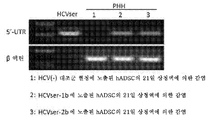
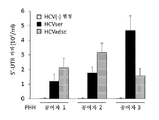
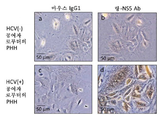
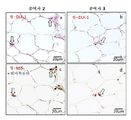
KR20170101998A - 혈청 유래 c형 간염 바이러스의 증식을 위해 이용되는 인간 지방 유래 줄기세포 및 그의 용도 - Google Patents
혈청 유래 c형 간염 바이러스의 증식을 위해 이용되는 인간 지방 유래 줄기세포 및 그의 용도 Download PDFInfo
- Publication number
- KR20170101998A KR20170101998A KR1020177022015A KR20177022015A KR20170101998A KR 20170101998 A KR20170101998 A KR 20170101998A KR 1020177022015 A KR1020177022015 A KR 1020177022015A KR 20177022015 A KR20177022015 A KR 20177022015A KR 20170101998 A KR20170101998 A KR 20170101998A
- Authority
- KR
- South Korea
- Prior art keywords
- hcv
- cells
- hadscs
- hadsc
- cell
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 241000711549 Hepacivirus C Species 0.000 title claims abstract description 270
- 210000000130 stem cell Anatomy 0.000 title claims abstract description 13
- 210000002966 serum Anatomy 0.000 title claims description 47
- 230000035755 proliferation Effects 0.000 title claims description 11
- 210000004027 cell Anatomy 0.000 claims abstract description 228
- 208000015181 infectious disease Diseases 0.000 claims abstract description 73
- 230000010076 replication Effects 0.000 claims abstract description 33
- 238000000034 method Methods 0.000 claims description 40
- 150000001875 compounds Chemical class 0.000 claims description 32
- 239000001963 growth medium Substances 0.000 claims description 23
- 108020004707 nucleic acids Proteins 0.000 claims description 10
- 102000039446 nucleic acids Human genes 0.000 claims description 10
- 150000007523 nucleic acids Chemical class 0.000 claims description 10
- 238000004458 analytical method Methods 0.000 claims description 9
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 9
- 210000004369 blood Anatomy 0.000 claims description 8
- 239000008280 blood Substances 0.000 claims description 8
- 238000012258 culturing Methods 0.000 claims description 8
- 210000001124 body fluid Anatomy 0.000 claims description 7
- 239000010839 body fluid Substances 0.000 claims description 7
- 238000001514 detection method Methods 0.000 claims description 7
- 239000012472 biological sample Substances 0.000 claims description 6
- 210000002381 plasma Anatomy 0.000 claims description 6
- 238000012216 screening Methods 0.000 claims description 6
- 230000000840 anti-viral effect Effects 0.000 claims description 5
- 238000003745 diagnosis Methods 0.000 claims description 5
- 238000004519 manufacturing process Methods 0.000 claims description 5
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 5
- 108090000623 proteins and genes Proteins 0.000 claims description 5
- 102000004169 proteins and genes Human genes 0.000 claims description 5
- -1 small molecule chemical compound Chemical class 0.000 claims description 5
- 230000000692 anti-sense effect Effects 0.000 claims description 4
- 229920001184 polypeptide Polymers 0.000 claims description 4
- 102000053642 Catalytic RNA Human genes 0.000 claims description 3
- 108090000994 Catalytic RNA Proteins 0.000 claims description 3
- 108091027967 Small hairpin RNA Proteins 0.000 claims description 3
- 238000012512 characterization method Methods 0.000 claims description 3
- 230000001902 propagating effect Effects 0.000 claims description 3
- 108091092562 ribozyme Proteins 0.000 claims description 3
- 239000004055 small Interfering RNA Substances 0.000 claims description 2
- 102100036467 Protein delta homolog 1 Human genes 0.000 abstract description 79
- 101710119301 Protein delta homolog 1 Proteins 0.000 abstract description 76
- 239000006228 supernatant Substances 0.000 abstract description 52
- 241000700605 Viruses Species 0.000 abstract description 43
- 230000003612 virological effect Effects 0.000 abstract description 36
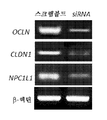
- 108090000600 Claudin-1 Proteins 0.000 abstract description 14
- 210000003494 hepatocyte Anatomy 0.000 abstract description 13
- 238000000338 in vitro Methods 0.000 abstract description 13
- 102000003940 Occludin Human genes 0.000 abstract description 12
- 108090000304 Occludin Proteins 0.000 abstract description 12
- 239000000427 antigen Substances 0.000 abstract description 11
- 102000036639 antigens Human genes 0.000 abstract description 11
- 108091007433 antigens Proteins 0.000 abstract description 11
- 102100027221 CD81 antigen Human genes 0.000 abstract description 10
- 101000914479 Homo sapiens CD81 antigen Proteins 0.000 abstract description 10
- 102000052116 epidermal growth factor receptor activity proteins Human genes 0.000 abstract description 9
- 108700015053 epidermal growth factor receptor activity proteins Proteins 0.000 abstract description 9
- YOHYSYJDKVYCJI-UHFFFAOYSA-N n-[3-[[6-[3-(trifluoromethyl)anilino]pyrimidin-4-yl]amino]phenyl]cyclopropanecarboxamide Chemical compound FC(F)(F)C1=CC=CC(NC=2N=CN=C(NC=3C=C(NC(=O)C4CC4)C=CC=3)C=2)=C1 YOHYSYJDKVYCJI-UHFFFAOYSA-N 0.000 abstract description 9
- 239000002245 particle Substances 0.000 abstract description 9
- 238000001727 in vivo Methods 0.000 abstract description 8
- 238000004113 cell culture Methods 0.000 abstract description 6
- 230000002458 infectious effect Effects 0.000 abstract description 5
- 239000000543 intermediate Substances 0.000 abstract description 5
- 230000001404 mediated effect Effects 0.000 abstract description 5
- 230000029812 viral genome replication Effects 0.000 abstract description 5
- 230000000704 physical effect Effects 0.000 abstract description 4
- 230000009385 viral infection Effects 0.000 abstract description 3
- 208000036142 Viral infection Diseases 0.000 abstract description 2
- 230000002293 adipogenic effect Effects 0.000 abstract description 2
- 150000001982 diacylglycerols Chemical class 0.000 abstract description 2
- 230000006394 virus-host interaction Effects 0.000 abstract description 2
- 102100040836 Claudin-1 Human genes 0.000 abstract 1
- 101710204837 Envelope small membrane protein Proteins 0.000 abstract 1
- 101000604005 Homo sapiens NPC1-like intracellular cholesterol transporter 1 Proteins 0.000 abstract 1
- 102100038441 NPC1-like intracellular cholesterol transporter 1 Human genes 0.000 abstract 1
- 101710088839 Replication initiation protein Proteins 0.000 abstract 1
- 230000000644 propagated effect Effects 0.000 abstract 1
- 230000006490 viral transcription Effects 0.000 abstract 1
- 238000002474 experimental method Methods 0.000 description 52
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 48
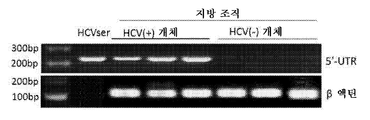
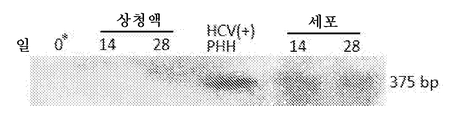
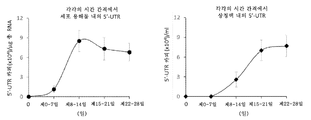
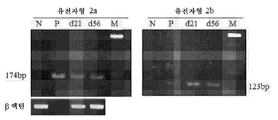
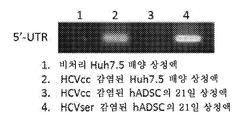
- 108020003589 5' Untranslated Regions Proteins 0.000 description 40
- 210000000577 adipose tissue Anatomy 0.000 description 35
- 238000010186 staining Methods 0.000 description 30
- 239000013592 cell lysate Substances 0.000 description 27
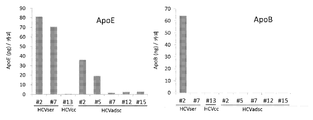
- 102000013918 Apolipoproteins E Human genes 0.000 description 22
- 108010025628 Apolipoproteins E Proteins 0.000 description 21
- 239000002609 medium Substances 0.000 description 21
- WZUVPPKBWHMQCE-UHFFFAOYSA-N Haematoxylin Chemical compound C12=CC(O)=C(O)C=C2CC2(O)C1C1=CC=C(O)C(O)=C1OC2 WZUVPPKBWHMQCE-UHFFFAOYSA-N 0.000 description 18
- 238000011529 RT qPCR Methods 0.000 description 18
- 210000001519 tissue Anatomy 0.000 description 17
- 241000283973 Oryctolagus cuniculus Species 0.000 description 15
- 238000011161 development Methods 0.000 description 15
- 239000003925 fat Substances 0.000 description 15
- 102000004162 Claudin-1 Human genes 0.000 description 13
- 108020004459 Small interfering RNA Proteins 0.000 description 13
- 239000013642 negative control Substances 0.000 description 12
- 239000000725 suspension Substances 0.000 description 12
- 238000005406 washing Methods 0.000 description 12
- 102000018616 Apolipoproteins B Human genes 0.000 description 11
- 108010027006 Apolipoproteins B Proteins 0.000 description 11
- 238000001890 transfection Methods 0.000 description 11
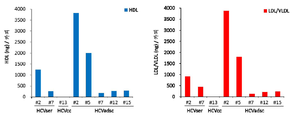
- 108010010234 HDL Lipoproteins Proteins 0.000 description 10
- 102000015779 HDL Lipoproteins Human genes 0.000 description 10
- 102000007330 LDL Lipoproteins Human genes 0.000 description 10
- 108010007622 LDL Lipoproteins Proteins 0.000 description 10
- 238000011534 incubation Methods 0.000 description 10
- 239000013641 positive control Substances 0.000 description 10
- 239000013615 primer Substances 0.000 description 10
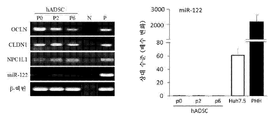
- 108091007780 MiR-122 Proteins 0.000 description 9
- 238000003365 immunocytochemistry Methods 0.000 description 9
- 150000002632 lipids Chemical class 0.000 description 9
- 108091051828 miR-122 stem-loop Proteins 0.000 description 9
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 8
- 239000008188 pellet Substances 0.000 description 8
- 239000000523 sample Substances 0.000 description 8
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 7
- 108010062497 VLDL Lipoproteins Proteins 0.000 description 7
- 230000000903 blocking effect Effects 0.000 description 7
- 239000000872 buffer Substances 0.000 description 7
- 210000003743 erythrocyte Anatomy 0.000 description 7
- 210000005228 liver tissue Anatomy 0.000 description 7
- 108020004999 messenger RNA Proteins 0.000 description 7
- 229930105110 Cyclosporin A Natural products 0.000 description 6
- PMATZTZNYRCHOR-CGLBZJNRSA-N Cyclosporin A Chemical compound CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O PMATZTZNYRCHOR-CGLBZJNRSA-N 0.000 description 6
- 108010036949 Cyclosporine Proteins 0.000 description 6
- 238000002123 RNA extraction Methods 0.000 description 6
- IWUCXVSUMQZMFG-AFCXAGJDSA-N Ribavirin Chemical compound N1=C(C(=O)N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 IWUCXVSUMQZMFG-AFCXAGJDSA-N 0.000 description 6
- 210000001789 adipocyte Anatomy 0.000 description 6
- 230000003833 cell viability Effects 0.000 description 6
- 230000001413 cellular effect Effects 0.000 description 6
- 238000005119 centrifugation Methods 0.000 description 6
- 229960001265 ciclosporin Drugs 0.000 description 6
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 6
- 238000000684 flow cytometry Methods 0.000 description 6
- 239000012737 fresh medium Substances 0.000 description 6
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 6
- 231100000844 hepatocellular carcinoma Toxicity 0.000 description 6
- 230000005764 inhibitory process Effects 0.000 description 6
- 238000010839 reverse transcription Methods 0.000 description 6
- HZCAHMRRMINHDJ-DBRKOABJSA-N ribavirin Natural products O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1N=CN=C1 HZCAHMRRMINHDJ-DBRKOABJSA-N 0.000 description 6
- 229960000329 ribavirin Drugs 0.000 description 6
- 238000012360 testing method Methods 0.000 description 6
- 238000002965 ELISA Methods 0.000 description 5
- 102000001708 Protein Isoforms Human genes 0.000 description 5
- 108010029485 Protein Isoforms Proteins 0.000 description 5
- GLNADSQYFUSGOU-GPTZEZBUSA-J Trypan blue Chemical compound [Na+].[Na+].[Na+].[Na+].C1=C(S([O-])(=O)=O)C=C2C=C(S([O-])(=O)=O)C(/N=N/C3=CC=C(C=C3C)C=3C=C(C(=CC=3)\N=N\C=3C(=CC4=CC(=CC(N)=C4C=3O)S([O-])(=O)=O)S([O-])(=O)=O)C)=C(O)C2=C1N GLNADSQYFUSGOU-GPTZEZBUSA-J 0.000 description 5
- 238000003556 assay Methods 0.000 description 5
- 239000002299 complementary DNA Substances 0.000 description 5
- 230000002401 inhibitory effect Effects 0.000 description 5
- 210000004185 liver Anatomy 0.000 description 5
- 239000003550 marker Substances 0.000 description 5
- 210000004379 membrane Anatomy 0.000 description 5
- 239000012528 membrane Substances 0.000 description 5
- 230000002062 proliferating effect Effects 0.000 description 5
- 239000000758 substrate Substances 0.000 description 5
- BBAWEDCPNXPBQM-GDEBMMAJSA-N telaprevir Chemical compound N([C@H](C(=O)N[C@H](C(=O)N1C[C@@H]2CCC[C@@H]2[C@H]1C(=O)N[C@@H](CCC)C(=O)C(=O)NC1CC1)C(C)(C)C)C1CCCCC1)C(=O)C1=CN=CC=N1 BBAWEDCPNXPBQM-GDEBMMAJSA-N 0.000 description 5
- 108010017101 telaprevir Proteins 0.000 description 5
- 229960002935 telaprevir Drugs 0.000 description 5
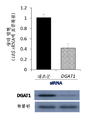
- 102100036869 Diacylglycerol O-acyltransferase 1 Human genes 0.000 description 4
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 4
- 206010019695 Hepatic neoplasm Diseases 0.000 description 4
- 208000002847 Surgical Wound Diseases 0.000 description 4
- 230000001464 adherent effect Effects 0.000 description 4
- 230000003321 amplification Effects 0.000 description 4
- 230000010261 cell growth Effects 0.000 description 4
- 239000003153 chemical reaction reagent Substances 0.000 description 4
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 4
- 201000010099 disease Diseases 0.000 description 4
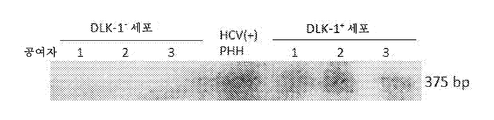
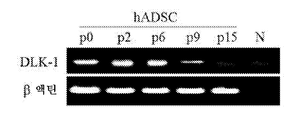
- 101150102995 dlk-1 gene Proteins 0.000 description 4
- 239000012091 fetal bovine serum Substances 0.000 description 4
- 239000012894 fetal calf serum Substances 0.000 description 4
- 238000003018 immunoassay Methods 0.000 description 4
- 210000005229 liver cell Anatomy 0.000 description 4
- 210000002901 mesenchymal stem cell Anatomy 0.000 description 4
- 238000003199 nucleic acid amplification method Methods 0.000 description 4
- 206010033675 panniculitis Diseases 0.000 description 4
- 238000003753 real-time PCR Methods 0.000 description 4
- 239000000243 solution Substances 0.000 description 4
- 210000004003 subcutaneous fat Anatomy 0.000 description 4
- 239000013589 supplement Substances 0.000 description 4
- 239000003981 vehicle Substances 0.000 description 4
- 210000002845 virion Anatomy 0.000 description 4
- 108010072220 Cyclophilin A Proteins 0.000 description 3
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 3
- 241000700721 Hepatitis B virus Species 0.000 description 3
- 101000771674 Homo sapiens Apolipoprotein E Proteins 0.000 description 3
- 101000927974 Homo sapiens Diacylglycerol O-acyltransferase 1 Proteins 0.000 description 3
- 108091026898 Leader sequence (mRNA) Proteins 0.000 description 3
- 102100034539 Peptidyl-prolyl cis-trans isomerase A Human genes 0.000 description 3
- 108091005487 SCARB1 Proteins 0.000 description 3
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 3
- 108010069201 VLDL Cholesterol Proteins 0.000 description 3
- 210000000593 adipose tissue white Anatomy 0.000 description 3
- 239000003443 antiviral agent Substances 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 238000003197 gene knockdown Methods 0.000 description 3
- 238000012606 in vitro cell culture Methods 0.000 description 3
- 239000002054 inoculum Substances 0.000 description 3
- NBQNWMBBSKPBAY-UHFFFAOYSA-N iodixanol Chemical compound IC=1C(C(=O)NCC(O)CO)=C(I)C(C(=O)NCC(O)CO)=C(I)C=1N(C(=O)C)CC(O)CN(C(C)=O)C1=C(I)C(C(=O)NCC(O)CO)=C(I)C(C(=O)NCC(O)CO)=C1I NBQNWMBBSKPBAY-UHFFFAOYSA-N 0.000 description 3
- 238000002350 laparotomy Methods 0.000 description 3
- 230000004060 metabolic process Effects 0.000 description 3
- 238000006386 neutralization reaction Methods 0.000 description 3
- 230000002829 reductive effect Effects 0.000 description 3
- 238000002271 resection Methods 0.000 description 3
- 238000012552 review Methods 0.000 description 3
- 238000004627 transmission electron microscopy Methods 0.000 description 3
- 102000040650 (ribonucleotides)n+m Human genes 0.000 description 2
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 2
- 206010006187 Breast cancer Diseases 0.000 description 2
- 208000026310 Breast neoplasm Diseases 0.000 description 2
- 238000008620 Cholesterol Assay Methods 0.000 description 2
- 102000012422 Collagen Type I Human genes 0.000 description 2
- 108010022452 Collagen Type I Proteins 0.000 description 2
- 239000006145 Eagle's minimal essential medium Substances 0.000 description 2
- 239000012981 Hank's balanced salt solution Substances 0.000 description 2
- 206010019708 Hepatic steatosis Diseases 0.000 description 2
- 101000600434 Homo sapiens Putative uncharacterized protein encoded by MIR7-3HG Proteins 0.000 description 2
- 101000622304 Homo sapiens Vascular cell adhesion protein 1 Proteins 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- 102100034343 Integrase Human genes 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- 101800001014 Non-structural protein 5A Proteins 0.000 description 2
- 102000003992 Peroxidases Human genes 0.000 description 2
- 229920002594 Polyethylene Glycol 8000 Polymers 0.000 description 2
- 102100037401 Putative uncharacterized protein encoded by MIR7-3HG Human genes 0.000 description 2
- 108010092799 RNA-directed DNA polymerase Proteins 0.000 description 2
- 102100023543 Vascular cell adhesion protein 1 Human genes 0.000 description 2
- 210000003486 adipose tissue brown Anatomy 0.000 description 2
- 210000004504 adult stem cell Anatomy 0.000 description 2
- 239000011324 bead Substances 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 239000012620 biological material Substances 0.000 description 2
- 230000005540 biological transmission Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 239000012888 bovine serum Substances 0.000 description 2
- 244000309464 bull Species 0.000 description 2
- 244000309466 calf Species 0.000 description 2
- 108091092328 cellular RNA Proteins 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 235000012000 cholesterol Nutrition 0.000 description 2
- 239000012228 culture supernatant Substances 0.000 description 2
- 208000035475 disorder Diseases 0.000 description 2
- 231100000673 dose–response relationship Toxicity 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 239000003797 essential amino acid Substances 0.000 description 2
- 235000020776 essential amino acid Nutrition 0.000 description 2
- 230000007717 exclusion Effects 0.000 description 2
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 2
- 238000005194 fractionation Methods 0.000 description 2
- 230000030279 gene silencing Effects 0.000 description 2
- 238000012226 gene silencing method Methods 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 230000012010 growth Effects 0.000 description 2
- 102000053020 human ApoE Human genes 0.000 description 2
- 238000003364 immunohistochemistry Methods 0.000 description 2
- 230000003834 intracellular effect Effects 0.000 description 2
- 229960004359 iodixanol Drugs 0.000 description 2
- 238000001738 isopycnic centrifugation Methods 0.000 description 2
- 210000002510 keratinocyte Anatomy 0.000 description 2
- 238000002372 labelling Methods 0.000 description 2
- 238000007443 liposuction Methods 0.000 description 2
- 208000014018 liver neoplasm Diseases 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 239000012139 lysis buffer Substances 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 235000013372 meat Nutrition 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 230000035772 mutation Effects 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- 239000012188 paraffin wax Substances 0.000 description 2
- 108040007629 peroxidase activity proteins Proteins 0.000 description 2
- 230000035479 physiological effects, processes and functions Effects 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 238000003752 polymerase chain reaction Methods 0.000 description 2
- 238000011084 recovery Methods 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 230000004083 survival effect Effects 0.000 description 2
- 108091035539 telomere Proteins 0.000 description 2
- 102000055501 telomere Human genes 0.000 description 2
- 210000003411 telomere Anatomy 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- AOFUBOWZWQFQJU-SNOJBQEQSA-N (2r,3s,4s,5r)-2,5-bis(hydroxymethyl)oxolane-2,3,4-triol;(2s,3r,4s,5s,6r)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O.OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@@H]1O AOFUBOWZWQFQJU-SNOJBQEQSA-N 0.000 description 1
- 108020004463 18S ribosomal RNA Proteins 0.000 description 1
- SFNLLCUAISZNRV-UHFFFAOYSA-N 2-chloro-n-(9h-fluoren-9-ylcarbamoyl)-6-fluorobenzamide Chemical compound FC1=CC=CC(Cl)=C1C(=O)NC(=O)NC1C2=CC=CC=C2C2=CC=CC=C21 SFNLLCUAISZNRV-UHFFFAOYSA-N 0.000 description 1
- 244000291564 Allium cepa Species 0.000 description 1
- 235000002732 Allium cepa var. cepa Nutrition 0.000 description 1
- 102100022749 Aminopeptidase N Human genes 0.000 description 1
- 102000007592 Apolipoproteins Human genes 0.000 description 1
- 108010071619 Apolipoproteins Proteins 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 102100032912 CD44 antigen Human genes 0.000 description 1
- 102100029761 Cadherin-5 Human genes 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 241000282836 Camelus dromedarius Species 0.000 description 1
- 101000947501 Camptotheca acuminata (S)-8-oxocitronellyl enol synthase CYC1 Proteins 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 101710132601 Capsid protein Proteins 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- 102000029816 Collagenase Human genes 0.000 description 1
- 108060005980 Collagenase Proteins 0.000 description 1
- 208000035473 Communicable disease Diseases 0.000 description 1
- 229940077097 Cyclophilin A inhibitor Drugs 0.000 description 1
- 108020004414 DNA Proteins 0.000 description 1
- 239000003155 DNA primer Substances 0.000 description 1
- 108050004099 Diacylglycerol O-acyltransferase 1 Proteins 0.000 description 1
- 102100023471 E-selectin Human genes 0.000 description 1
- 238000012286 ELISA Assay Methods 0.000 description 1
- 102100037241 Endoglin Human genes 0.000 description 1
- 101800003838 Epidermal growth factor Proteins 0.000 description 1
- 241000710781 Flaviviridae Species 0.000 description 1
- 241000287828 Gallus gallus Species 0.000 description 1
- 108010009202 Growth Factor Receptors Proteins 0.000 description 1
- 102000009465 Growth Factor Receptors Human genes 0.000 description 1
- 239000007995 HEPES buffer Substances 0.000 description 1
- 102100031573 Hematopoietic progenitor cell antigen CD34 Human genes 0.000 description 1
- 206010019668 Hepatic fibrosis Diseases 0.000 description 1
- 101000757160 Homo sapiens Aminopeptidase N Proteins 0.000 description 1
- 101000889953 Homo sapiens Apolipoprotein B-100 Proteins 0.000 description 1
- 101000868273 Homo sapiens CD44 antigen Proteins 0.000 description 1
- 101000622123 Homo sapiens E-selectin Proteins 0.000 description 1
- 101000881679 Homo sapiens Endoglin Proteins 0.000 description 1
- 101000777663 Homo sapiens Hematopoietic progenitor cell antigen CD34 Proteins 0.000 description 1
- 101000935043 Homo sapiens Integrin beta-1 Proteins 0.000 description 1
- 101001015004 Homo sapiens Integrin beta-3 Proteins 0.000 description 1
- 101001015006 Homo sapiens Integrin beta-4 Proteins 0.000 description 1
- 101000599852 Homo sapiens Intercellular adhesion molecule 1 Proteins 0.000 description 1
- 101000917858 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-A Proteins 0.000 description 1
- 101000917839 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-B Proteins 0.000 description 1
- 101001051093 Homo sapiens Low-density lipoprotein receptor Proteins 0.000 description 1
- 101001008874 Homo sapiens Mast/stem cell growth factor receptor Kit Proteins 0.000 description 1
- 101000946889 Homo sapiens Monocyte differentiation antigen CD14 Proteins 0.000 description 1
- 101000581981 Homo sapiens Neural cell adhesion molecule 1 Proteins 0.000 description 1
- 101000928535 Homo sapiens Protein delta homolog 1 Proteins 0.000 description 1
- 101000738771 Homo sapiens Receptor-type tyrosine-protein phosphatase C Proteins 0.000 description 1
- 101000914484 Homo sapiens T-lymphocyte activation antigen CD80 Proteins 0.000 description 1
- 101000800116 Homo sapiens Thy-1 membrane glycoprotein Proteins 0.000 description 1
- 101000835093 Homo sapiens Transferrin receptor protein 1 Proteins 0.000 description 1
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 1
- 241000725303 Human immunodeficiency virus Species 0.000 description 1
- 102100025304 Integrin beta-1 Human genes 0.000 description 1
- 102100032999 Integrin beta-3 Human genes 0.000 description 1
- 102100033000 Integrin beta-4 Human genes 0.000 description 1
- 102100037877 Intercellular adhesion molecule 1 Human genes 0.000 description 1
- 239000007760 Iscove's Modified Dulbecco's Medium Substances 0.000 description 1
- 102100029185 Low affinity immunoglobulin gamma Fc region receptor III-B Human genes 0.000 description 1
- 102100024640 Low-density lipoprotein receptor Human genes 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 102100027754 Mast/stem cell growth factor receptor Kit Human genes 0.000 description 1
- 108700011259 MicroRNAs Proteins 0.000 description 1
- 102100035877 Monocyte differentiation antigen CD14 Human genes 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 102000003729 Neprilysin Human genes 0.000 description 1
- 108090000028 Neprilysin Proteins 0.000 description 1
- 102100027347 Neural cell adhesion molecule 1 Human genes 0.000 description 1
- 241000282577 Pan troglodytes Species 0.000 description 1
- 208000037273 Pathologic Processes Diseases 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 1
- 102100024616 Platelet endothelial cell adhesion molecule Human genes 0.000 description 1
- 239000004353 Polyethylene glycol 8000 Substances 0.000 description 1
- 108010039918 Polylysine Proteins 0.000 description 1
- 102100033237 Pro-epidermal growth factor Human genes 0.000 description 1
- 230000006819 RNA synthesis Effects 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- 102100037422 Receptor-type tyrosine-protein phosphatase C Human genes 0.000 description 1
- 101710172711 Structural protein Proteins 0.000 description 1
- 102100027222 T-lymphocyte activation antigen CD80 Human genes 0.000 description 1
- 238000003917 TEM image Methods 0.000 description 1
- 108010017842 Telomerase Proteins 0.000 description 1
- 102100033523 Thy-1 membrane glycoprotein Human genes 0.000 description 1
- 102100026144 Transferrin receptor protein 1 Human genes 0.000 description 1
- 102000004142 Trypsin Human genes 0.000 description 1
- 108090000631 Trypsin Proteins 0.000 description 1
- 108020000999 Viral RNA Proteins 0.000 description 1
- 210000001015 abdomen Anatomy 0.000 description 1
- 230000003187 abdominal effect Effects 0.000 description 1
- 210000000579 abdominal fat Anatomy 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 102000005421 acetyltransferase Human genes 0.000 description 1
- 108020002494 acetyltransferase Proteins 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 238000011226 adjuvant chemotherapy Methods 0.000 description 1
- 238000011353 adjuvant radiotherapy Methods 0.000 description 1
- WLDHEUZGFKACJH-UHFFFAOYSA-K amaranth Chemical compound [Na+].[Na+].[Na+].C12=CC=C(S([O-])(=O)=O)C=C2C=C(S([O-])(=O)=O)C(O)=C1N=NC1=CC=C(S([O-])(=O)=O)C2=CC=CC=C12 WLDHEUZGFKACJH-UHFFFAOYSA-K 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 230000002155 anti-virotic effect Effects 0.000 description 1
- 239000007640 basal medium Substances 0.000 description 1
- 239000003012 bilayer membrane Substances 0.000 description 1
- 230000031018 biological processes and functions Effects 0.000 description 1
- 229960000074 biopharmaceutical Drugs 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 210000001185 bone marrow Anatomy 0.000 description 1
- 210000000481 breast Anatomy 0.000 description 1
- 210000001217 buttock Anatomy 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 210000005056 cell body Anatomy 0.000 description 1
- 239000006143 cell culture medium Substances 0.000 description 1
- 239000006285 cell suspension Substances 0.000 description 1
- 229920002301 cellulose acetate Polymers 0.000 description 1
- 239000000460 chlorine Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 230000001906 cholesterol absorption Effects 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 239000007979 citrate buffer Substances 0.000 description 1
- 238000010367 cloning Methods 0.000 description 1
- 230000008045 co-localization Effects 0.000 description 1
- 229960002424 collagenase Drugs 0.000 description 1
- 239000003636 conditioned culture medium Substances 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 210000002808 connective tissue Anatomy 0.000 description 1
- 239000000356 contaminant Substances 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- 238000007865 diluting Methods 0.000 description 1
- 239000013024 dilution buffer Substances 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 241001493065 dsRNA viruses Species 0.000 description 1
- 238000004043 dyeing Methods 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 229940116977 epidermal growth factor Drugs 0.000 description 1
- 206010015037 epilepsy Diseases 0.000 description 1
- 230000008472 epithelial growth Effects 0.000 description 1
- 239000003188 fatty acid synthesis inhibitor Substances 0.000 description 1
- 210000002950 fibroblast Anatomy 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 238000003205 genotyping method Methods 0.000 description 1
- 238000005469 granulation Methods 0.000 description 1
- 230000003179 granulation Effects 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 230000003394 haemopoietic effect Effects 0.000 description 1
- 208000006454 hepatitis Diseases 0.000 description 1
- 231100000283 hepatitis Toxicity 0.000 description 1
- 238000010842 high-capacity cDNA reverse transcription kit Methods 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 102000052249 human APOB Human genes 0.000 description 1
- 238000009396 hybridization Methods 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 238000011532 immunohistochemical staining Methods 0.000 description 1
- 238000005462 in vivo assay Methods 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- 238000011081 inoculation Methods 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 230000003902 lesion Effects 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 230000004132 lipogenesis Effects 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 210000001161 mammalian embryo Anatomy 0.000 description 1
- 230000035800 maturation Effects 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 238000003253 miRNA assay Methods 0.000 description 1
- 239000011325 microbead Substances 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 235000010755 mineral Nutrition 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 230000000877 morphologic effect Effects 0.000 description 1
- 239000012120 mounting media Substances 0.000 description 1
- 210000003205 muscle Anatomy 0.000 description 1
- 238000010899 nucleation Methods 0.000 description 1
- 210000004940 nucleus Anatomy 0.000 description 1
- 210000002747 omentum Anatomy 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 230000009054 pathological process Effects 0.000 description 1
- 229940049954 penicillin Drugs 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 210000004303 peritoneum Anatomy 0.000 description 1
- 230000035790 physiological processes and functions Effects 0.000 description 1
- 229940085678 polyethylene glycol 8000 Drugs 0.000 description 1
- 235000019446 polyethylene glycol 8000 Nutrition 0.000 description 1
- 229920000656 polylysine Polymers 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 238000001243 protein synthesis Methods 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000001172 regenerating effect Effects 0.000 description 1
- 230000017610 release of virus from host Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 150000004671 saturated fatty acids Chemical class 0.000 description 1
- 235000003441 saturated fatty acids Nutrition 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 238000012163 sequencing technique Methods 0.000 description 1
- 239000012679 serum free medium Substances 0.000 description 1
- 230000006807 siRNA silencing Effects 0.000 description 1
- 238000002791 soaking Methods 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 210000004304 subcutaneous tissue Anatomy 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 235000013619 trace mineral Nutrition 0.000 description 1
- 239000011573 trace mineral Substances 0.000 description 1
- 239000012096 transfection reagent Substances 0.000 description 1
- 230000014616 translation Effects 0.000 description 1
- 239000012588 trypsin Substances 0.000 description 1
- 210000004881 tumor cell Anatomy 0.000 description 1
- 238000005199 ultracentrifugation Methods 0.000 description 1
- 150000004670 unsaturated fatty acids Chemical class 0.000 description 1
- 235000021122 unsaturated fatty acids Nutrition 0.000 description 1
- VBEQCZHXXJYVRD-GACYYNSASA-N uroanthelone Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(C)C)[C@@H](C)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)CNC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CS)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O)C(C)C)[C@@H](C)CC)C1=CC=C(O)C=C1 VBEQCZHXXJYVRD-GACYYNSASA-N 0.000 description 1
- 230000007501 viral attachment Effects 0.000 description 1
- 230000007502 viral entry Effects 0.000 description 1
- 230000008957 viral persistence Effects 0.000 description 1
- 108010047303 von Willebrand Factor Proteins 0.000 description 1
- 102100036537 von Willebrand factor Human genes 0.000 description 1
- 229960001134 von willebrand factor Drugs 0.000 description 1
- 238000003260 vortexing Methods 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N7/00—Viruses; Bacteriophages; Compositions thereof; Preparation or purification thereof
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0652—Cells of skeletal and connective tissues; Mesenchyme
- C12N5/0662—Stem cells
- C12N5/0667—Adipose-derived stem cells [ADSC]; Adipose stromal stem cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/70—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving virus or bacteriophage
- C12Q1/701—Specific hybridization probes
- C12Q1/706—Specific hybridization probes for hepatitis
- C12Q1/707—Specific hybridization probes for hepatitis non-A, non-B Hepatitis, excluding hepatitis D
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/5005—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells
- G01N33/5008—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics
- G01N33/5044—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics involving specific cell types
- G01N33/5073—Stem cells
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/576—Immunoassay; Biospecific binding assay; Materials therefor for hepatitis
- G01N33/5767—Immunoassay; Biospecific binding assay; Materials therefor for hepatitis non-A, non-B hepatitis
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2502/00—Coculture with; Conditioned medium produced by
- C12N2502/70—Non-animal cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2770/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses positive-sense
- C12N2770/00011—Details
- C12N2770/24011—Flaviviridae
- C12N2770/24211—Hepacivirus, e.g. hepatitis C virus, hepatitis G virus
- C12N2770/24251—Methods of production or purification of viral material
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/136—Screening for pharmacological compounds
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/005—Assays involving biological materials from specific organisms or of a specific nature from viruses
- G01N2333/08—RNA viruses
- G01N2333/18—Togaviridae; Flaviviridae
- G01N2333/183—Flaviviridae, e.g. pestivirus, mucosal disease virus, bovine viral diarrhoea virus, classical swine fever virus (hog cholera virus) or border disease virus
- G01N2333/186—Hepatitis C; Hepatitis NANB
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Biomedical Technology (AREA)
- Organic Chemistry (AREA)
- Biotechnology (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Microbiology (AREA)
- Biochemistry (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- General Health & Medical Sciences (AREA)
- Hematology (AREA)
- Urology & Nephrology (AREA)
- Medicinal Chemistry (AREA)
- Cell Biology (AREA)
- Physics & Mathematics (AREA)
- Communicable Diseases (AREA)
- General Engineering & Computer Science (AREA)
- Analytical Chemistry (AREA)
- Virology (AREA)
- Food Science & Technology (AREA)
- Pathology (AREA)
- General Physics & Mathematics (AREA)
- Developmental Biology & Embryology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biophysics (AREA)
- Toxicology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Rheumatology (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/CN2015/070243 WO2016109947A1 (en) | 2015-01-07 | 2015-01-07 | Employing human adipose-derived stem cells to propagate serum-derived hepatitis c virus and use thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20170101998A true KR20170101998A (ko) | 2017-09-06 |
Family
ID=56355408
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020177022015A Withdrawn KR20170101998A (ko) | 2015-01-07 | 2015-01-07 | 혈청 유래 c형 간염 바이러스의 증식을 위해 이용되는 인간 지방 유래 줄기세포 및 그의 용도 |
Country Status (10)
| Country | Link |
|---|---|
| US (1) | US10273461B2 (enExample) |
| EP (1) | EP3242932A4 (enExample) |
| JP (1) | JP6670322B2 (enExample) |
| KR (1) | KR20170101998A (enExample) |
| CN (1) | CN107208060B (enExample) |
| AU (1) | AU2015376561A1 (enExample) |
| CA (1) | CA2973119A1 (enExample) |
| HK (1) | HK1246824A1 (enExample) |
| TW (1) | TWI616528B (enExample) |
| WO (1) | WO2016109947A1 (enExample) |
Families Citing this family (3)
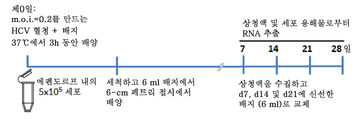
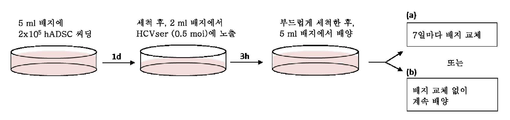
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| IL272145A (en) * | 2020-01-20 | 2021-07-29 | Stem Cell Medicine Ltd | Cosmetic preparations with protein concentrate from a conditioned growth medium of stem cells from adipose tissue |
| IL278473A (en) | 2020-11-03 | 2022-06-01 | Yeda Res & Dev | Methods for diagnosing and determining treatment in multiple myeloma |
| CN114686517A (zh) * | 2020-12-25 | 2022-07-01 | 中国医学科学院病原生物学研究所 | 一种hcv四受体转基因合并stat1敲除小鼠的制备方法 |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5786207A (en) | 1997-05-28 | 1998-07-28 | University Of Pittsburgh | Tissue dissociating system and method |
| CN1166773C (zh) | 1997-12-02 | 2004-09-15 | 泽恩比奥公司 | 分离的人脂肪组织衍生的基质细胞 |
| US6777231B1 (en) | 1999-03-10 | 2004-08-17 | The Regents Of The University Of California | Adipose-derived stem cells and lattices |
| US20050076396A1 (en) | 1999-03-10 | 2005-04-07 | Katz Adam J. | Adipose-derived stem cells and lattices |
| JP2009153383A (ja) * | 2006-04-03 | 2009-07-16 | Eci Inc | 成熟肝細胞様細胞の製造方法 |
| KR101224504B1 (ko) * | 2010-10-01 | 2013-01-22 | 가톨릭대학교 산학협력단 | 크립토탄신온의 신규 용도 |
| GB201119335D0 (en) * | 2011-11-09 | 2011-12-21 | Univ Leuven Kath | Hepatitis virus infectable stem cells |
| US20140179775A1 (en) * | 2012-02-29 | 2014-06-26 | Mead Johnson Nutrition Company | Neurogenesis screening method and uses thereof |
| US9402710B2 (en) * | 2012-07-16 | 2016-08-02 | The Board Of Trustees For The Leland Stanford Junior University | Macroporous 3-D scaffolds for tissue engineering |
-
2015
- 2015-01-07 EP EP15876456.3A patent/EP3242932A4/en not_active Withdrawn
- 2015-01-07 JP JP2017554627A patent/JP6670322B2/ja active Active
- 2015-01-07 US US15/541,913 patent/US10273461B2/en active Active
- 2015-01-07 CA CA2973119A patent/CA2973119A1/en not_active Abandoned
- 2015-01-07 AU AU2015376561A patent/AU2015376561A1/en not_active Abandoned
- 2015-01-07 HK HK18106252.0A patent/HK1246824A1/zh unknown
- 2015-01-07 WO PCT/CN2015/070243 patent/WO2016109947A1/en not_active Ceased
- 2015-01-07 CN CN201580072851.XA patent/CN107208060B/zh active Active
- 2015-01-07 KR KR1020177022015A patent/KR20170101998A/ko not_active Withdrawn
- 2015-05-19 TW TW104115953A patent/TWI616528B/zh active
Also Published As
| Publication number | Publication date |
|---|---|
| EP3242932A4 (en) | 2018-05-30 |
| EP3242932A1 (en) | 2017-11-15 |
| CA2973119A1 (en) | 2016-07-14 |
| AU2015376561A1 (en) | 2017-07-27 |
| JP2018501820A (ja) | 2018-01-25 |
| US10273461B2 (en) | 2019-04-30 |
| HK1246824A1 (zh) | 2018-09-14 |
| JP6670322B2 (ja) | 2020-03-18 |
| WO2016109947A1 (en) | 2016-07-14 |
| TWI616528B (zh) | 2018-03-01 |
| CN107208060A (zh) | 2017-09-26 |
| TW201625786A (zh) | 2016-07-16 |
| CN107208060B (zh) | 2021-06-18 |
| US20180023058A1 (en) | 2018-01-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Yu et al. | Exosomes derived from microRNA-199a-overexpressing mesenchymal stem cells inhibit glioma progression by down-regulating AGAP2 | |
| Roelandt et al. | Human pluripotent stem cell-derived hepatocytes support complete replication of hepatitis C virus | |
| Hassan et al. | Hepatitis C virus core protein triggers hepatic angiogenesis by a mechanism including multiple pathways | |
| Li et al. | Mesenchymal stem cells ameliorate hepatic ischemia/reperfusion injury via inhibition of neutrophil recruitment | |
| Wang et al. | TIM-1 promotes hepatitis C virus cell attachment and infection | |
| CN106795489A (zh) | 用于生产成人肝脏祖细胞的方法 | |
| Yuan et al. | A chimeric humanized mouse model by engrafting the human induced pluripotent stem cell-derived hepatocyte-like cell for the chronic hepatitis B virus infection | |
| Ranjan et al. | Glycosylation of the laminin receptor (α3β1) regulates its association with tetraspanin CD151: Impact on cell spreading, motility, degradation and invasion of basement membrane by tumor cells | |
| Azandeh et al. | Improvement of mesenchymal stem cell differentiation into the endoderm lineage by four step sequential method in biocompatible biomaterial | |
| Parent et al. | An immortalized human liver endothelial sinusoidal cell line for the study of the pathobiology of the liver endothelium | |
| KR20170101998A (ko) | 혈청 유래 c형 간염 바이러스의 증식을 위해 이용되는 인간 지방 유래 줄기세포 및 그의 용도 | |
| Banaudha et al. | Primary hepatocyte culture supports hepatitis C virus replication: a model for infection‐associated hepatocarcinogenesis | |
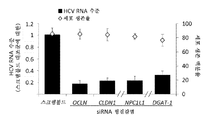
| KR102089477B1 (ko) | 1차 인간 간세포의 표현형을 갖는 세포를 생성하기 위한 방법 및 조성물 | |
| Lu et al. | Tree shrew bone marrow–derived mesenchymal stem cells express CD81, OCLN, and miR‐122, facilitating the entire hepatitis C virus life cycle | |
| Huang et al. | miR-96 regulates liver tumor-initiating cells expansion by targeting TP53INP1 and predicts Sorafenib resistance | |
| CN106148337A (zh) | 长非编码rna ay927503及其用途 | |
| KR101974509B1 (ko) | 보체 단백질의 간암 진단 용도 | |
| Suetsugu et al. | Differentiation of mouse hepatic progenitor cells induced by hepatocyte nuclear factor-4 and cell transplantation in mice with liver fibrosis | |
| Li et al. | Mouse mesenchymal stem cells from bone marrow differentiate into smooth muscle cells by induction of plaque-derived smooth muscle cells | |
| WO2023180122A1 (en) | Use of human allogenic liver-derived progenitor cells for treating and/or preventing cellular senescence | |
| Tsang et al. | Development of threedimensional human intestinal organoids as a physiologically relevant model for characterizing the viral replication kinetics and antiviral susceptibility of enteroviruses. Biomedicines, 2021, 9 (1), 88 | |
| JP5966172B2 (ja) | 培養細胞、及び、細胞系構築方法 | |
| Yuan et al. | Bone marrow stromal cells induce an ALDH+ stem cell-like phenotype in AML cells through TGF-β-p38-ALDH2 pathway | |
| KR20240085728A (ko) | 근육줄기세포의 분화능 예측을 위한 cd56의 용도 | |
| Galsinh | The role of stromal cells in Hepatitis C virus infection |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20170807 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |