
- HAWPXGHAZFHHAD-UHFFFAOYSA-N mechlorethamine Chemical compound ClCCN(C)CCCl HAWPXGHAZFHHAD-UHFFFAOYSA-N 0.000 description 2
- 229960004961 mechlorethamine Drugs 0.000 description 2
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 2
- 229960001924 melphalan Drugs 0.000 description 2
- GLVAUDGFNGKCSF-UHFFFAOYSA-N mercaptopurine Chemical compound S=C1NC=NC2=C1NC=N2 GLVAUDGFNGKCSF-UHFFFAOYSA-N 0.000 description 2
- 229920000609 methyl cellulose Polymers 0.000 description 2
- 235000010981 methylcellulose Nutrition 0.000 description 2
- 239000001923 methylcellulose Substances 0.000 description 2
- 230000000813 microbial effect Effects 0.000 description 2
- 244000005700 microbiome Species 0.000 description 2
- 229960004857 mitomycin Drugs 0.000 description 2
- 230000000394 mitotic effect Effects 0.000 description 2
- KKZJGLLVHKMTCM-UHFFFAOYSA-N mitoxantrone Chemical compound O=C1C2=C(O)C=CC(O)=C2C(=O)C2=C1C(NCCNCCO)=CC=C2NCCNCCO KKZJGLLVHKMTCM-UHFFFAOYSA-N 0.000 description 2
- 229960001156 mitoxantrone Drugs 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 239000001788 mono and diglycerides of fatty acids Substances 0.000 description 2
- 201000010879 mucinous adenocarcinoma Diseases 0.000 description 2
- 208000025113 myeloid leukemia Diseases 0.000 description 2
- 210000000822 natural killer cell Anatomy 0.000 description 2
- QZGIWPZCWHMVQL-UIYAJPBUSA-N neocarzinostatin chromophore Chemical compound O1[C@H](C)[C@H](O)[C@H](O)[C@@H](NC)[C@H]1O[C@@H]1C/2=C/C#C[C@H]3O[C@@]3([C@@H]3OC(=O)OC3)C#CC\2=C[C@H]1OC(=O)C1=C(O)C=CC2=C(C)C=C(OC)C=C12 QZGIWPZCWHMVQL-UIYAJPBUSA-N 0.000 description 2
- 230000000955 neuroendocrine Effects 0.000 description 2
- 210000002445 nipple Anatomy 0.000 description 2
- OSTGTTZJOCZWJG-UHFFFAOYSA-N nitrosourea Chemical compound NC(=O)N=NO OSTGTTZJOCZWJG-UHFFFAOYSA-N 0.000 description 2
- ZVVSSOQAYNYNPP-UHFFFAOYSA-N olaflur Chemical group F.F.CCCCCCCCCCCCCCCCCCN(CCO)CCCN(CCO)CCO ZVVSSOQAYNYNPP-UHFFFAOYSA-N 0.000 description 2
- 230000003287 optical effect Effects 0.000 description 2
- 230000002611 ovarian Effects 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 239000006072 paste Substances 0.000 description 2
- IZUPBVBPLAPZRR-UHFFFAOYSA-N pentachloro-phenol Natural products OC1=C(Cl)C(Cl)=C(Cl)C(Cl)=C1Cl IZUPBVBPLAPZRR-UHFFFAOYSA-N 0.000 description 2
- 230000010412 perfusion Effects 0.000 description 2
- 230000000144 pharmacologic effect Effects 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 2
- 239000010452 phosphate Substances 0.000 description 2
- XKJCHHZQLQNZHY-UHFFFAOYSA-N phthalimide Chemical compound C1=CC=C2C(=O)NC(=O)C2=C1 XKJCHHZQLQNZHY-UHFFFAOYSA-N 0.000 description 2
- 229910052697 platinum Inorganic materials 0.000 description 2
- 229920000747 poly(lactic acid) Polymers 0.000 description 2
- OXCMYAYHXIHQOA-UHFFFAOYSA-N potassium;[2-butyl-5-chloro-3-[[4-[2-(1,2,4-triaza-3-azanidacyclopenta-1,4-dien-5-yl)phenyl]phenyl]methyl]imidazol-4-yl]methanol Chemical compound [K+].CCCCC1=NC(Cl)=C(CO)N1CC1=CC=C(C=2C(=CC=CC=2)C2=N[N-]N=N2)C=C1 OXCMYAYHXIHQOA-UHFFFAOYSA-N 0.000 description 2
- 229920001592 potato starch Polymers 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 229960000624 procarbazine Drugs 0.000 description 2
- CPTBDICYNRMXFX-UHFFFAOYSA-N procarbazine Chemical compound CNNCC1=CC=C(C(=O)NC(C)C)C=C1 CPTBDICYNRMXFX-UHFFFAOYSA-N 0.000 description 2
- RXWNCPJZOCPEPQ-NVWDDTSBSA-N puromycin Chemical compound C1=CC(OC)=CC=C1C[C@H](N)C(=O)N[C@H]1[C@@H](O)[C@H](N2C3=NC=NC(=C3N=C2)N(C)C)O[C@@H]1CO RXWNCPJZOCPEPQ-NVWDDTSBSA-N 0.000 description 2
- 102000005962 receptors Human genes 0.000 description 2
- 108020003175 receptors Proteins 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 238000002271 resection Methods 0.000 description 2
- 201000009410 rhabdomyosarcoma Diseases 0.000 description 2
- 239000000523 sample Substances 0.000 description 2
- 230000035945 sensitivity Effects 0.000 description 2
- 238000007493 shaping process Methods 0.000 description 2
- 235000010339 sodium tetraborate Nutrition 0.000 description 2
- XOIQMTLWECTKJL-HXPDMXKUSA-M sodium;(3r,4s)-4-[(2s,5r,7s,8r,9s)-2-[(2r,5s)-5-ethyl-5-[(2r,3s,5r)-5-[(2s,3s,5r,6r)-6-hydroxy-6-(hydroxymethyl)-3,5-dimethyloxan-2-yl]-3-methyloxolan-2-yl]oxolan-2-yl]-7-hydroxy-2,8-dimethyl-1,10-dioxaspiro[4.5]decan-9-yl]-3-methoxy-2-methylpentanoate Chemical compound [Na+].C([C@@](O1)(C)[C@H]2CC[C@@](O2)(CC)[C@H]2[C@H](C[C@@H](O2)[C@@H]2[C@H](C[C@@H](C)[C@](O)(CO)O2)C)C)C[C@@]21C[C@H](O)[C@@H](C)[C@@H]([C@@H](C)[C@@H](OC)C(C)C([O-])=O)O2 XOIQMTLWECTKJL-HXPDMXKUSA-M 0.000 description 2
- 239000000600 sorbitol Substances 0.000 description 2
- 235000010356 sorbitol Nutrition 0.000 description 2
- 206010041823 squamous cell carcinoma Diseases 0.000 description 2
- 229940032147 starch Drugs 0.000 description 2
- 230000004936 stimulating effect Effects 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- 150000008163 sugars Chemical class 0.000 description 2
- 125000001174 sulfone group Chemical group 0.000 description 2
- 125000004434 sulfur atom Chemical group 0.000 description 2
- 239000013589 supplement Substances 0.000 description 2
- 239000004094 surface-active agent Substances 0.000 description 2
- 238000010189 synthetic method Methods 0.000 description 2
- 239000003826 tablet Substances 0.000 description 2
- 229960001196 thiotepa Drugs 0.000 description 2
- 229960003087 tioguanine Drugs 0.000 description 2
- 150000003613 toluenes Chemical class 0.000 description 2
- 229940034610 toothpaste Drugs 0.000 description 2
- 206010044412 transitional cell carcinoma Diseases 0.000 description 2
- 125000004044 trifluoroacetyl group Chemical group FC(C(=O)*)(F)F 0.000 description 2
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 2
- BSVBQGMMJUBVOD-UHFFFAOYSA-N trisodium borate Chemical compound [Na+].[Na+].[Na+].[O-]B([O-])[O-] BSVBQGMMJUBVOD-UHFFFAOYSA-N 0.000 description 2
- 230000003827 upregulation Effects 0.000 description 2
- 229960005088 urethane Drugs 0.000 description 2
- 125000003774 valeryl group Chemical group O=C([*])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 229960003048 vinblastine Drugs 0.000 description 2
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 2
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 2
- 229960004528 vincristine Drugs 0.000 description 2
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 2
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 2
- 239000000080 wetting agent Substances 0.000 description 2
- QBYIENPQHBMVBV-HFEGYEGKSA-N (2R)-2-hydroxy-2-phenylacetic acid Chemical compound O[C@@H](C(O)=O)c1ccccc1.O[C@@H](C(O)=O)c1ccccc1 QBYIENPQHBMVBV-HFEGYEGKSA-N 0.000 description 1
- YUKQVPZDQNCWOA-SECBINFHSA-N (2r)-1-butylpiperidin-1-ium-2-carboxylate Chemical compound CCCC[NH+]1CCCC[C@@H]1C([O-])=O YUKQVPZDQNCWOA-SECBINFHSA-N 0.000 description 1
- FLWWDYNPWOSLEO-HQVZTVAUSA-N (2s)-2-[[4-[1-(2-amino-4-oxo-1h-pteridin-6-yl)ethyl-methylamino]benzoyl]amino]pentanedioic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1C(C)N(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FLWWDYNPWOSLEO-HQVZTVAUSA-N 0.000 description 1
- URLVCROWVOSNPT-XOTOMLERSA-N (2s)-4-[(13r)-13-hydroxy-13-[(2r,5r)-5-[(2r,5r)-5-[(1r)-1-hydroxyundecyl]oxolan-2-yl]oxolan-2-yl]tridecyl]-2-methyl-2h-furan-5-one Chemical compound O1[C@@H]([C@H](O)CCCCCCCCCC)CC[C@@H]1[C@@H]1O[C@@H]([C@H](O)CCCCCCCCCCCCC=2C(O[C@@H](C)C=2)=O)CC1 URLVCROWVOSNPT-XOTOMLERSA-N 0.000 description 1
- CGMTUJFWROPELF-YPAAEMCBSA-N (3E,5S)-5-[(2S)-butan-2-yl]-3-(1-hydroxyethylidene)pyrrolidine-2,4-dione Chemical compound CC[C@H](C)[C@@H]1NC(=O)\C(=C(/C)O)C1=O CGMTUJFWROPELF-YPAAEMCBSA-N 0.000 description 1
- TVIRNGFXQVMMGB-OFWIHYRESA-N (3s,6r,10r,13e,16s)-16-[(2r,3r,4s)-4-chloro-3-hydroxy-4-phenylbutan-2-yl]-10-[(3-chloro-4-methoxyphenyl)methyl]-6-methyl-3-(2-methylpropyl)-1,4-dioxa-8,11-diazacyclohexadec-13-ene-2,5,9,12-tetrone Chemical compound C1=C(Cl)C(OC)=CC=C1C[C@@H]1C(=O)NC[C@@H](C)C(=O)O[C@@H](CC(C)C)C(=O)O[C@H]([C@H](C)[C@@H](O)[C@@H](Cl)C=2C=CC=CC=2)C/C=C/C(=O)N1 TVIRNGFXQVMMGB-OFWIHYRESA-N 0.000 description 1
- INAUWOVKEZHHDM-PEDBPRJASA-N (7s,9s)-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-7-[(2r,4s,5s,6s)-5-hydroxy-6-methyl-4-morpholin-4-yloxan-2-yl]oxy-4-methoxy-8,10-dihydro-7h-tetracene-5,12-dione;hydrochloride Chemical compound Cl.N1([C@H]2C[C@@H](O[C@@H](C)[C@H]2O)O[C@H]2C[C@@](O)(CC=3C(O)=C4C(=O)C=5C=CC=C(C=5C(=O)C4=C(O)C=32)OC)C(=O)CO)CCOCC1 INAUWOVKEZHHDM-PEDBPRJASA-N 0.000 description 1
- FPVKHBSQESCIEP-UHFFFAOYSA-N (8S)-3-(2-deoxy-beta-D-erythro-pentofuranosyl)-3,6,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol Natural products C1C(O)C(CO)OC1N1C(NC=NCC2O)=C2N=C1 FPVKHBSQESCIEP-UHFFFAOYSA-N 0.000 description 1
- OHILSKSRDDTCIR-WKSAPEMMSA-N (8s,9r,10s,11s,13s,14s,16r,17r)-9-fluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one;hydrochloride Chemical compound Cl.C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O OHILSKSRDDTCIR-WKSAPEMMSA-N 0.000 description 1
- AGNGYMCLFWQVGX-AGFFZDDWSA-N (e)-1-[(2s)-2-amino-2-carboxyethoxy]-2-diazonioethenolate Chemical compound OC(=O)[C@@H](N)CO\C([O-])=C\[N+]#N AGNGYMCLFWQVGX-AGFFZDDWSA-N 0.000 description 1
- GJGROPRLXDXIAN-UHFFFAOYSA-N 1,3-thiazol-4-one Chemical compound O=C1CSC=N1 GJGROPRLXDXIAN-UHFFFAOYSA-N 0.000 description 1
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- FNGNKTAPELTZSU-UHFFFAOYSA-N 2,2,2-trifluoroacetic acid hydroiodide Chemical compound I.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F FNGNKTAPELTZSU-UHFFFAOYSA-N 0.000 description 1
- XBNGYFFABRKICK-UHFFFAOYSA-N 2,3,4,5,6-pentafluorophenol Chemical compound OC1=C(F)C(F)=C(F)C(F)=C1F XBNGYFFABRKICK-UHFFFAOYSA-N 0.000 description 1
- BTOTXLJHDSNXMW-POYBYMJQSA-N 2,3-dideoxyuridine Chemical compound O1[C@H](CO)CC[C@@H]1N1C(=O)NC(=O)C=C1 BTOTXLJHDSNXMW-POYBYMJQSA-N 0.000 description 1
- AHDSRXYHVZECER-UHFFFAOYSA-N 2,4,6-tris[(dimethylamino)methyl]phenol Chemical compound CN(C)CC1=CC(CN(C)C)=C(O)C(CN(C)C)=C1 AHDSRXYHVZECER-UHFFFAOYSA-N 0.000 description 1
- QCXJFISCRQIYID-IAEPZHFASA-N 2-amino-1-n-[(3s,6s,7r,10s,16s)-3-[(2s)-butan-2-yl]-7,11,14-trimethyl-2,5,9,12,15-pentaoxo-10-propan-2-yl-8-oxa-1,4,11,14-tetrazabicyclo[14.3.0]nonadecan-6-yl]-4,6-dimethyl-3-oxo-9-n-[(3s,6s,7r,10s,16s)-7,11,14-trimethyl-2,5,9,12,15-pentaoxo-3,10-di(propa Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N=C2C(C(=O)N[C@@H]3C(=O)N[C@H](C(N4CCC[C@H]4C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]3C)=O)[C@@H](C)CC)=C(N)C(=O)C(C)=C2O2)C2=C(C)C=C1 QCXJFISCRQIYID-IAEPZHFASA-N 0.000 description 1
- SCVJRXQHFJXZFZ-KVQBGUIXSA-N 2-amino-9-[(2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-3h-purine-6-thione Chemical compound C1=2NC(N)=NC(=S)C=2N=CN1[C@H]1C[C@H](O)[C@@H](CO)O1 SCVJRXQHFJXZFZ-KVQBGUIXSA-N 0.000 description 1
- YOETUEMZNOLGDB-UHFFFAOYSA-N 2-methylpropyl carbonochloridate Chemical compound CC(C)COC(Cl)=O YOETUEMZNOLGDB-UHFFFAOYSA-N 0.000 description 1
- 125000000094 2-phenylethyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 1
- 125000006479 2-pyridyl methyl group Chemical group [H]C1=C([H])C([H])=C([H])C(=N1)C([H])([H])* 0.000 description 1
- YIMDLWDNDGKDTJ-QLKYHASDSA-N 3'-deamino-3'-(3-cyanomorpholin-4-yl)doxorubicin Chemical compound N1([C@H]2C[C@@H](O[C@@H](C)[C@H]2O)O[C@H]2C[C@@](O)(CC=3C(O)=C4C(=O)C=5C=CC=C(C=5C(=O)C4=C(O)C=32)OC)C(=O)CO)CCOCC1C#N YIMDLWDNDGKDTJ-QLKYHASDSA-N 0.000 description 1
- NDMPLJNOPCLANR-UHFFFAOYSA-N 3,4-dihydroxy-15-(4-hydroxy-18-methoxycarbonyl-5,18-seco-ibogamin-18-yl)-16-methoxy-1-methyl-6,7-didehydro-aspidospermidine-3-carboxylic acid methyl ester Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 NDMPLJNOPCLANR-UHFFFAOYSA-N 0.000 description 1
- PWMYMKOUNYTVQN-UHFFFAOYSA-N 3-(8,8-diethyl-2-aza-8-germaspiro[4.5]decan-2-yl)-n,n-dimethylpropan-1-amine Chemical compound C1C[Ge](CC)(CC)CCC11CN(CCCN(C)C)CC1 PWMYMKOUNYTVQN-UHFFFAOYSA-N 0.000 description 1
- UJZHYIMESNWEQA-UHFFFAOYSA-N 3-methoxycarbonylbicyclo[1.1.1]pentane-1-carboxylic acid Chemical compound C1C2(C(O)=O)CC1(C(=O)OC)C2 UJZHYIMESNWEQA-UHFFFAOYSA-N 0.000 description 1
- AOJJSUZBOXZQNB-VTZDEGQISA-N 4'-epidoxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-VTZDEGQISA-N 0.000 description 1
- NURJCIIHNXRHJV-UHFFFAOYSA-N 4,4-dimethyl-3h-pyridine Chemical compound CC1(C)CC=NC=C1 NURJCIIHNXRHJV-UHFFFAOYSA-N 0.000 description 1
- 229960000549 4-dimethylaminophenol Drugs 0.000 description 1
- ARRJEFLYRAVFJA-UHFFFAOYSA-N 4-methoxycarbonylcubane-1-carboxylic acid Chemical compound C12C3C4(C(=O)OC)C1C1C4C3C12C(O)=O ARRJEFLYRAVFJA-UHFFFAOYSA-N 0.000 description 1
- IDPUKCWIGUEADI-UHFFFAOYSA-N 5-[bis(2-chloroethyl)amino]uracil Chemical compound ClCCN(CCCl)C1=CNC(=O)NC1=O IDPUKCWIGUEADI-UHFFFAOYSA-N 0.000 description 1
- NMUSYJAQQFHJEW-KVTDHHQDSA-N 5-azacytidine Chemical compound O=C1N=C(N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 NMUSYJAQQFHJEW-KVTDHHQDSA-N 0.000 description 1
- WYXSYVWAUAUWLD-SHUUEZRQSA-N 6-azauridine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C=N1 WYXSYVWAUAUWLD-SHUUEZRQSA-N 0.000 description 1
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 1
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 1
- KDCGOANMDULRCW-UHFFFAOYSA-N 7H-purine Chemical class N1=CNC2=NC=NC2=C1 KDCGOANMDULRCW-UHFFFAOYSA-N 0.000 description 1
- ZGXJTSGNIOSYLO-UHFFFAOYSA-N 88755TAZ87 Chemical compound NCC(=O)CCC(O)=O ZGXJTSGNIOSYLO-UHFFFAOYSA-N 0.000 description 1
- HDZZVAMISRMYHH-UHFFFAOYSA-N 9beta-Ribofuranosyl-7-deazaadenin Natural products C1=CC=2C(N)=NC=NC=2N1C1OC(CO)C(O)C1O HDZZVAMISRMYHH-UHFFFAOYSA-N 0.000 description 1
- 208000003200 Adenoma Diseases 0.000 description 1
- 206010001233 Adenoma benign Diseases 0.000 description 1
- 208000005641 Adenomyosis Diseases 0.000 description 1
- 108010088751 Albumins Proteins 0.000 description 1
- 102000009027 Albumins Human genes 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- CEIZFXOZIQNICU-UHFFFAOYSA-N Alternaria alternata Crofton-weed toxin Natural products CCC(C)C1NC(=O)C(C(C)=O)=C1O CEIZFXOZIQNICU-UHFFFAOYSA-N 0.000 description 1
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 1
- 108091023037 Aptamer Proteins 0.000 description 1
- 241001120493 Arene Species 0.000 description 1
- NOWKCMXCCJGMRR-UHFFFAOYSA-N Aziridine Chemical compound C1CN1 NOWKCMXCCJGMRR-UHFFFAOYSA-N 0.000 description 1
- 102100024222 B-lymphocyte antigen CD19 Human genes 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 206010004146 Basal cell carcinoma Diseases 0.000 description 1
- 229940122361 Bisphosphonate Drugs 0.000 description 1
- 108010006654 Bleomycin Proteins 0.000 description 1
- 206010006187 Breast cancer Diseases 0.000 description 1
- 208000026310 Breast neoplasm Diseases 0.000 description 1
- 201000011057 Breast sarcoma Diseases 0.000 description 1
- 208000007690 Brenner tumor Diseases 0.000 description 1
- 206010073258 Brenner tumour Diseases 0.000 description 1
- 208000003170 Bronchiolo-Alveolar Adenocarcinoma Diseases 0.000 description 1
- 101710155857 C-C motif chemokine 2 Proteins 0.000 description 1
- 102100021943 C-C motif chemokine 2 Human genes 0.000 description 1
- 102100032367 C-C motif chemokine 5 Human genes 0.000 description 1
- 108010029697 CD40 Ligand Proteins 0.000 description 1
- 102100032937 CD40 ligand Human genes 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- GAGWJHPBXLXJQN-UORFTKCHSA-N Capecitabine Chemical compound C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1[C@H]1[C@H](O)[C@H](O)[C@@H](C)O1 GAGWJHPBXLXJQN-UORFTKCHSA-N 0.000 description 1
- GAGWJHPBXLXJQN-UHFFFAOYSA-N Capecitabine Natural products C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1C1C(O)C(O)C(C)O1 GAGWJHPBXLXJQN-UHFFFAOYSA-N 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 206010007275 Carcinoid tumour Diseases 0.000 description 1
- DLGOEMSEDOSKAD-UHFFFAOYSA-N Carmustine Chemical compound ClCCNC(=O)N(N=O)CCCl DLGOEMSEDOSKAD-UHFFFAOYSA-N 0.000 description 1
- 102100025064 Cellular tumor antigen p53 Human genes 0.000 description 1
- 229920000623 Cellulose acetate phthalate Polymers 0.000 description 1
- 206010050337 Cerumen impaction Diseases 0.000 description 1
- 108010055166 Chemokine CCL5 Proteins 0.000 description 1
- 108010012236 Chemokines Proteins 0.000 description 1
- 102000019034 Chemokines Human genes 0.000 description 1
- JWBOIMRXGHLCPP-UHFFFAOYSA-N Chloditan Chemical compound C=1C=CC=C(Cl)C=1C(C(Cl)Cl)C1=CC=C(Cl)C=C1 JWBOIMRXGHLCPP-UHFFFAOYSA-N 0.000 description 1
- 206010008583 Chloroma Diseases 0.000 description 1
- XJUZRXYOEPSWMB-UHFFFAOYSA-N Chloromethyl methyl ether Chemical compound COCCl XJUZRXYOEPSWMB-UHFFFAOYSA-N 0.000 description 1
- 206010008631 Cholera Diseases 0.000 description 1
- 241000251730 Chondrichthyes Species 0.000 description 1
- 201000009047 Chordoma Diseases 0.000 description 1
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 1
- QBXVXKRWOVBUDB-GRKNLSHJSA-N ClC=1C(=CC(=C(CN2[C@H](C[C@H](C2)O)C(=O)O)C1)OCC1=CC(=CC=C1)C#N)OCC1=C(C(=CC=C1)C1=CC2=C(OCCO2)C=C1)C Chemical compound ClC=1C(=CC(=C(CN2[C@H](C[C@H](C2)O)C(=O)O)C1)OCC1=CC(=CC=C1)C#N)OCC1=C(C(=CC=C1)C1=CC2=C(OCCO2)C=C1)C QBXVXKRWOVBUDB-GRKNLSHJSA-N 0.000 description 1
- 229920002785 Croscarmellose sodium Polymers 0.000 description 1
- 229930188224 Cryptophycin Natural products 0.000 description 1
- 108010069514 Cyclic Peptides Proteins 0.000 description 1
- 102000001189 Cyclic Peptides Human genes 0.000 description 1
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 1
- 101710112752 Cytotoxin Proteins 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- 230000005778 DNA damage Effects 0.000 description 1
- 231100000277 DNA damage Toxicity 0.000 description 1
- 230000004543 DNA replication Effects 0.000 description 1
- 206010011844 Dacryocystitis Diseases 0.000 description 1
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical compound [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- BUDQDWGNQVEFAC-UHFFFAOYSA-N Dihydropyran Chemical compound C1COC=CC1 BUDQDWGNQVEFAC-UHFFFAOYSA-N 0.000 description 1
- VYZAHLCBVHPDDF-UHFFFAOYSA-N Dinitrochlorobenzene Chemical compound [O-][N+](=O)C1=CC=C(Cl)C([N+]([O-])=O)=C1 VYZAHLCBVHPDDF-UHFFFAOYSA-N 0.000 description 1
- 241000255925 Diptera Species 0.000 description 1
- 206010059866 Drug resistance Diseases 0.000 description 1
- 206010014733 Endometrial cancer Diseases 0.000 description 1
- 206010014759 Endometrial neoplasm Diseases 0.000 description 1
- SAMRUMKYXPVKPA-VFKOLLTISA-N Enocitabine Chemical compound O=C1N=C(NC(=O)CCCCCCCCCCCCCCCCCCCCC)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 SAMRUMKYXPVKPA-VFKOLLTISA-N 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 206010014958 Eosinophilic leukaemia Diseases 0.000 description 1
- HEVGGTGPGPKZHF-UHFFFAOYSA-N Epilaurene Natural products CC1C(=C)CCC1(C)C1=CC=C(C)C=C1 HEVGGTGPGPKZHF-UHFFFAOYSA-N 0.000 description 1
- HTIJFSOGRVMCQR-UHFFFAOYSA-N Epirubicin Natural products COc1cccc2C(=O)c3c(O)c4CC(O)(CC(OC5CC(N)C(=O)C(C)O5)c4c(O)c3C(=O)c12)C(=O)CO HTIJFSOGRVMCQR-UHFFFAOYSA-N 0.000 description 1
- OBMLHUPNRURLOK-XGRAFVIBSA-N Epitiostanol Chemical compound C1[C@@H]2S[C@@H]2C[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CC[C@H]21 OBMLHUPNRURLOK-XGRAFVIBSA-N 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 101001039702 Escherichia coli (strain K12) Methyl-accepting chemotaxis protein I Proteins 0.000 description 1
- 229930189413 Esperamicin Natural products 0.000 description 1
- 102000015212 Fas Ligand Protein Human genes 0.000 description 1
- 108010039471 Fas Ligand Protein Proteins 0.000 description 1
- 102000009109 Fc receptors Human genes 0.000 description 1
- 108010087819 Fc receptors Proteins 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 206010053717 Fibrous histiocytoma Diseases 0.000 description 1
- KRHYYFGTRYWZRS-UHFFFAOYSA-N Fluorane Chemical group F KRHYYFGTRYWZRS-UHFFFAOYSA-N 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- 102100037813 Focal adhesion kinase 1 Human genes 0.000 description 1
- 208000004463 Follicular Adenocarcinoma Diseases 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- 208000003098 Ganglion Cysts Diseases 0.000 description 1
- 208000008999 Giant Cell Carcinoma Diseases 0.000 description 1
- 208000002966 Giant Cell Tumor of Bone Diseases 0.000 description 1
- 208000010412 Glaucoma Diseases 0.000 description 1
- 208000032612 Glial tumor Diseases 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 206010018498 Goitre Diseases 0.000 description 1
- 102000001895 Gonadotropin Receptors Human genes 0.000 description 1
- 108010040490 Gonadotropin Receptors Proteins 0.000 description 1
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 1
- 102100039620 Granulocyte-macrophage colony-stimulating factor Human genes 0.000 description 1
- 206010018691 Granuloma Diseases 0.000 description 1
- 229940121710 HMGCoA reductase inhibitor Drugs 0.000 description 1
- 102100031573 Hematopoietic progenitor cell antigen CD34 Human genes 0.000 description 1
- 108010068250 Herpes Simplex Virus Protein Vmw65 Proteins 0.000 description 1
- 208000017604 Hodgkin disease Diseases 0.000 description 1
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 1
- 101000980825 Homo sapiens B-lymphocyte antigen CD19 Proteins 0.000 description 1
- 101000721661 Homo sapiens Cellular tumor antigen p53 Proteins 0.000 description 1
- 101000777663 Homo sapiens Hematopoietic progenitor cell antigen CD34 Proteins 0.000 description 1
- 101000960954 Homo sapiens Interleukin-18 Proteins 0.000 description 1
- 101001055144 Homo sapiens Interleukin-2 receptor subunit alpha Proteins 0.000 description 1
- 101001034314 Homo sapiens Lactadherin Proteins 0.000 description 1
- 101001008874 Homo sapiens Mast/stem cell growth factor receptor Kit Proteins 0.000 description 1
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 description 1
- 101000611183 Homo sapiens Tumor necrosis factor Proteins 0.000 description 1
- 229920001479 Hydroxyethyl methyl cellulose Polymers 0.000 description 1
- VSNHCAURESNICA-UHFFFAOYSA-N Hydroxyurea Chemical compound NC(=O)NO VSNHCAURESNICA-UHFFFAOYSA-N 0.000 description 1
- 206010020649 Hyperkeratosis Diseases 0.000 description 1
- 102000026633 IL6 Human genes 0.000 description 1
- 102000018071 Immunoglobulin Fc Fragments Human genes 0.000 description 1
- 108010091135 Immunoglobulin Fc Fragments Proteins 0.000 description 1
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 1
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 1
- 102100026720 Interferon beta Human genes 0.000 description 1
- 102000006992 Interferon-alpha Human genes 0.000 description 1
- 108010047761 Interferon-alpha Proteins 0.000 description 1
- 102000003996 Interferon-beta Human genes 0.000 description 1
- 102000008070 Interferon-gamma Human genes 0.000 description 1
- 108010074328 Interferon-gamma Proteins 0.000 description 1
- 102000003814 Interleukin-10 Human genes 0.000 description 1
- 102000013691 Interleukin-17 Human genes 0.000 description 1
- 108050003558 Interleukin-17 Proteins 0.000 description 1
- 102100039898 Interleukin-18 Human genes 0.000 description 1
- 102100026878 Interleukin-2 receptor subunit alpha Human genes 0.000 description 1
- 108010002386 Interleukin-3 Proteins 0.000 description 1
- 102000010787 Interleukin-4 Receptors Human genes 0.000 description 1
- 108010038486 Interleukin-4 Receptors Proteins 0.000 description 1
- 108010002586 Interleukin-7 Proteins 0.000 description 1
- 108010002335 Interleukin-9 Proteins 0.000 description 1
- 102000015696 Interleukins Human genes 0.000 description 1
- 108010063738 Interleukins Proteins 0.000 description 1
- 208000007766 Kaposi sarcoma Diseases 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- 125000002061 L-isoleucyl group Chemical group [H]N([H])[C@]([H])(C(=O)[*])[C@](C([H])([H])[H])([H])C(C([H])([H])[H])([H])[H] 0.000 description 1
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 1
- 102000008238 LHRH Receptors Human genes 0.000 description 1
- 108010021290 LHRH Receptors Proteins 0.000 description 1
- 102100039648 Lactadherin Human genes 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 102000002297 Laminin Receptors Human genes 0.000 description 1
- 108010000851 Laminin Receptors Proteins 0.000 description 1
- 208000031671 Large B-Cell Diffuse Lymphoma Diseases 0.000 description 1
- 239000005639 Lauric acid Substances 0.000 description 1
- 229920001491 Lentinan Polymers 0.000 description 1
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 1
- 240000007472 Leucaena leucocephala Species 0.000 description 1
- 108090001030 Lipoproteins Proteins 0.000 description 1
- 102000004895 Lipoproteins Human genes 0.000 description 1
- 208000000265 Lobular Carcinoma Diseases 0.000 description 1
- 208000028018 Lymphocytic leukaemia Diseases 0.000 description 1
- 102000008072 Lymphokines Human genes 0.000 description 1
- 108010074338 Lymphokines Proteins 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 102000007651 Macrophage Colony-Stimulating Factor Human genes 0.000 description 1
- 108010046938 Macrophage Colony-Stimulating Factor Proteins 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 102100027754 Mast/stem cell growth factor receptor Kit Human genes 0.000 description 1
- 206010027145 Melanocytic naevus Diseases 0.000 description 1
- 102000018697 Membrane Proteins Human genes 0.000 description 1
- 108010052285 Membrane Proteins Proteins 0.000 description 1
- 201000009574 Mesenchymal Chondrosarcoma Diseases 0.000 description 1
- 239000012359 Methanesulfonyl chloride Substances 0.000 description 1
- 229930192392 Mitomycin Natural products 0.000 description 1
- 229920000881 Modified starch Polymers 0.000 description 1
- PCZOHLXUXFIOCF-UHFFFAOYSA-N Monacolin X Natural products C12C(OC(=O)C(C)CC)CC(C)C=C2C=CC(C)C1CCC1CC(O)CC(=O)O1 PCZOHLXUXFIOCF-UHFFFAOYSA-N 0.000 description 1
- 108010008707 Mucin-1 Proteins 0.000 description 1
- 102100034256 Mucin-1 Human genes 0.000 description 1
- 206010057269 Mucoepidermoid carcinoma Diseases 0.000 description 1
- 208000010357 Mullerian Mixed Tumor Diseases 0.000 description 1
- 101100400378 Mus musculus Marveld2 gene Proteins 0.000 description 1
- 241000186366 Mycobacterium bovis Species 0.000 description 1
- 206010028851 Necrosis Diseases 0.000 description 1
- JCXJVPUVTGWSNB-UHFFFAOYSA-N Nitrogen dioxide Chemical compound O=[N]=O JCXJVPUVTGWSNB-UHFFFAOYSA-N 0.000 description 1
- SYNHCENRCUAUNM-UHFFFAOYSA-N Nitrogen mustard N-oxide hydrochloride Chemical compound Cl.ClCC[N+]([O-])(C)CCCl SYNHCENRCUAUNM-UHFFFAOYSA-N 0.000 description 1
- 208000010505 Nose Neoplasms Diseases 0.000 description 1
- MXRIRQGCELJRSN-UHFFFAOYSA-N O.O.O.[Al] Chemical compound O.O.O.[Al] MXRIRQGCELJRSN-UHFFFAOYSA-N 0.000 description 1
- 102000004140 Oncostatin M Human genes 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 201000005702 Pertussis Diseases 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- 108091000080 Phosphotransferase Proteins 0.000 description 1
- 102100022427 Plasmalemma vesicle-associated protein Human genes 0.000 description 1
- 101710193105 Plasmalemma vesicle-associated protein Proteins 0.000 description 1
- 241000223960 Plasmodium falciparum Species 0.000 description 1
- 229920000954 Polyglycolide Polymers 0.000 description 1
- 229920001710 Polyorthoester Polymers 0.000 description 1
- 108090000412 Protein-Tyrosine Kinases Proteins 0.000 description 1
- 102000004022 Protein-Tyrosine Kinases Human genes 0.000 description 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-N R-2-phenyl-2-hydroxyacetic acid Natural products OC(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-N 0.000 description 1
- 201000000582 Retinoblastoma Diseases 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 229920001800 Shellac Polymers 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- ZSJLQEPLLKMAKR-UHFFFAOYSA-N Streptozotocin Natural products O=NN(C)C(=O)NC1C(O)OC(CO)C(O)C1O ZSJLQEPLLKMAKR-UHFFFAOYSA-N 0.000 description 1
- 208000005400 Synovial Cyst Diseases 0.000 description 1
- BXFOFFBJRFZBQZ-QYWOHJEZSA-N T-2 toxin Chemical compound C([C@@]12[C@]3(C)[C@H](OC(C)=O)[C@@H](O)[C@H]1O[C@H]1[C@]3(COC(C)=O)C[C@@H](C(=C1)C)OC(=O)CC(C)C)O2 BXFOFFBJRFZBQZ-QYWOHJEZSA-N 0.000 description 1
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 description 1
- 108010014401 TWEAK Receptor Proteins 0.000 description 1
- 102000016946 TWEAK Receptor Human genes 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- CGMTUJFWROPELF-UHFFFAOYSA-N Tenuazonic acid Natural products CCC(C)C1NC(=O)C(=C(C)/O)C1=O CGMTUJFWROPELF-UHFFFAOYSA-N 0.000 description 1
- 206010043276 Teratoma Diseases 0.000 description 1
- HEDRZPFGACZZDS-MICDWDOJSA-N Trichloro(2H)methane Chemical compound [2H]C(Cl)(Cl)Cl HEDRZPFGACZZDS-MICDWDOJSA-N 0.000 description 1
- UMILHIMHKXVDGH-UHFFFAOYSA-N Triethylene glycol diglycidyl ether Chemical compound C1OC1COCCOCCOCCOCC1CO1 UMILHIMHKXVDGH-UHFFFAOYSA-N 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- FYAMXEPQQLNQDM-UHFFFAOYSA-N Tris(1-aziridinyl)phosphine oxide Chemical compound C1CN1P(N1CC1)(=O)N1CC1 FYAMXEPQQLNQDM-UHFFFAOYSA-N 0.000 description 1
- YZCKVEUIGOORGS-NJFSPNSNSA-N Tritium Chemical compound [3H] YZCKVEUIGOORGS-NJFSPNSNSA-N 0.000 description 1
- 102000004243 Tubulin Human genes 0.000 description 1
- 108090000704 Tubulin Proteins 0.000 description 1
- 108010040002 Tumor Suppressor Proteins Proteins 0.000 description 1
- 102000001742 Tumor Suppressor Proteins Human genes 0.000 description 1
- 102100031988 Tumor necrosis factor ligand superfamily member 6 Human genes 0.000 description 1
- 108050002568 Tumor necrosis factor ligand superfamily member 6 Proteins 0.000 description 1
- 206010054094 Tumour necrosis Diseases 0.000 description 1
- 101710145727 Viral Fc-gamma receptor-like protein UL119 Proteins 0.000 description 1
- 208000008383 Wilms tumor Diseases 0.000 description 1
- PVNFMCBFDPTNQI-UIBOPQHZSA-N [(1S,2R,5S,6S,16E,18E,20R,21S)-11-chloro-21-hydroxy-12,20-dimethoxy-2,5,9,16-tetramethyl-8,23-dioxo-4,24-dioxa-9,22-diazatetracyclo[19.3.1.110,14.03,5]hexacosa-10,12,14(26),16,18-pentaen-6-yl] acetate [(1S,2R,5S,6S,16E,18E,20R,21S)-11-chloro-21-hydroxy-12,20-dimethoxy-2,5,9,16-tetramethyl-8,23-dioxo-4,24-dioxa-9,22-diazatetracyclo[19.3.1.110,14.03,5]hexacosa-10,12,14(26),16,18-pentaen-6-yl] 3-methylbutanoate [(1S,2R,5S,6S,16E,18E,20R,21S)-11-chloro-21-hydroxy-12,20-dimethoxy-2,5,9,16-tetramethyl-8,23-dioxo-4,24-dioxa-9,22-diazatetracyclo[19.3.1.110,14.03,5]hexacosa-10,12,14(26),16,18-pentaen-6-yl] 2-methylpropanoate [(1S,2R,5S,6S,16E,18E,20R,21S)-11-chloro-21-hydroxy-12,20-dimethoxy-2,5,9,16-tetramethyl-8,23-dioxo-4,24-dioxa-9,22-diazatetracyclo[19.3.1.110,14.03,5]hexacosa-10,12,14(26),16,18-pentaen-6-yl] propanoate Chemical compound CO[C@@H]1\C=C\C=C(C)\Cc2cc(OC)c(Cl)c(c2)N(C)C(=O)C[C@H](OC(C)=O)[C@]2(C)OC2[C@H](C)[C@@H]2C[C@@]1(O)NC(=O)O2.CCC(=O)O[C@H]1CC(=O)N(C)c2cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@H](OC(=O)N3)[C@@H](C)C3O[C@@]13C)cc(OC)c2Cl.CO[C@@H]1\C=C\C=C(C)\Cc2cc(OC)c(Cl)c(c2)N(C)C(=O)C[C@H](OC(=O)C(C)C)[C@]2(C)OC2[C@H](C)[C@@H]2C[C@@]1(O)NC(=O)O2.CO[C@@H]1\C=C\C=C(C)\Cc2cc(OC)c(Cl)c(c2)N(C)C(=O)C[C@H](OC(=O)CC(C)C)[C@]2(C)OC2[C@H](C)[C@@H]2C[C@@]1(O)NC(=O)O2 PVNFMCBFDPTNQI-UIBOPQHZSA-N 0.000 description 1
- 238000002679 ablation Methods 0.000 description 1
- QUHYUSAHBDACNG-UHFFFAOYSA-N acerogenin 3 Natural products C1=CC(O)=CC=C1CCCCC(=O)CCC1=CC=C(O)C=C1 QUHYUSAHBDACNG-UHFFFAOYSA-N 0.000 description 1
- KXKVLQRXCPHEJC-UHFFFAOYSA-N acetic acid trimethyl ester Natural products COC(C)=O KXKVLQRXCPHEJC-UHFFFAOYSA-N 0.000 description 1
- ZCHPKWUIAASXPV-UHFFFAOYSA-N acetic acid;methanol Chemical compound OC.CC(O)=O ZCHPKWUIAASXPV-UHFFFAOYSA-N 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 229930183665 actinomycin Natural products 0.000 description 1
- RJURFGZVJUQBHK-IIXSONLDSA-N actinomycin D Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)N[C@@H]4C(=O)N[C@@H](C(N5CCC[C@H]5C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-IIXSONLDSA-N 0.000 description 1
- 125000004442 acylamino group Chemical group 0.000 description 1
- 125000005035 acylthio group Chemical group 0.000 description 1
- 230000003044 adaptive effect Effects 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 238000009098 adjuvant therapy Methods 0.000 description 1
- BYRVKDUQDLJUBX-JJCDCTGGSA-N adozelesin Chemical compound C1=CC=C2OC(C(=O)NC=3C=C4C=C(NC4=CC=3)C(=O)N3C[C@H]4C[C@]44C5=C(C(C=C43)=O)NC=C5C)=CC2=C1 BYRVKDUQDLJUBX-JJCDCTGGSA-N 0.000 description 1
- 229950004955 adozelesin Drugs 0.000 description 1
- 208000020990 adrenal cortex carcinoma Diseases 0.000 description 1
- 208000007128 adrenocortical carcinoma Diseases 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 229940072056 alginate Drugs 0.000 description 1
- 125000002723 alicyclic group Chemical group 0.000 description 1
- 125000006323 alkenyl amino group Chemical group 0.000 description 1
- 125000003302 alkenyloxy group Chemical group 0.000 description 1
- 125000000033 alkoxyamino group Chemical group 0.000 description 1
- 125000004453 alkoxycarbonyl group Chemical group 0.000 description 1
- 150000003973 alkyl amines Chemical class 0.000 description 1
- 125000005277 alkyl imino group Chemical group 0.000 description 1
- 125000004390 alkyl sulfonyl group Chemical group 0.000 description 1
- 125000004656 alkyl sulfonylamino group Chemical group 0.000 description 1
- 125000004414 alkyl thio group Chemical group 0.000 description 1
- 125000006319 alkynyl amino group Chemical group 0.000 description 1
- 125000005133 alkynyloxy group Chemical group 0.000 description 1
- SHGAZHPCJJPHSC-YCNIQYBTSA-N all-trans-retinoic acid Chemical compound OC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-YCNIQYBTSA-N 0.000 description 1
- 230000000172 allergic effect Effects 0.000 description 1
- 230000000735 allogeneic effect Effects 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- SNAAJJQQZSMGQD-UHFFFAOYSA-N aluminum magnesium Chemical compound [Mg].[Al] SNAAJJQQZSMGQD-UHFFFAOYSA-N 0.000 description 1
- 206010065867 alveolar rhabdomyosarcoma Diseases 0.000 description 1
- ROBVIMPUHSLWNV-UHFFFAOYSA-N aminoglutethimide Chemical compound C=1C=C(N)C=CC=1C1(CC)CCC(=O)NC1=O ROBVIMPUHSLWNV-UHFFFAOYSA-N 0.000 description 1
- 229960003437 aminoglutethimide Drugs 0.000 description 1
- 229960002749 aminolevulinic acid Drugs 0.000 description 1
- 239000000908 ammonium hydroxide Substances 0.000 description 1
- 229960001220 amsacrine Drugs 0.000 description 1
- XCPGHVQEEXUHNC-UHFFFAOYSA-N amsacrine Chemical compound COC1=CC(NS(C)(=O)=O)=CC=C1NC1=C(C=CC=C2)C2=NC2=CC=CC=C12 XCPGHVQEEXUHNC-UHFFFAOYSA-N 0.000 description 1
- 239000003098 androgen Substances 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 230000000578 anorexic effect Effects 0.000 description 1
- 230000001093 anti-cancer Effects 0.000 description 1
- 230000000890 antigenic effect Effects 0.000 description 1
- 229940045687 antimetabolites folic acid analogs Drugs 0.000 description 1
- 239000004599 antimicrobial Substances 0.000 description 1
- 229940045719 antineoplastic alkylating agent nitrosoureas Drugs 0.000 description 1
- 201000007436 apocrine adenocarcinoma Diseases 0.000 description 1
- 239000008346 aqueous phase Substances 0.000 description 1
- 229910052786 argon Inorganic materials 0.000 description 1
- 239000012300 argon atmosphere Substances 0.000 description 1
- 150000001491 aromatic compounds Chemical class 0.000 description 1
- 125000005161 aryl oxy carbonyl group Chemical group 0.000 description 1
- 230000003140 astrocytic effect Effects 0.000 description 1
- 208000010668 atopic eczema Diseases 0.000 description 1
- 230000003305 autocrine Effects 0.000 description 1
- 229960002756 azacitidine Drugs 0.000 description 1
- 229950011321 azaserine Drugs 0.000 description 1
- 125000002393 azetidinyl group Chemical group 0.000 description 1
- 150000001541 aziridines Chemical class 0.000 description 1
- 125000004069 aziridinyl group Chemical group 0.000 description 1
- 210000003651 basophil Anatomy 0.000 description 1
- 235000013527 bean curd Nutrition 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 208000001119 benign fibrous histiocytoma Diseases 0.000 description 1
- LNSZVKGKTGXNRJ-UHFFFAOYSA-N benzene 2,2,2-trifluoroacetic acid Chemical compound C1=CC=CC=C1.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F LNSZVKGKTGXNRJ-UHFFFAOYSA-N 0.000 description 1
- WDMKMBJXZCMVQS-UHFFFAOYSA-N benzene;2,2,2-trifluoroacetic acid Chemical compound C1=CC=CC=C1.OC(=O)C(F)(F)F WDMKMBJXZCMVQS-UHFFFAOYSA-N 0.000 description 1
- 235000019445 benzyl alcohol Nutrition 0.000 description 1
- AGEZXYOZHKGVCM-UHFFFAOYSA-N benzyl bromide Chemical compound BrCC1=CC=CC=C1 AGEZXYOZHKGVCM-UHFFFAOYSA-N 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 229920002988 biodegradable polymer Polymers 0.000 description 1
- 229920013641 bioerodible polymer Polymers 0.000 description 1
- 239000003124 biologic agent Substances 0.000 description 1
- 239000003181 biological factor Substances 0.000 description 1
- 238000001815 biotherapy Methods 0.000 description 1
- 235000010290 biphenyl Nutrition 0.000 description 1
- 239000004305 biphenyl Substances 0.000 description 1
- 150000004663 bisphosphonates Chemical class 0.000 description 1
- 229960001561 bleomycin Drugs 0.000 description 1
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 description 1
- 238000004820 blood count Methods 0.000 description 1
- 208000007047 blue nevus Diseases 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- 201000011143 bone giant cell tumor Diseases 0.000 description 1
- 210000001185 bone marrow Anatomy 0.000 description 1
- 201000003163 breast adenoma Diseases 0.000 description 1
- 201000003714 breast lobular carcinoma Diseases 0.000 description 1
- 238000009395 breeding Methods 0.000 description 1
- 230000001488 breeding effect Effects 0.000 description 1
- 150000005752 bromopyridines Chemical class 0.000 description 1
- 229960005520 bryostatin Drugs 0.000 description 1
- MJQUEDHRCUIRLF-TVIXENOKSA-N bryostatin 1 Chemical compound C([C@@H]1CC(/[C@@H]([C@@](C(C)(C)/C=C/2)(O)O1)OC(=O)/C=C/C=C/CCC)=C\C(=O)OC)[C@H]([C@@H](C)O)OC(=O)C[C@H](O)C[C@@H](O1)C[C@H](OC(C)=O)C(C)(C)[C@]1(O)C[C@@H]1C\C(=C\C(=O)OC)C[C@H]\2O1 MJQUEDHRCUIRLF-TVIXENOKSA-N 0.000 description 1
- MUIWQCKLQMOUAT-AKUNNTHJSA-N bryostatin 20 Natural products COC(=O)C=C1C[C@@]2(C)C[C@]3(O)O[C@](C)(C[C@@H](O)CC(=O)O[C@](C)(C[C@@]4(C)O[C@](O)(CC5=CC(=O)O[C@]45C)C(C)(C)C=C[C@@](C)(C1)O2)[C@@H](C)O)C[C@H](OC(=O)C(C)(C)C)C3(C)C MUIWQCKLQMOUAT-AKUNNTHJSA-N 0.000 description 1
- 108700002839 cactinomycin Proteins 0.000 description 1
- 229950009908 cactinomycin Drugs 0.000 description 1
- FLJPGEWQYJVDPF-UHFFFAOYSA-L caesium sulfate Chemical compound [Cs+].[Cs+].[O-]S([O-])(=O)=O FLJPGEWQYJVDPF-UHFFFAOYSA-L 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- AXCZMVOFGPJBDE-UHFFFAOYSA-L calcium dihydroxide Chemical compound [OH-].[OH-].[Ca+2] AXCZMVOFGPJBDE-UHFFFAOYSA-L 0.000 description 1
- 239000000920 calcium hydroxide Substances 0.000 description 1
- 229910001861 calcium hydroxide Inorganic materials 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 208000035269 cancer or benign tumor Diseases 0.000 description 1
- 229960004117 capecitabine Drugs 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 125000003917 carbamoyl group Chemical group [H]N([H])C(*)=O 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 229920003123 carboxymethyl cellulose sodium Polymers 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 229940105329 carboxymethylcellulose Drugs 0.000 description 1
- 229940063834 carboxymethylcellulose sodium Drugs 0.000 description 1
- 208000002458 carcinoid tumor Diseases 0.000 description 1
- XREUEWVEMYWFFA-CSKJXFQVSA-N carminomycin Chemical compound C1[C@H](N)[C@H](O)[C@H](C)O[C@H]1O[C@@H]1C2=C(O)C(C(=O)C3=C(O)C=CC=C3C3=O)=C3C(O)=C2C[C@@](O)(C(C)=O)C1 XREUEWVEMYWFFA-CSKJXFQVSA-N 0.000 description 1
- 229930188550 carminomycin Natural products 0.000 description 1
- XREUEWVEMYWFFA-UHFFFAOYSA-N carminomycin I Natural products C1C(N)C(O)C(C)OC1OC1C2=C(O)C(C(=O)C3=C(O)C=CC=C3C3=O)=C3C(O)=C2CC(O)(C(C)=O)C1 XREUEWVEMYWFFA-UHFFFAOYSA-N 0.000 description 1
- 229960005243 carmustine Drugs 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 229950001725 carubicin Drugs 0.000 description 1
- BBZDXMBRAFTCAA-AREMUKBSSA-N carzelesin Chemical compound C1=2NC=C(C)C=2C([C@H](CCl)CN2C(=O)C=3NC4=CC=C(C=C4C=3)NC(=O)C3=CC4=CC=C(C=C4O3)N(CC)CC)=C2C=C1OC(=O)NC1=CC=CC=C1 BBZDXMBRAFTCAA-AREMUKBSSA-N 0.000 description 1
- 229950007509 carzelesin Drugs 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- CZTQZXZIADLWOZ-CRAIPNDOSA-N cefaloridine Chemical compound O=C([C@@H](NC(=O)CC=1SC=CC=1)[C@H]1SC2)N1C(C(=O)[O-])=C2C[N+]1=CC=CC=C1 CZTQZXZIADLWOZ-CRAIPNDOSA-N 0.000 description 1
- 230000022131 cell cycle Effects 0.000 description 1
- 230000030833 cell death Effects 0.000 description 1
- 230000022534 cell killing Effects 0.000 description 1
- 230000006727 cell loss Effects 0.000 description 1
- 235000010980 cellulose Nutrition 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229940081734 cellulose acetate phthalate Drugs 0.000 description 1
- 230000002490 cerebral effect Effects 0.000 description 1
- 210000002939 cerumen Anatomy 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 230000000973 chemotherapeutic effect Effects 0.000 description 1
- 229940044683 chemotherapy drug Drugs 0.000 description 1
- WIIZWVCIJKGZOK-RKDXNWHRSA-N chloramphenicol Chemical compound ClC(Cl)C(=O)N[C@H](CO)[C@H](O)C1=CC=C([N+]([O-])=O)C=C1 WIIZWVCIJKGZOK-RKDXNWHRSA-N 0.000 description 1
- 229960005091 chloramphenicol Drugs 0.000 description 1
- 229960004926 chlorobutanol Drugs 0.000 description 1
- 201000005217 chondroblastoma Diseases 0.000 description 1
- 210000004252 chorionic villi Anatomy 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- 239000011651 chromium Substances 0.000 description 1
- 201000010240 chromophobe renal cell carcinoma Diseases 0.000 description 1
- 230000002759 chromosomal effect Effects 0.000 description 1
- 210000000349 chromosome Anatomy 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 1
- 229960004316 cisplatin Drugs 0.000 description 1
- 208000009060 clear cell adenocarcinoma Diseases 0.000 description 1
- ACSIXWWBWUQEHA-UHFFFAOYSA-N clodronic acid Chemical compound OP(O)(=O)C(Cl)(Cl)P(O)(O)=O ACSIXWWBWUQEHA-UHFFFAOYSA-N 0.000 description 1
- 229960002286 clodronic acid Drugs 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 238000004440 column chromatography Methods 0.000 description 1
- 230000021615 conjugation Effects 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 239000012004 corey–bakshi–shibata catalyst Substances 0.000 description 1
- 230000001054 cortical effect Effects 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 229960001681 croscarmellose sodium Drugs 0.000 description 1
- 235000010947 crosslinked sodium carboxy methyl cellulose Nutrition 0.000 description 1
- 239000012043 crude product Substances 0.000 description 1
- 238000002681 cryosurgery Methods 0.000 description 1
- 108010006226 cryptophycin Proteins 0.000 description 1
- 108010089438 cryptophycin 1 Proteins 0.000 description 1
- 108010090203 cryptophycin 8 Proteins 0.000 description 1
- 150000001925 cycloalkenes Chemical class 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- MAWOHFOSAIXURX-UHFFFAOYSA-N cyclopentylcyclopentane Chemical compound C1CCCC1C1CCCC1 MAWOHFOSAIXURX-UHFFFAOYSA-N 0.000 description 1
- 208000002445 cystadenocarcinoma Diseases 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 description 1
- 231100000599 cytotoxic agent Toxicity 0.000 description 1
- 238000011393 cytotoxic chemotherapy Methods 0.000 description 1
- 239000002619 cytotoxin Substances 0.000 description 1
- 229960000640 dactinomycin Drugs 0.000 description 1
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 1
- 229960000975 daunorubicin Drugs 0.000 description 1
- 206010061428 decreased appetite Diseases 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 238000000151 deposition Methods 0.000 description 1
- URLVCROWVOSNPT-QTTMQESMSA-N desacetyluvaricin Natural products O=C1C(CCCCCCCCCCCC[C@@H](O)[C@H]2O[C@@H]([C@@H]3O[C@@H]([C@@H](O)CCCCCCCCCC)CC3)CC2)=C[C@H](C)O1 URLVCROWVOSNPT-QTTMQESMSA-N 0.000 description 1
- 230000000249 desinfective effect Effects 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 229910052805 deuterium Inorganic materials 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- 125000005265 dialkylamine group Chemical group 0.000 description 1
- WVYXNIXAMZOZFK-UHFFFAOYSA-N diaziquone Chemical compound O=C1C(NC(=O)OCC)=C(N2CC2)C(=O)C(NC(=O)OCC)=C1N1CC1 WVYXNIXAMZOZFK-UHFFFAOYSA-N 0.000 description 1
- 229950002389 diaziquone Drugs 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- BGRWYRAHAFMIBJ-UHFFFAOYSA-N diisopropylcarbodiimide Natural products CC(C)NC(=O)NC(C)C BGRWYRAHAFMIBJ-UHFFFAOYSA-N 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 239000004205 dimethyl polysiloxane Substances 0.000 description 1
- 239000003534 dna topoisomerase inhibitor Substances 0.000 description 1
- AMRJKAQTDDKMCE-UHFFFAOYSA-N dolastatin Chemical compound CC(C)C(N(C)C)C(=O)NC(C(C)C)C(=O)N(C)C(C(C)C)C(OC)CC(=O)N1CCCC1C(OC)C(C)C(=O)NC(C=1SC=CN=1)CC1=CC=CC=C1 AMRJKAQTDDKMCE-UHFFFAOYSA-N 0.000 description 1
- 229930188854 dolastatin Natural products 0.000 description 1
- ZWAOHEXOSAUJHY-ZIYNGMLESA-N doxifluridine Chemical compound O[C@@H]1[C@H](O)[C@@H](C)O[C@H]1N1C(=O)NC(=O)C(F)=C1 ZWAOHEXOSAUJHY-ZIYNGMLESA-N 0.000 description 1
- 229950005454 doxifluridine Drugs 0.000 description 1
- 230000002900 effect on cell Effects 0.000 description 1
- VLCYCQAOQCDTCN-UHFFFAOYSA-N eflornithine Chemical compound NCCCC(N)(C(F)F)C(O)=O VLCYCQAOQCDTCN-UHFFFAOYSA-N 0.000 description 1
- 229930185791 eluterin Natural products 0.000 description 1
- 201000009409 embryonal rhabdomyosarcoma Diseases 0.000 description 1
- 210000001671 embryonic stem cell Anatomy 0.000 description 1
- 125000001240 enamine group Chemical group 0.000 description 1
- 150000002081 enamines Chemical class 0.000 description 1
- 201000003914 endometrial carcinoma Diseases 0.000 description 1
- 201000009274 endometriosis of uterus Diseases 0.000 description 1
- 230000003511 endothelial effect Effects 0.000 description 1
- 229950011487 enocitabine Drugs 0.000 description 1
- 125000002587 enol group Chemical group 0.000 description 1
- 238000009505 enteric coating Methods 0.000 description 1
- 239000002702 enteric coating Substances 0.000 description 1
- 230000002327 eosinophilic effect Effects 0.000 description 1
- 230000001037 epileptic effect Effects 0.000 description 1
- 229960001904 epirubicin Drugs 0.000 description 1
- 210000002919 epithelial cell Anatomy 0.000 description 1
- 229950002973 epitiostanol Drugs 0.000 description 1
- 229930013356 epothilone Natural products 0.000 description 1
- 150000003883 epothilone derivatives Chemical class 0.000 description 1
- 230000008029 eradication Effects 0.000 description 1
- ITSGNOIFAJAQHJ-BMFNZSJVSA-N esorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)C[C@H](C)O1 ITSGNOIFAJAQHJ-BMFNZSJVSA-N 0.000 description 1
- 229950002017 esorubicin Drugs 0.000 description 1
- LJQQFQHBKUKHIS-WJHRIEJJSA-N esperamicin Chemical compound O1CC(NC(C)C)C(OC)CC1OC1C(O)C(NOC2OC(C)C(SC)C(O)C2)C(C)OC1OC1C(\C2=C/CSSSC)=C(NC(=O)OC)C(=O)C(OC3OC(C)C(O)C(OC(=O)C=4C(=CC(OC)=C(OC)C=4)NC(=O)C(=C)OC)C3)C2(O)C#C\C=C/C#C1 LJQQFQHBKUKHIS-WJHRIEJJSA-N 0.000 description 1
- 102000015694 estrogen receptors Human genes 0.000 description 1
- 108010038795 estrogen receptors Proteins 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- 125000001301 ethoxy group Chemical group [H]C([H])([H])C([H])([H])O* 0.000 description 1
- 229960005237 etoglucid Drugs 0.000 description 1
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 229960004887 ferric hydroxide Drugs 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 238000009501 film coating Methods 0.000 description 1
- 239000007888 film coating Substances 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 238000007667 floating Methods 0.000 description 1
- 108700014844 flt3 ligand Proteins 0.000 description 1
- 229960000390 fludarabine Drugs 0.000 description 1
- GIUYCYHIANZCFB-FJFJXFQQSA-N fludarabine phosphate Chemical compound C1=NC=2C(N)=NC(F)=NC=2N1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@@H]1O GIUYCYHIANZCFB-FJFJXFQQSA-N 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 125000001153 fluoro group Chemical group F* 0.000 description 1
- 239000004088 foaming agent Substances 0.000 description 1
- 102000006815 folate receptor Human genes 0.000 description 1
- 108020005243 folate receptor Proteins 0.000 description 1
- 230000037406 food intake Effects 0.000 description 1
- 238000013467 fragmentation Methods 0.000 description 1
- 238000006062 fragmentation reaction Methods 0.000 description 1
- 239000012458 free base Substances 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 125000002541 furyl group Chemical group 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 229940044658 gallium nitrate Drugs 0.000 description 1
- 208000015419 gastrin-producing neuroendocrine tumor Diseases 0.000 description 1
- 201000000052 gastrinoma Diseases 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 230000030279 gene silencing Effects 0.000 description 1
- 238000012226 gene silencing method Methods 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 210000004907 gland Anatomy 0.000 description 1
- 235000001727 glucose Nutrition 0.000 description 1
- 229940074045 glyceryl distearate Drugs 0.000 description 1
- 229940075507 glyceryl monostearate Drugs 0.000 description 1
- YMAWOPBAYDPSLA-UHFFFAOYSA-N glycylglycine Chemical compound [NH3+]CC(=O)NCC([O-])=O YMAWOPBAYDPSLA-UHFFFAOYSA-N 0.000 description 1
- 201000003872 goiter Diseases 0.000 description 1
- 201000009277 hairy cell leukemia Diseases 0.000 description 1
- 229910052736 halogen Inorganic materials 0.000 description 1
- 150000002367 halogens Chemical class 0.000 description 1
- 230000035876 healing Effects 0.000 description 1
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 1
- 208000006359 hepatoblastoma Diseases 0.000 description 1
- DMEGYFMYUHOHGS-UHFFFAOYSA-N heptamethylene Natural products C1CCCCCC1 DMEGYFMYUHOHGS-UHFFFAOYSA-N 0.000 description 1
- 229940022353 herceptin Drugs 0.000 description 1
- 125000005553 heteroaryloxy group Chemical group 0.000 description 1
- 208000029824 high grade glioma Diseases 0.000 description 1
- 210000003630 histaminocyte Anatomy 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 108091008039 hormone receptors Proteins 0.000 description 1
- 238000001794 hormone therapy Methods 0.000 description 1
- 150000004677 hydrates Chemical class 0.000 description 1
- 150000003840 hydrochlorides Chemical class 0.000 description 1
- 239000008172 hydrogenated vegetable oil Substances 0.000 description 1
- 125000002349 hydroxyamino group Chemical group [H]ON([H])[*] 0.000 description 1
- 229960001330 hydroxycarbamide Drugs 0.000 description 1
- 229920003132 hydroxypropyl methylcellulose phthalate Polymers 0.000 description 1
- 229940031704 hydroxypropyl methylcellulose phthalate Drugs 0.000 description 1
- 229920000639 hydroxypropylmethylcellulose acetate succinate Polymers 0.000 description 1
- 150000002466 imines Chemical class 0.000 description 1
- 125000001841 imino group Chemical group [H]N=* 0.000 description 1
- 210000002865 immune cell Anatomy 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 229940072221 immunoglobulins Drugs 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 125000003453 indazolyl group Chemical group N1N=C(C2=C1C=CC=C2)* 0.000 description 1
- 125000001041 indolyl group Chemical group 0.000 description 1
- 239000000411 inducer Substances 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 239000003701 inert diluent Substances 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 239000003978 infusion fluid Substances 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 239000007972 injectable composition Substances 0.000 description 1
- 150000007529 inorganic bases Chemical class 0.000 description 1
- 150000002484 inorganic compounds Chemical class 0.000 description 1
- 229910010272 inorganic material Inorganic materials 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 229940047122 interleukins Drugs 0.000 description 1
- 230000004068 intracellular signaling Effects 0.000 description 1
- 230000002601 intratumoral effect Effects 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- 206010073095 invasive ductal breast carcinoma Diseases 0.000 description 1
- 201000010985 invasive ductal carcinoma Diseases 0.000 description 1
- 206010073096 invasive lobular breast carcinoma Diseases 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- KLEAIHJJLUAXIQ-JDRGBKBRSA-N irinotecan hydrochloride hydrate Chemical compound O.O.O.Cl.C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 KLEAIHJJLUAXIQ-JDRGBKBRSA-N 0.000 description 1
- IEECXTSVVFWGSE-UHFFFAOYSA-M iron(3+);oxygen(2-);hydroxide Chemical compound [OH-].[O-2].[Fe+3] IEECXTSVVFWGSE-UHFFFAOYSA-M 0.000 description 1
- QRWOVIRDHQJFDB-UHFFFAOYSA-N isobutyl cyanoacrylate Chemical compound CC(C)COC(=O)C(=C)C#N QRWOVIRDHQJFDB-UHFFFAOYSA-N 0.000 description 1
- 125000003253 isopropoxy group Chemical group [H]C([H])([H])C([H])(O*)C([H])([H])[H] 0.000 description 1
- JJWLVOIRVHMVIS-UHFFFAOYSA-N isopropylamine Chemical compound CC(C)N JJWLVOIRVHMVIS-UHFFFAOYSA-N 0.000 description 1
- 239000000644 isotonic solution Substances 0.000 description 1
- 230000000155 isotopic effect Effects 0.000 description 1
- 125000000842 isoxazolyl group Chemical group 0.000 description 1
- 125000000468 ketone group Chemical group 0.000 description 1
- 208000022013 kidney Wilms tumor Diseases 0.000 description 1
- 230000002147 killing effect Effects 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 210000002429 large intestine Anatomy 0.000 description 1
- 238000011031 large-scale manufacturing process Methods 0.000 description 1
- 238000002430 laser surgery Methods 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 229940115286 lentinan Drugs 0.000 description 1
- 231100001231 less toxic Toxicity 0.000 description 1
- 125000003473 lipid group Chemical group 0.000 description 1
- 238000007443 liposuction Methods 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- PCZOHLXUXFIOCF-BXMDZJJMSA-N lovastatin Chemical compound C([C@H]1[C@@H](C)C=CC2=C[C@H](C)C[C@@H]([C@H]12)OC(=O)[C@@H](C)CC)C[C@@H]1C[C@@H](O)CC(=O)O1 PCZOHLXUXFIOCF-BXMDZJJMSA-N 0.000 description 1
- 229960004844 lovastatin Drugs 0.000 description 1
- QLJODMDSTUBWDW-UHFFFAOYSA-N lovastatin hydroxy acid Natural products C1=CC(C)C(CCC(O)CC(O)CC(O)=O)C2C(OC(=O)C(C)CC)CC(C)C=C21 QLJODMDSTUBWDW-UHFFFAOYSA-N 0.000 description 1
- 201000000014 lung giant cell carcinoma Diseases 0.000 description 1
- 201000010953 lymphoepithelioma-like carcinoma Diseases 0.000 description 1
- 208000003747 lymphoid leukemia Diseases 0.000 description 1
- 208000019420 lymphoid neoplasm Diseases 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 201000011614 malignant glioma Diseases 0.000 description 1
- 229960002510 mandelic acid Drugs 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 238000001819 mass spectrum Methods 0.000 description 1
- 208000000516 mast-cell leukemia Diseases 0.000 description 1
- 201000008749 mast-cell sarcoma Diseases 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 206010027191 meningioma Diseases 0.000 description 1
- 229960001428 mercaptopurine Drugs 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 229910052987 metal hydride Inorganic materials 0.000 description 1
- 150000004681 metal hydrides Chemical class 0.000 description 1
- 229910000000 metal hydroxide Inorganic materials 0.000 description 1
- 150000004692 metal hydroxides Chemical class 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- 229920003145 methacrylic acid copolymer Polymers 0.000 description 1
- 229940117841 methacrylic acid copolymer Drugs 0.000 description 1
- QARBMVPHQWIHKH-UHFFFAOYSA-N methanesulfonyl chloride Chemical compound CS(Cl)(=O)=O QARBMVPHQWIHKH-UHFFFAOYSA-N 0.000 description 1
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 1
- 125000004184 methoxymethyl group Chemical group [H]C([H])([H])OC([H])([H])* 0.000 description 1
- IFIIAGLCRNWWLX-JOYOIKCWSA-N methyl (2s,4r)-4-amino-5-(4-fluorophenyl)-2-methylpentanoate Chemical compound COC(=O)[C@@H](C)C[C@@H](N)CC1=CC=C(F)C=C1 IFIIAGLCRNWWLX-JOYOIKCWSA-N 0.000 description 1
- QRMNENFZDDYDEF-GOSISDBHSA-N methyl (8s)-8-(bromomethyl)-2-methyl-4-(4-methylpiperazine-1-carbonyl)oxy-6-(5,6,7-trimethoxy-1h-indole-2-carbonyl)-7,8-dihydro-3h-pyrrolo[3,2-e]indole-1-carboxylate Chemical compound C1([C@H](CBr)CN(C1=C1)C(=O)C=2NC3=C(OC)C(OC)=C(OC)C=C3C=2)=C2C(C(=O)OC)=C(C)NC2=C1OC(=O)N1CCN(C)CC1 QRMNENFZDDYDEF-GOSISDBHSA-N 0.000 description 1
- 125000000325 methylidene group Chemical group [H]C([H])=* 0.000 description 1
- HPNSFSBZBAHARI-UHFFFAOYSA-N micophenolic acid Natural products OC1=C(CC=C(C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-UHFFFAOYSA-N 0.000 description 1
- 239000003094 microcapsule Substances 0.000 description 1
- 239000004005 microsphere Substances 0.000 description 1
- 230000025090 microtubule depolymerization Effects 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- MXWHMTNPTTVWDM-NXOFHUPFSA-N mitoguazone Chemical compound NC(N)=N\N=C(/C)\C=N\N=C(N)N MXWHMTNPTTVWDM-NXOFHUPFSA-N 0.000 description 1
- 229960003539 mitoguazone Drugs 0.000 description 1
- 229960000350 mitotane Drugs 0.000 description 1
- 201000010225 mixed cell type cancer Diseases 0.000 description 1
- 208000029638 mixed neoplasm Diseases 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 125000002950 monocyclic group Chemical group 0.000 description 1
- 125000002757 morpholinyl group Chemical group 0.000 description 1
- 239000002324 mouth wash Substances 0.000 description 1
- 230000036457 multidrug resistance Effects 0.000 description 1
- HPNSFSBZBAHARI-RUDMXATFSA-N mycophenolic acid Chemical compound OC1=C(C\C=C(/C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-RUDMXATFSA-N 0.000 description 1
- 229960000951 mycophenolic acid Drugs 0.000 description 1
- 201000005987 myeloid sarcoma Diseases 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000001624 naphthyl group Chemical group 0.000 description 1
- 208000014761 nasopharyngeal type undifferentiated carcinoma Diseases 0.000 description 1
- 230000017074 necrotic cell death Effects 0.000 description 1
- 208000025426 neoplasm of thorax Diseases 0.000 description 1
- 210000005170 neoplastic cell Anatomy 0.000 description 1
- 201000008026 nephroblastoma Diseases 0.000 description 1
- 210000004126 nerve fiber Anatomy 0.000 description 1
- 230000001272 neurogenic effect Effects 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 239000002547 new drug Substances 0.000 description 1
- 229960001420 nimustine Drugs 0.000 description 1
- VFEDRRNHLBGPNN-UHFFFAOYSA-N nimustine Chemical compound CC1=NC=C(CNC(=O)N(CCCl)N=O)C(N)=N1 VFEDRRNHLBGPNN-UHFFFAOYSA-N 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 238000000655 nuclear magnetic resonance spectrum Methods 0.000 description 1
- 238000001668 nucleic acid synthesis Methods 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- OIPZNTLJVJGRCI-UHFFFAOYSA-M octadecanoyloxyaluminum;dihydrate Chemical compound O.O.CCCCCCCCCCCCCCCCCC(=O)O[Al] OIPZNTLJVJGRCI-UHFFFAOYSA-M 0.000 description 1
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 150000007530 organic bases Chemical class 0.000 description 1
- 239000012074 organic phase Substances 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 125000001979 organolithium group Chemical group 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- DWAFYCQODLXJNR-BNTLRKBRSA-L oxaliplatin Chemical compound O1C(=O)C(=O)O[Pt]11N[C@@H]2CCCC[C@H]2N1 DWAFYCQODLXJNR-BNTLRKBRSA-L 0.000 description 1
- 229960001756 oxaliplatin Drugs 0.000 description 1
- 125000002971 oxazolyl group Chemical group 0.000 description 1
- KJIFKLIQANRMOU-UHFFFAOYSA-N oxidanium;4-methylbenzenesulfonate Chemical compound O.CC1=CC=C(S(O)(=O)=O)C=C1 KJIFKLIQANRMOU-UHFFFAOYSA-N 0.000 description 1
- 125000000466 oxiranyl group Chemical group 0.000 description 1
- KDLHZDBZIXYQEI-UHFFFAOYSA-N palladium Substances [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 1
- 238000011499 palliative surgery Methods 0.000 description 1
- 201000010198 papillary carcinoma Diseases 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 125000000538 pentafluorophenyl group Chemical group FC1=C(F)C(F)=C(*)C(F)=C1F 0.000 description 1
- 229960002340 pentostatin Drugs 0.000 description 1
- FPVKHBSQESCIEP-JQCXWYLXSA-N pentostatin Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(N=CNC[C@H]2O)=C2N=C1 FPVKHBSQESCIEP-JQCXWYLXSA-N 0.000 description 1
- 210000003668 pericyte Anatomy 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 229960003742 phenol Drugs 0.000 description 1
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- 102000020233 phosphotransferase Human genes 0.000 description 1
- 230000001766 physiological effect Effects 0.000 description 1
- 230000035790 physiological processes and functions Effects 0.000 description 1
- 229940081066 picolinic acid Drugs 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 238000011020 pilot scale process Methods 0.000 description 1
- 125000004193 piperazinyl group Chemical group 0.000 description 1
- 125000003386 piperidinyl group Chemical group 0.000 description 1
- BLFWHYXWBKKRHI-JYBILGDPSA-N plap Chemical compound N([C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(=O)[C@@H]1CCCN1C(=O)[C@H](CO)NC(=O)[C@@H](N)CCC(O)=O BLFWHYXWBKKRHI-JYBILGDPSA-N 0.000 description 1
- 208000031223 plasma cell leukemia Diseases 0.000 description 1
- 239000004014 plasticizer Substances 0.000 description 1
- 229920000435 poly(dimethylsiloxane) Polymers 0.000 description 1
- 239000002745 poly(ortho ester) Substances 0.000 description 1
- 229920001610 polycaprolactone Polymers 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 208000015768 polyposis Diseases 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 150000004804 polysaccharides Chemical class 0.000 description 1
- 229940100467 polyvinyl acetate phthalate Drugs 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 235000015497 potassium bicarbonate Nutrition 0.000 description 1
- 229910000028 potassium bicarbonate Inorganic materials 0.000 description 1
- 239000011736 potassium bicarbonate Substances 0.000 description 1
- TYJJADVDDVDEDZ-UHFFFAOYSA-M potassium hydrogencarbonate Chemical compound [K+].OC([O-])=O TYJJADVDDVDEDZ-UHFFFAOYSA-M 0.000 description 1
- BDAWXSQJJCIFIK-UHFFFAOYSA-N potassium methoxide Chemical compound [K+].[O-]C BDAWXSQJJCIFIK-UHFFFAOYSA-N 0.000 description 1
- 239000012286 potassium permanganate Substances 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 238000012746 preparative thin layer chromatography Methods 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 238000002203 pretreatment Methods 0.000 description 1
- 150000003141 primary amines Chemical class 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 230000035755 proliferation Effects 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 150000003180 prostaglandins Chemical class 0.000 description 1
- 201000008520 protoplasmic astrocytoma Diseases 0.000 description 1
- 229950010131 puromycin Drugs 0.000 description 1
- 125000004309 pyranyl group Chemical group O1C(C=CC=C1)* 0.000 description 1
- 125000004076 pyridyl group Chemical group 0.000 description 1
- 150000003230 pyrimidines Chemical class 0.000 description 1
- 125000000714 pyrimidinyl group Chemical group 0.000 description 1
- 125000000719 pyrrolidinyl group Chemical group 0.000 description 1
- 125000000168 pyrrolyl group Chemical group 0.000 description 1
- 125000002294 quinazolinyl group Chemical group N1=C(N=CC2=CC=CC=C12)* 0.000 description 1
- 125000005493 quinolyl group Chemical group 0.000 description 1
- 125000001567 quinoxalinyl group Chemical group N1=C(C=NC2=CC=CC=C12)* 0.000 description 1
- UOWVMDUEMSNCAV-WYENRQIDSA-N rachelmycin Chemical compound C1([C@]23C[C@@H]2CN1C(=O)C=1NC=2C(OC)=C(O)C4=C(C=2C=1)CCN4C(=O)C1=CC=2C=4CCN(C=4C(O)=C(C=2N1)OC)C(N)=O)=CC(=O)C1=C3C(C)=CN1 UOWVMDUEMSNCAV-WYENRQIDSA-N 0.000 description 1
- 239000002534 radiation-sensitizing agent Substances 0.000 description 1
- 239000012857 radioactive material Substances 0.000 description 1
- 229940124553 radioprotectant Drugs 0.000 description 1
- GZUITABIAKMVPG-UHFFFAOYSA-N raloxifene Chemical compound C1=CC(O)=CC=C1C1=C(C(=O)C=2C=CC(OCCN3CCCCC3)=CC=2)C2=CC=C(O)C=C2S1 GZUITABIAKMVPG-UHFFFAOYSA-N 0.000 description 1
- 229960004622 raloxifene Drugs 0.000 description 1
- 230000008929 regeneration Effects 0.000 description 1
- 238000011069 regeneration method Methods 0.000 description 1
- 230000008439 repair process Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 229930002330 retinoic acid Natural products 0.000 description 1
- MBABCNBNDNGODA-WPZDJQSSSA-N rolliniastatin 1 Natural products O1[C@@H]([C@@H](O)CCCCCCCCCC)CC[C@H]1[C@H]1O[C@@H]([C@H](O)CCCCCCCCCC[C@@H](O)CC=2C(O[C@@H](C)C=2)=O)CC1 MBABCNBNDNGODA-WPZDJQSSSA-N 0.000 description 1
- VHXNKPBCCMUMSW-FQEVSTJZSA-N rubitecan Chemical compound C1=CC([N+]([O-])=O)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 VHXNKPBCCMUMSW-FQEVSTJZSA-N 0.000 description 1
- 208000014212 sarcomatoid carcinoma Diseases 0.000 description 1
- 238000013341 scale-up Methods 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 150000003335 secondary amines Chemical class 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 230000001235 sensitizing effect Effects 0.000 description 1
- 210000000717 sertoli cell Anatomy 0.000 description 1
- ZLGIYFNHBLSMPS-ATJNOEHPSA-N shellac Chemical compound OCCCCCC(O)C(O)CCCCCCCC(O)=O.C1C23[C@H](C(O)=O)CCC2[C@](C)(CO)[C@@H]1C(C(O)=O)=C[C@@H]3O ZLGIYFNHBLSMPS-ATJNOEHPSA-N 0.000 description 1
- 239000004208 shellac Substances 0.000 description 1
- 229940113147 shellac Drugs 0.000 description 1
- 235000013874 shellac Nutrition 0.000 description 1
- 125000005630 sialyl group Chemical group 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 238000009097 single-agent therapy Methods 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 208000000649 small cell carcinoma Diseases 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- 235000010413 sodium alginate Nutrition 0.000 description 1
- 239000000661 sodium alginate Substances 0.000 description 1
- 229940005550 sodium alginate Drugs 0.000 description 1
- MFRIHAYPQRLWNB-UHFFFAOYSA-N sodium tert-butoxide Chemical compound [Na+].CC(C)(C)[O-] MFRIHAYPQRLWNB-UHFFFAOYSA-N 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 230000003595 spectral effect Effects 0.000 description 1
- 238000004611 spectroscopical analysis Methods 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 229950006315 spirogermanium Drugs 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 230000001954 sterilising effect Effects 0.000 description 1
- 238000004659 sterilization and disinfection Methods 0.000 description 1
- ZSJLQEPLLKMAKR-GKHCUFPYSA-N streptozocin Chemical compound O=NN(C)C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O ZSJLQEPLLKMAKR-GKHCUFPYSA-N 0.000 description 1
- 229960001052 streptozocin Drugs 0.000 description 1
- 125000005017 substituted alkenyl group Chemical group 0.000 description 1
- 238000009495 sugar coating Methods 0.000 description 1
- PXQLVRUNWNTZOS-UHFFFAOYSA-N sulfanyl Chemical compound [SH] PXQLVRUNWNTZOS-UHFFFAOYSA-N 0.000 description 1
- 125000000475 sulfinyl group Chemical group [*:2]S([*:1])=O 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 206010042863 synovial sarcoma Diseases 0.000 description 1
- 238000001308 synthesis method Methods 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 101150047061 tag-72 gene Proteins 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 229960001603 tamoxifen Drugs 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 125000006633 tert-butoxycarbonylamino group Chemical group 0.000 description 1
- BCNZYOJHNLTNEZ-UHFFFAOYSA-N tert-butyldimethylsilyl chloride Chemical compound CC(C)(C)[Si](C)(C)Cl BCNZYOJHNLTNEZ-UHFFFAOYSA-N 0.000 description 1
- 125000001981 tert-butyldimethylsilyl group Chemical group [H]C([H])([H])[Si]([H])(C([H])([H])[H])[*]C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- BPEWUONYVDABNZ-DZBHQSCQSA-N testolactone Chemical compound O=C1C=C[C@]2(C)[C@H]3CC[C@](C)(OC(=O)CC4)[C@@H]4[C@@H]3CCC2=C1 BPEWUONYVDABNZ-DZBHQSCQSA-N 0.000 description 1
- 229960005353 testolactone Drugs 0.000 description 1
- 125000003718 tetrahydrofuranyl group Chemical group 0.000 description 1
- 125000004187 tetrahydropyran-2-yl group Chemical group [H]C1([H])OC([H])(*)C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- 125000001412 tetrahydropyranyl group Chemical group 0.000 description 1
- 125000004632 tetrahydrothiopyranyl group Chemical group S1C(CCCC1)* 0.000 description 1
- 125000003831 tetrazolyl group Chemical group 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 125000001544 thienyl group Chemical group 0.000 description 1
- RTKIYNMVFMVABJ-UHFFFAOYSA-L thimerosal Chemical compound [Na+].CC[Hg]SC1=CC=CC=C1C([O-])=O RTKIYNMVFMVABJ-UHFFFAOYSA-L 0.000 description 1
- 229940033663 thimerosal Drugs 0.000 description 1
- 125000003441 thioacyl group Chemical group 0.000 description 1
- 125000002813 thiocarbonyl group Chemical group *C(*)=S 0.000 description 1
- 125000004568 thiomorpholinyl group Chemical group 0.000 description 1
- 201000003957 thoracic cancer Diseases 0.000 description 1
- 208000030901 thyroid gland follicular carcinoma Diseases 0.000 description 1
- YFTWHEBLORWGNI-UHFFFAOYSA-N tiamiprine Chemical compound CN1C=NC([N+]([O-])=O)=C1SC1=NC(N)=NC2=C1NC=N2 YFTWHEBLORWGNI-UHFFFAOYSA-N 0.000 description 1
- 229950011457 tiamiprine Drugs 0.000 description 1
- 125000003944 tolyl group Chemical group 0.000 description 1
- 210000002105 tongue Anatomy 0.000 description 1
- 229940044693 topoisomerase inhibitor Drugs 0.000 description 1
- UCFGDBYHRUNTLO-QHCPKHFHSA-N topotecan Chemical compound C1=C(O)C(CN(C)C)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 UCFGDBYHRUNTLO-QHCPKHFHSA-N 0.000 description 1
- 229960000303 topotecan Drugs 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 239000003558 transferase inhibitor Substances 0.000 description 1
- 229960000575 trastuzumab Drugs 0.000 description 1
- IUCJMVBFZDHPDX-UHFFFAOYSA-N tretamine Chemical compound C1CN1C1=NC(N2CC2)=NC(N2CC2)=N1 IUCJMVBFZDHPDX-UHFFFAOYSA-N 0.000 description 1
- 229950001353 tretamine Drugs 0.000 description 1
- 229960001727 tretinoin Drugs 0.000 description 1
- 125000005270 trialkylamine group Chemical group 0.000 description 1
- 125000004306 triazinyl group Chemical group 0.000 description 1
- 125000001425 triazolyl group Chemical group 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- LZAJKCZTKKKZNT-PMNGPLLRSA-N trichothecene Chemical compound C12([C@@]3(CC[C@H]2OC2C=C(CCC23C)C)C)CO1 LZAJKCZTKKKZNT-PMNGPLLRSA-N 0.000 description 1
- 229930013292 trichothecene Natural products 0.000 description 1
- KVJXBPDAXMEYOA-CXANFOAXSA-N trilostane Chemical compound OC1=C(C#N)C[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CC[C@@]32O[C@@H]31 KVJXBPDAXMEYOA-CXANFOAXSA-N 0.000 description 1
- 229960001670 trilostane Drugs 0.000 description 1
- MBYLVOKEDDQJDY-UHFFFAOYSA-N tris(2-aminoethyl)amine Chemical compound NCCN(CCN)CCN MBYLVOKEDDQJDY-UHFFFAOYSA-N 0.000 description 1
- 229910052722 tritium Inorganic materials 0.000 description 1
- HDZZVAMISRMYHH-LITAXDCLSA-N tubercidin Chemical compound C1=CC=2C(N)=NC=NC=2N1[C@@H]1O[C@@H](CO)[C@H](O)[C@H]1O HDZZVAMISRMYHH-LITAXDCLSA-N 0.000 description 1
- 102000003390 tumor necrosis factor Human genes 0.000 description 1
- 150000003668 tyrosines Chemical class 0.000 description 1
- 238000009281 ultraviolet germicidal irradiation Methods 0.000 description 1
- 229960001055 uracil mustard Drugs 0.000 description 1
- 210000004291 uterus Anatomy 0.000 description 1
- 229960005486 vaccine Drugs 0.000 description 1
- 238000001291 vacuum drying Methods 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 239000003981 vehicle Substances 0.000 description 1
- UGGWPQSBPIFKDZ-KOTLKJBCSA-N vindesine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(N)=O)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1N=C1[C]2C=CC=C1 UGGWPQSBPIFKDZ-KOTLKJBCSA-N 0.000 description 1
- 229960004355 vindesine Drugs 0.000 description 1
- GBABOYUKABKIAF-GHYRFKGUSA-N vinorelbine Chemical compound C1N(CC=2C3=CC=CC=C3NC=22)CC(CC)=C[C@H]1C[C@]2(C(=O)OC)C1=CC([C@]23[C@H]([C@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC GBABOYUKABKIAF-GHYRFKGUSA-N 0.000 description 1
- 229960002066 vinorelbine Drugs 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 210000001835 viscera Anatomy 0.000 description 1
- XOOUIPVCVHRTMJ-UHFFFAOYSA-L zinc stearate Chemical compound [Zn+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O XOOUIPVCVHRTMJ-UHFFFAOYSA-L 0.000 description 1
- 229950009268 zinostatin Drugs 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K5/00—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof
- C07K5/04—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing only normal peptide links
- C07K5/06—Dipeptides
- C07K5/06139—Dipeptides with the first amino acid being heterocyclic
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/445—Non condensed piperidines, e.g. piperocaine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6801—Drug-antibody or immunoglobulin conjugates defined by the pharmacologically or therapeutically active agent
- A61K47/6803—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6835—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site
- A61K47/6851—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site the antibody targeting a determinant of a tumour cell
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D277/00—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings
- C07D277/02—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings not condensed with other rings
- C07D277/20—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D277/32—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D277/56—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings
- C07D417/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D453/00—Heterocyclic compounds containing quinuclidine or iso-quinuclidine ring systems, e.g. quinine alkaloids
- C07D453/02—Heterocyclic compounds containing quinuclidine or iso-quinuclidine ring systems, e.g. quinine alkaloids containing not further condensed quinuclidine ring systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K5/00—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof
- C07K5/02—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing at least one abnormal peptide link
- C07K5/021—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing at least one abnormal peptide link containing the structure -NH-(X)n-C(=0)-, n being 5 or 6; for n > 6, classification in C07K5/06 - C07K5/10, according to the moiety having normal peptide bonds
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K5/00—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof
- C07K5/04—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing only normal peptide links
- C07K5/06—Dipeptides
- C07K5/06139—Dipeptides with the first amino acid being heterocyclic
- C07K5/06165—Dipeptides with the first amino acid being heterocyclic and Pro-amino acid; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- Crystallography & Structural Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Immunology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Cell Biology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Nitrogen And Oxygen Or Sulfur-Condensed Heterocyclic Ring Systems (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Heterocyclic Carbon Compounds Containing A Hetero Ring Having Oxygen Or Sulfur (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562120613P | 2015-02-25 | 2015-02-25 | |
| US62/120,613 | 2015-02-25 | ||
| US201662275667P | 2016-01-06 | 2016-01-06 | |
| US62/275,667 | 2016-01-06 | ||
| PCT/US2016/019604 WO2016138288A1 (en) | 2015-02-25 | 2016-02-25 | Desacetoxytubulysin h and analogs thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20170137725A true KR20170137725A (ko) | 2017-12-13 |
Family
ID=56789084
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020177027116A Withdrawn KR20170137725A (ko) | 2015-02-25 | 2016-02-25 | 데스아세톡시투불리신 h 및 이의 유사체 |
Country Status (22)
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10829519B2 (en) | 2015-09-18 | 2020-11-10 | Wake Forest University Health Sciences | Angiotensin (1-7) analogs and methods relating thereto |
| CA2914601A1 (en) * | 2015-12-11 | 2017-06-11 | Wake Forest University Health Sciences | Angiotensin-(1-7) analogs and methods relating thereto |
| JP2020525513A (ja) * | 2017-07-03 | 2020-08-27 | グラクソスミスクライン、インテレクチュアル、プロパティー、ディベロップメント、リミテッドGlaxosmithkline Intellectual Property Development Limited | 癌および他の疾患を治療するためのatf4阻害剤としてのn−(3−(2−(4−クロロフェノキシ)アセトアミドビシクロ[1.1.1]ペンタン−1−イル)−2−シクロブタン−1−カルボキサミド誘導体および関連化合物 |
| PH12020550028B1 (en) | 2017-08-09 | 2024-05-24 | Denali Therapeutics Inc | Compounds, compositions and methods |
| DK3676297T3 (da) | 2017-09-01 | 2023-08-14 | Denali Therapeutics Inc | Forbindelser, sammensætninger og fremgangsmåder |
| CN111601803A (zh) | 2017-09-08 | 2020-08-28 | 西雅图基因公司 | 微管溶素及其中间体的制备方法 |
| WO2019108685A1 (en) * | 2017-11-29 | 2019-06-06 | William March Rice University | Tubulysin analogues as anticancer agents and payloads for antibody-drug conjugates and methods of treatment therewith |
| MY208067A (en) | 2018-12-21 | 2025-04-11 | Regeneron Pharma | Tubulysins and protein-tubulysin conjugates |
| US12091392B2 (en) | 2019-02-13 | 2024-09-17 | Denali Therapeutics Inc. | Compounds, compositions and methods |
| KR20210126036A (ko) | 2019-02-13 | 2021-10-19 | 데날리 테라퓨틱스 인크. | 화합물, 조성물 및 방법 |
| TWI750728B (zh) * | 2019-07-11 | 2021-12-21 | 大陸商蘇州亞盛藥業有限公司 | 2-吲哚啉螺環酮類化合物的製備方法及其中間體 |
| US11694341B2 (en) | 2019-12-23 | 2023-07-04 | Texas Instmments Incorporated | Cascaded architecture for disparity and motion prediction with block matching and convolutional neural network (CNN) |
| CN112645833A (zh) * | 2021-01-29 | 2021-04-13 | 上海吉奉生物科技有限公司 | 一种(s)-2,6-二氨基-5-氧己酸的合成方法 |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7776814B2 (en) * | 2002-07-09 | 2010-08-17 | R&D-Biopharmaceuticals Gmbh | Tubulysin conjugates |
| DK1523493T3 (da) | 2002-07-09 | 2013-12-02 | Alexander Doemling | Nye tubulysinanaloge |
| WO2008106080A2 (en) | 2007-02-27 | 2008-09-04 | University Of Pittsburgh - Of The Commonwealth System Of Higher Education | Synthesis of desacetoxytubulysin h and analogs thereof |
| EP2532673A3 (en) | 2007-05-10 | 2014-01-08 | R & D Biopharmaceuticals Gmbh | Tubulysine derivatives |
| WO2009012958A2 (en) | 2007-07-20 | 2009-01-29 | Helmholtz-Zentrum für Infektionsforschung GmbH | Tubulysin d analogues |
| CN101909441B (zh) * | 2007-10-25 | 2015-05-13 | 恩多塞特公司 | 微管蛋白抑制剂及其制备方法 |
| EP2174947A1 (en) | 2008-09-25 | 2010-04-14 | Universität des Saarlandes | Bioactive pre-tubulysins and use thereof |
| IT1394860B1 (it) | 2009-07-22 | 2012-07-20 | Kemotech S R L | Composti farmaceutici |
| US8394922B2 (en) * | 2009-08-03 | 2013-03-12 | Medarex, Inc. | Antiproliferative compounds, conjugates thereof, methods therefor, and uses thereof |
| EP2322537A1 (en) * | 2009-11-12 | 2011-05-18 | R & D Biopharmaceuticals Gmbh | Tubulin inhibitors |
| WO2011069116A1 (en) | 2009-12-04 | 2011-06-09 | Endocyte, Inc. | Binding ligand linked drug delivery conjugates of tubulysins |
| EP2409983A1 (en) * | 2010-07-19 | 2012-01-25 | Leibniz-Institut für Pflanzenbiochemie (IPB) | Tubulysin analogues |
| JP2013535220A (ja) | 2010-08-06 | 2013-09-12 | エンドサイト,インコーポレイテッド | ツブリシンを調製するためのプロセス |
| US20140080175A1 (en) * | 2012-03-29 | 2014-03-20 | Endocyte, Inc. | Processes for preparing tubulysin derivatives and conjugates thereof |
| BR112015019432A2 (pt) | 2013-02-14 | 2017-08-22 | Bristol Myers Squibb Co | Compostos de tubulisina, métodos de produção e uso |
| EA032203B1 (ru) | 2014-04-11 | 2019-04-30 | МЕДИММЬЮН ЭлЭлСи | Производные тубулизина |
-
2016
- 2016-02-25 EA EA201791896A patent/EA201791896A1/ru unknown
- 2016-02-25 US US15/553,674 patent/US10808007B2/en active Active
- 2016-02-25 BR BR112017018305A patent/BR112017018305A2/pt not_active Application Discontinuation
- 2016-02-25 EP EP16756378.2A patent/EP3261443B1/en active Active
- 2016-02-25 SG SG11201706820RA patent/SG11201706820RA/en unknown
- 2016-02-25 CR CR20170438A patent/CR20170438A/es unknown
- 2016-02-25 CA CA2977589A patent/CA2977589A1/en not_active Abandoned
- 2016-02-25 MX MX2017011024A patent/MX2017011024A/es unknown
- 2016-02-25 KR KR1020177027116A patent/KR20170137725A/ko not_active Withdrawn
- 2016-02-25 JP JP2017545239A patent/JP2018509404A/ja active Pending
- 2016-02-25 AU AU2016222575A patent/AU2016222575A1/en not_active Abandoned
- 2016-02-25 CN CN201680023813.XA patent/CN107529754A/zh active Pending
- 2016-02-25 WO PCT/US2016/019604 patent/WO2016138288A1/en not_active Ceased
- 2016-02-25 PE PE2017001465A patent/PE20180355A1/es unknown
- 2016-02-25 HK HK18107653.3A patent/HK1248069A1/zh unknown
-
2017
- 2017-08-22 IL IL254089A patent/IL254089A0/en unknown
- 2017-08-25 CO CONC2017/0008600A patent/CO2017008600A2/es unknown
- 2017-08-25 GT GT201700190A patent/GT201700190A/es unknown
- 2017-08-25 PH PH12017501539A patent/PH12017501539A1/en unknown
- 2017-08-25 CL CL2017002170A patent/CL2017002170A1/es unknown
- 2017-08-25 DO DO2017000202A patent/DOP2017000202A/es unknown
- 2017-08-30 EC ECIEPI201757131A patent/ECSP17057131A/es unknown
Also Published As
| Publication number | Publication date |
|---|---|
| CL2017002170A1 (es) | 2018-06-08 |
| SG11201706820RA (en) | 2017-09-28 |
| MX2017011024A (es) | 2017-10-20 |
| WO2016138288A1 (en) | 2016-09-01 |
| CA2977589A1 (en) | 2016-09-01 |
| EP3261443A1 (en) | 2018-01-03 |
| IL254089A0 (en) | 2017-10-31 |
| HK1248069A1 (zh) | 2018-10-12 |
| CR20170438A (es) | 2018-01-30 |
| DOP2017000202A (es) | 2017-09-29 |
| PE20180355A1 (es) | 2018-02-21 |
| US10808007B2 (en) | 2020-10-20 |
| ECSP17057131A (es) | 2019-03-29 |
| BR112017018305A2 (pt) | 2018-04-17 |
| GT201700190A (es) | 2018-11-26 |
| JP2018509404A (ja) | 2018-04-05 |
| AU2016222575A1 (en) | 2017-09-07 |
| EA201791896A1 (ru) | 2018-02-28 |
| EP3261443A4 (en) | 2018-09-26 |
| PH12017501539A1 (en) | 2018-02-05 |
| US20180127463A1 (en) | 2018-05-10 |
| CO2017008600A2 (es) | 2018-02-09 |
| EP3261443B1 (en) | 2021-04-28 |
| CN107529754A (zh) | 2018-01-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20170137725A (ko) | 데스아세톡시투불리신 h 및 이의 유사체 | |
| CN108271371B (zh) | 用于抑制精氨酸酶活性的组合物和方法 | |
| TWI893530B (zh) | Pd‐1/pd‐l1抑制劑 | |
| KR101596524B1 (ko) | 항바이러스 화합물 | |
| ES2903390T3 (es) | Métodos para preparar nuevos inhibidores de IDH1 | |
| US20240132505A1 (en) | Pyrrolopyrimidine itk inhibitors | |
| CN111032672B (zh) | 作为抗癌剂的环二核苷酸 | |
| JP2019518726A (ja) | アルギナーゼ阻害剤およびそれらの治療適用 | |
| KR20160068738A (ko) | 운시알라마이신의 유도체, 합성 방법 및 항종양 제제로서 이들의 용도 | |
| JP2024534184A (ja) | 新規ras阻害剤 | |
| CN119278201A (zh) | 效应t细胞的活化剂 | |
| JP2021531310A (ja) | ボロン酸誘導体 | |
| JP2020531510A (ja) | ボロン酸誘導体 | |
| US11274124B2 (en) | Tubulysin analogues as anticancer agents and payloads for antibody-drug conjugates and methods of treatment therewith | |
| KR20180053294A (ko) | 시시지미신 a 및 이의 유사체의 전체 합성 | |
| HK40125797A (zh) | 用於治疗癌症的组合疗法 | |
| WO2024074977A1 (en) | Substituted 1 h-pyrazolo-pyridine and-pyrimidine compounds | |
| EP4054591A1 (en) | Combination therapy for treating cancer | |
| JP2025506452A (ja) | Smarca2及び/又はsmarca4分解剤としての3-置換ピリダジン化合物 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20170925 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination |